Abstract
Memories are encoded in neural ensembles during learning and stabilized by post-learning reactivation. Integrating recent experiences into existing memories ensures that memories contain the most recently available information, but how neural ensembles accomplish this critical process remains unknown. Here we show that in mice, a strong aversive experience drives the offline ensemble reactivation of not only the recent aversive memory but also a neutral memory formed two days prior, spreading the fear from the recent aversive memory to the previous neutral memory. We find that fear specifically spreads retrospectively, but not prospectively, to neutral memories across days. Consistent with prior studies, we find reactivation of the recent aversive memory ensemble during the offline period following learning. However, a strong aversive experience also increases co-reactivation of the aversive and neutral memory ensembles during the offline period. Finally, inhibiting hippocampal reactivation during this offline period abolishes the spread of fear from the aversive experience to the neutral memory. Taken together, these results demonstrate that strong aversive experience can drive retrospective memory integration through the offline co-reactivation of recent memory ensembles with memory ensembles formed days prior, providing a neural mechanism by which memories can be integrated across days.
Keywords: hippocampus, memory integration, memory-linking, ensemble, reactivation, co-firing, offline periods, stress, PTSD
Individual memories are initially encoded by ensembles of cells active during a learning event1–5 and are stabilized during offline periods following learning through reactivation of those ensembles6–17. These reactivations often occur in brief bursts during sharp-wave ripples, which are necessary to drive memory consolidation18–20. However, animals are constantly aggregating new memories and updating past memories as new, relevant information is learned. Most studies of associative learning have focused on cues that directly precede or occur with an outcome. However, oftentimes in nature, a predictor may not immediately precede an outcome but animals are nonetheless capable of learning to make an inference about the association (e.g., conditioned taste aversion)21. It is unclear the environmental variables that could promote memories to be linked across long periods (i.e., days), and the neural mechanisms of memory integration across such disparate time periods are poorly understood. Moreover, while it has been shown that offline periods support memory consolidation, recent studies have suggested that offline periods following learning may be important for memory integration processes as well22–25.
Strong aversive experience drives retrospective memory-linking
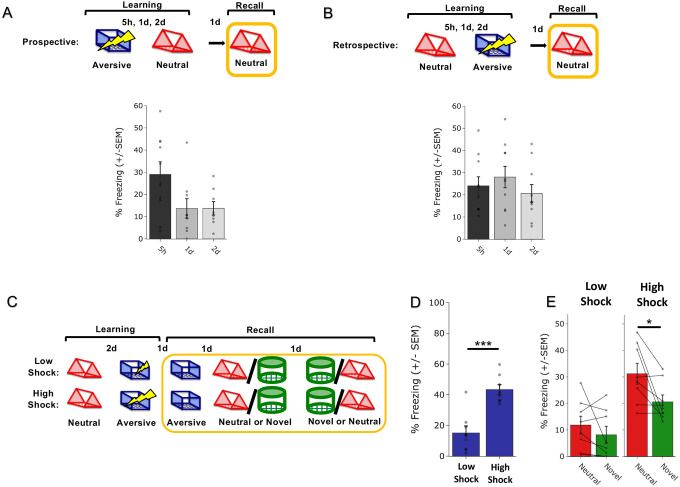
To investigate how memories are integrated across days, we first designed a behavioral experiment to test whether mice would spread fear from an aversive memory to a neutral memory formed two days prior (Retrospective memory-linking) or two days after (Prospective memory-linking). In the Retrospective group, mice first experienced a Neutral context followed by an Aversive context paired with a foot shock two days later. In the Prospective group, mice experienced an Aversive context followed by a Neutral context two days later. Both groups were then tested in the Aversive context to test for recall of the aversive memory, followed by testing in the previously experienced Neutral context or a new Novel context to test for non-specific fear generalization (Figure 1A). Memory-linking was defined as a selective increase in fear in the Neutral context compared to the Novel context, both contexts in which they had never been shocked. Mice froze no differently in the Aversive context in either group, suggesting that the perceived negative valence of the Aversive context was not different between groups (Figure 1B). Interestingly, in the Retrospective group, mice froze more in the Neutral context compared to the Novel context, suggesting that fear spread retrospectively from the Aversive context to the Neutral context experienced two days prior. However, in the Prospective group, there was no difference in freezing between the Neutral and Novel contexts, suggesting that fear did not spread prospectively to the Neutral context experienced two days after the Aversive context (Figure 1C). Consistent with prior studies, mice froze in the Neutral context in both Prospective and Retrospective conditions when the Neutral and Aversive contexts were experienced within one day26,27. However, when the contexts were separated by more than one day, mice froze in the Neutral context only in the Retrospective and not the Prospective condition (Extended Figure 1A,B).
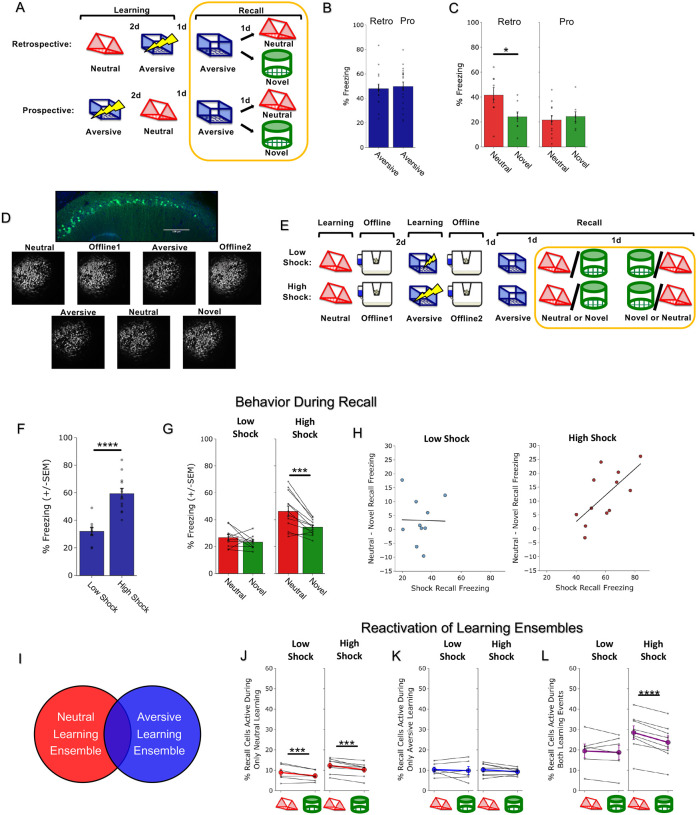
Figure 1. Strong aversive experience drives retrospective memory-linking to a neutral context learned days ago.
A) Schematic of prospective vs retrospective memory-linking behavior experiment. Mice either received a Neutral experience followed by an Aversive experience two days later (Retrospective) or the Aversive experience followed by Neutral (Prospective). One day after the second experience, mice were tested in the Aversive context they were shocked in. The following day, mice were tested in either the previously experienced Neutral context or a Novel context.
B) Freezing during Aversive recall in Prospective vs Retrospective groups. There was no difference in Aversive recall freezing between Prospective & Retrospective conditions (t34 = 0.36, p = 0.72).
C) Freezing during Neutral vs Novel recall in Prospective vs Retrospective groups. There was a significant interaction between freezing in Neutral vs Novel recall in the Retrospective vs Prospective groups, suggesting the Aversive experience retrospectively linked to the Neutral memory, but not prospectively. Significant interaction between Direction (Prospective vs Retrospective) and Context (Neutral vs Novel), (F1,32 = 4.90, p = 0.034). Post-hoc, Prospective (t16.76 = 0.512, p = 0.615), Retrospective (t14 = 2.34, p = 0.069).
D) Representative histology (top) of GCaMP6f expression in hippocampal CA1, and maximum intensity projections of an example mouse across all recorded sessions.
E) Schematic of Low Shock vs High Shock retrospective memory-linking experiment. Mice received a Neutral experience followed by a 1hr offline session in their homecage. Two days later, they received either 3 low shocks or 3 high shocks in an Aversive context, followed by another 1hr offline session in their homecage. The following day they were tested in the Aversive context, and for the following two days they were tested in either Neutral or Novel, counterbalanced. Calcium imaging was performed during all the sessions.
F) Freezing during Aversive recall in Low vs High Shock mice. Mice froze more in the Aversive context after receiving a high shock vs low shock (t18.8 = 5.877, p = 0.000012).
G) Freezing during Neutral vs Novel recall in Low vs High Shock mice. Mice only displayed enhanced freezing in Neutral vs Novel (i.e., retrospective memory-linking) after High Shock and not Low Shock. Significant effect of Context (Neutral vs Novel) (F1,20 = 17.32, p = 0.000048) and significant interaction between Context and Amplitude (F1,20 = 4.99, p = 0.037). High Shock mice froze more in the Neutral vs Novel contexts (t11 = 4.37, p = 0.002) while Low Shock mice froze no differently in the two contexts (t9 = 1.23, p = 0.249).
H) Correlation between Aversive recall freezing and Neutral – Novel recall freezing (measure of memory-linking). In High Shock mice, the strength of the aversive memory was correlated with the degree of memory-linking (R2 = 0.45, p = 0.016), while it did not in Low Shock mice (R2 = 0.0003, p = 0.963).
I) Schematic of the defined ensembles used to quantify J-L. We measured reactivation of cells exclusively active during Neutral learning and not Aversive learning (J), those exclusively active during Aversive learning and not Neutral learning (K), and those active during both Neutral and Aversive learning (L).
J) Cells active only during Neutral and not Aversive learning were selectively reactivated in the Neutral context compared to a Novel context. No effect of shock amplitude. Significant main effect of Context (Neutral vs Novel) (F1,12 = 24.44, p = 0.0003).
K) Cells active only during Aversive and not Neutral learning were no differently reactivated across Neutral vs Novel contexts. No main effects or interactions, Amplitude (F1,12 = 0.029, p = 0.869), Context (F1,12 = 1.39, p = 0.261).
L) Cells active during both Neutral and Aversive learning were selectively reactivated in the Neutral context compared to Novel context in High Shock mice. Significant interaction between Context (Neutral vs Novel) and Amplitude (Low vs High Shock) (F1,12 = 10.33, p = 0.007). Post-hoc tests: Low Shock (t5 = 0.55, p = 0.61), High Shock (t7 = 8.53, p = 0.00012).
