Abstract
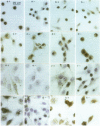
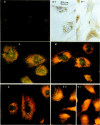
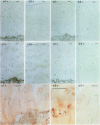
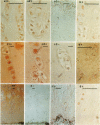
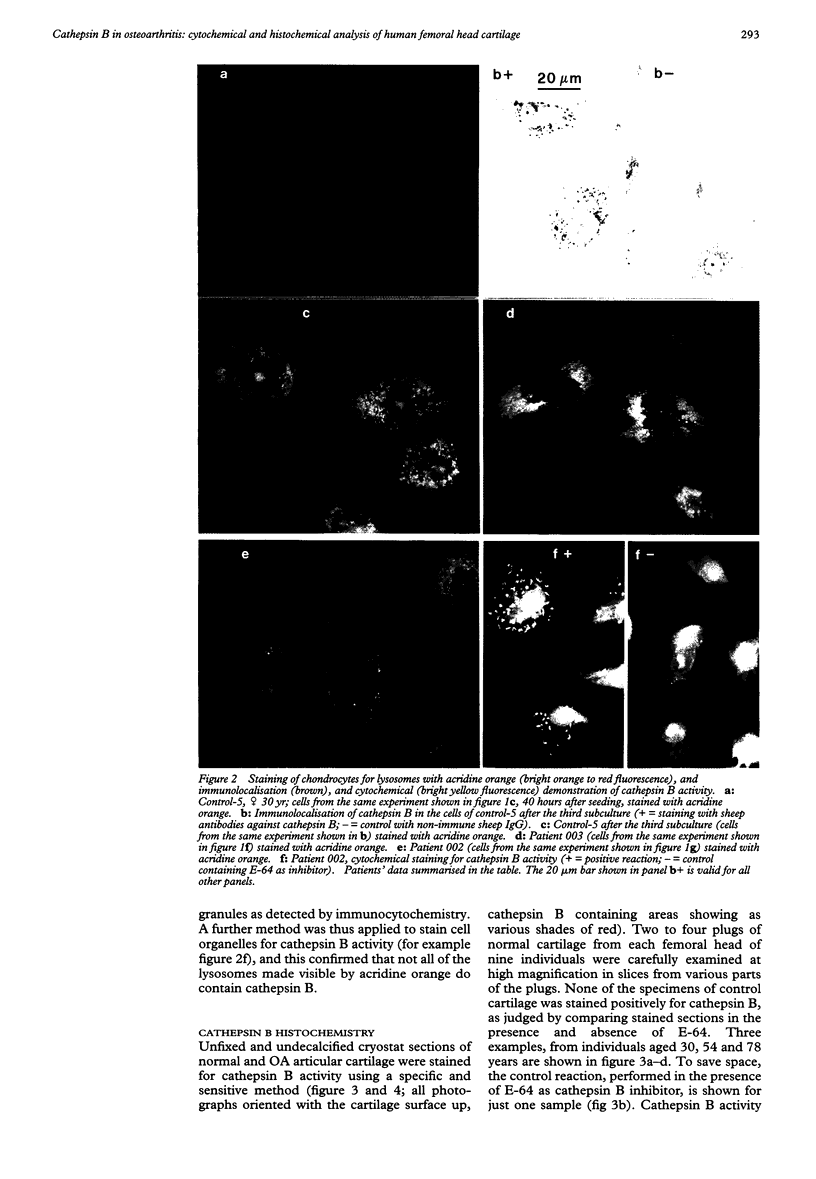
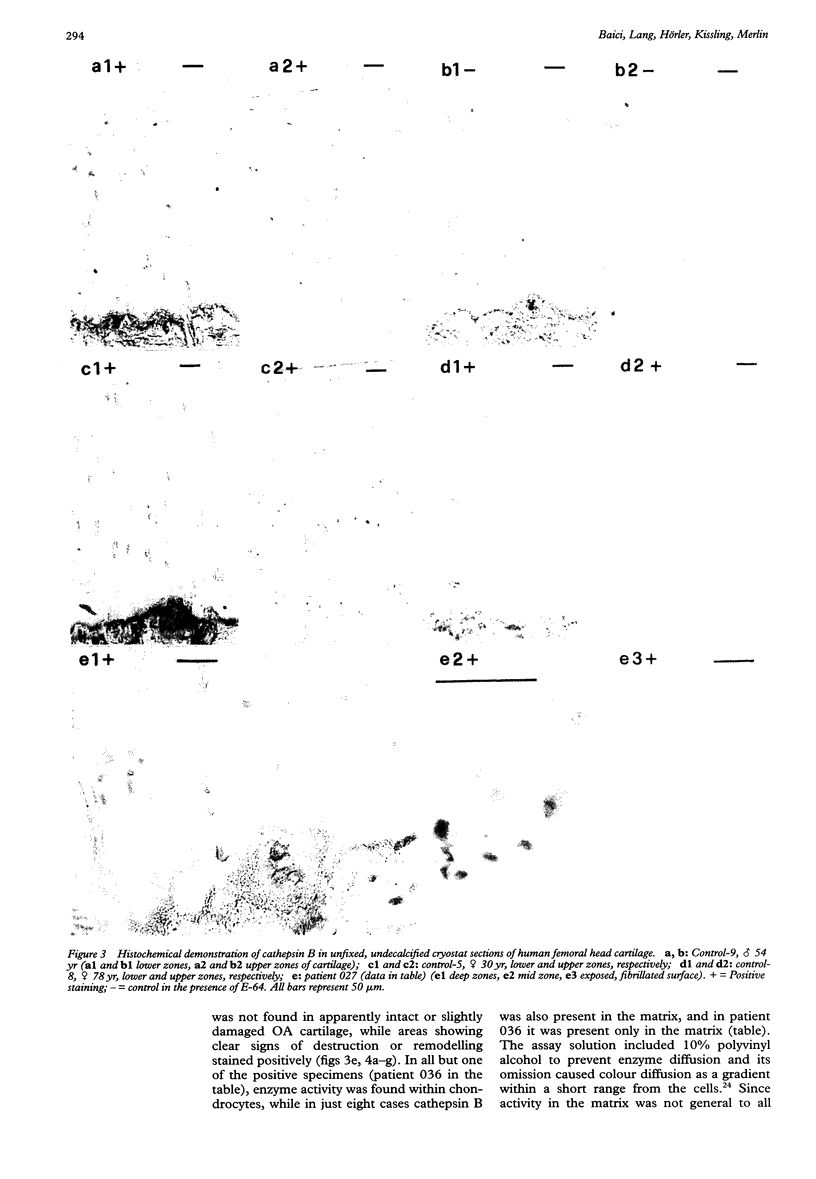
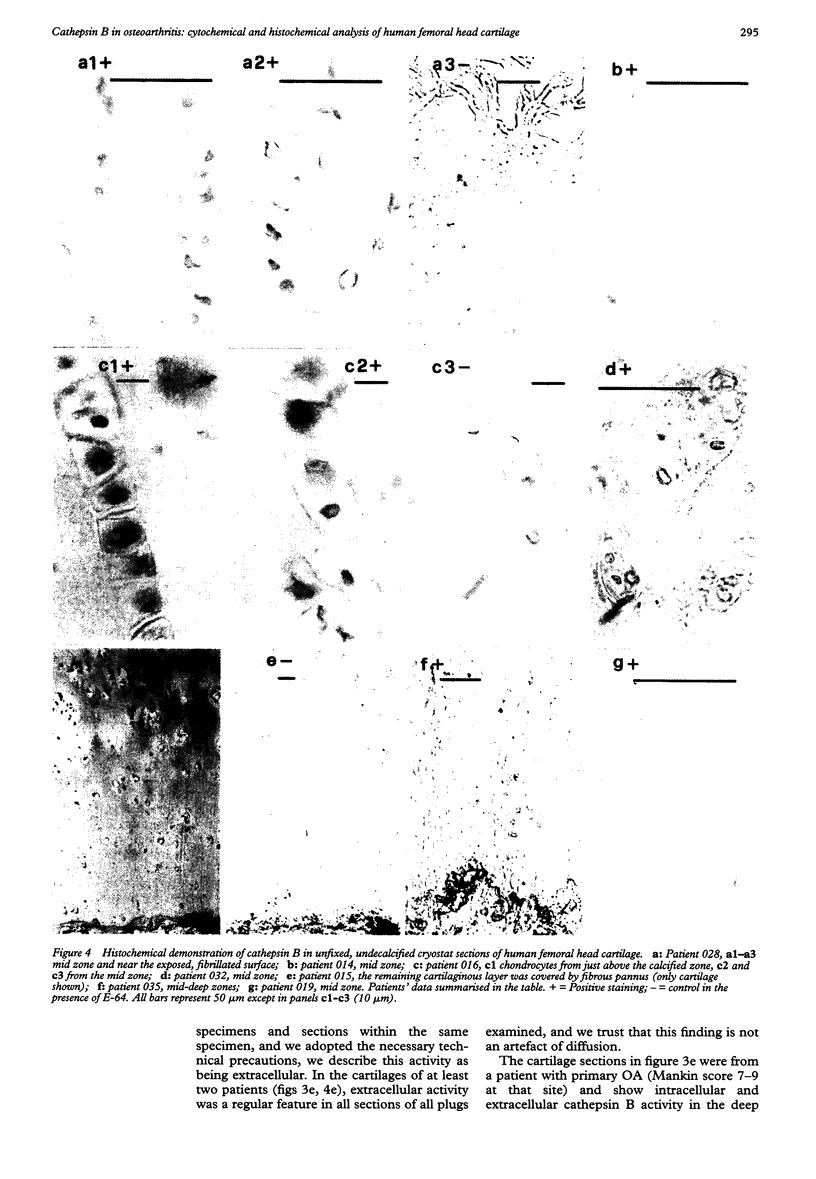
OBJECTIVE--To localise the cysteine endopeptidase cathepsin B in chondrocytes and cartilage from normal and osteoarthritic (OA) human femoral heads in order to provide qualitative information on its cellular expression and distribution at possible sites of action. METHODS--OA articular cartilage was obtained at surgery for total hip replacement; control cartilage was obtained at postmortem. Chondrocytes were isolated by sequential enzymatic digestion and cathepsin B analysed by immunocytochemistry and activity staining with a fluorogenic substrate. Lysosomes were visualised by fluorescence microscopy after staining of living cells with acridine orange. Using a histochemical reaction, enzyme activity was measured in cryosections of full thickness cartilage. RESULTS--Chondrocytes from normal cartilage contained very few lysosomes and only a minor cell population was cathepsin B positive. A high proportion of chondrocytes from active OA cartilage contained a large number of lysosomes and an excess of cathepsin B in intracellular organelles; the enzyme was stored in an active form. In this respect, OA chondrocytes closely resembled normal cells that had been phenotypically modulated by serial subcultures. No cathepsin B activity could be detected by histochemistry in either chondrocytes or matrix of normal cartilage. While apparently intact and severely degraded OA cartilage was also cathepsin B negative, tissue at sites of active destruction and, particularly, at repair sites was highly positive. CONCLUSION--The presence and the particular distribution of active cathepsin B in OA cartilage at 'more involved' sites suggest a pathological role for this enzyme in sustaining and perpetuating cartilage degradation. While other stimuli may also be responsible for cathepsin B expression in OA chondrocytes, the similarity with artificially modulated cells indicates fibroblastic metaplasia as a plausible mechanism.