Abstract
Background
Acute kidney injury (AKI) is a known complication of COVID-19 and is associated with an increased risk of in-hospital mortality. Unbiased proteomics using biological specimens can lead to improved risk stratification and discover pathophysiological mechanisms.
Methods
Using measurements of ~4000 plasma proteins in two cohorts of patients hospitalized with COVID-19, we discovered and validated markers of COVID-associated AKI (stage 2 or 3) and long-term kidney dysfunction. In the discovery cohort (N= 437), we identified 413 higher plasma abundances of protein targets and 40 lower plasma abundances of protein targets associated with COVID-AKI (adjusted p <0.05). Of these, 62 proteins were validated in an external cohort (p <0.05, N =261).
Results
We demonstrate that COVID-AKI is associated with increased markers of tubular injury (NGAL) and myocardial injury. Using estimated glomerular filtration (eGFR) measurements taken after discharge, we also find that 25 of the 62 AKI-associated proteins are significantly associated with decreased post-discharge eGFR (adjusted p <0.05). Proteins most strongly associated with decreased post-discharge eGFR included desmocollin-2, trefoil factor 3, transmembrane emp24 domain-containing protein 10, and cystatin-Cindicating tubular dysfunction and injury.
Conclusions
Using clinical and proteomic data, our results suggest that while both acute and long-term COVID-associated kidney dysfunction are associated with markers of tubular dysfunction, AKI is driven by a largely multifactorial process involving hemodynamic instability and myocardial damage.
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a novel coronavirus that has caused the coronavirus disease 2019 (COVID-19) pandemic. Although effective vaccines are available, novel variants that may evade neutralizing antibodies exist in the population and have led to high case counts and periodic case surges. COVID-19 most commonly presents with fever, cough, and dyspnea1,2 and is associated with acute respiratory distress syndrome (ARDS). However, the clinical syndrome resulting from SARS-CoV-2 infection is broad, ranging from asymptomatic infection to severe disease with extrapulmonary manifestations3, including acute kidney injury4, acute myocardial injury5,6 and thrombotic complications7–11. The CRIT-COV-U research group in Germany recently developed a urinary proteomics panel COV50 that could consider this variability in infection by generating biomarkers that can indicate adverse COVID-19 outcomes based on the WHO severity scale12
Acute kidney injury (AKI) is a particularly prominent complication. The rates of AKI vary greatly based on patient population, but evidence suggests that at least 30% of hospitalized patients and 50% of patients in the intensive care unit (ICU) develop AKI1,4,13–16. Although the rate of AKI in hospitalized COVID-19 patients has decreased since the initial surge in 2020, the incidence remains high17. Like community-acquired pneumonia18, AKI is increasingly recognized as a common complication of COVID-19 in the hospitalized setting and confers significantly increased morbidity and mortality19.
There is a limited understanding of the pathophysiology of COVID-19-associated AKI. A recent paper20 compared transcriptomics and proteomics of postmortem kidney samples of patients with severe COVID-19 and autopsy-derived control cohorts of sepsis-AKI and non-sepsis-AKI. The work found common inflammatory pathways and regulatory responses including the downregulation of oxidative signaling pathways between COVID-19 AKI and sepsis-AKI. They also confirmed the observation of tubular injury in almost all their COVID-19 AKI samples while drawing similarities between the inflammation response of sepsis-associated AKI and COVID-19 associated AKI. Histopathological reports from autopsy specimens have provided conflicting insights into the pathological changes in the kidney in COVID-19. A report of 26 patients who died with COVID-19 AKI revealed acute tubular injury as a prominent mechanism21. Additionally, the presence of viral particles in the tubular epithelium and podocytes in autopsy specimens has been reported21,22, which is evidence of direct viral invasion of the kidney. In addition, coagulopathy and endothelial dysfunction are hallmarks of COVID-1923 and may also contribute to AKI. Finally, SARS-CoV-2 may directly activate the complement system24. In addition to these mechanisms, systemic effects of critical illness (hypovolemia, mechanical ventilation) and derangements in cardiac function and volume may also contribute to COVID-19 AKI.
In addition to morbidity and mortality in the acute setting, COVID-19 is also associated with long term manifestations i.e., the post-acute sequelae of SARS-CoV2 (PASC)25. Kidney function decline is a major component of PASC and a study of more than 1 million individuals found that survivors of COVID-19 had an elevated risk of post-acute eGFR decline26, suggesting long term kidney dysfunction may occur following the acute infection.