How does the brain link recent aversive memories with past neutral memories formed days prior? It has previously been suggested that the emotional salience of an experience enhances its storage into memory28,29, as well as its likelihood of altering past neutral memories in humans30. Thus, we hypothesized that the more stressful the aversive experience, the more likely that fear would be retrospectively linked to a previous neutral memory. To test if increased stress would drive retrospective memory-linking, mice learned a Neutral context followed by an Aversive context paired with a low or high shock two days later (Low Shock group & High Shock group). Mice were then tested in the Aversive, Neutral, and a Novel context in the subsequent three days (Figure 1E). As expected, mice in the High Shock group froze more than mice in the Low Shock group during recall in the Aversive context (Figure 1F). We found that only High Shock mice exhibited a selective increase in freezing in the previously experienced Neutral context relative to the Novel context during recall (Figure 1G; Extended Figure 1C–E). If the intensity of the aversive experience influences the likelihood of memory-linking, we hypothesized that the strength of the aversive memory would correlate with the degree of retrospective memory-linking (as defined by the freezing in the Neutral context above the freezing in the Novel context). Indeed, in the High Shock mice, the freezing during Aversive context recall correlated with memory-linking strength (Figure 1H).
Since mice were freezing in a context in which they were not previously shocked, we hypothesized that retrospective memory-linking may be driven by the neural ensemble representing the aversive experience becoming associated with the neutral memory, and thus being reactivated during neutral memory recall to drive freezing26,27,31–33. To test this, we used open-source UCLA Miniscopes to measure hippocampal CA1 calcium dynamics (a brain area known to encode and represent episodic-like memories26,32) as mice learned the Neutral and Aversive contexts, during the offline homecage periods following each experience, and during each of the recall sessions (Figure 1D,E). During recall of the Neutral and Novel contexts, we measured the fraction of cells active during recall that were previously active during learning of the Neutral or Aversive contexts or both (Figure 1I–L; Extended Figure 3D,E). As expected, cells exclusively active during Neutral learning and not Aversive learning were more likely to be reactivated during Neutral recall than during Novel recall in both Low Shock and High Shock groups, suggesting a stable and selective neural population representing the neutral memory (Figure 1J). The cells exclusively active during Aversive learning were not selectively reactivated during Neutral or Novel recall in either group (Figure 1K). Interestingly, the cells active during both learning of the Neutral and Aversive contexts were more reactivated during Neutral recall than during Novel recall in the High Shock but not the Low Shock group (Figure 1L). This suggests that a strong aversive experience links the aversive memory with a prior neutral memory through a shared neural ensemble.
Strong aversive learning drives offline reactivation of a past neutral ensemble
We next asked what processes led to retrospective memory-linking. Previous work has suggested that offline periods following learning could promote not only the consolidation of recently formed memories, but also support the integration of memories22,24,25,34–36. Thus, we hypothesized that during the offline period following Aversive learning (while mice are in their homecage), not only would the ensemble active during aversive learning be reactivated to drive consolidation of the recently learned aversive memory, but if the aversive experience was strong enough, the ensemble active during the neutral experience from two days prior would be reactivated as well, integrating the two memories.
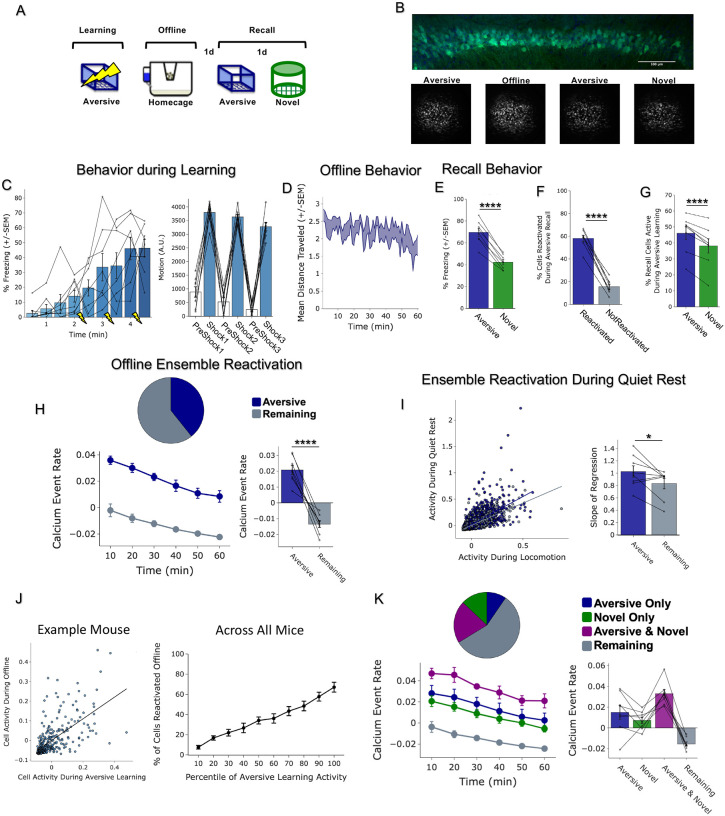
To first validate that we could detect ensemble reactivation after a salient experience using calcium imaging, we conducted a classical contextual fear conditioning experiment, recording hippocampal CA1 calcium dynamics using Miniscopes during Aversive learning, for the first hour offline following Aversive learning, and during recall of the Aversive context and of a Novel context (Extended Figure 2A,B). Consistent with the literature, we found that the ensemble of cells active during Aversive learning was reactivated offline and selectively reactivated during Aversive memory recall, suggesting a stable neural memory ensemble formed through offline ensemble reactivation(Extended Figure 2C–K).
To next investigate whether a strong aversive experience was driving offline reactivation of ensembles representing both the aversive and neutral memories, we performed calcium imaging recordings in CA1 during the offline periods following Neutral learning and subsequent Aversive learning in both Low and High Shock groups (Figure 2A). Consistent with the literature18,20 and with our previous experiment (Extended Figure 2), after Neutral learning mice showed greater calcium activity of the cells that were active during Neutral learning (Neutral ensemble) compared with cells not active during Neutral learning (Remaining ensemble) in both Low and High Shock groups, supporting the idea of ensemble reactivation to aid memory consolidation (Figure 2B, line graphs). To measure ensemble reactivation during the offline period after Aversive learning, we separated cells that were active during the offline period into four ensembles based on when those cells were previously active: Neutral ensemble represented cells active during Neutral learning and not Aversive learning; Aversive ensemble represented cells active during Aversive learning and not Neutral learning; Neutral ∩ Aversive ensemble represented cells that were active during both Neutral learning and Aversive learning; and Remaining ensemble represented cells not observed to be active prior to the offline period (Figure 2C). There was no difference in the fraction of cells that made up each ensemble across Low and High Shock groups (Figure 2C, pie charts). In the Low Shock group, consistent with prior literature, we found the Aversive ensemble and the Neutral ∩ Aversive ensemble had higher calcium activity than the Remaining ensemble and this activity decreased across the hour. The Neutral ensemble’s activity was not greater than that of the Remaining ensemble (Figure 2C, line graphs). These results are consistent with prior studies demonstrating offline reactivation of neural ensembles that were recently active during learning and that this reactivation decreases across time37. Interestingly, in the High Shock group, the Aversive, Neutral ∩ Aversive, and Neutral ensembles all had activity greater than the Remaining ensemble (Figure 2C, line graphs), suggesting that the high shock increased reactivation of the Neutral ensemble.
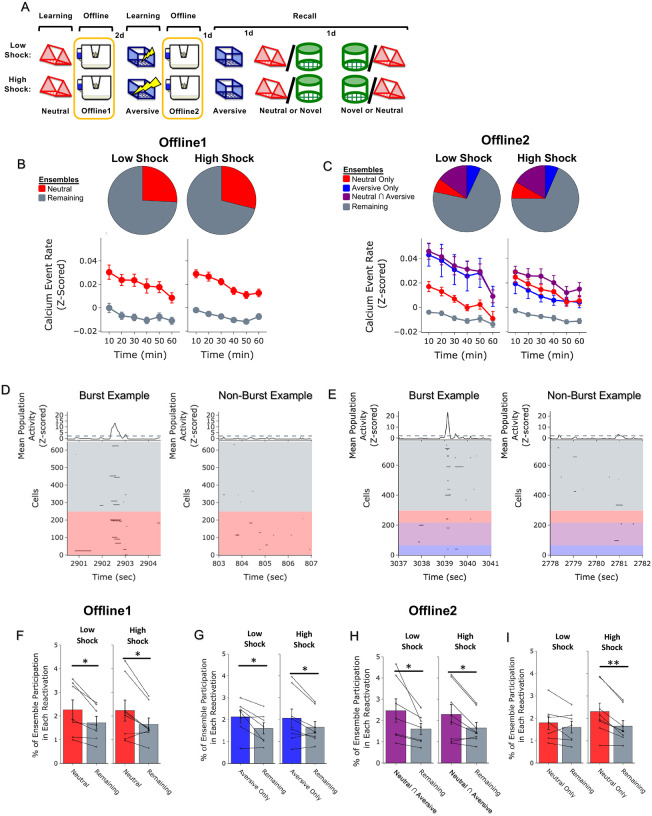
Figure 2. Strong aversive experience drives reactivation of a past neutral ensemble.
A) Schematic of experimental design, as in Figure 1D. In this figure, however, analyses are focused on the offline periods directly following learning.
B) During OfflineDay1, cells that were active during Neutral learning made up ~25–30% of the offline cell population (pie charts). This population was more highly active than the rest of the population (Remaining ensemble) across the hour (line graphs). For Low Shock: main effect of Ensemble (F1,6 = 13.81, p = 0.0099) and main effect of Time (F5,30 = 15.61, p = 0.00006). For High Shock: main effect of Ensemble (F1,7 = 53.61, p = 0.0002) and main effect of Time (F5,35 = 14.07, p = 0.0003).
C) During OfflineDay2, similar proportions of previously active cells were reactivated across Low and High shock groups (pie charts). In Low Shock mice, cells that were active during Aversive learning and not Neutral learning (Aversive ensemble), and cells that were active during both Aversive and Neutral learning (Neutral ∩ Aversive ensemble) were more highly reactivated than the rest of the population (Remaining ensemble); however, cells that were active during Neutral and not Aversive learning (Neutral ensemble) were not more highly reactivated than the Remaining ensemble (line graphs). In High Shock mice, the Aversive ensemble, the Neutral ∩ Aversive ensemble, and the Neutral ensemble all had higher activity than the Remaining ensemble. For Low Shock: main effect of Ensemble (F3,18 = 9.77, p = 0.0098) and main effect of Time (F5,30 = 8.77, p = 0.003). For High Shock: main effect of Ensemble (F3,21 = 7.88, p = 0.005) and main effect of Time (F5,35 = 9.15, p = 0.0009). Post-hoc Dunnett’s test comparing each ensemble’s activity to the Remaining ensemble as a reference. For Low Shock, the Aversive ensemble and Neutral ∩ Aversive ensemble had greater activity than the Remaining ensemble while the Neutral ensemble did not (p = 0.0016, 0.001, 0.356, respectively). In the High Shock group, the Aversive, Neutral ∩ Aversive, and Neutral ensembles were all different from the Remaining ensemble (p = 0.033, 0.0002, 0.006, respectively).
