Abstract
OBJECTIVE--To elucidate the relationship, if any, between lipid abnormalities and apolipoprotein E (apo E) polymorphism, by investigating apo E phenotype and allele frequency. METHODS--Fasting blood samples were taken for determination of apo E phenotype and serum lipids in 221 male patients with gout and 141 control male subjects. Apo E phenotype was determined by one dimensional flat gel isoelectric focusing. RESULTS--Frequencies of apo E phenotypes in gout were apo E3/3 67.9%, E4/3 18.1%, E4/4 2.3%, E4/2 1.8%, E3/2 9.5%, and E2/2 0.5%; those in control male subjects were 74.5%, 15.6%, 0%, 1.4%, 7.1%, and 1.4%, respectively. Frequencies of the e2, e3, and e4 alleles in gout were 0.061, 0.817 and 0.122, compared with the corresponding control frequencies of 0.057, 0.858 and 0.085. These differences in apo E phenotype and allele frequencies between gout and control subjects were not significant. The frequency of apo e4 allele in hyperlipidaemic gout subjects was significantly greater than that in normolipidaemic gout subjects; in contrast, its frequency was not different between hyperlipidaemic and normolipidaemic control subjects. Serum triglyceride, total cholesterol, apo B and E concentrations were significantly greater in gouty patients with the apo E4/3 phenotype than in those with gout having the apo E3/3 phenotype. CONCLUSIONS--These data suggest that gout subjects with hyperlipidaemia (hypertriglyceridaemia, hypercholesterolaemia or both) possess the apo e4 allele with higher frequency than those with normolipidaemia. They also suggest that apo e4 may induce some susceptibility to the development of hyperlipidaemia in gout in addition to that induced by obesity or excessive alcohol consumption, and may contribute to the high prevalence of atherosclerotic diseases in gout patients.
Full text
PDF
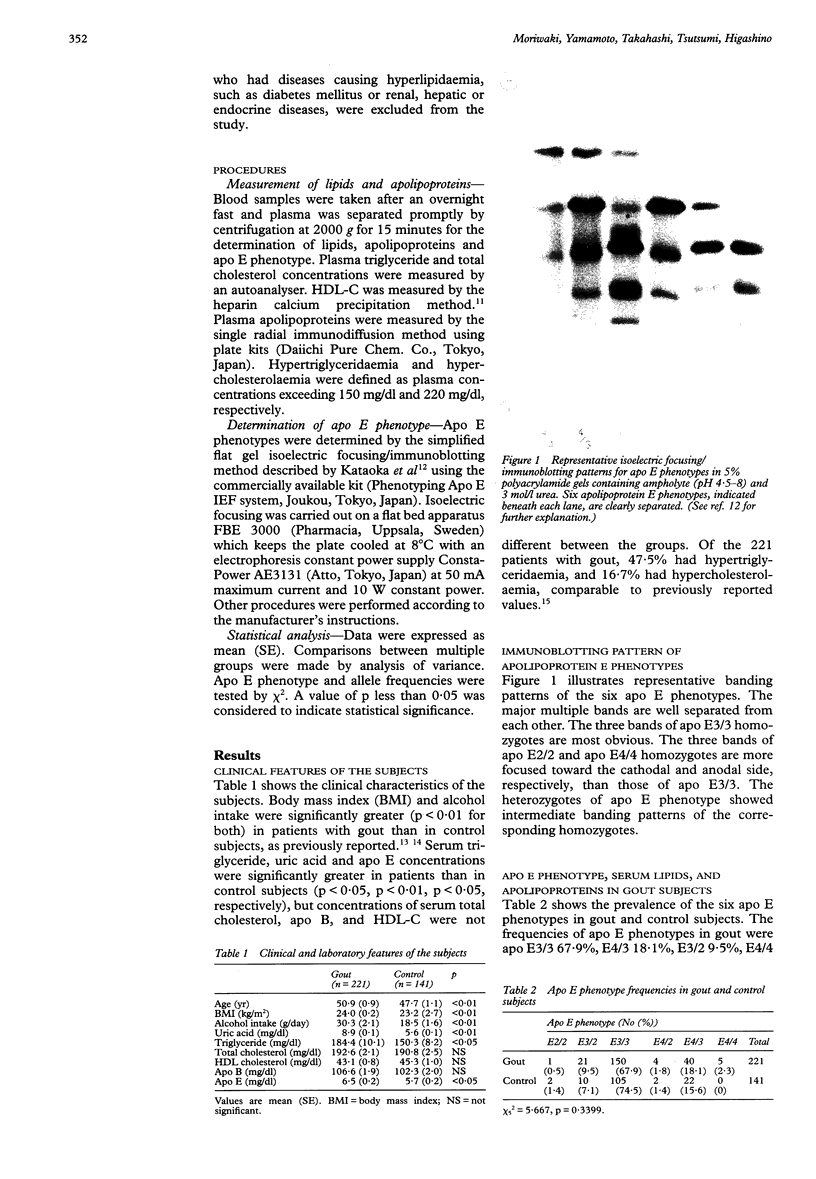
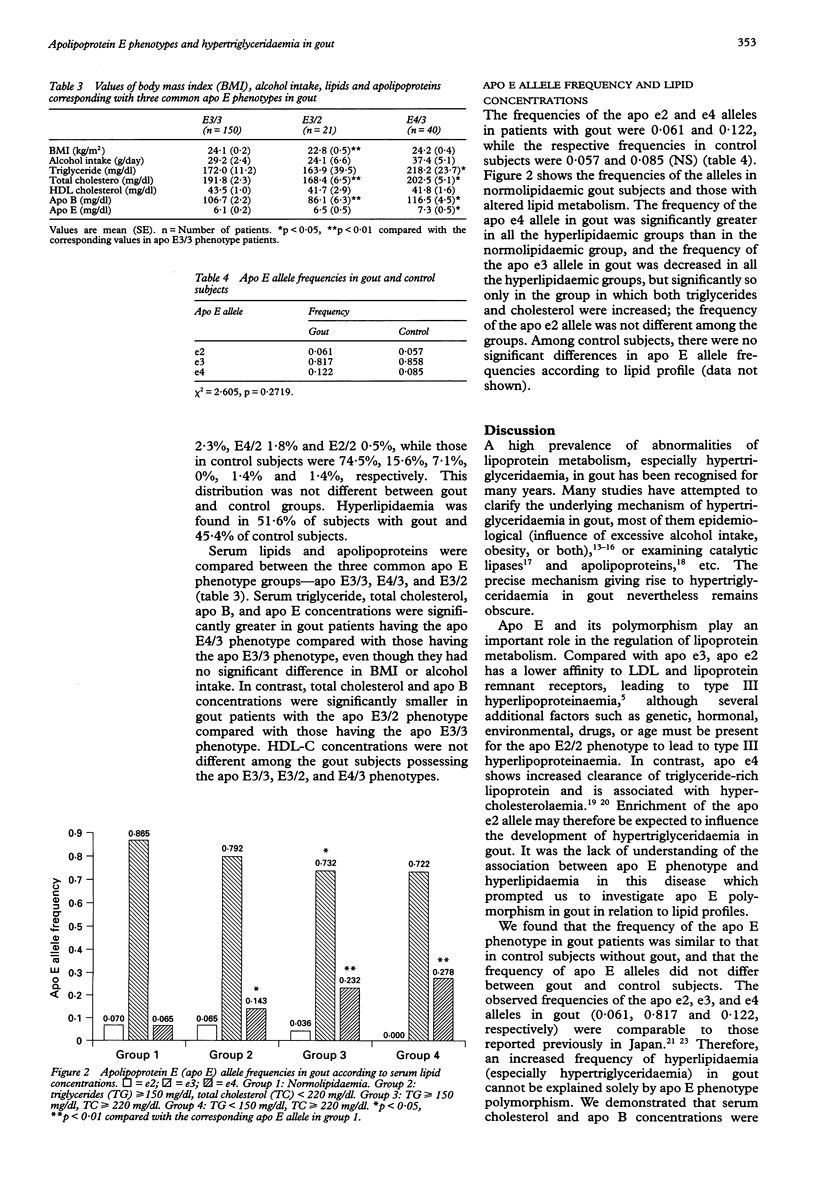

