Abstract
Background: While passive immunotherapy has been considered beneficial for patients with severe respiratory viral infections, the treatment of COVID-19 cases with convalescent plasma produced mixed results. Thus, there is a lack of certainty and consensus regarding its effectiveness. This meta-analysis aims to assess the role of convalescent plasma treatment on the clinical outcomes of COVID-19 patients enrolled in randomized controlled trials (RCTs). Methods: A systematic search was conducted in the PubMed database (end-of-search: 29 December 2022) for RCTs on convalescent plasma therapy compared to supportive care\standard of care. Pooled relative risk (RR) and 95% confidence intervals were calculated with random-effects models. Subgroup and meta-regression analyses were also performed, in order to address heterogeneity and examine any potential association between the factors that varied, and the outcomes reported. The present meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Results: A total of 34 studies were included in the meta-analysis. Per overall analysis, convalescent plasma treatment was not associated with lower 28-day mortality [RR = 0.98, 95% CI (0.91, 1.06)] or improved 28-day secondary outcomes, such as hospital discharge [RR = 1.00, 95% CI (0.97, 1.03)], ICU-related or score-related outcomes, with effect estimates of RR = 1.00, 95% CI (0.98, 1.05) and RR = 1.06, 95% CI (0.95, 1.17), respectively. However, COVID-19 outpatients treated with convalescent plasma had a 26% less risk of requiring hospital care, when compared to those treated with the standard of care [RR = 0.74, 95% CI (0.56, 0.99)]. Regarding subgroup analyses, COVID-19 patients treated with convalescent plasma had an 8% lower risk of ICU-related disease progression when compared to those treated with the standard of care (with or without placebo or standard plasma infusions) [RR = 0.92, 95% CI (0.85, 0.99)] based on reported outcomes from RCTs carried out in Europe. Finally, convalescent plasma treatment was not associated with improved survival or clinical outcomes in the 14-day subgroup analyses. Conclusions: Outpatients with COVID-19 treated with convalescent plasma had a statistically significantly lower risk of requiring hospital care when compared to those treated with placebo or the standard of care. However, convalescent plasma treatment was not statistically associated with prolonged survival or improved clinical outcomes when compared to placebo or the standard of care, per overall analysis in hospitalized populations. This hints at potential benefits, when used early, to prevent progression to severe disease. Finally, convalescent plasma was significantly associated with better ICU-related outcomes in trials carried out in Europe. Well-designed prospective studies could clarify its potential benefit for specific subpopulations in the post-pandemic era.
Keywords: COVID-19, convalescent plasma, meta-analysis, randomized controlled trials, mortality, intensive care unit
1. Introduction
At the end of 2019, a surge of pneumonia cases in Wuhan, a city in the Hubei Province of China, led to the identification of a novel coronavirus as the cause. Its rapid spread resulted in an epidemic throughout China, followed by an increasing number of cases around the world. On 11 March 2020, the World Health Organization declared the novel coronavirus outbreak a pandemic. The disease associated with it was designated as COVID-19, which stands for coronavirus disease 2019, and the virus that caused it was designated as Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) [1].
As of the end of 2022, COVID-19 disease management involves mostly supportive care, symptomatic treatment and prevention by vaccination. There have been only a few drugs or treatments proven to be effective specifically against the virus and the illness it causes, such as nirmatrelvir\ritonavir for high-risk patients.
Convalescent plasma, having been used to treat viral outbreaks of novel infectious diseases affecting the respiratory system in the past [2,3], was an early candidate [4,5]. The idea behind it is to transfuse blood plasma from a person who has recovered from a specific illness to someone who currently has the same illness in order to provide passive immunity and boost their fight against the pathogen, since such plasma contains antibodies to it [6,7].
With the potential for convalescent plasma to be beneficial, there was an urgency for clinical trials. The FDA provided emergency use authorization for its use and the WHO reinforced clinical trials to continue enrolment. Later updates included revisions on the matter, such as the focus and authorization being shifted to immunosuppressed patients or outpatients (FDA) or severe and high-risk patients in general (WHO) [8]. Moreover, the most up-to-date emergency authorization letter by the FDA states that convalescent plasma units used should be “high-titer”, based on studies showing the superiority of high-titer convalescent plasma in terms of preventing severe COVID-19-related outcomes [9].
Because studies and reviews yielded conflicting results, there has been a persistent lack of certainty and consensus regarding its efficacy [10,11]. Therefore, we conducted a meta-analysis, focusing strictly on RCTs, to assess the effect of convalescent plasma treatment on the clinical outcomes of patients with COVID-19.
2. Materials and Methods
2.1. Search Strategy and Eligibility of Studies
The present meta-analysis was performed following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [12]. The study protocol was discussed and agreed upon in advance by all authors.
A systematic search was conducted in the PubMed database, using the following algorithm:
(COVID-19 OR SARS-CoV-2 OR “novel coronavirus”) AND (convalescent OR convalescence) AND (plasma OR serum).
Eligible articles included randomized clinical trials on convalescent plasma treatment vs. supportive care or standard of care controls, with or without placebo. Case-control, cohort and cross-sectional studies, case series and case reports, reviews, in vitro and animal studies were not included in this meta-analysis. The selection of studies was conducted initially by two co-authors (CF and ANS) by independent work and any disagreements were resolved following consultation with a senior author (INS or TNS) and team consensus.
2.2. Data Abstraction and Effect Estimates
The data abstraction encompassed: general information (first author’s name, publication year, PubMed and CT database ID), study characteristics (time period, follow-up period, geographic region, multicenter status, control type, participant numbers, percentage of males, age), intervention characteristics (time to intervention from symptom onset and total CP dose) and outcomes (mortality and clinical outcomes with reported effect estimates or fourfolds with plain data, adjustment details).
If one of the above was not found in the main article, the Supplementary Material was thoroughly screened. There was no shortage of required data for the purposes of the meta-analysis. Data were independently extracted, analyzed and recorded in separate data extraction sheets by two authors (CF and KS). The finalized data form was reached after consultation with a senior author (TNS) and team consensus.
Extracted effect estimates included relative risks alongside their 95% Cis (per outcome) or any other form that could be mathematically transformed or translated to relative risk. Mortality was extracted as a primary outcome for our work and hospitalization, and hospital discharge, ICU-related outcomes and score-related outcomes were secondary outcomes.
As far as score-related outcomes are concerned, all of them were based on or using variations of the 9-point WHO score for COVID-19. This is defined as: 0: no clinical or virological evidence of infection; 1: ambulatory, no activity limitation; 2: ambulatory, activity limitation; 3: hospitalized, no oxygen therapy; 4: hospitalized, oxygen mask or nasal prongs; 5: hospitalized, noninvasive mechanical ventilation (NIMV) or high-flow nasal cannula (HFNC); 6: hospitalized, intubation and invasive mechanical ventilation (IMV); 7: hospitalized, IMV + additional support such as pressors or extracardiac membranous oxygenation (ECMO); 8: death.
Finally, a titer subgroup analysis was carried out, between studies that fulfilled the latest EUA/FDA cut-offs for high-titer plasma units versus the rest. This is defined as a neutralizing antibody titer of ≥250 in the Broad Institute’s neutralizing antibody assay or an S/C cutoff of ≥12 in the Ortho VITROS IgG assay.
In case the aforementioned information was not available, crude effect estimates and 95% CIs were calculated by means of fourfolds from plain data extracted from the articles.
2.3. Statistical Analyses
Statistical analyses included pooling of studies as well as post hoc meta-regression. Random-effects models were appropriately used to calculate the pooled effect estimates (relative risks). The convalescent plasma treatment arms were compared to the control arms. Between-study heterogeneity was assessed by Q-test and I2 estimations. Subgroup analyses were performed based on adjustment, multicenter status, blinding status and the geographic region of each study.
The post hoc meta-regression analysis was performed for subgroups with a total of 10 or more data entries for the variables to be analyzed. The aim was to assess whether gender, age, time from symptom onset to intervention or total convalescent plasma dose modified the association between convalescent plasma transfusion and each reported outcome.
All statistical analyses were performed using STATA/SE version 13 (Stata Corp, College Station, TX, USA).
2.4. Assessment of Study Quality and Risk of Bias
All records included randomized clinical trials, either blinded or open label. Risk was assessed with the implementation of the RoB:2 algorithm by Cochrane to our analysis tools [11]. Specifically, two authors (KS and ANS) carried out the assessment procedure independently, and upon inspection of the results by a third author (CF), consensus was met.
Publication bias was evaluated in the analyses that included 10 or more study arms [13]. For this purpose, Egger’s statistical test (statistical significance p < 0.1) [14,15] was implemented as well as the funnel plot inspection. The evaluation of publication bias was performed using STATA/SE version 13 (Stata Corp, College Station, TX, USA).
