Abstract
Advanced glycation end products (AGEs) are biologically active compounds formed physiologically throughout a sequence of chemical reactions, to generate highly oxidant-reactive aldehydes that combine covalently to proteins. They accumulate slowly in tissues during ageing but also in metabolic and selected inflammatory disorders. Accumulation of AGEs occurs more rapidly and intensely in the skin and serum of patients with type 2 diabetes, obesity, cardiovascular diseases, chronic renal insufficiency, and non-alcoholic fatty liver disease and also in the skin of patients with psoriasis. All of the above conditions are intimately associated with psoriasis. Interaction of AGEs with their receptors (RAGEs) stimulates cellular signaling with the formation of reactive oxygen species and activation of nuclear factor kappa light chain enhancer of activated B (NF-kB), which is a key regulator in the expression of inflammatory mediators and the production of oxidative stress. Thus, AGEs may play an interesting pathogenic role in the intersection of inflammatory and metabolic diseases, may represent a biomarker of inflammation and a potential target for novel therapeutic strategies. This is a narrative review with the objective to summarize current evidence on the role of AGEs in psoriasis.
Keywords: psoriasis, advanced glycation end products, AGEs, inflammation, autoimmunity
1. Introduction
Psoriasis is a chronic, immune-mediated skin disease that affects 1–4% of the Western countries’ population and about 14 million people in Europe [1,2,3]. Psoriasis is characterized by erythematous–desquamative plaques that typically affect the extensor surfaces such as elbows, knees, sacral region, and scalp. About 20–30% of patients with chronic plaque psoriasis have a moderate to severe disease characterized by extensive skin involvement (i.e., greater than 10% of the body surface area). Both genetic and environmental factors play a relevant role in psoriasis pathogenesis. Most psoriasis susceptibility loci are related to inflammatory and immunity genes [4,5,6]. Environmental and triggering factors include infections (including COVID), stressful life events, medications, skin trauma, and air pollution [4,5,6]. Early events in the development of psoriasis lesions include the recruitment and the activation of plasmacytoid dendritic, which together with natural killer cells and macrophages, secrete cytokines such as IFN-γ, IL-1β, and TNF-α. These cytokines activate myeloid dendritic cells to perform their migratory and antigen-presenting cell functions. Myeloid dendritic cells migrate to lymph nodes to activate naïve autoreactive T lymphocytes, which acquire the ability to recirculate in the skin microenvironment. Activated dendritic cells and macrophages secrete IL-23, a cytokine crucial for the survival and expansion of Th17 helper lymphocytes. These Th17 cells release the pathogenetic cytokines, IL-17, TNF-α and IL-22, which promote directly and indirectly the hyperproliferation of keratinocytes, and the release of chemokines and cytokines. The amplified inflammatory response, and the increased epidermal thickness and desquamation result in the typical skin changes of psoriasis plaque [4,7].
Psoriasis shares immunopathological pathways and genetic features with psoriatic arthritis (PsA), inflammatory bowel diseases (IBD), and uveitis. These diseases are frequently observed in patients with psoriasis. Moreover, psoriasis patients, particularly those with moderate to severe disease, are at greater risk of metabolic disorders, including type 2 diabetes, obesity, chronic renal insufficiency, non-alcoholic fatty liver disease (NAFLD), and the metabolic syndrome. The association between psoriasis and metabolic comorbidities is multifactorial and includes a common genetic background and environmental factors such unhealthy life habits and meta-inflammation [8,9]. Psychiatric diseases (depression, anxiety, suicidal ideation) have also been associated with severe psoriasis [1,2,3,4].
Advanced glycation end products (AGEs) are biologically active oxidant compounds that accumulate in tissues during ageing but also in metabolic and selected inflammatory disorders. AGEs act as pro-inflammatory mediators, and their accumulation occurs more rapidly and intensely in the skin and serum of patients with metabolic disorders and also in the skin of patients with psoriasis. Thus, AGEs may play a pathogenic role in the intersection of inflammatory and metabolic diseases [1,2,3]. In this narrative review, we highlight the role of AGEs in psoriasis.
2. Metabolic Comorbidities of Psoriasis
Psoriasis patients are frequently overweight or obese [10]. Obesity, body mass index, hip circumference, and waist–hip ratio are independent risk factors for psoriasis [11]. The risk increases with obesity severity, and there is also a direct correlation between psoriasis severity and obesity. Arterial hypertension is also more prevalent in psoriatic patients compared to the reference population [12]. Poorly controlled and severe hypertension appear to be more common in those patients with more severe psoriasis. Psoriasis is also an independent risk factor for type 2 diabetes mellitus, with a higher risk in patients with more severe skin disease [13]. In addition, diabetic patients with psoriasis are more likely to have micro- and macro-vascular complications, compared to patients without psoriasis. An atherogenic lipid profile, with high total cholesterol and reduced high-density lipoprotein (HDL), is more frequent in patients with psoriasis [14]. In addition, dyslipidemia is a risk factor for developing psoriasis. Metabolic syndrome describes the combination of central obesity, arterial hypertension, insulin resistance, and dyslipidemia. Metabolic syndrome is a very relevant risk factor for diabetes and cardiovascular diseases. The prevalence of metabolic syndrome is higher in patients with moderate to severe psoriasis compared to matched controls, and correlates directly with body surface area affected by psoriasis [15]. Analysis of each component of metabolic syndrome showed the strongest association with obesity, suggesting that the adiposity is the main factor in the association between psoriasis and metabolic syndrome. Although the major cardiovascular risk factors are more prevalent in psoriatic patients, moderate to severe psoriasis is an independent risk factor for major cardiovascular diseases, with the risk of myocardial infarction, stroke, and cardiovascular death greatest among patients with severe psoriasis and longer disease duration. NAFLD is commonly found in the general population and comprises a range from mild forms of steatosis to the more severe steato-hepatitis. Psoriasis is frequently associated with metabolic disorders that can favor liver steatosis. Indeed, patients with psoriasis have a higher prevalence of NAFLD compared with matched non-psoriatic patients, and psoriasis itself is an independent risk factor for NAFLD [16]. Finally, moderate-to-severe psoriasis may be an independent risk factor for chronic kidney disease and end-stage renal disease [9].
