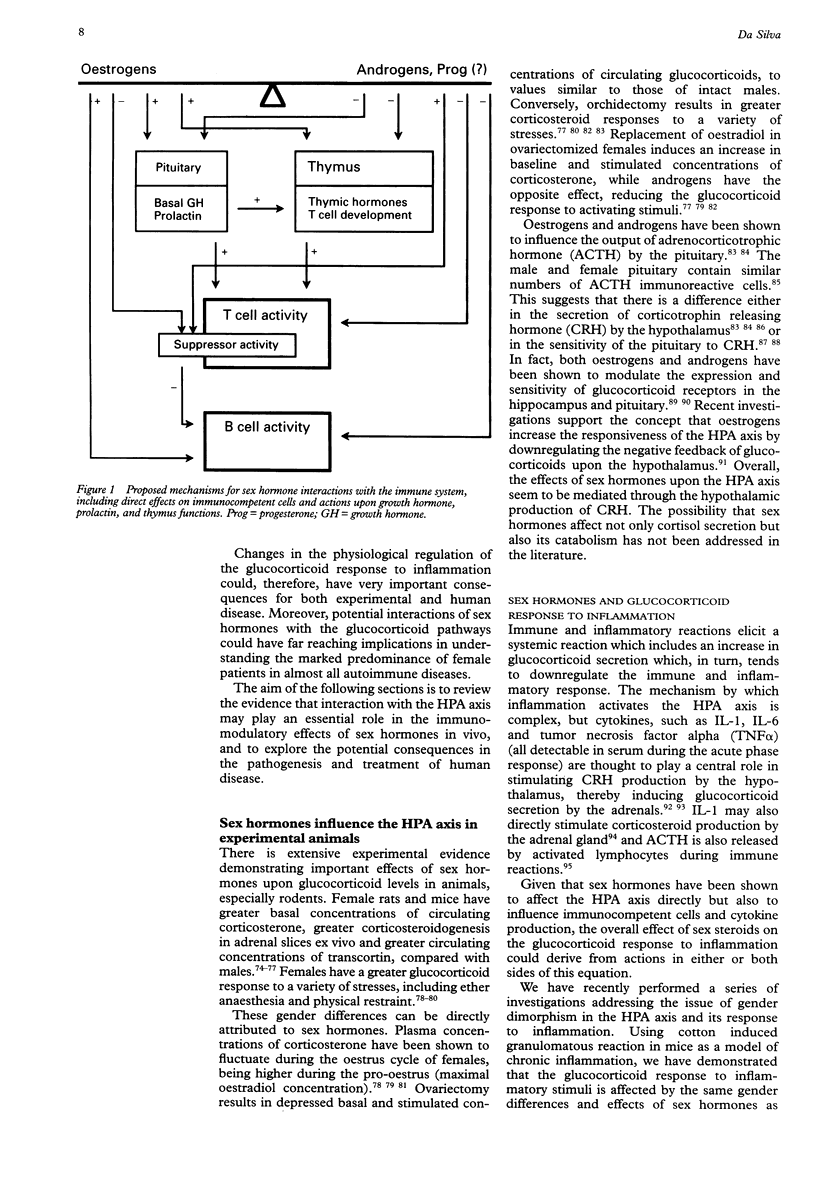
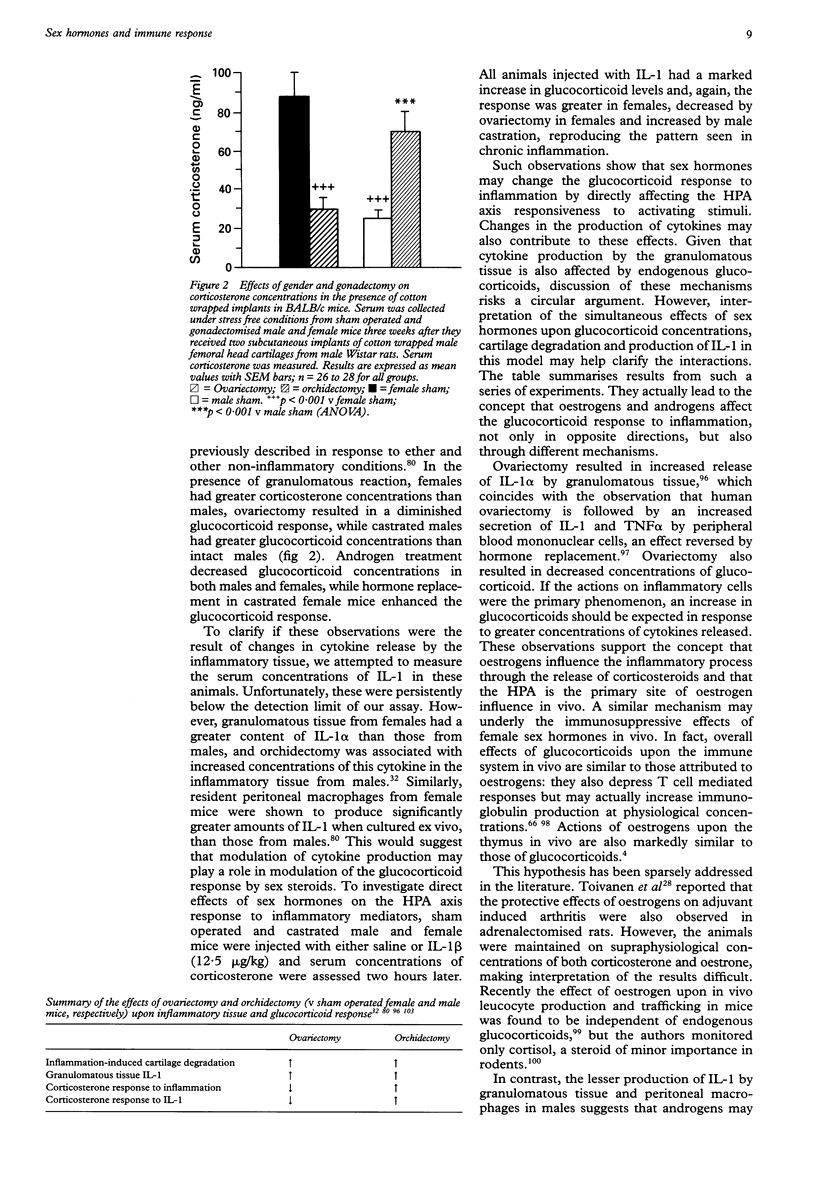
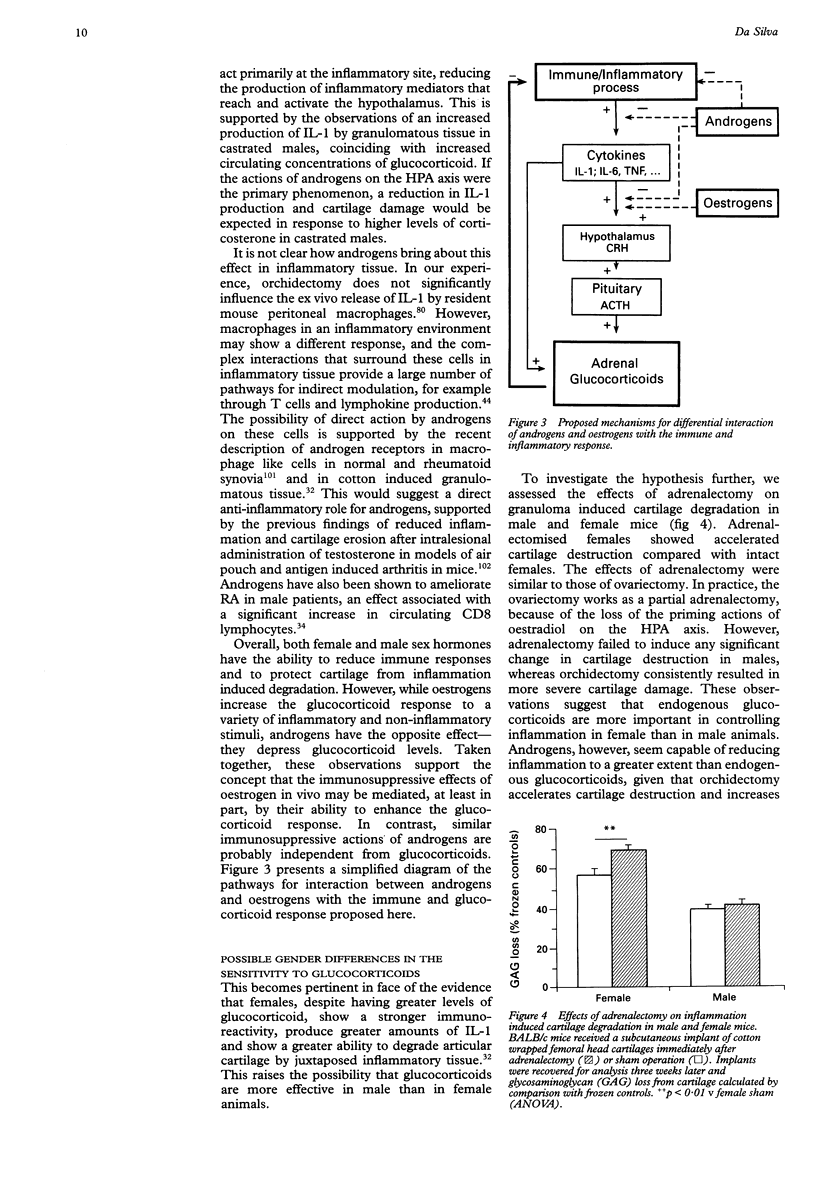
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ablin R. J., Bhatti R. A., Guinan P. D., Khin W. Modulatory effects of oestrogen on immunological responsiveness. II. Suppression of tumour-associated immunity in patients with prostatic cancer. Clin Exp Immunol. 1979 Oct;38(1):83–91. [PMC free article] [PubMed] [Google Scholar]
- Adler A. J., Scheller A., Hoffman Y., Robins D. M. Multiple components of a complex androgen-dependent enhancer. Mol Endocrinol. 1991 Nov;5(11):1587–1596. doi: 10.1210/mend-5-11-1587. [DOI] [PubMed] [Google Scholar]
- Agnello V., Pariser K., Gell J., Gelfand J., Turksoy R. N. Preliminary observations on danazol therapy of systemic lupus erythematosus: effects on DNA antibodies, thrombocytopenia and complement. J Rheumatol. 1983 Oct;10(5):682–687. [PubMed] [Google Scholar]
- Ahima R. S., Harlan R. E. Regulation of glucocorticoid receptor immunoreactivity in the rat hippocampus by androgenic-anabolic steroids. Brain Res. 1992 Jul 10;585(1-2):311–314. doi: 10.1016/0006-8993(92)91226-5. [DOI] [PubMed] [Google Scholar]
- Ahima R. S., Lawson A. N., Osei S. Y., Harlan R. E. Sexual dimorphism in regulation of type II corticosteroid receptor immunoreactivity in the rat hippocampus. Endocrinology. 1992 Sep;131(3):1409–1416. doi: 10.1210/endo.131.3.1505471. [DOI] [PubMed] [Google Scholar]
- Ahmed S. A., Penhale W. J. The influence of testosterone on the development of autoimmune thyroiditis in thymectomized and irradiated rats. Clin Exp Immunol. 1982 May;48(2):367–374. [PMC free article] [PubMed] [Google Scholar]
- Ahmed S. A., Talal N. Sex hormones and the immune system--Part 2. Animal data. Baillieres Clin Rheumatol. 1990 Apr;4(1):13–31. doi: 10.1016/s0950-3579(05)80241-9. [DOI] [PubMed] [Google Scholar]
- Allen-Rowlands C. F., Allen J. P., Greer M. A., Wilson M. Circadian rhythmicity of ACTH and corticosterone in the rat. J Endocrinol Invest. 1980 Oct-Dec;3(4):371–377. doi: 10.1007/BF03349373. [DOI] [PubMed] [Google Scholar]
- Allen J. B., Blatter D., Calandra G. B., Wilder R. L. Sex hormonal effects on the severity of streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1983 Apr;26(4):560–563. doi: 10.1002/art.1780260418. [DOI] [PubMed] [Google Scholar]
- Allen L. S., McClure J. E., Goldstein A. L., Barkley M. S., Michael S. D. Estrogen and thymic hormone interactions in the female mouse. J Reprod Immunol. 1984 Jan;6(1):25–37. doi: 10.1016/0165-0378(84)90039-1. [DOI] [PubMed] [Google Scholar]
- Ansar Ahmed S., Penhale W. J., Talal N. Sex hormones, immune responses, and autoimmune diseases. Mechanisms of sex hormone action. Am J Pathol. 1985 Dec;121(3):531–551. [PMC free article] [PubMed] [Google Scholar]
- Antakly T., Zhang C. X., Sarrieau A., Raquidan D. Cell-specific expression of the glucocorticoid receptor within granular convoluted tubules of the rat submaxillary gland. Endocrinology. 1991 Jan;128(1):617–622. doi: 10.1210/endo-128-1-617. [DOI] [PubMed] [Google Scholar]
- Araneo B. A., Dowell T., Diegel M., Daynes R. A. Dihydrotestosterone exerts a depressive influence on the production of interleukin-4 (IL-4), IL-5, and gamma-interferon, but not IL-2 by activated murine T cells. Blood. 1991 Aug 1;78(3):688–699. [PubMed] [Google Scholar]
- Ariga H., Edwards J., Sullivan D. A. Androgen control of autoimmune expression in lacrimal glands of MRL/Mp-lpr/lpr mice. Clin Immunol Immunopathol. 1989 Dec;53(3):499–508. doi: 10.1016/0090-1229(89)90011-1. [DOI] [PubMed] [Google Scholar]
- Arnason B. G., Richman D. P. Effect of oral contraceptives on experimental demyelinating disease. Arch Neurol. 1969 Jul;21(1):103–108. doi: 10.1001/archneur.1969.00480130117012. [DOI] [PubMed] [Google Scholar]
- Aroso Dias A., Lopes Vaz A., Hargreaves M., Afonso C., Araújø D., Bravo T. Biomarks in secondary osteoporosis. Clin Rheumatol. 1989 Jun;8 (Suppl 2):89–94. doi: 10.1007/BF02207241. [DOI] [PubMed] [Google Scholar]
