Abstract
For more than a century, Cannabis was considered a narcotic and has been banned by lawmakers all over the world. In recent years, interest in this plant has increased due to its therapeutic potential, in addition to a very interesting chemical composition, characterized by the presence of an atypical family of molecules known as phytocannabinoids. With this emerging interest, it is very important to take stock of what research has been conducted so far on the chemistry and biology of Cannabis sativa. The aim of this review is to describe the traditional uses, chemical composition and biological activities of different parts of this plant, as well as the molecular docking studies. Information was collected from electronic databases, namely SciFinder, ScienceDirect, PubMed and Web of Science. Cannabis is mainly popular for its recreational use, but it is also traditionally used as remedy for the treatment of several diseases, including diabetes, digestive, circulatory, genital, nervous, urinary, skin and respiratory diseases. These biological proprieties are mainly due to the presence of bioactive metabolites represented by more than 550 different molecules. Molecular docking simulations proved the presence of affinities between Cannabis compounds and several enzymes responsible for anti-inflammatory, antidiabetic, antiepileptic and anticancer activities. Several biological activities have been evaluated on the metabolites of Cannabis sativa, and these works have shown the presence of antioxidant, antibacterial, anticoagulant, antifungal, anti-aflatoxigenic, insecticidal, anti-inflammatory, anticancer, neuroprotective and dermocosmetic activities. This paper presents the up-to-date reported investigations and opens many reflections and further research perspectives.
Keywords: Cannabis sativa L., ethnobotany, chemical composition, biological activities, medicine, in silico, molecular docking
1. Introduction
Cannabis sativa L. is an herbaceous plant belonging to the Cannabaceae family. This plant species has many vernacular names and is known by many people as marijuana and hemp. Despite being native to Central Asia, this plant’s capacity of adaption to different climates lead to its spread all over the world [1]. The Cannabis genus is composed of a single specie named “sativa”, which regroup several subspecies or varieties including Cannabis sativa ssp. sativa, Cannabis sativa ssp. indica, Cannabis sativa ssp. ruderalis and Cannabis sativa ssp. afghanica. However, there is still controversy among the scientific community about the sub-classification of Cannabis species and varieties [2,3,4]. Cannabis sativa L. is one of the plants that have been used by humankind since antiquity, and many historians reported the different uses of this plant around the world [5,6,7,8]. The historical records show that this plant has been used as a source of fiber, food, oil, as well as for recreational and religious purposes. Additionally, several other uses have been developed through the centuries, such as livestock feed, skin and hair care [9]. Furthermore, many ethnobotanical surveys highlighted the therapeutic use of Cannabis sativa L. for the treatment of chronic pain, depression and inflammation. These activities have been justified by the original chemical composition, viz. Cannabis contains a large number of bioactive compounds with an estimation of more than 550 molecules [10]. Those compounds belong to the cannabinoid, terpenoid, stilbenoid, lignanamide, carotenoid, flavonoid and alkaloid classes [11]. The most notable compound of Cannabis remains the cannabinoids [12,13], a class of terpenolic compounds mainly found in the trichome cavity of female flowers [14,15]. Nowadays, Cannabis sativa L. is experiencing a renewed interest in many research fields, including microbiology and oncology [12,13]. In fact, the chemical diversity of cannabinoids proved to be very useful for targeting microorganisms such as bacteria, fungi, viruses, as well as cell components such as proteins and genes [16]. Additionally, their natural origin and low toxicity make them perfect candidates to treat hard-to-treat diseases, by solving therapeutic problems such as the resistance to antibiotics and, in the case of cancer treatment, the toxicity induced by ingestion and metabolization [17,18,19].
This review aims to present Cannabis sativa subspecies classification, description of the plant aspect and botany as well as a brief history and geographic distribution. This manuscript aims also to report and discuss the traditional uses as to both the preparation and administration modes of every part of the plant. It also aims to take into account the chemical composition of each part with the classification of the identified metabolites and their quantification, and the biological activities of Cannabis extracts and purified compounds. Finally, this review also includes the molecular docking studies of secondary metabolites previously identified in different parts of Cannabis sativa L.
2. Generalities about Cannabis sativa L.
2.1. Plant Nomenclature and Synonyms
Carolus Linnæus, also known as Carl von Linné (1707–1778), was the first person to frame principles for classification of living organisms into classes and sub-classes. His aim was to create a uniform international system for the identification of any living organism according to its morphological features. In this system, every organism is identified by his genera and specie names known as “binomial nomenclature”. In 1753, Carl von Linné mentioned the word Cannabis for the first time. This word comes from the Latin canna that means “reed” and bis that means “twice”, which means literally “reed with two sexes” [20]. Prior to the Linnæus nomenclature, Cannabis was widely used by different civilizations that gave it different names known as vernacular names [21,22,23]. At present, there are many local or vernacular names and various synonyms to name Cannabis. It is also known as hashish, marijuana, weed, Acapulco gold, ace, bat, bhang, log, hemp, Indian hemp, Colombian, doobie, dope (Cannabis), ganja, hydro, Jamaican, jive (sticks), joint, Maui wowie, Mexican, Panama gold, Panama red, pot, firecracker, ragweed, reefer, sativa, sinsemilla of California, spliff, Thai stick, etc. Those names and designations stay different depending on the region, country and culture. Cannabis sativa belongs to the Cannabaceae family, which includes 12 genera and 102 species, and with some species of economic importance, such as Humulus lupulus L. and Pteroceltis tatarinowii [24]. There are conflicting botanical classifications of Cannabis sativa, and the taxonomic classification of this plant has been the subject of divergences and debates. It is commonly accepted and recommended that Cannabis sativa is a single species [25], with four subspecies, namely indica, ruderalis, sativa and afghanica [2,3,4,26]. However, the classification criteria used for the differentiation of Cannabis sativa subspecies are often not very clear, since the chemical and morphological characteristics appear to vary according to the plant environment and pedology. In a study reported by Pacifico, et al. [27], the authors showed that the tetrahydrocannabinol (THC) content of a Cannabis sativa single species depends on the growing climate of the plant. In most cases, it is recommended to apply the name Cannabis sativa to all Cannabis plants encountered, since they all belong to the same species, and there is no agreement on the plant taxonomy [25].
2.2. Description and Botanical Aspect
Cannabis sativa L. is an annual, usually dioecious plant belonging to the Cannabaceae family [28]. It is now considered as the only species of the botanical genus Cannabis but divided into several phenotypes that can be described as subspecies or varieties [29]. Cannabis sativa has the particularity of being a fast-growing plant with a fluted stem that can reach 1 to 4 m with a diameter ranging between 1 and 3 cm (Figure 1a) [30]. The variation of height and diameter depends on the sub-species, environment, soil and climatic conditions [31,32]. The seeds are smooth, greyish ovoid or spherical in shape, 2.5 to 3.5 mm long and 2.5 to 3 mm in diameter (Figure 1c). Each seed contains two cotyledons rich in reserves (protein and oil), with an albumen considered particularly small compared to other plant species [33].
Figure 1.
Cannabis sativa L. General aspect (a); inflorescence (b); seed (c); leaf (d); stem (e).
This plant is also characterized by long, fine flowers (Figure 1b). It has glandular hairs that make it fragrant and sticky [34,35]. At post-germination, young male and female plants cannot be distinguished. It is only during the last phase of growth, when flowers start appearing, that sex determination becomes possible [24,36]. The female flowers have no petals and consist of two long white, yellow or pink stigmas. Their calyx (less than 3–6 mm) envelops the ovary containing a single ovule. The female flowers appear in pairs in the axils of small leaves named bracts, these bracts contain numerous glandular trichomes where cannabinoids, mainly THC, accumulate [34,37,38]. On the other hand, the male flowers have five sepals of approximately 5 mm length, with yellow, white or green color [33,39]. The male plants develop small pollen sacs that serve to fertilize the female plants with hairy, resinous stigmas [34,36,40]. The Cannabis leaves are stipulate and opposite, with palmate (five to seven unequal), elongated and spiny segments with toothed margins (Figure 1d). Towards the top of the axis, the leaves become alternate and are inserted on the stem in an opposite arrangement every 10–30 cm [39]. These plants have cystolithic, tectorial and resin-secreting hairs; the latter have a voluminous base ending in a cluster of several cells, with each one secreting resin [39]. The root is taproot with a length of up to 30 cm, but the lateral roots reach 20 to 100 cm. In addition, in peaty soils, the lateral roots are more strongly developed, and the main root grows to a depth of 10–20 cm [41]. The growth rate of the root system is quite slow in the initial stages of vegetation, in contrast to the aerial part of the Cannabis plant, which grows intensively and rapidly [41].
2.3. Geographic Distribution and History
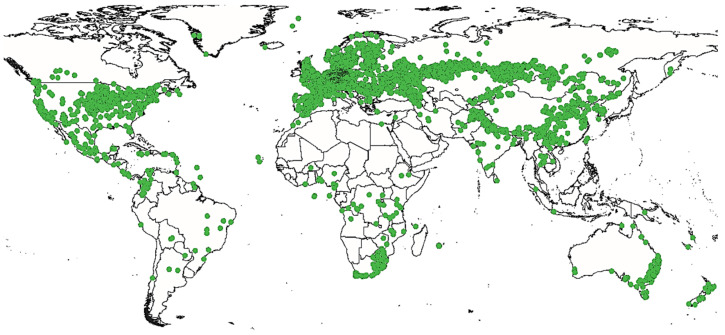
In nature, Cannabis is an annual flowering plant. This means that it completes its life cycle, from germination to seed production, in one year [42]. Cannabis can grow in a vast majority of climates (Figure 2). From its region of origin, it appreciates calcareous and nitrogenous soils with a neutral or slightly acidic pH [43,44].
Figure 2.
Geographic distribution of Cannabis sativa L.
This species originates from equatorial and subtropical regions, mainly from central Asia [1], where two places seem to be its cradle: the foothills of the Himalayas and the plains of the Pamir (a high mountain range centered in eastern Tajikistan with extensions into Afghanistan, the Republic of China and Kyrgyzstan) [45]. However, this plant has a wide geographical distribution growing up in Canada, United States of America, Europe and Africa. Cannabis is an ancient plant but the craze it has generated over (at least) the last century has greatly changed its face and even the face of the world. It is probably the first plant domesticated by humankind [46]. Many historical reports prove that this plant had been cultivated worldwide for thousands of years. The oldest documented evidence of Cannabis cultivation is a 26,900 B.C. hemp rope found in the Czech Republic [47]. Some of the earliest known prolific uses of Cannabis began in China around 10,000 B.C., where Cannabis was used to make clothing, rope and paper [48]. Further traces were reportedly found at the Neolithic site of Xianrendong on Chinese ceramics dating back to 8000 B.C. and decorated with hemp braided fibers. Between 8000 and 300 B.C., Cannabis was also cultivated in Japan and employed to make cloth fiber and paper [49,50]. However, the earliest reference of Cannabis psychotropic use goes back to 2700 B.C. It has been mentioned in the Chinese pharmacopoeia of the Emperor Chen Nong, where it is recommended as a sedative and remedy for insanity. Cannabis was also mentioned on the Ebers Papyrus of pharaonic Egypt back to 1550 B.C. as remedy for vaginal inflammations [51]. Yet, it was mentioned in Greek medicine, in the writings of Dioscorides, who underlines the psychotropic properties of the plant and already Galen fears that “it hurts the brain when we take too much” [52,53]. In India, it was one of the five magical plants used in religious rituals in the form of fumigation. In fact, around 1300 B.C., the stimulating and euphoric powers of bhanga (hemp in Sanskrit) were praised by the Indo-Aryans in one of the four holy books, the Atharva Veda [54]. Back to the European Continent, and around 700 B.C. in Marseille (France), Cannabis was used for rope manufacturing. The name Cannebiere (important avenue of the city) testifies of the importance of Cannabis at that time [35]. Jamestown settlers introduced Cannabis to colonial America in the early 1600s for the manufacture of rope, paper and other fiber products. This plant was so important that American presidents George Washington and Thomas Jefferson grew Cannabis [38]. The question of when and how Cannabis originated in the new world is still very controversial indeed. Cannabis was discovered in native American civilizations prior to Columbus’ arrival [55]. William Henry Holmes’ 1896 report “prehistoric textile art of the Eastern United States” indicated that Cannabis originated with native American tribes of the Great Lakes and Mississippi valley [56]. Cannabis products from pre-Columbian indigenous civilizations have also been found in Virginia [57]. Cannabis was an important crop in the United States until 1937, when the Marihuana Tax Act all but wiped out the American hemp industry. During World War II, Cannabis experienced a resurgence in the United States of America, as it was widely used to manufacture military items ranging from uniforms to canvas and rope [57]. At present, the most notable development in Cannabis production around the world is the rise of indoor cultivation, particularly in Europe, Australia and North America. This type of cultivation gives rise to a very lucrative trade, which is increasingly a source of profit for local organized crime groups [58].
3. Methodology
Relevant information about Cannabis sativa L. was collected from various scientific sources including SciFinder, ScienceDirect, PubMed and Web of Science. The targeted databases were probed with “Cannabis sativa”, “botany”, “history”, “ethnobotany”, “traditional use”, “phytochemistry”, “pharmacology”, “bioactivity”, “bioinformatic” and “in-silico prediction” as keywords. Thus, available articles were collected, summarized in tables and analyzed. In addition to that, we report up-to-date studies of Cannabis sativa ethnopharmacology, chemical composition, pharmacology and molecular docking simulations; this review aims to give a subjective critique to reported articles and offer perspectives for further investigations on Cannabis phytochemistry and pharmacology.
4. Results and Discussion
4.1. Traditional Uses of Cannabis sativa L.
Cannabis sativa L. has been used in a wide variety of fields and showed a high usability potential with many applications including manufacturing of tools, construction, cosmetics, medication, shelter insulation, papermaking, human nutrition, animal feed, agrofuels, composite materials in association with plastics, etc. [5,6,7,8]. Table 1 summarizes the parts of the plant and their traditional uses.
Table 1.
Traditional uses of different parts of Cannabis sativa.
| Plant Part | Traditional Use | Preparation | Administration | Reference |
|---|---|---|---|---|
| Seed | Nutrition | Powder | Oral | [59] |
| Seed | Narcotic, painkiller, treat nausea and vomiting, stimulate appetite in AIDS patients, hepatitis C, anxiety, seizure, muscle relaxants, anticancer and weight control | ND | ND | [60] |
| Seed | Hair fortification | Powder | External | [61] |
| Seed | Analgesic, antiarthritic and antirheumatic | Oil | External | [62] |
| Leaf | Eczema | Powder | External | [61] |
| Leaf | Bloating, cough, mucus | Leaf juice | Oral | [63] |
| Leaf | Central nervous system (CNS) depressant, gout, arthritic pain | Powder | Oral | [64] |
| Leaf | Schizophrenia-like psychotic problems | Oil | External | [65] |
| Leaf | Gastric disorders | ND | ND | [66] |
| Leaf | Skin and subcutaneous tissue disorders, circulatory system and blood disorders | Juice, paste or powder | ND | [62] |
| Inflorescence | Sedative, dysentery, diarrhea and appetite loss | ND | Oral | [62] |
| Stem | Firewood or torch wood | Raw | ND | [62] |
| Stem | Construction, materials, dress, papermaking, making ropes | Raw | ND | [5] |
| Seed, flower | Hair care | ND | ND | [67] |
| Leaf, inflorescence |
Soporific, abortifacient | ND | ND | [68] |
| Leaf, root | Cancer, hypertension, antidote to poison, itch, rheumatoid arthritis | ND | ND | [66] |
| Root | Fever | Maceration | External | [69] |
| Root | Gout, arthritis | Boiled roots | External (cataplasm) |
[70] |
| Root | Joint pain | Decoction | External (cataplasm) |
[70] |
| Root | Skin burns | Raw or decoction mixed with butter | External (topical) |
[70] |
| Root | Inflammation | Boiled roots, decoction |
External (cataplasm) |
[70] |
| Root | Childbirth, postpartum, hemorrhage | Decoction | Oral | [70] |
| Root | Erysipelas, toxins and infections | Pulverized and mixed with wine | Oral/external | [70] |
| Whole plant | Pain, gastric disorders, diabetes, scars and asthma | ND | ND | [71] |
The analysis of collected data shows that seeds and leaves are the most used parts for medication. Many studies reported the use of Cannabis seed as food. It is used for the production of pasta, gluten-free flour characterized by a nutty taste, beer and oil [72,73]. In addition to their use for human nutrition [59], the seeds are also used to treat nausea, vomiting, stimulate the appetite of AIDS patients, cancer and hepatitis C. It is also applied as a muscle relaxant, for weight control, lung capacity enhancer and as an analgesic, anxiolytic, antiepileptic, antiemetic and against neurological pain [60]. These seeds have also a cosmetic use, mainly for hair fortification as a hair serum by external application of seed powder [61]. Beyond their potential value as medicine or food, Cannabis sativa seeds have recently been used to treat contaminated groundwater, since hemp seed protein powder proved to be more effective than other plant protein sources for chelating perfluoroalkyl and polyfluoroalkyl substances, known as “eternal chemicals” [74]. Those Cannabis proteins proved to be very useful for the treatment of salt-contaminated soils as well [75].
The leaves are externally used as poultice to treat eczema [61] and subcutaneous tissue disorders [62]. They are also orally consumed by local people for the treatment of central nervous system (CNS) disorders such as schizophrenia, gout, arthritic pain, bloating, coughing and mucus [63,64]. Cannabis leaves are among the most iconic symbols of modern stoner culture; their shape is frequently associated with the recreative use of this plant. Several studies described the traditional uses of Cannabis leaves, and those applications include the treatment of a wide range of health problems such as hypertension, rheumatoid arthritis, itching, cancer, snake and scorpion poisoning [66], as well as gastric and circulatory system disorders [62]. These leaves have also been described as strong analgesics, sedatives and narcotics [76]. Other studies described the use of Cannabis sativa stem fibers as firewood [62], for construction, tools, clothes, paper and rope manufacturing [5,59]. These fibers are obtained by a process called defibration, which can be briefly described as a stem beating and grinding. During this process, two co-products are obtained, namely chenevotte and Cannabis dust. It is important to highlight that the quality of the fiber decreases with the maturity of the plant, since the fibers become harder and coarser. In addition to the aerial parts of Cannabis plant, the root parts are also used for medication. They are used in particular for the treatment of joint pain, skin burns, inflammation, vermin and erysipelas infection [70]. These roots are also orally used in the form of juice to relieve issues stemming from childbirth, postpartum and hemorrhage [70]. Among the most cited uses of Cannabis sativa, the psychoactive remains the most present. The leaves and inflorescences have been consumed as a narcotic in different forms and have been prepared using different methods; for instance, the leaves are smoked or prepared, the inflorescences or resin are processed into charas or attar, hashish, ganja and plant powder, whereas leaves, inflorescences and shoots are used to prepare drinks (e.g., bhang, thandai, tandai, etc.) [5,77]. Moreover, Klauke, et al. [62] reported the religious use of Cannabis drinks. This plant preparation, referred to as traditional bhang drink, is highly consumed during Indian festivals such as Shivaratri and Holi [78]. Some ethnobotanical surveys described the used of the whole Cannabis plant. The aerial parts are mostly used for the treatment of mental disorders and nervous-system-related conditions. However, the most common use of those parts is for the treatment of gastric disorders, diabetes, scarring and asthma [71]. Conversely, some studies reported the appetite-stimulating, antidysentery and antidiarrhea effects of Cannabis inflorescences, but omitted the mention of preparation and administration modes [62]. The analysis of ethnobotanical findings shows that Cannabis sativa is a plant that was integrally exploited by local populations; some traditional uses are common to many countries whereas others are specific to some cultures. One can cite the psychotropic, medicinal and cosmetic purposes found all over the world, in contrast with the religious uses exclusively reported in Asian and Latin American countries. The previously reported activities and proprieties are mainly due to the presence of metabolites with interesting chemical structures; the ethnobotanical uses of Cannabis attracted phytochemists to investigate its chemical composition. The first compound to be identified and isolated from Cannabis was cannabinol at the end of the 19th century [79].
