Abstract
Survival outcomes after radical cystectomy (RC) for bladder cancer (BCa) have not improved in recent decades; nevertheless, RC remains the standard treatment for patients with localized muscle-invasive BCa. Identification of the patients most likely to benefit from RC only versus a combination with systemic therapy versus systemic therapy first/only and bladder-sparing is needed. This systematic review and meta-analysis pools the data from published studies on blood-based biomarkers to help prognosticate disease recurrence after RC. A literature search on PubMed and Scopus was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement. Articles published before November 2022 were screened for eligibility. A meta-analysis was performed on studies investigating the association of the neutrophil-to-lymphocyte ratio (NLR), the only biomarker with sufficient data, with recurrence-free survival. The systematic review identified 33 studies, and 7 articles were included in the meta-analysis. Our results demonstrated a statistically significant correlation between elevated NLR and an increased risk of disease recurrence (HR 1.26; 95% CI 1.09, 1.45; p = 0.002) after RC. The systematic review identified various other inflammatory biomarkers, such as interleukin-6 or the albumin-to-globulin ratio, which have been reported to have a prognostic impact on recurrence after RC. Besides that, the nutritional status, factors of angiogenesis and circulating tumor cells, and DNA seem to be promising tools for the prognostication of recurrence after RC. Due to the high heterogeneity between the studies and the different cut-off values of biomarkers, prospective and validation trials with larger sample sizes and standardized cut-off values should be conducted to strengthen the approach in using biomarkers as a tool for risk stratification in clinical decision-making for patients with localized muscle-invasive BCa.
Keywords: urothelial carcinoma, radical cystectomy, prognostic factor, recurrence, neutrophil-to-lymphocyte ratio, biomarker
1. Introduction
Radical cystectomy (RC) affords a sustained local and distant disease control for patients with muscle-invasive bladder cancer (BCa) [1,2,3]. Nevertheless, overall recurrence-free survival (RFS) at 5 and 10 years after RC are only 68% and 66%, respectively [4]. Prognosis after RC is dependent on histopathologic features such as tumor stage and grade, metastasis status, lymph node involvement, histopathologic subtype, or lymphovascular and blood vessel invasion [5,6,7,8,9]. Given the morbidity and mortality of RC as well as its high recurrence rates, a better preoperative risk stratification and prognostic tool to select the optimal treatment strategy (i.e., local only versus local plus systemic therapy) are needed for optimal clinical decisions [3,10,11].
Much research has arisen on blood-based biomarkers to help guide clinical decision making in muscle-invasive BCa [12,13,14]. Despite substantial efforts, no biomarker is standard in the clinical setting so far, as the majority of studies suffer from inadequate study designs, analysis and reporting [15]. To assess the clinical role of biomarkers, predictive models are needed to show a significant improvement of performance, and the studies reporting a new biomarker should be highly regulated and well planned. As reported by Shariat et al., a study investigating biomarkers should be designed in such a way that it comprises and considers preclinical testing, assay development, feasibility and clinical prevalence, validation and standardization for clinical utility, independent confirmation studies, and impact assessments [15]. This standardized approach is often missing in the protocols of published studies in the past [16].
Since cytokine imbalances and inflammatory responses are described to act as a trigger for urothelial cancer cell proliferation and metastasis formation, blood levels of inflammatory cells seem easily accessible biomarkers that have become increasingly attractive in recent years [17,18,19]. Among these, the neutrophil-to-lymphocyte ratio (NLR) gained special interest as a marker of systemic inflammation, and several articles have been published reporting the NLR as a prognostic tool for survival outcomes in BCa patients [20,21,22].
The aim of this systematic review and meta-analysis is to pool the existing literature assessing the role of blood-based biomarkers regarding disease recurrence in BCa patients after RC because these are available preoperatively and may, therefore, help guide decision making.
2. Methods
2.1. Search Strategy
To define the research question, we used the PICO format and searched for all studies that included BCa patients (P) who underwent RC (I) and studies that reported prognostic factors (O), comparing patients with disease recurrence to those who did not have recurrence (C) [23]. The keywords used in our search strategy therefore included (((bladder cancer) AND ((cystectomy) OR (radical operation))) AND (prognostic)) AND (recurrence).
The electronic databases PubMed and Scopus were searched according to the PRISMA statement for articles published before November 2022. The study was registered on PROSPERO (ID CRD42023387840) [24].
2.2. Study Inclusion and Exclusion
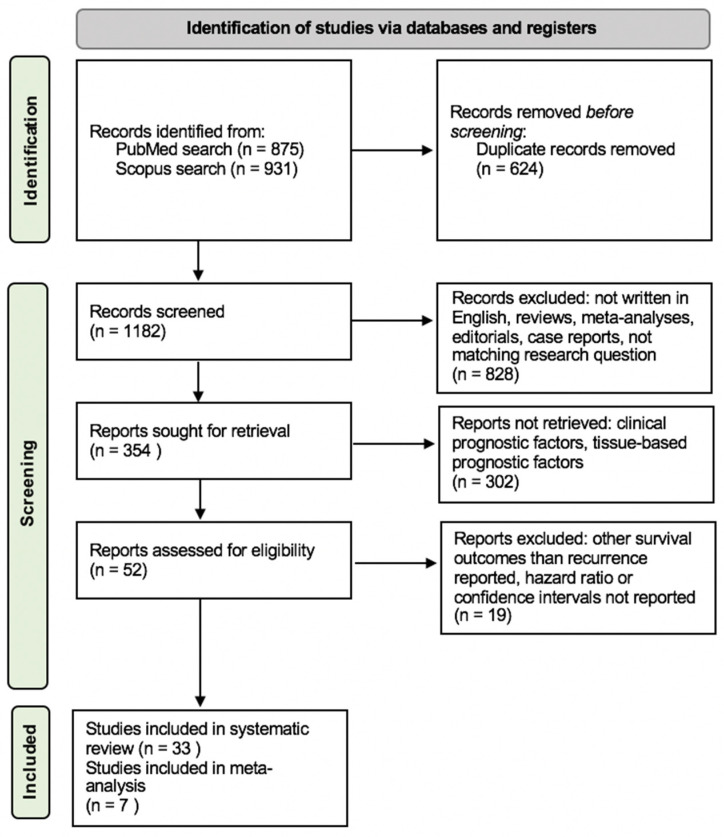
Two independent reviewers screened the detected studies initially by titles, then by abstracts. Afterwards, a full-text review was performed. Articles not written in English, case reports, editorials, reviews, and meta-analyses were excluded during the initial screening process, as demonstrated by Figure 1. During the conducted full-text review, publications not matching our research question were additionally excluded and the remaining articles were screened for content concerning blood-based prognostic factors. Articles with clinical factors or tissue-based biomarkers as prognostic tools were not retrieved. For the meta-analysis, the remaining articles were filtered for describing preoperatively measured NLR as a prognostic factor of recurrence. Articles not reporting recurrence or RFS as an endpoint or not stating hazard ratios (HRs) and confidence intervals (CIs) were excluded. References of selected studies were also screened for potentially relevant articles.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 flow diagram. From: Page et al. [25].
