Abstract
Background: Atrial fibrillation (AF) ablation is performed under deep sedation, which may cause inspiration-induced negative left atrial pressure (INLAP) associated with deep inspiration. INLAP could be the cause of periprocedural complications. Methods: We retrospectively enrolled 381 patients with AF (mean age, 63.9 ± 10.8 years; 76 women; 216 cases of paroxysmal AF) who underwent CA under deep sedation using an adaptive servo ventilator (ASV). Patients whose LAP was not obtained were excluded. INLAP was defined as <0 mmHg of mean LAP during inspiration immediately after the transseptal puncture. The primary and secondary endpoints were the presence of INLAP and the incidence of periprocedural complications. Results: Among 381 patients, INLAP was observed in 133 (34.9%). Patients with INLAP had higher CHA2DS2-Vasc scores (2.3 ± 1.5 vs. 2.1 ± 1.6) and 3% oxygen desaturation indexes (median 18.6 (interquartile range 11.2–31.1) vs. 15.7 (8.1–25.3)), and higher prevalence of diabetes mellitus (23.3 vs. 13.3%) than patients without INLAP. Air embolism occurred in four patients with INLAP (3.0 vs. 0.0%). Conclusion: INLAP is not rare in patients undergoing CA for AF under deep sedation with ASV. Much attention should be paid to the possibility of air embolism in patients with INLAP.
Keywords: atrial fibrillation, catheter ablation, air embolism, inspiration-induced negative left atrial pressure
1. Introduction
The incidence and prevalence of atrial fibrillation (AF) have increased in recent years. AF is associated with an increased risk of cerebrovascular accidents, heart failure, and all-cause death [1]. Early rhythm control therapy has been reported to improve the prognosis of patients with AF [2]. Additionally, rhythm control therapy with catheter ablation (CA) can improve a patient’s quality of life [3]. Currently, CA is recognized as a reasonable therapeutic option for AF [4].
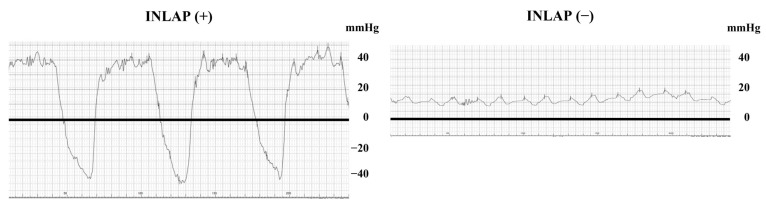
CA for AF is effective; however, the complication rate of CA in the acute phase is 7.48% [5]. Among the complications, air embolism is a crucial complication that can cause acute systemic embolism [6]. The most common embolisms are coronary air embolisms, with a prevalence of 2.6% in cryoballoon ablations [7]. Negative left atrial pressure (LAP), in conjunction with air-leaking sheaths, is essential for air intrusion into the left atrium (LA) [8]. In particular, chest wall expansion and diaphragm descent due to deep inspiration with airway obstruction induce negative atrial pressure [9]. However, the prevalence and clinical characteristics of inspiration-induced negative LAP (INLAP; Figure 1) in patients undergoing CA for AF are unknown. In this study, we aimed to evaluate the prevalence and clinical impact of INLAP during CA for AF.
Figure 1.
Representative LAP curve during inspiration with and without INLAP. The bold line indicates a level of 0 mmHg. The left panel shows a representative case with INLAP. LAP shows a significant respiratory change from −30 to 40 mmHg. Abbreviations: INLAP, inspiration-induced negative left atrial pressure; LAP, left atrial pressure.
2. Materials and Methods
2.1. Participants
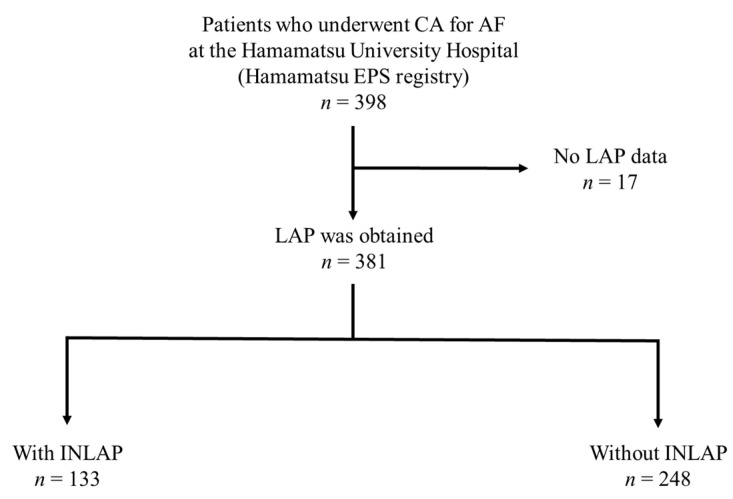
This study is a registry-based study and the registry itself prospectively collected the data. We retrospectively enrolled 398 Japanese patients who underwent CA for AF at the Hamamatsu University Hospital between March 2017 and March 2020, using a prospectively collected ablation database (Hamamatsu EPS registry). Patients aged 18 years or older were eligible. Seventeen patients whose LAP was not measured during CA were excluded from this study (Figure 2). The primary endpoint was the presence of INLAP measured immediately after a transseptal puncture, and the secondary endpoint was the incidence of periprocedural complications. Patients were divided into two groups according to the presence or absence of INLAP, and the procedural parameters were compared between the groups. Data on age, sex, body mass index, comorbidities, and medications were collected.
Figure 2.
