Summary
1. Pathogens and immune challenges can induce changes in host phenotype in ways that indirectly impact important community interactions, including those that affect host–pathogen interactions.
2. To explore host behavioural response to immune challenge, we exposed wild white-footed mice (Peromyscus leucopus) to an immunogen from an endemic, zoonotic pathogen, the spirochete Borrelia burgdorferi. White-footed mice are a major reservoir host of Lyme disease (LD) spirochetes in northeastern USA and an abundant member of forest communities. The activity patterns, foraging behaviour, and space use of white-footed mice have implications for population growth rates of community members upon which mice incidentally prey (i.e. gypsy moths and native thrushes), as well as potentially determining host-vector encounter rates and human risk of LD.
3. Immunochallenge led to specific humoral (antibody) and cellular (i.e. elevated neutrophils and eosinophils) immune responses, supporting use of the immunogen as a surrogate for infection.
4. Immunochallenged mice had reduced wheel-running activity early in the night when measured in the lab. However, mouse activity, as measured by track plates in natural field experiments, did not differ between mice exposed to the immunogen and unexposed mice.
5. Foraging behaviour of wild mice in the field – assessed with giving-up densities of seed at artificial feeding stations – was affected by exposure to the immunogen. Whereas immunochallenge did not influence whether foraging mice gained information on patch quality while foraging, it led to reductions in predator avoidance during foraging, suggesting that the proportion of space used by foraging mice may be greater as a result of immunochallenge. This increased space use is predicted to increase encounter rates with patchily distributed LD vectors (ticks) and with incidental prey items.
6. Thus, immunochallenge in white-footed mice, and potentially pathogenic infection, have the potential to indirectly impact community interactions, including those important for pathogen transmission.
Keywords: behavioural indicators, black-legged tick, Borrelia burgdorferi, GUDs, gypsy moth, indirect community effect, Ixodes scapularis, lyme disease ecology, quitting harvest rate
Introduction
Pathogen avoidance and investment in immune function are vital components of host evolutionary ecology (Sheldon & Verhulst 1996; Lochmiller & Deerenberg 2000; Zuk & Stoehr 2002; Lee 2006). Even non-pathogenic immune challenges can induce dramatic changes in traits as diverse as metabolic rate, competing immune function, food consumption, offspring feeding rate and reproductive output (Minchella & Loverde 1981; Adamo 1999; Lochmiller & Deerenberg 2000; Zuk & Stoehr 2002; Martin et al. 2006; Uller, Isaksson & Olsson 2006; Velando, Drummond & Torres 2006). In addition, these responses can potentially impact population dynamics and have cascading effects in community ecology through direct and indirect pathways (Anderson & May 1979; May & Anderson 1979; Dobson 1988; McCallum & Dobson 1995; Lafferty, Dobson & Kuris 2006; Collinge, Ray & Cully 2008; Lafferty 2008; Pederson & Grieves 2008). In particular, changes in host phenotype that alter population demographics and pathogen transmission rates could affect disease epidemiology. In addition, population regulation by pathogens can indirectly impact other community members through altered predatory or competitive interactions (Lafferty, Dobson & Kuris 2006; Collinge, Ray & Cully 2008; Lafferty 2008).
While appreciation has grown in the last decade for the indirect effects of host population regulation by pathogens, little is known about how pathogen-induced alterations of individual host phenotype (e.g. behaviour) impact community ecology, including interactions between hosts and pathogens. Space-use and foraging behaviour of animals reflect individual condition and behavioural motivation and can have extensive indirect impact on community ecology (e.g. Brown 1988; Schmidt 2004; Brown & Kotler 2007; Kotler & Brown 2007; Schmidt & Schauber 2007). For example, the quitting harvest rate (QHR) of a foraging animal is theorized to provide information on the energetic cost of foraging, missed opportunity costs and instantaneous predation risk (Brown 1988, 1992; Brown & Kotler 2007). If a forager has perfect information on patch quality, it will harvest patches of different quality to the same QHR in a density-dependent fashion (Valone & Brown 1989). In addition, QHR has immediate relevance for community ecology because it reflects the proportion of a home range used by a forager (restricted to ‘profitable’ space). Specifically, a higher QHR indicates that an animal forages only in high-quality patches (high density of primary prey items), ignores low-quality patches, and thus uses a smaller proportion of its home range for foraging. This leads to a reduced encounter rate with patchily distributed incidental prey as many prey go undiscovered in otherwise poor-quality patches (Schmidt, Goheen & Naumann 2001; Schmidt & Ostfeld 2003a; Schmidt 2004).
Pathogens and immune challenges may influence QHR if metabolic costs of immune response alter the energetic costs of foraging. If pathogen exposure leads to altered host life-history strategies (e.g. reproductive suppression or terminal investment; Forbes 1993; Clutton-Brock 1984), the missed reproductive opportunities costs would differ between exposed and unexposed individuals. With respect to the cost of predation, animals in good condition are predicted to avoid risky behaviours to protect their high reproductive value, whereas animals in poor condition should be less sensitive to risk because current energy intake is a priority (e.g. asset protection principle, Williams 1966; McNamara & Houston 1986; Brown 1992; Forbes 1993; Clark 1994; Morris & Davidson 2000; Kotler, Brown & Bouskila 2004; Brown & Kotler 2007). Finally, immune challenges may decrease a forager’s ability to assess patch quality, leading to under-exploitation of rich patches and an overall decrease in energy acquired (Valone & Brown 1989; Olsson & Brown 2006). In addition to providing information on individual motivation, such behavioural measures can serve as indicators of the indirect effect of pathogenic infection on wide-ranging community interactions.
The central role that hosts and their foraging behaviour can play in community interactions is exemplified by white-footed mice (Peromyscus leucopus) in the northeastern United States. White-footed mice serve as a major reservoir host for the aetiologic agent of Lyme disease (LD), the spirochete bacterium Borrelia burgdorferi (Burgdorfer et al. 1982; Levine, Wilson & Spielman 1985; Donahue, Piesman & Spielman 1987; LoGiudice et al. 2003). The bacterium is transmitted among vertebrate host species via the bite of the black-legged tick (Ixodes scapularis; Burgdorfer et al. 1982). White-footed mice display high natural infection rates and long infection duration (Anderson, Johnson & Magnarelli 1987; Donahue, Piesman & Spielman 1987; Hofmeister et al. 1999), which facilitates the persistence of infected mice in the community (Hofmeister et al. 1999; Schwanz et al. 2010; but see Burgess, French & Gendron-Fitzpatrick 1990, Moody et al. 1994). White-footed mice also have high reservoir competence (transmitting the bacterium to ~90% of ticks that feed on them) and successfully feed a high proportion of attached ticks (Levine, Wilson & Spielman 1985; Donahue, Piesman & Spielman 1987; LoGiudice et al. 2003; Keesing et al. 2009). As a consequence, population density of white-footed mice is a strong predictor of subsequent LD risk (Ostfeld et al. 2006) and human incidence rates (Schauber, Ostfeld & Evans 2005).
The interactions between white-footed mice, ticks and other community members may additionally be influenced by mouse behaviour (Schmidt & Schauber 2007). Patterns of activity, space use and foraging behaviour have been shown to influence encounter rates of mice with clumped incidental prey items (Schmidt, Goheen & Naumann 2001; Schmidt & Ostfeld 2003a; Connors et al. 2005). Pupae of introduced, pest gypsy moths (Lymantria dispar) and the nests of native thrushes (veeries and wood thrushes) are patchily distributed, incidental prey items whose abundance can be regulated by mouse density (Ostfeld, Jones & Wolff 1996a; Jones et al. 1998; Schmidt & Ostfeld 2003b; Schmidt, Ostfeld & Smyth 2006). More subtly, the proportion of space used by mice while foraging in a habitat is positively related to predation rates on thrush nests (Schmidt, Goheen & Naumann 2001; Schmidt & Ostfeld 2003a). Similarly local mouse activity influences the likelihood of encountering gypsy moth pupae (Connors et al. 2005). Because larval and nymphal ticks are also patchily distributed (Ostfeld, Hazler & Cepeda 1996b; Ostfeld, Miller & Hazler 1996c), it is likely that mouse space use would similarly affect mouse-tick encounter rates, altering host-vector dynamics and potentially disease epidemiology.