- BLISS E. L., MIGEON C. J., EIK-NES K., SANDBERG A. A., SAMUELS L. T. The effects of insulin, histamine, bacterial pyrogen, and the antabuse-alcohol reaction upon the levels of 17-hydroxycorticosteroids in the peripheral blood of man. Metabolism. 1954 Nov;3(6):493–501. [PubMed] [Google Scholar]
- Baron S., Brush F. R. Effects of acute and chronic restraint and estrus cycle on pituitary--adrenal function in the rat. Horm Behav. 1979 Jun;12(3):218–224. doi: 10.1016/0018-506x(79)90004-7. [DOI] [PubMed] [Google Scholar]
- Beato M., Chalepakis G., Schauer M., Slater E. P. DNA regulatory elements for steroid hormones. J Steroid Biochem. 1989 May;32(5):737–747. doi: 10.1016/0022-4731(89)90521-9. [DOI] [PubMed] [Google Scholar]
- Berczi I., Nagy E., Kovacs K., Horvath E. Regulation of humoral immunity in rats by pituitary hormones. Acta Endocrinol (Copenh) 1981 Dec;98(4):506–513. doi: 10.1530/acta.0.0980506. [DOI] [PubMed] [Google Scholar]
- Besedovsky H. O., del Rey A., Klusman I., Furukawa H., Monge Arditi G., Kabiersch A. Cytokines as modulators of the hypothalamus-pituitary-adrenal axis. J Steroid Biochem Mol Biol. 1991;40(4-6):613–618. doi: 10.1016/0960-0760(91)90284-c. [DOI] [PubMed] [Google Scholar]
- Bhalla A. K. Hormones and the immune response. Ann Rheum Dis. 1989 Jan;48(1):1–6. doi: 10.1136/ard.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazkovec A. A., Orsini M. W. Ontogenetic aspects of sexual dimorphism and the primary immune response to sheep erythrocytes in hamsters from prepuberty through senescence. Int Arch Allergy Appl Immunol. 1976;50(1):55–67. doi: 10.1159/000231480. [DOI] [PubMed] [Google Scholar]
- Bottomley K. M., Griffiths R. J., Rising T. J., Steward A. A modified mouse air pouch model for evaluating the effects of compounds on granuloma induced cartilage degradation. Br J Pharmacol. 1988 Mar;93(3):627–635. doi: 10.1111/j.1476-5381.1988.tb10320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brick J. E., Wilson D. A., Walker S. E. Hormonal modulation of responses to thymus-independent and thymus-dependent antigens in autoimmune NZB/W mice. J Immunol. 1985 Jun;134(6):3693–3698. [PubMed] [Google Scholar]
- Brinkmann A. O., Faber P. W., van Rooij H. C., Kuiper G. G., Ris C., Klaassen P., van der Korput J. A., Voorhorst M. M., van Laar J. H., Mulder E. The human androgen receptor: domain structure, genomic organization and regulation of expression. J Steroid Biochem. 1989;34(1-6):307–310. doi: 10.1016/0022-4731(89)90098-8. [DOI] [PubMed] [Google Scholar]
- Buckingham J. C. Effects of adrenocortical and gonadal steroids on the secretion in vitro of corticotrophin and its hypothalamic releasing factor. J Endocrinol. 1982 Apr;93(1):123–132. doi: 10.1677/joe.0.0930123. [DOI] [PubMed] [Google Scholar]
- Bulbrook R. D., Herian M., Tong D., Hayward J. L., Swain M. C., Wang D. Y. Effect of steroidal contraceptives on levels of plasma androgen sulphates and cortisol. Lancet. 1973 Mar 24;1(7804):628–631. doi: 10.1016/s0140-6736(73)92198-3. [DOI] [PubMed] [Google Scholar]
- Burgess L. H., Handa R. J. Chronic estrogen-induced alterations in adrenocorticotropin and corticosterone secretion, and glucocorticoid receptor-mediated functions in female rats. Endocrinology. 1992 Sep;131(3):1261–1269. doi: 10.1210/endo.131.3.1324155. [DOI] [PubMed] [Google Scholar]
- Butterworth M., McClellan B., Allansmith M. Influence of sex in immunoglobulin levels. Nature. 1967 Jun 17;214(5094):1224–1225. doi: 10.1038/2141224a0. [DOI] [PubMed] [Google Scholar]
- Carlström K., Stege R. Adrenocortical function in prostatic cancer patients: effects of orchidectomy or different modes of estrogen treatment on basal steroid levels and on the response to exogenous adrenocorticotropic hormone. Urol Int. 1990;45(3):160–163. doi: 10.1159/000281699. [DOI] [PubMed] [Google Scholar]