PsA is the major inflammatory comorbidity associated with psoriasis. The prevalence of PsA is around one third of Caucasian patients with psoriasis, in the range of 6–42%, and is highest among patients between 30 and 60 years [17]. It is possible for patients to present with psoriasis (i.e., skin lesions) without psoriatic arthritis and with psoriatic arthritis without psoriasis skin lesions. The severity of PsA is independent from the severity of psoriasis PsA, which has in most cases mild to moderate disease with a fluctuating course. However, bone erosions can occur early in the disease’s course and lead to disability and impaired function of affected joints and bones [18]. The Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) has recognized different domains of PsA including synovitis, enthesitis/dactylitis, nail, skin, and axial involvement [19]. Synovitis could potentially affect any joint, but it is more commonly observed in the hands or feet. Enthesitis is observed in 30–50% of patients and more frequently affects the plantar fascia and Achilles’ tendon, causing pain and/or functional impairment, and more rarely in the iliac crest and epicondyles. Dactylitis affects 40–50% of patients, involving either fingers and/or toes asymmetrically [20].
Psoriasis can also be associated with IBD, particularly with Crohn’s disease [21]. Patients with psoriasis and IBD are at a higher risk of comorbidities (seronegative arthritis, thyroiditis, diabetes, and lymphoma) than patients affected with only psoriasis. Psoriasis has also been associated to a lesser extent with other diseases, such as cancer, especially T-cell lymphoma, psychiatric disorders, chronic pulmonary disease and obstructive sleep apnea, peptic ulcer disease, hyperuricemia and gout, and osteoporosis [9].
3. Advanced Glycation End Products (AGEs)
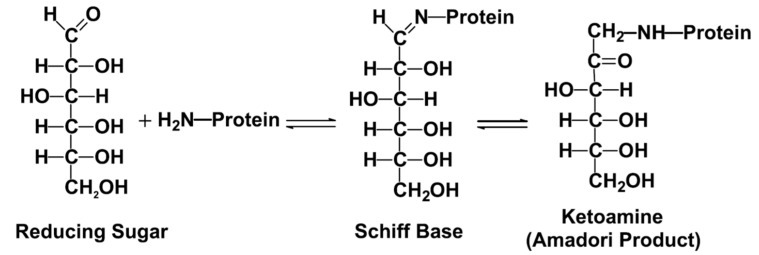
AGEs are highly oxidant, cross-linked irreversible ketone adducts created by a post-translational non-enzymatic modification between macromolecules, such as free amino groups of proteins, lipids or nucleic acids, and saccharides, such as glucose, fructose, and pentose [1,2,3,22]. Protein glycation is induced by the Maillard reaction in three steps: 1. formation of Schiff bases; 2. early creation of unstable AGE precursors that may trigger Amadori rearrangement; 3. the formation of late irreversible AGE products (Figure 1) [20,23]. Amadori compounds are non-reversible and a stable ketoamines that react with amino groups of proteins. Amadori products subsequently undergo a series of complex rearrangements yielding to AGEs. These rearrangements include oxidative degradation, which generates highly reactive intermediate dicarbonyl compounds, called glyoxal, methyglyoxal (MG), and deoxyglucosones. The principal AGEs are N-carboxylethylysine (CEL), N-carboxymethylysine (CML), N-lactatolysine, pyrraline, and pentosidine, and they have all been investigated in patients with psoriasis as well (Table 1). AGEs are formed primarily from glucose. On the contrary, advanced lipoxidation end products (ALEs) are generated from lipid peroxidation through a non-enzymatic reaction between reactive carbonyl species, the nucleophilic residues of macromolecules. ALEs exert their harmful bioactivity as a result of covalent modification of their target proteins and enzymes, which may result in changes in their biologic functions [2,3,24,25,26,27,28]. AGEs and ALEs are produced from the same precursors (glyoxal and methylglyoxal) and through the same intermediates (CML and N-carboxymethyl-cysteine-CMC). ALEs are generated from lipid peroxidation reactions, and AGEs from glycoxidation reactions [2,3,24,25,26,27,28]. Formation of endogenous AGEs is a physiological process in the normal metabolism over a lifetime and they accumulate slowly in tissues during ageing. In the case of hyperglycemia, obesity, selected autoimmune and inflammatory diseases, chronic renal insufficiency, and enhanced oxidative or carbonyl stress, there is a more rapid and intense accumulation of AGEs [24]. Exogenous AGEs derive from foods, called dietary AGEs, ultraviolet irradiation and ionizing radiation, air pollution, and cigarette smoking. Dietary AGEs are formed from Maillard’s reaction (Figure 1) and may be amplified 10–100 times during the cooking process involving high dry-heat temperatures in grilled and fried food, in particular, in the repeated heating of cooking oil as well as in red meat [24,29]. The oxidative stress deriving from skin exposure to ultraviolet light and smoking habit promotes AGEs accumulation by a greater production of oxygen free radicals [24].
Figure 1.
The classical pathway of the Maillard reaction. The open chain form of glucose reacts with lysine to form a Schiff base adduct, which undergoes an Amadori rearrangement to form a ketoamine (fructosamine) adduct to protein [23].
Table 1.