Given the high incidence of COVID-19 associated kidney dysfunction, the unknown pathophysiology, and the urgent need for better approaches for risk stratification for long term kidney function decline we aimed to characterize the proteomic changes associated with COVID associated AKI and long-term kidney function. Proteomic biomarkers have previously shown success in predicting COVID-19 outcomes27–29. Other work12 has also applied urinary proteomic profiling to predict worsening of COVID-19 at early stages of the infection. Prior research using minimally invasive proteomics assays supports the use of peripheral serum as a readily accessible source of proteins that accurately reflect the human disease state30–33. We measured protein expression of more than 4000 proteins from serum samples collected in a diverse large cohort of hospitalized patients with COVID-19 and validated significant results in an independent cohort and identified proteins that are significantly different between patients with and without AKI. We then determined whether these proteomic perturbations also characterize post-discharge kidney function decline as measured by estimated glomerular filtration rate (eGFR).
Materials And Methods
Patient cohort
An overview of the discovery cohort selection process is provided in Fig 1. We prospectively enrolled patients hospitalized with COVID-19 between March 24, and August 26, 2020, at five hospitals of a large urban, academic hospital system in New York City, NY into a cohort as previously described34. The cohort enrolled patients who were admitted to the health care system with a COVID-19 infection and had broad inclusion criteria without specific exclusion criteria. The Mount Sinai Institutional Review Board approved this study under a regulatory approval allowing for access to patient level data and biospecimen collection35. Peripheral blood specimens were collected at various points during the hospital admission for each patient.
Figure 1. Overview of the discovery cohort selection process.

a. Cohort selection strategy overview, b. eGFR measurements recorded post-discharge for returning patients until 12/21/2021.
A: Prospective cohort of patients enrolled between March 2020 – Aug 2020
B: Timeline of measurements taken
The validation cohort included a prospective biobank from Quebec, Canada that enrolled patients hospitalized with COVID-19, as previously described29. Patients were recruited from the Jewish General Hospital and Centre Hospitalier de l’niversité de Montréal. Peripheral blood specimens were collected at multiple time points after admission.
We defined an AKI cohort using proteomic data acquired at the last available timepoint during the hospital course for all individuals. Patients who developed AKI after the last specimen collection timepoint were excluded. Controls were defined as individuals who developed AKI stage 1 or did not develop AKI during their hospital course.
Serum collection and Processing
Blood samples were collected in Serum Separation Tubes (SST) with a polymer gel for serum separation as previously described35. Samples were centrifuged at 1200 g for 10 minutes at 20°C. After centrifugation, serum was pipetted to a 15 mL conical tube. Serum was then aliquoted into cryovials and stored at −80°C.
Definition of Acute Kidney Injury
We defined AKI (stage 2 or 3) as per Kidney Disease Improving Global Outcomes (KDIGO) criteria: an increase in serum creatinine of at least 2.0 times the baseline creatinine36. For patients with previous serum creatinine measurement available in the 365 days prior to admission, the minimum value in this period was considered the baseline creatinine. For patients without a baseline creatinine in this period, a baseline value was calculated based on an estimated glomerular filtration rate (eGFR) of 75 ml/min per 1.73 m2 as per the KDIGO AKI guidelines.
Clinical data collection
We collected demographic and laboratory data collected as part of standard medical care from an institutional electronic health record (EHR) database. We defined clinical comorbidities using diagnostic codes recorded in the EHR before the current hospital admission. To account for disease severity at the time of specimen collection, we defined supplemental oxygen requirement as 0 if the patient was not receiving supplemental oxygenation or on nasal cannula, 1 if the patient was receiving non-invasive mechanical ventilation (CPAP, BIPAP), or 2 if the person was receiving invasive mechanical ventilation.
Somalogic proteomic assay
We used the SomaScan discovery platform to quantify levels of protein expression37. The SomaScan platform is a highly multiplexed aptamer based proteomic assay based on Slow Off-rate Modified single-stranded DNA Aptamers (SOMAmers) capable of simultaneously detecting 4497 proteins in biological samples in the form of relative fluorescent units (RFUs). The assay was run using the standard 12 hybridization normalization control sequences to assess for variability in the Agilent plate quantification process, five human calibrator control pooled replicates, and 3 quality control pooled replicates to control for batch effects. Standard preprocessing protocols were applied as per Somalogic’s guidelines published previously37 The specificity and stability of the SOMAScan assay has been described previously38 . Briefly, the data was first normalized using the 12 hybridization controls to remove hybridization variation within a run. Then, median signal normalization is performed with calibrator samples across plates to remove variation in sample-to-sample differences attributable to variations due to pipetting, reagent concentrations, assay timings and other technical aspects. Data was then calibrated to remove assay differences between runs. Standard Somalogic acceptance criteria for quality control metrics were used (plate scale factor between 0.4 and 2.5 and 85% of QC ratios between 0.8 and 1.2). Samples with intrinsic issues such as reddish appearance or low sample volume were also removed as part of the Somalogic quality control protocol. After quality control and normalization procedures, the resulting relative fluorescence unit (RFU) values were log2 transformed.
