Abstract
The inner mitochondrial membrane (IMM) is the site of bulk ATP generation in cells and has a broadly conserved lipid composition enriched in unsaturated phospholipids and cardiolipin (CL). While proteins that shape the IMM and its characteristic cristae membranes (CM) have been defined, specific mechanisms by which mitochondrial lipids dictate its structure and function have yet to be elucidated. Here we combine experimental lipidome dissection with multi-scale modeling to investigate how lipid interactions shape CM morphology and ATP generation. When modulating fatty acid unsaturation in engineered yeast strains, we observed that loss of di-unsaturated phospholipids (PLs) led to a breakpoint in IMM topology and respiratory capacity. We found that PL unsaturation modulates the organization of ATP synthases that shape cristae ridges. Based on molecular modeling of mitochondrial-specific membrane adaptations, we hypothesized that conical lipids like CL buffer against the effects of saturation on the IMM. In cells, we discovered that loss of CL collapses the IMM at intermediate levels of PL saturation, an effect that is independent of ATP synthase oligomerization. To explain this interaction, we employed a continuum modeling approach, finding that lipid and protein-mediated curvatures are predicted to act in concert to form curved membranes in the IMM. The model highlighted a snapthrough instability in cristae tubule formation, which could drive IMM collapse upon small changes in composition. The interaction between CL and di-unsaturated PLs suggests that growth conditions that alter the fatty acid pool, such as oxygen availability, could define CL function. While loss of CL only has a minimal phenotype under standard laboratory conditions, we show that its synthesis is essential under microaerobic conditions that better mimic natural yeast fermentation. Lipid and protein-mediated mechanisms of curvature generation can thus act together to support mitochondrial architecture under changing environments.
Keywords: Mitochondria, Lipids, Cristae, Mechanics, Cardiolipin
Introduction
Mitochondria are ubiquitous eukaryotic organelles whose membrane architectures play central roles in both metabolic and non-metabolic functions1. The inner mitochondrial membrane (IMM) is the site of the Electron Transport Chain (ETC) and consists of two regions defined by their curvature: the flat inner boundary membrane (IBM), adjacent to the outer mitochondrial membrane (OMM), and cristae membranes (CM), which invaginate into the matrix and are connected to the IBM by crista junctions (CJs)2,3. CM structure is dependent on organism, tissue and physiological state3,4 but is commonly composed of tubular and lamellar membranes4–74–68. A clear function of CMs is to effectively increase the surface area for ETC reactions within the membrane. It has also been proposed that cristae invaginations act as a ‘proton sink’ where protons accumulate and expeditiously flow from the ETC to F1F0 adenosine-triphosphate (ATP) synthase9–11. Another model is that cristae act as diffusion barriers for metabolites between the intracristal space (ICS) and the intermembrane space (IMS), controlling the flux of ADP/ATP through the adenine nucleotide translocase (ANT)12–15. All these potential functions of cristae are dependent on their high intrinsic membrane curvature.
The IMM is shaped by proteins that drive cristae assembly and maintenance. The molecular determinants of CMs are best understood in Saccharomyces cerevisiae, where ATP synthases form ribbon-like rows of dimers which induce curvature along tubule and lamellar rims9,16–18. Loss of the dimerization subunit g, Atp20p, results in monomeric ATP synthases and onion-like mitochondria with flat layers of IMM running parallel with the OMM19–22. When reconstituted, ATP synthase dimers spontaneously assemble into rows driven by changes to elastic membrane bending energies23 and are sufficient to form tubular liposomes18. At CJs, Optic atrophy protein 1 (OPA1)/Mgm1p are dynamin-related GTPases that interact with the mitochondrial contact site and cristae organizing system (MICOS) complex24–29. Cells lacking Mgm1 feature a completely flat IMM30,31, while loss of the major MICOS subunit Mic60p results in elongated cristae sheets that do not contain CJs22,24.
In addition to its proteinaceous determinants, mitochondrial lipids are also hypothesized to also play key roles in shaping cristae structure. The predominant phospholipids (PLs) of the IMM are phosphatidylethanolamine (PE), phosphatidylcholine (PC) and cardiolipin (CL)32,33. IMM phospholipids, or their phosphatidylserine (PS) precursors in the case of PE, are imported from the ER at contact sites34. A notable exception is CL and its precursor phosphatidylglycerol (PG), which are synthesized in and remain localized to the IMM. Among PLs, CL is unique in featuring four acyl chains whose larger cross sectional area contributes to an overall conical shape35,36. CL can be in a dianion or monoanion form37–39. While the latter features a larger curvature, it is only present in acidic conditions. In liposomes, CL localizes to regions of high curvature and can drive the pH-dependent formation of invaginations40–42, which suggests a role in promoting highly curved membrane topologies. The curvature of CL varies in magnitude depending on the local lipid and chemical environments36,43. Molecular simulations predict key roles for CL ionization state44 and binding of counter ions45 in its curvature. Despite these biophysical data, the fundamental mitochondrial functions of CL are not fully resolved. In the genetic disorder Barth syndrome, loss of the acyl chain remodeler Taffazin causes reduced amounts and altered composition of CL46,47 leading to abnormal cristae48, which have also been observed in cell lines lacking CL synthesis42,49–51. In yeast, however, loss of cardiolipin synthase (Crd1p) does not render a respiratory or morphological phenotype under regular growth temperatures52,53. It thus remains unknown if CL serves a mechanical role in the IMM, or has more organism-specific functions relating to ETC enzymes54 and their organization55.
The acyl chain composition of mitochondrial PLs also differs from the rest of the cell56 and broadly regulates membrane biophysical properties. The IMM is especially enriched in unsaturated and polyunsaturated PLs, which promote membrane fluidity, and lacks sterols and saturated sphingolipids, which promote membrane ordering57,58. During synthesis, lipid unsaturation is controlled by the activity of fatty acid desaturases, such as Ole1p in yeast59,60. OLE1 was discovered in genetic screens for both unsaturated fatty acid auxotrophy and mitochondrial distribution and morphology genes (MDM). Mutations in mdm2 resulted in abnormal mitochondrial morphology and inheritance61,62 but were later identified as OLE1 alleles, indicating an unexplained link between desaturase activity and mitochondrial structure. In mammalian cells, addition of exogenous saturated fatty acids (e.g. palmitate) has long been observed to drive mitochondrial abnormality63–65, but the underlying mechanisms of these perturbations have not been identified. Metabolic diseases including obesity and type-2 diabetes mellitus have been associated with both saturated fat accumulation and mitochondrial stress66,67. Saturated PLs could modulate respiration through diffusional effects on electron carriers68, but these are unlikely to drive large changes in mitochondrial morphology.
Computational modeling can provide a framework to interrogate how changes in lipid composition impact membrane properties and influence cellular structures69–73. Molecular dynamic simulations can be used to predict changes to membrane properties as a function of composition74–76, while continuum modeling can be used to obtain the governing equations for the shape of the membrane given its material properties77–79. In the Helfrich-Canham-Evans model80–82, membranes are treated as a two-dimensional surface with an elastic bending energy given by:
where кc is the bending modulus of the membrane (stiffness), H is the mean curvature of the structure, C0 is the net spontaneous curvature across the bilayer, кG is the Gaussian modulus, K is the Gaussian curvature, and λ is the membrane tension. The total energy of the membrane (E) is obtained by integrating the energy density of the manifold over the area Ω. When this energy is minimized, the shape of the membrane corresponding to mechanical equilibrium is obtained. Key parameters of this model can be modulated by lipid chemistry. For example, the stiffness (кc) of saturated PL bilayers is higher than those with unsaturated (Table S1A, B) and polyunsaturated chains83,84. Reconstituted membranes from porcine IMM lipids have been observed to be especially soft (low кc)85. Curvature induced by differences in lipid abundance or intrinsic spontaneous curvatures (c0) of lipids across the two leaflets of the bilayer can be modeled as a net membrane spontaneous curvature C0. PL composition is a major determinant of c0, which ranges from near zero for cylindrical lipids like PC, to negative values for conical lipids like PE or CL, and positive in the case of lyso-PLs86. PL acyl composition also modulates c0, with more voluminous unsaturated chains favoring negative spontaneous curvatures87.
Here we combine experimental perturbations with mutli-scale modeling to elucidate new roles for conserved mitochondrial lipids in IMM morphology. We first used genetic manipulation of the desaturase Ole1p to control PL saturation in yeast and observed a surprising lipidic breakpoint, in which the IMM abruptly loses its CMs and mitochondria lose their ATP synthesis capacity. This transition is controlled by the IMM lipidome in two distinct ways: through modulation of ATP synthase oligomerization by PL saturation and through loss of CL. We develop a mathematical model to explain these effects by considering the energetics of lipid and protein-mediated membrane curvature. We then show CL function is dependent on growth conditions that modulate PL saturation, most notably oxygenation, and that it has an essential role in natural yeast growth environments.
Results
Systematic modulation of the yeast PL double bond profile reveals a critical mitochondrial breakpoint in IMM curvature and ATP generation
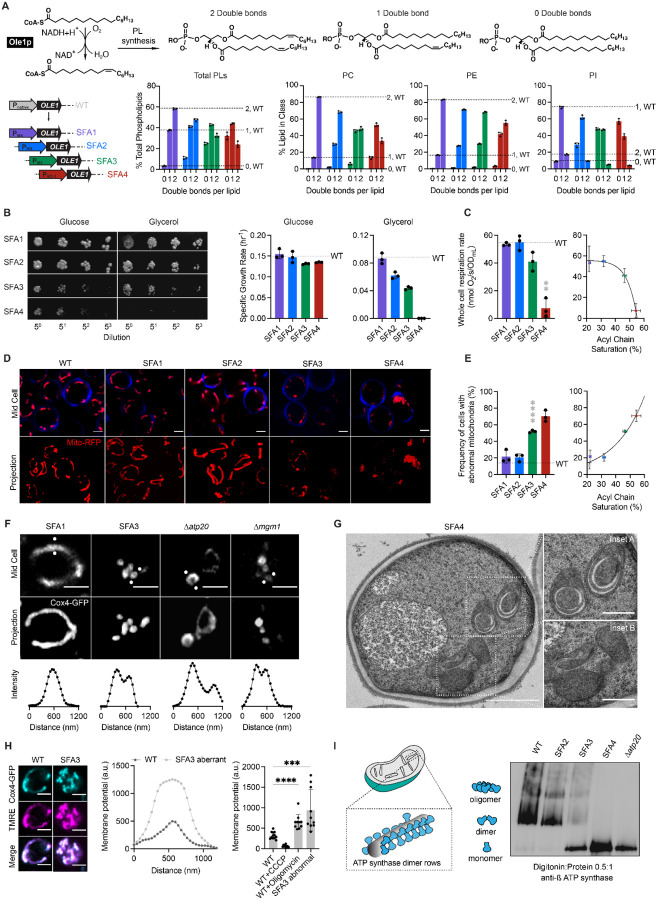
Bulk membrane properties are regulated by PL composition, most notably the stoichiometry between saturated and unsaturated acyl chains. To modulate lipid saturation of budding yeast we utilized a library of promoters controlling the expression of Ole1p (Figure 1A). We focused on four strains, saturated fatty acid (SFA) 1–4, which showed a range in lipid saturation (Figure 1A). SFA1 features a wild-type (WT) PL composition while SFA2–4 have consecutively increasing levels of PL acyl chain saturation due to lower levels of OLE1 expression (Figure S1A). Among PC and PE lipids, WT and SFA1 strains possess predominantly di-unsaturated PLs, while SFA 2,3 and 4 (weaker OLE1 expression) show an increasing ratio of mono to di-unsaturated species and incorporation of fully saturated PLs (Figure 1A). We observed potentially compensatory adaptations to increasing saturation in whole cell PLs (Figure S1), including a decrease in the PE/PC ratio (Figure S1B), used by several organisms to increase membrane fluidity88,89, an increase in PI (Figure S1B), and shortening of acyl chains length (Figure S1C), which also occurs during yeast cold adaptation90.
Figure 1:
Modulation of OLE1 expression results in a critical level of PL saturation driving loss of ATP generation and mitochondrial morphology.
(A) The yeast desaturase, Ole1p, is an oxygen-dependent enzyme that introduces cis double bonds at the C9 position (top left). SFA strains were generated via promoter substitution, resulting in progressively decreasing levels of OLE1 expression (bottom left). Lipidomics analysis showing double bond distributions of the total PL pool as well as of individual PLs within SFA strains; the wild-type distribution is depicted with dotted lines. Error bars indicate SD from biological replicates n=3.