Schematic diagram for patient selection in the study. Abbreviations: CA, catheter ablation; AF, atrial fibrillation; LAP, left atrium pressure; INLAP, inspiration-induced negative left atrium pressure.
Paroxysmal (terminated within 7 days) and persistent AF (lasting > 7 days) were defined according to the current guidelines [1]. Before ablation, patients underwent a blood examination, transthoracic echocardiography, and electrocardiogram-gated contrast-enhanced computed tomography (CT) scanning. The LA volume index was obtained by dividing the LA volume by body surface area (BSA). BSA was calculated by the following formula: BSA = (height)0.725 ×(body weight)0.425 × 0.007184. Overnight pulse oximetry was performed 1 day after CA for AF. Written informed consent was obtained from all the patients before enrollment. The protocol was performed according to the Declaration of Helsinki and approved by the Human Investigations Committee of the Hamamatsu University School of Medicine (approval number #20-361).
2.2. Measurement of Intracardiac Pressure and the Definition of INLAP and Inspiration-Induced Negative Right Atrial Pressure
Inferior vena cava pressure, right atrial pressure (RAP), and LAP were measured under sedation and adaptive servo-ventilator (ASV) support without the use of the nasal airway by connecting a pressure transducer with a T-shaped stopcock of the SL0TM 8.5 Fr Swartz Braided Sheath (Abbott, St. Paul, MN, USA). These pressures were measured at an average of four to five respiratory cycles. RAP and LAP were measured immediately after the sheath’s tip reached the right atrium (RA) and LA. INLAP or inspiration-induced negative right atrial pressure (INRAP) was defined as a mean LAP or RAP < 0 mmHg during inspiration. Representative cases of patients with and without INLAP are shown in Figure 1. The pressure data during the procedure was obtained by an electrophysiological specialist independent of this study.
2.3. Electrophysiological Study and CA
Rivaroxaban, apixaban, and edoxaban treatments were omitted on the morning of the ablation procedure; however, dabigatran and warfarin treatments were uninterrupted. Antiarrhythmic drugs prescribed before the ablation were continued. The procedure was performed under intravenous sedation with midazolam (0.1 mg/kg/h) and dexmedetomidine (0.2 µg/kg/h following 10 min of bolus infusion of 4 µg/kg/h). The sedation level was monitored using the Richmond Agitation–Sedation Scale (RASS) and a bispectral index (BIS). The infusion rate of sedatives (midazolam and dexmedetomidine) was adjusted to maintain a BIS level between 50 and 80. Respiration was supported by ASV under the uniform settings (S/T mode, inspiratory positive airway pressure at 8 cm H2O, expiratory positive airway pressure at 4 cm H2O, fraction of inspired oxygen (FiO2) 40%, and respirate 15 cycles/min) at the beginning of the procedure. Confirming mask fitting, changing mask size or raising the oxygen concentration level were offered according to the operator’s judgement when there were problems with oxygenation or other problems. Intravenous heparin was administered to maintain an activated clotting time of 300–400 s during the procedure.
Electroanatomical mapping was performed in sinus rhythm or atrial pacing, and electrical cardioversion was performed when the patient presented with AF during voltage mapping. Electroanatomical voltage maps of the LA were created using a 20-pole circular mapping catheter (Optima or Advisor-VL, Abbott, St. Paul, MN, or LASSO, Biosense Webster, Diamond Bar, CA, USA) or a multi-electrode high-density mapping catheter (HD grid, Abbott, or PENTARAY, Biosense Webster), with three-dimensional electroanatomical mapping systems (Navx-Ensite Velocity, Abbott, or CARTO 3, Biosense Webster). In both mapping systems, low voltage zones (LVZs) were defined as sites with a peak-to-peak electrogram amplitude of < 0.50 mV. Pulmonary vein isolation (PVI) was performed in all patients with de novo AF procedures using open-irrigated radiofrequency catheters, cryoballoons (Arctic Front Advance, Medtronic, Minneapolis, MN, USA), or laser balloons (HeartLight, CardioFocus, Marlborough, MA, USA). A water bucket developed to prevent air intrusion (AirTray, NISSHO, Shizuoka, Japan) was used in balloon catheter insertion. PVI was confirmed in patients with repeated procedures, and the pulmonary vein (PV) was re-isolated if PV reconnection was observed. Additional ablation, consisting of LA posterior box isolation [10], superior vena cava isolation, or ablation of the spatiotemporal dispersion area [11], was performed at the operator’s discretion. Air embolisms during CA for AF were defined as coronary air embolisms with ST segment elevation in inferior leads confirmed with coronary angiography or air intrusion images (e.g., in the LA or aorta).
2.4. Statistical Analysis
Continuous variables are expressed as mean ± standard deviation or median (interquartile range (IQR)). Between-group comparisons were performed using an unpaired t-test or Mann–Whitney U test. All categorical variables were presented as numbers and percentages for each group and were compared using the chi-square test or Fisher’s exact test. Logistic regression analysis was performed to detect any independent, significant predictors by adjusting the variables with multivariable models (reported as odds ratios (ORs) with 95% confidence intervals (Cis)). Variables that achieved statistical significance (p < 0.05) or were close to significance (p < 0.1) in the Spearman’s rank correlation coefficient and possible factors were included in the multiple linear regression analysis. Correlations between the INLAP and INRAP scores were analyzed using Spearman’s correlation coefficient, and freedom from AF recurrence was assessed using the Kaplan–Meier method. Survival curves were compared between the groups using a log-rank test. To evaluate the predictive value of the mean RAP during inspiration for INLAP, a receiver operating characteristic analysis was performed, calculating the area under the curve and evaluating possible cutoff points. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SPSS version 26.0 (IBM, Armonk, NY, USA). Graphs were compiled using Prism 7.03 (GraphPad, La Jolla, CA, USA).