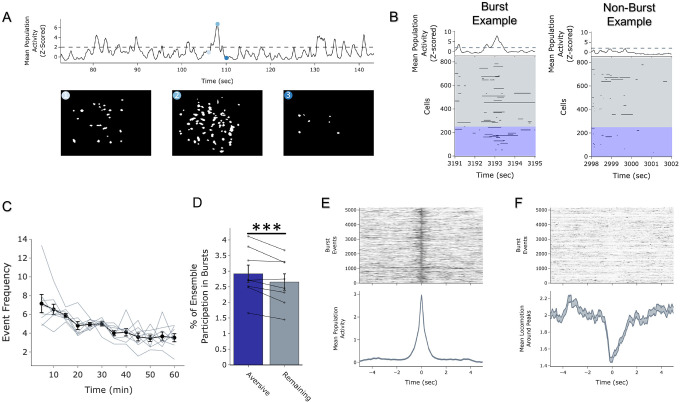
D) During the offline periods, hippocampal activity displayed brief bursts of neural activity. To detect these bursts, we computed the z-scored mean activity of the entire recorded population and thresholded it with z=2 and defined burst periods as all the timepoints above this threshold. The left raster represents an example burst period during Offline1, during which mean population activity briefly reached above threshold. Each row of the raster represents the activity of every recorded neuron, color-coded based on the ensemble it was a part of. The top black trace represents the z-scored mean population activity. The right represents an example non-burst period.
E) Same as D but an example burst and non-burst period for Offline2.
F) During OfflineDay1, a larger fraction of the Neutral ensemble participated in bursts than the Remaining ensemble did. No effect of amplitude or interaction. Significant main effect of Ensemble (Neutral vs Remaining) (F1,13 = 16.33, p = 0.001).
G) During OfflineDay2, a larger fraction of the Aversive ensemble participated in bursts than the Remaining ensemble. No effect of amplitude. Significant main effect of Ensemble (Aversive vs Remaining) (F1,13 = 13.57, p = 0.0028).
H) During OfflineDay2, a larger fraction of the Neutral ∩ Aversive ensemble participated in bursts than the Remaining ensemble. No effect of amplitude. Significant main effect of Ensemble (Neutral ∩ Aversive vs Remaining) (F1,13 = 13.95, p = 0.0025).
I) During OfflineDay2, only in High Shock mice, the Neutral ensemble also participated more than the Remaining ensemble in these burst events. Significant main effect of Ensemble (Neutral vs Remaining) (F1,13 = 19.58, p = 0.0007), and significant interaction between Ensemble and Amplitude (Low vs High Shock) (F1,13 = 5.186, p = 0.040). Post-hoc tests: Low Shock (t6 = 1.33, p = 0.23), High Shock (t7 = 4.88, p = 0.0036).
Since the Neutral ensemble was being highly reactivated after high shock, we next investigated whether these ensembles might be firing together on a finer temporal scale. Hippocampal activity is known to exhibit organized bursts, oftentimes accompanied by sharp-wave ripples in the local field potential, during which cells active during learning are preferentially reactivated18. We observed that during the offline recordings, hippocampal activity periodically exhibited brief bursts of activity during which numerous cells were co-active (Extended Figure 4A). We isolated these brief burst periods to ask whether ensembles that were previously active during learning were selectively participating in these brief burst events, to support memory consolidation and integration (Figure 2D,E; Extended Figure 4A,B; see Methods). We first measured these burst events after a single Aversive learning experience and found that a larger fraction of Aversive ensemble cells participated in these events than Remaining non-ensemble cells (Extended Figure 4D). Interestingly, these burst events coincided with the mouse briefly slowing down about 1 second prior to the event, and about 1 second after its onset resuming its locomotion, suggesting that these bursts occurred during periods of brief quiescence (Extended Figure 4F)18.
We then asked whether a strong shock paired with an Aversive context would drive the Neutral ensemble to also participate within these bursts after Aversive learning. In both Low and High Shock mice and after both Neutral and Aversive learning, frequencies of burst events (defined by periods when the mean activity of the entire recorded population reached above a required threshold, see Methods) were comparable across groups and slightly decreased across the hour (Extended Figure 3G,H). As expected, after Neutral learning, both Low and High Shock groups had a larger fraction of the Neutral ensemble participating in these burst events than the Remaining ensemble (Figure 2D,F). After Aversive learning, both groups again showed selective participation of the Aversive ensemble that was most recently active (Figure 2G) as well as of the Neutral ∩ Aversive ensemble that was previously active during both learning events (Figure 2H). However, only in the High Shock group (and not the Low Shock group) the Neutral ensemble selectively participated in these burst events as well (Figure 2I), suggesting that a strong aversive experience drove the recruitment of the Neutral ensemble into these burst events.
Strong aversive experience drives co-bursting of the Neutral ensemble with the Neutral ∩ Aversive ensemble
Since after High Shock, the Neutral and Aversive ensembles were both participating in burst events, we next asked whether the two ensembles co-participated within the same bursts, or whether they participated separately in different bursts. Co-bursting between the Neutral ensemble and Aversive ensemble could suggest a process through which the two ensembles can become integrated into a cell assembly likely to co-fire during memory recall thereafter. This process could occur through Hebbian plasticity38 or through behavioral timescale synaptic plasticity, which has been proposed to drive the formation of place fields in hippocampal neurons39. Previous work has shown that hippocampal neurons become highly co-active during recall of a fear memory but not during initial learning40, that co-activity relationships among hippocampal neurons can distinguish between contexts that a mouse has experienced41, and that ensembles that are highly co-active during an offline period following learning are more likely to be reactivated during memory recall than non-co-active neurons15.
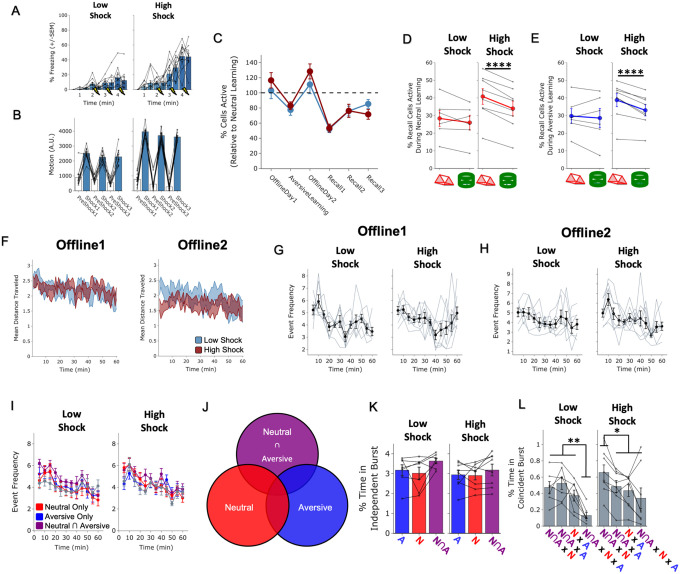
To ask whether the Neutral and Aversive ensembles were co-bursting after Aversive learning, we measured the fraction of burst events that the Aversive ensemble participated in alone, that the Neutral ensemble participated in alone, or that the two ensembles co-participated in (Figure 3A,B, see Methods). We hypothesized that high shock would increase the occurrence of co-bursting between the two ensembles. Surprisingly, we found that the High Shock mice did not exhibit an increase in co-bursting between the Neutral ensemble and Aversive ensemble. Instead, we found that high shock led the Neutral ensemble to participate independently more frequently than the Aversive ensemble during these burst events (Figure 3C).
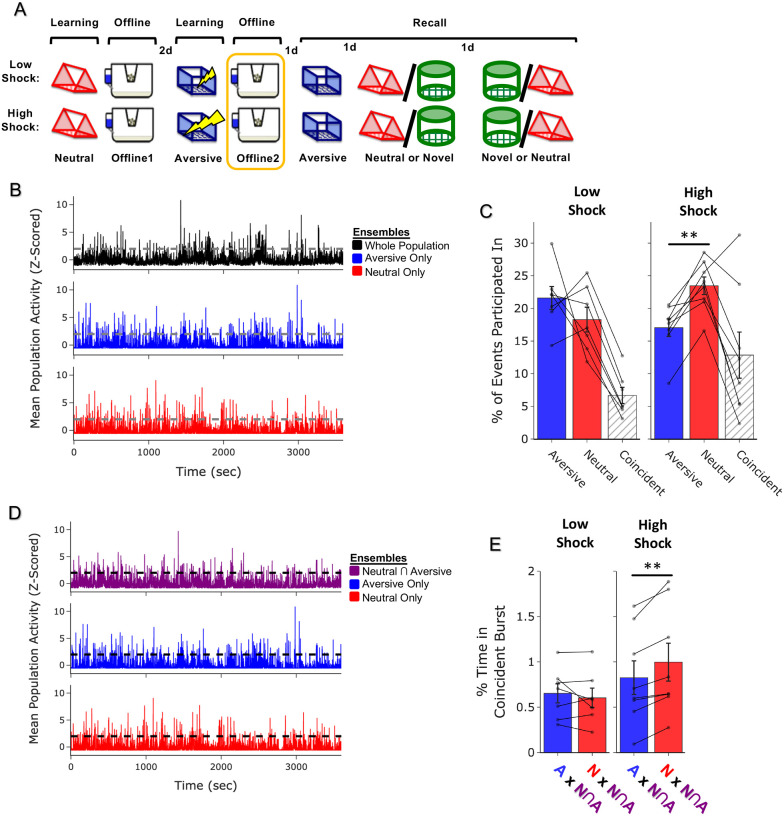
Figure 3. Strong aversive experience drives co-reactivation of the Neutral ensemble with the Neutral ∩ Aversive ensemble.
A) Schematic of experimental design, as in Figure 2A, but now focusing only on OfflineDay2, following Aversive learning.
B) Representation of the quantification of independent or coincident burst participation. Burst events were defined by the whole recorded population, as in Figure 2E. However, now the z-scored mean population activity of the Aversive and Neutral ensembles was computed to ask how frequently either ensemble or both ensembles participated in bursts.