2.3. Data Extraction
One reviewer conducted the data extraction independently into a datasheet and the process was verified by a second reviewer. Discrepancies were resolved by consensus between co-authors. Data containing the following information were obtained: name of the first author; year of publication; whether a univariable and/or multivariable analysis was conducted; sample size, gender and the median age of subjects; median follow-up; HR in multivariable analysis with a CI of 95%; p-values; cut-off values and the timepoint of measurement; and whether neoadjuvant chemotherapy or radiation was received. The primary endpoint investigated was recurrence or RFS; other reported survival outcomes were also listed in the data sheet.
2.4. Risk of Bias Assessment
We evaluated the risk of bias using the risk of bias in non-randomized studies of interventions (ROBINS-I) tool, which is based on seven domains: bias due to confounding, participant selection, classification of interventions, deviations from intended intervention, missing data, measurement of outcomes, and selection of the reported results [26]. The detailed data are added as Supplementary Table S1.
2.5. Statistical Analysis
To perform statistical analysis, data were extracted from the collected articles eligible for the meta-analysis on NLR only (n = 7), as described above. In addition, p-values < 0.05 were considered statistically significant. Heterogeneity among the outcomes of included studies in this meta-analysis was evaluated using Cochrane’s Q test and the I2 statistic. Significant heterogeneity was indicated by a p < 0.05 in Cochrane’s Q tests and a ratio > 50% in I2 statistics. A random effect model was used in case of heterogeneity. The statistical analysis was performed with Review Manager version 5.4.1 (The Cochrane Collaboration, London, UK).
3. Results
3.1. Search Results
In total, 1806 studies published between 1976 and 2022 were found during the literature search. Duplicates were removed and 1182 publications were screened by title and abstract, leaving 354 articles. As shown in Figure 1, thirty-three articles were eligible for the systematic review and seven articles for meta-analysis.
3.2. Study Characteristics
Thirty-three articles reporting pre-treatment blood-based biomarkers as prognostic factors for disease recurrence in BCa patients treated with RC were retrieved for this systematic review. The characteristics of the included studies are listed in Table 1. The studies were published between 2004 and 2022, and the sample size ranged between 26 and 4335 patients. Six research groups conducted prospective single-center studies, whereas the other articles reported retrospective data analyses. Thirteen of the eligible studies investigated inflammatory biomarkers as a prognostic factor of survival outcomes. All eligible studies reported RFS as an outcome, whereas cancer-specific survival (CSS) and overall survival (OS) were reported by 23 and 28 articles, respectively.
Table 1.
Eligible studies for the systematic review and their study characteristics.
| Author | Year | Blood-Based Biomarker | Sample Size | Study Design | Survival Outcomes |
|---|---|---|---|---|---|
| Chang et al. [27] | 2004 | CA 125 | n = 287 | retrospective single-center | RFS, OS |
| Kouba et al. [28] | 2009 | CA 125 | n = 92 | prospective single-center | recurrence |
| Hazzaa et al. [29] | 2010 | clusterin | n = 26 | prospective single-center | RFS, OS |
| Rink et al. [30] | 2012 | HER2 expression of circulating tumor cells | n = 100 | prospective single-center | OS, CSS, RFS |
| Ahmadi et al. [31] | 2014 | CA 19-9, CA 125, CEA | n = 186 | prospective single-center | OS, RFS |
| Hermanns et al. [32] | 2014 | NLR | n = 424 | retrospective single-center | OS, CSS, RFS |
| Viers et al. [33] | 2014 | NLR | n = 899 | retrospective single-center | OS, CSS, RFS |
| Djaladat et al. [34] | 2014 | albumin level, ASA score | n = 1471 | retrospective single-center | RFS, OS |
| Morizawa et al. [22] | 2016 | NLR | n = 110 | retrospective single-center | OS, CSS, RFS |
| Bhindi et al. [20] | 2016 | hemoglobin, individual cell counts, NLR, MLR, LMR, PLR | n = 418 | retrospective single-center | OS, CSS, RFS |
| Liu et al. [35] | 2016 | albumin/globulin ratio | n = 296 | retrospective multicenter | RFS, CSS |
| Tan et al. [36] | 2017 | NLR | n = 84 | retrospective single-center | OS, CSS, RFS |
| D’Andrea et al. [21] | 2017 | LMR, NLR | n = 4198 | retrospective multicenter | OS, CSS, RFS |
| Christensen et al. [37] | 2019 | circulating tumor DNA | n = 68 | prospective single-center | RFS, OS |
| Yuk et al. [38] | 2019 | De Ritis ratio | n = 771 | retrospective single-center | OS, CSS, RFS |
| Ninomiya et al. [39] | 2020 | NLR, MLR, LPR, De Ritis ratio, PNI | n = 107 | retrospective single-center | OS, RFS |
| Fallah et al. [40] | 2020 | Myeloid-derived suppressor cells | n = 109 | retrospective single-center | OS, RFS |
| Su et al. [41] | 2020 | lactate dehydrogenase | n = 263 | retrospective single-center | OS, CSS, DFS |
| Dohn et al. [42] | 2021 | uPA | n = 107 | prospective single-center | OS, CSS, RFS |
| Schuettfort et al. [43] | 2021 | albumin-globulin ratio | n = 4335 | retrospective multicenter | OS, CSS, RFS |
| Li et al. [44] | 2021 | albumin-to-alkaline phosphatase ratio | n = 199 | retrospective single-center | OS, CSS, RFS |
| Mari et al. [45] | 2021 | inflammatory biomarkers | n = 255 | retrospective single-center | recurrence, CSM, OM |
| Schuettfort et al. [46] | 2021 | uPA, SuPAR, PAI-one | n = 1036 | retrospective multicenter | RFS, CSS |
| Schuettfort et al. [47] | 2022 | panel of SIR markers | n = 4199 | retrospective multicenter | CSS, RFS |
| Grossmann et al. [48] | 2022 | systemic immune-inflammation index | n = 4335 | retrospective multicenter | OS, CSS, RFS |
| Laukhtina et al. [49] | 2022 | endoglin | n = 1036 | retrospective multicenter | OS, CSS, RFS |
| Sari Motlagh et al. [50] | 2022 | IGF-I, IGFBP-2, IGFBP-3 | n = 1036 | retrospective multicenter | OS, CSS, RFS |
| Mori et al. [51] | 2022 | VCAM-1 | n = 1036 | retrospective multicenter | OS, CSS, RFS |
| Lei et al. [52] | 2022 | NLR, PLR, LMR | n = 186 | retrospective single-center | RFS |
| Schuettfort et al. [53] | 2022 | Interleukin-6 and its soluble receptor | n = 1036 | retrospective multicenter | OS, CSS, RFS |
| Urabe et al. [54] | 2022 | Serum microRNA | n = 81 | retrospective single-center | OS, PFS |
| Katayama et al. [55] | 2022 | hepatocyte growth factor | n = 565 | retrospective multicenter | OS, CSS, RFS |
| Mori et al. [51] | 2022 | VEGF plasma levels | n = 1036 | retrospective multicenter | OS, CSS, RFS |
uPA = urokinase-type plasminogen activator, CA = carbohydrate antigen, CEA = carcinoembryonic antigen, HER = human epidermal growth factor receptor, SIR = systemic inflammatory response, LMR = lymphocyte-to-monocyte ratio, NLR = neutrophil-to-lymphocyte ratio, IGF = insulin-like growth factor, IGFBP = insulin-like growth factor-I binding protein, suPAR = urokinase-type plasminogen activator soluble receptor, PAI = urokinase-type plasminogen inhibitor, VCAM = vascular cell adhesion molecule, MLR = monocyte-to-lymphocyte ratio, LPR = lymphocyte-to-platelet ratio, PNI = prognostic nutritional index, PLR = platelet-to-lymphocyte ratio, ASA = American Society of Anesthesiologists, OS = overall survival, CSS = cancer-specific survival, RFS = recurrence-free survival, CSM = cancer-specific mortality, OM = overall mortality, VEGF = vascular endothelial growth factor, PFS = progression-free survival, DFS = disease-free survival.
