Abstract
Chagas disease is a significant public health risk in rural and semi-rural areas of Venezuela. Triatomine infection by the aetiological agent Trypanosoma cruzi is also observed in the Metropolitan District of Caracas (MDC), where foodborne T. cruzi outbreaks occasionally occur but active vector-to-human transmission (infection during triatomine bloodmeal) is considered absent. Citizen science-based domiciliary triatomine collection carried out between 2007 and 2013 in the MDC has advanced understanding of urban T. cruzi prevalence patterns and represents an important public awareness-building tool. The present study reports on the extension of this triatomine collection program from 2014 to 2019 and uses mitochondrial metabarcoding to assess feeding behavior in a subset of specimens. The combined, thirteen-year dataset (n = 4872) shows a high rate of T. cruzi infection (75.2%) and a predominance of Panstrongylus geniculatus (99.01%) among triatomines collected in domiciliary areas by MDC inhabitants. Collection also involved nymphal stages of P. geniculatus in 18 of 32 MDC parishes. Other collected species included Triatoma nigromaculata, Triatoma maculata, Rhodnius prolixus, and Panstrongylus rufotuberculatus. Liquid intestinal content indicative of bloodmeal was observed in 53.4% of analyzed specimens. Dissection pools representing 108 such visually blooded P. geniculatus specimens predominantly tested positive for human cytochrome b DNA (22 of 24 pools). Additional bloodmeal sources detected via metabarcoding analysis included key sylvatic T. cruzi reservoirs (opossum and armadillo), rodents, and various other synanthropic and domesticated animals. Results suggest a porous sylvatic-domiciliary transmission interface and ongoing adaptation of P. geniculatus to the urban ecotope. Although P. geniculatus defecation traits greatly limit the possibility of active T. cruzi transmission for any individual biting event, the cumulation of this low risk across a vast metropolitan population warrants further investigation. Efforts to prevent triatomine contact with human food sources also clearly require greater attention to protect Venezuela’s capital from Chagas disease.
Author summary
Vector-borne diseases are a significant cause of morbidity and mortality in tropical and subtropical countries. Efficient control strategies require detailed understanding of vector species distribution and behavior in human environments. General public awareness-building that selected animal species carry and transmit dangerous human-infective parasites is also key. This study details long-term spatiotemporal prevalence patterns of hematophagous Chagas disease vectors known as triatomines based on the collection of specimens from domiciliary areas of greater Caracas, the capital of Venezuela. A citizen science-based sample collection approach is used to simultaneously help educate the public on Chagas disease and prevention. Microscopy indicates that triatomines found inside homes by inhabitants of the city are often positive for the Chagas disease parasite Trypanosoma cruzi. Specimens found inside homes also occasionally involve nymphs. Such evidence of indoor reproduction is found in various parts of the city. Genetic analyses of triatomine intestinal content further indicate that domiciliary triatomine occurrence is facilitated by feeding on human blood. Bloodmeals on various other domesticated and wild animal species are also identified, suggesting an extensive T. cruzi transmission network spanning urban and sylvatic environments. Results compel greater attention toward Chagas disease surveillance in Caracas. A human serological survey for T. cruzi, especially in the pediatric population, would help clarify the consequences of triatomine infection and human-triatomine contact events demonstrated from this study’s citizen science-based dataset.
Introduction
Chagas disease, also known as American trypanosomiasis, is a life-threatening vector-borne zoonosis caused by Trypanosoma cruzi, a protozoan parasite endemic to various regions of the tropical and subtropical Americas [1]. Transmission occurs when infected hematophagous triatomine insects (Reduviidae, subfamily Triatominae [2,3]) defecate during bloodmeal, releasing metacyclic parasite stages into the bite wound or intact mucosal membranes. Apart from this active (stercorarian) mode of transmission, human infection can result congenitally (mother to fetus), via blood transfusion from an infected donor, or upon ingestion of food (often fruit juices) contaminated with triatomines or their feces [1]. Predation is also considered an important alternative to stercorarian forms of T. cruzi transmission among a vast range of non-human hosts. The parasite has been recorded in at least 73 mammalian genera occurring in diverse sylvatic, peri-domiciliary, and domiciliary ecotopes [4].
Venezuela currently belongs to the countries most heavily burdened by Chagas disease. Recent estimates of T. cruzi seroprevalence exceed 10% nationwide, with highest values suggested for hotspots of active transmission in Barinas, Lara, Portuguesa, and Trujillo states [5,6]. Although case burden is primarily concentrated to the country’s rural Andean piedmont, the possibility that human T. cruzi infections are also being contracted in larger urban centers deserves greater attention. Urban infection events become possible when cities encroach onto wild T. cruzi habitat without effective barriers against human-vector contact and when environmental conditions allow vectors not only to temporarily survive in domiciliary settings but also to colonize these long-term without needing to return to the wild for food and reproduction. Regular urban circulation of T. cruzi may then begin to establish if urban triatomine food sources represent T. cruzi-competent host species or when competent host species commonly enter the urban space from the wild.
The above conditions appear relevant to the Metropolitan District of Caracas (MDC), Venezuela’s capital region of several million inhabitants and little to no regular T. cruzi sero-surveillance in recent decades [6,7]. The MDC features some of the largest shantytowns in Latin America (both along its perimeter and stretching into the city center) and borders directly on expansive sylvatic environment such as Waraira Repano National Park. Multiple foodborne outbreaks have occurred in and around the MDC in the past and have recurrently involved Panstrongylus geniculatus, one of 24 triatomine species found across Venezuela [8–11]. Despite its extensive geographic distribution (mid-Central America to northern Argentina), P. geniculatus has historically been considered a predominantly sylvatic species of low epidemiological importance in the Americas relative to species presenting high levels of (peri-) domiciliation in multiple populations [12]. These include Rhodnius prolixus and Triatoma dimidiata in northern South America and Central America, Triatoma infestans and Panstrongylus megistus in the Southern Cone, and Triatoma brasiliensis in Brazil [12]. Possibly influenced by intervention campaigns targeting these species and other processes of climate and environmental change [13], changes in ecological niche and dispersal behaviors are increasingly reported for P. geniculatus, also including evidence of domiciliary adaptation in parts of Venezuela and Brazil [14–17]. Molecular evidence of human blood feeding by P. geniculatus in the MDC and the adjacent states of Miranda and La Guaira (formerly called Vargas) was first shown in 2005, as intestinal contents from 53 of 88 analyzed specimens reacted positively to human antiserum. Of these, 41% were positive for T. cruzi infection [18]. Panstrongylus geniculatus is considered a poor T. cruzi vector on account of its slow digestion (ca. 1 hour between start of feeding and excretion in adults) [19], but the assumption that active Chagas disease transmission is therefore absent from the MDC may need reconsideration if human blood feeding by T. cruzi-infected triatomines recurrently affects many MDC residents.
To improve general understanding of urban triatomine ecology and help measure the public health risk posed by species such as P. geniculatus in the MDC, the Instituto de Medicina Tropical (IMT) at Universidad Central de Venezuela (UCV) has operated a citizen science-based triatomine collection program since the year 2000. MDC inhabitants that observe triatomines in domiciliary areas are encouraged to submit specimens to the program for taxonomic identification and T. cruzi infection analysis [20]. Safe collection protocols and various other educational materials are provided at http://www.chipo.chagas.ucv.ve. The first program report describes results for the collection years 2007 to 2013 [21]. Of 3551 triatomines analyzed for this time period, 75.2% were infected with T. cruzi and nearly all (98.96%) identified as P. geniculatus. Nymphal triatomine stages, a strong indicator of local reproduction, were also found inside homes in 15 of the MDC’s 32 parishes [21].
The present work represents an update on results from the IMT triatomine collection program, now covering data spanning 2007 to 2019. This update also describes triatomine biting reports by MDC inhabitants and uses cytochrome b (cytb) metabarcoding to provide a snapshot of triatomine blood feeding patterns in the parishes with highest triatomine collection yield. Bloodmeal metabarcoding (most often targeting cytb and 12S) is widely used in the study of Chagas disease vector ecology [22–30]. For example, this tool has helped infer triatomine dispersal rates between wild and human-inhabited ecotopes (e.g., cases of high connectivity and re-infestation potential shown for T. infestans in Bolivia [22] and T. dimidiata in Ecuador [23]), or gauge triatomine diet specialization and overlap (e.g., within [24] and across triatomine species in Colombia [25]).
Results of this study suggest that P. geniculatus has continuously occurred in domiciliary spaces throughout most of the MDC over a surveillance period of 13 years. Metabarcoding analyses highlight humans as a primary blood source among domiciliary specimens collected via the citizen science approach. The domiciliary sample set shows exceptionally high rates of T. cruzi infection, possibly facilitated by contact with various reservoir species (e.g., Didelphis marsupialis, Dasypus novemcinctus, and Rattus spp.) also detected via bloodmeal analysis. Results call for greater attention to human infection rates in the MDC, where T. cruzi seroprevalence has not been directly assessed in recent years. Although acute Chagas disease can be highly symptomatic, it is possible that autochthonous cases are occurring without report and treatment given limited access to medical care. We hope that this study stimulates greater attention towards the study and management of Chagas disease and numerous other public health burdens that Venezuela has been facing in the past decade.
Materials and methods
Triatomine collection
The present study summarizes previously published triatomine collection metrics representing the period 01/01/2007–12/31/2013 [21] in conjunction with results of new collection analyses representing the period 01/01/2014–12/31/2019. The study was performed under approval of the IMT ethical committee and the National Council of Scientific and Technological Research of Venezuela. All triatomines were brought to the IMT by MDC inhabitants as part of the citizen science-based collection program introduced above. Information recorded from contributors included name, address, and phone number for all collection years. Beginning in 2014, contributors were also asked whether the specimen was collected following a suspected biting event or collected independently of any such interaction. Suspected bites were reviewed by medical staff. The MDC is a political administrative division composed of 5 municipalities and 32 parishes covering an area of 810 km2 at an average of 900 meters altitude with a population of 2.95 million inhabitants according to the 2011 census [7] (present day population size has not been officially recorded but may differ significantly due to extensive population movement in and out of Venezuela over the past decade).
