Abstract
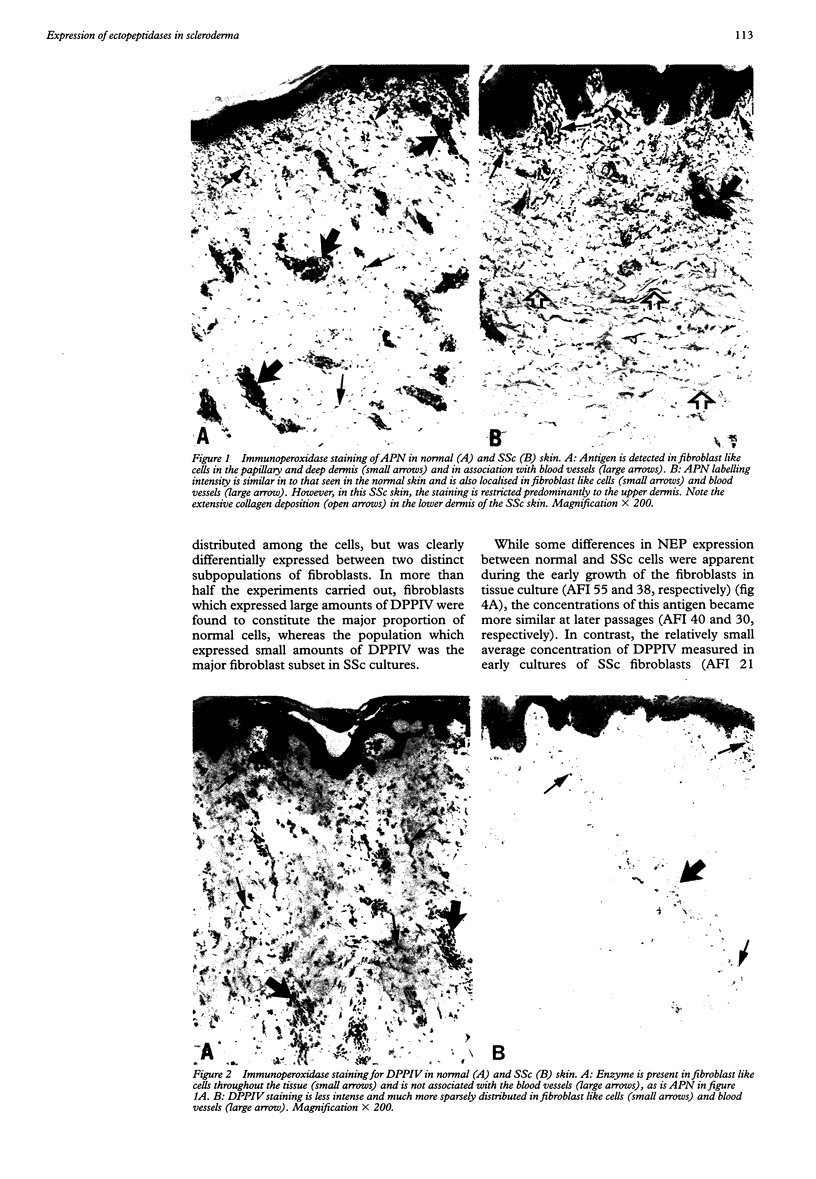
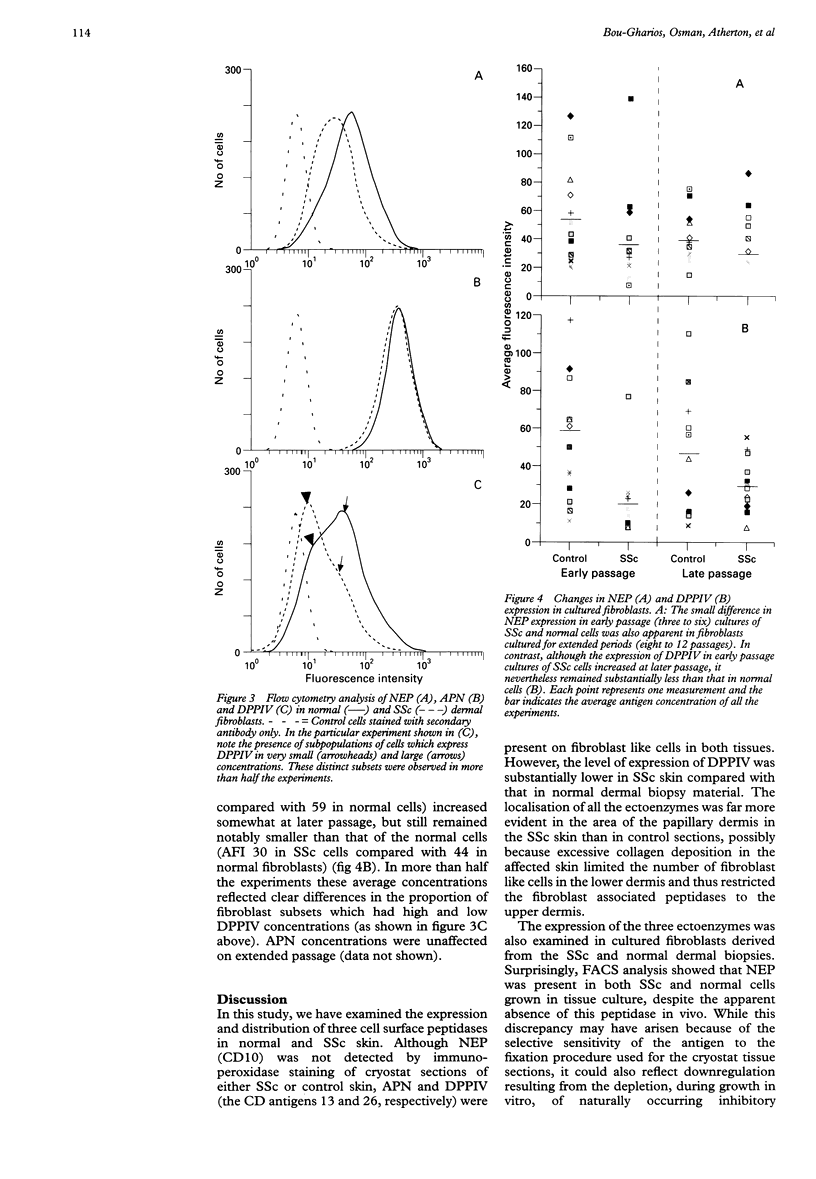
OBJECTIVES--To examine the expression and concentrations of three ectopeptidases likely to be involved in regulating the functional levels of adhesion molecules and the turnover of connective tissue components, in patients with scleroderma (systemic sclerosis) (SSc) and in normal individuals. METHODS--Monoclonal antibodies against these antigens were used for immunoperoxidase staining of cryostat skin sections and for flow cytometric (fluorescence activated cell sorter) analysis of cultured dermal fibroblasts grown from SSc patients and normal controls. RESULTS--Although neutral endopeptidase-24.11 (NEP) (CD10) was not detected in either SSc or normal skin, aminopeptidase N (APN) (CD13) and dipeptidyl peptidase IV (DPPIV) (CD26) were both readily visualised. However, DPPIV appeared to be present in smaller concentrations in the SSc biopsy specimens. Moreover, while fibroblasts grown in vitro from both SSc and normal skin also had similar concentrations of APN, the expression of DPPIV in the cultured SSc cells was found to be very much less than that present in the normal fibroblasts. It is noteworthy that NEP, which was not detected in the tissue sections, was nevertheless readily detected in fibroblasts in culture. CONCLUSIONS--These results show that a number of cell surface proteases are expressed by dermal fibroblasts both in vivo and in vitro, and it is suggested that the marked downregulation of DPPIV in SSc could be at least partly responsible for the increased concentrations of adhesion molecules and matrix proteins associated with the molecular pathology of this disease.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham D., Lupoli S., McWhirter A., Plater-Zyberk C., Piela T. H., Korn J. H., Olsen I., Black C. Expression and function of surface antigens on scleroderma fibroblasts. Arthritis Rheum. 1991 Sep;34(9):1164–1172. doi: 10.1002/art.1780340913. [DOI] [PubMed] [Google Scholar]
- Bauvois B. A collagen-binding glycoprotein on the surface of mouse fibroblasts is identified as dipeptidyl peptidase IV. Biochem J. 1988 Jun 15;252(3):723–731. doi: 10.1042/bj2520723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bylinkina V. S., Lokshina L. A., Samojlova R. S., Lubkova O. N., Gureeva T. A., Golubeva N. V. Dipeptidylpeptidase IV in human leukemic B- and T-cells. Biochem Int. 1992 Oct;28(1):31–39. [PubMed] [Google Scholar]
