Abstract
Viruses are the most abundant biological entities on Earth and play a significant role in the evolution of many organisms and ecosystems. In pathogenic protozoa, the presence of endosymbiotic viruses has been linked to an increased risk of treatment failure and severe clinical outcome. Here, we studied the molecular epidemiology of the zoonotic disease cutaneous leishmaniasis in Peru and Bolivia through a joint evolutionary analysis of Leishmania braziliensis parasites and their endosymbiotic Leishmania RNA virus. We show that parasite populations circulate in isolated pockets of suitable habitat and are associated with single viral lineages that appear in low prevalence. In contrast, groups of hybrid parasites were geographically and ecologically dispersed, and commonly infected from a pool of genetically diverse viruses. Our results suggest that parasite hybridization, likely due to increased human migration and ecological perturbations, increased the frequency of endosymbiotic interactions known to play a key role in disease severity.
INTRODUCTION
Viruses have the ability to infect virtually any cellular life form on Earth. Particularly fascinating are RNA viruses that infect simple eukaryotes 1–3, some of which have important biological roles such as limiting fungal pathogenicity 4 or increasing protist fecundity 5. Among the RNA viruses, the double-stranded RNA (dsRNA) totivirids have evolved significant diversity and are present in phyla separated by billion years of evolution, with closely related viruses identified in almost all genera of yeasts, fungi and protozoa studied 6. Totivirids have no lytic infectious phase and thus adopted a lifestyle of coexistence favoring long-term symbiotic persistence, passing from cell to cell mainly through mating and cell division 7. Because of this intimate association, it is postulated that these viruses have a mutualistic co-evolutionary history with their hosts 8.
Totivirids encompass most viral endosymbionts identified in pathogenic protozoa causing widespread severe illnesses such as trichomoniasis, giardiasis and leishmaniasis 6. An icon group of human pathogenic parasites is the genus Leishmania (Family Trypanosomatidae), causing the vector-borne disease leishmaniasis in about 88 countries, mainly in the tropics and subtropics 9. Members of the Leishmania genus are associated with the Leishmania RNA virus (LRV) (Family Totiviridae), forming a tripartite symbiosis with the mammalian or arthropod host. Phylogenetic studies suggest that the virus was most likely present in the common ancestor of Leishmania, prior to the divergence of these parasites into different species around the world 8,10. This is reflected by the two types of viruses (LRV1 and LRV2) that are carried by members of the subgenera Viannia and Leishmania, respectively 11. It was shown that the dsRNA of LRV1 is recognized by Toll-like receptor 3 (TLR3) which directly activates a hyperinflammatory response causing increased disease pathology, parasite numbers and immune response in murine models 12. In human infections, the presence of LRV1 has been associated with an increased risk of drug-treatment failures and acute pathology 13,14. The virus thus confers survival advantage to Leishmania and plays a key role in the severity of human leishmaniasis 15,16.
Given the epidemiological and biomedical relevance of the Leishmania-LRV symbiosis 17, there is a clear need to understand the diversity and dissemination of the virus in parasite populations. To this end, we investigated the joint evolutionary history of L. braziliensis (Lb) parasites and LRV1 using whole genome sequencing data. The Lb parasite is a zoonotic pathogen circulating mainly in rodents and other wild mammals (e.g. marsupials) in Neotropical rainforests 18. The parasite is the major cause of cutaneous leishmaniasis (CL) in Central and South America and occasionally develops the disfiguring mucocutaneous disease where the parasite spreads to mucosal tissue. Our previous work in Peru has shown that LRV1 was present in >25% of sampled Lb parasites, and that the virus was significantly associated with an increased risk of treatment failure 14. Here, we characterize the co-evolutionary dynamics between Lb and LRV1 from Peru and Bolivia.
RESULTS
Natural genome diversity of Lb parasites
Our study included 79 Lb isolates (Supp. Table 1; Supp. Fig. 1) from Peru (N=55) and Bolivia (N=24) that were sampled during various studies on the genetics and epidemiology of leishmaniasis 14,19–23, and for which the LRV infection status was previously characterized 14. Sequence reads of Lb were aligned against the Lb M2904 reference genome, resulting in a median coverage of 58x (min=35x, max=121x).
Variant discovery was done with GATK HaplotypeCaller to uncover high-quality Single Nucleotide Polymorphisms (SNPs) and small insertions/deletions (INDELs). The number of SNPs identified between each genome and the M2904 reference genome was relatively consistent across the panel, varying between 97,777 SNPs in CEN002 and 109,798 SNPs in LC2319 (median = 105,096; mean = 106,530). Exceptions were isolates PER231 (126,178), LC2318 (128,544) and CUM68 (134,882) that showed larger SNP densities and double the number of heterozygous sites compared to the other isolates (Supp. Fig. 2). When investigating the genome-wide distribution of normalized allele frequencies at heterozygous sites (which should be centered around 0.5 in diploid individuals) 24, we discovered that isolates CUM68, LC2318 and PER231 showed unbalanced read counts, symptomatic of tetraploidy 24, although we could not rule out contamination or a mixed infection (Supp. Fig. 3). Removing these three isolates for downstream analyses, the resulting dataset comprised a total of 407,070 SNPs and 69,604 bi-allelic INDELs called across 76 Lb genomes. The SNP allele frequency spectrum was dominated by low-frequency variants, with over 66% of SNPs being at <= 1% MAF. Respectively 41.5% and 5.7% of the SNPs and INDELs were found in the coding region of the genome, including 437 SNPs and 55 INDELs with a deleterious impact (e.g. introducing stop codons). Most of these deleterious mutations were rare in our panel of parasites (83.6% being at <= 1% MAF), and the remaining 80 mutations were not linked to Lb population structure or LRV infection status (Supp. Table 2).
Chromosome and gene copy numbers were investigated using normalized median read depths. This revealed that the majority of chromosomes were diploid (Supp. Fig. 4). Chromosome 31 was highly polysomic with at least four copies present in all isolates, a consistent observation in Leishmania 25,26. Chromosomes 10, 26, 32 and 34 were diploid in all Lb isolates (Supp. Fig. 4). Excluding chromosome 31, we found 21 Lb isolates that were entirely diploid and 25 Lb isolates with at least 5 polysomic chromosomes, including 6 Lb isolates with at least 10 polysomic chromosomes (Supp. Fig. 4). When investigating variation in copy numbers for 8,573 coding DNA sequences, we found 201–286 amplifications and 13–33 deletions per Lb isolate (Supp. Table. 3), as expected for Leishmania and other eukaryotic genomes 24. Variation in chromosome and gene copy numbers was not associated with Lb population structure or LRV infection status.
Epidemic clones against a background of prevalent recombination in Lb
It has been postulated since 1990 that protozoan parasites may have a predominantly clonal mode of reproduction and that sexual recombination events are rare 27, although this theory has been the subject of intense debate for more than 30 years 28. For Lb, studies using multilocus microsatellite profiles revealed contradictory results, including moderate degrees of inbreeding in populations from Peru and Bolivia 19,29 and significant levels of recombination in populations from the Brazilian Atlantic Coast 30. These results may be biased due to the presence of population substructure (Wahlund effect) or because of the low discriminatory power of the molecular markers used 28.