In this study, we assessed the extent to which the cost of an immunochallenge in white-footed mice alters behaviour, and the potential indirect impacts on community and disease ecology. We measured individual immune response of mice exposed to varying doses of a known B. burgdoferi-based immunogen (Gomes-Solecki, Brisson & Dattwyler 2006). We assessed the behavioural effects of immunochallange in individual mice in a lab setting and in populations of mice in the field. For the field study, we assessed activity and foraging behaviour as behavioural indicators of important community interactions, including incidental predation on gypsy moth pupae and nests of native thrushes and encounter rates with (tick) disease vectors (Connors et al. 2005; Schmidt & Schauber 2007).
Materials and methods
The experiment was conducted in eastern deciduous forests on the property of the Cary Institute of Ecosystem Studies in Dutchess County, New York (41°50′N 73°45′W). As part of long-term studies, portions of these forests have been monitored for densities of small mammals and ticks, and infection of hosts and vectors with B. burgdorferi for 19 years (Ostfeld et al. 2006). Six small-mammal trapping grids were monitored in 2008. Each grid had 8 × 8 trapping stations, with two Sherman live traps at every station and 15 m between each station to conduct capture–recapture studies of small mammals. Three of the grids (experimental grids) received oat bait containing an immunogen (Cary Drive; Canoe Gap; Field Lab; see immunogen details below), while the remaining three grids (control grids) did not receive any immunogen in their bait (Henry X; Green C; Green X). The bait immunogen was deployed on experimental grids every trapping night (Monday-Thursday) between 31 May and 12 September. Trapping on the control grids occurred for two nights every 3 weeks between 8 May and 31 October. Captured white-footed mice were given ear tags and information on sex, age, body mass, tick burden and proportion of bait eaten (for experimental grids) was recorded.
For analyses of the data from our field experiment, population size on each trapping grid was estimated using the Jolly-Seber open population estimator (POPAN-5; Schwarz & Arnason 1996). All trapping data in 2008 from control grids was included (8–92-day trapping sessions). Because the trapping schedule on the experimental grids was continuous, the trapping data were filtered to include captures recorded only on the first two trapping nights every 3 weeks (72-day trapping sessions). Population size for each grid was then estimated for the trapping week preceding the field experiment and the trapping week following the field experiment (Table 1). The average of these two estimates was used as the estimated population size during the field experiment.
Table 1.
Grid population sizes. Population size estimates for the six trapping grids in weeks preceding and following the field experiment
| Grid | Weeks | Population size | Average |
|---|---|---|---|
| Control | |||
| Henry X (8 × 8 subplot) | 10–11 July | 18·86 | 17·765 |
| 5–6 August | 16·67 | ||
| Green C (8 × 8 subplot) | 3–4 July | 28·66 | 26·85 |
| 31 August – 1 July | 25·04 | ||
| Green X (8 × 8 subplot) | 1–2 July | 27·5 | 24·13 |
| 29–30 July | 20·76 | ||
| Experimental | |||
| Cary Drive | 8–9 July | 21·75 | 20·715 |
| 29–30 July | 19·68 | ||
| Canoe Gap | 8–9 July | 13·5 | 14·54 |
| 29–30 July | 15·58 | ||
| Field Lab | 8–9 July | 31 | 20·745 |
| 29–30 July | 21·18 | ||
BAIT IMMUNOGEN
Wild white-footed mice were exposed to a B. burgdorferi-based immunogen – outer surface protein A (OspA) – shown to induce a specific antibody response in lab mice when orally consumed (Gomes-Solecki, Brisson & Dattwyler 2006). Use of an immunogen rather than a live pathogen was necessary to avoid ethical concerns involving experimental exposure of free-ranging animals to pathogens, while providing a surrogate for such exposure. Although OspA naturally occurs in B. burgdorferi, its down-regulation when spirochetes enter vertebrate hosts reduces natural exposure rates in mice (and other hosts; De Silva et al. 1996). Consequently, mice do not naturally produce antibody to OspA even in LD-endemic areas (Hofmeister et al. 1999; Bunikis et al. 2004). Escherichia coli transformed with recombinant B. burgdorferi outer surface protein A, was induced with IPTG for protein expression. Cells were harvested, resuspended in TBY containing 24% sucrose and quickly frozen in a dry ice bath. The antigen was placed in a lyophilizer (Labconco, Kansas City, MO, USA) overnight and stored at −70 °C for future use. A total of 200 mg of immunogen was weighed and mixed with 10% regular oatmeal, wrapped in a c. 2 cm diameter ball of whole oat and water in wax paper. Single bait balls were deployed each trapping night for ingestion ad libitum by trapped animals. For control plots, similar quantities oat bait were deployed without the immunogen.
IMMUNOLOGY
Between 21 August and 11 September 2008, wild-caught mice from experimental (N = 28) and control (N = 21) grids were transferred to the lab on the morning of trapping. Mass (g) was recorded at the time of trapping and, based on trapping records, immunogen dose was known for each mouse (# baits eaten). Blood was collected via submandibular puncture with lancets to measure complete blood cell counts and the strength of the (anti-OspA) antibody response to immunogen exposure. Blood was collected between the hours of 13.30 and 15.00, from adult and sub-adult males and females. Ten microlitres of whole blood was collected in a heparinized capillary tube for blood cell counts. The remainder of the blood was collected in eppendorf tubes and allowed to coagulate at room temperature for a day. Subsequently, the serum was frozen for antibody assays.
To perform blood cell counts, 2 μL of the whole blood collected in capillary tubes were used to determine volume of red and white blood cells as in Beldomenico et al. (2008a). Two microlitres of whole blood sample were immediately mixed with 18 μL of 0·01 M PBS (1 : 10 dilution). Within three hours of blood sampling, 2 μL of the 1 : 10 blood dilution were mixed with 1 mL of PBS (1 : 5000 dilution) to determine red blood cell volume. The remaining 1 : 10 blood dilution was used to prepare a 1 : 20 dilution in 4% acetic acid with 1% crystal blue to determine white blood cell volume. Samples were loaded into Kova Glasstic® slides with grids (Item 87144; Hycor Biomedical Inc., Garden Grove, CA, USA) to count blood cells in pre-determined grids, and the number of cells per μL of whole blood was calculated. Five to 10 μL of the remaining whole blood were used to prepare a blood smear for blood cell differentials. For the blood cell differential, blood smears were air-dried and stained with Wright Stain (Sigma 45253, St. Louis, MO, USA). Hundred cells were counted per slide and identified as lymphocytes, neutrophils, monocytes, eosinophils or basophils (Feldman, Zinkl & Jain 2000).
Antibody (anti-OspA) titres (OD at 410 nm) in serum of immunochallenged wild white-footed mice were measured using enzyme-linked immunosorbent assays (ELISA). To prepare ELISA plates, a solution of purified OspA in 140 mM sodium carbonate, pH 9·0 was used to coat commercial microwell plates (Nunc Maxisorp®, Thermo Fisher Scientific, Pittsburgh, PA, USA). The coating procedure was as follows: 100 μL of a solution containing 0·5 μg mL−1 antigen was added to each well and the microwell plate incubated either 1 h at room temperature or at 4 °C overnight. The antigen solution was removed from the wells, the plate washed three times with 140 mm sodium carbonate, pH 9·0 and 200 μL of blocking solution added [2% BSA fraction V (Sigma) in 140 mm sodium carbonate pH 9·0]. Following a 30 min incubation at 37 °C the plates were washed three times with sodium carbonate pH 9·0, wrapped in plastic and stored at 4 °C until used (up to a week).