Dimensionality reduction
Principal component analysis (PCA) was performed using log2 transformed RFU values of all proteins. Pairwise plots of the top three principal components were plotted.
Differential expression analysis for prevalent AKI
Using data from the AKI cohort, log2 transformed normalized protein values were modelled using multivariable linear regression in the Limma framework39 Models were adjusted for age, sex, history of chronic kidney disease (CKD), and supplemental oxygen requirement (0,1, or 2 [see above]) at the time of specimen collection. P-values were adjusted using the Benjamin-Hochberg procedure to control the false discovery rate (FDR) at 5%.
Proteomic characterization of long-term kidney function in discovery cohort
Outpatient creatinine values measured after discharge were used to compute estimated glomerular filtration rate (eGFR) values the CKD-EPI equation. All values were taken from the EHR as part of routine clinical care with follow-up until 12/2/2021. To determine whether AKI associated protein expression correlated with post-discharge kidney function, we fit a mixed effects linear regression model with random intercept. Using the discovery cohort, protein expression of AKI-associated proteins measured at the last available timepoint during admission was used. The dependent variable was eGFR and the model was adjusted for age, sex, baseline creatinine, history of CKD, maximum AKI stage during the hospital admission, and day of eGFR measurement after hospital discharge. Models included a random effect of patient ID to adjust for correlation between eGFR values taken from the same individuals. Significance was evaluated using a t-test with Satterthwaite degrees of freedom implemented in the ImerTest R package40. P values were adjusted using the Benjamin-Hochberg procedure to control the false discovery rate (FDR) at 5%.
We then plotted the post-discharge eGFR values over time for individuals separated by protein expression tertiles (bottom 33rd percentile, middle 33rd percentile, and top 33rd percentile). We transformed data using the LOESS smoothing function as implemented in the ggplot R package.
Data analysis and visualization
We performed all statistical analysis using R version 4.0.3. Protein-protein interaction (PPI) network was constructed using the Network X package in Python v3.4.10 to display a Minimum Spanning Tree (MST) using Prim’s algorithm. Network clustering was conducted using the MCL cluster algorithm and functional enrichment was carried out using the STRING41 database in Cytoscape42. Using results from a recent publication30, we also annotated protein quantitative trait loci (pQTLs) for the set of COVID AKI-associated proteins. For each AKI-associated protein, we determined whether cis and trans pQTL associations had been reported.
Discussion
Using proteomic profiling in two large groups of patients hospitalized with COVID-19, we report several observations. First, we identified specific protein markers of AKI and post-discharge kidney dysfunction, both well-documented sequelae of COVID-194,43. Second, in the acute phase, tubular injury and hemodynamic perturbation may play a role. Thus, characterization of the peripheral blood suggests specific large-scale perturbations of the proteome that accompany both AKI and long-term eGFR decline with implications for more specific prognostic models and targeted therapeutic development.
Based on our results, we hypothesize that COVID AKI may involve several mechanisms: tubular injury, neutrophil activation, and hemodynamic perturbation. First, we found significantly higher plasma abundances of NGAL (LCN2), a canonical marker of tubular injury that is also involved in neutrophil activation. NGAL is secreted by circulating neutrophils and kidney tubular epithelium in response to systemic inflammation or ischemia. Since renal tubular epithelial cells express the angiotensin-converting enzyme 2 (ACE2) receptor which enables SARS-CoV2 viral entry into cells, direct tubular infection may cause the release of NGAL into the serum and urine. This potential mechanism is supported by our results and remains a testable hypothesis. Although NGAL is a known marker for intrinsic AKI accompanied by tubular injury, it is relatively insensitive to pre-renal AKI caused by hemodynamic disturbance44,45. However, our results demonstrate higher plasma abundance of BNP, a protein released in the setting of volume overload as well as several cardiac structural proteins (cardiac troponin T, titin, myosin light chain 1, and sarcalumenin). This proteomic signature may represent either myocardial injury leading to decreased renal perfusion or impaired filtration by the kidney in response to injury. Myocardial injury has been previously reported in patients hospitalized with COVID-196 and thus may contribute to the multifactorial nature of COVID-AKI. It is worth nothing that in addition to myocardial injury, BNP may also be increased in critical illness due to pro-inflammatory cytokine release.