Principal advanced glycation end products (AGEs) investigated in patients with psoriasis.
| AGEs | Other RAGE Ligands |
|---|---|
| Carboxymethylysine (CML) | Psoriasin (S100A7) |
| Carboxyethyllysine (CEL) | Koebnerisin (S100A15) |
| Pyrraline | High-mobility group protein B1 (HMGB1) |
| Pentosidine | |
| Glyoxal-lysine dimer (GOLD) | |
| Deoxyglucasone-lysine dimer (DOLD) | |
| Methyl glyoxal-lysine dimer (MOLD) | |
| N-lactatolysine |
The receptors for AGEs (RAGEs) belong to the immunoglobulin superfamily. They are expressed on the surface of many cell types, including epithelial cells (keratinocytes, hepatocytes), dendritic cells, endothelial cells, and macrophages. RAGEs are also present in the serum in a soluble form [24,30].
4. Methods of Assessing AGEs
Several methods for quantifying AGEs have been developed without any real consensus on a gold standard technique. AGEs can be measured in biological fluids or in tissue samples, in particular, on serum or plasma [31]. The use of urinary or saliva samples has been less common. The evaluation of tissue AGEs poses the main problem of obtaining tissue samples, which may require invasive procedures, such as skin biopsies. The patients are usually more reluctant to give their consent to a skin biopsy than to provide blood samples. To overcome this problem, non-invasive measurement methods have been developed, such as skin autofluorescence (SA) (Table 2) [32,33,34]. It is still difficult to compare results in AGE quantification from different studies because of the different modes of AGE measurements and the lack of method standardization.
Table 2.
Characteristics of the main analytical methods currently used for AGEs quantification.
| Methods | AGEs | Sample |
|---|---|---|
| ELISA | Carboxymethylysine (CML) | Blood |
| Total AGEs | Blood | |
| LC-MS/MS | Carboxymethylysine (CML) | Blood |
| AGEs panels | Blood | |
| SA | Total fluorescent AGEs | Skin |
AGEs, advanced glycation end products; LC-MS/MS, liquid chromatography coupled to tandem mass spectrometry; ELISA, enzyme-linked immunoassay; SA, skin autofluorescence.
4.1. Serum Detection with Enzyme-Linked Immunoassay (ELISA)
AGEs can be easily measured in the serum. In particular, serum total AGEs are derived from venous sample collection, centrifuged, and subsequently measured using enzyme-linked immunoassay (ELISA) kit. In addition, the soluble RAGEs can be measured throughout serum using the ELISA technique and comprise both the extracellular domain of wild-type full-length RAGE and the endogenous-secreted isoform lacking a transmembrane domain (esRAGE) [2,3]. As glycation occurs continuously over the lifetime of the protein, the AGEs’ concentration reflects the average blood glucose value over a period of time [32].
4.2. Liquid Chromatography Coupled to Tandem Mass Spectrometry (LC-MS/MS)
Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) methods are the gold standard, where AGEs are measured throughout serum, and serve as reference methods owing to their excellent analytical performances. Nevertheless, they are not easy to perform in daily practice or in large-scale studies. The results of LC-MS/MS assays are expressed in mmol/mol of lysine. Such unit of measure is used because protein glycation in biological systems occurs on lysine, arginine, and N-terminal residues of proteins [32] and lysine is a substrate of the glycation.
4.3. Cutaneous AGEs
Cutaneous AGEs can also be measured as skin autofluorescence (SA) with the AGE ReaderTM. This is a standardized, non-invasive, and reproducible technique, and closely reflects serum AGEs values [1,2,3]. Detection of AGEs with SA is generally performed on the forearm [32]. A limitation of this method is that skin pigments, such as melanin, may interfere with the quantification, which limits its use in individuals with a darker skin complexion [32]. Cutaneous AGEs are predominantly bound to skin collagen, which has been estimated to have a half-life of approximately 14 years; therefore, their levels are assumed to remain quite stable over time. The results of SA values are expressed in arbitrary units [32].
5. AGEs and Psoriasis
5.1. AGEs in Psoriasis
AGEs have been investigated in patients with psoriasis and this disease may represent a model to exemplify how AGEs may be associated not only with metabolic but also inflammatory diseases. Papagrigoraki et al. showed that levels of skin AGEs were significantly higher in patients with severe psoriasis (16.84 × 103 μg/mL) compared to patients with mild psoriasis (6.33 × 103 μg/mL), and age, sex, and body mass index matched controls with severe atopic eczema (14.81 × 103 μg/mL) or healthy individuals (12.29 × 103 μg/mL) [2], suggesting a specific accumulation of these compounds in psoriasis (Th17 dominated) as compared to atopic dermatitis (Th2 dominated). No differences in levels of AGEs between normal and lesional skin in psoriasis and eczema patients have been detected. A direct correlation between serum and/or cutaneous AGEs levels and psoriasis severity was also shown. Cutaneous AGEs (p < 0.04), serum AGEs (p < 0.03), and pentosidine (p < 0.05) were higher in patients with severe psoriasis. Cutaneous AGE levels correlated with serum AGEs (r = 0.93, p < 0.0001) and with psoriasis area and severity score (r = 0.91, p < 0.0001). RAGE levels were lower (p < 0.001) in severe psoriasis, and inversely correlated with disease severity [35]. In other studies, serum amount of pentosidine and psoriasin were shown to be significantly higher in patients with severe psoriasis compared to patients with mild psoriasis or eczema, and healthy individuals. In these studies, controls and patients with severe AD were also matched for age, sex, and BMI in order to minimize the selection bias [36,37]. The amount of both compounds correlated with psoriasis severity [2]. Psoriasin is a proinflammatory protein that belongs to the S100 family of small calcium-binding proteins. Psoriasin can bind RAGEs and stimulates further production of AGEs. A direct correlation between intima-media thickness and serum psoriasin levels has been shown in patients with psoriasis [36]. Furthermore, a direct correlation between psoriasis severity and serum levels of koebnerisin has been described [38]. Instead, serum levels of RAGEs were lower in patients with psoriasis compared to patients with eczema or healthy subjects. RAGEs levels correlated inversely with disease severity [2]. More recently, we found that effective treatment of moderate to severe psoriasis with biological agents is associated with significant reduction in the levels of cutaneous AGEs [1]. In particular, a marked and sustained improvement in psoriasis with IL-17 and IL-23 inhibitors was associated with marked decrease in cutaneous AGEs. To what extent this effect may also have a favorable impact on metabolic comorbidities needs further investigation. Our findings are consistent with previous studies that have shown a reduction in SA in patients with psoriasis or PsA treated with adalimumab [39], or in patients with inactive disease [40]. Damasiewicz-Bodzek A et al. showed that blood concentration of AGEs is higher during the active disease phase and decreases during remission [40].