3.3. Systematic Review of Blood-Based Biomarkers
3.3.1. Inflammatory Biomarkers
Thirteen eligible articles investigated inflammatory biomarkers in patients treated with RC for BCa. Schuettfort et al. showed an association of elevated interleukin-6 and its soluble receptor with RFS, with a HR of 1.04 (95% CI 1.03–1.05) and 1.32 (95% CI 1.23–1.41), respectively. Their attempt to create a prognostic panel of systemic inflammatory response (SIR) biomarkers as a tool for patient selection failed, however, as it did not improve prognostic accuracy beyond the established clinicopathologic characteristics [47,53]. Besides the already mentioned inflammatory markers, the lymphocyte-to-monocyte ratio and lymphocyte-to-platelet ratio have been investigated as prognostic factors in different publications [20,39]. Grossmann et al. calculated a systemic inflammatory index (SII) by using neutrophil, platelet, and lymphocyte counts [48]. A high preoperatively measured SII was associated with worse RFS, OS, and CSS, but decision curve analysis did not show a clinical net benefit for decision-making.
Another inflammatory marker investigated by Fallah et al. is the presence of myeloid-derived suppressor cells, which are reported to increase through inflammatory cytokines released by cancer cells [40]. An elevated monocytic myeloid-derived suppressor cell count in blood was shown to be a negative prognostic factor for OS and RFS (HR 5.95, p = 0.0004 and HR 7.487, p = 00004, respectively). A low albumin-to-globulin ratio as a sign of systemic inflammation was reported to be negatively associated with RFS, OS, and CSS by two studies [35,43].
Therefore, abnormal levels of inflammatory biomarkers seem to have a negative correlation with oncologic outcomes after RC for BCa, but the attempts of creating prognostic panels for clinical decision-making failed due to the lack of a clinical added value of these biomarkers.
3.3.2. Serum Carbohydrate Antigen and Carcinoembryonic Antigen
Serum carbohydrate antigen (CA) has been studied in several articles [27,28,31]. Kouba et al. report that higher mean CA-125 serum levels in patients with pT2/T3 BCa were associated with recurrence after RC (20.1 vs. 10.8 U/mL, respectively; p = 0.224) [28]. Contradictory to these results, Chang et al. and Ahmadi et al. did not find an association between the preoperative blood levels of CA-125 or carcinoembryonic antigen (CEA) with RFS after RC [27,31]. Ahmadi et al. reported a negative association between high preoperative CA 19-9 levels and 3-year RFS in patients with BCa.
3.3.3. Circulating Tumor Cells and DNA
Christensen et al. investigated the prognostic impact of circulating tumor DNA in BCa patients and reported an overall recurrence rate after RC of 76% and a 12-month recurrence rate of 59% in ctDNA-positive patients [37]. The recurrence rate for ctDNA-negative patients was 0% for both time points. The published results seem very promising, yet the study was conducted with a small sample size (n = 68) and needs further validation.
The expression of the human epidermal growth factor receptor 2 (HER-2) on circulating tumor cells was assessed by Rink et al. through fluorescein-labeled antibodies [30]. In 23% of patients, circulating tumor cells were detected and 25% of subjects with HER-2 positive tumors expressed HER-2 on their circulating tumor cells. The presence of circulating tumor cells was associated with a worse RFS, CSS, and overall mortality (OM), but a correlation between HER-2 expression and clinical outcomes was not reported and the study was limited by a small sample size (n = 100).
3.3.4. Nutritional Status Biomarkers
Three studies reported the association of impaired nutritional status with worse survival outcomes [34,39,44]. Higher albumin-to-alkaline phosphatase ratios, as a sign of better nutritional status, correlated with better RFS, OS, and CSS. Djaladat et al. reported that higher serum albumin levels and the American Society of Anesthesiologists (ASA) score were both associated with a higher 90-day complication rate after radical surgery. Similarly, Ninomiya et al. demonstrated that a worse prognostic nutritional status was associated with a higher recurrence rate (p = 0.028) [39].
3.3.5. Factors of Angiogenesis and Vascular Endothelial Growth
Three articles investigating different markers associated with angiogenesis and vascular endothelial growth were identified. Laukhtina et al. conducted a retrospective analysis of 1036 patients, showing that a higher preoperatively measured plasma level of endoglin was associated with worse RFS (HR 1.85, p < 0.001), CSS (HR 2.02, p < 0.001), and OS (HR 1.63, p < 0.001) [49].
Similarly, higher preoperative plasma levels of both vascular cell adhesion molecule-1 (VCAM-1) and the vascular endothelial growth factor (VEGF) were associated with worse RFS, CSS, and OS after RC [42,50].
3.3.6. Other Blood-Based Biomarkers
The level of urokinase plasminogen activator proteins correlates with aggressive disease and worse survival outcomes after RC for BCa, as reported by Dohn et al. and Schuettfort et al. [42,46]. Hazza et al. measured serum and urine clusterin levels and showed a negative correlation with recurrence and OS [29].
Sari Motlagh et al. investigated the prognostic impact of the insulin-like growth factor-I and its binding proteins 2 and 3 in another retrospective cohort study [50]. There was no association between the insulin-like growth factor-I and clinical outcomes, but the authors report a favorable prognosis in subjects with elevated binding proteins. Katayama et al. and Yuk et al. proposed the integration of the hepatocyte growth factor and the De Ritis ratio into a prognostic panel for risk stratification [38,55].
Another interesting blood-based biomarker is serum lactate dehydrogenase. Since cancer cells prioritize glycolysis and lactate dehydrogenase catalyzes pyruvate and lactic acid, the enzyme’s serum level might reflect the metabolic changes seen in tumor progression. An elevated lactate dehydrogenase level was associated with a shorter disease-free survival (HR 2.051; p = 0.019) [41]. With its feasibility and easy access, the measurement of serum lactate dehydrogenase might therefore be a promising part of a risk stratification panel.
