Abstract
Resistance to nirmatrelvir (Paxlovid) has been shown by multiple groups and may already exist in clinical SARS-CoV-2 isolates. Here a panel of SARS-CoV-2 main protease (Mpro) variants and a robust cell-based assay are used to compare the resistance profiles of nirmatrelvir, ensitrelvir, and FB2001. The results reveal distinct resistance mechanisms (“fingerprints”) and indicate that these next-generation drugs have the potential to be effective against nirmatrelvir-resistant variants and vice versa.
Antiviral drugs are necessary to combat SARS-CoV-2/COVID-19, particularly with waning interest in the repeated vaccination boosts necessary to keep-up with virus evolution. The main protease (Mpro) of SARS-CoV-2 is essential for virus replication and, accordingly, a proven therapeutic target as evidenced by Paxlovid (active component: nirmatrelvir; Figure 1A). However, as for drugs developed to treat other viruses1 and for first-generation SARS-CoV-2 vaccines, there is a high probability that variants will emerge that resist nirmatrelvir. Indeed, a flurry of recent studies has described a variety of candidate nirmatrelvir-resistance mutations2-9. Thus, considerable urgency exists to develop next-generation Mpro inhibitors with different resistance mechanisms and, in parallel, robust systems to rapidly assess the potential impact of candidate resistance mutations.
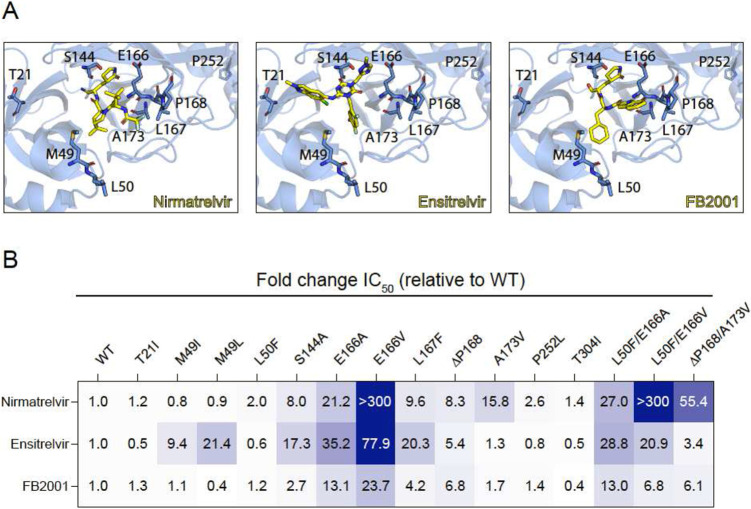
Figure 1. Resistance profiles of nirmatrelvir, ensitrelvir, and FB2001.
(A) Co-crystal structures of SARS-CoV-2 Mpro in complex with nirmatrelvir (PDB:7SI9), ensitrelvir (PDB:7VU6), or FB2001 (PDB:6LZE). Labeled residues are interrogated in panel B.
(B) Fold-change in IC50 relative to WT for the indicated mutants using the live cell gain-of-signal assay in 293T cells.
Ensitrelvir (Xocova) and FB2001 are being evaluated in clinical trials, and the former drug also recently received EUA in Japan10,11 (Figure 1A). We recently developed a gain-of-signal system for facile quantification of Mpro inhibition12, and subsequently used it together with an evolution- and structure-guided approach to characterize candidate nirmatrelvir- and ensitrelvir-resistance mutations2. Here, an expanded panel of Mpro single and double mutants based on recent studies by our group and others2-9 is leveraged to determine resistance profiles of these two drugs, as well as FB2001, a potential next-generation therapy (heatmap of results in Figure 1B; quantification summary in Table 1; representative dose responses in Figure S1).
Table 1. IC50 values of nirmatrelvir, ensitrelvir, and FB2001 against Mpro resistance variants.