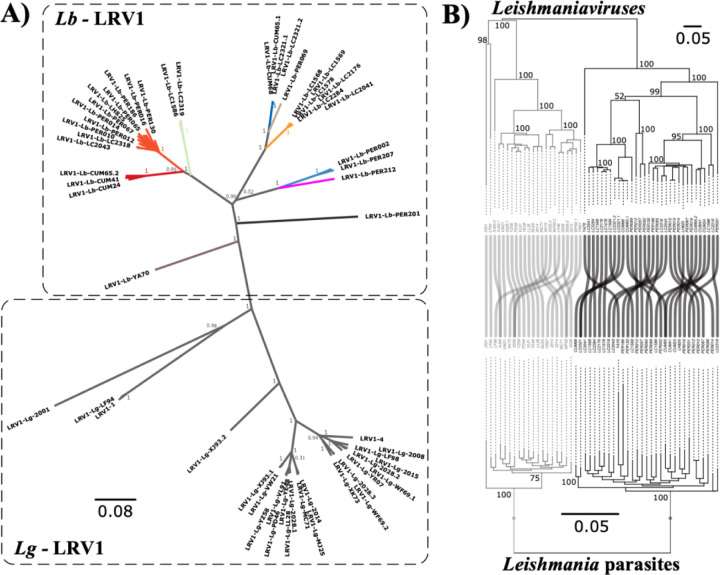
Our phylogenetic network based on genome-wide SNPs revealed a star-like topology whereby the majority of isolates were separated by long branches (Fig. 1A), a pattern symptomatic of recombination. Indeed, levels of linkage-disequilibrium (LD) were low (r2 decayed to <0.1 within <20bp) (Supp. Fig. 5) and distributions of per-site inbreeding coefficients per population were unimodal and centered around zero (Supp. Fig. 6; Supp. Table 4), after correcting for population structure and spatio-temporal Wahlund effects. Our genome-scale data thus indicate that the distinct populations in Peru and Bolivia are approximately in Hardy-Weinberg and linkage equilibrium, suggesting that recombination may be a prevalent and universal process in this species.
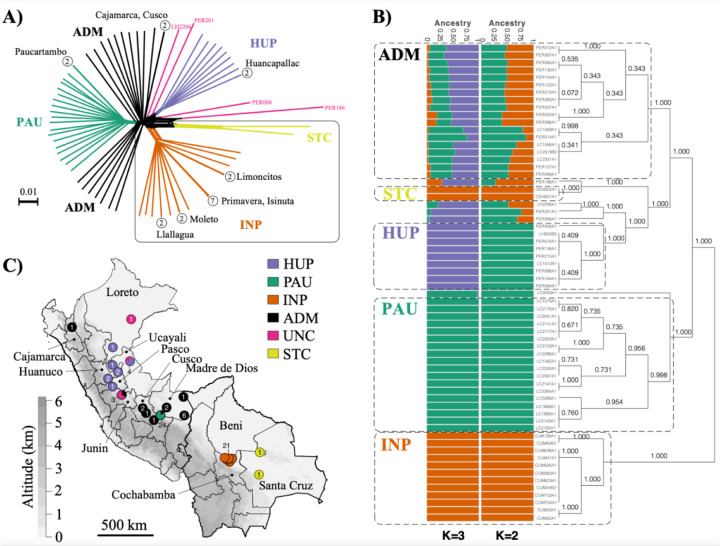
Figure 1. Population genomic structure and admixture in Lb parasites from Peru and Bolivia.
A) Phylogenetic network as inferred with SPLITSTREE based on uncorrected p-distances between 76 Lb genomes (excluding isolates CUM68, LC2318 and PER231) typed at 407,070 bi-allelic SNPs. Branches are coloured according to groups of Lb parasites as inferred with ADMIXTURE and fineSTRUCTURE (panel B). Box indicates the position of the Bolivian Lb genomes; all other genomes were sampled in Peru. Circles at the tips of seven branches point to groups of near-identical Lb genomes, showing the number of isolates and their geographical origin. B) ADMIXTURE barplot summarizing the ancestry components inferred at K = 2 or K = 3 populations in 65 Lb genomes. The phylogenetic tree summarizes the fineSTRUCTURE clustering results based on the haplotype co-ancestry matrix. Numbers indicate the MCMC posterior probability of a given clade. Dashed boxes indicate the three main parasite groups (INP, HUP, PAU) and the two main groups of admixed parasites (ADM, STC); the remainder of the parasites were of uncertain ancestry (UNC). C) Geographic map of Peru and Bolivia showing the origin of 76 Lb genomes. Dots are coloured according to groups of Lb parasites as inferred with ADMIXTURE and fineSTRUCTURE (panel B). Gray-scale represents altitude in kilometers, indicating the position of the Andes along the Peruvian and Bolivian Coast. Names are given for those Peruvian departments where a Lb parasite was isolated.
While the majority of Lb genomes differed at an average 9,866 fixed SNP differences (median 9,494), we also identified seven small groups of isolates with near-identical genomes that cluster terminally in the phylogenetic network (Fig. 1A, circles with numbers). Isolates within each of these groups displayed no or few fixed SNP differences and a relatively small amount of heterozygous SNP differences (Supp. Table 5), and are thus likely the result of clonal propagation in the wild. With the exception of a pair of near-identical genomes found in two distant Departments of Peru (Cajamarca and Cusco), each of the clonal lineages were geographically confined (Fig. 1A; Supp. Table 1). This is best exemplified by four clonal lineages that each circulated in a different location in the National Park of Isiboro in Bolivia (Fig. 1A). One of these lineages was sampled over a period of 12 years during an epidemic outbreak of leishmaniasis in Cochabamba (Bolivia), suggesting that the observed clonal population structure is temporally stable (Supp. Table 5).
Our data thus suggest that Lb population structure follows an epidemic/semi-clonal model of evolution 31,32, as proposed for other protozoan parasites 33. This model assumes frequent recombination within all members of a given population, but that occasionally a successful individual increases in frequency to produce an epidemic clone 31,32.
Parasite populations are isolated in pockets of suitable habitat
Divergent Lb ecotypes were described in Peru 20,21 and Eastern Brazil 34 that are each associated with particular ecological niches, suggesting that the environment may play a key role in Lb population structure. In Peru, our previous work revealed the existence of two Andean and one Amazonian Lb lineage 20. Here, we characterized in more detail the ancestry of the Amazonian Lb parasites.
We included one isolate from each clonal lineage and removed SNPs showing strong Linkage Disequilibrium (LD), leaving a total of 176,143 SNPs for investigating population structure. We identify three distinct ancestry components (PAU, HUP, INP) corresponding to groups of Lb parasites with geographically restricted distributions (Fig. 1B,C; Supp. Fig 7). These three populations showed signatures of spatial and temporal genetic structure: PAU (N=19) was sampled between 1991 and 1994 in Paucartambo (Southern Peru), INP (N=21) was sampled between 1994 and 2002 in the Isiboro National Park (Central Bolivia) and HUP (N=10) was sampled between 1990 and 2003 in Huanuco, Ucayali and Pasco (Central Peru) (Fig. 1B,C; Supp. Fig 7). Assuming K = 2 populations, the two Peruvian populations PAU and HUP were grouped as one population (Fig. 1B). While our results indicate that Lb may be genetically clustered at sampling sites, we also identify several groups of Lb parasites with uncertain ancestries (ADM, UNC and STC), one of which contained a total of 19 Lb isolates sampled between 1991 and 2003 across Central and Southern Peru (ADM) (Fig. 1B,C).
The strong signatures of geographical isolation of the three inferred ancestry components (PAU, INP and HUP) prompted us to investigate the geographical and/or environmental variables that impacted the population structure of Lb in the region. This was done through redundancy analysis (RDA) and generalized dissimilarity modeling (GDM), including geographic distance and 19 bioclimatic variables (Suppl Table 6). Variable selection using two approaches consistently pinpointed differences in isothermality (bio3) and precipitation of driest month (bio14) between sampling locations as the environmental contributors to parasite genetic distance (Suppl Table 7; Suppl. Results). The RDA model revealed that environmental differences and geographic distance explained one-third (27.3%) of the genomic variability, of which 10.2% was contributed by environmental differences and 7.5% by geographic distance (Suppl Fig 8A; Suppl. Table 8; Suppl. Results). Similar results were obtained with the GDM model (Suppl. Results).
