Abstract
Background: Since the advent of global COVID-19 vaccination, several studies reported cases of encephalitis with its various subtypes following COVID-19 vaccinations. In this regard, we conducted a systematic review to investigate and characterize the clinical settings of these reported cases to aid in physician awareness and proper care provision. Methods: We systematically searched PubMed, Web of Science, and Scopus and manually searched Google Scholar. Studies published until October 2022 were included. Demographic data, clinical features, vaccine data, treatment lines, and outcomes were extracted. Results: A total of 65 patients from 52 studies were included. The mean age of patients was 46.82 ± 19.25 years, 36 cases (55.4%) were males. AstraZeneca was the most-reported vaccine associated with encephalitis (38.5%) followed by Pfizer (33.8%), Moderna (16.9%), and others. Moat encephalitis cases occurred after the first dose of vaccination in 41/65 (66.1%). The mean time between vaccination and symptom onset was 9.97 ± 7.16 days. Corticosteroids (86.2 %) and immunosuppressants (81.5 %) were the most used lines of treatment. The majority of affected individuals experienced a full recovery. Conclusion: Our study summarizes the current evidence of reported post-vaccination encephalitis, regarding clinical presentation, symptoms onset, management, outcomes, and comorbid conditions; however, it fails to either acknowledge the incidence of occurrence or establish a causal relationship between various COVID-19 vaccines and encephalitis.
Keywords: COVID-19 vaccine, adverse effects, encephalitis, systematic review
1. Introduction
Encephalitis is an inflammation of the brain tissues and is most usually caused by a viral infection (mainly herpes simplex virus), which represents about 75% of diagnosed cases; however, autoimmune causes such as N-methyl D-aspartate receptor (NMDAR) antibody encephalitis are also common [1]. Encephalitis is a neurological emergency that can cause severe cognitive impairment or death if not treated promptly. It can be diagnosed by at least two of the following criteria: fever, seizures, focal neurological findings by a cause of brain parenchymal damage, EEG findings indicative of encephalitis, lumbar puncture pleocytosis (more than four white cells per μL), or neuroimaging findings suggestive of encephalitis [2].
Recently, COVID-19 emerged as a new public health crisis affecting worldwide populations. As of the date of this review, 664 million confirmed cases and 6.7 million deaths have been reported since the outbreak in late 2019 [3]. To curtail the development of this disease, research on coronavirus diagnosis, prevention, treatment techniques, and vaccines was launched. The burden was heavily lifted when COVID-19 vaccines emerged. The mechanism of action of various vaccines aim to elicit immune response: the mRNA-based vaccines (PfiZerBioNTech and Moderna) are made up of genetically modified viruses RNA or DNA that produces a viral protein [4,5,6]. The genetically modified non-mRNA adenovirus vector vaccines (Janssen/Johnson and Johnson, Sputnik V, and AstraZeneca) also produce coronavirus proteins [5]. The spike protein or its fragments that resemble COVID-19 are introduced in protein subunit vaccines (Corbevax, Novavax). A killed or weakened COVID-19 virus is introduced in the attenuated viral vaccines Sinopharm and Sinovac Corona Vaccine [6].
Due to the urgency, vaccinations were approved based merely on the initial stages of clinical trials, without completion of all phases [7]. However, adverse reactions to vaccinations, including myelitis and severe disseminated encephalomyelitis, have been identified, although poorly documented [8].
Variable neurological complications after the COVID-19 vaccination, despite the unproven causes, have been reported. These include functional neurological disorder symptoms, such as altered mental status, autoimmune encephalitis (AE), acute disseminated encephalomyelitis (ADEM), dizziness, myalgia, fatigue, cognitive impairment, gait instability, facial palsy, Guillain–Barré syndrome (GBS), convulsions, strokes, transverse myelitis, chronic fatigue syndrome, and acute encephalopathy [9,10]. Recently, major neurological complications indicative of vaccination-related autoimmune encephalitis and acute encephalitis after the first dose of mRNA COVID-19 vaccines were reported [11,12,13,14,15]. Notably, acute disseminated encephalomyelitis (ADEM) was consistently reported after the viral vector-based vaccines or inactivated viral vaccine (AstraZeneca, Sputnik V, Sinopharm) [16,17,18,19,20,21,22].
Dutta et al. reported 19,529 neurological adverse events after COVID-19 vaccination, including encephalitis [23]. Zuhorn et al. demonstrated a temporal association between ChAdOx1 nCov-19 vaccination (AstraZeneca) and encephalitic symptoms [24]. The diagnosis of mRNA-1273 vaccine-induced encephalitis and status epilepticus was made by Fan et al. [25] in several recent cases.
The underlying mechanism of such symptomatology is not clearly understood; some researchers theorized that SARS-CoV-2 spike protein produced by mRNA-based vaccines may act as a catalyst for the inflammatory processes that ensue, particularly in autoimmune encephalitis [26].
Globally, vaccine hesitation is linked to a lack of trust in the COVID-19 vaccine’s safety and doubts about its effectiveness. However, vaccination acceptance rates increased to 75.2% last year according to an international survey [27]. Continuous vaccine improvement efforts and modifications are ongoing with the expanding range of immunity and adverse events in vaccinated populations. In this study, we aim to characterize clinical and laboratory features and the diagnostic and management implications of encephalitis cases following COVID-19 vaccinations to aid in physician awareness and proper care provision.
2. Methods
2.1. Database Search
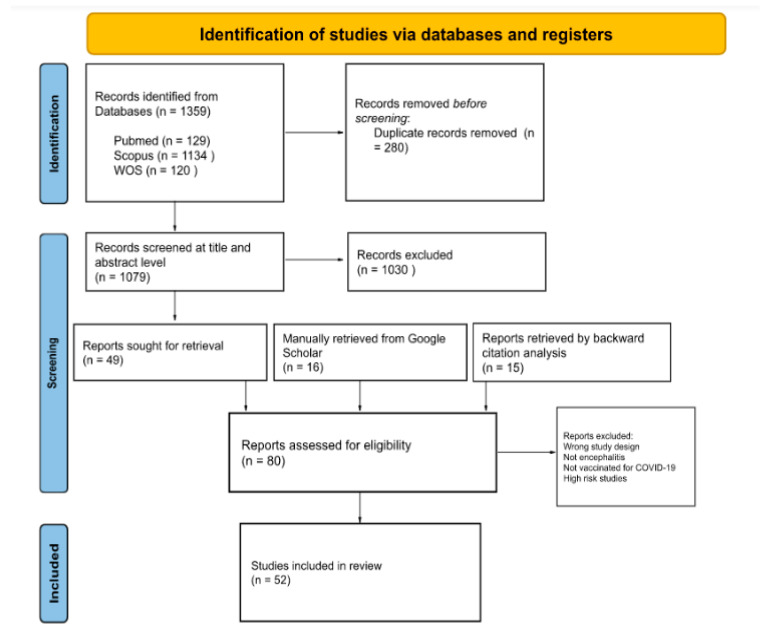
Our systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) checklist. It is registered in PROSPERO database with ID number: CRD42023389901. We performed a systematic literature search of PubMed, Scopus, and Web of Science databases, from inception until October 2022, following the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) [28]. The following search strategy was used (COVID-19 vaccination OR SARS-CoV-2 vaccine OR COVID-19 vaccine) AND (encephalitis). To increase our chances of identifying all relevant studies, we manually retrieved other studies from Google Scholar and performed backward citation analysis.
2.2. Screening and Inclusion Criteria
We included all published or pre-published papers presenting cases of any type of encephalitis in individuals who received any type of COVID-19 vaccination either as case reports, case series, or letters to editors. No language restrictions were applied. Secondary studies including reviews and meta-analyses, book chapters, and press releases were excluded from our study. Studies underwent title and abstract blind screening by two reviewers using Rayyan Artificial Intelligence [29]. After the removal of duplicates, the identified full-text articles were examined, and we manually assessed retrieved full-text records from Google Scholar and related references of further studies (Figure 1).
Figure 1.
PRISMA flow diagram for included studies.
2.3. Statistical Analysis
To provide a comprehensive understanding of the data included in those studies, we extracted patients’ characteristics (i.e., age and gender), the type of encephalitis, the type and dose of the vaccine, and the latency period before the onset of symptoms. We extracted symptoms, either relating to the nervous system or any other systems, and whether these patients had any other comorbidities. Investigations, treatment, and treatment outcomes were likewise extracted (Table 1). Extracted data were pooled into mean and standard deviation for continuous variables or frequency and percentage for categorical variables (Table 2).
Table 1.