C) Only in High Shock mice, the Neutral ensemble participated independently in a larger fraction of events than the Aversive ensemble did. The Aversive and Neutral ensembles participated coincidentally no differently in Low vs High shock mice. Significant main effect of Ensemble (F2,26 = 22.71, p = 0.0000002) and significant interaction between Ensemble & Amplitude (F2,26 = 5.64, p = 0.009). Post-hoc tests demonstrate significantly higher Neutral vs Aversive independent participation in High Shock mice (t7 = 6.04, p = 0.0016). No difference in co-participation between Low vs High Shock mice (t8.69 = 1.65, p = 0.13), and a trend toward lower independent participation of the Neutral ensemble in Low vs High Shock mice (t11.16 = 2.23, p = 0.095) and a trend toward higher independent participation of the Aversive ensemble in Low vs High Shock (t11.6 = 2.05, p = 0.095).
D) Burst events were now defined instead of on the entire population, now based on the mean activity of each ensemble reaching above threshold, to ask how frequently the Neutral ∩ Aversive ensemble co-bursted with either the Neutral ensemble or the Aversive ensemble.
E) In High Shock mice, the Neutral ∩ Aversive ensemble co-bursted with the Neutral ensemble more than with the Aversive ensemble. In Low Shock mice, the Neutral ∩ Aversive ensemble co-bursted no differently with the two ensembles. Significant interaction between Ensemble & Amplitude (F1,13 = 11.68, p = 0.0046). Post-hoc tests demonstrate higher co-participation with the Neutral ensemble in High Shock mice (t7 = 4.398, p = 0.006) and not in Low Shock mice (t6 = 0.940, p = 0.383).
Next, we asked whether the Neutral ensemble might be participating in bursts with other cells. Previously, we had found that the Neutral ∩ Aversive cells (those active during both Neutral and Aversive learning) were highly reactivated during Neutral memory recall in the High Shock group, and that this ensemble was the most highly active during the offline period (Figure 2C). Highly active subpopulations of neurons have been proposed to form a ‘hub-like’ population of neurons that may orchestrate the activity of other neurons in a larger network42. Therefore, these highly active neurons could be organizing the activity of other neurons in the hippocampus to drive activity during this offline period. Thus, we defined burst events separately for the Neutral ∩ Aversive ensemble, the Neutral ensemble, and the Aversive ensemble, to ask how frequently the Neutral ∩ Aversive ensemble was co-bursting with either of the other two ensembles (Figure 3D). Burst event frequencies did not vary across ensembles or across Low or High Shock groups (Extended Figure 3I). Surprisingly, we found that only in the High Shock group, the Neutral ∩ Aversive ensemble was selectively co-bursting with the Neutral ensemble, more than with the Aversive ensemble (Figure 3E). We further separated fractions of time co-bursting to ask how frequently the three ensembles fired independently and together, in all the possible combinations (Extended Figure 3J). We found that the three ensembles did not display different amounts of independent bursting in the Low Shock compared to High Shock group (Extended Figure 3K). Interestingly, in the High Shock group, the Neutral ∩ Aversive ensemble preferentially co-bursted with the Neutral ensemble, compared to the other combinations of ensembles (Extended Figure 3L). Collectively, these results suggested that high shock led the Neutral ensemble to co-burst more frequently with the Neutral ∩ Aversive ensemble.
Inhibition of hippocampal reactivation abolishes retrospective memory-linking
Finally, to test whether hippocampal reactivation was necessary to drive retrospective memory-linking, we inhibited hippocampal activity during the offline period following Aversive learning paired with a strong shock. We predicted that this would disrupt retrospective memory-linking. Mice were injected with a pan-neuronal, PSAM4-GlyR-expressing virus bilaterally in hippocampus and allowed 4 weeks to recover. PSAM4-GlyR (PSAM) is an inhibitory ionotropic receptor with no endogenous ligand, and binding of the PSEM ligand with the PSAM receptor causes robust hyperpolarization in neurons43. After 4 weeks of recovery from surgery, mice were given a Neutral experience followed by Aversive learning two days later. Immediately following Aversive learning, half the mice were administered PSEM (PSEM Group) and the other half saline as a control (Saline Group). These injections were repeated four times, every three hours, to cover inhibition across a 12-hour period. Two days later (to allow for washout of the drug), mice were tested in either the Neutral or a Novel context (Figure 4A,B). Saline group exhibited a selective increase in freezing in the Neutral over the Novel context, demonstrating retrospective memory-linking. In contrast, mice that received hippocampal inhibition no longer showed this increase in freezing in the Neutral context (Figure 4C). To ensure that this effect on retrospective memory-linking was not due to a disrupted memory for the Aversive context, we repeated the experiment, where mice experienced the Neutral and Aversive experiences as before, and half the mice received PSEM during the offline period while the other half received saline. Two days following Aversive learning, mice were tested in the Aversive context to test for the integrity of the aversive memory (Figure 4D). During Aversive memory recall, mice that received hippocampal inhibition froze no differently compared to saline controls, suggesting that the strong aversive memory was left intact (Figure 4C). These results suggest that hippocampal reactivation during the offline period is necessary to drive retrospective memory-linking.
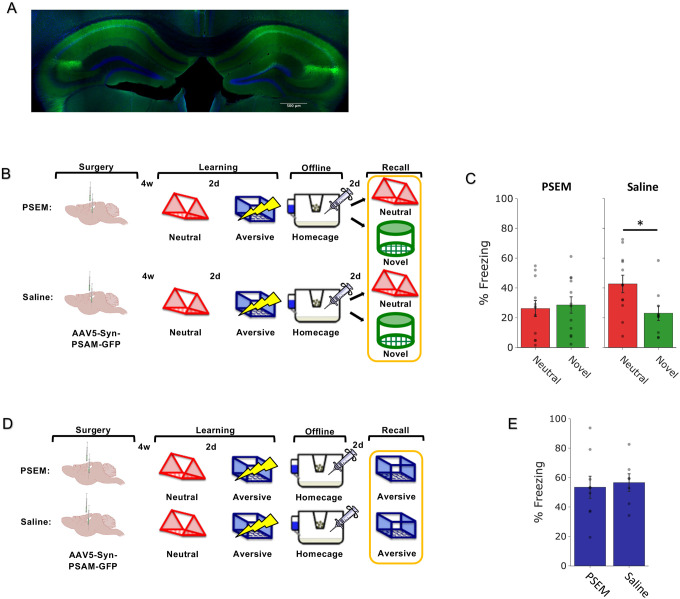
Figure 4. Hippocampal inhibition during the offline period abolishes retrospective memory-linking.
A) Representative histological verification of viral expression. Blue represents DAPI and green represents AAV5-Syn-PSAM-GFP.
B) Schematic of the behavioral experiment disrupting hippocampal activity during the offline period. Mice were injected with AAV5-Syn-PSAM-GFP into dorsal and ventral hippocampus. Mice all had a Neutral experience and two days later a strong Aversive experience. Right after Aversive learning, mice either had the hippocampus inactivated for 12hrs using the PSAM agonist, PSEM, or were given saline as a control. Two days later, mice were tested in the Aversive context, the Neutral context, or a Novel context for freezing.
C) Mice froze no differently in the Aversive context whether they had received hippocampal inhibition or not (t13.9 = 0.32, p = 0.748).
D) Schematic of the behavioral experiment as above, but this time to test the effects of hippocampal inactivation on memory-linking. Mice all had the Neutral and Aversive experiences as before, as well as PSEM or saline injections following Aversive learning; however, two days following Aversive learning, mice were either tested in the Neutral or Novel contexts.
E) Control (saline-treated) mice displayed retrospective memory-linking (i.e., higher freezing during Neutral vs Novel recall), while mice that received hippocampal inhibition (PSEM-treated) no longer displayed retrospective memory-linking. Significant interaction between Experimental Group (PSEM vs Sal) and Context (Neutral vs Novel) (F1,42 = 4.00, p = 0.05). Post-hoc tests demonstrate higher freezing in Neutral vs Novel contexts in the Sal group (t19.84 = 2.57, p = 0.03) and no difference in freezing in Neutral vs Novel contexts in the PSEM group (t22 = 0.31, p = 0.76).
Discussion
How animals actively update memories as they encounter new information remains a fundamental question in neuroscience44. Past work has shown that individual experiences are encoded by subpopulations of neurons across the brain that are highly active during learning45. These neuronal ensembles undergo synaptic modifications after learning to support memory storage46–49. After learning, activity of these ensembles is necessary50 and sufficient2 to drive memory recall, and their reactivation during memory recall is correlated with the strength of memory recall1. How memories encoded across time are integrated remains a critical and unanswered question in neuroscience. The memory allocation hypothesis suggests that neurons with high intrinsic excitability at the time of learning are likely to be allocated to a memory trace5,51. Prior studies suggest that two memories encoded within a day are likely to be linked because they share an overlapping population of highly excitable neurons during the initial learning. This shared neural ensemble links the two temporally related memories, such that the recall of one memory is more likely to trigger the recall of another memory that was encoded close in time4,26,27,33. Here we demonstrate that memories can be dynamically updated even days after they have been encoded and consolidated, and that this process is driven by ensemble co-reactivation during a post-learning period.
Whether linking memories across days is an adaptive or maladaptive process may depend on the environmental conditions. Under everyday circumstances, memories that are encoded far apart in time and which share no features in common may typically not need to be linked, and memories must also be segregated to allow for proper recall of distinct memories. Notably, hippocampus has been shown to successfully discriminate between distinct memories52,53. However, after a potentially life-threatening experience, especially one where the source of the aversive outcome is ambiguous (as in the aversive experience employed here), it could benefit an animal to link fear from that aversive experience to prior events, particularly if the event is rare and novel as seen in conditioned taste aversion21. Our results suggest that a highly aversive experience is more likely to drive memory-linking than a mild aversive experience is (Figure 1D–H), consistent with this intuition. Moreover, our results suggest that fear is more likely to be linked retrospectively to past events rather than prospectively to future events (Figure 1A–C). This is consistent with the notion that cues that occurred before an outcome can predict that outcome. On a shorter timescale, it has been well established that when a neutral cue directly precedes a footshock by seconds, this drives associative learning between the cue and the footshock to drive cue-elicited freezing54. Interestingly, however, if the cue instead occurs directly after the footshock, the animal no longer freezes in response to cue presentation thereafter55. Though the difference in timescale suggests that different mechanisms are likely at play in these two scenarios, our results are consistent with the idea that cues occurring prior to an outcome can be interpreted as predictive cues to the animal. A recent review has also suggested that animals use “retrospective cognitive maps” to infer the states that precede an outcome, to draw causal associations between those stimuli56. Our results suggest that offline periods are responsible for driving this retrospective inference (Figure 5).
Figure 5. Offline ensemble reactivation drives retrospective memory-linking across days.
After single experiences, the cells active during learning are reactivated to support their consolidation. After a strong aversive experience, memories are linked retrospectively across days by the co-reactivation of the ensembles representing both the recent and the past neutral memory ensembles.
