Abstract
Globally, tuberculosis (TB) was the leading cause of death from a single infectious agent until the coronavirus (COVID-19) pandemic. In 2020, an estimated 10 million people fell ill with TB and a total of 1.5 million people died from the disease. About one-quarter of the global population, almost two billion people, is estimated to be latently infected with Mycobacterium tuberculosis (MTB). Although latent TB infection (LTBI) is asymptomatic and noncontagious, about 5–10% of LTBI patients have a lifetime risk of progression to active TB. The diagnosis and treatment of active cases are extremely vital for TB control programs. However, achieving the End TB goal of 2035 without the ability to identify and treat the pool of latently infected individuals will be a big challenge. To do so, improved technology to provide more accurate diagnostic tools and accessibility are crucial. Therefore, this chapter covers the current WHO-endorsed tests and advances in diagnostic and screening tests for active and latent TB.
Keywords: Tuberculosis, Latent TB infection, Mycobacterium tuberculosis, Nucleic acid amplification test, Tuberculin skin test, Interferon gamma release assays, Tuberculosis antigen-based skin test
Abbreviations
- TB
Tuberculosis
- LTBI
Latent TB infection
- MTB
Mycobacterium tuberculosis
- PTB
pulmonary TB
- AFB
Acid-fast bacillus
- WHO
World Health Organization
- NAAT
Nucleic acid amplification test
- TST
Tuberculin skin test
- IGRA
Interferon gamma release assays
- PPD
Purified protein derivative
- QFT-Plus
QuantFERON-TB gold plus
- TBST
uberculosis antigen-based skin test
- IFN-γ
Interferon gamma
1. Introduction
Tuberculosis (TB), an ancient disease, remains a major global killer. In 1882, Robert Koch reported the successful isolation of the causative agent, Mycobacterium tuberculosis [1], an aerobic, non-motile, rod-shaped acid-fast bacterium in the family Mycobacteriaceae [2]. Although M. tuberculosis (MTB) is primarily a contagious respiratory pathogen, it can also spread to other parts of the body [3]. As a result, TB claimed the lives of billions over the centuries and continues with 1.5 million deaths in 2020 worldwide [4]. The high mortality associated with TB has been documented archeologically, i.e., Pott’s disease of the spine, in Egyptian mummies [5]. More recently, molecular studies confirmed the presence of the genetic material of the pathogen in specimens from ancient skeletons [6].
Infection with MTB causes patients to develop one of two disease conditions: latent or active TB ( Table 1). Active TB is caused by the inability of the host immune system to defend against the invading pathogen. In this scenario, the disease is symptomatic due to a recent infection and can spread to others. Alternatively, active TB can be due to a reactivation of a latent infection. In fact, more than 80% of active TB cases nowadays are attributed to reactivation of untreated latent TB in low-incidence countries such as the United States [7]. Latent TB infection (LTBI) is a noninfective state where the bacteria is contained immunologically. In LTBI, the disease is asymptomatic and can’t spread to others [8]. Although only 5–10% of non-treated LTBI patients may develop active TB, the risk of progression to active TB disease becomes significantly greater in high-risk immunocompromised individuals [9]. Therefore, diagnosis and treatment of LTBI is critical to reduce the rate of TB below the elimination threshold [10]. In this chapter, we review the current World Health Organization (WHO)-endorsed tests and advances in diagnostic and screening tools for active and latent TB.
Table 1.
Characteristics of latent TB infection vs TB disease.
| Latent TB infection | TB disease | |
|---|---|---|
| Symptoms | None | Hemoptysis, chest pain, unexplained weight loss, night sweats, persistent cough and fever |
| Physical appearance | Well-looking, feels healthy | Unwell, feels sick |
| Skin or blood test | Positive result indicating TB infection | Positive result indicating TB infection |
| TB bacterial infection | Previous infection Cannot spread the disease to others | Recent infection Can spread the disease to others |
| Chest X-ray | Normal | Abnormal |
| Sputum smear or culture | Negative | Positive |
| Treatment | Encouraged to decrease the risk of progression to active disease | Recommended to treat TB disease |
2. Active tuberculosis
Tuberculosis disease can occur in various anatomical sites, but can be divided into two main groups, pulmonary TB disease and extrapulmonary disease. Because TB disease predominantly affects the lungs, this form is commonly referred to as pulmonary disease. A person with TB pulmonary disease may present symptoms of hemoptysis, chest pain, unexplained weight loss, night sweats, persistent cough and fever. Although these symptoms are not specific to TB disease, TB should be included in the differential diagnosis especially in geographic regions of high prevalence. The 2006 WHO TB diagnostic criteria rely on clinical data vs laboratory testing. Clinical diagnosis may be based on epidemiologic exposure with physical findings, radiographic findings, positive tuberculin skin test or interferon-gamma release assay, analysis of sputum or bronchoscopy specimens, or histopathology analysis [11], [12]. In some cases, bacteriologic confirmation is not possible. In resource limited countries like Kenya, treatment is generally initiated without laboratory evidence that is largely based on clinical symptoms and disease prevalence [13]. Meta-analysis of this approach demonstrated clinical sensitivity and specificity of 61% and 69%, respectively [14]. Mortality rate is double in clinically vs bacteriologically diagnosed patients [13]. Furthermore, empiric treatment could also increase unnecessary administration of TB drugs resulting in increased antimicrobial resistance. Although radiographic anomalies found in screening are inadequate for initiation of treatment, supplemental sputum testing and bronchoscopy improves diagnostic accuracy [15].
Extrapulmonary TB (EPTB) disease symptoms exhibit the same general symptoms of pulmonary disease, i.e., fatigue, unexplained weight loss, night sweats and fever. But additional symptoms may be present depending on disease location within the body [16]. WHO defined EPTB as any bacteriologically confirmed or clinically diagnosed case of TB involving organs other than the lungs and tuberculous intra-thoracic lymphadenopathy (mediastinal and/or hilar) or tuberculous pleural effusion, without radiographic abnormalities in the lungs [17]. Renal TB may present with pyuria [18], back pain is the most common symptom for spinal TB [19], and hoarseness if the larynx is affected [20]. Prevalence factors associated with EPTB include female gender, young age, Asian or African origin and HIV infection [21]. Further discussion will focus on pulmonary TB (PTB) disease.
In patients suspected of PTB disease, testing is initiated by analysis of sputum acid-fast bacillus (AFB) smear and culture. Unfortunately, low- and middle-income countries often have high TB prevalence and rely heavily on outdated diagnostic tests [22]. In 2010, the WHO endorsed nucleic acid amplification and drug resistance testing for TB screening and diagnosis. In 2021, the WHO updated the TB diagnosis guidelines, reported on commercially available nucleic acid amplification test (NAAT) platforms and developed an algorithm for disease identification ( Fig. 1). NAAT provides accurate and rapid turn-around time and identifies the species and antibiotic susceptibility. Accordingly, the WHO recommends NAAT in addition to existing TB tests such as AFB smear, bacterial culture and antibiotic susceptibility.
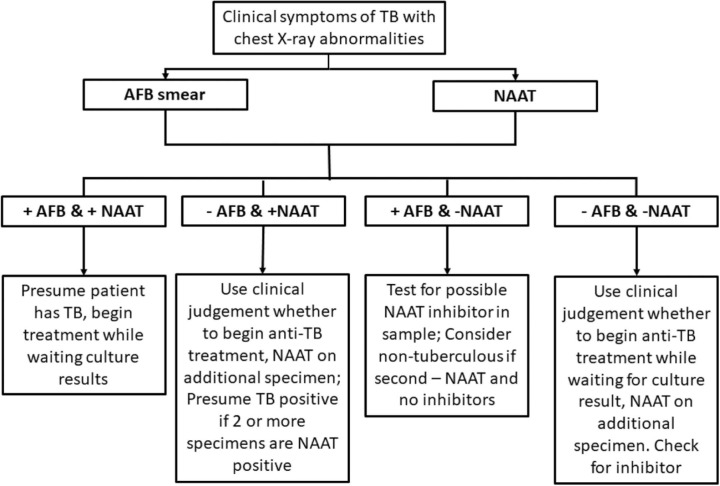
Fig. 1.
TB testing algorithm and interpretation using AFB and NAAT results [54]. AFB and NAAT has faster turn-around time compared to bacterial culture. These two tests can be used to guide the management of patients suspected of having TB based on clinical symptoms and chest x-ray abnormalities.
2.1. Sample types and collection methods
Sample type and collection are essential components in obtaining optimal results. Sputum, a thick sticky mucus which accumulates during infection and is expectorated from the respiratory tract, is most appropriate for identification of pulmonary TB disease.
As can be appreciated, analytic sensitivity is dependent on sample type. Sputum, the most common, can be obtained spontaneously by coughing or induction. However, in infants and children spontaneous sputum collection is not generally feasible [23]. Also, about one third of HIV-positive individuals with suspected TB are sputum-scarce [24]. Other more invasive and costly approaches such as bronchoscopic sampling and gastric washing may be utilized as alternative sample types [25]. It should be noted, however, the specimens not indicated in the manufacturer instructions will require validation prior to use.
Sputum induction with nebulized hypertonic saline can help produce adequate sputum for diagnostic testing [26], [27]. In this procedure, the patient inhales the nebulized solution which promotes coughing and enables expectoration of respiratory secretions. Induced sputum tends to be waterier than spontaneously obtained sputum and could be misidentified as saliva. Unfortunately, a side effect of hypertonic solution exposure is bronchoconstriction. In general, induction is an effective alternative and generally produces adequate specimen volume (>1.0 mL) for testing [28].
In indicated above, HIV patients tend to be sputum-scarce, so testing for TB in this population can be problematic. Furthermore, HIV patients tend to have lower MTB load in respiratory secretions [29]. A non-invasive oral swab collection was evaluated in TB positive patients with or without HIV co-infection [30]. The study found that PCR signal amplification was weaker in HIV-positive patients. Tongue swabs yielded the strongest signal (vs gum and cheek swabs) and demonstrated a sensitivity and specificity of 78% and 96%, respectively.