3. Results
3.1. Subject Characteristics
A total of 381 patients (76 (19.9%) women; mean age, 63.9 ± 10.8 years) were analyzed. Table 1 presents the patients’ baseline characteristics. There were 216 (56.7%) patients with paroxysmal AF. A history of PVI was present in 40 patients (10.5%). The mean LA diameters assessed by echocardiography and the LA volume index by cardiac CT were 39.1 mm and 71.5 mL/m2, respectively.
Table 1.
Demographic and baseline characteristics in patients with and without inspiration-induced negative left atrial pressure.
| All (n = 381) | INLAP (n = 133) |
No INLAP (n = 248) |
p-Value | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 63.9 ± 10.8 | 65.2 ± 9.3 | 63.2 ± 11.5 | 0.077 |
| Female, n (%) | 76 (19.9) | 24 (18.0) | 52 (21.0) | 0.496 |
| Body mass index, kg/m2 | 24.6 ± 4.1 | 25.0 ± 4.4 | 24.4 ± 4.0 | 0.173 |
| Current smoker, n (%) | 41 (12.0) | 17 (13.8) | 24 (11.0) | 0.443 |
| Use of alcohol, n (%) | 194 (56.6) | 72 (58.5) | 122 (55.5) | 0.581 |
| Paroxysmal AF, n (%) | 216 (56.7) | 77 (57.9) | 139 (56.0) | 0.729 |
| History of prior PVI, n (%) | 40 (10.5) | 5 (3.8) | 35 (14.1) | 0.002 |
| Clinical characteristics | ||||
| HT, n (%) | 198 (52.0) | 75 (56.4) | 123 (49.6) | 0.206 |
| Dyslipidemia, n (%) | 98 (25.7) | 36 (27.1) | 62 (25.0) | 0.660 |
| DM, n (%) | 64 (16.8) | 31 (23.3) | 33 (13.3) | 0.013 |
| Stroke, n (%) | 38 (10.0) | 16 (12.0) | 22 (8.9) | 0.327 |
| Heart failure, n (%) | 101 (26.5) | 36 (27.1) | 65 (26.2) | 0.856 |
| 3% ODI | 17.0 (9.5–26.7) | 18.6 (11.2–31.1) | 15.7 (8.1–25.3) | 0.009 |
| CHADS2 score, points | 1.3 ± 1.2 | 1.5 ± 1.2 | 1.3 ± 1.2 | 0.071 |
| CHA2DS2-Vasc score, points | 2.2 ± 1.5 | 2.3 ± 1.5 | 2.1 ± 1.6 | 0.043 |
| Medications | ||||
| ACE-Is / ARBs, n (%) | 137 (36.0) | 54 (40.6) | 83 (33.5) | 0.167 |
| Beta-blockers, n (%) | 225 (59.1) | 83 (62.4) | 142 (57.3) | 0.330 |
| Type I AADs, n (%) | 153 (40.2) | 45 (33.8) | 108 (43.5) | 0.065 |
| Type III AADs, n (%) | 31 (8.1) | 9 (6.8) | 22 (8.9) | 0.474 |
| DOAC, n (%) | 350 (88.5) | 126 (94.7) | 224 (90.3) | 0.133 |
| Laboratory data | ||||
| Hb, g/dL | 14.2 ± 1.6 | 14.2 ± 1.6 | 14.2 ± 1.6 | 0.957 |
| eGFR, ml/min/1.73 m2 | 63.7 ± 16.9 | 62.7 ± 13.8 | 64.2 ± 18.3 | 0.462 |
| NT-proBNP, pg/ml | 243.0 (72.0–610.0) | 240.0 (82.0–683.0) | 249.0 (70.0–575.0) | 0.409 |
| Echocardiography | ||||
| LVEF, % | 62.7 ± 10.2 | 62.4 ± 11.0 | 62.8 ± 9.9 | 0.765 |
| LAD, mm | 39.1 ± 7.1 | 39.8 ± 7.3 | 38.7 ± 7.0 | 0.159 |
| LVDd, mm | 47.7 ± 5.9 | 48.0 ± 5.9 | 47.5 ± 5.9 | 0.434 |
| LVDs, mm | 31.3 ± 6.3 | 31.5 ± 6.6 | 31.2 ± 6.2 | 0.683 |
| Computed tomography | ||||
| LA volume index, ml/m2 | 71.5 ± 39.1 | 70.6 ± 24.2 | 72.1 ± 45.1 | 0.727 |
Data are presented as mean ± SD, median (IQR), or number (%). Abbreviations: AADs, antiarrhythmic drugs; ACE-Is, angiotensin-converting enzyme inhibitors; AF, atrial fibrillation; ARBs, angiotensin receptor blockers; DM, diabetes mellitus; DOAC, direct oral anticoagulant; eGFR, estimated glomerular filtration rate; Hb, hemoglobin; HT, hypertension; INLAP, inspiration-induced negative left atrial pressure; IQR, interquartile range; LA, left atrium; LAD, left atrial diameter; LVDd, left ventricular end-diastolic diameter; LVDs, left ventricular end-systolic diameter; LVEF, left ventricular ejection fraction; NT-proBNP, N-terminal prohormone B-type natriuretic peptide; ODI, oxygen desaturation index; PVI, pulmonary vein isolation; SD, standard deviation.