(B) SFA4 cells lose viability under respiratory conditions. Shown are serial dilutions of yeast cells plated on media containing fermentable (glucose) and non-fermentable (glycerol) carbon sources and specific growth rates for each in liquid cultures. Error bars indicate SD from n=3 independent cultures.
(C) (Left) SFA3 and SFA4 cells show a drop in whole cell respiration, measured using a Clark electrode. Error bars indicate SD from n=3 independent cultures. **p=0.004, unpaired two-tailed t-test of SFA4 compared against wild-type. (Right) Scatter plot depicting a fitted single exponential (R2 =0.99) for the decrease in respiration as a function of acyl chain saturation.
(D) SFA3 and SFA4 cells lose tubular mitochondrial morphology. Shown are representative Airyscan confocal micrographs of yeast expressing matrix-localized RFP (mts-RFP). Cells were stained with cell wall-binding calcofluor white (blue) for clarity. Scale bars, 2 μm.
(E) Mitochondrial morphology changes between SFA2 and SFA3 strains, as assayed by confocal microscopy of cells harboring an mts-RFP plasmid. N>50 cells were counted in biological triplicate in each condition. Error bars indicate SD from n=3 independent cultures. ****p < 0.0001, unpaired two-tailed t-test of SFA3 compared against wild-type. (Right) Scatter plot depicting a fitted single exponential (R2 =0.97) for the increase in frequency of abnormal mitochondria as a function of acyl chain saturation.
(F) Airyscan confocal micrographs of yeast expressing IMM protein Cox4-GFP showing hollow mitochondria in SFA3 cells, as is also observed in mutants of CM shaping proteins. Scale bars, 2 μm. Profiling analysis (below) depicts fluorescence intensity across the indicated mitochondria; two peaks indicate a lack of fenestrated IMM.
(G) Thin section TEM micrographs of high-pressure frozen (HPF) SFA4 yeast showing the appearance of an onion-like (Inset A) IMM and total loss of CM (Inset B). Scale bars, 1 μm (full image), 400 nm (insets A and B).
(H) Abnormal mitochondria show an increase in membrane potential, consistent with loss of ATP synthase, but not ETC, activity. (Left) Representative micrographs are shown of individual cells expressing Cox4-GFP and stained with TMRE. (Center) Example membrane potential plots showing an example of an abnormal SFA3 cell with higher TMRE intensity compared to WT. (Right) Quantification of TMRE peak intensities from N>10 cells per condition showing a higher membrane potential in aberrant mitochondria, as well as those where ATP synthase is inhibited by oligomycin (5 μM), and lower potential in cells treated with the uncoupler CCCP (20 μM). Scale bars, 2 μm. ***p<0.0005, ****p<0.0001 unpaired two-tailed t-test of SFA3 aberrant and WT+oligomycin compared against WT.
(I) ATP synthase oligomerization is lost with increasing PL saturation. SFA2 mitochondria lose higher order oligomers observed in WT cells, while SFA3 and SFA4 mitochondria possess predominantly monomeric ATP synthase, similar to Δatp20 cells. Shown are BN-PAGE western blots of digitonin-solubilized purified mitochondria. 2500 pmol of mitochondrial lipid was loaded per well.
When handling these strains, we observed that SFA3 and SFA4 cells lost viability in non-fermentable carbon sources (Figure 1B). While SFA1 and SFA2 cells respired at WT-levels, but SFA3/4 showed a reduction and loss of respiratory function (Figure 1C), respectively, indicating that a modest increase in saturation confers a sudden loss in respiratory function. Microscopy of cells expressing a matrix-localized RFP revealed a striking loss in tubular mitochondrial networks and prevalence of aberrant mitochondria in SFA3 cells (Figure 1D, 1E, Figure S2B), while other organelles remained unperturbed (Figure S2A). SFA3 and SFA4 mitochondria also featured gaps in Cox4-GFP, a subunit of cytochrome c oxidase in the IMM, as in Δatp20 and Δmgm1 cells that lack CMs (Figure 1F). Transmission electron microscopy (TEM) analysis of SFA4 cells prepared by high-pressure freeze substitution (HPFS) (Figure 1G, S3A,B) showed mitochondria with flat IMM membranes similar to those previously observed in Δatp20 cells19 or Δmgm1 cells30. The moderate increase in saturation in SFA2 cells, which does not cause aberrancy independently, also showed epistasis with loss of shaping proteins at both the cristae ridge and rim: Atp20p and Mic60p of the MICOS complex, respectively (Figure S2C, S2D). We thus hypothesized that PL saturation has a specific role in cristae membrane formation.
We next asked how increasing lipid saturation resulted in the aberrant mitochondrial morphologies. We observed that aberrant mitochondria in SFA strains showed elevated membrane potential compared to non-aberrant mitochondria (Figure 1H), as measured by imaging of cells stained with tetramethylrhodamine ethyl ester (TMRE). This phenotype was inconsistent with a loss of proton-pumping reactions in the ETC. It instead suggested an impediment in ATP synthesis itself; TMRE fluorescence of aberrant mitochondria was similar to that when ATP synthase was inhibited by oligomycin in WT cells. We analyzed ATP synthase in isolated mitochondria with blue native PAGE (BN-PAGE) western blotting, which assays both enzyme levels and organization. In mitochondria from SFA strains, increasing saturation did not reduce ATP synthase levels, but instead altered its organization. SFA1 mitochondria show WT-levels of ATP synthase dimers and oligomers, while in SFA2 oligomers are lost, and SFA3 and SFA4 contain predominantly contained monomeric ATP synthases, similar similar to Δatp20 (Figure 1I). Thus, unsaturated lipid metabolism modulates the ATP synthase oligomerization.
Modeling of transport processes in aberrant mitochondria suggest a mechanism for loss of ATP generation
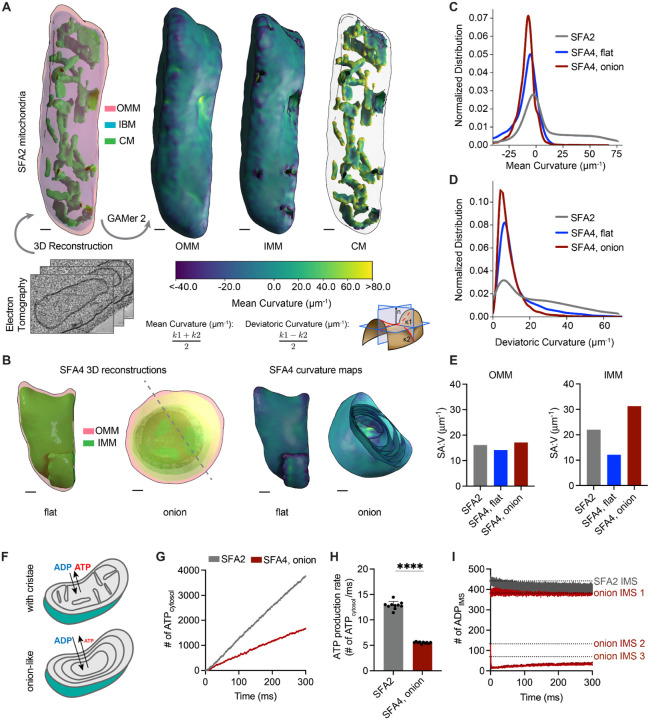
The loss of ATP synthase oligomerization in more saturated lipidomes provides one possible mechanism for loss of CM morphology, but does not fully explain loss of respiration given that monomeric ATP synthases largely retain ATPase activity19,20. To further investigate the function of IMM morphology, we sought to characterize the morphological and surface area changes to the IMM of SFA strains (Figure 2). For this, 3D reconstructions generated from multi-tilt TEM were meshed using the GAMer2 platform7,91,92 (Figure S3D) and local membrane curvature was computed across their surfaces. SFA2 tomograms displayed tubular CMs in the IMM similar to WT cells; these invaginated regions contain regions of high mean and deviatoric curvatures (Figure 2A, 2C–D). In contrast, the two types of aberrant morphologies in SFA4 cells showed a loss of high (>25 μm−1) mean and deviatoric curvatures (Figure 2B, 2C–D). Flat (Δmgm1-like) IMM were defined by an identical curvature profile as the OMM and a reduced surface Δatp20-like) possessed an increased SA:V ratio, but still lacked any of the high curvature regimes of the CMs (Figure 2E, 2C–D). Wide-field TEM showed that mitochondria with multiple IMMs made up at least 40% of SFA4 mitochondria (Figure S3A). Tomography of onion mitochondria revealed that the membrane layers showed connection vertices (Figure S3C), suggesting a continuous IMM. We also noted a distinct alternating shading pattern of high contrast matrix regions alternating with low contrast IMS layers (Figure S3B), which were previously observed in Δatp20 cells19. These two features are consistent with a model in which mitochondria with multiple layers could arise from repeated low curvature buddings from a continuous, single IMM (Figure S3C).
Figure 2:
PL saturation shifts the IMM to a regime of low membrane curvature, which reduces IMM surface area and modeled transport rates needed for ATP generation.
(A) 3D reconstruction of mitochondrial membrane topology and quantification of curvature using the GAMer 2 pipeline. Shown are electron tomograms of mitochondria from SFA2 cells, which show regular, tubular CM. CM are highlighted alongside the OMM and the inner boundary membrane (IBM) in the 3D reconstruction. (Right) Maps of mean curvature of OMM, IMM and CM computed using GAMer2. Scale bars, 50 nm.
(B) 3D reconstructions of SFA4 mitochondria showing flat (Δmgm1-like) and onion (Δatp20-like) abnormal morphologies. Also shown are the maps of mean curvature showing the IMM of these abnormal mitochondria, using the same color scale as in A. The onion-like IMM is sliced at an angle to illustrate the many layers; each layer is spherical in nature. Scale bars, 50 nm.
(C) Histograms of mean curvature distributions of inner membranes generated from SFA2 and SFA4 reconstructions, highlighting that high mean curvature areas in SFA2 IMM are lost in SFA4 cells.
(D) Histograms of deviatoric curvature distributions of inner membranes generated from SFA2 and SFA4 reconstructions, which are mechanical corollaries to curvature induced by ATP synthase dimerization, highlight that high deviatoric curvature areas in SFA2 IMM are lost in SFA4 cells.
(E) The IMMs of onion mitochondria show increased surface area:volume (SA:V) ratio compared to SFA2 while flat mitochondria show a decrease in the surface area:volume without major changes to the OMM.
(F) Schematic of ATP production in mitochondria containing normal CMs and those showing a multi-layer, online-like IMM, highlighting how multiple membrane layers could impede in trafficking of ADP and ATP.
(G) Modeled cytosolic ATP generation from mitochondria with a CM-containing morphology (taken from SFA2 tomogram) vs. an onion-like (taken from SFA4 tomogram) IMM. ATP generated in the cytosol in each condition is an average from 10 simulations. Details of the model equations and simulations are provided in the Supplementary Text.
(H) Comparison of cytosolic ATP generation rates derived from multiple Monte Carlo simulations shown in G. 10 simulations were run for each morphology. Error bars indicate SD; ****p<0.0001, unpaired two-tailed t-test.
(I) Modeled substrate depletion in onion-like mitochondria. In the CM-containing mitochondrion, the ADP level in the IMS remains constant at ~440 ADP throughout the 300 ms simulation. In the onion-like SFA4 mitochondrion, ADP remains constant only in the first IMS layer and is rapidly depleted in layers 2 and 3, indicating that the multi-layer structure could be limiting for ATP/ADP trafficking. Dotted lines represent initial values of ADP in each IMS layer.
While flat IMMs show a reduced surface area for ETC reactions compared to CM-containing ones, onion-like IMMs with multiple layers instead show a high surface area (Figure 2E). Previous modeling studies have shown that surface-to-volume ratio is an important determinant of flux of molecules between different compartments93–95. We hypothesized that multiple concentric layers would likely impede transport of ATP out of, and ADP into, the mitochondria of SFA3/SFA4 and Δatp20 cells. To explore this hypothesis, we employed an MCell-based reaction-diffusion simulation pipeline for modeling ATP generation using EM-derived morphologies96–98. These simulations showed that mitochondria with multiple IMM layers cause lower ATP generation when cytosolic ADP concentration was kept constant (Figure 2F, 2G, 2H). We also observed that predicted ATP production was inhibited by depletion of ADP as a substrate in the inner layers of the onion-like IMM, highlighting that ATP/ADP exchange by ANT could be limiting for high surface area IMM morphologies with low membrane curvature (Figure 2I). These simulations suggest that efficient substrate transport could be dependent on the architecture of CMs in addition to IMM surface area.