- Diamond M. S., Staunton D. E., de Fougerolles A. R., Stacker S. A., Garcia-Aguilar J., Hibbs M. L., Springer T. A. ICAM-1 (CD54): a counter-receptor for Mac-1 (CD11b/CD18). J Cell Biol. 1990 Dec;111(6 Pt 2):3129–3139. doi: 10.1083/jcb.111.6.3129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischmajer R., Perlish J. S., Reeves J. R. Cellular infiltrates in scleroderma skin. Arthritis Rheum. 1977 May;20(4):975–984. doi: 10.1002/art.1780200410. [DOI] [PubMed] [Google Scholar]
- Gay S., Trabandt A., Moreland L. W., Gay R. E. Growth factors, extracellular matrix, and oncogenes in scleroderma. Arthritis Rheum. 1992 Mar;35(3):304–310. doi: 10.1002/art.1780350309. [DOI] [PubMed] [Google Scholar]
- Gorrell M. D., Wickson J., McCaughan G. W. Expression of the rat CD26 antigen (dipeptidyl peptidase IV) on subpopulations of rat lymphocytes. Cell Immunol. 1991 Apr 15;134(1):205–215. doi: 10.1016/0008-8749(91)90343-a. [DOI] [PubMed] [Google Scholar]
- Gruschwitz M., von den Driesch P., Kellner I., Hornstein O. P., Sterry W. Expression of adhesion proteins involved in cell-cell and cell-matrix interactions in the skin of patients with progressive systemic sclerosis. J Am Acad Dermatol. 1992 Aug;27(2 Pt 1):169–177. doi: 10.1016/0190-9622(92)70165-c. [DOI] [PubMed] [Google Scholar]
- Hanski C., Huhle T., Reutter W. Involvement of plasma membrane dipeptidyl peptidase IV in fibronectin-mediated adhesion of cells on collagen. Biol Chem Hoppe Seyler. 1985 Dec;366(12):1169–1176. doi: 10.1515/bchm3.1985.366.2.1169. [DOI] [PubMed] [Google Scholar]
- Harris E. D., Jr Rheumatoid arthritis. Pathophysiology and implications for therapy. N Engl J Med. 1990 May 3;322(18):1277–1289. doi: 10.1056/NEJM199005033221805. [DOI] [PubMed] [Google Scholar]
- Heymann E., Mentlein R. Liver dipeptidyl aminopeptidase IV hydrolyzes substance P. FEBS Lett. 1978 Jul 15;91(2):360–364. doi: 10.1016/0014-5793(78)81210-1. [DOI] [PubMed] [Google Scholar]
- Higley H., Persichitte K., Chu S., Waegell W., Vancheeswaran R., Black C. Immunocytochemical localization and serologic detection of transforming growth factor beta 1. Association with type I procollagen and inflammatory cell markers in diffuse and limited systemic sclerosis, morphea, and Raynaud's phenomenon. Arthritis Rheum. 1994 Feb;37(2):278–288. doi: 10.1002/art.1780370218. [DOI] [PubMed] [Google Scholar]
