Abstract
We investigated the relationship between neighborhood disadvantage (area deprivation index [ADI]) and intracortical myelination (T1-weighted/T2-weighted ratio at deep to superficial cortical levels), and the potential mediating role of the body mass index (BMI) and perceived stress in 92 adults. Worse ADI was correlated with increased BMI and perceived stress (p's<.05). Non-rotated partial least squares analysis revealed associations between worse ADI and decreased myelination in middle/deep cortex in supramarginal, temporal, and primary motor regions and increased myelination in superficial cortex in medial prefrontal and cingulate regions (p<.001); thus, neighborhood disadvantage may influence the flexibility of information processing involved in reward, emotion regulation, and cognition. Structural equation modelling revealed increased BMI as partially mediating the relationship between worse ADI and observed myelination increases (p=.02). Further, trans-fatty acid intake was correlated with observed myelination increases (p=.03), suggesting the importance of dietary quality. These data further suggest ramifications of neighborhood disadvantage on brain health.
Introduction
Living in a disadvantaged neighborhood (area deprivation) is linked to worse health outcomes, including poor brain health1. Disadvantaged neighborhoods can be stressful, and which can then alter brain structure and function, including decreased brain volume2. The key mechanisms that underlying the link between neighborhood conditions and brain health remain unclear, but one possible pathway might be through obesity. People who live in disadvantaged neighborhoods are at higher risk of obesity due to the poor quality of available foods and environments that hamper physical activity3, 4, 5. In particular, neighborhood disadvantage is associated with an increased intake of calories from trans-fatty acids (TFAs) and sodium6. TFAs (high in fried fast food) are known to contribute to obesity, especially abdominal obesity7, 8. Additionally, chronic neighborhood stressors (allostatic load) can impact eating behaviors9, 10, increasing desire for highly palatable, but unhealthy foods used as a coping response11, 12. Numerous neuroimaging studies have demonstrated that stress can alter brain structure and function, leading to food cravings, contributing to an increased risk for obesity13, 14, 15. Further, high body mass index (BMI) has been shown to mediate the impact of living in a disadvantaged neighborhood on reduced brain volume, suggesting its importance in the negative impact of neighborhood disadvantage on brain health16.
Neighborhood disadvantage, high BMI, and chronic stress have also been shown to impact intracortical myelination17, 18, 19. Intracortical myelination, which refers to the myelination of axons in the cortical gray matter, affects the timing and integration of signals from multiple axons, and is critical for neural synchrony and fine-tuning of cortical circuits, affecting cognitive functioning20, 21, 22. Additionally, intracortical myelination is actively involved in brain remodeling and plasticity throughout the lifespan23, 24. Myelinated components vary by cortical layer, with a large fraction of intracortical myelin ensheathing the axons of inhibitory internerons in upper cortical layers (layers 1-3); specifically, parvalbumin-positive basket cells, which play an important role in gamma network oscillations that support a variety of cognitive processes25. In deeper cortical layers, myelin predominantly ensheaths non-gamma-aminobutyric acid (non-GABA) axons, presumably from long-distance excitatory pyramidal neurons, which integrate thousands of synaptic inputs and act on targets in other cortical regions and subcerebral structures26, 27, 28. Further, cortical layers vary in terms of specific inputs and outputs and information processing functions (e.g., subcortical vs. intercortical input, feedback vs. forward processes)29. Accordingly, examining intracortical myelination at different cortical layers can inform how alterations in cell populations, processes, and communication routes may be affected by adverse or stressful environments, such as living in a disadvantaged neighborhood.
We, therefore, investigated the relationship between the area deprivation index (ADI) and intracortical myelination at multiple cortical levels, as well as the role of potential mediators, including BMI and stress (Graphical Abstract). In addition, we investigated the relationship between TFA intake and intracortical myelination in a subset of participants with diet data. We hypothesized that worse ADI would be associated with higher BMI and stress levels, with negative effects on intracortical myelination in reward-related, emotion regulation, and cognitive regions, related to poor dietary quality characterized by high TFA intake.
Results
Sample characteristics
Participant characteristics, including TFA intake, are summarized in Table 1. In this sample, ADI was positively correlated with BMI (r=0.27, p=0.01) and the Perceived Stress Scale (PSS) score (r=0.22, p=0.04), but BMI and PSS were not correlated (r=0.04, p=0.73).
Table 1.