Timing of collection is also important. The WHO recommends collecting two sputum samples with at least one being early morning [31]. The morning specimen is preferred because respiratory secretions pool overnight. Smear microscopy has shown that morning sputum samples yielded higher positivity rates for acid fast bacilli vs random samples [32].
In summary, sample type, collection method and timing must be appropriate for the type of assay being employed. Evaluation of diagnostic accuracy is necessary for different sample types and is obligatory.
2.2. Smear microscopy
Microbial confirmation of pulmonary TB is vital to reduce empiric antibiotic use that potentially contributes to multi-drug resistance. Microscopic analysis of sputum smear is a quick and easy to assess bacteriologic evidence of mycobacteria. Acid-Fast staining, i.e., Ziehl-Neelsen, Kinyoun and Auramin-Rhodamine Fluorochrome, takes advantage of the mycolic acids present in the outer membrane of acid-fast mycobacteria.
Ziehl-Neelsen and Kinyoun methods use carbolfuchsin. The main difference is the use of heat in the former while no heat and higher dye concentration is used in the latter. Acid-Fast bacillus stain red when exposed to carbolfuchsin even after washing with acid alcohol. Slides can be analyzed using standard bright-field microscopy. Auramine O and rhodamine B are fluorescent dyes that also bind mycolic acids. Stained acid-fast organisms are identified by fluorescence microscopy which requires specific filters for proper visualization. Acid-fast bacteria fluoresce yellow-orange.
Both fluorescence microscopy and Ziehl-Neelsen staining had 100% specificity using the Xpert MTB/RIF as the reference test [32]. In addition, the former demonstrated higher sensitivity (84.5% vs 54.8%) and shorter reading time.
Smear microscopy is a fast and simple approach for detecting mycobacteria. Although fluorescence microscopy demonstrated better diagnostic values, cost was substantially higher. Smear results depend on bacterial burden, samples with low bacterial burden may be missed. Positive smears require further testing for speciation. In addition, other mycolic acid containing species, i.e., Rhodococcus, Nocardia and Corynebacterium, can be incorrectly identified as mycobacteria [33]. As such, smear microscopy is generally insufficient and further testing is needed to differentiate MTB from other mycobacteria and other species with mycolic acids. Conventional selective bacterial culture can be subsequently used to identify the mycobacterial species.
2.3. Bacterial culture
MTB is slow growing. Due to the challenges of cultivation, the use of one liquid and one solid media is recommended to maximize recovery [34]. A two year study published in 1975 compared four culture media that included Lowenstein-Jensen (LJ), Middlebrook (7H10), Petragnani and ribonucleic acid (RNA) media and found that LJ and RNA media were best for routine practice [35]. The recommended media for isolating MTB includes egg- or agar-based media supplemented with green malachite and Middlebrook broths or solid media [36]. Culture typically requires 3–8 weeks to detect growth from clinical samples.
Although bacterial culture is considered the gold standard, contamination with other bacteria and fungi could undermine its effectiveness [37]. Samples are processed using N-acetyl-L-cysteine-sodium hydroxide, a common chemical used for decontamination, prior to culturing. The use of selective media such as LJ medium is most common, especially in resource limited settings. Addition of antibiotics to LJ media dramatically decreases contamination (32–2.3%) when supplemented with Selectatab-MB [38].
Drug susceptibility of MTB isolate is performed by observing growth or metabolic inhibition in a medium containing an anti-tuberculosis drug. Unfortunately, manual culturing extends resulting time especially in high TB burden countries. However, newer techniques are now available to shorten detection of growth inhibition. The most widely used US FDA-cleared automated systems for rapid detection of mycobacteria using liquid media are BacT/ALERT 3D (Biomerieux), BACTEC MGIT (Becton Dickinson) and VersaTREK (Thermo Fisher). These employ automated continuous monitoring of carbon dioxide (CO2) production or oxygen (O2) consumption to detect MTB. BACTEC and VersaTrek are also FDA-cleared for susceptibility testing of the MT complex (MTBC). Due to the possibility of instrument negative mycobacterial growth, some manufacturers recommend visual inspection [39].
BACTEC MGIT 960, a non-radiometric version of BACTEC 460 and fully automated system, detects growth by measuring O2 consumption in the culture vial via an O2-sensitive fluorescent indicator [40]. Using this approach, mean detection time improved from 38.6 to 21.4 days for abscess samples vs those cultured in LJ media [41]. Smear negative sputum, bronchial aspirate and pleural fluid had higher positivity rate (16.2% vs 9.8%) and shorter detection time (11.1 vs 30.7 days) when BACTEC MFIT was compared to LJ culture [42]. A 3-year Massachusetts General Hospital study reported that found that 10% of all cultures yielded mycobacteria using BACTEC MGIT. Visual inspection of instrument-negative samples revealed colony-like clumps at the bottom of the tube and hence recommended supplementary procedures after 42 days incubation. This included comparing AFB smears, visual inspection and performing an additional AFB smear for the colony-like clumps.
The BacT/ALERT system utilizes a colorimetric CO2 sensor. Parallel testing of the BacT/ALERT and BACTEC MGIT 960 systems demonstrated the latter had shorter detection time (13.5 vs 25.2 days) [43], lower contamination rate [44] and higher sensitivity (100% vs 66.6%) [43]. The authors suggested that longer incubation times may improve recovery using BacT/ALERT system. VersaTREK monitors headspace pressure due to O2 consumption to detect bacterial growth. An MTB susceptibility study that compared the VersaTREK and BACTEC MGIT 960 reported 100% concordance for isoniazid, rifampin and ethambutol and 97% concordance for pyrazinamide [45].
One difficulty in containing and managing TB is patient follow-up compliance that disrupts continuity of care and promotes disease spread. The ability to decrease bacterial culture time is important to stop TB spread by enabling earlier use of anti-tuberculosis medication. Unfortunately, culturing has limitations. Positive-smear culture-negative specimens underscore the possibility of non-cultivable MTB. In addition, MTB is also prone to transitioning to a dormant non-replicative phenotype believed the source of latent TB infection [46]. Clinical and experimental studies indicate that the composition of MTB is likely heterogenous [47]. Active replicating bacilli are killed effectively by first-line drugs such as isoniazid while dormant MTB may not be eliminated. The latter further underscores the limitation of culturing as a diagnostic test.
Nucleic acid tests can be used in symptomatic patients with AFB positive or negative culture results. NAAT does not replace microbiologic tests and is considered complementary.
2.4. Nucleic acid test
Nucleic acid testing (NAT) identifies a species or subspecies of an organism by detecting the genetic make-up, i.e., ribonucleic acid (RNA) or deoxyribonucleic acid (DNA). NATs coupled with PCR, i.e., nucleic acid amplification testing (NAAT), enables trace or ultra-low detection.
In 2010, the WHO endorsed the Xpert MTB/RIF assay (Cepheid, USA) for TB testing [48]. It is the most commonly used test worldwide (>130 countries) [49]. In 2013, the US FDA approved the Xpert MTB/RIF for TB diagnosis [4]. Although this assay simultaneously detects MTBC and its susceptibility to rifampicin, it does not detect resistance to isoniazid. The Xpert MTB/RIF utilizes nested real-time PCR for the qualitative detection of the MTB complex and RIF resistance [50]. The WHO Consolidated Guidelines on Tuberculosis Module 3 recommends that Xpert MTB/RIF be used in adults and children with signs and symptoms of pulmonary TB as an initial diagnostic test rather than smear microscopy/culture and phenotypic drug susceptibility testing [51]. The diagnostic algorithm using NAAT as an initial diagnostic test pending culturing is shown (Fig. 1). Although NAAT increases diagnostic specificity, sensitivity is still too low to rule out disease, especially in paucibacillary cases [52]. The predictive value in smear positive specimens is 95%. However, false-negative NAAT can occur in the presence of PCR inhibitor(s) [53]. Rapid turn-around time of NAAT and AFB is necessary for diagnosis and the prompt initiation of therapy pending culture results. An algorithm that includes AFB, NAAT and bacterial culture is shown ( Fig. 2). NAAT results should always be assessed in respect to other diagnostic tests, clinical symptoms and disease prevalence [54].
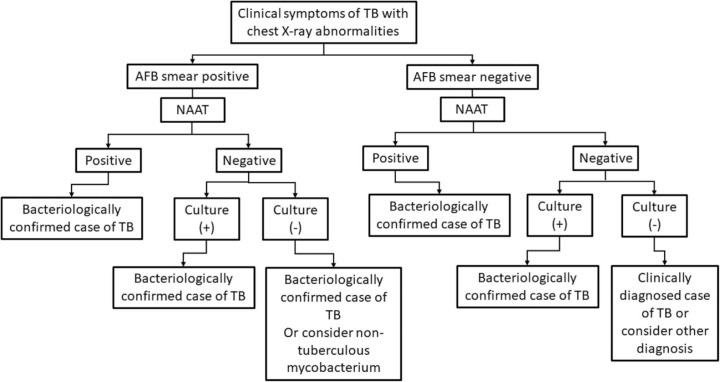
Fig. 2.
Summary of diagnostic algorithm utilizing smear, bacterial culture and NAAT as recommended by WHO TB diagnosis guideline in 2010.
A Taiwanese study where NAAT using Enhance Amplified MTB Direct Test [E-MTD] (Gen-Probe, USA) was used long-term (>20 y) showed that NAAT was useful in identifying MTB and excluding infections caused by nontuberculous mycobacteria [55]. After inclusion of NAAT in the testing protocol, the study showed a decrease in unnecessary isolations and treatment of suspected cases of PTB especially among patients with non-PTB, i.e., those infected with hepatitis B and C. Therefore, NAAT may be useful in regions with high hepatitis B and C prevalence. The authors reported that the sensitivity, specificity, positive predictive value, and negative predictive value of NAAT were 99.0%, 92.3%, 99.0% and 92.3%, when compared to culture, respectively.
An independent study in South Africa, i.e., a country with high TB burden, compared four commercial platforms (Abbott RealTime MTB and MTB rifampicin and isoniazid resistance assays (RIF/INH), Hain Lifescience FluoroType MTBDR assay, BD Max MDR-TB assay, and the Roche cobas MTB and MTB-RIF/INH) for detecting MTBC and resistance to rifampicin and isoniazid. They concluded that the clinical performance of these methods are similar to WHO-recommended molecular TB and MDR-TB assays [56]. The average limit of detection, defined as number of genomes/mL at the 95% hit rate was 3781, 322, 826, 2416 and 10398 for Xpert MTB/RIF, Abbott RealTime MTB, BD Max MDR-TB, Roche cobas MTB and Hain Lifescience FlouroType MTBDR, respectively.