- Cash J. M., Crofford L. J., Gallucci W. T., Sternberg E. M., Gold P. W., Chrousos G. P., Wilder R. L. Pituitary-adrenal axis responsiveness to ovine corticotropin releasing hormone in patients with rheumatoid arthritis treated with low dose prednisone. J Rheumatol. 1992 Nov;19(11):1692–1696. [PubMed] [Google Scholar]
- Castro J. E. Orchidectomy and the immune response. II. Response of orchidectomized mice to antigens. Proc R Soc Lond B Biol Sci. 1974 Feb 26;185(1081):437–451. doi: 10.1098/rspb.1974.0028. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Chikanza I. C., Petrou P., Kingsley G., Chrousos G., Panayi G. S. Defective hypothalamic response to immune and inflammatory stimuli in patients with rheumatoid arthritis. Arthritis Rheum. 1992 Nov;35(11):1281–1288. doi: 10.1002/art.1780351107. [DOI] [PubMed] [Google Scholar]
- Cidlowski J. A., Cidlowski N. B. Regulation of glucocorticoid receptors by glucocorticoids in cultured HeLa S3 cells. Endocrinology. 1981 Dec;109(6):1975–1982. doi: 10.1210/endo-109-6-1975. [DOI] [PubMed] [Google Scholar]
- Cohen J. H., Danel L., Cordier G., Saez S., Revillard J. P. Sex steroid receptors in peripheral T cells: absence of androgen receptors and restriction of estrogen receptors to OKT8-positive cells. J Immunol. 1983 Dec;131(6):2767–2771. [PubMed] [Google Scholar]
- Coyne M. D., Kitay J. I. Effect of orchiectomy on pituitary secretion of ACTH. Endocrinology. 1971 Oct;89(4):1024–1028. doi: 10.1210/endo-89-4-1024. [DOI] [PubMed] [Google Scholar]
- Cupps T. R., Fauci A. S. Corticosteroid-mediated immunoregulation in man. Immunol Rev. 1982;65:133–155. doi: 10.1111/j.1600-065x.1982.tb00431.x. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Accardo S., Villaggio B., Clerico P., Bagnasco M., Coviello D. A., Carruba G., lo Casto M., Castagnetta L. Presence of estrogen-binding sites on macrophage-like synoviocytes and CD8+, CD29+, CD45RO+ T lymphocytes in normal and rheumatoid synovium. Arthritis Rheum. 1993 Aug;36(8):1087–1097. doi: 10.1002/art.1780360809. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Accardo S., Villaggio B., Clerico P., Indiveri F., Carruba G., Fecarotta E., Castagnetta L. Evidence for the presence of androgen receptors in the synovial tissue of rheumatoid arthritis patients and healthy controls. Arthritis Rheum. 1992 Sep;35(9):1007–1015. doi: 10.1002/art.1780350905. [DOI] [PubMed] [Google Scholar]
- Cutolo M., Balleari E., Giusti M., Intra E., Accardo S. Androgen replacement therapy in male patients with rheumatoid arthritis. Arthritis Rheum. 1991 Jan;34(1):1–5. doi: 10.1002/art.1780340102. [DOI] [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. I. Protein-over loading paralysis. Immunology. 1962 Jan;5:161–168. [PMC free article] [PubMed] [Google Scholar]
- Da Silva J. A., Larbre J. P., Seed M. P., Cutolo M., Villaggio B., Scott D. L., Willoughby D. A. Sex differences in inflammation induced cartilage damage in rodents. The influence of sex steroids. J Rheumatol. 1994 Feb;21(2):330–337. [PubMed] [Google Scholar]
- Da Silva J. A., Larbre J. P., Spector T. D., Perry L. A., Scott D. L., Willoughby D. A. Protective effect of androgens against inflammation induced cartilage degradation in male rodents. Ann Rheum Dis. 1993 Apr;52(4):285–291. doi: 10.1136/ard.52.4.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Silva J. A., Peers S. H., Perretti M., Willoughby D. A. Sex steroids affect glucocorticoid response to chronic inflammation and to interleukin-1. J Endocrinol. 1993 Mar;136(3):389–397. doi: 10.1677/joe.0.1360389. [DOI] [PubMed] [Google Scholar]
- De Brito F. B., Holmes M. J., Carney S. L., Willoughby D. A. Drug effects on a novel model of connective tissue breakdown. Agents Actions. 1987 Aug;21(3-4):287–289. doi: 10.1007/BF01966493. [DOI] [PubMed] [Google Scholar]
- De Brito F. B., Moore A. R., Holmes M. J., Willoughby D. A. Cartilage damage by a granulomatous reaction in a murine species. Br J Exp Pathol. 1987 Oct;68(5):675–686. [PMC free article] [PubMed] [Google Scholar]