The standard procedure for the ELISA tests was as follows: serum samples were diluted 1 : 100 in filter sterilized specimen diluent (10% fetal bovine serum – Gibco, Carlsbad, CA, USA – in PBS, 1 mm KH2PO4, 10 mm Na2HPO4, 137 mM NaCl, 2·7 mM KCl, pH 7·4 – Boehringer Mannheim, Germany) and 100 μL of each sample added to ELISA plate microwells. Following incubation for 1 h at 37 °C the samples were removed and the plates washed three times in PBS-T (PBS – 0·05% Tween 20). Alkaline phosphatase conjugated goat anti-P. leucopus IgG was used as secondary antibody (KPL). It was diluted 1 : 1000 in PBS, pH 7·4 and 100 μL of the solution added to each well. Following incubation for 30 min at 37 °C, the plates were washed three times with PBS-T and 100 μL of substrate solution (5 mg of p-nitrophenylphosphate tablets dissolved in 1× diethanolamine substrate buffer to yield a 2 mg mL−1 solution – Kirkegaard Perry Laboratory) was added to each well. The plates were incubated for 30 min at 37 °C and 100 μL of stop solution (5% EDTA) was added to each well. The absorbance (OD) at 410 nm was read on a microplate reader (Dynatech, Dynex Technologies, Chantilly, VA, USA). As negative controls we used five serum samples from normal individuals. The same negative controls were included in each plate. A sample was considered positive if it produced an average absorbance greater than the mean of the negative controls plus three standard deviations.
WHEEL RUNNING ACTIVITY
Between 29 July and 19 September 2008, wild-caught mice of known immunogen dose from experimental (N = 27) and control (N = 24) grids were transferred to the lab on the morning of trapping. Mice were weighed and placed individually in polycarbonate activity wheel chambers (24 × 35 × 20 cm) affixed with automated wheel revolution counters (Model 80820 Mouse Single Activity Wheel System with Model 86061 Activity Wheel Counter; Lafayette Instruments, Lafayette, IN, USA). Wheel revolutions were recorded automatically every minute from roughly 17.00 to 07.30 h using manufacturer-supplied software (Activity Wheel Monitor v. 10.4 Software; Lafayette Instruments). Food and water were provided ad libitum, and chambers contained aspen shavings for bedding. Mice were returned to the site of trapping the following morning. Chambers were washed with warm water and soap after each use. The animal room was kept on a 14 : 10 L : Dcycle, with the lights out between 20:00 and 06:00 h.
FIELD EXPERIMENT
Field activity of mice on control and experimental grids was measured using track plates, which provide a measure of local activity density and correlate with predation on gypsy moth pupae (Connors et al. 2005). Foraging behaviour was assayed using giving-up densities of seed at artificial seed trays. Giving-up densities (GUDs) provide a measure of QHR and space use by white-footed mice (Brown 1988; Schmidt & Ostfeld 2003a).
On nights when the field experiment was running, no trapping occurred. Eight trap stations on each grid were chosen to establish experimental stations. These experimental stations consisted of 16 track plates (4 × 4 grid), separated from each other by 2 m. Four seed trays (2 × 2 grid), separated from each other by 1 m, were placed in the centre of the track plate grid. The seed trays were located 2 m from the traps at the trap station (in one of eight randomly chosen cardinal directions).
Track plates were prepared with a graphite solution painted on acetate sheets (14 × 22 cm) as in Connors et al. (2005), and presented in the field on top of pieces of aluminium flashing. Plates were checked for animal tracks after each night for four total nights (13, 14, 24 and 25 July 2008). Tracks of P. leucopus on each track plate were recorded as 0 footprints, 1–4 footprints, 5–10 footprints or > 10 footprints, and given scores 0, 1, 2 or 3 respectively. On the first night, sustained rain prevented confidently assessing tracks on a portion of the track plates, so this first night was removed from analysis. Scores for the 16 track plates at each station were summed for each of the three nights to get a nightly summed track plate score for each station (maximum score 48).
Four treatments of seed tray were presented at each station: (i) Low food – uncovered, LU; (ii) Low food – covered, LC; (iii) High food – uncovered, HU; and (iv) High food – covered, HC. The tray treatments were randomly assigned to the four tray locations at each station. All seed trays (20 × 28 cm; Perma-nest Plant Tray, Growers Supply Co., Inc., Dexter, MI, USA) contained 1·5 L of natural play sand. Low-food seed trays received 2 g of millet seed mixed in the sand, and high-food trays received 4 g of millet seed. Covered trays were placed under an opaque black shade cloth suspended 5–10 cm above the top edge of the tray, whereas uncovered trays had no shade.
Because immunochallenge could impact QHR in many ways, we had no a priori expectations for the overall change in GUD. Rather, GUDs were measured to assess the effects of immunochallenge on behaviours likely to affect space use and related community interactions. Comparisons of GUDs between covered and uncovered trays allow assessment of the risk sensitivity of foraging mice. Comparisons between high-food and low-food trays (rich and poor patches) allow assessment of foraging strategy (Valone & Brown 1989). Specifically, mice with perfect knowledge of patch quality (prescient strategy) were expected to forage with positive density dependence and equalize GUDs in the two patch types (ratio of rich GUD : poor GUD = 1 : 1). Mice with no information about patch quality should forage density-independently, producing GUDs of the same ratio as before foraging (2 : 1; fixed time strategy). Mice that update information about patch quality while foraging (Bayesian strategy) should forage with positive density dependence, but would not equalize GUDs (ratio of rich GUD : poor GUD between 2 : 1 and 1 : 1).
To record foraging behaviour of P. leucopus in the seed trays, the trays were accessible from dusk (18.00–20.00 h) until sunrise the following morning (06.00–08.00 h). In the morning, each tray was recorded for footprints and diggings in the sand. Trays with sand disturbance were sieved to collect seed, which was transferred to the lab and weighed to the nearest 0·01 g. Trays were replenished with seed and closed during the daytime. Trays were recorded as visited if the sand was disturbed and the weighed seed was depleted. Stations were recorded as visited if at least one tray was visited. The experiment was conducted over three nights (16, 18 and 21 July) following a pre-bait night (15 July) when all trays received 6 g of millet. The pre-bait was established to increase the likelihood that mice would locate and visit the stations during the experiment. Seed was provided in equal amounts in all trays during pre-bait so that assessment and learning of different patch qualities would initiate during the experiment and not prior. From 576 total tray-nights, 556 tray-nights passed a data quality test (all seed bags were properly labelled and accounted for). The moon was full 18 July 2008.
In August, vegetation above the seed trays was measured to account for the effect of vegetation on mouse risk perception (Schmidt, Goheen & Naumann 2001). A 1·4 × 1·4 m plot was settled around the seed tray grid and divided visually into quadrants. Each quadrant was scored for the cover of vegetation up to 1·5 m above the ground (scores: 1 = < 5% cover; 2 = 5–25% cover; 3 = 2550% cover; 4 = 50–75% cover; 5 = > 75% cover). Quadrant scores were summed to provide a station-specific local vegetation measure.
ANALYSIS
Antibody titres and all blood cell components were analysed with ancova, with mouse mass, immunogen dose, and sex entered as predictors. Wheel running behaviour between 20.00 and 07.00 h was analysed. We limited the data set only to wheel-naïve, adult or sub-adult males. We first calculated the total distance (m), the time moving (min, number of minutes in which the wheel revolved; max. 660), and the average moving speed (m min−1, total distance per time moving). Wheel running data were log-transformed to normalize the distributions. We examined the influence of immunogen dose and body mass with multiple regression. Because mass was not significant in any of these models, it was removed as a predictor. We also examined changes in wheel running speed over the course of night to determine whether activity timing differed according to immunogen dose. For this analysis, running speed for each minute of record between 20.00 and 07.00 h was entered as the response variable, and dose, time and dose × time were entered as fixed effects. Mouse ID was entered as a random effect to account for repeated measures.