Participant characteristics
| Variable | N (%) or Mean±SD |
|---|---|
| Male | 27 (29.3%) |
| Age (yrs) | 28.0±10.3 |
| Hispanic (%) | 37 (40.2%) |
| Race (%) | |
| Asian | 34 (37.0%) |
| Black | 5 (5.4%) |
| White | 28 (30.4%) |
| Mixed | 11 (112.0%) |
| Other | 19 (20.7%) |
| Income (%) | |
| <20k | 8 (8.7%) |
| 20k-39k | 14 (15.2%) |
| 40k-59k | 15 (16.3%) |
| 60k-79k | 17 (18.5%) |
| 80k + | 32 (34.8%) |
| Body mass index (kg/m2) | 24.9±4.7 |
| Perceived Stress Scale score | 15.05±7.5 |
| National ADI | 11.4±10.6 |
| California ADI | 4.0±2.4 |
| Trans-fatty acid intake (unit?) | |
| trans-hexadecenoic acid | .03±.02 |
| trans-octadecenoic (elaidic) acid | 1.38±.80 |
| trans-octadecadienoic (linolelaidic) acid | .24±.14 |
| Total | 1.65±.93 |
Data are mean±standard deviation, unless otherwise indicated.
ADI, area deprivation index
Regions showing a relationship between ADI and intracortical myelination
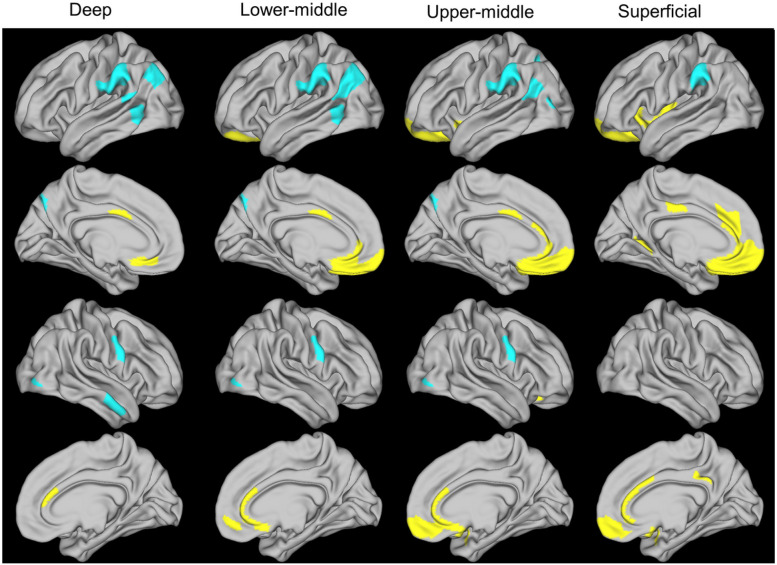
Non-rotated partial least squares correlational (PLSC) analysis revealed worse ADI as associated with increased myelination in medial prefrontal and cingulate regions (hereupon referred to as ADI-positive regions), involved in reward-related processing, emotional regulation, and higher cognition30, 31, 32, and decreased myelination in supramarginal, middle temporal, and primary motor regions (hereupon referred to as ADI-negative regions), components of the extended mirror system involved in social interaction (p<0.001) (Figure 1)33, 34, 35. Regions with a positive association were more extensive at middle/superficial cortical levels, while those with a negative association were more extensive at middle/deep cortical levels. Intracortical myelination was averaged across all ADI-positive regions, and across all ADI-positive regions, for further analysis.
Figure 1.
Partial least squares analysis of the relationship between worse ADI and intracortical myelination at 4 cortical ribbon levels
The 4 cortical ribbon levels were as follows: deep, 5%-25% of cortical thickness from white-pial boundary; lower-middle, 30%-50%; upper-middle, 55%-75%, superficial, 80%-100%.
Worse ADI was associated with increased myelination (yellow) in medial prefrontal and cingulate regions, and decreased myelination (blue) in supramarginal, middle temporal, and primary motor regions (p<.001). ADI, area deprivation index
Mediation of the relationship between ADI and intracortical myelination
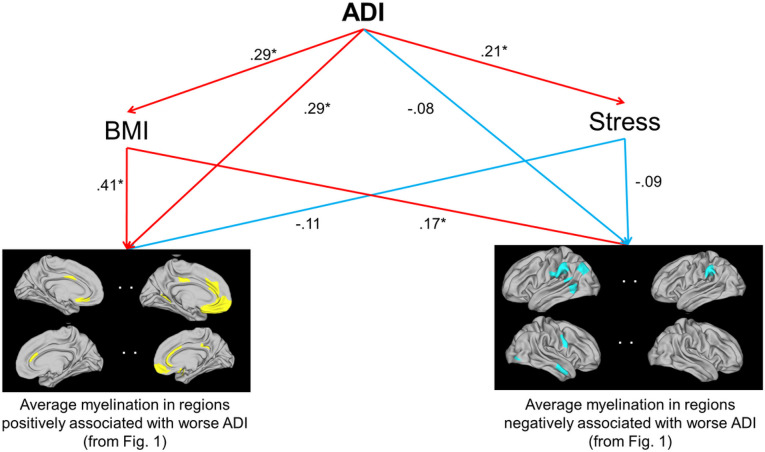
The final model in the structural equation modelling (SEM) analysis is shown in Figure 2; for simplicity, sex and age are not shown in the model, but were used as control variables. The model had a high chi-squared p-value (χ2(2)=0.025, p=0.988), comparative fit index of 1.0, and standardized root mean square residual of 0.002, indicating good model fit. BMI had significant positive direct effects on both brain variables (ADI-positive regions: p<0.001; ADI-positive regions: p=0.03). Additionally, BMI partially mediated the relationship between ADI and the average intracortical myelination in ADI-positive regions, but not ADI-negative regions (indirect effect of ADI on ADI-positive regions via BMI: p=0.02; on ADI-negative regions: p=0.09). Although stress (PSS score) had a negative direct effect on both brain variables, statistical significance was not reached (ADI-positive regions: p=0.33; ADI-positive regions: p=0.31). Stress also failed to reach significance as a mediator of the relationship between ADI and the average intracortical myelination in these regions (indirect effect of ADI on ADI-positive regions via stress: p=0.36; on ADI-negative regions: p=0.32).