Triatomine identification and parasite screening
Specimens collected between 2007 and 2019 were identified to species and sex using the morphological key by Lent and Wygodzinsky [11]. Stomach and intestines were drawn into isotonic saline solution (0.85%) on microscope slides using watchmaker’s forceps. Samples were then stained with Giemsa and examined microscopically for the presence of parasites. Characteristic morphological forms of T. cruzi were identified as described by Hoare [4]. The presence or absence of liquid content within the intestines (potential bloodmeal) was also noted during the dissection process.
Bloodmeal barcoding sample set origin
Mitochondrial DNA metabarcoding was used to profile the bloodmeal content of 108 adult P. geniculatus specimens (40 male and 68 female) obtained from citizen science contributions to the IMT between 2009 and 2016 (S1 Table). The sample represents a random subset of geo-referenced specimens presenting blood-like intestinal content from Petare (n = 47), Baruta (n = 29), and Sucre (n = 32). Sample size reflects study budget. Petare, Baruta, and Sucre parishes differ socioeconomically and in the extent and quality of urban development (Instituto Nacional de Estadística (INE) [7]). Petare on the northeastern side of the MDC contains one of the largest, most densely populated shantytowns in Latin America (ca. 11200 inhabitants per km2 (INE)). Much of Petare is characterized by uncontrolled building practices with limited (green) space between units. Baruta in the southern MDC features higher levels of civil planning in most urban areas, with significantly lower population densities (ca. 3600 inhabitants per km2 (INE)). Baruta more often features larger property units separated from others by areas of green space. Urban aggregations are also more often interrupted by extensive areas of sparsely occupied, naturally forested land. Sucre occupies the northwestern corner of the MDC and is likewise less densely populated (ca. 6700 inhabitants per km2 (INE)) and more environmentally heterogeneous than Petare, extending from its high-density core (Catia neighborhood) into sparsely populated montane areas on the southern edge of Waraira Repano National Park. Urban aggregations also occur along subsections of La Guaira highway leading northwest out of Sucre to the international airport [31]. Aside from these broad characterizations from the literature, this study did not attempt to define environmental differences between parishes given substantial heterogeneity within parishes.
Cytochrome b amplicon library construction
Total DNA extracted from triatomine digestive tracts [32] (Promega Wizard Genomic Purification Kit, USA) was brought to equimolar concentrations and consolidated into 24 pools according to T. cruzi infection status, collection period (2009–2012 vs. 2013–2016), parish, and sex (S1 Table).
A conserved, ~359 bp region of the cytb gene [33] was amplified by polymerase chain reaction (PCR) using forward primer 5’-ACACTGACGACATGGTTCTACACCATCCAACATCTCAGCATGATGAAA-3’ and reverse primer 5’-TACGGTAGCAGAGACTTGGTCTGCCCCTCAGAATGATATTTGTCCTCA-3’. This first-round PCR reaction creates 22 nt overhangs required for subsequent barcoding PCR using universal forward primer 5’-AATGATACGGCGACCACCGAGATCTACACTGACGACATGGTTCTA-3’ and barcoded (*X*) reverse primer (5’-CAAGCAGAAGACGGCATACGAGAT*X*TACGGTAGCAGAGACTTGGTCT-3’. Two DNA extraction controls (dH2O) were also subjected to cytb- and barcoding PCR. The final library was purified using AMPure XP paramagnetic beads (Beckman Coulter).
Cytochrome b amplicon sequence analysis
The 24 amplicon pools and 2 negative controls underwent paired-end 2 x 300 nt sequencing on the Illumina MiSeq platform using Reagent Kit v3, yielding between 159907 and 476984 read-pairs for all pools except pool 12 (9188 read-pairs) and 17 (60926 read-pairs). After quality-filtering by 5’-3’ windowed trimming (-q 25) with sickle v1.33 [34], forward and reverse reads were merged and clustered to consensus haplotypes with the ‘cluster_otus’ command (-otu_radius_pct 3) in USEARCH v8.1 [35]. Potential cross-talk signal was mitigated by discarding consensus haplotypes from a pool if the consensus haplotype was supported by less than 200 reads within the pool but by more reads in other pools. Consensus haplotypes were then defined to species by searching for ≥90% matches to cytb sequences databased by the National Center for Biotechnology Information at https://www.ncbi.nlm.nih.gov/nucleotide/. The 2 negative controls introduced during DNA extraction showed no signal following the procedures described above.
Apart from the 97% similarity threshold-based clustering methods used for between-species classification, we also resolved unique haplotypes from human DNA signal found in the dataset using DADA2 v1.2.2 [36] in R v3.4.1. The DADA2 algorithm infers a parametric model of substitution errors from the data and then determines whether variation around abundant sequence types is statistically consistent or inconsistent with the expected error distribution. Inconsistent reads form new clusters that represent distinct sources of DNA. We applied standard filtering parameters (truncQ = 2, maxEE = c(2, 2)) and performed 3’-trimming using truncLen = c(230, 230).
The positions of mutations distinguishing human haplotypes found using DADA2 were manually identified via sequence alignment and consistency to known human mitochondrial diversity reviewed at https://www.mitomap.org [37].
Finally, we also assessed the relationship between human sample size and number of segregating sites in cytb target alignment by randomly subsampling Latin American mitogenomes sequenced by the 1000 Genomes Project [38] accessible at ftp.1000genomes.ebi.ac.uk. We downloaded multi-fasta files representing 3 available Latin American foci: Lima, Peru; Medellín, Colombia; and Puerto Rico (all municipalities). The dataset from Puerto Rico might constitute the best available proxy for human cytb target diversity in Caracas given similar mitochondrial haplotype distribution observed in prior studies [39,40]. Greater mitochondrial divergence is possible for Lima and Medellín due to limited historic gene flow between populations of the Caribbean region and those of the Andes and Pacific coast [40]. We used custom scripts to draw 100 random cytb target segments (excluding primer binding sites) for every possible sample size from each multi-fasta file. Segregating sites were extracted after each draw using SNP-sites v2.1.3 [41] and average counts plotted with standard deviations using base graphics in R v3.4.1.
Results
Geographical distribution of triatomines in Caracas
General collection results are browsable in a dynamic dashboard provided in S1 File.
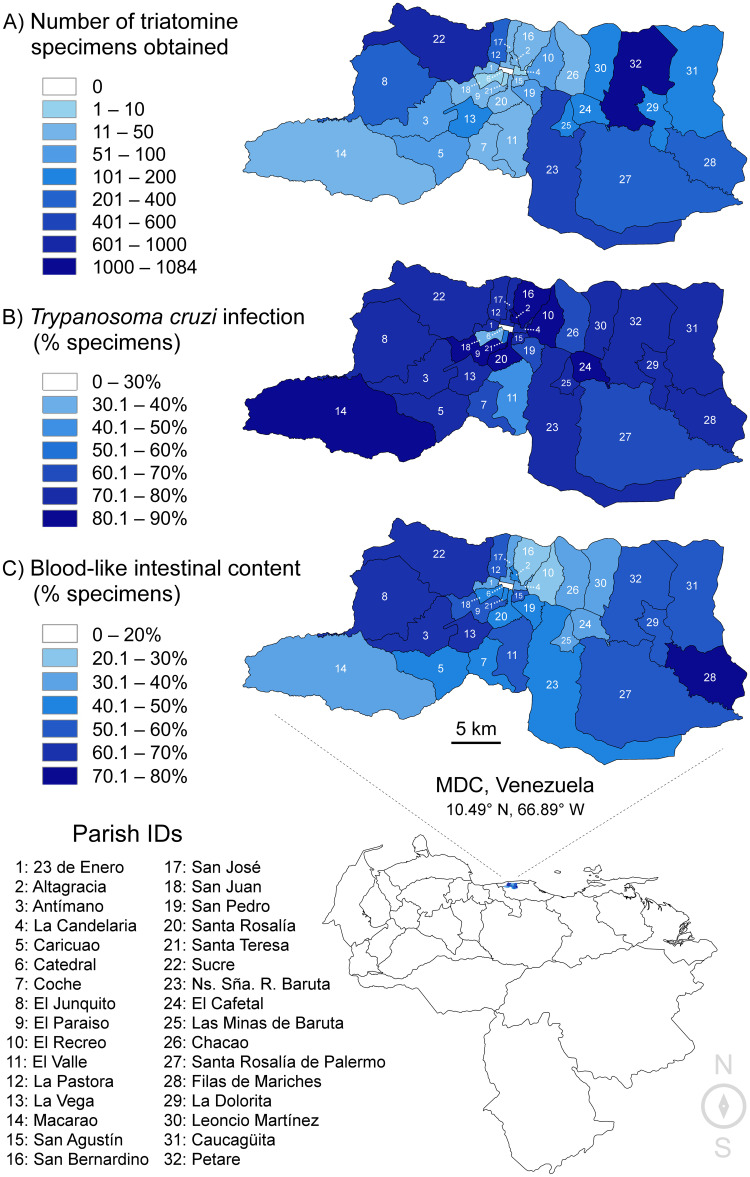
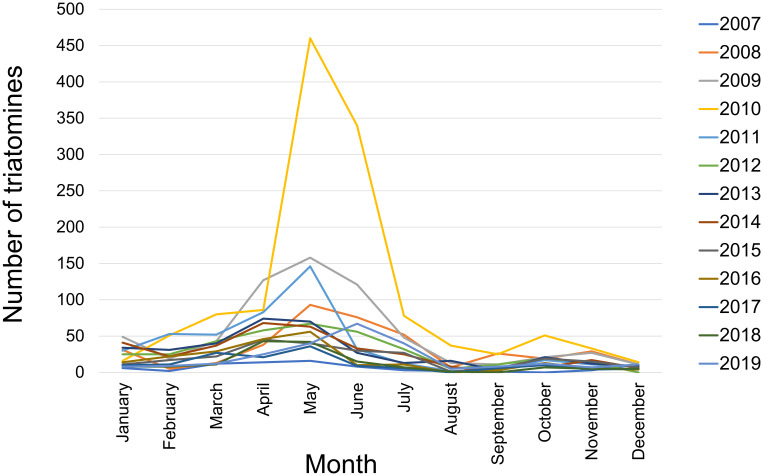
A total of 1321 triatomines were analyzed for the period 2014–2019. Of these, 1310 (99.17%) were taxonomically identified as P. geniculatus, 3 (0.23%) as Triatoma maculata, 5 (0.38%) as Triatoma nigromaculata, 1 (0.08%) as R. prolixus, and 2 (0.15%) as Panstrongylus rufotuberculatus. These metrics are highly similar to those demonstrated previously for the period 2007–2013. Species composition for the combined, 2007–2019 dataset (4872 triatomines) was 4824 (99.01%) P. geniculatus, 17 (0.35%) T. maculata, 26 (0.53%) T. nigromaculata, 3 (0.06%) R. prolixus, and 2 (0.04%) P. rufotuberculatus. The geographical distribution of triatomines collected between 2007 and 2019 spans 31 of 32 MDC parishes. Only Catedral parish yielded no specimens during the study period. The highest numbers of specimens were obtained from Petare, Sucre, and Baruta parishes (Fig 1A and S1 File and Table 1). Fig 2 shows the monthly record of triatomines for the period 2007–2019, the time of highest collection rates occurring between April and July.