Since COVID-AKI increases the risk of long-term eGFR decline43, we then sought to determine whether these two phenomena shared common proteomic markers. Surprisingly, we found that although almost half of the AKI-associated proteins were also significantly associated with post-discharge eGFR decline, the strengths of associations were not correlated. While COVID-AKI is likely caused by a combination of intrinsic tubular injury and hemodynamic disturbance in the setting of critical illness, long term eGFR decline was associated with increased expression of trefoil factor 3 (TFF3), a known prognostic marker for incident CKD46. Trefoil factors are a class of small peptides expressed in colonic and urinary tract epithelia that play essential roles in regeneration and repair of epithelial tissue47,48.
Immunohistochemistry reveals TFF3 expression is localized to the tubular epithelial cells in kidney specimens from patients with CKD46, suggesting that long term eGFR decline may be associated with renal tubular epithelial damage. The exact pathological role of TFF3 in the renal tubules is unclear but it has been hypothesized to play a role in repair of kidney damage49. Additionally, TFF3 release from the renal interstitium has also been hypothesized to direct the epithelial-to-mesenchymal transition (EMT) in renal interstitial fibrosis, a main pathway that leads to ESKD46. Our results implicate tubular damage in both AKI and long term eGFR decline suggesting that SARS-CoV2 may preferentially target this region of the nephron. While AKI in the acute setting may be a result of ischemia and decreased renal perfusion associated with critical illness, the specific elevation of TFF3 associated with eGFR decline implicates a more general pattern of tubular injury that underlies COVID mediated kidney dysfunction. Since the ACE2 is preferentially expressed in the tubular epithelial cells of the kidney50,51, the elevation of markers of tubular damage in the plasma may represent direct viral invasion of tubular epithelia cells. However, again this would need to be tested using biopsy/autopsy specimens or other mechanistic studies. Direct viral entry into the kidney remains controversial and using our current data we are not able to comment on this mechanism.
Our study should be interpreted in the context of certain limitations. First, samples were collected during the hospital course of patients with confirmed COVID-19. However, the timepoints were not systematic due to logistical challenges during the peak of the COVID-19 pandemic and thus are not standardized between patients. Since a subset of patients had AKI at the time of admission, these patients were excluded from our analysis since specimens were collected after admission. Additionally, we did not include patients who developed AKI without COVID and were unable to determine whether COVID-AKI has unique proteomic markers compared to other forms of sepsis-AKI. Thus, our AKI cases may be biased towards less severe presentations. Second, since kidney injury is usually not an isolated phenomenon in critically ill patients, the protein expression changes observed may have been partially due to damage to other organs, such as the lung, liver, and heart. However, we accounted for non-kidney damage by adjusting for the highest level of ventilatory support and thus our results are likely a reflection of kidney injury. Specifically, our results do show the importance of crosstalk between the cardiac system and the kidneys. In addition, we did not include proteomic measurements from urine specimens and thus it is unclear whether poor filtration or resorption of proteins plays a role in peripheral blood protein concentrations. For example, poor resorption of cystatin-C in the setting of AKI may have led to the increased peripheral blood cystatin-C that we report. Our study was adequately powered to detect effect sizes of greater than or equal to 1.6. Also, since we enrolled patients only from March-October 2020, we cannot generalize our findings to other COVID-19 variants and time periods. Although we adjusted our regression models for history of CKD, it is possible that unmeasured confounding due to preexisting impaired kidney function has not completely been controlled our in our analyses. Another big limitation of our study is the inability to significantly distinguish between effects of impaired filtration and true pathogenic differences in protein levels without having to conduct additional validation tests.
We were not able to exclude individuals who were lost to follow-up or died because the data was extracted from an institutional EHR. Some patients accessed care at other hospitals after discharge. This remains a limitation as well. Finally, our cohort did not include autopsy or kidney biopsy specimens. Histopathological analysis of kidney specimens is necessary to determine the mechanism of AKI and whether viral particles are present in the kidney.