Lipid saturation modulates membrane mechanical properties which are buffered by mitochondrial-specific head group changes
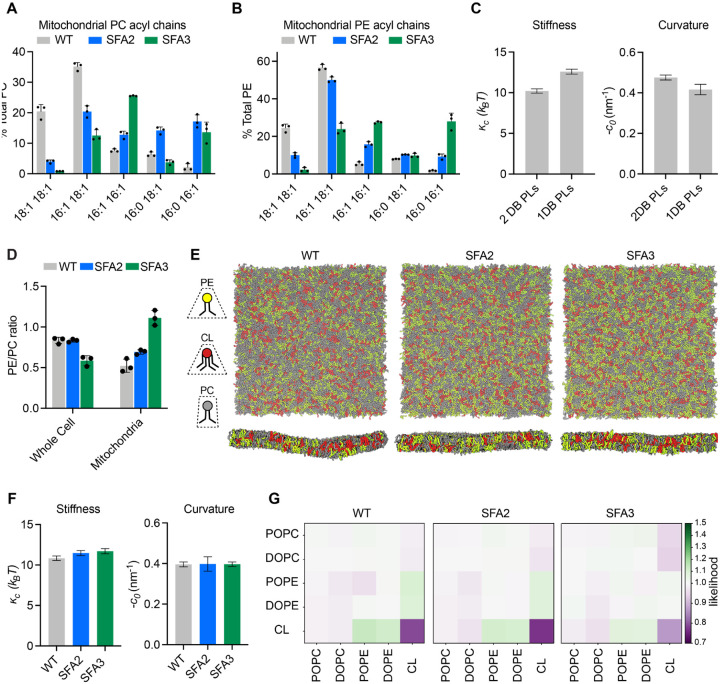
To define the lipid stoichiometry that drives CM loss, we analyzed biochemically isolated mitochondria from SFA strains. As in the whole cell lipidome, increasing Ole1p activity in SFA strains increased mitochondria PL saturation, but few fully saturated species were observed. The lipidome of isolated inner membranes was similar to that of intact mitochondria from the same sample, with the notable exception of CL levels (Figure S1F). The lipidic breakpoint for CMs in SFA3 cells corresponded to changes in the double bond distribution from predominantly di-unsaturated to monounsaturated PLs, e.g. PE-16:1/18:1 to PE-16:0/16:1 (Figure 3A, 3B), a surprisingly modest shift given the magnitude of the morphological change.
Figure 3:
Modeling of lipid-driven mechanical changes in the IMM and their homeostatic responses.
(A) Lipidomic profile of isolated mitochondria showing a transition from di-unsaturated to monounsaturated PC, n=3. Error bars represent SD.
(B) Mitochondrial PE shows a similar transition from di-unsaturated species to monounsaturated species, n=3. Error bars represent SD.
(C) Changes to membrane mechanical properties predicted by Martini 2 CG-MD simulations of mitochondrial-like lipid mixtures that shift from di-unsaturated (2 double bonds, DB) to mono-unsaturated (1 DB) PLs. Stiffness (bending modulus) increases while negative spontaneous curvature, derived from the first moment of the lateral pressure profile and bending modulus, decreases. Compositions of these ‘ideal’ systems are shown in Figure S4A.
(D) Increasing lipid saturation reduces the PE/PC ratio in whole cells, but increases it in isolated mitochondria, suggesting a curvature based adaptation to increasing saturation.
(E) Top-down and side-on snapshots of CG-MD bilayers showing headgroup adaptation to increasing saturation in SFA strains.
(F) Simulations of the lipid systems derived from SFA mitochondria that incorporate homeostatic head-group changes. In contrast to the systems in (C), the complex systems show only small changes to membrane mechanical properties, suggesting that headgroup adaptations may offset the changes to mechanical properties associated with increased saturation.
(G) Likelihood relative to random that we observe lipids (x-axis) around a given lipid type (y-axis) in a 1.5 nm neighborhood. PO/DOPE molecules are enriched around CL, indicating the potential association between conically shaped lipids in the IMM.
To explore the effects of this change in acyl chains on membrane properties, we set up simulations of lipid bilayers mimicking the mitochondrial composition of SFA strains. We employed coarse-grained molecular dynamics (CG-MD) simulations, which forego atomistic detail in favor of enabling improved sampling of complex mixtures and the modeling of larger domains and shape transformations99,100. To test whether the CG-MD simulations capture known mechanical properties of saturated PLs, we first simulated simplified mixtures of inner mitochondrial lipidomes (50:30:20 PC:PE:CL) containing either monounsaturated or diunsaturated PC & PE. Simulations of large membranes (~40 nm × 40 nm, 5000 lipids) were conducted and the thermally induced height undulations were analyzed to derive the bending stiffness (кc) of each membrane composition. In parallel, small membranes (~15 nm × 15 nm, 700 lipids) of identical compositions were used to compute stress (lateral pressure) profiles, whose first moment is equal to the product of кc and the monolayer spontaneous curvature, c0. Using кc values derived from the large systems, c0 values can thus be extracted. Shifting from diunsaturated to monounsaturated PLs led to a ~30% increase in stiffness, кc, (Figure 3C), consistent with previous experimental measurements in monocomponent liposomes (Table S1A–B). Similarly, monounsaturated mixtures showed a ~25% increase in c0, which was consistent with previous measurements in monocomponent mixtures measured by small angle x-ray scattering (Table S1C). We concluded that CG-MD using the Martini 2 force field is capable of reproducing the expected changes to mechanical properties modulated by PL lipid saturation. We thus proceeded to more complex mixtures derived from mitochondrial lipidomes.
In lipidomics of SFA strains, we observed a mitochondrial-specific change in head group composition: PE levels increased with increasing saturation at the expense of PC, resulting in an increase in the PE/PC ratio (Figure 3D, Figure S1E). In contrast, the PE/PC ratio decreased in the corresponding whole cell lipidome (Figure 3D, Figure S1B). Because PE has a higher melting temperature than PC88, its increase in SFA strains argues against a fluidity-specific stress of saturation on the mitochondria. Instead, the high negative spontaneous curvature of PE suggested an adaptation to membrane spontaneous curvature itself. To understand the biophysical basis for this adaptation, we employed the CG-MD workflow described above using more complex lipid mixtures that mimic the changes in head-group composition in SFA strains (Figure 3E). In these systems, shifting from the WT mitochondrial lipidomes to those of the SFA3 mitochondria resulted in only a modest increase in stiffness (кc) and no change to the magnitude of c0, despite the increase in PL saturation (Figure 3F). These findings suggested that mitochondrial specific adaptations in SFA strains act to buffer membrane mechanical properties relevant to curvature generation.
Beyond changes in the PE/PC ratio, our membrane simulations highlighted the key role of CL in dictating properties relevant to curvature generation. Replacement of CL with its precursor lipid PG resulted in an overall stiffening of simulated IMMs (Figure S4D–E) and loss of spontaneous curvature. Switching CL from the dianion form predominant at neutral pH to the monoanion form had the opposite effect, dramatically softening the bilayer and increasing spontaneous curvature (Figure S4D–E). In simulations without CL, the increase in PE in SFA strains could only partially compensate for curvature lost when CL was removed (Figure S4F). CL preferentially clustered with PE lipids in the simulated bilayers (Figure 3G), highlighting how conical lipids sort together in areas of high curvature101. This association was maintained in SFA lipidomes, though the extent of CL-PE association was reduced in the saturated SFA3 bilayers (Figure 3G).
An epistasis between PL saturation and CL abundance underlies IMM structure
The observation that mitochondria in SFA2 cells responded to increased PL saturation by increasing the abundance of high-curvature PE and CL lipids compared to WT (Figure S1D) led us to consider the interplay of lipid curvature and saturation. While PL saturation modulates intrinsic lipid curvature (c0) (Table S1C), there is no evidence that acyl chain composition differs across the two leaflets of the IMM, which would be needed to generate net membrane curvature (C0). We instead hypothesized that the effects of CL on predicted membrane curvature described above and the established asymmetric localization of CL in the IMM102 could provide membrane curvature independent of cristae shaping proteins, such as ATP synthase dimers. To explore this possibility, we tested out compositions based on previously measured asymmetries of CL in the yeast IMM. The simulations predicted that enrichment of CL in the IMM outer leaflet increased c0 and reduced кc (Figure S4G). Based on values for the former, we estimated that CL asymmetry could contribute at least a −0.05 nm−1 net bilayer curvature (C0) (Figure S4G).
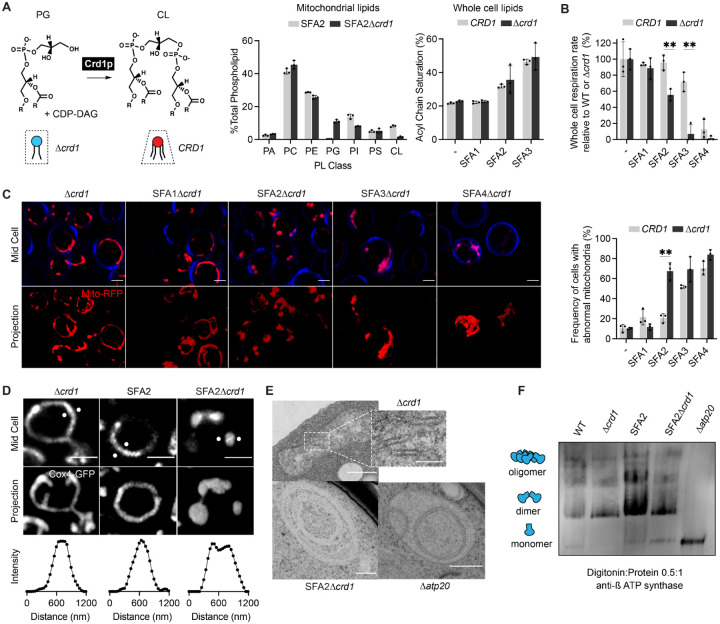
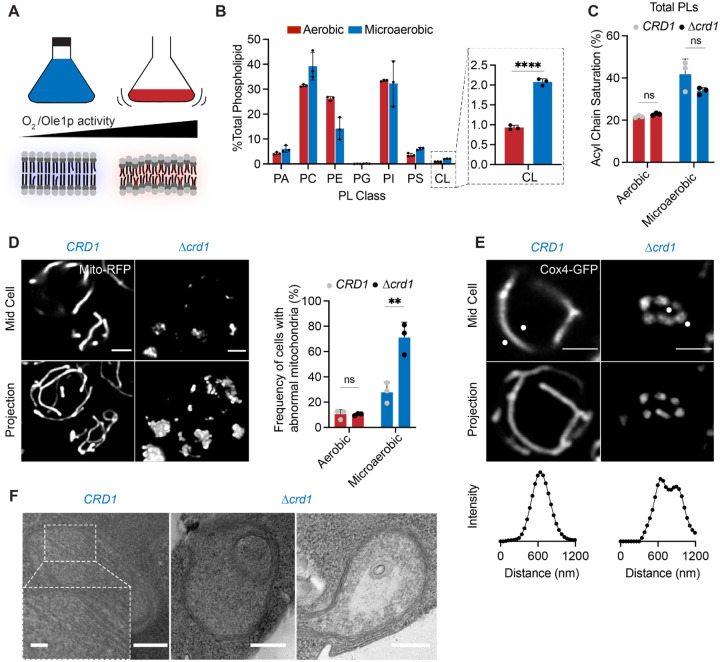
We next asked whether IMM curvature imparted by CL could compensate for loss of curvature imparted by lipid saturation and loss of ATP synthase oligomerization. For this, we evaluated how loss of Crd1p, which catalyzes the condensation of PG with a cytidine diphosphate diacylglycerol (CDP-DAG) molecule to form CL, affects mitochondrial morphology in SFA1–4 strains (Figure 4A). CL was absent in Δcrd1 strains in isolated mitochondria, which also showed a substantial increase in PG (Fig 4A). We also confirmed that loss of CL in SFA strains did not result in modification of PL acyl chain saturation (Figure 4A). As previously observed52, Δcrd1 cells did not show defects in mitochondrial morphology or cellular respiration in WT (CRD1) or SFA1 backgrounds (Figure S6A–B). However, in the SFA2 background, which shows increased saturation and reduced ATP synthase oligomerization but normal mitochondrial morphology, loss of CL had a dramatic phenotype (Figure 4B–E). SFA2Δcrd1 exhibited aberrant mitochondria (Figure 4C) that lack cristae membranes (Figure 4D–E) as previously observed in SFA3/SFA4. Respirometry measurements confirmed these results: Δcrd1 and SFA1Δcrd1 strains were able to respire at the same rate as CRD1 cells, SFA2Δcrd1 exhibited a perturbed oxygen consumption rate (Figure 4B). Loss of morphology and respiration in SFA2Δcrd1 was completely rescued by supplementation with oleic acid, demonstrating that Δcrd1 has a specific interaction with PL saturation (Figure S6C).