Fig 1.
A. Numbers of triatomine specimens obtained from each MDC parish for the collection period 2007–2019. Fill color in the choropleth map represents the total number of specimens obtained per parish, increasing from lighter to darker blues. No specimens were obtained from Catedral parish (#6, white fill). A total of 4872 specimens were obtained across 31 parishes. B. Rates of natural T. cruzi infection in triatomine specimens from each MDC parish for the collection period 2007–2019. Fill color in the choropleth map represents the rate of T. cruzi infection (by microscopy) per parish, increasing from lighter to darker blues. Infection occurred in all parishes from which triatomine specimens were obtained. The analyzed sample set represents 3845 specimens. C. Rates of observing liquid material (potential bloodmeal) in triatomine intestinal content from each MDC parish for the collection period 2007–2019. Fill color in the choropleth map represents visually blooded triatomine occurrence per parish, increasing from lighter to darker blues. The visually blooded condition occurred in all parishes from which triatomine specimens were obtained. The analyzed sample set represents 4422 specimens. A random subset of visually blooded specimens from Petare (#32), Baruta (#23), and Sucre (#22) feature in bloodmeal barcoding analysis. The country map at bottom was created using 1:10 m cultural vectors from https://www.naturalearthdata.com. MDC maps above contain information from OpenStreetMap and OpenStreetMap Foundation, which is made available under the Open Database License. Specifically, parish boundaries were plotted using administrative level 7 lines downloaded with the OpenStreetMap Overpass API [42].
Table 1. Numbers of triatomine specimens obtained from each MDC parish for the collection period 2007–2019, tabulated by species.
Slashes separate values for adult (left of slash) and nymphal triatomine specimens (right of slash). Hyphens (-) are used when no specimens of the indicated species were obtained from the parish. The IDs used for parishes correspond to those used in Fig 1.
| ID | Parish | Panstrongylus geniculatus | Triatoma maculata | Triatoma nigromaculata | Rhodnius prolixus | Panstrongylus rufotuberculatus | Total |
|---|---|---|---|---|---|---|---|
| 1 | 23 de Enero | 15/1 | - | - | - | - | 16 |
| 2 | Altagracia | 38/1 | - | - | - | - | 39 |
| 3 | Antímano | 91/7 | - | - | 1/0 | - | 99 |
| 4 | La Candelaria | 5/0 | - | - | - | - | 5 |
| 5 | Caricuao | 83/2 | 1/0 | - | - | - | 86 |
| 6 | Catedral | - | - | - | - | - | 0 |
| 7 | Coche | 19/0 | 1/0 | - | - | - | 20 |
| 8 | El Junquito | 286/5 | 2/0 | 4/0 | - | - | 297 |
| 9 | El Paraiso | 36/0 | - | - | - | - | 36 |
| 10 | El Recreo | 52/0 | - | - | - | - | 52 |
| 11 | El Valle | 22/0 | - | - | - | - | 22 |
| 12 | La Pastora | 311/8 | - | - | - | - | 319 |
| 13 | La Vega | 115/4 | 2/0 | - | - | - | 121 |
| 14 | Macarao | 25/0 | - | - | - | - | 25 |
| 15 | San Agustín | 15/0 | - | - | - | - | 15 |
| 16 | San Bernardino | 29/0 | - | - | - | - | 29 |
| 17 | San José | 50/0 | - | - | - | - | 50 |
| 18 | San Juan | 4/0 | - | - | 1/0 | - | 5 |
| 19 | San Pedro | 50/0 | 1/0 | - | - | - | 51 |
| 20 | Santa Rosalía | 11/0 | - | - | - | - | 11 |
| 21 | Santa Teresa | 7/0 | - | - | - | - | 7 |
| 22 | Sucre | 578/40 | 1/0 | - | - | - | 619 |
| 23 | Ns. Sña. R. Baruta | 526/9 | 2/0 | 4/0 | - | - | 541 |
| 24 | El Cafetal | 171/3 | - | - | - | - | 174 |
| 25 | Las Minas de Baruta | 96/8 | 1/0 | - | - | - | 105 |
| 26 | Chacao | 35/2 | 0/1 | - | 1/0 | - | 39 |
| 27 | Sta. Rosalía de Palermo | 307/9 | 3/0 | 9/0 | - | 1/0 | 329 |
| 28 | Filas de Mariches | 254/14 | 2/0 | 2/0 | - | - | 272 |
| 29 | La Dolorita | 98/7 | - | - | - | - | 105 |
| 30 | Leoncio Martínez | 185/5 | - | - | - | - | 190 |
| 31 | Caucagüita | 104/2 | - | 3/0 | - | 109 | |
| 32 | Petare | 1033/46 | - | 4/0 | - | 1/0 | 1084 |
| Total | 4651/173 | 16/1 | 26/0 | 3/0 | 2/0 | 4872 |
Fig 2. Monthly triatomine yield for the MDC collection period 2007–2019.
Line color indicates collection year. Media attention to an oral Chagas disease outbreak in May 2010 may help explain the high collection numbers of May and June 2010.
T. cruzi infection rates in triatomines
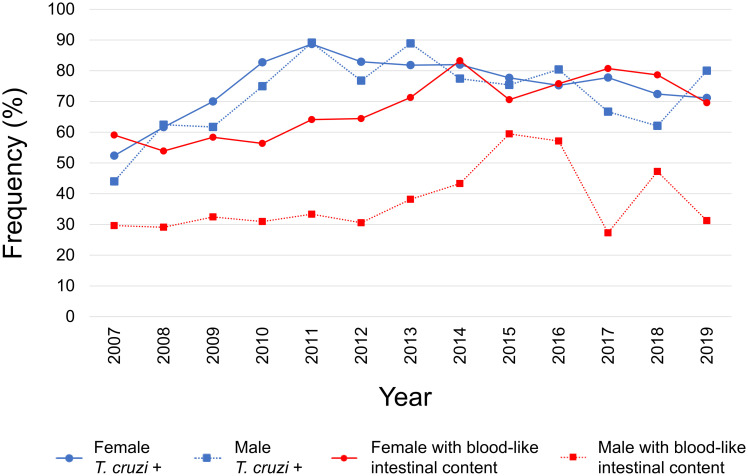
Of 4872 studied triatomines, 3845 (78.9%) could be evaluated for natural infection with T. cruzi. The remaining insects were either in a state of internal decomposition or too dry at the time of microscopic analysis. These 3845 specimens included 3711 adults, of which 55.8% (2146) were females and 40.7% (1565) were males. Rates of natural infection by T. cruzi were 77.2% (1657/2146) for females and 73.9% (1156/1565) for males. These rates are categorized by year in Fig 3 and S2 Table. They are categorized by parish in Fig 1B and S3 Table.
Fig 3. Annual rates of natural T. cruzi infection and observations of blood-like intestinal content in male and female triatomine specimens for the MDC collection period 2007–2019.
Blue color indicates the rate of T. cruzi infection among 3845 specimens analyzed. Red color indicates the rate at which blood-like intestinal content was observed among 4422 specimens analyzed. Circles and squares indicate values for females and males, respectively.
Infection by T. cruzi was 75.4% (2873/3810) for P. geniculatus, 47.4% (9/19) for T. nigromaculata, 50% (6/12) for T. maculata, 50% (1/2) for R. prolixus, and 100% (2/2) for P. rufotuberculatus (S2 Table).
Presence of blood in triatomine digestive tracts
Presence or absence of liquid content in the intestines (potential bloodmeal) was determined for 4422 (90.8%) of 4872 triatomines. These 4422 specimens included 4270 adults, of which 2513 were females (58.9%) and 1757 were males (41.1%). Blood occurred in 65% (1633/2513) of these females and 34.5% (607/1757) of these males. The distribution of blood-containing triatomines is categorized by sex and year in Fig 3 and S4 Table, and by parish in Fig 1C and S5 Table. Only in the species T. nigromaculata did blood occur more often in the digestive tracts of males (5/7) than in those of females (7/15).
Nymphal triatomine stages
Of the 4872 triatomines collected between 2007 and 2019, 174 (3.6%) were identified as nymphs (173 P. geniculatus and 1 T. maculata) (Table 1 and S1 File). These nymphs were found in 18 of the MDC’s 32 parishes, varying in number over the years. Follow-up questioning clarified that all nymphs were found inside houses, not in peripheral areas.
Of 134 nymphs received in adequate condition for the determination of infection with parasites, 78 (58.2%) were positive for T. cruzi. Rates of infection were 0% (0/3) for nymphal stage II (NII), 63.3% (19/30) for nymphal stage III (NIII), 60.3% (41/68) for nymphal stage 4 (NIV), and 54.5% (18/33) for nymphal stage V (NV) (S2 Table).