In all these studies, those patients with a smoking habit, diabetes, dyslipidemia, hypercholesterolemia, hypertension and/or any other systemic inflammatory or autoimmune diseases were excluded to avoid confounding factors affecting levels of AGEs [36,37,38,39]. Having methodologically excluded these confounding factors, it could be speculated that the association between AGEs and psoriasis is linked to the specific inflammation of psoriasis. AGEs act both directly on skin tissue and through their RAGE receptors. Accumulation of AGEs in the skin may cause an increased production of free radicals, oxidized LDL, and consequently favor skin peroxidation. Proteins modified by glycation or glycosylation can become immunogenic, thus amplifying pathogenic immune responses [41]. The AGE–RAGE interaction on inflammatory cells such as monocytes, macrophages, neutrophils, and endothelial cells stabilizes the receptor in the active state and favors the release of cytokines and chemokines, the production of reactive oxygen species, and the activation of metalloproteases and migration of T lymphocytes and other inflammatory cells into the inflammation site [41,42,43]. RAGEs can also bind the S100 proteins, including psoriasin and koebnerisin [2,3]. To what extent these phenomena may play a role in the pathogenetic mechanisms of psoriasis needs to be investigated.
5.2. AGEs as Biomarker of Cardiovascular Risk in Patients with Psoriasis
Moderate to severe psoriasis has been associated with an increased cardiovascular risk. In particular, an increased relative risk of myocardial infarction (1.17; 95% CI 1.11–1.24) and cardiovascular death (1.46; 95% CI 1.26–1.69) has been reported in patients with severe psoriasis [44]. This may be related to the association with classical cardiovascular risk factors including type 2 diabetes, obesity, hypertension, chronic renal insufficiency, and NAFLD (Table 3). In all these disorders, higher levels of AGEs have been measured [44]. The interaction of AGEs with RAGE in endothelial and smooth muscle cells as well as in platelets, activates intracellular signaling, and leads to endothelial cell injury, modulation of vascular smooth muscle cell function, and altered platelet activity [44]. In addition, the activation of RAGE can induce complex signaling pathways leading to enhanced calcium deposition, and increased vascular smooth muscle apoptosis, concurring to the development of atherosclerosis [45]. A relationship between psoriasis, AGEs, and atherosclerosis has been studied. Ergun T et al. found that SA was significantly increased in psoriasis patients (N = 52) compared to healthy controls (N = 20) and correlated with carotid intima-media thickness, supporting the hypothesis of a role for AGEs in atherosclerosis in patients with psoriasis [46]. Batycka-Baran A et al. found decreased soluble RAGE levels in patients with chronic plaque psoriasis compared to controls and an inverse correlation with psoriasis severity as assessed with the psoriasis area severity index. This correlation was independent of other variables. They concluded that decreased levels of soluble RAGE may contribute to the chronic inflammatory process and atherosclerosis [47]. Measuring cutaneous AGEs by SA may be a surrogate marker of systemic AGE burden as well as systemic inflammation and may thus represent a non-invasive method to identify patients with psoriasis with an increased cardiovascular risk [48].
Table 3.
Psoriasis comorbidities associated with an increased cardiovascular risk.
| Comorbidities | Measure of Association * between Psoriasis and Comorbidities |
|---|---|
| Obesity | 20.7% (OR, 1.79; 95% CI, 1.55–2.05) [49,50,51] |
| Type 2 diabetes | 7.0–11.4% [52] |
| Metabolic syndrome | 20–50% (OR, 2.26; 95% CI, 1.70–3.01) [53] |
| Dyslipidemia | 62.8% (OR, 1.04–5.55; 95% CI, 3.53–10.36) [54] |
| Non-alcoholic fatty liver disease | 47% (OR, 1.7; 95% CI, 1.1–2.6) [53] |
| Chronic renal insufficiency | 15.2% [55,56,57] |
* Prevalence and/or odds ratio of the association.