In conclusion, we provide, to the best of our knowledge, the first comprehensive characterization of the plasma proteome of AKI and long term eGFR decline in hospitalized COVID-19 patients. Our results suggest in the setting of COVID-AKI and post-discharge kidney dysfunction there is evidence of tubular damage in the peripheral blood but that in the acute setting, several factors including hemodynamic disturbance and myocardial injury also play a role.
Figure 2. Analysis of the clinical and proteomic data.

a. Top 3 Principal Components show separation of the sample by AKI (stage 2 or 3) case status. b. External validation of AKI associated proteins in the discovery cohort shows high correlation with increased risk of AKI with significance of p<0.05. (n=62) c. Expression heatmap shows a distinct separation of the cases and controls using the 62 significant proteins identified from the validation cohort in the discovery cohort.
Figure 3.

Nested Venn diagram of the analyses performed.
Figure 4. Protein-protein interaction (PPI) and clustering analysis for functional annotation of the 62 differentially expressed proteins.
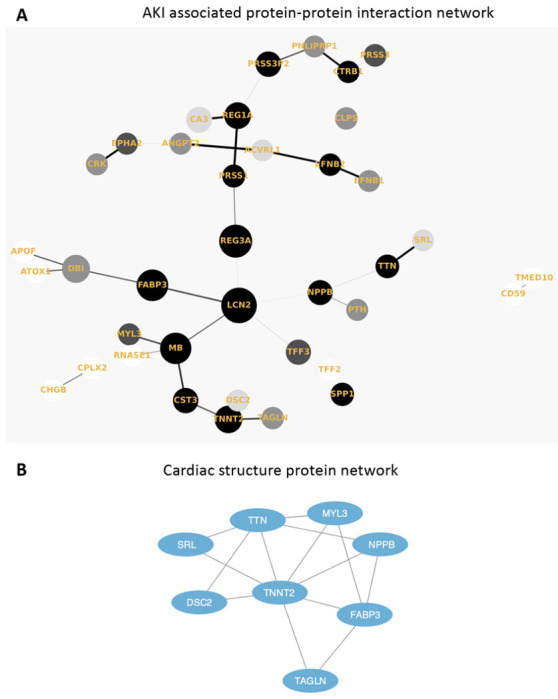
a. Protein–protein interaction (PPI) network (Minimum Spanning Tree) of the 62 overlapping AKI associated proteins with a score >0.4. The size of each node corresponds to number of interactions and the thickness of the edges represent the weight of the interactions between the nodes. b. MCL algorithm was used to identify tightly connected cluster of proteins which was functionally enriched for cardiac structure proteins using the STRING database.
Figure 5. Proteomic characterization of long-term eGFR decline.
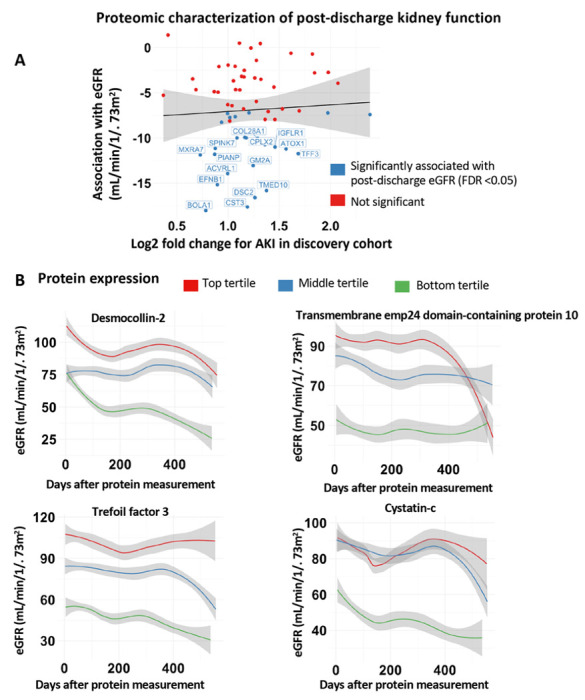
a. Comparison of strengths of association with AKI and long term eGFR for proteins associated with AKI in both the discovery and validation cohorts (n=62). b. Trend in eGFR values separated by protein expression for tertiles for proteins most significantly (by P value) associated with eGFR trend.
Acknowledgements:
We would like to thank the scientists at Somalogic for their assistance with technical and scientific questions about the assay.
Funding:
EUA, GNN, JCH and SGC are partially funded by R01 DK118222. GNN also is supported by R01DK127139, R01HL155915 and R56DK126930.