Activity measured by track plates over three nights was examined with a mixed-effect model. The nightly summed track plate score was log-transformed to normalize the data. Predictor variables in the model were Grid Treatment (experimental or control), Date, Grid Treatment × Date and Grid Population Size as fixed effects, and Grid and Station as independent random effects to account for the multiple stations per grid and the multiple nights recorded for each station.
We examined the effect of immunogen deployment on GUD values in visited stations using a mixed-effect model, with seed mass remaining in each tray as the response variable, and the predictor variables Grid Treatment, Tray Treatment, Date, Local Vegetation, Grid Treatment × Tray Treatment, Tray Treatment × Local Vegetation, Grid Treatment × Date, and Tray Treatment × Date. Grid and Station were entered as independent random effects into the model to account for non-independence of data across time and within grids and stations. Estimated population size was not entered into the model because previous research has shown that white-footed mouse density does not influence GUDs at our field sites (Schmidt, Goheen & Naumann 2001; Schmidt & Ostfeld 2003a). To determine whether immunochallenge influenced foraging strategies (i.e. degree of density-dependent foraging), the slope of the relationship between GUDs in paired rich and poor patches was assessed (intercept constrained at zero) and compared to the starting patch-quality ratio of 2 : 1. We tested for differences in slopes between experimental and control grids for covered and uncovered trays separately. The GUD of the rich patch was the response variable and the predictors were the GUD in the poor patch, grid treatment, date, poor-patch GUD × grid treatment (to test for different slopes), station (random) and grid (random). Untransformed GUD data were used in these analyses because the data did not appear to violate the assumptions of normality and heteroscedasticity. Tukey’s post-hoc pairwise tests were implemented to compare among factor levels for significant predictors. All statistics were performed in JMP v.8 (SAS Institute Inc., Cary, NC, USA).
Results
IMMUNOLOGY
Anti-OspA response appeared to increase at low doses of the immunogen and then reach a plateau (Fig. 1a). As such, the dosage of the immunogen did not influence the level of anti-OspA response (Table 2; Fig. 1a). However, mice that consumed at least five doses of the immunogen had a significantly higher level of anti-OspA antibodies than mice that had received fewer than 5 doses [0·67 ± 0·56 (≥5 doses, n = 19) vs. 0·25 ± 0·16 (<5 doses, n = 15); Wilcoxon 2-sample test: Z = −3·61, P = 0·0003]. A dosage-dependent cellular immune response to the immunogen was observed. Mice with higher doses of the immunogen had higher white blood cell counts due to increases in neutrophils and eosinophils (Table 2; Fig. 1). Immunogen dosage did not influence counts of red blood cells, lymphocytes, monocytes or basophils (Table 2; Fig. 1).
Fig. 1.

Immune function in response to immunogen dosage for males (filled circles) and females (open circles), including (a) antibody response to the OspA protein expressed in the immunogen (absorbance at OD 410 nm), and (b–h) volume of red blood cells (RBCs), total white blood cells (WBCs) and different types of white blood cells separately.
Table 2.
Immune response to immunochallenge. Influence of mouse immunochallenge dose, sex, mass and all two-way interactions on antibody response to immunogen (measured as absorbance at 410 nm; N = 34) and blood cell volumes (N = 49). Values presented are F-statistics (P-values) for predictors in ancova models. Predictors significant at the P = 0·05 level are bolded. The direction of response is indicated if P < 0·1
| Dose | Sex | Mass | Dose × Sex | Dose × Mass | Sex × Mass | |
|---|---|---|---|---|---|---|
| Absorbance | 1·42 (0·24) | 2·12 (0·16) | 0·98 (0·33) | 0·30 (0·59) | 1·83 (0·19) | 0·22 (0·64) |
| RBC | 0·47 (0·50) | 1·54 (0·22) | 10·52 (0·002) (−) | 0·01 (0·92) | 2·79 (0·10) | 2·90 (0·10) |
| WBC | 7·93 (0·007) ( + ) | 3·76 (0·06) (F>M) | 0·04 (0·85) | 5·24 (0·03) | 0·77 (0·39) | 0·60 (0·44) |
| Neutrophils | 9·64 (0·003) ( + ) | 4·44 (0·04) (F>M) | 0·15 (0·70) | 6·04 (0·02) | 0·14 (0·71) | 1·01 (0·32) |
| Lymphocytes | 1·46 (0·23) | 0·72 (0·40) | 0·08 (0·78) | 1·20 (0·28) | 0·59 (0·45) | 0·23 (0·64) |
| Monocytes | 0·49 (0·49) | 0·17 (0·68) | 0·29 (0·60) | 0·24 (0·62) | 5·95 (0·02) | 0·32 (0·58) |
| Eosinophils | 10·70 (0·002) ( + ) | 3·69 (0·06) (F>M) | 0·28 (0·60) | 5·37 (0·03) | 0·36 (0·55) | 0·003 (0·96) |
| Basophils | 0·78 (0·38) | 4·89 (0·03) (F>M) | 5·85 (0·02) ( + ) | 0·92 (0·34) | 16·8 (0·0002) | 4·13 (0·05) |
WHEEL RUNNING BEHAVIOUR
Wheel running behaviour varied substantially among mice (Table 3). Total distance run overnight (r2 = 0·06, F1,49 = 2·92, P = 0·09), moving speed (r2 = 0·05, F1,49 = 2·41, P = 0·13) and time moving (r2 = 0·05, F1,49 = 2·34, P = 0·13; n = 51 for all) were not related to immunogen dose. When examining running speed for each minute over the night, we saw a significant interaction between dose and time (Table 4). Running speed declined over the course of the night for mice with no or low immunogen dose, whereas mice that had received high immunogen dose started the night at a slower running speed and declined less strongly over the night (Table 4; Fig. 2). To address the concern that a partial correlation between immunogen dose and the number of trappings may confound a dosage effect (if trap-happy animals exhibit altered levels of activity), we added the number of trappings of an individual as a predictor into the model. The dose × time interaction remained significant and the predictive equation was qualitatively similar with respect to dose and time terms in this larger model, so we left the number of trappings out of the model to simplify interpretation of the significant interaction term.
Table 3.
Wheel-running behaviour summary statistics. Average wheel running behaviour of wild mice from 20.00 to 07.00 h in a lab setting
| Immunogen dosage |
||
|---|---|---|
| <5 doses, Mean ± SD (range) | ≥5 doses, Mean ± SD (range) | |
| Total distance (m) | 1571 ± 1888 (44–8035) | 1696 ± 2540 (43–10 321) |
| Average nightly moving speed (m min−1) | 6·8 ± 4·6 (0·7–17·4) | 6·7 ± 5·7 (0·8–23·9) |
| Time moving (min) | 183 ± 110 (64–463) | 174 ± 123 (29–431) |
Table 4.
ancova of wheel-running behaviour. Change in the wheel running speed of mice over the course of a single night of running, where time represents each minute between 20.00 and 07.00 h. Mouse identity was entered into the model as a random effect
| Source | d.f. | d.f.Den | F | P |
|---|---|---|---|---|
| Dose | 1 | 49 | 0·24 | 0·62 |
| Time | 1 | 33 658 | 131·57 | <0·0001 |
| Dose × Time | 1 | 33 658 | 9·93 | 0·0016 |
Fig. 2.