3. Results
3.1. Description of Eligible Studies
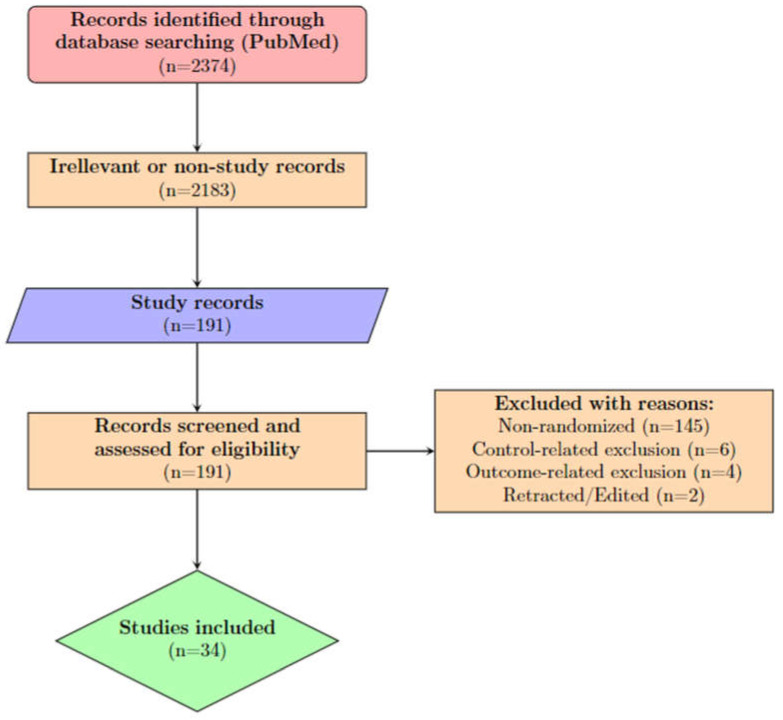
A total of 2374 records were identified from PubMed using the search algorithm (Section 2.1) and were assessed for eligibility. The flowchart (Figure 1) portrays the successive steps in the selection of eligible studies.
Figure 1.
Study selection flowchart.
For the 28-day main cohort, 34 randomized controlled trials were included [16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49]. For the 14-day secondary cohort, 10 articles on randomized controlled trials provided the necessary data. All studies had convalescent plasma therapy arms vs. standard of care or supportive care arms, with some including standard plasma, non-convalescent plasma or fresh-frozen plasma to the control arms.
From the 28-day cohort studies, all of them reported mortality figures, except one (Alemany, 2022) [17]. Regarding secondary clinical outcomes, hospital discharge was reported on nine records, ICU-related outcomes were reported on twenty-one records, hospitalization was a reported outcome in six studies and score-related outcomes (WHO score for COVID-19) in six studies.
Table 1 and Table 2 present the characteristics of the included studies regarding study design, patient and disease characteristics and interventions.
Table 1.
General characteristics of the included studies.
| Author and Year | Setting | Geographic Region | Multicenter | Blinded | Control |
|---|---|---|---|---|---|
| Abani (2021) | Hospitalized | More than one area | Yes | No | SoC |
| Agarwal (2020) | Hospitalized | India | Yes | No | SoC |
| Alemany (2022) | Outpatients | Europe | Yes | Yes | Placebo |
| AlQahtani (2021) | Hospitalized | Middle East | No | No | SoC |
| Avendaño-Solá (2021) | Hospitalized | Europe | Yes | No | SoC |
| Bajpai (2020) | Hospitalized | India | No | No | SoC |
| Bajpai (2022) | Hospitalized | India | Yes | No | SoC |
| Baldeón (2022) | Hospitalized | Latin America | Yes | Yes | Placebo |
| Bar (2021) | Hospitalized | USA | No | No | SoC |
| Bégin (2021) | Hospitalized | More than one area | Yes | No | SoC |
| Bennett-Guerrero (2021) | Hospitalized | USA | No | Yes | Placebo |
| Dekinger (2022) | Hospitalized | Europe | Yes | No | SoC |
| Devos (2022) | Hospitalized | Europe | Yes | No | SoC |
| Estcourt (2021) | Hospitalized | More than one area | Yes | No | SoC |
| Gharbharan (2021) | Hospitalized | Europe | Yes | No | SoC |
| Gharbharan (2022) | Outpatients | Europe | Yes | Yes | Placebo |
| Holm (2021) | Hospitalized | Europe | No | No | SoC |
| Kirenga (2021) | Mixed | Africa | No | No | SoC |
| Korley (2021) | Outpatients | USA | Yes | Yes | SoC |
| Li (2020) | Hospitalized | East Asia | Yes | No | SoC |
| Libster (2021) | Outpatients | Latin America | Yes | Yes | Placebo |
| Manzini (2022) | Hospitalized | Europe | No | Yes | SoC |
| Menichetti (2021) | Hospitalized | Europe | Yes | No | SoC |
| O’Donnell (2021) | Hospitalized | More than one area | Yes | Yes | Placebo |
| Ortigoza (2022) | Hospitalized | USA | Yes | Yes | Placebo |
| Ray (2022) | Hospitalized | India | No | No | SoC |
| Rojas (2022) | Hospitalized | Latin America | Yes | Yes | SoC |
| Santis (2022) | Hospitalized | Latin America | Yes | No | SoC |
| Sekine (2022) | Hospitalized | Latin America | No | Yes | SoC |
| Self (2022) | Hospitalized | USA | Yes | Yes | Placebo |
| Simonovich (2021) | Hospitalized | Latin America | No | Yes | Placebo |
| Sullivan (2022) | Outpatients | USA | Yes | Yes | Placebo |
| Thorlacius-Ussing (2022) | Hospitalized | Europe | Yes | No | Placebo |
| van de Berg (2022) | Hospitalized | Africa | No | Yes | Placebo |
SoC: standard of care; placebo control includes SoC.
Table 2.
Intervention characteristics of the included studies.
| Author and Year | CP (n) | Control (n) | Male % | Age (μ ± σ) |
Time from Symptom Onset to Intervention (μ ± σ) |
CP Dose (mL) |
|---|---|---|---|---|---|---|
| Abani (2021) | 5795 | 5763 | 64% | 63.50 ± 14.70 | 9.00 ± 4.45 | 550 |
| Agarwal (2020) | 235 | 229 | 76% | 51.13 ± 19.53 | 8.35 ± 3.73 | 400 |
| Alemany (2022) | 188 | 188 | 54% | 56.70 ± 7.44 | 4.40 ± 1.40 | 275 |
| AlQahtani (2021) | 20 | 20 | 80% | 51.65 ± 19.45 | 10.00 | 400 |
| Avendaño-Solá (2021) | 179 | 171 | 65% | 63.00 ± 15.30 | 5.65 ± 2.23 | 275 |
| Bajpai (2020) | 14 | 15 | 73% | 48.20 ± 9.80 | 3.00 | 500 |
| Bajpai (2022) | 200 | 200 | 67% | 55.52 ± 1.17 | - | 500 |
| Baldeón (2022) | 63 | 95 | 68% | 74.34 ± 18.39 | 10.60 ± 4.90 | - |
| Bar (2021) | 40 | 39 | 46% | - | 7.71 ± 4.53 | - |
| Bégin (2021) | 625 | 313 | 59% | 67.50 ± 15.60 | 7.90 ± 3.70 | 500 |
| Bennett-Guerrero (2021) | 59 | 15 | 60% | 65.70 ± 23.50 | 11.12 ± 9.12 | 480 |
| Denkinger (2022) | 68 | 66 | 68% | 68.50 ± 11.30 | 7.00 ± 4.50 | 575 |
| Devos (2022) | 320 | 163 | 69% | 62.00 ± 14.00 | 7.00 ± 4.46 | 450 |
| Estcourt (2021) | 1078 | 909 | 68% | 60.77 ± 18.38 | - | 550 |
| Gharbharan (2021) | 43 | 43 | 72% | 64.40 ± 13.45 | 10.35 ± 6.72 | 300 |
| Gharbharan (2022) | 207 | 209 | 78% | 60.00 ± 7.44 | 5.00 ± 1.49 | 400 |
| Holm (2021) | 17 | 14 | 61% | 69.95 ± 40.64 | 7.00 ± 3.23 | 675 |
| Kirenga (2021) | 69 | 67 | 71% | 50.18 ± 17.61 | 6.30 ± 3.00 | - |
| Korley (2021) | 257 | 254 | 46% | 51.90 ± 16.35 | 3.65 ± 2.24 | 250 |
| Li (2020) | 52 | 51 | 58% | 70.00 ± 12.03 | 29.65 ± 14.29 | - |
| Libster (2021) | 80 | 80 | 38% | 77.20 ± 8.60 | 1.65 ± 0.58 | 250 |
| Manzini (2022) | 60 | 60 | 72% | 65.48 ± 11.96 | 8.35 ± 5.23 | 600 |
| Menichetti (2021) | 232 | 241 | 64% | 64.00 ± 14.87 | 7.21 ± 2.98 | 400 |
| O’Donnell (2021) | 150 | 73 | 66% | 60.30 ± 17.91 | 10 ± 4.49 | - |
| Ortigoza (2022) | 468 | 473 | 59% | 62.65 ± 15.59 | 6.65 ± 3.71 | 250 |
| Ray (2022) | 40 | 40 | 71% | - | 4.20 ± 2.21 | 400 |
| Rojas (2022) | 46 | 45 | 70% | 51.76 ± 18.68 | 10.65 ± 2.96 | 500 |
| Santis (2022) | 36 | 71 | 72% | 56.00 ± 16.16 | 9.00 ± 1.50 | 1800 |
| Sekine (2022) | 80 | 80 | 41% | 58.74 ± 14.96 | 10.00 ± 3.00 | 300 |
| Self (2022) | 487 | 473 | 57% | 59.65 ± 15.59 | 7.65 ± 3.72 | 300 |
| Simonovich (2021) | 228 | 105 | 67% | 62.00 ± 14.89 | 7.65 ± 3.72 | 500 |
| Sullivan (2022) | 592 | 589 | 57% | 43.35 ± 23.12 | 5.65 ± 2.23 | 250 |
| Thorlacius-Ussing (2022) | 98 | 46 | 72% | 65.00 ± 14.98 | 10.65 ± 3.76 | 600 |
| van de Berg (2022) | 52 | 51 | 41% | 77.20 ± 8.60 | 8.65 ± 3.76 | 250 |
Missing values were not reported either in the article or in the supplementary material. CP dose is the total convalescent plasma transfused.