Figure 2. Structural equation modeling analysis of the mediation of the relationships between worse ADI and intracortical myelination.
BMI partially mediated the relationship between ADI and intracortical myelination in regions positively associated with worse ADI. *p<.05; ADI, area deprivation index; BMI, body mass index
Relationship between TFA intake and intracortical myelination
Correlations between TFA intake and intracortical myelination are shown in Table 2. Significant positive correlations were observed between the average intracortical myelination in ADI-positive regions and trans-octadecenoic (elaidic) acid and total TFA intake (p’s<0.05). No significant correlations were observed for the average intracortical myelination in ADI-positive regions.
Table 2.
Correlations between TFA Intake and intracortical myelination, controlling for sex and age
| trans- hexadecenoic acid |
trans-octadecenoic (elaidic) acid |
trans-octadecadienoic (linolelaidic) acid |
Total TFA |
|
|---|---|---|---|---|
| Regions positively associated with ADI | .10 | .29 * | .18 | .28 * |
| Regions negatively associated with ADI | .09 | .20 | .12 | .19 |
| BMI | .24* | .27* | .24* | .27* |
ADI, area deprivation index; BMI, body mass index; TFA, tans-fatty acid
p<.05
Discussion
We examined the relationship between ADI and myelin content, as assessed by the T1-weighted/T2-weighted (T1w/T2w) ratio, at different levels of the cortical ribbon, as well as potential mediation by factors associated with ADI, namely, BMI and stress. We found that ADI was associated with increased myelination in medial prefrontal and cingulate cortices at more superficial levels (we refer to these regions as ADI-positive regions); these regions are involved in reward-related, emotion regulation, and higher cognitive processes30, 31, 32. This association was partially mediated by increased BMI. We also found ADI associated with decreased myelination in supramarginal, middle temporal, and primary motor cortices at deeper levels (we refer to these regions as ADI-negative regions); these regions are components of the mirror neuron system, involved in social interaction33, 34, 35. BMI did not appear to mediate this relationship. Further, perceived stress was associated with ADI but not myelination. Cortical layers differ in the type of axons myelinated and information processing functions, with superficial cortex receiving top-down information that can modulate feed-forward and feed-back processes in deeper layers26, 27, 28, 29. Thus, our findings provide new insights as to the nature of affected information processing pathways under worse ADI, as discussed below.
ADI and increased superficial intracortical myelination
In the present study, worse ADI was associated with increased myelination signal in more superficial cortex in medial prefrontal and cingulate regions. These results are largely similar to a previous developmental study on the relationship between disadvantage due to low socioeconomic status (SES) and intracortical myelination as assessed by the T1w/T2w ratio. This previous study found that lower parental SES was associated with increased intracortical myelination in frontal, temporal, medial parietal, and occipital regions in children and adolescents36. Thus, disadvantage due to ADI or individual SES may be associated with increased intracortical myelination in overlapping regions.
The maturation of intracortical myelination is protracted in humans, especially in prefrontal regions, peaking at 30-45 years of age depending on the brain region37. Accordingly, intracortical myelination within a normative range may be needed for optimal function, with both reduced and excessive myelination being problematic38. Consistent with the latter, animal studies have found that under conditions of excessive myelination (i.e., beyond axonal demand) mistargeting to cell bodies occurs readily39. Thus, our finding that worse ADI and increased BMI are associated with increased myelination in more superficial cortex in medial prefrontal and cingulate regions may imply excessive and disorganized myelination in the upper layers of the cortex. Superficial cortex receives top-down information from subcortical and cortical regions and is thought to enable flexible and state-dependent processing of feedforward sensory input arriving in deeper layers29, 40. One may speculate that myelination mistargeting in superficial cortex could negatively influence the context and flexibility of information processing in affected regions, which in the present study, comprised the prefrontal and cingulate cortices. Given the involvement of these regions in reward-related processing, emotional regulation, and higher cognition, this interpretation is similar to previous behavioral studies showing an impact of neighborhood disadvantage on these functions throughout the lifespan41, 42, 43, 44.