Disclosures:
GNN and SGC reports grants, personal fees, and non-financial support from Renalytix. GNN reports non-financial support from Pensieve Health, personal fees from AstraZeneca, personal fees from BioVie, personal fees from GLG Consulting, personal fees from Siemens Healthineers from outside the submitted work. IP receives personal fees from Character Biosciences.
Footnotes
Data and code for figures:
Fig 2. Source data for the PCA plots in Fig 2a. are found in the Synapse data repository syn35874390 generated using Code for fig 2 on GitHub. This has also been updated in the Data Availability section in the manuscript. Source Data for Fig 2b. are provided in Main Manuscript Table 2 in Supplementary Data 1. Source Data for Fig 2c. is from the raw data matrix provided in syn35874390 but filtered for the 62 proteins documented in the Main Manuscript Table 2 in Supplementary Data 1.
Fig 3. Source data for Fig 3. Results Overview is acquired from Supplementary Table3. in the Supplementary Information document and Main Manuscript Table 3. from Supplementary Data 1.
Fig 4. Source data for Fig 4a. AKI-associated protein-protein interaction network is in our Code for fig 4a on GitHub.
Fig 5. Source data for Fig 5a. Proteomic characterization of post-discharge kidney function is derived from Main Manuscript Table 3. from Supplementary Data 1 and is created using this code: Code for fig 5 on GitHub. The input data used to derive Main Manuscript Table 3. is in Supplementary Data 3.xlsx.
Contributor Information
Girish Nadkami, Icahn School of Medicine at Mount Sinai.
Ishan Paranjpe, Hasso Plattner Institute for Digital Health at Mount Sinai, Icahn School of Medicine at Mount Sinai, New York, NY USA.
Pushkala Jayaraman, Icahn School of Medicine at Mount Sinai.
Chen-Yang Su, McGill University.
Sirui Zhou, Lady Davis Institute.
Steven Chen, Icahn School of Medicine at Mount Sinai.
Diane Del Valle, Icahn School of Medicine at Mount Sinai.
Ryan Thompson, Icahn School of Medicine at Mount Sinai.
Ephraim Kenigsberg, Icahn School of Medicine at Mount Sinai.
Shan Zhao, Icahn School of Medicine at Mount Sinai.
Suraj Jaladanki, Icahn School of Medicine at Mount Sinai.
Kumardeep Chaudhary, Icahn School of Medicine at Mount Sinai.
Steven Ascolillo, Icahn School of Medicine at Mount Sinai.
Akhil Vaid, Icahn School of Medicine at Mount Sinai.
Edgar Gonzalez-Kozlova, Mt Sinai School of Medicine.
Arvind Kumar, Icahn School of Medicine at Mount Sinai.
Manish Paranjpe, Harvard Medical School.
Ross O’Hagan, Icahn School of Medicine at Mount Sinai.
Samir Kamat, Icahn School of Medicine at Mount Sinai.
Faris Gulamali, Icahn School of Medicine at Mount Sinai.
Justin Kauffman, Icahn School of Medicine at Mount Sinai.
Hui Xie, Icahn School of Medicine at Mount Sinai.
Joceyln Harris, Icahn School of Medicine at Mount Sinai.
Manishkumar Patel, Icahn School of Medicine at Mount Sinai.
Kimberly Argueta, Icahn School of Medicine at Mount Sinai.
Craig Batchelor, Icahn School of Medicine at Mount Sinai.
Kai Nie, Icahn School of Medicine at Mount Sinai.
Sergio Dellepiane, Icahn School of Medicine at Mount Sinai.
Leisha Scott, Icahn School of Medicine at Mount Sinai.
Matthew Levin, Icahn School of Medicine at Mount Sinai.
John He, Mount Sinai School of Medicine.
Mayte Suárez-Fariñas, Icahn School of Medicine at Mount Sinai.
Lili Chan, Icahn School of Medicine at Mount Sinai.
Evren Azeloglu, Icahn School of Medicine at Mount Sinai.
Eric Schadt, Icahn School of Medicine at Mount Sinai.
Noam Beckmann, Icahn School of Medicine at Mount Sinai.
Sacha Gnjatic, Icahn School of Medicine at Mount Sinai.
Miriam Merad, Icahn School of Medicine at Mount Sinai.
Seunghee Kim-Schulze, Icahn School of Medicine at Mt. Sinai.