Due to the expense of NAAT, it is important to use sample types that offer the best diagnostic performance. Meta-analysis for the Xpert MTB/RIF assay concluded that expectorated sputum provided the best diagnostic performance, i.e., 90% sensitivity and 98% specificity, in adults [57]. Sensitivity and specificity for bronchoalveolar lavage and induced sputum was 87% and 91% and 86% and 97%, respectively. In the pediatric population, however, gastric aspiration was superior to expectorated sputum, induced sputum and nasopharyngeal aspirates.
2.4.1. Common target genes for TB by NAAT
Various genes are targeted by NAAT. Commercially available assays and the target genes are listed ( Table 2). The MTBC gene targets are IS6110, protein antigen B (pab), rpoB and IS1081. IS6110, an insertion element, can be found multiple times in the MTB genome and is considered a sensitive diagnostic target. IS6110, thought to be found exclusively within the members of the MTBC, is also present in the genome of mycobacterium smegmatis [58]. The pab gene encodes a 38 kDa protein specific for MTB [59]. Rifampicin resistance is detected through the mutations in rpoB gene, which codes for the Beta subunit of the RNA polymerase, while detection of katG gene mutations, coding for the catalase peroxidase, is used to identify isoniazid resistance [60]. Inh A gene, which codes for 2-trans-enoyl-acyl carrier protein reductase [61], was identified as important for isoniazid and ethionamide resistance [62].
Table 2.
Commercial platforms and target genes for TB detection and drug resistance.
| Method | MTBC gene targets | Rifampicin/isoniazid targets |
|---|---|---|
| RealTime MTB assay (Abbott) | IS6110, pab | rpoB, katG, inhA |
| FluoroType MTBDR assay (Hain Lifescience) | rpoB | rpoB, katG, inhA |
| Max MDR-TB (BD) | IS6110, IS1081 | rpoB, katG, inhA |
| Roche cobas MTB (Roche) | 16sRNA, esxJ, esxK, esxM, esxP, esxW | rpoB, katG, inhA |
| Xpert MTB/RIF (Cephied) | rpoB | rpoB |
| GenoType MTBDRplus (Hain Lifescience) | rpoB, katG, inhA | |
Advantages of NAAT include multiplex capability to increase specificity without dramatic increase in analytical run time and the ability to amplify low copy genomes to improve sensitivity. Despite these advances, the use of laboratory-bound instrumentation limits remote testing in geographic regions with high TB burden.
2.5. Future NAAT
A better approach in TB testing would enable point-of-care for immediate isolation and initiation of therapy thus mitigating loss of patient contact especially in remote areas.
For example, isothermal amplification methods enable nucleic acid detection at constant temperature thus eliminating the constraint of thermal cycling. These include loop-mediated isothermal amplification, strand displacement amplification, helicase-dependent amplification, and recombinase polymerase amplification. All provide analytical sensitivity and specificity comparable to traditional thermal cycling.
In point-of-care molecular assays, choosing the appropriate enzyme is critical for isothermal amplification-based MTB detection. Helicase-dependent amplification unwinds the double-stranded DNA target thus eliminating the need for heat denaturation and thermal cycling. A proof-of-concept study reported that isothermal amplification with a single primer for IS6110 in MTB had a limit of detection of 2.5 × 105 copies/µL [63]. A loop mediated isothermal amplification study that used an inexpensive device reported an analytical sensitivity of 10 genomic copies [64]. A non-NAAT point-of-care test using lateral flow immunoassay to detect urinary mycobacterial lipoarabinomannan demonstrated improved sensitivity for TB in those coinfected with HIV [51].
The development of decentralized TB test devices has received much commercial interest. For example, Cepheid has developed a battery operated GeneXpert Omni system for Xpert cartridge designed for remote low-throughput settings. This system demonstrated high concordance with the traditional Xpert MTB/RIF assay (99.5%; 198/199) [65]. Technologies based on microfluidic devices, extensively used in diagnostic testing [66], [67], likely provide innovative solutions applicable to TB [68].
Future development of these novel tools will overcome geographic, economic and resource limitations as part of the WHO End TB strategy [69].
3. Latent tuberculosis infection
Latent tuberculosis infection is defined by the WHO as “a state of persistent immune response to stimulation by MTB antigens with no evidence of clinically manifest active TB” [70], a definition that has evolved over a span of two centuries. The term “latent” TB was described for the first time by Louis and Laennec in the early 19th century [71], [72]. The authors independently reported the presence of TB lesions on autopsy of asymptomatic individuals who died of other causes and referred to this condition as latent TB. In the mid-1950s, bacteriologic studies on mice infected with MTB recovered tubercle bacilli from the infected sites despite the absence of TB pathology [73]. A half-century later, the TB “immunoreactive” concept was introduced by the American Thoracic Society (ATS). The ATS guidelines described latent TB as an infected host who is TB immunoreactive without symptomatic disease [74]. Nevertheless, no gold standard test for the diagnosis of LTBI exists yet. At present, the tuberculin skin test (TST) and the interferon gamma release assays (IGRA) are the only testing methods endorsed by the WHO to establish the diagnosis of latent TB infection in combination with chest X-ray, physical examination and medical history [75]. Different algorithmic approaches for targeted diagnosis and treatment of latent cases and exclusion of active TB are in use. The WHO recommended approach is shown ( Fig. 3) [70]. Both endorsed tests evaluate cell-mediated immunity response. The characteristics of the TST and IGRAs tests along with advances in LTBI diagnostic tools are discussed in this section.
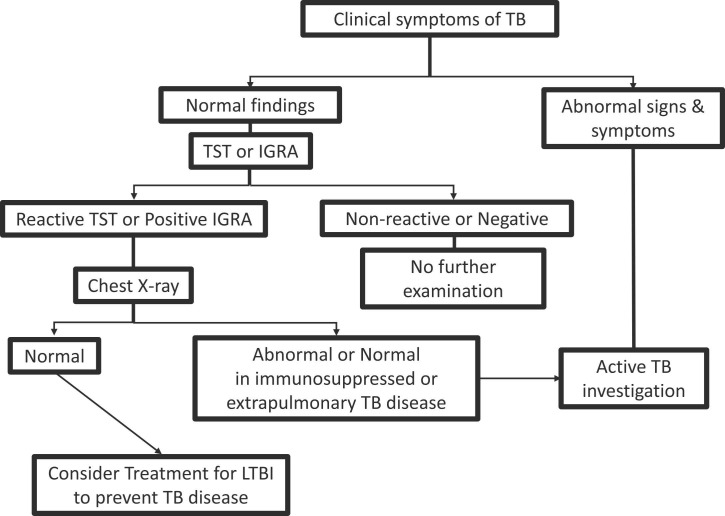
Fig. 3.
Diagnostic algorithm for LTBI utilizing clinical symptoms, chest X-rays, TST or IGRA as recommended by WHO’s updated and consolidated guidelines, 2018.
3.1. Tuberculin skin test (TST)
The TST was originally introduced by Koch as a live vaccine, i.e., “old tuberculin”, to cure TB [76], [77]. Although not therapeutically effective [78], it became one of the oldest diagnostic tools still in use albeit with some modifications [79]. The old tuberculin was prepared by heating tubercle bacillus culture broth and concentrate before mixing the filtrate with a glycerin-based solution. Pirquet introduced the cutaneous tuberculin scratch test to diagnose latent TB in 1907 [79], [80]. A couple of years later, Charles Mantoux improved the tuberculin administration technique and established the diagnostic criteria for reading a TST [80]. Nowadays, the standard procedure for TST requires the Mantoux intradermal injection method of tuberculin purified protein derivative (PPD) [74]. The PPD is a sterile solution of a purified protein fractions precipitated from a filtrate of mycobacterial culture developed by Seibert and Long in the 1930s and subsequently became the reference standard for the US Public Health Service [81], [82]. Later, the standard lot PPD-S was adopted by the WHO as an international standard [83]. Currently there are several formulations such as PPD-S2 (Aplisol; JHP Pharmaceuticals, Inc, Rochester, USA) and Tubersol (Sanofi Pasteur Limited, Swiftwater, USA), which are mainly used in the US and Canada, and PPD RT23 (AJ Vaccines, Denmark) which is the most commonly used worldwide [81]. Although terminology involving the TB skin test, tuberculin skin test, PPD and Mantoux are often used interchangeably, TST is preferred.
The TST is an indirect method used to evaluate cell-mediated immunity in response to mycobacterial antigens [84]. In brief, a delayed hypersensitivity reaction due to the presence of immune cells at the site of injection indicates current or previous exposure to MTB. Therefore, TST can be used to screen for TB infection or to assist in diagnosis. Unfortunately, false positive reactions are common.
The TST is performed by injecting 0.1 mL of 5 tuberculin units (TU) of PPD-S or 2 TU of PPD RT-23 intradermally on the flexor surface of the forearm [61], [84]. The injection is administered at a 5- to 15-degree angle using a tuberculin syringe with the needle bevel facing upward [61]. If the needle angle is too deep or too shallow, testing will be compromised. The correct administration creates a palpable, raised, swollen spot of 6–10 mm in diameter on the skin surface. If the raised spot is<6 mm in diameter, the test must be re-administered at a different site. The formed induration should be read at 48–72-h by measuring the diameter of the elevated spot [61]. It is critical to that the induration be viewed in this time period.
Interpretation of TST reaction should not solely be based on the measurement of the induration but rather within the context of the associated risk factors for TB infection [61], [85]. Thus, CDC guidelines determined three diameter cut-points (5, 10 and 15 mm) to define a positive reaction based on certain risk factors for TB infection ( Table 3) [84]. It is important to note that the specificity of the test increases as the diameter increases. Thus, an induration of 15 mm or more is considered positive even in a healthy individual without any risk factors. Therefore, a positive result is diagnostic of latent TB infection if medical evaluation does not indicate TB disease. Otherwise, the diagnosis of TB disease, meaning current infection with MTB, is established. Conversely, a measurement below the cut-points for each category is considered negative.