However, in contrast with the current results, studies using markers of intracortical myelination other than the T1w/T2w ratio have mainly found reductions associated with neighborhood or socioeconomic disadvantage. For example, a study using magnetization transfer as a myelin-sensitive marker, found that living in a disadvantaged neighborhood before the age of 12 years was associated with slower myelin growth in adolescents and young adults in sensorimotor, cingulate, and prefrontal cortices18. Another study using magnetization transfer found that SES in adulthood was associated with decreased entorhinal cortical myelination45. Although the T1w/T2w ratio is sensitive to intracortical myelin content, accumulating evidence suggests that it is not a straightforward proxy for intracortical myelin46, 47. Given our finding that increased intracortical myelination in more superficial, but not deeper, cortical levels of prefrontal and cingulate regions was mediated by BMI, we entertained an alternative explanation of our results. We considered it possible that the T1w/T2w signal at the upper BMI range was affected by a fatty acid-rich cortical environment, with lipid droplet accumulation and lipid-laden astrocytes, due to blood-brain barrier disruption and increased transport of fatty acids under obese conditions48, 49, 50. In support of this, we found that total TFA intake, largely driven by trans-octadecenoic (elaidic) acid intake, was correlated with increased superficial cortical myelination in ADI-positive regions. Although industrial TFAs such as partially hydrogenated oil have been banned in the United States because of health concerns (effective 2020), the process of repeatedly cooking oil at high temperatures can cause high levels of TFAs in fried fast foods51. Additionally, some meat and dairy products naturally contain small amounts of TFA52. Higher intake of TFA, including elaidic acid, is associated with an increased risk of dementia53, 54. TFAs can be incorporated into cell membranes, including myelin55. Poor-quality diet, such as a diet high in fried fast foods, is thought to be one of the factors of worse ADI that contributes to obesity and worse health outcomes3, 4, 5, 56. Thus, our results could suggest that a diet high in TFA under worse ADI may create a fatty acid-rich environment in superficial cortical layers, become incorporated into cell membranes and disrupt information processing in affected regions.
ADi and decreased intracortical myelination
We also found that worse ADI was associated with decreased myelination in middle/deep cortex in supramarginal, middle temporal, and primary motor regions. These regions are components of the mirror neuron system, involved in understanding the actions of others and in interpersonal coordination in activities (e.g. imitation, cooperation)33, 34, 35. As middle/deep levels were involved, feed-forward and feed-back processes and intercortical and subcortical-cortical communication in these regions may be affected with worse ADI29. Animal studies suggest that myelin plasticity is a major component in the response to stress57. In addition, a previous study using the T1w/T2w ratio as a measure of intracortical myelination found that higher perceived stress was associated with lower intracortical myelination in the right supramarginal gyrus19. However, in the present study, perceived stress was not significantly associated with decreased myelination in ADI-negative regions, which included the left supramarginal gyrus. Thus, mediation of the association between worse ADI and decreased intracortical myelination in these regions remains unclear.
Limitations
The present study has several limitations. Although the T1w/T2w ratio is sensitive to myelin, it may not be a straightforward proxy, as it does not correlate well with myelin-related gene expression and other measures58. Confirmation studies using other acquisition protocols sensitive to intracortical myelin are needed. Additionally, we assessed current ADI at one point in time; we did not have information regarding the length of residence, nor did we have historical data on ADI in younger ages. A previous study found that, although both child and adult SES showed correlations with intracortical myelination, childhood SES showed robust associations even after controlling for adult SES, suggesting a lasting imprint, which may also hold for neighborhood-level factors such as ADI42, 45.
Conclusions
We found that worse ADI was associated with decreased myelination in middle/deep cortex in supramarginal, middle temporal, and primary motor regions, potentially impacting intercortical and subcortical-cortical communication of the mirror neuron system, important for understanding the actions of others and cooperative behavior. ADI was also associated with increased myelination in the superficial cortex in medial prefrontal and cingulate regions, which was partially mediated by increased BMI. Further, this increased myelination was positively correlated with TFA intake. Thus, obesogenic features of neighborhood disadvantage may disrupt the flexibility of information processing involved in reward, emotion regulation, and cognition. These results provide new information regarding the ramifications of living in a disadvantaged neighborhood on brain health. Further research on the mediating factors involved in the impact of ADI on the brain during development and adulthood is needed.