- Emilie D., Crevon M. C., Auffredou M. T., Galanaud P. Glucocorticosteroid-dependent synergy between interleukin 1 and interleukin 6 for human B lymphocyte differentiation. Eur J Immunol. 1988 Dec;18(12):2043–2047. doi: 10.1002/eji.1830181226. [DOI] [PubMed] [Google Scholar]
- Endres D. B., Milholland R. J., Rosen F. Sex differences in the concentrations of glucocorticoid receptors in rat liver and thymus. J Endocrinol. 1979 Jan;80(1):21–26. doi: 10.1677/joe.0.0800021. [DOI] [PubMed] [Google Scholar]
- Fukata J., Martin J. B. Influence of sex steroid hormones on rat growth hormone-releasing factor and somatostatin in dispersed pituitary cells. Endocrinology. 1986 Nov;119(5):2256–2261. doi: 10.1210/endo-119-5-2256. [DOI] [PubMed] [Google Scholar]
- Gaillard-Moguilewsky M. Pharmacology of antiandrogens and value of combining androgen suppression with antiandrogen therapy. Urology. 1991;37(2 Suppl):5–12. doi: 10.1016/0090-4295(91)80095-o. [DOI] [PubMed] [Google Scholar]
- Gala R. R., Westphal U. Further studies on the corticosteroid-binding globulin in the rat: proposed endocrine control. Endocrinology. 1966 Jul;79(1):67–76. doi: 10.1210/endo-79-1-67. [DOI] [PubMed] [Google Scholar]
- Giguère V., Meunier H., Veilleux R., Labrie F. Direct effects of sex steroids on prolactin release at the anterior pituitary level: interactions with dopamine, thyrotropin-releasing hormone, and isobutylmethylxanthine. Endocrinology. 1982 Sep;111(3):857–862. doi: 10.1210/endo-111-3-857. [DOI] [PubMed] [Google Scholar]
- Glover J. F., Irwin J. T., Darbre P. D. Interaction of phenol red with estrogenic and antiestrogenic action on growth of human breast cancer cells ZR-75-1 and T-47-D. Cancer Res. 1988 Jul 1;48(13):3693–3697. [PubMed] [Google Scholar]
- Golsteyn E. J., Fritzler M. J. The role of the thymus-hypothalamus-pituitary-gonadal axis in normal immune processes and autoimmunity. J Rheumatol. 1987 Oct;14(5):982–990. [PubMed] [Google Scholar]
- Graff R. J., Lappé M. A., Snell G. D. The influence of the gonads and adrenal glands on the immune response to skin grafts. Transplantation. 1969 Feb;7(2):105–111. doi: 10.1097/00007890-196902000-00003. [DOI] [PubMed] [Google Scholar]
- Grossman C. J., Roselle G. A., Mendenhall C. L. Sex steroid regulation of autoimmunity. J Steroid Biochem Mol Biol. 1991;40(4-6):649–659. doi: 10.1016/0960-0760(91)90287-f. [DOI] [PubMed] [Google Scholar]
- Grossman C. Possible underlying mechanisms of sexual dimorphism in the immune response, fact and hypothesis. J Steroid Biochem. 1989;34(1-6):241–251. doi: 10.1016/0022-4731(89)90088-5. [DOI] [PubMed] [Google Scholar]
- Guerne P. A., Carson D. A., Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol. 1990 Jan 15;144(2):499–505. [PubMed] [Google Scholar]
- Haas D. A., George S. R. Estradiol or ovariectomy decreases CRF synthesis in hypothalamus. Brain Res Bull. 1989 Sep;23(3):215–218. doi: 10.1016/0361-9230(89)90150-0. [DOI] [PubMed] [Google Scholar]
- Hall G. M., Daniels M., Huskisson E. C., Spector T. D. A randomised controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal rheumatoid arthritis. Ann Rheum Dis. 1994 Feb;53(2):112–116. doi: 10.1136/ard.53.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedman M., Nilsson E., de la Torre B. Low blood and synovial fluid levels of sulpho-conjugated steroids in rheumatoid arthritis. Clin Exp Rheumatol. 1992 Jan-Feb;10(1):25–30. [PubMed] [Google Scholar]
- Helgason S., Carlström K., Damber M. G., Damber J. E., Selstam G., von Schoultz B. Effects of various oestrogens on circulating androgens and cortisol during replacement therapy in post-menopausal women. Maturitas. 1981 Dec;3(3-4):301–308. doi: 10.1016/0378-5122(81)90038-4. [DOI] [PubMed] [Google Scholar]
- Holdstock G., Chastenay B. F., Krawitt E. L. Effects of testosterone, oestradiol and progesterone on immune regulation. Clin Exp Immunol. 1982 Feb;47(2):449–456. [PMC free article] [PubMed] [Google Scholar]
- Hou J., Zheng W. F. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol. 1988;10(1):15–22. doi: 10.1016/0192-0561(88)90145-2. [DOI] [PubMed] [Google Scholar]