Characteristics of included patients.
| No. | Author | Vaccine | Age/Sex | Type of Encephalitis | Onset Time (Days) | Dose | Clinical Features | Comorbidities | Other Complaints | Neuroimaging and CSF Analysis | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Ahmed et al. | Pfizer BioNTech | 61/F | ADEM | 7 | 1 | Progressive generalized weakness and difficulty with communication | Hypertension | - | MRI: nonspecific acute versus subacute leukoencephalopathy involving the brainstem and deep white matter | Methylprednisolone, IVIG | Full recovery |
| 2 | Ahmed et al. | Pfizer BioNTech | 62/F | Meningoencephalitis | 5 | 2 | Headache, fever, and rigor for 4 days, inability to stand up and walk, did not obey commands | Ceftriaxone allergy | - | CSF: high protein, pleocytosis | Acyclovir | Full recovery |
| 3 | Ahn et al. | AstraZeneca | 53/M | AE | 7 | 2 | Gait disturbance, dysarthria, cognitive decline, Hoffman sign, and ankle clonus | - | - | MRI: Increased FLAIR signal intensity in the bilateral hippocampus, Multiple enhanced lesions of the spinal cord (C6, T1 level) CSF: pleocytosis, elevated protein, oligoclonal band type 2 (+) | Acyclovir, IVIG, and rituximab | Favorable |
| 4 | Albsheer et al. | Moderna | 35/F | LE | 2 | 2 | Seizures | - | Anisocoria | CSF: pleocytosis CT: temporal lobe hypodensities ANA (+) |
Steroids, IVIG, and rituximab | Responded well with no additional neurological sequelae |
| 5 | Aljamea et al. | Pfizer BioNTech | 38/M | Bickerstaff Brainstem Encephalitis | 10 | 1 | Generalized fatigue, weakness, slowing of movement, hypophonia | - | Aspiration pneumonia, constipation | CSF: elevated proteins and albumin, GD1a (+), MRI: T2 hyperintensity within the distal spinal cord/conus medullaris | IVIG, plasmapheresis, and corticosteroids | Management and rehabilitation in a long-term care facility |
| 6 | Aljamea et al. | Pfizer BioNTech | 54/M | Bickerstaff Brainstem Encephalitis | 14 | 2 | Dysphagia, altered mental status, progressive weakness, limb ataxia, disturbed conscious level | DM, hypertension | Aspiration pneumonia, ophthalmoplegia | CSF: pleocytosis, oligoclonal bands (+), GQ1b and GM1 antibodies (+) | IVIG, plasmapheresis, Rituximab | Extensive rehabilitation in a long-term care facility |
| 7 | Al-Quliti et al. | AstraZeneca | 56/F | ADEM | 10 | Generalized weakness, lower extremity myalgia, difficulty in the articulation of speech, dysmetria | - | Anorexia | MRI: T2 and FLAIR showed hyperintensities in the subcortical and deep white matter involving basal ganglia CSF: protein and glucose elevated |
Hypertonic saline, methylprednisolone | Neck stiffness and bilateral-adduction-gaze deficit were resolved, as well as minimal improvement in her lower limbs’ weakness was observed, able to mobilize freely without assistance | |
| 8 | Ancau et al. | AstraZeneca | 61/M | AHEM | 4 | 1 | Fever, headache, apathy, generalized seizure, unconsciousness, bedridden, foaming around the mouth | Hypothyroidism, polymyalgia rheumatica | CT: diffuse hypodense areas in the right subcortical, frontotemporal, and right thalamic region. MRI: bilateral confluent cortical and subcortical FLAIR hyperintense lesions with hemorrhagic involvement of the basal ganglia CSF: moderate disturbance of the BBB |
Methylprednisolone, plasmapheresis | Vegetative state | |
| 9 | Ancau et al. | AstraZeneca | 25/F | AHEM | 2 | 1 | Cephalgia, fatigue, lack of sensation in legs, paraplegic syndrome, absent tendon reflexes, detrusor areflexia, difficulty urinating, mild weakness, ascending numbness in legs | - | Thoracic back pain | MRI: longitudinal edema along the thoracic spinal cord with contrast enhancement, focal central hemorrhage, bi-hemispheric white matter lesions with focal contrast enhancement CSF: pleocytosis, increased albumin, intrathecal IgM synthesis |
Methylprednisolone, Plasmapheresis | Persistent paraplegia |
| 10 | Ancau et al. | AstraZeneca | 55/F | AHEM | 9 | 1 | Meningism, spastic tetraparesis, coma | - | Nausea, dizziness | MRI: multiple FLAIR hyperintense hemorrhagic lesions in the right temporal and parietal lobes, bilaterally in fronto-temporal distribution and in the right occipital lobe and left fronto-basal region CSF: pleocytosis, intrathecal IgM, IgG and IgA, trans-tenetorial herniation, and hydrocephalus occlusion |
Right-sided decompressive hemicraniectomy, Methylprednisolone | Death |
| 11 | Asaduzzaman et al. | Pfizer BioNTech | 15/F | Autoimmune encephalitis | 1 | 2 | Fever, agitation, altered consciousness, convulsions | - | Diarrhea, dehydration, palpitation, myocarditis | CSF: pleocytosis, raised protein level, and normal glucose level MRI: no abnormality | Acyclovir, Ceftriaxone, methylprednisolone | Full recovery after 4 weeks |
| 12 | Asioli et al. | AstraZeneca | 73/F | Anti-LGI1 encephalitis | 14 | 1 | FBDS, behavioral disturbances | - | - | CSF: Anti-LGI1 (+), EEG: Bilateral fronto-temporal sharp waves; electrographic temporal seizures, MRI: Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling in the left hippocampus | Methylprednisolone, Valproate | Seizure-free, normal mental status |
| 13 | Asioli et al. | Pfizer BioNTech | 66/M | Anti-LGI1 encephalitis | 6 | 2 | Cognitive impairment, behavioral disturbances | Hypertension | - | CSF: Anti-LGI1 (+), high protein, RBCs (+), pleocytosis MRI: Bilateral mesial temporal lobe T2-weighted hyper-intensity with swelling and contrast enhancement in the right amygdala and hippocampus, EEG: Right fronto-temporal sharp waves; electrographic temporal seizures |
Methylprednisolone, Levetiracetam | Normal mental status in 7 months |
| 14 | Asioli et al. | Pfizer BioNTech | 66/M | Anti-LGI1 encephalitis | 9 | 2 | FBDS, focal seizures, behavioral disturbances | Polyallergy | Hypersomnia | CSF: Anti-LGI1 (+), EEG: Bilateral fronto-temporal epileptiform discharges | Methylprednisolone, Lacosamide | Seizure-free, normal mental status in 3 months |
| 15 | Asioli et al. | Moderna | 18/F | Anti-LGI1 encephalitis | 23 | 3 | Focal seizures, short-term memory impairment | - | - | MRI: Right fronto-temporal sharp waves, CSF: Anti-LGI1 (+) | Methylprednisolone, Lacosamide | Seizure-free, normal mental status in 3 months |
| 16 | Autjimanon et al. | Pfizer BioNTech | 14/F | Acute encephalopathy | 9 | 1 | Fever, headaches, drowsiness, tonic-clonic focal and generalized seizures, status epilepticus |
- | - | ANA (+) | Anti-convulsants, immunosuppressants | Discharged home but had residual memory problems |
| 17 | Ballout et al. | Pfizer BioNTech | 27/M | Autoimmune Encephalitis | 6 | 1 | Fatigue, confusion and anxiety, headache, agitation, dysfluent speech with paraphasic errors, and difficulty with writing | - | - | CSF: Pleocytosis with lymphocytic predominance MRI: no abnormalities EEG: mild generalized slowing without epileptiform abnormalities |
Methylprednisolone | Full recovery after 1 month |
| 18 | Ballout et al. | Moderna | 81/M | ADEM | 13 | 1 | Change in mental status with severe encephalopathy, fever, absent pupillary response to light, absent right corneal reflex, diffuse hypertonicity, extensor plantar responses bilaterally | - | Fatigue, myalgia | CSF: pleocytosis, elevated protein, elevated myelin basic protein (MBP) MRI: diffusion restriction in the right dorsal medulla with corresponding T2 FlAIR hyperintensity, faint T2 hyperintensities in the left pons, midbrain, and thalamus, and minimal T2 sulcal hyperintensity without contrast enhancement | Methylprednisolone, IVIG therapy, plasmapheresis | Death |
| 19 | Bastide et al. | AstraZeneca | 49/F | ADEM | 2 | 1 | Fever, flu-like symptoms, Lhermitte’s phenomenon, sensory ataxia, Romberg sign, impaired tandem walking, paraparesis, pallesthesia, hypoesthesia, and Sphincter dysfunction | - | - | Brain MRI: large ill-defined T2 FLAIR hyperintensities of periventricular and deep white matter with smaller lesions infratentorially, spared cortex, deep gray matter and subcortical U fibers; SSEPs: abnormal conduction above the sensory decussation in the lower brainstem CSF: mild pleocytosis |
Methylprednisolone, rituximab | Improved and MRI showed stability or regression of most lesions |