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ali S. Y., Evans L., Stainthorpe E., Lack C. H. Characterization of cathepsins in cartilage. Biochem J. 1967 Nov;105(2):549–557. doi: 10.1042/bj1050549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali S. Y. The degradation of cartilage matrix by an intracellular protease. Biochem J. 1964 Dec;93(3):611–618. doi: 10.1042/bj0930611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer C. W., McDowell J., Bayliss M. T., Stephens M. D., Bentley G. Phenotypic modulation in sub-populations of human articular chondrocytes in vitro. J Cell Sci. 1990 Oct;97(Pt 2):361–371. doi: 10.1242/jcs.97.2.361. [DOI] [PubMed] [Google Scholar]
- Baici A., Gyger-Marazzi M. The slow, tight-binding inhibition of cathepsin B by leupeptin. A hysteretic effect. Eur J Biochem. 1982 Dec;129(1):33–41. doi: 10.1111/j.1432-1033.1982.tb07017.x. [DOI] [PubMed] [Google Scholar]
- Baici A., Hörler D., Lang A., Merlin C., Kissling R. Cathepsin B in osteoarthritis: zonal variation of enzyme activity in human femoral head cartilage. Ann Rheum Dis. 1995 Apr;54(4):281–288. doi: 10.1136/ard.54.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baici A., Lang A. Cathepsin B secretion by rabbit articular chondrocytes: modulation by cycloheximide and glycosaminoglycans. Cell Tissue Res. 1990 Mar;259(3):567–573. doi: 10.1007/BF01740785. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A. Effect of interleukin-1 beta on the production of cathepsin B by rabbit articular chondrocytes. FEBS Lett. 1990 Dec 17;277(1-2):93–96. doi: 10.1016/0014-5793(90)80816-2. [DOI] [PubMed] [Google Scholar]