6. AGEs in Metabolic Diseases
Comorbidities associated with psoriasis, including metabolic disorders such as type 2 diabetes, obesity, hypertension, chronic renal insufficiency, and NAFLD, have been linked to increased serum levels of AGEs. An increased AGEs formation and accumulation in human tissues may cause modifications in the structure and function of the cells, leading to the amplification of inflammation, accelerating the development of the comorbidities associated with psoriasis, and correlating with their prognosis [2,3,24,58]. In particular, obesity and hyperglycemia strongly contribute to AGE production and tissue accumulation [8,36]. Indeed, serum levels of AGEs are predictors of heart failure and the development of cardiovascular events. AGEs increase vascular permeability, and arterial stiffness and promote neoangiogenesis. In addition, they inhibit vasodilation by interfering with nitric oxide, and thus promote endothelial cell dysfunction, ultimately favoring atherosclerosis. In patients with type 2 diabetes, serum levels of AGEs correlate with the risk and severity of cardiovascular disease. Higher expression of RAGE is associated with a stronger inflammation in the atherosclerotic carotid plaque, and treatment with statins decreases inflammation and expression of RAGE [59]. The activation of the AGE–RAGE axis causes the liberation and accumulation of reactive oxygen species (ROS) and activation of nuclear factor kappa light chain enhancer of activated (NF-κB), which is a master regulator of inflammation [59]. The binding of AGEs to RAGEs also modifies LDLs and promotes their uptake by macrophages and thus foam cell formation. AGEs are involved in the pathomechanism of diabetes, and they may be a crucial diagnostic marker, due to the long time remaining in the body. Their determination may allow for monitoring the progression of the disease and the effectiveness of the treatment [60]. In addition, measuring cutaneous AGEs in diabetic patients has been proposed to be important to identify those who have a major risk in developing vascular complications. High SA is an independent marker of acute myocardial infarction, cardiovascular disease, inflammation, and oxidative stress; it is also a strong predictor of survival in diabetic patients [2,3,24,58].
7. Conclusions
AGEs are biologically active products involved in the amplification and perpetuation of chronic immune-mediated inflammatory process possibly by the sustained activation of NF-κB. RAGE-mediated signaling triggers the expression of several inflammatory mediators, including adhesion molecules, chemokines, proinflammatory cytokines, such as TNF-α, IL-1, -6, and -8, and the consequent increased recruitment of inflammatory cells to the site of inflammation [2,3]. AGE formation and accretion in human tissues may cause modifications with a prominent role in the pathophysiology of inflammatory and metabolic diseases. In patients with psoriasis, the intensified protein glycation in the skin may have a role in the amplification of skin inflammation and may provide a link between cutaneous inflammation and increased prevalence of associated metabolic diseases. Increasing evidence supports a possible role for the AGE/RAGE axis also in the development, severity, and progression of cardiovascular diseases in patients with psoriasis. Additional prospective multicenter randomized controlled studies are needed to further evaluate the possibility that circulating AGEs or soluble RAGE levels can serve as a biomarker for cardiovascular diseases risk and to identify patients who may benefit from early prevention and treatment. More convincingly, AGEs and RAGEs may become a biomarker of systemic inflammation, metabolic abnormalities, and cardiovascular risk in patients with psoriasis. Moreover, AGEs and RAGEs may represent a potential therapeutic target, and investigating the role of AGEs in the crosstalk between psoriasis and its metabolic comorbidities may pave the way to new therapeutic targets and strategies.
Author Contributions
Conceptualization, M.M., P.G. and G.G.; writing, M.M. and P.G.; supervision, G.G. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marella M., Bellinato F., Gisondi P., Girolomoni G. Reduction of cutaneous advanced glycation end products levels after effective psoriasis treatment. J. Eur. Acad. Dermatol. Venereol. 2022;36:e629–e631. doi: 10.1111/jdv.18090. [DOI] [PubMed] [Google Scholar]
- 2.Papagrigoraki A., Del Giglio M., Cosma C., Maurelli M., Girolomoni G., Lapolla A. Advanced Glycation End Products are increased in the skin and blood of patients with severe psoriasis. Acta Derm. Venereol. 2017;97:782–787. doi: 10.2340/00015555-2661. [DOI] [PubMed] [Google Scholar]
- 3.Papagrigoraki A., Maurelli M., Del Giglio M., Gisondi P., Girolomoni G. Advanced Glycation End Products in the pathogenesis of psoriasis. Int. J. Mol. Sci. 2017;18:2471. doi: 10.3390/ijms18112471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maurelli M., Gisondi P., Girolomoni G. Tailored biological treatment for patients with moderate to severe psoriasis. Expert Rev. Clin. Immunol. 2022;9:1–7. doi: 10.1080/1744666X.2023.2141226. [DOI] [PubMed] [Google Scholar]
- 5.Gisondi P., Geat D., Armeni P., Bellinato F., Maurelli M., Girolomoni G. Cost per responder of Adalimumab biosimilars MSB11022 and ABP 501 versus the originator and methotrexate in chronic plaque psoriasis. Expert. Opin. Biol. Ther. 2022;26:1–6. doi: 10.1080/14712598.2022.2070428. [DOI] [PubMed] [Google Scholar]