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asakawa J., Takahashi N., Rosenblum B. B., Neel J. V. Two-dimensional gel studies of genetic variation in the plasma proteins of Amerindians and Japanese. Hum Genet. 1985;70(3):222–230. doi: 10.1007/BF00273446. [DOI] [PubMed] [Google Scholar]
- Beard J. T., 2nd Serum uric acid and coronary heart disease. Am Heart J. 1983 Aug;106(2):397–400. doi: 10.1016/0002-8703(83)90209-0. [DOI] [PubMed] [Google Scholar]
- Dallongeville J., Lussier-Cacan S., Davignon J. Modulation of plasma triglyceride levels by apoE phenotype: a meta-analysis. J Lipid Res. 1992 Apr;33(4):447–454. [PubMed] [Google Scholar]
- Eto M., Watanabe K., Ishii K. Reciprocal effects of apolipoprotein E alleles (epsilon 2 and epsilon 4) on plasma lipid levels in normolipidemic subjects. Clin Genet. 1986 Jun;29(6):477–484. doi: 10.1111/j.1399-0004.1986.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Fox I. H., John D., DeBruyne S., Dwosh I., Marliss E. B. Hyperuricemia and hypertriglyceridemia: metabolic basis for the association. Metabolism. 1985 Aug;34(8):741–746. doi: 10.1016/0026-0495(85)90025-3. [DOI] [PubMed] [Google Scholar]
- Gibson T., Grahame R. Gout and hyperlipidaemia. Ann Rheum Dis. 1974 Jul;33(4):298–303. doi: 10.1136/ard.33.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T., Kilbourn K., Horner I., Simmonds H. A. Mechanism and treatment of hypertriglyceridaemia in gout. Ann Rheum Dis. 1979 Feb;38(1):31–35. doi: 10.1136/ard.38.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg R. E., Zech L. A., Schaefer E. J., Stark D., Wilson D., Brewer H. B., Jr Abnormal in vivo metabolism of apolipoprotein E4 in humans. J Clin Invest. 1986 Sep;78(3):815–821. doi: 10.1172/JCI112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innerarity T. L., Mahley R. W. Enhanced binding by cultured human fibroblasts of apo-E-containing lipoproteins as compared with low density lipoproteins. Biochemistry. 1978 Apr 18;17(8):1440–1447. doi: 10.1021/bi00601a013. [DOI] [PubMed] [Google Scholar]