Liquid content within the intestines (potential bloodmeal) was detected in 123 (80.9%) of 152 evaluated nymphs. The presence of liquid content within the intestines was 100% (3/3) for NII, 80.6% (29/36) for NIII, 76.9% (60/78) for NIV, and 88.6% (31/35) for NV (S4 Table).
S6 Table shows the percentage of nymphs collected in relation to the total number of triatomines collected by parish per year. The parishes with the highest percentage of nymph collection across years were Chacao (7.7%), Minas de Baruta (7.6%), Antímano (7.1%), La Dolorita (6.7%), and Sucre (6.5%). The highest absolute numbers of collected nymphs across years were obtained from Petare (46), Sucre (40), and Filas de Mariche (14) parishes. It is important to emphasize that all nymphs were found within houses, most of them in the bedrooms.
Blood feeding patterns
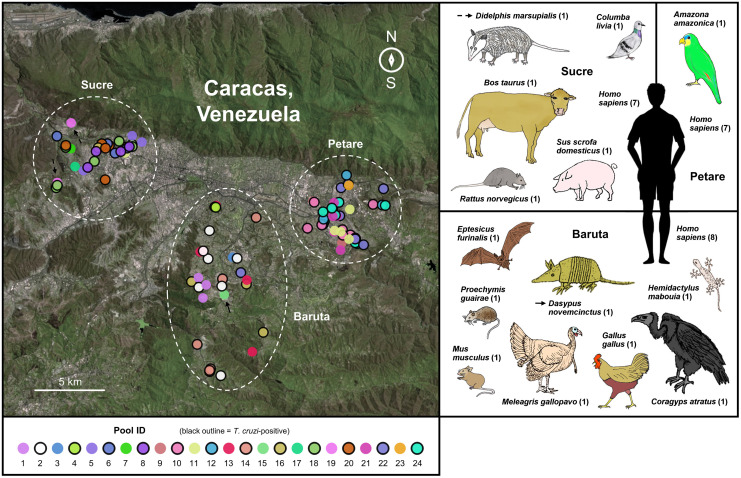
Fifteen distinct bloodmeal sources were identified among the 24 sample pools (see Methods) representing 108 randomly selected, blood-containing adult P. geniculatus specimens collected between 2009 and 2016 (Tables 2 and 3, S1 Table and Fig 4 and S2 File). These included the major sylvatic T. cruzi reservoir species Dasypus novemcinctus (nine-banded armadillo) and Didelphis marsupialis (common opossum) (see arrows in Fig 4), 4 livestock/poultry species (Sus scrofa domesticus, Bos taurus, Meleagris gallopavo, Gallus gallus), 3 wild avian species (Columba livia, Coragyps atratus, Amazona amazonica), 3 rodent species (Rattus norvegicus, Mus musculus, Proechimys guairae), the brown bat Eptesicus furinalis, and the house gecko Hemidactylus mabouia.
Table 2. Vertebrate sources of cytb DNA identified in P. geniculatus bloodmeals for the MDC collection period 2009–2012.
Dissection pools 1–12 are organized in columns based on the parish from which contributing samples were obtained. Pool sizes (i.e., the number of samples contributing to each pool) range from 1–7. Vertebrate species with cytb sequences matching pool sequences at ≥90% similarity are listed in rows numbered 1–4. Corresponding read support values are provided at right prior to the ‘Match’ column indicating the fraction of nucleotides matching between pool sequences and the reference (excluding primer binding sites).
| Parish → | Baruta | Sucre | Petare | Match → | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pool size → | 3 | 7 | 1 | 1 | 4 | 4 | 1 | 4 | 3 | 7 | 5 | 4 | |
| Pool ID → | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
| 1) Homo sapiens | 1625 | 7122 | 512 | 8268 | 5153 | 5220 | 0 | 7205 | 5983 | 6015 | 7334 | 0 | 307/307 |
| 2) Amazona amazonica | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 302/307 |
| 3) Mus musculus | 0 | 834 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 307/307 |
| 4) Columba livia | 0 | 0 | 0 | 0 | 0 | 0 | 4075 | 0 | 0 | 0 | 0 | 0 | 307/307 |
Table 3. Vertebrate sources of cytb DNA identified in P. geniculatus bloodmeals for the MDC collection period 2013–2016.
Dissection pools 13–24 are organized in columns based on the parish from which contributing samples were obtained. Pool sizes (i.e., the number of samples contributing to each pool) range from 1–12. Vertebrate species with cytb sequences matching pool sequences at ≥90% similarity are listed in rows numbered 1–12. Corresponding read support values are provided at right prior to the ‘Match’ column indicating the fraction of nucleotides matching between pool sequences and the reference (excluding primer binding sites).
| Parish → | Baruta | Sucre | Petare | Match → | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pool size → | 5 | 6 | 1 | 5 | 2 | 8 | 2 | 7 | 7 | 12 | 1 | 8 | |
| Pool ID → | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | |
| 1) Homo sapiens | 6618 | 10100 | 8649 | 3309 | 355 | 10100 | 2426 | 8632 | 11900 | 9026 | 10100 | 9035 | 307/307 |
| 2) Dasypus novemcinctus | 0 | 0 | 47 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 306/307 |
| 3) Sus scrofa domesticus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 45 | 0 | 0 | 0 | 0 | 307/307 |
| 4) Proechimys guairae | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 304/307 |
| 5) Eptesicus furinalis | 18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 300/306 |
| 6) Didelphis marsupialis | 0 | 0 | 0 | 0 | 0 | 0 | 15400 | 0 | 0 | 0 | 0 | 0 | 305/307 |
| 7) Hemidactylus mabouia | 0 | 0 | 0 | 20 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 307/307 |
| 8) Meleagris gallopavo | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 306/307 |
| 9) Bos taurus | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5 | 0 | 0 | 0 | 0 | 307/307 |
| 10) Coragyps atratus | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 304/306 |
| 11) Rattus norvegicus | 0 | 0 | 0 | 0 | 0 | 642 | 0 | 0 | 0 | 0 | 0 | 0 | 307/307 |
| 12) Gallus gallus | 0 | 0 | 517 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 307/307 |
Fig 4. Vertebrate DNA sources identified in 108 pooled P. geniculatus bloodmeals from Petare, Baruta, and Sucre parishes for the MDC collection period 2009–2016.
Points in the map indicate the geographical origin of the 108 samples contributing to the 24 dissection pools used in cytb analysis. Point fill color indicates pool membership. Pools composed of T. cruzi-positive samples are outlined in black. Arrows indicate pools presenting cytb DNA from the T. cruzi reservoir species D. marsupialis and D. novemcinctus. Numbers in parentheses indicate the number of pools in which the corresponding vertebrate blood source was detected. The map base layer represents Scene ID LC80040532015120LGN01 from the Landsat 8 OLI/TIRS C1 Level-1 dataset–courtesy of the U.S. Geological Survey.
While the above-listed species were detected in only 1 pool each and predominantly in Baruta parish, human blood was detected in all but 2 of the 24 pools. Human sequences also dominated non-human sequences in abundance by at least an order of magnitude in most pools (Tables 2 and 3). Nine distinct human haplotypes were identified, and these differed from one another by 8 common single-nucleotide polymorphisms (SNPs) catalogued at https://www.mitomap.org (S1A Fig) [37]. The observed SNP diversity was compared with that obtained when subsampling sequences from the 1000 Genomes Project (1KGP) [38] to help gauge how many humans were fed upon by the 108 triatomines from which bloodmeals were pooled. Randomly subsampling 1KGP sequences available for Lima (Peru), Medellín (Colombia), and Puerto Rico (USA), an average subsample size of at least 75 individuals was required to observe 8 SNPs within the cytb locus amplified herein (S1B Fig). If SNP diversity in these populations is consistent with that in Caracas, results suggest that more than 2 human bloodmeals occurred for every 3 triatomines in the barcoded sample set.
Given that human cytb DNA was detected in 22 of 24 pools, no relationship between T. cruzi infection status and human vs. non-human blood feeding events was observed. Numbers of non-human bloodmeal detections were also similar between T. cruzi-positive (11 of 12) and T. cruzi-negative (11 of 12) pools. Infection status was distinct, however, in pools involving the 5 avian species detected in this study. These 4 pools were exclusively negative for T. cruzi infection.
Eleven of 14 non-human bloodmeal sources corresponded to the 2013–2016 collection period, and 9 of 14 non-human bloodmeal sources corresponded to male triatomines.
Non-human bloodmeal sources were most diverse in Baruta (8 different sources), followed by Sucre (5) and Petare parish (1).
Reports of triatomine biting
During the 2014–2019 study time period, contributors were also asked whether or not they noticed having been bitten near the time of triatomine discovery. A total of 1153 triatomine submissions included responses to this question and were additionally assayed for the presence/absence of liquid content within the intestines (potential bloodmeal). Of these, 721 were submitted by contributors reporting bites. These bites were verified as triatomine-like by medical staff, and 85.4% (616/721) of corresponding triatomine specimens were positive for the presence of liquid content within the intestines. Forty-five of these bite- and liquid content-positive specimens also featured in the above bloodmeal metabarcoding analysis, and all belonged to pools positive for human cytb DNA. Of the 337 submissions by contributors reporting that no triatomine bite had occurred, 27.3% (92/337) were positive for the presence of liquid content within the intestines (S1 File). A chi-squared test indicates that these contributor responses on biting events predict triatomine feeding status (presence or absence of liquid content within the intestines) (p < 0.001).
Additionally, 95 specimens were associated with contributors that reported not knowing if they had been bitten or not. Of these, 61.1% (58/95) were positive for the presence of liquid content within the intestines.
Discussion
This study assessed spatiotemporal triatomine prevalence, T. cruzi infection, and blood feeding patterns in the capital of Venezuela by analyzing > 4000 triatomine specimens encountered in domiciliary areas by inhabitants of the city and submitted to the IMT between 2007 and 2019.