3.2. Meta-Analysis
3.2.1. 28-Day Results
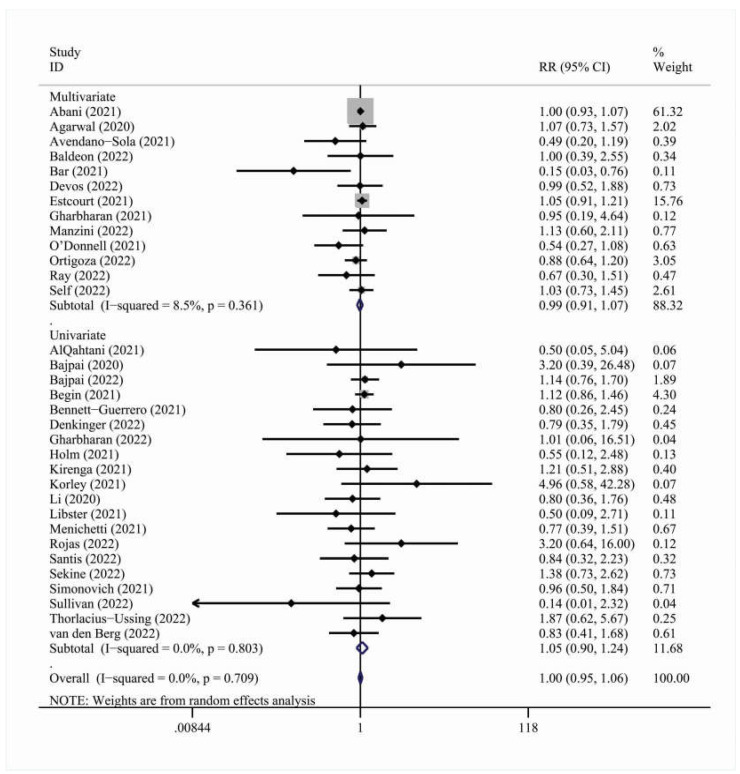
In total, 34 studies were included in the overall meta-analysis for the 28-day cohort [13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46]. The effect outcome for 28-day mortality was not statistically significant [RR = 1.00, 95% C.I. (0.95, 1.06)] (Figure 2). There were no statistically significant results in the adjustment, multicenter status, blinding status and geographic region subgroup analyses (Table 3).
Figure 2.
Forest plot describing the association between convalescent plasma treatment and 28-day mortality. Apart from the overall analysis, the subanalysis on adjustment type is presented.
Table 3.
Results of the meta-analyses examining the association between convalescent plasma therapy and mortality (28-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 33 | 0.98 (0.91, 1.06) | 0.0%, 0.709 |
| Subgroups by adjustment | |||
| Multivariate | 13 | 0.99 (0.91, 1.07) | 8.5%, 0.361 |
| Univariate | 20 | 1.05 (0.95, 1.06) | 0.0%, 0.803 |
| Subgroups by multicenter status | |||
| Multicenter | 22 | 1.00 (0.95, 1.06) | 0.0%, 0.703 |
| Single-center | 11 | 0.95 (0.74, 1.24) | 0.0, 0.451 |
| Subgroups by blinding status | |||
| Blinded | 15 | 0.97 (0.82, 1.15) | 0.0%, 0.524 |
| Open label | 18 | 1.01 (0.95, 1.07) | 0.0%, 0.667 |
| Subgroups by geographic region | |||
| Africa | 2 | 0.96 (0.56, 1.67) | 0.0%, 0.509 |
| East Asia | 1 | 0.80 (0.36, 1.76) | Not calculable |
| Europe | 9 | 0.89 (0.67, 1.19) | 0.0%, 0.778 |
| India | 4 | 1.06 (0.82, 1.38) | 0.0%, 0.493 |
| Latin America | 6 | 1.10 (0.77, 1.57) | 0.0%, 0.623 |
| Middle East | 1 | 0.50 (0.05, 5.04) | Not calculable |
| USA | 6 | 0.85 (0.55, 1.31) | 47.1%, 0.092 |
| More than one area | 4 | 1.02 (0.93, 1.12) | 26.8%, 0.251 |
Highlighted rows denote statistically significant associations.
A meta-analysis for the secondary clinical outcomes showed no statistically significant association between convalescent plasma therapy and hospital discharge [RR = 0.99, 95% C.I. (0.96, 1.03)] or score-related outcomes [RR = 1.06, 95% C.I. (0.97, 1.16)] (Table 4 and Table 5). The ICU-related outcomes analysis yielded no statistically significant overall result [RR = 0.98, 95% C.I. (0.93, 1.02)] as well (Table 6).
Table 4.
Results of the meta-analyses examining the association between convalescent plasma therapy and hospital discharge (28-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 9 | 0.99 (0.96, 1.03) | 0.0%, 0.955 |
| Subgroups by adjustment | |||
| Multivariate | 2 | 0.98 (0.94, 1.03) | 0.0%, 0.455 |
| Univariate | 7 | 1.01 (0.96, 1.05) | 0.0%, 0.949 |
| Subgroups by multicenter status | |||
| Multicenter | 7 | 1.00 (0.96, 1.03) | 0.0%, 0.859 |
| Single-center | 2 | 0.95 (0.74, 1.24) | 0.0, 0.451 |
| Subgroups by blinding status | |||
| Blinded | 15 | 0.97 (0.82, 1.15) | 0.0%, 0.524 |
| Open label | 18 | 0.98 (0.87, 1.11) | 0.0%, 1.000 |
| Subgroups by geographic region | |||
| Africa | 1 | 0.98 (0.72, 1.34) | Not calculable |
| Europe | 1 | 1.06 (0.87, 1.30) | Not calculable |
| India | 1 | 1.03 (0.88, 1.21) | Not calculable |
| Latin America | 2 | 1.00 (0.92, 1.09) | 0.0%, 0.652 |
| USA | 2 | 0.99 (0.94, 1.06) | 0.0%, 0.529 |
| More than one area | 2 | 1.00 (0.92 1.08) | 21.5%, 0.259 |
Highlighted rows denote statistically significant associations.
Table 5.
Results of the meta-analyses examining the association between convalescent plasma therapy and score-related outcomes (28-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 7 | 1.06 (0.97, 1.16) | 17.2%, 0.299 |
| Subgroups by adjustment | |||
| Multivariate | 2 | 1.25 (0.87, 1.78) | 61.0%, 0.110 |
| Univariate | 5 | 1.01 (0.94, 1.09) | 0.0%, 0.601 |
| Subgroups by multicenter status | |||
| Multicenter | 4 | 1.15 (1.02, 1.29) | 0.0%, 0.451 |
| Single-center | 3 | 0.99 (0.91, 1.07) | 0.0%, 0.854 |
| Subgroups by blinding status | |||
| Blinded | 4 | 1.12 (0.98, 1.27) | 0.0%, 0.394 |
| Open label | 3 | 1.03 (0.92, 1.17) | 21.5%, 0.280 |
| Subgroups by geographic region | |||
| Africa | 2 | 0.98 (0.90, 1.07) | 0.0%, 0.749 |
| East Asia | 1 | 1.20 (0.82, 1.75) | Not calculable |
| Europe | 1 | 1.16 (0.91, 1.47) | Not calculable |
| Latin America | 1 | 1.60 (1.03, 2.49) | Not calculable |
| USA | 1 | 1.33 (0.37, 4.77) | Not calculable |
| More than one area | 1 | 1.09 (0.93, 1.27) | Not calculable |
Highlighted rows denote statistically significant associations.
Table 6.
