Abstract
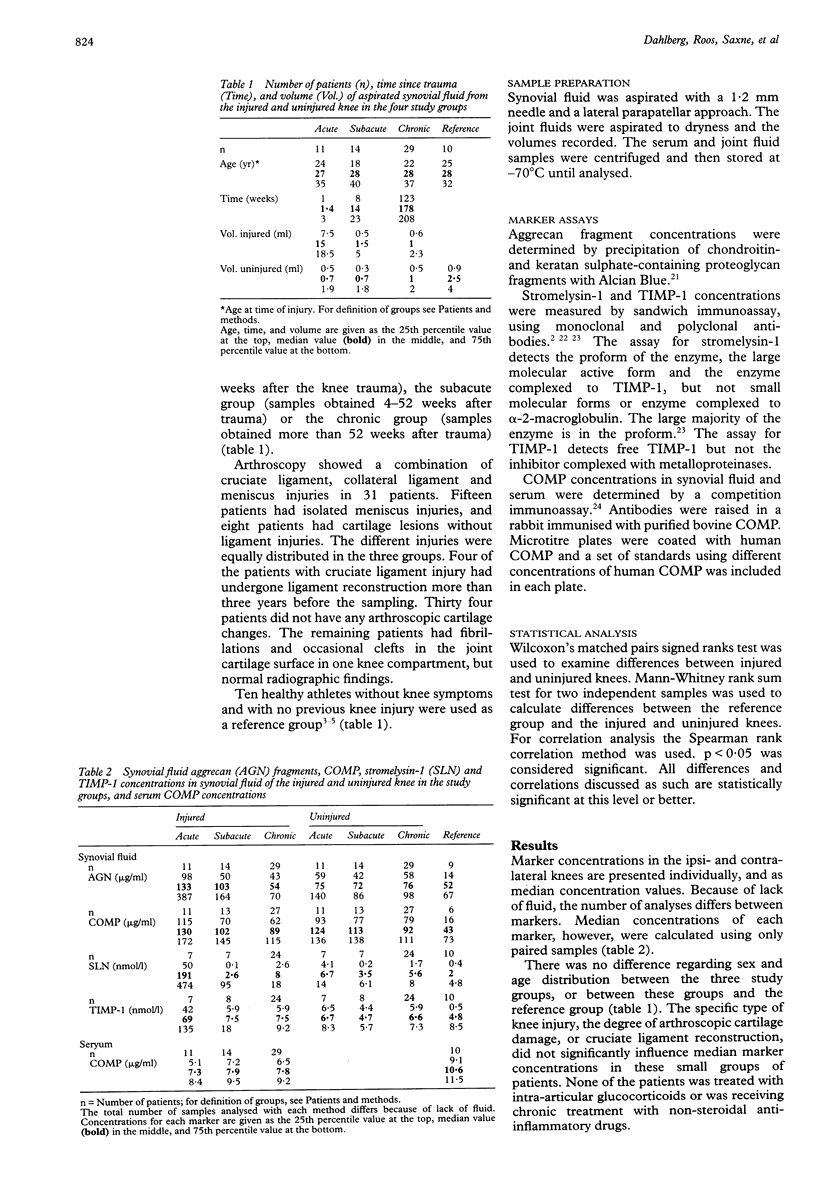
OBJECTIVE--To examine if unilateral knee injury affects the synovial fluid concentrations of aggrecan fragments, cartilage oligomeric matrix protein (COMP) fragments, stromelysin-1, and tissue inhibitor of metalloproteinases-1 (TIMP-1) in the contralateral uninjured knee. METHODS--Synovial fluids from the injured and uninjured knees were obtained at different times in a group of patients after unilateral knee trauma. Serum samples were obtained on the same occasion. Concentrations of aggrecan fragments were determined by precipitation with Alcian Blue; those of COMP fragments, stromelysin-1, and TIMP-1 were measured by immunoassay. Concentrations were compared with those in a reference group of 10 healthy volunteers. RESULTS--Immediately after knee injury, concentrations of aggrecan fragments, COMP fragments, stromelysin-1 and TIMP-1 were increased in the synovial fluid of the injured knee. However, concentrations of aggrecan and COMP fragments, and stromelysin-1 increased also in the contralateral uninjured knee immediately after injury, but less than in the injured knee. Subsequently, the concentrations of all markers decreased in the synovial fluid of the injured knee, but remained unchanged in the uninjured knee. The concentration of aggrecan fragments in the injured knee decreased to less than that in the uninjured knee in the chronic phase. Serum concentrations of COMP were much smaller than those in synovial fluid. CONCLUSIONS--The increased concentrations of aggrecan and COMP fragments and stromelysin-1 in the joint fluid of the contralateral, uninjured knee following unilateral knee injury, compared with concentrations in healthy reference knees, suggest changes in joint cartilage metabolism in both knees following unilateral knee injury. The mechanisms for these changes are unclear. The low serum concentration of COMP makes it less likely that there is any significant 'exchange' of molecular makers between the knees. A further consequence of these findings is that the contralateral knee cannot be recommended as the only control joint in studies of matrix metabolism in patients with unilateral knee injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Björnsson S. Simultaneous preparation and quantitation of proteoglycans by precipitation with alcian blue. Anal Biochem. 1993 May 1;210(2):282–291. doi: 10.1006/abio.1993.1197. [DOI] [PubMed] [Google Scholar]
- Carney S. L., Billingham M. E., Muir H., Sandy J. D. Demonstration of increased proteoglycan turnover in cartilage explants from dogs with experimental osteoarthritis. J Orthop Res. 1984;2(3):201–206. doi: 10.1002/jor.1100020301. [DOI] [PubMed] [Google Scholar]