3.2. Feature of Patients with INLAP
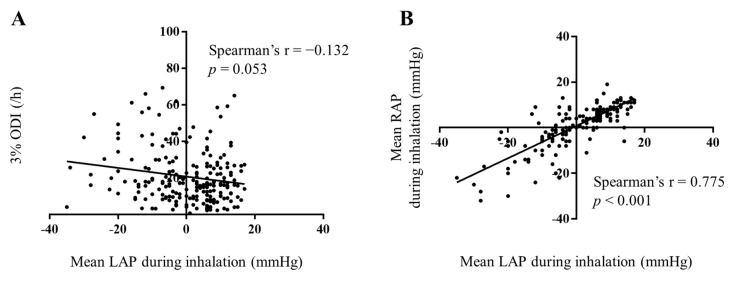
Within the entire cohort, 133 patients (34.9%) had INLAP. Patients with INLAP had a higher prevalence of diabetes mellitus (p = 0.013) than those without INLAP. Higher CHA2DS2-Vasc scores (p = 0.043) and 3% oxygen desaturation indexes (ODIs; p = 0.009) were observed in patients with INLAP. However, 3% ODI correlated very weakly with the mean LAP during inspiration (Figure 3A). There were no significant between-group differences regarding medications and the results of laboratory testing, echocardiography, and CT findings (Table 1).
Figure 3.
Association between the mean LAP during inspiration and 3% ODI value (A) or mean RAP during inspiration (B). The correlation is significant between mean LAP and mean RAP during inspiration (r = 0.775). Abbreviations: LAP, left atrial pressure; ODI, oxygen desaturation index; RAP, right atrial pressure.
No significant differences were observed in inferior vena cava (IVC) pressure during expiration; however, IVC (p < 0.001), RA (p < 0.001), and LA pressure (p < 0.001) during inspiration were lower in patients with INLAP. A higher prevalence of INRAP was detected in patients with INLAP (p < 0.001). Moreover, the mean RAP during inspiration correlated well with the mean LAP during inspiration (Figure 3B). Additional ablation for the cavotricuspid isthmus line and mitral isthmus was conducted less frequently in patients with INLAP (p = 0.007 and 0.040, respectively). Air embolism occurred in four patients with INLAP. Air embolism was suspected from ST segment elevation in the inferior leads of an electrocardiogram, fall in blood pressure, or bradycardia. Air embolism was observed after inserting the 20-pole circular mapping catheter into the LA of two patients, a multi-electrode, high-density mapping catheter into the LA of one patient, and cryoballoon into the LA of one patient. Emergent coronary angiography showed an air embolism in the right coronary artery. Fortunately, proper treatment, such as air aspiration via a catheter, ensured that there was no residual damage, including focal symptoms of stroke and myocardial wall motion impairment, in any of the cases. In contrast, no patients without INLAP experienced air embolism during CA for AF (p = 0.014). Median LA pressure during the inspiration period did not differ significantly between INLAP patients with or without air embolism (−9.5 (−4.5–−13.8) mmHg vs. −8.0 (−3.5–−14.0) mmHg; p = 0.0812). The incidence of cardiac tamponade tended to be higher in patients with INLAP than in those without (p = 0.053). The RASS and BIS index did not differ between patients with and without INLAP. No significant between-group difference was observed in the type of ablation technology and procedural and fluoroscopic times (Table 2).
Table 2.
Procedural characteristics in patients with and without INLAP.
| All (n = 381) | INLAP (n = 133) |
No INLAP (n = 248) |
p-Value | |
|---|---|---|---|---|
| INRAP, n (%) | 55 (31.2) | 51 (73.9) | 4 (3.7) | < 0.001 |
| RA pressure | ||||
| Inspiration period, mmHg | 1.2 ± 9.3 | −6.2 ± 9.9 | 6.1 ± 4.5 | < 0.001 |
| Expiration period, mmHg | 11.4 ± 5.6 | 12.0 ± 7.0 | 11.1 ± 4.5 | 0.273 |
| LA pressure | ||||
| Inspiration period, mmHg | 1.5 ± 10.4 | −10.1 ± 8.0 | 7.8 ± 4.4 | < 0.001 |
| Expiration period, mmHg | 15.9 ± 6.5 | 17.9 ± 7.3 | 14.9± 5.8 | < 0.001 |
| IVC pressure | ||||
| Inspiration period, mmHg | 5.8 ± 5.8 | 2.8 ± 6.8 | 7.9 ± 3.9 | < 0.001 |
| Expiration period, mmHg | 11.2 ± 6.0 | 11.5 ± 7.7 | 11.0 ± 4.5 | 0.539 |
| Low voltage zones, n (%) | 77 (20.2) | 33 (24.8) | 44 (17.7) | 0.101 |
| Type of ablation technology | ||||
| Radiofrequency catheters, n (%) | 106 (27.8) | 43 (32.3) | 63 (25.4) | 0.150 |
| Cryoballoons and laser balloons, n (%) | 275 (72.2) | 90 (67.7) | 185 (74.6) | 0.150 |
| Additional ablation, n (%) | ||||
| CTI, n (%) | 179 (47.0) | 50 (37.6) | 129 (52.0) | 0.007 |
| Bottom line, n (%) | 123 (32.3) | 39 (29.3) | 84 (33.9) | 0.365 |
| Mitral isthmus, n (%) | 25 (6.6) | 4 (3.0) | 21 (8.5) | 0.040 |
| Complications | ||||
| Air embolism, n (%) | 4 (1.0) | 4 (3.0) | 0 (0.0) | 0.014 |
| Diaphragmatic paralysis, n (%) | 9 (2.4) | 4 (3.1) | 5 (2.0) | 0.725 |
| Bleeding from puncture site, n (%) | 6 (1.6) | 2 (1.5) | 4 (1.6) | 1.000 |
| Cardiac tamponade, n (%) | 7 (1.8) | 5 (3.8) | 2 (0.8) | 0.053 |
| Sedation level | ||||
| RASS | −4.0 (−4.0–−5.0) | −4.0 (−4.0–−5.0) | −4.0 (−4.0–−4.0) | 0.131 |
| BIS | 57.0 (48.0–70.0) | 58.5 (50.8–73.0) | 56.0 (47.0–68.0) | 0.098 |
| Procedure time, minute | 207.3 ± 83.8 | 208.5 ± 81.2 | 206.6 ± 85.3 | 0.834 |
| Fluoroscopic time, minute | 53.4 ± 34.8 | 50.5 ± 32.4 | 54.9 ± 35.9 | 0.234 |
Data are presented as mean ± SD, median (IQR), or number (%). Abbreviations: BIS, bispectral index; CBs, cryoballoons; CTI, cavotricuspid isthmus line; INLAP, inspiration-induced negative left atrial pressure; INRAP, inspiration-induced negative right atrial pressure; IQR, interquartile range; IVC, inferior vena cava; RASS, Richmond Agitation–Sedation Scale; SD, standard deviation.