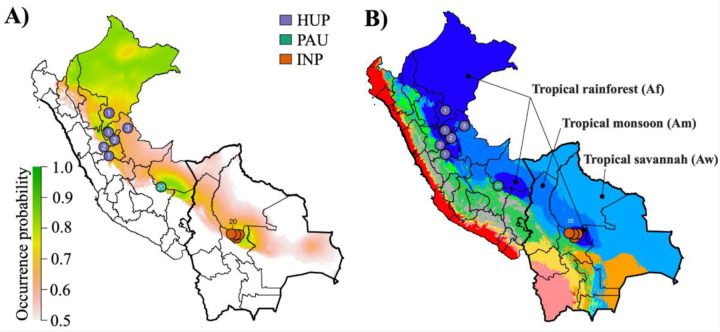
Environmental niche modeling (ENM) using present and past bioclimatic variables predicted the putative suitable habitat of the Lb populations during the Last Glacial Maximum (LGM; 21 kya) and the LIG (130 kya). Present-day predicted suitability regions for Lb coincided with tropical rainforests as predicted by the Köppen-Geiger (KG) climate classification, and shows that the three Lb populations are surrounded by the less suitable tropical monsoon forests and the non-suitable Andean ecoregions (Fig 2; Supp. Fig. 9, 10). Suitability predictions for the LGM and the LIG periods revealed largely similar ecological niches, suggesting that Lb suitable habitats have been relatively stable over the past 130,000 years (Supp. Fig. 9, 10).
Figure 2. Distribution of Lb parasite populations and their endosymbiotic LRV1 lineages in Peru and Bolivia.
A) Present-day predicted suitability regions for Lb parasites as revealed with ecological niche modeling. The continuous-scale legend represents habitat suitability (probability of occurrence). Map shows the distribution of isolates belonging to the three Lb parasite populations HUP, PAU and INP. B) Ecological regions in Peru and Bolivia as per Köppen-Geiger climate classification. Only three ecological regions were labeled for visibility reasons. The distribution is shown for Lb isolates belonging to the three Lb parasite populations HUP, PAU and INP.
Hybrid parasites are geographically and ecologically dispersed
In addition to the three Lb populations, we also identified three groups of Lb parasites with mixed ancestries: one large group of 19 Lb isolates (ADM) sampled between 1991 and 2003 mainly from Southern Peru (Junin, Cusco and Madre de Dios), four Lb isolates (UNC) sampled between 2001 and 2003 from Central/Northern Peru (Junin, Ucayali and Loreto) and one group of two isolates (STC) sampled in 1984/1985 from the Santa Cruz Department in Central Bolivia (Fig. 1). The genetic diversity of the largest group of hybrid parasites (ADM) was much larger compared to the three Lb populations: the number of mitochondrial haplotypes and nuclear segregating sites was 1.4–4 times higher in the ADM group (309,543 SNPs; 8 haplotypes) compared to that of the PAU (189.748 SNPs; 4 haplotypes), INP (185.927 SNPs; 4 haplotypes) and HUP (215.741 SNPs; 2 haplotypes) populations. The observation of a large number of mitochondrial haplotypes in the ADM group suggests that these parasites are descendants from multiple independent hybridization events (Supp. Fig. 11).
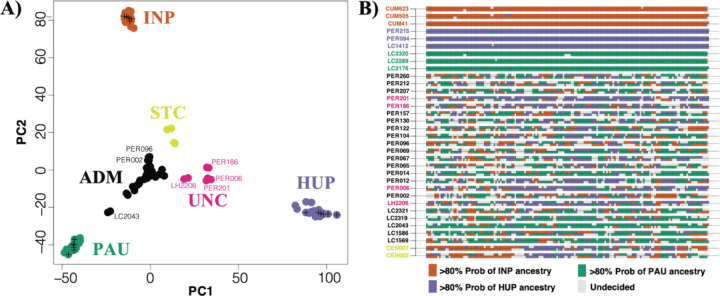
We used PCAdmix to infer the genome-wide ancestry of all admixed individuals and three control individuals from each source (Fig. 3). Ancestry was assigned to phased haplotype blocks of 30 SNPs by comparing them to reference panels of PAU, INP and HUP. While the control samples were assigned 92.9–99.4% to their respective populations, the admixed individuals showed mixed ancestries between PAU (21.1–73.1%), INP (14.3–36.7%) and HUP (0.1–54.4%). Plots of local ancestry revealed a complex and heterogeneous pattern of mosaic ancestry between the three sources (Fig. 3B, Supp. Fig. 12), suggesting that the hybrid parasites experienced many cycles of recombination following the initial admixture event(s). We next used the three-population statistic f3 to formally test the potential source populations for introgressed alleles in the ADM and STC groups. When testing (test; A,B), a negative result indicates that the test group is an admixed population from A and B. We found a significantly negative f3 statistic when ADM was the test group with PAU/HUP (f3 = −0.0013, Z-score = −18.2) and PAU/INP (f3 = −0.0002, Z-score = −2.8) as sources, but not with INP/HUP (f3 = 0.0013, SD=, Z-score = 14.8) as sources. All estimated f3 statistics were positive when STC was used as the test group. Hence, the ancestry of STC remains largely unresolved, but may involve admixture with divergent Lb parasites not captured in this study, as indicated by their distant position in a haplotype network of mitochondrial maxicircle SNPs (Supp. Fig. 11) and by the comparatively large number of fixed SNP differences between STC and other Lb groups (Supp. Fig. 13).
Figure 3. Mosaic ancestry in hybrid Lb parasites.
A) PCA-based ancestry assignment of parasites of uncertain ancestry (ADM, UNC, STC) assuming three Lb populations (ADM, INP, HUP). Plus signs indicate three randomly selected genomes of each source population that were used as controls. B) PCA-based local ancestry assignment to PAU, INP and HUP source populations of the 23 ADM isolates, 2 STC isolates and three randomly selected isolates from each source population. Ancestry was assigned in windows of 30 SNPs along chromosome 35 (examples for other chromosomes are shown in Supp. Fig. 12).
The distribution of the three groups of uncertain ancestries largely pair with the distribution of the Lb populations: the ADM group is mainly found within ecological regions surrounding the location of the PAU population (Southern Peru), the UNC group is found within ecological regions surrounding the location of the HUP population (Central/Northern Peru) and the STC group is found near the INP population (Central Bolivia) (Fig. 1C). In addition, the median distance between hybrids from the ADM group (310 km), STC group (203.8 km) and UNC group (349 km) was much larger compared to the median distance between parasites of each of the populations PAU (0 km), HUP (155 km) and INP (37 km).
Altogether, our observations show that hybrid parasites are geographically and ecologically more widespread compared to the three parasite populations, suggesting that secondary contacts occurred following migration out of the suitable rainforest habitats.
High viral prevalence and lineage diversity within group of hybrid parasites
A total of 31 out of 79 included Lb isolates (39%) were LRV1+, as revealed by previous work 14. While these originated mainly from Peru (N=27), the geographic distribution of LRV1+ and LRV1− Lb isolates was approximately similar (Supp. Fig. 1). Here, we recovered LRV1 genomes for 29/31 LRV1+ Lb isolates from Peru and Bolivia following a de novo assembly of total RNA sequencing reads (Supp. Table 9; Supp. Results). The procedure failed for two Lb isolates, either because of difficulties in growing cultures (PER096) or because assembly yielded a partial LRV1 genome (PER231). Two Lb isolates (CUM65 and LC2321) each harbored two LRV1 genomes (Supp. Results), differing at 999 (for CUM65) and 60 (for LC2321) nucleotides, bringing the total to 31 viral genomes. While only 0.04% (0.004%−0.1%) of the RNA sequencing reads aligned against the LRV1 assemblies, median coverages were relatively high, ranging between 31X and 868X (median = 372X) (Supp. Table 9). Analogous genomic regions in the assembled sequences were identical to ~1 kb sequences as obtained with conventional Sanger sequencing, confirming the high quality of our assemblies (Supp. Results).
Sequences were 4,738 – 5,285 bp long, covering the full-length coding sequence of the virus, and showing an average GC content of 46% (45.4%−46.8%) (Supp. Table 9). All but two genomes included two open reading frames without internal stop codons, encoding the Capsid Protein (CP; 2,229bp) and the RNA-dependent RNA Polymerase (RDRP; 2,637bp). Two isolates (PER014 and PER201) each contained 1 internal stop codon at amino acid positions 875 (TAA) and 882 (TAG), respectively. Sequence identities between these novel LRV1 genomes from Peru and Bolivia, and a previously published LRV1 genome from French Guiana (YA70; KY750610) ranged between 80% and 81%. Amino acid identity of both genes against YA70 ranged between 94%−96% for CP, and 85%−87% for RDRP (Supp. Table 9).
