Abstract
Background: in recent years, the role of positron emission tomography (PET) and PET/computed tomography (PET/CT) has emerged as a reliable diagnostic tool in a wide variety of pathological conditions. This review aims to collect and review PET criteria developed for interpretation and treatment response assessment in cases of non-[18F]fluorodeoxyglucose ([18F]FDG) imaging in oncology. Methods: A wide literature search of the PubMed/MEDLINE, Scopus and Google Scholar databases was made to find relevant published articles about non-[18F]FDG PET response criteria. Results: The comprehensive computer literature search revealed 183 articles. On reviewing the titles and abstracts, 149 articles were excluded because the reported data were not within the field of interest. Finally, 34 articles were selected and retrieved in full-text versions. Conclusions: available criteria are a promising tool for the interpretation of non-FDG PET scans, but also to assess the response to therapy and therefore to predict the prognosis. However, oriented clinical trials are needed to clearly evaluate their impact on patient management.
Keywords: non-FDG PET criteria, PSMA-RADS, miTNM, Pro-PET, PRIMARY, PPP, RECIP 1.0, SSTR-RADS, NETPET, NAFCIST, FuMeGa
1. Introduction
Positron emission tomography (PET) and PET/computed tomography (PET/CT) have emerged in recent years as pivotal tools for the non-invasive assessment of a high number of pathological conditions. The most used tracer to perform this imaging modality is [18F]fluorodeoxyglucose ([18F]FDG) which can evaluate the glycolytic activity of cells [1,2], but other radiopharmaceuticals that explore different metabolic pathways or localize to particular targets because of specific binding interactions are available nowadays. The role of PET/CT imaging for the assessment of neoplasia has emerged in several steps of oncological patients’ work-up, such as in the diagnosis, staging, re-staging after therapy and follow-up. During the last 10 years, the use of many criteria has emerged in order to standardize the initial interpretation of oncological disease or their response after or during therapy. Imaging criteria can help in the interpretation of the scan, guiding the reader to better define its status or diagnosis. Moreover, since PET/CT is able to reflect different metabolic pathways of the tissues, functional changes can occur early in the course of therapy, preceding reduction in the size of tumors. Therefore, PET/CT can also be useful for the assessment of response to a specific therapy, thus guiding the future diagnostic and therapeutic work-up of the patient [3]. Different approaches measuring the response rate of neoplasms with morphological imaging modalities have been classically developed, such as the “Response Evaluation Criteria in Solid Tumors” (RECIST) [4]. Pointing our attention to [18F]FDG PET/CT, some specific response criteria have been formulated to improve its diagnostic predictive value, such as the EORTC, “PET/CT Criteria for early prediction of Response to Immune checkpoint inhibitor Therapy” (PECRIT), the “PET Response Evaluation Criteria for ImmunoTherapy” (PERCIMT) or the “PET Response Criteria in Solid Tumors” (PERCIST) [4,5,6,7]. In this scenario most of the PET criteria that have been proposed in the literature focus on [18F]FDG PET/CT, while only a small number of them were thought suitable for other radiopharmaceuticals. The aim of this systematic review is, therefore, to collect and discuss PET criteria developed for non-[18F]FDG probes in the oncological setting both for imaging interpretation and treatment response assessment.
2. Research Strategy
A bibliographic literature search up to 30 November 2022 was performed on three electronic databases (PubMed/MEDLINE, Scopus, Google Scholar) in order to find articles concerning the use of non-[18F]FDG PET criteria for the assessment of solid tumors. The search algorithms were different combinations of the following terms: “PET criteria”, “PET score”, “positron emission tomography”, “imaging interpretation”, “response”, “post-treatment evaluation criteria”, “osteosarcoma”, “Ewing sarcoma”, “bone tumor”, “brain”, “glioma”, “meningioma”, “breast cancer”, “pancreatic carcinoma”, “gastric cancer”, “esophageal cancer”, “anal cancer”, “GIST”, “cervical cancer”, “bladder cancer”, “head and neck squamous cell carcinoma”, “head and neck carcinoma”, “lung cancer”, “NSCLC”, “small cell lung cancer”, “mesothelioma”, “pleural mesothelioma”, “medullary thyroid cancer”, “thyroid cancer”, “anaplastic thyroid cancer”, “papillary thyroid cancer”, “adrenal gland tumor”, “neuroendocrine tumor”, “neuroendocrine”, “paraganglioma”, “prostate”, “prostate cancer”, “18F”, “68Ga”, “11C”, “MET”, “FET”, “DOPA”, “FCH”, “choline”, “DOTA”, “DOTATOC” “DOTATATE”, “DOTANOC”, “PSMA” and “NaF”. To identify supplementary eligible articles, the references of the retrieved articles were also screened for additional papers. Two reviewers (A.L. and J.G.) screened, retrieved, and selected data from each report. Original articles edited in the English language and performed on humans which evaluated non-[18F]FDG PET criteria for the evaluation of solid cancers were finally included in this review. Exclusion criteria were the assessment of [18F]FDG PET criteria and the assessment of semiquantitative parameters without the production of criteria. Review, meta-analysis, conference proceedings, case reports and case series were also excluded from the present analysis. The included studies were finally divided per cancer type classification, and they were also distinguished, if necessary, between interpretation and response assessment criteria in order to compare and evaluate mean differences between studies. From the included studies, the following information was extracted: characteristics of the studies (first author, year of publication), non-[18F]FDG PET criteria/score name, description of non-[18F]FDG PET criteria/score, type of cancer, radiopharmaceutical, sample size, and main findings.
3. Research Strategy Results
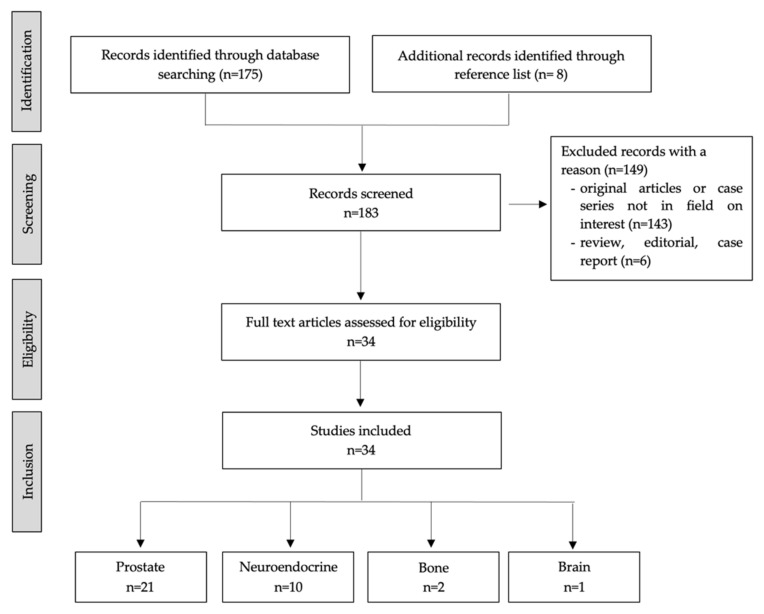
The Preferred Reporting Items for Systematic reviews and Meta-analyses (PRISMA) flowchart of research strategy and studies selection is summarized in Figure 1. The literature search revealed 183 articles; among them, a total of 149 were excluded after reviewing titles, abstracts, and full texts because the reported data were not within the field of interest of this review. Therefore, 34 studies were considered suitable for the analysis and subsequently divided following different cancer types: Prostate Cancer (PCa) in 21 studies, Neuroendocrine tumors (NET) in 10, primary bone cancer in 2, and brain tumors in 1.
Figure 1.
PRISMA flowchart of research strategy and studies selection.
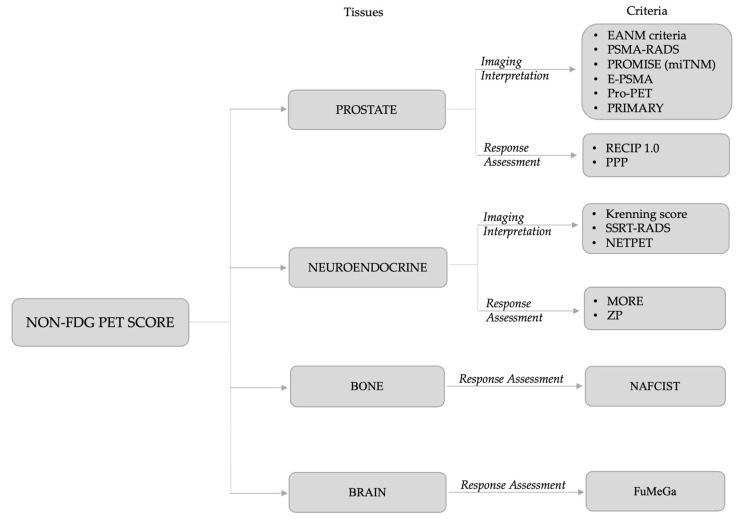
Finally, the research revealed 15 different non-[18F]FDG PET criteria: the European Association of Nuclear Medicine (EANM) criteria, the Prostate-Specific Membrane Antigen- Reporting and Data Systems (PSMA-RADS), the PROMISE (miTNM) criteria, the E-PSMA reporting system, the Pro-PET score, and the PRIMARY for PSMA PET imaging interpretation of PCa; PSMA PET Progression (PPP) and RECIP 1.0 as treatment response evaluation criteria for PCa. For NET, the PET-based Krenning score (KS), the somatostatin receptor (SSTR)-RADS and the NETPET grade were proposed for SSTR PET-based imaging interpretation and the selection of eligible patients for [177Lu]Lu-DOTA radioligand therapy (RLT), whereas MORE and ZP score were recently proposed for the assessment of response to RLT in NET patients. In primary bone cancer, the NAFCIST emerged as a response assessment criterion with [18F]sodium-fluoride ([18F]NaF) PET in patients affected by osteosarcoma or osteosarcoma-like tumor who underwent Radium223 dichloride (223RaCl2) therapy. Finally, the FuMeGa score was proposed to evaluate post-surgery response in high-grade glioma (HGG) patients with [18F]Fluorocholine.
The proposed criteria per cancer type are reported in Figure 2 and detailed in Table 1. Data about the studies included in the review and their main findings are summarized in Table 2.
Figure 2.
The fifteen non-[18F]FDG PET criteria retrieved from literature research and categorized following different tissues.
Table 1.