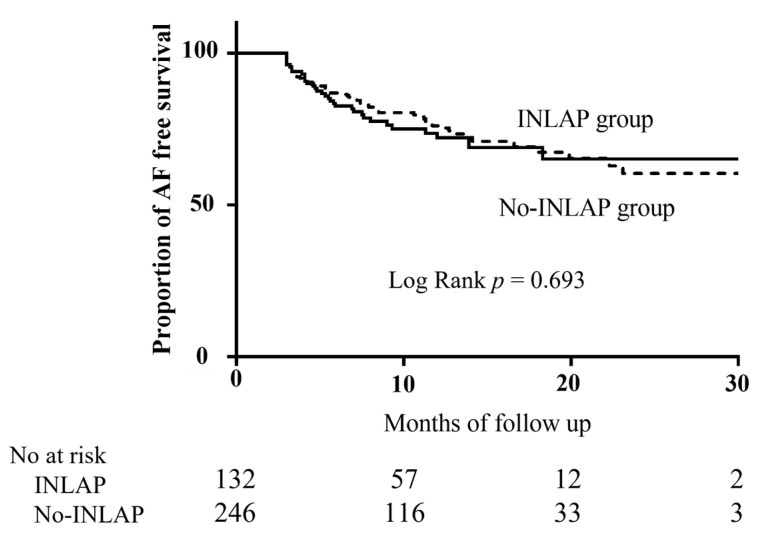
During a median follow-up period of 8.7 (IQR 6.16–13.3) months, AF recurrence occurred in 92 (24.1%) patients. The Kaplan–Meier survival curve showed no significant difference in AF recurrence between patients with and without INLAP (p = 0.693 by log-rank test; Figure 4).
Figure 4.
Kaplan–Meier curve of freedom from AF recurrence. Abbreviations: AF, atrial fibrillation; INLAP, inspiration-induced negative left atrial pressure.
3.3. Prediction of INLAP
Multiple linear regression analysis was performed to identify the factors associated with the decrease in LA pressure in the inspiration period. Age, body mass index, diabetes mellitus, INRAP, 3% ODI (categorized into the following three groups according to their value: <5, 5–10, and >10), and history of prior PVI were included as factors. Multiple linear regression analysis demonstrated that the presence of INRAP was independently associated with the decrease in LA pressure in the inspiration period (p < 0.001; Table 3).
Table 3.
Multiple linear regression analysis of variables associated with predictors of the decrease in LA pressure during the inspiration period (presence of INRAP predicts the decrease in LA pressure during inspiration period).
| Variable | Beta | Standard Error | T Value | p-Value |
|---|---|---|---|---|
| (Constant) | 1.785 | 6.358 | 0.281 | 0.779 |
| Age | 0.002 | 0.062 | 0.034 | 0.973 |
| BMI | 0.201 | 0.158 | 1.267 | 0.207 |
| DM | 0.954 | 1.524 | 0.626 | 0.532 |
| INRAP | −16.795 | 1.238 | −13.571 | < 0.001 |
| 3% ODI | −0.780 | 0.876 | −0.890 | 0.375 |
| History of prior PVI | 0.063 | 2.225 | 0.028 | 0.978 |
| R2 = 0.532 (p < 0.001) |
The 3% ODI is categorized into the following three groups according to its value: <5, 5–10, and >10. Abbreviations: BMI, body mass index; CI, confidence interval; DM, diabetes mellitus; INLAP, inspiration-induced negative left atrial pressure; INRAP, inspiration-induced negative right atrial pressure; ODI, oxygen desaturation index; PVI, pulmonary vein isolation.