The IMT received an average of 375 specimens per year during the 13-year study period, with collection highs generally occurring between April and July. The vast majority (99.01%) of these specimens were P. geniculatus and most were positive for T. cruzi infection (75.2%), with similar rates observed between sexes. Infection rates appear to increase between 2007 and 2011, then remain relatively constant until 2019. A majority (52.5%) of adult specimens, more often females (65%) than males (34.5%), also presented intestinal content suggestive of vertebrate blood. This visually blooded condition was especially likely when contributors reported having been bitten near the time of triatomine discovery. Human cytb DNA was detected in 22 of 24 visually blooded dissection pools subjected to barcoding analysis. Polymorphism levels among the detected haplotypes suggest that more than two human bloodmeals occurred for every three triatomines in the barcoded sample set. The DNA of 14 other animal species was also detected, but only in a single dissection pool each. The study additionally documented triatomine nymphs (stages II–V) in domiciliary areas in 18 of the MDC’s 32 parishes. Nymphs accounted for >5% collected specimens in various parishes with strong total triatomine collection yields (e.g., Sucre and Filas de Mariches). Nymphs were also generally positive for T. cruzi infection (58.2%) and frequently in a visually blooded state (80.9%).
This study’s recurrent observation of P. geniculatus in domiciliary areas adds to accumulating evidence for domiciliary habitat use by this primarily sylvatic species [9]. Domiciliary P. geniculatus occurrence was noted as early as 1986 in the MDC [43] and in at least seven other Chagas disease-endemic countries in later years [9,14,44–51]. Intrusive domiciliary behavior by P. geniculatus is facilitated by adult flight ranges of at least 2 km and is generally associated with nocturnal attraction to light. Climatic influences on intrusion behavior have been suggested in some triatomine species but are not well resolved for P. geniculatus [52–56]. This study’s highest collection yields generally occurred during months presenting higher rainfall in northern Venezuela (April–July), possibly reflecting climatic influences on triatomine proliferation and dispersal behavior as shown for many others insect species [57,58]. Future studies using matched data on sylvatic and domiciliary prevalence patterns may help determine whether collection peaks reflect overall abundance phenology or are specific to domiciliary intrusion events.
Numerous encounters of triatomine nymphs inside houses, now recorded in a total of 18 MDC parishes (previously 15 parishes for the period 2007–2013) [21], represents one of the key findings of this study. Given their lack of wings, nymphs are very likely to have hatched in near proximity to where they are discovered (in this study often in bedrooms and specifically beneath mattresses). Similar is true for the location of nymphal blood feeding and parasite infection. The presence of blood-fed nymphs in domiciliary areas thus strongly indicates successful courses of domiciliary reproduction and the possibility that long-term domiciliation is developing or established. The occurrence of P. geniculatus eggs and nymphal stages within domiciliary areas has been documented in various countries [14,59–61]. Some laboratory and field studies also suggest that adaptive processes are occurring in different microhabitats within the MDC [62–64]. Aldana et al., for example, suggest that intra-domiciliary specimens collected in 2008–2009 from Altagracia and Petare parishes exhibit adaptive wing size reductions relative to rural and sylvatic controls from Lara state [63]. It will be important to further quantify rates of successful reproduction and identify microniches where eggs are deposited in domiciliary areas of the MDC. Long-term triatomine domiciliation is pivotal epidemiologically because it suggests that domiciliary conditions and resources suffice for survival (food, shelter from predators, etc.) and reproduction without necessary connectivity to sylvatic ecotopes. This scenario can discouple domiciliary and sylvatic transmission cycles and implies different intervention priorities, particularly when considering the significant presence of synanthropic T. cruzi reservoirs like opossums and rats [65–67] that facilitate an urban enzootic cycle of the parasite. Measures to reduce urban habitat suitability, e.g., eliminating indoor breeding niches or limiting the availability of synanthropic feeding sources such as rodents in urban areas, may show greater success in mitigating disease risk than measures focusing on inhibiting intrusion from peripheral sylvatic environment.
Domiciliary habitat intrusion and potentially also long-term domiciliation processes become more concerning in conjunction with the high rates of T. cruzi infection this study reports from domiciliary collection. Triatomine infection rates of similar magnitude are sporadically reported in more rural or exclusively sylvatic areas [9,68,69] but very rarely in such a highly urban context. The city of Cochabamba in Bolivia is a rare example of comparable urban scale with similar levels of triatomine infection in some districts [50]. Cochabamba also suffers from high human seropositivity for T. cruzi [70]. Rates of human infection in Caracas, however, are currently unknown.
This study’s bloodmeal analysis offers insights on the transmission context underlying high rates of T. cruzi infection in domiciliary triatomines of the MDC. For example, results illuminate possible avenues of connectivity between sylvatic and urban T. cruzi transmission cycles, exposing bloodmeals on didelphid opossum and armadillo (D. novemcinctus) in samples from neighborhoods on the perimeter of the city (e.g., Barrio El Progreso). These two host mammals are strongly associated with T. cruzi lineages TcI and TcIII (respectively) in Venezuela [65,71]. Triatomine contact to such canonical reservoir hosts, perhaps primarily in semi-forested urban areas characteristic of Baruta parish, combined with large rodent reservoir populations in much of the city (especially impoverished regions such as Petare) likely contribute to the high rates of T. cruzi infection documented herein. Previous evidence from the MDC that P. geniculatus infection by T. cruzi primarily involves TcI (e.g., 269 of 270 previously analyzed infections presenting TcI and 1 of 270 presenting TcIII [21]), with TcI also often found in rats [65], further aligns with the bloodmeal barcoding results of this study. Importantly, however, bloodmeals on human hosts were detected far more often than those on common synanthropic animals such as mice/rats (identified in three pools), pigeons (one pool), or dogs (not detected). While very prominent, multiple observation of human blood feeding is not unexpected; similar has been reported from various less urban environments where human density relative to that of other animals is lower than in the MDC [69,72,73]. This study’s bloodmeal analysis also specifically addressed domiciliary triatomines found in a visually blooded state. Human blood detection occurred most often in pools of female triatomines of the sample set, with these accounting for only five of 14 non-human bloodmeal sources identified in cytb analysis (despite accounting for 68 of 108 analyzed specimens). This trend could potentially indicate sex-specific behavioral differences, for example, that female triatomines are more likely to focus activity on indoor areas that favor egg deposition and species survival as opposed to moving back and forth between indoor and outdoor areas.
Further investigation with larger and more systematically obtained intra- and extra-domiciliary sample sets is certainly required to determine the extent to which patterns observed in bloodmeal analysis reflect vector behavior and/or ecological circumstances (prey abundance, accessibility, etc.) as opposed to various methodological details–e.g., this study’s sampling focus on visually blooded specimens, and its single-marker metabarcoding approach: although the cytb fragment sequenced at high depth in this study (median of >260000 read-pairs per sample pool) represents one of the best validated markers of arthropod bloodmeal diversity [74], a multi-marker method involving also shorter DNA targets may have enhanced amplification from degraded (digested) DNA fragments and could have provided additional control against stochastic dropout or amplification bias that may affect some taxa. For example, while observed bloodmeal diversity (15 species) and hit depths are generally similar to those reported in other studies of triatomine feeding behaviour [27], the absence of dog DNA in this study’s sample set was unexpected (dogs represent a common triatomine feeding source in various contexts [27,75,76]) and could have been further verified with additional marker screening. Inferences from bloodmeal analysis were also limited by the decision to pool sets of intestinal samples before indexing. This minimized the extent to which identified bloodmeals could be tied to individual specimens. For example, while human haplotype diversity analysis helped estimate that ≥75 distinct human sources contributed to the human DNA signal detected across 108 triatomine specimens, it was not possible to delineate how these sources were distributed across individual specimens, e.g., to determine how often individual specimens fed on more than a single human individual. One reason why resolving such details is valuable relates to the probability of P. geniculatus defecation during host contact, which depends on whether feeding to repletion generally occurs via one complete bloodmeal on a single host or via multiple incomplete bloodmeals across multiple hosts [19]. A controlled experiment evaluating blood source DNA detection half-life would further improve inferences on individual triatomine bloodmeal diversity across multiple feeds and potentially help characterize individual triatomine movements between habitats associated with different types of prey. Such higher-resolution analysis might also help better understand whether the occurrence of T. cruzi-refractory animals (e.g., this study detected bloodmeals from 5 avian species as well as one species of gecko) has a significant effect on the stability of enzootic T. cruzi transmission.
We note various further complexities surrounding the citizen science-based sample collection approach. Spatial patterns in triatomine collection yield, for example, may be influenced by variable awareness of the IMT collection program and more generally of Chagas disease. Similar citizen science-based work in Texas (USA) has shown that triatomine submissions vary temporally in response to media attention [77], and this effect may have contributed to occasional aberrations in collection yield in this study (e.g., exceptional collection yield in May 2010 coincides with an oral Chagas disease outbreak among children in Caracas [78]). The probability of triatomine encounter and collection likely also differs by species and circumstances such as whether a human biting event previously occurred. Aside from representative sampling, data completion and precision levels are challenging to maintain in citizen science. As yet, only a subset of this study’s sample set could be assigned specific geographic coordinates due to difficulties in describing addresses, especially from areas where standard naming and numbering systems do not apply. Stigma likely also plays a role in the report of microgeographic details such as the precise domiciliary space (e.g., type of room or extent of insulation from the outside) in which a specimen is found or personal information such as the possible occurrence of triatomine biting events.