Figure 4:
Epistasis between PL saturation and CL synthesis in shaping mitochondrial morphology.
(A) Loss of Crd1p decreases mitochondrial CL content, but otherwise does not affect the lipidome of SFA strains. (Left) Reaction schematic depicting cylindrical PG and CDP-DAG converted into conical CL by Crd1p. (Right) Head-group stoichiometry of lipidomes from isolated mitochondria from SFA2 and SFA2Δcrd1 cells and acyl chain saturation (% of acyl chains with a double bond) for CRD1 and Δcrd1 cells across the SFA series. Error bars indicate SD from n=3 biological replicates.
(B) Loss of CL causes a loss of respiration in SFA2 cells. Whole cell respiration rates are shown for SFA strains, comparing CRD1 with Δcrd1 cells. Respirometry was conducted in biological triplicates (n=3) by Clark electrode. Error bars indicate SD. **p<0.005 unpaired two-tailed t-test between SFA2 and SFA3 strains and their corresponding Δcrd1 mutants.
(C) The morphological breakpoint shifts to SFA2 upon loss of CL. (Left) Representative Airyscan confocal micrographs of yeast expressing matrix-localized RFP (mts-RFP). Cells were stained cell wall-binding calcofluor white (blue) for clarity. Scale bars, 2 μm. (Right) Frequency of mitochondrial abnormality, N>50 cells were counted in n=3 independent cultures for each condition. Error bars indicate SD. **p=0.0011 unpaired two-tailed t-test between CRD1 and Δcrd1 in the SFA2 background.
(D) SFA2Δcrd1 cells show hollow mitochondria, imaged with Cox4-GFP. Scale bars, 2 μm. Line profile analysis (below) depicts fluorescent intensity across the indicated mitochondria.
(E) Thin section TEM micrographs of Δcrd1 show IMM with cristae invaginations (see inset, scale bars 100 nm), while SFA2Δcrd1 cells show an onion-like IMM similar to what has been observed in Δatp20. Scale bars, 400 nm.
(F) SFA2Δcrd1 retains ATP synthase dimerization. Shown are BN-PAGE western blots run from digitonin-solubilized isolated mitochondria of SFA strains and WT with and without CL. Δatp20 is the monomeric ATP synthase control.
While loss of CL modulated CM formation under increased PL saturation, it did so independently of ATP synthase dimerization. Δcrd1 cells showed no defect in ATP synthase oligomerization compared to CRD1 cells, while SFA2Δcrd1 cells maintained an identical level of oligomerization found in SFA2 cells (Figure 4F). However, loss of CL still showed a strong epistasis with the saturation induced loss of ATP synthase oligomerization in SFA2 cells, as well as with complete loss of dimerization in Δatp20 cells (Figure S6D). Thus, CL acts orthogonally to PL saturation and ATP synthase oligomerization to modulate IMM morphology, but only under conditions where the latter is reduced compared to WT cells grown in laboratory conditions.
Modeling predicts compensatory roles for lipid and protein-encoded curvature in shaping cristae tubule formation
To understand the interaction between CL, which contributes to net membrane spontaneous curvature, and ATP synthase oligomers, whose induced curvature is localized specifically at cristae ridges, we employed continuum modeling framework based on previous efforts to model membrane tubule formation73,103–105. We modeled the simplest CM structure — cristae tubules — as axisymmetric tubes that bud from a flat membrane (Figure 5A). We added a pre-defined coat area of ATP synthase oligomers that contribute an anisotropic curvature (D0). An isotropic membrane curvature (C0) was then applied across the entire membrane to model the effects of asymmetrically localized CL. In simulations, we varied the magnitude of D0 and C0 based on data from simulations of curvature induced by ATP synthase dimers23 and CL effects on mitochondrial membrane compositions estimated by simulations of outer and inner IMM leaflets (Figure S4G). In both cases, the approximate ranges were set to 0 to 0.035 nm−1. The stiffness of the membrane was also varied across biologically reasonable values (10–20 kBT) and the membrane tension was set at 0.01 pN/nm104. Finally, to incorporate the stresses due to the proposed roles of the MICOS complex and Mgm1 at the CJs, we used a localized collar force density of 8 pN/nm around the base of the tubule neck. Additional details of the governing equations and parameters of the model can be found in the Supplementary Text.
Figure 5:
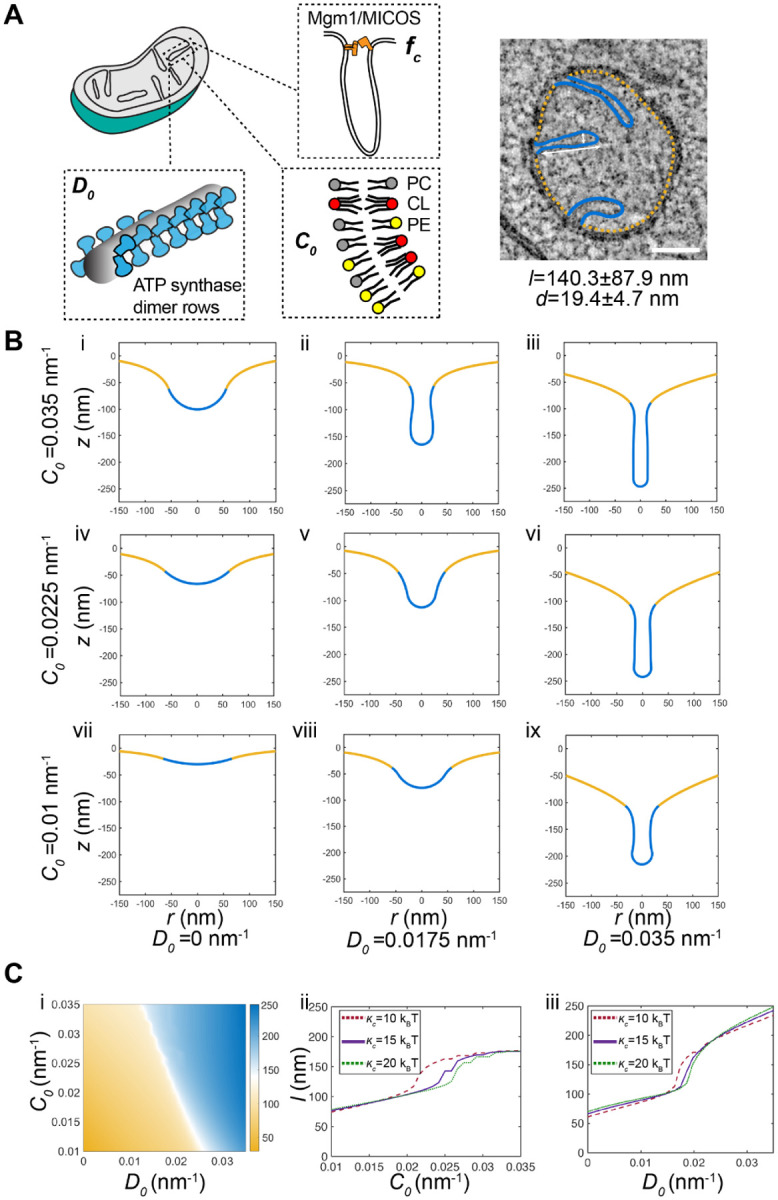
Continuum modeling of CM tubule formation reveals a snap-through instability mediated by both lipid and ATP synthase generated curvatures.
(A) Schematic depiction of yeast CM tubules containing a modeled neck force (fc) induced by Mgm1p and MICOS complexes, a deviatoric curvature imposed by ATP synthase at tubule ridges (D0), and a spontaneous curvature along the entire membrane imposed by asymmetric distribution of CL across the IMM (C0). (Right) TEM image showing typical yeast CM tubules in a single mitochondrion. Scale bars, 100 nm. The average tubule length (140.3±87.9nm) and diameter (19.4±4.7nm) of 15 cristae tubules were analyzed from tomograms of n=3 SFA2 mitochondria.
(B) Changes in deviatory and spontaneous curvatures modulates tubule morphology. Panels i-ix show shapes of the membranes from simulations of the continuum model. For these simulations, the bending modulus of the membrane was maintained at 15 kBT and the tension was set at 0.01 pN/nm. The total membrane area was set to 5.65 × 105 nm2 and the coated area was 1.413 × 104 nm2. The values of C0 and D0 were varied as shown and fc was set to 8 pN/nm at the base of the coat.
(C) CM tubule formation shows a snap-through behavior. (i) Length of the tube as a function of C0 and D0 for the same values of bending modulus, tension, and areas as shown in (B). The white region shows the transition from the short to the long tube. The color bar shows the length of the tube in nm. Line graphs in (ii) show the length of the tube as a function of C0 for D0 fixed at 0.0175 nm−1 and and line graphs in (iii) show the length of the tube as a function of D0 for C0 fixed at 0.0225 nm−1 for three different values of к. In both these graphs, the abrupt change in length is indicative of a snapthrough instability.
We observed that the combination of isotropic and anisotropic spontaneous curvatures is sufficient to deform the membrane into different shapes reminiscent of flat membranes, buds that could be relevant for onion-like IMM formation, and CM-like tubules (Figure 5B). High values of anisotropic spontaneous curvature (Figure 5B, third column) promoted the formation of long tubular structures for all values of isotropic spontaneous curvatures and bending moduli, and did so independently of the presence of the collar force (Figure S5). Tubule length and diameter were generally consistent with that of CMs in cells (Figure 5B). Low values of anisotropic curvature (e.g. no ATP synthase oligomerization), did not allow for tubule formation and instead led to flat or bud-like structures (Figure 5B, i-iii). The latter was observed even when isotropic spontaneous curvature, potentially encoded by CL, remained high. For intermediate values of anisotropic curvature, which could approximate the state in SFA2 cells that show partial loss ATP synthase oligomerization, higher values of isotropic curvature resulted in shapes that still resembled cristae (Figure 5B, ii and v), while lower values of isotropic curvature resulted in shallow invaginations. From these simulations, we predicted that a combination of either high isotropic or anisotropic curvatures are sufficient for forming cristae-like structures relevant to observed phenotypes in cells. Specifically, the dependency of tubule formation on isotropic spontaneous curvature when anisotropic spontaneous curvature is partially lost could explain the interaction observed between CL and PL saturation. We note that similar calculations in the absence of the collar force (Figure S5), show bud-like and tubular structures with a wider neck, consistent with the model that local forces exerted by the MICOS and Mgm1p shape CJs22,28,106,107.
Further investigation into the parameter space for cristae-like tubular structures revealed a transition from the short U-shaped invaginations to the long tubular structures in an abrupt manner (Figure 5Ci). This transition was observed as the anisotropic curvature (e.g. via ATP synthase oligomerization) increased for a fixed value of isotropic curvature (Figure 5Cii) or as the isotropic curvature (e.g. via CL-encoded membrane spontaneous) was increased for a fixed value of anisotropic curvature (Figure 5Cii) for the different values of bending moduli (see also Figure S5 for additional simulations). This sudden change in length is characterized by a snapthrough transition, where a small amount of thermodynamic work done on the system, by changing either material properties or curvatures induced, results in a change in the minimal energy state of the system where a tube-like shape is a low energy configuration103,104,108,109. We observed that the curvature values at which this transition occurs was modulated by the presence of a collar force at the tubular neck, suggesting that mechanics could underlie the functional interactions observed between Mic60p at the CJ and PL saturation (Figure S2C, Figure S5). These simulations thus predict that the formation of cristae-like structures in mitochondrial membranes can result from mechanical instabilities and that these instabilities are governed by the composition and mechanical properties of the IMM.