The non-[18F]FDG PET criteria main characteristics and description.
| Reference | Year | Radiotracer | Criteria | Description |
|---|---|---|---|---|
| Fanti et al. [8] | 2017 | PSMA-ligand | EANM criteria | All “anomalous” findings suggestive of recurrent PCa were noticed and categorized as “pathologic” unless another explanation could be hypothesized. |
| Rowe et al. [9] | 2018 | PSMA-ligand | PSMA-RADS | 5-point scale framework reflecting the level of confidence in interpreting PSMA-PET imaging in PCa. |
| Eiber et al. [10] | 2019 | PSMA-ligand | PROMISE (miTNM) | Standardized reporting framework based on miTNM system for PSMA-ligand PET/CT or PET/MRI imaging in PCa. |
| Ceci et al. [11] | 2021 | PSMA-ligand | E-PSMA | The E-PSMA standardized reporting guidelinesprovide consensus statements to develop a structuredreport for PSMA-PET in PCa. |
| Adnan et al. [12] | 2021 | PSMA/FDG | Pro-PET | An integrated dual tracer PET/CT (PSMA and FDG) image scoring system for mCRPCa patients referred to [177Lu]Lu-PSMA therapy. |
| Emmett et al. [13] | 2022 | PSMA-ligand | PRIMARY | A 5-level score for the diagnosis of clinically significant PCa. |
| Fanti et al. [14] | 2020 | PSMA-ligand | PSMA PET Progression (PPP) | PSMA-driven response evaluation criteria in patients with metastatic PCa. |
| Grafita et al. [15] | 2022 | PSMA-ligand | RECIP 1.0 | PSMA-driven response evaluation criteria in mCRPCa patients who underwent [177Lu]Lu-PSMA therapy. |
| Krenning et al. [16] * | 1999 | SSTR-ligand | Krenning | Visual interpretation criteria for evaluating NETs patients’ eligibility for [177Lu]Lu DOTA therapy. |
| Werner et al. [17] | 2018 | SSTR-ligand | SSTR-RADS | 5-point scale framework reflecting the level of confidence of interpreting SSTR-PET imaging in NET assessing the eligibility to [177Lu]Lu-DOTA therapy. |
| Chan et al. [18] | 2017 | SSTR/FDG | NETPET | An integrated dual tracer PET/CT (SSTR and FDG) image scoring system for NET patients. |
| Zwirtz et al. [19] | 2021 | SSTR-ligand | MORE; ZP | Response evaluation criteria in NETs patients who underwent [177Lu]Lu-DOTA therapy. |
| Kairemo et al. [20] | 2019 | [18F]NaF | NAFCIST | Response evaluation criteria for high-risk osteosarcoma patients who underwent [223Ra]Cl2 therapy. |
| García Vicente et al. [21] | 2020 | [18F]Fluorocholine | FuMeGa | Visual interpretation criteria for post-surgery evaluation of HGG patients |
* Adapted from [111In]Pentetreotide imaging to SSTR PET-imaging. Abbreviations: PSMA, prostate-specific membrane antigen; EANM, European Association of Nuclear Medicine; PCa, prostate cancer; RADS, Reporting and Data Systems; PET, positron emission tomography; mi, molecular imaging; CT, computed tomography; MRI, magnetic resonance imaging; FDG, fluorodeoxyglucose; mCRPCa, metastatic castration-resistant PCa; SSTR, somatostatin receptor; NET, neuroendocrine tumor; HGG, high-grade glioma.
Table 2.
Selected studies main characteristics and key point by cancer type.
| Reference | Radiotracer(s) | n pts | Key Point | |
|---|---|---|---|---|
| Prostate | Fanti et al. [8] | [68Ga]Ga-PSMA | 104 | EANM criteria showed a moderate interobserver agreement among multiple readers. |
| Werner et al. [22] | [18F]DCFPyL | 50 | PSMA-RADS showed excellent interobserver agreement for lymph nodes and for the overall scan impression (ICC 0.79 and 0.84, respectively). | |
| Chiu et al. [23] | [68Ga]Ga-PSMA-11 | 56 | PSMA-RADS ratings showed perfect interrater reliability to detect prostate bone metastasis. A SUVmax ratio (lesion-to-blood pool) > 2.2 presented a superior lesion detection rate and specificity when compared to PSMA-RADS. | |
| Letang et al. [24] | [68Ga]Ga-PSMA-11 | 53 | PSMA-RADS had a significantly higher AUROC than the initial reading for the assessment of bone metastasis. | |
| Yin et al. [25] | [18F]DCFPyL | 36 | PSMA-RADS-3A lesions are more likely than PSMA-RADS-3B lesions to represent sites of PCa. | |
| Kuten et al. [26] | [68Ga]Ga-PSMA-11[18F]PSMA-1007 | 15 | In intermediate and high-risk patients staged prior to radical prostatectomy, most PSMA-RADS-3B lesions are of no clinical relevance. | |
| Bhoil et al. [27] | [18F]PSMA-1007 | 203 | A structured reporting with PSMA-RADS grading helps in the proper classification of lesions and standardization of reports. | |
| Garg et al. [28] | [18F]DCFPyL | 28 | A PSA level ≥ 0.6–1.0 ng/mL can be used as a marker for a high index of suspicion for true positivity in PSMA-RADS-3A lesions. A Gleason score ≥ 7 showed a higher rate of PSMA-RADS-3A lesion positivity. | |
| Khatri et al. [29] | [18F]DCFPyL | 30 | The PSF reconstructions allowed the re-categorization of a small number of indeterminate PSMA-RADS-3A soft tissue lesions as more definitive PSMA-RADS-4 lesions. | |
| Mihatsch et al. [30] | [18F]PSMA-1007 | 18 | SUVmax was significantly higher in PSMA-RADS-5 lesions compared to all other categories. PSMA-RADS-3A lesions showed significantly lower SUVmax and SUVpeak compared to the entire PSMA-RADS-4 or -5 cohort. | |
| Gültekin et al. [31] | [68Ga]Ga-PSMA-I&T | 80 | The miN and miM categories showed almost perfect interobserver agreement (K = 0.93 and 0.94, respectively) as well as miM (K = 0.84). | |
| Wang et al. [32] | [68Ga]Ga-PSMA-11 | 187 | Preoperative miTNM correlated with postoperative Gleason score, surgical margin status and time to biochemical recurrence. | |
| Hoberück et al. [33] | [68Ga]Ga-PSMA-11; [18F]PSMA-1007 | 46 | In terms of miTNM staging, both [68Ga]Ga-PSMA-11 and [18F]PSMA-1007 appeared exchangeable, as no tracer outperformed the other. | |
| Koehler et al. [34] | [68Ga]Ga-PSMA-I&T | 297 | Additional late scans of the pelvis in [68Ga]Ga-PSMA-I&T PET/CT detected more lesions and an increasing contrast compared to early imaging, influencing the final miTNM-staging. | |
| Demirci et al. [35] | [68Ga]Ga-PSMA-11 | 133 | The miTNM provides a high level of concordance among readers, with the highest agreement level in miM and lowest agreement in miT. PSMA-RADS showed almost-perfect agreement between readers in terms of scoring (ICC analysis). However, if the results were grouped as benign (score of 1/2), indeterminate (3), and malignant (4/5), Fleiss’ κ analysis showed a moderate level of agreement. | |
| Toriihara et al. [36] | [68Ga]Ga-PSMA-11 | 104 | Comparing the 3 proposed interpretation criteria for PCa, intrareader agreement was moderate to almost perfect. The intercriteria agreement for each site was moderate to almost perfect. | |
| Adnan et al. [12] | [68Ga]Ga-PSMA-11 + [18F]FDG | 47 | The Pro-PET score significantly correlated with symptomatic, biochemical, metabolic, and anatomical responses, as well as with PFS (p = 0.03) and OS (p = 0.027). | |
| Emmett et al. [13] | [68Ga]Ga-PSMA-11 | 291 | The estimated AUC of the PRIMARY score was 0.85 (95%CI: 0.81–0.89) and exceeded that of PI-RADS 0.76 (95%CI: 0.71–0.81) (p = 0.003). Sensitivity, specificity, PPV and NPV for PRIMARY score 3 to 5 (high risk patterns) vs. PRIMARY score 1,2 (low risk patterns) was 88%, 64%, 76% and 81%, compared to 83%, 53%, 69% and 72% for PI-RADS 3–5 vs. 2. | |
| Michalski et al. [37] | [68Ga]Ga-PSMA-11; [18F]PSMA-1007 | 46 | Modified PPP criteria showed high reproducibility. A significant correlation was shown between progressive and non-progressive disease defined by PPP and OS. | |
| Gafita et al. [15] | PSMA-ligand | 124 | Patients with RECIP-PD had shorter OS compared to patients with RECIP-SD/PR | |
| Gafita et al. [38] | PSMA-ligand | 124 | PD patients according to RECIP 1.0 had a higher risk of death compared to non-PD, also in comparison with other response criteria (PPP, RECIST 1.1, aPERCIST) | |
| Neurendocrine | Hope et al. [39] | [68Ga]Ga-DOTA-TATE | 150 | The detection rate of SSTR-positive disease (KS 2–4) resulted of 23%, 38%, and 72% with [111In]Pentetreotide planar scintigraphy, SPECT and PET, respectively. Lesion size did not affect SSTR PET-based KS. |
| Purandare et al. [40] | [68Ga]Ga-DOTA-NOC | 119 | [68Ga]Ga-DOTA-NOC showed a high sensitivity for tumor detection (92.4%) and can help differentiate between typical and atypical carcinoid variants. | |
| Menon et al. [41] | [68Ga]Ga-DOTA-TATE | 32 | No significant change in KS was seen between the delayed scan compared to the standard one (1–1.5 h). | |
| Werner et al. [42] | [68Ga]Ga-DOTA-TOC | 51 | The interobserver agreement for overall scan impression based on SSTR-RADS was excellent (ICC, 0.88), as well as for lymph node and liver lesions (ICC, 0.91 and 0.77, respectively). | |
| Chan et al. [18] | [68Ga]Ga-DOTATATE + [18F]FDG | 62 | NETPET grade showed statistically significant correlation with OS at univariate analysis. NETPET grade was significantly associated with histological grade. | |
| Chan et al. [43] | [68Ga]Ga-DOTATATE + [18F]FDG | 319 | NETPET score significantly correlated with OS and TTP. NETPET showed both high inter-rater (kappa = 0.8) and intra-rater (kappa = 0.9) reliability. | |
| Hayes et al. [44] | [68Ga]Ga-DOTATATE + [18F]FDG | 87 | The dual-tracer PET classification was an independent predictor of OS (multivariate p = 0.016) and predicted PFS (univariate p = 0.030). | |
| Karfis et al. [45] | [68Ga]Ga-DOTATATE + [18F]FDG | 85 | Combined [68Ga]Ga-DOTATATE and [18F]FDG PET imaging classification reached high prognostic value in terms of PFS in GEPNET patients. | |
| Chan et al. [46] | [68Ga]Ga-DOTATATE + [18F]FDG | 38 | The NETPET score and histology were significantly correlated with OS (p = 0.003, p = 0.01) | |
| Zwirtz et al. [19] | [68Ga]Ga-DOTA-TATE/TOC | 34 | Only patients showing progressive disease after two PRRT cycles according to MORE criteria had a worse prognosis, while baseline ZP and ZPnormalized performed best in predicting lesion progression after three cycles of PRRT. | |
| Bone | Kairemo et al. [20] | [18F]NaF | 18 | NAFCIST correlates with changes in bone alkaline phosphatase levels, cumulative dose of [223Ra]Cl2 and OS. |
| Kairemo et al. [47] | [18F]NaF | 18 | Due to mixed response, neither PERCIST nor RECIST were able to predict response in osteosarcoma patients treated with [223Ra]Cl2. Radiomics can help in the assessment of intra-tumoral and inter-tumoral heterogeneity in the response to bone-forming osteosarcoma to [223Ra]Cl2 therapy. | |
| Brain | García Vicente et al. [48] | [18F]Fluorocholine | 47 | Significant differences were found for PFS and OS between incomplete versus complete metabolic resections assessed by the FuMeGa score |
Abbreviations: AUROC, area under the receiver operating characteristic curve; DCFPyL, 2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid; GEP, gastroenteropancreatic; ICC, intraclass correlation coefficient; KS, Krenning score; mi, molecular imaging; NAFCIST, [18F]NaF PET response Criteria in Solid Tumors; NET, neuroendocrine tumor; NPV, negative predictive value; OS, overall survival; PCa, prostate cancer; PI, prostate imaging; PD, progressive disease; aPERCIST, adapted PET response criteria in solid tumors; PET/CT, positron emission tomography/computed tomography; PFS, progression free survival; PPP, PSMA PET progression; PPV, positive predictive value; PR, partial response; PRRT, peptide-receptor radionuclide therapy; PSA, prostate-specific antigen; PSF, point-spread function; PSMA, prostate-specific membrane antigen; RADS, Reporting and Data Systems; RECIST, Response Evaluation Criteria in Solid Tumors; SD, stable disease; SPECT, single photon emission tomography; SSTR, somatostatin receptor; SUV, standardized uptake value; TTP, time-to-progression.