- Hu S. K., Mitcho Y. L., Rath N. C. Effect of estradiol on interleukin 1 synthesis by macrophages. Int J Immunopharmacol. 1988;10(3):247–252. doi: 10.1016/0192-0561(88)90055-0. [DOI] [PubMed] [Google Scholar]
- Imura H., Fukata J., Mori T. Cytokines and endocrine function: an interaction between the immune and neuroendocrine systems. Clin Endocrinol (Oxf) 1991 Aug;35(2):107–115. doi: 10.1111/j.1365-2265.1991.tb03506.x. [DOI] [PubMed] [Google Scholar]
- JENKINS J. S., ELKINGTON S. G. METYRAPONE AND PYROGEN IN THE ASSESSMENT OF PITUITARY-ADRENAL FUNCTION AFTER REMOVAL OF PITUITARY ADENOMA. Lancet. 1964 Nov 7;2(7367):991–994. doi: 10.1016/s0140-6736(64)90934-1. [DOI] [PubMed] [Google Scholar]
- Jansson J. O., Ekberg S., Isaksson O. G., Edén S. Influence of gonadal steroids on age- and sex-related secretory patterns of growth hormone in the rat. Endocrinology. 1984 Apr;114(4):1287–1294. doi: 10.1210/endo-114-4-1287. [DOI] [PubMed] [Google Scholar]
- Jansson L., Holmdahl R. The Y chromosome-linked "autoimmune accelerating" yaa gene suppresses collagen-induced arthritis. Eur J Immunol. 1994 May;24(5):1213–1217. doi: 10.1002/eji.1830240531. [DOI] [PubMed] [Google Scholar]
- Jia X. C., Ny T., Hsueh A. J. Synergistic effect of glucocorticoids and androgens on the hormonal induction of tissue plasminogen activator activity and messenger ribonucleic acid levels in granulosa cells. Mol Cell Endocrinol. 1990 Jan 22;68(2-3):143–151. doi: 10.1016/0303-7207(90)90187-d. [DOI] [PubMed] [Google Scholar]
- Josefsson E., Tarkowski A., Carlsten H. Anti-inflammatory properties of estrogen. I. In vivo suppression of leukocyte production in bone marrow and redistribution of peripheral blood neutrophils. Cell Immunol. 1992 Jun;142(1):67–78. doi: 10.1016/0008-8749(92)90269-u. [DOI] [PubMed] [Google Scholar]
- Jungers P., Dougados M., Pélissier C., Kuttenn F., Tron F., Lesavre P., Bach J. F. Influence of oral contraceptive therapy on the activity of systemic lupus erythematosus. Arthritis Rheum. 1982 Jun;25(6):618–623. doi: 10.1002/art.1780250603. [DOI] [PubMed] [Google Scholar]
- KITAY J. I. PITUITARY-ADRENAL FUNCTION IN THE RAT AFTER GONADECTOMY AND GONADAL HORMONE REPLACEMENT. Endocrinology. 1963 Aug;73:253–260. doi: 10.1210/endo-73-2-253. [DOI] [PubMed] [Google Scholar]
- Kincl F. A., Ciaccio L. A. Suppression of immune responses by progesterone. Endocrinol Exp. 1980 Mar;14(1):27–33. [PubMed] [Google Scholar]
- Klein A., Buskila D., Gladman D., Bruser B., Malkin A. Cortisol catabolism by lymphocytes of patients with systemic lupus erythematosus and rheumatoid arthritis. J Rheumatol. 1990 Jan;17(1):30–33. [PubMed] [Google Scholar]
- Komm B. S., Terpening C. M., Benz D. J., Graeme K. A., Gallegos A., Korc M., Greene G. L., O'Malley B. W., Haussler M. R. Estrogen binding, receptor mRNA, and biologic response in osteoblast-like osteosarcoma cells. Science. 1988 Jul 1;241(4861):81–84. doi: 10.1126/science.3164526. [DOI] [PubMed] [Google Scholar]
- Kovacs W. J., Olsen N. J. Androgen receptors in human thymocytes. J Immunol. 1987 Jul 15;139(2):490–493. [PubMed] [Google Scholar]
- Le Mevel J. C., Abitbol S., Beraud G., Maniey J. Temporal changes in plasma adrenocorticotropin concentration after repeated neurotropic stress in male and female rats. Endocrinology. 1979 Sep;105(3):812–817. doi: 10.1210/endo-105-3-812. [DOI] [PubMed] [Google Scholar]
- Leśniewska B., Miśkowiak B., Nowak M., Malendowicz L. K. Sex differences in adrenocortical structure and function. XXVII. The effect of ether stress on ACTH and corticosterone in intact, gonadectomized, and testosterone- or estradiol-replaced rats. Res Exp Med (Berl) 1990;190(2):95–103. doi: 10.1007/pl00020011. [DOI] [PubMed] [Google Scholar]
