ABSTRACT
Vaginal infections continue to be a serious public health issue, and developing new approaches to address antibiotic-resistant pathogens is an urgent task. The dominant vaginal Lactobacillus species and their active metabolites (e.g., bacteriocins) have the potential to defeat pathogens and help individuals recover from disorders. Here, we describe for the first time a novel lanthipeptide, inecin L, a bacteriocin from Lactobacillus iners with posttranslational modifications. The biosynthetic genes of inecin L were actively transcribed in the vaginal environment. Inecin L was active against the prevailing vaginal pathogens, such as Gardnerella vaginalis and Streptococcus agalactiae, at nanomolar concentrations. We demonstrated that the antibacterial activity of inecin L was closely related to the N terminus and the positively charged His13 residue. In addition, inecin L was a bactericidal lanthipeptide that showed little effect on the cytoplasmic membrane but inhibited the cell wall biosynthesis. Thus, the present work characterizes a new antimicrobial lanthipeptide from a predominant species of the human vaginal microbiota.
IMPORTANCE The human vaginal microbiota plays essential roles in preventing pathogenic bacteria, fungi, and viruses from invading. The dominant vaginal Lactobacillus species show great potential to be developed as probiotics. However, the molecular mechanisms (such as bioactive molecules and their modes of action) involved in the probiotic properties remain to be determined. Our work describes the first lanthipeptide molecule from the dominant Lactobacillus iners. Additionally, inecin L is the only lanthipeptide found among the vaginal lactobacilli thus far. Inecin L shows strong antimicrobial activity toward the prevalent vaginal pathogens and antibiotic-resistant strains, suggesting that inecin L is a potent antibacterial molecule for drug development. In addition, our results show that inecin L exhibits specific antibacterial activity related to the residues in the N-terminal region and ring A, which will contribute to structure-activity relationship studies in lacticin 481-like lanthipeptides.
KEYWORDS: vaginal infections, Lactobacillus iners, lanthipeptides, antimicrobial activity, mode of action
INTRODUCTION
The human vaginal microbiota is closely associated with the health of women and newborns (1, 2). The vaginal microbiota of healthy women is usually dominated by Lactobacillus spp., such as Lactobacillus crispatus, Lactobacillus iners, Lactobacillus gasseri, and Lactobacillus jensenii (3). When lactobacilli become the minority, some pathogens, such as Gardnerella vaginalis, Streptococcus agalactiae, and Candida albicans, will overgrow in the vaginal tract and cause a variety of diseases. G. vaginalis is a major pathogen (these pathogens also include Prevotella spp., Atopobium vaginae, Bacteroides spp., and Mobiluncus spp.) that causes bacterial vaginosis (BV) (4, 5), while S. agalactiae mainly leads to premature birth and neonatal sepsis (6). Vaginal infections and dysbiosis are pressing clinical challenges that are commonly addressed by the administration of antibiotics (such as metronidazole and clindamycin) (7). However, the overuse of antibiotics exacerbates the dysbiosis and leads to the expansion of drug-resistant microbes (4).
Developing new antimicrobial substances is essential for addressing the antibiotic resistance problem and improving the effectiveness of treatments for vaginal infections. Lanthipeptides, a family of ribosomally synthesized and posttranslationally modified peptides (RiPPs) (8), are potential alternatives due to their high antimicrobial activities and low susceptibility to drug resistance. Many lanthipeptides (e.g., nisin, mersacidin, and mutacin B-Ny266) are highly active against antibiotic-resistant microbes, such as methicillin-resistant Staphylococcus aureus (MRSA), vancomycin-resistant enterococci (VRE), and multidrug-resistant Mycobacterium tuberculosis (9, 10).
Lanthipeptides are divided into five classes according to their modification enzymes (I to IV) (11, 12). Taking class II as an example, the biosynthetic gene cluster generally includes lanA (precursor gene), lanM (modification enzyme gene), lanT (bifunctional processing and transport gene), lanFEG (immunity genes), and lanKR (sensor and regulatory genes). Lanthipeptides are primarily synthesized as prepeptides, which are composed of leader peptides and core peptides. Guided by leader peptides, LanMs act on serine and threonine residues in core peptides to generate dehydroalanine (Dha) and dehydrobutyrine (Dhb), which further react with cysteine residues to form intramolecular thioether bridges through Michael-type addition. Then, modified precursors are processed to remove leader peptides and transferred to the periplasmic space by LanT (13, 14).
Since the antimicrobial activities of lanthipeptides are closely related to their structures, the structure-activity relationships (SARs) have been explored (15). In most cases, thioether bonds are essential to the activities of lanthipeptides. Each group of lanthipeptides exhibits similar SRAs. For example, in the nisin group, the N-terminal AB rings are necessary to bind to lipid II, while the C-terminal DE rings are crucial to the attachment to the cytoplasmic membrane and the formation of pores. For the lacticin 481 group, ring A, especially the negatively charged Asp or Glu, is vital for interactions with lipid II; the positively charged residues or the amphiphilicity of the N-terminal regions significantly affect the inhibition activities (16, 17).
Different antimicrobial mechanisms have been revealed among lanthipeptides (18, 19). In general, lanthipeptides target the cell wall, the plasma membrane, or both. By hijacking lipid II, a peptidoglycan precursor, many lanthipeptides (such as nisin, nukacin ISK-1, and mersacidin) can inhibit the biosynthesis of the cell wall. Furthermore, a portion of lanthipeptides (e.g., nisin, lacticin 3147, and haloduracin) form pores in the cytoplasmic membrane using lipid II as a membrane receptor. In addition, cinnamycin-like lanthipeptides target phosphatidylethanolamine in the membranes, while pep5 group lanthipeptides employ non-lipid II molecules to exhibit antibacterial activities (20).
Lanthipeptides from the dominant lactobacilli have the potential to kill antibiotic-resistant strains leading to vaginal diseases. However, they have rarely been characterized in vaginal microbes thus far. Notably, L. iners, unlike other predominant Lactobacillus species in the vagina, not only prevails in the vaginal microbiota of healthy women but also colonizes widely in patients with vaginal infections (21). Therefore, compared to other species, L. iners is thought to exhibit a stronger ability to occupy the vaginal tract. In the present study, we describe a novel lanthipeptide from L. iners for the first time, and this lanthipeptide is called inecin L. Inecin L is shown to be highly active against S. agalactiae and G. vaginalis, the prevalent vaginal pathogens. The key residues related to the specific activity of inecin L are revealed. We also demonstrate that inecin L is a bactericidal lanthipeptide with activity that inhibits cell wall biosynthesis.
RESULTS
Biosynthesis and structural dissection of the lanthipeptide inecin L.