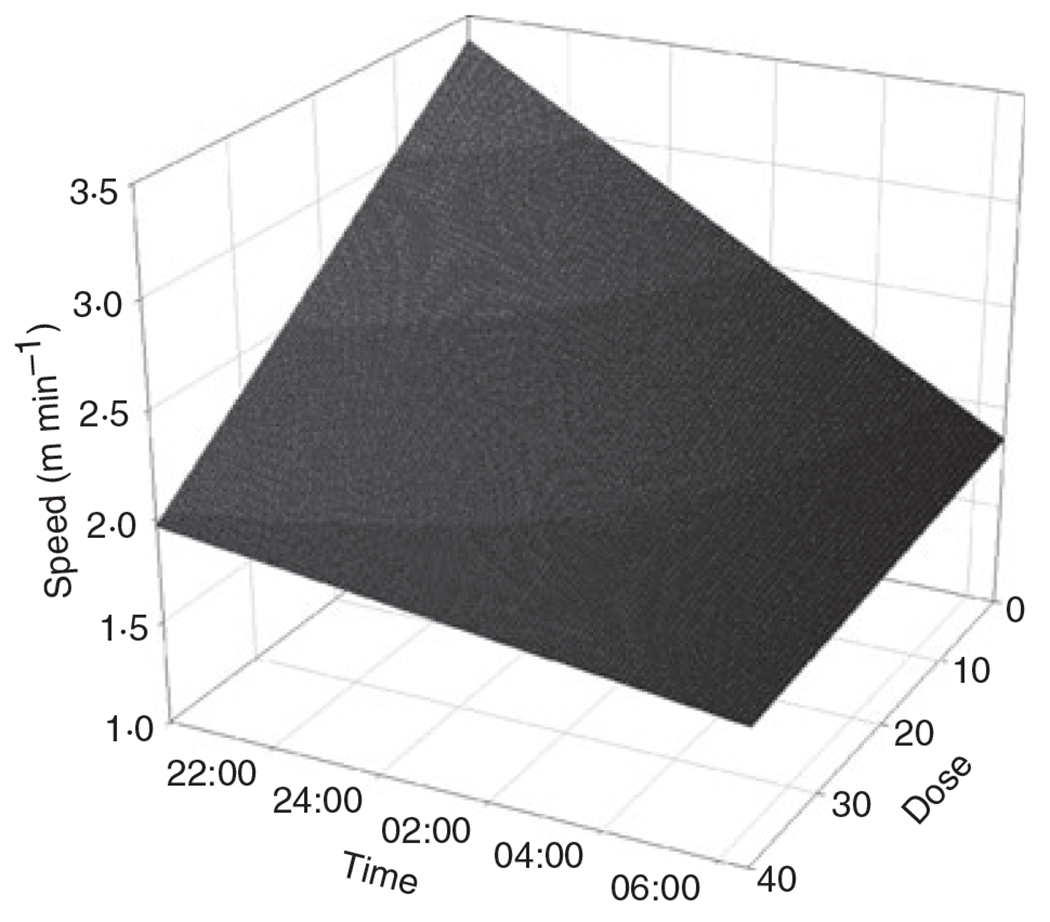
Wheel running behaviour (20:00–07:00 h) as a function of immunogen dose of wild-caught mice. The plot shows the predictive equation for the influence of time of the night and dose on running speed.
FIELD BEHAVIOUR: TRACK PLATES AND GIVING-UP DENSITY
Field activity assessed by track plates was not significantly different on experimental (immunochallenged) and control grids, and did not depend on grid population size (Table 5). At the feeding trays, 73% of tray-nights (404/556) and 81% of station-nights had foraging activity. Giving-up-densities differed according to tray treatment (Table 5). All four treatments were significantly different from each other, with the highest GUDs in the HU (high-food, uncovered) trays, followed by HC (high-food, covered) trays, then LU trays and the lowest GUDs in LC trays. GUDs declined significantly each successive night.
Table 5.
Mixed-effect models of field behaviour. Influence of field parameters on field track plate activity and seed tray giving-up densities (GUDs). Track plate activity was analysed as log-transformed daily summed track plate score. Grid and station were entered as independent random effects in both models
| d.f. | d.f.Den | F | P | |
|---|---|---|---|---|
| Track plates (N = 144 station-nights) | ||||
| Grid treatment | 1 | 3 | 0·00 | 0·98 |
| Date | 3 | 92 | 13·82 | <0·0001 |
| Grid treatment × Date | 3 | 92 | 0·12 | 0·88 |
| Grid population size | 1 | 3 | 2·01 | 0·25 |
| Seed tray GUDs (N = 452 tray-nights) | ||||
| Grid treatment | 1 | 4 | 1·14 | 0·35 |
| Tray treatment | 3 | 388·9 | 113·30 | <0·0001 |
| Local vegetation | 1 | 39·8 | 1·86 | 0·18 |
| Date | 2 | 399·9 | 24·32 | <0·0001 |
| GridTrt × TrayTrt | 3 | 388·9 | 4·77 | 0·003 |
| TrayTrt × LocalVeg | 3 | 388·9 | 0·46 | 0·71 |
| TrayTrt × Date | 6 | 388·9 | 1·75 | 0·11 |
| GridTrt × Date | 2 | 400 | 0·15 | 0·86 |
There was a significant interaction between tray treatment and grid treatment, indicating that mice on experimental grids displayed different foraging behaviour compared to mice on the control grids (Table 5; Fig. 3). In particular, immunochallenge reduced the response to tray cover. On control grids, mice depleted seed to lower GUDs under covered trays compared to uncovered trays for both densities. On experimental grids, mice harvested seed to approximately the same GUD in the covered and uncovered trays when food density was low.
Fig. 3.

Giving-up densities (GUDs) of seed from four tray treatments on experimental (E) and control (C) grids. Values are Least Squares Means ± SE for all dates combined. Tukey’s post-hoc pairwise comparisons revealed differences between covered and uncovered trays except for immunochallenged mice in low density food trays (Experimental: HU > HC, LU~LC; Control: HU > HC, LU > LC). Mice in experimental and control grids did not equalize GUDs between food patches of different quality (Experimental: HU > LU, HC > LC; Control: HU > LU, HC > LC).
In contrast, immunochallenge did not influence foraging in response to patch quality. The slopes of the relationship between the GUDs of rich patches and poor patches (intercept constrained to be at zero) were 1·48 (control) and 1·77 (experimental) at covered trays and 1·72 (control) and 1·98 (experimental) at uncovered trays (Fig. 4). With the ratio of GUDs in rich and poor patches lying above 1 : 1, mice clearly did not forage with a prescient strategy that equalized GUDs in the different-quality patches. Instead, the ratio indicate a fixed time (slope near 2) or Bayesian (slope between 1 and 2) foraging strategy. Mixed-effect models revealed no significant differences in slope between experimental and control grids (no significant GUD poor patch × Grid treatment effect; Table 6).
Fig. 4.

Giving-up densities (GUD) of seeds on experimental (filled circles) and control (open circles) grids for paired rich (high-density seed) and poor (low-density seed) patches, shown separately for covered (a) and uncovered (b) trays. In both panels, the solid line represents a forager with perfect knowledge that equalizes GUDs across patch quality, and the dotted line represents a forager that forages density-independently (fixed time foraging strategy) and produces GUD ratio that match the starting ratio (2 : 1). The GUD ratio data suggest either a fixed time foraging strategy or a Bayesian foraging strategy (ratios between 2 and 1).
Table 6.
ancova of paired GUDs. Significance of terms in a linear relationship between GUDs in paired rich patches (response variable) and poor patches (predictor), analysed in separate ancova for covered and uncovered trays. Grid and stations were entered as random effects into the model. N = 113 pairs for both models
| Predictor |
P
|
|
|---|---|---|
| Uncovered trays | Covered trays | |
| GUD poor patch | <0·0001 | <0·0001 |
| Grid treatment | 0·27 | 0·13 |
| Date | 0·18 | 0·003 |
| GUD poor × Grid treatment | 0·42 | 0·48 |
Discussion
We examined the effect of an immunochallenge on behaviours of white-footed mice that are linked to important community interactions. Altered host phenotypes have been observed repeatedly in response to non-pathogenic immune challenges and are recorded in immune cell counts, metabolic rate, body mass, feeding behaviour and reproductive output (Ilmonen, Taarna & Hasselquist 2000; Ots et al. 2001; Zuk & Stoehr 2002; Velando, Drummond & Torres 2006). Our immunogen clearly triggered an immune response in mice, resulting in the generation of anti-OspA antibodies and an elevated cellular immune response (greater counts of neutrophils and eosinophils). We confirmed that immunochallenge did not alter the proportion of mice infected with B. burgdorferi in each of our grids, determined via xenodiagnosis (infected/tested: 4/4, Henry X; 11/11, Green C; 9/10, Green X; 9/9, Cary Drive; 15/15, Canoe Gap; 29/31, Field Lab). Immunochallenged mice maintained their infection with B. burgdorferi despite producing anti-OspA antibodies because B. burgdorferi only expresses ospA in the tick and not in the vertebrate host (De Silva et al. 1996). Thus, the responses we recorded in mice were the result of exposure to the immunogen and not infection with B. burgdorferi.