3.1. Prostate Cancer
3.1.1. Interpretation Criteria: EANM Criteria, PSMA-RADS, PROMISE (miTNM), E-PSMA, Pro-PET and PRIMARY Score
On behalf of the EANM, Fanti et al. proposed the first standardized imaging interpretation system for [68Ga]Ga-PSMA PET. The criteria are structured as follows: first, “anomalous” findings, defined as suggestive radiotracer uptake above physiologic background, are recorded. Thus, all these sites are classified as “pathologic” for PCa, unless another explanation is possible. Third, the anatomic localization is considered (up to 5 lesions). However, in the same document, the authors observed a moderate interobserver agreement among multiple readers. Namely, inter-reader agreement for the presence of anomalous findings was 0.47 but became substantial when readers judged the anomalous findings as suggestive for a pathologic, uncertain, or non-pathologic image (K’s alpha: 0.64) [8].
The second proposed interpretation criterion, termed PSMA-RADS Version 1.0, was introduced by Rowe et al. in 2018 [9]. The PSMA-RADS can be applied on both individual target lesions (maximum of 5 per-scan) or on the overall impression of the imaging study. As shown in Table 3, PSMA-RADS is based on a 5-point scale that reflects the confidence of the interpreting imaging specialist that a given lesion represents a site of PCa, scoring from PSMA-RADS-1 (=definitively benign) to PSMA-RADS-5 (=high degree of certainty that PCa is present).
Table 3.
Summary of PSMA-RADS Version 1.0 for the interpretation of PSMA-PET imaging.
| PSMA-RADS 1.0 | ||
|---|---|---|
| Category | Findings | Action |
| PSMA-RADS-1 (benign) | ||
| PSMA-RADS-1A | Benign lesion characterized by biopsy or pathognomonic finding on anatomic imaging and without abnormal uptake | |
| PSMA-RADS-1B | Benign lesion characterized by biopsy or pathognomonic finding on anatomic imaging and with focal radiotracer uptake | |
| PSMA-RADS-2 (likely benign) |
Equivocal uptake (focal, but low level such as blood pool) in soft-tissue or in a bone site atypical of PCa involvement | |
| PSMA-RADS-3 (equivocal) | Consider further work-up | |
| PSMA-RADS-3A | Equivocal uptake in soft-tissue site typical of PCa involvement. | Lesion targetable: biopsy is suggested. Alternatively, follow-up imaging (either anatomic or PSMA-targeted PET/CT) after 3–6 months is recommended. |
| PSMA-RADS-3B | Equivocal uptake in bone lesion not definitive but also not atypical of PCa on anatomic imaging. | Comparison to other imaging modalities (bone scan, [18F]NaF PET, or tumor-protocol MRI images) or bone biopsy. Alternatively, follow-up imaging (either anatomic or PSMA-targeted PET/CT) after 3–6 months is recommended. |
| PSMA-RADS-3C | Lesions that would be atypical for PCa but have high levels of uptake. The likelihood of non-prostatic malignancy or another benign tumor is high. | Biopsy to histologically confirm the diagnosis is often preferred. Alternatively, organ-specific follow-up imaging may be done (e.g., liver-protocol MRI to evaluate possible primary hepatocellular carcinoma). |
| PSMA-RADS-3D | Lesion suggestive of malignancy on anatomic imaging but lacking uptake. | Consider non-prostatic malignancy, neuroendocrine PCa, and an uncommon case of prostate adenocarcinoma that fails to express PSMA. Biopsy to histologically confirm the diagnosis is often preferred; alternatively, organ-specific follow-up imaging may be done. |
| PSMA-RADS-4 (Pca highly likely) |
Intense uptake in sites typical of PCa but lacking definitive findings on conventional imaging. | No biopsy confirmation will be needed, although tissue for genomic analysis (or other purposes) may be useful. |
| PSMA-RADS-5 (Pca almost certainly present) |
Intense uptake in sites typical of PCand having corresponding findings on conventional imaging. | No biopsy confirmation will be needed, although tissue for genomic analysis (or other purposes) may be useful. |
Adapted from Rowe SP et al. [9]. Abbreviations: PSMA, Prostate-specific membrane antigen; RADS, Reporting and Data Systems; PCa, prostate cancer; NaF, sodium fluoride; MRI, magnetic resonance imaging; PET/CT, positron emission tomography/computed tomography.
The interobserver reliability of PSMA-RADS was proved in a prospective study by Werner and colleagues, both on a per-lesion level and on an organ-based analysis by four readers with different levels of experience, evaluating 50 [18F]2-(3-{1-carboxy-5-[(6-18F-fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (DCFPyL) PET/CT scans. Notably, an excellent interobserver agreement was reported for lymph node assessment (ICC 0.79 95% CI, 0.66–0.89) and when considering the overall PSMA-RADS (ICC = 0.84 95% CI, 0.77–0.90) [22]. Similarly, in a retrospective single-center study of 56 PCa patients, patient- and lesion-based PSMA-RADS ratings showed great interrater reliability for bone metastasis (patient-based Cohen’s K = 0.88; lesion-based Cohen’s K = 0.82) [23]. More recently Letang et al. reported that PSMA-RADS had a significantly higher area under the curve (AUC) at receiver operating characteristic (ROC) analysis than the initial reading in clinical practice when assessing metastatic patients [24]. The PSMA-RADS-3 category (the most discussed in the literature) reflects the uncertainty level of the lesion to be compatible with PCa. In these regards, Yin et al. performed a longitudinal follow-up of 46 lesions considered indeterminate for PCa on [18F]DCFPyL PET/CT scans. During the follow-up, 58.7% of these lesions resulted true-positive for PCa. Moreover, the authors showed that PSMA-RADS-3A lesions were more likely to represent PCa than PSMA-RADS-3B ones [25]. Consistent with this evidence, another study reported that debatable lesions proved to have no clinical relevance in 84.6% of cases, and only 11% of equivocal PSMA-RADS-3B bone lesions were true positive [26]. In addition, Bhoil et al. reported that in the skeletal system only a minority of equivocal lesions findings will have characteristic changes in PCa involvement on follow-up imaging [27]. In the context of indeterminate lesions, an elevated prostate-specific antigen (PSA) value was shown in 81.9% of patients with true positive PSMA-RADS-3A lesions [28]. In this setting, the point-spread function (PSF) reconstruction, a modern reconstruction model with higher spatial resolution, was able to solve the diagnostic uncertainty of PSMA-RADS-3A lesions in 7.6% of cases, re-categorized as PSMA-RADS-4 [29]. PSMA-RADS categories were also correlated to maximum standardized uptake value (SUVmax) by Mihatsch and colleagues, who reported a significantly higher SUVmax in PSMA-RADS-5 lesions. Furthermore, the challenging PSMA-RADS-3A lesions showed significantly lower SUVmax and SUVpeak compared to the PSMA-RADS-4 or -5 [30].
In 2019, Eiber et al. published the Prostate Cancer Molecular Imaging Standardized Evaluation, so-called PROMISE criteria. This molecular imaging TNM system (miTNM, version 1.0) consists of a standardized reporting framework for PSMA-ligand PET/CT or PET/magnetic resonance imaging (MRI) and provides a standardized system for the presence, location, and extent of local PCa and its pelvic and extrapelvic spread (Table 4) [10].
Table 4.
miTNM version 1.0 system for Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE) using PSMA-PET tracer.
| miTNM | |
|---|---|
| Class | Description |
| Local tumor (T) | |
| miT0 | No local tumor |
| miT2 | Organ confined tumor (unifocal or multifocal) |
| miT3 | Non-organ confined tumor |
| miT3a | Extracapsular extension |
| miT3b | Tumor invading seminal vesicles |
| miT4 | Tumor invading adjacent structures other than the seminal vesicles, such as external sphincter, rectum, bladder, elevator muscles, or pelvic wall |
| miTr | Presence of local recurrence after radical prostatectomy |
| Regional nodes (N) | |
| miN0 | No positive regional lymph nodes |
| miN1a | Single lymph node region with lymph node metastases |
| miN1b | Multiple lymph node regions with lymph node metastases |
| Distant metastases (M) | |
| miM0 | No distant metastasis |
| miM1 | Distant metastasis |
| miM1a | Extrapelvic lymph nodes |
| miM1b | Bones |
| miM1c | Other sites |
Adapted from Eiber M et al. [10]. Abbreviation: mi, molecular imaging.
As suggested by the authors, the diagnosis of a PCa lesion should be assessed considering PSMA-uptake, location, and CT or MRI findings, as well as the specific clinical scenario. For this purpose, a standardized method for the PSMA expression level of tumor lesions was introduced (Table 5). Notably, the molecular imaging PSMA (miPSMA) score described the PSMA expression in relation to the mean uptake in the blood pool, liver (or spleen as reference organ instead of liver, for PSMA ligands with liver-dominant excretion e.g., [18F]PSMA-1007), and parotid gland. Scores 2 and 3 are considered typical for PCa lesions and favorable for PSMA-directed RLT [10].
Table 5.
miPSMA score describing the PSMA-expression level of tumor lesions.
| miPSMA Score | |||
|---|---|---|---|
| Category | Score | PSMA Expression | Uptake |
| Negative | Score 0 | No | Below blood pool |
| Score 1 | Low | Equal to or above blood pool and lower than liver (or spleen) | |
| Positive | Score 2 | Intermediate | Equal to or above liver (or spleen) and lower than parotid gland |
| Score 3 | High | Equal to or above parotid gland | |
Adapted from Eiber M et al. [10]. Abbreviation: mi, molecular imaging.
In this setting, a prospective study determined the intra- and inter-observer agreement of [68Ga]Ga-PSMA-I&T PET/CT in 80 patients according to miTNM, reporting great interobserver agreement in the miN (Cohen’s K = 0.74) and miM (Cohen’s K = 0.84) categories. Differently, a lower interobserver agreement in the miT (Cohen’s K = 0.52) category was observed, especially in patients who had bladder or surrounding soft-tissue invasion [31]. Recently, Wang et al. evaluated the predictive role of preoperative miTNM from [68Ga]Ga-PSMA-11 PET in 187 patients with primary PCa who underwent radical prostatectomy. They showed that miTNM correlated with postoperative Gleason score, surgical margin status and time to biochemical recurrence [32]. Comparing [68Ga]Ga-PSMA-11 to [18F]PSMA-1007 PET in terms of miTNM staging, the miM staging was shown to be more concordant than the miT and miN; however, both tracers appeared widely exchangeable [33]. Koehler et al. evaluated the influence of a late scan of the pelvis at [68 Ga]Ga-PSMA-I&T PET/CT, reporting a change in 19.5% of the cases in miTNM in comparison with the early scan, and a change in staging in a not negligible number of subjects [34]. The inter- and intra-observer agreements were also evaluated according to the miTNM and PSMA-RADS in the study by Demirici et al. A substantial agreement was reported for miTNM system, while the PSMA-RADS showed almost-perfect agreement among readers. However, in the case of benign lesions authors observed more discordant results for PSMA-RADS than miTNM [35]. Finally, the 3 PSMA PET interpretation criteria (EANM, PSMA-RADS and PROMISE) demonstrated substantial to almost perfect interreader, intrareader, and intercriteria agreement in most situations as described by the group of Stanford [36].
In 2019, a consensus statement for standardized reporting of the PSMA-ligand PET was proposed by a panel of worldwide experts. The E-PSMA provides a structured report including what needs to be included in a report, considering different clinical settings, and including elements from the PROMISE and RADS systems [11].
More recently a dual-tracers scoring system combining [68Ga]Ga-PSMA and FDG was proposed for patients referred to [177Lu]Lu-PSMA RLT, termed Pro-PET score (Table 6). The scoring scheme, a 5-point categorical scale, is based on the single lesion that was the most FDG avid relative to its [68Ga]Ga-PSMA uptake (most discordant lesion). The concept proposal study retrospectively recruited 47 patients and showed that Pro-PET significantly correlated with the symptomatic response (p = 0.05), biochemical tumor marker response (p = 0.05), metabolic response (p = 0.001), anatomical response (p = 0.012), PFS (p = 0.03) and OS (p = 0.027). The trend observed showed unfavorable outcome when the disease shifted more towards the high grade of the Pro-PET scoring system [12].
Table 6.