- 6.Gisondi P., Geat D., Maurelli M., Degli Esposti L., Bellinato F., Girolomoni G. Cost per responder analysis of Secukinumab versus Adalimumab in the treatment of psoriatic disease. Vaccines. 2022;10:646. doi: 10.3390/vaccines10050646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Morelli M., Galluzzo M., Madonna S., Scarponi C., Scaglione G.L., Galluccio T., Andreani M., Pallotta S., Girolomoni G., Bianchi L., et al. HLA-Cw6 and other HLA-C alleles, as well as MICB-DT, DDX58, and TYK2 genetic variants associate with optimal response to anti-IL-17A treatment in patients with psoriasis. Expert. Opin. Biol. Ther. 2021;21:259–270. doi: 10.1080/14712598.2021.1862082. [DOI] [PubMed] [Google Scholar]
- 8.Abramczyk R., Queller J.N., Rachfal A.W., Schwartz S.S. Diabetes and psoriasis: Different sides of the same prism. Diabetes Metab. Syndr. Obes. 2020;13:3571–3577. doi: 10.2147/DMSO.S273147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gisondi P., Bellinato F., Girolomoni G., Albanesi C. Pathogenesis of chronic plaque psoriasis and its intersection with cardio-metabolic comorbidities. Front. Pharmacol. 2020;11:117. doi: 10.3389/fphar.2020.00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bu J., Ding R., Zhou L., Chen X., Shen E. Epidemiology of psoriasis and comorbid diseases: A narrative review. Front. Immunol. 2022;13:880201. doi: 10.3389/fimmu.2022.880201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aune D., Snekvik I., Schlesinger S., Norat T., Riboli E., Vatten L.J. Body mass index, abdominal fatness, weight gain and the risk of psoriasis: A systematic review and dose-response meta-analysis of prospective studies. Eur. J. Epidemiol. 2018;33:1163–1178. doi: 10.1007/s10654-018-0366-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correa T.L., Quitete M.A.C., de Azevedo L.T., Fraga I.A.C., Teixeira L.C. Infarctus du myocarde, accident vasculaire cérébral et psoriasis: Une revue systématique des études observationnelles [Myocardial infarction, stroke, and psoriasis: A systematic review of observational studies] Ann. Cardiol. Angeiol. 2022;16 doi: 10.1016/j.ancard.2022.11.009. [DOI] [PubMed] [Google Scholar]
- 13.Mamizadeh M., Tardeh Z., Azami M. The association between psoriasis and diabetes mellitus: A systematic review and meta-analysis. Diabetes Metab. Syndr. 2019;13:1405–1412. doi: 10.1016/j.dsx.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 14.Ma C., Harskamp C.T., Armstrong E.J., Armstrong A.W. The association between psoriasis and dyslipidaemia: A systematic review. Br. J. Dermatol. 2013;168:486–495. doi: 10.1111/bjd.12101. [DOI] [PubMed] [Google Scholar]
- 15.Langan S.M., Seminara N.M., Shin D.B., Troxel A.B., Kimmel S.E., Mehta N.N., Margolis D.J., Gelfand J.M. Prevalence of metabolic syndrome in patients with psoriasis: A population-based study in the United Kingdom. J. Investig. Dermatol. 2012;132:556–562. doi: 10.1038/jid.2011.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bellinato F., Gisondi P., Mantovani A., Girolomoni G., Targher G. Risk of non-alcoholic fatty liver disease in patients with chronic plaque psoriasis: An updated systematic review and meta-analysis of observational studies. J. Endocrinol. Investig. 2022;45:1277–1288. doi: 10.1007/s40618-022-01755-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alinaghi F., Calov M., Kristensen L.E., Gladman D.D., Coates L.C., Jullien D., Gottlieb A.B., Gisondi P., Wu J.J., Thyssen J.P., et al. Prevalence of psoriatic arthritis in patients with psoriasis: A systematic review and meta-analysis of observational and clinical studies. J. Am. Acad. Dermatol. 2019;80:251–265. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 18.Gessl I., Hana C.A., Deimel T., Durechova M., Hucke M., Konzett V., Popescu M., Studenic P., Supp G., Zauner M., et al. Tenderness and radiographic progression in rheumatoid arthritis and psoriatic arthritis. Ann. Rheum. Dis. 2023;82:344–350. doi: 10.1136/ard-2022-222787. [DOI] [PubMed] [Google Scholar]
- 19.De Marco G., Berekméri A., Coates L.C., Dubash S., Emmel J., Gladman D.D., Lubrano E., McGonagle D.G., Mahmood F., Marchesoni A., et al. Systematic literature review of non-topical treatments for early, untreated (systemic therapy naïve) psoriatic disease: A GRAPPA initiative. Rheumatol. Adv. Pract. 2020;4:rkaa032. doi: 10.1093/rap/rkaa032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gisondi P., Bellinato F., Maurelli M., Geat D., Zabotti A., McGonagle D., Girolomoni G. Reducing the risk of developing psoriatic arthritis in patients with psoriasis. Psoriasis. 2022;12:213–220. doi: 10.2147/PTT.S323300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alinaghi F., Tekin H.G., Burisch J., Wu J.J., Thyssen J.P., Egeberg A. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease-a systematic review and meta-analysis. J. Crohns Colitis. 2020;14:351–360. doi: 10.1093/ecco-jcc/jjz152. [DOI] [PubMed] [Google Scholar]
- 22.Singh R., Barden A., Mori T., Beilin L. Advanced glycation endproducts: A review. Diabetologia. 2001;44:129–146. doi: 10.1007/s001250051591. [DOI] [PubMed] [Google Scholar]
- 23.Thorpe S.R., Baynes J.W. Maillard reaction products in tissue proteins: New products and new perspectives. Amino Acids. 2003;25:275–281. doi: 10.1007/s00726-003-0017-9. [DOI] [PubMed] [Google Scholar]