Taken together, this study from the capital of Venezuela shows widespread domiciliary habitat use (including cases of reproduction) by P. geniculatus triatomines exhibiting high rates of T. cruzi infection. Many specimens are encountered in a blood-fed state which participant surveys as well as molecular analyses suggest often to correspond to a recent human blood feeding event. But how do these observations translate to human risk of active vectorial infection? Unlike highly effective vectors such as adult R. prolixus and T. infestans which generally defecate within ca. 30 minutes of initiating a bloodmeal [79,80], defecation is suggested to occur within approximately one hour after bloodmeal initiation for adult P. geniculatus [19]. This interval is only slightly shorter than the average time needed to complete a full bloodmeal without interruption [19]. The likelihood that P. geniculatus feces containing infective parasites are released during human contact is therefore expected to be lower than in several other triatomine species. Vectorial capacity and human infection risk are however highly context-specific, conditioned by a multitude of parameters such as host/vector densities, infection rates, and infection intensities whose complex interplay in the unique urban epidemiological setting represented by the MDC must be further investigated. A systematic human serological survey for T. cruzi occurrence in the MDC is important to this end. Other important investigative and precautionary measures include developing a better understanding of domiciliary niches where triatomine reproduction occurs, reducing urban T. cruzi reservoir populations, promoting housing materials that reduce triatomine intrusion, tracking and intervening against patterns of parasite-food source contamination [81], further educating the public on Chagas disease epidemiology, and increasing cognizance among medical workers towards the possibility of acute T. cruzi infection and associated clinical profiles. All of these objectives are especially challenging in Venezuela’s current socioeconomic situation, and broad support in funding and resources is key.
Supporting information
This dataset contains triatomine ID code, species, state, parish, sex, age, parasitology result, and presence of liquid material (potential bloodmeal) in the intestinal content. The file features interactive dashboard elements (users may subset collection statistics by selecting categories of interest).
(XLSX)
Sequences include primer-binding regions.
(TXT)
Specimens represent a random visually blooded subset from Petare, Baruta, and Sucre parishes. Nymphal stages were not analyzed.
(XLSX)
Abbreviations ‘NEG’ and ‘POS’ denote negative vs. positive T. cruzi infection status inferred via microscopy.
(XLSX)
Infection status was inferred via microscopy.
(XLSX)
Abbreviations ‘B’ and ‘NB’ denote presence and absence of blood-like intestinal content, respectively.
(XLSX)
The presence of blood-like intestinal content is also referred to as the ‘visually blooded’ condition elsewhere in this work.
(XLSX)
Values represent percentages of collected specimens representing nymphs.
(XLSX)
A) Human cytb target diversity in pooled P. geniculatus bloodmeals. The sequence positions and nucleotide changes distinguishing the 9 human haplotypes (green circles) detected in this study are shown in white boxes. Labels outside boxes indicate the number of times each mutation has been recorded at https://www.mitomap.org [37] to help verify sequence denoising performed using the R package DADA2 [36]. B) Relationship between human sample size and number of segregating sites in cytb target alignment using publicly available datasets from the 1000 Genomes Project. One hundred random cytb target segments (excluding primer binding sites) were drawn for every possible sample size from multi-fasta files representing American mitogenomes from Lima, Peru; Medellín, Colombia; and Puerto Rico (all municipalities) [38]. Segregating sites were extracted after each draw using SNP-sites v2.1.3 [41].
(TIF)
Acknowledgments
We thank all the kind inhabitants of the MDC who have contributed data to this project. We thank Julie Galbraith at Glasgow Polyomics, University of Glasgow, for sequencing work. Thanks also to A. Schwabl for illustrations and A. Busetti for help with library preparation.
Data Availability
All Revelant data are withn the manuscript and its Supporting information files.
Funding Statement
This work was supported by the Fondo Nacional de Ciencia, Tecnología e Innovación (FONACIT-Venezuela) (grant G-2005000827 to HJC) and the Scottish Funding Council (Small Grants Fund SFC/AN/12/2017 to MSL). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Rassi A, Marin-Neto JA. Chagas disease. Lancet. 2010;375:1388–402. doi: 10.1016/S0140-6736(10)60061-X [DOI] [PubMed] [Google Scholar]
- 2.Monteiro FA, Weirauch C, Felix M, Lazoski C, Abad-Franch F. Evolution, systematics, and biogeography of the Triatominae, vectors of Chagas disease. Adv Parasitol. 2018;99:265–344. doi: 10.1016/bs.apar.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 3.Galvão C. Taxonomy. In: Guarneri A, Lorenzo M, editors. Triatominae—the biology of Chagas disease vectors. Cham: Springer; 2021. p. 15–38. [Google Scholar]
- 4.Hoare CA. The trypanosomes of mammals. A zoological monograph. Blackwell Scientific Publications; 1972.
- 5.Grillet ME, Hernández-Villena J V., Llewellyn MS, Paniz-Mondolfi AE, Tami A, Vincenti-Gonzalez MF, et al. Venezuela’s humanitarian crisis, resurgence of vector-borne diseases, and implications for spillover in the region. Lancet Infect Dis. 2019;19:e149–61. doi: 10.1016/S1473-3099(18)30757-6 [DOI] [PubMed] [Google Scholar]
- 6.Añez N, Crisante G, Rojas A, Segnini S, Espinoza-Álvarez O, Teixeira MMG. Update on Chagas disease in Venezuela during the period 2003–2018. A review. Acta Tropica. 2020;203:105310. doi: 10.1016/j.actatropica.2019.105310 [DOI] [PubMed] [Google Scholar]
- 7.Instituto Nacional de Estadística. XIV Censo nacional de población y vivienda 2011. http://www.ine.gob.ve/CENSO2011/
- 8.Soto-vivas A. Clave pictórica de triatóminos (Hemiptera:Triatominae) de Venezuela. Bol Mal Salud Amb. 2009;49:259–74. [Google Scholar]
- 9.Vivas RJ, García JE, Guhl F, Hernández C, Velásquez N, Ramírez JD, et al. Systematic review on the biology, ecology, genetic diversity and parasite transmission potential of panstrongylus geniculatus (Latreille 1811) in Latin America. Mem Inst Oswaldo Cruz. 2021;116:e200528. doi: 10.1590/0074-02760200528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galvão C, Carcavallo RU, da Silva Rocha D, Jurberg J, Cleber galvão, carcavallo rodolfo da silva rocha dayse & jurberg josé. A checklist of the current valid species of the subfamily Triatominae Jeannel, 1919 (Hemiptera, Reduviidae) and their geographical distribution, with nomenclatural and taxonomic notes. Zootaxa. 2003;36:1–2. [Google Scholar]
- 11.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae), and their significance as vectors of Chagas disease. Bull Am Mus Nat Hist. 1979;163:123–520. [Google Scholar]
- 12.Guhl F. Geographical distribution of Chagas Disease. In: Telleria J, Tibayrenc M, editors. American Trypanosomiasis, Chagas Disease. 2nd ed. Amsterdam: Elsevier; 2017. p. 89–112. [Google Scholar]
- 13.Costa J, Dale C, Galvão C, Almeida CE, Dujardin JP. Do the new triatomine species pose new challenges or strategies for monitoring Chagas disease? An overview from 1979–2021. Mem Inst Oswaldo Cruz. 2021;116:e210015. doi: 10.1590/0074-02760210015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reyes-Lugo M, Rodriguez-Acosta A. Domiciliation of the sylvatic Chagas disease vector Panstrongylus geniculatus Latreille, 1811 (Triatominae: Reduviidae) in Venezuela. Trans R Soc Trop Med Hyg. 2000;94:508. doi: 10.1016/s0035-9203(00)90068-3 [DOI] [PubMed] [Google Scholar]
- 15.Feliciangeli MD, Carrasco H, Patterson JS, Suarez B, Martínez C, Medina M. Mixed domestic infestation by Rhodnius prolixus StäL, 1859 and Panstrongylus geniculatus Latreille, 1811, vector incrimination, and seroprevalence for Trypanosoma cruzi among inhabitants in El Guamito, Lara State, Venezuela. American Journal of Tropical Medicine and Hygiene. 2004;71:501–5. [PubMed] [Google Scholar]
- 16.Valente VC, Valente SA, Noireau F, Carrasco HJ, Miles MA. Chagas disease in the Amazon Basin: association of Panstrongylus geniculatus (Hemiptera: Reduviidae) with domestic pigs. J Med Entomol. 1998;35:99–103. doi: 10.1093/jmedent/35.2.99 [DOI] [PubMed] [Google Scholar]
- 17.da C Valente V. Potential for domestication of Panstrongylus geniculatus (Latreille, 1811) (Liemiptera, Reduviidae, Triatominae) in the municipality of Muaná, Marajó Island, state of Pará, Brazil. Mem Inst Oswaldo Cruz. 1999;94:399–400. [DOI] [PubMed] [Google Scholar]
- 18.Carrasco HJ, Torrellas A, García C, Segovia M, Feliciangeli MD. Risk of Trypanosoma cruzi I (Kinetoplastida: Trypanosomatidae) transmission by Panstrongylus geniculatus (Hemiptera: Reduviidae) in Caracas (Metropolitan District) and neighboring States, Venezuela. Int J Parasitol. 2005;35:1379–84. doi: 10.1016/j.ijpara.2005.05.003 [DOI] [PubMed] [Google Scholar]
- 19.Wolff M., Castillo D. Evidencias de domesticación y aspectos biológicos de Panstrongylus geniculatus (Latreille, 1811) (Hemiptera: Reduviidae). Acta Entomol Chil. 2000;24:77–83. [Google Scholar]
- 20.SIGSEM. http://www.chipo.chagas.ucv.ve/vista/index.php
- 21.Carrasco HJ, Segovia M, Londoño JC, Ortegoza J, Rodríguez M, Martínez CE. Panstrongylus geniculatus and four other species of triatomine bug involved in the Trypanosoma cruzi enzootic cycle: high risk factors for Chagas’ disease transmission in the Metropolitan District of Caracas, Venezuela. Parasit Vectors. 2014;7:602. doi: 10.1186/s13071-014-0602-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buitrago R, Bosseno MF, Depickère S, Waleckx E, Salas R, Aliaga C, et al. Blood meal sources of wild and domestic Triatoma infestans (Hemiptera: Reduviidae) in Bolivia: connectivity between cycles of transmission of Trypanosoma cruzi. Parasit Vectors. 2016;9:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong YY, Sornosa Macias KJ, Guale Martínez D, Solorzano LF, Ramirez-Sierra MJ, Herrera C, et al. Molecular epidemiology of Trypanosoma cruzi and Triatoma dimidiata in costal Ecuador. Infection, Genetics and Evolution. 2016;41:207–12. doi: 10.1016/j.meegid.2016.04.001 [DOI] [PubMed] [Google Scholar]
- 24.Erazo D, Gottdenker NL, González C, Guhl F, Cuellar M, Kieran TJ, et al. Generalist host species drive Trypanosoma cruzi vector infection in oil palm plantations in the Orinoco region, Colombia. Parasit Vectors. 2019;12:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Arias-Giraldo LM, Muñoz M, Hernández C, Herrera G, Velásquez-Ortiz N, Cantillo-Barraza O, et al. Identification of blood-feeding sources in Panstrongylus, Psammolestes, Rhodnius and Triatoma using amplicon-based next-generation sequencing. Parasit Vectors. 2020;13:434. doi: 10.1186/s13071-020-04310-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pessanha TS, Herrera HM, Jansen AM, Iñiguez AM. “Mi Casa, Tu Casa”: the coati nest as a hub of Trypanosoma cruzi transmission in the southern Pantanal biome revealed by molecular blood meal source identification in triatomines. Parasit Vectors. 2023;16: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Owens D, Glenn TC, Varian CP, Means N, Kieran TJ, Saldaña A, et al. Blood meal source characterization using Illumina sequencing in the Chagas disease vector Rhodnius pallescens (Hemiptera: Reduviidae) in Panamá. J Med Entomol. 2017;54:1786–9. [DOI] [PubMed] [Google Scholar]
- 28.Balasubramanian S, Curtis-Robles R, Chirra B, Auckland LD, Mai A, Bocanegra-Garcia V, et al. Characterization of triatomine bloodmeal sources using direct Sanger sequencing and amplicon deep sequencing methods. Sci Rep. 2022;12:10234. doi: 10.1038/s41598-022-14208-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quiroga N, Correa JP, Campos-Soto R, Juan ES, Araya-Donoso R, González CR, et al. Blood-meal sources and Trypanosoma cruzi infection in coastal and insular triatomine bugs from the Atacama Desert of Chile. Microorganisms. 2022;10:785. doi: 10.3390/microorganisms10040785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Almeida CE, Faucher L, Lavina M, Costa J, Harry M. Molecular individual-based approach on Triatoma brasiliensis: inferences on triatomine foci, Trypanosoma cruzi natural infection prevalence, parasite diversity and feeding sources. PLoS Negl Trop Dis. 2016;10:e0004447. doi: 10.1371/journal.pntd.0004447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naranjo N. MetroCable San Agustin Caracas, Venezuela. Gobernanza, movilidad, reducción de la pobreza: lecciones de Medellín, Colombia. Instituto de Urbanismo FAU-UCV. Medellín, 2011.