| 20 | Cao et al. | Sinopharm and Sputnik V | 24/F | ADEM | 14 | 1 | Reduced memory, headache, fever, spasticity, weakness in extremities | - | Loss of appetite | CSF: pleocytosis, oligoclonal band (+) MRI: abnormal signals in the B/L temporal cortex, lesions EEG: epileptiform waves |
Immunoglobulin, diazepam, levetiracetam | Full recovery after 1 month |
| 21 | Escola et al. | AstraZeneca | 43/F | Encephalomyelitis | 9 | 1 | Headache, meningism, and fever, sensorimotor tetraparesis, subacute sensorimotor paraparesis, urinary retention, hyperreflexia | Migraine | - | MRI: T2 hyperintense lesions involving frontal cortex, periventricular space, pulvinar thalamic nuclei, brain stem, and cerebellar peduncles CSF: extensive predominant granulocytic pleocytosis, elevated lactate and protein |
Methylprednisolone, ceftriaxone, ampicillin, plasma exchange, meropenem, tocilizumab | Patient developed a stuporous to a comatose state and was discharged to a rehabilitation center; after 3 months, she improved with a light cerebellar syndrome (Rt hand intention tremor) |
| 22 | Etemadifar et al. | Sinopharm and Sputnik V | 50/F | Anti-NMDAR encephalitis | 20 | 2 | Behavioral disturbances, muscle pain, limb weakness, ataxia, dizziness, weakness, agitation, (+) Babinski sign | Rituximab-treated MS | Vomiting | MRI: plaques in the periventricular, juxtacortical, and cortical area | Methylprednisolone | Full recovery |
| 23 | Fan et al. | Moderna | 22/M | AE | 6 | 2 | Fever, blurry vision, consciousness disturbance, status epilepticus, slurred speech, memory loss | - | Sinus tachycardia | CSF: no pleocytosis, elevated protein, SARS-CoV-2 spike S1 RBD IgG; EEG: continuous diffuse slowing in theta and delta ranges; CT: mild hypoperfusion in right temporal region | Levetiracetam, acyclovir, valproate sodium, and methylprednisolone | Full recovery |
| 24 | Fernandes et al. | Pfizer BioNTech | 16/M | Anti-GAD encephalitis | 7 | 1 | Generalized tonic clonic seizures | DM | - | EEG: bitemporal focal slowing with admixed sharp waves CSF: pleocytosis, protein elevation |
Dexamethasone | Improved with minimal right focal slowing |
| 25 | Flannery et al. | Pfizer BioNTech | 20–30/F | Anti-NMDAR encephalitis | 7 | 1 | Anxiety, decreased mental acuity, insomnia, COVID-19 hypochondria, motor dysfunction, aphasia, accusatory auditory hallucinations, spontaneous defecation, psychosis, catatonia, grand mal seizure, lethargy | Irritable bowels and kidney disease | Tachycardia, hypertension | CSF: mild lymphocyte pleocytosis, anti-NMDA titer 1:20 | Olanzapine, haloperidol, lithium, Risperidone, IVIG, methylprednisolone, metoprolol, and rituximab | Improved with minor neurological deficits after 45 days |
| 26 | Gao et al. | Moderna | 82/F | AE | 5 | 1 | Fever, headache, behavioral changes | DM, hypertension | - | EEG: slow waves in the right frontoparietal regions MRI: signal change in the right middle and posterior temporal lobe CSF: elevated protein |
Pulse steroid | Full recovery |
| 27 | Garibashvili et al. | AstraZeneca | 71/M | Anti-LGI1 encephalitis | 28 | 1 | Faciobrachial dystonic seizures, mild pallhypaethesia | Heart disease, hypertension and hyperlipidemia | Cardiac pauses | LG1 antibodies: marked increase in serum, normal in CSF | Prednisolone | Full recovery |
| 28 | Gogu et al. | Johnson & Johnson | 45/M | AHEM | 30 | 1 | Right hemiparesis, mixed aphasia, severe headache, agitation, depressed level of consciousness, coma | SARS-CoV-2 (+), DM, Tolosa–Hunt Syndrome | Incomplete left ophthalmoplegia | MRI: multiple ischemic strokes, meningitis, infectious vasculitis, and hemorrhagic encephalitis with extension of the lesion to left fronto-parieto-temporal lobes with hypersignal aspects on the T2, Flair, and DWI images | Methylprednisolone | Death |
| 29 | Grossi et al. | Pfizer BioNTech | /M | AE | 17 | 1 | Fever, agitation, confusion, headache, and Tonic-Clonic seizures | CLL, herpetic trigeminal rash | Vomiting | CSF: pleocytosis, high albumin, oligoclonal bands (+) | Dexamethasone Acyclovir, Ceftriaxone, Vancomycin, and Levetiracetam | Free from focal neurological impairment after 4 months |
| 30 | Huang et al. | AstraZeneca | 38/F | Autoimmune Encephalitis | 14 | 1 | Acute-onset amnesia, fever, and general malaise for 2 days, incoherent speech, difficulty typing using communication software, tonic–clonic seizure | - | - | MRI: a subacute infarction at the right internal capsule and irregular vascular contour, which indicated a vasculopathy, such as vasculitis CSF: inflammation without pleocytosis EEG: diffuse background slowing with sharp transients at the right temporal region |
Levetiracetam + steroid pulse therapy | Full recovery without neurological deficit or sequelae |
| 31 | Jarius et al. | Pfizer BioNTech | 67/M | MOG encephalomyelitis | 10 | Color desaturation in the left eye associated with left-sided temporal headache and pain upon eye movement | Arterial hypertension and benign prostate hyperplasia | Optic neuritis | MRI showed swelling and contrast enhancement of the anterior part of the left optic nerves. Visual evoked potentials (VEP) demonstrated prolonged absolute and relative P100 latency and marked amplitude reduction in the left eye (by 40 ms and 73%, respectively, compared with the right eye) | Methylprednisolone | Favorable | |
| 32 | Kania et al. | Moderna | 19/F | ADEM | 14 | 1 | Headache, fever, urinary retention | - | Nausea, vomiting, back and neck pain | MRI: multiple hyperintense lesions in T2 weighted and FLAIR images located in both brain hemispheres, pons, the medulla oblongata, and cerebellum CSF: pleocytosis, elevated protein and RBC |
Methylprednisolone | Full recovery |
| 33 | Kobayashi et al. | Pfizer BioNTech | 46/F | Brainstem encehalitis | 5 | 2 | Diplopia | Vasculitis | - | MRI: lesion on the dorsal pons across the midline and no gadolinium enhancement | Methyprednisolone | Full recovery |
| 34 | Kwon et al. | AstraZeneca | 57/F | Autoimmune encephalitis | 5 | 1 | Headache, fever, generalized convulsive seizure, cognitive decline including attention and memory deficits along with gradually worsening dysphasia | Hypertension | Myalgia | MRI: restricted diffusion through the left insular and mesial temporal cortices, contrast enhancement CSF: pleocytosis, elevated protein, oligoclonal band (+) EEG: intermittent generalized delta activity |
Methylprednisolone, Immunoglobulin, Rituximab | The patient’s language function slowly improved substantially following rituximab therapy, but the memory dysfunction hardly improved. Encephalomalacia change was observed in the left temporal lobe |
| 35 | Lazaro et al. | Sinopharm and Sputnik V | 26/F | ADEM | 28 | 1 | Disorientation, inappropriate behavior, headache, gait imbalance declined memory, hypoprosexia, anosognosia, incoherent speech, visuospatial failures, right upper limb weakness, gait ataxia | - | - | CSF: normal, OCB (+) MRI: nodular hyperintense lesions on T2/FLAIR without restricted diffusion | Methylprednisolone | Full recovery and clear MRI after 3 months |
| 36 | Li et al. | AstraZeneca | 55/M | AE | 7 | 1 | Fever, progressive weakness, consciousness disturbance | Hypertension, hyperlipidemia, and sleep apnea under medication | - | CSF: pleocytosis, elevated protein, ANA (+); high D-dimer | Dexamethasone and subcutaneous fondaparinux | Normal, 4 months later he received Moderna vaccine with no sequelae |
| 37 | Maramattom et al. | AstraZeneca | 64/M | LE | 10 | 1 | Headache, altered sensorium, fever | Glomerulonephritis, Subsegmental pulmonary embolism | - | CT chest; normal MRI brain: hyperintensities in bilateral medial temporal lobe and head and proximal body of hippocampus (L > R) CSF: pleocytosis | Methylprednisolone, plasma exchange, and rituximab | Improved |
| 38 | Maramattom et al. | AstraZeneca | 46/M | ADEM | 5 | 1 | Fever, urinary complaints, progressive paraparesis | A brisk jaw jerk, spastic quadriparesis paresis, loss of posterior column sensations till the T6 level | - | MRI spine: longitudinally extensive transverse myelitis MRI brain: T2, FLAIR hyperintensities in bilateral middle cerebellar peduncle (left > right), pontine tegmentum, right paramedian medulla, and left thalamocapsular region CSF: encephalitis panel: negative |
methylprednisolone, plasma exchange | Improved |