- MacPhee I. A., Antoni F. A., Mason D. W. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989 Feb 1;169(2):431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malendowicz L. K., Stachowiak A., Zabel M. Sex differences in adrenocortical structure and function. XXV. Quantitative analysis of ACTH-immunoreactive cells in the anterior pituitary of gonadectomized and gonadal hormone replaced male and female rats. Exp Clin Endocrinol. 1987 Aug;90(1):1–8. doi: 10.1055/s-0029-1210666. [DOI] [PubMed] [Google Scholar]
- Mason D. Genetic variation in the stress response: susceptibility to experimental allergic encephalomyelitis and implications for human inflammatory disease. Immunol Today. 1991 Feb;12(2):57–60. doi: 10.1016/0167-5699(91)90158-P. [DOI] [PubMed] [Google Scholar]
- Milich D. R., Gershwin M. E. Murine autoimmune hemolytic anemia induced via xenogeneic erythrocyte immunization. III. Influences of sex. Clin Immunol Immunopathol. 1981 Jan;18(1):1–11. doi: 10.1016/0090-1229(81)90001-5. [DOI] [PubMed] [Google Scholar]
- Miśkowiak B., Leśniewska B., Nowak M., Malendowicz L. K. Studies on hypothalamo-pituitary corticoliberin system. V. The effects of gonadectomy and sex hormones on plasma ACTH and on the reactivity of the anterior pituitary gland to CRF. Exp Clin Endocrinol. 1988 Sep;92(1):1–6. doi: 10.1055/s-0029-1210773. [DOI] [PubMed] [Google Scholar]
- Myles A. B., Schiller L. F., Glass D., Daly J. R. Single daily dose corticosteroid treatment. Ann Rheum Dis. 1976 Feb;35(1):73–76. doi: 10.1136/ard.35.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy E., Berczi I., Friesen H. G. Regulation of immunity in rats by lactogenic and growth hormones. Acta Endocrinol (Copenh) 1983 Mar;102(3):351–357. doi: 10.1530/acta.0.1020351. [DOI] [PubMed] [Google Scholar]
- Neeck G., Federlin K., Graef V., Rusch D., Schmidt K. L. Adrenal secretion of cortisol in patients with rheumatoid arthritis. J Rheumatol. 1990 Jan;17(1):24–29. [PubMed] [Google Scholar]
- Nichols D. J., Chevins P. F. Plasma corticosterone fluctuations during the oestrous cycle of the house mouse. Experientia. 1981 Mar 15;37(3):319–320. doi: 10.1007/BF01991678. [DOI] [PubMed] [Google Scholar]
- Nolten W. E., Rueckert P. A. Elevated free cortisol index in pregnancy: possible regulatory mechanisms. Am J Obstet Gynecol. 1981 Feb 15;139(4):492–498. doi: 10.1016/0002-9378(81)90331-8. [DOI] [PubMed] [Google Scholar]
- Okayasu I., Kong Y. M., Rose N. R. Effect of castration and sex hormones on experimental autoimmune thyroiditis. Clin Immunol Immunopathol. 1981 Aug;20(2):240–245. doi: 10.1016/0090-1229(81)90182-3. [DOI] [PubMed] [Google Scholar]
- Paavonen T., Andersson L. C., Adlercreutz H. Sex hormone regulation of in vitro immune response. Estradiol enhances human B cell maturation via inhibition of suppressor T cells in pokeweed mitogen-stimulated cultures. J Exp Med. 1981 Dec 1;154(6):1935–1945. doi: 10.1084/jem.154.6.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paavonen T. Hormonal regulation of lymphocyte functions. Med Biol. 1987;65(5-6):229–240. [PubMed] [Google Scholar]
- Pacifici R., Brown C., Puscheck E., Friedrich E., Slatopolsky E., Maggio D., McCracken R., Avioli L. V. Effect of surgical menopause and estrogen replacement on cytokine release from human blood mononuclear cells. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5134–5138. doi: 10.1073/pnas.88.12.5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandian M. R., Talwar G. P. Effect of growth hormone on the metabolism of thymus and on the immune response against sheep erythrocytes. J Exp Med. 1971 Nov 1;134(5):1095–1113. doi: 10.1084/jem.134.5.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgorski M. R., Goulding N. J., Hall N. D., Flower R. J., Maddison P. J. Autoantibodies to lipocortin-1 are associated with impaired glucocorticoid responsiveness in rheumatoid arthritis. J Rheumatol. 1992 Nov;19(11):1668–1671. [PubMed] [Google Scholar]
- Polan M. L., Daniele A., Kuo A. Gonadal steroids modulate human monocyte interleukin-1 (IL-1) activity. Fertil Steril. 1988 Jun;49(6):964–968. [PubMed] [Google Scholar]