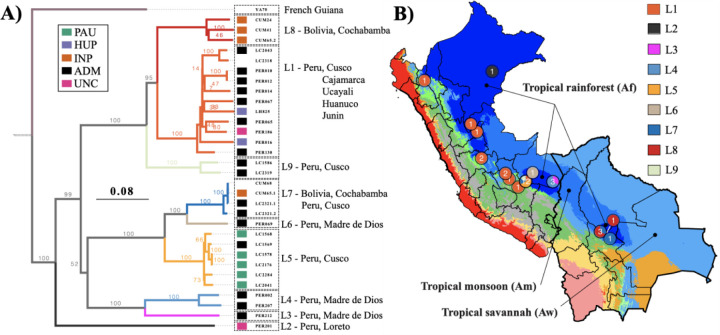
We defined a total of nine divergent viral lineages in Peru and Bolivia (L1–9), all supported by high bootstrap values (Fig. 4) and low pairwise genetic distances (<0.09; Supp. Table 10; Supp. Table 11). No evidence of recombination was found in our set of LRV1 sequences, following pairwise homoplasy index (PHI) tests (p=0.99). The number of LRV1 lineages sampled per locality was associated with the number of sampled parasites (t=5.72; p < 0.001) (Supp. Figure 14). For instance, the most densely sampled location (Paucartambo, Cusco, Peru) in terms of Lb parasites (N = 25) also contained the most viral lineages (Supp. Figure 14), two of which (L5 and L9) were only found in this location (Fig. 4). Other locations that include multiple viral lineages are Tambopata (Madre de Dios, Peru) (L3 and L4) and Moleto (Cochabamba, Bolivia) (L7, L8) (Fig. 4; Supp. Figure 14). These results show that multiple divergent viral lineages could co-occur within the same geographic region, and that a more dense sampling of parasites may uncover more viral lineages.
Figure 4. Evolutionary history of LRV1 in Peru and Bolivia.
A) Midpoint-rooted maximum likelihood phylogenetic tree based on 31 LRV1 genomes of Lb from Peru and Bolivia, one LRV1 genome (YA70) of Lb from French Guiana and 26 LRV1 genomes of Leishmania guyanensis from Brazil, Suriname and French Guiana (the latter were omitted for visibility reasons). The position of the root is indicated with a dashed line. Clades are colored according to the different viral lineages (L1-L9). Colored boxes at the tips of each branch represent the population structure of Lb (see legend), with colors matching the different groups of parasites as shown in Figure 1. Note that the tetraploid hybrid parasites LC2318 and CUM68 from Peru and Bolivia, and isolate YA70 from French Guiana were omitted from the analyses of parasite population structure; these were thus not assigned to any parasite group. B) Ecological regions in Peru and Bolivia as per Köppen-Geiger climate classification. Only three ecological regions were labeled for visibility reasons. The distribution is shown for LRV+ Lb isolates belonging to the nine viral lineages L1-L9.
The majority of viral lineages (L2–6, L8–9) were found in a single locality (Fig. 4), suggesting that the geographic spread of most LRV1 lineages is restricted. Two viral lineages were more widely distributed: one large group of viral strains (L1) was found along the Andes from Northern Peru to Southern Peru, and one group (L7) was found in Cusco (Peru) and Cochabamba (Bolivia) (Fig. 4). Three viral strains of lineage L7 were virtually identical: viral sequences of the Bolivian Lb parasites CUM68 and CUM65 were identical, and differed by one nucleotide from a viral sequence of the Peruvian Lb strain LC2321. The distal position of the two Bolivian L7 strains within a clade of Peruvian viral lineages suggests that the L7 viral lineage was introduced in Bolivia from Southern Peru (Fig. 4). Finally, all early-diverging lineages (L2-L9) were found in the tropical rainforests (KG-Af) of Peru and Bolivia, except for the widespread lineage L1 that was found in tropical rainforests (KG-Af), tropical monsoon (KG-Am), tropical savannah (KG-Aw) and temperate climate (KG-Cwb) (Fig. 4). This suggests that LRV1 predominantly evolved in the lowland tropical rainforests before spreading to other ecological regions.
When investigating the distribution of viral lineages across the different Lb parasite groups, we found two impactful observations. First, LRV1 prevalence differs between the different Lb groups, with a significantly lower prevalence in the Lb populations PAU (30%; 6/20), INP (14.3%; 3/21) and HUP (20%; 2/10) compared to the ADM (78.9%; 15/19) and UNC (50%; 2/4) groups that included Lb parasites of uncertain ancestry. Second, the three Lb populations PAU, INP and HUP (comprising a total of 50 Lb isolates) each consisted of strictly one viral lineage: the two LRV+ Lb isolates from HUP comprised the L1 viral lineage, the five LRV+ Lb isolates from PAU comprised the L5 viral lineage and the three LRV+ Lb isolates from INP comprised the L8 viral lineage, with the exception of isolate CUM65 that was coinfected with a viral strain from lineage L7 (Fig. 4). These observations strongly suggest that the three isolated Lb populations are each associated with a unique viral lineage. In contrast, the group of widespread hybrid Lb parasites (ADM) comprised almost all viral lineages (L1, L3-L9), four of which were found exclusively in the ADM Lb group (Fig. 4). The extensive viral lineage diversity within the ADM group suggests that parasite gene flow and hybridization has promoted the dissemination of viral lineages. This is best exemplified by the widespread L1 viral lineage that is mainly associated with Lb parasites from the ADM group, suggesting that the spread of L1 viruses along the Andes was mediated by parasite hybridization.
Leishmaniaviruses co-diverge with Leishmania (Viannia) host species
Phylogenetic studies indicate that LRV co-diverged with Leishmania spp. 35, though the virus is not identified in all Leishmania species 11,36 and the presence of viruses in some Leishmania species may be due to relatively recent horizontal transfer 37–39. The latter observation corroborates experimental evidence that LRV transfer among Leishmania species may occur via exosomes that are shed by infected Leishmania cells 40. The degree of LRV1 co-divergence between Leishmania Viannia species has not yet been documented.
Viral evolutionary analyses were done using partial (N = 70) and full-length genome (N = 57) sequences, including publicly available LRV1 genomes of Lb, L. guyanensis (Lg) and L. shawi (Ls) from Brazil, French Guiana and Suriname. Despite the allopatric sample, a phylogeny based on LRV1 genomes revealed two groups of viral strains: one including Lb viral strains from Peru, Bolivia and French Guiana, and one including Lg viral strains from Brazil, French Guiana and Suriname (Fig. 5A). Similar results were obtained based on partial sequences of LRV1, whereby viral strains from Brazil clustered either with the Lb or Lg viral strains (Supp. Fig. 15). In addition, nucleotide differences were on average higher between Lb and Lg viral genomes (median = 1,114, min = 1,065, max = 1,208) than between viral genomes of Lb (median = 908, min = 0, max = 991) and Lg (median = 647, min = 24, max = 1,156) (Supp. Fix. 16; Supp. Table 12). These results show that LRV1 consists of divergent viral lineages that are grouped by Leishmania host species.
Figure 5. Co-divergence between Leishmania and LRV1.
A) Unrooted maximum likelihood phylogenetic tree based on 31 LRV1 genomes of Lb from Peru and Bolivia, one LRV1 genome (YA70) of Lb from French Guiana and 26 LRV1 genomes of Lg from Brazil, Suriname and French Guiana. LRV1 clades of Lb are coloured according to the different viral lineages identified in this study (L1-L9) (see Figure 4). B) Phylogenetic tangle plot revealing clear patterns of virus-parasite co-divergence between Lb and Lg and their respective monophyletic LRV1 clades. The gray and black branches and tree links correspond to Lg and Lb, respectively.