3.4. Meta-Analysis
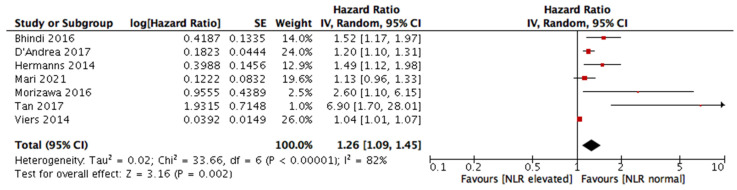
The seven studies eligible for meta-analysis were published between 2014 and 2021, as presented in Table 2. All of them report preoperatively measured NLR as a prognostic factor of RFS. The sample sizes ranged between 84 and 4198 patients, reporting 6388 patients in total. The median follow-up time ranged from 30.1 months to 10.9 years. Two studies did not report their median follow-up times. The patient median age ranged between 67 and 79 years. More male than female subjects were included in the eligible studies. Except for one, all studies reported statistically significant results regarding disease recurrence. The reported NLR cut-off values ranged between 2.6 and 3. Study characteristics in more detail are listed in Supplementary Table S2.
Table 2.
Eligible studies for the meta-analysis and their study characteristics.
| Author | Year | Sample Size | Age, Years, Median (Range) | Pathological Stage | Median Follow-Up (Range) | HR, Multivariate Analysis (CI) | NLR Cut-Off | p-Value |
|---|---|---|---|---|---|---|---|---|
| Viers et al. [33] | 2014 | 899 | 69 (62–76) | T ≤ 1–T4, Nx, N0–N3 | 10.9 years (8.3–13.9) | Recurrence: HR 1.04 (1.01–1.08) | NLR > 2.7 | p = 0.02 |
| Hermanns et al. [32] | 2014 | 424 | 70.1 (60.6–76.3) | T0–T4, Ta, Tis, Nx, N0, N+ | 58.4 months (21.3–94.5) | Recurrence: HR 1.49 (1.12–2.00) | NLR ≥ 3 | p = 0.007 |
| Bhindi et al. [20] | 2016 | 418 | 70 (61–76) | T0– T4, Ta, Tis, N0, N+, Nx | 40 months (14–72) | RFS: HR 1.52 (1.17–1.98) | NLR < 2.9 | p = 0.002 |
| Morizawa et al. [22] | 2016 | 110 | 72 (65–76) | T0–T4, N+ | 37.5 months (11–65) | RFS: HR 2.6 (1.1–6.0) | NLR < 2.6 | p = 0.02 |
| D’Andrea et al. [21] | 2017 | 4198 | 67 (60–73) | T0–T4, Ta, Tis; N+ | not reported | RFS: HR 1.2 (1.1–1.3) | NLR < 2.7 | p < 0.001 |
| Tan et al. [36] | 2017 | 84 | 67 (37–82) | T1–T4, N+ | 30.1 months (3.2–161.7) | Recurrence: HR 6.999 (1.712–28.606) | NLR ≥ 2.7 | p = 0.007 |
| Mari et al. [45] | 2021 | 255 | 79 (75–83) | T1–T4, Nx, N0–N3 | Not reported | Recurrence: HR 1.13 (0.96–1.32) | NLR > 3 | p = 0.14 |
HR = hazard ratio, CI = confidence interval, NLR = neutrophil-to-lymphocyte ratio, RFS = recurrence-free survival.
The meta-analysis reports worse RFS for patients with an elevated preoperative NLR with a HR of 1.26 (95% CI 1.09, 1.45), p = 0.002. Since a statistically significant heterogeneity (I2 = 82%) was shown between studies, a random effect model was used. The statistical results visualized by a forest plot are shown in Figure 2.
Figure 2.
Meta-analysis results of neutrophil-to-lymphocyte ratio (NLR) and its impact on the recurrence-free survival (RFS) of urothelial carcinoma patients after RC [20,21,22,32,33,36,45].
4. Discussion
This systematic review summarizes the available data of the last two decades on preoperative blood-based biomarkers as prognostic factors of recurrence in RC patients, with special focus on inflammatory biomarkers. In our meta-analysis, we confirmed the strong prognostic impact of preoperative NLR on RFS after RC.
We identified various blood-based biomarkers as predictors of recurrence after RC for BCa through a systematic review. Inflammatory biomarkers were reported to be negatively correlated with recurrence, but attempts to create prognostic panels for clinical decision-making have failed [48,53]. Two studies evaluated the presence of ctDNA and circulating tumor cells with promising results, but were limited by a small sample size [30,37]. Further reports evaluated serum carbohydrate antigen, nutritional status, and factors of angiogenesis as prognostic biomarkers.
Inflammation and cancer have been linked since Rudolf Virchow reported the presence of leukocytes in tumor biopsies in 1863 [56]. Inflammatory reactions play important roles in tumor development, cancer promotion, and growth, as well as in invasion and metastasis [57]. In particular, urothelial cancer is known to be an immunogenic malignancy with tumor-infiltrating cytotoxic cells inducing BCa cell proliferation caused by the release of excessive amounts of cytokines [17]. This, together with the ease of access and measurement of inflammatory biomarkers in peripheral blood, make the latter an obvious candidate for prognostic biomarker research.
Nevertheless, it should be mentioned that studies evaluating biomarkers show an association between the marker and cancer outcomes rather than an actual predictive accuracy [15]. Since the use of a biomarker should add a benefit in clinical practice compared to an already existing standard model, internal and external validation is needed for all reported biomarkers. The incorporation of statistical tools such as decision curve analysis has not been performed in most studies, therefore limiting the use of these biomarkers in guiding therapeutic decisions [15].
Using meta-analysis methodology, we found an association between elevated NLR and decreased RFS. Urothelial cancer patients with preoperatively increased NLR exhibited increased rates of disease recurrence after radical surgery compared to patients with lower NLR. Except for one publication by Mari et al., six articles included in this meta-analysis reported a statistically significant negative correlation of elevated NLR with RFS [20,21,22,32,33,36,45]. These results support the integration of NLR for risk stratification tools and could help guide decision-making, especially regarding treatment intensification. Since patterns of NLR change are reported to vary significantly between responders and non-responders to systemic therapies, this ratio might also be investigated as a predictive tool to identify patients likely to respond to systemic therapy [58].