Offline periods offer an opportunity for the brain to draw inferences about relationships that were not necessarily formed at the time of learning. In humans, it has been shown that an emotional experience can retrospectively increase memory for previously experienced neutral objects, only after a period of consolidation30. A separate study demonstrated that this retrospective memory enhancement coincided with increased fMRI BOLD activity in the ventral tegmental area and increased functional hippocampal-cortical coupling34. Moreover, a recent study in mice showed that two contexts with strongly shared geometrical features can be integrated right after learning, whereas two contexts with subtly shared geometrical features require an offline period after learning to drive their integration. During this offline period, cortical ensemble co-reactivation drives this memory integration57. Our study demonstrates that a highly aversive experience can alter the likelihood of retrospective memory-linking, that this is dependent upon post-learning hippocampal activity, and is accompanied by co-reactivation of the ensembles for the two memories.
Past studies have shown that ensemble reactivation occurs during both sleep (NREM and REM sleep) and wake states. Reactivation during different states have been proposed to support different memory processes. For instance, classical studies demonstrated that following a salient experience, the patterns of neuronal activity that were present during learning are replayed in the same sequential order offline, and this replay has been observed during both NREM9 and REM8 sleep. This sleep-dependent replay was proposed to support memory consolidation, and indeed, manipulation of sharp-wave ripples (during which most of these replay events occur) disrupts the storage of memories such that memory recall is disrupted thereafter16,19. It has also been observed that hippocampal replay occurs while animals are awake and engaged in an experimental task, and it can occur in a forward or reverse direction10,12. This led to the idea that different forms of replay may serve different functions, from memory consolidation to planning and decision-making18,58, though this remains a debate59. More generally, sleep has been shown to strongly benefit learning in both rodents17,60–62 and in humans63–66, and neurophysiological events during sleep, such as sharp wave ripples and sleep spindles, have been suggested to support learning16,19,62. Whether ensemble co-reactivation supporting integration is a sleep state specific phenomenon and whether distinct sleep states differentially support memory consolidation versus integration has yet to be answered. A recent study demonstrated that in a neural network model with autonomous offline reactivation, interleaved periods of NREM and REM sleep were critical for the integration of memories24. However, a previous study in rats suggested that offline reactivation and modification of a past neutral memory occurred during wake periods23. Thus, resolving whether and how different sleep states support memory integration processes will be an interesting future direction.
Finally, these results have implications for the interpretation of the clinical manifestation of memory-related conditions such as post-traumatic stress disorder (PTSD). PTSD transpires from a single traumatic event and is hallmarked by uncontrollable fear in non-life-threatening contexts67. A common form of behavioral treatment for PTSD is exposure therapy, whereby the patient is carefully re-exposed to the trauma-associated conditioned stimuli, seeking to detach the association between those stimuli and fear. In many cases, exposure therapy successfully decreases fear, but patients are often prone to relapse thereafter68. Our results suggest that highly salient aversive experiences can drive fear to be associated with seemingly unrelated stimuli that were not present at the time of the aversive experience. This predicts that while exposure therapy may successfully inhibit fear to the trauma stimuli, the fear from the trauma may have spread to other stimuli that were not directly targeted by the therapy. Thus, it may be useful to consider stimuli that were experienced close in time to a trauma that may have insidiously become linked with the trauma. Ultimately, our results point to the offline period after an aversive event as a potential intervention timepoint to unlink memories separated across days.
Methods
Subjects
Adult C57BL/6J mice from Jackson Laboratories were used in all experiments. Mice arrived group-housed in cages of 4 mice/cage and were singly housed for the experiment. For behavioral experiments where mice did not undergo surgery, mice were ordered to arrive at 12 weeks of age and underwent behavioral testing 1–2 weeks from then. For experiments where mice underwent surgery, mice were ordered to arrive at 8–9 weeks of age and underwent behavioral testing about 4–6 weeks after the arrival date.
Viral constructs
For calcium imaging experiments, AAV1-Syn-GCaMP6f-WPRE-SV40 (titer: 2.8 × 10^13 GC/mL) was purchased from AddGene and was diluted 1:4 in sterile 1x PBS. Mice had 300nL of the diluted virus injected into the right hemisphere of dorsal CA1. For PSAM experiments, AAV5-Syn-PSAM4-GlyR-IRES-eGFP (2.4 × 10^13 GC/mL) was purchased from AddGene. Mice had the virus injected at stock titer bilaterally into dorsal and ventral hippocampus, 300nL per injection site.
Surgery
Mice were anesthetized with 1 to 2% isoflurane for surgical procedures and placed into a stereotaxic frame (David Kopf Instruments, Tujunga, CA). Eye ointment was applied to prevent desiccation, and mice were kept on a heated pad to prevent hypothermia. Surgery was performed with aseptic technique. After surgery, carprofen (5 mg/kg) was administered every day for the following three days, and ampicillin (20 mg/kg) was administered every day for the following 7 days. For calcium imaging experiments, dexamethasone (0.2 mg/kg) was also administered for the following 7 days.
For PSAM experiments, AAV5-Syn-PSAM4-GlyR-IRES-eGFP was injected at stock concentration. Mice had 300nL of the virus injected bilaterally into dorsal hippocampus (AP: −2mm, ML: +/−1.5mm, DV: −1.5mm) and 300nL injected bilaterally into ventral hippocampus (AP: −3mm, ML: +/−3.2mm, DV: −4mm), for a total of 4 injections and 1.2uL injected per mouse, using a glass pipette and Nanoject injector. The pipette was slowly lowered to the injection site, the virus was injected at 2nL/sec, and then the pipette remained for 5min before being removed to allow for virus diffusion. Mice had their incision sutured following surgery and had betadine applied to the site to prevent infection.
For calcium imaging experiments, mice underwent two serial surgeries spaced one month apart, as described before (Cai et al., 2016). During the first surgery, a 1mm diameter craniotomy was made above the dorsal hippocampus on the right hemisphere (centered at AP −2mm, ML +1.5mm from bregma). An anchor screw was screwed into the skull on the contralateral hemisphere at approximately AP −1mm and ML −2.5mm from bregma. 300nL of AAV1-Syn-GCaMP6f was injected into dorsal CA1 of the hippocampus on the right hemisphere (AP −2mm, ML +1.5mm, DV −1.2mm). Virus was injected as described in PSAM experiments above. After the pipette was removed, the mouse remained on the stereotaxic frame for 20min to allow for complete diffusion of the virus. After the 20min of diffusion, the cortex below the craniotomy was aspirated with a 25-gauge blunt syringe needle attached to a vacuum pump, while constantly being irrigated with cortex buffer. When the striations of the corpus collosum were visible, the 25-gauge needle was replaced with a 27-gauge needle for finer tuned aspiration. Once most of corpus collosum was removed, bleeding was controlled using surgical foam (Surgifoam), and then a 1mm diameter × 4mm length GRIN lens (GRINTECH) was slowly lowered into the craniotomy. The lens was fixed with cyanoacrylate, and then dental acrylic was applied to cement the implant in place and cover the rest of the exposed skull. The top of the exposed lens was covered with Kwik-Sil (World Precision Instruments) to protect it and the Kwik-Sil was covered with dental cement. Four weeks later, mice were returned to attach the baseplate, visually guided by a Miniscope. The overlying dental cement was drilled off and the Kwik-Sil was removed to reveal the top of the lens. The Miniscope with an attached baseplate was lowered near the implanted lens and the field of view was monitored in real-time on a computer. The Miniscope was rotated until a well-exposed field of view was observed, at which point the baseplate was fixed to the implant with cyanoacrylate and dental cement. The mouse did not receive post-operative drugs after this surgery since it was not invasive.
Behavioral procedures
Prior to all experiments, mice were handled for one minute each day for at least one week. On at least four of those days, mice were transported to the testing room and handled there. On the rest of the days, the mice were handled in the vivarium. In calcium imaging experiments, mice were handled and habituated for 2 weeks instead of 1, during which they were habituated to having the Miniscope attached and detached from its head. To become accustomed to the weight of the Miniscope, they were placed in their homecage with the Miniscope attached for 5min per day for at least 5 days.
In Retrospective and Prospective memory-linking behavioral experiments, mice were exposed to the Neutral context for 10 minutes to explore. During Aversive learning, mice were placed in a novel context and allowed to explore for 2 minutes. Then, mice received a 2-second footshock of either 0.25mA (low shock) or 1.5mA (high shock). One minute after the first shock, they received a second shock of the sample duration and amplitude, with a third shock following 1 minute after the second. 30 seconds after the third shock, the mice were removed and placed back in their homecage. On the following three days, mice were tested in the previously experienced Aversive and Neutral contexts, as well as a completely Novel context that they had not been exposed to prior. The features of the Neutral and Novel contexts were counter-balanced and were made up of different olfactory, auditory, lighting, and tactile cues. The Aversive context was always the same with distinct cues from the Neutral and Novel contexts. In the PSAM experiment, mice were tested in either the Aversive, Neutral, or Novel context. In the Prospective versus Retrospective memory-linking experiment, mice were tested in the Aversive context first, and then half the mice were tested in the Neutral context and the other half in the Novel context. In the Low vs High Shock experiments, mice were tested in the Aversive context first, followed by testing in the Neutral and Novel context counter-balanced; half the mice received Neutral recall and then Novel recall the next day, and the other half Novel and then Neutral recall. All testing was done in Med Associates chambers. Behavioral data were analyzed using the Med Associates software for measuring freezing. In experiments where mice were tethered with a Miniscope, behavioral data were analyzed using our previously published open-source behavioral tracking pipeline, ezTrack69. In the Prospective versus Retrospective memory-linking timecourse experiments, the Aversive learning experience was distinct: mice explored for 2min, then administered one 0.75mA, 2-second long footshock and removed from the context 30sec following this shock.
Drug injections
uPSEM-817 tartrate was made in a solution of 0.1mg/mL in saline and injected intraperitoneally at a dose of 1mg/kg (10mL/kg injection volume). Saline was used as a vehicle. The first injection was done as soon as the mice were brought back to the vivarium after Aversive learning (~3min after the end of Aversive learning). The next 3 injections were done every 3 hours to cover a 12-hour timespan of inhibition.
Calcium imaging Miniscope recordings
Open-source V4 Miniscopes (https://github.com/Aharoni-Lab/Miniscope-v4) were connected to a coaxial cable which connected to a Miniscope data acquisition board (DAQ) 3.3. The DAQ connected to a computer via a USB3.0. Data was collected via the Miniscope QT Software (https://github.com/Aharoni-Lab/Miniscope-DAQ-QT-Software) at 30 frames per second. Miniscopes and DAQ boards were all purchased from Open Ephys.