J. Brent Richards, Lady Davis Institute for Medical Research, Jewish General Hospital.
Benjamin Glicksberg, Icahn School of Medicine at Mount Sinai.
Alexander Charney, Icahn School of Medicine at Mount Sinai.
Data and Code Availability
The Data is available in Synapse syn35874390 and can also be accessed by contacting the senior author, Girish Nadkarni (girish.nadkarni@mountsinai.org).
Code is available at our GitHub repository Nadkarni-Lab: aki_covid_proteomics.
References
- 1.Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA - J Am Med Assoc 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal R Choi JJ, Pinheiro LC, et al. Clinical characteristics of COVID-19 in New York City. N. Engl. J. Med. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Madhavan M V., Sehgal K, et al. Extrapulmonary manifestations of COVID-19. Nat. Med. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan L, Chaudhary K, Saha A, et al. AKI in Hospitalized Patients with COVID-19. J Am Soc Nephrol [Internet] 2020;ASN.2020050615. Available from: http://jasn.asnjournals.org/content/early/2020/09/02/ASN.2020050615.abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sala S, Peretto G, Gramegna M, et al. Acute myocarditis presenting as a reverse Tako-Tsubo syndrome in a patient with SARS-CoV-2 respiratory infection. Eur. Heart J. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lala A, Johnson KW, Januzzi JL, et al. Prevalence and Impact of Myocardial Injury in Patients Hospitalized With COVID-19 Infection. J Am Coll Cardiol 2020;76(5):533–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui S, Chen S, Li X, Liu S, Wang F. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bilaloglu S, Aphinyanaphongs Y, Jones S, Iturrate E, Hochman J, Berger JS. Thrombosis in Hospitalized Patients with COVID-19 in a New York City Health System. JAMA - J. Am. Med. Assoc. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lodigiani C, lapichino G, Carenzo L, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helms J, Tacquard C, Severac F, et al. High risk of thrombosis in patients with severe SARS-CoV-2 infection: a multicenter prospective cohort study. Intensive Care Med 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paranjpe I, Fuster V, Lala A, et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wendt R, Thijs L, Kalbitz S, et al. A urinary peptidomic profile predicts outcome in SARS-CoV-2-infected patients. eClinicalMedicine 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Argenzian MG, Bruc SL, Slate CL, et al. Characterization and clinical course of 1000 patients with coronavirus disease 2019 in New York: Retrospective case series. BMJ 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohamed MMB, Lukitsch I, Torres-Ortiz AE, et al. Acute Kidney Injury Associated with Coronavirus Disease 2019 in Urban New Orleans. Kidney 360 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cao J, Tu WJ, Cheng W, et al. Clinical features and short-term outcomes of 102 patients with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dellepiane S, Vaid A, Jaladanki SK, et al. Acute Kidney Injury in Patients Hospitalized With COVID-19 in New York City: Temporal Trends From March 2020 to April 2021. Kidney Med 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murugan R, Karajala-Subramanyam V, Lee M, et al. Acute kidney injury in non-severe pneumonia is associated with an increased immune response and lower survival. Kidney Int 2010; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nadim MK, Forni LG, Mehta RL, et al. COVID-19-associated acute kidney injury: consensus report of the 25th Acute Disease Quality Initiative (ADQI) Workgroup. Nat. Rev. Nephrol. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alexander MP, Mangalaparthi KK, Madugundu AK, et al. Acute Kidney Injury in Severe COVID-19 Has Similarities to Sepsis-Associated Kidney Injury: A Multi-Omics Study. Mayo Clin Proc 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su H, Yang M, Wan C, et al. Renal histopathological analysis of 26 postmortem findings of patients with COVID-19 in China. Kidney Int 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Farkash EA, Wilson AM, Jentzen JM. Ultrastructural evidence for direct renal infection with sars-cov-2. J Am Soc Nephrol 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Noris M, Benigni A, Remuzzi G. The case of complement activation in COVID-19 multiorgan impact. Kidney Int. 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Al-Aly Z, Xie Y, Bowe B. High-dimensional characterization of post-acute sequelae of COVID-19. Nature 2021; [DOI] [PubMed] [Google Scholar]