Results of the meta-analyses examining the association between convalescent plasma therapy and ICU-related outcomes (28-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 20 | 0.98 (0.93, 1.02) | 0.0%, 0.542 |
| Subgroups by adjustment | |||
| Multivariate | 3 | 1.00 (0.86, 1.15) | 70.7%, 0.033 |
| Univariate | 17 | 0.98 (0.93, 1.02) | 0.0%, 0.542 |
| Subgroups by multicenter status | |||
| Multicenter | 14 | 0.98 (0.93, 1.04) | 8.0%, 0.365 |
| Single-center | 6 | 0.84 (0.57, 1.24) | 0.0%, 0.703 |
| Subgroups by blinding status | |||
| Blinded | 10 | 0.96 (0.71, 1.31) | 8.0%, 0.807 |
| Open label | 10 | 0.99 (0.92, 1.06) | 27.4%, 0.192 |
| Subgroups by geographic region | |||
| Africa | 1 | 0.33 (0.04, 2.88) | Not calculable |
| Europe | 7 | 0.92 (0.85, 0.99) | 0.0%, 0.897 |
| India | 1 | 0.88 (0.63, 1.23) | Not calculable |
| Latin America | 3 | 0.77 (0.46, 1.29) | 0.0%, 0.529 |
| Middle East | 1 | 0.67 (0.22, 2.03) | Not calculable |
| USA | 3 | 0.84 (0.43, 1.64) | 0.0%, 0.460 |
| More than one area | 4 | 1.05 (0.96, 1.15) | 32.8%, 0.215 |
| Subgroups by ICU-related outcome | |||
| ICU admission | 4 | 0.97 (0.74, 1.26) | 0.0%, 0.501 |
| IMV or ECMO or death | 1 | 1.10 (0.98, 1.24) | Not calculable |
| Intubation or death | 1 | 1.16 (0.94, 1.43) | Not calculable |
| IMV | 1 | 0.33 (0.04, 2.88) | Not calculable |
| Invasive ventilatory support | 1 | 0.88 (0.42, 1.83) | Not calculable |
| MV | 1 | 0.50 (0.09, 2.74) | Not calculable |
| MV or ICU admission | 1 | 0.75 (0.17, 3.31) | Not calculable |
| MV or death | 1 | 1.10 (0.62, 1.97) | Not calculable |
| MV or ECMO | 1 | 0.49 (0.15, 1.58) | Not calculable |
| NIV or high flow O2 or IMV or ECMO or death | 1 | 0.91 (0.84, 0.99) | Not calculable |
| PaO2/FiO2 of <150 mm Hg or death | 1 | 0.91 (0.67, 1.23) | Not calculable |
| Ventilation treatment | 6 | 0.98 (0.90, 1.06) | 0.0%, 0.784 |
Highlighted rows denote statistically significant associations.
Furthermore, a subgroup analysis was conducted according to the levels of anti-SARS-CoV-2 antibodies in the CP. Studies were grouped as “high-titer” or “non-high-titer” as per the latest EUA/FDA guideline cut-offs. The subgroup analysis for the titer level did not reveal any statistically significant associations (Supplementary Figures S5, S10, S14, S20 and S25).
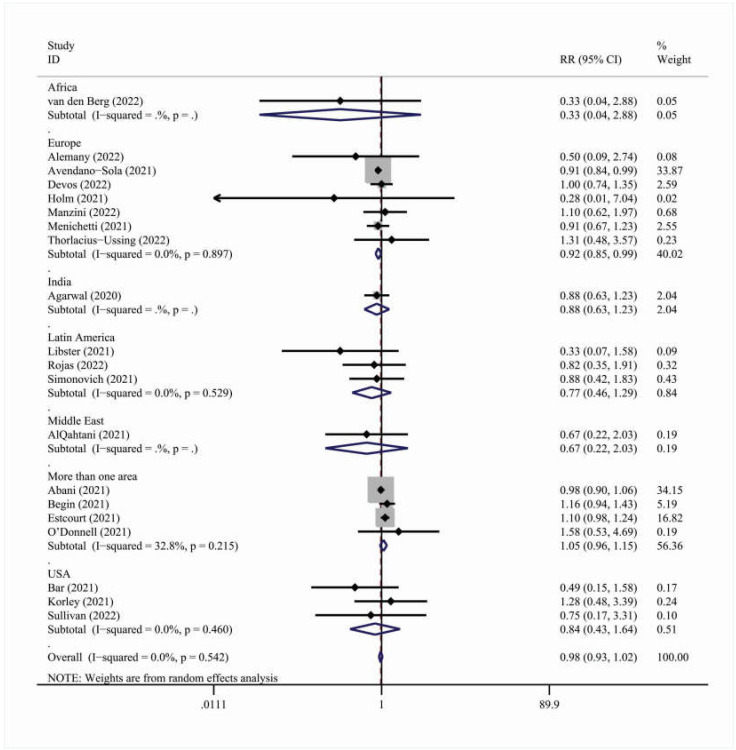
However, when analyzing by geographic region, studies carried out in Europe [RR = 0.92, 95% C.I. (0.85, 0.99)] showed a statistically significant association between convalescent plasma therapy and ICU-related outcomes (Table 6 and Figure 3).
Figure 3.
Forest plot describing the association between convalescent plasma treatment and 28-day ICU-related outcomes. Apart from the overall analysis, the subanalysis per geographic region is presented.
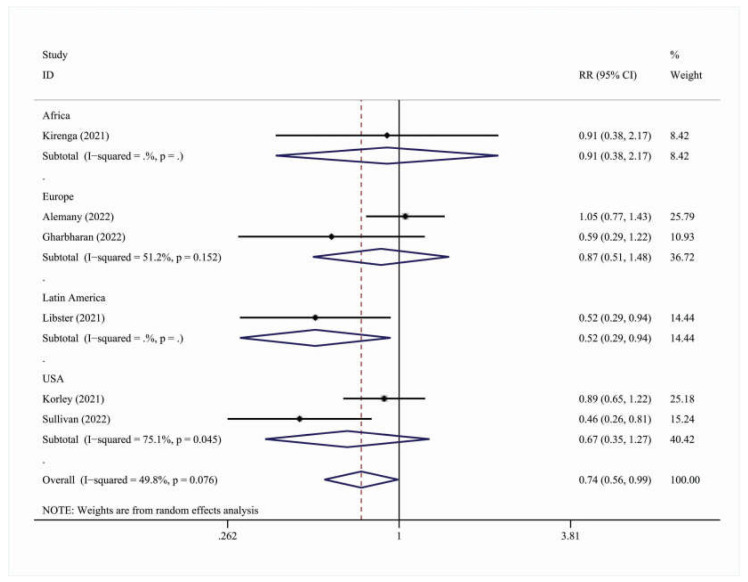
The subanalysis on hospitalization outcomes [RR = 0.74, 95% C.I. (0.56, 0.99)] was also statistically significant, showing that outpatients treated with convalescent plasma had a 26% less risk of needing hospital care than those treated with the standard of care (Table 7, Figure 4).
Table 7.
Subanalysis on hospitalization (28-day).
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 6 | 0.74 (0.56, 0.99) | 49.8%, 0.076 |
| Subgroups by adjustment | |||
| Multivariate | 0 | - | - |
| Univariate | 6 | 0.74 (0.56, 0.99) | 49.8%, 0.076 |
| Subgroups by multicenter status | |||
| Multicenter | 5 | 0.72 (0.52, 1.00) | 59.6%, 0.042 |
| Single-center | 1 | 0.91 (0.38, 2.17) | Not calculable |
| Subgroups by blinding status | |||
| Blinded | 5 | 0.72 (0.52, 1.00) | 59.6%, 0.042 |
| Open label | 1 | 0.91 (0.38, 2.17) | Not calculable |
| Subgroups by geographic region | |||
| Africa | 1 | 0.91 (0.38, 2.17) | Not calculable |
| Europe | 2 | 0.87 (0.51, 1.48) | 51.2%, 0.152 |
| Latin America | 1 | 0.52 (0.29, 0.94) | Not calculable |
| USA | 2 | 0.67 (0.35, 1.27) | 75.1%, 0.045 |
Table 7 Results of the meta-analyses examining the association between convalescent plasma therapy and hospitalization outcomes (28-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented. Highlighted rows denote statistically significant associations.
Figure 4.
Forest plot describing the association between convalescent plasma treatment and 28-day hospitalization outcomes. Apart from the overall analysis, the subanalysis on geographic region is presented.
3.2.2. 14-Day Results
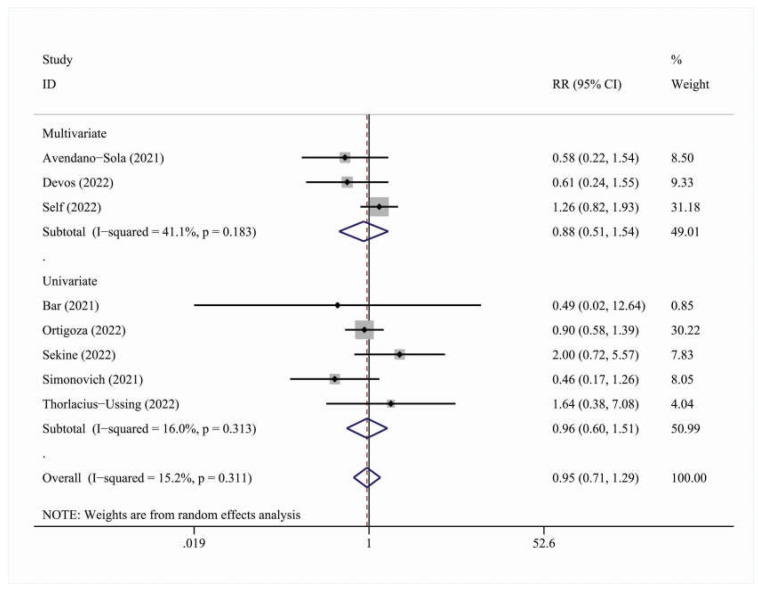
In total, 10 studies were included in the overall meta-analysis for the 14-day cohort. The effect outcome for 14-day mortality was not statistically significant [RR = 0.98, 95% C.I. (0.91, 1.06)] (Figure 5 and Table 8). There were no statistically significant results in the adjustment, multicenter status, blinding status or geographic region subgroups (Table 8).
Figure 5.
Forest plot describing the association between convalescent plasma treatment and 14-day mortality. Apart from the overall analysis, the subanalysis on adjustment type is presented.
Table 8.