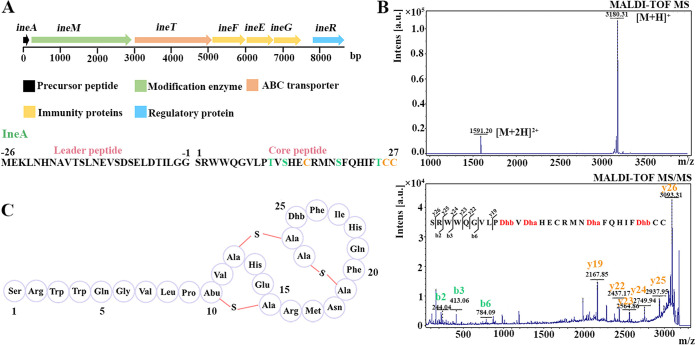
To assess the distribution of lanthipeptides among L. iners strains, their genomic sequences from NCBI were analyzed using antiSMASH. A new class II lanthipeptide biosynthetic gene cluster (named ine) was present in 10.3% (21/204) of the strains (Table S1 in the supplemental material). The gene cluster comprises ineA, ineM, ineT, ineFEG, and ineR (Fig. 1A). The ineA gene encodes the precursor peptide, composed of a 26-amino-acid-residue leader peptide with a predicted double-glycine cleavage site and a 27-amino-acid-residue core peptide that includes 3 Ser residues, 2 Thr residues, and 3 Cys residues. IneM contains two domains, an N-terminal dehydratase domain and a C-terminal cyclase domain, indicating that IneM is capable of performing dehydration and cyclization. IneT is annotated as an ABC transporter with an N-terminal C39 peptidase domain, a central transmembrane domain, and a C-terminal ATP-binding domain, suggesting that IneT is involved in the leader peptide cleavage and mature peptide transport. In addition, IneFEG and IneR are predicted to be immune proteins and a regulatory protein, respectively. Furthermore, we found that the biosynthetic genes (such as ineA, ineM, and ineT) were expressed in vaginal swab samples (Table S2) by analyzing the metatranscriptomic data (PRJEB21446 and PRJNA797778), illustrating that the gene cluster was active in the vaginal environment.
FIG 1.
Biosynthetic gene cluster and structural dissection of inecin L. (A) Gene cluster and precursor peptide sequence of inecin L. (B) Mass spectrum (MS) and tandem MS (MS/MS, 3180.31 Da) of inecin L. (C) Primary structure of inecin L.
We have tried to isolate inecin L-producing L. iners strains from vaginal swab samples (>80). Unfortunately, neither an ineA- nor an ineM-positive strain has yet been obtained. Although 10.3% of L. iners strains contain ineA or ineM, the actual ratio in the vaginal samples may be lower due to prediction deviations (caused by small sample size in the database, the preference of the sequenced strains, etc.) and no L. iners-containing samples were obtained. As an alternative, heterologous expression in Escherichia coli was employed to produce the lanthipeptide. By coexpressing ineA and ineM, we obtained the modified precursor peptide (His6-IneAm). Furthermore, we recombinantly expressed and purified the N-terminal peptidase structural domain (IneT150) of IneT to remove the leader peptide of His6-IneAm. The product showed a clear inhibition zone on the indicator (Micrococcus luteus strain NCIB 8166) plate. After purification by high-performance liquid chromatography (HPLC), the active peak was examined by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS). Its molecular weight ([M+H]+) was 3,180.3 Da (Fig. 1B), 72.2 Da lower than the theoretical molecular weight (3,252.5 Da), which corresponded to the dehydration of four potential residues (Thr10, Ser12, Ser19, and Thr25) in the core peptide. To confirm the dehydration and subsequent cyclization, the mass peak at 3,180.3 was subsequently analyzed by tandem MS (MS/MS) (Fig. 1B). Many N-terminal fragments were identified, but the C-terminal part was not split, implying that the C-terminal region probably formed a whole piece. Based on the MS fragments, as well as the predicted sites of modification by IneM, the intramolecular thioether bonds of the peptide were determined to be Thr10-Cys15, Ser12-Cys26, and Ser19-Cys27 (Fig. 1C). As the sequence of IneA is new and never reported before, we named the new lanthipeptide inecin L. Inecin L contains a linear N-terminal region and a globular C-terminal region similar to those of lacticin 481-like lanthipeptides.
Antimicrobial spectra and antibiofilm activities of inecin L.
To test the inhibitory activity of inecin L, we selected several indicator strains, including two vaginal pathogenic species, S. agalactiae and G. vaginalis, and four vaginal Lactobacillus species, L. iners, Lactobacillus crispatus, Lactobacillus gasseri, and Lactobacillus jensenii. It was shown that inecin L was active against Streptococcus pyogenes (group A streptococcus [GAS]) strain CGMCC 1.8868 and S. agalactiae (group B streptococcus [GBS]) strains L29 and W53 (Table 1). Notably, strain L29 was highly resistant to clindamycin (MIC of ≥94.1 μM), a drug frequently used in clinical practice (22). The MIC of inecin L against strain L29 was 666.7 ± 144.3 nM, which was comparable to that of nisin (500 nM). Inecin L also exhibited high antibacterial activity against G. vaginalis (MIC between 100 nm and 800 nM). However, most of the lactobacilli strains other than the L. iners strains were insensitive to inecin L (MIC of ≥1 μM). Generally, lanthipeptides are active against strains (excluding those with immunity-associated genes) from sibling species of the producers. The two L. iners strains were ineFEG free, which may be the reason they were sensitive (MICs of 100 ± 40 nM) to inecin L. In addition, inecin L strongly inhibited the growth of M. luteus strain NCIB 8166 (MICs of 163.3 ± 75.06 nM). The nanomolar level of antibacterial activity suggested that inecin L was a lanthipeptide with potential for drug development.
TABLE 1.
MICs of the lanthipeptides against different indicator strains
| Strain | Mean MIC ± SD (nM) |
||
|---|---|---|---|
| Inecin L | Nisin | Mutacin II | |
| Streptococcus pyogenes CGMCC 1.8868 | 6,000 ± 1732 | 3,833 ± 288.7 | >8,000 |
| Micrococcus luteus NCIB 8166 | 163.3 ± 75.06 | 93.33 ± 23.09 | 250 |
| Streptococcus agalactiae L29 | 666.7 ± 144.3 | 500 | 1,667 ± 577.4 |
| S. agalactiae W53 | 1,000 | 750 | |
| Gardnerella vaginalis ATCC 14018 | 130 ± 17.32 | 53.33 ± 23.09 | 140 ± 17.32 |
| G. vaginalis HR4 | 666.7 ± 115.5 | 400 | |
| Lactobacillus iners X1 | 100 | 40 | |
| L. iners X4 | 100 | 40 | |
| Lactobacillus crispatus L49 | 1,667 ± 144.3 | 66.67 ± 23.09 | |
| L. crispatus 48 | 200 | 20 | |
| Lactobacillus gasseri 24 | >4,000 | 120 | |
| L. gasseri 1Y10 | >4,000 | 333.3 ± 144.3 | |
| Lactobacillus jensenii 8-21 | 1,000 | 40 | |
| L. jensenii 21-21 | >4,000 | 500 | |
Moreover, we measured the antibiofilm activities of inecin L, since the major vaginal pathogens, such as S. agalactiae and G. vaginalis, persisted in the vaginal tract through biofilm formation (23, 24). The results indicated that inecin L was able to reduce the biofilms formed by S. agalactiae L29 and G. vaginalis ATCC 14018 at 300 nM (0.4× MIC) and 10 nM (0.083× MIC), respectively (Fig. 2A and B). The inhibition of biofilm formation was enhanced along with increased concentrations of inecin L. The total optical densities at 600 nm (OD600) of the cultures in the presence of inecin L were obviously lower than that of the control without inecin L, indicating that inecin L probably inhibited the biofilm formation through a growth inhibition effect (Fig. S1). Furthermore, we examined the activity of inecin L on established biofilms. It was shown that inecin L had little effect on the already-formed biofilms, and thus, inecin L possessed a weak ability to disrupt mature biofilms, unlike nisin (Fig. 2C).