The NLR is calculated using peripheral blood measurements and reflects the balance between acute/chronic inflammation or adaptive immunity, as neutrophil counts rise in acute and chronic inflammatory reactions [59]. Besides urologic cancers, blood-based NLR was also shown to exhibit a prognostic value in gastrointestinal and gynecological cancers [60]. An isolated rise in neutrophils is also observed in bacterial and fungal infections, strokes and myocardial infarctions, atherosclerosis, and tissue damage that activates a systemic inflammatory response [61,62,63,64,65]. Although NLR is relevant for many different clinical scenarios, the cut-off value for its upper normal limit is still under debate. Beyond oncology, Song et al. reported an association of elevated NLR with overall mortality in the United States general population between 1999 and 2014 [59]. They also showed increased NLR values in patients that died due to heart disease, chronic lower respiratory disease, pneumonia, and kidney disease. Since NLR elevation can be caused by various maladies and circumstances, a very precise patient selection should be conducted in studies with survival outcomes as primary endpoints. Karakonstantis et al. reported several confounders in NLR values such as age, steroid intake, endogenous sexual hormones, and hematological disorders [66]. In a study by Fest et al., a significant difference in NLR values between female and male subjects was shown [67]. Hence, the above-mentioned factors might contribute to a bias in the results of conducted studies. A patient-selection approach for NLR utilization as a prognostic factor of recurrence after RC could be used to exclude patients with immunological or hematological diseases that could alter the NLR (i.e., leukemia, viral infections and chronic inflammatory diseases, malignant lymphoma, autoimmune diseases) as well as other malignancies [20,45]. Besides that, blood collection in patients receiving neoadjuvant chemotherapy should be carried out before the initiation of chemotherapy, since this could also be an influencing factor [22].
Our study is limited by several factors. NLR cut-off values differed between articles (ranging from 2.6 to 3.0), which makes the data less comparable. A consensus for standardized cut-off values has not been found yet. Additionally, every eligible article for this meta-analysis was conducted as a retrospective single- or multicenter study, thereby having an inferior level of evidence compared to prospective studies. The number of eligible articles for our meta-analysis was low, as only seven studies met our search criteria, and a high heterogeneity between these articles was found. Articles not written in English were excluded during the screening process, which could also lead to bias. In summary, prospectively conducted trials with larger sample sizes are needed to validate the most promising blood-based biomarker candidates that could be used to optimize risk stratification regarding treatment selection for patients with muscle-invasive BCa.
5. Conclusions
This study, focusing on disease recurrence, pooled available data on preoperative blood-based biomarkers as prognostic factors for survival outcomes in patients treated with RC for BCa. Several blood-based biomarkers such as inflammatory markers, factors of angiogenesis, circulating tumor cells and DNA, nutritional factors, and serum carbohydrate antigen seem to be promising tools for the prediction of recurrence after RC. Using a meta-analysis, we found a RFS benefit in patients with preoperatively measured NLR values below the upper limit of normal. However, to use these biomarkers as a part of a risk stratification tool in clinical decision-making, further investigation based on well-designed prospective clinical trials with larger sample sizes and strict patient inclusion criteria to reduce confounders are needed.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24065846/s1.
Author Contributions
Conceptualization, H.O., E.L. and S.F.S.; methodology H.O. and E.L.; software H.O. and E.L.; validation E.L., M.R.H. and S.F.S.; formal analysis H.O. and E.L.; investigation H.O., data curation, H.O.; writing – original draft preparation, H.O.; writing – review and editing, E.L. and M.R.H. and S.F.S.; visualization H.O.; supervision, S.F.S.; All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Ethical review and approval were waived for this study due to the nature of the study (systematic review and meta-analysis), only publicly accessible documents were used.
Informed Consent Statement
Patient consent was waived due to the nature of the study (systematic review and meta-analysis), only publicly accessible documents were used.
Data Availability Statement
Data used in this systematic review and meta-analysis is cited in the references. Used electronic databases include PubMed (https://pubmed.ncbi.nlm.nih.gov) and Scopus (https://www.scopus.com).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Xylinas E., Robinson B., Kluth L., Volkmer B., Hautmann R., Küfer R., Zerbib M., Kwon E., Thompson R., Boorjian S., et al. Association of T-cell co-regulatory protein expression with clinical outcomes following radical cystectomy for urothelial carcinoma of the bladder. Eur. J. Surg. Oncol. EJSO. 2014;40:121–127. doi: 10.1016/j.ejso.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 2.Shariat S.F., Karakiewicz P.I., Palapattu G.S., Lotan Y., Rogers C.G., Amiel G.E., Vazina A., Gupta A., Bastian P.J., Sagalowsky A.I., et al. Outcomes of Radical Cystectomy for Transitional Cell Carcinoma of the Bladder: A Contemporary Series from the Bladder Cancer Research Consortium. Pt 1J. Urol. 2006;176:2414–2422;. doi: 10.1016/j.juro.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Aziz A., May M., Burger M., Palisaar R.-J., Trinh Q.-D., Fritsche H.-M., Rink M., Chun F., Martini T., Bolenz C., et al. Prediction of 90-day Mortality after Radical Cystectomy for Bladder Cancer in a Prospective European Multicenter Cohort. Eur. Urol. 2014;66:156–163. doi: 10.1016/j.eururo.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Stein J.P., Lieskovsky G., Cote R., Groshen S., Feng A.-C., Boyd S., Skinner E., Bochner B., Thangathurai D., Mikhail M., et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1054 Patients. J. Clin. Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 5.Shariat S.F., Ashfaq R., Karakiewicz P.I., Saeedi O., Sagalowsky A.I., Lotan Y. Survivin expression is associated with bladder cancer presence, stage, progression, and mortality. Cancer. 2007;109:1106–1113. doi: 10.1002/cncr.22521. [DOI] [PubMed] [Google Scholar]