The 5-point of Pro-PET score based on FDG avid relative to PSMA uptake of the reference lesion.
| Pro-PET Score | |||
|---|---|---|---|
| Score | PSMA-PET | FDG-PET | Description of Reference Lesion * (n° of Lesions) |
| Pro-PET 0 | − | − | NA |
| Pro-PET 1 | + | − | NA |
| Pro-PET 2a | + | + | FDG uptake less than PSMA (≤4 lesions) |
| Pro-PET 2b | + | + | FDG uptake less than PSMA (≤5 lesions) |
| Pro-PET 3a | + | + | FDG uptake equivalent to PSMA (≤4 lesions) |
| Pro-PET 3b | + | + | FDG uptake equivalent to PSMA (≥5 lesions) |
| Pro-PET 4a | + | + | FDG uptake more than PSMA (≤4 lesions) |
| Pro-PET 4b | + | + | FDG uptake more than PSMA (≥5 lesions) |
| Pro-PET 5 | − | + | NA |
* lesion most FDG avid relative to PSMA uptake. Adapted from Adnan et al. [12] Abbreviations: FDG, fluorodeoxyglucose; NA, not available; PSMA, prostate-specific membrane antigen.
In November 2022, a new score for primary PCa diagnosis based on intraprostatic PSMA-uptake pattern was published by Emmett and his group. The PRIMARY combined different pattern information and SUVmax assigned to each patient (Table 7). The specific patterns were described as follows: Pattern A, defined as diffuse transition zone (TZ) activity if centrally placed within the prostate, with no PSMA activity extending to the edge of the prostate margin; Pattern B, symmetrical central zone (CZ) activity with no PSMA activity extending to the prostate margin; Pattern C, focal TZ activity defined visually as more than twice background TZ activity; Pattern D, focal peripheral zone (PZ) activity. Table 7 shows the 5-point PRIMARY score classification [13].
Table 7.
The schematic description of the PRIMARY score.
| PRIMARY Score | |
|---|---|
| Score | Description |
| Score 1 | No pattern, low grade activity. |
| Score 2 | Diffuse TZ or symmetrical CZ activity without focal uptake (including diffuse TZ activity with irregular focal uptake not above background TZ activity). |
| Score 3 | Focal TZ activity (must be visually above twice background TZ activity). |
| Score 4 | Focal PZ activity |
| Score 5 | Any pattern with SUVmax ≥ 12 |
Abbreviations: TZ, transition zone; CZ, central zone; PZ, peripheral zone; SUV, standardized uptake value.
The estimated AUC of the five-level PRIMARY score was 0.85 (95%CI: 0.81–0.89) and exceeded that of PI-RADS 0.76 (95%CI: 0.71–0.81) (p = 0.003). Furthermore, the authors showed a substantial inter-rater reproducibility for differentiating PRIMARY score low-risk from high-risk patterns between independent readers [13].
3.1.2. Response Assessment: PPP and RECIP 1.0 Criteria
To date, two specific criteria for treatment response assessment in PCa patients have been developed. First, Fanti et al. proposed the PPP criteria [14] that defined progressive disease (PD) according to three different classes based on the appearance of new PSMA-positive lesions, clinical or laboratory data and, eventually, biopsy or imaging confirmation (Table 8).
Table 8.
PSMA PET Progression (PPP) criteria for treatment response evaluation in patients with metastatic prostate cancer.
| PPP Criteria | ||
|---|---|---|
| Category | Progression Criterion | Description and Recommendations |
| A | ≥2 new PSMA-positive lesions | ≥2 new PSMA-positive distant lesions |
| B | 1 new PSMA-positive lesion | 1 new PSMA-positive lesion plus clinical or laboratory data. Recommended confirmation by biopsy or correlative imaging within 3 months from PSMA |
| C | No new lesions but size increase | Increase by ≥30% in size or uptake plus consistent clinical or laboratory data. Confirmation by biopsy or correlative imaging within 3 months of PSMA PET |
Adapted from Fanti et al. [14]. Abbreviations: PPP, PSMA PET Progression; PSMA, prostate-specific membrane antigen; PET, positron emission tomography.
Michalski et al. evaluated the feasibility of PPP criteria in patients undergoing [177Lu]Lu-PSMA therapy and their prognostic implications. The authors reported that inter-observer agreement was substantial, and that progression of disease evaluated by score was a significant prognostic marker for overall survival (OS) [37].
Considering the same scenario, Gafita et al. proposed a PSMA-PET response evaluation criteria for metastatic castration-resistant PCa (mCRPCa) patients treated with [177Lu]Lu-PSMA therapy, termed RECIP. Namely, the appearance of new lesions (=any new focal uptake of PSMA-ligand higher than surrounding background, and each tumor SUVmax > mean SUVmean) and changes in PSMA-VOL (the total positive PSMA volume) were combined to develop RECIP 1.0, which included the classifications of response to therapy presented in Table 9. The authors reported that RECIP-PD had a prognostic impact and the combination of PSA values and RECIP 1.0 criteria may result in a more reliable prognostic evaluation [15].
Table 9.
Standardized framework for response evaluation criteria in PSMA PET/CT (RECIP) in mCRPCa patients.
| RECIP 1.0 | |
|---|---|
| Progression Criterion | Description |
| RECIP-CR | Absence of any PSMA-ligand uptake on PET after 12 weeks from RLT |
| RECIP-PR | Decline ≥ 30% in PSMA-VOL and no appearance of new lesions |
| RECIP-PD | Increase ≥ 20% in PSMA-VOL and the appearance of new lesions |
| RECIP-SD | Any condition but RECIP-PR or RECIP-PD |
Adapted from Grafita et al. [15]. Abbreviations: CR, complete response; PR, partial response; PD, progression disease; SD, stable disease; PSMA, prostate-specific membrane antigen, PET, positron emission tomography; RLT, radioligand therapy; PSMA-VOL, total positive PSMA volume.
More recently, the same group compared the available response criteria for PCa, both specific (aPCWG3, PPP and RECIP 1.0) and non-specific (RECIST 1.1, aPERCIST), in the response evaluation of patients treated with [177Lu]Lu-PSMA. As a result, a better accuracy and inter-reader agreement were obtained with PSMA-specific criteria (PPP and RECIP 1.0). Moreover, a significant lower percentage of patients was defined as having PD according to RECIP 1.0 and these subjects had a higher risk of death in comparison with other response criteria [38].
3.2. Neuroendocrine Tumors
3.2.1. Interpretation Criteria: PET-Based Krenning Score, SSTR-RADS, and NETPET
The most used method for determining SSTR-ligand uptake on imaging and the eligibility of NET patients for RLT is the Krenning Score (KS), based on the lesion with the highest uptake at [111In]Pentetreotide (OctreoScan) scintigraphy or SSTR-PET images (Table 10) [16]. However, the PET-based KS showed a higher sensitivity compared to [111In]Pentetreotide one, applied both to planar and single emission tomography (SPECT) scintigraphy [49,50,51].
Table 10.
PET-based Krenning score for eligibility of NETs patients to RLT.
| PET-Based Krenning Score | ||
|---|---|---|
| Category | Score | Interpretation |
| Negative | Score 0 | no uptake |
| Score 1 | very low uptake | |
| Positive | Score 2 | uptake less than or equal to that of the liver |
| Score 3 | uptake greater than the liver | |
| Score 4 | uptake greater than that of the spleen | |
Adapted from Krenning et al. [16].
Hope et al. performed a comparison of [68Ga]Ga-DOTA-TATE-based vs. [111In]Pentetreotide-based KS [39], reporting a detection rate of SSTR-positive disease (KS 2–4) of 23%, 38%, and 72% with [111In]Pentetreotide planar scintigraphy, SPECT and PET, respectively. Moreover, an influence of the size of the lesion on the KS for the three modalities and a correlation between SUVmax and KS were reported. These results imply that patients with lesions <2 cm would not have qualified for RLT based on [111In]Pentetreotide but appear as candidates on SSTR PET. The predictive role of KS from [68Ga]Ga-DOTA-NOC PET/CT was also demonstrated in lung carcinoids, with high sensitivity [40]. Finally, Menon and colleagues explored a dual-time-point [68Ga]Ga-DOTA-TATE PET/CT imaging protocol, but no significant changes in KS were reported [41]. The KS was created starting from [111In]Pentetreotide scintigraphy; therefore, reliable standards and criteria for SSTR PET are still lacking since many pitfalls can influence PET scans [52,53,54,55,56].
In this scenario, Werner et al. proposed a structured reporting system based on a 5-point scale for SSTR PET imaging, named SSTR-RADS Version 1.0, which might serve as a standardized assessment for both diagnosis and treatment planning in NET. The uptake levels for SSTR-RADS were established by a three-point qualitative assessment as shown in Table 11. For the clinical report, they suggested an overall interpretation of the SSTR PET scan with a minimum of clinical and imaging acquisition information and the number of lesions. Moreover, RLT may be considered in the case of an overall SSTR-RADS score of 4 or 5 (Table 12). The interobserver reliability of SSTR-RADS in [68Ga]Ga-DOTA-TOC PET/CT was evaluated by the same group, observing a high agreement in establishing the level of uptake and in the appropriateness of choosing RLT for both inexperienced and experienced readers [17].
Table 11.
A three-point visual score for defining the uptake level of SSTR-avid lesion.
| SSTR-Expression | |
|---|---|
| Level 1 | ≤bloodpool |
| Level 2 | Uptake > bloodpool, but ≤physiological liver uptake |
| Level 3 | uptake > physiological liver uptake |
Table 12.
Summary of SSTR-RADS version 1.0 system for the interpretation of SSTR-PET imaging and RLT eligibility.
| SSTR-RADS | ||||
|---|---|---|---|---|
| Category | Findings | Uptake Level |
Action | RLT |
| SSTR-RADS 1 (benign) |
Benign lesion confirmed by biopsy or with a pathognomonic appearance on anatomic imaging | |||
| SSTR-RADS 1A | Benign lesion, characterized by biopsy or by anatomic imaging and without any abnormal uptake | 1 | Not to be considered | |
| SSTR-RADS 1B | Benign lesion, characterized by biopsy or by anatomic imaging but with increased (focal) uptake | 2–3 | Not to be considered | |
| SSTR-RADS 2 (likely benign) |
Soft-tissue site atypical of metastatic NET or equivocal uptake in bone lesion atypical for NET | 1 | Not to be considered | |
| SSTR-RADS 3 | Further work-up might be required | |||
| SSTR-RADS 3A | Suggestive but not definitive for NET. Equivocal uptake in soft-tissue sites typical for NET metastases (e.g., regional lymph nodes). |
1–2 | Biopsy or initial fu imaging (SSTR-PET or whole-body MRI after 3 months), also depending on Ki-67/Grading. | Not to be considered |
| SSTR-RADS 3B | Suggestive but not definitive for NET. Uptake in bone lesions not atypical for NET. |
1–2 | Initial fu imaging (SSTR-PET or whole-body MRI after 3 months) might confirm diagnosis, also depending on Ki-67/Grading. | Single lesions: locoregional procedure. Increased number of lesions: RLT |
| SSTR-RADS 3C | Intense uptake in a site highly atypical for NET. Suggestive of an SSTR-expressing, non-NET benign tumor or malignant process. | 3 | Tissue confirmation of tumor histology should be considered. | Not to be considered |
| SSTR-RADS 3D | High likelihood of malignant NET lesion, but negative on an SSTR-PET scan (de-differentiated NET or another type of malignancy). | n/a | [18F]FDG PET should be considered. Tissue confirmation of tumor histology should be considered. |
Not to be considered |
| SSTR-RADS 4 NET highly likely |
Intense uptake in common site typical for NET lesion, but without confirmatory findings on anatomic imaging. | 3 | Further confirmation by biopsy might be not necessary. | To be considered |
| SSTR-RADS 5 NET almost certainly present |
Intense uptake in site typical for NET with corresponding findings on conventional imaging. | 3 | Further confirmation by biopsy might be not necessary. | Definitely to be considered. |
Adapted from Werner et al. [17] Abbreviations: SSTR, somatostatin receptors; RADS, Reporting and Data Systems; RLT, radioligand therapy; NET, neuroendocrine tumor; fu, follow-up; PET, positron emission tomography; MRI, magnetic resonance imaging; FDG, fluorodeoxyglucose.