CL is an essential mitochondrial lipid in natural yeast environments
Given the role of CL in cristae morphology of more saturated IMMs and the conservation of CL synthesis among eukaryotes110, we considered the physiological function for CL in yeast. The lack of phenotype for Δcrd1 strains under standard laboratory growth conditions has long been perplexing, given the demonstrated importance of CL for mitochondrial function in other organisms111. However, natural yeast environments, such as rotting fruits or fermentation tanks, differ starkly from laboratory conditions, especially with regards to the availability of molecular oxygen. Like other lipid desaturases, Ole1p consumes oxygen as an electron acceptor, which binds to a low-affinity (KM ~60 μM) di-iron site112; thus the enzyme’s activity is sensitive to environmental oxygenation113,114. Cells grown in microaerobic fermenters show a lower level of di-unsaturated phospholipids than those grown under highly aerated conditions (low volume shake flask); their lipidomes are an intermediate between that of aerated SFA2 and SFA3 cells (Figure S6E). CRD1 cells grown under microaerobic conditions also showed similar whole cell headgroup compensations (a decrease in the PE/PC ratio) as SFA2 strains (Figure S6F). We hypothesized that in these less aerated environments, CL metabolism could have evolved to support an essential mechanical function.
To test the role of oxygenation on CL function, we grew CRD1 and Δcrd1 yeast strains in microaerobic chambers, comparing their lipidomes and mitochondrial morphologies to those grown in highly aerated shake flasks (Figure 6A). Under limited oxygenation, CRD1 cells increased the abundance of CL twofold compared to highly aerated conditions (Figure 6B), counter to the canonical role of CL in respiratory metabolism. Both CRD1 and Δcrd1 cells showed increased levels of PL saturation in the total PL pool (Figure 6C), and in major Pl classes PC and PE (Figure S6F), under microaerobic conditions. Importantly, loss of CL did not have an effect on the levels of acyl chain saturation (Figure 6C). After microaerobic growth, yeast containing CL still presented tubular mitochondrial morphology, but Δcrd1 cells displayed a stark increase in aberrant mitochondrial morphologies (Figure 6D, 6E). We also observed that Δcrd1 cells showed increased abundance of PE lipids under microaerobic conditions, potentially as a curvature compensation for lack of CL in the cell (Figure S6F). Ultrastructural analysis of IMM structure revealed that CRD1 cells grown under microaerobic conditions displayed tubular cristae morphologies while Δcrd1 cells displayed a mixture of flat and onion-like IMM structures (Figure 6F). CL is thus required for mitochondrial biogenesis during yeast fermentation, but not in the highly oxygenated laboratory conditions used by most mitochondrial research.
Figure 6:
Cardiolipin synthesis is essential for mitochondria in yeast growth conditions characterized by low oxygenation.
(A) Schematic representation of different oxygen concentrations in different yeast growth environments. Microaerobic conditions cause increased saturation of membranes due to lower desaturate activity of Ole1p, an oxygen dependent enzyme.
(B) WT cells increase the abundance of CL under microaerobic conditions. Shown are abundances of each PL class, n=3. Error bars indicate SD. ****p < 0.0001, unpaired two-tailed t-test compared against wild-type.
(C) Microaerobic growth conditions cause an increase in acyl chain saturation in the total PL pool, which is not affected by loss of CL in Δcrd1 strains. Error bars indicate SD from n=3 biological replicates.
(D) Under microaerobic conditions, loss of CL (Δcrd1) causes loss of tubular mitochondrial structure. WT (CRD1) and Δcrd1 cells were grown in microaerobic chambers for 48 hours prior to imaging, n=3. Scale bars, 2 μm. **p < 0.005, unpaired two-tailed t-test compared against WT.
(E) Microaerobic Δcrd1 show hollow mitochondria, imaged with Cox4-GFP. Scale bars, 2 μm. Line profile analysis (below) depicts fluorescent intensity across the indicated mitochondria.
(F) Under microaerobic conditions, CRD1 cells contain long, sheet-like cristae structures while Δcrd1 cells lack cristae, and display both onion-like and flat abnormal IMM structures as visualized by thin-section TEM. Scale bars, 250 nm. Inset showing abundant cristae sheets in CRD1 cells, scale bar, 125 nm.
Discussion
While a large body of research has identified specific proteins that shape the IMM, analogous roles for lipids have been less defined. Here we explored an initial observation that small changes to the unsaturation of PL acyl chains in yeast specifically causes a loss of CMs, similar to what has been observed upon loss of cristae shaping proteins. We found that di-unsaturated PLs promotes ATP synthase oligomerization, which is progressively lost when the activity of the lipid desaturase Ole1p decreases. We then explored a key interaction between 1) the curvature at cristae ridges induced by ATP synthase oligomerization and modulated by PL saturation and 2) lipid-encoded curvature across the IMM, which is promoted by conical lipids like CL. Both of these define whether the IMM exists in a high or low curvature state, which in turns controls ATP production.
We propose that intrinsic membrane curvature can be generated both by proteins and by asymmetric distributions of lipids across the bilayer, which contributes net spontaneous curvature between the two leaflets. For CL, there are at least three potential sources by which net curvature is generated. First, extensive measurements of compositional asymmetry of CL have been performed in yeast using nonyl acridine orange, which preferentially interacts with CL on the outer (IMS-facing) leaflet at low concentrations102,115. These suggest that the yeast IMM features up to 2-fold more CL in the outer leaflet, which would optimize its negative curvature at cristae ridges. Second, the differences in local pH between the matrix and intermembrane space could favor the monoanion species in the leaflet facing the latter, which would also promote negative curvature at the cristae ridge. Finally, CL and other conical lipids would segregate into deformed membrane regions, further promoting curvature. Such a phenomenon has been observed experimentally when CL localized to thin, high curvature tubules that have been pulled from large, low curvature vesicles36. In simulations, this effect is apparent in localized CL concentrations in thermal fluctuations (Figure 3G) and those induced by compression44,116. In the IMM, it is likely that local deformations induced by ATP synthase18 cause the local concentration of conical lipids (e.g. CL and di-unsaturated PE), which further act to deform the IMM. The action of membrane shaping lipids and proteins are therefore likely to be intrinsically linked.
In response to perturbations, we observed mitochondrial-specific lipidomes that are consistent with curvature-based effects of both PL saturation and CL. Increasing saturation through lowering of OLE1 expression in SFA strains causes an increase of PC in cells, which increases fluidity compared to PE and can thus be understood in a homeoviscous adaptation framework. However, in the mitochondria, increased saturation has the opposite effect, increasing PE levels to counteract the loss in lipid spontaneous curvature. Analogously, under microaerobic conditions, CL levels increase compared to highly aerobic cells, despite limited usage of the ETC for energy production in the former. Cells rely on mitochondria for other metabolic tasks, such as amino acid and cofactor biosynthesis117, and so maintenance of IMM structure is still required under fermentation conditions. Additional CL could supplement the loss of di-unsaturated PLs due to limited Ole1p activity. We observe a similar change in the CL content of SFA2 mitochondria. Though SFA3 mitochondria show a loss of CL, this corresponds to dramatic increase in saturation specifically in CL, which could limit spontaneous curvature and promote its degradation118. These examples of mitochondrial lipid adaptation suggest unidentified mechanisms in the IMM to sense and respond to changes in membrane curvature.
The interplay with PL saturation provides a biophysical rationale for long-standing questions regarding the cellular roles of CL. Despite its ubiquity among eukaryotes, the reported functions of CL have differed strikingly across experimental systems. While loss of CL is lethal in mice52,54,119 and drives CM loss in mammalian cell lines120, it has only minor phenotypes in yeast grown under common laboratory conditions. In Drosophila, loss of CL reduces the density of ETC components in the IMM and thus modulates respiration54, but also does not cause loss of CMs. We propose that the mechanical functions of CL function are dependent on the surrounding lipid environment in the IMM, which can change depending on growth conditions. Under yeast growth conditions that cause higher PL saturation, we find that CL is essential for CM formation. These are likely to better mimic the oxygenation of natural yeast environments, such as rotting fruits. Yeast grown under highly aerated laboratory conditions (shake flasks or culture tubes shaking at >100 rpm) instead show an unusually high level of di-unsaturated PLs. The acyl chain composition of PE and PC in mammalian cell lines and tissues, where CL is essential, more closely resemble those of microaerobically grown yeast or the aerobically grown SFA2 strain (Figure S1F). In the case of Drosophila, an elevated PE/PC ratio121,122 might also compensate for the loss of CL. It is notable that these model systems are being widely utilized to study the mechanisms underlying Barth syndrome, a disease caused by altered CL metabolism. In humans, lipid saturation is highly influenced by diet and other metabolic states123 and so these could be relevant to the pathology of CL loss. Global analysis and modeling of mitochondrial lipidomes could be required to understand the function of CL in different organisms and metabolic conditions.
To understand how membrane curvature generated by CL can act independently of ATP synthase dimerization, we carried out mechanical modeling of tubules as the simplest CM structure. Modeling predicted that intermediate levels of anisotropic curvature at the tubule ridge can be compensated by high isotropic curvature encoded by the lipidome, which is consistent with observations of how CL modulates IMM structure in SFA2 vs. SFA2Δcrd1 cells. An additional motivation for employing continuum models for biological structures is to identify the physical principles that could underlie their formation in cells. CM formation in yeast is an example of a bimodal process: cells either show high curvature tubules or flat structures due to both lipidome changes or loss of cristae-shaping proteins. Our simulations suggest that tubule formation is defined by a snap-through instability, in which a system can shift between two morphological states via modest perturbations in its properties or forces applied. Such buckling events are intrinsic to the Helfrich model depending on choice of coordinates124,125 and have previously been shown for other curved structures, such as buds in endocytosis104,108. They are thus likely to apply for other CM structures. The IMM is just one example of a highly curved membrane in cells; others include narrow tubules in the peripheral ER126 and rims of Golgi cisternae127. A common feature across these organelles is that regions of very high curvature are present alongside those with low curvatures7,128,129. While relevant curvature-inducing proteins have been identified in these structures, how bimodal distributions of curvature are stabilized within the same continuous lipid membrane is less clear. Membrane compositions that border snapthrough instabilities could provide a mechanism for this arrangement.
An outstanding question of this work is how specific lipid perturbations modulate the dimerization and higher order organization of ATP synthases. PL saturation, which influences both membrane stiffness and curvature, reduces ATP synthase oligomerization in cells. Among membrane protein dimers, the yeast ER sensors Mga2/Spt23 show a similar dimer to monomer transition when their host membrane shifts di-unsaturated to mono-unsaturated PLs130,131. While Mga2/Spt23 are small, single pass transmembrane proteins, the CLC Cl−/H+ antiporter dimer has a similar buried surface area as ATP synthase (~2400 Å2) and a modest dimerization free energy (19 kBT132) that is also highly sensitive to its lipidic environment133. In CLC, short chain PLs, which notably accumulate in more saturated lipidomes, have been shown to stabilize the monomer conformation and through this mechanism weaken its dimerization. Unlike CLC, dimerization of ATP synthase is associated with an extreme local membrane deformation (a ~90° bend in S. cerevisiae134). An alternative hypothesis is that the changes in mechanical properties encoded by PL saturation, especially stiffness, alters the energetics of the local membrane deformation, reducing the dimer:monomer equilibrium by increasing the elastic cost of this deformation. Although adaptive changes in the mitochondrial lipidome could buffer against membrane stiffness (Figure 3D), they are likely to do so incompletely in ways that are not captured by our simplified MD simulations. Distinguishing between these models will require future experiments and modeling, which would be aided by structures of monomeric ATP synthases with fully resolved dimerization subunits.
During the evolution of eukaryotic cells, the machinery for ATP generation in the ETC adopted a secondary function in the shaping of the IMM. The emergence of ATP synthase dimers and other cristae-shaping protein complexes occurred alongside a specialization in the inner membrane lipidome, including the proliferation of CL from the proteobacterial inner membrane135,136. Among extant eukaryotes, ATP synthase organization varies widely; for example, in mammalian mitochondria ATP synthases range from primarily monomeric137 to mixtures of monomers and dimers138. It is notable that CL is essential for proper IMM structure in these systems, unlike in aerobic yeast in which dimers are predominant. The composition of other cristae-shaping proteins, such as the MICOS complex139, also differ across eukaryotes, despite the ubiquity of CMs in mitochondria. Such variability in IMM shaping proteins could have necessitated the utilization of alternative forms of curvature generation encoded by the mitochondrial lipidome.