4.2. Chemical Composition of Cannabis sativa L.
Numerous studies have shown the importance of Cannabis secondary metabolites as well as their roles. This plant offers a rich reservoir of bioactive molecules that can be used for the production of pharmaceutical, nutraceutical and cosmetic products. Table 2 regroups the chemical composition of Cannabis sativa seeds, flowers, leaves and resin.
Table 2.
Chemical composition of Cannabis sativa different plant parts.
| Plant Part | Extraction Method (Type of Extract) | Class of Metabolites | Compounds (Quantities) | Reference |
|---|---|---|---|---|
| Seed | Maceration (hexane) |
Fatty acids | Linoleic acid (47.06%) Oleic acid (43.20%) Palmitic acid (4.88%) Stearic acid (3.32%) |
[80] |
| Seed | Soxhlet (petroleum benzine) |
Fatty acids | Linoleic acid (58.41 ± 0.04%) α-Linolenic Acid (16.26 ± 0.03%) Oleic acid (16.05 ± 0.02%) Palmitic acid (5.59 ± 0.12%) Stearic acid (2.46 ± 0.01%) |
[81] |
| Seed | Soxhlet (ethanol–water 80:20) |
Polyphenols | Gallic acid (12.9 ± 18.28 mg/100 g) (+)-Catechin (5.995 ± 5.23 mg/100 g) 1,2-Dihydroxybenzene (5.155 ± 4.59 mg/100 g) 3,4-Dihydroxybenzoic acid (4.89 ± 4.68 mg/100 g) Syringic acid (3.795 ± 1.99 mg/100 g) Caffeic acid (2.475 ± 3.53 mg/100 g) Quercetin (2.08 ± 3.36 mg/100 g) Rutin trihydrate (0.915 ± 1.15 mg/100 g) Isorhamnetin (0.765 ± 0.89 mg/100 g) trans-Ferulic acid (0.685 ± 0.61 mg/100 g) Apigenin-7-glucoside (0.55 ± 0.38 mg/100 g) Naringenin (0.255 ± 0.33 mg/100 g) trans-Cinnamic acid (0.19 ± 0.24 mg/100 g) Resveratrol (0.17 ± 0.24 mg/100 g) p-Coumaric acid (0.165 ± 0.17 mg/100 g) |
[81] |
| Seed | Ultrasound-assisted extraction (ethanol) |
Polyphenols | Cannabisin A (105.1 ± 54 mg/100 g DW) N-trans-caffeoyltyramine (49 ± 34.2 mg/100 g DW) Cinnamic acid (3.75 ± 3.55 mg/100 g DW) p-hydroxybenzoic acid (2.1 ± 0.9 mg/100 g DW) Protocatechuic acid (1 ± 0.6 mg/100 g DW) |
[82] |
| Seed | Maceration (methanol–water 80:20) |
Polyphenols | Quercetin-O-glucoside (ND) N-trans-caffeoyltyramine (ND) Rutin (ND) |
[83] |
| Seed | Maceration (methanol–water 80:20) |
Polyphenols | Cannabisin A, B, C, D, E, F, G, I, M, N, O (ND) | [83] |
| Seed | Soxhlet (hexane) |
Tocopherols | γ-tocopherol (426 mg/kg) δ-tocopherol (33 mg/kg) α-tocopherol (13 mg/kg) β-tocopherol (2 mg/kg) |
[84] |
| Seed | Ultrasound-assisted extraction (ethanol) |
Tocopherols | γ-tocopherol (7.95 ± 3.35 mg/100 g DW) δ-tocopherol (0.95 ± 0.35 mg/100 g DW) |
[82] |
| Seed | Maceration (hexane) |
Phytosterols | β-Sitosterol (90.75 ± 0.42%) Campesterol (6.20 ± 0.00%) Stigmasterol (2.88 ± 0.17%) |
[85] |
| Seed | Soxhlet (hexane) |
Phytosterols | β-sitosterol (68.0%) Campesterol (17.1%) Δ-5-avenasterol (7.8%) Stigmasterol (3.8%) Δ-5,25-stigmastadienol (1.1%) |
[84] |
| Seed | Ultrasound-assisted extraction (ethanol) |
Carotenoids | Lutein (2.45 ± 0.95 mg/100 g DW) β-Carotene (0.5 ± 0.3 mg/100 g DW) |
[82] |
| Seed | Supercritical fluid extraction (CO2) |
Aldehydes | Hexanal (39.57 ± 0.91 mg/kg) Octadienal (10.29 ± 3.18 mg/kg) Heptadienal (9.38 ± 1.41 mg/kg) Nonenal (8.77 ± 1.27 mg/kg) Nonanal (8.34 ± 1.28 mg/kg) |
[85] |
| Seed | Supercritical fluid extraction (CO2) |
Alcohols | Hexanol (30.66 ± 0.95 mg/kg) | [85] |
| Seed | Maceration (hexane) |
Hydrocarbons | Dodecane (112.5 ± 2.06 mg/kg) Tetradecane (69.0 ± 1.40 mg/kg) 1.3-Di-tert-butylbenzene (46.6 ± 2.25 mg/kg) |
[85] |
| Leaf | Hydrodistillation | Terpenes | (E)-Caryophyllene (28.3 ± 4.1%) α-Humulene (9.3 ± 1.1%) β-Selinene (4.7 ± 0.9%) Caryophyllene oxide (4.3 ± 0.9%) α-Selinene (3.1± 0.6%) |
[86] |
| Leaf | Ultrasound-assisted extraction (methanol) |
Polyphenols | Apigenin C-(hexoside-O-rhamnoside) (0.83 mg/g) Luteolin C-(hexoside-O-rhamnoside) (0.67 mg/g) Luteolin di-C-hexoside (0.60 mg/g) Luteolin glucuronide(0.60 mg/g) Apigenin di-C-hexoside (0.54 mg/g) |
[86] |
| Leaf | Ultrasound-assisted extraction (methanol) |
Cannabinoids | CBD (11.2 ± 1.9%) CBDV (0.8 ± 0.2%) THC (0.7 ± 0.2%) CBC (0.5 ± 0.1%) |
[86] |
| Leaf | Ultrasound-assisted extraction (water–ethanol 20:80) |
Cannabinoids | Cannabidiol acid (150.00 ± 16.84 mg/g DW) Cannabidiol (31.00 ± 2.86 mg/g DW) THCA (6.50 ± 0.52mg/g DW) Cannabigerolic acid (6.30 ± 0.52 mg/g DW) Δ9-THC (4.00 ± 0.34 mg/g DW) |
[87] |
| Leaf | Maceration (ethanol–acetic acid 90:10) |
Alkaloids | Cannabisativine (410.30 μg/g) Cannabimine C (376.12 μg/g) Anhydrocannabisativine (218.11 μg/g) Aconitine (160.43 μg/g) Boldine (103.41 μg/g) Strychnine (72.63 μg/g) |
[88] |
| Leaf | Vacuum liquid chromatography (ethanol–water 95:05) |
Stilbenoids | Canniprene (ND); Combretastatin B-2 (ND) α,α′-dihydro-3,4′,5-trihydroxy-4,5′-diisopentenylstilbene (ND) |
[89] |
| Flower | Hydrodistillation | Terpenes | (E)-Caryophyllene (28.5 ± 3.1%) α-Humulene (9.2 ± 1.7%); β-Selinene (4.3 ± 0.8%); α-Selinene (2.9 ± 0.6%) Caryophyllene oxide (2.3 ± 0.5%); α-trans-Bergamotene (1.9 ± 0.4%) |
[86] |
| Flower | Ultrasound-assisted extraction (methanol) |
Polyphenols | Quercetin di-C-hexoside (2.55 mg/g) Luteolin C-hexoside-2″-O-hexoside (1.01 mg/g) Apigenin di-C-hexoside (0.68 mg/g) Apigenin C-(hexoside-O-rhamnoside) (0.51 mg/g) |
[86] |
| Flower | Ultrasound-assisted extraction (methanol) |
Cannabinoids | CBD (24.9 ± 3.9%) THC (1.4 ± 0.3%) CBDV (1.4 ± 0.3%) CBTC (0.9 ± 0.2%) |
[86] |
| Inflorescence | Hydrodistillation | Terpenes | Transcaryophyllene (38.2 ± 1.7%) Nerolidol (12.7 ± 1.2%) α-pinene (11.8 ± 0.4%) β-pinene (3.4 ± 0.3%) Cedrol (2.2 ± 0.0%) Myrcene (1.7 ± 0.5%) α-bisabolol (0.6 ± 0.1%) γ-terpinene (0.5 ± 0.3%) Camphene (0.2 ± 0.0%) α-humulene (0.2 ± 0.0%) α-terpinene (0.1 ± 0.0%) Menthol (0.1 ± 0.0%) |
[90] |
| Inflorescence | Hydrodistillation | Terpenes | β-caryophyllene (14.4 ± 0.89%) Caryophyllene oxide (7.0 ± 1.06%) α-humulene (5.3 ± 0.10%) Selina-3,7(11)-diene (3.4 ± 0.33%) α-pinene (3.0 ± 0.04%) Myrcene (2.6 ± 0.16%) 14-hydroxy-9-epi-(E) -caryophyllene (2.5 ± 0.12%) Humulene oxide II (2.4 ± 0.24%) Caryophylla-4(14),8(5)-dien-5-ol (1.4 ± 0.15%) β-selinene (1.2 ± 0,07%) α-bisabolol (1.1 ± 0.13%) Selin-6-en-4-ol (0.8 ± 0.23%) |
[91] |
| Inflorescence | Maceration (methanol) |
Cannabinoids | CBDA (9.515 ± 1.085%) tCBD (8.695 ± 0.955%) tTHC (0.545 ± 0.075%) CBGA (0.535 ± 0.365%) CBG (0.535 ± 0.355%) tCBG (0.490 ± 0.080%) THCA (0.490 ± 0.080%) CBD (0.345 ± 0.005%) Δ9-THC (0.080 ± 0.000%) Δ8-THC (0.045 ± 0.005%) |
[90] |
| Inflorescence | Microwave-assisted hydrodistillation | Cannabinoids | THCA (0.66 ± 0.04%) THC (0.34 ± 0.02%) CBDA (0.05 ± 0.005%) |
[92] |
| Leaf and flower | Soxhlet (methanol–water 75:25) |
Flavonoids | Luteolin-O-β-D-glucuronide (3.75 ± 0.75 mg/g DW) Apigenin-O-β-D-glucuronide (1.25 ± 0.25 mg/g DW) Vitexin (1.25 ± 0.25 mg/g DW) |
[93] |
| Leaf and inflorescence | Hydrodistillation | Terpenes | (E)-Caryophyllene (28%) Caryophyllene oxide (15%) Humulene (13%) β-Myrcene (11%) α-Pinene (8%) |
[94] |
| Leaf and inflorescence | Hydrodistillation | Phenolic compounds | Naringenin (706 µg/mL) Naringin (83 µg/mL) Catechin (60 µg/mL) Epicatechin (56 µg/mL) |
[94] |
| Leaf and stem | Rapid solid–liquid dynamic extraction (ethanol) |
Terpenes | Caryophyllene (52.78 ± 2.61%) Humulene (13.49 ± 0.14%) Linalool (9.42 ± 0.24%) α-bergamotene (6.14 ± 0.51%) cis-β-farnesene (3.54 ± 0.42%) Aromadendrene (2.86 ± 0.12%) |
[95] |
| Leaf and stem | Rapid solid–liquid dynamic extraction (ethanol) |
Polyphenols | Luteolin (304.37 ± 1.10 µg/g DW) Ferulic acid (247.77 ± 0.64 µg/g DW) Gallic acid (52.29 ± 0.98 µg/g DW) Apigenin (51.43 ± 0.48 µg/g DW) p-OH-benzoic acid (47.70 ± 0.75 µg/g DW) Rosmarinic acid (27.09 ± 0.85 µg/g DW) |
[95] |
| Resin | Supercritical fluid (CO2) |
Cannabinoids | CBD (72.12 μg/mL) THC (48.02 μg/mL) CBC (4.78 μg/mL) CBDA (2.34 μg/mL) CBN (0.40 μg/mL) |
[96] |
| Resin | Ultrasound-assisted extraction (ethanol) |
Cannabinoids | Δ9-THC > 20% | [97] |
Cannabidiol (CBD); cannabidivarine (CBDV); cannabicitran (CBTC); cannabigerol (CBG); cannabichromene (CBC); cannabinol (CBN); δ9-tetrahydrocannabinol (δ9-THC); cannabidiolic acid (CBDA); tetrahydrocannabinolic acid (THCA); delta-8-tetrahydrocannabinol (δ8-THC); cannabigerol (CBG); cannabigerol acid (CBGA); cannabielsoin (CBE); cannabicitran (CBTC); cannabiripsol (CBR); total cannabidiol (tCBD); total cannabigerol (tCBG); total tetrahydrocannabinol (tTHC).
The chemical investigations conducted in different Cannabis sativa plant parts shows that terpenes, polyphenols and cannabinoids are the main represented secondary metabolites. Terpenes are represented by more than 100 molecules identified in the flowers, roots and leaves, as well as in the secretory glandular hairs considered as the main production site [11,98]. Furthermore, more than 20 polyphenols have been identified, and they are mainly flavonoids belonging to the flavone and flavonol subclasses [99]. Concerning the cannabinoids, they are among the most represented metabolites of Cannabis despite being represented by less than 20 molecules.
Cannabis seeds contain approximately 40% oil, 30% fibers and 25% proteins [85,100]. Those oils are rich in triacylglycerols (TAGs) represented by 18 different molecules; the predominating tags were LLL and OLLD with respective values of 23 and 19% [100]. Moreover, those oils contain high amounts of polyunsaturated fatty acids, which represent approximately 80% total fatty acids [101]. The fatty acid composition is characterized by the predominance of linoleic acid with range values of 45–60%, followed by oleic acid and palmitic with respective range values of 15–40% and 5–6% [80,81].
Hemp seeds contains also considerable amounts of polyphenols and tocopherols. According to Babiker, et al. [81], the hydroalcoholic extract of Cannabis seeds contained many polyphenols such as gallic acid (12.9 ± 18.3 mg/100 g) and catechin (6.0 ± 5.2 mg/100 g), whereas Moccia, et al. [83] reported the presence of additional polyphenols, namely quercetin-o-glucoside, n-trans-caffeoyltyramine and rutin. Moreover, another sub-class of polyphenols known as cannabisins have been reported on the seed hydroalcoholic maceration by Moccia, et al. [83]. The last authors described the presence of 11 molecules, namely cannabisin A, B, C, D, E, F, G, I, M, N and O, although these compounds have not been quantified. Concerning the tocopherols, four different isomers have been identified: the lead tocopherols are γ- and δ-tocopherols with 426 and 33 mg/kg, respectively [82,84].
Cannabinoids are a group of C21 or C22 terpenolic compounds mainly produced in Cannabis. They have also been reported in other plant species of the genus Radula and Helichrysum [102]. These cannabinoids are mainly present in leaves and inflorescences. However, Marzorati, et al. [103] reported the presence of some cannabinoids in the hydroalcoholic maceration of seeds, and Stambouli, et al. [104] reported the presence of mainly the cannabinoids tetrahydrocannabinol (THC), cannabidiol (CBD) and cannabigerol (CBG) in seed oil. These results are probably due to contamination of seeds by inflorescence resins, since cannabinoids are not produced or transported to the seeds [105]. Moreover, phytosterols, a group of lipids with a structure similar to cholesterols, mainly found in vegetable oils, have been identified in seed oils. Aiello, et al. [85] and Stambouli, et al. [84] mentioned the presence of this class of metabolites represented by the β-sitosterol, with 65–90%, and campesterol, with 6–17%. Furthermore, Stambouli, et al. [84] described the presence of an additional phytosterol known as δ-5-avenasterol, with a value of 7.8%. The carotenoids are among the less represented compounds in Cannabis seeds. They have been identified in the seed ethanolic extract, whereas lutein and β-carotene have been reported as major sterols, with 2.5 and 0.5 mg/100 g of dry weight, respectively [82].
The composition in hemp seeds of fatty acids, polyphenols, phytosterols, proteins and fibers, and precisely the presence of insoluble fibers in addition to a wide variety of minerals represented by phosphorus, potassium, magnesium, sulfur and calcium, as well as modest amounts of iron and zinc (an important enzyme cofactor for immunity and food absorption), widely justifies its biological proprieties and importance for human nutrition [106,107].
The Cannabis leaves contain terpenes, polyphenols, cannabinoids and alkaloids. The leaf essential oils are characterized by the presence of (e)-caryophyllene (28.3 ± 4.1%), α-humulene (9.3 ± 1.1%), β-selinene (4.7 ± 0.9%), caryophyllene oxide (4.3 ± 0.9%), α-selinene (3.1± 0.6%) and α-trans-bergamotene (2.7 ± 0.5%) [86]. These volatile terpenes are generally found in photosynthetic plant leaves and plays the role of protection from parasites and water loss [108]. The polyphenols of Cannabis leaves are mainly flavonoids and glycosides with apigenin and luteolin. These flavonoids represent 4 mg per g of the plant material. Elsewhere, the cannabinoids of Cannabis leaves had been described by Nagy, et al. [86]. The authors reported the presence of cannabidiol (CBD), cannabidivarine (CBDV), tetrahydrocannabinol (THC) and cannabichromene (CBC) with 11, 0.8, 0.7 and 0.5%, respectively. Moreover, Zagórska-Dziok, et al. [87] reported the lead presence of CBDA and CBD, with respective values of 150 and 31 mg/g of dry matter. Similarly to the cannabinoids, the alkaloids protect plants from predators and regulate plant growth [109]. These alkaloids have been described in cannabis leaves; Fasakin, et al. [88] described the presence of some alkaloids, with cannabisativine (410.30 μg/g), cannabimine C (376.12 μg/g) and anhydrocannabisativine (218.11 μg/g) as lead compounds.
The flowers show a chemical composition qualitatively similar to the leaves. They are mainly composed of terpenes, polyphenols and cannabinoids. The Cannabis flower terpenes have been identified in the essential oils obtained by hydrodistillation. According to Nagy, et al. [86], these essential oils are mainly composed of (E)-caryophyllene, α-humulene, β-selinene and α-selinene, with values of 29, 10, 4 and 3%, respectively. Additionally, Fischedick, et al. [110] reported the presence of mono- and sesquiterpenes in Cannabis flower essential oils. This study featured the quantification of monoterpenes and proved that they dominate the chemical composition, with a concentration of 28.3 mg/g of dry weight. These monoterpenes are represented by d-limonene, β-myrcene, α- and β-pinene. Furthermore, sesquiterpenes are represented by β-caryophyllene and α- humulene. These sesquiterpenes represent a concentration ranging between 0.5 and 10.1 mg/g of dry weight [110]. Polyphenols and cannabinoids have also been identified in the methanolic extract of flowers. The main polyphenols of flowers are quercetin di-c-hexoside and luteolin c-hexoside-2″-o-hexoside with 2.55 mg/g and 1.01 mg/g, respectively [86]. On the other hand, the cannabinoids are represented by cannabidiol (CBD), tetrahydrocannabinol (THC), cannabidivarine (CBDV), cannabicitran (CBTC) and cannabichromene (CBC), with respective values of 25, 1.5, 1.5, 1 and 0.2% [86]. The cannabinoidic composition of flowers fluctuates due to several environmental factors (e.g., temperature, soil nutrients, desiccation, insect predation and ultraviolet radiation) and genetic factors (varieties and heredity) [111]. According to Yang, et al. [112], the total THC, CBD and CBG increases significantly as the flowers matures, reaching the highest concentration during 6 to 7 weeks after anthesis.
