Abstract
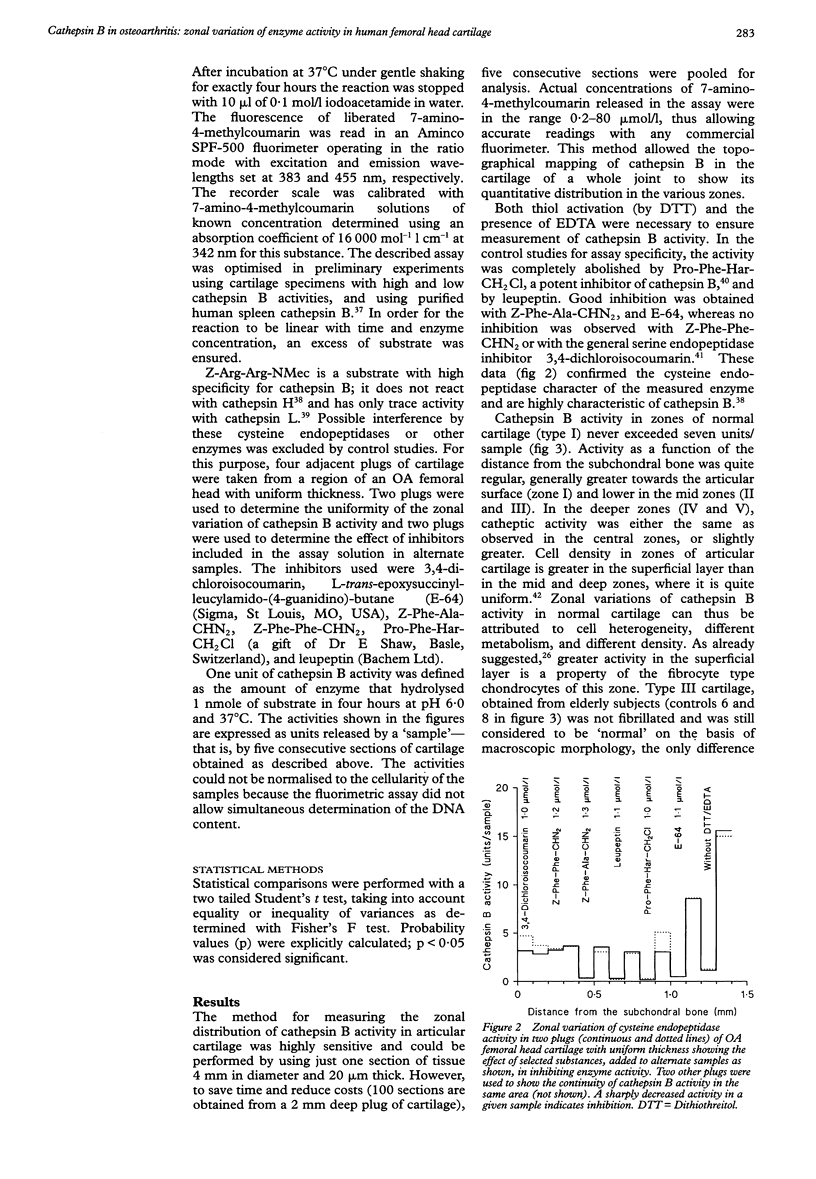
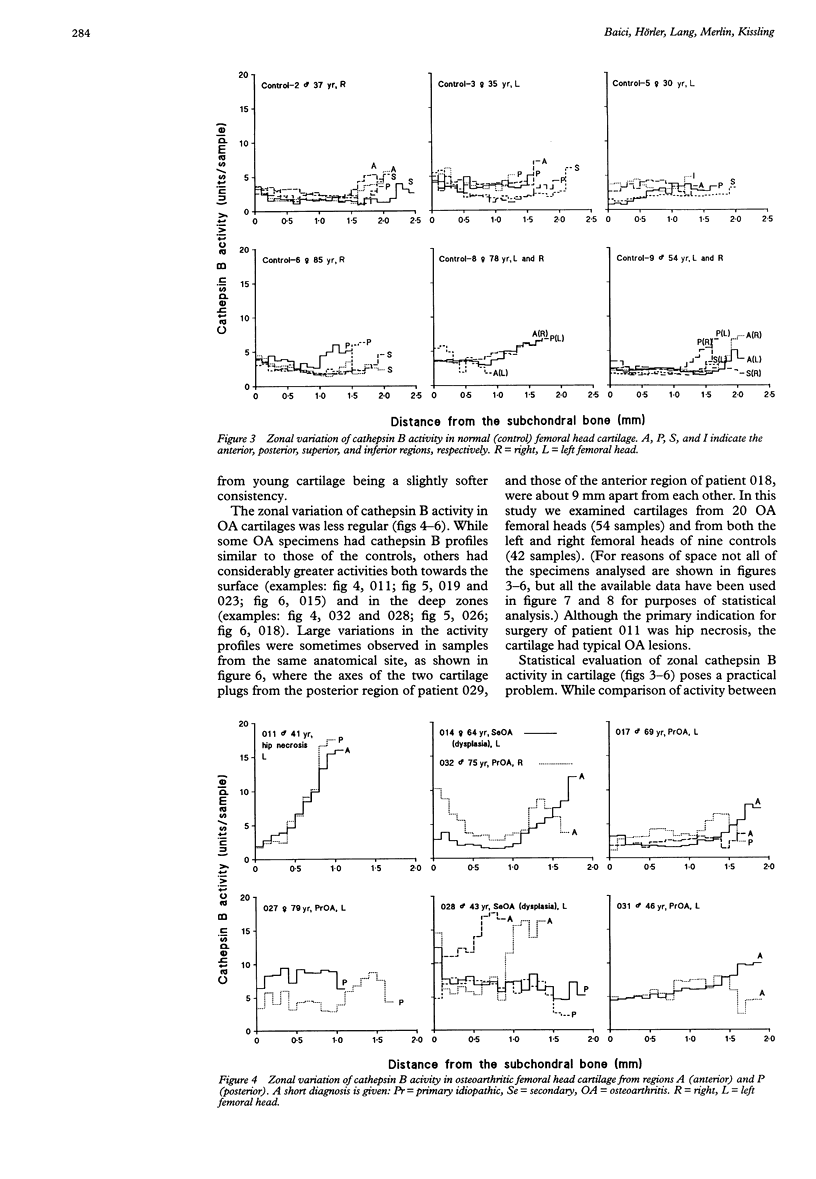
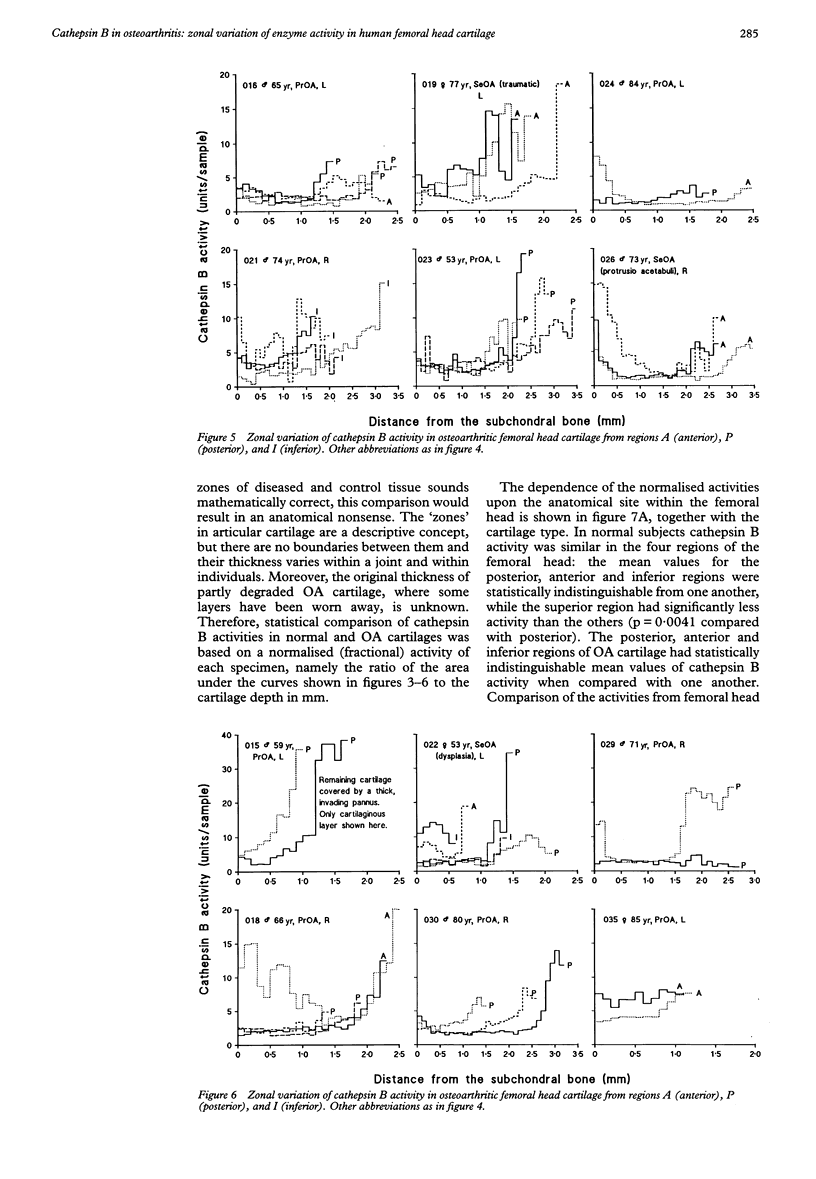
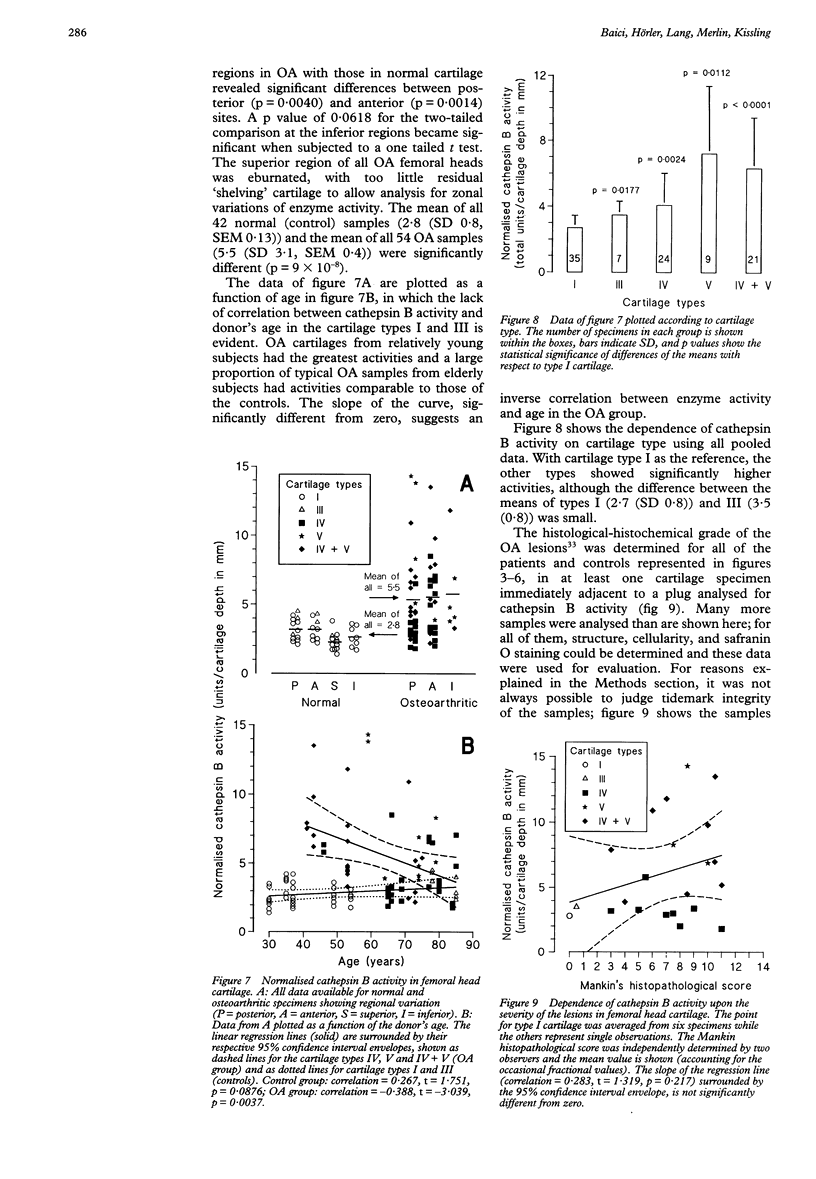
OBJECTIVES--To determine the quantitative topographical distribution of cathepsin B in human femoral head cartilage by measuring the zonal variation of enzyme activity in specimens taken from various anatomical regions of normal and osteoarthritic (OA) tissues, and to correlate this parameter with the severity of the OA lesions. METHODS--OA articular cartilage was obtained at surgery for total hip replacement and control cartilage obtained at postmortem. Cylinders of full thickness cartilage with underlying bone were retrieved with a biopsy trephine. Sections of cartilage were produced by cryocutting the tissue as slices parallel to the articular surface and assayed for cathepsin B with a specific, highly sensitive fluorogenic substrate. The severity of the OA lesions was graded according to the histopathological-histochemical method of Mankin. RESULTS--Zonal cathepsin B activity of normal cartilage was uniform and low in all regions of the femoral head. In apparently intact OA cartilage and in severely degraded tissue the zonal distribution and the amounts of enzyme were similar to control values. At sites with active disease, cathepsin B activity was much greater than in controls and its irregular zonal distribution correlated with tissue degeneration, hypercellularity, or cloning of chondrocytes as determined histochemically. Particularly high enzyme levels were observed at sites with regenerating cartilage, where some zonal peaks attained 20-fold activity with respect to controls. CONCLUSION--Cathepsin B may play a role in sustaining the chronicity of OA, not as an initiator, but rather as a perpetuator of the disease and as an antagonist of regeneration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L. Enzymic degradation of cartilage in osteoarthritis. Fed Proc. 1973 Apr;32(4):1494–1498. [PubMed] [Google Scholar]