- 6.Kluth L.A., Black P.C., Bochner B.H., Catto J., Lerner S.P., Stenzl A., Sylvester R., Vickers A.J., Xylinas E., Shariat S.F. Prognostic and Prediction Tools in Bladder Cancer: A Comprehensive Review of the Literature. Eur. Urol. 2015;68:238–253. doi: 10.1016/j.eururo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 7.Shariat S.F., Zlotta A.R., Ashfaq R., Sagalowsky A.I., Lotan Y. Cooperative effect of cell-cycle regulators expression on bladder cancer development and biologic aggressiveness. Mod. Pathol. 2007;20:445–459. doi: 10.1038/modpathol.3800757. [DOI] [PubMed] [Google Scholar]
- 8.Mathieu R., Lucca I., Rouprêt M., Briganti A., Shariat S.F. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat. Rev. Urol. 2016;13:471–479. doi: 10.1038/nrurol.2016.126. [DOI] [PubMed] [Google Scholar]
- 9.Moschini M., D’Andrea D., Korn S., Irmak Y., Soria F., Compérat E., Shariat S.F. Characteristics and clinical significance of histological variants of bladder cancer. Nat. Rev. Urol. 2017;14:651–668. doi: 10.1038/nrurol.2017.125. [DOI] [PubMed] [Google Scholar]
- 10.Svatek R.S., Shariat S.F., Lasky R.E., Skinner E.C., Novara G., Lerner S.P., Fradet Y., Bastian P.J., Kassouf W., Karakiewicz P.I., et al. The Effectiveness of Off-Protocol Adjuvant Chemotherapy for Patients with Urothelial Carcinoma of the Urinary Bladder. Clin. Cancer Res. 2010;16:4461–4467. doi: 10.1158/1078-0432.CCR-10-0457. [DOI] [PubMed] [Google Scholar]
- 11.Isbarn H., Jeldres C., Zini L., Perrotte P., Baillargeon-Gagne S., Capitanio U., Shariat S.F., Arjane P., Saad F., McCormack M., et al. A Population Based Assessment of Perioperative Mortality After Cystectomy for Bladder Cancer. J. Urol. 2009;182:70–77. doi: 10.1016/j.juro.2009.02.120. [DOI] [PubMed] [Google Scholar]
- 12.Grossmann N.C., Rajwa P., Quhal F., König F., Mostafaei H., Laukhtina E., Mori K., Katayama S., Motlagh R.S., Fankhauser C.D., et al. Comparative Outcomes of Primary versus Recurrent High-risk Non–muscle-invasive and Primary versus Secondary Muscle-invasive Bladder Cancer after Radical Cystectomy: Results from a Retrospective Multicenter Study. Eur. Urol. Open Sci. 2022;39:14–21. doi: 10.1016/j.euros.2022.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Necchi A., Pond G.R., Moschini M., Plimack E.R., Niegisch G., Yu E.Y., Bamias A., Agarwal N., Vaishampayan U., Theodore C., et al. Development of a Prediction Tool for Exclusive Locoregional Recurrence after Radical Cystectomy in Patients with Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer. 2019;17:7–14.e3. doi: 10.1016/j.clgc.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ru Y., Dancik G.M., Theodorescu D. Biomarkers for prognosis and treatment selection in advanced bladder cancer patients. Curr. Opin. Urol. 2011;21:420–427. doi: 10.1097/MOU.0b013e32834956d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shariat S.F., Lotan Y., Vickers A., Karakiewicz P.I., Schmitz-Dräger B.J., Goebell P.J., Malats N. Statistical consideration for clinical biomarker research in bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2010;28:389–400. doi: 10.1016/j.urolonc.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bensalah K., Montorsi F., Shariat S.F. Challenges of Cancer Biomarker Profiling. Eur. Urol. 2007;52:1601–1609. doi: 10.1016/j.eururo.2007.09.036. [DOI] [PubMed] [Google Scholar]
- 17.Gakis G. The Role of Inflammation in Bladder Cancer. Adv. Exp. Med. Biol. 2014;816:183–196. doi: 10.1007/978-3-0348-0837-8_8. [DOI] [PubMed] [Google Scholar]
- 18.Nabavizadeh R., Bobrek K., Master V.A. Risk stratification for bladder cancer: Biomarkers of inflammation and immune activation. Urol. Oncol. Semin. Orig. Investig. 2020;38:706–712. doi: 10.1016/j.urolonc.2020.04.006. [DOI] [PubMed] [Google Scholar]
- 19.Cancer-Related Inflammation|Nature. [(accessed on 10 December 2022)]. Available online: https://www-nature-com.ez.srv.pmu.ac.at/articles/nature07205.
- 20.Bhindi B., Hermanns T., Wei Y., Yu J., O Richard P., Wettstein M.S., Templeton A., Li K., Sridhar S.S., Jewett M.A.S., et al. Identification of the best complete blood count-based predictors for bladder cancer outcomes in patients undergoing radical cystectomy. Br. J. Cancer. 2016;114:207–212. doi: 10.1038/bjc.2015.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Andrea D., Moschini M., Gust K.M., Abufaraj M., Özsoy M., Mathieu R., Soria F., Briganti A., Rouprêt M., Karakiewicz P.I., et al. Lymphocyte-to-monocyte ratio and neutrophil-to-lymphocyte ratio as biomarkers for predicting lymph node metastasis and survival in patients treated with radical cystectomy. J. Surg. Oncol. 2017;115:455–461. doi: 10.1002/jso.24521. [DOI] [PubMed] [Google Scholar]
- 22.Morizawa Y., Miyake M., Shimada K., Hori S., Tatsumi Y., Nakai Y., Anai S., Tanaka N., Konishi N., Fujimoto K. Neutrophil-to-lymphocyte ratio as a detection marker of tumor recurrence in patients with muscle-invasive bladder cancer after radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2016;34:257.e11–257.e17. doi: 10.1016/j.urolonc.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Riva J.J., Malik K.M.P., Burnie S.J., Endicott A.R., Busse J.W. What is your research question? An introduction to the PICOT format for clinicians. J. Can. Chiropr. Assoc. 2012;56:167–171. [PMC free article] [PubMed] [Google Scholar]
- 24.Liberati M., Tetzlaff J., Altman D.G., PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sterne J.A.C., Hernán M.A., Reeves B.C., Savović J., Berkman N.D., Viswanathan M., Henry D., Altman D.G., Ansari M.T., Boutron I., et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chang A., Cai J., Miranda G., Groshen S., Skinner D., Stein J.P. Usefulness of CA 125 as a preoperative prognostic marker for transitional cell carcinoma of the bladder. Pt 1J. Urol. 2004;172:2182–2186. doi: 10.1097/01.ju.0000143487.20280.ed. [DOI] [PubMed] [Google Scholar]
- 28.Kouba E.J., Lentz A., Wallen E.M., Pruthi R.S. Clinical use of serum CA-125 levels in patients undergoing radical cystectomy for transitional cell carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2009;27:486–490. doi: 10.1016/j.urolonc.2008.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Hazzaa S.M., Elashry O.M., Afifi I.K. Clusterin as a Diagnostic and Prognostic Marker for Transitional Cell Carcinoma of the Bladder. Pathol. Oncol. Res. 2010;16:101–109. doi: 10.1007/s12253-009-9196-3. [DOI] [PubMed] [Google Scholar]