- Jiao S., Kameda K., Matsuzawa Y., Tarui S. Hyperlipoproteinaemia in primary gout: hyperlipoproteinaemic phenotype and influence of alcohol intake and obesity in Japan. Ann Rheum Dis. 1986 Apr;45(4):308–313. doi: 10.1136/ard.45.4.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka S., Paidi M., Howard B. V. Simplified isoelectric focusing/immunoblotting determination of apoprotein E phenotype. Clin Chem. 1994 Jan;40(1):11–13. [PubMed] [Google Scholar]
- Kodama T., Murase T., Itakura H., Akanuma Y., Takaku F., Nishida Y. Postheparin plasma lipoprotein lipase and hepatic triglyceride lipase activities in patients with primary asymptomatic gout. Clin Chem. 1983 Dec;29(12):2124–2125. [PubMed] [Google Scholar]
- Kuusi T., Taskinen M. R., Solakivi T., Kauppinen-Mäkelin R. Role of apolipoproteins E and C in type V hyperlipoproteinemia. J Lipid Res. 1988 Mar;29(3):293–298. [PubMed] [Google Scholar]
- Leren T. P., Børresen A. L., Berg K., Hjermann I., Leren P. Increased frequency of the apolipoprotein E-4 isoform in male subjects with multifactorial hypercholesterolemia. Clin Genet. 1985 May;27(5):458–462. doi: 10.1111/j.1399-0004.1985.tb00231.x. [DOI] [PubMed] [Google Scholar]
- Macfarlane D. G., Midwinter C. A., Dieppe P. A., Bolton C. H., Hartog M. Demonstration of an abnormality of C apoprotein of very low density lipoprotein in patients with gout. Ann Rheum Dis. 1985 Jun;44(6):390–394. doi: 10.1136/ard.44.6.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito H. K., Mackenzie A. H. Secondary hypertriglyceridemia and hyperlipoproteinemia in patients with primary asymptomatic gout. Clin Chem. 1979 Mar;25(3):371–375. [PubMed] [Google Scholar]
- Noma A., Nezu-Nakayama K., Kita M., Okabe H. Simultaneous determination of serum cholesterol in high- and low-density lipoproteins with use of heparin, Ca2+, and an anion-exchange resin. Clin Chem. 1978 Sep;24(9):1504–1508. [PubMed] [Google Scholar]