| 39 | Maramattom et al. | AstraZeneca | 64/M | ADEM | 20 | 2 | Progressive paresthesia of legs, followed by UL, spastic paraparesis | - | - | MRI brain and spine: bilateral corticospinal tract hyperintensities Dorsal cord hyperintensity at D8–9 CT: normal |
methylprednisolone and Rituximab | Improved |
| 40 | Maramattom et al. | AstraZeneca | 42/F | LE | 5 | 1 | Persistent daily headache | - | Papilledema | CSF: opening pressure 32 cm H O2 CSF parameters normal serum and CSF autoimmune MRI: initial MRI: leptomeningeal and sulcal enhancement; 25 days later: large right temporal irregular enhancing lesion with significant perilesional edema |
Decompression of lesion Excisional biopsy, prednisolone | Good recovery with some symptoms |
| 41 | Monte et al. | Pfizer BioNTech | 15/F | Bickerstaff brainstem encephalitis | 3 | 2 | Diplopia, dysarthria, consciousness disturbance, fever, asthenia, limb paresthesia, cranial nerve paresis, and gait unsteadiness | Previous recent mycoplasma pneumoniae infection | Cough, vomiting, ophthalmoplegia, abnormal blink reflex | Both normal | Methylprednisolone, immunoglobulins | After 2 weeks, he was able to walk without assistance and the neurological examination was normal. Six weeks after the disease onset, the blink reflex was normal |
| 42 | Moslemi et al. | AstraZeneca | 27/M | HSE | 8 | 1 | Agitation, headache delirium, and disorientation to time, location, and people, slowed psychomotor activity, and loss of alertness | - | Vomiting | CT scans: normal findings with no evidence of involvement MRI: all normal |
Acyclovir | Improved |
| 43 | Nagaratnam et al. | AstraZeneca | 36/F | ADEM | 14 | 1 | Headache, fatigue, photophobia, bilateral visual disablement, subjective color desaturation, aching eye movements | - | Photophobia, blurred vision in the right eye | MRI: T1/FLAIR hyperintense lesions involving the subcortical white matter, posterior limb of bilateral internal capsules, pons and left middle cerebellar peduncle, multiple internal punctuate foci of gadolinium contrast enhancement |
Methylprednisolone | Improvement in response bilaterally with responses being detectable on the left eye |
| 44 | Naz et al. | Sinopharm and Sputnik V | 33/F | Anti-NMDAR encephalitis | 4 | 2 | Constitutional symptoms, memory disturbance, confusion, fish mouthing movement and seizures | - | - | CSF: pleocytosis, oligoclonal band (+), CT and MRI (−), blood NMDA test (−) | Steroids, IVIG, T-PLEX, and Rituximab | Full recovery |
| 45 | Permezel et al. | AstraZeneca | 63/M | ADEM | 12 | 1 | Vertigo, fatigue, disorientation, declining cognition, impaired attention, poor responsiveness | DM, ischemic heart disease and atrial fibrillation | Abdominal pain, fatigue, ketoacidosis, and silent myocardial infarction | MRI: bilateral foci (>20) of high T2 and FLAIR signal in the white matter | Corticosteroids, plasmapheresis | Death |
| 46 | Rastogi et al. | Moderna | 59/F | Rhombencephalitis | 12 | 2 | Binocular diplopia, paresthesia, hand numbness, decreased sensation, cerebellar dysfunction | Fibromyalgia, migraines, and carpel tunnel syndrome | Dizziness, lethargy | CSF: elevated protein, elevated glucose, lymphocytic pleocytosis; MRI: multiple focal poorly defined regions of contrast enhancement in the cerebral cortex, deep grey matter, brainstem, and cerebellum | Expectant, without empiric corticosteroids or antimicrobials | Ongoing gradual improvement |
| 47 | Rinaldi et al. | AstraZeneca | 45/M | ADEM | 12 | 1 | Numbness of limbs, trunk and legs, slurred speech, difficulty swallowing, clumsy right-hand movements, dysarthria, dysphagia, urge incontinence | - | Reduced visual acuity | MRI: large, poorly marginated T2-weighted hyperintensities in the pons, right cerebellar peduncle, right thalamus, and multiple spinal cord segments. All lesions, except the thalamic one and a single dorsal spinal area, showed blurred gadolinium enhancement on T1-weighted images CSF: pleocytosis |
Methylprednisolone | Complete clinical recovery and no relapses, almost entire resolution of the brainstem and spinal cord lesions at the dorsal/conus medullaris level, and further shrinkage of cervical areas |
| 48 | Saad et al. | Pfizer BioNTech | 69/F | Acute encephalopathy | 5 | 1 | Coma, seizure, status epilepticus | - | - | CSF: high protein, MRI: pyriform-pattern diffusion restriction in the right hemisphere and left frontoparietal region | Methylprednisolone, antibiotics, and antivirals | Discharged in a deeply comatose status on day 30 of hospital admission |
| 49 | Sawczyńska et al. | Unknown | 77/F | AE | 14 | 1 | Fever, attention and cognition disturbances, confusion, hyperactivity, delusions, chorea, orofacial dyskinesia, psychomotor slowing, seizures, hemiparesis, loss of consciousness | COVID-19 infection, hypertension, DM, hypothyroidis, urinary incontinence, and multiple malignancies in remission, slight cognition disturbances | Atrial fibrillation, pneumonia, sepsis, pulmonary embolism, urinary tract infection, reactive arthritis | MRI: features of cerebral small vessel disease, diffuse white matter hyperintensities, cortical and subcortical atrophy EEG: FIRDA pattern |
Methylprednisolone, diazepam, remdesivir, IVIG, and antiepileptic | Hospitalization for non-neurological complications |
| 50 | Senda et al. | Pfizer BioNTech | 72/F | Acute Meningoencephalitis | 3 | 1 | Depressed level of consciousness, headache | Rheumatoid vasculitis, DM, and hyperlipidemia | General fatigue | CSF: a cell count of four cells/mm3 (all mononuclear leukocytes), an increased protein level, IgG index was elevated (1.13) MRI: hyperintensities in white matter of the bilateral frontotemporal areas on DWI, more on the right side FLAIR images: diffuse cerebral cortex swelling in bilateral frontotemporal areas, also stronger on the right side |
Intravenous steroid pulse and gammaglobulin therapies | Improved |
| 51 | Shinet al. | AstraZeneca | 35/F | Autoimmune encephalitis | 5 | 1 | Dysarthria, abnormal movements, anxiety, fever, rigidity, dystonia, catatonia, motor aphasia, jaw-opening dystonia, hypophonia, drooling, reduced voluntary movements | - | Sinus tachycardia | MRI: swelling of the hippocampus, encephalomalacia in frontoparietal lobes EEG: diffuse beta wave activity, intermittent generalized delta activity |
Methylprednisolone, immunoglobulins, rituximab | After 1 week, her catatonia, rigidity, and drooling had improved, and she could walk for a short distance without assistance; however, she still had significant rigidity |
| 52 | Shyu et al. | Moderna | 58/F | AE | 7 | 1 | Fever, cognitive deficits, left deviation of the head and eyeballs, and mild weakness of the right UL | - | - | CSF: pleocytosis, elevated protein, CSF/serum albumin ratio of 19.7 | Dexamethasone | Regained normal cognitive function and was discharged in 13 days |
| 53 | Shyu et al. | Moderna | 21/M | AE | 7 | 1 | Coma, status epilepticus | - | - | CSF: elevated protein and microalbumin EEG: continuous diffuse slowing in the theta and delta ranges, indicating moderate diffuse cerebral dysfunction SPECT: hypoperfusion in the right temporal region |
Methylprednisolone | Healthy and seizure free after 3 months |
| 54 | Sluyts et al. | Moderna | 48/3 | AE | 6 | booster dose | Agitation, physical aggression, mutism, left arm: paretic and atactic, confusion | - | Bradyphrenia | CSF: pleocytosis, elevated protein MRI: small left internal capsule developmental venous anomaly |
Ceftriaxone, amoxicilline, and acyclovir | Full recovery after 3 days from steroids admission |
| 55 | Takata et al. | AstraZeneca | 22/F | Autoimmune encephalitis | Few | 2 | Headache, fatigue, confusion, agitation, hallucinations, fever, disorientation |
- | - | CSF: opening pressure of 30 cm H2O, pleocytosis, IgG oligoclonal bands (+ve) | Ceftriaxone, acyclovir, lorazepam, and olanzepine | She remains on low-dose olanzapine and is functionally well with independent activities of daily living, but her family reports that she has not recovered back to her pre-morbid state |