The inflorescences of Cannabis have also been evaluated for their composition, and similar classes of metabolites have been described. These compounds belong to the same classes previously reported for the leaves and flowers. The essential oils of inflorescences have been reported in two research manuscripts. The first, published by Laznik, et al. [90], reported the presence of transcaryophyllene (38.2 ± 1.7%), nerolidol (12.7 ± 1.2%) and α-pinene (11.8 ± 0.4%), whereas the second, published by Pieracci et al. [91], reported the presence of β-caryophyllene (14.4 ± 0.89%), caryophyllene oxide (7.0 ± 1.06%) and α-humulene (5.3 ± 0.10%) as lead compounds. This variation is probably due to the variation of Cannabis sativa subspecies, cultivars and/or geographic, climatic and pedologic parameters. Laznik, et al. [90] also reported the chemical composition of inflorescence methanolic extract and concluded that this extract contained mainly cannabinoids represented by cannabidiolic acid (CBDA) and cannabidiol (CBD) with 9.5 and 8.7%, respectively.
Several studies have also described the composition of other Cannabis sativa L. parts, including roots, stem and resin. Sakakibara, et al. [113] and Lesma, et al. [114] reported the presence of phenolic amides and lignanamides in the fruits and roots of Cannabis. These compounds belong to the classes of lignans and polyphenols and are mainly cannabisin (A, B, C, D, E, F and G) and grossamide [99]. Triterpenes have also been found in Cannabis roots in form of friedelin and epifriedelanol [115]. The glandular secretory hairs are the main site of resin production. The latter is a yellow and sticky substance which contains the active principles. Some studies have shown its chemical composition. Stambouli, et al. [97] reported a high concentration of THC with a value higher than 20%. More recently, Elkins, et al. [96] identified and confirmed the high content of cannabinoids including cannabidiol (CBD) with a concentration of 72.12 μg/mL, followed by tetrahydrocannabinol (THC) with 48.02 μg/mL and cannabichromene (CBC) with 4.78 μg/mL. The concentrations of Cannabis sativa secondary metabolites depend on tissue type, age, variety, growing conditions (soil nutrition, temperature, humidity, UV radiation or light), harvest time (maturity) and storage conditions [80,111,116,117]. The Cannabis seeds are rich in oils and starch mainly composed of terpenes [118]. The seed oils serve as food for the young plant during the early stages of germination. In fact, the plant embryo needs a source of nutrition prior to its contact with soil and air [119]. Moreover, the leaves, flowers and inflorescences are rich in volatile terpenes, polyphenols and cannabinoids. These metabolites are generally involved in defense against ultraviolet radiation or aggression by pathogens. Unlike polyphenols, cannabinoids are more interesting due to their chemical structures and representativeness of Cannabis genus. The study of chemical composition of Cannabis plant parts is very important for the determination of biological activities. It is possible to predict a biological activity from the exhaustive chemical composition of an extract using bioinformatics tools such as molecular docking. This approach has been widely applied on Cannabis secondary metabolites, as described in the next section.
4.3. Molecular Docking Studies of Cannabis sativa L.
Molecular docking is a computational approach aiming to predict potential interactions between one or more ligands and a protein [120]. This approach predicts the optimal spatial conformation and orientation of the ligand within a protein active site, in addition to the determination of the interaction mode and binding affinity represented by a score. Molecular docking is the in silico equivalent of real high-throughput screening in which many molecules are tested against biological targets. The main goal of this approach is to discriminate active and inactive agents, in order to identify new molecules that will serve as a starting point for medicinal chemists [121].
The process of molecular docking can be subdivided into two basic steps, namely docking and scoring [122]. Docking is the step in which all possible spatial interactions between a ligand and a receptor are tested in order to identify the optimal interactions, whereas the binding affinity between the ligand and the receptor are quantified in scoring, and a score is given to the poses recorded after the docking phase.
Currently, there are more than 30 available docking software packages [123]. Most of them are also designed for virtual screening (independent dockings of multiple ligands with a protein). The three most frequently cited docking tools are AutoDock, GOLD and flex; they represent 27, 15 and 11% of the references, respectively [124,125].
Despite being very useful for guiding the selection of bioactive molecules for in vitro testing, the molecular docking simulations remains a prediction and can sometimes give erroneous results. They can be expressed either as a false negative, when an active molecule gives low docking affinity, or a false positive, when a non-active molecule is identified as a strong ligand [126]. However, this approach remains useful as a pre-investigation predictive tool. Concerning the Cannabis plant, several molecular docking studies have been reported on the different classes of metabolites, namely cannabinoids, terpenes, polyphenols, flavonoids, lignanamides, alkaloids, vitamins and proteins. These classes have been probed against a large number of specific enzymes that instigate important roles in different physiological processes (digestion, nerve conduction, hormone synthesis, etc.). Results of molecular docking are expressed in kcal/mol, and the lowest values correspond to higher affinity between a ligand and a protein. The studies reported in the bibliography are summarized in Table 3.
Table 3.
Molecular docking of Cannabis sativa L. compounds.
| Enzyme | Ligand | Docking Tool |
Score (kcal/mol) |
Reference | ||||
|---|---|---|---|---|---|---|---|---|
| Name | ID (pdb = p / Unipr = up) |
Class of Protein |
Biological Activity |
Name | Class of Metabolite |
|||
| Acetylcholine esterase (ACHE) | 1EVE (p) | Hydrolase | Pesticidal | Cannabioxepane | Cannabinoids | AutoDock vina | –10.4 | [127] |
| Δ-9-THCA | Cannabinoids | –10.3 | ||||||
| Δ-8-THC | Cannabinoids | –10.1 | ||||||
| CBN | Cannabinoids | –10.1 | ||||||
| CBT | Cannabinoids | –9.8 | ||||||
| CBD | Cannabinoids | –9.8 | ||||||
| CBL | Cannabinoids | –9.6 | ||||||
| Isocannabispiradienone | Cannabinoids | –9.4 | ||||||
| CBC | Cannabinoids | –9.4 | ||||||
| CBCA | Cannabinoids | –9.3 | ||||||
| CBGA | Cannabinoids | –9.2 | ||||||
| Cannabispiradienone | Terpenes | –9.2 | ||||||
| Cannabispirol | Polyphenols | –9.2 | ||||||
| Butyrylcholinesterase (BCHE) |
1P0I (p) | Hydrolase | Pesticidal | Cannabioxepane | Cannabinoids | AutoDock vina | –9.8 | [127] |
| CBL | Cannabinoids | –8.9 | ||||||
| CBN | Cannabinoids | –8.8 | ||||||
| CBT | Cannabinoids | –8.7 | ||||||
| Δ-8-THC | Cannabinoids | –8.7 | ||||||
| CBL | Cannabinoids | –8.4 | ||||||
| Isocannabispiradienone | Cannabinoids | –8.3 | ||||||
| CBD | Cannabinoids | –8.2 | ||||||
| Cannabispiradienone | Terpenes | –8.2 | ||||||
| Cannabispiran | Polyphenols | –8.2 | ||||||
| Acetylcholine esterase (ACHE) | 1EVE (p) | Hydrolase | Pesticidal | CBD | Cannabinoids | AutoDock vina | −14.38 | [128] |
| CBN | Cannabinoids | −13.91 | ||||||
| Δ-9-THC | Cannabinoids | −13.82 | ||||||
| Plasmodium falciparum dihydrofolate reductase-thymidinesynthase (pfdhfr-ts) | 1J3I (p) | Lyase | Antimalarial | 7-oxo-9a-hydroxyhexahydrocannabinol | Cannabinoids | AutoDock vina | −9.40 | [129] |
| 10ar-hydroxyhexahydrocannabinol | Cannabinoids | −9.20 | ||||||
| 10-oxo-delta6a,10a-tetrahydrocannabinol | Cannabinoids | −9.20 | ||||||
| 8-oxo-delta9-tetrahydrocannabinol | Cannabinoids | −9.10 | ||||||
| 10a-hydroxyhexahydrocannabinol | Cannabinoids | −9.10 | ||||||
| Leishmania major pteridine reductase 1 (lmajptr1) | 1E7W, 1W0C, 2BF7 and 3H4V (p) | Oxido-reductase | Anti-leishmania | 4,5,4’5’-dimethylenedioxy-3,3’-dimethoxy-7,7’-epoxylignan | Polyphenols | Molegro virtual docker | −35.01 | [130] |
| Cannflavin A | Flavonoids | −34.42 | ||||||
| Leishmania mexicana glycerol-3-phosphate dehydrogenase (lmexgpdh) | 1EVZ, 1M66, 1N1E and 1N1G (p) | Oxido-reductase | Anti-leishmania | 4-terpenylcannabinolate | Cannabinoids | Molegro virtual docker | −35.90 | [130] |
| 3’-o-methyldiplacone | Flavonoids | −35.44 | ||||||
| 4’-o-methyldiplacone | Flavonoids | −34.18 | ||||||
| Sophoronol E | Flavonoids | −33.89 | ||||||
| Leishmania major methionyl-transynthetase (lmajmetrs) | 3KFL (p) | Ligase | Anti-leishmania | 4,6-dibenzoyl-2-[phenylhydroxymethyl]-3(2h)-benzofuranone | Polyphenols | Molegro virtual docker | −38.81 | [130] |
| Leishmania donovani cyclophilin (ldoncyp) | 2HAQ and 3EOV (p) | Isomerase | Anti-leishmania | 3’-o-methyldiplacone | Flavonoids | Molegro virtual docker | −30.23 | [130] |
| Leishmania major oligopeptidase b (lmajopb) | 2XE4 (p) | Protease | Anti-leishmania | 3-methoxycitrunobin-4-methyl ether | Polyphenols | Molegro virtual docker | −30.74 | [130] |
| 4’-o-methylglycyrrhisoflavone | Flavonoids | −30.47 | ||||||
| Leishmania major uridine 2p-glucose pyrophosphorylase (lmajugpase) | 2OEF and 2OEG (p) | Transferase | Anti-leishmania | 4’,6-dihydroxy-2-[phenylmethylene]-3(2h)-benzofuranone | Polyphenols | Molegro virtual docker | −33.94 | [130] |
| Leishmania major n-myristoyl-transferase (lmajnmt) | 2WSA, 3H5Z and 4A30 (p) | Transferase | Anti-leishmania | Diplacone | Flavonoids | Molegro virtual docker | −32.43 | [130] |
| Trans-4-isopentenyl-3,5,2,4 -tetrahydroxystilbene | Polyphenols | −30.45 | ||||||
| Leishmania infantum nicotinamidase (linfpnc1) | 3R2J (p) | Hydrolase | Anti-leishmania | 5,8-dihydroxy-1-hydroxymethylnaphtho-[2,3-c] furan-4,9-dione | Polyphenols | Molegro virtual docker | −23.45 | [130] |
| Umckalin | Polyphenols | −21.85 | ||||||
| Scoparone | Flavonoids | −21.77 | ||||||
| Leishmania major dihydroorotate dehydrogenase (lmajdhodh) | 3GYE, 3MHU and 3MJY (p) | Oxido-reductase | Anti-leishmania | Aristolignin | Flavonoids | Molegro virtual docker | −31.21 | [130] |
| Crotaorixin | Flavonoids | −31.84 | ||||||
| Mammea B/BA | Polyphenols | −29.09 | ||||||
| Leishmania mexicana pyruvate kinase (lmexpyk) | 1PKL, 3HQP and 3PP7 (p) | Protein kinase | Anti-leishmania | Kusunokinin | Polyphenols | Molegro virtual docker | −31.19 | [130] |
| Leishmania major phosphodiesterase 1 (lmajpde1) | 2R8Q (p) | Hydrolase | Anti-leishmania | Machaeriol B | Cannabinoids | Molegro virtual docker | −28.97 | [130] |
| Leishmania major tyrosyl-trna synthetase (lmajtyrrs) | 3P0H and 3P0J (p) | Ligase | Anti-leishmania | Bractein triacetate | Polyphenols | Molegro virtual docker | −33.08 | [130] |
| COVID-19 protease | 6LU7 (p) | Protease | Antiviral | CBD | Cannabinoids | AutoDock vina | −7.10 | [131] |
| Hiv-1 protease | ND | Protease | Antiviral | Cannflavin | Flavonoids | AutoDock vina | −9.70 | [132] |
| Human angiotensin-converting enzyme (ACE2) | 1R4L (p) | Protease | Antiviral | THC | Cannabinoids | AutoDock vina | −9.2 | [133] |
| 2019-ncov spike protein s2 subunit | 6LXT (p) | Surface glycoprotein | Antiviral | THC | Cannabinoids | AutoDock vina | −4.2 | [133] |
| SARS-CoV-2 mpro | ND | Protease | Antiviral | Cannabisin A | Lignanamide | AutoDock vina | −12.76 | [134] |
| 3c-like protease (C) | 6LU7 (p) | Protease | Antiviral | Hesperidin | Flavonoids | AutoDock vina | −8.3 | [135] |
| Nabiximols | Cannabinoids | −8 | ||||||
| Spike glycoprotein (S) | 6VXX (p) | Surface glycoprotein | Antiviral | Hesperidin | Flavonoids | AutoDock vina | −10.4 | [135] |
| Nabiximols | Cannabinoids | −10.2 | ||||||
| Inhibitor of nuclear factor kappa-b kinase subunit β (IKKbeta) | 3BRT (p) | Inhibitor kappa kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −7.99 | [136] |
| Mitogen-activated protein kinase 14 | 4IDT (p) | Map kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −7.35 | [136] |
| Cellular tumor antigen p53 | IAIE (p) | Tumor suppressor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −6.08 | [136] |
| Nf-kappa-b inhibitor alpha | 1IKN (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.82 | [136] |
| Tnf receptor-associated factor 6 | 1IB6 (p) | Traf protein | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.74 | [136] |
| Transcription factor p65 | 1NFI (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.66 | [136] |
| Epidermal growth factor receptor | 1MOX (p) | Epidermal growth factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.64 | [136] |
| Nf-kappa-b essential modulator | 3BRV (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.36 | [136] |
| Rac-alpha serine/threonine-protein kinase | 1UNQ (p) | Transferase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.34 | [136] |
| Mitogen-activated protein kinase 3 | 2O2V (p) | Map kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −5.34 | [136] |
| Poly [adp-ribose] polymerase 1 | 2COK (p) | DNA polymerase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.88 | [136] |
| Hypoxia-inducible factor 1-alpha | 1H2K (p) | Hypoxia inducible factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.41 | [136] |
| Inhibitor of nuclear factor kappa-b kinase subunit a | 3BRT (p) | Inhibitor kappa kinase | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.32 | [136] |
| Nuclear factor kappa-b p105 subunit | IMDI (p) | Nfkb inhibitor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −4.23 | [136] |
| G1/s-specific cyclin-d1 | 5VZU (p) | Proto-oncogene regulator | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | −3.44 | [136] |
| Activator protein-1 (AP-1) | 1JUN (p) | Transcription factor | Anti-inflammatory | CBD | Cannabinoids | AutoDock-tools | -3.41 | [136] |
| Crystal structure of the DLC1 RhoGAP domain in liver cancer 1 | 3KUQ (p) | GTPase-activating proteins | Anticancer | CBC | Cannabinoids | Docking-server | −3.89 | [137] |
| THCV | Cannabinoids | −3.25 | ||||||
| CBGV | Cannabinoids | −3.21 | ||||||
| CBDA | Cannabinoids | −3.34 | ||||||
| Placental aromatase cytochrome p450 | 3EQM (p) | Cytochrome | Anticancer | CBDC1 | Cannabinoids | Molegro (Glide) | −9.03 | [138] |
| CBGV | Cannabinoids | −7.8 | ||||||
| CBCA | Cannabinoids | −7.73 | ||||||
| CBCVA | Cannabinoids | −7.45 | ||||||
| CBCV | Cannabinoids | −8.29 | ||||||
| CBDV | Cannabinoids | −8.34 | ||||||
| CBT | Cannabinoids | −7.86 | ||||||
| (Δ−9-THC) | Cannabinoids | −7.43 | ||||||
| CBR | Cannabinoids | −6.93 | ||||||
| Heat shock protein 90 (hsp90) | 2QG2 (p) | Chaperone protein | Anticancer | Guaiol | Terpenes | AutoDock vina | −10.80 | [139] |
| Topoisomerase II alpha | 5GWK (p) | Nuclear enzyme | Anticancer | 7-o-methylcyanidin | Flavonoids | Molegro (Glide) | −10.395 | [140] |
| Rutin | Flavonoids | −9.847 | ||||||
| Luteolin-7-o-glucoside | Flavonoids | −9.563 | ||||||
| Myricetin 7-glucoside | Flavonoids | −9.383 | ||||||
| Tumor necrosis factor-α (TNF-α) | 2E7A (p) | Tumor necrosis factor | Anticancer | Ascorbic acid | Vitamins | AutoDock vina | −5.4 | [141] |
| Linoleic acid | Terpenes | −3.8 | ||||||
| Tryptophan | Amino acids | −5.6 | ||||||
| Arachidonate 5-lypoxygenase | 5IR4 (p) | Lipoxygenase | Anticancer and anti-inflammatory | Δ-9-THC | Cannabinoids | Molecular operating environment | −4.57 | [142] |
| Δ-8-THC | Cannabinoids | −4.87 | ||||||
| CBC | Cannabinoids | −5.14 | ||||||
| CBG | Cannabinoids | −5.59 | ||||||
| CBL | Cannabinoids | −4.83 | ||||||
| CBD | Cannabinoids | −4.97 | ||||||
| Human myeloperoxidase (MPO) | 3F9P (p) | Peroxidase | Anti-inflammatory and degenerative processes | Peptide YGRDEISV | Proteins | Discovery studio | −114.6 | [143] |
| Peptide LDLVKPQ | Proteins | −82.8 | ||||||
| Angiotensin-converting enzyme (ACE) | 1O8A (p) | Protease | Hypertension | Peptide WVYY | Proteins | Accelrys discovery studio | −27.25 | [144] |
| Peptide WYT | Proteins | −21.99 | ||||||
| Renin | 2V0Z (p) | Protease | Hypertension | Peptide SVYT | Proteins | Accelrys discovery studio | −25.33 | [144] |
| Peptide WYT | Proteins | −19.12 | ||||||
| Angiotensin-converting enzyme (ACE) | 1O8A (p) | Protease | Hypertension | Peptide ALVY | Proteins | Accelrys discovery studio | −69.23 | [145] |
| Peptide LLVY | Proteins | −65.97 | ||||||
| Peptide LSTSTDVR | Proteins | −105.59 | ||||||
| Peptide LLAPHY | Proteins | −86.86 | ||||||
| Cannabinoid receptor 1 (CNR1) | P21554 (up) | G-protein coupled receptor | Epilepsy | 8b-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | Molegro (Glide) | −8.039 | [146] |
| Epilepsy | 10aa-hydroxy-10-oxo-d8-tetrahydrocannabinol | Cannabinoids | −8.52 | |||||
| Epilepsy | 10aα-hydroxyhexahydrocannabinol | Cannabinoids | −8.55 | |||||
| Epilepsy | CBNM | Cannabinoids | −8.761 | |||||
| Epilepsy | Cannabichromanone D | Cannabinoids | −8.446 | |||||
| Epilepsy | CBL | Cannabinoids | −8.336 | |||||
| Epilepsy | 5-acetoxy-6-geranyl-3-npentyl-1,4-benzoquinone | Cannabinoids | −8.571 | |||||
| Epilepsy | CBTC | Cannabinoids | −8.574 | |||||
| Androgen receptor (AR) | P10275 (up) | Nuclear receptor | Epilepsy | CBNM | Cannabinoids | Molegro (Glide) | −8.706 | [146] |
| Epilepsy | 5-acetoxy-6-geranyl-3-npentyl-1,4-benzoquinone | Cannabinoids | −8.262 | |||||
| Epilepsy | CBTC | Cannabinoids | −8.015 | |||||
| Glycogen synthase kinase-3 beta (GSK3B) | P49841 (up) | Protein kinase | Epilepsy | 8a-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | Molegro (Glide) | −6.439 | [146] |
| Epilepsy | 8b-hydroxy-δ9-trans-tetrahydrocannabinolate | Cannabinoids | −5.876 | |||||
| Epilepsy | CBL | Cannabinoids | −6.183 | |||||
| Albumin | P02768 (up) | Albumin | Epilepsy | (-)-trans-10-ethoxy-9-hydroxy-d6a(10a)-tetrahydrocannabinol | Cannabinoids | Molegro (Glide) | −6.429 | [146] |
| Epilepsy | CBNM | Cannabinoids | −6.306 | |||||
| Epilepsy | CBL | Cannabinoids | −6.057 | |||||
| Neurokinin 3 receptor (NK3R) | 1F88 (p) | Neurokinin receptor | Neuro-protective | CBD | Cannabinoids | AutoDock | −6.72 | [147] |
| CBG | Cannabinoids | −10.36 | ||||||
| Angiotensin-converting enzyme 2 (ACE2) | 6CS2 (p) | Protease | Neuro-protective | CBD | Cannabinoids | AutoDock vina | −8.9 | [148] |
| Interleukin-6 (IL6) | 1ALU (p) | Cytokine | Neuro-protective | CBD | Cannabinoids | AutoDock vina | −8.2 | [148] |
| Transmembrane protease serine 2 (TMPRSS2) | 3NPS (p) | Protease | Neuro-protective | CBN | Cannabinoids | AutoDock vina | −8.7 | [148] |
| Nrp1 protein | 7BP6 (p) | Semaphorin receptor | Neuro-protective | CBN | Cannabinoids | AutoDock vina | −8.5 | [148] |
| Tyrosine phosphatase-1b (PTP1B) | 1NWE (p) | Hydrolase | Dermo-cosmetic | Chrysophanol | Polyphenols | Charmm-based docker | −24.34 | [149] |
| P glycoprotein (P-GP) | 4Q9H (p) | GTPase | Multi-drug resistance | Cannabisin M | Lignanamide | AutoDock vina | −10.2 | [150] |
| Cannabisin N | Lignanamide | −10.2 | ||||||
| Cannabisin A | Lignanamide | −10.1 | ||||||
| Cannabisin B | Lignanamide | −10.1 | ||||||
| Cannabisin C | Lignanamide | −10.1 | ||||||
| Cannabisin D | Lignanamide | −10.1 | ||||||
Cannabichromene (CBC); cannabichromenic acid (CBCA); cannabichromevarin (CBCV); cannabichromevarinic acid (CBCVA); cannabicitran (CBTC); cannabicoumaronone (CBCN); cannabicyclol (CBL); cannabidiol (CBD); cannabidiol-c4 (CBDC4); cannabidiolic acid (CBDA); cannabidiorcol (CBDC); cannabidivarin (CBDV); cannabielsoin (CBE); cannabielsoin (CBL); cannabigerol (CBG); cannabigerolic acid (CBGA); cannabigerovarin (CBGV); cannabinodiol (CBND); cannabinodivarin (CBVD); cannabinol (CBN); cannabinol methyl ether (CBNM); cannabiripsol (CBR); cannabitriol (CBT); cannabivarin (CVN); tetrahydrocannabinol (THC); tetrahydrocannabivarin (THCV); δ-8-tetrahydrocannabinol (Δ-8-THC); δ-9-tetrahydrocannabinol (Δ-9-THC); δ9-tetrahydrocannabinolic acid (Δ-9-THCA).