FIG 2.
Quantification of antibiofilm activity of inecin L. (A and B) Biofilms formed by S. agalactiae L29 and G. vaginalis ATCC 14018 in the presence of different concentrations of inecin L. (C) Inhibitory activities of inecin L and nisin against established biofilms formed by strain L29. Error bars show standard deviations. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.
Key residues associated with the antibacterial activity of inecin L.
To characterize the specific activity of inecin L, a multiple-sequence alignment was employed to analyze the sequence differences between inecin L and other class II lanthipeptides. It was shown that inecin L shared the highest identity (81.5%) with mutacin II, which differs in 5 amino acid residues from inecin L (Fig. S2). We wondered whether the 5 residues would alter the antimicrobial activity between inecin L and mutacin II. Since a mutacin II-producing strain was not available, we tried to produce mutacin II by heterologous expression in E. coli. Based on the interaction between LanMs and the corresponding leader peptides of LanAs (8, 25), we utilized IneM to modify a hybrid peptide composed of the leader peptide of IneA and the core peptide of MutA (precursor of mutacin II) (Fig. 3A). After the hybrid peptide was modified, IneT150 was used to remove the leader peptide. Mass spectrometry analysis indicated that the synthesized mature peptide possessed the same molecular mass (Fig. 3B) and ring topology (Fig. 3C) as mutacin II isolated from the producing strain (26). The results suggested that we successfully synthesized mutacin II in E. coli. We subsequently determined the antimicrobial activity of mutacin II by MIC assay. Mutacin II exhibited higher activity against M. luteus NCIB 8166 and G. vaginalis ATCC 14018 than against S. agalactiae L29 and S. pyogenes CGMCC 1.8868 (Table 1). The MICs also demonstrated that inecin L was superior to mutacin II in terms of inhibiting the Streptococcus strains.
FIG 3.
Hybrid precursor sequence for biosynthesis of mutacin II and its structural dissection. (A) Hybrid precursor consisting of the leader peptide of inecin L and the core peptide of mutacin II. MutA is the natural precursor of mutacin II. (B) Mass spectrometry analysis of mutacin II synthesized in E. coli. (C) Primary structure of mutacin II, identified by mass spectrometry.
We noticed that obviously different amino acid residues (His or Tyr) were present at the 13th site of inecin L and mutacin II (Fig. 4A), since His was distinctive from Tyr in its charged state and hydrophilic/hydrophobic property. Therefore, we assumed that His13 might be partially responsible for the better inhibitory activity of inecin L. To verify this hypothesis, we separately mutated the His of inecin L to Tyr to obtain an H13Y mutant (Fig. S3A) and the Tyr of mutacin II to His to produce a Y13H variant (Fig. S4). The antibacterial activity assay indicated that the MICs of the mutants (H13Y or Y13H) were not significantly different from those of the equivalent wild types (inecin L or mutacin II) when the indicator strain was S. agalactiae L29 (data not shown). However, when the sensitive strain M. luteus NCIB 8166 was used as the indicator, the H13Y mutant displayed decreased activity and the Y13H mutant showed increased activity compared with those of the matching wild types (Fig. 4B and C). Therefore, the His13 residue was important for the antibacterial activity of inecin L. To further verify the role of His13, we replaced the His residue of inecin L with Asp (H13D) and Ala (H13A). The H13D variant showed no inhibitory activity toward strain NCIB 8166, mainly because of the presence of an unbonded thioether ring (Fig. S5). Unexpectedly, the antibacterial activity of the H13A mutant (Fig. S3D) was slightly higher than that of the wild type (Fig. 4C), suggesting that the positively charged residue was not essential to the antimicrobial activity.
FIG 4.
Mutants of inecin L and mutacin II and comparison of their antibacterial activities against M. luteus NCIB 8166. (A) Mutation sites of inecin L. The amino acid variants are marked in green. (B) Antibacterial activities of mutacin II, Y13H mutant (mutacin II), and H13Y mutant (inecin L). (C) Inhibitory activities of inecin L and its mutants. Error bars show standard deviations. *, P < 0.05; **, P < 0.01; ****, P < 0.0001.
Since the H13Y mutant of inecin L still exhibited higher activity than mutacin II (Fig. 4B), we evaluated the contributions of the other four residues of inecin L to the difference in activities. Mutants of inecin L with S1N, L8V, F20W, and I23V mutations were constructed by individually exchanging these four residues of inecin L for the matching residues of mutacin II (Fig. 4A). Except for the F20W mutant (which failed to be processed by IneT150), the variants were fully modified (Fig. S3B, C, and E) and prepared. Compared with inecin L, the I23V mutant led to no significant change in activity. However, the antimicrobial activities of the S1N and L8V N-terminal mutants increased by 15.4% and 40.7% compared with that of inecin L (Fig. 4C). The two mutants exhibited reduced levels of hydrophobicity, as the retention times of the S1N (25.7 min) and L8V (25.4 min) mutants in a C18 column were all shorter than that of the wild type (25.8 min); the reduced hydrophobicity may affect their antibacterial activities.
Mode of action of inecin L.
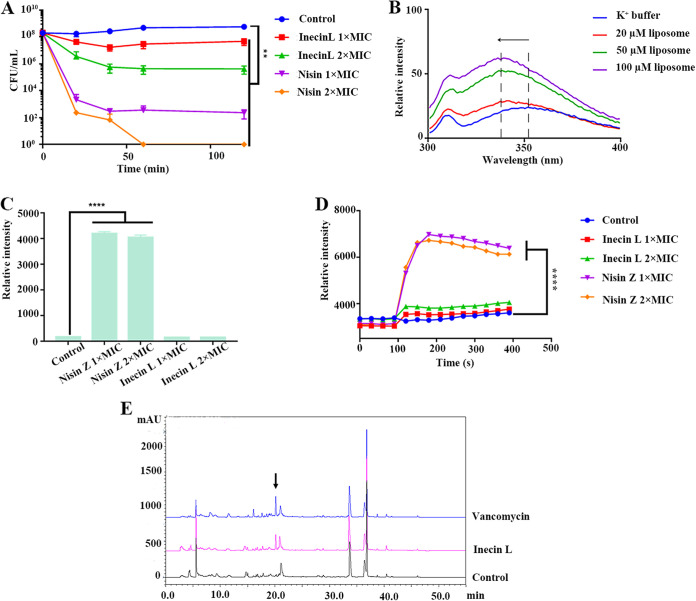
To elucidate the antimicrobial mechanism of inecin L, we initially assessed whether the action of inecin L was bacteriostatic or bactericidal. Viable cell counting showed that inecin L at 1× MIC quickly killed the S. agalactiae cells, and the killing activity increased with increasing concentrations of inecin L (Fig. 5A). The action of inecin L was similar to that of nisin, a bactericidal lanthipeptide, which led to an immediate decrease in viable cells (Fig. 5A). Consequently, we determined that inecin L was a bactericidal lanthipeptide.
FIG 5.