Supplementary Material
Acknowledgments
José Faraldo-Gómez, Edward Lyman, and Nicolas Frédéric-Lipp provided helpful discussions. Daniel Degreif assisted in initial strain development. Alexander Tzagoloff, Leticia Franco, and Mário Barros supplied reagents. The National Institutes of Health (R35-GM142960 to I.B, R01AG065549, R24GM137200 and U24NS120055 to M.H.E.), the Office of Naval Research (ONR N00014-20-1-2469 to P.R.), the National Science Foundation (DBI-2014862 to M.H.E), and the Moore-Simons Project on the Origin of the Eukaryotic Cell (GBMF-9734 to I.B., P.R., and M.H.E.) provided financial support. K.V. was supported by the NIH Molecular Biophysics Training Grant (T32-GM008326C). C.L. was supported by a Kavli Institute for Brain and Mind Postdoctoral Fellowship. Howard Hughes Medical Institute supported initial project conception through the Janelia Visiting Scientist Program. Molecular dynamics simulations were run on hardware hosted by the Triton Shared Computing Cluster.
References
- 1.Nunnari J., and Suomalainen A. (2012). Mitochondria: In Sickness and in Health. Cell 148, 1145–1159. 10.1016/j.cell.2012.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daems W.T., and Wisse E. (1966). Shape and attachment of the cristae mitochondriales in mouse hepatic cell mitochondria. J. Ultrastruct. Res. 16, 123–140. [DOI] [PubMed] [Google Scholar]
- 3.Perkins G., Renken C., Martone M.E., Young S.J., Ellisman M., and Frey T. (1997). Electron tomography of neuronal mitochondria: three-dimensional structure and organization of cristae and membrane contacts. J. Struct. Biol. 119, 260–272. [DOI] [PubMed] [Google Scholar]
- 4.Revel J.P., Fawcett D.W., and Philpott C.W. (1963). OBSERVATIONS ON MITOCHONDRIAL STRUCTURE. Journal of Cell Biology 16, 187–195. 10.1083/jcb.16.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mannella C.A. (2006). Structure and dynamics of the mitochondrial inner membrane cristae. Biochim. Biophys. Acta 1763, 542–548. [DOI] [PubMed] [Google Scholar]
- 6.Zick M., Rabl R., and Reichert A.S. (2009). Cristae formation—linking ultrastructure and function of mitochondria. Biochimica et Biophysica Acta (BBA) - Molecular Cell Research 1793, 5–19. [DOI] [PubMed] [Google Scholar]
- 7.Mendelsohn R., Garcia G.C., Bartol T.M., Lee C.T., Khandelwal P., Liu E., Spencer D.J., Husar A., Bushong E.A., Phan S., et al. (2022). Morphological principles of neuronal mitochondria. Journal of Comparative Neurology 530, 886–902. 10.1002/cne.25254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pánek T., Eliáš M., Vancová M., Lukeš J., and Hashimi H. (2020). Returning to the Fold for Lessons in Mitochondrial Crista Diversity and Evolution. Current Biology 30, R575–R588. 10.1016/j.cub.2020.02.053. [DOI] [PubMed] [Google Scholar]
- 9.Davies K.M., Strauss M., Daum B., Kief J.H., Osiewacz H.D., Rycovska A., Zickermann V., and Kühlbrandt W. (2011). Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl. Acad. Sci. U. S. A. 108, 14121–14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rieger B., Junge W., and Busch K.B. (2014). Lateral pH gradient between OXPHOS complex IV and F0F1 ATP-synthase in folded mitochondrial membranes. Nature Communications 5. 10.1038/ncomms4103. [DOI] [PubMed] [Google Scholar]
- 11.Cogliati S., Enriquez J.A., and Scorrano L. (2016). Mitochondrial Cristae: Where Beauty Meets Functionality. Trends in Biochemical Sciences 41, 261–273. 10.1016/j.tibs.2016.01.001. [DOI] [PubMed] [Google Scholar]
- 12.Mannella C.A., Marko M., and Buttle K. (1997). Reconsidering mitochondrial structure: new views of an old organelle. Trends in Biochemical Sciences 22, 37–38. 10.1016/s0968-0004(96)30050-9. [DOI] [PubMed] [Google Scholar]
- 13.Mannella C.A. (2006). The relevance of mitochondrial membrane topology to mitochondrial function. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease 1762, 140–147. 10.1016/j.bbadis.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Mannella C.A., Marko M., Penczek P., Barnard D., and Frank J. (1994). The internal compartmentation of rat-liver mitochondria: Tomographic study using the high-voltage transmission electron microscope. Microscopy Research and Technique 27, 278–283. 10.1002/jemt.1070270403. [DOI] [PubMed] [Google Scholar]
- 15.Frey T.G., and Mannella C.A. (2000). The internal structure of mitochondria. Trends in Biochemical Sciences 25, 319–324. 10.1016/s0968-0004(00)01609-1. [DOI] [PubMed] [Google Scholar]
- 16.Dudkina N.V., Heinemeyer J., Keegstra W., Boekema E.J., and Braun H.-P. (2005). Structure of dimeric ATP synthase from mitochondria: an angular association of monomers induces the strong curvature of the inner membrane. FEBS Lett. 579, 5769–5772. [DOI] [PubMed] [Google Scholar]
- 17.Strauss M., Hofhaus G., Schröder R.R., and Kühlbrandt W. (2008). Dimer ribbons of ATP synthase shape the inner mitochondrial membrane. EMBO J. 27, 1154–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Blum T.B., Hahn A., Meier T., Davies K.M., and Kühlbrandt W. (2019). Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc. Natl. Acad. Sci. U. S. A. 116, 4250–4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paumard P., Vaillier J., Coulary B., Schaeffer J., Soubannier V., Mueller D.M., Brèthes D., di Rago J.-P., and Velours J. (2002). The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 21, 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arnold I., Pfeiffer K., Neupert W., Stuart R.A., and Schägger H. (1998). Yeast mitochondrial F1F0-ATP synthase exists as a dimer: identification of three dimer-specific subunits. EMBO J. 17, 7170–7178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Arselin G., Vaillier J., Salin B., Schaeffer J., Giraud M.-F., Dautant A., Brèthes D., and Velours J. (2004). The modulation in subunits e and g amounts of yeast ATP synthase modifies mitochondrial cristae morphology. J. Biol. Chem. 279, 40392–40399. [DOI] [PubMed] [Google Scholar]
- 22.Rabl R., Soubannier V., Scholz R., Vogel F., Mendl N., Vasiljev-Neumeyer A., Körner C., Jagasia R., Keil T., Baumeister W., et al. (2009). Formation of cristae and crista junctions in mitochondria depends on antagonism between Fcj1 and Su e/g. J. Cell Biol. 185, 1047–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Anselmi C., Davies K.M., and Faraldo-Gómez J.D. (2018). Mitochondrial ATP synthase dimers spontaneously associate due to a long-range membrane-induced force. J. Gen. Physiol., jgp.201812033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harner M., Körner C., Walther D., Mokranjac D., Kaesmacher J., Welsch U., Griffith J., Mann M., Reggiori F., and Neupert W. (2011). The mitochondrial contact site complex, a determinant of mitochondrial architecture. EMBO J. 30, 4356–4370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoppins S., Collins S.R., Cassidy-Stone A., Hummel E., Devay R.M., Lackner L.L., Westermann B., Schuldiner M., Weissman J.S., and Nunnari J. (2011). A mitochondrial-focused genetic interaction map reveals a scaffold-like complex required for inner membrane organization in mitochondria. J. Cell Biol. 195, 323–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glytsou C., Calvo E., Cogliati S., Mehrotra A., Anastasia I., Rigoni G., Raimondi A., Shintani N., Loureiro M., Vazquez J., et al. (2016). Optic Atrophy 1 Is Epistatic to the Core MICOS Component MIC60 in Mitochondrial Cristae Shape Control. Cell Rep. 17, 3024–3034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G.V., Rudka T., Bartoli D., Polishuck R.S., Danial N.N., De Strooper B., et al. (2006). OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell 126, 177–189. [DOI] [PubMed] [Google Scholar]
- 28.Patten D.A., Wong J., Khacho M., Soubannier V., Mailloux R.J., Pilon-Larose K., MacLaurin J.G., Park D.S., McBride H.M., Trinkle-Mulcahy L., et al. (2014). OPA1-dependent cristae modulation is essential for cellular adaptation to metabolic demand. EMBO J. 33, 2676–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu C., Shu L., Huang X., Yu J., Li L., Gong L., Yang M., Wu Z., Gao Z., Zhao Y., et al. (2020). OPA1 and MICOS Regulate mitochondrial crista dynamics and formation. Cell Death Dis. 11, 940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harner M.E., Unger A.-K., Geerts W.J., Mari M., Izawa T., Stenger M., Geimer S., Reggiori F., Westermann B., and Neupert W. (2016). An evidence based hypothesis on the existence of two pathways of mitochondrial crista formation. Elife 5. 10.7554/eLife.18853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sesaki H., Southard S.M., Yaffe M.P., and Jensen R.E. (2003). Mgm1p, a dynamin-related GTPase, is essential for fusion of the mitochondrial outer membrane. Mol. Biol. Cell 14, 2342–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinser E., Sperka-Gottlieb C.D., Fasch E.V., Kohlwein S.D., Paltauf F., and Daum G. (1991). Phospholipid synthesis and lipid composition of subcellular membranes in the unicellular eukaryote Saccharomyces cerevisiae. Journal of Bacteriology 173, 2026–2034. 10.1128/jb.173.6.2026-2034.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mejia E.M., and Hatch G.M. (2016). Mitochondrial phospholipids: role in mitochondrial function. Journal of Bioenergetics and Biomembranes 48, 99–112. 10.1007/s10863-015-9601-4. [DOI] [PubMed] [Google Scholar]
- 34.Horvath S.E., and Daum G. (2013). Lipids of mitochondria. Progress in Lipid Research 52, 590–614. 10.1016/j.plipres.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 35.LeCocq J., and Ballou C.E. (1964). On the Structure of Cardiolipin*. Biochemistry 3, 976–980. 10.1021/bi00895a023. [DOI] [PubMed] [Google Scholar]
- 36.Beltrán-Heredia E., Tsai F.-C., Salinas-Almaguer S., Cao F.J., Bassereau P., and Monroy F. (2019). Membrane curvature induces cardiolipin sorting. Communications Biology 2. 10.1038/s42003-019-0471-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Olofsson G., and Sparr E. (2013). Ionization constants pKa of cardiolipin. PLoS One 8, e73040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kates M., Syz J.Y., Gosser D., and Haines T.H. (1993). pH-dissociation characteristics of cardiolipin and its 2’-deoxy analogue. Lipids 28, 877–882. [DOI] [PubMed] [Google Scholar]
- 39.Sturm M., Gutowski O., and Brezesinski G. (2022). The effect of pH on the structure and lateral organization of cardiolipin in Langmuir monolayers. Chemphyschem 23, e202200218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khalifat N., Puff N., Bonneau S., Fournier J.-B., and Angelova M.I. (2008). Membrane Deformation under Local pH Gradient: Mimicking Mitochondrial Cristae Dynamics. Biophysical Journal 95, 4924–4933. 10.1529/biophysj.108.136077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khalifat N., Fournier J.-B., Angelova M.I., and Puff N. (2011). Lipid packing variations induced by pH in cardiolipin-containing bilayers: The driving force for the cristae-like shape instability. Biochimica et Biophysica Acta (BBA) - Biomembranes 1808, 2724–2733. 10.1016/j.bbamem.2011.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Ikon N., and Ryan R.O. (2017). Cardiolipin and mitochondrial cristae organization. Biochimica et Biophysica Acta (BBA) - Biomembranes 1859, 1156–1163. 10.1016/j.bbamem.2017.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Y.-F., Tsang K.-Y., Chang W.-F., and Fan Z.-A. (2015). Differential dependencies on [Ca2] and temperature of the monolayer spontaneous curvatures of DOPE, DOPA and cardiolipin: effects of modulating the strength of the inter-headgroup repulsion. Soft Matter 11, 4041–4053. 10.1039/c5sm00577a. [DOI] [PubMed] [Google Scholar]
- 44.Dahlberg M., and Maliniak A. (2010). Mechanical Properties of Coarse-Grained Bilayers Formed by Cardiolipin and Zwitterionic Lipids. J. Chem. Theory Comput. 6, 1638–1649. [DOI] [PubMed] [Google Scholar]