When performing calcium imaging with concurrent behavior in the Med Associates boxes, mice were brought into the testing room from the vivarium, taken out of their homecage, and had the Miniscope attached. They were placed back into their homecage for 1min. Then, they were removed from their homecage and placed in the testing chamber. To record calcium and behavior, the Med Associates software sent a continuous TTL pulse to record from the Miniscope while the behavior was concurrently tracked via Med Associates cameras. After the session was complete, the mice were immediately returned to their homecage, then the Miniscope was removed, and the mouse was returned to the vivarium. One mouse was brought to the testing room at a time so that mice did not idly wait in the testing room with partial recall cues from the room present.
Offline calcium imaging recordings were done in the mouse’s homecage for the 1 hour following the first Neutral context exposure and following Aversive learning. During these recordings, mice were placed back in their homecage and the homecage was placed in a large rectangular and opaque storage bin to occlude distal cues, with a webcam (Logitech C920e) overlying the homecage to track behavior during the recording. Using the Miniscope QT Software with two devices connected (Miniscope and webcam), calcium imaging and behavior were concurrently tracked. After the offline recording was complete, mice were removed from their homecage, the Miniscope was removed, they were returned to their homecage and returned to the vivarium immediately thereafter.
Miniscope data processing and data alignment
To extract calcium transients from the calcium imaging data, we employed our previously published open-source calcium imaging data processing pipeline, Minian70. Briefly, videos were pre-processed for background fluorescence and sensor noise, and motion corrected. Then, putative cell bodies were detected to feed into a constrained non-negative matrix factorization algorithm to decompose the 3-dimensional video array into a 3-dimensional array representing the spatial footprint of each cell, as well as a 2-dimensional matrix representing the calcium transients of each cell. The calcium transients were then deconvolved to extract the estimated time of each calcium transient. These deconvolved calcium activities were analyzed in these studies, after undergoing various transformations depending on the specific analysis (see below). Cells recorded across sessions within a mouse were cross-registered using a previously published open-source cross-registration algorithm, CellReg, using the spatial correlations of nearby cells to determine whether highly correlated footprints close in space are likely to be the same cell across sessions71.
To align calcium imaging data with behavior, behavior recordings were first aligned to an idealized template assuming a perfect sampling rate. This meant that if a recording session was 5min long, this meant that there should be 300sec * 30frames/sec = 9000frames. All behavior recordings were within 4 frames of this perfect template. Calcium recordings recorded with a much more variable and dynamic sampling rate. Then, for each behavior frame, the closest calcium imaging frame was aligned to that frame. No calcium imaging frame was re-used more than twice.
General statistics and code/data availability
All analyses and statistics were done using custom-written Python scripts. Code detailing all the analysis in this manuscript will be made available upon publication (https://github.com/denisecailab). Calcium imaging data used in this manuscript will be made available using the Neurodata Without Borders framework to seamlessly share data across institutions72. Statistical significance was assessed with two-tailed paired and unpaired t-tests, as well as one-way or two-way ANOVAs where appropriate. Significant effects or interaction were followed with post-hoc testing with the use of Benjamini-Hochberg corrections for multiple comparisons. Significance levels were set to α=0.05. Significance for comparisons: *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001. Sample sizes were chosen based on previous similar studies. The investigators were not blinded to behavioral testing in calcium imaging studies but were blinded to behavioral testing in all other experiments.
Ensemble reactivation analysis
To measure ensemble reactivation across the offline period, for each mouse, all cells that were recorded during that session were z-scored along both axes. Cells were then broken up into ensembles based on whether they were previously observed to be active. Previously active cells were defined based on whether they had a corresponding matched cell via CellReg. On OfflineDay1 after neutral learning, cells were either previously matched to an active cell during neutral learning (Neutral ensemble) or had no previously matched cell (Remaining ensemble). On OfflineDay2, cells had a matched cell only with neutral learning and not aversive learning (Neutral ensemble), a matched cell with aversive learning and not neutral learning (Aversive ensemble), a matched cell on both neutral learning and aversive learning (Neutral ∩ Aversive ensemble), or no matched cell (Remaining ensemble). For each ensemble, the activity of cells was averaged across cells, and then averaged across time for each timebin.
Burst participation analysis
To measure population bursts, for each mouse, all cells that were recorded during that session were z-scored along the time dimension, such that each cell was normalized to its own activity. By doing this, no cell overly contributed to population bursts by having a very high amplitude event. Then, the mean population activity across the whole population was computed across the session and that 1-dimensional trace was z-scored. Time periods above a threshold of z=2 were considered burst events. During each of these burst events, each cell was considered to have “participated” if its activity was above z=2 during the event. For each ensemble (as defined in the previous section), the fraction of the ensemble that participated in each event was computed, and then this was averaged across all events. The average participation of each ensemble was compared across ensembles and across Low vs High Shock groups.
Ensemble co-bursting analysis
Ensemble co-bursting was computed in two ways. First, bursts were defined based on the whole population. Then, for each burst event, the z-scored mean population activity was computed for the Neutral ensemble and for the Aversive ensemble (see Ensemble reactivation analysis for ensemble definitions). For each population-level burst event, the “participation” of the Neutral ensemble or Aversive ensemble was measured based on whether the ensemble’s mean population activity was above the z=2 threshold during the population level event. The fraction of events where the Neutral ensemble participated without the Aversive ensemble, the Aversive ensemble participated without the Neutral ensemble, or both ensembles co-participated, was computed. For the second method of calculating co-bursting across ensembles, now burst events were calculated separately for each ensemble (i.e., for the Neutral ∩ Aversive, Neutral, and Aversive ensembles). Then, the percentage of time spent co-bursting in each possible combination of ensembles was computed.
Extended Data
Extended Figure 1. Behavioral experiment controls.
A) Schematic to test the timecourse of prospective memory-linking (top). Mice underwent Aversive learning and then either 5h, 1d, or 2d later they experienced a Neutral context. The following day, mice were tested in the previously experienced Neutral context. Mice froze significantly more in the Neutral context when the Neutral context occurred within 5h of the Aversive context, compared to when it occurred one day or more after Aversive learning (bottom). Main effect of timepoint (F2,24 = 3.689, p = 0.04). Post-hoc tests revealed a trend for higher freezing in the 5h timepoint compared to the 1d or 2d timepoints: 1d (t16.38 = 2.137, p = 0.07), 2d (t13.45 = 2.38, p = 0.07).
B) Schematic to test the timecourse of retrospective memory-linking (top). Mice experienced a Neutral context, followed by Aversive learning in a separate context 5h, 1d, or 2d later. The day following Aversive learning, they were tested in the previously experienced Neutral context. Mice froze no differently in the Neutral context regardless of how long before Aversive learning the Neutral context was experienced (bottom). No main effect of timepoint (F2,27 = 0.73, p = 0.49).
C) Schematic of low vs high shock retrospective memory-linking experiment (without calcium imaging as a replication). Mice received a Neutral experienced followed by a low or high shock Aversive learning two days later. In the subsequent 3 days, mice were tested in the Aversive context, and then Neutral and Novel contexts, counterbalanced.
D) Mice froze more in the Aversive context in High Shock vs Low Shock mice (t14 = 5.04, p = 0.00018).
E) High Shock mice exhibited higher freezing in Neutral vs Novel recall, while Low Shock mice did not. A priori post-hoc test: High Shock (t7 = 2.65, p = 0.033), Low Shock (t7 = 1.21, p = 0.133).
Extended Figure 2. Neurons active during Aversive learning are selectively reactivated offline and during Aversive recall.
A) Schematic of a single aversive experience. Mice had an Aversive experience followed by a 1hr offline session in the homecage. The next day, mice were tested in the Aversive context, followed by a test in a Novel context one day later. Calcium imaging in hippocampal CA1 was performed during all sessions.
B) Representative histology of AAV1-Syn-GCaMP6f expression (top) and representative maximum intensity projections of the field-of-view across all the recording days (bottom).
C) Mice acquired within-session freezing during Aversive learning (left); main effect of time (F8,56 = 12.59, p = 3.87e-10). And mice responded robustly to all three footshocks, though their locomotion generally decreased across shocks, driven by increased freezing (right); main effect of shock number (F2,14 = 7.45, p = 0.0154) and main effect of PreShock vs Shock (F1,7 = 581, p = 5.38e-8), and no interaction.
D) Mice displayed a modest decrease in locomotion across the 1hr offline period (R2 = 0.064, p = 1.9e-8).
E) Mice froze significantly more in the Aversive context than in a Novel context during recall (t7 = 165, p = 4e-6).
F) Cells that were active during Aversive learning and reactivated offline were significantly more likely to be reactivated during Aversive recall than cells active during Aversive learning and not reactivated offline (t7 = 19.41, p = 2e-7).
G) A larger fraction of cells active during Aversive recall than during Novel recall were previously active during Aversive learning (t7 = 6.897, p = 0.0002).
H) During the offline period, ~40% of the population was made up of cells previously active during Aversive learning (top). This Aversive ensemble was much more highly active than the rest of the population during the offline period (bottom) (t7 = 8.538, p = 0.00006).
I) Each cell’s activity was compared during locomotion vs during quiet rest (left). A regression line was fit to the cells in the Aversive ensemble and in the Remaining ensemble separately, for each mouse. The Remaining ensemble showed greater activity during locomotion than during quiet rest (i.e., a less positive slope). The Aversive ensemble showed relatively greater activity during quiet rest than locomotion (i.e., a more positive slope) across mice (right) (t7 = 5.76, p = 0.047).
J) Cells that had high levels of activity during Aversive learning continued to have high levels of activity during the offline period (example mouse; left). There was a linear relationship between how active a cell was during Aversive learning and how likely it was to be reactivated during the offline period (all mice; right) (R2 = 0.726, p = 1.25e-23).
K) During the offline period, cells that would go on to become active during recall were more highly active than the Remaining ensemble during the offline period. The top represents the proportion of each ensemble (legend to its right). The cells that would become active during both Aversive and Novel recall were most highly active. There was no difference in activity in the cells that would go on to be active in Aversive or Novel. Main effect of Ensemble (F3,21 = 27.81, p = 1.65e-7). Post-hoc tests: for Aversive vs Novel (t7 = 1.33, p = 0.22), for Remaining vs Aversive ∩ Novel (t7 = 11.95, p = 0.000007), for Remaining vs Aversive (t7 = 3.97, p = 0.005), for Remaining vs Novel (t7 = 7.47, p = 0.0001).
Extended Figure 3. Low vs High Shock calcium imaging controls.