4.3.1. Pesticidal Activity
Cholinesterase is an enzyme that catalyzes the hydrolysis reaction of a choline ester (acetylcholine, butyrylcholine) into choline and acetic acid. Acetylcholine is a well-known excitatory neurotransmitter that causes muscle contraction and stimulates the release of certain hormones. The inhibition of choline esterase causes the disfunction of nerve impulse transmission inducing mortality, and this activity is highly coveted for the elimination of pests and insects. The molecular docking of cannabis secondary metabolites against two types of cholinesterase, namely acetylcholinesterase (ACHE) and butyrylcholinesterase (BCHE), was described by Karimi, et al. [127]. In this article, the authors tested compounds belonging to the cannabinoid, flavonoid, terpene and phytosterol classes. For acetylcholinesterase, cannabioxepane, δ-9-THCA, Δ-8-THC and CBN showed scores lower than −10 kcal/mol, whereas cannabioxepane, CBL, CBN, CBT and Δ-8-THC showed scores lower than −8.5 kcal/mol against butyrylcholinesterase. Another investigation reported by Nasreen, et al. [128] reported the acetylcholinesterase docking with some cannabinoids, and CBD showed the best score with a value of −14.38 kcal/mol. Despite using the same docking software and acetylcholinesterase three-dimensional structure, the CBD score is better than the ones previously reported by Karimi, et al. [127]. This difference of results can be explained by the variation of docking parameters, such as the gridbox dimensions, position and the exhaustiveness.
4.3.2. Antimalarial and Anti-Leishmania Activities
The antimalarial activity of cannabinoids from Cannabis sativa was reported by Quan, et al. [129]. Their studied cannabinoids were docked against plasmodium falciparum dihydrofolate reductase-thymidinesynthase to recognize the potential binding affinities of these phytochemicals. Furthermore, the in silico antileishmanial activity of phytochemicals from Cannabis sativa has been well reported in the literature. In the studies conducted by Ogungbe, et al. [130], a molecular docking analysis was performed to examine the potential leishmania protein targets of plant-derived antiprotozoal polyphenolic compounds. A total of 352 phenolic phytochemicals—including 10 aurones, 6 cannabinoids, 34 chalcones, 20 chromenes, 52 coumarins, 92 flavonoids, 41 isoflavonoids, 52 lignans, 25 quinones, 8 stilbenoids, 9 xanthones and 3 miscellaneous phenolic compounds—were used in the virtual screening study with 24 leishmania enzymes (52 different protein structures from the protein data bank). Notable target proteins were leishmania dihydroorotate dehydrogenase, n-myristoyl transferase, phosphodiesterase b1, pteridine reductase, methionyl-trna synthetase, tyrosyl-trna synthetase, uridine diphosphate-glucose pyrophosphorylase, nicotinamidase and glycerol-3-phosphate dehydrogenase. The results showed that docked polyphenols can be considered as promising drug leads deserving further investigation.
4.3.3. Antiviral Activity
Despite great advances in medical and pharmaceutical research in recent years, diseases caused by viruses have remained a huge burden on public health like coronavirus, in particular SARS-CoV-2. In silico studies, including molecular docking, have repeatedly proved to be useful in addressing the particular challenges of antiviral drug discovery. A study published by Srivastava et al. [131] showed that cannabidiol may have an good affinity with a COVID-19 protease, and such affinity is represented by a score value of −7.10 kcal/mol. In another study, cannflavin exhibited a better score against an HIV-protease with a score of −9.70 kcal/mol [132].
4.3.4. Anti-Inflammatory Activity
Inflammation is a natural body reaction to injury and infection. It is mainly due to the deployment of immune system cells to the site of the injury or infection. The four symptoms of inflammation are heat, redness, swelling and pain. However, anti-inflammatory drugs are used to combat inflammation regardless of the cause of the inflammation. They are symptomatic treatments, i.e., they do not eliminate the cause of the inflammation but only its consequence and have an analgesic action. In a recent study, a panel of proteins, including the cellular tumor antigen p53, the essential modulator of NF-KB, the tumor necrosis factor (TNF) receptor, the transcription factor p65, NF-KB p105, the NF-k-b α inhibitor, the inhibitor of nuclear factor k-b kinase α subunit and the epidermal growth factor receptor, were identified as a primary target implicated in cannabidiol (CBD) anti-inflammatory activity. This finding was supported by molecular docking, which showed interactions between the major proteins and CBD. In addition, several signaling pathways, including TCF, toll-like receptors, mitogen-activated protein kinases, nuclear factor kappa, activated b-cell light chain activator and nucleotide-binding oligomerization domain receptors, were identified as key regulators in mediating the anti-inflammatory activity of CBD [136].
4.3.5. Anticancer Activity
Several molecular docking studies were interested in the potential anticancer activity of molecules derived from Cannabis sativa L. The molecular docking performed on placental aromatase cytochrome p450 was reported by Baroi, et al. [138]. These authors reported that cannabinoids, mainly cannabidiorcol and cannabidivarin, potentially bind with the best binding energies of −9.03 kcal/mol and −8.34 kcal/mol, respectively. In another study, molecular docking calculations were also performed to investigate the binding affinity of cannabinoids in the active site of crystal structure of the DLC1 RhoGAP domain in liver cancer 1. According to the performed calculations, cannabichromene and cannabidiolic acid showed promising results with regard to binding affinity to the target GTPase-activating proteins [137]. These compounds are held within the active site by a variety of non-covalent interactions, in particular hydrogen bonds, involving important amino acids. Similarly, cannabinoids were docked to predict their anti-inflammatory and anticancer activity. The researchers reported that cannabigerol and cannabichromene potentially bind to arachidonate 5-lypoxygenase with a binding energy score of −5.34 kcal/mol and −5.14 kcal/mol, respectively [142]. Cannabis flavonoids were also docked against topoisomerase II α to investigate the binding affinity of flavonoids in the active site of topoisomerase II α. The results showed that docked flavonoids can be considered as promising drug leads, thus deserving further investigation.
4.3.6. Antiepileptic Activity
A study published by Li, et al. [146] aimed to examine the mechanism of action of Cannabis on epilepsy, focusing on key compounds, targets and pathways. The molecular docking simulations were applied to identify the active ingredients and potential targets of Cannabis in the treatment of epilepsy. Topological analysis showed that cannabinoid receptor 1, albumin and glycogen synthase kinase-3 β (cnr1, alb and gsk3b) were the key targets with intense interaction. The results showed that cannabinol methyl ether could be the lead compound on the basis of molecular docking against docked protein targets. Therefore, these studies shed holistic light on the active components of Cannabis, which contributes to the search for lead compounds and the development of new drugs for the treatment of neurological diseases.
4.3.7. Neuroprotective Activity
Neuroprotective agents target the various deleterious mechanisms that occur in cerebral ischemia, with the aim of limiting the extension of the ischemic heart. It has been shown that the cannabinoid substances contained in the Cannabis sativa plant have great potential in a wide variety of therapeutic applications. However, its neuroprotective capacity has been the most studied in diseases such as Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, multiple sclerosis and amyotrophic lateral sclerosis [151]. The only example of proteins as infectious agents leading to neurodegenerative disorders was the prion protein. Since then, the characteristic self-seeding mechanism of the prion protein has also been attributed to other proteins associated with neurodegenerative diseases, notably amyloid-β (aβ). Modelling with the aβ monomer and pentamer revealed that cannabinoids interacted with the aβ protein mainly through steric interactions and hydrogen bonds. The results showed that CBG bound with the highest affinity of all the docked cannabinoids. The authors of this study reported that this was mainly due to the presence of a geranyl side chain in the CBG structure, as this side chain is associated with increased lipophilicity and may, therefore, increase the propensity to bind in the hydrophobic groove of the pentamer [152].
4.3.8. Dermocosmetic Activities
Dermocosmetic products are on the borderline between cosmetic products and medicines. Considered as cosmetic products, they are mainly used to ensure photoprotection of the skin, i.e., to limit the effects of its exposure to solar radiation, but also to improve the appearance of dry or aged skin, reduce inflammatory dermatological conditions (acne, couperose, seborrheic and atopic dermatitis, psoriasis, etc.), as well as for the care of nails and hair [153]. Furthermore, tyrosinase is a key enzyme in the process of melanogenesis (the biosynthesis of melanin). The dermocosmetic potential of Cannabis polyphenols has been reported, and the results showed a good affinity with the tyrosine phosphatase-1b with a free energy score of −24.34 kcal/mol [149]. Similarly, Cannabis alkaloids have been shown to possess affinity with tyrosinase with score a value of −3 kcal/mol [154].
4.4. Biological Activities of Cannabis sativa L.
Studies with Cannabis sativa L. have shown the presence of several biological activities, such as antioxidant, antibacterial, anticoagulant, insecticide, anticancer, anti-aflatoxigenic, antifungal, cytotoxic, anti-elastase, anti-collagenase, anti-acetylcholinesterase, anti-inflammatory, neuroprotective (anti-Alzheimer’s, anti-epilepsy and anti-Parkinson’s) and dermocosmetic (anti-tyrosinase, anti-collagenase and anti-elastase). Table 4 summarizes the biological activities of different Cannabis sativa parts according to the literature.
Table 4.
Biological activities of different parts of Cannabis sativa L.
| Plant Part | Extraction Method (Solvent) | Bioactive Metabolites |
Biological Activity | Results | Reference |
|---|---|---|---|---|---|
| Seed | Ultrasound-assisted extraction (methanol–water 80:20) |
Polyphenols |
Antioxidant—DPPH Standard: (not used) |
Inhibition activity = 74 ± 1% at 500 µL/mL |
[83] |
| Seed | Maceration (ethanol–water 95:05) |
Cannabinoids and polyphenols |
Antioxidant—DPPH Standard: Ascorbic acid (IC50 = 0.012 ± 0.002 mg/mL) |
IC50 = 14.39 ± 2.27 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) |
Cannabinoids and polyphenols |
Antioxidant—metal ion chelating assay Standard: EDTA (CC50 = 0.15 ± 0.002 mg/mL) |
CC50 = 1.92 ± 1.05 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) |
Cannabinoids and polyphenols |
Antioxidant—lipid peroxidation inhibition Standard: α-tocopherol (IPC50 = 0.045 ± 0.002 mg/mL) |
IPC50 = 92.68 ± 30.77 mg/mL | [155] |
| Seed | Maceration (ethanol–water 75:25) |
Lignanamides |
Antioxidant—DPPH Standard: Quercetin (IC50 = 25.5 µm) |
Cannabisin M (IC50 = 69.5 µm) Cannabisin N and O (IC50 = ND) 3,3′-demethyl-heliotropamide (IC50 = 39.3 µm) |
[156] |
| Seed | Maceration (ethanol–water 75:25) |
Lignanamides |
Antioxidant—ORAC Standard: Quercetin (IC50 = 0.40 µm) |
Cannabisin M (IC50 = 6.61 µm) Cannabisin N and O (IC50 = ND) 3,3′-demethyl-heliotropamide (IC50 = 0.56 µm) |
[156] |
| Seed | Maceration (ethanol–water 75:25) |
Lignanamides |
Antioxidant—ABTS Standard: Quercetin (IC50 = 9.19 µm) |
Cannabisin M (IC50 = 74.70 µm) 3,3′-demethyl-heliotropamide (IC50 = 16.41 µm) |
[156] |
| Seed oil | Acid/alkali extraction | Proteins |
Antioxidant—DPPH Standard: (not used) |
Alkali soluble proteins: - inhibition value = 73.33% after ht enzyme hydrolysis. Acid soluble proteins: - inhibition value = 68.67% after ht enzyme hydrolysis. |
[157] |
| Seed flour | Ultrasound-assisted extraction (methanol–water 80:20) |
Polyphenols |
Antioxidant—DPPH Standard: (not used) |
Inhibition activity = 67 ± 1% at 500 µL/mL | [83] |
| Seed oil | Ultrasound-assisted extraction (methanol–water 80:20) |
Polyphenols |
Antioxidant—DPPH Standard: (not used) |
Inhibition activity = 22 ± 2% at 500 µL/mL | [112] |
| Seed | Maceration (ethanol–water 80:20) |
Polyphenols |
Antibacterial (against Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Enterobacter aerogenes and Enterococcus faecalis) Standard: Gentamycin and vancomycin (MIC < 0.05 mg/mL) |
All bacterial strains showed MIC values superior to 1 mg/mL | [158] |
| Seed | Maceration (ethanol–water 95:05) |
Cannabinoids and polyphenols |
Antibacterial (against Staphylococcus aureus) Standard: Erythromycin (ID = 26.67 ± 1.15 mm) |
Inhibition diameter = 1.00 mm | [155] |
| Seed | Maceration (ethanol–water 80:20) |
Polyphenols |
Antibacterial (against Lactobacillus paracasei, Lactobacillus reuteri, Lactobacillus brevis, Lactobacillus plantarum, Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacterium breve) Standard: Gentamycin and vancomycin (MIC < 0.05 mg/mL) |
All bacterial strains showed MIC values superior to 1 mg/mL | [158] |
| Seed | Maceration (ethanol–water 95:05) |
Terpenoid,
phytocannabinoids and unsaturated fatty acids |
Cytotoxicity—SRB assay Standard: (not used) |
Percentages of human skin fibroblast viability 66.12 ± 7.63% at 1 mg/mL | [155] |
| Seed | Maceration (ethanol–water 95:05) |
Terpenoids and flavonoids |
Antiproliferative—SRB assay Standard: Doxorubicin (IC50 = 0.0042 ± 0.0025 mg/mL) |
On hepg2 cell lines: IC50 = 12.07 ± 1.18 mg/mL |
[155] |
| Seed oil | Ultrasound-assisted extraction (methanol–water 80:20) |
Polyphenols |
Antiproliferative Standard: (not used) |
Reduction in caco-2 and ht-29 cell viability to less than 40% from 150 mg/mL | [83] |
| Seed | Maceration (ethanol–water 95:05) |
Terpenoids and flavonoids |
Anti-tyrosinase Standard: Kojic acid (IC50 = 0.005 ± 0.004 mg/mL) |
IC50 = 0.07 ± 0.06 mg/mL | [155] |
| Seed | Maceration (ethanol–water 75:25) |
Lignanamides |
Acetyl choline esterase inhibition Standard: Galantamine (IC50 = 2.76 μm) |
Cannabisin M, N and O (IC50 = nd) 3,3′-demethyl-heliotropamide (IC50 = 46.2 µm) |
[156] |
| Leaf | Maceration (ethanol–water 95:05) |
Cannabinoids and
polyphenols |
Antioxidant—DPPH Standard: Ascorbic acid (IC50 = 0.012 ± 0.002 mg/mL) |
IC50 = 2.73 ± 0.422 mg/mL | [155] |
| Leaf | Maceration (ethanol–water 95:05) |
Cannabinoids and
polyphenols |
Antioxidant—Metal ion chelating assay Standard: EDTA (CC50 = 0.15 ± 0.002 mg/mL) |
CC50 = 0.93 ± 0.20 mg/mL | [155] |
| Leaf | Maceration (ethanol–water 95:05) |
Cannabinoids and
polyphenols |
Antioxidant—Lipid peroxidation inhibition Standard: α-tocopherol (IPC50 = 0.045 ± 0.002 mg/mL) |
IPC50 = 246.32 ± 69.38 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) |
Polyphenols and
flavonoids |
Antioxidant—DPPH Standard: (not used) |
Inhibition activity of 40% at 1000 µg/mL | [87] |
| Leaf | Maceration (acetone) |
ND |
Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) |
Inhibition diameters: Escherichia coli = 24.7 ± 1.5 mm Staphylococcus aureus = 19.6 ± 2.1 mm Pseudomonas aeruginosa = 19.0 ± 2.6 mm |
[159] |
| Leaf | Maceration (chloroform) |
ND |
Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) |
Inhibition diameters: Escherichia coli = 23.0 ± 2.0 mm Staphylococcus aureus = 18.6 ± 2.08 mm Pseudomonas aeruginosa = 22.3 ± 1.52 mm |
[159] |
| Leaf | Maceration (ethanol–water 60:40) |
ND |
Antibacterial Standard: Ampicillin (ID = 17.3 to 19.6 mm) |
Inhibition diameters: Escherichia coli = 19.3 ± 1.2 mm Staphylococcus aureus = 18.6 ± 3.05 mm |
[159] |
| Leaf | Maceration (ethanol–water 95:05) |
Polyphenols, flavones and polyholozides |
Antibacterial (against Staphylococcus mutans) Standard: Erythromycin (ID = 23 mm) |
Inhibition diameter: 1.33 ± 0.58 mm | [155] |
| Leaf | Maceration (acetone) |
ND |
Antifungal Standard: (not used) |
Inhibition diameters: Aspergillus niger = 21.3 ± 2 mm Fusarium spp. = 20 ± 2.64 mm |
[159] |
| Leaf | Maceration (chloroform) |
ND |
Antifungal Standard: (not used) |
Inhibition diameters: Aspergillus niger = 20.6 ± 1.5 mm Fusarium spp. = 18.3 ± 1.52 mm |
[159] |
| Leaf | Maceration (ethanol–water 60:40) |
ND |
Antifungal Standard: (not used) |
Inhibition diameters: Aspergillus niger = 23 ± 2 mm Fusarium spp. = 21.3 ± 3.21 mm |
[159] |
| Leaf | Maceration (water) |
ND |
Antifungal Standard: (not used) |
Inhibition diameters: Aspergillus niger = 21 ± 2.6 mm Fusarium spp. = 24.3 ± 3.51 mm |
[159] |
| Leaf | Maceration (ethanol–water 95:05) |
Polyphenols, flavones and polyholozides |
Cytotoxicity—SRB assay Standard: (not used) |
Percentages of human skin fibroblast viability 73.02 ± 3.57% at 1 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) |
Polyphenols and flavonoids |
Cytotoxicity Standard: (not used) |
Viability of: HaCaT keratinocytes cells with 120% at 100 g/mL. BJ fibroblasts cells with 188% at 500 µg/mL. |
[87] |
| Leaf | Maceration (ethanol–water 95:05) |
Polyphenols, flavones and polyholozides |
Antiproliferative—SRB assay Standard: Doxorubicin (IC50 = 0.0042 to 0.0274 mg/mL) |
On hepg2 cell lines: IC50 = 13.17 ± 1.53mg/mL On kb cell lines: IC50 = 5.16 ± 1.66 mg/mL |
[155] |
| Leaf | Maceration (ethanol–water 95:05) |
Terpenoids and flavonoids |
Anti-tyrosinase Standard: Kojic acid (IC50 = 0.005 ± 0.004 mg/mL) |