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A. Cathepsin B secretion by rabbit articular chondrocytes: modulation by cycloheximide and glycosaminoglycans. Cell Tissue Res. 1990 Mar;259(3):567–573. doi: 10.1007/BF01740785. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A. Effect of interleukin-1 beta on the production of cathepsin B by rabbit articular chondrocytes. FEBS Lett. 1990 Dec 17;277(1-2):93–96. doi: 10.1016/0014-5793(90)80816-2. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A., Hörler D., Kissling R., Merlin C. Cathepsin B in osteoarthritis: cytochemical and histochemical analysis of human femoral head cartilage. Ann Rheum Dis. 1995 Apr;54(4):289–297. doi: 10.1136/ard.54.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A., Lang A., Hörler D., Knöpfel M. Cathepsin B as a marker of the dedifferentiated chondrocyte phenotype. Ann Rheum Dis. 1988 Aug;47(8):684–691. doi: 10.1136/ard.47.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kirschke H. Cathepsin B, Cathepsin H, and cathepsin L. Methods Enzymol. 1981;80(Pt 100):535–561. doi: 10.1016/s0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Studies on cathepsin B in human articular cartilage. Biochem J. 1978 Apr 1;171(1):149–154. doi: 10.1042/bj1710149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burleigh M. C., Barrett A. J., Lazarus G. S. Cathepsin B1. A lysosomal enzyme that degrades native collagen. Biochem J. 1974 Feb;137(2):387–398. doi: 10.1042/bj1370387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshmukh-Phadke K., Lawrence M., Nanda S. Synthesis of collagenase and neutral proteases by articular chondrocytes: stimulation by a macrophage-derived factor. Biochem Biophys Res Commun. 1978 Nov 14;85(1):490–496. doi: 10.1016/s0006-291x(78)80068-0. [DOI] [PubMed] [Google Scholar]