| 56 | Torrealb a-Acosta G et al. | Moderna | 77/M | Meningoencephalitis | 2 | 1 | Dizziness, fever, rashes, headache, double vision, confusion | Coronary artery disease, hyperlipidemia, and hypothyroidism | Edematous erythematous papules and plaques with overlying pustules on the trunk and abdomen | CSF: pleocytosis, increased protein vEEG: generalized slow theta range with state changes and reactivity |
Methylprednisolone following prednisone | Full recovery |
| 57 | Vences et al. | Pfizer BioNTech | 72/M | AE | 1 | 1, relapse in 4 2 | Malaise, headache, fever, confusion, aggressiveness, and gait alterations | - | - | CSF: elevated protein MRI: circumscribed encephalitis at the anterior frontal and bilateral temporal lobes |
Methylprednisolone | Favorable |
| 58 | Vogrig et al. | Pfizer BioNTech | 56/F | ADEM | 14 | 1 | Malaise, chills, unsteadiness of gait | - | - | MRI: hyperintensities on FLAIR sequences involving the left cerebellar peduncle, with moderate mass effect on the fourth ventricle | Prednisone | Full recovery |
| 59 | Walter et al. | Pfizer BioNTech | 30/M | RE | 21 | 2 | Malaise, headache, taste disorder, facial paralysis (left side), gait disturbance by ataxia, hypoglossal nerve paralysis | - | - | MRI: weak FLAIR hyperintensity of the brainstem, mesencephalon and cerebellar around the fourth ventricle without contrast enhancement CSF: pleocytosis |
Methylprednisolone | Full recovery within a few weeks |
| 60 | Werner et al. | Pfizer BioNTech | 35/F | Autoimmune encephalitis | 2 | 2 | Fever, headache, visual impairment, behavioral changes, recurrent focal to bilateral tonic-clonic seizures, reduced level of consciousness, and choreatic movements | - | Skin rash | Cerebral magnetic resonance imaging: swelling in the (para-) hippocampal region predominantly on the left hemisphere and bilateral subcortical subinsular FLAIR hyperintensities. Cerebrospinal fluid analysis: a lymphocytic pleocytosis of 7 cells/μL and normal protein and immunoglobulin parameters | Levetiracetam, lacosamide, methylprednisolone, and plasma exchange | Partial recovery |
| 61 | Yazdanpanah et al. | Sinopharm and Sputnik V | 37/M | ADEM | Few | 1 | Intermittent myalgia, drooling, progressive weakness of 4 limbs, bilateral f, dysphagia | - | Nausea, vomiting | Brain MRI: Hyperintense foci within the left cerebral peduncle, left corticospinal tract, right and left sides of pons and medulla Spine MRI: unremarkable Magnetic resonance spectroscopy (MRS): confirmed the demyelination process by the presence of Myoinositol and Choline peaks | Heparin, Pantoprazole, Clindamycin, Paracetamol, and Methylprednisolone | Full recovery |
| 62 | Zlotnik et al. | Pfizer BioNTech | 48/M | Anti-LGI1 encephalitis | 18 | 2 | Fatigue, memory deficit, anterograde amnesia |
- | - | MRI: hyperintense signal on both medial temporal lobes | Methylprednisolone | Recovered, but still faces some executive skills difficulties |
| 63 | Zuhorn et al. | AstraZeneca | 21/F | Autoimmune encephalitis | 5 | 1 | Headache, concentration difficulties, fever, malaise, epileptic seizure | - | - | CSF: pleocytosis EEG: diffuse slow theta rhythm |
Dexamethasone | Normal state of the parenchyma without sequelae |
| 64 | Zuhorn et al. | AstraZeneca | 63/F | Autoimmune encephalitis | 6 | - | Gait deterioration, twitching, opsoclonus-myoclonus syndrome |
‘ | Oral anticoagulation, vigilance disorder | EEG: diffuse slow theta rhythm CSF: pleocytosis |
Methylprednisolone | Normal state of the parenchyma |
| 65 | Zuhorn et al. | AstraZeneca | 63/M | Autoimmune encephalitis | 8 | - | Fever, aphasia | - | - | CSF: pleocytosis | - | Further improvement could be observed, no evidence of structural lesions |
Normal IgG index < 0.66. UL: upper limb, FBDS: Faciobrachial dystonic seizures, DM: diabetes mellitus, PD: Parkinson disease, MS: Multiple sclerosis.
Table 2.
Summary of patients’ characteristics, common symptoms, laboratory and imaging findings, treatment, and treatment outcomes of patients with post-COVID-19 vaccine encephalitis.
| Variable | Descriptive Statistics | |
|---|---|---|
| Sex (%) | Male | 36 (55.4%) |
| Female | 28 (43.1%) | |
| Transgender male | 1 (1.5%) | |
| Age (mean ± SD) | 46.82 ± 19.25 | |
| Period after vaccination in days (mean ± SD) | 9.97 ± 7.16 | |
| Vaccine Subtypes (%) | AstraZeneca | 25 (38.5%) |
| Pfizer BioNTech | 22 (33.8%) | |
| Moderna | 11 (16.9%) | |
| Sinopharm and Sputnik | 5 (7.7%) | |
| Johnson & Johnson | 1 (1.5%) | |
| Unknown | 1 (1.5%) | |
| Vaccine dose (%) | 1st | 41 (66.1%) |
| 2nd | 18 (29%) | |
| 3rd | 1 (1.6%) | |
| 4th | 2 (3.2%) | |
| Encephalitis Subtypes (%) | Acute encephalitis | 11 (16.9%) |
| ADEM | 14 (21.5%) | |
| AHEM | 4 (6.2%) | |
| Other | 36 (55.4%) | |
| Headache (%) | 20 (30.8 %) | |
| Fever (%) | 23 (35.4 %) | |
| Seizure (%) | 15 (23.1 %) | |
| Abnormal movement (%) | 24 (36.9 %) | |
| CSF findings (%) | ||
| Pleocytosis | 32 (49.2 %) | |
| High protein | 7 (10.8 %) | |
| Antibodies positive | 6 (9.2 %) | |
| MRI findings (%) | ||
| Abnormal | 40 (61.5 %) | |
| Treatment (%) | Steroids | 56 (86.2 %) |
| Immunoglobulins | 15 (23.1 %) | |
| Plasmapheresis | 9 (13.8 %) | |
| Antiviral | 10 (15.4 %) | |
| Immunosuppressive drug | 53 (81.5 %) | |
| Treatment outcome (%) | Full recovery | 41 (63.1 %) |
| Residual Symptoms | 11 (16.9 %) | |
| Extensive rehabilitation | 9 (13.8 %) | |
| Death | 4 (6.2 %) | |
| Death-associated comorbidities | Hemicraniectomy, Tolosa Hunt Syndrome, Diabetes type 2, ischemic heart disease, atrial fibrillation |
2.4. Quality Assessment
For included case reports and case series, we used the Joanna Briggs Institute (JBI) quality assessment tools, based on the clinical features, history, diagnoses, interventions, and management plans. Fourteen letters to the editor were excluded from the assessment due to a lack of appropriate assessment tools. Two authors assessed the quality of included studies and resolved conflicts by consensus. For case reports, JBI domains included eight questions, and the case series checklist assessed ten domains. Grades were assigned such that; Low risk: 75–100%, Moderate: 50–74%, High risk < 50%. High-risk studies will be excluded.
3. Results
Out of 1395 studies identified from databases, 280 were excluded as duplicates. We screened 1079 records at a title and abstract level, through which we excluded 1030 records for irrelevancy. At this point, 15 studies retrieved from previous studies and 16 records identified manually through a Google Scholar search were compared with studies eligible for full-text screening (n = 49), excluding duplicates. In total, 80 records were assessed through full-text screening, from which 11 studies were excluded for reporting CNS infections other than encephalitis and non-COVID-19 vaccination; 14 systematic reviews, literature reviews, and meta-analyses were excluded; 3 studies were excluded for being high risk on quality assessment. In total, 52 studies were included in the final qualitative synthesis, see Figure 1.
3.1. Patient Characteristics
Patients’ mean age was (46.82 SD 19.25) years, 55.4% of patients were males (37/28), and one case presented as a transgender male. Twenty-four patients (36.9%) had several comorbidities, including hypertension, DM, PD, heart disease, hypothyroidism, polymyalgia rheumatica, polyallergy, herpes simplex, migraine, MS, irritable bowel, kidney disease, hyperlipidemia, SARS-CoV-2, Tolosa–Hunt syndrome, CLL, benign prostate hyperplasia, pulmonary embolism, mycoplasma pneumonia, vasculitis, and fibromyalgia. The mean time for symptoms appearance post-vaccination was 9.97 ± 7.16, which was mostly reported after the first dose (66.1%), followed by the second dose (29%), the booster dose (3.2%) and the third dose (1.6%), see Table 2.
3.2. Clinical Presentation
Of all 65 patients, 11 presented with Acute encephalitis (16.9%), 15 with Acute disseminated encephalomyelitis (ADEM) (23.1%), 4 with Acute hemorrhagic encephalitis (AHEM) (6.2 %), and 35 cases with other types of encephalitis (53.8%) encompassing: unspecified Autoimmune encephalitis, Anti-LGI1 encephalitis, Anti-NMDAR encephalitis, Meningoencephalitis, Acute encephalopathy, Herpes simplex encephalitis, Rasmussen encephalitis, Limbic encephalitis, Bickerstaff Brainstem Encephalitis, Brainstem encephalitis, Encephalomyelitis, Multifocal Necrotizing Encephalitis, Anti-GAD encephalitis, and MOG encephalomyelitis, see Table 1.