- 26.Bowe B, Xie Y, Xu E, Al-Aly Z. Kidney Outcomes in Long COVID. J Am Soc Nephrol 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shu T, Ning W, Wu D, et al. Plasma Proteomics Identify Biomarkers and Pathogenesis of COVID-19. Immunity 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park J, Kim H, Kim SY, et al. In-depth blood proteome profiling analysis revealed distinct functional characteristics of plasma proteins between severe and non-severe COVID-19 patients. Sci Rep 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Su C-Y, Zhou S, Gonzalez-Kozlova E, et al. Circulating proteins to predict adverse COVID-19 outcomes. medRxiv [Internet] 2021;2021.10.04.21264015. Available from: http://medrxiv.org/content/early/2021/10/05/2021.10.04.21264015.abstract [Google Scholar]
- 30.Ferkingstad E, Sulem R Atlason BA, et al. Large-scale integration of the plasma proteome with genetics and disease. Nat Genet 2021; [DOI] [PubMed] [Google Scholar]
- 31.Yang J, Brody EN, Murthy AC, et al. Impact of kidney function on the blood proteome and on protein cardiovascular risk biomarkers in patients with stable coronary heart disease. J Am Heart Assoc 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold L, Walker JJ, Wilcox SK, Williams S. Advances in human proteomics at high scale with the SOMAscan proteomics platform. N Biotechnol 2012; [DOI] [PubMed] [Google Scholar]
- 33.Yu LR, Sun J, Daniels JR, et al. Aptamer-Based Proteomics Identifies Mortality-Associated Serum Biomarkers in Dialysis-Dependent AKI Patients. Kidney Int Reports 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Charney AW, Simons NW, Mouskas K, et al. Sampling the host response to SARS-CoV-2 in hospitals under siege. Nat. Med. 2020; [DOI] [PubMed] [Google Scholar]
- 35.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med 2020; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron - Clin. Pract. 2012; [DOI] [PubMed] [Google Scholar]
- 37.Williams SA, Kivimaki M, Langenberg C, et al. Plasma protein patterns as comprehensive indicators of health. Nat Med 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim CH, Tworoger SS, Stampfer MJ, et al. Stability and reproducibility of proteomic profiles measured with an aptamer-based platform. Sci Rep 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ritchie ME, Phipson B, Wu D, et al. Limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 2015;43(7):e47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuznetsova A, Brockhoff PB, Christensen RHB. ImerTest Package: Tests in Linear Mixed Effects Models . J Stat Softw 2017; [Google Scholar]
- 41.Szklarczyk D, Gable AL, Lyon D, et al. STRING v11: Protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic Acids Res 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doncheva NT, Morris JH, Gorodkin J, Jensen LJ. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J Proteome Res 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nugent J, Aklilu A, Yamamoto Y, et al. Assessment of Acute Kidney Injury and Longitudinal Kidney Function after Hospital Discharge among Patients with and without COVID-19. JAMA Netw Open 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Basu RK, Wong HR, Krawczeski CD, et al. Combining functional and tubular damage biomarkers improves diagnostic precision for acute kidney injury after cardiac surgery. J Am Coll Cardiol 2014; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nickolas TL, Schmidt-Ott KM, Canetta P, et al. Diagnostic and prognostic stratification in the emergency department using urinary biomarkers of nephron damage: A multicenter prospective cohort study. J Am Coll Cardiol 2012; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du TY, Luo HM, Qin HC, et al. Circulating serum trefoil factor 3 (TFF3) is dramatically increased in chronic kidney disease. PLoS One 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Madsen J, Nielsen O, Tornøe I, Thim L, Holmskov U. Tissue localization of human trefoil factors 1, 2, and 3. J Histochem Cytochem 2007; [DOI] [PubMed] [Google Scholar]
- 48.Kjellev S. The trefoil factor family - Small peptides with multiple functionalities. Cell. Mol. Life Sci. 2009; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Astor BC, Köttgen A, Hwang SJ, Bhavsar N, Fox CS, Coresh J. Trefoil factor 3 predicts incident chronic kidney disease: A case-control study nested within the Atherosclerosis Risk in Communities (ARIC) study. Am J Nephrol 2011; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Z, Hu J, Liu L, et al. SARS-CoV-2 Causes Acute Kidney Injury by Directly Infecting Renal Tubules. Front Cell Dev Biol 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lin W, Fan J, Hu LF, et al. Single-cell analysis of angiotensin-converting enzyme II expression in human kidneys and bladders reveals a potential route of 2019 novel coronavirus infection. Chin Med J (Engl) 2021; [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The Data is available in Synapse syn35874390 and can also be accessed by contacting the senior author, Girish Nadkarni (girish.nadkarni@mountsinai.org).
Code is available at our GitHub repository Nadkarni-Lab: aki_covid_proteomics.