Table 3.
Interpretation of TST based on CDC guidelines.
| TST reaction size (mm) | Reaction is considered positive |
|---|---|
| ≥ 5 | HIV infected individuals |
| Recent contact with active TB patients | |
| TB signs on X-ray such as fibrotic changes | |
| organ transplant patients & immunosuppressed individuals | |
| ≥ 10 | Immigrants from endemic/high prevalence countries in the last 5 years |
| Injection drug abusers | |
| High-risk congregate settings (e.g., nursing homes, homeless shelters, or prisons, Mycobacteriology laboratory workers) | |
| Chronic medical conditions that increase the risk for TB | |
| Children younger than 5 years of age | |
| ≥ 15 | People with no known risk factors for TB |
| TST reaction size (mm) | Reaction is considered negative |
| 0 or <5, 10, 15 | No induration or a measurement below the defined cut point for each category |
Although TST is cost effective, it requires rigorous standardization on administration, reading and interpretation to ensure reliability and accuracy [86]. Unfortunately, it also requires a second visit within the specified time which may be inconvenient. In addition, failure to stringently follow established protocols may consequently result in a falsely positive or falsely negative test result [87]. Furthermore, the TST is not absolutely sensitive or specific and is unable to distinguish current from past infection. The TST also cross-reacts with the Bacillus Calmette-Guerin (BCG) vaccine strain and nontuberculous mycobacteria producing falsely positive results [84]. Test sensitivity is reduced in immunocompromised individuals, i.e., patients on immunosuppressants or HIV positive, leading to inadequate response or no reaction [80]. Falsely negative reactions may also be seen in recent exposure to TB infection or viral illness, anergy, recent live-virus vaccination and in children younger than six months of age [88].
3.2. Interferon-gamma release assay (IGRA)
The interferon-gamma release assay (IGRA) was developed to mitigate some of the limitations associated with the TST, particularly, the high false-positive rates due to BCG vaccination. Therefore, IGRAs were designed to quantify the immune response to antigens unique to the organism [75], [89]. The progress in genomic research along with elucidation of the innate and adoptive immune pathways led to the development of IGRAs [90]. Interferon-gamma (IFN-γ) is a cytokine released by activated effector T cells, namely CD4 + and CD8 + cells [91]. Briefly, antigen-presenting cells such as macrophages detect, engulf and process the mycobacterial pathogen then stimulate adaptive immune response through MTB antigens. There are two testing scenarious. The first approach measures the total amount of IFN-γ produced by sensitized T-cells, a test pioneered by Qiagen through a family of QuantiFERON TB assays. The QuantiFERON TB test, the first FDA-approved IGRA, came to the US market in 2001, followed by the QuantiFERON TB Gold (FDA 2004) and the QuantiFERON TB Gold in Tube (FDA 2007), each replacing the latter. At present, the QuantiFERON Gold Plus is the 4th generation of this test line and was FDA-approved in 2017 [92]. The second approach detects the number of T-cells secreting IFN-γ by a proprietary T-SPOT technology, known as the T-SPOT.TB assay (Oxford Immunotec, FDA approval 2008), offered exclusively through Quest Diagnostics [93]. Both approaches used the same antigen peptides, i.e., the early secreted antigenic target 6 (ESAT-6) and the culture filtrate protein 10 (CFP10), to stimulate IFN-γ release from T cells previously exposed to MTB. Because these peptide antigens are present in MTBC and absent in the vaccine strain BCG, IGRAs are more specific than TST. Unfortunately, both are incapable of detecting MTB directly and hence are considered indirect markers of TB exposure.
3.2.1. T-SPOT.TB
The T-SPOT.TB is an enzyme-linked immunospot (ELISpot) assay for the detection of IFN-γ producing T cells (count of spot forming cells) in response to stimulation with MTB specific antigens, ESAT-6 and CFP10 [93]. The test is performed on separated and counted peripheral blood mononuclear cells (PBMCs). It is an indirect test for MTB infection (including disease) and is intended for use in conjunction with risk assessment, radiography and other medical and diagnostic evaluations.
A single whole blood specimen is collected by venipuncture into a lithium or sodium heparin blood collection tube. The tube must be gently inverted (8–10 times) to ensure proper mixing of blood and anticoagulant. A subset of white blood cells, including monocytes and lymphocytes, then are isolated. The cells are washed, counted and normalized to create a standard cell suspension. A standard number of cells (250,000 PBMCs per well) are added into specially designed 96 well plastic microtiter plate. A total of four wells are required for each patient. Two quality control wells which include a Nil control and a Positive control are run with each individual sample. The remaining two wells are spared for the TB antigens, ESAT-6 in one and CFP-10 in the other. The plate is incubated in a humidified incubator at 37 °C with 5% CO2 for 16–20 h. Cells that respond to stimulation release IFN-γ which is immediately captured by IFN-γ antibodies pre-coated on the wells. A secondary enzyme labeled antibody is then added and the number of IFN-γ producing cells quantified, i.e., spot number.
Results are reported as number of IFN-γ producing T cells calculated by subtracting spot-forming cells in Nil well from either antigen containing well. Accordingly, an individual is considered positive if counts in either (ESAT-6 – Nil) or (CFP10 – Nil) are ≥8, negative if ≤ 4 and borderline (equivocal) if counts are in between (5, 6 or 7 spots). A typical result would be expected to have few or no spots in the Nil Control. A Nil control spot count>10 spots should be reported as ‘Invalid’. A typical positive (Mitogen) control spot count is ≥ 20 spots. If the reaction in the positive control well is insufficient (<20 spots), a subset of patients may have T cells with limited response to PHA, and has ≤ 4 spots in either antigen well after subtraction, then it should also be reported as ‘Invalid’. It is recommended to retest by collecting another patient specimen if results are equivocal or invalid.
The T-SPOT.TB test limitations include the requirement of a blood specimen that must be processed within a specific time after collection, i.e., while white blood cells are viable. Incorrect blood sample collection or improper handling may affect lymphocyte function leading to falsely negative results. While ESAT-6 and CFP10 antigens are absent from BCG strains of M. bovis and most environmental mycobacteria, it is possible that a positive result may be due to infection with other mycobacterial species.
3.2.2. QuantiFERON-TB Gold Plus
The QuantFERON-TB Gold Plus (QFT-Plus) is an enzyme-linked immunosorbent assay (ELISA) for the detection of IFN-γ using a peptide cocktail, composed of ESAT-6 and CFP-10 proteins, to stimulate immune cells in heparinized whole blood [92]. QFT-Plus is an indirect test for MTB infection (including disease) and is intended for use in conjunction with risk assessment, radiography and other medical and diagnostic evaluations.
Whole blood can be collected by venipuncture into one heparin plasma tube for transfer or collected directly into the four specialized tubes composed of a gray cap Nil tube, a green cap TB Antigen Tube 1 (TB1), a yellow cap TB Antigen Tube 2 (TB2) and a purple cap Mitogen tube. Antigen tubes contain peptide antigens from the MTBC-associated antigens, ESAT-6 and CFP-10. The peptide antigens are designed to prompt cell mediated immune response from CD4 + T-helper cells in TB1 and TB2 while inducing immune response from CD8 + cytotoxic T lymphocytes in TB2. The QFT-Plus blood collection tubes must be properly mixed to ensure complete solubilization of antigens on tube walls prior to incubation. Following a 16–24 h incubation at 37 °C, tubes are centrifuged, the plasma is harvested and IFN-γ measured by ELISA. The QFT-Plus ELISA uses a recombinant human IFN-γ standard, which has been assayed against a reference IFN-γ preparation (NIH Ref: Gxg01–902–535). Results are reported in International Units per mL (IU/mL) relative to a standard curve prepared by testing dilutions of the standard supplied with the kit.
Although the QFT-Plus test is quantitative, results should only be reported qualitatively, i.e., positive, negative or indeterminate. The measured values from each of the four tubes are evaluated and a final result is calculated using an FDA-approved algorithm. Basically, the Nil tube value is subtracted from the values of the Mitogen, TB1 and TB2 tubes to provide a numeric final value for each. The Nil tube adjusts for background, i.e., excessive circulating IFN-γ due to a preexisting immune response or the presence of heterophile antibodies. The Nil tube must have a value of ≤8.0 IU/mL for a test to be valid. The Mitogen tube serves as a control for correct blood handling and incubation. It contains phytohemagglutinin-P (PHA), a nonspecific T cell stimulator that induces production of IFN-γ. The Mitogen tube must have an IFN-γ value of at least 0.5 IU/mL higher than the Nil tube to be considered valid. It is used to detect falsely negative results that occur due to insufficient lymphocyte number, reduced activity due to improper handling, incorrect filling/mixing of the Mitogen tube, or an inability to generate IFN-γ. The cut-off value of 0.35 IU/mL in TB1 and/or TB2 tubes is considered as a decision level when combined with the Nil and Mitogen tubes.
Similar to T-SPOT.TB test, fresh blood must be collected in specialized tubes and processed expeditiously. Inappropriate specimen collection, transportation and preparation affect test performance. In addition, the stimulation step increases test turnaround time and impacts workflow. Test complexity requires adequate equipment, trained personnel, and a rigorous quality assurance program to ensure reliability. Finally, the QFT-Plus test is unable to distinguish recent from past infection.
Numerous studies have evaluated the performance of IGRA tests and TST for LTBI. Because no gold standard has been established, it is difficult to reliably assess sensitivity and specificity. As such, studies usually estimate sensitivity among culture-confirmed TB cases (active TB) while specificity is estimated among low-risk individuals with no known TB exposure. Because LTBI and active TB are inherently different, estimating sensitivity for LTBI is highly inaccurate and likely underperforms in medium-risk individuals. This is also true for estimating specificity because there is no known method to exclude low-risk participants with LTBI currently. In general, IGRA has similar sensitivity and specificity to TST in healthy, non-BCG vaccinated populations. Accordingly, the WHO recommends that TST or IGRA be used for LTBI as the situation merits [70].