- Cooksley S., Hipkiss J. B., Tickle S. P., Holmes-Ievers E., Docherty A. J., Murphy G., Lawson A. D. Immunoassays for the detection of human collagenase, stromelysin, tissue inhibitor of metalloproteinases (TIMP) and enzyme-inhibitor complexes. Matrix. 1990 Oct;10(5):285–291. doi: 10.1016/s0934-8832(11)80183-6. [DOI] [PubMed] [Google Scholar]
- Dahlberg L., Ryd L., Heinegård D., Lohmander L. S. Proteoglycan fragments in joint fluid. Influence of arthrosis and inflammation. Acta Orthop Scand. 1992 Aug;63(4):417–423. doi: 10.3109/17453679209154758. [DOI] [PubMed] [Google Scholar]
- Floman Y., Eyre D. R., Glimcher M. J. Induction of osteoarthrosis in the rabbit knee joint: biochemical studies on the articular cartilage. Clin Orthop Relat Res. 1980 Mar-Apr;(147):278–286. [PubMed] [Google Scholar]
- Gauffin H., Pettersson G., Tegner Y., Tropp H. Function testing in patients with old rupture of the anterior cruciate ligament. Int J Sports Med. 1990 Feb;11(1):73–77. doi: 10.1055/s-2007-1024766. [DOI] [PubMed] [Google Scholar]
- Geborek P., Saxne T., Heinegård D., Wollheim F. A. Measurement of synovial fluid volume using albumin dilution upon intraarticular saline injection. J Rheumatol. 1988 Jan;15(1):91–94. [PubMed] [Google Scholar]
- Glant T. T., Mikecz K., Arzoumanian A., Poole A. R. Proteoglycan-induced arthritis in BALB/c mice. Clinical features and histopathology. Arthritis Rheum. 1987 Feb;30(2):201–212. doi: 10.1002/art.1780300211. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Inerot S., Wieslander J., Lindblad G. A method for the quantification of cartilage proteoglycan structures liberated to the synovial fluid during developing degenerative joint disease. Scand J Clin Lab Invest. 1985 Sep;45(5):421–427. doi: 10.1080/00365518509155238. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Saxne T. Molecular markers of processes in cartilage in joint disease. Br J Rheumatol. 1991;30 (Suppl 1):21–24. [PubMed] [Google Scholar]
- Johnson R. J., Kettelkamp D. B., Clark W., Leaverton P. Factors effecting late results after meniscectomy. J Bone Joint Surg Am. 1974 Jun;56(4):719–729. [PubMed] [Google Scholar]
- Kannus P., Järvinen M. Posttraumatic anterior cruciate ligament insufficiency as a cause of osteoarthritis in a knee joint. Clin Rheumatol. 1989 Jun;8(2):251–260. doi: 10.1007/BF02030082. [DOI] [PubMed] [Google Scholar]
- Kiviranta I., Tammi M., Jurvelin J., Sämänen A. M., Helminen H. J. Moderate running exercise augments glycosaminoglycans and thickness of articular cartilage in the knee joint of young beagle dogs. J Orthop Res. 1988;6(2):188–195. doi: 10.1002/jor.1100060205. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Dahlberg L., Ryd L., Heinegård D. Increased levels of proteoglycan fragments in knee joint fluid after injury. Arthritis Rheum. 1989 Nov;32(11):1434–1442. doi: 10.1002/anr.1780321113. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Dahlberg L., Roos H., Björnsson S., Lark M. W. Stromelysin, tissue inhibitor of metalloproteinases and proteoglycan fragments in human knee joint fluid after injury. J Rheumatol. 1993 Aug;20(8):1362–1368. [PubMed] [Google Scholar]