- Roh M. S., Drazenovich K. A., Barbose J. J., Dinarello C. A., Cobb C. F. Direct stimulation of the adrenal cortex by interleukin-1. Surgery. 1987 Aug;102(2):140–146. [PubMed] [Google Scholar]
- Roubinian J. R., Papoian R., Talal N. Androgenic hormones modulate autoantibody responses and improve survival in murine lupus. J Clin Invest. 1977 Jun;59(6):1066–1070. doi: 10.1172/JCI108729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saldanha C., Tougas G., Grace E. Evidence for anti-inflammatory effect of normal circulating plasma cortisol. Clin Exp Rheumatol. 1986 Oct-Dec;4(4):365–366. [PubMed] [Google Scholar]
- Schauenstein K., Fässler R., Dietrich H., Schwarz S., Krömer G., Wick G. Disturbed immune-endocrine communication in autoimmune disease. Lack of corticosterone response to immune signals in obese strain chickens with spontaneous autoimmune thyroiditis. J Immunol. 1987 Sep 15;139(6):1830–1833. [PubMed] [Google Scholar]
- Schlaghecke R., Kornely E., Wollenhaupt J., Specker C. Glucocorticoid receptors in rheumatoid arthritis. Arthritis Rheum. 1992 Jul;35(7):740–744. doi: 10.1002/art.1780350704. [DOI] [PubMed] [Google Scholar]
- Schuurs A. H., Verheul H. A. Effects of gender and sex steroids on the immune response. J Steroid Biochem. 1990 Feb;35(2):157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Schöneshöfer M., Wagner G. G. Sex differences in corticosteroids in man. J Clin Endocrinol Metab. 1977 Oct;45(4):814–817. doi: 10.1210/jcem-45-4-814. [DOI] [PubMed] [Google Scholar]
- Screpanti I., Morrone S., Meco D., Santoni A., Gulino A., Paolini R., Crisanti A., Mathieson B. J., Frati L. Steroid sensitivity of thymocyte subpopulations during intrathymic differentiation. Effects of 17 beta-estradiol and dexamethasone on subsets expressing T cell antigen receptor or IL-2 receptor. J Immunol. 1989 May 15;142(10):3378–3383. [PubMed] [Google Scholar]
- Shimizu K., Amagaya S., Ogihara Y. Analysis of corticosterone in the serum of mice and rats using high-performance liquid chromatography. J Chromatogr. 1983 Jan 14;272(1):170–175. doi: 10.1016/s0378-4347(00)86114-9. [DOI] [PubMed] [Google Scholar]
- Shull J. D., Gorski J. Estrogen regulation of prolactin gene transcription in vivo: paradoxical effects of 17 beta-estradiol dose. Endocrinology. 1989 Jan;124(1):279–285. doi: 10.1210/endo-124-1-279. [DOI] [PubMed] [Google Scholar]
- Simard J., de Launoit Y., Haagensen D. E., Labrie F. Additive stimulatory action of glucocorticoids and androgens on basal and estrogen-repressed apolipoprotein-D messenger ribonucleic acid levels and secretion in human breast cancer cells. Endocrinology. 1992 Mar;130(3):1115–1121. doi: 10.1210/endo.130.3.1537279. [DOI] [PubMed] [Google Scholar]
- Sobhon P., Jirasattham C. Effect of sex hormones on the thymus and lymphoid tissue of ovariectomized rats. Acta Anat (Basel) 1974;89(2):211–225. doi: 10.1159/000144285. [DOI] [PubMed] [Google Scholar]
- Spector T. D., Perry L. A., Tubb G., Silman A. J., Huskisson E. C. Low free testosterone levels in rheumatoid arthritis. Ann Rheum Dis. 1988 Jan;47(1):65–68. doi: 10.1136/ard.47.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staub J. J., Jenkins J. S., Ratcliffe J. G., Landon J. Comparison of corticotrophin and corticosteroid response to lysine vasopressin, insulin, and pyrogen in man. Br Med J. 1973 Feb 3;1(5848):267–269. doi: 10.1136/bmj.1.5848.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg A. D., Smathers P. A., Boegel W. B. Effects of sex hormones on autoantibody production by NZB mice and modification by environmental factors. Clin Immunol Immunopathol. 1980 Dec;17(4):562–572. doi: 10.1016/0090-1229(80)90152-x. [DOI] [PubMed] [Google Scholar]