Immune challenges can enhance or depress alternative branches of the immune system, potentially leading to facilitation or prevention of further parasitic infection and indirectly influencing parasite-host interactions (Martin et al. 2006; Gasparini et al. 2009; Beldomenico & Begon 2010). Beldomenico et al. (2008a,b) reported that parasitic infection and physiological ‘condition’ may operate in a vicious cycle, whereby mounting defenses against current infections (i.e. elevated neutrophil and monocyte counts) leads to poorer future condition (lower red blood cell and lymphocyte counts), which in turn leads to a greater likelihood of future infection. Our study was not longitudinal, but we found no decrease in red blood cell or lymphocyte counts in immunochallenged mice to suggest that the elevation of other white blood cells would lead to a cycle of increasing pathogen infection. The majority of immunochallenged and control white-footed mice in our study was concurrently infected with B. burgdorferi and was almost certainly exposed to additional pathogens. In fact, mice of all levels of immunogen dose had high neutrophil counts compared to lab-reared P. leucopus (roughly 0·4–0·8 × 103 per μL whole blood, Wu et al. 1999; Schwanz et al. 2010), suggesting that wild mice carry more pathogens than lab conspecifics. Examining the abundance of common micro-and macro-parasites of wild white-footed mice would provide better indication as to the influence of immunochallenge on physiological pathogen susceptibility.
If the elevation of immunity seen in our immunochallenged mice represents a physiological cost (Lochmiller & Deerenberg 2000; Derting & Compton 2003; Demas 2004; Martin et al. 2006), immunochallenge effectively reduced the ‘condition’ of the mice in our study. The cost of immunochallenge appeared to lead to a reduction in wheel running activity. However, we saw no measurable effect in field activity measured by track plates.
The putative decrease in condition in immunochallenged mice was supported by the finding that mice on experimental grids showed a significant, although modest, decrease in risk sensitivity when foraging compared to control grids, although they did not change their foraging strategy otherwise. Foragers in good condition should be risk averse and have higher GUDs in risky patches compared to foragers in poor condition and compared to safe patches (Brown 1988, 1992; Clark 1994; Brown & Kotler 2007). Empirical support has been found, for example, in gerbils that are food supplemented and have higher overall GUDs, spend less time foraging, and are more sensitive to microhabitat and the presence of predators (Kotler 1997). In contrast, gerbils with flea infestations (presumably in poor condition) apparently are distracted by flea bites and have higher GUDs and respond more strongly to predation risk (A. Raveh, pers. comm.). Here, we show for the first time that manipulating individual condition by challenging the immune system may affect risk sensitivity during foraging.
This subtle behavioural shift may indicate important changes in individual condition and reproductive value. Because organisms maximize lifetime fitness by trading-off investment in survival and reproduction, a pathogenic attack that reduces survival can lead to the host abandoning investment in survival and diverting energy into risky behaviour and reproductive effort (Minchella & Loverde 1981; Forbes 1993; Schwanz 2008). For example, crickets and snails increased reproductive output when exposed to bacterial LPS and schisotome parasites respectively (Minchella & Loverde 1981; Adamo 1999). In our study, exposing white-footed mice to an immune challenge appeared to lead to an increase in risky foraging behaviour, suggesting that the costs of our immunochallenge led to a lower perceived reproductive value in mice (Forbes 1993; Clark 1994).
Given the reduced responsiveness to predation risk, immunochallenged mice might be predicted to have reductions in perceived costs of predation at all tray types, leading to lower GUDs in general (riskier foragers will forage anywhere for a longer time). That GUDs were not universally reduced on experimental grids compared to control grids suggests that immunochallenge also influences the energetic cost of foraging or the missed opportunity costs in a manner that cancelled out the effect on the costs of predation. If immunochallenged white-footed mice are adopting a riskier, terminal investment strategy, the missed reproductive opportunities could represent a substantive and conflicting cost of foraging (Minchella & Loverde 1981; Clutton-Brock 1984; Forbes 1993). It is worth noting that the behavioural indicators recorded on experimental grids in the field (track plates and GUDs) would have sampled mice of variable immunogen dose. Without knowledge of individual mouse identity in our field samples, we cannot determine whether the quantitative effects of immunochallenge were dampened by the presence of low-dose mice. In addition, the different trapping frequencies on control and experimental grids could have affected resource acquisition and perceived predation risk in mice, suggesting caution is warranted in the interpretation of foraging results.
In Peromyscus spp., live pathogens have variable influences on individual fitness and population dynamics. Infection with B. burgdorferi itself appears to have minimal impacts on individual host activity and survival (Hofmeister et al. 1999; Schwanz et al. 2010; but see Burgess, French & Gendron-Fitzpatrick 1990), a result that is mirrored in studies with many macroparasites (e.g. Munger & Karasov 1991; Meagher 1998). In contrast, parasites as diverse as protists, trematodes, nematodes, and dipterans can all have dramatic impacts on survival and life history of Peromyscus hosts (Poirier, Rau & Wang 1995; Fuller & Blaustein 1996; Burns, Goodwin & Ostfeld 2005; Pederson & Grieves 2008; Schwanz 2008). Even non-pathogenic immune challenge in P. leucopus can lead to altered investment in reproductive organs (Derting & Compton 2003; Derting & Virk 2005).Given the diverse response of hosts to pathogens, connecting pathogens to relevant ecological outcomes such as host fitness and community interactions can be difficult without intensive population surveys.
In this study, we have connected the cost of the immune responses to community ecology through the measure of behavioural indicators that provide insight into host behavioural motivation and reveal the nature of important community interactions (Schmidt & Schauber 2007). The consequences for community ecology of individual host responses to challenges are rarely explored. Here, we show that immunochallenged mice demonstrate reduced risk aversion, which may have far-reaching implications for community ecology. Greater use of ‘risky’ microhabitats by mice increases the proportion of their home range that is profitable for foraging. One implication of using riskier microhabitats is that mice may become more vulnerable to predation and removal from the population. In addition, changes in space use may link our immune challenge to interactions between white-footed mice and patchily distributed community members (e.g. gypsy moth pupae, thrush nests and disease vectors such as larval ticks). If space use by mice increases in response to immune challenges, the amount of space that is “predator-free” from the perspective of incidental prey would decrease, although the quantitative effect of decreased risk aversion on predation of incidental prey remains to be measured (Schmidt, Goheen & Naumann 2001; Schmidt & Ostfeld 2003a,b; Schmidt 2004; Connors et al. 2005; Schmidt & Schauber 2007).
Immunochallenge with OspA is not a perfect surrogate for infection with B. burgdorferi given that it does not lead to pathogen proliferation in host tissues or expose hosts to multiple antigens present in whole B. burgdorferi. Therefore, we cannot say, presently, whether white-footed mice infected with B. burgdorferi would show similar behavioural responses as the immunochallenged mice in this study. However, the results raise the possibility of vital links within disease and community ecology. If mice respond to pathogens in the same manner as they do to our immunochallenge, infected white-footed mice are predicted to encounter and prey upon a greater number of gypsy moth pupae and thrush nests. Given the role white-footed mice play in controlling the population density of these species (Ostfeld, Jones & Wolff 1996a; Jones et al. 1998; Schmidt & Ostfeld 2003b; Schmidt, Ostfeld & Smyth 2006), the effect of altered predation rates could be a substantial indirect community effect of disease.