Methods
Participants
Participants comprised 92 adults (27 men; 65 women) recruited from the Los Angeles area who completed a neuroimaging session including both T1-weighted (T1w) and T2-weighted (T2w) scans (enabling the calculation of intracortical myelination) and provided residential address information. Exclusion criteria were as follows: major neurological condition, current or past psychiatric illness, vascular disease, weight loss/abdominal surgery, substance use disorder, use of medications that interfere with the central nervous system, pregnant or breastfeeding, strenuous exercise regimen (> 8 h/week of continuous exercise), weight > 400 pounds, or metal implants. In addition, individuals with poor quality images were excluded. Image quality was evaluated using Qi1, which reflects the proportion of voxels with intensity corrupted by artifacts normalized by the number of voxels in the background, from the MRI Quality Control tool (MRIQC)30. Qi1 > 0.00506 for T1w images and > 0.0030 for T2w images was considered to indicate poor image quality31.
All procedures were approved by the Institutional Review Board at the University of California, Los Angeles’s Office of Protection for Research Subjects (Nos. 16–000187, 15-001591). All participants provided written informed consent.
Assessments
Basic demographic data, as well as weight and height, were collected. BMI was calculated as weight divided by the square of the height (kg/m2). ADI was originally developed by the Health Resources and Services Administration several decades ago and is updated periodically. We used the 2020 ADI in the Neighborhood Atlas®32, based on the residential address provided by the participant. This atlas ranks census block groups, which are considered as similar to neighborhoods, on 17 neighborhood-level measures reflecting income, education, employment, and housing quality, within state and nationally. We employed the California State ADI, which is provided in deciles (scores range 1–10), with higher values indicating greater deprivation/disadvantage.
Participants also completed the Perceived Stress Scale (PSS), which is a 10-item questionnaire that assesses feelings of stress during the prior month33. Scores range from 0 to 40, with higher scores indicating greater stress. Diet information was collected using the VioScreen Graphical Food Frequency System (Viocare Technologies, Inc., Princeton, NJ), and was available in a subset of participants (N = 81) as 11 participants did not provide this information. The VioScreen System provides information on nutrient intake, including fatty acid intake. We focused on the intake of individual TFAs (trans-hexadecenoic acid, trans-octadecenoic acid [elaidic acid], and trans-octadecadienoic acid [linolelaidic acid]), as well as the total TFA intake. TFA intake was measured as a component of a poor-quality diet known to contribute to obesity, especially abdominal obesity, and have harmful effects on brain cell membranes, including myelin7, 8, 34.
Imaging acquisition and preprocessing
T1w and T2w structural images were obtained for the non-invasive assessment of intracortical myelination using a 3.0T Siemens Prisma MRI scanner (Siemens, Erlangen, Germany). Spin echo fieldmaps were also acquired in anterior-posterior and posterior-anterior directions for distortion correction. The acquisition parameters for high-resolution T1w images were as follows: echo time, 1.81 ms; repetition time, 2500 ms; slice thickness, 0.8 mm; number of slices, 208; voxel matrix, 320×300; and voxel size, 1.0×1.0×0.8 mm. The parameters for the T2w images were as follows: echo time, 564 ms; repetition time, 3200 ms; slice thickness, .8 mm; number of slices, 208; voxel matrix, 320×300; and voxel size, 1.0×1.0×0.8 mm.
Imaging data were preprocessed using Human Connectome Project (HCP) pipelines, including volume segmentation and cortical surface reconstruction with FreeSurfer 6.035, 36. Intracortical myelination was estimated by the T1w/T2w ratio at multiple levels within the cortical ribbon37, 38. Specifically, the T1w/T2w ratio was calculated at 5% increments in cortical thickness from the gray-white boundary to the gray-cerebrospinal fluid boundary and averaged within 4 cortical ribbon levels as follows: 5%-25% (deep cortex), 30%-50% (lower-middle cortex), 55%-75% (upper-middle cortex), and 80%-100% (superficial cortex). The 4 resulting myelin maps were parcellated using the HCP multi-modal parcellation 1.0 atlas, resulting in 360 cortical regions with 4 intracortical myelination estimates each39.
Statistical analysis
Partial correlation coefficients, controlling for sex and age, were calculated to determine individual factors associated with worse ADI using SPSS version 28 (IBM Crop., Albany, NY, USA), with bootstrapping (5000 samples). P-values < .05 were considered statistically significant.
Non-rotated partial least squares correlational (PLSC) analysis was applied to identify regions correlated with ADI according to cortical level, using freely available code (http://www.rotman-baycrest.on.ca/pls)40. PLSC is a multivariate analytical technique that identifies weighted patterns of variables in two blocks of variables that maximally covary with each other40, 41. In this study, one block comprised demographic variables (ADI, age, sex) and one block comprised parcellated intracortical myelin values (at a specific cortical level). Weights were preset to identify brain regions sensitive to ADI but not sex or age. Reliability of identified regions was assessed using bootstrap estimation (5000 samples); regions with a bootstrap ratio > 3.1 (p < .001) were considered significant. Myelin data was extracted from significant brain clusters to calculate an average of all regions/levels with a positive relationship with ADI and an average of all regions/levels with a negative relationship with ADI, for further analysis.