- Dingle J. T., Saklatvala J., Hembry R., Tyler J., Fell H. B., Jubb R. A cartilage catabolic factor from synovium. Biochem J. 1979 Oct 15;184(1):177–180. doi: 10.1042/bj1840177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etherington D. J. The purification of bovine cathepsin B1 and its mode of action on bovine collagens. Biochem J. 1974 Mar;137(3):547–557. doi: 10.1042/bj1370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrijelcic D., Annan-Prah A., Rodic B., Rozman B., Cotic V., Turk V. Determination of cathepsins B and H in sera and synovial fluids of patients with different joint diseases. J Clin Chem Clin Biochem. 1990 Mar;28(3):149–153. doi: 10.1515/cclm.1990.28.3.149. [DOI] [PubMed] [Google Scholar]
- Grushko G., Schneiderman R., Maroudas A. Some biochemical and biophysical parameters for the study of the pathogenesis of osteoarthritis: a comparison between the processes of ageing and degeneration in human hip cartilage. Connect Tissue Res. 1989;19(2-4):149–176. doi: 10.3109/03008208909043895. [DOI] [PubMed] [Google Scholar]
- Harper J. W., Hemmi K., Powers J. C. Reaction of serine proteases with substituted isocoumarins: discovery of 3,4-dichloroisocoumarin, a new general mechanism based serine protease inhibitor. Biochemistry. 1985 Apr 9;24(8):1831–1841. doi: 10.1021/bi00329a005. [DOI] [PubMed] [Google Scholar]
- Hembry R. M., Knight C. G., Dingle J. T., Barrett A. J. Evidence that extracellular cathepsin D is not responsible for the resorption of cartilage matrix in culture. Biochim Biophys Acta. 1982 Feb 2;714(2):307–312. doi: 10.1016/0304-4165(82)90338-5. [DOI] [PubMed] [Google Scholar]
- Huet G., Flipo R. M., Richet C., Thiebaut C., Demeyer D., Balduyck M., Duquesnoy B., Degand P. Measurement of elastase and cysteine proteinases in synovial fluid of patients with rheumatoid arthritis, sero-negative spondylarthropathies, and osteoarthritis. Clin Chem. 1992 Sep;38(9):1694–1697. [PubMed] [Google Scholar]
- IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). Nomenclature and symbolism for amino acids and peptides. Recommendations 1983. Biochem J. 1984 Apr 15;219(2):345–373. doi: 10.1042/bj2190345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H., Kembhavi A. A., Bohley P., Barrett A. J. Action of rat liver cathepsin L on collagen and other substrates. Biochem J. 1982 Feb 1;201(2):367–372. doi: 10.1042/bj2010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LUCY J. A., DINGLE J. T., FELL H. B. Studies on the mode of action of excess of vitamin A. 2. A possible role of intracellular proteases in the degradation of cartilage matrix. Biochem J. 1961 Jun;79:500–508. doi: 10.1042/bj0790500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenarcic B., Gabrijelcic D., Rozman B., Drobnic-Kosorok M., Turk V. Human cathepsin B and cysteine proteinase inhibitors (CPIs) in inflammatory and metabolic joint diseases. Biol Chem Hoppe Seyler. 1988 May;369 (Suppl):257–261. [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Martel-Pelletier J., Cloutier J. M., Pelletier J. P. Cathepsin B and cysteine protease inhibitors in human osteoarthritis. J Orthop Res. 1990 May;8(3):336–344. doi: 10.1002/jor.1100080305. [DOI] [PubMed] [Google Scholar]
- Meats J. E., McGuire M. B., Russell R. G. Human synovium releases a factor which stimulates chondrocyte production of PGE and plasminogen activator. Nature. 1980 Aug 28;286(5776):891–892. doi: 10.1038/286891a0. [DOI] [PubMed] [Google Scholar]
- Morrison R. I., Barrett A. J., Dingle J. T., Prior D. Cathepsins BI and D. Action on human cartilage proteoglycans. Biochim Biophys Acta. 1973 Apr 12;302(2):411–419. doi: 10.1016/0005-2744(73)90170-8. [DOI] [PubMed] [Google Scholar]