Mode of action of inecin L. (A) Viable cell counting of S. agalactiae L29 under the action of different concentrations (1× MIC and 2× MIC) of inecin L or nisin. (B) Tryptophan fluorescence spectra of inecin L in K+ buffer and in liposomes (DOPC/DOPG in a ratio of 4:1) with different concentrations. (C) Propidium iodide staining of S. agalactiae L29 after incubation with different concentrations (1× MIC and 2× MIC) of inecin L or nisin Z. (D) Transmembrane potential changes of S. agalactiae L29 in the presence of different concentrations (1× MIC and 2× MIC) of inecin L or nisin Z. (E) HPLC spectra of intracellular extracts of M. luteus NCIB 8166 after treatment with sterile water (control), vancomycin, or inecin L (10× MIC). The arrow indicates the UDP-N-acetylmuramyl pentapeptide peak. Error bars show standard deviations. **, P < 0.01; ****, P < 0.0001.
Next, we examined the effect of inecin L on the cytoplasmic membrane. There are two tryptophan residues in the N-terminal region of inecin L, and their fluorescence emission spectra in liposomal vesicles were examined to reveal the interaction between inecin L and the membrane in vitro. As shown by the results in Fig. 5B, an obvious blueshift of the maximum emission wavelength was present in the liposomes, and their fluorescence intensities were much higher than those in the aqueous solution, suggesting that inecin L possessed strong membrane-binding capacity. Moreover, propidium iodide (PI), a nucleic acid-staining dye, was used to detect the membrane permeabilization. Additionally, nisin was employed as a positive control, since it is capable of forming pores on the cytoplasmic membrane, which is followed by the inflow of PI and an increase in fluorescence intensity. However, no change in fluorescence was observed when inecin L (1× MIC and 2× MIC) acted upon strain L29 (Fig. 5C). In addition, DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide), a potentiometric probe, was used to determine the change in transmembrane potential of strain L29 under the action of inecin L. We found that inecin L did not cause apparent fluorescence changes at different concentrations (1× MIC and 2× MIC), while nisin immediately boosted the fluorescence intensity (Fig. 5D). Accordingly, inecin L hardly affected the integrity of the cytoplasmic membrane.
Furthermore, we evaluated the inhibitory activity of inecin L on cell wall biosynthesis. Generally, cell wall-inhibiting antibiotics (e.g., vancomycin) lead to the accumulation of cell wall precursors, such as UDP-N-acetylmuramyl pentapeptide (UDP-MurNAc-pp) (27). After incubation with inecin L for 1 h, the bacterial cells were collected, and the intracellular components were extracted and analyzed by reverse-phase (RP-HPLC). Compared with the spectrum of the blank control, an extra peak was observed in the spectrum from the inecin L treatment that was similar to that of vancomycin (Fig. 5E). The extra peak was further identified as UDP-MurNAc-pp by mass spectrometry (Fig. S6). Collectively, the above-described results suggested that inecin L showed weak activity against the cytoplasmic membrane but inhibited the biosynthesis of cell wall.
DISCUSSION
Antibiotics are commonly used to treat vaginal infections, but long-term treatment with antibiotics leads to the development of drug-resistant microbes and disorders of the vaginal microbial community (22). The dominant Lactobacillus species should be capable of maintaining the homeostasis of the vaginal microbiome and preventing the overgrowth of pathogens in multiple ways, such as by producing secondary metabolites (28). Among them, lanthipeptides have received much attention because of their high antimicrobial activities and specificities. In the human skin, mouth, and gut, many lanthipeptides have been discovered, and some of them exhibit strong antimicrobial activities against antibiotic-resistant bacteria, such as MRSA and VRE (29–31). Several lanthipeptides (e.g., nisin and staphylococcin Au-26) from the human vagina have also been described previously (32, 33). However, the lanthipeptide-producing strain was either a minor species (Lactococcus lactis) or a pathogen (S. aureus). In the present study, we characterize the first lanthipeptide, inecin L, from L. iners, a dominant species of the vaginal microbiota of healthy women. Inecin L was highly active against vaginal pathogens, including GBS and G. vaginalis. In particular, inecin L also inhibited biofilm formation by S. agalactiae and G. vaginalis at sub-MICs. This property was different from that of clinical antibiotics, such as clindamycin and metronidazole, which enhanced biofilm formation at subinhibitory concentrations (34). Therefore, inecin L has the potential as a new tool to address vaginal infections.
The antibacterial activity of inecin L is closely related to its structure. Similar to lacticin 481-like lanthipeptides, inecin L contains a linear N-terminal region and an intertwined C-terminal region. Generally, the N-terminal parts of the lacticin 481 group are amphipathic, and maintaining amphipathicity is important to their antimicrobial activities. For example, in nukacin ISK-1 and salivaricin A2, removing most of the N-terminal hydrophilic residues or changing them to hydrophobic residues resulted in dramatic decreases in their antibacterial activities (35, 36). Among the N-terminal sequences of inecin L, there are more residues (Trp3, Trp4, Val7, Leu8, and Pro9) with hydrophobic side chains than in other members of the lacticin 481 group (≤3) (Fig. S2), except for mutacin II. We showed that the S1N and L8V N-terminal mutants of inecin L exhibited decreased hydrophobicity but increased antimicrobial activity compared with those of the wild type. As the N-terminal region was shown to be involved in binding to the cytoplasmic membrane (Fig. 5B), we inferred that the increased antibacterial activities of the N-terminal mutants might have been caused by enhanced membrane-binding abilities.
In addition to the N termini, the conserved A rings of lacticin 481-like lanthipeptides were closely related to the antimicrobial activities. Ring A of nukacin ISK-1 was essential for binding with lipid II, and residue D13 was mainly involved in the interaction (16, 37). Notably, a positively charged His was prevalent near the conserved Asp or Glu, except for Tyr (with a large side chain), in mutacin II, and Asp (negatively charged) in salivaricin A/A2 (Fig. S2). We demonstrated that the conserved His significantly affected the antibacterial activities of inecin L and the corresponding mutant of mutacin II. However, replacing His with Ala led to slightly elevated antimicrobial activity of inecin L, while this mutation resulted in a very large reduction in the antibacterial activity of nukacin ISK-1 (16). The difference in activity implied that the site in inecin L played a distinct role in the antimicrobial action. As mentioned above, a totally different residue, Asp, is present in the matching site in salivaricin A2, which displayed low affinity to lipid II (36). The N-terminal region of salivaricin A2 was structured and also involved in the interaction with lipid II (36). Since a similar amphipathic property was present, the N-terminal region of inecin L may also form a structure to cooperate with the adjacent residues (e.g., His13) on ring A. This interaction partially explains the specific activity of inecin L and needs to be further determined.
The antimicrobial mechanisms vary in different groups of lanthipeptides. To date, most lacticin 481-like lanthipeptides can inhibit the biosynthesis of cell wall (16, 36, 38–40). The inhibition is largely accomplished by blocking the cell wall precursor lipid II, since the lipid II-binding ring A is conserved in the group. Several members of the group, such as streptococcin A-FF22 and nukacin ISK-1, also form unstable or small-sized pores on the membrane under certain circumstances (41, 42). We show that inecin L exerts bactericidal activity and inhibits cell wall biosynthesis. Similarly, mutacin II displays bactericidal activity toward sensitive bacterial cells by interrupting the energy metabolism (43), which may lead to the inhibition of cell wall biosynthesis.