- Kameoka J., Tanaka T., Nojima Y., Schlossman S. F., Morimoto C. Direct association of adenosine deaminase with a T cell activation antigen, CD26. Science. 1993 Jul 23;261(5120):466–469. doi: 10.1126/science.8101391. [DOI] [PubMed] [Google Scholar]
- Kreisel W., Volk B. A., Büchsel R., Reutter W. Different half-lives of the carbohydrate and protein moieties of a 110,000-dalton glycoprotein isolated from plasma membranes of rat liver. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1828–1831. doi: 10.1073/pnas.77.4.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy E. C., Smith E. A., Kahaleh M. B., Trojanowska M., Silver R. M. A strategy for determining the pathogenesis of systemic sclerosis. Is transforming growth factor beta the answer? Arthritis Rheum. 1989 Jul;32(7):817–825. [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Look A. T., Ashmun R. A., Shapiro L. H., Peiper S. C. Human myeloid plasma membrane glycoprotein CD13 (gp150) is identical to aminopeptidase N. J Clin Invest. 1989 Apr;83(4):1299–1307. doi: 10.1172/JCI114015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S., Hunzelmann N., Johnson J. P., Jung C., Mauch C., Ziegler-Heitbrock H. W., Riethmüller G., Krieg T. Expression of intercellular adhesion molecule-1 (ICAM-1) in the skin of patients with systemic scleroderma. J Invest Dermatol. 1991 Oct;97(4):667–671. doi: 10.1111/1523-1747.ep12483739. [DOI] [PubMed] [Google Scholar]
- Marlin S. D., Springer T. A. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1). Cell. 1987 Dec 4;51(5):813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Mattern T., Ansorge S., Flad H. D., Ulmer A. J. Anti-CD26 monoclonal antibodies can reversibly arrest human T lymphocytes in the late G1 phase of the cell cycle. Immunobiology. 1993 Jun;188(1-2):36–50. doi: 10.1016/S0171-2985(11)80485-7. [DOI] [PubMed] [Google Scholar]
- Needleman B. W., Wigley F. M., Stair R. W. Interleukin-1, interleukin-2, interleukin-4, interleukin-6, tumor necrosis factor alpha, and interferon-gamma levels in sera from patients with scleroderma. Arthritis Rheum. 1992 Jan;35(1):67–72. doi: 10.1002/art.1780350111. [DOI] [PubMed] [Google Scholar]