The newly published WHO policy on alternative IFN-γ release assays for TB infection reviewed sensitivity, specificity, and agreement of QFT-Plus test with reference methods. Overall, QFT-Plus was found to be highly comparable to its immediate predecessor QFT-GIT. In comparison to T-SPOT.TB, no significant difference in sensitivity or specificity was reported. In addition, good agreement between QFT-Plus and T-SPOT.TB was noted (kappa = 0.74; 95% CI, 0.60, 0.88) but was much lower with TST (kappa = 0.33; 95% CI, 0.22, 0.45). Accordingly, QFT-Plus was equivalent to QFT-GIT and considered a good alternative to existing and WHO-endorsed tests [94].
3.3. Advances in diagnostic tools for latent TB infection
The development of more accurate tests to diagnose TB infection is key in achieving the WHO End TB Strategy [69]. Although testing for TB has evolved over the past 15 years, these were primarily focused on diagnosis of active TB. Development of tests for detection of latent infection was lacking. Currently, there are only two IGRAs FDA-approved in the USA. In 2018, the WHO recommended the use of both IGRAs along with the previously endorsed TST to aid in the diagnosis of LTBI. Several new TB skin tests and IGRAs have been developed since then with some undergoing WHO evaluation [95].
3.3.1. Novel emerging skin tests
In April 2022, the WHO announced tuberculosis antigen-based skin tests (TBST) as a new test class for diagnosis of TB infection [96]. This class is defined as skin tests for the detection of TB infection that use MTB specific antigens (ESAT6 and CFP10). The WHO Guideline Development Group (GDG) has recently evaluated data from three novel technologies in this class, i.e., C-Tb (Serum Institute of India, India), Creative-TST or C-TST (formerly known as EC-Test), Anhui Zhifei Longcom, China and Diaskintest (Generium, Russian Federation). The GDG concluded that the new TBST class of tests were accurate, acceptable, feasible and cost-effective. Specificity was comparable to IGRAs and superior to TST particularly in populations with prior BCG vaccination history.
Diaskintest, the first test in TBST class that utilizes recombinant fusion protein CFP10-ESAT6, has been widely used in Russia since 2009 [97], [98]. These proteins are present in virulent strains of MTB and absent in other mycobacteria including BCG. Diaskintest, like TST, is injected intradermally and causes specific induration of delayed hyperresponsiveness in people with TB infection. Following dosing (0.2 μg in 0.1 mL), the result is recorded at 48–72 h by measuring papule diameter at the injection site. The test is interpreted as negative, doubtful or positive based on the presence or absence of infiltration and hyperemia [99].
C-Tb skin test is another MTB-specific skin test that contains recombinant ESAT-6 and CFP-10 antigens mixed in an equivalent weight ratio [100]. The C-Tb skin test is applied intradermally (0.1 μg per 0.1 mL) by the Mantoux technique similar to TST [101]. In contrast to variable TST cut-offs, the C-Tb test has a fixed cut-off value of 5 mm of induration. Reactions are classified as positive if the induration size is 5 mm or more, otherwise it is considered negative.
C-TST, the newest TBST for diagnosis of TB infection, is also based on recombinant fusion protein ESAT-6-CFP-10 of MTB [102]. Clinical trials of the C-Test have been recently completed followed by the Chinese State Drug Administration approval for commercial use [103]. The C-TST is applied intradermally, the induration must be measured with 48–72 h and the results are considered positive when the diameter of induration is equal or greater than 5 mm [104].
3.3.2. Novel IGRA tests
Newer versions of IGRAs using different immunoassay approaches have simplified workflow and reduced turnaround time thus improving ease of use with minimal training. Recently, Qiagen and DiaSorin announced the FDA approval of the Liaison QuantiFERON-TB Gold Plus (Liaison QFT-Plus) assay, a fully automated chemiluminescence immunoassay, on the LIAISON platform [105]. This approach reduces manual hands-on time and increases throughput for the Qiagen blood-based assay for latent TB detection. The Liaison QFT-Plus assay is an IGRA technology assessing cell-mediated response by measurement of IFN-γ in whole blood. It is designed to work with QIAGEN QFT-Plus Blood Collection Tubes which employ TB-specific antigens that elicit both CD8 + and CD4 + T cell responses for accurate assessment of cell-mediated immune response to TB infection.
The Liaison QFT-Plus assay detects IFN-γ by direct sandwich chemiluminescence immunoassay [30]. Anti IFN-γ monoclonal (mouse) antibodies are used for coating magnetic particles (solid phase) and IFN-γ monoclonal (mouse) antibodies are linked to an isoluminol derivative forming an isoluminol-antibody conjugate. Binding between the monoclonal antibodies and isoluminol in the conjugate is mediated by a biotin-streptavidin immunocomplex. In the first incubation, IFN-γ binds the solid phase and conjugated monoclonal antibodies to form a sandwich. Unbound material is removed by washing. In the second incubation, flash chemiluminescent reaction is initiated. The light signal is measured by a photomultiplier as relative light units (RLU) proportional to IFN-γ.
Interpretation is performed using an algorithm that combines the results from each of the four QFT-Plus Blood Collection Tubes. The concentration of IFN-γ in each tube is reported in IU/mL. Although the assay detects IFN-γ quantitatively, interpretation is qualitative. Unfortunately, IFN-γ concentration cannot be correlated to stage or degree of infection, level of immune responsiveness or likelihood for progression to active disease.
Similar to conventional IGRAs, Liaison QFT-Plus does not differentiate active and LTBI. In addition, it should not be used solely for the diagnosis of active TB infection given suboptimal sensitivity. Therefore, Liaison QFT-Plus assay should be used as an aid in diagnosing infection with MTB. The automated Liaison QFT-Plus assay has comparable diagnostic performance to the Qiagen QFT-Plus assay for LTBI diagnosis with an overall agreement of 97.8% [106].
Another novel IGRA test, LIOFeron TB/LTBI assay (Lionex GmbH, Germany), has become commercially available late 2019 [107]. Although LIOFeron TB/LTBI test is an ELISA-based whole blood IGRA, it exclusively contains alanine dehydrogenase (Ala DH) antigen of MTB that can be used to differentiate active TB from LTBI diagnosis based on IL-2 [108]. The LIOFeron TB/LTBI assay is a cytokine release assay for quantitative determination of IFN-γ produced by human blood cells stimulated with MTB antigens. Therefore, it is intended for diagnosing LTBI and TB.
The test kit consists of two main components, the human blood stimulation tubes and the human IFN-γ ELISA [109]. The stimulation tubes include a positive control tube, a negative control tube and two different TB antigen tubes, TB A and TB B for each sample. TB A tube contains ESAT-6, CFP-10, and TB7.7 antigens, i.e., known to be missing in BCG. While TB B tube contains Ala-DH, a proprietary antigen of LIONEX, with CD8 + epitopes. Each stimulation tube requires at least 1 mL of heparinized blood sample before an overnight incubation (16–24 h, 37 °C). The plasma is subsequently analyzed using a standard ELISA which measures IFN-γ produced in response to the specific antigens in each tube. Results are interpreted as positive, negative or intermediate. Overall sensitivity and specificity was comparable to the QuantiFERON-TB Gold Plus test in diagnosing MTB infection/TB disease [108].
The newest IGRA is VIDAS TB-IGRA (bioMérieux, France), a fully automated enzyme-linked immunofluorescent assay (ELFA), for the detection of LTBI [110]. The test process is fully automated including the stimulation step and requires a single whole blood sample tube without manual intervention. Each sample is incubated in a negative control (NIL), a mitogenic response positive control (MIT-NIL) and an antigen response (AG-NIL) (16 h, 37 °C). IFN-γ results are interpreted as positive, negative or indeterminant. AG-NIL ≥0.35 IU/mL indicates a positive result, whereas ≤0.35 IU/mL indicates a negative result. When MIT-NIL is<1.1 IU/mL or NIL is ≥ 6.4 IU/mL, the result is considered indeterminant. For clinical performance, Diagbouga et al. reported higher sensitivity in individuals with TB disease vs QFT-Plus while maintaining comparable specificity in healthy blood donors [111]. In high-risk groups, both assays demonstrated comparable performance.
Alternatively, fluorescent immunoassay using lateral flow technology offers a relatively simple rapid and cost-effective approach for point-of-care testing. The QIAreach QuantiFERON-TB (QIAGEN, The Netherlands), the ichroma IGRA-TB (Boditech Med Inc., Republic of Korea), and the STANDARD TB-Feron FIA (SD Biosensor, Republic of Korea) are the most advanced technologies in this category.
The ichroma IGRA-TB is a qualitative point-of-care fluorescence immunoassay for detection of IFN-γ released in response to in-vitro stimulation by MTB specific antigen [112]. Like most IGRAs, the assay consists of a stimulation and IFN-γ detection step [113]. Briefly, whole blood is pipetted into three specific tubes (Nil, Mitogen and TB antigen containing ESAT-6 and CFP-10 peptides) and incubated for 16–24 hours. Following centrifugation, the sample is applied to a lateral flow cartridge and IFN-γ detected by sandwich immunoassay. Antigen antibody complexes are detected using a portable fluorescence detector. Performance was found to be comparable to QFT-Plus for detection of LTBI in a large cohort of health care workers [114]. In this study, the total agreement rate and kappa values between the two methods were 95.20% and 0.70, respectively. Unfortunately, the ichroma IGRA-TB produced higher frequency of indeterminate results.
The QIAreach QuantiFERON-TB assay measures IFN-γ in plasma released by CD4 and CD8 T cells with single use cartridges (named eStick) on a portable lateral flow platform (eHub) using nanoparticle fluorescence technology [115]. Testing requires a single blood tube, with the same antigens as the TB2 tube of QFT Plus, to detect TB infection. It has the capacity of performing up to eight tests and providing a final qualitative (positive or negative) result within 20 min after sample incubation. The QIAreach QFT test is designed to reach high burden low resource remote regions due to its simplicity and portability [116] and clinical performance [117], [118]. In these two studies, the assay demonstrated 93–100% sensitivity and 97.7–97.6% specificity with 95.7–98.8% overall concordance vs the QFT-Plus test. Both studies recognized the advantages of being an objective (positive/negative) test, quicker to perform and requiring smaller sample volume (1 mL vs 4 mL). In addition, both reports agreed on the need for further studies to evaluate assay performance in various populations/settings. This has recently been echoed in WHO policy statement [94].