In order to investigate whether LRV1 co-diverged with Lb and Lg parasites, we reconstructed phylogenies based on Lb+Lg viral genomes and their corresponding Lb+Lg host genotypes. Leishmania genotypes were based on SNPs called with GATK Haplotypecaller across our set of 79 Lb genomes and a set of 20 publicly available Lg genomes (see methods). Despite the relatively low coverage of Lg genomes (median 3x, min 1x, max 4x), genotyping recovered a total of 7,571 high-quality bi-allelic SNPs called across 99 Leishmania genomes. Similar to results obtained for LRV1 (Fig. 5A), a phylogenetic network revealed a clear dichotomy between Lb and Lg parasites (Supp. Fig. 17). Co-phylogenetic analyses revealed a split of both viral and parasite genomes at the deepest phylogenetic node, confirming that LRV1 viruses cluster with their Leishmania host species (Fig. 5B). A consecutive permutation test for co-speciation confirmed the topological similarity between both phylogenetic trees (RF=70, p-value = 0.001). The extremely low coverage of publicly available sequence data for Lg precluded us to study intraspecific patterns of co-divergence and host-switching within a co-phylogenetic framework.
DISCUSSION
Our main goal was to understand the evolution and dissemination of endosymbiotic viruses (here LRV1) in an important group of human pathogenic parasites (here Lb). Genetic studies have shown that both Lb 20,29,30,34,41 and LRV1 43 populations are structured according to their geographical origin. Here, we characterized the joint ancestry of the two species in Peru and Bolivia based on whole genome sequence analysis. Our results show that both Lb and LRV1 are genetically highly heterogeneous and predominantly evolved within the lowland tropical rainforests. Models of landscape genomics reveal that geographic distance and in particular environmental differences between sampling locations contributed to partitioning Lb diversity. This indicates that the large diversity and population substructure of Lb and LRV1 may have been driven by the extremely diversified ecosystem of the Amazonian rainforest, including various host–vector communities 44.
The degree of LRV1 co-divergence and host-switching between and within Leishmania species has not yet been documented. We show that LRV1 lineages cluster according to different Leishmania species, indicating that horizontal transfer of LRV1 between parasite species is rare 43,45. The agreement between LRV1 and Leishmania phylogenies corroborates the general hypothesis that LRV1 is an ancient virus that has co-evolved with its parasite 8,11,35. While we observed a clear pattern of co-divergence at the species level, we did not detect such a pattern when investigating co-phylogenies of LRV1 and Lb. Our data suggest that recombination in Lb may drive prevalent horizontal transmission of LRV1 at the intraspecific level. Such intraspecific phylogenetic incongruences have also been observed for other endosymbionts, such as Wolbachia 46.
One third of our panel showed signatures of mixed ancestry, supporting a growing body of genomic evidence for extensive genetic exchange in protozoan parasites in the wild 20,28,47–52. We propose that increased human migration and ecological perturbations over the past decades, including movement of hemerophile reservoirs/hosts such as dogs and rats, may have resulted in the displacement of Lb parasites out of the tropical rainforests 29,53. Probably the most exciting outcome of our study is that hybrid parasites were geographically and ecologically widely distributed and commonly infected from a pool of genetically diverse LRV1, in contrast to the Lb populations that were confined to tropical rainforests and infrequently associated with a single LRV1 lineage. Our data thus suggests that parasite hybridization increased the frequency of Lb-LRV1 symbiotic interactions, which play a key role in the severity of human leishmaniasis 15,16 and which may have profound epidemiological consequences in the region.
METHODS
Nucleic acid extraction and sequencing of DNA parasites and their dsRNA viruses
This study included 79 Lb isolates (Supp. Table 1) from Peru (N=55) and Bolivia (N=24) that were sampled within the context of various studies on the genetics and epidemiology of leishmaniasis. In Bolivia, the majority of Lb isolates (N=21) were sampled between 1994 and 2002 within the context of a CL outbreak in the Isiboro National Park (Department of Cochabamba). Two Lb isolates were sampled between 1984 and 1985 within the Santa Cruz Department and one is of unknown origin (Supp. Fig. 1). In Peru, half of the Lb isolates were sampled between 1991 and 2003 in the Cusco state (N=29), mainly from the Paucartambo province (N=25). The remaining 26 Lb isolates originated from Madre de Dios (N=9), Ucayali (N=5), Huanuco (N=4), Junin (N=4), Loreto (N=2), Pasco (N=1) and Cajamarca (N=1) (Supp. Fig. 1).
All 79 Lb isolates were cultured for 3–4 days on a HOMEM medium with Fetal Bovine Serum (FBS) at the Antwerp Institute of Tropical Medicine. Parasites were subjected to a small number of passages (mean = 18 ± 5) to reduce potential culture-related biases in parasite genomic characterization 54. Parasite cells were centrifuged into dry pellets and their DNA was extracted using the QIAGEN QIAmp DNA Mini kit following the manufacturer’s protocol. At the Wellcome Sanger Institute, genomic DNA was sheared into 400 to 600 base pair fragments by focused ultrasonication (Covaris Inc.), and amplification-free Illumina libraries were prepared 55. One hundred base pair paired-end reads were generated on the HiSeq 2000, and 150 base pair paired end reads were generated on the HiSeq ×10 according to the manufacturer’s standard sequencing protocol.
Previous work has shown that 31 out of the 79 Lb isolates were positive for LRV1 14. The majority of the LRV1-positive Lb isolates originated from Peru (N=27) while the remaining four isolates were sampled in the Isiboro National Park (Cochabamba, Bolivia) (Supp. Fig 1). More than half of the LRV1-positive isolates in Peru originated from Cusco (N=15) of which 11 were sampled in the Paucartambo province. LRV1 was also sampled in Madre de Dios (N=5), Junin (N=3), Cajamarca (N=1), Huanuco (N=1), Loreto (N=1) and Ucayali (N=1) (Supp. Fig 1).
The 31 LRV1-positive Lb isolates 14 were grown on a HOMEM medium with FBS for 2–3 weeks (mean passage number = 18 ± 5) at the Antwerp Institute of Tropical Medicine, ensuring high parasite yields (ca. 107-108 parasites/ml). Isolation of dsRNA was performed as previously described 56. In short, isolation involved a TRIZOL reagent (Invitrogen) RNA extraction followed by a RNase-free DNase I (NEB.) and a S1 nuclease (Sigma-Aldrich) treatment along with an additional purification step (Zymoclean). Double-stranded RNA of approximately 5.2kb. was visualized on 0.8% agarose gel (TAE buffer) stained with ethidium bromide. Extracts of dsRNA were sequenced at Genewiz (Leipzig, Germany) using the NovaSeq platform (Illumina) generating 150bp paired end reads.
Bioinformatics and population genomics of Lb parasites
Sequencing reads were mapped against the Lb M2904 reference genome using SMALT v.0.7.4 (https://www.sanger.ac.uk/tool/smalt-0/) as previously described 20. The reference genome comprises 35 chromosomes (32.73 Mb) and a complete mitochondrial maxicircle sequence (27.69 kb), and is available on https://tritrypdb.org/ as LbraziliensisMHOM/BR/75/M2904_2019. Genome wide variant calling (SNPs, INDELs) was done using GATK v.4.0.1.0 57,58. More specifically, we used GATK HaplotypeCaller for generating genotype VCF files (gVCF) for each Lb isolate. Individual gVCF files were combined and jointly genotyped using CombineGVCFs and GenotypeGVCFs, respectively. SNPs and INDELs were separated using SelectVariants. Low quality variants were excluded using GATK VariantFiltration following GATK’s best practices59 and BCFtools v.1.10.2 60 view and filter. Specifically, SNPs were excluded when QD < 2, FS > 60.0, SOR > 3.0, MQ < 40.0, MQRankSum < −12.5, ReadPosRankSum < −8.0, QUAL < 250, format DP < 10, format GQ < 25, or when SNPs occurred in clusters (ClusterSize=3, clusterWindowSize=10). INDELs were excluded when QD < 2, FS < 200.0, ReadPosRankSum < −20.0. The final set of SNPs and INDELs were annotated using the Lb M2904 annotation file with SNPEFF v4.5 61. At heterozygous SNP sites, the frequencies of the alternate allele read depths 24 were calculated using the vcf2freq.py script (available at github.com/FreBio/mytools).