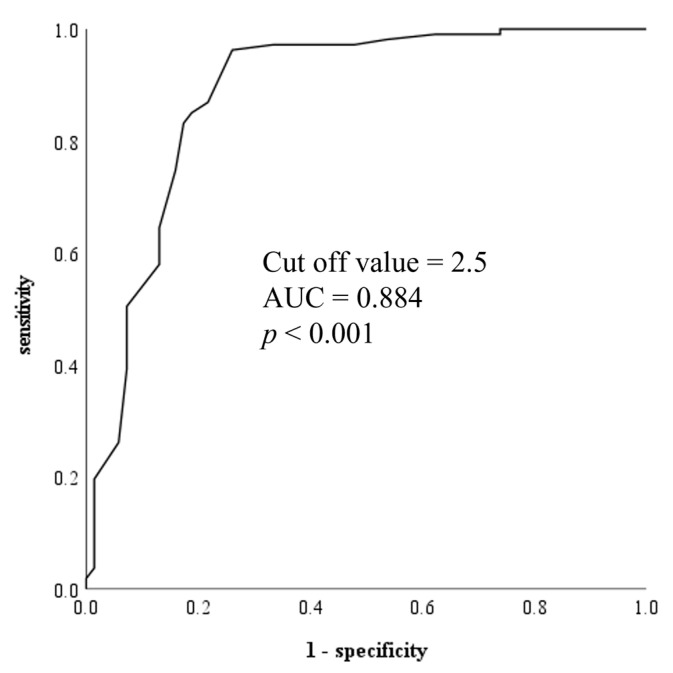
The receiver operating characteristic curve used to evaluate the predictive value of the mean RAP during inspiration in distinguishing between those with and without INLAP during PVI revealed an area under the curve of 0.884 (p < 0.001). An RAP during inspiration that was less than 2.5 mmHg identified the presence of INLAP with a sensitivity and specificity of 83.2% and 82.6%, respectively (Figure 5).
Figure 5.
Receiver operating characteristic curve of RAP during inspiration. Abbreviations: AUC, area under the curve; RAP, right arterial pressure.
4. Discussion
4.1. Main Findings
The main findings of this study were as follows: 1) approximately one third of the patients undergoing PVI under deep sedation with ASV had INLAP during the ablation procedure, 2) the presence of INLAP can be predicted from the RAP, 3) the presence of INLAP could cause air embolisms during PVI, and 4) the presence of INLAP was not associated with AF recurrence after PVI.
4.2. Clinical Impact and Importance of Air Embolism
The Japanese Heart Rhythm Society emphasized the risk of air embolism during cryoballoon ablation in 2018. In addition, a comprehensive review suggested the importance of detecting and managing air embolism [12]. An ex vivo study revealed that air intrusion, the cause of air embolism, is associated with the following two factors: the catheter system (comprising catheters of different vascular diameters) and the negative pressure in the sheath [13]. It was reported that negative LAP was an important factor in air embolism [8], and ex vivo experiments that evaluated air-tightness in response to negative pressures revealed air aspiration at −11 to −13 mmHg of suction [13]. All four cases of air embolism in this study were observed in patients with INLAP. Air intrusion in the left heart system can lead to more severe conditions or after-effects (i.e., myocardial or brain infarctions) than that of the right heart system (pulmonary embolism). Therefore, much attention should be paid to air embolisms in patients with INLAP.
4.3. Possible Mechanisms of INLAP
In this study, INLAP was observed in 133 (34.9%) patients under deep sedation with ASV, signifying that INLAP is not rare and could even occur with ASV in patients undergoing PVI. Patients with obstructive sleep apnea (OSA) show negative LA pressure [13]. Although the 3% ODI values were higher in patients with INLAP, the correlation was weak. In addition, this study revealed that INLAP was not associated with LA volume and the presence of LVZs, which indicates LA remodeling [14].
Furthermore, the presence of INLAP did not affect the recurrence of AF after PVI. From the above, it seems that INLAP is not associated with AF’s pathophysiology or PVI’s effectiveness. Additionally, since OSA was associated with the presence of LA enlargement [15] and recurrence of AF after CA [16], the severity of OSA could not be associated with the presence of INLAP.
A previous study that reports LAP during atrial septal defect/patent foramen ovale closure revealed that sedation provoked a marked decline in the mean inspiratory LAP compared to non-sedated patients [8]. Deep sedation using propofol or midazolam is associated with a profound drop in LAP [17,18]. In addition, a previous report revealed that patients that require airway management tools due to airway obstruction during CA showed substantial negative esophageal pressure compared to those without airway obstruction [19]. Since our findings showed no significant differences in IVC pressure during expiration, the patients’ circulating plasma volume statuses, including overflow and dehydration, did not affect the presence of INLAP. Thus, airway obstruction due to the decrease in muscle tonus under deep sedation, which could not be removed using ASV, could cause INLAP.
A recent report showed that the prevalence of INLAP significantly reduced after ASV (pre-ASV 73% vs. post-ASV 14%) [20], which is lower than our result of 35%. A possible reason for the difference in the prevalence of INLAP in patients during PVI with ASV between the two studies could be that the BIS level was higher in the previous report than in the present study (68.5 ± 12.7 vs. 59.3 ± 14.4). This finding that deeper sedation was associated with a higher prevalence of INLAP could support this as a suspected mechanism of INLAP rather than the severity of sleep apnea. Deep sedation was mainly responsible for INLAP development in patients undergoing PVI under deep sedation with ASV.
4.4. Prevention of Complications Due to INLAP
We found that RAP could predict the decrease in LA pressure during the inspiration period, which is a crucial finding. Predicting the presence of INLAP and taking proper provisions before a transseptal puncture enables us to prevent systemic air embolisms. The RAP can be measured easily by connecting a long sheath to a pressure transducer. We should pay great attention to the occurrence of systemic air embolisms if a mean RAP during inspiration < 2.5 mmHg is observed before a transseptal puncture. It has been reported that a water bucket developed to prevent air intrusion (AirTray, NISSHO, Shizuoka, Japan or SAFE BOAT, DVx, Tokyo, Japan) could reduce the incidence of air embolisms [7]. Such equipment should be used for patients with INLAP in the insertion of any catheters, as we reported air embolism even when a catheter other than a balloon catheter was inserted. Furthermore, since the incidence of INLAP was high (34.9% in this study) under deep sedation with ASV, the routine use of such devices may be recommended. Furthermore, our findings demonstrated that patients with INLAP have an increased risk of cardiac tamponade than those without INLAP. An unstable respiration pattern is often observed in patients with INLAP due to airway obstruction, which could be responsible for the occurrence of cardiac tamponade because of the sudden increase in the contact force of an ablation catheter.