All 65 patients presented with both neurologic symptoms. Most occurring neurologic symptoms were fever in 23/65 (35.4%) and abnormal movements in 24/65 (36.9%), headache occurred in 20/65 (30.8%) patients, and seizure occurred in 15/65 (23.1%) patients, see Table 2. Other reported neurologic symptoms included dysarthria, aphasia, dysphasia, altered mental status, gait disturbance, cognitive decline, general weakness, hypophonia, ataxia, disturbed conscious level, paraplegia, numbness, areflexia, agitation, spasms, FBDS, behavioral disturbances, memory impairment, status epilepticus, Lhermitte’s phenomenon, paraparesis, hypoesthesia, sphincter dysfunction, Babinski sign, hallucinations, spontaneous defecation, psychosis, coma, urinary retention, diplopia, photophobia, psychological changes, hypoglossal nerve paralysis, dizziness, taste disorder, and facial nerve paralysis. Non-neurologic symptoms were present in 36 patients (55.3%) and included: ophthalmoparesis, ophthalmoplegia, papilledema, optic neuritis, photophobia, blurred vision, aspiration pneumonia, cough, palpitation, myocarditis, bradyphrenia, sinus tachycardia, cardiac pauses, silent myocardial infarction, atrial fibrillation, abdominal pain, constipation, diarrhea, dehydration, hypersomnia, myalgia, dizziness, back pain, fatigue, loss of appetite, ketoacidosis, sepsis, urinary tract infection, reactive arthritis, and skin rash. Three patients (4.6%) were hospitalized, see Table 1.
3.3. Investigations and Diagnostic Results
Diagnostic test results were primarily available for CSF and MRI findings; 61.5% (40/60) of MRI results were abnormal with the following findings: FLAIR, T2, and DWI hyperintensities in various regions, central focal hemorrhage, bilateral white matter lesions, minimal T2 sulcal hyperintensity without contrast enhancement, plaques in periventricular, juxtacortical and cortical areas, swelling and hyperintensities of the anterior part of the optic nerves, restricted diffusion through insular and mesial temporal cortices, swelling of the hippocampus, encephalomalacia in frontoparietal lobes, blurred gadolinium enhancement on T1-weighted images; for MRI spine: multiple enhanced lesions of the spinal cord were found in addition to longitudinal edema along the thoracic spinal cord with contrast enhancement and longitudinally extensive transverse myelitis. In CSF, the most common finding was pleocytosis (48.5 %). Seven patients (10.8%) had high protein in their CSF samples, and six had positive CSF antibodies, including ANA, Anti-LGI1, SARS-CoV-2 spike S1 RBD IgG, anti-NMDA, intrathecal IgA, and IgM.
3.4. Treatment Plan and Its Outcomes
All 65 patients received a spectrum of medical treatments (steroids, IVIG, antivirals, immunosuppressive drugs, plasmapheresis, antibiotics, anticonvulsants, and analgesics), and 2 patients received anticoagulants. Most patients, i.e., 56 (86.2%), received steroids and immunosuppressive drugs, while 53 (81.5%) received drugs such as rituximab and tocilizumab. In total, 15 received immunoglobulins (23.1%) and 10 received an antiviral treatment (15.4%). Only 9 patients received plasmapheresis (13.8%).
Overall, 41 patients made a full recovery (63.1%), 11 had residual symptoms (16.9%), and 9 were transferred to a rehabilitation facility for extensive residual symptoms, including hypophonia, disturbed conscious level, dysphagia, vegetative state or coma, short-term memory loss, and tonic-clonic seizures. Four patients (6.2%) died, see Table 2.
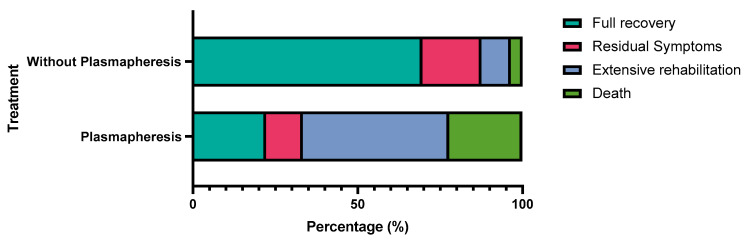
The type of vaccine administered was not statistically associated with any specific outcome (p = 0.124), see Table 3; however, patients with autoimmune encephalitis and other subtypes were more likely to undergo full recovery (p < 0.001), see Table 4. Patients who did not receive plasmapheresis were more likely to make a full recovery (p = 0.002), see Table 5 and Figure 2.
Table 3.
Relation between the type of vaccine and the outcome of treatment.
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Vaccine | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| AstraZeneca | 13 | 4 | 6 | 2 | 25 | 0.124 * |
| 52.0% | 16.0% | 24.0% | 8.0% | 100.0% | ||
| Pfizer BioNTech | 14 | 5 | 3 | 0 | 22 | |
| 63.6% | 22.7% | 13.6% | 0.0% | 100.0% | ||
| Moderna | 9 | 1 | 0 | 1 | 11 | |
| 81.8% | 9.1% | 0.0% | 9.1% | 100.0% | ||
| Sinopharm and Sputnik V | 5 | 0 | 0 | 0 | 5 | |
| 100.0% | 0.0% | 0.0% | 0.0% | 100.0% | ||
| Johnson & Johnson | 0 | 0 | 0 | 1 | 1 | |
| 0.0% | 0.0% | 0.0% | 100.0% | 100.0% | ||
| Unknown mRNA vaccine | 0 | 1 | 0 | 0 | 1 | |
| 0.0% | 100.0% | 0.0% | 0.0% | 100.0% | ||
* Fischer’s exact test.
Table 4.
Relation between the subtypes of encephalitis and the outcome of treatment.
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Encephalitis Subtype | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| Acute encephalitis | 10 | 1 | 0 | 0 | 11 | <0.001 * |
| 90.9% | 9.1% | 0.0% | 0.0% | 100.0% | ||
| ADEM | 10 | 2 | 1 | 2 | 15 | |
| 66.7% | 13.3% | 6.7% | 13.3% | 100.0% | ||
| AHEM | 0 | 0 | 2 | 2 | 4 | |
| 0.0% | 0.0% | 50.0% | 50.0% | 100.0% | ||
| Other | 21 | 8 | 6 | 0 | 35 | |
| 60.0% | 22.9% | 17.1% | 0.0% | 100.0% | ||
* Chi-square test.
Table 5.
Comparison between the outcome of patients who underwent plasmapheresis versus patients who did not undergo plasmapheresis.
| Outcome | ||||||
|---|---|---|---|---|---|---|
| Plasmapheresis | Full Recovery | Residual Symptoms | Extensive Rehabilitation | Death | Total | p-Value |
| Plasmapheresis | 2 | 1 | 4 | 2 | 9 | 0.002 * |
| 22.2% | 11.1% | 44.4% | 22.2% | 100.0% | ||
| Without Plasmapheresis | 39 | 10 | 5 | 2 | 56 | |
| 69.6% | 17.9% | 8.9% | 3.6% | 100.0% | ||
* Chi-square test.
Figure 2.
Comparison between the outcome of patients who underwent plasmapheresis versus patients who did not undergo plasmapheresis.
3.5. Quality Assessment
Fifty-two case reports and three case series were assessed using the JBI checklist, see Table 6. Most case reports (n = 40) had a low risk of bias, nine had a moderate risk, and three had a high risk of bias. All three case series had a moderate risk of bias; data are shown in Table 7. Three high-risk studies were excluded.
Table 6.
Joanna Brigg’s institute critical appraisal checklist for case reports.
| Author’s Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Total | % | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Ahmed et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Ahmed et al. | No | No | Yes | Yes | Yes | Yes | No | Yes | 5 | 63% | Moderate risk |
| Ahn et al. | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 6 | 75% | Low risk |
| Albsheer et al. | No | Yes | Yes | Yes | No | No | No | Yes | 4 | 50% | Moderate risk |
| Aljamea et al. | UC | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Al-Quliti et.al | No | Yes | Yes | Yes | Yes | No | No | Yes | 5 | 63% | Moderate risk |
| Ancau et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Asaduzzaman et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Autjimanon et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Bastide et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Cao et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Ebadi et al. | No | Yes | Yes | No | No | No | No | Yes | 3 | 38% | High risk |
| Escolà et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Etemadifaret et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Fan et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Fernandes et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Flannery et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Gao et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Garibashvili et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Gogu et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Grossi et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Huang et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Jarius et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 | 88% | Low risk |
| K. Kania et al. | No | Yes | Yes | Yes | No | Yes | UC | Yes | 5 | 63% | Moderate risk |
| Kobayashi et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 7 | 88% | Low risk |
| Kobayashi et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Kwon et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Lagioia et al. | No | Yes | No | No | No | No | No | No | 1 | 13% | High risk |
| Lazaro et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Li et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Mörz et al. | No | Yes | Yes | No | No | No | No | Yes | 3 | 38% | High risk |
| Moslemi et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Nagaratnam et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Naz et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Permezel et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Rastogi et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Rinaldi et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
| Saad et al. | No | No | Yes | No | Yes | Yes | Yes | Yes | 5 | 63% | Moderate risk |
| Sawczyńska et al. | No | No | Yes | Yes | Yes | Yes | Yes | Yes | 6 | 75% | Low risk |
| Senda et al. | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | 8 | 100% | Low risk |
| Shin HR et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Shyu et al. | No | No | Yes | Yes | Yes | Yes | No | Yes | 5 | 63% | Moderate risk |
| Sluyts et al. | No | Yes | Yes | Yes | No | No | No | Yes | 4 | 50% | Moderate risk |
| Takata et al. | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 8 | 100% | Low risk |
| Torrealba-Acosta et al. | No | Yes | Yes | Yes | No | Yes | UC | Yes | 5 | 63% | Moderate risk |
| Vences et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Vogrig et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Walter et al. | No | No | Yes | Yes | Yes | Yes | NA | Yes | 5 | 63% | Moderate risk |
| Werner et al. | No | Yes | Yes | Yes | Yes | Yes | UC | Yes | 6 | 75% | Low risk |
| Yazdanpanah et al. | Yes | Yes | Yes | Yes | No | Yes | No | Yes | 6 | 75% | Low risk |
| Zlotnik et al. | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | 7 | 88% | Low risk |
| Zuhorn et al. | No | Yes | Yes | Yes | Yes | Yes | No | Yes | 6 | 75% | Low risk |
Table 7.