In 2017, Chan et al. proposed a novel dual tracers SSTR/FDG PET grading scheme, the NETPET grade, a 5-point visual scale based on the characteristics of the reference lesions, grading from P1 (=purely SSTR-avid disease without FDG uptake in any lesions), to P5 (=significant FDG-positive/SSTR-negative disease) [18]. The score is presented in Table 13.
Table 13.
The 5-point of NETPET grade based on FDG avid relative to SSTR uptake of the reference lesion.
| NETPET Grade | ||||
|---|---|---|---|---|
| Grade | SSTR-PET | FDG-PET | Description of Reference Lesion * (n° of Lesions) | Secondary Characteristics |
| P0 | − | − | NA | |
| P1 | + | − | NA | |
| P2a | + | + | FDG uptake less than SSRT (1–2 lesions) | |
| P2b | + | + | FDG uptake less than SSRT (≥3 lesions) | |
| P3a | + | + | FDG uptake equivalent to SSRT (1–2 lesions) | |
| P3b | + | + | FDG uptake equivalent to SSRT (≥3 lesions) | |
| P4a | + | + | FDG uptake more than SSRT (1–2 lesions) | |
| P4b | + | + | FDG uptake more than SSRT (≥3 lesions) | |
| + | + | FDG+/SSRT- (1 lesion) | One additional lesion FDG > SSRT | |
| P5 | + | + | FDG+/SSRT- (1 lesion) | ≥2 additional lesions FDG > >SSRT |
| + | + | FDG+/SSRT- (≥2 lesions) | ||
| − | + | NA | ||
* lesion most FDG avid relative to SSRT uptake. Adapted from Chan et al. [18] Abbreviations: SSRT, somatostatin receptor; FDG, fluorodeoxyglucose; NA, not available.
The authors found a statistically significant correlation between NETPET grade and OS at the univariate analysis. Furthermore, the score correlated with WHO 2010 histological grade (p < 0.00001) [18]. Some years later, the same group of authors validated the grading system in a bigger cohort of 319 metastatic/unresectable gastroenteropancreatic (GEP) NET patients, confirming its prognostic value in terms of OS and time-to-progression as well as with the histological grade [43]. The impact of NETPET was subsequently evaluated by other studies, and its potential use as a prognostic marker was confirmed [44,45] even in patients with bronchial neuroendocrine neoplasms (NENs) [46].
3.2.2. Response Assessment: ZP and MORE
A standardized and reliable response assessment system after RLT is an unmet clinical need, as both morphological and functional imaging have shown limitations [57,58]. Recently, Zwirtz et al. compared the response evaluation with respect to OS in patients treated with at least two cycles of RLT introducing a new metabolic criterion, based on modified EORTC, so-called MORE criteria (Table 14). In addition, to identify other possible predictors of response, they generated two new combined parameters named ZP and ZPnormalized, using baseline CT and SSTR-PET data and summarized as follow [19]:
| P (Target) = SUVmean (Target) × Hounsfield Unit (HU) (Target) |
| ZPnormalized (Target) = normalized SUVmean (Target) × HU (Target) |
The concept proposal study, including 34 GEPNET patients, demonstrated that baseline ZP and ZPnormalized with overlapping sensitivity and specificity were the only predictive parameters of lesion progression after three RLT cycles. Moreover, patients who presented a progressive disease after the second cycle of RLT according to MORE criteria showed a significantly shorter OS [19,59].
Table 14.
Response assessment criteria for NET patients underwent RLT.
| MORE | ZP | ||
|---|---|---|---|
| Category | Description | Description | |
| Non PD | CR | Complete uptake disappearance in all lesions | No lesion (CT or PET) |
| PR | ≥25% reduction in the sum of SUVmax after more than one RLT cycle |
≥25% reduction in the product of SUVmean and HU | |
| SD | Does not meet other criteria | Does not meet other criteria | |
| PD | PD | ≥25% increase in the sum of SUVmax or at least one new lesion | ≥25% increase in the product of SUVmean and HU |
Adapted from Zwirtz et al. [19]. Abbreviations: PD, progressive disease; CR, complete response; CT, computed tomography; PET, positron emission tomography; PR, partial response; SUV, standardized uptake value; RLT, radioligand therapy; HU, Hounsfield Unit; SD, stable disease.
3.3. Bone Primary Cancer: [18F]NaF PET Response Criteria in Solid Tumors (NAFCIST)
Kairemo et al. proposed an [18F]NaF PET imaging criterion for the response assessment of osteosarcoma in a Radium223 dichloride ([223Ra]Cl2) phase I clinical trial, termed “Na18F PET response Criteria in Solid Tumors” (NAFCIST) [20]. NAFCIST is based on the measurement of the mean SUVpeak in 1 cm3. The single most active bone avid lesion on each scan and the summed activity of up to the five most active ones (no more than two per organ) were considered for the category’s determination (Table 15). A significant correlation between the changes in NAFCIST and in alkaline phosphatase and bone alkaline phosphatase was shown, as well as a negative correlation between the cumulative dose of [223Ra]Cl2 and changes in NAFCIST, thus demonstrating that the more [223Ra]Cl2 administered, the more NAFCIST value decreased. Moreover, NAFCIST correlated with OS. Interestingly, in the same sample, PERCIST applied to [18F]FDG PET/CT did not show any significant correlations with outcomes [20]. In a subsequent work, the same authors applied radiomics to [18F]NaF PET imaging. A decrease in 18F- concentration in metastatic areas was associated with an increase in intra-metastatic disorder after [223Ra]Cl2 treatment. Even with a small sample, these findings suggested that the more cycles of treatment were administered, the more radiotracer concentration and entropy decreased [47].
Table 15.
[18F]NaF response criteria in primary bone tumors (NAFCIST).
| NAFCIST | |
|---|---|
| Category | Description |
| Complete metabolic response | Normalization of all lesions (target and non-target) to SUV less than the mean skeletal SUV and equal to the normal surrounding tissue SUV; verification with a follow-up study in 1 month if anatomical criteria indicate disease progression. |
| Partial metabolic response | >30% decrease in SUVpeak *; verification with follow-up study if anatomical criteria indicate disease progression. |
| Stable metabolic disease | Does not meet other criteria. |
| Progressive metabolic disease | >30% increase in SUVpeak *; >75% increase in total [18F]NaF burden of the five most active lesions; visible increase in the extent of [18F]NaF uptake; new lesions; verification with follow-up study if anatomical criteria indicate complete or partial response. |
* Primary outcome determination is measured on the single most active lesion on each scan (not necessarily the same lesion). Secondary outcome determination is the summed activity of up to the five most intense lesions (no more than two lesions per organ). Adapted from Kairemo et al. [20]. Abbreviations: NAFCIST, Na18F PET response Criteria in Solid Tumors; Na18F, sodium fluoride-18; SUV, standardized uptake value.
3.4. Glioma: Functional and Metabolic Glioma Analysis (FuMeGA)
García Vicente et al. presented the “Functional and Metabolic Glioma Analysis” (FuMeGA) score criteria for the visual interpretation of [18F]Fluorocholine PET/CT in patients with resected HGG, classifying complete versus incomplete metabolic tumor resection, as shown in Table 16 [21]. The prognostic value of the FuMeGa score on [18F]Fluorocholine PET/CT in HGGs was validated in a multicentric prospective study. Analyzing the postoperative score, significant differences were found for progression free survival (PFS) and OS for incomplete versus complete metabolic resections, respectively. The authors found that postoperative positive [18F]Fluorocholine PET/CT localizations correlated with the sites of tumor recurrence. Furthermore, on preoperative PET/CT, they observed that lesions with higher tracer uptake were followed by higher metabolic residual lesions after surgery [48].
Table 16.
Functional and Metabolic Glioma Analysis (FuMeGa) criteria for post-operative assessment of HGG patients.
| FuMeGa | ||
|---|---|---|
| Category | Score | Description |
| Metabolic complete resection | Score 1 | no uptake |
| Score 2 | faint uptake (lower than the contralateral skull) around the resection cavity | |
| Incomplete metabolic resection | Score 3 | moderate uptake around the resection cavity (like the contralateral skull) |
| Score 4 | moderate/high uptake around the resection cavity with focal deposits (unique) | |
| Score 5 | moderate/high uptake around the resection cavity with focal deposits (multiple) | |
Adapted from García Vicente et al. [21].
4. Discussion
From its introduction in the 2000s, PET/CT gained space for the molecular imaging-based assessment of tumors in clinical practice [60,61]. In this scenario, many efforts have been made to develop standardized image analyses through the introduction of reliable, easy-to-use, and practical criteria. The existing scores were introduced mainly to assess treatment response, but the advent of new specific radiopharmaceuticals (e.g., PSMA-ligands compounds) underlined the need for a standardized method for imaging interpretation too. In this setting, PSMA showed to be the most promising tracer for PCa with high sensitivity and specificity, even if its role in different pathological conditions has been reported [62,63,64,65,66,67,68]. In this scenario, several criteria for interpreting PSMA-ligand PET were proposed. The first introduced EANM criteria demonstrated moderate consensus among readers, probably due to the absence of a scale, thus underlining the importance of categories in a structured reporting system. As consequence, the PSMA-RADS and the miTNM were proposed: the first, a 5-point visual scale, demonstrated a high interobserver agreement, even with different levels of experience; however, it lacks a reference uptake scale as introduced by the miTNM. The latter is the only score endorsed by the EANM guidelines and seems to pave the way for its introduction in large clinical trials [11]. However, considering the recent approvement of [177Lu]Lu-PSMA RLT for PSMA-positive mCRPCa patients [69], neither PSMA-RADS nor miTNM include treatment recommendations for this therapy needing warranting improvements. In this scenario, the recently proposed Pro-PET score combined both PSMA-ligand and [18F]FDG PET/CT performed before RLT to improve patients’ selection and serving as a prognostic marker. Among interpretation criteria, PRIMARY score is lastly emerged in literature as a promising criterion for the use of PSMA-ligand PET/CT also in the diagnosis of primary tumor. Considering instead the evaluation of response to treatment in PCa patients, it is usually performed by applying RECIST version 1.1 to CT, alongside the assessment of the PSA trend. However, these criteria have several limitations and are not enough to fully evaluate PCa patients [70,71]. [18F]Fluorocholine PET/CT or PSMA-ligands PET/CT are not currently recommended by most updated guidelines for this purpose, mainly due to the absence of prospective, randomized, large sample size trials [72,73,74,75]. In this scenario, Fanti et al. introduced the PPP score, based on PCWG3 guidelines principles, for the evaluation of progression in PCa patients. However, this criterion missed the definition of complete, partial, and stable metabolic patterns and does not consider changes in PSMA expression (as neuroendocrine differentiation) [76,77]. The traditional four response categories were re-introduced with RECIP 1.0 used to assess the response to [177Lu]Lu-PSMA. According to preliminary data, both criteria seem to correlate with OS and appear superior to assess responses compared to their non-specific counterpart (RECIST 1.1, aPERCIST).