IC50 = 0.049 ± 0.02 mg/mL | [155] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) |
Cannabinoids |
Anti-elastase Standard (not used) |
Inhibition value of 30% at 1000 µg/mL | [87] |
| Leaf | Ultrasound-assisted extraction (ethanol–water 80:20) |
Cannabinoids |
Anti-collagenase Standard: (not used) |
Inhibition value of 80% at 1000 µg/mL | [87] |
| Leaf | Maceration (chloroform) |
Cannabinoids (THC, CBD and CBN) |
Anticoagulant Standard: (not used) |
Inhibition values at 1 mg/mL: THC = 34.87% (IC50 = 1.79 mg/mL) CBN = 7.3% (IC50 = high value) |
[160] |
| Flowers | Maceration (acetone–water 50:50) |
Polyphenols |
Antifungal (against Aspergillus favus) |
Total inhibition at > 0.225 mg of DM/mL of medium | [161] |
| Flowers | Maceration (acetone–water 50:50) |
Polyphenols |
Anti-aflatoxigenic Standard: (not used) |
Reduction in fungal growth by 36% at 7.2 mg DM/mL of medium | [161] |
| Inflorescences | Hydrodistillation (essential oil) |
Terpenes |
Insecticidal Standard: (not used) |
Lethal concentration of LC50: Anopheles stephensi = 73.50 to 78.80 ppm for larvae. Anopheles gambiae = 20.13 to 67.19 ppm for pupae. |
[162] |
| Inflorescences | Hydrodistillation (essential oil) |
Terpenes |
Cytotoxicity Standard: (not used) |
IC50 values of: HaCaT cells = 2.23 ± 0.09 mg/mL Nhf a12 cells = 3.71 ± 0.2 mg/mL |
[162] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antioxidant—DPPH Standard: (not used) |
Inhibition activity: Essential oil = 5.6 mg TE/g sample Aromatic water = 40 mg TE/g sample. |
[94] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes |
Antioxidant—DPPH Standard: Quercetin (IC50 = 1.1 ± 0.0 µg/mL) |
IC50 = 1.6 ± 0.1 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antioxidant—FRAP Standard: (not used) |
Inhibition activity: Essential oil = 57 mg TE/g sample Aromatic water = 83 mg TE/g sample. |
[94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antioxidant—metal chelating activity Standard: (not used) |
Inhibition activity: Essential oil = 19.3 mg EDTAE/g sample Aromatic water = 3.8 mg mg EDTAE/g DW |
[94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antioxidant—phosphomolybdenum Standard: (not used) |
Inhibition activity: Essential oil = 35.1 mmol TE/g of oil Aromatic water = 1.4 mmol TE/g DW |
[94] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes |
Antioxidant—reducing power assay Standard: Quercetin (EC50 = 2.3 ± 0.1 µg/mL) |
EC50 = 1.8 ± 0.2 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes |
Antioxidant—β-carotene/linoleic acid Standard: Quercetin (EC50 = 0.9 ± 0.0 µg/mL) |
EC50 = 0.9 ± 0.1 mg/mL | [163] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antioxidant—CUPRAC Standard: (not used) |
Inhibition activity: Essential oil = 141 mg TE/g sample Aromatic water = 109 mg TE/g sample. |
[94] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes and polyphenols |
Antibacterial (against Helicobacter pylori strains) Standard: Naringenin (MIC and MBC = 8–32 µg/mL) |
MIC = 8–64 µg/mL MBC = 8–32 µg/mL |
[94] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes and polyphenols |
Antibacterial (against Staphylococcus aureus) Standard: (not used) |
MIC = 8 mg/mL MBC = 16 mg/mL MBEC = 16–24 mg/mL |
[94] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes |
Antibacterial Standard: Ciprofloxacin (MIC = 0.015–1.00 mm) |
Micrococcus luteus and Staphylococcus aureus: MIC = 4.7 mg/mL Bacillus subtilis, Escherichia coli and Pseudomonas aeruginosa: MIC = 1.2 mg/mL |
[163] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes |
Antifungal Standard: Fluconazole (MIC = 1.00 mm) |
Candida albicans, Candida glabrata, Candida krusei and Candida parapsilosis: MIC = 9.5 mg/mL |
[163] |
| Aerial parts | Hydrodistillation (essential oil) |
Terpenes and polyphenols |
Antifungal (against Candida spp. and Malassezia spp.) Standard: (not used) |
MIC value > 12,460 µg/mL | [94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Cytotoxicity Standard: Doxorubicin (IC50 = 3.1–23.3 µg/mL) |
Inhibition activity of 50%: IC50 (mda-mb-468) = 53.0 µg/mL IC50 (caco-2) = 28.7 µg/mL IC50 (mz-cha-1) = 22.3 µg/mL |
[94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Antidiabetic Standard: (not used) |
α-amylase inhibition: Essential oil = nd Aromatic water = 0.10 mmol ACAE/g extract α-glucosidase inhibition: Essential oil = 3.77 mmol ACAE/g oil Aromatic water = 0.17 mmol ACAE/g extract |
[94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Acetyl- and butyryl-choline esterase inhibition Standard: (not used) |
Acetyl-choline esterase inhibition: Essential oil = nd Aromatic water = 2.56 mg GALAE/g extract Butyryl-choline esterase inhibition: Essential oil = 3.4 mg GALAE/g oil Aromatic water = 3.48 mg GALAE/g extract |
[94] |
| Aerial parts | Hydrodistillation (essential oil + aromatic water) |
Terpenes and polyphenols |
Lipase inhibition Standard: (not used) |
Inhibition activity: - essential oil = 70.14 mg OE/g oil - aromatic water = nd |
[94] |
IPC50: concentration providing 50% inhibition of lipid peroxidation/CC50: concentration providing 50% metal chelating activity. IC50: concentration of the sample that inhibits 50% expressed in mg/mL/ID: inhibition diameter (mm). EDTA: ethylenediaminetetraacetic acid./GAE: gallic acid equivalent./RE: rutin equivalent./CE: caffeic acid equivalent./TE: trolox equivalent. EDTAE: ethylenediaminetetraacetic acid equivalent./MIC: minimum inhibitory concentration./MBC: minimum bactericidal concentration. MCF-7: estrogen-dependent breast cancer cells./MDA-MB-468: triple-negative breast cancer cells./CACO-2: colorectal adenocarcinoma cells./MZ-CHA-1: cholangiocarcinoma cells.
4.4.1. Antioxidant Activity
An antioxidant is a molecule that slows down or prevents the oxidation of molecules that can play an important role in an organism metabolism. Cannabis sativa L. proved to have plenty of antioxidant substances. The antioxidant activity of this plant has been widely reported in the literature and it was determined by using many assays, including the free radical scavenging method (DPPH), oxygen radical absorbance capacity (ORAC), ferric reducing ability of plasma (FRAP) and 2,2′-azino-bis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), as well as other methods such as phosphomolybdenum and metal chelation. The antioxidant activity of Cannabis sativa has been reported in the plant seeds, leaves and aerial parts. Research on the antioxidant effects of Cannabis sativa L. seeds have been well reported in the literature. Manosroi, et al. [155] described the antioxidant activity of ethanolic extract using different tests, namely DPPH, chelating assay and lipid peroxidation inhibition. The obtained results showed that the seed organic extract exhibited strong antioxidant capacity, suggested by IC50 (low inhibitory concentration at 50%) values with 14.5 mg/mL for DPPH, 1.9 mg/mL for chelating assay and 92.7 mg/mL for lipid peroxidation inhibition assay. In another study, the methanolic extract of the seeds showed an average activity against DPPH with an inhibition value of 75% at 500 µL/mL [83]. According to these authors, this activity is probably due to the presence of polyphenols and cannabinoids, known for their strong antioxidant capacity [164,165]. Moreover, other seed metabolites, such as lignamides [156] and proteins [157], exhibited average antioxidant activities. However, they are lower than the results obtained with polyphenols and cannabinoids. Considering the high amount of polyphenols in the leaves, the extracts obtained from these plant parts have generally shown strong antioxidant activity [87,155]. For example, the hydroalcoholic extract of the leaves showed a DPPH IC50 value of 2.7 mg/mL [155]. This value is relatively lower than the result obtained in the seed DPPH assay, which suggests a higher antioxidant potential. Moreover, the aerial essential parts showed also excellent antioxidant activity, with values near to the positive control [163]. As previously mentioned, polyphenols are generally considered as the main group of antioxidant molecules that work through different mechanisms, such as the suppression of free radicals that initiate oxidative damage and inhibit the oxidation process, via chelation of catalytic metals or metal ions and the inhibition of lipoxygenase [166,167]. Furthermore, some volatile terpenes exhibits potent antioxidant and anti-free-radical properties [168]. This can explain the antioxidant activity of the essential oils of the aerial parts proven through different tests.
4.4.2. Antimicrobial Activity
An antimicrobial is a molecule with microbicidal (kills microorganisms) or microbiostatic (slow the microbial growth and/or development) activity. These substances have different names depending on the type of targeted microorganism, such as antibacterial (for bacteria), antifungal (for fungi), antiviral (for virus) or antiparasitic (for parasites). The antimicrobial activity of essential oils and organic extracts of different parts of Cannabis sativa L. against several microorganisms has been reported. However, the degree of antimicrobial activity varies from cultivar to cultivar [169], as well as according to the part of the plant used, the extraction method and type of extract. The seed hydroalcoholic extract was evaluated against Gram-positive and -negative bacteria, namely Staphylococcus aureus, Escherichia coli, Salmonella typhimurium, Enterobacter aerogenes, Enterococcus faecalis, Lacticaseibacillus paracasei, Limosilactobacillus reuteri, Levilactobacillus brevis, Lactiplantibacillus plantarum, Bifidobacterium bifidum, Bifidobacterium longum and Bifidobacterium breve, and the obtained results showed low antibacterial activity with MIC values superior to 1 mg/mL [158]. Regarding antibacterial tests on Cannabis leaves, a study published by Anjum [159] compared the efficacy of four extracts obtained with acetone, chloroform, ethanol and water against three bacterial strains, namely Escherichia coli, Staphylococcus aureus and Pseudomonas aeruginosa. The results showed similarities between the four extracts with values near to 19 mm. Moreover, the results published by Manosroi, et al. [155] showed the effect of ethanolic extract as an antibacterial agent against Staphylococcus mutans with an inhibition diameter of 1.33 ± 0.58 mm. The essential oils of the aerial parts were evaluated as well for their antibacterial activity. The volatile terpenes of Cannabis exerted diverse activity intensities according to the targeted bacterial strains, where the weakest antibacterial activity was observed against Helicobacter pylori and Klebsiella pneumonia strains, with MICs values of 64 and 38 mg/mL, respectively. Moreover, a weak antibacterial activity was observed against Micrococcus luteus and Staphylococcus aureus, with an MIC of 4.7 mg/mL for both strains, whereas a more moderate inhibitory activity was observed against Escherichia coli, Pseudomonas aeruginosa and Bacillus subtilis, with an MIC of 1.2 mg/mL [163]. These results are explained by the fact that volatile terpenes are known to be strong antibacterial compounds, according to many biological investigations [170,171,172].
Concerning the antifungal activity, Anjum [159] compared the efficacy of four Cannabis leaf extracts, obtained with acetone, chloroform, ethanol and water, against two fungi, namely Aspergillus niger and Fusarium spp. The extracts showed similar results with inhibition diameter values ranging between 20.6 and 23 mm for Aspergillus niger, and 18.3 to 24.3 mm for Fusarium spp. In another studies, the acetone extract of Cannabis flowers showed a significant effect on the growth of Aspergillus favus, the flower hydroalcoholic extract led to the inhibition of 36% of fungi mycelium at concentration of 7.2 mg of dry matter per mL of culture medium [161]. Moreover, aerial part essential oils showed interesting antifungal potential. Nafis, et al. [163] reported an MIC value of 9.5 mg/mL against four fungi species, namely Candida albicans, Candida glabrata, Candida krusei and Candida parapsilosis. However, Zengin, et al. [94] discovered a weak antifungal activity against a group of clinically relevant and multidrug-resistant microorganisms belonging to Candida spp. and Malassezia spp., with essential oil MIC values superior to 12.460 µg/mL.
4.4.3. Insecticidal Activity
Insecticides are active substances with the property of killing insects, their larvae and/or eggs. Insecticides act either by contact or after penetration into the digestive tract or into the respiratory system. Essential oils from inflorescences were evaluated for their mosquitocidal activities on larvae and pupae of two main malaria vectors known as Anopheles gambiae and Anopheles stephensi. The results showed that Cannabis inflorescence essential oils showed toxicity against mosquitoes with LC50 values of 73.5 to 78.8 ppm for Anopheles stephensi larvae, and 20.13 to 67.19 ppm for pupae of Anopheles gambiae. Their natural origin and volatile propriety makes the use of these essential oils very attractive for the formulation of stable and safe biocontrol products [162]. The cholinesterase inhibition activity had also been reported in the literature. Furthermore, a molecule purified from the ethanolic extract of Cannabis seeds, namely 3,3′-demethyl-heliotropamide, showed a moderate activity with an IC50 value of 46.2 µm [156], whereas the aerial part essential oils showed stronger activity against butyryl-choline esterase, with a concentration of 3.4 mg GALAE/g oil [94].
4.4.4. Anticoagulant Activity
It has been suggested that plants with anticoagulant activities act as herbal remedies that could lead to the discovery of new therapeutic agents to treat thrombosis-related diseases. Blood clotting studies were conducted to determine the possible antiprothrombotic effect of Cannabis leaf metabolites, with three main cannabinoids, THC, CBD and CBN, targeted. The in vitro effect of Cannabis extract on thrombin activity was evaluated by Coetzee, et al. [160]. In their publication, two cannabinoids, namely THC and CBN, showed interesting IC50 values. The highest activity was obtained by THC, with a value of 1.79 mg/mL, whereas CBN showed weaker activity suggested by high IC50 value. However, this study also featured an in vivo test applied on obese rats in order to determine the clotting times. As a result, the Cannabis-treated rats showed an efficiency of 50% with clotting two times higher than the control groups, thus proving that cannabinoids may have a good anticoagulant activity.
4.4.5. Antidiabetic Activity
Diabetes is a chronic, progressive and complex metabolic disorder characterized by abnormally high blood glucose levels. This condition is also known as hyperglycemia. Worldwide, approximately 90% of affected patients are non-insulin-dependent, classified as type 2 diabetes [173]. According to the World Health Organization (WHO), diabetes is a chronic disease that occurs when the pancreas does not produce enough insulin, or the body does not properly use the produced insulin. Insulin is a hormone that regulates the concentration of sugar in the blood. Antidiabetic activity is generally evaluated by the quantification of α-amylase inhibition [174]. This enzyme is generally produced in the pancreas and plays a key enzyme role in the increase in blood sugar by breaking down dietary carbohydrates, such as starch, into simple monosaccharides in the digestive system, followed by the further α-glucosidase degradation into glucose which, upon absorption, enters in the bloodstream. Therefore, inhibition of the enzymes α-amylase and α-glucosidase can suppress carbohydrate digestion, delay glucose absorption and, consequently, reduce blood glucose levels [175]. The study published by Zengin, et al. [94] proved that Cannabis aerial part essential oils exhibited antidiabetic properties against the α-glucosidase enzyme with a value of 3.77 mmol ACAE/g oil. This essential oil has also been evaluated against α-amylase but showed no significant result.
4.4.6. Anticancer Activity
Cancer remains a major cause of morbidity and mortality worldwide. It is currently treated using classical approaches such as surgery, chemotherapy and radiotherapy. The toxic side effects associated with chemotherapy and radiotherapy often lead to adverse health effects. This explains the huge need for new drugs, safer to use with less side effects. Experimentally, several Cannabis-derived compounds demonstrated conclusive efficacy in vitro and in vivo on a wide range of cancer cell lines, including breast [176], prostate [177], cervix [178], brain [179], colon [180] and leukemia/lymphoma [181]. A number of in vitro and in vivo studies have demonstrated the effects of phytocannabinoids on tumor progression. These studies suggest that specific cannabinoids such as Δ9-THC and CBD induce apoptosis and inhibit proliferation in various cancer cell lines at concentrations ranging between 5 and 65 µm [176,182,183,184,185,186,187]. Moreover, combination of certain phytocannabinoids improved the anticancer activity of Cannabis preparations; for example, Armstrong, et al. [182] revealed that the combination of CBD and Δ9 -THC exhibited a stronger melanoma cell mortality in comparison with Δ9-THC alone. In general, phytochemicals in the Cannabis plant, and especially cannabinoids, are non-selective in their functions and limited in their differential activity on cancer cells with normal cells. Therefore, researchers show interest in isolating bioactive phytochemicals from Cannabis with potent anticancer properties and generating lead compounds based on the natural backbone of a molecule as a synthetic approach.