Finally, larval and nymphal ticks are also patchily distributed (Ostfeld, Hazler & Cepeda 1996b; Ostfeld, Miller & Hazler 1996c), which suggests that encounter rates between white-footed mice and ticks could similarly increase in response to immune challenge. If a mouse’s behavioural response to live pathogens is similar to that following immunochallenge, infection with other pathogens could increase the likelihood of exposure to B. burgdorferi-carrying nymphs, and infection with B. burgdorferi could increase the likelihood of encountering the vector necessary for pathogen transmission (larval ticks). Both scenarios, if correct and substantive, indicate strong positive feedbacks within disease ecology.
References
- Adamo SA (1999) Evidence for adaptive changes in egg laying in crickets exposed to bacteria and parasites. Animal Behavior, 57, 117–124. [DOI] [PubMed] [Google Scholar]
- Anderson JF, Johnson RC & Magnarelli LA (1987) Seasonal prevalence of Borrelia burgdorferi in natural populations of white-footed mice, Peromyscus leucopus. Journal of Clinical Microbiology, 25, 1564–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM & May RM (1979) Population biology of infectious diseases: part I. Nature, 280, 361–367. [DOI] [PubMed] [Google Scholar]
- Beldomenico PM & Begon M (2010) Disease spread, susceptibility and infection intensity: vicious circles? Trends in Ecology & Evolution, 25, 21–27. [DOI] [PubMed] [Google Scholar]
- Beldomenico PM,Telfer S, Gebert S, Lukomski L, Bennett M & Begon M (2008a) The dynamics of health in wild field vole populations: a haematological perspective. Journal of Animal Ecology, 77, 984–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldomenico PM, Telfer S, Gebert S, Lukomski L, Bennett M & Begon M (2008b) Poor condition and infection: a vicious circle in natural populations. Proceedings B – Biological Sciences, 275, 1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JS (1988) Patch use as an indicator of habitat preference, predation risk, and competition. Behavioral Ecology and Sociobiology, 22, 37–47. [Google Scholar]
- Brown JS (1992) Patch use under predation risk: I. Models and predictions. Annales Zoologici Fennici, 29, 301–309. [Google Scholar]
- Brown JS & Kotler BP (2007) Foraging and the ecology of fear. Foraging Behavior and Ecology (eds D.W. Stephen, J.S. Brown & R.C. Ydenberg), pp. 437–480. University of Chicago Press, Chicago. [Google Scholar]
- Bunikis J, Tsao J, Luke CJ, Luna MG, Fish D & Barbour AG (2004) Borrelia burgdorferi infection in a natural population of Peromyscus leucopus mice: a longitudinal study in an area where Lyme borreliosis is highly endemic. Journal of Infectious Diseases, 189, 1515–1523. [DOI] [PubMed] [Google Scholar]
- Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E & Davis JP (1982) Lyme disease: a tick-borne spirochetosis? Science, 216, 1317–1319. [DOI] [PubMed] [Google Scholar]
- Burgess EC, French JB Jr &, Gendron-Fitzpatrick A (1990) Systemic disease in Peromyscus leucopus associated with Borrelia burgdorferi infection. American Journal of Tropical Medicine and Hygiene, 42, 254–259. [DOI] [PubMed] [Google Scholar]
- Burns CE, Goodwin BJ & Ostfeld RS (2005) A prescription for longer life? Bot fly parasitism of the white-footed mouse. Ecology, 86, 753–761. [Google Scholar]
- Clark CW (1994) Antipredator behavior and the asset-protection principle. Behavioral Ecology, 5, 159–170. [Google Scholar]
- Clutton-Brock TH (1984) Reproductive effort and terminal investment in iteroparous animals. American Naturalist, 123, 212–229. [Google Scholar]
- Collinge SK, Ray C & Cully JF Jr (2008) Effects of disease on keystone species, dominant species, and their communities. Infectious Disease Ecology (eds Ostfeld RS, Keesing F & Eviner VT), pp. 129–144. Princeton University Press, Princeton. [Google Scholar]
- Connors MJ, Schauber EM, Forbes A, Jones CG, Goodwin BJ & Ostfeld RS (2005) Use of track plates to quantify predation risk at small spatial scales. Journal of Mammalogy, 86, 991–996. [Google Scholar]
- De Silva AM, Telford SR III, Brunet LR, Barthold SW & Fikrig E (1996) Borrelia burgdorferi OspA is an arthropod-specific transmissionblocking Lyme disease vaccine. Journal of Experimental Medicine, 183, 271–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE (2004) The energetics of immunity: a neuroendocrine link between energy balance and immune function. Hormones and Behavior, 45, 173–180. [DOI] [PubMed] [Google Scholar]
- Derting TL & Compton S (2003) Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus). Physiological and Biochemical Zoology, 76, 744–752. [DOI] [PubMed] [Google Scholar]
- Derting TL & Virk MK (2005) Positive effects of testosterone and immunochallenge on energy allocation to reproductive organs. Journal of Comparative Physiology B, 175, 543–556. [DOI] [PubMed] [Google Scholar]
- Dobson AP (1988) The population biology of parasite-induced changes in host behavior. Quarterly Review of Biology, 63, 139–165. [DOI] [PubMed] [Google Scholar]
- Donahue JG, Piesman J & Spielman A (1987) Reservoir competence of white-footed mice for Lyme disease spirochetes. American Journal of Tropical Medicine and Hygiene, 36, 92–96. [DOI] [PubMed] [Google Scholar]
- Feldman BF, Zinkl JG & Jain NC (2000) Schalm’s Veterinary Hematology, 5th edn, Lippincott, Williams and Wilkins, New York. [Google Scholar]
- Forbes MRL (1993) Parasitism and host reproductive effort. Oikos, 67, 444–450. [Google Scholar]
- Fuller CA & Blaustein AR (1996) Effects of the parasite Eimeria arizonensis on survival of deer mice (Peromyscus maniculatus). Ecology, 77, 2196–2202. [Google Scholar]
- Gasparini J, Piault R, Bize P & Roulin A (2009) Synergistic and antagonistic interaction between different branches of the immune system is related to melanin-based coloration in nesting tawny owls. Journal of Evolutionary Biology, 22, 2348–2353. [DOI] [PubMed] [Google Scholar]
- Gomes-Solecki MJC, Brisson DR & Dattwyler RJ (2006) Oral vaccine that breaks the transmission cycle of the Lyme disease spirochete can be delivered via bait. Vaccine, 24, 4440–4449. [DOI] [PubMed] [Google Scholar]
- Hofmeister EK, Ellis BA, Glass GE & Childs JE (1999) Longitudinal study of infection with Borrelia burdorferi in a population of Peromyscus leucopus at a Lyme disease-enzootic site in Maryland. American Journal of Tropical Medicine and Hygiene, 60, 598–609. [DOI] [PubMed] [Google Scholar]
- Ilmonen P, Taarna T & Hasselquist D (2000) Experimentally activates immune defence in females pied flycatchers results in reduced breeding success. Proceedings B – Biological Sciences, 267, 665–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Ostfeld RS, Richard MP, Schauber EM & Wolff JO (1998) Chain reactions linking acorns to gypsy moth outbreaks and Lyme disease risk. Science, 279, 1023–1026. [DOI] [PubMed] [Google Scholar]
- Keesing F, Brunner J, Duerr S, Killilea M, LoGiudice K, Schmidt K, Vuong H & Ostfeld RO (2009) Hosts as ecological traps for the vector of Lyme disease. Proceedings B – Biological Sciences, 276, 3911–3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotler BP (1997) Patch use by gerbils in a risky environment: manipulating food and safety to test four models. Oikos, 78, 274–282. [Google Scholar]
- Kotler BP & Brown JS (2007) Community Ecology. Foraging Behavior and Ecology (eds D.W. Stephen, J.S. Brown & R.C. Ydenberg), pp. 397–434. University of Chicago Press, Chicago. [Google Scholar]