Nowadays, theragnostic represents the driving force to introduce generalizable framework systems for standardized reporting as well as for treatment response assessment both for PCa and NET patients. In the last few years, the SSTR-RADS, structured in a reciprocal fashion of PSMA-RADS, were introduced to convey to the nuclear medicine scan reader the level of certainty that an equivocal finding is a site of disease, avoiding common pitfalls in interpreting SSTR imaging [52,53,54,55]. Such a framework also allowed identifying appropriate candidates for treatment with [177Lu]Lu-DOTA compounds. Our systematic review pointed out the few data available for the SSRT-RADS, probably due to the absence of dual-tracer incorporation into the score. Namely, dual PET imaging should be considered for all patients with a diagnosis of metastatic GEPNET (grade 2–3). In this regard, Chan and colleagues devised a novel scheme for dual SSTR/FDG grading, termed NETPET grade, which showed a significant correlation with OS, time to progression, and histological grade, serving as a predictor of outcome. Despite the introduction of RLT many years ago, no reliable treatment response assessment criteria were introduced [78,79]. In this setting, some authors evaluated different PET-derived parameters for response evaluation, with contradictory results [80,81]. The newly introduced scores that emerged in our review (MORE, ZP) need to be validated, but preliminary results demonstrated their possible role in prognostication and in prediction of response to RLT.
Other minor investigated non-[18F]FDG PET criteria deserve mention. The NAFCIST criteria, introduced to assess treatment response in patients undergoing [223Ra]Cl2 therapy on [18F]NaF PET imaging, seem to better correlated with outcome than PERCIST. Moreover, compared to RECIST, known to be suboptimal for the evaluation of osteosarcoma with calcified bone-forming tumors often not shrinking even if responding, NAFCIST could represent a more accurate method of categorizing osteosarcoma owing to its better ability in reflecting bone-forming component [20]. Finally, the FuMeGa criteria emerged as the first metabolic criteria introduced for post-operative PET interpretation in HGG patients. It is known that postoperative assessment in glioma patients is crucial in the imaging follow-up and for prognostic considerations [82]. Even for the evaluation of recurrence, PET imaging demonstrated its added value over MRI, given its ability to assess tumor metabolism and reduce pseudo-progression pitfalls [83]. However, [18F]Fluorocholine is not the standard of choice because amino acid tracers (such as [11C]MET, [18F]FET) demonstrated better diagnostic performance. However due to the limited availability of the amino acid PET tracers, [18F]Fluorocholine PET could be useful in blood–brain alterations, namely HGG.
To sum up, this systematic review showed a trend to standardization particularly evident in PCa because of its incidence, with promising evidence for SSTR-PET imaging and preliminary experiences for other oncological scenarios which warrant further and larger applications.
5. Conclusions
In conclusion, many criteria regarding the use of non-[18F]FDG PET imaging in oncology have been proposed in the literature, but the majority of them are not integrated into clinical practice. Even if more data are needed to clearly evaluate their impact on the management of patients, these criteria represent promising tools for the interpretation of PET scans and standardization of reporting, but also to assess the response to therapy and, therefore, to guide the prognosis.
Author Contributions
Conceptualization, L.E., R.L. and G.S.; methodology, F.D., A.L., J.G. and G.S.; literature search: S.P., R.C., M.C. and N.O.; article selection: A.L. and J.G.; writing—original draft preparation, P.G., F.B., R.F., A.V., M.S.D.F., P.M., V.F., E.F., L.U. and R.L.; writing—review and editing, F.D. and G.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Basu S., Hess S., Nielsen Braad P.E., Olsen B.B., Inglev S., Høilund-Carlsen P.F. The Basic Principles of FDG-PET/CT Imaging. PET Clin. 2014;9:355–370. doi: 10.1016/j.cpet.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 2.Dondi F., Pasinetti N., Gatta R., Albano D., Giubbini R., Bertagna F. Comparison between Two Different Scanners for the Evaluation of the Role of 18F-FDG PET/CT Semiquantitative Parameters and Radiomics Features in the Prediction of Final Diagnosis of Thyroid Incidentalomas. J. Clin. Med. 2022;11:615. doi: 10.3390/jcm11030615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kim J.H. Comparison of the EORTC criteria and PERCIST in solid tumors: A pooled analysis and review. Oncotarget. 2016;7:58105–58110. doi: 10.18632/oncotarget.11171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wahl R.L., Jacene H., Kasamon Y., Lodge M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009;50:122S–150S. doi: 10.2967/jnumed.108.057307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annovazzi A., Vari S., Giannarelli D., Pasqualoni R., Sciuto R., Carpano S., Cognetti F., Ferraresi V. Comparison of 18F-FDG PET/CT Criteria for the Prediction of Therapy Response and Clinical Outcome in Patients With Metastatic Melanoma Treated With Ipilimumab and PD-1 Inhibitors. Clin. Nucl. Med. 2020;45:187–194. doi: 10.1097/RLU.0000000000002921. [DOI] [PubMed] [Google Scholar]
- 6.Cho S.Y., Lipson E.J., Im H.J., Rowe S.P., Gonzalez E.M., Blackford A., Chirindel A., Pardoll D.M., Topalian S.L., Wahl R.L. Prediction of Response to Immune Checkpoint Inhibitor Therapy Using Early-Time-Point 18F-FDG PET/CT Imaging in Patients with Advanced Melanoma. J. Nucl. Med. 2017;58:1421–1428. doi: 10.2967/jnumed.116.188839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anwar H., Sachpekidis C., Winkler J., Kopp-Schneider A., Haberkorn U., Hassel J.C., Dimitrakopoulou-Strauss A. Absolute number of new lesions on 18F-FDG PET/CT is more predictive of clinical response than SUV changes in metastatic melanoma patients receiving ipilimumab. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:376–383. doi: 10.1007/s00259-017-3870-6. [DOI] [PubMed] [Google Scholar]
- 8.Fanti S., Minozzi S., Morigi J.J., Giesel F., Ceci F., Uprimny C., Hofman M.S., Eiber M., Schwarzenbock S., Castellucci P., et al. Development of standardized image interpretation for 68Ga-PSMA PET/CT to detect prostate cancer recurrent lesions. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1622–1635. doi: 10.1007/s00259-017-3725-1. [DOI] [PubMed] [Google Scholar]
- 9.Rowe S.P., Pienta K.J., Pomper M.G., Gorin M.A. Proposal for a Structured Reporting System for Prostate-Specific Membrane Antigen-Targeted PET Imaging: PSMA-RADS Version 1.0. J. Nucl. Med. 2018;59:479–485. doi: 10.2967/jnumed.117.195255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eiber M., Herrmann K., Calais J., Hadaschik B., Giesel F.L., Hartenbach M., Hope T., Reiter R., Maurer T., Weber W.A., et al. Prostate Cancer Molecular Imaging Standardized Evaluation (PROMISE): Proposed miTNM Classification for the Interpretation of PSMA-Ligand PET/CT. J. Nucl. Med. 2018;59:469–478. doi: 10.2967/jnumed.117.198119. [DOI] [PubMed] [Google Scholar]
- 11.Ceci F., Oprea-Lager D.E., Emmett L., Adam J.A., Bomanji J., Czernin J., Eiber M., Haberkorn U., Hofman M.S., Hope T.A., et al. E-PSMA: The EANM standardized reporting guidelines v1.0 for PSMA-PET. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1626–1638. doi: 10.1007/s00259-021-05245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Adnan A., Basu S. Concept proposal for a six-tier integrated dual tracer PET-CT (68Ga-PSMA and FDG) image scoring system (‘Pro-PET’ score) and examining its potential implications in metastatic castration-resistant prostate carcinoma theranostics and prognosis. Nucl. Med. Commun. 2021;42:566–574. doi: 10.1097/MNM.0000000000001371. [DOI] [PubMed] [Google Scholar]
- 13.Emmett L., Papa N., Buteau J., Ho B., Liu V., Roberts M., Thompson J., Moon D., Sheehan-Dare G., Alghazo O., et al. The PRIMARY Score: Using Intraprostatic 68Ga-PSMA PET/CT Patterns to Optimize Prostate Cancer Diagnosis. J. Nucl. Med. 2022;63:1644–1650. doi: 10.2967/jnumed.121.263448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fanti S., Hadaschik B., Herrmann K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J. Nucl. Med. 2020;61:678–682. doi: 10.2967/jnumed.119.233817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gafita A., Rauscher I., Weber M., Hadaschik B., Wang H., Armstrong W.R., Tauber R., Grogan T.R., Czernin J., Rettig M.B., et al. Novel Framework for Treatment Response Evaluation Using PSMA PET/CT in Patients with Metastatic Castration-Resistant Prostate Cancer (RECIP 1.0): An International Multicenter Study. J. Nucl. Med. 2022;63:1651–1658. doi: 10.2967/jnumed.121.263072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krenning E.P., Valkema R., Kooij P.P., Breeman W.A., Bakker W.H., de Herder W.W., van Eijck C.H., Kwekkeboom D.J., deJong M., Pauwels S. Scintigraphy and radionuclide therapy with [indium-111-labelled-diethyl triamine penta-acetic acid-D-Phe1]-octreotide. Ital. J. Gastroenterol. Hepatol. 1999;31:S219–S223. [PubMed] [Google Scholar]
- 17.Werner R.A., Solnes L.B., Javadi M.S., Weich A., Gorin M.A., Pienta K.J., Higuchi T., Buck A.K., Pomper M.G., Rowe S.P., et al. SSTR-RADS Version 1.0 as a Reporting System for SSTR PET Imaging and Selection of Potential PRRT Candidates: A Proposed Standardization Framework. J. Nucl. Med. 2018;59:1085–1091. doi: 10.2967/jnumed.117.206631. [DOI] [PubMed] [Google Scholar]
- 18.Chan D.L., Pavlakis N., Schembri G.P., Bernard E.J., Hsiao E., Hayes A., Barnes T., Diakos C., Khasraw M., Samra J., et al. Dual Somatostatin Receptor/FDG PET/CT Imaging in Metastatic Neuroendocrine Tumours: Proposal for a Novel Grading Scheme with Prognostic Significance. Theranostics. 2017;7:1149–1158. doi: 10.7150/thno.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zwirtz K., Hardt J., Acker G., Baur A.D.J., Pavel M., Huang K., Brenner W., Prasad V. Comparison of Choi, RECIST and Somatostatin Receptor PET/CT Based Criteria for the Evaluation of Response and Response Prediction to PRRT. Pharmaceutics. 2022;14:1278. doi: 10.3390/pharmaceutics14061278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kairemo K., Rohren E.M., Anderson P.M., Ravizzini G., Rao A., Macapinlac H.A., Subbiah V. Development of sodium fluoride PET response criteria for solid tumours (NAFCIST) in a clinical trial of radium-223 in osteosarcoma: From RECIST to PERCIST to NAFCIST. ESMO Open. 2019;4:e000439. doi: 10.1136/esmoopen-2018-000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.García Vicente A.M., Pena Pardo F.J., Lozano Setien E., Sandoval Valencia H., Villena Martín M. FuMeGA Criteria for Visual Assessment of Postoperative 18F-Fluorocholine PET in Patients With Glioma. Clin. Nucl. Med. 2020;45:448–450. doi: 10.1097/RLU.0000000000003034. [DOI] [PubMed] [Google Scholar]