- 30.Rink M., Chun F.K., Dahlem R., Soave A., Minner S., Hansen J., Stoupiec M., Coith C., Kluth L.A., Ahyai S.A., et al. Prognostic Role and HER2 Expression of Circulating Tumor Cells in Peripheral Blood of Patients Prior to Radical Cystectomy: A Prospective Study. Eur. Urol. 2012;61:810–817. doi: 10.1016/j.eururo.2012.01.017. [DOI] [PubMed] [Google Scholar]
- 31.Ahmadi H., Djaladat H., Cai J., Miranda G., Daneshmand S. Precystectomy serum levels of carbohydrate antigen 19-9, carbohydrate antigen 125, and carcinoembryonic antigen: Prognostic value in invasive urothelial carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2014;32:648–656. doi: 10.1016/j.urolonc.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 32.Hermanns T., Bhindi B., Wei Y., Yu J., Noon A., O Richard P., Bhatt J.R., Almatar A., Jewett M.A.S., E Fleshner N., et al. Pre-treatment neutrophil-to-lymphocyte ratio as predictor of adverse outcomes in patients undergoing radical cystectomy for urothelial carcinoma of the bladder. Br. J. Cancer. 2014;111:444–451. doi: 10.1038/bjc.2014.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Viers B., Boorjian S.A., Frank I., Tarrell R.F., Thapa P., Karnes R.J., Thompson R.H., Tollefson M.K. Pretreatment Neutrophil-to-Lymphocyte Ratio Is Associated with Advanced Pathologic Tumor Stage and Increased Cancer-specific Mortality Among Patients with Urothelial Carcinoma of the Bladder Undergoing Radical Cystectomy. Eur. Urol. 2014;66:1157–1164. doi: 10.1016/j.eururo.2014.02.042. [DOI] [PubMed] [Google Scholar]
- 34.Djaladat H., Bruins H.M., Miranda G., Cai J., Skinner E.C., Daneshmand S. The association of preoperative serum albumin level and American Society of Anesthesiologists (ASA) score on early complications and survival of patients undergoing radical cystectomy for urothelial bladder cancer. BJU Int. 2014;113:887–893. doi: 10.1111/bju.12240. [DOI] [PubMed] [Google Scholar]
- 35.Liu J., Dai Y., Zhou F., Long Z., Li Y., Liu B., Xie D., Tang J., Tan J., Yao K., et al. The prognostic role of preoperative serum albumin/globulin ratio in patients with bladder urothelial carcinoma undergoing radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2016;34:484.e1–484.e8. doi: 10.1016/j.urolonc.2016.05.024. [DOI] [PubMed] [Google Scholar]
- 36.Tan Y.G., Eu E., On W.L.K., Huang H.H. Pretreatment neutrophil-to-lymphocyte ratio predicts worse survival outcomes and advanced tumor staging in patients undergoing radical cystectomy for bladder cancer. Asian J. Urol. 2017;4:239–246. doi: 10.1016/j.ajur.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Christensen E., Birkenkamp-Demtröder K., Sethi H., Shchegrova S., Salari R., Nordentoft I.K., Wu H.-T., Knudsen M., Lamy P., Lindskrog S.V., et al. Early Detection of Metastatic Relapse and Monitoring of Therapeutic Efficacy by Ultra-Deep Sequencing of Plasma Cell-Free DNA in Patients With Urothelial Bladder Carcinoma. J. Clin. Oncol. 2019;37:1547–1557. doi: 10.1200/JCO.18.02052. [DOI] [PubMed] [Google Scholar]
- 38.Yuk H.D., Jeong C.W., Kwak C., Kim H.H., Ku J.H. De Ritis Ratio (Aspartate Transaminase/Alanine Transaminase) as a Significant Prognostic Factor in Patients Undergoing Radical Cystectomy with Bladder Urothelial Carcinoma: A Propensity Score-Matched Study. Dis. Markers. 2019;2019:6702964. doi: 10.1155/2019/6702964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ninomiya S., Kawahara T., Miyoshi Y., Yao M., Uemura H. A retrospective study on the possible systematic inflammatory response markers to predict the prognosis of patients with bladder cancer undergoing radial cystectomy. Mol. Clin. Oncol. 2020;13:47. doi: 10.3892/mco.2020.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fallah J., Diaz-Montero C.M., Rayman P., Wei W., Finke J.H., Kim J.S., Pavicic P.G., Lamenza M., Dann P., Company D., et al. Myeloid-Derived Suppressor Cells in Nonmetastatic Urothelial Carcinoma of Bladder Is Associated with Pathologic Complete Response and Overall Survival. Clin. Genitourin. Cancer. 2020;18:500–508. doi: 10.1016/j.clgc.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 41.Su S., Liu L., Sun C., Yang L., Nie Y., Chen Y., Zhang J., Li S. Prognostic significance of serum lactate dehydrogenase in patients undergoing radical cystectomy for bladder cancer. Urol. Oncol. Semin. Orig. Investig. 2020;38:852.e1–852.e9. doi: 10.1016/j.urolonc.2020.05.031. [DOI] [PubMed] [Google Scholar]
- 42.Dohn L., Thind P., Salling L., Lindberg H., Oersted S., Christensen I., Laerum O., Illemann M., von der Maase H., Høyer-Hansen G., et al. Circulating Forms of Urokinase-Type Plasminogen Activator Receptor in Plasma Can Predict Recurrence and Survival in Patients with Urothelial Carcinoma of the Bladder. Cancers. 2021;13:2377. doi: 10.3390/cancers13102377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuettfort V.M., D’andrea D., Quhal F., Mostafaei H., Laukhtina E., Mori K., Motlagh R.S., Rink M., Abufaraj M., Karakiewicz P.I., et al. Impact of preoperative serum albumin-globulin ratio on disease outcome after radical cystectomy for urothelial carcinoma of the bladder. Urol. Oncol. Semin. Orig. Investig. 2021;39:235.e5–235.e14. doi: 10.1016/j.urolonc.2020.11.005. [DOI] [PubMed] [Google Scholar]
- 44.Li S., Lu S., Liu X., Chen X. Association between the Pretreatment Albumin-to-Alkaline Phosphatase Ratio and Clinical Outcomes in Patients With Bladder Cancer Treated With Radical Cystectomy: A Retrospective Cohort Study. Front. Oncol. 2021;11:664392. doi: 10.3389/fonc.2021.664392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mari A., Muto G., Di Maida F., Tellini R., Bossa R., Bisegna C., Campi R., Cocci A., Viola L., Grosso A., et al. Oncological impact of inflammatory biomarkers in elderly patients treated with radical cystectomy for urothelial bladder cancer. Arab. J. Urol. 2021;19:2–8. doi: 10.1080/2090598X.2020.1814974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schuettfort V.M., Pradere B., D’Andrea D., Grossmann N.C., Quhal F., Mostafaei H., Laukhtina E., Mori K., Rink M., Karakiewicz P.I., et al. Prognostic Impact of Preoperative Plasma Levels of Urokinase Plasminogen Activator Proteins on Disease Outcomes after Radical Cystectomy. J. Urol. 2021;206:1122–1131. doi: 10.1097/JU.0000000000001936. [DOI] [PubMed] [Google Scholar]
- 47.Schuettfort V.M., D’Andrea D., Quhal F., Mostafaei H., Laukhtina E., Mori K., König F., Rink M., Abufaraj M., Karakiewicz P.I., et al. A panel of systemic inflammatory response biomarkers for outcome prediction in patients treated with radical cystectomy for urothelial carcinoma. BJU Int. 2022;129:182–193. doi: 10.1111/bju.15379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grossmann N.C., Schuettfort V.M., Pradere B., Rajwa P., Quhal F., Mostafaei H., Laukhtina E., Mori K., Motlagh R.S., Aydh A., et al. Impact of preoperative systemic immune-inflammation Index on oncologic outcomes in bladder cancer patients treated with radical cystectomy. Urol. Oncol. Semin. Orig. Investig. 2022;40:106.e11–106.e19. doi: 10.1016/j.urolonc.2021.10.006. [DOI] [PubMed] [Google Scholar]