- 32.Gonzalez-Britez NE, Carrasco HJ, Purroy CEM, Dora Feliciangeli M, Maldonado M, López E, et al. Genetic and morphometric variability of Triatoma sordida (Hemiptera: Reduviidae) from the eastern and western regions of Paraguay. Front Public Health. 2014;2:149. doi: 10.3389/fpubh.2014.00149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kocher TD, Thomas WK, Meyer A, Edwards SV., Paabo S, Villablanca FX, et al. Dynamics of mitochondrial DNA evolution in animals: amplification and sequencing with conserved primers. Proc Natl Acad Sci USA. 1989;86:6196–200. doi: 10.1073/pnas.86.16.6196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi N, Fass J. Sickle: a sliding-window, adaptive, quality-based trimming tool for FastQ files (Version 1.33). https://github.com/najoshi/sickle.
- 35.Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–1. doi: 10.1093/bioinformatics/btq461 [DOI] [PubMed] [Google Scholar]
- 36.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJA, Holmes SP. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–3. doi: 10.1038/nmeth.3869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.MITOMAP. https://www.mitomap.org/MITOMAP.
- 38.Clarke L, Fairley S, Zheng-Bradley X, Streeter I, Perry E, Lowy E, et al. The international Genome sample resource (IGSR): a worldwide collection of genome variation incorporating the 1000 Genomes Project data. Nucleic Acids Res. 2017;45:D854–9. doi: 10.1093/nar/gkw829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fleskes RE, Vilar MG, Melendez C, Schurr TG. Genetic diversity of A2 and C1 haplotypes in Puerto Rico: implications for initial migration and settlement patterns of the Caribbean. 85th Annual Meeting of the American Association of Physical Anthropologists. Atlanta, 2016.
- 40.Gómez-Carballa A, Pardo-Seco J, Brandini S, Achilli A, Perego UA, Coble MD, et al. The peopling of South America and the trans-Andean gene flow of the first settlers. Genome Res. 2018;28:767–79. doi: 10.1101/gr.234674.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page AJ, Taylor B, Delaney AJ, Soares J, Seemann T, Keane JA, et al. SNP-sites: rapid efficient extraction of SNPs from multi-FASTA alignments. Microb Genom. 2016;2:e000056. doi: 10.1099/mgen.0.000056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Overpass API. http://overpass-api.de/.
- 43.Pifano F. El potencial enzootico silvestre del complejo ecológico Schizotrypanum cruzi–Didelphis marsupialis–Panstrongylus geniculatus y sus incursiones a la vivienda humana del valle de Caracas. Boletín de la Academia de Ciencias Físicas, Matemáticas y Naturales (Caracas). 1986;46:9–37. [Google Scholar]
- 44.Santana KDSO, Bavia ME, Lima AD, Guimarães ICS, Soares ES, Silva MMN, et al. Spatial distribution of triatomines (Reduviidae: Triatominae) in urban areas of the city of Salvador, Bahia, Brazil. Geospat Health. 2011;5:199–203. doi: 10.4081/gh.2011.172 [DOI] [PubMed] [Google Scholar]
- 45.Javitt M, Traviezo L, Rodríguez R, Perdomo R. Hallazgo de Panstrongylus geniculatus en urbanización de la zona este de Barquisimeto, estado Lara, Venezuela. Revista Electrónica de Veterinaria. 2012;13:1–19. [Google Scholar]
- 46.Ceretti-Junior W, Vendrami DP, de Matos-Junior MO, Rimoldi-Ribeiro A, Alvarez JV, Marques S, et al. Occurrences of triatomines (Hemiptera: Reduviidae) and first reports of Panstrongylus geniculatus in urban environments in the city of Sao Paulo, Brazil. Rev Inst Med Trop Sao Paulo. 2018;60:e33. doi: 10.1590/S1678-9946201860033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mesa-Arciniegas P, Parra-Henao G, Carrión-Bonifacio Á, Casas-Cruz A, Patiño-Cuellar A, Díaz-Rodríguez K, et al. Trypanosoma cruzi infection in naturally infected dogs from an endemic region of Cundinamarca, Colombia. Vet Parasitol Reg Stud Reports. 2018;14:212–6. doi: 10.1016/j.vprsr.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 48.Reyes M, Torres Á, Esteban L, Flórez M, Angulo VM, Reyes M, et al. Risk of transmission of Chagas disease by intrusion of triatomines and wild mammals in Bucaramanga, Santander, Colombia. Biomédica. 2017;37:68. doi: 10.7705/biomedica.v37i1.3051 [DOI] [PubMed] [Google Scholar]
- 49.Ribeiro G, Gurgel-Gonçalves R, Reis RB, dos Santos CGS, Amorim A, Andrade SG, et al. Frequent house invasion of Trypanosoma cruzi-infected triatomines in a suburban area of Brazil. Gürtler RE, editor. PLoS Negl Trop Dis. 2015;9:e0003678. doi: 10.1371/journal.pntd.0003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rojas-Cortez M, Pinazo MJ, Garcia L, Arteaga M, Uriona L, Gamboa S, et al. Trypanosoma cruzi-infected Panstrongylus geniculatus and Rhodnius robustus adults invade households in the Tropics of Cochabamba region of Bolivia. Parasit Vectors. 2016;9:158. doi: 10.1186/s13071-016-1445-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Reyes-Lugo M. Panstrongylus geniculatus Latreille 1811 (Hemiptera: Reduviidae: Triatominae), vector de la enfermedad de Chagas en el ambiente domiciliario del centro-norte de Venezuela. Revista Biomédica. 2009;20:180–205. [Google Scholar]
- 52.di Iorio O, Gürtler RE. Seasonality and temperature-dependent flight dispersal of Triatoma infestans (Hemiptera: Reduviidae) and other vectors of Chagas disease in Western Argentina. J Med Entomol. 2017;54:1285–92. doi: 10.1093/jme/tjx109 [DOI] [PubMed] [Google Scholar]
- 53.de Souza O Santana K, Bavia ME, Soares ÊS, Nascimento Silva MM, Mendonça J, Guimarães ICS, et al. Spatial distribution of triatomines (Reduviidae: Triatominae) in urban areas of the city of Salvador, Bahia, Brazil. Geospat Health. 2014;5:199. [DOI] [PubMed] [Google Scholar]
- 54.Maeda Maicon Hitoshi, Knox Monique Britto, Gurgel-Gonçalves R. Occurrence of synanthropic triatomines (Hemiptera: Reduviidae) in the Federal District of Brazil. Rev Soc Bras Med Trop. 2012;45:71–6. doi: 10.1590/s0037-86822012000100014 [DOI] [PubMed] [Google Scholar]
- 55.Dias JVL, Fernandes EG, Pires HHR, Dias JCP. Occurrence and distribution of triatomines (Hemiptera: Reduviidae) in municipalities of the Northeastern region of Minas Gerais State, Brazil. Rev Soc Bras Med Trop. 2016;49:502–7. doi: 10.1590/0037-8682-0142-2016 [DOI] [PubMed] [Google Scholar]
- 56.Curtis-Robles R, Hamer SA, Lane S, Levy MZ, Hamer GL. Bionomics and spatial distribution of triatomine vectors of Trypanosoma cruzi in Texas and other southern states, USA. Am J Trop Med Hyg. 2018;98:113–21. doi: 10.4269/ajtmh.17-0526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kuussaari M, Rytteri S, Heikkinen RK, Heliölä J, von Bagh P. Weather explains high annual variation in butterfly dispersal. Proc Biol Sci. 2016;283:20160413. doi: 10.1098/rspb.2016.0413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morsello SC, Groves RL, Nault BA, Kennedy GG. Temperature and precipitation affect seasonal patterns of dispersing tobacco thrips, Frankliniella fusca, and onion thrips, Thrips tabaci (Thysanoptera: Thripidae) caught on sticky traps. Environ Entomol. 2008;37:79–86. doi: 10.1603/0046-225x(2008)37[79:tapasp]2.0.co;2 [DOI] [PubMed] [Google Scholar]
- 59.Feliciangeli MD, Carrasco H, Patterson JS, Suarez B, Martínez C, Medina M. Mixed domestic infestation by Rhodnius prolixus Stal, 1859 and Panstrongylus geniculatus Latreille, 1811, vector incrimination, and seroprevalence for Trypanosoma cruzi among inhabitants in El Guamito, Lara State, Venezuela. Am J Trop Med Hyg. 2004;71:501–5. [PubMed] [Google Scholar]
- 60.Maestre-Serrano R, Eyes-Escalante M. Actualización de la presencia y distribución de triatominos en el departamento del Atlántico-Colombia: 2003–2010. Bol Malariol Salud Ambient. 2012;52:125–8. [Google Scholar]
- 61.Carrasco HJ, Miles MA, Valente SAS, Noireau F, Valente VC. Chagas disease in the Amazon basin: association of Panstrongylus geniculatus (Hemiptera: Reduviidae) with domestic pigs. J Med Entomol. 2015;35:99–103. [DOI] [PubMed] [Google Scholar]