The STANDARD F TB-Feron FIA is a three tube based fluorescent immunoassay with the TB antigen tube containing recombinant protein antigens (ESAT-6, CFP-10, and TB7.7) [119]. After 16 hours of incubation, the test procedure involves mixing the plasma from each tube with labeled fluorescent conjugate before applying on a single use lateral flow-based cartilage. The test cartilage is then inserted into the STANDARD F2400 analyzer for reading fluorescent signals and providing quantitative results within 15 min. The results are interpreted in the same way as QFT- gold in-tube assay. Unfortunately, the STANDARD F2400 analyzer is a bench-top device and cannot be used remotely. Clinical data is pending on its performance.
4. Summary and concluding remarks
Although preventable and curable, TB remains a leading cause of morbidity and mortality worldwide. Diagnosis of TB is established through medical history, physical examination, chest-radiography and laboratory testing. Culture remains the gold standard for laboratory confirmation of TB disease and growing bacteria are required to perform drug-susceptibility. However, culture methods have long turnaround-time and require laboratory infrastructure. With advances in molecular testing, WHO endorsed the use of rapid molecular diagnostic tests, i.e., NAAT, that can accurately detect MTB and rifampicin resistance simultaneously. However, complexity and cost of molecular tests has hampered its use in many resource limited and remote regions. Affordability and portability are deemed essential especially in high burden geographies.
Although rapid identification of active TB cases is crucial to prevent transmission and initiate treatment, early diagnosis and treatment of latent TB cases are critical. The TST and two IGRA tests are the only WHO endorsed methods for TB infection. Unfortunately, neither distinguish latent from active disease or recent from remote infection. Therefore, better tools to identify those that progress from infection to TB disease are necessary. Emerging TB antigen-based skin tests and advanced versions of IGRAs may offer some advantages over conventional tests but the problem remains. To conclude, future tools must be developed and validated to improve diagnostic accuracy in the context of clinical impact, affordability and feasibility in resource limited settings.
References
- 1.Burke D.S. Of postulates and peccadilloes: Robert Koch and vaccine (tuberculin) therapy for tuberculosis. Vaccine. 1993;11(8):795–804. doi: 10.1016/0264-410x(93)90354-z. [DOI] [PubMed] [Google Scholar]
- 2.Grange J. Mycobacterium bovis infection in human beings. Tuberculosis. 2001;81(1–2):71–77. doi: 10.1054/tube.2000.0263. [DOI] [PubMed] [Google Scholar]
- 3.Banta J.E., et al. Pulmonary vs. extra-pulmonary tuberculosis hospitalizations in the US [1998–2014] J. Infect. Public Health. 2020;13(1):131–139. doi: 10.1016/j.jiph.2019.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Centers for Disease, C. and Prevention Availability of an assay for detecting Mycobacterium tuberculosis, including rifampin-resistant strains, and considerations for its use – United States, 2013. MMWR Morb. Mortal. Wkly. Rep. 2013;62(41):821–827. [PMC free article] [PubMed] [Google Scholar]
- 5.Morse D., Brothwell D.R., Ucko P.J. Tuberculosis in ancient Egypt. Am. Rev. Respir. Dis. 1964;90(4):524–541. doi: 10.1164/arrd.1964.90.4.524. [DOI] [PubMed] [Google Scholar]
- 6.Donoghue H.D., et al. Tuberculosis in Dr Granville’s mummy: a molecular re-examination of the earliest known Egyptian mummy to be scientifically examined and given a medical diagnosis. Proc. Biol. Sci. 2010;277(1678):51–56. doi: 10.1098/rspb.2009.1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Latent tuberculosis infection: a guide for primary health care providers, Centers for Disease Control and Prevention, Atlanta, GA, 2013. Available: 〈http://www.cdc.gov/tb/publications/ltbi/diagnosis.htm〉 (accessed April 26, 2021).
- 8.Pai M., et al. Tuberculosis. Nat. Rev. Dis. Primers. 2016;2:16076. doi: 10.1038/nrdp.2016.76. [DOI] [PubMed] [Google Scholar]
- 9.CDC, Reported tuberculosis in the United States, 2018, US Department of Health and Human Services, CDC, Atlanta, GA, 2019. 〈https://www.cdc.gov/tb/statistics/reports/2018/default.htm〉.
- 10.LoBue P.A., Mermin J.H. Latent tuberculosis infection: the final frontier of tuberculosis elimination in the USA. Lancet Infect. Dis. 2017;17(10):e327–e333. doi: 10.1016/S1473-3099(17)30248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UpToDate, Diagnosis of pulmonary tuberculosis in adults, 2022. Available from: 〈https://www.uptodate.com/contents/diagnosis-of-pulmonary-tuberculosis-in-adults〉.
- 12.Chisti M.J., et al. Diagnosis of tuberculosis following world health organization-recommended criteria in severely malnourished children presenting with pneumonia. Global Pediatr. Health. 2017;4 doi: 10.1177/2333794X16686871. p. 2333794×16686871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdullahi O., et al. The effect of empirical and laboratory-confirmed tuberculosis on treatment outcomes. Sci. Rep. 2021;11(1):14854. doi: 10.1038/s41598-021-94153-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walusimbi S., et al. Meta-analysis to compare the accuracy of GeneXpert, MODS and the WHO 2007 algorithm for diagnosis of smear-negative pulmonary tuberculosis. BMC Infect. Dis. 2013;13:507. doi: 10.1186/1471-2334-13-507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schoch O.D., et al. Diagnostic yield of sputum, induced sputum, and bronchoscopy after radiologic tuberculosis screening. Am. J. Respir. Crit. Care Med. 2007;175(1):80–86. doi: 10.1164/rccm.200608-1092OC. [DOI] [PubMed] [Google Scholar]
- 16.Lee J.Y. Diagnosis and treatment of extrapulmonary tuberculosis. Tuberc. Respir. Dis. (Seoul) 2015;78(2):47–55. doi: 10.4046/trd.2015.78.2.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eurosurveillance editorial T. WHO revised definitions and reporting framework for tuberculosis. Eurosurveillance. 2013;18(16):20455. [PubMed] [Google Scholar]
- 18.Eastwood J.B., Corbishley C.M., Grange J.M. Tuberculosis and the kidney. J. Am. Soc. Nephrol. 2001;12(6):1307–1314. doi: 10.1681/ASN.V1261307. [DOI] [PubMed] [Google Scholar]
- 19.Garg R.K., Somvanshi D.S. Spinal tuberculosis: a review. J. Spinal Cord Med. 2011;34(5):440–454. doi: 10.1179/2045772311Y.0000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kiakojuri K., Hasanjani Roushan M.R. Laryngeal tuberculosis without pulmonary involvement. Caspian J. Intern. Med. 2012;3(1):397–399. [PMC free article] [PubMed] [Google Scholar]
- 21.Kruijshaar M.E., Abubakar I. Increase in extrapulmonary tuberculosis in England and Wales 1999–2006. Thorax. 2009;64(12):1090–1095. doi: 10.1136/thx.2009.118133. [DOI] [PubMed] [Google Scholar]
- 22.Lawn S.D., et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect. Dis. 2013;13(4):349–361. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Grant L.R., et al. Procedures for collection of induced sputum specimens from children. Clin. Infect. Dis. 2012;54(Suppl 2):S140–S145. doi: 10.1093/cid/cir1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabur N.F., et al. Diagnosing tuberculosis in hospitalized HIV-infected individuals who cannot produce sputum: is urine lipoarabinomannan testing the answer? BMC Infect. Dis. 2017;17(1):803. doi: 10.1186/s12879-017-2914-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prevention, C.f.D.C.a., Interactive Core Curriculum on Tuberculosis, March 17, 2022.
- 26.Bart I.Y., et al. Sputum induction in children is feasible and useful in a bustling general hospital practice. Global Pediatr. Health. 2016;3 doi: 10.1177/2333794X16636504. p. 2333794×16636504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gonzalez-Angulo Y., et al. Sputum induction for the diagnosis of pulmonary tuberculosis: a systematic review and meta-analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2012;31(7):1619–1630. doi: 10.1007/s10096-011-1485-6. [DOI] [PubMed] [Google Scholar]
- 28.Peter J.G., et al. Comparison of two methods for acquisition of sputum samples for diagnosis of suspected tuberculosis in smear-negative or sputum-scarce people: a randomised controlled trial. Lancet Respir. Med. 2013;1(6):471–478. doi: 10.1016/S2213-2600(13)70120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mendelson M. Diagnosing tuberculosis in HIV-infected patients: challenges and future prospects. Br. Med. Bull. 2007;81–82:149–165. doi: 10.1093/bmb/ldm009. [DOI] [PubMed] [Google Scholar]
- 30.Luabeya A.K., et al. Noninvasive detection of tuberculosis by oral swab analysis. J. Clin. Microbiol. 2019;57(3) doi: 10.1128/JCM.01847-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ssengooba W., et al. An early morning sputum sample is necessary for the diagnosis of pulmonary tuberculosis, even with more sensitive techniques: a prospective cohort study among adolescent TB-suspects in Uganda. Tuberc. Res. Treat. 2012;2012 doi: 10.1155/2012/970203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dzodanu E.G., et al. Diagnostic yield of fluorescence and Ziehl-Neelsen staining techniques in the diagnosis of pulmonary tuberculosis: a comparative study in a district health facility. Tuberc. Res. Treat. 2019;2019:4091937. doi: 10.1155/2019/4091937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nataraj V., et al. Mycolic acids: deciphering and targeting the Achilles’ heel of the tubercle bacillus. Mol. Microbiol. 2015;98(1):7–16. doi: 10.1111/mmi.13101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.CLSI, CLSI M48-ED2: 2018 Laboratory Detection and Identification of Mycobacteria, Wayne, PA, 2018.