4.4.7. Anti-Inflammatory and Analgesic Activities
Many compounds of Cannabis proved to have strong anti-inflammatory activity. The Cannabis seeds showed an inflammation-reducing capacity, especially on primary human monocytes treated with LPS. The results proved that the compounds of these seeds decreased the respective expression and secretion of IL-6 genes and TNF-α. Additionally, cannabinoids proved to be strong anti-inflammatory agents; in fact, they can suppress the production of pro-inflammatory cytokines and chemokines and may have therapeutic applications in health conditions underlying inflammatory components [188,189]. Analgesic action is defined as any procedure whose principle of activity is to reduce pain. This can be not only a drug but also any other method aimed at achieving analgesia, i.e., the abolition of the sensation of pain [190]. Clinical and experimental studies showed that Cannabis-derived compounds act as analgesic agents. However, the effectiveness of each product is variable and depends on the administration mode. With opioids being the only therapy for severe pain, the analgesic capacity of cannabinoids could provide a much-needed alternative to opioids [191]. Furthermore, cannabinoids act synergistically with opioids and act as opioid-sparing agents, allowing lower doses and fewer side effects of chronic opioid treatment [192]. Thus, the rational use of Cannabis-based medicines deserves to be seriously considered to alleviate patients’ suffering from severe pain.
4.4.8. Neuroprotective Activity
The terpenes and cannabinoids of Cannabis proved also to have neuroprotective proprieties. The neuroprotective effects of 17 compounds present in the aerial parts of Cannabis sativa L. were evaluated in PC-12 cells including p-hydroxybenzaldehyde, (e)-methyl p-hydroxycinnamate and ferulic acid—which showed additional protective effects against H2O2-induced damage [193]. Furthermore, di Giacomo, et al. [147] reported the neuroprotective and neuromodulatory effects induced by cannabidiol and cannabigerol in rat Hypo-E22 cells and isolated hypothalamus, whereas Landucci, et al. [194] proved that appropriate concentrations of CBD or CBD/THC ratios can represent a valid therapeutic intervention in the treatment of post-ischemic neuronal death. In another study, Esposito, et al. [195] highlighted the importance of CBD as a promising new drug able to reduce neuroinflammatory responses evoked by β-amyloid. Furthermore, the study published by Perez, et al. [196] described the neuronal counting of both motor and sensory neurons after CBD treatment using immunohistochemical analysis. The obtained results showed an increase by 30% of synaptic preservation on the spinal cord for the CBD-treated group, suggesting an average neuroprotective effect.
4.4.9. Antiepileptic and Anticonvulsant Activities
Despite being well-known for its psychoactive proprieties, Cannabis sativa L. has been investigated for additional effects on the central nervous system. A recently published study showed that CBD has a high efficacy in epilepsy with hippocampal focus than with the extrahippocampal amygdala and parvalbumin, implying a protective role in regulating hippocampal seizures and neurotoxicity at a juvenile age [197]. The efficiency of CBD has been further replicated in human populations, including adolescents and young adults with severe childhood-onset epilepsy [198]. In a 12-week open-label trial, a group of patients aged between 1 and 30 years were treated with 25 and 50 mg/kg of CBD. The treated groups showed a reduction in epileptic attack severity by 36.5%. Preclinical research has also attempted a more chronic elucidation of the efficacy of CBD as an anticonvulsant. Using the PTZ model of epilepsy, it was found that a reduction in seizure activity could be achieved with varying doses between 20 and 50 mg/kg of CBD over a 28-day treatment period [199]. Other cannabinoids, such as cannabigerol, cannabidivarin, cannabichromene, δ 9 -tetrahydrocannabinolic acid and tetrahydrocannabivarin, showed efficacy in models of Huntington’s disease and epilepsy. It is important to note that these phytocannabinoids and their combinations are warranted in a range of other neurodegenerative disorders such as Parkinson’s [200].
4.4.10. Dermocosmetic Activity
Lipids in human skin play a very important role in preserving the structure of the dermis, protecting it from dehydration. However, during menopause, hormonal changes negatively affect the skin’s balance, making it more prone to developing dryness [201]. The underlying tissues, such as subcutaneous adipose tissue and muscles, undergo atrophy due to the overproduction of some enzymes—among them tyrosinase, elastase and collagenase. Tyrosinase is mainly responsible for the production of melanin on the skin, whereas collagenase and elastase target the skin structural proteins collagen and elastin and degrade them, respectively. The results published by Manosroi, et al. [155] showed that leaf extracts have anti-tyrosinase activity with an IC50 value of 0.07 ± 0.06 mg/mL, which suggest a strong tyrosinase inhibitory activity. Similarly, Zagórska-Dziok et al. [87] showed respective collagenase and elastase inhibitory activities of 80 and 30% at 1000 µg/mL. These activities are mainly due to the presence of polyphenols and cannabinoids.
4.5. Drugs Based on Cannabis sativa L.
Due to the importance of the biological evaluation’s findings, several Cannabis-based commercial pharmaceuticals have been produced. These products have many biological proprieties and were produced to treat a wide range of conditions. The first reported product has been commercialized under the name “marinol”. This drug was developed 40 years ago, in 1985, by an American pharmaceutical company, and used dronabinol and synthetic THC as active agents [202]. Likewise, “syndros” is another drug marketed in the USA in 2016 and it contains the same active ingredients [202]. Both drugs are indicated for the treatment of severe nausea and vomiting related to cancer chemotherapy and AIDS-anorexia associated with weight loss. Another active compound known as “nabilone” is used as ingredient of two drugs, “casamet” and “canemes” [203]. The “nabilone” is a synthetic analogue of THC approved by the U.S. Food and Drug Administration (U.S. FDA) for the treatment of chemotherapy and AIDS symptoms [204]. Two other cannabinoids, namely “CBD” and “THC”, are also used in two drugs commercialized under the name of Bourneville and used against two types of severe epilepsy (Lennox–Gastaut syndrome and Dravet syndrome) [205], whereas Sativex, generally known as “nabiximols”, is used to alleviate muscle spasms in multiple sclerosis disease.
5. Conclusions
This manuscript has reviewed and analyzed the historical, botanical, ethnopharmacological, chemical, bioinformatics and biological knowledge of Cannabis sativa from the earliest human communities to current medical applications, with a critical analysis of the multiple potential applications of cannabinoids in the contemporary scientific context.
At present, more than 545 phytochemicals have been described in the different parts of the Cannabis plant. The most represented metabolite class is the phytocannabinoids and they exhibit enormous structural diversity and bioactivities. Cannabis sativa is found in a wide variety of forms and environments on all continents and its pharmacological properties seem to go far beyond psychotic effects, with the ability to address needs such as the treatment and relief of many symptoms and diseases.
Furthermore, the relaxation of regulatory standards for therapeutic Cannabis and the conduct of more controlled clinical trials suggests that the Cannabis sativa plant has interesting therapeutic potential as an antiemetic, appetite stimulant in debilitating diseases (cancer and AIDS), analgesic, as well as in the treatment of multiple sclerosis, spinal cord injury, Tourette syndrome, epilepsy and glaucoma. Further clinical research is needed to investigate the potential therapeutic uses of this plant in specific medical conditions. Scientifically designed trials will help establish which of the cannabinoids produce the various beneficial effects described, or whether these are the result of a combination of cannabinoids. The research would also help to better characterize the adverse effects of each cannabinoid.
Acknowledgments
We would like to thank COST Action 18101 SOURDOMICS—Sourdough biotechnology network towards novel, healthier and sustainable food and bioprocesses (https://sourdomics.com/; https://www.cost.eu/actions/CA18101/, accessed on 7 February 2023), where the author N.E.A. is member of the working groups 3, 4 and 7, and the author J.M.R. is the Chair and Grant Holder Scientific Representative and is supported by COST (European Cooperation in Science and Technology) (https://www.cost.eu/, accessed on 7 February 2023). COST is a funding agency for research and innovation networks. Regarding the author J.M.R., he is financially supported by LA/P/0045/2020 (ALiCE) and UIDB/00511/2020-UIDP/00511/2020 (LEPABE) funded by national funds through FCT/MCTES (PIDDAC).
Author Contributions
Conceptualization: N.E.A. and S.H.; Data collection: S.H., A.Y.B. and H.M.; Writing—original draft preparation: S.H. and H.M.; Writing—review and editing: H.M., J.M.R. and N.E.A.; Supervision: N.E.A. and J.M.R.; Funding acquisition: N.E.A. and J.M.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
“Agence National des Plantes Médicinales et Aromatiques-Taounate, ANPMA” Morocco and University Abdelmalek Essaadi-Tetouan Morocco (VPMA4).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.McPartland J.M., Hegman W., Long T. Cannabis in Asia: Its center of origin and early cultivation, based on a synthesis of subfossil pollen and archaeobotanical studies. Veg. Hist. Archaeobot. 2019;28:691–702. doi: 10.1007/s00334-019-00731-8. [DOI] [Google Scholar]
- 2.Anderson L.C. Leaf variation among Cannabis species from a controlled garden. Bot. Mus. Leafl. Harv. Univ. 1980;28:61–69. doi: 10.5962/p.168641. [DOI] [Google Scholar]
- 3.McPartland J.M. Cannabis systematics at the levels of family, genus, and species. Cannabis Cannabinoid Res. 2018;3:203–212. doi: 10.1089/can.2018.0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McPartland J.M., Small E. A classification of endangered high-THC cannabis (Cannabis sativa subsp. indica) domesticates and their wild relatives. PhytoKeys. 2020;144:81. doi: 10.3897/phytokeys.144.46700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke R., Merlin M. Cannabis: Evolution and Ethnobotany. University of California Press; Berkeley, CA, USA: 2016. [Google Scholar]
- 6.Gedik G., Avinc O. Sustainability in the Textile and Apparel Industries. Springer; Berlin/Heidelberg, Germany: 2020. Hemp fiber as a sustainable raw material source for textile industry: Can we use its potential for more eco-friendly production? pp. 87–109. [Google Scholar]
- 7.Gomez F.P., Hu J., Clarke M.A. Cannabis as a Feedstock for the Production of Chemicals, Fuels, and Materials: A Review of Relevant Studies To Date. Energy Fuels. 2021;35:5538–5557. doi: 10.1021/acs.energyfuels.0c04121. [DOI] [Google Scholar]
- 8.Rupasinghe H.V., Davis A., Kumar S.K., Murray B., Zheljazkov V.D. Industrial hemp (Cannabis sativa subsp. sativa) as an emerging source for value-added functional food ingredients and nutraceuticals. Molecules. 2020;25:4078. doi: 10.3390/molecules25184078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleming M.P., Clarke R.C. Physical evidence for the antiquity of Cannabis sativa L. J. Int. Hemp Assoc. 1998;5:80–95. [Google Scholar]
- 10.Lowe H., Steele B., Bryant J., Toyang N., Ngwa W. Non-cannabinoid metabolites of Cannabis sativa L. with therapeutic potential. Plants. 2021;10:400. doi: 10.3390/plants10020400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andre C.M., Hausman J.-F., Guerriero G. Cannabis sativa: The plant of the thousand and one molecules. Front. Plant Sci. 2016;7:19. doi: 10.3389/fpls.2016.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Giupponi L., Leoni V., Carrer M., Ceciliani G., Sala S., Panseri S., Pavlovic R., Giorgi A. Overview on Italian hemp production chain, related productive and commercial activities and legislative framework. Ital. J. Agron. 2020;15:194–205. doi: 10.4081/ija.2020.1552. [DOI] [Google Scholar]
- 13.Pollastro F., Minassi A., Fresu L.G. Cannabis phenolics and their bioactivities. Curr. Med. Chem. 2018;25:1160–1185. doi: 10.2174/0929867324666170810164636. [DOI] [PubMed] [Google Scholar]
- 14.Taura F., Sirikantaramas S., Shoyama Y., Shoyama Y., Morimoto S. Phytocannabinoids in Cannabis sativa: Recent studies on biosynthetic enzymes. Chem. Biodivers. 2007;4:1649–1663. doi: 10.1002/cbdv.200790145. [DOI] [PubMed] [Google Scholar]
- 15.Brenneisen R. Marijuana and the Cannabinoids. Humana Press; Clifton, NJ, USA: 2007. Chemistry and analysis of phytocannabinoids and other Cannabis constituents; pp. 17–49. [Google Scholar]
- 16.Whiting P.F., Wolff R.F., Deshpande S., Di Nisio M., Duffy S., Hernandez A.V., Keurentjes J.C., Lang S., Misso K., Ryder S. Cannabinoids for medical use: A systematic review and meta-analysis. Jama. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 17.Mansoori B., Mohammadi A., Davudian S., Shirjang S., Baradaran B. The different mechanisms of cancer drug resistance: A brief review. Adv. Pharm. Bull. 2017;7:339. doi: 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zaman S.B., Hussain M.A., Nye R., Mehta V., Mamun K.T., Hossain N. A review on antibiotic resistance: Alarm bells are ringing. Cureus. 2017;9:e1403. doi: 10.7759/cureus.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kennedy L.B., Salama A.K. A review of cancer immunotherapy toxicity. CA A Cancer J. Clin. 2020;70:86–104. doi: 10.3322/caac.21596. [DOI] [PubMed] [Google Scholar]
- 20.Pollio A. The name of Cannabis: A short guide for nonbotanists. Cannabis Cannabinoid Res. 2016;1:234–238. doi: 10.1089/can.2016.0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bruneau D. Ph.D. Thesis. University of Rennes; Rennes, France: 2016. Le Cannabis sativa: Une plante psychotrope ayant des intérêtsthérapeutiques. [Google Scholar]
- 22.Russo E.B. History of cannabis and its preparations in saga, science, and sobriquet. Chem. Biodivers. 2007;4:1614–1648. doi: 10.1002/cbdv.200790144. [DOI] [PubMed] [Google Scholar]
- 23.Wujastyk D. Cannabis in Traditional Indian Herbal Medicine. Ayurveda at the Crossroads of Care and Cure, Centro de Historia del Alêmm-Mar, Universidade Nova de Lisboa; Lisbon, Portugal: 2002. pp. 45–73. [Google Scholar]
- 24.Hazekamp A. Cannabis Review. Volume 2009 Department of Plant Metobolomics, Leiden University; Leiden, The Netherlands: 2008. [Google Scholar]
- 25.Farag S., Kayser O. Handbook of Cannabis and Related Pathologies. Elsevier; Amsterdam, The Netherlands: 2017. The cannabis plant: Botanical aspects; pp. 3–12. [Google Scholar]
- 26.Schultes R.E., Klein W.M., Plowman T., Lockwood T.E. Cannabis: An example of taxonomic neglect. In: Rubin V., editor. Cannabis and Culture. De Gruyter Mouton; Berlin, NY, USA: 1975. pp. 21–38. [Google Scholar]
- 27.Pacifico D., Miselli F., Carboni A., Moschella A., Mandolino G. Time course of cannabinoid accumulation and chemotype development during the growth of Cannabis sativa L. Euphytica. 2008;160:231–240. doi: 10.1007/s10681-007-9543-y. [DOI] [Google Scholar]
- 28.Small E., Cronquist A. A practical and natural taxonomy for Cannabis. Taxon. 1976;25:405–435. doi: 10.2307/1220524. [DOI] [Google Scholar]
- 29.Clarke R.C., Merlin M.D., Small E. Evolution and classification of Cannabis sativa (Marijuana, Hemp) in relation to human utilization. Bot. Rev. 2015;81:189–294. [Google Scholar]
- 30.Amaducci S., Zatta A., Raffanini M., Venturi G. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions. Plant Soil. 2008;313:227–235. doi: 10.1007/s11104-008-9695-0. [DOI] [Google Scholar]
- 31.Amaducci S., Zatta A., Pelatti F., Venturi G. Influence of agronomic factors on yield and quality of hemp (Cannabis sativa L.) fibre and implication for an innovative production system. Field Crops Res. 2008;107:161–169. doi: 10.1016/j.fcr.2008.02.002. [DOI] [Google Scholar]
- 32.Campiglia E., Radicetti E., Mancinelli R. Plant density and nitrogen fertilization affect agronomic performance of industrial hemp (Cannabis sativa L.) in Mediterranean environment. Ind. Crops Prod. 2017;100:246–254. doi: 10.1016/j.indcrop.2017.02.022. [DOI] [Google Scholar]
- 33.Bouloc P. Le Chanvre Industriel: Production et Utilisations. France Agricole Editions; Paris, France: 2006. [Google Scholar]
- 34.Anwar F., Latif S., Ashraf M. Analytical characterization of hemp (Cannabis sativa) seed oil from different agro-ecological zones of Pakistan. J. Am. Oil Chem. Soc. 2006;83:323–329. doi: 10.1007/s11746-006-1207-x. [DOI] [Google Scholar]
- 35.Bouloc P. Hemp: Industrial Production and Uses. CABI; Wallingford, UK: 2013. [Google Scholar]
- 36.Schilling S., Melzer R., McCabe P.F. Cannabis sativa. Curr. Biol. 2020;30:R8–R9. doi: 10.1016/j.cub.2019.10.039. [DOI] [PubMed] [Google Scholar]
- 37.Lynch R.C., Vergara D., Tittes S., White K., Schwartz C., Gibbs M.J., Ruthenburg T.C., DeCesare K., Land D.P., Kane N.C. Genomic and chemical diversity in Cannabis. Crit. Rev. Plant Sci. 2016;35:349–363. doi: 10.1080/07352689.2016.1265363. [DOI] [Google Scholar]
- 38.Newton D.E. Marijuana: A Reference Handbook. Abc-Clio; Santa Barbara, CA, USA: 2013. [Google Scholar]
- 39.Botineau M. Botanique Systématique et Appliquée des Plantes à Fleurs. Tec & doc; Paris, France: 2010. [Google Scholar]
- 40.Richard D., Senon J.-L. Le Cannabis. Presses universitaires de France; Paris, France: 2010. [Google Scholar]
- 41.Strzelczyk M., Lochynska M., Chudy M. Systematics and botanical characteristics of industrial hemp Cannabis sativa L. J. Nat. Fibers. 2021;19:5804–5826. doi: 10.1080/15440478.2021.1889443. [DOI] [Google Scholar]
- 42.Radosevich S.R., Holt J.S., Ghersa C. Weed Ecology: Implications for Management. John Wiley & Sons; Hoboken, NJ, USA: 1997. [Google Scholar]
- 43.Citterio S., Santagostino A., Fumagalli P., Prato N., Ranalli P., Sgorbati S. Heavy metal tolerance and accumulation of Cd, Cr and Ni by Cannabis sativa L. Plant Soil. 2003;256:243–252. doi: 10.1023/A:1026113905129. [DOI] [Google Scholar]
- 44.Magnusson K., Svennerstedt B. Influence of temperature on the water retting process of hemp (Cannabis sativa L.) cultivated under Swedish climate conditions. J. Ind. Hemp. 2007;12:3–17. doi: 10.1300/J237v12n02_02. [DOI] [Google Scholar]
- 45.Matthieu M.L. Les Cannabinoïdes Dans La Prise en Charge des Patients Sous Anticancereux et Antiretroviraux: Connaissances Actuelles et Perspectives D’avenir en France. Faculty of Pharmacy, University of Lille; Lille, France: 2015. [Google Scholar]
- 46.Li H.-L. An archaeological and historical account of cannabis in China. Econ. Bot. 1974;28:437–448. doi: 10.1007/BF02862859. [DOI] [Google Scholar]
- 47.Hill B. Legalized Marijuana: Canada Comes Round to the Wisdom of Ages, Ancient-Origins. [(accessed on 6 March 2023)]. Available online: https://www.ancient-origins.net/history/cannabis-journey-through-ages-003084.