Structural equation modeling (SEM) was applied to investigate the mediation of relationships between ADI and significant findings from the PLSC analysis. SEM was performed in R Studio using the lavaan package42. Input variables comprised ADI, BMI, PSS score, average myelination in ADI-positive regions, and average myelination in ADI-negative regions, as well as sex and age as control variables. Data were standardized prior to fitting the model. Missing values were estimated using the maximum likelihood (4 participants had missing PSS scores). Model fit was assessed using the chi-squared p-value, comparative fit index, and standardized root mean square residual. Model paths with a p-value < .05 were considered significant.
Acknowledgements:
We would like to acknowledge the assistance of the Neuroimaging Core, Bioinformatics and Statistics Core, Microbiome Core, and the Biorepository Core of the UCLA Microbiome Center for their assistance with various processing, storage, and analytic assistance in the current study. We also thank the participants for their time and beautiful brains.
Funding:
This research was supported by grants from the National Institutes of Health including R01 MD015904 (AG), K23 DK106528 (AG), R03 DK121025(AG), T32 DK07180 (TD), ULTR001881/DK041301 (UCLA CURE/CTSI Pilot and Feasibility Study (AG), R01 DK048351 (EAM), P30 DK041301; and pilot funds provided for brain scanning by the Ahmanson-Lovelace Brain Mapping Center. These funders played no role in study design, or the collection, analysis, and interpretation of the data.
Footnotes
Competing Interests: AG is a scientific consultant to Yamaha. All other authors have nothing to disclose.
Data Availability:
The datasets generated during and/or analyzed during the current study are not publicly available due to an ongoing collaboration with multiple principal investigators involving participant identifiers at the G. Oppenheimer Center for Neurobiology of Stress and Resilience. However, data is available from the corresponding author on reasonable request.
References
- 1.Kim P, Evans GW, Chen E, Miller G, Seeman T. How Socioeconomic Disadvantages Get Under the Skin and into the Brain to Influence Health Development Across the Lifespan. In: Handbook of Life Course Health Development(eds Halfon N, Forrest CB, Lerner RM, Faustman EM) (2018). [PubMed] [Google Scholar]
- 2.Hunt JFV, et al. Association of Neighborhood-Level Disadvantage With Cerebral and Hippocampal Volume. JAMA Neurol 77, 451–460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ludwig J, et al. Neighborhoods, obesity, and diabetes--a randomized social experiment. N Engl J Med 365, 1509–1519(2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zick CD, Smith KR, Fan JX, Brown BB, Yamada I, Kowaleski-Jones L. Running to the store? The relationship between neighborhood environments and the risk of obesity. Soc Sci Med 69, 1493–1500 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saelens BE, et al. Obesogenic neighborhood environments, child and parent obesity: the Neighborhood Impact on Kids study. Am J Prev Med 42, e57–64 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Keita AD, Casazza K, Thomas O, Fernandez JR. Neighborhood-level disadvantage is associated with reduced dietary quality in children. J Am Diet Assoc 109, 1612–1616 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dhaka V, Gulia N, Ahlawat KS, Khatkar BS. Trans fats-sources, health risks and alternative approach - A review. J Food Sci Technol 48, 534–541 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kavanagh K, et al. Trans fat diet induces abdominal obesity and changes in insulin sensitivity in monkeys. Obesity (Silver Spring) 15, 1675–1684 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Steptoe A, Feldman PJ. Neighborhood problems as sources of chronic stress: development of a measure of neighborhood problems, and associations with socioeconomic status and health. Ann Behav Med 23, 177–185 (2001). [DOI] [PubMed] [Google Scholar]
- 10.Karb RA, Elliott MR, Dowd JB, Morenoff JD. Neighborhood-level stressors, social support, and diurnal patterns of cortisol: the Chicago Community Adult Health Study. Soc Sci Med 75, 1038–1047 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sinha R. Role of addiction and stress neurobiology on food intake and obesity. Biological psychology 131, 5–13(2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y, Cubbin C, Oh S. A systematic review of neighbourhood economic context on child obesity and obesity-related behaviours. Obesity reviews : an official journal of the International Association for the Study of Obesity 20, 420–431 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudenga K, Sinha R, Small D. Acute stress potentiates brain response to milkshake as a function of body weight and chronic stress. International journal of obesity 37, 309–316 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tryon MS, Carter CS, DeCant R, Laugero KD. Chronic stress exposure may affect the brain's response to high calorie food cues and predispose to obesogenic eating habits. Physiology & behavior 120, 233–242 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Maier SU, Makwana AB, Hare TA. Acute stress impairs self-control in goal-directed choice by altering multiple functional connections within the brain’s decision circuits. Neuron 87, 621–631 (2015). [DOI] [PubMed] [Google Scholar]