Lanthipeptides with antimicrobial activities are thought to be beneficial for producers against competitors in the same environment (30). Therefore, L. iners has an advantage over other species, such as pathogenic G. vaginalis and S. agalactiae, by synthesizing inecin L. Unlike traditional antibiotics, lanthipeptides usually possess narrow antimicrobial spectra mainly against susceptible strains (44), which reduces the effects on the microbial community and the frequency of developing multiresistant strains. Notably, the role (friend or foe) of L. iners in vaginal health is unclear thus far (21). The species is thought to be composed of different variant strains, which may separately contribute to health and disease (21). In L. iners, the immune genes ineFEG are absent in approximately 50% (data not shown) of the strains in the NCBI database, which are potential targets of inecin L. In addition, inecin L also showed inhibitory activities against some strains of beneficial Lactobacillus species, such as L. crispatus (Table 1). Therefore, other research on inecin L (e.g., the working concentrations and the effects on the vaginal microbiota) should be performed before inecin L can be considered a candidate to address vaginal diseases.
In summary, we have characterized the first lanthipeptide, inecin L, from Lactobacillus iners, a dominant species in the human vaginal microbiota. Inecin L is capable of killing some of the major vaginal pathogens and inhibiting biofilm formation. We have also described the structure-activity relationships and the mode of action of inecin L. The present work will contribute to the discovery of novel antimicrobial substances from the vaginal microbiota.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli strain DH5α was used for vector construction, and E. coli strain BL21(DE3) was used for protein expression. Micrococcus luteus strain NCIB 8166, Gardnerella vaginalis strains ATCC 14018 and HR4, Streptococcus agalactiae strains L29 and W53, S. pyogenes strain CGMCC 1.8868, and eight vaginal lactobacilli strains were used to indicate the antimicrobial activities of lanthipeptides. Recombinant E. coli strains were maintained in Luria-Bertani (LB) broth at 37°C with 50 μg/mL kanamycin. M. luteus NCIB 8166 was cultured in S1 medium (17) at 30°C. G. vaginalis was cultured anaerobically in Columbia blood (CB) medium at 37°C, streptococci were inoculated into Todd-Hewitt broth (THB) at 37°C, and lactobacilli were cultured in de Man, Rogosa, and Sharpe (MRS) medium at 37°C. The vaginal strains were isolated from vaginal swab samples of women, and the sampling procedure was performed by professional doctors. The study was approved by the Medical Ethics Committee of Second Affiliated Hospital of Guangxi Medical University, and informed-consent forms were signed by the participants.
Plasmid construction.
The sequences of ineA, ineM, ineT150, and the core peptide-encoding gene of mutacin II were synthesized by Beijing Generaybiotech Co., Ltd. The ineAM fragment was obtained by fusion PCR and amplified by primers with restriction sites. Plasmids pET28a-ineAM and pET28a-ineT150 were obtained through the digestion-ligation method. The leader peptide sequence of ineA and core peptide sequences of mutA and ineM were also fused by fusion PCR, and the digestion-ligation method was employed to construct plasmid pET28a-mutAM. Site-directed ligase-independent mutagenesis (SLIM) was used to generate variants of inecin L and mutacin II (45). The plasmids and primers used in the study are listed in Table 2.
TABLE 2.
Primers used in the study
| Primer | Sequence (5′–3′) | Purpose |
|---|---|---|
| ineAM-F | CGCCATATGATGGAAAAACTGAACCATAACG | Amplifying the ineAM fragment |
| ineAM-R | CGCGGATCCTTAGAAAAAGCTCAGATTCAG | |
| ineT150-F | GGAATTCCATATGTTGAAACTAGTATTACAAAATAATG | Amplifying the ineT150 fragment |
| ineT150-R | ACGCGTCGACTTATTTTTTAATTAAATCAACCAAAG | |
| PineA-F | CGCGGATCCATGGAAAAACTGAACCATAACG | Amplifying the ineA leader peptide sequence |
| PineA-R | CACCAACGATTGCCGCCCAGGATGGTATC | |
| MmutA-F | CATCCTGGGCGGCAATCGTTGGTGGCAAGGTG | Amplifying the mutA core peptide sequence |
| MmutA-R | ACTTCAACTTCTTAACAGCAAGTGAAAACATG | |
| H13Y-L1 | CGACAGTTAGCTATGAATGTCGTATGAACTCCTTTC | Constructing the inecin L (H13Y) expression vector |
| H13Y-S2 | GCAGCACGCCCTGCCACCAAC | |
| H13Y-L2 | TCATACGACATTCATAGCTAACTGTCGGCAGCACG | |
| H13Y-S1 | ACTCCTTTCAGCATATCTTTAC | |
| Y13H-L1 | ACGGTCTCACATGAGTGTCGCATGAATTCATGGC | Constructing the mutacin II (Y13H) expression vector |
| Y13H-S2 | TGGCACAACACCTTGCCACCAACG | |
| Y13H-L2 | GACACTCATGTGAGACCGTTGGCACAACACCTTGCC | |
| Y13H-S1 | GCATGAATTCATGGCAACATG | |
| S1N-L1 | TGGGCGGCAACCGTTGGTGGCAGGGCGTGCTGCCGACAG | Constructing the inecin L (S1N) expression vector |
| S1N-S2 | GGATGGTATCCAGTTCGCTATCG | |
| S1N-L2 | ACCAACGGTTGCCGCCCAGGATGGTATCCAGTTCGCTATC | |
| S1N-S1 | GGCAGGGCGTGCTGCCGACAGTTAG | |
| L8V-L1 | AGGGCGTGGTGCCGACAGTTAGCCATGAATGTCGTATG | Constructing the inecin L (L8V) expression vector |
| L8V-S2 | GCCACCAACGTGAGCCGCCCAGGATG | |
| L8V-L2 | ACTGTCGGCACCACGCCCTGCCACCAACGTGAGCCGCCCAG | |
| L8V-S1 | TAGCCATGAATGTCGTATGAACTC | |
| F20W-L1 | ATGAACTCCTGGCAGCATATCTTTACCTGCTGTTAAGAAG | Constructing the inecin L (F20W) expression vector |
| F20W-S2 | ACGACATTCATGGCTAACTGTCG | |
| F20W-L2 | ATATGCTGCCAGGAGTTCATACGACATTCATGGCTAACTG | |
| F20W-S1 | CTTTACCTGCTGTTAAGAAGTTG | |
| I23V-L1 | TTCAGCATGTGTTTACCTGCTGTTAAGAAGTTGAAGTAAAG | Constructing the inecin L (I23V) expression vector |
| I23V-S2 | AGGAGTTCATACGACATTCATGG | |
| I23V-L2 | AGGTAAACACATGCTGAAAGGAGTTCATACGACATTCATG | |
| I23V-S1 | GCTGTTAAGAAGTTGAAGTAAAG |
Protein expression and purification.
Heterologous expression in E. coli (46) was used to produce inecin L, mutacin II, and their mutants, and proteins were expressed and purified as mentioned previously (47). Briefly, recombinant E. coli BL21(DE3) strains with the different expression vectors were incubated in LB medium at 37°C. When the OD600 reached 0.6 to 0.8, the strains were induced with 0.5 mM IPTG (isopropyl-d-1-thiogalactopyranoside) at 18°C for 24 h. After that, the modified precursor peptides were purified by nickel ion chelate affinity chromatography, while the processing protein IneT150 was purified by using immobilized cobalt ion affinity chromatography. SDS-PAGE was used to evaluate the purified proteins. Purified precursors and peptidases were coincubated to remove the leader peptides, and the mature peptides produced were purified by reverse-phase HPLC using a C18 column. Matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS) was used to determine the molecular masses and intramolecular thioether bonds of the peptides. A bicinchoninic acid (BCA) assay kit (Thermo Scientific, USA) was used to quantify the peptide concentrations.