- Kotler BP, Brown JS & Bouskila A (2004) Apprehension and time allocation in gerbils: the effects of predatory risk and energetic state. Ecology, 85, 917–922. [Google Scholar]
- Lafferty KD (2008) Effects of disease on community interactions and food web structure. Infectious Disease Ecology (eds R.S. Ostfeld, F. Keesing & V.T. Eviner), pp. 205–222. Princeton University Press, Princeton. [Google Scholar]
- Lafferty KD, Dobson AP & Kuris AM (2006) Parasites dominate food web links. Proceedings of the National Academy of Sciences of the United States of America, 103, 11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KA (2006) Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology, 46, 1000–1015. [DOI] [PubMed] [Google Scholar]
- Levine JF, Wilson ML & Spielman A (1985) Mice as reservoirs of the Lyme disease spirochete. American Journal of Tropical Medicine and Hygiene, 34, 355–360. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL & Deerenberg C (2000) Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos, 88, 87–98. [Google Scholar]
- LoGiudice K, Ostfeld RS, Schmidt KA & Keesing F (2003) The ecology of infectious disease: effects of host diversity and community composition on Lyme disease risk. Proceedings of the National Academy of Sciences of the United States of America, 100, 567–571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LB II, Weil ZM, Kuhlman JR & Nelson RJ (2006) Trade-offs within the immune systems of female white-footed mice, Peromyscus leucopus. Functional Ecology, 20, 630–636. [Google Scholar]
- May RM & Anderson RM (1979) Population biology of infectious diseases: part II. Nature, 280, 455–461. [DOI] [PubMed] [Google Scholar]
- McCallum H & Dobson A (1995) Detecting disease and parasite threats to endangered species and ecosystems. Trends in Ecology & Evolution, 10, 190–194. [DOI] [PubMed] [Google Scholar]
- McNamara JM & Houston AI (1986) The common currency for behavioral decisions. American Naturalist, 127, 358–378. [Google Scholar]
- Meagher S (1998) Physiological responses of deer mice (Peromyscus maniculatus) to infection with Capillaria hepatica (Nematoda). Journal of Parasitology, 84, 1112–1118. [PubMed] [Google Scholar]
- Minchella DJ & Loverde PT (1981)A cost of increased early reproductive effort in the snail Biomphalaria glabrata. American Naturalist, 118, 876–881. [Google Scholar]
- Moody KD, Terwilliger GA, Hansen GH & Barthold SW (1994) Experimental Borrelia burgdorferi infection in Peromyscus leucopus. Journal of Wildlife Diseases, 30, 155–161. [DOI] [PubMed] [Google Scholar]
- Morris DW & Davidson DL (2000) Optimally foraging mice match patch use with habitat differences in fitness. Ecology, 81, 2061–2066. [Google Scholar]
- Munger JC & Karasov WH (1991) Sublethal parasites in white-footed mice: impact on survival and reproduction. Canadian Journal of Zoology, 69, 398–404. [Google Scholar]
- Olsson O & Brown JS (2006) The foraging benefits of information and the penalty of ignorance. Oikos, 112, 260–273. [Google Scholar]
- Ostfeld RS, Hazler KR & Cepeda OM (1996b) Temporal and spatial dynamics of Ixodes scapularis (Acari: Ixodidae) in a rural landscape. Journal of Medical Entomology, 33, 90–95. [DOI] [PubMed] [Google Scholar]
- Ostfeld RS, Jones CG & Wolff JO (1996a) Of mice and mast. Bioscience, 46, 323–330. [Google Scholar]
- Ostfeld RS, Miller MC & Hazler KR (1996c) Causes and consequences of tick (Ixodes scapularis) burdens on white-footed mice (Peromyscus leucopus). Journal of Mammalogy, 77, 266–273. [Google Scholar]
- Ostfeld RS, Canham CD, Oggenfuss K, Winchcombe RJ & Keesing F (2006) Climate, deer, rodents, and acorns as determinants of variation in Lyme-disease risk. PLoS Biology, 4, e–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ots I, Kerimov AB, Ivankina EV, Ilyina TA & Hõrak P (2001) Immune challenge affects basal metabolic activity in wintering great tits. Proceedings B – Biological Sciences, 268, 1175–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pederson AB & Grieves TJ (2008) The interaction of parasites and resource cause crashes in wild mouse population. Journal of Animal Ecology, 77, 370–377. [DOI] [PubMed] [Google Scholar]
- Poirier SR, Rau ME & Wang X (1995) Diel locomotory activity of deer mice (Peromyscus maniculatus) infected with Trichinella nativa or Trichinella pseudospiralis. Canadian Journal of Zoology, 73, 1323–1334. [Google Scholar]
- Schauber EM, Ostfeld RS & Evans AS Jr (2005) What is the best predictor of annual Lyme disease incidence: weather, mice or acorns? Ecological Applications, 15, 575–586. [Google Scholar]
- Schmidt KA (2004) Incidental predation, enemy-free space and the coexistence of incidental prey. Oikos, 106, 335–343. [Google Scholar]
- Schmidt KA, Goheen JR & Naumann R (2001) Incidental nest predation in songbirds: behavioral indicators detect ecological scale and processes. Ecology, 82, 2937–2947. [Google Scholar]
- Schmidt KA & Ostfeld RS (2003a) Mice in space: space use predicts the interaction between mice and songbirds. Ecology, 84, 3276–3283. [Google Scholar]
- Schmidt KA & Ostfeld RS (2003b) Songbird populations in fluctuating environments: predator responses to pulsed resources. Ecology, 84, 406–415. [Google Scholar]
- Schmidt KA, Ostfeld RS & Smyth KN (2006) Spatial heterogeneity in predator activity, nest survivorship, and nest-site selection in two forest thrushes. Oecologia, 148, 22–29. [DOI] [PubMed] [Google Scholar]
- Schmidt KA & Schauber EM (2007) Behavioral indicators of predator space use: studying species interactions through the behavior of predators. Israel Journal of Ecology and Evolution, 53, 389–406. [Google Scholar]
- Schwanz LE (2008) Chronic parasitic infection alters reproductive output in deer mice. Behavioral Ecology and Sociobiology, 62, 1351–1358. [Google Scholar]
- Schwanz LE, Voordouw M, Brisson B & Ostfeld RS (2010) Borrelia burgdorferi has minimal impact on the Lyme disease reservoir host Peromyscus leucopus. Vector-Borne and Zoonotic Diseases, in press. [DOI] [PubMed] [Google Scholar]
- Schwarz CJ & Arnason AN (1996) A general methodology for the analysis of capture-recapture experiments in open populations. Biometrics, 52, 860–873. [Google Scholar]
- Sheldon BC & Verhulst S (1996) Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends in Ecology & Evolution, 11, 317–321. [DOI] [PubMed] [Google Scholar]
- Uller T, Isaksson C & Olsson M (2006) Immune challenge reduces reproductive output and growth in a lizard. Functional Ecology, 20, 873–879. [Google Scholar]
- Valone TJ & Brown JS (1989) Measuring patch assessment abilities of desert granivores. Ecology, 70, 1800–1810. [Google Scholar]
- Velando A, Drummond H & Torres R (2006) Senescent birds redouble reproductive effort when ill: confirmation of the terminal investment hypothesis. Proceedings B – Biological Sciences, 273, 1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GC (1966) Natural selection, the costs of reproduction, and a refinement of Lack’s principle. American Naturalist, 100, 687–690. [Google Scholar]
- Wu P-J, Greeley EH, Hansen LG & Segre M (1999) Hematology values from clinically healthy Peromyscus leucopus. Journal of Zoo and Wildlife Medicine, 30, 589–590. [PubMed] [Google Scholar]
- Zuk M & Stoehr AM (2002) Immune defense and host life history. American Naturalist, 160, S9–S22. [DOI] [PubMed] [Google Scholar]