Clear examples of single amino acid substitution mutations conferring selective resistance to nirmatrelvir and ensitrelvir are highlighted in gray; similar mutations have yet to be found for FB2001. The relative values in brackets are reflected in the heatmap in Figure 1B.
| Mpro variant |
IC50 [nM] (Fold-change relative to WT) | ||
|---|---|---|---|
| Nirmatrelvir | Ensitrelvir | FB2001 | |
| WT | 29.4 (1.0) | 35.9 (1.0) | 27.2 (1.0) |
| T21I | 36.0 (1.2) | 16.3 (0.5) | 34.0 (1.3) |
| M49I | 23.0 (0.8) | 338 (9.4) | 29.8 (1.1) |
| M49L | 27.1 (0.9) | 769 (21.4) | 10.7 (0.4) |
| L50F | 58.4 (2.0) | 21.0 (0.6) | 33.2 (1.2) |
| S144A | 236 (8.0) | 623 (17.3) | 74.7 (2.7) |
| E166A | 622 (21.2) | 126 (35.2) | 355 (13.1) |
| E166V | >10000 (>300) | 2800 (77.9) | 645 (23.7) |
| L167F | 282 (9.6) | 728 (20.3) | 115 (4.2) |
| ΔP168 | 243 (8.3) | 193 (5.4) | 184 (6.8) |
| A173V | 460 (15.8) | 45.9 (1.3) | 45.7 (1.7) |
| P252L | 76.9 (2.6) | 28.8 (0.8) | 38.9 (1.4) |
| T304I | 40.7 (1.4) | 19.0 (0.5) | 10.5 (0.4) |
| L50F/E166A | 793 (27) | 1040 (28.8) | 355 (13) |
| L50F/E166V | >10000 (>300) | 751 (20.9) | 185 (6.8) |
| ΔP168/A173V | 1630 (55.4) | 122 (3.4) | 166 (6.1) |
Several single amino acid substitution variants including T21I, L50F, P252L, and T304I show minimal resistance to nirmatrelvir, ensitrelvir, or FB2001. Selective resistance to ensitrelvir is conferred by M49I and M49L, whereas selective resistance to nirmatrelvir is caused by A173V (highlighted in gray in Table 1). ΔP168 elicits similar resistance to all inhibitors, and synergistic resistance to nirmatrelvir when combined with A173V. S144A and L167F show the greatest resistance to ensitrelvir, intermediate resistance to nirmatrelvir, and lower resistance toward FB2001. In contrast to E166A and L50F/E166A, which cause a similar broad-spectrum resistance, E166V and L50F/E166V elicit very high resistance to nirmatrelvir, intermediate resistance to ensitrelvir, and substantially lower resistance to FB2001.
In addition to providing a method to rapidly profile candidate resistance mutations in living cells, our gain-of-signal assay also provides a quantitative metric for Mpro functionality12 (Methods). This system is based on the fact that overexpression of wildtype SARS-CoV-2 Mpro results in the cleavage of multiple substrates in cells13,14 including at least one required for RNA Polymerase II-dependent gene expression12. Therefore, expression of the Src-Mpro-Tat-Luc reporter itself is rapidly shut down following transfection and can only be recovered by chemical or genetic inhibition of Mpro. Thus, genetic mutations effectively phenocopy the chemical dose-responsiveness of the system, with some variations showing wildtype Mpro activity (background luminescence) and others compromising activity weakly or strongly depending on the nature of the mutation (low to high luminescence). For example, in comparison to wildtype Mpro, catalytic mutants such as C145A yields 50- to 100-fold higher luminescence2,12. The Mpro variant constructs used here display a range of luminescence levels in the absence of drug indicative of near-normal Mpro activity (notably, M49I and M49L), weakly compromised Mpro activity (notably, A173V), and strongly compromised Mpro activity (notably, E166V) (Figure S2). These results suggest that several variants can confer at least partial drug resistance with little loss in Mpro functionality (and accordingly high viral fitness), whereas others such as E166V require suppressor mutations such as L50F to restore Mpro function to a level that enables virus replication (evidenced by recent resistance studies with pathogenic SARS-CoV-2 in culture and in vivo in animal models3,5).