- 45.Konar S., Arif H., and Allolio C. (2023). Mitochondrial Membranes: Model Lipid Compositions, Material Properties and the Changing Curvature of Cardiolipin. bioRxiv, 2023.02.06.527315. 10.1101/2023.02.06.527315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Adès L.C., Gedeon A.K., Wilson M.J., Latham M., Partington M.W., Mulley J.C., Nelson J., Lui K., and Sillence D.O. (1993). Barth syndrome: Clinical features and confirmation of gene localisation to distal Xq28. American Journal of Medical Genetics 45, 327–334. 10.1002/ajmg.1320450309. [DOI] [PubMed] [Google Scholar]
- 47.Bione S., D’Adamo P., Maestrini E., Gedeon A.K., Bolhuis P.A., and Toniolo D. (1996). A novel X-linked gene, G4.5. is responsible for Barth syndrome. Nature Genetics 12, 385–389. 10.1038/ng0496-385. [DOI] [PubMed] [Google Scholar]
- 48.Acehan D., Xu Y., Stokes D.L., and Schlame M. (2007). Comparison of lymphoblast mitochondria from normal subjects and patients with Barth syndrome using electron microscopic tomography. Laboratory Investigation 87, 40–48. 10.1038/labinvest.3700480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Claypool S.M., and Koehler C.M. (2012). The complexity of cardiolipin in health and disease. Trends in Biochemical Sciences 37, 32–41. 10.1016/j.tibs.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ren M., Phoon C.K.L., and Schlame M. (2014). Metabolism and function of mitochondrial cardiolipin. Progress in Lipid Research 55, 1–16. 10.1016/j.plipres.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Paradies G., Paradies V., Ruggiero F.M., and Petrosillo G. (2019). Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 8, 728. 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baile M.G., Sathappa M., Lu Y.-W., Pryce E., Whited K., Michael McCaffery J., Han X., Alder N.N., and Claypool S.M. (2014). Unremodeled and Remodeled Cardiolipin Are Functionally Indistinguishable in Yeast. Journal of Biological Chemistry 289, 1768–1778. 10.1074/jbc.m113.525733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jiang F., Rizavi H.S., and Greenberg M.L. (1997). Cardiolipin is not essential for the growth of Saccharomyces cerevisiae on fermentable or non-fermentable carbon sources. Mol. Microbiol. 26, 481–491. [DOI] [PubMed] [Google Scholar]
- 54.Xu Y., Erdjument-Bromage H., Phoon C.K.L., Neubert T.A., Ren M., and Schlame M. (2021). Cardiolipin remodeling enables protein crowding in the inner mitochondrial membrane. EMBO J. 40, e108428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang M., Mileykovskaya E., and Dowhan W. (2005). Cardiolipin Is Essential for Organization of Complexes III and IV into a Supercomplex in Intact Yeast Mitochondria. Journal of Biological Chemistry 280, 29403–29408. 10.1074/jbc.m504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harayama T., and Riezman H. (2019). Author Correction: Understanding the diversity of membrane lipid composition. Nature Reviews Molecular Cell Biology 20, 715–715. 10.1038/s41580-019-0171-x. [DOI] [PubMed] [Google Scholar]
- 57.Filippov A., Orädd G., and Lindblom G. (2003). The Effect of Cholesterol on the Lateral Diffusion of Phospholipids in Oriented Bilayers. Biophysical Journal 84, 3079–3086. 10.1016/s0006-3495(03)70033-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vance J.E. (2015). Phospholipid Synthesis and Transport in Mammalian Cells. Traffic 16, 1–18. 10.1111/tra.12230. [DOI] [PubMed] [Google Scholar]
- 59.Bard M. (1972). Biochemical and Genetic Aspects of Nystatin Resistance in Saccharomyces cerevisiae. Journal of Bacteriology 111, 649–657. 10.1128/jb.111.3.649-657.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stukey J.E., McDonough V.M., and Martin C.E. (1989). Isolation and characterization of OLE1, a gene affecting fatty acid desaturation from Saccharomyces cerevisiae. Journal of Biological Chemistry 264, 16537–16544. 10.1016/s0021-9258(19)84740-3. [DOI] [PubMed] [Google Scholar]
- 61.McConnell S.J., Stewart L.C., Talin A., and Yaffe M.P. (1990). Temperature-sensitive yeast mutants defective in mitochondrial inheritance. Journal of Cell Biology 111, 967–976. 10.1083/jcb.111.3.967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stewart L.C., and Yaffe M.P. (1991). A role for unsaturated fatty acids in mitochondrial movement and inheritance. The Journal of Cell Biology 115, 1249–1257. 10.1083/jcb.115.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penzo D., Tagliapietra C., Colonna R., Petronilli V., and Bernardi P. (2002). Effects of fatty acids on mitochondria: implications for cell death. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1555, 160–165. 10.1016/s0005-2728(02)00272-4. [DOI] [PubMed] [Google Scholar]
- 64.Sparagna G.C., Hickson-Bick D.L., Maximilian Buja L., and McMillin J.B. (2000). A metabolic role for mitochondria in palmitate-induced cardiac myocyte apoptosis. American Journal of Physiology-Heart and Circulatory Physiology 279, H2124–H2132. 10.1152/ajpheart.2000.279.5.h2124. [DOI] [PubMed] [Google Scholar]
- 65.Jheng H.-F., Tsai P.-J., Guo S.-M., Kuo L.-H., Chang C.-S., Su I.-J., Chang C.-R., and Tsai Y.-S. (2012). Mitochondrial Fission Contributes to Mitochondrial Dysfunction and Insulin Resistance in Skeletal Muscle. Molecular and Cellular Biology 32, 309–319. 10.1128/mcb.05603-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Petersen K.F., Dufour S., Befroy D., Garcia R., and Shulman G.I. (2004). Impaired Mitochondrial Activity in the Insulin-Resistant Offspring of Patients with Type 2 Diabetes. New England Journal of Medicine 350, 664–671. 10.1056/nejmoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lowell B.B., and Shulman G.I. (2005). Mitochondrial Dysfunction and Type 2 Diabetes. Science 307, 384–387. 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 68.Budin I., de Rond T., Chen Y., Chan L.J.G., Petzold C.J., and Keasling J.D. (2018). Viscous control of cellular respiration by membrane lipid composition. Science 362, 1186–1189. 10.1126/science.aat7925. [DOI] [PubMed] [Google Scholar]
- 69.Raote I., Chabanon M., Walani N., Arroyo M., Garcia-Parajo M.F., Malhotra V., and Campelo F. (2020). A physical mechanism of TANGO1-mediated bulky cargo export. Elife 9. 10.7554/eLife.59426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alimohamadi H., Bell M.K., Halpain S., and Rangamani P. (2021). Mechanical Principles Governing the Shapes of Dendritic Spines. Front. Physiol. 12, 657074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alimohamadi H., Smith A.S., Nowak R.B., Fowler V.M., and Rangamani P. (2020). Non-uniform distribution of myosin-mediated forces governs red blood cell membrane curvature through tension modulation. PLoS Comput. Biol. 16, e1007890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kozlov M.M., Campelo F., Liska N., Chernomordik L.V., Marrink S.J., and McMahon H.T. (2014). Mechanisms shaping cell membranes. Curr. Opin. Cell Biol. 29, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yuan F., Alimohamadi H., Bakka B., Trementozzi A.N., Day K.J., Fawzi N.L., Rangamani P., and Stachowiak J.C. (2021). Membrane bending by protein phase separation. Proc. Natl. Acad. Sci. U. S. A. 118. 10.1073/pnas.2017435118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Fowler P.W., Hélie J., Duncan A., Chavent M., Koldsø H., and Sansom M.S.P. (2016). Membrane stiffness is modified by integral membrane proteins. Soft Matter 12, 7792–7803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lindahl E., and Sansom M.S.P. (2008). Membrane proteins: molecular dynamics simulations. Curr. Opin. Struct. Biol. 18, 425–431. [DOI] [PubMed] [Google Scholar]
- 76.Venable R.M., Brown F.L.H., and Pastor R.W. (2015). Mechanical properties of lipid bilayers from molecular dynamics simulation. Chem. Phys. Lipids 192, 60–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chabanon M., Stachowiak J.C., and Rangamani P. (2017). Systems biology of cellular membranes: a convergence with biophysics. Wiley Interdiscip. Rev. Syst. Biol. Med. 9, e1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rangamani P. (2022). The many faces of membrane tension: Challenges across systems and scales. Biochim. Biophys. Acta Biomembr. 1864, 183897. [DOI] [PubMed] [Google Scholar]
- 79.Steigmann D.J. (2017). The Role of Mechanics in the Study of Lipid Bilayers (Springer; ). [Google Scholar]
- 80.Helfrich W. (1973). Elastic Properties of Lipid Bilayers: Theory and Possible Experiments. Zeitschrift für Naturforschung C 28, 693–703. 10.1515/znc-1973-11-1209. [DOI] [PubMed] [Google Scholar]
- 81.Canham P.B. (1970). The minimum energy of bending as a possible explanation of the biconcave shape of the human red blood cell. J. Theor. Biol. 26, 61–81. [DOI] [PubMed] [Google Scholar]
- 82.Evans E.A. (1973). A new material concept for the red cell membrane. Biophys. J. 13, 926–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Manni M.M., Tiberti M.L., Pagnotta S., Barelli H., Gautier R., and Antonny B. (2018). Acyl chain asymmetry and polyunsaturation of brain phospholipids facilitate membrane vesiculation without leakage. Elife 7. 10.7554/eLife.34394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rawicz W., Olbrich K.C., McIntosh T., Needham D., and Evans E. (2000). Effect of Chain Length and Unsaturation on Elasticity of Lipid Bilayers. Biophysical Journal 79, 328–339. 10.1016/s0006-3495(00)76295-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schiaffarino O., Valdivieso González D., García-Pérez I.M., Peñalva D.A., Almendro-Vedia V.G., Natale P., and López-Montero I. (2022). Mitochondrial membrane models built from native lipid extracts: Interfacial and transport properties. Front Mol Biosci 9, 910936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dymond M.K. (2021). Lipid monolayer spontaneous curvatures: A collection of published values. Chem. Phys. Lipids 239, 105117. [DOI] [PubMed] [Google Scholar]
- 87.Szule J.A., Fuller N.L., and Rand R.P. (2002). The effects of acyl chain length and saturation of diacylglycerols and phosphatidylcholines on membrane monolayer curvature. Biophys. J. 83, 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dawaliby R., Trubbia C., Delporte C., Noyon C., Ruysschaert J.-M., Van Antwerpen P., and Govaerts C. (2016). Phosphatidylethanolamine Is a Key Regulator of Membrane Fluidity in Eukaryotic Cells. Journal of Biological Chemistry 291, 3658–3667. 10.1074/jbc.m115.706523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Janssen M.J.F.W., Janssen M.J.F., Koorengevel M.C., de Kruijff B., and de Kroon A.I.P.M. (2000). The phosphatidylcholine to phosphatidylethanolamine ratio ofSaccharomyces cerevisiae varies with the growth phase. Yeast 16, 641–650. . [DOI] [PubMed] [Google Scholar]