A) Mice acquired within-session freezing during the Aversive learning paradigm. Mice that received high shocks (1.5mA) displayed more freezing than mice that received low shocks (0.25mA).
B) Mice responded robustly to each footshock. High shock mice responded more strongly to each shock than low mice did.
C) Relative to the first calcium imaging recording, mice showed comparable fractions of observed cells across the remaining sessions.
D) In High Shock mice, Neutral recall cells were composed of more Neutral learning cells being reactivated, compared to Novel recall cells. In Low Shock mice, Neutral recall cells and Novel recall cells were composed of similar fractions of Neutral learning cells being reactivated. Significant interaction between Context (Neutral vs Novel) and Amplitude (Low vs High Shock) (F1,12 = 6.81, p = 0.022). Post-hoc tests, Low Shock (t5 = 1.34, p = 0.24), High Shock (t7 = 10.22, p = 0.000037).
E) In High Shock mice, Neutral recall cells were composed of more Aversive learning cells being reactivated, compared to Novel recall cells. In Low Shock mice, Neutral recall cells and Novel recall cells were composed of similar fractions of Aversive learning cells being reactivated. Significant interaction between Context (Neutral vs Novel) and Amplitude (Low vs High Shock) (F1,12 = 4.75, p = 0.0499). Post-hoc tests, Low Shock (t5 = 0.59, p = 0.58), High Shock (t7 = 5.46, p = 0.0019).
F) Locomotion across the 1hr offline period after Neutral learning (OfflineDay1) and after Aversive learning (OfflineDay2) in Low and High Shock mice. Mice showed decreased locomotion across the offline period on both days. Low Shock mice did not locomote differently from High Shock mice during either offline period.
G) During OfflineDay1, burst events occurred more frequently right after learning and gradually decreased across the hour. No difference across shock amplitudes.
H) During OfflineDay2, burst event frequency was similar to on OfflineDay1. No difference across shock amplitudes.
I) During OfflineDay2, burst events as defined by each ensemble (rather than by the whole population) decreased across the hour, with comparable event frequencies across ensembles and amplitudes.
J) Schematic of how ensemble co-firing combinations were defined for K and L. A burst was considered independent if one ensemble fired and the other two did not. Otherwise, it was considered a coincident burst.
K) Quantification of amount of time independently bursting across ensembles and amplitudes. Significant main effect of Ensemble (Aversive vs Neutral vs Neutral ∩ Aversive) (F2,26 = 4.40, p = 0.02). Post-hoc tests revealed that the Neutral ∩ Aversive ensemble burst more frequently than the Neutral ensemble (t14 = 2.96, p = 0.03) and there was a trend for more bursting in the Neutral ∩ Aversive ensemble compared with the Aversive ensemble (t14 = 2.15, p = 0.075). No effect of shock amplitude.
L) Quantification of amount of time coincidentally bursting across all combinations of ensembles. Significant main effect of EnsembleCombo (F3,39 = 19.38, p = 7.5e-8) and significant interaction between EnsembleCombo and Amplitude (F3,39 = 3.17, p = 0.035). Post-hoc tests revealed that in Low Shock mice, co-bursting of all three ensembles occurred less frequently than the other combinations (N∩A × N × A compared with: N∩A × N, p = 0.001; N∩A × A, p = 0.002; N × A, p = 0.009). In High Shock mice, co-bursting between the Neutral ∩ Aversive ensemble and the Neutral ensemble was more frequent than the other three combinations (N∩A × N compared with: N∩A × A, p = 0.009; N × A, p = 0.013; N∩A × N × A, p = 0.010). Meanwhile, co-bursting of all three ensembles (i.e., N∩A × N × A) now occurred just as frequently as co-bursting with the Aversive ensemble (N∩A × N × A compared with: N∩A × A, p = 0.21, N × A, p = 0.38).
Extended Figure 4. Neurons active during Aversive learning selectively participate in burst events offline.
A) Example of a burst event quantified in this figure. The top trace represents the z-scored mean population activity within one of the offline recordings. Three timepoints were chosen (overlaid in circles), the middle representing the peak of a burst event, and the timepoints to its left and right representing t−2sec and t+2sec from the peak, respectively. The bottom three matrices represent binarized spatial footprints depicting the spatial footprints of the cells sufficiently active to participate in a burst (z>2). The matrices represent the timepoints of the three datapoints above it, ordered by time.
B) Representative process of extracting ensemble participations (one mouse example). The left is an example burst period, with the rows in the heatmap representing the activity of the recorded cells during that session, binarized by z>2 and color-coded by whether they were previously active during Aversive learning (Aversive ensemble, blue) or if they were not previously active (Remaining ensemble, grey). The black trace above represents the z-scored mean population activity during this period, demonstrating a brief burst in activity accompanied by participation by a significant fraction of neurons. On the right is an example non-burst period, where mean population activity remains below threshold.
C) The burst event frequency decreased across the hour.
D) A larger fraction of the Aversive ensemble vs the Remaining ensemble participated in each burst event (left) (t7 = 3.68, p = 0.0079).
E) The top matrix is every reactivation event stacked and centered on time t=0. The bottom is the average mean population activity around each burst event. There is no periodicity to when these burst events occur. Note that an example mouse is shown here but all mice showed this effect.
F) The top matrix is the locomotion of an example mouse during each burst event. The bottom is the mean locomotion around burst events. Mice showed a robust and brief slowing down ~1sec before each burst event, before increasing locomotion back up ~2sec later. Note that an example mouse is shown here but all mice showed this effect.
Acknowledgements
This work was supported by the DP2 MH122399, R01 MH120162, Brain Research Foundation Award, Klingenstein-Simons Fellowship, NARSAD Young Investigator Award, McKnight Memory and Cognitive Disorder Award, One Mind-Otsuka Rising Star Research Award, Hirschl/Weill-Caulier Award, Mount Sinai Distinguished Scholar Award, and Friedman Brain Institute Award, to DJC; the CURE Taking Flight Award, American Epilepsy Society Junior Investigator Award, R03 NS111493, and R21 DA049568 to TS; NIMH F31MH126543 to YZ; NIMH K99 MH131792 and BBRF Young Investigator Award to ZTP; NIMH R01 MH113071, NIA R01 AG013622, and Dr. Miriam and Sheldon G. Adelson Medical Research Foundation to AJS. We would like to thank Brandon Wei, Mimi La-Vu, Christopher Lee for experimental support, and the members of the Cai and Shuman labs for their feedback throughout the duration of the project. We would like to thank Dr. Daniel Aharoni and Federico Sangiuliano Jimka for Miniscope-related support. We thank Dr. Margot Tirole, Dr. Claudia Clopath, Geoffroy Delamare, Dr. Matthew van der Meer, and Hung-Tu Chen for thoughtful discussions and input regarding analyses. We thank Dr. Patrick Davis for discussions throughout the project and for comments on the manuscript. We thank William Janssen for microscopy support. We thank MazeEngineers for the development of a custom-made homecage to support long-term offline Miniscope recordings.
Footnotes
Competing Interests
The authors declare no competing interests.
References
- 1.Reijmers L. G., Perkins B. L., Matsuo N. & Mayford M. Localization of a stable neural correlate of associative memory. Science 317, 1230–1233, doi: 10.1126/science.1143839 (2007). [DOI] [PubMed] [Google Scholar]
- 2.Liu X. et al. Optogenetic stimulation of a hippocampal engram activates fear memory recall. Nature 484, 381–385, doi: 10.1038/nature11028 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Han J. H. et al. Neuronal competition and selection during memory formation. Science 316, 457–460, doi: 10.1126/science.1139438 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Rogerson T. et al. Synaptic tagging during memory allocation. Nat Rev Neurosci 15, 157–169, doi: 10.1038/nrn3667 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Josselyn S. A. & Frankland P. W. Memory Allocation: Mechanisms and Function. Annu Rev Neurosci 41, 389–413, doi: 10.1146/annurev-neuro-080317-061956 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavlides C. & Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci 9, 2907–2918, doi: 10.1523/JNEUROSCI.09-08-02907.1989 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilson M. A. & McNaughton B. L. Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679, doi: 10.1126/science.8036517 (1994). [DOI] [PubMed] [Google Scholar]
- 8.Louie K. & Wilson M. A. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron 29, 145–156, doi: 10.1016/s0896-6273(01)00186-6 (2001). [DOI] [PubMed] [Google Scholar]
- 9.Lee A. K. & Wilson M. A. Memory of sequential experience in the hippocampus during slow wave sleep. Neuron 36, 1183–1194, doi: 10.1016/s0896-6273(02)01096-6 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Foster D. J. & Wilson M. A. Reverse replay of behavioural sequences in hippocampal place cells during the awake state. Nature 440, 680–683, doi: 10.1038/nature04587 (2006). [DOI] [PubMed] [Google Scholar]
- 11.Ji D. & Wilson M. A. Coordinated memory replay in the visual cortex and hippocampus during sleep. Nat Neurosci 10, 100–107, doi: 10.1038/nn1825 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Diba K. & Buzsaki G. Forward and reverse hippocampal place-cell sequences during ripples. Nat Neurosci 10, 1241–1242, doi: 10.1038/nn1961 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carr M. F., Jadhav S. P. & Frank L. M. Hippocampal replay in the awake state: a potential substrate for memory consolidation and retrieval. Nat Neurosci 14, 147–153, doi: 10.1038/nn.2732 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Girardeau G., Inema I. & Buzsaki G. Reactivations of emotional memory in the hippocampus-amygdala system during sleep. Nat Neurosci 20, 1634–1642, doi: 10.1038/nn.4637 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Ghandour K. et al. Orchestrated ensemble activities constitute a hippocampal memory engram. Nat Commun 10, 2637, doi: 10.1038/s41467-019-10683-2 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gridchyn I., Schoenenberger P., O’Neill J. & Csicsvari J. Assembly-Specific Disruption of Hippocampal Replay Leads to Selective Memory Deficit. Neuron 106, 291–300 e296, doi: 10.1016/j.neuron.2020.01.021 (2020). [DOI] [PubMed] [Google Scholar]