Regardless of the details of each molecular mechanism, the results here demonstrate that nirmatrelvir, ensitrelvir, and FB2001 have distinct resistance profiles and that the latter inhibitors (with appropriate formulations) may be effective in patients suffering from Paxlovid rebound15 or bona fide resistance2. FB2001 may additionally have a higher resistance barrier given that no fully functional single Mpro variants tested to-date confer a strong resistance to this compound. Importantly, the gain-of-signal live cell assay recapitulates recent findings using replication competent viruses and provides a safe and rapid method for assessing resistance. As the SARS-CoV-2 variant pool deepens, this assay and variant panel can be expanded in lock-step to provide early resistance “fingerprints” of candidate next-generation Mpro inhibitors. Such an early profiling strategy has the potential to minimize the risks of developing drugs prone to cross-resistance and, importantly, to help identify inhibitors with the highest barriers to resistance.
Supplementary Material
Acknowledgments
This work was supported by National Institute of Allergy and Infectious Disease grant U19-AI171954. RSH is an Investigator of the Howard Hughes Medical Institute, a CPRIT Scholar, and the Ewing Halsell President’s Council Distinguished Chair at University of Texas Health San Antonio.
Footnotes
Additional Declarations: There is a conflict of interest Competing Interests The Mpro gain-of-signal system is the subject of U.S. Provisional Application Serial No. 63/108,611, filed on November 2, 2020, with RSH and SAM as inventors. The authors declare that there are no additional competing interests.
References
- 1.Majerova T. & Konvalinka J. Viral proteases as therapeutic targets. Mol Aspects Med 88, 101159 (2022). 10.1016/j.mam.2022.101159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moghadasi S. A. et al. Transmissible SARS-CoV-2 variants with resistance to clinical protease inhibitors. Sci Adv in press (2023). 10.1101/2022.08.07.503099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou Y. et al. Nirmatrelvir-resistant SARS-CoV-2 variants with high fitness in an infectious cell culture system. Sci Adv 8, eadd7197 (2022). 10.1126/sciadv.add7197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iketani S. et al. Functional map of SARS-CoV-2 3CL protease reveals tolerant and immutable sites. Cell Host Microbe 30, 1354–1362 e1356 (2022). 10.1016/j.chom.2022.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iketani S. et al. Multiple pathways for SARS-CoV-2 resistance to nirmatrelvir. Nature 613, 558–564 (2023). 10.1038/s41586-022-05514-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jochmans D. et al. The substitutions L50F, E166A, and L167F in SARS-CoV-2 3CLpro are selected by a protease inhibitor in vitro and confer resistance to nirmatrelvir. mBio, e0281522 (2023). 10.1128/mbio.02815-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heilmann E. et al. SARS-CoV-2 3CL(pro) mutations selected in a VSV-based system confer resistance to nirmatrelvir, ensitrelvir, and GC376. Sci Transl Med 15, eabq7360 (2023). 10.1126/scitranslmed.abq7360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Noske G. D. et al. Structural basis of nirmatrelvir and ensitrelvir activity against naturally occurring polymorphisms of the SARS-CoV-2 Main Protease. J Biol Chem, 103004 (2023). 10.1016/j.jbc.2023.103004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan S. et al. Nirmatrelvir resistance in SARS-CoV-2 Omicron_BA.1 and WA1 replicons and escape strategies. BioRxiv doi: 10.1101/2022.12.31.522389 (2023). [DOI] [Google Scholar]
- 10.Dai W. et al. Structure-based design of antiviral drug candidates targeting the SARS-CoV-2 main protease. Science 368, 1331–1335 (2020). 10.1126/science.abb4489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Unoh Y. et al. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. Journal of medicinal chemistry 65, 6499–6512 (2022). 10.1021/acs.jmedchem.2c00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moghadasi S. A. et al. Gain-of-signal assays for probing inhibition of SARS-CoV-2 M(pro)/3CL(pro) in living cells. mBio 13, e0078422 (2022). 10.1128/mbio.00784-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyers J. M. et al. The proximal proteome of 17 SARS-CoV-2 proteins links to disrupted antiviral signaling and host translation. PLoS Pathog 17, e1009412 (2021). 10.1371/journal.ppat.1009412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon D. E. et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468 (2020). 10.1038/s41586-020-2286-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson A. S., Caubel P., Rusnak J. M. & Investigators, E.-H. T. Nirmatrelvir-ritonavir and viral load rebound in Covid-19. N Engl J Med 387, 1047–1049 (2022). 10.1056/NEJMc2205944 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.