- 35.Martin R.S., Sumarah R.K., Robart E.M. Comparison of four culture media for the isolation of Mycobacterium tuberculosis: a 2-year study. J. Clin. Microbiol. 1975;2(5):438–440. doi: 10.1128/jcm.2.5.438-440.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drancourt M., et al. Blood agar and Mycobacterium tuberculosis: the end of a dogma. J. Clin. Microbiol. 2003;41(4):1710–1711. doi: 10.1128/JCM.41.4.1710-1711.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandjean L., et al. Tuberculosis diagnosis and multidrug resistance testing by direct sputum culture in selective broth without decontamination or centrifugation. J. Clin. Microbiol. 2008;46(7):2339–2344. doi: 10.1128/JCM.02476-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kassaza K., et al. Lowenstein-Jensen selective medium for reducing contamination in Mycobacterium tuberculosis culture. J. Clin. Microbiol. 2014;52(7):2671–2673. doi: 10.1128/JCM.00749-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pena J.A., et al. Growth detection failures by the nonradiometric Bactec MGIT 960 mycobacterial culture system. J. Clin. Microbiol. 2012;50(6):2092–2095. doi: 10.1128/JCM.00108-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leitritz L., et al. Evaluation of BACTEC MGIT 960 and BACTEC 460TB systems for recovery of mycobacteria from clinical specimens of a university hospital with low incidence of tuberculosis. J. Clin. Microbiol. 2001;39(10):3764–3767. doi: 10.1128/JCM.39.10.3764-3767.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ma Y., et al. Comparison of Lowenstein-Jensen medium and MGIT culture system for recovery of Mycobacterium tuberculosis from abscess samples. Diagn. Microbiol. Infect. Dis. 2020;96(4) doi: 10.1016/j.diagmicrobio.2019.114969. [DOI] [PubMed] [Google Scholar]
- 42.Chien H.P., et al. Comparison of the BACTEC MGIT 960 with Lowenstein-Jensen medium for recovery of mycobacteria from clinical specimens. Int. J. Tuberc. Lung Dis. 2000;4(9):866–870. [PubMed] [Google Scholar]
- 43.Parrish N., et al. Differences in time to detection and recovery of Mycobacterium spp. between the MGIT 960 and the BacT/ALERT MB automated culture systems. Diagn. Microbiol. Infect. Dis. 2009;63(3):342–345. doi: 10.1016/j.diagmicrobio.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 44.Dionne K., et al. Methods for reducing bacterial contamination in the BacT/Alert mycobacterial culture detection system. J. Clin. Microbiol. 2005;43(5):2523–2525. doi: 10.1128/JCM.43.5.2523-2525.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Espasa M., et al. Evaluation of the VersaTREK system compared to the Bactec MGIT 960 system for first-line drug susceptibility testing of Mycobacterium tuberculosis. J. Clin. Microbiol. 2012;50(2):488–491. doi: 10.1128/JCM.06432-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trutneva K.A., et al. One-year old dormant, “non-culturable” Mycobacterium tuberculosis preserves significantly diverse protein profile. Front. Cell Infect. Microbiol. 2020;10:26. doi: 10.3389/fcimb.2020.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dusthackeer A., et al. Differential culturability of Mycobacterium tuberculosis in culture-negative sputum of patients with pulmonary tuberculosis and in a simulated model of dormancy. Front. Microbiol. 2019;10:2381. doi: 10.3389/fmicb.2019.02381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Albert H., et al. Development, roll-out and impact of Xpert MTB/RIF for tuberculosis: what lessons have we learnt and how can we do better? Eur. Respir. J. 2016;48(2):516–525. doi: 10.1183/13993003.00543-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Tanna G.L., et al. Effect of Xpert MTB/RIF on clinical outcomes in routine care settings: individual patient data meta-analysis. Lancet Global Health. 2019;7(2):e191–e199. doi: 10.1016/S2214-109X(18)30458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lawn S.D., Nicol M.P. Xpert(R) MTB/RIF assay: development, evaluation and implementation of a new rapid molecular diagnostic for tuberculosis and rifampicin resistance. Future Microbiol. 2011;6(9):1067–1082. doi: 10.2217/fmb.11.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.WHO, WHO consolidated guidelines on tuberculosis. Module 3: Dianosis Rapid Diagnostics for Tuberculosis Detection, WHO, 2021.
- 52.Dinnes J., et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol. Assess. 2007;11(3):1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 53.Amicosante M., et al. Inactivation of polymerase inhibitors for Mycobacterium tuberculosis DNA amplification in sputum by using capture resin. J. Clin. Microbiol. 1995;33(3):629–630. doi: 10.1128/jcm.33.3.629-630.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.CDC, Updated Guidelines for the Use of Nucleic Acid Amplification Tests in the Diagnosis of Tuberculosis, 2009. [PubMed]
- 55.Wu C.W., et al. Impact of nucleic acid amplification test on pulmonary tuberculosis notifications and treatments in Taiwan: a 7-year single-center cohort study. BMC Infect. Dis. 2019;19(1):726. doi: 10.1186/s12879-019-4358-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Vos M., et al. Comparative analytical evaluation of four centralized platforms for the detection of Mycobacterium tuberculosis complex and resistance to rifampicin and isoniazid. J. Clin. Microbiol. 2021;59(3) doi: 10.1128/JCM.02168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lyu M., et al. Which sample type is better for Xpert MTB/RIF to diagnose adult and pediatric pulmonary tuberculosis? Biosci. Rep. 2020;40(8) doi: 10.1042/BSR20200308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Soolingen D., et al. Insertion element IS1081-associated restriction fragment length polymorphisms in Mycobacterium tuberculosis complex species: a reliable tool for recognizing Mycobacterium bovis BCG. J. Clin. Microbiol. 1992;30(7):1772–1777. doi: 10.1128/jcm.30.7.1772-1777.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Negi S.S., et al. Protein antigen b (Pab) based PCR test in diagnosis of pulmonary and extra-pulmonary tuberculosis. Indian J. Med. Res. 2006;124(1):81–88. [PubMed] [Google Scholar]
- 60.Isakova J., et al. Mutations of rpoB, katG, inhA and ahp genes in rifampicin and isoniazid-resistant Mycobacterium tuberculosis in Kyrgyz Republic. BMC Microbiol. 2018;18(1):22. doi: 10.1186/s12866-018-1168-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Phusi N., et al. Specific interactions between 2-trans enoyl-acyl carrier protein reductase and its ligand: Protein-ligand docking and ab initio fragment molecular orbital calculations. J. Mol. Graph Model. 2019;88:299–308. doi: 10.1016/j.jmgm.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 62.Banerjee A., et al. inhA, a gene encoding a target for isoniazid and ethionamide in Mycobacterium tuberculosis. Science. 1994;263(5144):227–230. doi: 10.1126/science.8284673. [DOI] [PubMed] [Google Scholar]
- 63.Shanmugakani R.K., et al. An isothermal amplification-based point-of-care diagnostic platform for the detection of Mycobacterium tuberculosis: a proof-of-concept study. Curr. Res. Biotechnol. 2021;3:154–159. doi: 10.1016/j.crbiot.2021.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaur N., Michael J.S., Toley B.J. A modular paper-and-plastic device for tuberculosis nucleic acid amplification testing in limited-resource settings. Sci. Rep. 2019;9(1):15367. doi: 10.1038/s41598-019-51873-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Georghiou S.B., et al. Equivalence of the GeneXpert System and GeneXpert Omni System for tuberculosis and rifampicin resistance detection. PLoS One. 2021;16(12) doi: 10.1371/journal.pone.0261442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pagaduan J.V., Sahore V., Woolley A.T. Applications of microfluidics and microchip electrophoresis for potential clinical biomarker analysis. Anal Bioanal. Chem. 2015;407(23):6911–6922. doi: 10.1007/s00216-015-8622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pagaduan J.V., et al. 3D hybrid small scale devices. Small. 2018;14(27) doi: 10.1002/smll.201702497. [DOI] [PubMed] [Google Scholar]
- 68.Hong J.M., et al. Point-of-care diagnostic tests for tuberculosis disease. Sci. Transl. Med. 2022;14(639):eabj4124. doi: 10.1126/scitranslmed.abj4124. [DOI] [PubMed] [Google Scholar]
- 69.World Health Organization, Gear up to end TB: introducing the end TB strategy, World Health Organization, 2015. 〈https://apps.who.int/iris/handle/10665/156394〉.
- 70.World Health Organization, Latent tuberculosis infection: updated and consolidated guidelines for programmatic management, World Health Organization, 2018. 〈https://apps.who.int/iris/handle/10665/260233〉. License: CC BY-NC-SA 3.0 IGO. [PubMed]
- 71.P.C.A. Louis, Recherches anatomico-pathologiques sur la Phthisie, Precedees du rapport fait a l′academie royale de medecine, Gabon, 1825.
- 72.Laennec R.T.H. vol. 1. Société typographique belge; 1837. (Traité de l′auscultation médiate, et des maladies des poumons et du coeur). [Google Scholar]
- 73.F.G. Harbitz, Studies in the frequency, localization, and modes of dissemination of tuberculosis with special reference to its occurrence in the lymph nodes and during childhood, 1905.