Chromosomal and gene copy number variation were estimated using normalized read depths. To this end, per-site read depths were calculated with SAMtools depth (-a option). Haploid copy numbers (HCN) were obtained for each chromosome by dividing the median chromosomal read depth by the median genome-wide read depth. Somy variation was then obtained by multiplying HCN by two (assuming diploidy). To obtain gene HCN, the median read depth per coding DNA sequence (CDS) was divided by the median genome-wide read depth. The HCN per CDS were summed up per orthologous gene group. Gene copy number variations were then defined where the z-score was lower than −1 (deletions) or larger than 1 (amplifications).
A NeighborNet tree was reconstructed based on uncorrected p-distances with SplitsTree v.4.17.062. The population genomic structure of Lb was examined with ADMIXTURE v.1.3.063 and fineSTRUCTURE v.4.1.164. ADMIXTURE was run assuming 1 to 10 populations (K), performing a 5-fold cross-validation procedure, and after removing SNPs with strong LD with plink v.1.9 65 (--indep-pairwise 50 10 0.3). CHROMOPAINTER analysis (as part of fineSTRUCTURE) was run to infer the genetic ancestry based on haplotype similarity. To this end, individual genotypes were computationally phased with BEAGLE v.5.2 66 using default parameters, after which fineSTRUCTURE was run using 8e06 MCMC iterations with 50% as burn-in, and 2e06 maximization steps for finding the best state for building the tree 48. Local ancestry was assigned with PCAdmix 67 using phased genotype data (i.e. haplotypes) as obtained with the BEAGLE v5.4 66. F3-statistics were calculated with Treemix v1.13 68. Finally, Hardy-Weinberg equilibrium (HWE) was examined by calculating per site the inbreeding coefficient as Fis = 1 – Ho/He; with Ho representing the observed heterozygosity and He the expected heterozygosity. Decay of LD was calculated and visualized using PopLDdecay 69. To control for spatio-temporal Wahlund effects, we calculated Fis and LD decay using subsets of isolates that were isolated close in time (year of isolation) and space (locality), and taking into account population genomic structure (Supp. Table 4).
To investigate the spatio-environmental impact on genetic variation among the three Lb populations we included geographic distances among sampling locations and extracted 19 bioclimatic variables of the WorldClim2 database 70. We firstly investigated the role of geography on the genomic differentiation of the Lb components through linear and non-linear regression analyses of distance matrices (Supp. Methods). Secondly, we used redundancy analysis (RDA) 71 and generalized dissimilarity modeling (GDM) 72 to test the impact of environmental differences and geographic distance on parasite genetic distance. To account for model overfitting and multicollinearity, we performed two variable selection approaches (mod-A, mod-M) (Supp. Methods). Environmental Niche Modeling (ENM) was done based on present-day and past bioclimatic variables using Maxent, as implemented in the ‘dismo’ R package 73,74. A more detailed description on the landscape genomics analyses (variable selection, RDA, GDM and ENM) is presented in the Supplementary method section.
Bioinformatics and phylogenetic analyses of LRV1
Raw sequencing reads were trimmed and filtered with fastp75 using the following settings: a minimum base quality (-q) of 30; the percentage of unqualified bases (-u) set to 10; per read sliding window trimming based on mean quality scores (−5, front to tail; −3, tail to front) with a window size (-W) of 1 and mean quality score (-M) of 30; right-cutting reads ( -- cut_right) per 10bp windows (--cut_right_window_size) when mean quality score (-- cut_right_mean_quality) is below 30; only considering reads between 100 (-l) and 150bp (-b). LRV1 sequences were assembled de novo with MEGAHIT76 and identified using BLASTn77 against conventional LRV1 reference genomes LRV1–178 and LRV1–479 (accession numbers M92355 and U01899, respectively). In order to check and improve the quality of the assemblies, trimmed reads were mapped against the LRV1 contigs with SMALT as described above, with the minimal nucleotide identity (-y) set to 95%. Alignments were examined with Artemis80 and used to improve assemblies with Pilon v.1.2381. Genome sequences were aligned using MAFFT v.7.49 82.
For comparative purposes, we included 26 (near-) complete LRV1 genomes and 13 partial LRV1 sequences of Lb (N=8), Lg (N=26) and Lsh (L. shawi) (N=1) from French Guiana, Brazil and Suriname 43,45,78,79. Multiple sequence alignments were generated by trimming and re-aligning i) all available genomes to 5,189 bp sequences (N=57) and ii) all available sequences to 755 bp sequences (N=70). Maximum likelihood (ML) trees were generated using IQtree v.1.6.12 83 with 100 bootstrap replicates and implementation of the ModelFinder function 84 to determine the best substitution model based on the lowest Bayesian Information Criterion (BIC). The best substitution model identified for the genome alignment was the GTR+F+R4 model, which was also applied on the partial sequence alignment for consistency. Pairwise genetic distance among LRV1 genomes were calculated with the ‘ape’ R-package 85 (model= ‘raw’). Viral genomes with a genetic distance below 0.09 (i.e. 9% of the sites that are dissimilar among two genomes) were grouped into distinct viral lineages. Nucleotide diversity and Fst statistics were calculated within and between viral lineages using the ‘PopGenome’ package in R86 while recombination was tested by pairwise homoplasy index (PHI) tests implemented in SplitsTree 62,87.
Co-phylogenetic analysis of LRV1 and Leishmania
Co-phylogenetic analyses were done at both the species (between LRV1 infecting Lb and Lg) and at the population (LRV1 infecting Lb from Peru and Bolivia) level. These analyses were performed using the phytools R package 88. To assess the co-evolutionary history of LRV1 with Lb and Lg, we added 24 LRV1 genomes and 19 Lg and 1 Lb SNP genotypes from French Guiana (N=19) and Brazil (N=1)45 (Lg reads accession: PRAJNA371487; LRV1 genome accessions: KY750607 to KY750630). The phylogenetic trees were tested for topological similarity calculating the Robinson-Foulds distance 89 between both trees with comparison against a null distribution of 1,000 permuted un-correlated trees. For LRV1 we reconstructed a ML tree as described above, including the 23 LRV1 genomes of Lg and 32 LRV1 genomes of Lb. For Leishmania, sequence reads of Lg were mapped against the M2904 reference genome and GATK Haplotypecaller was run as described above. We then performed joint genotyping on a dataset including the 19 Lg genomes and 80 Lb genomes. SNPs were filtered following similar criteria as described above (QD<2, FS>200.0, SOR>3.0, MQ<40.0, MQRankSum<−12.5, ReadPosRankSum<−8.0, QUAL<250, info DP < 10, ClusterSize=3 and ClusterWindowSize=10). The Leishmania ML tree was generated using IQtree based on 7,571 jointly called bi-allelic SNPs with 100 bootstrap replicates and GTR+F+R5 as best performing substitution model 83,84. For the co-phylogenetic reconciliation at the intraspecific level, we focused on phylogenies generated for our dataset of LRV1 (GTR+F+R4 ML tree generated with IQtree) and Lb (bifurcating tree generated by fineSTRUCTURE ) from Peru and Bolivia.
Supplementary Material
FUNDING
This work received financial support from the European Commission (Contracts TS2-CT90–0315 and TS3-CT92–0129) and Directie-Generaal Ontwikkelingssamenwerking en Humanitaire Hulp (DGD) (Belgian cooperation). FVDB and SH acknowledge support from the Research Foundation Flanders (Grants 1226120N and G092921N). LFL and SMB acknowledge support from the National Institute of Health (5R01AI130222 and 2R01AI029646).
Footnotes
DATA AVAILABILITY
Genomic sequence reads of the 79 sequenced Lb genomes are available on the European Nucleotide Archive (https://www.ebi.ac.uk/ena/browser/home) under accession number PRJEB4442. The assembled sequences of the 31 LRV1 genomes are available on GenBank (https://www.ncbi.nlm.nih.gov/genbank/) under accession numbers OQ673070-OQ673100.