- Baici A., Lang A., Hörler D., Knöpfel M. Cathepsin B as a marker of the dedifferentiated chondrocyte phenotype. Ann Rheum Dis. 1988 Aug;47(8):684–691. doi: 10.1136/ard.47.8.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barratt M. E. Role of the chondrocytes in the breakdown of pig articular cartilage induced by complement-sufficient antiserum to pig erythrocytes. Int Arch Allergy Appl Immunol. 1975;48(4):547–563. doi: 10.1159/000231342. [DOI] [PubMed] [Google Scholar]
- Barratt M. E. The role of soft connective tissue in the response of pig articular cartilage in organ culture to excess of retinol. J Cell Sci. 1973 Jul;13(1):205–219. doi: 10.1242/jcs.13.1.205. [DOI] [PubMed] [Google Scholar]
- Bayliss M. T., Ali S. Y. Studies on cathepsin B in human articular cartilage. Biochem J. 1978 Apr 1;171(1):149–154. doi: 10.1042/bj1710149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Handley C. J., Ilic M. Z., Saklatvala J., Murata M., Barrett A. J. Inhibition of cartilage proteoglycan release by a specific inactivator of cathepsin B and an inhibitor of matrix metalloproteinases. Evidence for two converging pathways of chondrocyte-mediated proteoglycan degradation. Arthritis Rheum. 1993 Dec;36(12):1709–1717. doi: 10.1002/art.1780361210. [DOI] [PubMed] [Google Scholar]
- Buttle D. J., Saklatvala J. Lysosomal cysteine endopeptidases mediate interleukin 1-stimulated cartilage proteoglycan degradation. Biochem J. 1992 Oct 15;287(Pt 2):657–661. doi: 10.1042/bj2870657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Saklatvala J., Tamai M., Barrett A. J. Inhibition of interleukin 1-stimulated cartilage proteoglycan degradation by a lipophilic inactivator of cysteine endopeptidases. Biochem J. 1992 Jan 1;281(Pt 1):175–177. doi: 10.1042/bj2810175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolbeare F., Vanderlaan M. A fluorescent assay of proteinases in cultured mammalian cells. J Histochem Cytochem. 1979 Nov;27(11):1493–1495. doi: 10.1177/27.11.512330. [DOI] [PubMed] [Google Scholar]
- Fell H. B., Barratt M. E., Welland H., Green R. The capacity of pig articular cartilage in organ culture to regenerate after breakdown induced by complement-sufficient antiserum to pig erythrocytes. Calcif Tissue Res. 1976 Apr 13;20(1):3–21. doi: 10.1007/BF02546393. [DOI] [PubMed] [Google Scholar]