- 48.Abel E.L. Marihuana: The First Twelve Thousand Years. Springer Science & Business Media; Berlin, Germany: 2013. [Google Scholar]
- 49.Ramamoorthy S.K., Skrifvars M., Persson A. A review of natural fibers used in biocomposites: Plant, animal and regenerated cellulose fibers. Polym. Rev. 2015;55:107–162. doi: 10.1080/15583724.2014.971124. [DOI] [Google Scholar]
- 50.Turner C.E., Elsohly M.A., Boeren E.G. Constituents of Cannabis sativa L. XVII. A review of the natural constituents. J. Nat. Prod. 1980;43:169–234. doi: 10.1021/np50008a001. [DOI] [PubMed] [Google Scholar]
- 51.Veiga P. Oncology and Infectious Diseases in Ancient Egypt: The Ebers Papyrus’ Treatise on Tumours 857–877 and the Cases Found in Ancient Egyptian Human Material. University of Manchester; Manchester, UK: 2009. [Google Scholar]
- 52.Dawson W.R. Studies in the Egyptian Medical Texts—III. J. Egypt. Archaeol. 1934;20:41–46. doi: 10.1177/030751333402000108. [DOI] [Google Scholar]
- 53.Faulkner R.O. The Ancient Egyptian Pyramid Texts. Aris & Phillips; Wiltshire, England: 1969. [Google Scholar]
- 54.Ferrara M.S. Peak-experience and the entheogenic use of cannabis in world religions. J. Psychedelic Stud. 2021;4:179–191. doi: 10.1556/2054.2020.00122. [DOI] [Google Scholar]
- 55.Gately I. Tobacco: A Cultural History of How an Exotic Plant Seduced Civilization. Open Road and Grove Atlantic; New York, NY, USA: 2007. [Google Scholar]
- 56.Holmes W.H. Prehistoric Textile Art of Eastern United States. Volume 13. Independently published; Washington, DC, USA: 1896. p. 44. [Google Scholar]
- 57.Deitch R. Hemp: American History Revisited: The Plant with a Divided History. Algora Publishing; New York, NY, USA: 2003. [Google Scholar]
- 58.Chouvy P.-A. Cannabis cultivation in the world: Heritages, trends and challenges. EchoGéo. 2019;48:21. doi: 10.4000/echogeo.17591. [DOI] [Google Scholar]
- 59.Liu F.-H., Hu H.-R., Du G.-H., Deng G., Yang Y. Ethnobotanical research on origin, cultivation, distribution and utilization of hemp (Cannabis sativa L.) in China. Indian J. Tradit. Knowl. 2017;16:235–242. [Google Scholar]
- 60.Singh R., Upadhyay S.K., Rani A., Kumar P., Sharma P., Sharma I., Singh C., Chauhan N., Kumar M. Ethnobotanical study of weed flora at district Ambala, Haryana, India: Comprehensive medicinal and pharmacological aspects of plant resources. Int. J. Pharm. Res. 2020;12:1941–1956. [Google Scholar]
- 61.El Khomsi M., Dandani Y., Chaachouay N., Hmouni D. Ethnobotanical study of plants used for medicinal, cosmetic, and food purposes in the region of Moulay Yacoub, Northeast of Morocco. J. Pharm. Pharmacogn. Res. 2022;10:13–29. doi: 10.56499/jppres21.1084_10.1.13. [DOI] [Google Scholar]
- 62.Klauke A.L., Racz I., Pradier B., Markert A., Zimmer A.M., Gertsch J., Zimmer A. The cannabinoid CB2 receptor-selective phytocannabinoid beta-caryophyllene exerts analgesic effects in mouse models of inflammatory and neuropathic pain. Eur. Neuropsychopharmacol. 2014;24:608–620. doi: 10.1016/j.euroneuro.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 63.Rahmatullah M., Mollik M.A.H., Azam A., Islam M.R., Chowdhury M.A.M., Jahan R., Chowdhury M.H., Rahman T. Ethnobotanical survey of the Santal tribe residing in Thakurgaon District, Bangladesh. Am. Eurasian J. Sustain. Agric. 2009;3:889–898. [Google Scholar]
- 64.Mawla F., Khatoon S., Rehana F., Jahan S., Shelley M.M.R., Hossain S., Haq W.M., Rahman S., Debnath K., Rahmatullah M. Ethnomedicinal plants of folk medicinal practitioners in four villages of Natore and Rajshahi districts, Bangladesh. Am. Eur. J. Sustain. Agric. 2012;6:406–416. [Google Scholar]
- 65.Ahmed M., Azam K., Nur M. Traditional knowledge and formulations of medicinal plants used by the traditional medical practitioners of Bangladesh to treat schizophrenia like psychosis. Schizophr. Res. Treat. 2014;2014:679810. doi: 10.1155/2014/679810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rahmatullah M., Mollik M.A.H., Khatun M.A., Jahan R., Chowdhury A.R., Seraj S., Hossain M.S., Nasrin D., Khatun Z. A survey on the use of medicinal plants by folk medicinal practitioners in five villages of Boalia sub-district, Rajshahi district, Bangladesh. Adv. Nat. Appl. Sci. 2010;4:39–44. [Google Scholar]
- 67.Benkhnigue O., Zidane L., Fadli M., Elyacoubi H., Rochdi A., Douira A. Etude ethnobotanique des plantes médicinales dans la région de Mechraâ Bel Ksiri (Région du Gharb du Maroc) Acta Botánica Barcinonensia. 2010;53:191–216. [Google Scholar]
- 68.Kona S., Rahman A. Inventory of medicinal plants at Mahadebpur upazila of Naogaon district, Bangladesh. Appl. Ecol. Environ. Sci. 2016;4:75–83. [Google Scholar]
- 69.Rahmatullah M., Azam M.N.K., Rahman M.M., Seraj S., Mahal M.J., Mou S.M., Nasrin D., Khatun Z., Islam F., Chowdhury M.H. A survey of medicinal plants used by Garo and non-Garo traditional medicinal practitioners in two villages of Tangail district, Bangladesh. Am. Eurasian J. Sustain. Agric. 2011;5:350–357. [Google Scholar]
- 70.Ryz N.R., Remillard D.J., Russo E.B. Cannabis roots: A traditional therapy with future potential for treating inflammation and pain. Cannabis Cannabinoid Res. 2017;2:210–216. doi: 10.1089/can.2017.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bouarfa M., Lebtar S., Boukhira S., Bousta D. An ethnobotanical and ethnopharmacological survey of Cannabis sativa of Taounate Region in Northern Morocco. Int. J. Pharm. Sci. Rev. Res. 2020;64:116–122. doi: 10.47583/ijpsrr.2020.v64i02.019. [DOI] [Google Scholar]
- 72.Callaway J.C. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140:65–72. doi: 10.1007/s10681-004-4811-6. [DOI] [Google Scholar]
- 73.Carus M., Sarmento L. The European Hemp Industry: Cultivation, processing and applications for fibres, shivs, seeds and flowers. Eur. Ind. Hemp Assoc. 2016;5:1–9. [Google Scholar]
- 74.Turner B.D., Sloan S.W., Currell G.R. Novel remediation of per-and polyfluoroalkyl substances (PFASs) from contaminated groundwater using Cannabis sativa L.(hemp) protein powder. Chemosphere. 2019;229:22–31. doi: 10.1016/j.chemosphere.2019.04.139. [DOI] [PubMed] [Google Scholar]
- 75.Cosarca S., Rosca I., Coman A., Bota M.C.N., Tanase C. Effect of treatment with saline solution (NaCl) on rape plants in presence of the hemp shives. Rev. Chim. 2017;68:1843–1846. doi: 10.37358/RC.17.8.5777. [DOI] [Google Scholar]
- 76.Hussain W., Ullah M., Dastagir G., Badshah L. Quantitative ethnobotanical appraisal of medicinal plants used by inhabitants of lower Kurram, Kurram agency, Pakistan. Avicenna J. Phytomed. 2018;8:313. [PMC free article] [PubMed] [Google Scholar]
- 77.Knapp A.A., Lee D.C., Borodovsky J.T., Auty S.G., Gabrielli J., Budney A.J. Emerging trends in cannabis administration among adolescent cannabis users. J. Adolesc. Health. 2019;64:487–493. doi: 10.1016/j.jadohealth.2018.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chopra I., Chopra R.N. The use of the cannabis drugs in India. Bull. Narc. 1957;9:4–29. [Google Scholar]
- 79.Wood T.B., Spivey W.N., Easterfield T.H. III.—Cannabinol. Part I. J. Chem. Soc. Trans. 1899;75:20–36. doi: 10.1039/CT8997500020. [DOI] [Google Scholar]
- 80.Ross S.A., ElSohly H.N., ElKashoury E.A., ElSohly M.A. Fatty acids of cannabis seeds. Phytochem. Anal. 1996;7:279–283. doi: 10.1002/(SICI)1099-1565(199611)7:6<279::AID-PCA322>3.0.CO;2-P. [DOI] [Google Scholar]
- 81.Babiker E.E., Uslu N., Al Juhaimi F., Ahmed I.A.M., Ghafoor K., Özcan M.M., Almusallam I.A. Effect of roasting on antioxidative properties, polyphenol profile and fatty acids composition of hemp (Cannabis sativa L.) seeds. LWT. 2021;139:110537. doi: 10.1016/j.lwt.2020.110537. [DOI] [Google Scholar]
- 82.Irakli M., Tsaliki E., Kalivas A., Kleisiaris F., Sarrou E., Cook C.M. Effect οf genotype and growing year on the nutritional, phytochemical, and antioxidant properties of industrial hemp (Cannabis sativa L.) seeds. Antioxidants. 2019;8:491. doi: 10.3390/antiox8100491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Moccia S., Siano F., Russo G.L., Volpe M.G., La Cara F., Pacifico S., Piccolella S., Picariello G. Antiproliferative and antioxidant effect of polar hemp extracts (Cannabis sativa L., Fedora cv.) in human colorectal cell lines. Int. J. Food Sci. Nutr. 2020;71:410–423. doi: 10.1080/09637486.2019.1666804. [DOI] [PubMed] [Google Scholar]
- 84.Stambouli H., El Bouri A., Bouayoun T., Bellimam A. Caractérisation de l’huile de graines de Cannabis sativa L. cultivé au nord du Maroc. Ann. Toxicol. Anal. 2006;18:119–125. doi: 10.1051/ata:2006004. [DOI] [Google Scholar]
- 85.Aiello A., Pizzolongo F., Scognamiglio G., Romano A., Masi P., Romano R. Effects of supercritical and liquid carbon dioxide extraction on hemp (Cannabis sativa L.) seed oil. Int. J. Food Sci. Technol. 2020;55:2472–2480. doi: 10.1111/ijfs.14498. [DOI] [Google Scholar]
- 86.Nagy D.U., Cianfaglione K., Maggi F., Sut S., Dall’Acqua S. Chemical characterization of leaves, male and female flowers from spontaneous Cannabis (Cannabis sativa L.) growing in Hungary. Chem. Biodivers. 2019;16:e1800562. doi: 10.1002/cbdv.201800562. [DOI] [PubMed] [Google Scholar]
- 87.Zagórska-Dziok M., Bujak T., Ziemlewska A., Nizioł-Łukaszewska Z. Positive effect of Cannabis sativa L. herb extracts on skin cells and assessment of cannabinoid-based hydrogels properties. Molecules. 2021;26:802. doi: 10.3390/molecules26040802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Fasakin O.W., Oboh G., Ademosun A.O., Lawal A.O. The modulatory effects of alkaloid extracts of Cannabis sativa, Datura stramonium, Nicotiana tabacum and male Carica papaya on neurotransmitter, neurotrophic and neuroinflammatory systems linked to anxiety and depression. Inflammopharmacology. 2022;30:2447–2476. doi: 10.1007/s10787-022-01006-x. [DOI] [PubMed] [Google Scholar]
- 89.Guo T., Liu Q., Hou P., Li F., Guo S., Song W., Zhang H., Liu X., Zhang S., Zhang J. Stilbenoids and cannabinoids from the leaves of Cannabis sativa f. sativa with potential reverse cholesterol transport activity. Food Funct. 2018;9:6608–6617. doi: 10.1039/C8FO01896K. [DOI] [PubMed] [Google Scholar]
- 90.Laznik Ž., Košir I.J., Košmelj K., Murovec J., Jagodič A., Trdan S., Ačko D.K., Flajšman M. Effect of Cannabis sativa L. root, leaf and inflorescence ethanol extracts on the chemotrophic response of entomopathogenic nematodes. Plant Soil. 2020;455:367–379. doi: 10.1007/s11104-020-04693-z. [DOI] [Google Scholar]
- 91.Pieracci Y., Ascrizzi R., Terreni V., Pistelli L., Flamini G., Bassolino L., Fulvio F., Montanari M., Paris R. Essential Oil of Cannabis sativa L: Comparison of Yield and Chemical Composition of 11 Hemp Genotypes. Molecules. 2021;26:4080. doi: 10.3390/molecules26134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Gunjević V., Grillo G., Carnaroglio D., Binello A., Barge A., Cravotto G. Selective recovery of terpenes, polyphenols and cannabinoids from Cannabis sativa L. inflorescences under microwaves. Ind. Crops Prod. 2021;162:113247. doi: 10.1016/j.indcrop.2021.113247. [DOI] [Google Scholar]
- 93.Vanhoenacker G., Van Rompaey P., De Keukeleire D., Sandra P. Chemotaxonomic features associated with flavonoids of cannabinoid-free cannabis (Cannabis sativa subsp. sativa L.) in relation to hops (Humulus lupulus L.) Nat. Prod. Lett. 2010;16:57–63. doi: 10.1080/1057563029001/4863. [DOI] [PubMed] [Google Scholar]
- 94.Zengin G., Menghini L., Di Sotto A., Mancinelli R., Sisto F., Carradori S., Cesa S., Fraschetti C., Filippi A., Angiolella L. Chromatographic analyses, in vitro biological activities, and cytotoxicity of Cannabis sativa L. essential oil: A multidisciplinary study. Molecules. 2018;23:3266. doi: 10.3390/molecules23123266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Palmieri S., Pellegrini M., Ricci A., Compagnone D., Lo Sterzo C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods. 2020;9:1221. doi: 10.3390/foods9091221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Elkins A.C., Deseo M.A., Rochfort S., Ezernieks V., Spangenberg G. Development of a validated method for the qualitative and quantitative analysis of cannabinoids in plant biomass and medicinal cannabis resin extracts obtained by super-critical fluid extraction. J. Chromatogr. B. 2019;1109:76–83. doi: 10.1016/j.jchromb.2019.01.027. [DOI] [PubMed] [Google Scholar]
- 97.Stambouli H., El Bouri A., Bouayoun T. Évolution de la teneur en Δ9-THC dans les saisies de résines de cannabis au Maroc de 2005 à 2014. Toxicol. Anal. et Clin. 2016;28:146–152. doi: 10.1016/j.toxac.2015.11.001. [DOI] [Google Scholar]
- 98.ElSohly M.A. Marijuana and the Cannabinoids. Springer Science & Business Media; Berlin, Germany: 2007. [Google Scholar]
- 99.Flores-Sanchez I.J., Verpoorte R. Secondary metabolism in cannabis. Phytochem. Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- 100.Taaifi Y., Benmoumen A., Belhaj K., Aazza S., Abid M., Azeroual E., Elamrani A., Mansouri F., Serghini Caid H. Seed composition of non-industrial hemp (Cannabis sativa L.) varieties from four regions in northern Morocco. Int. J. Food Sci. Technol. 2021;56:5931–5947. doi: 10.1111/ijfs.15136. [DOI] [Google Scholar]
- 101.Da Porto C., Decorti D., Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012;36:401–404. doi: 10.1016/j.indcrop.2011.09.015. [DOI] [Google Scholar]
- 102.Appendino G., Gibbons S., Giana A., Pagani A., Grassi G., Stavri M., Smith E., Rahman M.M. Antibacterial cannabinoids from Cannabis sativa: A structure− activity study. J. Nat. Prod. 2008;71:1427–1430. doi: 10.1021/np8002673. [DOI] [PubMed] [Google Scholar]
- 103.Marzorati S., Friscione D., Picchi E., Verotta L. Cannabidiol from inflorescences of Cannabis sativa L.: Green extraction and purification processes. Ind. Crops Prod. 2020;155:112816. doi: 10.1016/j.indcrop.2020.112816. [DOI] [Google Scholar]
- 104.Stambouli H., El Bouri A., Bouayoun T., El Karni N., Naciri Z., Johar A., Saoura A., Saidi S. Expérimentation de la culture de chanvre industriel à fibres au Maroc. Ann. Toxicol. Anal. 2011;23:15–20. doi: 10.1051/ata/2011105. [DOI] [Google Scholar]
- 105.Jang E., Kim H., Jang S., Lee J., Baeck S., In S., Kim E., Kim Y.-U., Han E. Concentrations of THC, CBD, and CBN in commercial hemp seeds and hempseed oil sold in Korea. Forensic Sci. Int. 2020;306:110064. doi: 10.1016/j.forsciint.2019.110064. [DOI] [PubMed] [Google Scholar]
- 106.Deferne J.-L., Pate D.W. Hemp seed oil: A source of valuable essential fatty acids. J. Int. Hemp. Assoc. 1996;3:1–7. [Google Scholar]
- 107.Orhan İ., Küsmenoǧlu Ş., Şener B. GC-MS analysis of the seed oil of Cannabis sativa L. cultivated in Turkey. Gazi Univ. Eczaci. Fak. Derg. 2000;17:79–81. [Google Scholar]
- 108.Cunningham S.A., Summerhayes B., Westoby M. Evolutionary divergences in leaf structure and chemistry, comparing rainfall and soil nutrient gradients. Ecol. Monogr. 1999;69:569–588. doi: 10.1890/0012-9615(1999)069[0569:EDILSA]2.0.CO;2. [DOI] [Google Scholar]
- 109.Chik S.C., Or T.C., Luo D., Yang C.L., Lau A.S. Pharmacological effects of active compounds on neurodegenerative disease with gastrodia and uncaria decoction, a commonly used poststroke decoction. Sci. World J. 2013;2013:896873. doi: 10.1155/2013/896873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fischedick J.T., Hazekamp A., Erkelens T., Choi Y.H., Verpoorte R. Metabolic fingerprinting of Cannabis sativa L., cannabinoids and terpenoids for chemotaxonomic and drug standardization purposes. Phytochemistry. 2010;71:2058–2073. doi: 10.1016/j.phytochem.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 111.Pate D.W. Chemical ecology of Cannabis. J. Int. Hemp. Assoc. 1994;2:32–37. [Google Scholar]
- 112.Yang R., Berthold E.C., McCurdy C.R., da Silva Benevenute S., Brym Z.T., Freeman J.H. Development of cannabinoids in flowers of industrial hemp (Cannabis sativa L.): A pilot study. J. Agric. Food Chem. 2020;68:6058–6064. doi: 10.1021/acs.jafc.0c01211. [DOI] [PubMed] [Google Scholar]
- 113.Sakakibara I., Ikeya Y., Hayashi K., Mitsuhashi H. Three phenyldihydronaphthalene lignanamides from fruits of Cannabis sativa. Phytochemistry. 1992;31:3219–3223. doi: 10.1016/0031-9422(92)83479-I. [DOI] [Google Scholar]