Antimicrobial activity assay.
The antimicrobial activities of the lanthipeptides and their mutants were initially evaluated by agar diffusion assay with M. luteus NCIB 8166 as the indicator strain (17). The peptides were dissolved in a 10% acetonitrile-water solution, and a final concentration of 4 μM was used in the assay. In addition, the MICs of lanthipeptides against different indicator strains were determined as described previously (48). The bacterial cells were collected in stationary phase and resuspended in fresh medium to a concentration of 5 × 105 CFU per mL. Each peptide, in a gradient of concentrations, was separately incubated with 200 μL of the resuspended bacterial cells in a 96-well plate for 24 h at 37°C. Sterile water and 10% acetonitrile-water solution were used as blank controls. MICs were defined as the lowest concentrations to totally inhibit the growth of the indicators.
Measurement of biofilm inhibition activity.
The biofilm formation of S. agalactiae L29 and G. vaginalis ATCC 14018 in 96-well polystyrene plates was determined by crystal violet staining as described in a previous study (49). Strain L29, taken as an example, was grown overnight in THB medium at 37°C and reinoculated into fresh LB medium with 1% glucose until the OD600 reached 0.1. Then, 200-μL amounts of the culture were mixed separately with different concentrations of each lanthipeptide and dispensed into a 96-well plate. After incubation at 37°C for 24 h, the unbound cultures were removed, and each well was washed twice with phosphate-buffered saline (PBS). The wells were air dried and stained with 0.1% crystal violet for 30 min, followed by two washes with PBS. For quantification, the stained biofilms were resuspended in ethanol-acetone (80/20 [vol/vol]) solution, and the A595 values of the resuspended solutions were measured. The effect of inecin L on established S. agalactiae L29 biofilm was examined according to a method described previously (50), with some modifications. Briefly, strain L29 (OD600 of 0.1) was cultured at 37°C for 12 h to form biofilms. After the unattached cells were moved, 150-μL amounts of inecin L and nisin at 4× and 10× MIC were separately added to wells with established biofilms. The biofilms were quantified after the wells were incubated at 37°C for 12 h.
Viable-cell counting.
S. agalactiae cells were cultured in THB medium with an initial concentration of 1 × 108 CFU per mL. The cultures were separately incubated with inecin L and nisin at 1× and 2× MIC, and the viable cells were determined every 20 min for 2 h by serial-dilution assays.
Membrane integrity analysis.
Propidium iodide (PI) was used to measure the membrane integrity of S. agalactiae L29, as described previously (51). Briefly, S. agalactiae L29 was cultured overnight in THB medium and transferred to fresh medium to obtain an OD600 of 0.4 to 0.6. Then, the cells were washed with PBS, and the bacterial concentration was adjusted to 1 × 108 CFU per mL. The cell suspensions were separately incubated with inecin L (0.75 μM and 1.5 μM, 1× and 2× MIC) or nisin (0.5 μM and 1.0 μM, 1× and 2× MIC) (positive control) at 37°C for 5 min. Subsequently, the cells were washed and resuspended in PBS and then incubated with PI (2 μg/mL) at 37°C for 10 min. After that, the fluorescence was recorded using a Synergy H4 microplate reader with excitation and emission wavelengths of 535 nm and 615 nm, respectively.
Membrane depolarization assay.
Membrane depolarization was determined by using the membrane potential-sensitive cyanine probe DiSC3(5) (3,3′-dipropylthiadicarbocyanine iodide) (52). S. agalactiae L29 cells were prepared with the same procedure used in the membrane integrity analysis. Bacterial suspensions were incubated with 2.5 μM DiSC3(5) at 37°C for 5 min, and then inecin L (0.75 μM and 1.5 μM, 1× and 2× MIC) or nisin (0.5 μM and 1.0 μM, 1× and 2× MIC) (positive control) was added to the suspensions. The changes in fluorescence were detected immediately. The excitation and emission wavelengths were set at 651 nm and 675 nm, respectively.
UDP-MurNAc-pp accumulation.
The UDP-N-acetylmuramyl pentapeptide (UDP-MurNAc-pp) was extracted mainly according to previous methods, with some modifications (17, 53). Simply, M. luteus NCIB 8166 was cultured in S1 medium until the OD600 reached 1.0 to 1.5. After preprocessing with 130 μg/mL chloramphenicol, the bacterial cells were separately incubated with sterile water (blank control), inecin L (1.2 μM, 10× MIC), and vancomycin (5 μM, 10× MIC) (positive control) at 37°C for 1 h. The extracts were purified by RP-HPLC and identified by mass spectrometry as described previously.
Tryptophan fluorescence spectroscopy.
The liposomal vesicles were prepared as previously described with slight modifications (17). DOPC (1,2-dioleoyl-sn-glycero-3-phosphocholine) (4 mM) and DOPG (1,2-dioleoyl-sn-glycero-3-phosphoglycerol) (1 mM) were dissolved in chloroform. The mixture was completely volatilized and then resuspended in K+ buffer (5 mM MES [4-Morpholineethanesulfonic acid], 10 mM K2SO4, pH 6.0). Next, the mixture was maintained at 55°C for 5 min and then vortexed vigorously for 1 min. The steps described above were repeated 4 times. Liposomes were prepared from the mixture by extrusion through polycarbonate membranes with a pore size of 100 nm using an Avanti miniextruder (Avanti Polar Lipids). An F-7000 spectrophotometer was used to determine the fluorescence intensities. Inecin L was dissolved in the K+ buffer or the liposomal solution at a final concentration of 1 μM. The different concentrations of liposomes were set at 20, 50, and 100 μM. The solutions were excited at 280 nm, and spectra were recorded from 300 nm to 400 nm.
Statistics.
Statistical assays were performed using one-way analysis of variance (ANOVA) in GraphPad Prism. The data are expressed as the mean values ± standard deviations.
ACKNOWLEDGMENTS
This study was supported by grants from the National Natural Science Foundation of China (grants number 31900025, 31972049, and 31570114) and the Strategic Priority Research Program of the Chinese Academy of Sciences (grant number XDA26040201).
Footnotes
Supplemental material is available online only.
Contributor Information
Jie Zhang, Email: jzhang@im.ac.cn.
Jin Zhong, Email: zhongj@im.ac.cn.