- 22.Werner R.A., Bundschuh R.A., Bundschuh L., Javadi M.S., Leal J.P., Higuchi T., Pienta K.J., Buck A.K., Pomper M.G., Gorin M.A., et al. Interobserver Agreement for the Standardized Reporting System PSMA-RADS 1.0 on 18F-DCFPyL PET/CT Imaging. J. Nucl. Med. 2018;59:1857–1864. doi: 10.2967/jnumed.118.217588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chiu L.W., Lawhn-Heath C., Behr S.C., Juarez R., Perez P.M., Lobach I., Bucknor M.D., Hope T.A., Flavell R.R. Factors Predicting Metastatic Disease in 68Ga-PSMA-11 PET-Positive Osseous Lesions in Prostate Cancer. J. Nucl. Med. 2020;61:1779–1785. doi: 10.2967/jnumed.119.241174. [DOI] [PubMed] [Google Scholar]
- 24.Letang A., Crombé A., Rousseau C., Sargos P., Merlin C., Cantarel C., Cazeau A.L. Bone Uptake in Prostate Cancer Patients: Diagnostic Performances of PSMA-RADS v1.0, Clinical, Biological, and 68 Ga-PSMA-11 PET Features to Predict Metastasis After Biochemical Recurrence. Clin. Nucl. Med. 2022;47:e529–e539. doi: 10.1097/RLU.0000000000004259. [DOI] [PubMed] [Google Scholar]
- 25.Yin Y., Werner R.A., Higuchi T., Lapa C., Pienta K.J., Pomper M.G., Gorin M.A., Rowe S.P. Follow-up of Lesions with Equivocal Radiotracer Uptake on PSMA-Targeted PET in Patients with Prostate Cancer: Predictive Values of the PSMA-RADS-3A and PSMA-RADS-3B Categories. J. Nucl. Med. 2019;60:511–516. doi: 10.2967/jnumed.118.217653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuten J., Dekalo S., Mintz I., Yossepowitch O., Mano R., Even-Sapir E. The significance of equivocal bone findings in staging PSMA imaging in the preoperative setting: Validation of the PSMA-RADS version 1.0. EJNMMI Res. 2021;11:3. doi: 10.1186/s13550-020-00745-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bhoil A., Seshadri N., Vinjamuri S. Indeterminate skeletal and lymph node lesion on 18F PSMA 1007 PET/CT scanning: Lessons from a review at 12 months with PSMA-RADS. Nucl. Med. Commun. 2022;43:1034–1041. doi: 10.1097/MNM.0000000000001600. [DOI] [PubMed] [Google Scholar]
- 28.Garg T., Werner R.A., Chung H.W., Khatri W., Pienta K.J., Pomper M.G., Gorin M.A., Saad E., Rowe S.P. Association of True Positivity with Serum Prostate-Specific Antigen Levels and Other Clinical Factors in Indeterminate PSMA-RADS-3A Lesions Identified on 18F-DCFPyL PET/CT Scans. Tomography. 2022;8:220. doi: 10.3390/tomography8060220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khatri W., Chung H.W., Werner R.A., Leal J.P., Pienta K.J., Lodge M.A., Gorin M.A., Pomper M.G., Rowe S.P. Effect of Point-Spread Function Reconstruction for Indeterminate PSMA-RADS-3A Lesions on PSMA-Targeted PET Imaging of Men with Prostate Cancer. Diagnostics. 2021;11:665. doi: 10.3390/diagnostics11040665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mihatsch P.W., Beissert M., Pomper M.G., Bley T.A., Seitz A.K., Kübler H., Buck A.K., Rowe S.P., Serfling S.E., Hartrampf P.E., et al. Changing Threshold-Based Segmentation Has No Relevant Impact on Semi-Quantification in the Context of Structured Reporting for PSMA-PET/CT. Cancers. 2022;14:270. doi: 10.3390/cancers14020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gültekin A., Yaylalı O., Şengöz T., Yüksel D., Şahin B. Intraobserver and interobserver agreement for the interpretation of 68Ga-prostate-specific membrane antigen-I&T positron emission tomography/computed tomography imaging. Nucl. Med. Commun. 2019;40:1250–1255. doi: 10.1097/MNM.0000000000001097. [DOI] [PubMed] [Google Scholar]
- 32.Wang H., Amiel T., Würnschimmel C., Langbein T., Steiger K., Rauscher I., Horn T., Maurer T., Weber W., Wester H.J., et al. PSMA-ligand uptake can serve as a novel biomarker in primary prostate cancer to predict outcome after radical prostatectomy. EJNMMI Res. 2021;11:76. doi: 10.1186/s13550-021-00818-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hoberück S., Löck S., Borkowetz A., Sommer U., Winzer R., Zöphel K., Fedders D., Michler E., Kotzerke J., Kopka K., et al. Intraindividual comparison of [68Ga]-Ga-PSMA-11 and [18F]-F-PSMA-1007 in prostate cancer patients: A retrospective single-center analysis. EJNMMI Res. 2021;11:109. doi: 10.1186/s13550-021-00845-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koehler D., Sauer M., Karimzadeh A., Apostolova I., Klutmann S., Adam G., Knipper S., Maurer T., Berliner C. Evaluation of [68 Ga]Ga-PSMA-I&T PET/CT with additional late scans of the pelvis in prostate-specific antigen recurrence using the PROMISE criteria. EJNMMI Res. 2022;12:66. doi: 10.1186/s13550-022-00938-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Demirci E., Akyel R., Caner B., Alan-Selçuk N., Güven-Meşe Ş., Ocak M., Kabasakal L. Interobserver and intraobserver agreement on prostate-specific membrane antigen PET/CT images according to the miTNM and PSMA-RADS criteria. Nucl. Med. Commun. 2020;41:759–767. doi: 10.1097/MNM.0000000000001219. [DOI] [PubMed] [Google Scholar]
- 36.Toriihara A., Nobashi T., Baratto L., Duan H., Moradi F., Park S., Hatami N., Aparici C.M., Davidzon G., Iagaru A. Comparison of 3 Interpretation Criteria for 68Ga-PSMA11 PET Based on Inter- and Intrareader Agreement. J. Nucl. Med. 2020;61:533–539. doi: 10.2967/jnumed.119.232504. [DOI] [PubMed] [Google Scholar]
- 37.Michalski K., Klein C., Brueggemann T., Meyer P.T., Jilg C.A., Ruf J. Assessing Response to [177Lu]PSMA Radioligand Therapy using modified PSMA PET Progression Criteria. J. Nucl. Med. 2021;62:1741–1746. doi: 10.2967/jnumed.120.260836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gafita A., Rauscher I., Fendler W.P., Murthy V., Hui W., Armstrong W.R., Herrmann K., Weber W.A., Calais J., Eiber M., et al. Measuring response in metastatic castration-resistant prostate cancer using PSMA PET/CT: Comparison of RECIST 1.1, aPCWG3, aPERCIST, PPP, and RECIP 1.0 criteria. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:4271–4281. doi: 10.1007/s00259-022-05882-x. [DOI] [PubMed] [Google Scholar]
- 39.Hope T.A., Calais J., Zhang L., Dieckmann W., Millo C. 111In-Pentetreotide Scintigraphy Versus 68Ga-DOTATATE PET: Impact on Krenning Scores and Effect of Tumor Burden. J. Nucl. Med. 2019;60:1266–1269. doi: 10.2967/jnumed.118.223016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Purandare N.C., Puranik A., Agrawal A., Shah S., Kumar R., Jiwnani S., Karimundackal G., Pramesh C.S., Rangarajan V. Does 68Ga-DOTA-NOC-PET/CT impact staging and therapeutic decision making in pulmonary carcinoid tumors? Nucl. Med. Commun. 2020;41:1040–1046. doi: 10.1097/MNM.0000000000001248. [DOI] [PubMed] [Google Scholar]
- 41.Menon B.K., Kalshetty A., Bhattacharjee A., Basu S. Standardized uptake values and ratios on 68Ga-DOTATATE PET-computed tomography for normal organs and malignant lesions and their correlation with Krenning score in patients with metastatic neuroendocrine tumors. Nucl. Med. Commun. 2020;41:1095–1099. doi: 10.1097/MNM.0000000000001253. [DOI] [PubMed] [Google Scholar]
- 42.Werner R.A., Derlin T., Rowe S.P., Bundschuh L., Sheikh G.T., Pomper M.G., Schulz S., Higuchi T., Buck A.K., Bengel F.M., et al. High Interobserver Agreement for the Standardized Reporting System SSTR-RADS 1.0 on Somatostatin Receptor PET/CT. J. Nucl. Med. 2021;62:514–520. doi: 10.2967/jnumed.120.245464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chan D.L., Hayes A.R., Karfis I., Conner A., Furtado O’Mahony L., Mileva M., Bernard E., Roach P., Marin G., Pavlakis N., et al. Dual [68Ga]DOTATATE and [18F]FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasms: A multicentre validation of the NETPET score. Br. J. Cancer. 2022 doi: 10.1038/s41416-022-02061-5. Epub ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hayes A.R., Furtado O’Mahony L., Quigley A.M., Gnanasegaran G., Caplin M.E., Navalkissoor S., Toumpanakis C. The Combined Interpretation of 68Ga-DOTATATE PET/CT and 18F-FDG PET/CT in Metastatic Gastroenteropancreatic Neuroendocrine Tumors: A Classification System With Prognostic Impact. Clin. Nucl. Med. 2022;47:26–35. doi: 10.1097/RLU.0000000000003937. [DOI] [PubMed] [Google Scholar]
- 45.Karfis I., Marin G., Levillain H., Drisis S., Muteganya R., Critchi G., Taraji-Schiltz L., Guix C.A., Shaza L., Elbachiri M., et al. Prognostic value of a three-scale grading system based on combining molecular imaging with 68Ga-DOTATATE and 18F-FDG PET/CT in patients with metastatic gastroenteropancreatic neuroendocrine neoplasias. Oncotarget. 2020;11:589–599. doi: 10.18632/oncotarget.27460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan D.L., Ulaner G.A., Pattison D., Wyld D., Ladwa R., Kirchner J., Li B.T., Lai W.V., Pavlakis N., Roach P.J., et al. Dual PET Imaging in Bronchial Neuroendocrine Neoplasms: The NETPET Score as a Prognostic Biomarker. J. Nucl. Med. 2021;62:1278–1284. doi: 10.2967/jnumed.120.257659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kairemo K., Roszik J., Anderson P., Ravizzini G., Rao A., Macapinlac H.A., Subbiah V. 18F-sodium fluoride positron emission tomography (NaF-18-PET/CT) radiomic signatures to evaluate responses to alpha-particle Radium-223 dichloride therapy in osteosarcoma metastases. Curr. Probl. Cancer. 2021;45:100797. doi: 10.1016/j.currproblcancer.2021.100797. [DOI] [PubMed] [Google Scholar]
- 48.García Vicente A.M., Pena Pardo F.J., Amo-Salas M., Villena Martín M., López Menéndez C., Soriano Castrejón Á.M., Pérez-Beteta J. Prognostic Potential of Postoperative 18F-Fluorocholine PET/CT in Patients With High-Grade Glioma. Clinical Validation of FuMeGA Postoperative PET Criteria. Clin. Nucl. Med. 2022;47:480–487. doi: 10.1097/RLU.0000000000004127. [DOI] [PubMed] [Google Scholar]
- 49.Deppen S.A., Blume J., Bobbey A.J., Shah C., Graham M.M., Lee P., Delbeke D., Walker R.C. 68Ga-DOTATATE Compared with 111In-DTPA-Octreotide and Conventional Imaging for Pulmonary and Gastroenteropancreatic Neuroendocrine Tumors: A Systematic Review and Meta-Analysis. J. Nucl. Med. 2016;57:872–878. doi: 10.2967/jnumed.115.165803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gabriel M., Decristoforo C., Kendler D., Dobrozemsky G., Heute D., Uprimny C., Kovacs P., Von Guggenberg E., Bale R., Virgolini I.J. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: Comparison with somatostatin receptor scintigraphy and CT. J. Nucl. Med. 2007;48:508–518. doi: 10.2967/jnumed.106.035667. [DOI] [PubMed] [Google Scholar]