- Shelburne F., Hanks J., Meyers W., Quarfordt S. Effect of apoproteins on hepatic uptake of triglyceride emulsions in the rat. J Clin Invest. 1980 Mar;65(3):652–658. doi: 10.1172/JCI109710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrill B. C., Innerarity T. L., Mahley R. W. Rapid hepatic clearance of the canine lipoproteins containing only the E apoprotein by a high affinity receptor. Identity with the chylomicron remnant transport process. J Biol Chem. 1980 Mar 10;255(5):1804–1807. [PubMed] [Google Scholar]
- Takahashi S., Yamamoto T., Moriwaki Y., Tsutsumi Z., Higashino K. Impaired lipoprotein metabolism in patients with primary gout--influence of alcohol intake and body weight. Br J Rheumatol. 1994 Aug;33(8):731–734. doi: 10.1093/rheumatology/33.8.731. [DOI] [PubMed] [Google Scholar]
- Utermann G., Hees M., Steinmetz A. Polymorphism of apolipoprotein E and occurrence of dysbetalipoproteinaemia in man. Nature. 1977 Oct 13;269(5629):604–607. doi: 10.1038/269604a0. [DOI] [PubMed] [Google Scholar]
- Utermann G., Kindermann I., Kaffarnik H., Steinmetz A. Apolipoprotein E phenotypes and hyperlipidemia. Hum Genet. 1984;65(3):232–236. doi: 10.1007/BF00286508. [DOI] [PubMed] [Google Scholar]
- Wallace S. L., Robinson H., Masi A. T., Decker J. L., McCarty D. J., Yü T. F. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977 Apr;20(3):895–900. doi: 10.1002/art.1780200320. [DOI] [PubMed] [Google Scholar]
- Weisgraber K. H., Innerarity T. L., Mahley R. W. Abnormal lipoprotein receptor-binding activity of the human E apoprotein due to cysteine-arginine interchange at a single site. J Biol Chem. 1982 Mar 10;257(5):2518–2521. [PubMed] [Google Scholar]
- Zannis V. I., Breslow J. L., Utermann G., Mahley R. W., Weisgraber K. H., Havel R. J., Goldstein J. L., Brown M. S., Schonfeld G., Hazzard W. R. Proposed nomenclature of apoE isoproteins, apoE genotypes, and phenotypes. J Lipid Res. 1982 Aug;23(6):911–914. [PubMed] [Google Scholar]