Christopher A. Elkins, Centers for Disease Control and Prevention
REFERENCES
- 1.Chee WJY, Chew SY, Than LTL. 2020. Vaginal microbiota and the potential of Lactobacillus derivatives in maintaining vaginal health. Microb Cell Fact 19:203. 10.1186/s12934-020-01464-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, Mistry SD, Duckworth RA, III, Bradley SP, Koparde VN, Orenda XV, Milton SH, Rozycki SK, Matveyev AV, Wright ML, Huzurbazar SV, Jackson EM, Smirnova E, Korlach J, Tsai YC, Dickinson MR, Brooks JL, Drake JI, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Wijesooriya NR, Hendricks-Muñoz KD, Jefferson KK, Strauss JF, III, Buck GA. 2019. The vaginal microbiome and preterm birth. Nat Med 25:1012–1021. 10.1038/s41591-019-0450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ravel J, Gajer P, Abdo Z, Schneider GM, Koenig SS, McCulle SL, Karlebach S, Gorle R, Russell J, Tacket CO, Brotman RM, Davis CC, Ault K, Peralta L, Forney LJ. 2011. Vaginal microbiome of reproductive-age women. Proc Natl Acad Sci USA 108(Suppl 1):4680–4687. 10.1073/pnas.1002611107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turovskiy Y, Sutyak Noll K, Chikindas ML. 2011. The aetiology of bacterial vaginosis. J Appl Microbiol 110:1105–1128. 10.1111/j.1365-2672.2011.04977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muzny CA, Łaniewski P, Schwebke JR, Herbst-Kralovetz MM. 2020. Host-vaginal microbiota interactions in the pathogenesis of bacterial vaginosis. Curr Opin Infect Dis 33:59–65. 10.1097/QCO.0000000000000620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vornhagen J, Adams Waldorf KM, Rajagopal L. 2017. Perinatal group B streptococcal infections: virulence factors, immunity, and prevention strategies. Trends Microbiol 25:919–931. 10.1016/j.tim.2017.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chico RM, Mayaud P, Ariti C, Mabey D, Ronsmans C, Chandramohan D. 2012. Prevalence of malaria and sexually transmitted and reproductive tract infections in pregnancy in sub-Saharan Africa: a systematic review. JAMA 307:2079–2086. 10.1001/jama.2012.3428. [DOI] [PubMed] [Google Scholar]
- 8.Repka LM, Chekan JR, Nair SK, van der Donk WA. 2017. Mechanistic understanding of lanthipeptide biosynthetic enzymes. Chem Rev 117:5457–5520. 10.1021/acs.chemrev.6b00591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jangra M, Kaur M, Nandanwar H. 2019. In-vitro studies on a natural lantibiotic, paenibacillin: a new-generation antibacterial drug candidate to overcome multi-drug resistance. Int J Antimicrob Agents 53:838–843. 10.1016/j.ijantimicag.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 10.Kim SG, Becattini S, Moody TU, Shliaha PV, Littmann ER, Seok R, Gjonbalaj M, Eaton V, Fontana E, Amoretti L, Wright R, Caballero S, Wang Z-MX, Jung H-J, Morjaria SM, Leiner IM, Qin W, Ramos RJJF, Cross JR, Narushima S, Honda K, Peled JU, Hendrickson RC, Taur Y, van den Brink MRM, Pamer EG. 2019. Microbiota-derived lantibiotic restores resistance against vancomycin-resistant Enterococcus. Nature 572:665–669. 10.1038/s41586-019-1501-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Q, Yu Y, Vélasquez JE, van der Donk WA. 2012. Evolution of lanthipeptide synthetases. Proc Natl Acad Sci USA 109:18361–18366. 10.1073/pnas.1210393109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu M, Zhang F, Cheng Z, Bashiri G, Wang J, Hong J, Wang Y, Xu L, Chen X, Huang SX, Lin S, Deng Z, Tao M. 2020. Functional genome mining reveals a class V lanthipeptide containing a d-amino acid introduced by an F420 H2 -dependent reductase. Angew Chem Int Ed Engl 59:18029–18035. 10.1002/anie.202008035. [DOI] [PubMed] [Google Scholar]
- 13.Li B, Yu JP, Brunzelle JS, Moll GN, van der Donk WA, Nair SK. 2006. Structure and mechanism of the lantibiotic cyclase involved in nisin biosynthesis. Science 311:1464–1467. 10.1126/science.1121422. [DOI] [PubMed] [Google Scholar]
- 14.Paul M, Patton GC, van der Donk WA. 2007. Mutants of the zinc ligands of lacticin 481 synthetase retain dehydration activity but have impaired cyclization activity. Biochemistry 46:6268–6276. 10.1021/bi7000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ross AC, Vederas JC. 2011. Fundamental functionality: recent developments in understanding the structure-activity relationships of lantibiotic peptides. J Antibiot (Tokyo) 64:27–34. 10.1038/ja.2010.136. [DOI] [PubMed] [Google Scholar]
- 16.Islam MR, Nishie M, Nagao J, Zendo T, Keller S, Nakayama J, Kohda D, Sahl HG, Sonomoto K. 2012. Ring A of nukacin ISK-1: a lipid II-binding motif for type-A(II) lantibiotic. J Am Chem Soc 134:3687–3690. 10.1021/ja300007h. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Feng Y, Teng K, Lin Y, Gao Y, Wang J, Zhong J. 2014. Type AII lantibiotic bovicin HJ50 with a rare disulfide bond: structure, structure-activity relationships and mode of action. Biochem J 461:497–508. 10.1042/BJ20131524. [DOI] [PubMed] [Google Scholar]
- 18.Chatterjee C, Paul M, Xie L, van der Donk WA. 2005. Biosynthesis and mode of action of lantibiotics. Chem Rev 105:633–684. 10.1021/cr030105v. [DOI] [PubMed] [Google Scholar]
- 19.Bierbaum G, Sahl HG. 2009. Lantibiotics: mode of action, biosynthesis and bioengineering. Curr Pharm Biotechnol 10:2–18. 10.2174/138920109787048616. [DOI] [PubMed] [Google Scholar]
- 20.Wang X, Gu Q, Breukink E. 2020. Non-lipid II targeting lantibiotics. Biochim Biophys Acta Biomembr 1862:183244. 10.1016/j.bbamem.2020.183244. [DOI] [PubMed] [Google Scholar]
- 21.Petrova MI, Reid G, Vaneechoutte M, Lebeer S. 2017. Lactobacillus iners: friend or foe? Trends Microbiol 25:182–191. 10.1016/j.tim.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 22.Bradshaw CS, Sobel JD. 2016. Current treatment of bacterial vaginosis—limitations and need for innovation. J Infect Dis 214:S14–S20. 10.1093/infdis/jiw159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shabayek S, Spellerberg B. 2018. Group B streptococcal colonization, molecular characteristics, and epidemiology. Front Microbiol 9:437. 10.3389/fmicb.2018.00437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu X, Zhang S, Li H, Shen L, Dong C, Sun Y, Chen H, Xu B, Zhuang W, Deighton M, Qu Y. 2020. Biofilm formation of Candida albicans facilitates fungal infiltration and persister cell formation in vaginal candidiasis. Front Microbiol 11:1117. 10.3389/fmicb.2020.01117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang Q, Yang X, Wang H, van der Donk WA. 2014. High divergence of the precursor peptides in combinatorial lanthipeptide biosynthesis. ACS Chem Biol 9:2686–2694. 10.1021/cb500622c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krull RE, Chen P, Novak J, Kirk M, Barnes S, Baker J, Krishna NR, Caufield PW. 2000. Biochemical structural analysis of the lantibiotic mutacin II. J Biol Chem 275:15845–15850. 10.1074/jbc.275.21.15845. [DOI] [PubMed] [Google Scholar]