- 51.Sadowski S.M., Neychev V., Millo C., Shih J., Nilubol N., Herscovitch P., Pacak K., Marx S.J., Kebebew E. Prospective Study of 68Ga-DOTATATE Positron Emission Tomography/Computed Tomography for Detecting Gastro-Entero-Pancreatic Neuroendocrine Tumors and Unknown Primary Sites. J. Clin. Oncol. 2016;34:588–596. doi: 10.1200/JCO.2015.64.0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bodei L., Ambrosini V., Herrmann K., Modlin I. Current Concepts in 68Ga-DOTATATE Imaging of Neuroendocrine Neoplasms: Interpretation, Biodistribution, Dosimetry, and Molecular Strategies. J. Nucl. Med. 2017;58:1718–1726. doi: 10.2967/jnumed.116.186361. [DOI] [PubMed] [Google Scholar]
- 53.Ma G., Du J., Zhang X., Liu J., Xu X., Xu B., Guan Z. Quantitative analysis of 68Ga-DOTA(0)-Tyr(3)-octreotate positron emission tomography/computed tomography imaging for the differential diagnosis of primary pheochromocytoma and paraganglioma. Quant. Imaging. Med. Surg. 2022;12:2427–2440. doi: 10.21037/qims-21-652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bodei L., Mueller-Brand J., Baum R.P., Pavel M.E., Hörsch D., O’Dorisio M.S., O’Dorisio T.M., Howe J.R., Cremonesi M., Kwekkeboom D.J., et al. The joint IAEA, EANM, and SNMMI practical guidance on peptide receptor radionuclide therapy (PRRNT) in neuroendocrine tumours. Eur. J. Nucl. Med. Mol. Imaging. 2013;40:800–816. doi: 10.1007/s00259-013-2454-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bozkurt M.F., Virgolini I., Balogova S., Beheshti M., Rubello D., Decristoforo C., Ambrosini V., Kjaer A., Delgado-Bolton R., Kunikowska J., et al. Guideline for PET/CT imaging of neuroendocrine neoplasms with 68Ga-DOTA-conjugated somatostatin receptor targeting peptides and 18F-DOPA. Eur. J. Nucl. Med. Mol. Imaging. 2017;44:1588–1601. doi: 10.1007/s00259-017-3728-y. [DOI] [PubMed] [Google Scholar]
- 56.Fueger B.J., Hamilton G., Raderer M., Pangerl T., Traub T., Angelberger P., Baumgartner G., Dudczak R., Virgolini I. Effects of chemotherapeutic agents on expression of somatostatin receptors in pancreatic tumor cells. J. Nucl. Med. 2001;42:1856–1862. [PubMed] [Google Scholar]
- 57.Bartolomei M., Berruti A., Falconi M., Fazio N., Ferone D., Lastoria S., Pappagallo G., Seregni E., Versari A. Clinical Management of Neuroendocrine Neoplasms in Clinical Practice: A Formal Consensus Exercise. Cancers. 2022;14:2501. doi: 10.3390/cancers14102501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Urso L., Panareo S., Castello A., Ambrosio M.R., Zatelli M.C., Caracciolo M., Tonini E., Valpiani G., Boschi A., Uccelli L., et al. Glucose Metabolism Modification Induced by Radioligand Therapy with [177Lu]Lu/[90Y]Y-DOTATOC in Advanced Neuroendocrine Neoplasms: A Prospective Pilot Study within FENET-2016 Trial. Pharmaceutics. 2022;14:2009. doi: 10.3390/pharmaceutics14102009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Luo Y., Chen J., Huang K., Lin Y., Chen M., Xu L., Li Z.P., Feng S.T. Early evaluation of sunitinib for the treatment of advanced gastroenteropancreatic neuroendocrine neoplasms via CT imaging: RECIST 1.1 or Choi Criteria? BMC Cancer. 2017;17:154. doi: 10.1186/s12885-017-3150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Duclos V., Iep A., Gomez L., Goldfarb L., Besson F.L. PET Molecular Imaging: A Holistic Review of Current Practice and Emerging Perspectives for Diagnosis, Therapeutic Evaluation and Prognosis in Clinical Oncology. Int. J. Mol. Sci. 2021;22:4159. doi: 10.3390/ijms22084159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lindsay M.J., Siegel B.A., Tunis S.R., Hillner B.E., Shields A.F., Carey B.P., Coleman R.E. The National Oncologic PET Registry: Expanded medicare coverage for PET under coverage with evidence development. Am. J. Roentgenol. 2007;188:1109–1113. doi: 10.2214/AJR.06.1175. [DOI] [PubMed] [Google Scholar]
- 62.Maurer T., Gschwend J.E., Rauscher I., Souvatzoglou M., Haller B., Weirich G., Wester H.J., Heck M., Kübler H., Beer A.J., et al. Diagnostic Efficacy of (68)Gallium-PSMA Positron Emission Tomography Compared to Conventional Imaging for Lymph Node Staging of 130 Consecutive Patients with Intermediate to High Risk Prostate Cancer. J. Urol. 2016;195:1436–1443. doi: 10.1016/j.juro.2015.12.025. [DOI] [PubMed] [Google Scholar]
- 63.Eiber M., Maurer T., Souvatzoglou M., Beer A.J., Ruffani A., Haller B., Graner F.P., Kübler H., Haberkorn U., Eisenhut M., et al. Evaluation of Hybrid ⁶⁸Ga-PSMA Ligand PET/CT in 248 Patients with Biochemical Recurrence After Radical Prostatectomy. J. Nucl. Med. 2015;56:668–674. doi: 10.2967/jnumed.115.154153. [DOI] [PubMed] [Google Scholar]
- 64.Rowe S.P., Macura K.J., Mena E., Blackford A.L., Nadal R., Antonarakis E.S., Eisenberger M., Carducci M., Fan H., Dannals R.F., et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol. Imaging Biol. 2016;18:411–419. doi: 10.1007/s11307-016-0957-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dondi F., Albano D., Cerudelli E., Gazzilli M., Giubbini R., Treglia G., Bertagna F. Radiolabelled PSMA PET/CT or PET/MRI in Hepatocellular Carcinoma (HCC): A Systematic Review. Clin. Transl. Imaging. 2020;8:461–467. doi: 10.1007/s40336-020-00396-8. [DOI] [Google Scholar]
- 66.Urso L., Castello A., Rocca G.C., Lancia F., Panareo S., Cittanti C., Uccelli L., Florimonte L., Castellani M., Ippolito C., et al. Role of PSMA-ligands imaging in Renal Cell Carcinoma management: Current status and future perspectives. J. Cancer Res. Clin. Oncol. 2022;148:1299–1311. doi: 10.1007/s00432-022-03958-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rizzo A., Dall’Armellina S., Pizzuto D.A., Perotti G., Zagaria L., Lanni V., Treglia G., Racca M., Annunziata S. PSMA Radioligand Uptake as a Biomarker of Neoangiogenesis in Solid Tumours: Diagnostic or Theragnostic Factor? Cancers. 2022;14:4039. doi: 10.3390/cancers14164039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Werner R.A., Bundschuh R.A., Bundschuh L., Fanti S., Javadi M.S., Higuchi T., Weich A., Pienta K.J., Buck A.K., Pomper M.G., et al. Novel Structured Reporting Systems for Theranostic Radiotracers. J. Nucl. Med. 2019;60:577–584. doi: 10.2967/jnumed.118.223537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hennrich U., Eder M. [177Lu]Lu-PSMA-617 (PluvictoTM): The First FDA-Approved Radiotherapeutical for Treatment of Prostate Cancer. Pharmaceuticals. 2022;15:1292. doi: 10.3390/ph15101292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scher H.I., Morris M.J., Stadler W.M., Higano C., Basch E., Fizazi K., Antonarakis E.S., Beer T.M., Carducci M.A., Chi K.N., et al. Prostate Cancer Clinical Trials Working Group 3. Trial Design and Objectives for Castration-Resistant Prostate Cancer: Updated Recommendations From the Prostate Cancer Clinical Trials Working Group 3. J. Clin. Oncol. 2016;34:1402–1418. doi: 10.1200/JCO.2015.64.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Eisenhauer E.A., Therasse P., Bogaerts J., Schwartz L.H., Sargent D., Ford R., Dancey J., Arbuck S., Gwyther S., Mooney M., et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur. J. Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 72.Ceci F., Castellucci P., Graziani T., Schiavina R., Renzi R., Borghesi M., Di Tullio P., Brunocilla E., Ardizzoni A., Fanti S. (11)C-Choline PET/CT in castration-resistant prostate cancer patients treated with docetaxel. Eur. J. Nucl. Med. Mol. Imaging. 2016;43:84–91. doi: 10.1007/s00259-015-3177-4. [DOI] [PubMed] [Google Scholar]
- 73.Urso L., Lancia F., Ortolan N., Frapoli M., Rauso M., Artioli P., Cittanti C., Uccelli L., Frassoldati A., Evangelista L., et al. 18F-Choline PET/CT or PET/MR and the evaluation of response to systemic therapy in prostate cancer: Are we ready? Clin. Transl. Imaging. 2022;10:687–695. doi: 10.1007/s40336-022-00515-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Seitz A.K., Rauscher I., Haller B., Krönke M., Luther S., Heck M.M., Horn T., Gschwend J.E., Schwaiger M., Eiber M., et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur. J. Nucl. Med. Mol. Imaging. 2018;45:602–612. doi: 10.1007/s00259-017-3887-x. [DOI] [PubMed] [Google Scholar]
- 75.Alongi P., Laudicella R., Lanzafame H., Farolfi A., Mapelli P., Picchio M., Burger I.A., Iagaru A., Minutoli F., Evangelista L. PSMA and Choline PET for the Assessment of Response to Therapy and Survival Outcomes in Prostate Cancer Patients: A Systematic Review from the Literature. Cancers. 2022;14:1770. doi: 10.3390/cancers14071770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shahrokhi P., Emami-Ardekani A., Karamzade-Ziarati N. SSTR-based theranostics in neuroendocrine prostate cancer (NEPC) Clin. Transl. Imaging. 2022;1:1–8. doi: 10.1007/s40336-022-00535-3. [DOI] [Google Scholar]
- 77.Fanti S., Goffin K., Hadaschik B.A., Herrmann K., Maurer T., MacLennan S., Oprea-Lager D.E., Oyen W.J., Rouvière O., Mottet N., et al. Consensus statements on PSMA PET/CT response assessment criteria in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:469–476. doi: 10.1007/s00259-020-04934-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Strosberg J., El-Haddad G., Wolin E., Hendifar A., Yao J., Chasen B., Mittra E., Kunz P.L., Kulke M.H., Jacene H., et al. NETTER-1 Trial Investigators. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. N. Engl. J. Med. 2017;376:125–135. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kwekkeboom D.J., de Herder W.W., Kam B.L., van Eijck C.H., van Essen M., Kooij P.P., Feelders R.A., van Aken M.O., Krenning E.P. Treatment with the radiolabeled somatostatin analog [177 Lu-DOTA 0,Tyr3]octreotate: Toxicity, efficacy, and survival. J. Clin. Oncol. 2008;26:2124–2130. doi: 10.1200/JCO.2007.15.2553. [DOI] [PubMed] [Google Scholar]
- 80.Haug A.R., Auernhammer C.J., Wängler B., Schmidt G.P., Uebleis C., Göke B., Cumming P., Bartenstein P., Tiling R., Hacker M. 68Ga-DOTATATE PET/CT for the early prediction of response to somatostatin receptor-mediated radionuclide therapy in patients with well-differentiated neuroendocrine tumors. J. Nucl. Med. 2010;51:1349–1356. doi: 10.2967/jnumed.110.075002. [DOI] [PubMed] [Google Scholar]
- 81.Gabriel. M., Oberauer A., Dobrozemsky G., Decristoforo C., Putzer D., Kendler D., Uprimny C., Kovacs P., Bale R., Virgolini I.J. 68Ga-DOTA-Tyr3-octreotide PET for assessing response to somatostatin-receptor-mediated radionuclide therapy. J. Nucl. Med. 2009;50:1427–1434. doi: 10.2967/jnumed.108.053421. [DOI] [PubMed] [Google Scholar]
- 82.Cui M., Zorrilla-Veloz R.I., Hu J., Guan B., Ma X. Diagnostic Accuracy of PET for Differentiating True Glioma Progression From Post Treatment-Related Changes: A Systematic Review and Meta-Analysis. Front. Neurol. 2021;12:671867. doi: 10.3389/fneur.2021.671867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Santo G., Laudicella R., Linguanti F., Nappi A.G., Abenavoli E., Vergura V., Rubini G., Sciagrà R., Arnone G., Schillaci O., et al. The Utility of Conventional Amino Acid PET Radiotracers in the Evaluation of Glioma Recurrence also in Comparison with MRI. Diagnostics. 2022;12:844. doi: 10.3390/diagnostics12040844. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.