- Mort J. S., Recklies A. D., Poole A. R. Extracellular presence of the lysosomal proteinase cathepsin B in rheumatoid synovium and its activity at neutral pH. Arthritis Rheum. 1984 May;27(5):509–515. doi: 10.1002/art.1780270505. [DOI] [PubMed] [Google Scholar]
- Ollivierre F., Gubler U., Towle C. A., Laurencin C., Treadwell B. V. Expression of IL-1 genes in human and bovine chondrocytes: a mechanism for autocrine control of cartilage matrix degradation. Biochem Biophys Res Commun. 1986 Dec 30;141(3):904–911. doi: 10.1016/s0006-291x(86)80128-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg L. Chemical basis for the histological use of safranin O in the study of articular cartilage. J Bone Joint Surg Am. 1971 Jan;53(1):69–82. [PubMed] [Google Scholar]
- Shaw E., Kettner C. The specificity of cathepsin B. Acta Biol Med Ger. 1981;40(10-11):1503–1511. [PubMed] [Google Scholar]
- Trabandt A., Gay R. E., Fassbender H. G., Gay S. Cathepsin B in synovial cells at the site of joint destruction in rheumatoid arthritis. Arthritis Rheum. 1991 Nov;34(11):1444–1451. doi: 10.1002/art.1780341116. [DOI] [PubMed] [Google Scholar]
- Tyler J. A. Chondrocyte-mediated depletion of articular cartilage proteoglycans in vitro. Biochem J. 1985 Jan 15;225(2):493–507. doi: 10.1042/bj2250493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittorio N., Crissman J. D., Hopson C. N., Herman J. H. Histologic assessment of cathepsin D in osteoarthritic cartilage. Clin Exp Rheumatol. 1986 Jul-Sep;4(3):221–230. [PubMed] [Google Scholar]
- Watanabe S., Georgescu H. I., Kuhns D. B., Evans C. H. Chondrocyte activation by a putative interleukin-1 derived from lapine polymorphonuclear leukocytes. Arch Biochem Biophys. 1989 Apr;270(1):69–76. doi: 10.1016/0003-9861(89)90008-8. [DOI] [PubMed] [Google Scholar]
- Weiss C. Ultrastructural characteristics of osteoarthritis. Fed Proc. 1973 Apr;32(4):1459–1466. [PubMed] [Google Scholar]