- Sternberg E. M., Hill J. M., Chrousos G. P., Kamilaris T., Listwak S. J., Gold P. W., Wilder R. L. Inflammatory mediator-induced hypothalamic-pituitary-adrenal axis activation is defective in streptococcal cell wall arthritis-susceptible Lewis rats. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2374–2378. doi: 10.1073/pnas.86.7.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg E. M., Young W. S., 3rd, Bernardini R., Calogero A. E., Chrousos G. P., Gold P. W., Wilder R. L. A central nervous system defect in biosynthesis of corticotropin-releasing hormone is associated with susceptibility to streptococcal cell wall-induced arthritis in Lewis rats. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4771–4775. doi: 10.1073/pnas.86.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward A., Bayley D. L. Effects of androgens in models of rheumatoid arthritis. Agents Actions. 1992 Mar;35(3-4):268–272. doi: 10.1007/BF01997510. [DOI] [PubMed] [Google Scholar]
- Stimson W. H., Hunter I. C. Oestrogen-induced immunoregulation mediated through the thymus. J Clin Lab Immunol. 1980 Jul;4(1):27–33. [PubMed] [Google Scholar]
- Stimson W. H. Oestrogen and human T lymphocytes: presence of specific receptors in the T-suppressor/cytotoxic subset. Scand J Immunol. 1988 Sep;28(3):345–350. doi: 10.1111/j.1365-3083.1988.tb01459.x. [DOI] [PubMed] [Google Scholar]
- Takasu N., Komiya I., Nagasawa Y., Asawa T., Yamada T. Exacerbation of autoimmune thyroid dysfunction after unilateral adrenalectomy in patients with Cushing's syndrome due to an adrenocortical adenoma. N Engl J Med. 1990 Jun 14;322(24):1708–1712. doi: 10.1056/NEJM199006143222404. [DOI] [PubMed] [Google Scholar]
- Tazuke S., Khaw K. T., Barrett-Connor E. Exogenous estrogen and endogenous sex hormones. Medicine (Baltimore) 1992 Jan;71(1):44–51. doi: 10.1097/00005792-199201000-00004. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Siikala H., Laiho P., Paavilainen T. Suppression of adjuvant arthritis by estrone in adrenalectomized and ovariectomized rats. Experientia. 1967 Jul 15;23(7):560–561. doi: 10.1007/BF02137970. [DOI] [PubMed] [Google Scholar]
- Turner B. B. Sex difference in glucocorticoid binding in rat pituitary is estrogen dependent. Life Sci. 1990;46(19):1399–1406. doi: 10.1016/0024-3205(90)90340-w. [DOI] [PubMed] [Google Scholar]
- Viau V., Meaney M. J. Variations in the hypothalamic-pituitary-adrenal response to stress during the estrous cycle in the rat. Endocrinology. 1991 Nov;129(5):2503–2511. doi: 10.1210/endo-129-5-2503. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Calandra G. B., Garvin A. J., Wright K. D., Hansen C. T. Strain and sex variation in the susceptibility to streptococcal cell wall-induced polyarthritis in the rat. Arthritis Rheum. 1982 Sep;25(9):1064–1072. doi: 10.1002/art.1780250906. [DOI] [PubMed] [Google Scholar]
- Wilder R. L., Sternberg E. M. Neuroendocrine hormonal factors in rheumatoid arthritis and related conditions. Curr Opin Rheumatol. 1990 Jun;2(3):436–440. doi: 10.1097/00002281-199002030-00004. [DOI] [PubMed] [Google Scholar]
- Willoughby D. A., Ryan G. B. Evidence for a possible endogenous antigen in chronic inflammation. J Pathol. 1970 Jul;101(3):233–239. doi: 10.1002/path.1711010305. [DOI] [PubMed] [Google Scholar]
- Wyle F. A., Kent J. R. Immunosuppression by sex steroid hormones. The effect upon PHA- and PPD-stimulated lymphocytes. Clin Exp Immunol. 1977 Mar;27(3):407–415. [PMC free article] [PubMed] [Google Scholar]
- Zachmann M., Manella B., Eiholzer U., Bucher H., Prader A. Influence of oestrogen in high and low doses on plasma steroid concentrations in girls with tall stature and Turner syndrome. Acta Endocrinol (Copenh) 1984 Jul;106(3):368–373. doi: 10.1530/acta.0.1060368. [DOI] [PubMed] [Google Scholar]
- da Silva J. A., Colville-Nash P., Spector T. D., Scott D. L., Willoughby D. A. Inflammation-induced cartilage degradation in female rodents. Protective role of sex hormones. Arthritis Rheum. 1993 Jul;36(7):1007–1013. doi: 10.1002/art.1780360719. [DOI] [PubMed] [Google Scholar]
- de Weerdt O., Gooren L. J. Patterns of serum cortisol levels in ovariectomized females with and without androgen administration. Horm Metab Res. 1992 Feb;24(2):82–84. doi: 10.1055/s-2007-1003261. [DOI] [PubMed] [Google Scholar]