Joanna Brigg’s institute critical appraisal checklist for case series.
| Author’s Name | Q1 | Q2 | Q3 | Q4 | Q5 | Q6 | Q7 | Q8 | Q9 | Q10 | Total | % | ROB |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Asioli et al. | Yes | Yes | Yes | Yes | Yes | No | UC | Yes | Yes | NA | 7 | 70% | Moderate risk |
| Ballout et al. | Yes | No | Yes | Yes | Yes | No | Yes | No | Yes | NA | 6 | 60% | Moderate risk |
| Maramattom et al. | Yes | UC | Yes | No | UC | UC | No | Yes | Yes | Yes | 5 | 50% | Moderate risk |
4. Discussion
In our sampled data, the majority of affected cases were males. According to the demographic distribution, encephalitis was likely to affect all ages, with the highest incidence in patients in their 40s. Most patients complained of encephalitis after the first dose 1–2 weeks post-vaccination. AstraZeneca was the most reported vaccine followed by Pfizer, Moderna, and others. Unlike typical diagnostic criteria for encephalitis (major criteria of patients presenting with altered mental status lasting ≥24 h with no alternative cause identified), the most occurring symptoms in our study were abnormal movements, fever, headache, and seizures [30]. CSF encephalitic findings and MRI abnormalities were evident in almost two-thirds of included patients. Most patients received corticosteroids as a part of the immunosuppressive regimen and achieved full recovery. However, death was common in patients who received plasmapheresis, which, to our understanding, could be contributed to the already late presentation and severe symptoms, which prompted plasma exchange therapy in the first place.
Although the exact etiology of vaccine-induced encephalitis is still not fully understood, it could be attributed to different potential mechanisms of vaccine-induced autoimmune diseases. COVID-19 vaccine was shown to trigger proinflammatory cytokine expression and the response of T-cells as in other vaccines [31]. This is important because these cytokines may reach the brain and activate microglial cells resulting in neuroinflammation [32]. However, the possible molecular mimicry between the vaccine antigens and self-antigens, or the acceleration of an ongoing autoimmune process caused by vaccines are still considered potential mechanisms [33].
Myalgia and general weakness were considered the most reported symptoms in a previous clinical trial assessing the safety of the AstraZeneca vaccine; notably, only two cases presented with neurologic symptoms, such as demyelinating polyradiculoneuropathy and hypoesthesia. Nonetheless, no encephalitis-associated symptoms were reported [34]. To our knowledge, no other clinical trials reported encephalitis post-vaccination. As the vaccinated population increased, several SARS-CoV-2 vaccines have been associated with neurological side effects on follow-up observational studies [35]. Most reported cases in the literature were immunized by AstraZeneca and Pfizer. The incidence of encephalitis after vaccination with the AstraZeneca and Pfizer-Biontech mRNA vaccines was estimated to be 8 per 10 million and 2 per 10 million vaccination doses, respectively, which denotes an extremely rare incidence of adverse event occurrence in comparison to other well-established vaccines such as hepatitis vaccines [24]. A previous systematic review addressing 11 patients with autoimmune encephalitis also corresponds with a greater majority of AstraZeneca and Pfizer predominant case reporting (8/15) [31]. Consistent with our findings, in which AstraZeneca and Pfizer comprised almost two-thirds of available cases. However, no statistical correlation between vaccine subtype and encephalitis outcomes could be established in our study. Generally, performing a lumbar puncture can suggest the presence of encephalitic involvement [36]. Huang et al. presented 9/11 cases with lumbar puncture findings indicating encephalitic changes. In our study, CSF sample abnormalities were indeed reported in most cases followed by MRI abnormalities. Full recovery is indicated in a greater percentage of all the aforementioned studies and also in our study.
Strengths and Limitations
The main strength of this systematic review is the use of a thorough search strategy to locate studies for evaluation, minimizing selection bias. These studies were evaluated using established critical appraisal tools and individually assessed by two authors to estimate the risk of bias in each study. We were able to synthesize comprehensive descriptive statistics. On the other hand, however, only 65 patients were included, which was considered insufficient to reach a precise conclusion. For a clear association between encephalitis and COVID-19 vaccination, a larger sample size with consistent reporting of clinical phases is required. To reduce the reporting bias, more clinical trials with appropriate follow-up of the treatment protocol must be analyzed. This would give a better insight into the severity and prognosis of the condition. Other limitations inherent to the nature of our systematic review of case reports and series follow naturally and must be considered.
5. Conclusions
With the increasing population of vaccinated individuals, a growing body of literature introduced a variety of rare side effects including encephalitis with its various subtypes. Studies included in our report should prompt awareness of possible encephalitis cases with presenting symptoms of abnormal movements, fever, and seizures, particularly 2–3 weeks post-vaccination. Physicians must pay attention to such adverse effects as they can be easily managed if noticed promptly and with excellent recovery rates. Further studies are needed to understand the underlying pathophysiologic mechanism and investigate an association relationship. Due to rarity of reported cases and good overall recovery, we still recommend COVID-19 vaccination.
Author Contributions
Conceptualization, M.A.; Screening, M.A., M.A.E., M.A.H., M.M.A., M.M. and L.S.M.; Protocol, M.A.E.; Formal analysis, M.A. and A.A.; Data extraction, M.A., A.A., Y.H., M.A.E., M.A.H., M.M.A., L.S.M. and M.M.; Writing—original draft preparation, M.A.H., M.A.E. and Y.H.; Writing—review and editing, A.N., A.A., M.A., M.M.A., Y.H. and L.S.M.; Visualization, M.M.A. and A.A.; Supervision, A.N., A.A. and M.A.; Validation: A.A., A.N. and M.A.; Software: A.A., A.N. and M.A.; Resources A.N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study as this is a secondary study incorporating data from previously published studies.
Informed Consent Statement
Patient consent was waived as no patient identification data were included for this is a secondary study incorporating data from previously published studies.
Data Availability Statement
The data that support the findings of this systematic review are available from the original studies, but restrictions may apply. Some authors may not have provided open access to their data. All data are available upon reasonable request and with permission of the original authors.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Granerod J., Ambrose H.E., Davies N.W.S., Clewley J.P., Walsh A.L., Morgan D., Cunningham R., Zuckerman M., Mutton K.J., Solomon T., et al. Causes of encephalitis and differences in their clinical presentations in England: A multicentre, population-based prospective study. Lancet Infect. Dis. 2010;10:835–844. doi: 10.1016/S1473-3099(10)70222-X. [DOI] [PubMed] [Google Scholar]
- 2.Ellul M., Solomon T. Acute encephalitis—Diagnosis and management. Clin. Med. 2018;18:155–159. doi: 10.7861/clinmedicine.18-2-155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coronavirus Disease (COVID-19) [(accessed on 12 November 2022)]. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019?adgroupsurvey={adgroupsurvey}&gclid=Cj0KCQiAiJSeBhCCARIsAHnAzT88OV4oFdsSZEUH8dm2dTrqizrL4qFUaSGociASz3fTLjYEhyp3vw0aAmayEALw_wcB.