Additionally, we hypothesize that the use of the nasal airway in addition to ASV could help to relieve airway obstruction in patients with INLAP under deep sedation with ASV and we are conducting further research to assess this hypothesis.
4.5. Study Limitations
This study had several limitations. First, this was a single-center retrospective study, and the small sample size limited its predictive power. Second, four cases of air embolism were observed in this study. Although the CA procedures were performed by well-trained operators, the incidence of air embolisms and cardiac tamponade may still be affected by the skill of the operators. In addition, it was reported that the asymptomatic cerebral embolism during cryoballoon ablation of AF was observed in 22.9% of patients [21]. Since brain magnetic resonance imaging was not performed routinely after ablation, silent air embolisms were not evaluated. In addition, as the coronary angiography was conducted for patients with ST elevation in ECG, slight changes that are difficult to recognize, including transient ST elevation, could be underestimated. Third, since all the patients received CA procedures under deep sedation using an ASV, we have no control group without sedation and the use of ASV. Fourth, we did not evaluate the detailed cardiac morphology and hemodynamics of the patients, including interventricular septum thickness, trans mitral flow velocity pattern, and blood pressure that could affect the presence of INLAP. Although we did not find any relationship between INLAP and BNP levels in the present study, there is a report in the literature that discusses the relationship between BNP and left atrial morphology [22]. Further investigation that explores the relationships among LA morphology, biomarkers, and the presence of INLAP is needed.
5. Conclusions
INLAP during CA was not rare in patients undergoing CA for AF under deep sedation, even with ASV. Much attention should be paid to air embolisms in patients with INLAP.
Author Contributions
Conceptualization, T.I. and Y.N.; methodology, Y.N.; formal analysis, T.I.; investigation, Y.K., T.S., T.N., M.S. (Makoto Sano) and T.U.; data curation, Y.K., T.S., T.N., M.S. (Makoto Sano) and T.U.; writing—original draft preparation, T.I.; writing—review and editing, Y.N., S.M., K.S., H.O. and M.S. (Masao Saotome); supervision, Y.M. All authors reviewed the manuscript draft and critically revised the intellectual content. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the Human Investigations Committee of the Hamamatsu University School of Medicine (approval number #20-361, date of approval: 3/3/2021).
Informed Consent Statement
Written informed consent was obtained from all patients before enrollment.
Data Availability Statement
The deidentified participant data will not be shared.
Conflicts of Interest
There are no conflict of interest to declare.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.-A., Dilaveris P.E., et al. ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020;42:373–498. doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 2.Kirchhof P., Camm A.J., Goette A., Brandes A., Eckardt L., Elvan A., Fetsch T., van Gelder I.C., Haase D., Haegeli L.M., et al. Early rhythm-control therapy in patients with atrial fibrillation. N. Engl. J. Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 3.Mark D.B., Anstrom K.J., Sheng S., Piccini J.P., Baloch K.N., Monahan K.H., Daniels M.R., Bahnson T.D., Poole J.E., Rosenberg Y., et al. Effect of catheter ablation vs medical therapy on quality of life among patients with atrial fibrillation: The CABANA randomized clinical trial. JAMA. 2019;321:1275–1285. doi: 10.1001/jama.2019.0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hakalahti A., Biancari F., Nielsen J.C., Raatikainen M.J. Radiofrequency ablation vs. antiarrhythmic drug therapy as first line treatment of symptomatic atrial fibrillation: Systematic review and meta-analysis. Europace. 2015;17:370–378. doi: 10.1093/europace/euu376. [DOI] [PubMed] [Google Scholar]
- 5.Deshmukh A., Patel N.J., Pant S., Shah N., Chothani A., Mehta K., Grover P., Singh V., Vallurupalli S., Savani G.T., et al. In-hospital complications associated with catheter ablation of atrial fibrillation in the United States between 2000 and 2010: Analysis of 93 801 procedures. Circulation. 2013;128:2104–2112. doi: 10.1161/CIRCULATIONAHA.113.003862. [DOI] [PubMed] [Google Scholar]
- 6.Calkins H., Hindricks G., Cappato R., Kim Y.H., Saad E.B., Aguinaga L., Akar J.G., Badhwar V., Brugada J., Camm J., et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation. Heart Rhythm. 2017;14:e275–e444. doi: 10.1016/j.hrthm.2017.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miyazaki S., Hasegawa K., Mukai M., Ishikawa E., Aoyama D., Nodera M., Kaseno K., Ishida K., Uzui H., Tada H. Clinically manifesting air embolisms in cryoballoon ablation: Can novel water buckets reduce the risk? JACC Clin. Electrophysiol. 2020;6:1067–1072. doi: 10.1016/j.jacep.2020.07.012. [DOI] [PubMed] [Google Scholar]