- 49.Laukhtina E., Schuettfort V.M., D’andrea D., Pradere B., Mori K., Quhal F., Motlagh R.S., Mostafaei H., Katayama S., Grossmann N., et al. Preoperative plasma level of endoglin as a predictor for disease outcomes after radical cystectomy for nonmetastatic urothelial carcinoma of the bladder. Mol. Carcinog. 2022;61:5–18. doi: 10.1002/mc.23355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Motlagh R.S., Schuettfort V.M., Mori K., Katayama S., Rajwa P., Aydh A., Grossmann N.C., Laukhtina E., Pradere B., Mostafai H., et al. Prognostic impact of insulin-like growth factor-I and its binding proteins, insulin-like growth factor-I binding protein-2 and -3, on adverse histopathological features and survival outcomes after radical cystectomy. Int. J. Urol. 2022;29:676–683. doi: 10.1111/iju.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mori K., Schuettfort V.M., Katayama S., Laukhtina E., Pradere B., Quhal F., Motlagh R.S., Mostafaei H., Grossmann N.C., Rajwa P., et al. Prognostic Role of Preoperative Vascular Cell Adhesion Molecule-1 Plasma Levels in Urothelial Carcinoma of the Bladder Treated With Radical Cystectomy. Ann. Surg. Oncol. 2022;29:5307–5316. doi: 10.1245/s10434-022-11575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lei Y., Jiao D., Yao Z., Wang L., Zhao Z. Prognostic values of preoperative neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio and lymphocyte- to-monocyte ratio for patients with muscle- invasive bladder cancer undergoing radical cystectomy. Arch. Esp. Urol. 2022;75:287–294. [PubMed] [Google Scholar]
- 53.Schuettfort V.M., Pradere B., Trinh Q.-D., D’Andrea D., Quhal F., Mostafaei H., Laukhtina E., Mori K., Motlagh R.S., Rink M., et al. Impact of preoperative plasma levels of interleukin 6 and interleukin 6 soluble receptor on disease outcomes after radical cystectomy for bladder cancer. Cancer Immunol. Immunother. 2022;71:85–95. doi: 10.1007/s00262-021-02953-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Urabe F., Matsuzaki J., Ito K., Takamori H., Tsuzuki S., Miki J., Kimura T., Egawa S., Nakamura E., Matsui Y., et al. Serum microRNA as liquid biopsy biomarker for the prediction of oncological outcomes in patients with bladder cancer. Int. J. Urol. 2022;29:968–976. doi: 10.1111/iju.14858. [DOI] [PubMed] [Google Scholar]
- 55.Katayama S., Schuettfort V.M., Pradere B., Mori K., Mostafaei H., Quhal F., Motlagh R.S., Laukhtina E., Grossmann N.C., Aydh A., et al. Prognostic value of hepatocyte growth factor for muscle-invasive bladder cancer. J. Cancer Res. Clin. Oncol. 2022;148:3091–3102. doi: 10.1007/s00432-021-03887-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Inflammation and Cancer: Back to Virchow?-ScienceDirect. [(accessed on 11 December 2022)]. Available online: https://www-sciencedirect-com.ez.srv.pmu.ac.at/science/article/pii/S0140673600040460?via%3Dihub?ezproxy=true.
- 57.Fernandes J.V., Cobucci R., Jatobá C.A.N., Fernandes T., De Azevedo J.W.V., De Araújo J.M.G. The Role of the Mediators of Inflammation in Cancer Development. Pathol. Oncol. Res. POR. 2015;21:527–534. doi: 10.1007/s12253-015-9913-z. [DOI] [PubMed] [Google Scholar]
- 58.Seah J.-A., Leibowitz-Amit R., Atenafu E.G., Alimohamed N., Knox J.J., Joshua A.M., Sridhar S.S. Neutrophil-Lymphocyte Ratio and Pathological Response to Neoadjuvant Chemotherapy in Patients with Muscle-Invasive Bladder Cancer. Clin. Genitourin. Cancer. 2015;13:e229–e233. doi: 10.1016/j.clgc.2015.02.001. [DOI] [PubMed] [Google Scholar]
- 59.Song M., Graubard B.I., Rabkin C.S., Engels E.A. Neutrophil-to-lymphocyte ratio and mortality in the United States general population. Sci. Rep. 2021;11:464. doi: 10.1038/s41598-020-79431-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Guthrie G.J.K., Charles K.A., Roxburgh C.S.D., Horgan P.G., McMillan D.C., Clarke S.J. The systemic inflammation-based neutrophil–lymphocyte ratio: Experience in patients with cancer. Crit. Rev. Oncol. Hematol. 2013;88:218–230. doi: 10.1016/j.critrevonc.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 61.Lowsby R., Gomes C., Jarman I., Lisboa P., Nee P.A., Vardhan M., Eckersley T., Saleh R., Mills H. Neutrophil to lymphocyte count ratio as an early indicator of blood stream infection in the emergency department. Emerg. Med. J. 2015;32:531–534. doi: 10.1136/emermed-2014-204071. [DOI] [PubMed] [Google Scholar]
- 62.Adamstein N.H., MacFadyen J.G., Rose L.M., Glynn R.J., Dey A.K., Libby P., Tabas I.A., Mehta N.N., Ridker P.M. The neutrophil–lymphocyte ratio and incident atherosclerotic events: Analyses from five contemporary randomized trials. Eur. Heart J. 2021;42:896–903. doi: 10.1093/eurheartj/ehaa1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Park J.M. Neutrophil-to-lymphocyte ratio in trauma patients. J. Trauma Acute Care Surg. 2017;82:225–226. doi: 10.1097/TA.0000000000001266. [DOI] [PubMed] [Google Scholar]
- 64.Li W., Hou M., Ding Z., Liu X., Shao Y., Li X. Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Stroke: A Systematic Review and Meta-Analysis. Front. Neurol. 2021;12:686983. doi: 10.3389/fneur.2021.686983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee M.-J., Park S.-D., Kwon S.W., Woo S.-I., Lee M.-D., Shin S.-H., Kim D.-H., Kwan J., Park K.-S. Relation between Neutrophil-to-Lymphocyte Ratio and Index of Microcirculatory Resistance in Patients with ST-Segment Elevation Myocardial Infarction Undergoing Primary Percutaneous Coronary Intervention. Am. J. Cardiol. 2016;118:1323–1328. doi: 10.1016/j.amjcard.2016.07.072. [DOI] [PubMed] [Google Scholar]
- 66.Karakonstantis S., Kalemaki D., Tzagkarakis E., Lydakis C. Pitfalls in studies of eosinopenia and neutrophil-to-lymphocyte count ratio. Infect. Dis. 2018;50:163–174. doi: 10.1080/23744235.2017.1388537. [DOI] [PubMed] [Google Scholar]
- 67.Fest J., Ruiter R., Ikram M.A., Voortman T., van Eijck C.H.J., Stricker B.H. Reference values for white blood-cell-based inflammatory markers in the Rotterdam Study: A population-based prospective cohort study. Sci. Rep. 2018;8:10566. doi: 10.1038/s41598-018-28646-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used in this systematic review and meta-analysis is cited in the references. Used electronic databases include PubMed (https://pubmed.ncbi.nlm.nih.gov) and Scopus (https://www.scopus.com).