REFERENCES
- 1.Wang A. L. & Wang C. C. VIRUSES OF THE PROTOZOA. Annu. Rev. Microbiol. 45, 251–263 (1991). [DOI] [PubMed] [Google Scholar]
- 2.Banik G., Stark D., Rashid H. & Ellis J. Recent Advances in Molecular Biology of Parasitic Viruses. Infectious Disorders - Drug Targets 14, 155–167 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Ghabrial S. A., Castón J. R., Jiang D., Nibert M. L. & Suzuki N. 50-plus years of fungal viruses. Virology 479–480, 356–368 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Chen B., Geletka L. M. & Nuss D. L. Using Chimeric Hypoviruses To Fine-Tune the Interaction between a Pathogenic Fungus and Its Plant Host. J. Virol. 74, 7562–7567 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jenkins M. C. et al. Fecundity of Cryptosporidium parvum is correlated with intracellular levels of the viral symbiont CPV. Int. J. Parasitol. 38, 1051–1055 (2008). [DOI] [PubMed] [Google Scholar]
- 6.Bruenn J. A. Viruses of Fungi and Protozoans: Is Everyone Sick? Viral Ecology 297–317 (2000).
- 7.Hillman B. I. & Cohen A. B. Totiviruses (Totiviridae). in Encyclopedia of Virology (Fourth edition) (eds. Bamford D. H. & Zuckerman M.) 648–657 (Academic Press, 2021). [Google Scholar]
- 8.Widmer G. & Dooley S. Phylogenetic analysis of Leishmania RNA virus and Leishmania suggests ancient virus-parasite association. Nucleic Acids Res. 23, 2300–2304 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres-Guerrero E., Quintanilla-Cedillo M. R., Ruiz-Esmenjaud J. & Arenas R. Leishmaniasis: a review. F1000Res. 6, 750 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scheffter S. M., Ro Y. T., Chung I. K. & Patterson J. L. The complete sequence of Leishmania RNA virus LRV2–1, a virus of an Old World parasite strain. Virology 212, 84–90 (1995). [DOI] [PubMed] [Google Scholar]
- 11.Cantanhêde L. M. et al. The Maze Pathway of Coevolution: A Critical Review over the Leishmania and Its Endosymbiotic History. Genes 12, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ives A. et al. Leishmania RNA virus controls the severity of mucocutaneous leishmaniasis. Science 331, 775–778 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bourreau E. et al. Presence of Leishmania RNA Virus 1 in Leishmania guyanensis Increases the Risk of First-Line Treatment Failure and Symptomatic Relapse. J. Infect. Dis. 213, 105–111 (2016). [DOI] [PubMed] [Google Scholar]
- 14.Adaui V. et al. Association of the Endobiont Double-Stranded RNA Virus LRV1 With Treatment Failure for Human Leishmaniasis Caused by Leishmania braziliensis in Peru and Bolivia. J. Infect. Dis. 213, 112–121 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartley M.-A., Ronet C., Zangger H., Beverley S. M. & Fasel N. Leishmania RNA virus: when the host pays the toll. Front. Cell. Infect. Microbiol. 2, 99 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brettmann E. A. et al. Tilting the balance between RNA interference and replication eradicates Leishmania RNA virus 1 and mitigates the inflammatory response. Proc. Natl. Acad. Sci. U. S. A. 113, 11998–12005 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lafleur A. & Olivier M. Viral endosymbiotic infection of protozoan parasites: How it influences the development of cutaneous leishmaniasis. PLoS Pathog. 18, e1010910 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roque A. L. R. & Jansen A. M. Wild and synanthropic reservoirs of Leishmania species in the Americas. Int. J. Parasitol. Parasites Wildl. 3, 251–262 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rougeron V. et al. Extreme inbreeding in Leishmania braziliensis. Proc. Natl. Acad. Sci. U. S. A. 106, 10224–10229 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van den Broeck F. et al. Ecological divergence and hybridization of Neotropical Leishmania parasites. Proc. Natl. Acad. Sci. U. S. A. 117, 25159–25168 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odiwuor S. et al. Evolution of the Leishmania braziliensis species complex from amplified fragment length polymorphisms, and clinical implications. Infect. Genet. Evol. 12, 1994–2002 (2012). [DOI] [PubMed] [Google Scholar]
- 22.Arevalo J. et al. Influence of Leishmania (Viannia) species on the response to antimonial treatment in patients with American tegumentary leishmaniasis. J. Infect. Dis. 195, 1846–1851 (2007). [DOI] [PubMed] [Google Scholar]
- 23.Adaui V. et al. Multilocus genotyping reveals a polyphyletic pattern among naturally antimony-resistant Leishmania braziliensis isolates from Peru. Infect. Genet. Evol. 11, 1873–1880 (2011). [DOI] [PubMed] [Google Scholar]
- 24.Rogers M. B. et al. Chromosome and gene copy number variation allow major structural change between species and strains of Leishmania. Genome Res. 21, 2129–2142 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imamura H. et al. Evolutionary genomics of epidemic visceral leishmaniasis in the Indian subcontinent. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franssen S. U. et al. Global genome diversity of the Leishmania donovani complex. Elife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tibayrenc M. & Ayala F. J. Reproductive clonality of pathogens: a perspective on pathogenic viruses, bacteria, fungi, and parasitic protozoa. Proc. Natl. Acad. Sci. U. S. A. 109, E3305–13 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ramírez J. D. & Llewellyn M. S. Reproductive clonality in protozoan pathogens-truth or artefact? Mol. Ecol. 23, 4195–4202 (2014). [DOI] [PubMed] [Google Scholar]
- 29.De los Santos M. B., Ramírez I. M., Rodríguez J. E., Beerli P. & Valdivia H. O. Genetic diversity and population structure of Leishmania (Viannia) braziliensis in the Peruvian jungle. PLoS Negl. Trop. Dis. 16, e0010374 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuhls K. et al. Population structure and evidence for both clonality and recombination among Brazilian strains of the subgenus Leishmania (Viannia). PLoS Negl. Trop. Dis. 7, e2490 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maiden M. C. J. Multilocus sequence typing of bacteria. Annu. Rev. Microbiol. 60, 561–588 (2006). [DOI] [PubMed] [Google Scholar]
- 32.Smith J. M., Smith N. H., O’Rourke M. & Spratt B. G. How clonal are bacteria? Proceedings of the National Academy of Sciences 90, 4384–4388 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van den Broeck F., Tavernier L. J. M., Vermeiren L., Dujardin J.-C. & Van Den Abbeele J. Mitonuclear genomics challenges the theory of clonality in Trypanosoma congolense: Reply to Tibayrenc and Ayala. Molecular ecology vol. 27 3425–3431 (2018). [DOI] [PubMed] [Google Scholar]
- 34.S L Figueiredo de Sá B., Rezende A. M., Melo Neto O. P. de, Brito M. E. F. de & Brandão Filho S. P. Identification of divergent Leishmania (Viannia) braziliensis ecotypes derived from a geographically restricted area through whole genome analysis. PLoS Negl. Trop. Dis. 13, e0007382 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zangger H. et al. Leishmania aethiopica field isolates bearing an endosymbiontic dsRNA virus induce pro-inflammatory cytokine response. PLoS Negl. Trop. Dis. 8, e2836 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shita E. Y. et al. Prevalence of Leishmania RNA virus in Leishmania parasites in patients with tegumentary leishmaniasis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 16, e0010427 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hajjaran H. et al. Detection and molecular identification of leishmania RNA virus (LRV) in Iranian Leishmania species. Arch. Virol. 161, 3385–3390 (2016). [DOI] [PubMed] [Google Scholar]
- 38.Saberi R. et al. Presence and diversity of Leishmania RNA virus in an old zoonotic cutaneous leishmaniasis focus, northeastern Iran: haplotype and phylogenetic based approach. Int. J. Infect. Dis. 101, 6–13 (2020). [DOI] [PubMed] [Google Scholar]
- 39.Nalçacı M. et al. Detection of Leishmania RNA virus 2 in Leishmania species from Turkey. Trans. R. Soc. Trop. Med. Hyg. 113, 410–417 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Atayde V. D. et al. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat Microbiol 4, 714–723 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Patino L. H., Muñoz M., Cruz-Saavedra L., Muskus C. & Ramírez J. D. Genomic Diversification, Structural Plasticity, and Hybridization in Leishmania (Viannia) braziliensis. Front. Cell. Infect. Microbiol. 10, 582192 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oddone R. et al. Development of a multilocus microsatellite typing approach for discriminating strains of Leishmania (Viannia) species. J. Clin. Microbiol. 47, 2818–2825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cantanhêde L. M. et al. New insights into the genetic diversity of Leishmania RNA Virus 1 and its species-specific relationship with Leishmania parasites. PLoS One 13, e0198727 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rotureau B. et al. Diversity and ecology of sand flies (Diptera: Psychodidae: Phlebotominae) in coastal French Guiana. Am. J. Trop. Med. Hyg. 75, 62–69 (2006). [PubMed] [Google Scholar]
- 45.Tirera S. et al. Unraveling the genetic diversity and phylogeny of Leishmania RNA virus 1 strains of infected Leishmania isolates circulating in French Guiana. PLoS Negl. Trop. Dis. 11, e0005764 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Scholz M. et al. Large scale genome reconstructions illuminate Wolbachia evolution. Nat. Commun. 11, 5235 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rogers M. B. et al. Genomic confirmation of hybridisation and recent inbreeding in a vector-isolated Leishmania population. PLoS Genet. 10, e1004092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tihon E., Imamura H., Dujardin J.-C., Van Den Abbeele J. & Van den Broeck F. Discovery and genomic analyses of hybridization between divergent lineages of Trypanosoma congolense, causative agent of Animal African Trypanosomiasis. Mol. Ecol. 26, 6524–6538 (2017). [DOI] [PubMed] [Google Scholar]
- 49.Schwabl P. et al. Meiotic sex in Chagas disease parasite Trypanosoma cruzi. Nat. Commun. 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glans H. et al. High genome plasticity and frequent genetic exchange in Leishmania tropica isolates from Afghanistan, Iran and Syria. PLoS Negl. Trop. Dis. 15, e0010110 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van den Broeck F. et al. Emerging hybrid uncovers cryptic Leishmania lineage diversity in the Neotropics. under review (2022).