- Lohmander L. S., Hoerrner L. A., Lark M. W. Metalloproteinases, tissue inhibitor, and proteoglycan fragments in knee synovial fluid in human osteoarthritis. Arthritis Rheum. 1993 Feb;36(2):181–189. doi: 10.1002/art.1780360207. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S. Markers of cartilage metabolism in arthrosis. A review. Acta Orthop Scand. 1991 Dec;62(6):623–632. doi: 10.3109/17453679108994513. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Roos H., Dahlberg L., Hoerrner L. A., Lark M. W. Temporal patterns of stromelysin-1, tissue inhibitor, and proteoglycan fragments in human knee joint fluid after injury to the cruciate ligament or meniscus. J Orthop Res. 1994 Jan;12(1):21–28. doi: 10.1002/jor.1100120104. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S., Saxne T., Heinegård D. K. Release of cartilage oligomeric matrix protein (COMP) into joint fluid after knee injury and in osteoarthritis. Ann Rheum Dis. 1994 Jan;53(1):8–13. doi: 10.1136/ard.53.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz R. W., Goldberg V. M., Malemud C. J. Metabolic responses of cartilage in experimentally induced osteoarthritis. Ann Rheum Dis. 1981 Dec;40(6):584–592. doi: 10.1136/ard.40.6.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. P., Gore D. R., Sepic S. B., Mollinger L. A. Antalgic maneuvers during walking in men with unilateral knee disability. Clin Orthop Relat Res. 1985 Oct;(199):192–200. [PubMed] [Google Scholar]
- Palmoski M., Perricone E., Brandt K. D. Development and reversal of a proteoglycan aggregation defect in normal canine knee cartilage after immobilization. Arthritis Rheum. 1979 May;22(5):508–517. doi: 10.1002/art.1780220511. [DOI] [PubMed] [Google Scholar]
- Saxne T., Heinegård D. Cartilage oligomeric matrix protein: a novel marker of cartilage turnover detectable in synovial fluid and blood. Br J Rheumatol. 1992 Sep;31(9):583–591. doi: 10.1093/rheumatology/31.9.583. [DOI] [PubMed] [Google Scholar]
- Sherman M. F., Warren R. F., Marshall J. L., Savatsky G. J. A clinical and radiographical analysis of 127 anterior cruciate insufficient knees. Clin Orthop Relat Res. 1988 Feb;227:229–237. [PubMed] [Google Scholar]
- Walakovits L. A., Moore V. L., Bhardwaj N., Gallick G. S., Lark M. W. Detection of stromelysin and collagenase in synovial fluid from patients with rheumatoid arthritis and posttraumatic knee injury. Arthritis Rheum. 1992 Jan;35(1):35–42. doi: 10.1002/art.1780350106. [DOI] [PubMed] [Google Scholar]