- 27.Breukink E, de Kruijff B. 2006. Lipid II as a target for antibiotics. Nat Rev Drug Discov 5:321–332. 10.1038/nrd2004. [DOI] [PubMed] [Google Scholar]
- 28.Petrova MI, van den Broek M, Balzarini J, Vanderleyden J, Lebeer S. 2013. Vaginal microbiota and its role in HIV transmission and infection. FEMS Microbiol Rev 37:762–792. 10.1111/1574-6976.12029. [DOI] [PubMed] [Google Scholar]
- 29.Donia MS, Fischbach MA. 2015. Human microbiota. Small molecules from the human microbiota. Science 349:1254766. 10.1126/science.1254766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Piper C, Cotter PD, Ross RP, Hill C. 2009. Discovery of medically significant lantibiotics. Curr Drug Discov Technol 6:1–18. 10.2174/157016309787581075. [DOI] [PubMed] [Google Scholar]
- 31.O’Sullivan JN, Rea MC, O’Connor PM, Hill C, Ross RP. 2019. Human skin microbiota is a rich source of bacteriocin-producing staphylococci that kill human pathogens. FEMS Microbiol Ecol 95:fiy241. 10.1093/femsec/fiy241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scott JC, Sahl HG, Carne A, Tagg JR. 1992. Lantibiotic-mediated anti-Lactobacillus activity of a vaginal Staphylococcus aureus isolate. FEMS Microbiol Lett 72:97–102. 10.1111/j.1574-6968.1992.tb05047.x. [DOI] [PubMed] [Google Scholar]
- 33.Gao Y, Lu Y, Teng KL, Chen ML, Zheng HJ, Zhu YQ, Zhong J. 2011. Complete genome sequence of Lactococcus lactis subsp. lactis CV56, a probiotic strain isolated from the vaginas of healthy women. J Bacteriol 193:2886–2887. 10.1128/JB.00358-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsu CY, Lin MH, Chen CC, Chien SC, Cheng YH, Su IN, Shu JC. 2011. Vancomycin promotes the bacterial autolysis, release of extracellular DNA, and biofilm formation in vancomycin-non-susceptible Staphylococcus aureus. FEMS Immunol Med Microbiol 63:236–247. 10.1111/j.1574-695X.2011.00846.x. [DOI] [PubMed] [Google Scholar]
- 35.Asaduzzaman SM, Nagao J, Aso Y, Nakayama J, Sonomoto K. 2006. Lysine-oriented charges trigger the membrane binding and activity of nukacin ISK-1. Appl Environ Microbiol 72:6012–6017. 10.1128/AEM.00678-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Geng M, Austin F, Shin R, Smith L. 2018. Covalent structure and bioactivity of the type AII lantibiotic salivaricin A2. Appl Environ Microbiol 84:e02528-17. 10.1128/AEM.02528-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fujinami D, Mahin AA, Elsayed KM, Islam MR, Nagao JI, Roy U, Momin S, Zendo T, Kohda D, Sonomoto K. 2018. The lantibiotic nukacin ISK-1 exists in an equilibrium between active and inactive lipid-II binding states. Commun Biol 1:150. 10.1038/s42003-018-0150-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Asaduzzaman SM, Nagao J, Iida H, Zendo T, Nakayama J, Sonomoto K. 2009. Nukacin ISK-1, a bacteriostatic lantibiotic. Antimicrob Agents Chemother 53:3595–3598. 10.1128/AAC.01623-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Knerr PJ, Oman TJ, Garcia De Gonzalo CV, Lupoli TJ, Walker S, van der Donk WA. 2012. Non-proteinogenic amino acids in lacticin 481 analogues result in more potent inhibition of peptidoglycan transglycosylation. ACS Chem Biol 7:1791–1795. 10.1021/cb300372b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barbour A, Tagg J, Abou-Zied OK, Philip K. 2016. New insights into the mode of action of the lantibiotic salivaricin B. Sci Rep 6:31749. 10.1038/srep31749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jack R, Benz R, Tagg J, Sahl HG. 1994. The mode of action of SA-FF22, a lantibiotic isolated from Streptococcus pyogenes strain FF22. Eur J Biochem 219:699–705. 10.1111/j.1432-1033.1994.tb19986.x. [DOI] [PubMed] [Google Scholar]
- 42.Roy U, Islam MR, Nagao J, Iida H, Mahin AA, Li M, Zendo T, Nakayama J, Sonomoto K. 2014. Bactericidal activity of nukacin ISK-1: an alternative mode of action. Biosci Biotechnol Biochem 78:1270–1273. 10.1080/09168451.2014.918485. [DOI] [PubMed] [Google Scholar]
- 43.Chikindas ML, Novák J, Driessen AJ, Konings WN, Schilling KM, Caufield PW. 1995. Mutacin II, a bactericidal antibiotic from Streptococcus mutans. Antimicrob Agents Chemother 39:2656–2660. 10.1128/AAC.39.12.2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mills S, Ross RP, Hill C. 2017. Bacteriocins and bacteriophage; a narrow-minded approach to food and gut microbiology. FEMS Microbiol Rev 41:S129–S153. 10.1093/femsre/fux022. [DOI] [PubMed] [Google Scholar]
- 45.Joyce C, March PE, Ryan L, Daniel T. 2004. Site-directed, ligase-independent mutagenesis (SLIM): a single-tube methodology approaching 100% efficiency in 4 h. Nucleic Acids Res 32:e174. 10.1093/nar/gnh172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin Y, Teng K, Huan L, Zhong J. 2011. Dissection of the bridging pattern of bovicin HJ50, a lantibiotic containing a characteristic disulfide bridge. Microbiol Res 166:146–154. 10.1016/j.micres.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Wang J, Zhang L, Teng K, Sun S, Sun Z, Zhong J. 2014. Cerecidins, novel lantibiotics from Bacillus cereus with potent antimicrobial activity. Appl Environ Microbiol 80:2633–2643. 10.1128/AEM.03751-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Levengood MR, Knerr PJ, Oman TJ, van der Donk WA. 2009. In vitro mutasynthesis of lantibiotic analogues containing nonproteinogenic amino acids. J Am Chem Soc 131:12024–12025. 10.1021/ja903239s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stepanovic S, Vukovic D, Dakic I, Savic B, Vabic-Vlahovic M. 2000. A modified microtiter-plate test for quantification of staphylococcal biofilm formation. J Microbiol Methods 40:175–179. 10.1016/s0167-7012(00)00122-6. [DOI] [PubMed] [Google Scholar]
- 50.Thangamani S, Mohammad H, Abushahba MF, Hamed MI, Sobreira TJ, Hedrick VE, Paul LN, Seleem MN. 2015. Exploring simvastatin, an antihyperlipidemic drug, as a potential topical antibacterial agent. Sci Rep 5:16407. 10.1038/srep16407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brackman G, Breyne K, De Rycke R, Vermote A, Van Nieuwerburgh F, Meyer E, Van Calenbergh S, Coenye T. 2016. The quorum sensing inhibitor hamamelitannin increases antibiotic susceptibility of Staphylococcus aureus biofilms by affecting peptidoglycan biosynthesis and eDNA release. Sci Rep 6:20321. 10.1038/srep20321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie J, Zhou M, Qian Y, Cong Z, Chen S, Zhang W, Jiang W, Dai C, Shao N, Ji Z, Zou J, Xiao X, Liu L, Chen M, Li J, Liu R. 2021. Addressing MRSA infection and antibacterial resistance with peptoid polymers. Nat Commun 12:5898. 10.1038/s41467-021-26221-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Münch D, Müller A, Schneider T, Kohl B, Wenzel M, Bandow JE, Maffioli S, Sosio M, Donadio S, Wimmer R, Sahl H-G. 2014. The lantibiotic NAI-107 binds to bactoprenol-bound cell wall precursors and impairs membrane functions. J Biol Chem 289:12063–12076. 10.1074/jbc.M113.537449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material. Download aem.02123-22-s0001.pdf, PDF file, 1.6 MB (1.6MB, pdf)