Results of the meta-analyses examining the association between convalescent plasma therapy and overall mortality (14-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 8 | 0.95 (0.71, 1.29) | 15.2%, 0.311 |
| Subgroups by adjustment | |||
| Multivariate | 3 | 0.88 (0.51, 1.54) | 41.1%, 0.183 |
| Univariate | 5 | 0.96 (0.60, 1.51) | 6.0%, 0.313 |
| Subgroups by multicenter status | |||
| Multicenter | 5 | 0.98 (0.75, 1.30) | 1.4%, 0.398 |
| Single-center | 3 | 0.89 (0.28, 2.83) | 51.9%, 0.125 |
| Subgroups by blinding status | |||
| Blinded | 4 | 1.03 (0.68, 1.57) | 43.1%, 0.153 |
| Open label | 4 | 0.70 (0.38, 1.28) | 0.0%, 0.666 |
| Subgroups by geographic region | |||
| Europe | 3 | 0.71 (0.31, 1.31) | 0.0%, 0.467 |
| Latin America | 2 | 0.96 (0.23, 4.04) | 75.1%, 0.045 |
| USA | 3 | 1.06 (0.78, 1.44) | 0.0%, 0.500 |
Highlighted rows denote statistically significant associations.
A meta-analysis for the secondary clinical outcomes showed no statistically significant association between convalescent plasma therapy and hospital discharge [RR = 0.96, 95% C.I. (0.89, 1.03)] (Table 9). A subgroup analysis for the titer level was not statistically significant as well (Supplementary Figures S30 and S35).
Table 9.
Results of the meta-analyses examining the association between convalescent plasma therapy and hospital discharge (14-day); subgroup analyses by adjustment, multicenter status, blinding status and geographic region are presented.
| n | RR | Heterogeneity I2, p | |
|---|---|---|---|
| Overall analysis | 4 | 0.96 (0.89, 1.03) | 0.0%, 0.995 |
| Subgroups by adjustment | |||
| Multivariate | - | - | - |
| Univariate | 4 | 0.96 (0.89, 1.03) | 0.0%, 0.995 |
| Subgroups by multicenter status | |||
| Multicenter | 2 | 0.96 (0.88, 1.04) | 0.0%, 0.795 |
| Single-center | 2 | 0.96 (0.81, 1.14) | 0.0%, 0.964 |
| Subgroups by blinding status | |||
| Blinded | 3 | 0.96 (0.88, 1.04) | 0.0%, 0.999 |
| Open label | 1 | 0.93 (0.75, 1.16) | Not calculable |
| Subgroups by geographic region | |||
| Europe | 1 | 0.93 (0.75, 1.16) | Not calculable |
| Latin America | 2 | 0.96 (0.81, 1.14) | 0.0%, 0.964 |
| USA | 1 | 0.96 (0.87, 1.05) | Not calculable |
Highlighted rows denote statistically significant associations.
3.3. Meta-Regression Analysis
The post hoc meta-regression aimed to assess whether gender, age, time from symptom onset to intervention or total cp dose modified the association between convalescent plasma treatment and each reported outcome. This analysis yielded no statistically significant associations (Table 10 and Table 11). It was carried out only for the 28-day analysis cohort and specifically only for the overall mortality, hospital discharge and ICU-related outcomes, as other categories had less than 10 study arms.
Table 10.
Meta-regression on mortality (28-day). Results of meta-regression analysis examining the role of potential modifiers in the association between convalescent plasma treatment and 28-day mortality.
| Variables | Increment | n | Exponentiated Coefficient | p |
|---|---|---|---|---|
| Male% | 10% increase | 33 | 1.06 (0.93, 1.21) | 0.368 |
| Mean age | 10 y increase | 31 | 0.92 (0.76, 1.12) | 0.405 |
| Time from symptom onset to intervention | 1 day more | 31 | 1.00 (0.97, 1.04) | 0.945 |
| Total CP dose | 100 mL more | 27 | 1.01 (0.96, 1.07) | 0.691 |
Table 11.
Results of meta-regression analysis examining the role of potential modifiers in the association between convalescent plasma treatment and 28-day ICU-related outcomes.
| Variables | Increment | n | Exponentiated Coefficient | p |
|---|---|---|---|---|
| Male% | 10% increase | 20 | 1.02 (0.85, 1.23) | 0.789 |
| Mean age | 10 y increase | 19 | 1.07 (0.86, 1.35) | 0.514 |
| Time from symptom onset to intervention | 1 day more | 19 | 1.03 (0.99, 1.06) | 0.157 |
| Total CP dose | 100 mL more | 18 | 1.05 (1.00, 1.11) | 0.064 |
3.4. Quality Assessment and Risk of Bias
All included studies were randomized control trials, blinded or open label. For the evaluation of quality and risk of bias of each one, the RoB:2 tool by Cochrane was used [13]. Table 12 presents the risk of bias assessment for the included studies.
Table 12.
Risk of bias assessment based on the RoB:2 algorithm.
| Randomization Process | Deviations from Intended Interventions | Missing Outcome Data | Measurement of the Outcome | Selection of the Reported Result | Overall | |
|---|---|---|---|---|---|---|
| Abani (2021) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Agarwal (2020) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Alemany (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| AlQahtani (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Avendaño-Solá (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bajpai (2020) | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Bajpai (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Baldeón (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bar (2021) | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Bégin (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Bennett-Guerrero (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Denkinger (2022) | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Devos (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Estcourt (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Gharbharan (2021) | Low risk | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Gharbharan (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Holm (2021) | Some concerns | Some concerns | Low risk | Low risk | Low risk | Some concerns |
| Kirenga (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Korley (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Li (2020) | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Libster (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Manzini (2022) | Some concerns | Low risk | Low risk | Low risk | Low risk | Some concerns |
| Menichetti (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| O’Donnell (2021) | Low risk | Low risk | Low risk | Low risk | Some concerns | Some concerns |
| Ortigoza (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Ray (2022) | High risk | Some concerns | Low risk | Low risk | Low risk | High risk |
| Rojas (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Santis (2022) | Low risk | Some concerns | Some concerns | Low risk | Low risk | Some concerns |
| Sekine (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Self (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Simonovich (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Sullivan (2021) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| Thorlacius-Ussing (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
| van de Berg (2022) | Low risk | Low risk | Low risk | Low risk | Low risk | Low risk |
In total, 23/34 studies (67.7%) were assessed as having a low risk of bias, 10/34 studies (29.4%) raised some concerns and only one was deemed to have a high risk of bias [40]. More specifically:
Six studies (17.7%) raised some concerns on their randomization process, mostly due to lack of information on allocation concealment;
Five studies (14.7%) raised some concerns on whether there were deviations from the intended interventions;
Only one study raised concerns on potential selection of the reported result;
One study had a high risk of bias due to vital randomization process concerns.
3.5. Publication Bias
A publication bias assessment was performed on outcomes reported in 10 or more studies with the use of Egger’s test [14,15]. These were the 28-day mortality and 28-day ICU-related outcomes.
For the 28-day mortality analysis, for a total of 33 studies, the p-value for the bias coefficient generated by Egger’s regression test for small-study effects was p = 0.247 (Supplemental Figure S35). For the 28-day ICU-related outcomes, for a total of 20 studies, the aforementioned p-value was p = 0.337 (Supplemental Figure S36). In both cases, this means that there were no small-study effects, and thus no publication bias.
4. Discussion
The present meta-analysis, comprising data from 34 individual randomized controlled trials, found no statistically significant association between convalescent plasma treatment and 28-day or 14-day mortality, hospital discharge, hospitalization, ICU-related or score-related outcomes. When analyzing by subgroups, though, the European cohort for the ICU-related outcomes yielded a statistically significant result [RR = 0.92, 95% CI (0.85, 0.99)], showing that convalescent plasma treatment was beneficial in protecting patients from ICU-related disease progression. Specifically, patients treated with convalescent plasma had an 8% less risk of presenting an ICU-related outcome (such as the need for ventilation treatment, intubation, ECMO), when compared to those treated with the standard of care or supportive care (with or without placebo/standard plasma infusion). While this result is interesting and significant, it can largely be attributed to the contribution of the weight of the Avendaño-Solá (2021) study [17]. Moreover, convalescent plasma was found to be beneficial in protecting outpatients from hospitalization. After analyzing hospitalization outcomes, a statistically significant result [RR = 0.74, 95% C.I. (0.56, 0.99)] was yielded, meaning that outpatients treated with convalescent plasma had a 26% lower risk of needing to be hospitalized than those treated with the standard of care.
Carrying out a subgroup analysis for titer levels (high-titer vs. non-high titer) was challenging, as each study used different antibody measurements and cut-off levels for high-titer labeling. Moreover, achieving in-study heterogeneity among the titers of the plasma units administered was also significant. These led to a statistically nonsignificant and mostly inconclusive result. There was a scarcity of outcome data regarding secondary clinical outcomes, such as hospital discharge (9/34 studies), hospitalization (6/34 studies) and score-related outcomes (6/34 studies). The plasma titer between studies varied and so did COVID-19 disease severity at randomization and study size. Serostatus at the time of treatment was not possible to assess and analyze, as only a percentage of studies provided robust and uniform data for it. Furthermore, records were extracted solely from the PubMed database.
In addition, the RECOVERY trial (Abani, 2021) has raised some concerns during our risk of bias assessment and is worth mentioning, as its weight skewed the results. This is due to the fact that it failed to completely adhere to its design, as 9% of the patients did not receive the allocated intervention (plasma infusion). While this raises questions about the robustness of the results, the aforementioned population percentage was excluded from the comparison analysis between the convalescent plasma group and the control group.