- 52.Inbar E. et al. Whole genome sequencing of experimental hybrids supports meiosis-like sexual recombination in leishmania. PLoS Genet. 15, 1–28 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marzochi M. C. de A. et al. Anthropogenic Dispersal of Leishmania (Viannia) braziliensis in the Americas: A Plausible Hypothesis. Frontiers in Tropical Diseases 2, 21 (2021). [Google Scholar]
- 54.Domagalska M. A. et al. Genomes of Leishmania parasites directly sequenced from patients with visceral leishmaniasis in the Indian subcontinent. PLoS Negl. Trop. Dis. 13, e0007900 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kozarewa I. et al. Amplification-free Illumina sequencing-library preparation facilitates improved mapping and assembly of (G+C)-biased genomes. Nat. Methods 6, 291–295 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kleschenko Y. et al. Molecular Characterization of Leishmania RNA virus 2 in Leishmaniamajor from Uzbekistan. Genes 10, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McKenna A. et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 20, 1297–1303 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Van der Auwera G. A. & OConnor B. D. Genomics in the Cloud Using Docker, GATK, and WDL in Terra (First edition). (O’Reilly Media, 2020). [Google Scholar]
- 59.Van der Auwera G. A. et al. From FastQ data to high confidence variant calls: the Genome Analysis Toolkit best practices pipeline. Curr. Protoc. Bioinformatics 43, 11.10.1–11.10.33 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Danecek P. et al. Twelve years of SAMtools and BCFtools. Gigascience 10, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cingolani P. et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly 6, 80–92 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huson D. H. & Bryant D. Application of phylogenetic networks in evolutionary studies. Mol. Biol. Evol. 23, 254–267 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Alexander D. H., Novembre J. & Lange K. Fast model-based estimation of ancestry in unrelated individuals. Genome Res. 19, 1655–1664 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lawson D. J., Hellenthal G., Myers S. & Falush D. Inference of population structure using dense haplotype data. PLoS Genet. 8, e1002453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Browning B. L., Tian X., Zhou Y. & Browning S. R. Fast two-stage phasing of large-scale sequence data. Am. J. Hum. Genet. 108, 1880–1890 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Brisbin A. et al. PCAdmix: principal components-based assignment of ancestry along each chromosome in individuals with admixed ancestry from two or more populations. Hum. Biol. 84, 343–364 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pickrell J. K. & Pritchard J. K. Inference of population splits and mixtures from genome-wide allele frequency data. PLoS Genet. 8, e1002967 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang C., Dong S.-S., Xu J.-Y., He W.-M. & Yang T.-L. PopLDdecay: a fast and effective tool for linkage disequilibrium decay analysis based on variant call format files. Bioinformatics 35, 1786–1788 (2019). [DOI] [PubMed] [Google Scholar]
- 70.Fick S. E. & Hijmans R. J. WorldClim 2: new 1‐km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017). [Google Scholar]
- 71.Oksanen J. et al. Vegan Community Ecology Package Version 2.5–7. (2020).
- 72.Fitzpatrick M., Mokany K., Manion G., Nieto-Lugilde D. & Ferrier S. gdm: Generalized Dissimilarity Modeling. (2022).
- 73.Hijmans R. J., Phillips S., Leathwick J., Elith J. & Hijmans M. R. J. Package ‘dismo’. Circles 9, 1–68 (2017). [Google Scholar]
- 74.Phillips S. J., Anderson R. P. & Schapire R. E. Maximum entropy modeling of species geographic distributions. Ecol. Modell. 190, 231–259 (2006). [Google Scholar]
- 75.Chen S., Zhou Y., Chen Y. & Gu J. fastp: an ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 34, i884–i890 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li D., Liu C.-M., Luo R., Sadakane K. & Lam T.-W. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de Bruijn graph. Bioinformatics 31, 1674–1676 (2015). [DOI] [PubMed] [Google Scholar]
- 77.Altschul S. F., Gish W., Miller W., Myers E. W. & Lipman D. J. Basic local alignment search tool. J. Mol. Biol. 215, 403–410 (1990). [DOI] [PubMed] [Google Scholar]
- 78.Widmer G., Comeau A. M., Furlong D. B., Wirth D. F. & Patterson J. L. Characterization of a RNA virus from the parasite Leishmania. Proc. Natl. Acad. Sci. U. S. A. 86, 5979–5982 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scheffter S., Widmer G. & Patterson J. L. Complete sequence of Leishmania RNA virus 1–4 and identification of conserved sequences. Virology 199, 479–483 (1994). [DOI] [PubMed] [Google Scholar]
- 80.Carver T., Harris S. R., Berriman M., Parkhill J. & McQuillan J. A. Artemis: an integrated platform for visualization and analysis of high-throughput sequence-based experimental data. Bioinformatics 28, 464–469 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walker B. J. et al. Pilon: an integrated tool for comprehensive microbial variant detection and genome assembly improvement. PLoS One 9, e112963 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Katoh K. & Standley D. M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nguyen L.-T., Schmidt H. A., von Haeseler A. & Minh B. Q. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 32, 268–274 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A. & Jermiin L. S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14, 587–589 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Paradis E., Claude J. & Strimmer K. APE: Analyses of Phylogenetics and Evolution in R language. Bioinformatics 20, 289–290 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Pfeifer B., Wittelsbürger U., Ramos-Onsins S. E. & Lercher M. J. PopGenome: an efficient Swiss army knife for population genomic analyses in R. Mol. Biol. Evol. 31, 1929–1936 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bruen T. C., Philippe H. & Bryant D. A simple and robust statistical test for detecting the presence of recombination. Genetics 172, 2665–2681 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Revell L. J. phytools: an R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012). [Google Scholar]
- 89.Robinson D. F. & Foulds L. R. Comparison of phylogenetic trees. Math. Biosci. 53, 131–147 (1981). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.