- 90.Al-Fageeh M.B., and Mark Smales C. (2006). Control and regulation of the cellular responses to cold shock: the responses in yeast and mammalian systems. Biochemical Journal 397, 247–259. 10.1042/bj20060166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lee C.T., Laughlin J.G., Angliviel de La Beaumelle N., Amaro R.E., McCammon J.A., Ramamoorthi R., Holst M., and Rangamani P. (2020). 3D mesh processing using GAMer 2 to enable reaction-diffusion simulations in realistic cellular geometries. PLoS Comput. Biol. 16, e1007756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lee C.T., Laughlin J.G., Moody J.B., Amaro R.E., McCammon J.A., Holst M.J., and Rangamani P. (2020). An Open-Source Mesh Generation Platform for Biophysical Modeling Using Realistic Cellular Geometries. Biophys. J. 118, 1003–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Rangamani P., Lipshtat A., Azeloglu E.U., Calizo R.C., Hu M., Ghassemi S., Hone J., Scarlata S., Neves S.R., and Iyengar R. (2013). Decoding information in cell shape. Cell 154, 1356–1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Calizo R.C., Bell M.K., Ron A., Hu M., Bhattacharya S., Wong N.J., Janssen W.G.M., Perumal G., Pederson P., Scarlata S., et al. (2020). Cell shape regulates subcellular organelle location to control early Ca2+ signal dynamics in vascular smooth muscle cells. Sci. Rep. 10, 17866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cugno A., Bartol T.M., Sejnowski T.J., Iyengar R., and Rangamani P. (2019). Geometric principles of second messenger dynamics in dendritic spines. Sci. Rep., 444489. 10.1038/s41598-019-48028-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Bell M.K., Holst M.V., Lee C.T., and Rangamani P. (2022). Dendritic spine morphology regulates calcium-dependent synaptic weight change. bioRxiv, 2021.05.06.442994. 10.1101/2021.05.06.442994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Garcia G.C., Bartol T.M., Phan S., Bushong E.A., Perkins G., Sejnowski T.J., Ellisman M.H., and Skupin A. (2019). Mitochondrial morphology provides a mechanism for energy buffering at synapses. Sci. Rep. 9, 18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husar A., Ordyan M., Garcia G.C., Yancey J.G., Saglam A.S., Faeder J.R., Bartol T.M., and Sejnowski T.J. (2022). MCell4 with BioNetGen: A Monte Carlo Simulator of Rule-Based Reaction-Diffusion Systems with Python Interface. bioRxiv, 2022.05.17.492333. 10.1101/2022.05.17.492333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Marrink S.J., and Tieleman D.P. (2013). Perspective on the Martini model. Chem. Soc. Rev. 42, 6801–6822. [DOI] [PubMed] [Google Scholar]
- 100.Marrink S.J., Corradi V., Souza P.C.T., Ingólfsson H.I., Tieleman D.P., and Sansom M.S.P. (2019). Computational Modeling of Realistic Cell Membranes. Chem. Rev. 119, 6184–6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Callan-Jones A., Sorre B., and Bassereau P. (2011). Curvature-driven lipid sorting in biomembranes. Cold Spring Harb. Perspect. Biol. 3. 10.1101/cshperspect.a004648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gallet P.F., Petit J.M., Maftah A., Zachowski A., and Julien R. (1997). Asymmetrical distribution of cardiolipin in yeast inner mitochondrial membrane triggered by carbon catabolite repression. Biochem. J 324 (Pt 2), 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mahapatra A. (2022). Formation of protein-mediated tubes is governed by a snapthrough transition. bioRxiv, 2022.06.07.494774. 10.1101/2022.06.07.494774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hassinger J.E., Oster G., Drubin D.G., and Rangamani P. (2017). Design principles for robust vesiculation in clathrin-mediated endocytosis. Proc. Natl. Acad. Sci. U. S. A. 114, E1118–E1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Alimohamadi H., Vasan R., Hassinger J.E., Stachowiak J.C., and Rangamani P. (2018). The role of traction in membrane curvature generation. Mol. Biol. Cell 114, 2024–2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chabanon M., and Rangamani P. (2018). Gaussian curvature directs the distribution of spontaneous curvature on bilayer membrane necks. Soft Matter 14, 2281–2294. [DOI] [PubMed] [Google Scholar]
- 107.Powers T.R., Huber G., and Goldstein R.E. (2002). Fluid-membrane tethers: minimal surfaces and elastic boundary layers. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 65, 041901. [DOI] [PubMed] [Google Scholar]
- 108.Walani N., Torres J., and Agrawal A. (2015). Endocytic proteins drive vesicle growth via instability in high membrane tension environment. Proceedings of the National Academy of Sciences 112, 201418491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Irajizad E., Ramachandran R., and Agrawal A. (2018). Geometric instability catalyzes mitochondrial fission. Mol. Biol. Cell, mbcE18010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Schlame M., Brody S., and Hostetler K.Y. (1993). Mitochondrial cardiolipin in diverse eukaryotes. Comparison of biosynthetic reactions and molecular acyl species. European Journal of Biochemistry 212, 727–733. 10.1111/j.1432-1033.1993.tb17711.x. [DOI] [PubMed] [Google Scholar]
- 111.Paradies G., Paradies V., De Benedictis V., Ruggiero F.M., and Petrosillo G. (2014). Functional role of cardiolipin in mitochondrial bioenergetics. Biochimica et Biophysica Acta (BBA) - Bioenergetics 1837, 408–417. 10.1016/j.bbabio.2013.10.006. [DOI] [PubMed] [Google Scholar]
- 112.McKeon T.A., and Stumpf P.K. (1982). Purification and characterization of the stearoyl-acyl carrier protein desaturase and the acyl-acyl carrier protein thioesterase from maturing seeds of safflower. Journal of Biological Chemistry 257, 12141–12147. 10.1016/s0021-9258(18)33690-1. [DOI] [PubMed] [Google Scholar]
- 113.Vasconcelles M.J., Jiang Y., McDaid K., Gilooly L., Wretzel S., Porter D.L., Martin C.E., and Goldberg M.A. (2001). Identification and Characterization of a Low Oxygen Response Element Involved in the Hypoxic Induction of a Family ofSaccharomyces cerevisiae Genes. Journal of Biological Chemistry 276, 14374–14384. 10.1074/jbc.m009546200. [DOI] [PubMed] [Google Scholar]
- 114.Kwast K.E., Burke P.V., Staahl B.T., and Poyton R.O. (1999). Oxygen sensing in yeast: Evidence for the involvement of the respiratory chain in regulating the transcription of a subset of hypoxic genes. Proceedings of the National Academy of Sciences 96, 5446–5451. 10.1073/pnas.96.10.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Petit J.M., Huet O., Gallet P.F., Maftah A., Ratinaud M.H., and Julien R. (1994). Direct analysis and significance of cardiolipin transverse distribution in mitochondrial inner membranes. Eur. J. Biochem. 220, 871–879. [DOI] [PubMed] [Google Scholar]
- 116.Boyd K.J., Alder N.N., and May E.R. (2017). Buckling Under Pressure: Curvature-Based Lipid Segregation and Stability Modulation in Cardiolipin-Containing Bilayers. Langmuir 33, 6937–6946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spinelli J.B., and Haigis M.C. (2018). The multifaceted contributions of mitochondria to cellular metabolism. Nat. Cell Biol. 20, 745–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yadav P.K., and Rajasekharan R. (2016). Misregulation of a DDHD Domain-containing Lipase Causes Mitochondrial Dysfunction in Yeast. J. Biol. Chem. 291, 18562–18581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kasahara T., Kubota-Sakashita M., Nagatsuka Y., Hirabayashi Y., Hanasaka T., Tohyama K., and Kato T. (2020). Cardiolipin is essential for early embryonic viability and mitochondrial integrity of neurons in mammals. FASEB J. 34, 1465–1480. [DOI] [PubMed] [Google Scholar]
- 120.Choi S.-Y., Gonzalvez F., Jenkins G.M., Slomianny C., Chretien D., Arnoult D., Petit P.X., and Frohman M.A. (2007). Cardiolipin deficiency releases cytochrome c from the inner mitochondrial membrane and accelerates stimuli-elicited apoptosis. Cell Death Differ. 14, 597–606. [DOI] [PubMed] [Google Scholar]
- 121.Carvalho M., Sampaio J.L., Palm W., Brankatschk M., Eaton S., and Shevchenko A. (2012). Effects of diet and development on the Drosophila lipidome. Mol. Syst. Biol. 8, 600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.van der Veen J.N., Kennelly J.P., Wan S., Vance J.E., Vance D.E., and Jacobs R.L. (2017). The critical role of phosphatidylcholine and phosphatidylethanolamine metabolism in health and disease. Biochim. Biophys. Acta Biomembr. 1859, 1558–1572. [DOI] [PubMed] [Google Scholar]
- 123.Montoya M.T., Porres A., Serrano S., Fruchart J.C., Mata P., Gerique J.A.G., and Castro G.R. (2002). Fatty acid saturation of the diet and plasma lipid concentrations, lipoprotein particle concentrations, and cholesterol efflux capacity. Am. J. Clin. Nutr. 75, 484–491. [DOI] [PubMed] [Google Scholar]
- 124.Ou-Yang Z.-C., Liu J.-X., Xie Y.-Z., and Yu-Zhang X. (1999). Geometric Methods in the Elastic Theory of Membranes in Liquid Crystal Phases (World Scientific; ). [Google Scholar]
- 125.Vasan R., Rudraraju S., Akamatsu M., Garikipati K., and Rangamani P. (2020). A mechanical model reveals that non-axisymmetric buckling lowers the energy barrier associated with membrane neck constriction. Soft Matter 16, 784–797. [DOI] [PubMed] [Google Scholar]
- 126.Hu J., Shibata Y., Voss C., Shemesh T., Li Z., Coughlin M., Kozlov M.M., Rapoport T.A., and Prinz W.A. (2008). Membrane proteins of the endoplasmic reticulum induce high-curvature tubules. Science 319, 1247–1250. [DOI] [PubMed] [Google Scholar]
- 127.Ladinsky M.S., Mastronarde D.N., McIntosh J.R., Howell K.E., and Staehelin L.A. (1999). Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J. Cell Biol. 144, 1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shibata Y., Shemesh T., Prinz W.A., Palazzo A.F., Kozlov M.M., and Rapoport T.A. (2010). Mechanisms Determining the Morphology of the Peripheral ER. Cell 143, 774–788. 10.1016/j.cell.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Derganc J. (2007). Curvature-driven lateral segregation of membrane constituents in Golgi cisternae. Physical Biology 4, 317–324. 10.1088/1478-3975/4/4/008. [DOI] [PubMed] [Google Scholar]
- 130.Covino R., Ballweg S., Stordeur C., Michaelis J.B., Puth K., Wernig F., Bahrami A., Ernst A.M., Hummer G., and Ernst R. (2016). A Eukaryotic Sensor for Membrane Lipid Saturation. Mol. Cell 63, 49–59. [DOI] [PubMed] [Google Scholar]
- 131.Ballweg S., Sezgin E., Doktorova M., Covino R., Reinhard J., Wunnicke D., Hänelt I., Levental I., Hummer G., and Ernst R. (2020). Regulation of lipid saturation without sensing membrane fluidity. Nat. Commun. 11, 756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chadda R., Krishnamani V., Mersch K., Wong J., Brimberry M., Chadda A., Kolmakova-Partensky L., Friedman L.J., Gelles J., and Robertson J.L. (2016). The dimerization equilibrium of a ClC Cl(−)/H(+) antiporter in lipid bilayers. Elife 5. 10.7554/eLife.17438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Chadda R., Bernhardt N., Kelley E.G., Teixeira S.C., Griffith K., Gil-Ley A., Öztürk T.N., Hughes L.E., Forsythe A., Krishnamani V., et al. (2021). Membrane transporter dimerization driven by differential lipid solvation energetics of dissociated and associated states. Elife 10. 10.7554/eLife.63288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Guo H., Bueler S.A., and Rubinstein J.L. (2017). Atomic model for the dimeric FO region of mitochondrial ATP synthase. Science 358, 936–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Rowlett V.W., Mallampalli V.K.P.S., Karlstaedt A., Dowhan W., Taegtmeyer H., Margolin W., and Vitrac H. (2017). Impact of Membrane Phospholipid Alterations in Escherichia coli on Cellular Function and Bacterial Stress Adaptation. J. Bacteriol. 199. 10.1128/JB.00849-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sohlenkamp C., and Geiger O. (2016). Bacterial membrane lipids: diversity in structures and pathways. FEMS Microbiol. Rev. 40, 133–159. [DOI] [PubMed] [Google Scholar]
- 137.Galber C., Valente G., von Stockum S., and Giorgio V. (2019). Purification of Functional F-ATP Synthase from Blue Native PAGE. Methods Mol. Biol. 1925, 233–243. [DOI] [PubMed] [Google Scholar]
- 138.Bisetto E., Di Pancrazio F., Simula M.P., Mavelli I., and Lippe G. (2007). Mammalian ATPsynthase monomer versus dimer profiled by blue native PAGE and activity stain. Electrophoresis 28, 3178–3185. [DOI] [PubMed] [Google Scholar]
- 139.Huynen M.A., Mühlmeister M., Gotthardt K., Guerrero-Castillo S., and Brandt U. (2016). Evolution and structural organization of the mitochondrial contact site (MICOS) complex and the mitochondrial intermembrane space bridging (MIB) complex. Biochim. Biophys. Acta 1863, 91–101. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.