- 16.Hall PA, Best J, Beaton EA, Sakib MN, Danckert J. Morphology of the Prefrontal Cortex Predicts Body Composition in Early Adolescence: Cognitive Mediators and Environmental Moderators in the ABCD Study. Soc Cogn Affect Neurosci, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong D, et al. The Association between Body Mass Index and Intra-Cortical Myelin: Findings from the Human Connectome Project. Nutrients 13, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ziegler G, et al. Childhood socio-economic disadvantage predicts reduced myelin growth across adolescence and young adulthood. Hum Brain Mapp 41, 3392–3402 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo Y, et al. Stress and the brain: Emotional support mediates the association between myelination in the right supramarginal gyrus and perceived chronic stress. Neurobiol Stress 22, 100511 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt BA, et al. Relationships between cortical myeloarchitecture and electrophysiological networks. Proc Natl Acad Sci U S A 113, 13510–13515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vandewouw MM, Hunt BAE, Ziolkowski J, Taylor MJ. The developing relations between networks of cortical myelin and neurophysiological connectivity. Neuroimage 237, 118142 (2021). [DOI] [PubMed] [Google Scholar]
- 22.Grydeland H, Walhovd KB, Tamnes CK, Westlye LT, Fjell AM. Intracortical myelin links with performance variability across the human lifespan: results from T1- and T2-weighted MRI myelin mapping and diffusion tensor imaging. J Neurosci 33, 18618–18630 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fields RD. A new mechanism of nervous system plasticity: activity-dependent myelination. Nat Rev Neurosci 16, 756–767 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang SM, Michel K, Jokhi V, Nedivi E, Arlotta P. Neuron class-specific responses govern adaptive myelin remodeling in the neocortex. Science 370, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kriener B, Hu H, Vervaeke K. Parvalbumin interneuron dendrites enhance gamma oscillations. Cell Rep 39, 110948 (2022). [DOI] [PubMed] [Google Scholar]
- 26.Micheva KD, et al. A large fraction of neocortical myelin ensheathes axons of local inhibitory neurons. Elife 5, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Micheva KD, et al. Distinctive Structural and Molecular Features of Myelinated Inhibitory Axons in Human Neocortex. eNeuro 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stedehouder J, et al. Fast-spiking Parvalbumin Interneurons are Frequently Myelinated in the Cerebral Cortex of Mice and Humans. Cereb Cortex 27, 5001–5013 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Larkum ME, Petro LS, Sachdev RNS, Muckli L. A Perspective on Cortical Layering and Layer-Spanning Neuronal Elements. Front Neuroanat 12, 56 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rogers RD, et al. Distinct portions of anterior cingulate cortex and medial prefrontal cortex are activated by reward processing in separable phases of decision-making cognition. Biol Psychiatry 55, 594–602 (2004). [DOI] [PubMed] [Google Scholar]
- 31.Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15, 85–93 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jobson DD, Hase Y, Clarkson AN, Kalaria RN. The role of the medial prefrontal cortex in cognition, ageing and dementia. Brain Commun 3, fcab125 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pineda JA. Sensorimotor cortex as a critical component of an 'extended' mirror neuron system: Does it solve the development, correspondence, and control problems in mirroring? Behav Brain Funct 4, 47 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endedijk HM, Meyer M, Bekkering H, Cillessen AHN, Hunnius S. Neural mirroring and social interaction: Motor system involvement during action observation relates to early peer cooperation. Dev Cogn Neurosci 24, 33–41 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Molenberghs R, Brander C, Mattingley JB, Cunnington R. The role of the superior temporal sulcus and the mirror neuron system in imitation. Hum Brain Mapp 31, 1316–1326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Norbom LB, et al. Parental socioeconomic status is linked to cortical microstructure and language abilities in children and adolescents. Dev Cogn Neurosci 56, 101132 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grydeland H, et al. Waves of Maturation and Senescence in Micro-structural MRI Markers of Human Cortical Myelination over the Lifespan. Cereb Cortex 29, 1369–1381 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snaidero N, Simons M. The logistics of myelin biogenesis in the central nervous system. Glia 65, 1021–1031 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Almeida RG, et al. Myelination of Neuronal Cell Bodies when Myelin Supply Exceeds Axonal Demand. Curr Bioi 28, 1296–1305 e1295 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.D'Souza RD, Burkhalter A. A Laminar Organization for Selective Cortico-Cortical Communication. Front Neuroanat 11, 71 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vargas T, Damme KSF, Mittal VA. Neighborhood deprivation, prefrontal morphology and neurocognition in late childhood to early adolescence. Neuroimage 220, 117086 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gonzalez MZ, Allen JR, Coan JA. Lower neighborhood quality in adolescence predicts higher mesolimbic sensitivity to reward anticipation in adulthood. Dev Cogn Neurosci 22, 48–57 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackman DA, et al. Neighborhood environments influence emotion and physiological reactivity. Sci Rep 9, 9498 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Settels J, Leist AK. Changes in neighborhood-level socioeconomic disadvantage and older Americans' cognitive functioning. Health Place 68, 102510 (2021). [DOI] [PubMed] [Google Scholar]