- 4.Sahin U., Muik A., Derhovanessian E., Vogler I., Kranz L.M., Vormehr M., Baum A., Pascal K., Quandt J., Maurus D., et al. COVID-19 vaccine BNT162b1 elicits human antibody and TH1 T-cell responses. Nature. 2020;586:594–599. doi: 10.1038/s41586-020-2814-7. [DOI] [PubMed] [Google Scholar]
- 5.Mascellino M.T., Di Timoteo F., De Angelis M., Oliva A. Overview of the Main Anti-SARS-CoV-2 Vaccines: Mechanism of Action, Efficacy and Safety. Infect. Drug Resist. 2021;14:3459–3476. doi: 10.2147/IDR.S315727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abufares H.I., Oyoun Alsoud L., Alqudah M.A.Y., Shara M., Soares N.C., Alzoubi K.H., El-Huneidi W., Bustanji Y., Soliman S.S.M., Semreen M.H., et al. COVID-19 Vaccines, Effectiveness, and Immune Responses. Int. J. Mol. Sci. 2022;23:15415. doi: 10.3390/ijms232315415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skowronski D.M., De Serres G. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2021;384:1576–1578. doi: 10.1056/nejmc2036242. [DOI] [PubMed] [Google Scholar]
- 8.Kaur R.J., Dutta S., Bhardwaj P., Charan J., Dhingra S., Mitra P., Singh K., Yadav D., Sharma P., Misra S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021;36:427–439. doi: 10.1007/s12291-021-00968-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Garg R.K., Paliwal V.K. Spectrum of neurological complications following COVID-19 vaccination. Neurol. Sci. 2022;43:3–40. doi: 10.1007/s10072-021-05662-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meppiel E., Peiffer-Smadja N., Maury A., Bekri I., Delorme C., Desestret V., Gorza L., Hautecloque-Raysz G., Landre S., Lannuzel A., et al. Neurologic manifestations associated with COVID-19: A multicentre registry. Clin. Microbiol. Infect. 2021;27:458–466. doi: 10.1016/j.cmi.2020.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vences M.A., Canales D., Albujar M.F., Barja E., Araujo-Chumacero M.M., Cardenas E., Alvarez A., Urrunaga-Pastor D. Post-Vaccinal Encephalitis with Early Relapse after BNT162b2 (COMIRNATY) COVID-19 Vaccine: A Case Report. Vaccines. 2022;10:1065. doi: 10.3390/vaccines10071065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao J.-J., Tseng H.-P., Lin C.-L., Hsu R.-F., Lee M.-H., Liu C.-H. Acute encephalitis after COVID-19 vaccination: A case report and literature review. Hum. Vaccines Immunother. 2022;18:2082206. doi: 10.1080/21645515.2022.2082206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shyu S., Fan H.-T., Shang S.-T., Chan J.-S., Chiang W.-F., Chiu C.-C., Chen M.-H., Shyu H.-Y., Hsiao P.-J. Clinical Manifestation, Management, and Outcomes in Patients with COVID-19 Vaccine-Induced Acute En-cephalitis: Two Case Reports and a Literature Review. Vaccines. 2022;10:1230. doi: 10.3390/vaccines10081230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zlotnik Y., Gadoth A., Abu-Salameh I., Horev A., Novoa R., Ifergane G. Case Report: Anti-LGI1 Encephalitis Following COVID-19 Vaccination. Front. Immunol. 2021;12:813487. doi: 10.3389/fimmu.2021.813487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asaduzzaman M., Purkayastha B., Alam M.M.J., Chakraborty S.R., Roy S., Ahmed N. COVID-19 mRNA vaccine-associated encephalopathy, myocarditis, and thrombocytopenia with ex-cellent response to methylprednisolone: A case report. J. Neuroimmunol. 2022;368:577883. doi: 10.1016/j.jneuroim.2022.577883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nagaratnam S.A., Ferdi A.C., Leaney J., Lee R.L.K., Hwang Y.T., Heard R. Acute disseminated encephalomyelitis with bilateral optic neuritis following ChAdOx1 COVID-19 vaccination. BMC Neurol. 2022;22:54. doi: 10.1186/s12883-022-02575-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinaldi V., Bellucci G., Romano A., Bozzao A., Salvetti M. ADEM after ChAdOx1 nCoV-19 vaccine: A case report. Mult. Scler. J. 2022;28:1151–1154. doi: 10.1177/13524585211040222. [DOI] [PubMed] [Google Scholar]
- 18.Permezel F., Borojevic B., Lau S., de Boer H.H. Acute disseminated encephalomyelitis (ADEM) following recent Ox-ford/AstraZeneca COVID-19 vaccination. Forensic Sci. Med. Pathol. 2022;18:74–79. doi: 10.1007/s12024-021-00440-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Al-Quliti K., Qureshi A., Quadri M., Abdulhameed B., Alanazi A., Alhujeily R. Acute Demyelinating Encephalomyelitis Post-COVID-19 Vaccination: A Case Report and Literature Review. Diseases. 2022;10:13. doi: 10.3390/diseases10010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazaro L.G., Cossio J.E.P., Luis M.B., Tamagnini F., Mejia D.A.P., Solarz H., Liguori N.A.F., Alonso R.N. Acute disseminated encephalomyelitis following vaccination against SARS-CoV-2: A case report. Brain Behav. Immun. Health. 2022;20:100439. doi: 10.1016/j.bbih.2022.100439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yazdanpanah F., Iranpour P., Haseli S., Poursadeghfard M., Yarmahmoodi F. Acute disseminated encephalomyelitis (ADEM) after SARS-CoV-2 vaccination: A case report. Radiol. Case Rep. 2022;17:1789–1793. doi: 10.1016/j.radcr.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao L., Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: A case report. Acta Neurol. Belg. 2022;122:793–795. doi: 10.1007/s13760-021-01608-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutta S., Kaur R., Charan J., Bhardwaj P., Ambwani S.R., Babu S., Goyal J.P., Haque M. Analysis of Neurological Adverse Events Reported in VigiBase From COVID-19 Vaccines. Cureus. 2022;14:e21376. doi: 10.7759/cureus.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuhorn F., Graf T., Klingebiel R., Schäbitz W., Rogalewski A. Postvaccinal Encephalitis after ChAdOx1 nCov-19. Ann. Neurol. 2021;90:506–511. doi: 10.1002/ana.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fan H.-T., Lin Y.-Y., Chiang W.-F., Lin C.-Y., Chen M.-H., Wu K.-A., Chan J.-S., Kao Y.-H., Shyu H.-Y., Hsiao P.-J. COVID-19 vaccine-induced encephalitis and status epilepticus. Qjm Int. J. Med. 2022;115:91–93. doi: 10.1093/qjmed/hcab335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al-Mashdali A.F., Ata Y.M., Sadik N. Post-COVID-19 vaccine acute hyperactive encephalopathy with dramatic response to methylprednisolone: A case report. Ann. Med. Surg. 2021;69:102803. doi: 10.1016/j.amsu.2021.102803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lazarus J.V., Wyka K., White T.M., Picchio C.A., Rabin K., Ratzan S.C., Leigh J.P., Hu J., El-Mohandes A. Revisiting COVID-19 vaccine hesitancy around the world using data from 23 countries in 2021. Nat. Commun. 2022;13:3801. doi: 10.1038/s41467-022-31441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Page M.J., McKenzie J.E., Bossuyt P.M. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Br. Med. J. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ouzzani M., Hammady H., Fedorowicz Z., Elmagarmid A. Rayyan—A web and mobile app for systematic reviews. Syst. Rev. 2016;5:210. doi: 10.1186/s13643-016-0384-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Venkatesan A., Tunkel A.R., Bloch K.C., Lauring A.S., Sejvar J., Bitnun A., Stahl J.-P., Mailles A., Drebot M., Rupprecht C.E., et al. Case definitions, diagnostic algorithms, and priorities in encephalitis: Consensus statement of the international encephalitis consortium. Clin. Infect. Dis. 2013;57:1114–1128. doi: 10.1093/cid/cit458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y.-F., Ho T.-C., Chang C.-C., Shen D.H.-Y., Chan H.-P., Chuang K.-P., Tyan Y.-C., Yang M.-H. A Rare Adverse Effect of the COVID-19 Vaccine on Autoimmune Encephalitis. Vaccines. 2022;10:1114. doi: 10.3390/vaccines10071114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hervé C., Laupèze B., Del Giudice G., Didierlaurent A.M., Tavares Da Silva F. The how’s and what’s of vaccine reac-togenicity. Npj Vaccines. 2019;4:39. doi: 10.1038/s41541-019-0132-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Khan E., Shrestha A.K., Colantonio M.A., Liberio R.N., Sriwastava S. Acute transverse myelitis following SARS-CoV-2 vaccination: A case report and review of literature. J. Neurol. 2022;269:1121–1132. doi: 10.1007/s00415-021-10785-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsey A.R., Sobieszczyk M.E., Hirsch I., Sproule S., Robb M.L., Corey L., Neuzil K.M., Hahn W., Hunt J., Mulligan M.J., et al. Phase 3 Safety and Efficacy of AZD1222 (ChAdOx1 nCoV-19) COVID-19 Vaccine. N. Engl. J. Med. 2021;385:2348–2360. doi: 10.1056/NEJMoa2105290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maramattom B.V., Lotlikar R.S., Sukumaran S. Central nervous system adverse events after ChAdOx1 vaccination. Neurol. Sci. 2022;43:3503–3507. doi: 10.1007/s10072-022-06000-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liotta E.M., Batra A., Clark J.R., Shlobin N.A., Hoffman S.C., Orban Z.S., Koralnik I.J. Frequent neurologic manifestations and encephalopathy-associated morbidity in COVID-19 patients. Ann. Clin. Transl. Neurol. 2020;7:2221–2230. doi: 10.1002/acn3.51210. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this systematic review are available from the original studies, but restrictions may apply. Some authors may not have provided open access to their data. All data are available upon reasonable request and with permission of the original authors.