Despite the aforementioned notable limitations, the present work possesses a plethora of important strengths. Overall heterogeneity was low and not significant both in the 28-day (I2 = 0.0%, p = 0.709) and 14-day (I2 = 15.2%, p = 0.311) cohorts. In the statistically significant ICU-related European subgroup, heterogeneity was also low and not significant (I2 = 0.0%, p = 0.897). Overall heterogeneity was 49.8% for the hospitalization outcomes subanalysis, but it was marginally not statistically significant (p = 0.076). While region, sex, age, time from symptom onset to intervention and total convalescent plasma dose can be considerable sources of heterogeneity, subgroup analyses and meta-regression showed no statistically significant association between them and treatment effectiveness. The extensive abstraction and analysis of separate and discrete clinical outcomes and thorough risk of bias assessment are also parts of this study’s strengths. Contrary to other meta-analyses [50,51,52], our work focuses strictly on randomized controlled trials, thus lying in the highest part of the hierarchy of evidence pyramid.
Moreover, screening was extensive and detailed, pairing information from each trial article and its official registry page. This led to avoiding errors such as misclassifying [50,51,52] the article record by Rasheed et al. [53] as an RCT, when it was a control-matched cohort study. Furthermore, thorough auditing led to excluding two trials, which were retracted/edited as far as their patient allocation method was concerned.
When comparing our work to others, the results for overall mortality (a commonly reported primary outcome) are similar. Axfors et al. conducted a systematic review and meta-analysis on 33 published and unpublished trial papers and showed a non-statistically significant association as well [54]. Other published meta-analyses were comprised of considerably fewer studies, such as the study by Piscova et al. [50] with five trials and six cohorts, the analysis by Snow et al. [51] with seventeen trials and the study by Janiaud et al. including ten trials [55]. The meta-analysis by Kloypan et al. [52] showed a statistically significant association between convalescent plasma therapy and overall mortality but it was subject to notable limitations. The primary outcome of all-cause mortality at any given time point included nonrandomized trials and observational studies, whereas the Rasheed trial was misclassified. Another difference lies in our secondary outcomes analysis, where the aforementioned systematic reviews and meta-analyses failed to yield statistically significant results. This can be attributed to the big pool of studies (and thus variety and data available), outcome assessment and categorization and extensive subgroup analyses.
Finally, subgroup analyses on immunocompromised patients were not feasible due to the scarcity of available data from randomized studies in the field. A recently published randomized controlled trial by Dekinger et al. [49] evaluated the role of convalescent plasma in a subgroup of 56 patients with hematological or solid cancer and severe COVID-19. The administration of convalescent plasma significantly improved survival and reduced the time to clinical improvement. Patients with cancer under active treatment present attenuated humoral responses to COVID-19 vaccination, and thus they are at high risk for severe SARS-CoV-2 infection [56,57,58,59]. Other trials on vulnerable populations for severe COVID-19-related outcomes showed signs of benefits with [35] or without statistically significant results [30,33]. A recent systematic review and meta-analysis including trials, cohort studies, case series and case reports found that convalescent plasma therapy was associated with a mortality benefit in patients who were immunocompromised and were diagnosed with COVID-19 [60,61].
5. Conclusions
Convalescent plasma treatment was not associated with a statistically significant reduced risk of overall 28-day or 14-mortality or any other clinical outcome. It was associated, though, with a statistically significant beneficial effect on 28-day ICU-related outcomes in the European study cohort and 28-day hospitalization. The aforementioned evidence hints against the use of convalescent plasma for the treatment of COVID-19 in the general population, but it highlights potential clinical benefits when studying subpopulations (e.g., European ICU cohorts, outpatients). As such, further study on specific subpopulations and outcomes could establish consensus on determining the clinical benefits of convalescent plasma therapy.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/v15030765/s1. Figure S1. 28-day mortality, by adjustment; Figure S2. 28-day mortality, by multicenter status; Figure S3. 28-day mortality, by blinding status; Figure S4. 28-day mortality, by geographic region; Figure S5. 28-day mortality, by titer; Figure S6. 28-day hospitalization, by adjustment; Figure S7. 28-day hospitalization, by multicenter status; Figure S8. 28-day hospitalization, by blinding status; Figure S9. 28-day hospitalization, by geographic region; Figure S10. 28-day hospitalization, by titer; Figure S11. 28-day hospital discharge, by adjustment; Figure S12. 28-day hospital discharge, by multicenter status; Figure S13. 28-day hospital discharge, by blinding status; Figure S14. 28-day hospital discharge, by geographic region; Figure S15. 28-day hospital discharge, by titer; Figure S16. 28-day ICU-related outcomes, by adjustment; Figure S17. 28-day ICU-related outcomes, by multicenter status; Figure S18. 28-day ICU-related outcomes, by adjustment; Figure S19. 28-day ICU-related outcomes, by geographic region; Figure S20. 28-day ICU-related outcomes, by ICU status; Figure S21. 28-day ICU-related outcomes, by titer; Figure S22. 28-day score-related outcomes, by adjustment; Figure S23. 28-day score-related outcomes, by multicenter status; Figure S24. 28-day score-related outcomes, by blinding status; Figure S25. 28-day score-related outcomes, by geographic region; Figure S26. 28-day score-related outcomes, by titer; Figure S27. 14-day mortality, by adjustment; Figure S28. 14-day mortality, by multicenter status; Figure S29. 14-day mortality, by blinding status; Figure S30. 14-day mortality, by geographic region; Figure S31. 14-day mortality, by titer; Figure S32. 14-day hospital discharge, by adjustment; Figure S33. 14-day hospital discharge, by multicenter status; Figure S34. 14-day hospital discharge, by blinding status; Figure S35. 14-day hospital discharge, by multicenter status; Figure S36. 14-day hospital discharge, by titer; Figure S37. Funnel plot, portraying publication bias for 28-day mortality; Figure S38. Funnel plot, portraying publication bias for 28-day ICU-related outcomes
Author Contributions
Conceptualization, T.P. and E.T.; methodology, T.N.S. and I.N.-S.; software, C.F. and T.N.S.; validation, T.N.S., G.D., M.G. and T.P.; formal analysis, C.F. and T.N.S.; investigation, C.F., I.N.-S., K.S. and A.N.-S.; data curation, C.F., K.S. and A.N.-S.; writing—original draft preparation, C.F. and I.N.-S.; writing—review and editing, K.S., A.N.-S., M.G., T.P., G.D., T.N.S. and E.T.; visualization, T.N.S.; supervision, T.N.S. and E.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data available upon reasonable request from the corresponding author.
Conflicts of Interest
The authors declare no relevant conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kenneth McIntosh COVID-19: Clinical Features. [(accessed on 30 December 2022)]. Available online: https://www.uptodate.com/contents/covid-19-clinical-features.