- 74.American Thoracic Society Diagnostic Standards and Classification of Tuberculosis in Adults and Children. This official statement of the American Thoracic Society and the Centers for Disease Control and Prevention was adopted by the ATS Board of Directors, July 1999. This statement was endorsed by the Council of the Infectious Disease Society of America, September 1999. Am. J. Respir. Crit. Care Med. 2000;161(4 Pt 1):1376–1395. doi: 10.1164/ajrccm.161.4.16141. [DOI] [PubMed] [Google Scholar]
- 75.Lewinsohn D.M., et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2017;64(2):e1–e33. doi: 10.1093/cid/ciw694. [DOI] [PubMed] [Google Scholar]
- 76.Daniel T.M. The history of tuberculosis. Respir. Med. 2006;100(11):1862–1870. doi: 10.1016/j.rmed.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 77.Behr M.A., Kaufmann E., Duffin J. Latent tuberculosis: two centuries of confusion. 2021;204(2):142–148. doi: 10.1164/rccm.202011-4239PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parish H.J. A history of immunization. A History of Immunization. 1965 [Google Scholar]
- 79.von Pirquet C. Frequency of tuberculosis in childhood. J. Am. Med. Assoc. 1909;52(9):675–678. [Google Scholar]
- 80.Goldstein E.J.C., Lee E., Holzman R.S. Evolution and current use of the tuberculin test. Clin. Infect. Dis. 2002;34(3):365–370. doi: 10.1086/338149. [DOI] [PubMed] [Google Scholar]
- 81.Yang H., Kruh-Garcia N.A., Dobos K.M. Purified protein derivatives of tuberculin—Past, present, and future. FEMS Immunol. Med. Microbiol. 2012;66(3):273–280. doi: 10.1111/j.1574-695X.2012.01002.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seibert F.B., Glenn J.T. Tuberculin purified protein derivative. Am. Rev. Tuberc. 1941;44(1):9–25. [Google Scholar]
- 83.Affronti L.F., et al. What is PPD-S? Am. Rev. Respir. Dis. 1969;99(3):460–461. doi: 10.1164/arrd.1969.99.3.460. [DOI] [PubMed] [Google Scholar]
- 84.Lewinsohn D.M., et al. Official American Thoracic Society/Infectious Diseases Society of America/Centers for Disease Control and Prevention Clinical Practice Guidelines: Diagnosis of Tuberculosis in Adults and Children. Clin. Infect. Dis. 2016;64(2):e1–e33. doi: 10.1093/cid/ciw694. [DOI] [PubMed] [Google Scholar]
- 85.Huebner R.E., Schein M.F., Bass J.B., Jr The tuberculin skin test. Clin. Infect. Dis. 1993:968–975. doi: 10.1093/clinids/17.6.968. [DOI] [PubMed] [Google Scholar]
- 86.Nayak S., Acharjya B. Mantoux test and its interpretation. Indian Dermatol. Online J. 2012;3(1):2–6. doi: 10.4103/2229-5178.93479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Coros A., DeConno E., Derbyshire K.M. IS6110, a Mycobacterium tuberculosis complex-specific insertion sequence, is also present in the genome of Mycobacterium smegmatis, suggestive of lateral gene transfer among mycobacterial species. J. Bacteriol. 2008;190(9):3408–3410. doi: 10.1128/JB.00009-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hartman-Adams H., Clark K., Juckett G. Update on latent tuberculosis infection. Am. Fam. Phys. 2014;89(11):889–896. [PubMed] [Google Scholar]
- 89.Lalvani A., Millington K.A. T-cell interferon-γ release assays: can we do better? Eur. Respir. J. 2008;32(6):1428–1430. doi: 10.1183/09031936.00148308. [DOI] [PubMed] [Google Scholar]
- 90.Lalvani A., Whitworth H.S. Progress in interferon-gamma release assay development and applications: an unfolding story of translational research. Ann. Transl. Med. 2019;7(Suppl. 3) doi: 10.21037/atm.2019.05.76. S128-S128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Andersen P., et al. Specific immune-based diagnosis of tuberculosis. Lancet. 2000;356(9235):1099–1104. doi: 10.1016/s0140-6736(00)02742-2. [DOI] [PubMed] [Google Scholar]
- 92.QuantiFERON®-TB Gold Plus [package insert]. QuantiFERON. 〈http://www.quantiferon.com/wp-content/uploads/2017/04/English_QFTPlus_ELISA_R04_022016.pdf〉 (accessed June 14, 2022).
- 93.T-SPOT®.TB [Package Insert]. Oxford Immunotec. PI-TB-US-0001 V7. 〈https://www.tspot.com/wp-content/uploads/2020/01/The-T-SPOT.TB-test-package-insert.pdf〉 (accessed June 14, 2022).
- 94.Web Annex. Study report. In: Use of alternative interferon-gamma release assays for the diagnosis of TB infection: WHO policy statement, World Health Organization, Geneva, 2022. Licence: CC BY-NC-SA 3.0 IGO.
- 95.Global tuberculosis report 2020, World Health Organization, Geneva, 2020.
- 96.Rapid communication: TB antigen-based skin tests for the diagnosis of TB infection, World Health Organization, Geneva, 2022. (WHO/UCN/TB/2022.1). License: CC BY-NC-SA 3.0 IGO.
- 97.Kiselev V.I., et al. [Clinical trials of the new skin test Diaskintest for the diagnosis of tuberculosis] Probl. Tuberk Bolezn Legk. 2009;2:11–16. [PubMed] [Google Scholar]
- 98.[DETECTION OF TUBERCULOSIS AND TACTICS FOR FOLLOWING UP TUBERCULOSIS RISK GROUP SUBJECTS, BY USING DIASKINTEST® (RECOMBINANT TUBERCULOSIS ANTIGEN)]. Tuberk Biolezni Legkih, 2010 (2) p. 13-9. https://pubmed.ncbi.nlm.nih.gov/27529924/. [PubMed]
- 99.Aksenova V.A., et al. Latent tuberculosis infection in children and adolescents in Russia. Int. J. Infect. Dis.: IJID. 2020;92S:S26–S30. doi: 10.1016/j.ijid.2020.02.038. [DOI] [PubMed] [Google Scholar]
- 100.Aggerbeck H., et al. Randomised clinical trial investigating the specificity of a novel skin test (C-Tb) for diagnosis of M. tuberculosis infection. PLoS One. 2013;8(5) doi: 10.1371/journal.pone.0064215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bergstedt W., et al. First-in-man open clinical trial of a combined rdESAT-6 and rCFP-10 tuberculosis specific skin test reagent. PLoS One. 2010;5(6) doi: 10.1371/journal.pone.0011277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang W., et al. The effect of BCG vaccination and risk factors for latent tuberculosis infection among college freshmen in China. Int. J. Infect. Dis. 2022;122:321–326. doi: 10.1016/j.ijid.2022.06.010. [DOI] [PubMed] [Google Scholar]
- 103.Li F., et al. Recombinant fusion ESAT6-CFP10 immunogen as a skin test reagent for tuberculosis diagnosis: an open-label, randomized, two-centre phase 2a clinical trial. Clin. Microbiol. Infect. 2016;22(10):889.e9–889.e16. doi: 10.1016/j.cmi.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 104.Zhang H., et al. Induration or erythema diameter not less than 5 mm as results of recombinant fusion protein ESAT6-CFP10 skin test for detecting M. tuberculosis infection. BMC Infect. Dis. 2020;20(1):685. doi: 10.1186/s12879-020-05413-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.U.S. Food and Drug Administration. Premarket Approval Order, 2019. 〈https://www.accessdata.fda.gov/cdrh_docs/pdf18/P180047A.pdf〉.
- 106.Altawallbeh G., et al. Performance of an advanced interferon-gamma release assay for Mycobacterium tuberculosis detection. J. Appl. Lab. Med. 2021;6(5):1287–1292. doi: 10.1093/jalm/jfab012. [DOI] [PubMed] [Google Scholar]
- 107.LIONEX GmbH. LIOFeron®TB/LTBI IGRA [Online]. Germany: LIONEX, 2021. Available online at: 〈https://lionex.de/product/lioferon/〉 (accessed June 28, 2022).
- 108.Della Bella C., et al. LIOFeron®TB/LTBI: a novel and reliable test for LTBI and tuberculosis. Int. J. Infect. Dis. 2020;91:177–181. doi: 10.1016/j.ijid.2019.12.012. [DOI] [PubMed] [Google Scholar]
- 109.LIOFeron®TB/LTBI IGRA [package insert]. LIONEX GmbH. 〈https://lionex.de/wp-content/uploads/2019/11/LIOFeron%C2%AETB-LTBI_EN_Instructions-for-use-rev.–6.0.pdf〉 (accessed July 14, 2022).
- 110.BioMérieux. VIDAS® TB-IGRA [Online]. France: BIOMÉRIEUX, 2022. Available online at: 〈https://www.biomerieux-diagnostics.com/vidasr-tb-igra〉 (accessed June 29, 2022).
- 111.S. Diagbouga, et al., Preliminary diagnostic performance of the VIDAS® TB-IGRA for the detection of Mycobacterium tuberculosis infection and disease. medRxiv, 2022, p. 2022.04.01.22271763.
- 112.Boditech Med Inc. ichroma IGRA-TB [Online]. Korea: Boditech, 2021. Available online at: 〈https://www.boditech.co.kr/en/product/reagents/id/342〉 (accessed July 14, 2022).
- 113.Ichroma IGRA-TB [package insert]. Boditech Med Inc. 〈http://web.labindustrias.com/wp-content/uploads/2021/03/INS-TBE-EN-ichroma-IGRA-TB-25_Rev.00_2002111.pdf〉 (accessed July 14, 2022).
- 114.Kweon O.J., et al. Performance evaluation of newly developed fluorescence immunoassay-based interferon-gamma release assay for the diagnosis of latent tuberculosis infection in healthcare workers. J. Microbiol. Immunol. Infect. 2022;55(2):328–331. doi: 10.1016/j.jmii.2021.05.007. [DOI] [PubMed] [Google Scholar]
- 115.QIAGEN. QIAreach QuantiFERON-TB [Online]. The Netherlands: QIAGEN, 2022. Available online at: 〈https://www.qiagen.com/ca/applications/tb-management/products/qiareach-quantiferon-tb-test〉 (accessed July 14, 2022).
- 116.Kaaba C., et al. Assessing usability of QIAreach QuantiFERON-TB platform in a high tuberculosis prevalence, low-resource setting. ERJ Open Res. 2021;7(4) doi: 10.1183/23120541.00511-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fukushima K., et al. First clinical evaluation of the QIAreachTM QuantiFERON-TB for tuberculosis infection and active pulmonary disease. Pulmonology. 2022;28(1):6–12. doi: 10.1016/j.pulmoe.2021.07.003. [DOI] [PubMed] [Google Scholar]
- 118.Saluzzo F., Mantegani P., Poletti de Chaurand V. QIAreach QuantiFERON-TB for the diagnosis of Mycobacterium tuberculosis infection. Eur Respir J. 2022;59(3) doi: 10.1183/13993003.02563-2021. https://pubmed.ncbi.nlm.nih.gov/34675051/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.S.D. Biosensor, STANDARD F TB-Feron FIA [Online]. SD Biosensor Inc., Korea, 2021. Available online at: 〈https://www.sdbiosensor.com/product/product_view?product_no=167〉 (accessed July 14, 2022).