- 24.Shen C.Y., Lu C.H., Wu C.H., Li K.J., Kuo Y.M., Hsieh S.C., Yu C.L. The development of Maillard reaction, and Advanced Glycation End Product (AGE)-Receptor for AGE (RAGE) signaling inhibitors as novel therapeutic strategies for patients with AGE-related diseases. Molecules. 2020;25:5591. doi: 10.3390/molecules25235591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dyer D.G., Blackledge J.A., Thorpe S.R., Baynes J.W. Formation of pentosidine during nonenzymatic browning of proteins by glucose. Identification of glucose and other carbohydrates as possible precursors of pentosidine in vivo. J. Biol. Chem. 1991;266:11654–11660. doi: 10.1016/S0021-9258(18)99007-1. [DOI] [PubMed] [Google Scholar]
- 26.Pizzimenti S., Ciamporcero E., Daga M., Pettazzoni P., Arcaro A., Cetrangolo G., Minelli R., Dianzani C., Lepore A., Gentile F., et al. Interaction of aldehydes derived from lipid peroxidation and membrane proteins. Front. Physiol. 2013;4:242. doi: 10.3389/fphys.2013.00242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaur S., Zilmer K., Leping V., Zilmer M. Serum methylglyoxal level and its association with oxidative stress and disease severity in patients with psoriasis. Arch. Dermatol. Res. 2013;305:489–494. doi: 10.1007/s00403-013-1362-5. [DOI] [PubMed] [Google Scholar]
- 28.Vistoli G., De Maddis D., Cipak A., Zarkovic N., Carini M., Aldini G. Advanced glycoxidation and lipoxidation end products (AGEs and ALEs): An overview of their mechanisms of formation. Free Radic. Res. 2013;47((Suppl. 1)):13–27. doi: 10.3109/10715762.2013.815348. [DOI] [PubMed] [Google Scholar]
- 29.Damasiewicz-Bodzek A., Wielkoszynski T. Advanced protein glycation in psoriasis. J. Eur. Acad. Dermatol. Venereol. 2012;26:172–179. doi: 10.1111/j.1468-3083.2011.04024.x. [DOI] [PubMed] [Google Scholar]
- 30.Sparvero L.J., Asafu-Adjei D., Kang R., Tang D., Amin N., Im J., Rutledge R., Lin B., Amoscato A.A., Zeh H.J., et al. RAGE (receptor for advanced glycation end products), RAGE ligands, and their role in cancer and inflammation. J. Transl. Med. 2009;7:17. doi: 10.1186/1479-5876-7-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Perrone A., Giovino A., Benny J., Martinelli F. Advanced Glycation End Products (AGEs): Biochemistry, signaling, analytical methods, and epigenetic effects. Oxid. Med. Cell Longev. 2020;2020:3818196. doi: 10.1155/2020/3818196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jaisson S., Gillery P. Methods to assess advanced glycation end-products. Curr. Opin. Clin. Nutr. Metab. Care. 2021;24:411–415. doi: 10.1097/MCO.0000000000000774. [DOI] [PubMed] [Google Scholar]
- 33.Da Moura Semedo C., Webb M., Waller H., Khunti K., Davies M. Skin autofluorescence, a non-invasive marker of advanced glycation end products: Clinical relevance and limitations. Postgrad. Med. J. 2017;93:289–294. doi: 10.1136/postgradmedj-2016-134579. [DOI] [PubMed] [Google Scholar]
- 34.Koetsier M., Lutgers H.L., de Jonge C., Links T.P., Smit A.J., Graaff R. Reference values of skin autofluorescence. Diabetes Technol. Ther. 2010;12:399–403. doi: 10.1089/dia.2009.0113. [DOI] [PubMed] [Google Scholar]
- 35.Shakoei S., Nakhjavani M., Mirmiranpoor H., Motlagh M.A., Azizpour A., Abedini R. The serum level of oxidative stress and antioxidant markers in patients with psoriasis: A cross-sectional study. J. Clin. Aesthet. Dermatol. 2021;14:38–41. [PMC free article] [PubMed] [Google Scholar]
- 36.Maurelli M., Gisondi P., Danese E., Gelati M., Papagrigoraki A., Del Giglio M., Lippi G., Girolomoni G. Psoriasin (S100A7) is increased in the serum of patients with moderate-to-severe psoriasis. Br. J. Dermatol. 2020;182:1502–1503. doi: 10.1111/bjd.18807. [DOI] [PubMed] [Google Scholar]
- 37.Awad S.M., Attallah D.A., Salama R.H., Mahran A.M., Abu El-Hamed E. Serum levels of psoriasin (S100A7) and koebnerisin (S100A15) as potential markers of atherosclerosis in patients with psoriasis. Clin. Exp. Dermatol. 2018;43:262–267. doi: 10.1111/ced.13370. [DOI] [PubMed] [Google Scholar]
- 38.Batycka-Baran A., Hattinger E., Zwicker S., Summer B., Zack Howard O.M., Thomas P., Szepietowski J.C., Ruzicka T., Prinz J.C., Wolf R. Leucocyte derived koebnerisin (S100A15) and psoriasin (S100A7) are systemic mediators of inflammation in psoriasis. J. Dermatol. Sci. 2015;79:214–221. doi: 10.1016/j.jdermsci.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 39.Lanna C., Zangrilli A., Bavetta M., Diluvio L., Campione E., Bianchi L. Skin advanced glycation end products as a diagnostic and monitoring tool among psoriatic patients: How the therapy helps reduce cardiovascular disease risk. Int. J. Dermatol. 2022;61:577–581. doi: 10.1111/ijd.15851. [DOI] [PubMed] [Google Scholar]
- 40.Damasiewicz-Bodzek A., Nowak A. Concentrations of N6-carboxymethyllysine (CML), N6-carboxyethyllysine (CEL), and soluble receptor for Advanced Glycation End-Products (sRAGE) are increased in psoriatic patients. Biomolecules. 2022;12:1870. doi: 10.3390/biom12121870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mezentsev A.V., Bruskin S.A., Soboleva A.G., Sobolev V.V., Piruzian E.S. Pharmacological control of receptor of advanced glycation end-products and its biological effects in psoriasis. Int. J. Biomed. Sci. 2013;9:112–122. [PMC free article] [PubMed] [Google Scholar]