- 2.Mair-Jenkins J., Saavedra-Campos M., Baillie J.K., Cleary P., Khaw F.M., Lim W.S., Makki S., Rooney K.D., Nguyen-Van-Tam J.S., Beck C.R., et al. The Effectiveness of Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of Severe Acute Respiratory Infections of Viral Etiology: A Systematic Review and Exploratory Meta-analysis. J. Infect. Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.van Griensven J., De Weiggheleire A., Delamou A., Smith P.G., Edwards T., Vandekerckhove P., Bah E.I., Colebunders R., Herve I., Lazaygues C., et al. The Use of Ebola Convalescent Plasma to Treat Ebola Virus Disease in Resource-Constrained Settings: A Perspective from the Field. Clin. Infect. Dis. 2016;62:69–74. doi: 10.1093/cid/civ680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barone P., DeSimone R.A. Convalescent plasma to treat coronavirus disease 2019 (COVID-19): Considerations for clinical trial design. Transfusion. 2020;60:1123–1127. doi: 10.1111/trf.15843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rojas M., Rodríguez Y., Monsalve D.M., Acosta-Ampudia Y., Camacho B., Gallo J.E., Rojas-Villarraga A., Ramírez-Santana C., Díaz-Coronado J.C., Manrique R., et al. Convalescent plasma in COVID-19: Possible mechanisms of action. Autoimmun. Rev. 2020;19:102554. doi: 10.1016/j.autrev.2020.102554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Psaltopoulou T., Sergentanis T.N., Pappa V., Politou M., Terpos E., Tsiodras S., Pavlakis G.N., Dimopoulos M.A. The Emerging Role of Convalescent Plasma in the Treatment of COVID-19. HemaSphere. 2020;4:e409. doi: 10.1097/HS9.0000000000000409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosati M., Terpos E., Ntanasis-Stathopoulos I., Agarwal M., Bear J., Burns R., Hu X., Korompoki E., Donohue D., Venzon D.J., et al. Sequential Analysis of Binding and Neutralizing Antibody in COVID-19 Convalescent Patients at 14 Months After SARS-CoV-2 Infection. Front. Immunol. 2021;12:793953. doi: 10.3389/fimmu.2021.793953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.WHO . 7th Update of WHO’s Living Guidelines on COVID-19 Therapeutics. WHO; Geneva, Switzerland: 2022. [Google Scholar]
- 9.Pappa V., Bouchla A., Terpos E., Thomopoulos T.P., Rosati M., Stellas D., Antoniadou A., Mentis A., Papageorgiou S.G., Politou M., et al. A Phase II Study on the Use of Convalescent Plasma for the Treatment of Severe COVID-19—A Propensity Score-Matched Control Analysis. Microorganisms. 2021;9:806. doi: 10.3390/microorganisms9040806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estcourt L., Callum J. Convalescent Plasma for COVID-19—Making Sense of the Inconsistencies. N. Engl. J. Med. 2022;386:1753–1754. doi: 10.1056/NEJMe2204332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sullivan D.J., Gebo K.A., Hanley D.F. Correspondence: Convalescent Plasma for COVID-19—Making Sense of the Inconsistencies. N. Engl. J. Med. 2022;387:955–956. doi: 10.1056/NEJMc2208338. [DOI] [PubMed] [Google Scholar]
- 12.Higgins J.P., Thomas J., Chandler J., Cumpston M., Li T., Page M.J., Welch V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.3. Cochrane; Oxford, UK: 2022. [Google Scholar]
- 13.Sterne J.A., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 14.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne J.A.C., Egger M. Funnel plots for detecting bias in meta-analysis: Guidelines on choice of axis. J. Clin. Epidemiol. 2001;54:1046–1055. doi: 10.1016/S0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 16.RECOVERY Collaborative Group Convalescent plasma in patients admitted to hospital with COVID-19 (RECOVERY): A randomised controlled, open-label, platform trial. Lancet. 2021;397:2049–2059. doi: 10.1016/S0140-6736(21)00897-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agarwal A., Mukherjee A., Kumar G., Chatterjee P., Bhatnagar T., Malhotra P. Convalescent plasma in the management of moderate COVID-19 in adults in India: Open label phase II multicentre randomized controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alemany A., Millat-Martinez P., Corbacho-Monné M., Malchair P., Ouchi D., Ruiz-Comellas A., Ramírez-Morros A., Codina J.R., Simon R.A., Videla S., et al. High-titre methylene blue-treated convalescent plasma as an early treatment for outpatients with COVID-19: A randomised, placebo-controlled trial. Lancet. 2022;10:278–288. doi: 10.1016/S2213-2600(21)00545-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.AlQahtani M., Abdulrahman A., Almadani A., Alali S.Y., Al Zamrooni A.M., Hejab A.H., Conroy R.M., Wasif P., Otoom S., Atkin S.L., et al. Randomized controlled trial of convalescent plasma therapy against standard therapy in patients with severe COVID-19 disease. Sci. Rep. 2021;11:9927. doi: 10.1038/s41598-021-89444-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Avendaño-Solá C., Ramos-Martínez A., Muñez-Rubio E., Ruiz-Antorán B., de Molina R.M., Torres F., Fernández-Cruz A., Calderón-Parra J., Payares-Herrera C., de Santiago A.D., et al. A multicenter randomized open-label clinical trial for convalescent plasma in patients hospitalized with COVID-19 pneumonia. J. Clin. Investig. 2021;131:e152740. doi: 10.1172/JCI152740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bajpai M., Maheshwari A., Kumar S., Chhabra K., Kale P., Narayanan A., Gupta A., Gupta E., Trehanpati N., Agarwal R., et al. Comparison of safety and efficacy of convalescent plasma with fresh frozen plasma in severe COVID-19 patients. SciELO. 2020 doi: 10.1590/0001-3765202220210202. [DOI] [PubMed] [Google Scholar]
- 22.Bajpai M., Maheshwari A., Dogra V., Kumar S., Gupta E., Kale P., Saluja V., Thomas S.S., Trehanpati N., Bihari C., et al. Efficacy of convalescent plasma therapy in the patient with COVID-19: A randomised control trial (COPLA-II trial) BMJ Open. 2022;12:e055189. doi: 10.1136/bmjopen-2021-055189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baldeón M.E., Maldonado A., Ochoa-Andrade M., Largo C., Pesantez M., Herdoiza M., Granja G., Bonifaz M., Espejo H., Mora F., et al. Effect of convalescent plasma as complementary treatment in patients with moderate COVID-19 infection. Transfus. Med. 2022;32:153–161. doi: 10.1111/tme.12851. [DOI] [PubMed] [Google Scholar]
- 24.Bar K.J., Shaw P.A., Choi G.H., Aqui N., Fesnak A., Yang J.B., Soto-Calderon H., Grajales L., Starr J., Andronov M., et al. A randomized controlled study of convalescent plasma for individuals hospitalized with COVID-19 pneumonia. J. Clin. Investig. 2021;131:e155114. doi: 10.1172/JCI155114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bégin P., Callum J., Jamula E., Cook R., Heddle N.M., Tinmouth A., Zeller M.P., Beaudoin-Bussières G., Amorim L., Bazin R., et al. Convalescent plasma for hospitalized patients with COVID-19: An open-label, randomized controlled trial. Nat. Med. 2021;27:2012–2024. doi: 10.1038/s41591-021-01488-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bennett-Guerrero E., Romeiser J.L., Talbot L.R., Ahmed T., Mamone L.J., Singh S.M., Hearing J.C., Salman H., Holiprosad D.D., Freedenberg A.T., et al. Severe Acute Respiratory Syndrome Coronavirus 2 Convalescent Plasma Versus Standard Plasma in Coronavirus Disease 2019 Infected Hospitalized Patients in New York: A Double-Blind Randomized Trial. Crit. Care Med. 2021;49:1015–1025. doi: 10.1097/CCM.0000000000005066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devos T., Van Thillo Q., Compernolle V., Najdovski T., Romano M., Dauby N., Jadot L., Leys M., Maillart E., Loof S., et al. Early high antibody titre convalescent plasma for hospitalised COVID-19 patients: DAWn-plasma. Eur. Respir. J. 2022;59:2101724. doi: 10.1183/13993003.01724-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Writing Committee for the REMAP-CAP Investigators Effect of Convalescent Plasma on Organ Support-Free Days in Critically Ill Patients with COVID-19: A Randomized Clinical Trial. JAMA. 2021;326:1690–1702. doi: 10.1001/jama.2021.18178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gharbharan A., Jordans C.C.E., GeurtsvanKessel C., Hollander J.G.D., Karim F., Mollema F.P.N., Schukken J.E.S., Dofferhoff A., Ludwig I., Koster A., et al. Effects of potent neutralizing antibodies from convalescent plasma in patients hospitalized for severe SARS-CoV-2 infection. Nat. Commun. 2021;12:3189. doi: 10.1038/s41467-021-23469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gharbharan A., Jordans C., Zwaginga L., Papageorgiou G., van Geloven N., van Wijngaarden P., Hollander J.D., Karim F., van Leeuwen-Segarceanu E., Soetekouw R., et al. Outpatient convalescent plasma therapy for high-risk patients with early COVID-19: A randomized placebo-controlled trial. Clin. Microbiol. Infect. 2023;29:208–214. doi: 10.1016/j.cmi.2022.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Holm K., Lundgren M.N., Kjeldsen-Kragh J., Ljungquist O., Böttiger B., Wikén C., Öberg J., Fernström N., Rosendal E., Överby A.K., et al. Convalescence plasma treatment of COVID-19: Results from a prematurely terminated randomized controlled open-label study in Southern Sweden. BMC Res. Notes. 2021;14:440. doi: 10.1186/s13104-021-05847-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kirenga B., Byakika-Kibwika P., Muttamba W., Kayongo A., Loryndah N.O., Mugenyi L., Kiwanuka N., Lusiba J., Atukunda A., Mugume R., et al. Efficacy of convalescent plasma for treatment of COVID-19 in Uganda. BMJ Open Respir. Res. 2021;8:e001017. doi: 10.1136/bmjresp-2021-001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Korley F.K., Durkalski-Mauldin V., Yeatts S.D., Schulman K., Davenport R.D., Dumont L.J., El Kassar N., Foster L.D., Hah J.M., Jaiswal S., et al. Early Convalescent Plasma for High-Risk Outpatients with COVID-19. N. Engl. J. Med. 2021;385:1951–1960. doi: 10.1056/NEJMoa2103784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li L., Zhang W., Hu Y., Tong X., Zheng S., Yang J., Kong Y., Ren L., Wei Q., Mei H. Effect of Convalescent Plasma Therapy on Time to Clinical Improvement in Patients With Severe and Life-threatening COVID-19: A Randomized Clinical Trial. JAMA. 2020;324:460–470. doi: 10.1001/jama.2020.10044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Libster R., Marc G.P., Wappner D., Coviello S., Bianchi A., Braem V., Esteban I., Caballero M.T., Wood C., Berrueta M., et al. Early High-Titer Plasma Therapy to Prevent Severe COVID-19 in Older Adults. N. Engl. J. Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manzini P.M., Ciccone G., De Rosa F.G., Cavallo R., Ghisetti V., D’Antico S., Galassi C., Saccona F., Castiglione A., Birocco N., et al. Convalescent or standard plasma versus standard of care in the treatment of COVID-19 patients with respiratory impairment: Short and long-term effects. A three-arm randomized controlled clinical trial. BMC Infect. Dis. 2022;22:879. doi: 10.1186/s12879-022-07716-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Menichetti F., Popoli P., Puopolo M., Alegiani S.S., Tiseo G., Bartoloni A., De Socio G.V., Luchi S., Blanc P., Puoti M., et al. Effect of High-Titer Convalescent Plasma on Progression to Severe Respiratory Failure or Death in Hospitalized Patients with COVID-19 Pneumonia: A Randomized Clinical Trial. JAMA Netw. Open. 2021;4:e2136246. doi: 10.1001/jamanetworkopen.2021.36246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O’Donnell M.R., Grinsztejn B., Cummings M.J., Justman J.E., Lamb M.R., Eckhardt C.M., Philip N.M., Cheung Y.K., Gupta V., João E., et al. A randomized double-blind controlled trial of convalescent plasma in adults with severe COVID-19. J. Clin. Investig. 2021;131:e150646. doi: 10.1172/JCI150646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortigoza M.B., Yoon H., Goldfeld K.S., Troxel A.B., Daily J.P., Wu Y., Li Y., Wu D., Cobb G.F., Baptiste G., et al. Efficacy and Safety of COVID-19 Convalescent Plasma in Hospitalized Patients: A Randomized Clinical Trial. JAMA Intern. Med. 2022;182:115–126. doi: 10.1001/jamainternmed.2021.6850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ray Y., Paul S.R., Bandopadhyay P., D’Rozario R., Sarif J., Raychaudhuri D., Bhowmik D., Lahiri A., Vasudevan J.S., Maurya R., et al. A phase 2 single center open label randomised control trial for convalescent plasma therapy in patients with severe COVID-19. Nat. Commun. 2022;13:383. doi: 10.1038/s41467-022-28064-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rojas M., Rodríguez Y., Hernández J.C., Díaz-Coronado J.C., Vergara J.A.D., Vélez V.P., Mancilla J.P., Araujo I., Yepes J.T., Ricaurte O.B., et al. Safety and efficacy of convalescent plasma for severe COVID-19: A randomized, single blinded, parallel, controlled clinical study. BMC Infect. Dis. 2022;22:575. doi: 10.1186/s12879-022-07560-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.De Santis G.C., Oliveira L.C., Garibaldi P.M., Almado C.E., Croda J., Arcanjo G.G., Oliveira A., Tonacio A.C., Langhi D.M., Bordin J.O., et al. High-Dose Convalescent Plasma for Treatment of Severe COVID-19. Emerg. Infect. Dis. 2022;28:548–555. doi: 10.3201/eid2803.212299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sekine L., Arns B., Fabro B.R., Cipolatt M.M., Machado R.R.G., Durigon E.L., Parolo E., Pellegrini J.A.S., Viana M.V., Schwarz P., et al. Convalescent plasma for COVID-19 in hospitalised patients: An open-label, randomized clinical trial. Eur. Respir. J. 2022;59:2101471. doi: 10.1183/13993003.01471-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Self W.H., Wheeler A.P., Stewart T.G., Schrager H., Mallada J., Thomas C.B., Cataldo V.D., O’Neal H.R., Jr., Shapiro N.I., Higgins C., et al. Neutralizing COVID-19 Convalescent Plasma in Adults Hospitalized with COVID-19 A Blinded, Randomized, Placebo-Controlled Trial. Chest Infect. Orig. Res. 2022;162:982–994. doi: 10.1016/j.chest.2022.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonovich V.A., Pratx L.D.B., Scibona P., Beruto M.V., Vallone M.G., Vázquez C., Savoy N., Giunta D.H., Pérez L.G., Sánchez M.D.L., et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. N. Engl. J. Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sullivan D.J., Gebo K.A., Shoham S., Bloch E.M., Lau B., Shenoy A.G., Mosnaim G.S., Gniadek T.J., Fukuta Y., Patel B., et al. Early Outpatient Treatment for COVID-19 with Convalescent Plasma. N. Engl. J. Med. 2022;386:1700–1711. doi: 10.1056/NEJMoa2119657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Thorlacius-Ussing L., Brooks P.T., Nielsen H., Jensen B.A., Wiese L., Sækmose S.G., Johnsen S., Gybel-Brask M., Johansen I.S., Bruun M.T., et al. A randomized placebo-controlled trial of convalescent plasma for adults hospitalized with COVID-19 pneumonia. Sci. Rep. 2022;12:16385. doi: 10.1038/s41598-022-19629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van den Berg K., Glatt T.N., Vermeulen M., Little F., Swanevelder R., Barrett C., Court R., Bremer M., Nyoni C., Swarts A., et al. Convalescent plasma in the treatment of moderate to severe COVID-19 pneumonia: A randomized controlled trial (PROTECT-Patient Trial) Sci. Rep. 2022;12:2552. doi: 10.1038/s41598-022-06221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Denkinger C.M., Janssen M., Schäkel U., Gall J., Leo A., Stelmach P., Weber S.F., Krisam J., Baumann L., Stermann J., et al. Anti-SARS-CoV-2 antibody-containing plasma improves outcome in patients with hematologic or solid cancer and severe COVID-19: A randomized clinical trial. Nat. Cancer. 2022 doi: 10.1038/s43018-022-00503-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Piscoya A., Ng-Sueng L.F., del Riego A.P., Cerna-Viacava R., Pasupuleti V., Thota P., Roman Y.M., Hernandez A.V. Efficacy and harms of convalescent plasma for treatment of hospitalized COVID-19 patients: A systematic review and meta-analysis. Arch. Med. Sci. 2021;17:1251–1261. doi: 10.5114/aoms/132492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Snow T.A.C., Saleem N., Ambler G., Nastouli E., McCoy L.E., Singer M., Arulkumaran N. Convalescent plasma for COVID-19: A meta-analysis, trial sequential analysis, and meta-regression. Br. J. Anaesth. 2021;127:834–844. doi: 10.1016/j.bja.2021.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kloypan C., Saesong M., Sangsuemoon J., Chantharit P., Mongkhon P. CONVALESCENT plasma for COVID-19: A meta-analysis of clinical trials and real-world evidence. Eur. J. Clin. Investig. 2021;51:e13663. doi: 10.1111/eci.13663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasheed A.M., Fatak D.F., Hashim H.A., Maulood M.F., Kabah K.K., Almusawi Y.A., Abdulamir A.S. The therapeutic potential of convalescent plasma therapy on treating critically-ill COVID-19 patients residing in respiratory care units in hospitals in Baghdad, Iraq. Infez. Med. 2020;28:357–366. [PubMed] [Google Scholar]
- 54.Axfors C., Janiaud P., Schmitt A.M., Hooft J.V., Smith E.R., Haber N.A., Abayomi A., Abduljalil M., Abdulrahman A., Acosta-Ampudia Y., et al. Association between convalescent plasma treatment and mortality in COVID-19: A collaborative systematic review and meta-analysis of randomized clinical trials. BMC Infect. Dis. 2021;21:1170. doi: 10.1186/s12879-021-06829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janiaud P., Axfors C., Schmitt A.M., Gloy V., Ebrahimi F., Hepprich M., Smith E.R., Haber N.A., Khanna N., Moher D., et al. Association of Convalescent Plasma Treatment with Clinical Outcomes in Patients With COVID-19: A Systematic Review and Meta-analysis. JAMA. 2021;325:1185–1195. doi: 10.1001/jama.2021.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rosati M., Terpos E., Bear J., Burns R., Devasundaram S., Ntanasis-Stathopoulos I., Gavriatopoulou M., Kastritis E., Dimopoulos M.-A., Pavlakis G.N., et al. Low Spike Antibody Levels and Impaired BA.4/5 Neutralization in Patients with Multiple Myeloma or Waldenstrom’s Macroglobulinemia after BNT162b2 Booster Vaccination. Cancers. 2022;14:5816. doi: 10.3390/cancers14235816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Terpos E., Fotiou D., Karalis V., Ntanasis-Stathopoulos I., Sklirou A.D., Gavriatopoulou M., Malandrakis P., Iconomidou V.A., Kastritis E., Trougakos I.P., et al. SARS-CoV-2 humoral responses following booster BNT162b2 vaccination in patients with B-cell malignancies. Am. J. Hematol. 2022;97:1300–1308. doi: 10.1002/ajh.26669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ntanasis-Stathopoulos I., Karalis V., Gavriatopoulou M., Malandrakis P., Sklirou A.D., Eleutherakis-Papaiakovou E., Migkou M., Roussou M., Fotiou D., Alexopoulos H., et al. Second Booster BNT162b2 Restores SARS-CoV-2 Humoral Response in Patients With Multiple Myeloma, Excluding Those Under Anti-BCMA Therapy. HemaSphere. 2022;6:e764. doi: 10.1097/HS9.0000000000000764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Levine A.C., Fukuta Y., Huaman M., Ou J., Meisenberg B., Patel B., Paxton J., Hanley D.F., Rijnders B., Gharbharan A., et al. COVID-19 Convalescent Plasma Outpatient Therapy to Prevent Outpatient Hospitalization: A Meta-analysis of Individual Participant Data From Five Randomized Trials. medRxiv. 2022 doi: 10.1093/cid/ciad088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Senefeld J.W., Franchini M., Mengoli C., Cruciani M., Zani M., Gorman E.K., Focosi D., Casadevall A., Joyner M.J. COVID-19 Convalescent Plasma for the Treatment of Immunocompromised Patients: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2023;6:e2250647. doi: 10.1001/jamanetworkopen.2022.50647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson M.A., Henderson J.P., Shah P.K., Rubinstein S.M., Joyner M.J., Choueiri T.K., Flora D.B., Griffiths E.A., Gulati A.P., Hwang C., et al. Association of Convalescent Plasma Therapy With Survival in Patients With Hematologic Cancers and COVID-19. JAMA Oncol. 2021;7:1167–1175. doi: 10.1001/jamaoncol.2021.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data available upon reasonable request from the corresponding author.