- Graf F. M., Sträuli P. Use of the avidin-biotin-peroxidase complex (ABC) method for the localization of rabbit cathepsin B in cells and tissues. J Histochem Cytochem. 1983 Jun;31(6):803–810. doi: 10.1177/31.6.6341462. [DOI] [PubMed] [Google Scholar]
- Graf M., Baici A., Sträuli P. Histochemical localization of cathepsin B at the invasion front of the rabbit V2 carcinoma. Lab Invest. 1981 Dec;45(6):587–596. [PubMed] [Google Scholar]
- Graf M., Leeman U., Ruch F., Sträuli P. The fluorescence and bright field microscopic demonstration of cathepsin B in human fibroblasts. Histochemistry. 1979;64(3):319–322. doi: 10.1007/BF00495033. [DOI] [PubMed] [Google Scholar]
- Mankin H. J., Dorfman H., Lippiello L., Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971 Apr;53(3):523–537. [PubMed] [Google Scholar]
- Martel-Pelletier J., Cloutier J. M., Pelletier J. P. Cathepsin B and cysteine protease inhibitors in human osteoarthritis. J Orthop Res. 1990 May;8(3):336–344. doi: 10.1002/jor.1100080305. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M. Enzyme histochemical reactions in unfixed and undecalcified cryostat sections of mouse knee joints with special reference to arthritic lesions. Histochemistry. 1986;86(2):127–133. doi: 10.1007/BF00493377. [DOI] [PubMed] [Google Scholar]
- Van Noorden C. J., Vogels I. M., Smith R. E. Localization and cytophotometric analysis of cathepsin B activity in unfixed and undecalcified cryostat sections of whole rat knee joints. J Histochem Cytochem. 1989 May;37(5):617–624. doi: 10.1177/37.5.2703699. [DOI] [PubMed] [Google Scholar]
- Weiss C., Mirow S. An ultrastructural study of osteoarthritis changes in the articular cartilage of human knees. J Bone Joint Surg Am. 1972 Jul;54(5):954–972. [PubMed] [Google Scholar]
- Weiss C. Ultrastructural characteristics of osteoarthritis. Fed Proc. 1973 Apr;32(4):1459–1466. [PubMed] [Google Scholar]