- 8.Franzen O.W., Klemm H., Hamann F., Koschyk D., Von Kodolitsch Y., Weil J., Meinertz T., Baldus S. Mechanisms underlying air aspiration in patients undergoing left atrial catheterization. Catheter. Cardiovasc. Interv. 2008;71:553–558. doi: 10.1002/ccd.21445. [DOI] [PubMed] [Google Scholar]
- 9.Aksu T., Guler T.E., Yalin K., Golcuk S.E., Ozcan K.S., Guler N. A novel deep inspiration maneuver for difficult transseptal puncture. Am. J. Cardiol. 2017;119:428–433. doi: 10.1016/j.amjcard.2016.10.023. [DOI] [PubMed] [Google Scholar]
- 10.Thiyagarajah A., Kadhim K., Lau D.H., Emami M., Linz D., Khokhar K., Munawar D.A., Mishima R., Malik V., O’Shea C., et al. Feasibility, safety, and efficacy of posterior wall isolation during atrial fibrillation ablation: A systematic review and meta-analysis. Circ. Arrhythm. Electrophysiol. 2019;12:e007005. doi: 10.1161/CIRCEP.118.007005. [DOI] [PubMed] [Google Scholar]
- 11.Seitz J., Bars C., Théodore G., Beurtheret S., Lellouche N., Bremondy M., Ferracci A., Faure J., Penaranda G., Yamazaki M., et al. AF ablation guided by spatiotemporal electrogram dispersion without pulmonary vein isolation: A wholly patient-tailored approach. J. Am. Coll. Cardiol. 2017;69:303–321. doi: 10.1016/j.jacc.2016.10.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aksu T., Yalin K., Guler T.E., Bozyel S., Heeger C.H., Tilz R.R. Acute procedural complications of cryoballoon ablation: A comprehensive review. J. Atr. Fibrillation. 2019;12:2208. doi: 10.4022/jafib.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsukahara K., Oginosawa Y., Fujino Y., Ohe H., Yamagishi Y., Iwataki M., Sonoda S., Kohno R., Otsuji Y., Abe H. Prevention of serious air embolism during cryoballoon ablation; risk assessment of air intrusion into the sheath by catheter selection and change in intrathoracic pressure: An ex vivo study. J. Cardiovasc. Electrophysiol. 2019;30:2944–2949. doi: 10.1111/jce.14208. [DOI] [PubMed] [Google Scholar]
- 14.Dinov B., Kosiuk J., Kircher S., Bollmann A., Acou W.-J., Arya A., Hindricks G., Rolf S. Impact of metabolic syndrome on left atrial electroanatomical remodeling and outcomes after radiofrequency ablation of nonvalvular atrial fibrillation. Circ. Arrhythm. Electrophysiol. 2014;7:483–489. doi: 10.1161/CIRCEP.113.001185. [DOI] [PubMed] [Google Scholar]
- 15.Somers V.K., Mark A.L., Zavala D.C., Abboud F.M. Influence of ventilation and hypocapnia on sympathetic nerve responses to hypoxia in normal humans. J. Appl. Physiol. 1989;67:2095–2100. doi: 10.1152/jappl.1989.67.5.2095. [DOI] [PubMed] [Google Scholar]
- 16.Ng C.Y., Liu T., Shehata M., Stevens S., Chugh S.S., Wang X. Meta-analysis of obstructive sleep apnea as predictor of atrial fibrillation recurrence after catheter ablation. Am. J. Cardiol. 2011;108:47–51. doi: 10.1016/j.amjcard.2011.02.343. [DOI] [PubMed] [Google Scholar]
- 17.Eastwood P.R., Platt P.R., Shepherd K., Maddison K., Hillman D.R. Collapsibility of the upper airway at different concentrations of propofol anesthesia. Anesthesiology. 2005;103:470–477. doi: 10.1097/00000542-200509000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Norton J.R., Ward D.S., Karan S., Voter W.A., Palmer L., Varlese A., Rackovsky O., Bailey P. Differences between midazolam and propofol sedation on upper airway collapsibility using dynamic negative airway pressure. Anesthesiology. 2006;104:1155–1164. doi: 10.1097/00000542-200606000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Iwasaki Y.-K., Fujimoto Y., Oka E., Hagiwara K.I., Takahashi K., Tsuboi I., Hayashi H., Yodogawa K., Hayashi M., Miyauchi Y., et al. Esophageal pressure monitoring for airway management during catheter ablation of atrial fibrillation. Int. J. Cardiol. Heart Vasc. 2021;33:100771. doi: 10.1016/j.ijcha.2021.100771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawaguchi N., Suzuki A., Usui M., Yoshikawa S., Watanabe S., Maeno R., Kujiraoka H., Sato K., Goya M., Sasano T. Clinical effect of adaptive servo-ventilation on left atrial pressure during catheter ablation in sedated patients with atrial fibrillation. Circ. J. 2021;85:1321–1328. doi: 10.1253/circj.CJ-20-1263. [DOI] [PubMed] [Google Scholar]
- 21.Yokoyama M., Tokuda M., Tokutake K., Sato H., Oseto H., Yokoyama K., Kato M., Narui R., Tanigawa S.-I., Yamashita S., et al. Effect of air removal with extracorporeal balloon inflation on incidence of asymptomatic cerebral embolism during cryoballoon ablation of atrial fibrillation: A prospective randomized study. Int. J. Cardiol. Heart Vasc. 2022;40:101020. doi: 10.1016/j.ijcha.2022.101020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sielski J., Grabowska U., Kaziród-Wolski K., Matejko M. Correlation analysis of the relationship between B-type natriuretic peptide and selected echocardiographic parameters in patients with permanent pacemakers. Med. Stud./Stud. Med. 2015;31:241–248. doi: 10.5114/ms.2015.56665. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The deidentified participant data will not be shared.