- 45.Loued-Khenissi L, et al. Signatures of life course socioeconomic conditions in brain anatomy. Hum Brain Mapp 43, 2582–2606 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uddin MN, Figley TD, Solar KG, Shatil AS, Figley CR. Comparisons between multi-component myelin water fraction, T1w/T2w ratio, and diffusion tensor imaging measures in healthy human brain structures. Sci Rep 9, 2500 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hagiwara A, et al. Myelin Measurement: Comparison Between Simultaneous Tissue Relaxometry, Magnetization Transfer Saturation Index, and T(1)w/T(2)w Ratio Methods. Sci Rep 8, 10554 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhea EM, Salameh TS, Logsdon AF, Hanson AJ, Erickson MA, Banks WA. Blood-Brain Barriers in Obesity. AAPS J 19, 921–930 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwon YH, et al. Hypothalamic lipid-laden astrocytes induce microglia migration and activation. FEBS Lett 591, 1742–1751 (2017). [DOI] [PubMed] [Google Scholar]
- 50.Karmi A, et al. Increased brain fatty acid uptake in metabolic syndrome. Diabetes 59, 2171–2177 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Song J, et al. Analysis of Trans Fat in Edible Oils with Cooking Process. Toxicol Res 31, 307–312 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wanders AJ, Zook PL, Brouwer IA. Trans Fat Intake and Its Dietary Sources in General Populations Worldwide: A Systematic Review. Nutrients 9, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Honda T, et al. Serum elaidic acid concentration and risk of dementia: The Hisayama Study. Neurology 93, e2053–e2064 (2019). [DOI] [PubMed] [Google Scholar]
- 54.Morris MC, Tangney CC. Dietary fat composition and dementia risk. Neurobiol Aging 35 Suppl 2, S59–64 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ginter E, Simko V. New data on harmful effects of trans-fatty acids. Bratisl Lek Listy 117, 251–253 (2016). [DOI] [PubMed] [Google Scholar]
- 56.Link BG, Phelan J. Social conditions as fundamental causes of disease. J Health Soc Behav Spec No, 80–94 (1995). [PubMed] [Google Scholar]
- 57.Laine MA, et al. Genetic Control of Myelin Plasticity after Chronic Psychosocial Stress. eNeuro 5, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ritchie J, Pantazatos SP, French L. Transcriptomic characterization of MRI contrast with focus on the T1-w/T2-w ratio in the cerebral cortex. Neuroimage 174, 504–517 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Esteban O, Birman D, Schaer M, Koyejo OO, Poldrack RA, Gorgolewski KJ. MRIQC: Advancing the automatic prediction of image quality in MRI from unseen sites. PLoS One 12, e0184661 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mortamet B, et al. Automatic quality assessment in structural brain magnetic resonance imaging. Magn Reson Med 62, 365–372 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kind AJH, Golden RN. Social Determinants of Health: Fundamental Drivers of Health Inequity. WMJ 117, 231–232 (2018). [PubMed] [Google Scholar]
- 62.Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 24, 385–396 (1983). [PubMed] [Google Scholar]
- 63.Glasser MF, et al. The minimal preprocessing pipelines for the Human Connectome Project. Neuroimage 80, 105–124 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage 9, 179–194 (1999). [DOI] [PubMed] [Google Scholar]
- 65.Glasser MF, Van Essen DC. Mapping human cortical areas in vivo based on myelin content as revealed by T1-and T2-weighted MRI. J Neurosci 31, 11597–11616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kilpatrick LA, et al. Impact of prenatal alcohol exposure on intracortical myelination and deep white matter in children with attention deficit hyperactivity disorder. Neuroimage: Reports 2, 100082 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Glasser MF, et al. A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.McIntosh AR, Lobaugh NJ. Partial least squares analysis of neuroimaging data: applications and advances. Neuroimage 23 Suppl 1, S250–263 (2004). [DOI] [PubMed] [Google Scholar]
- 69.Krishnan A, Williams LJ, McIntosh AR, Abdi H. Partial Least Squares (PLS) methods for neuroimaging: a tutorial and review. Neuroimage 56, 455–475 (2011). [DOI] [PubMed] [Google Scholar]
- 70.Rosseel Y. lavaan: An R Package for Structural Equation Modeling. Journal of Statistical Software 48, 1–36 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to an ongoing collaboration with multiple principal investigators involving participant identifiers at the G. Oppenheimer Center for Neurobiology of Stress and Resilience. However, data is available from the corresponding author on reasonable request.