- 42.Lohwasser C., Neureiter D., Weigle B., Kirchner T., Schuppan D. The receptor for advanced glycation end products is highly expressed in the skin and upregulated by advanced glycation end products and tumor necrosis factor-alpha. J. Investig. Dermatol. 2006;126:291–299. doi: 10.1038/sj.jid.5700070. [DOI] [PubMed] [Google Scholar]
- 43.Soboleva A.G., Bruskin S.A., Nikolaev A.A., Sobolev V.V., Mezentsev A.V. Role of receptor for advanced glycation end-products in pathogenesis of psoriasis. Mol. Biol. 2013;47:743–753. doi: 10.1134/S0026893313050191. [DOI] [PubMed] [Google Scholar]
- 44.Liu L., Cui S., Liu M., Huo X., Zhang G., Wang N. Psoriasis increased the risk of adverse cardiovascular outcomes: A new systematic review and meta-analysis of cohort study. Front. Cardiovasc. Med. 2022;9:829709. doi: 10.3389/fcvm.2022.829709. Erratum in: Front. Cardiovasc. Med. 2022, 9, 929149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosmopoulos M., Drekolias D., Zavras P.D., Piperi C., Papavassiliou A.G. Impact of advanced glycation end products (AGEs) signaling in coronary artery disease. Biochim. Biophys. Acta Mol. Basis Dis. 2019;1865:611–619. doi: 10.1016/j.bbadis.2019.01.006. [DOI] [PubMed] [Google Scholar]
- 46.Fishman S.L., Sonmez H., Basman C., Singh V., Poretsky L. The role of advanced glycation end-products in the development of coronary artery disease in patients with and without diabetes mellitus: A review. Mol. Med. 2018;24:59. doi: 10.1186/s10020-018-0060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ergun T., Yazici V., Yavuz D., Seckin-Gencosmanoglu D., Ozen G., Salman A., Direskeneli H., Inanc N. Advanced Glycation End Products, a potential link between psoriasis and cardiovascular disease: A case-control study. Indian J. Dermatol. 2019;64:201–206. doi: 10.4103/ijd.IJD_396_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Batycka-Baran A., Baran W., Nowicka-Suszko D., Szepietowski J.C. Soluble receptor for Advanced Glycation End Products: A novel biomarker for psoriasis severity with therapeutic implications? Acta Derm. Venereol. 2018;98:797–798. doi: 10.2340/00015555-2933. [DOI] [PubMed] [Google Scholar]
- 49.Kopeć-Pyciarz K., Makulska I., Zwolińska D., Łaczmański Ł., Baran W. Skin autofluorescence, as a measure of AGE accumulation in individuals suffering from chronic plaque psoriasis. Mediat. Inflamm. 2018;2018:4016939. doi: 10.1155/2018/4016939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Masson W., Lobo M., Molinero G. Psoriasis and cardiovascular risk: A comprehensive review. Adv. Ther. 2020;37:2017–2033. doi: 10.1007/s12325-020-01346-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Egeberg A., Gisondi P., Carrascosa J.M., Warren R.B., Mrowietz U. The role of the interleukin-23/Th17 pathway in cardiometabolic comorbidity associated with psoriasis. J. Eur. Acad. Dermatol. Venereol. 2020;34:1695–1706. doi: 10.1111/jdv.16273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chiricozzi A., Gisondi P., Girolomoni G. The pharmacological management of patients with comorbid psoriasis and obesity. Expert Opin. Pharmacother. 2019;20:863–872. doi: 10.1080/14656566.2019.1583207. [DOI] [PubMed] [Google Scholar]
- 53.Holm J.G., Thomsen S.F. Type 2 diabetes and psoriasis: Links and risks. Psoriasis. 2019;9:1–6. doi: 10.2147/PTT.S159163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gisondi P., Fostini A.C., Fossà I., Girolomoni G., Targher G. Psoriasis and the metabolic syndrome. Clin. Dermatol. 2018;36:21–28. doi: 10.1016/j.clindermatol.2017.09.005. [DOI] [PubMed] [Google Scholar]
- 55.Takeshita J., Grewal S., Langan S.M., Mehta N.N., Ogdie A., Van Voorhees A.S., Gelfand J.M. Psoriasis and comorbid diseases: Epidemiology. J. Am. Acad Dermatol. 2017;76:377–390. doi: 10.1016/j.jaad.2016.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Conti A., Giovannini L., Mandel V.D., Odorici G., Lasagni C., Bigi L., Pellacani G., Cappelli G. Chronic kidney disease in psoriasis: A cohort study. J. Dtsch Dermatol. Ges. 2020;18:438–445. doi: 10.1111/ddg.14087. [DOI] [PubMed] [Google Scholar]
- 57.Munera-Campos M., Ferrándiz C., Mateo L., Prior-Español Á., Carrascosa J.M. Prevalence and stages of chronic kidney disease in psoriasis and psoriatic arthritis: A cross-sectional study. Indian J. Dermatol. Venereol. Leprol. 2021;87:321. doi: 10.25259/IJDVL_372_19. [DOI] [PubMed] [Google Scholar]
- 58.Sohouli M.H., Fatahi S., Sharifi-Zahabi E., Santos H.O., Tripathi N., Lari A., Pourrajab B., Kord-Varkaneh H., Găman M.A., Shidfar F. The impact of low Advanced Glycation End Products diet on metabolic risk factors: A systematic review and meta-analysis of randomized controlled trials. Adv. Nutr. 2021;12:766–776. doi: 10.1093/advances/nmaa150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schmidt A.M. 22016 ATVB plenary lecture: Receptor for advanced glycation endproducts and implications for the pathogenesis and treatment of cardiometabolic disorders: Spotlight on the macrophage. Arterioscler. Thromb. Vasc. Biol. 2017;37:613–621. doi: 10.1161/ATVBAHA.117.307263. Erratum in: Arterioscler. Thromb. Vasc. Biol. 2017, 37, e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Indyk D., Bronowicka-Szydełko A., Gamian A., Kuzan A. Advanced glycation end products and their receptors in serum of patients with type 2 diabetes. Sci. Rep. 2021;11:13264. doi: 10.1038/s41598-021-92630-0. [DOI] [PMC free article] [PubMed] [Google Scholar]