- 62.Nakad Bechara CC, Londoño JC, Segovia M, Leon Sanchez MA, Martínez P CE, Rodríguez R MM, et al. Genetic variability of Panstrongylus geniculatus (Reduviidae: Triatominae) in the Metropolitan District of Caracas, Venezuela. Infection, Genetics and Evolution. 2018;66:236–44. doi: 10.1016/j.meegid.2018.09.011 [DOI] [PubMed] [Google Scholar]
- 63.Aldana E, Heredia-Coronado E, Avendaño-Rangel F, Lizano E, Concepción JL, Bonfante-Cabarcas R, et al. Análisis morfométrico de Panstrongylus geniculatus de Caracas, Venezuela. Biomedica. 2011;31:108–17. [DOI] [PubMed] [Google Scholar]
- 64.Caicedo-Garzón V, Salgado-Roa FC, Sánchez-Herrera M, Hernández C, Arias-Giraldo LM, García L, et al. Genetic diversification of Panstrongylus geniculatus (Reduviidae: Triatominae) in northern South America. PLoS One. 2019;14:e0223963. doi: 10.1371/journal.pone.0223963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Carrasco HJ, Segovia M, Llewellyn MS, Morocoima A, Urdaneta-Morales S, Martínez C, et al. Geographical distribution of Trypanosoma cruzi genotypes in Venezuela. PLoS Negl Trop Dis. 2012;6:e1707. doi: 10.1371/journal.pntd.0001707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Urdaneta-Morales S. Chagas disease: an emergent urban zoonosis. The Caracas Valley (Venezuela) as an epidemiological model. Front Public Health. 2014;2:265. doi: 10.3389/fpubh.2014.00265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Herrera L, Urdaneta-Morales S. Synanthropic rodent reservoirs of Trypanosoma (Schizotrypanum) cruzi in the valley of Caracas, Venezuela. Rev Inst Med Trop Sao Paulo. 1997;39:279–82. doi: 10.1590/s0036-46651997000500006 [DOI] [PubMed] [Google Scholar]
- 68.Hernández C, Vera MJ, Cucunubá Z, Flórez C, Cantillo O, Buitrago LS, et al. High-resolution molecular typing of Trypanosoma cruzi in 2 large outbreaks of acute Chagas disease in Colombia. J Infect Dis. 2016;214:1252–5. doi: 10.1093/infdis/jiw360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hylton A, Fitzpatrick DM, Suepaul R, Dobson AP, Charles RA, Peterson JK. Preliminary characterization of triatomine bug blood meals on the island of Trinidad reveals opportunistic feeding behavior on both human and animal hosts. Trop Med Infect Dis. 2020;5:166. doi: 10.3390/tropicalmed5040166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Medrano-Mercado N, Ugarte-Fernandez R, Butrón V, Uber-Busek S, Guerra HL, de Araújo-Jorge TC, et al. Urban transmission of Chagas disease in Cochabamba, Bolivia. Mem Inst Oswaldo Cruz. 2008;103:423–30. doi: 10.1590/s0074-02762008000500003 [DOI] [PubMed] [Google Scholar]
- 71.Llewellyn MS, Lewis MD, Acosta N, Yeo M, Carrasco HJ, Segovia M, et al. Trypanosoma cruzi IIc: phylogenetic and phylogeographic insights from sequence and microsatellite analysis and potential impact on emergent Chagas disease. PLoS Negl Trop Dis. 2009;3:e510. doi: 10.1371/journal.pntd.0000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gourbière S, Dumonteil E, Ramirez-Sierra M-J, Pérez-Carrillo S, Herrera C, Teh-Poot C, et al. Detailed ecological associations of triatomines revealed by metabarcoding and next-generation sequencing: implications for triatomine behavior and Trypanosoma cruzi transmission cycles. Sci Rep. 2018;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Beatty NL, Behrens-Bradley N, Love M, McCants F, Smith S, Schmidt JO, et al. Rapid detection of human blood in triatomines (Kissing bugs) utilizing a lateral flow immunochromatographic assay—A pilot study. Mem Inst Oswaldo Cruz. 2019;114:e190047. doi: 10.1590/0074-02760190047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kent RJ. Molecular methods for arthropod bloodmeal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour. 2009;9:4–18. doi: 10.1111/j.1755-0998.2008.02469.x [DOI] [PubMed] [Google Scholar]
- 75.Rodriguez F, Luna BS, Calderon O, Manriquez-Roman C, Amezcua-Winter K, Cedillo J, et al. Surveillance of Trypanosoma cruzi infection in triatomine vectors, feral dogs and cats, and wild animals in and around El Paso County, Texas, and New Mexico. PLoS Negl Trop Dis. 2021;15:e0009147. doi: 10.1371/journal.pntd.0009147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Orantes LC, Monroy C, Dorn PL, Stevens L, Rizzo DM, Morrissey L, et al. Uncovering vector, parasite, blood meal and microbiome patterns from mixed-DNA specimens of the Chagas disease vector Triatoma dimidiata. PLoS Negl Trop Dis. 2018;12:e0006730. doi: 10.1371/journal.pntd.0006730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Curtis-Robles R, Wozniak EJ, Auckland LD, Hamer GL, Hamer SA. Combining public health education and disease ecology research: using citizen science to assess Chagas disease entomological risk in Texas. PLoS Negl Trop Dis. 2015;9:e0004235. doi: 10.1371/journal.pntd.0004235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alarcón de Noya B, Díaz-Bello Z, Colmenares C, Ruiz-Guevara R, Mauriello L, Muñoz-Calderón A, et al. Update on oral chagas disease outbreaks in Venezuela: epidemiological, clinical and diagnostic approaches. Mem Inst Oswaldo Cruz. 2015;110:377–86. doi: 10.1590/0074-02760140285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nogueda-Torres B, Alejandre-Aguilar R, Isita-Tornell L, Camacho AD. Defaecation pattern in seven species of triatomines (Insecta, Reduviidae) present in México. Rev Latinoam Microbiol. 2000;42:145–8. [Google Scholar]
- 80.Zeledón R, Alvarado R, Jirón LF. Observations on the feeding and defecation patterns of three triatomine species (Hemiptera: Reduviidae). Acta Trop. 1977;34:65–77. [PubMed] [Google Scholar]
- 81.Segovia M, Carrasco HJ, Martínez CE, Messenger LA, Nessi A, Londoño JC, et al. Molecular epidemiologic source tracking of orally transmitted Chagas disease, Venezuela. Emerg Infect Dis. 2013;19:1098–101. doi: 10.3201/eid1907.121576 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This dataset contains triatomine ID code, species, state, parish, sex, age, parasitology result, and presence of liquid material (potential bloodmeal) in the intestinal content. The file features interactive dashboard elements (users may subset collection statistics by selecting categories of interest).
(XLSX)
Sequences include primer-binding regions.
(TXT)
Specimens represent a random visually blooded subset from Petare, Baruta, and Sucre parishes. Nymphal stages were not analyzed.
(XLSX)
Abbreviations ‘NEG’ and ‘POS’ denote negative vs. positive T. cruzi infection status inferred via microscopy.
(XLSX)
Infection status was inferred via microscopy.
(XLSX)
Abbreviations ‘B’ and ‘NB’ denote presence and absence of blood-like intestinal content, respectively.
(XLSX)
The presence of blood-like intestinal content is also referred to as the ‘visually blooded’ condition elsewhere in this work.
(XLSX)
Values represent percentages of collected specimens representing nymphs.
(XLSX)
A) Human cytb target diversity in pooled P. geniculatus bloodmeals. The sequence positions and nucleotide changes distinguishing the 9 human haplotypes (green circles) detected in this study are shown in white boxes. Labels outside boxes indicate the number of times each mutation has been recorded at https://www.mitomap.org [37] to help verify sequence denoising performed using the R package DADA2 [36]. B) Relationship between human sample size and number of segregating sites in cytb target alignment using publicly available datasets from the 1000 Genomes Project. One hundred random cytb target segments (excluding primer binding sites) were drawn for every possible sample size from multi-fasta files representing American mitogenomes from Lima, Peru; Medellín, Colombia; and Puerto Rico (all municipalities) [38]. Segregating sites were extracted after each draw using SNP-sites v2.1.3 [41].
(TIF)
Data Availability Statement
All Revelant data are withn the manuscript and its Supporting information files.