- Piazza G. A., Callanan H. M., Mowery J., Hixson D. C. Evidence for a role of dipeptidyl peptidase IV in fibronectin-mediated interactions of hepatocytes with extracellular matrix. Biochem J. 1989 Aug 15;262(1):327–334. doi: 10.1042/bj2620327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynaud F., Bauvois B., Gerbaud P., Evain-Brion D. Characterization of specific proteases associated with the surface of human skin fibroblasts, and their modulation in pathology. J Cell Physiol. 1992 May;151(2):378–385. doi: 10.1002/jcp.1041510219. [DOI] [PubMed] [Google Scholar]
- Roques B. P., Noble F., Daugé V., Fournié-Zaluski M. C., Beaumont A. Neutral endopeptidase 24.11: structure, inhibition, and experimental and clinical pharmacology. Pharmacol Rev. 1993 Mar;45(1):87–146. [PubMed] [Google Scholar]
- Schön E., Ansorge S. Dipeptidyl peptidase IV in the immune system. Cytofluorometric evidence for induction of the enzyme on activated T lymphocytes. Biol Chem Hoppe Seyler. 1990 Aug;371(8):699–705. doi: 10.1515/bchm3.1990.371.2.699. [DOI] [PubMed] [Google Scholar]
- Sollberg S., Peltonen J., Uitto J., Jimenez S. A. Elevated expression of beta 1 and beta 2 integrins, intercellular adhesion molecule 1, and endothelial leukocyte adhesion molecule 1 in the skin of patients with systemic sclerosis of recent onset. Arthritis Rheum. 1992 Mar;35(3):290–298. doi: 10.1002/art.1780350307. [DOI] [PubMed] [Google Scholar]
- Subramanyam M., Gutheil W. G., Bachovchin W. W., Huber B. T. Mechanism of HIV-1 Tat induced inhibition of antigen-specific T cell responsiveness. J Immunol. 1993 Mar 15;150(6):2544–2553. [PubMed] [Google Scholar]
- Tanaka T., Duke-Cohan J. S., Kameoka J., Yaron A., Lee I., Schlossman S. F., Morimoto C. Enhancement of antigen-induced T-cell proliferation by soluble CD26/dipeptidyl peptidase IV. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3082–3086. doi: 10.1073/pnas.91.8.3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanham G., Kestens L., De Meester I., Vingerhoets J., Penne G., Vanhoof G., Scharpé S., Heyligen H., Bosmans E., Ceuppens J. L. Decreased expression of the memory marker CD26 on both CD4+ and CD8+ T lymphocytes of HIV-infected subjects. J Acquir Immune Defic Syndr. 1993 Jul;6(7):749–757. [PubMed] [Google Scholar]
- de Meester I., Scharpé S., Vanham G., Bosmans E., Heyligen H., Vanhoof G., Corte G. Antibody binding profile of purified and cell-bound CD26. Designation of BT5/9 and TA5.9 to the CD26 cluster. Immunobiology. 1993 Jun;188(1-2):145–158. doi: 10.1016/S0171-2985(11)80494-8. [DOI] [PubMed] [Google Scholar]