- 17.Clawson B. C. et al. Causal role for sleep-dependent reactivation of learning-activated sensory ensembles for fear memory consolidation. Nat Commun 12, 1200, doi: 10.1038/s41467-021-21471-2 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buzsaki G. Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188, doi: 10.1002/hipo.22488 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van de Ven G. M., Trouche S., McNamara C. G., Allen K. & Dupret D. Hippocampal Offline Reactivation Consolidates Recently Formed Cell Assembly Patterns during Sharp Wave-Ripples. Neuron 92, 968–974, doi: 10.1016/j.neuron.2016.10.020 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Colgin L. L. Rhythms of the hippocampal network. Nat Rev Neurosci 17, 239–249, doi: 10.1038/nrn.2016.21 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chambers K. C. Conditioned taste aversions. World J Otorhinolaryngol Head Neck Surg 4, 92–100, doi: 10.1016/j.wjorl.2018.02.003 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai D. J., Mednick S. A., Harrison E. M., Kanady J. C. & Mednick S. C. REM, not incubation, improves creativity by priming associative networks. Proc Natl Acad Sci U S A 106, 10130–10134, doi: 10.1073/pnas.0900271106 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jezek K. et al. Stress-induced out-of-context activation of memory. PLoS Biol 8, e1000570, doi: 10.1371/journal.pbio.1000570 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singh D., Norman K. A. & Schapiro A. C. A model of autonomous interactions between hippocampus and neocortex driving sleep-dependent memory consolidation. Proc Natl Acad Sci U S A 119, e2123432119, doi: 10.1073/pnas.2123432119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurth-Nelson Z. et al. Replay and compositional computation. Neuron, doi: 10.1016/j.neuron.2022.12.028 (2023). [DOI] [PubMed] [Google Scholar]
- 26.Cai D. J. et al. A shared neural ensemble links distinct contextual memories encoded close in time. Nature 534, 115–118, doi: 10.1038/nature17955 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rashid A. J. et al. Competition between engrams influences fear memory formation and recall. Science 353, 383–387, doi: 10.1126/science.aaf0594 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Phelps E. A. & Sharot T. How (and Why) Emotion Enhances the Subjective Sense of Recollection. Curr Dir Psychol Sci 17, 147–152, doi: 10.1111/j.1467-8721.2008.00565.x (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.LaBar K. S. & Cabeza R. Cognitive neuroscience of emotional memory. Nat Rev Neurosci 7, 54–64, doi: 10.1038/nrn1825 (2006). [DOI] [PubMed] [Google Scholar]
- 30.Dunsmoor J. E., Murty V. P., Davachi L. & Phelps E. A. Emotional learning selectively and retroactively strengthens memories for related events. Nature 520, 345–348, doi: 10.1038/nature14106 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramirez S. et al. Creating a false memory in the hippocampus. Science 341, 387–391, doi: 10.1126/science.1239073 (2013). [DOI] [PubMed] [Google Scholar]
- 32.Ohkawa N. et al. Artificial association of pre-stored information to generate a qualitatively new memory. Cell Rep 11, 261–269, doi: 10.1016/j.celrep.2015.03.017 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Yokose J. et al. Overlapping memory trace indispensable for linking, but not recalling, individual memories. Science 355, 398–403, doi: 10.1126/science.aal2690 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Clewett D., Dunsmoor J., Bachman S. L., Phelps E. A. & Davachi L. Survival of the salient: Aversive learning rescues otherwise forgettable memories via neural reactivation and post-encoding hippocampal connectivity. Neurobiol Learn Mem 187, 107572, doi: 10.1016/j.nlm.2021.107572 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghandour K. & Inokuchi K. Memory reactivations during sleep. Neurosci Res, doi: 10.1016/j.neures.2022.12.018 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Lewis P. A., Knoblich G. & Poe G. How Memory Replay in Sleep Boosts Creative Problem-Solving. Trends Cogn Sci 22, 491–503, doi: 10.1016/j.tics.2018.03.009 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giri B., Miyawaki H., Mizuseki K., Cheng S. & Diba K. Hippocampal Reactivation Extends for Several Hours Following Novel Experience. J Neurosci 39, 866–875, doi: 10.1523/JNEUROSCI.1950-18.2018 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buzsaki G. Neural syntax: cell assemblies, synapsembles, and readers. Neuron 68, 362–385, doi: 10.1016/j.neuron.2010.09.023 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bittner K. C., Milstein A. D., Grienberger C., Romani S. & Magee J. C. Behavioral time scale synaptic plasticity underlies CA1 place fields. Science 357, 1033–1036, doi: 10.1126/science.aan3846 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rajasethupathy P. et al. Projections from neocortex mediate top-down control of memory retrieval. Nature 526, 653–659, doi: 10.1038/nature15389 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gava G. P. et al. Integrating new memories into the hippocampal network activity space. Nat Neurosci 24, 326–330, doi: 10.1038/s41593-021-00804-w (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Buzsaki G. & Mizuseki K. The log-dynamic brain: how skewed distributions affect network operations. Nat Rev Neurosci 15, 264–278, doi: 10.1038/nrn3687 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Magnus C. J. et al. Chemical and genetic engineering of selective ion channel-ligand interactions. Science 333, 1292–1296, doi: 10.1126/science.1206606 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mau W., Hasselmo M. E. & Cai D. J. The brain in motion: How ensemble fluidity drives memory-updating and flexibility. Elife 9, doi: 10.7554/eLife.63550 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Josselyn S. A. & Tonegawa S. Memory engrams: Recalling the past and imagining the future. Science 367, doi: 10.1126/science.aaw4325 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bocchio M., Nabavi S. & Capogna M. Synaptic Plasticity, Engrams, and Network Oscillations in Amygdala Circuits for Storage and Retrieval of Emotional Memories. Neuron 94, 731–743, doi: 10.1016/j.neuron.2017.03.022 (2017). [DOI] [PubMed] [Google Scholar]
- 47.Ryan T. J., Roy D. S., Pignatelli M., Arons A. & Tonegawa S. Memory. Engram cells retain memory under retrograde amnesia. Science 348, 1007–1013, doi: 10.1126/science.aaa5542 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Choi J. H. et al. Interregional synaptic maps among engram cells underlie memory formation. Science 360, 430–435, doi: 10.1126/science.aas9204 (2018). [DOI] [PubMed] [Google Scholar]
- 49.Abdou K. et al. Synapse-specific representation of the identity of overlapping memory engrams. Science 360, 1227–1231, doi: 10.1126/science.aat3810 (2018). [DOI] [PubMed] [Google Scholar]
- 50.Denny C. A. et al. Hippocampal memory traces are differentially modulated by experience, time, and adult neurogenesis. Neuron 83, 189–201, doi: 10.1016/j.neuron.2014.05.018 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Silva A. J., Zhou Y., Rogerson T., Shobe J. & Balaji J. Molecular and cellular approaches to memory allocation in neural circuits. Science 326, 391–395, doi: 10.1126/science.1174519 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van Dijk M. T. & Fenton A. A. On How the Dentate Gyrus Contributes to Memory Discrimination. Neuron 98, 832–845 e835, doi: 10.1016/j.neuron.2018.04.018 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lohnas L. J. et al. Time-resolved neural reinstatement and pattern separation during memory decisions in human hippocampus. Proc Natl Acad Sci U S A 115, E7418–E7427, doi: 10.1073/pnas.1717088115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.LeDoux J. E. Emotion circuits in the brain. Annu Rev Neurosci 23, 155–184, doi: 10.1146/annurev.neuro.23.1.155 (2000). [DOI] [PubMed] [Google Scholar]
- 55.Moscovitch A. & LoLordo V. M. Role of safety in the Pavlovian backward fear conditioning procedure. J Comp Physiol Psychol 66, 673–678, doi: 10.1037/h0026548 (1968). [DOI] [PubMed] [Google Scholar]
- 56.VM K. N. & Stuber G. D. The learning of prospective and retrospective cognitive maps within neural circuits. Neuron 109, 3552–3575, doi: 10.1016/j.neuron.2021.09.034 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Aly M. H., Abdou K., Okubo-Suzuki R., Nomoto M. & Inokuchi K. Selective engram coreactivation in idling brain inspires implicit learning. Proc Natl Acad Sci U S A 119, e2201578119, doi: 10.1073/pnas.2201578119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Joo H. R. & Frank L. M. The hippocampal sharp wave-ripple in memory retrieval for immediate use and consolidation. Nat Rev Neurosci 19, 744–757, doi: 10.1038/s41583-018-0077-1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gillespie A. K. et al. Hippocampal replay reflects specific past experiences rather than a plan for subsequent choice. Neuron 109, 3149–3163 e3146, doi: 10.1016/j.neuron.2021.07.029 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Havekes R. et al. Sleep deprivation causes memory deficits by negatively impacting neuronal connectivity in hippocampal area CA1. Elife 5, doi: 10.7554/eLife.13424 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cai D. J., Shuman T., Harrison E. M., Sage J. R. & Anagnostaras S. G. Sleep deprivation and Pavlovian fear conditioning. Learn Mem 16, 595–599, doi: 10.1101/lm.1515609 (2009). [DOI] [PubMed] [Google Scholar]
- 62.Varga A. W., Kang M., Ramesh P. V. & Klann E. Effects of acute sleep deprivation on motor and reversal learning in mice. Neurobiol Learn Mem 114, 217–222, doi: 10.1016/j.nlm.2014.07.001 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Diekelmann S. & Born J. The memory function of sleep. Nat Rev Neurosci 11, 114–126, doi: 10.1038/nrn2762 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Denis D. et al. The roles of item exposure and visualization success in the consolidation of memories across wake and sleep. Learn Mem 27, 451–456, doi: 10.1101/lm.051383.120 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wagner U., Gais S., Haider H., Verleger R. & Born J. Sleep inspires insight. Nature 427, 352–355, doi: 10.1038/nature02223 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Klinzing J. G., Niethard N. & Born J. Mechanisms of systems memory consolidation during sleep. Nat Neurosci 22, 1598–1610, doi: 10.1038/s41593-019-0467-3 (2019). [DOI] [PubMed] [Google Scholar]
- 67.Ressler K. J. et al. Post-traumatic stress disorder: clinical and translational neuroscience from cells to circuits. Nat Rev Neurol 18, 273–288, doi: 10.1038/s41582-022-00635-8 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boschen M. J., Neumann D. L. & Waters A. M. Relapse of successfully treated anxiety and fear: theoretical issues and recommendations for clinical practice. Aust N Z J Psychiatry 43, 89–100, doi: 10.1080/00048670802607154 (2009). [DOI] [PubMed] [Google Scholar]
- 69.Pennington Z. T. et al. ezTrack: An open-source video analysis pipeline for the investigation of animal behavior. Sci Rep 9, 19979, doi: 10.1038/s41598-019-56408-9 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dong Z. et al. Minian, an open-source miniscope analysis pipeline. Elife 11, doi: 10.7554/eLife.70661 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sheintuch L. et al. Tracking the Same Neurons across Multiple Days in Ca(2+) Imaging Data. Cell Rep 21, 1102–1115, doi: 10.1016/j.celrep.2017.10.013 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Rubel O. et al. The Neurodata Without Borders ecosystem for neurophysiological data science. Elife 11, doi: 10.7554/eLife.78362 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]