- 114.Lesma G., Consonni R., Gambaro V., Remuzzi C., Roda G., Silvani A., Vece V., Visconti G. Cannabinoid-free Cannabis sativa L. grown in the Po valley: Evaluation of fatty acid profile, antioxidant capacity and metabolic content. Nat. Prod. Res. 2014;28:1801–1807. doi: 10.1080/14786419.2014.926354. [DOI] [PubMed] [Google Scholar]
- 115.Slatkin D.J., Doorenbos N.J., Harris L.S., Masoud A.N., Quimby M.W., Schiff P.L. Chemical constituents of Cannabis sativa L. root. J. Pharm. Sci. 1971;60:1891–1892. doi: 10.1002/jps.2600601232. [DOI] [PubMed] [Google Scholar]
- 116.Keller A., Leupin M., Mediavilla V., Wintermantel E. Influence of the growth stage of industrial hemp on chemical and physical properties of the fibres. Ind. Crops Prod. 2001;13:35–48. doi: 10.1016/S0926-6690(00)00051-0. [DOI] [Google Scholar]
- 117.Kushima H., Shoyama Y., Nishioka I. Cannabis. Xii. Variations of cannabinoid contents in several strains of Cannabis sativa L. with leaf-age, season and sex. Chem. Pharm. Bull. 1980;28:594–598. doi: 10.1248/cpb.28.594. [DOI] [Google Scholar]
- 118.Mechqoq H., El Yaagoubi M., Momchilova S., Msanda F., El Aouad N. Comparative study on yields and quality parameters of argan oils extracted by conventional and green extraction techniques. Grain Oil Sci. Technol. 2021;4:125–130. doi: 10.1016/j.gaost.2021.08.002. [DOI] [Google Scholar]
- 119.Copeland L.O., McDonald M.F. Principles of Seed Science and Technology. Springer Science & Business Media; Berlin, Germany: 2012. [Google Scholar]
- 120.Morris G.M., Lim-Wilby M. Molecular Modeling of Proteins. Springer; Berlin/Heidelberg, Germany: 2008. Molecular docking; pp. 365–382. [DOI] [PubMed] [Google Scholar]
- 121.Hourfane S., Mechqoq H., Errajouani F., Rocha J.M., El Aouad N. In Vitro and In Silico Evaluations of Boswellia carterii Resin Dermocosmetic Activities. Cosmetics. 2022;9:131. doi: 10.3390/cosmetics9060131. [DOI] [Google Scholar]
- 122.Kitchen D.B., Decornez H., Furr J.R., Bajorath J. Docking and scoring in virtual screening for drug discovery: Methods and applications. Nat. Rev. Drug Discov. 2004;3:935–949. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 123.Sousa S.F., Fernandes P.A., Ramos M.J. Protein–ligand docking: Current status and future challenges. Proteins Struct. Funct. Bioinform. 2006;65:15–26. doi: 10.1002/prot.21082. [DOI] [PubMed] [Google Scholar]
- 124.Chen G., Seukep A.J., Guo M. Recent advances in molecular docking for the research and discovery of potential marine drugs. Mar. Drugs. 2020;18:545. doi: 10.3390/md18110545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Grosdidier A., Zoete V., Michielin O. EADock: Docking of small molecules into protein active sites with a multiobjective evolutionary optimization. Proteins Struct. Funct. Bioinform. 2007;67:1010–1025. doi: 10.1002/prot.21367. [DOI] [PubMed] [Google Scholar]
- 126.Muegge I., Rarey M. Small molecule docking and scoring. Rev. Comput. Chem. 2001;17:1–60. [Google Scholar]
- 127.Karimi I., Yousofvand N., Hussein B.A. In vitro cholinesterase inhibitory action of Cannabis sativa L. Cannabaceae and in silico study of its selected phytocompounds. Silico Pharmacol. 2021;9:1–15. doi: 10.1007/s40203-021-00075-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Nasreen N., Niaz S., Khan A., Zaman M.A., Ayaz S., Naeem H., Khan N., Elgorban A.M. The potential of Allium sativum and Cannabis sativa extracts for anti-tick activities against Rhipicephalus (Boophilus) microplus. Exp. Appl. Acarol. 2020;82:281–294. doi: 10.1007/s10493-020-00540-z. [DOI] [PubMed] [Google Scholar]
- 129.Quan P.M., Toan T.Q., Hung N.P., Nam P.H., Kiet N.T., Ha N.X., Le D.T.T., An T.N.T., Show P.L., Thi H.H.P. Cannabis sativa L. chemical compositions as potential plasmodium falciparum dihydrofolate reductase-thymidinesynthase enzyme inhibitors: An in silico study for drug development. Open Chem. 2021;19:1244–1250. doi: 10.1515/chem-2021-0102. [DOI] [Google Scholar]
- 130.Ogungbe I.V., Erwin W.R., Setzer W.N. Antileishmanial phytochemical phenolics: Molecular docking to potential protein targets. J. Mol. Graph. Model. 2014;48:105–117. doi: 10.1016/j.jmgm.2013.12.010. [DOI] [PubMed] [Google Scholar]
- 131.Srivastava A.K., Kumar A., Misra N. On the inhibition of COVID-19 protease by Indian herbal plants: An in silico investigation. arXiv. 20202004.03411 [Google Scholar]
- 132.Akhtar A., Hussain W., Rasool N. Probing the pharmacological binding properties, and reactivity of selective phytochemicals as potential HIV-1 protease inhibitors. Univ. Sci. 2019;24:441–464. doi: 10.11144/Javeriana.SC24-3.artf. [DOI] [Google Scholar]
- 133.Nouadi B., Ezaouine A., El Messal M., Blaghen M., Bennis F., Chegdani F. Prediction of anti-COVID 19 therapeutic power of medicinal Moroccan plants using molecular docking. Bioinform. Biol. Insights. 2021;15:11779322211009199. doi: 10.1177/11779322211009199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ngo S.T., Quynh Anh Pham N., Thi Le L., Pham D.-H., Vu V.V. Computational determination of potential inhibitors of SARS-CoV-2 main protease. J. Chem. Inf. Model. 2020;60:5771–5780. doi: 10.1021/acs.jcim.0c00491. [DOI] [PubMed] [Google Scholar]
- 135.Tallei T.E., Tumilaar S.G., Niode N.J., Kepel B.J., Idroes R., Effendi Y., Sakib S.A., Emran T.B. Potential of plant bioactive compounds as SARS-CoV-2 main protease (Mpro) and spike (S) glycoprotein inhibitors: A molecular docking study. Scientifica. 2020;2020:6307457. doi: 10.1155/2020/6307457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ma H., Xu F., Liu C., Seeram N.P. A Network Pharmacology Approach to Identify Potential Molecular Targets for Cannabidiol’s Anti-Inflammatory Activity. Cannabis Cannabinoid Res. 2021;6:288–299. doi: 10.1089/can.2020.0025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.TÜZÜN B. Investigation of the molecules obtained from marijuana: Computational study of spectral, structural and docking. J. Phys. Theor. Chem. (IAU Iran) 2020;16:59–74. [Google Scholar]
- 138.Baroi S., Saha A., Bachar R., Bachar S.C. Cannabinoid as potential aromatase inhibitor through molecular modeling and screening for anti-cancer activity. Dhaka Univ. J. Pharm. Sci. 2020;19:47–58. doi: 10.3329/dujps.v19i1.47818. [DOI] [Google Scholar]
- 139.Metibemu D.S. 3D-QSAR and Molecular Docking Approaches for the Identification of Phyto-Inhibitors of Hsp90. LIANBS. 2022;11:3871–3886. [Google Scholar]
- 140.Adeniran O.Y., Ayorinde O., Boboye S.O. Virtual high-throughput screening (VHTS), three-dimensional quantitative structure-activity and relationship (3D-QSAR) and molecular docking studies of novel phyto-inhibtors of topoisomerase II alpha. GSC Biol. Pharm. Sci. 2021;15:072–082. doi: 10.30574/gscbps.2021.15.2.0099. [DOI] [Google Scholar]
- 141.Zaka M., Sehgal S.A., Shafique S., Abbasi B.H. Comparative in silico analyses of Cannabis sativa, Prunella vulgaris and Withania somnifera compounds elucidating the medicinal properties against rheumatoid arthritis. J. Mol. Graph. Model. 2017;74:296–304. doi: 10.1016/j.jmgm.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 142.Bouchentouf S., Ghalem S., Allali H., Missoum N. Investigating the Inhibition of 5-LO Enzyme by Main Cannabinoids Contained in Cannabis safiva and Seized Resin Cannabis by Using Molecular Modeling. Insights Enzym. Res. 2017;1:10. [Google Scholar]
- 143.Gao J., Li T., Chen D., Gu H., Mao X. Identification and molecular docking of antioxidant peptides from hemp seed protein hydrolysates. Lwt. 2021;147:111453. doi: 10.1016/j.lwt.2021.111453. [DOI] [Google Scholar]
- 144.Girgih A.T., He R., Aluko R.E. Kinetics and molecular docking studies of the inhibitions of angiotensin converting enzyme and renin activities by hemp seed (Cannabis sativa L.) peptides. J. Agric. Food Chem. 2014;62:4135–4144. doi: 10.1021/jf5002606. [DOI] [PubMed] [Google Scholar]
- 145.Ngamsuk S., Huang T.-C., Hsu J.-L. ACE inhibitory activity and molecular docking of gac seed protein hydrolysate purified by HILIC and RP-HPLC. Molecules. 2020;25:4635. doi: 10.3390/molecules25204635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li Y., Ding Y., Xiao W., Zhu J.-B. Investigation on the active ingredient and mechanism of Cannabis sativa L. for treating epilepsy based on network pharmacology. Biotechnol. Biotechnol. Equip. 2021;35:994–1009. doi: 10.1080/13102818.2021.1942208. [DOI] [Google Scholar]
- 147.di Giacomo V., Chiavaroli A., Recinella L., Orlando G., Cataldi A., Rapino M., Di Valerio V., Ronci M., Leone S., Brunetti L. Antioxidant and neuroprotective effects induced by cannabidiol and cannabigerol in rat CTX-TNA2 astrocytes and isolated cortexes. Int. J. Mol. Sci. 2020;21:3575. doi: 10.3390/ijms21103575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sarkar I., Sen G., Bhattacharya M., Bhattacharyya S., Sen A. In silico inquest reveals the efficacy of Cannabis in the treatment of post-Covid-19 related neurodegeneration. J. Biomol. Struct. Dyn. 2022;40:8030–8039. doi: 10.1080/07391102.2021.1905556. [DOI] [PubMed] [Google Scholar]
- 149.Onoda T., Li W., Sasaki T., Miyake M., Higai K., Koike K. Identification and evaluation of magnolol and chrysophanol as the principle protein tyrosine phosphatase-1B inhibitory compounds in a Kampo medicine, Masiningan. J. Ethnopharmacol. 2016;186:84–90. doi: 10.1016/j.jep.2016.03.063. [DOI] [PubMed] [Google Scholar]
- 150.Kazemi F., Karimi I., Yousofvand N. Molecular docking study of lignanamides from Cannabis sativa against P-glycoprotein. Silico Pharmacol. 2021;9:1–7. doi: 10.1007/s40203-020-00066-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Holgado M., Martín-Banderas L., Álvarez-Fuentes J., Fernández-Arévalo M. Neuroprotective effect of cannabinoids nanoplatforms in neurodegenerative diseases. J. Drug Deliv. Sci. Technol. 2017;42:84–93. doi: 10.1016/j.jddst.2017.04.023. [DOI] [Google Scholar]
- 152.Marsh D.T., Smid S.D. Cannabis Phytochemicals: A Review of Phytocannabinoid Chemistry and Bioactivity as Neuroprotective Agents. Aust. J. Chem. 2020;74:388–404. doi: 10.1071/CH20183. [DOI] [Google Scholar]
- 153.Dreno B., Araviiskaia E., Berardesca E., Bieber T., Hawk J., Sanchez-Viera M., Wolkenstein P. The science of dermocosmetics and its role in dermatology. J. Eur. Acad. Dermatol. Venereol. 2014;28:1409–1417. doi: 10.1111/jdv.12497. [DOI] [PubMed] [Google Scholar]
- 154.Kim J.K., Heo H.-Y., Park S., Kim H., Oh J.J., Sohn E.-H., Jung S.-H., Lee K. Characterization of Phenethyl Cinnamamide Compounds from Hemp Seed and Determination of Their Melanogenesis Inhibitory Activity. ACS Omega. 2021;6:31945–31954. doi: 10.1021/acsomega.1c04727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Manosroi A., Chankhampan C., Kietthanakorn B.O., Ruksiriwanich W., Chaikul P., Boonpisuttinant K., Sainakham M., Manosroi W., Tangjai T., Manosroi J. Pharmaceutical and cosmeceutical biological activities of hemp (Cannabis sativa L. var. sativa) leaf and seed extracts. Chiang Mai J. Sci. 2019;46:180–195. [Google Scholar]
- 156.Yan X., Tang J., dos Santos Passos C., Nurisso A., Simoes-Pires C.A., Ji M., Lou H., Fan P. Characterization of lignanamides from hemp (Cannabis sativa L.) seed and their antioxidant and acetylcholinesterase inhibitory activities. J. Agric. Food Chem. 2015;63:10611–10619. doi: 10.1021/acs.jafc.5b05282. [DOI] [PubMed] [Google Scholar]
- 157.Teh S.-S., Bekhit A.E.-D.A., Carne A., Birch J. Antioxidant and ACE-inhibitory activities of hemp (Cannabis sativa L.) protein hydrolysates produced by the proteases AFP, HT, Pro-G, actinidin and zingibain. Food Chem. 2016;203:199–206. doi: 10.1016/j.foodchem.2016.02.057. [DOI] [PubMed] [Google Scholar]
- 158.Frassinetti S., Gabriele M., Moccia E., Longo V., Di Gioia D. Antimicrobial and antibiofilm activity of Cannabis sativa L. seeds extract against Staphylococcus aureus and growth effects on probiotic Lactobacillus spp. Lwt. 2020;124:109149. doi: 10.1016/j.lwt.2020.109149. [DOI] [Google Scholar]
- 159.Anjum M. 35. Evaluation of antimicrobial activity and ethnobotanical study of Cannabis sativa L. Pure Appl. Biol. (PAB) 2018;7:706–713. [Google Scholar]
- 160.Coetzee C., Levendal R.-A., Van de Venter M., Frost C. Anticoagulant effects of a Cannabis extract in an obese rat model. Phytomedicine. 2007;14:333–337. doi: 10.1016/j.phymed.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 161.Al Khoury A., Sleiman R., Atoui A., Hindieh P., Maroun R.G., Bailly J.-D., El Khoury A. Antifungal and anti-aflatoxigenic properties of organs of Cannabis sativa L.: Relation to phenolic content and antioxidant capacities. Arch. Microbiol. 2021;203:4485–4492. doi: 10.1007/s00203-021-02444-x. [DOI] [PubMed] [Google Scholar]
- 162.Rossi P., Cappelli A., Marinelli O., Valzano M., Pavoni L., Bonacucina G., Petrelli R., Pompei P., Mazzara E., Ricci I. Mosquitocidal and anti-inflammatory properties of the essential oils obtained from monoecious, male, and female inflorescences of hemp (Cannabis sativa L.) and their encapsulation in nanoemulsions. Molecules. 2020;25:3451. doi: 10.3390/molecules25153451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Nafis A., Kasrati A., Jamali C.A., Mezrioui N., Setzer W., Abbad A., Hassani L. Antioxidant activity and evidence for synergism of Cannabis sativa (L.) essential oil with antimicrobial standards. Ind. Crops Prod. 2019;137:396–400. doi: 10.1016/j.indcrop.2019.05.032. [DOI] [Google Scholar]
- 164.Mechqoq H., Hourfane S., Yaagoubi M.E., Hamdaoui A.E., Msanda F., Almeida J.R.G.d.S., Rocha J.M., Aouad N.E. Phytochemical Screening, and In Vitro Evaluation of the Antioxidant and Dermocosmetic Activities of Four Moroccan Plants: Halimium antiatlanticum, Adenocarpus artemisiifolius, Pistacia lentiscus and Leonotis nepetifolia. Cosmetics. 2022;9:94. doi: 10.3390/cosmetics9050094. [DOI] [Google Scholar]
- 165.Mirzamohammad E., Alirezalu A., Alirezalu K., Norozi A., Ansari A. Improvement of the antioxidant activity, phytochemicals, and cannabinoid compounds of Cannabis sativa by salicylic acid elicitor. Food Sci. Nutr. 2021;9:6873–6881. doi: 10.1002/fsn3.2643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Saura-Calixto F., Serrano J., Pérez-Jiménez J. Beer in Health and Disease Prevention. Elsevier; Amsterdam, The Netherlands: 2009. What contribution is beer to the intake of antioxidants in the diet; pp. 441–448. [Google Scholar]
- 167.Shahidi F., Janitha P., Wanasundara P. Phenolic antioxidants. Crit. Rev. Food Sci. Nutr. 1992;32:67–103. doi: 10.1080/10408399209527581. [DOI] [PubMed] [Google Scholar]
- 168.Smeriglio A., Alloisio S., Raimondo F.M., Denaro M., Xiao J., Cornara L., Trombetta D. Essential oil of Citrus lumia Risso: Phytochemical profile, antioxidant properties and activity on the central nervous system. Food Chem. Toxicol. 2018;119:407–416. doi: 10.1016/j.fct.2017.12.053. [DOI] [PubMed] [Google Scholar]
- 169.Novak J., Zitterl-Eglseer K., Deans S.G., Franz C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001;16:259–262. doi: 10.1002/ffj.993. [DOI] [Google Scholar]
- 170.El Hamdaoui A., Msanda F., Boubaker H., Leach D., Bombarda I., Vanloot P., El Aouad N., Abbad A., Boudyach E., Achemchem F. Essential oil composition, antioxidant and antibacterial activities of wild and cultivated Lavandula mairei Humbert. Biochem. Syst. Ecol. 2018;76:1–7. doi: 10.1016/j.bse.2017.11.004. [DOI] [Google Scholar]
- 171.Prabuseenivasan S., Jayakumar M., Ignacimuthu S. In vitro antibacterial activity of some plant essential oils. BMC Complement. Altern. Med. 2006;6:1–8. doi: 10.1186/1472-6882-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.El Yaagoubi M., Mechqoq H., Ortiz S., Cavaleiro C., Lecsö-Bornet M., Pereira C.G., Rodrigues M.J., Custódio L., El Mousadik A., Picot L. Chemical Composition and Biological Screening of the Essential Oils of Micromeria macrosiphon and M. arganietorum (Lamiaceae) Chem. Biodivers. 2021;18:e2100653. doi: 10.1002/cbdv.202100653. [DOI] [PubMed] [Google Scholar]
- 173.Xiong W.-T., Gu L., Wang C., Sun H.-X., Liu X. Anti-hyperglycemic and hypolipidemic effects of Cistanche tubulosa in type 2 diabetic db/db mice. J. Ethnopharmacol. 2013;150:935–945. doi: 10.1016/j.jep.2013.09.027. [DOI] [PubMed] [Google Scholar]
- 174.Agarwal P., Gupta R. Alpha-amylase inhibition can treat diabetes mellitus. Res. Rev. J. Med. Health Sci. 2016;5:1–8. [Google Scholar]
- 175.Kajaria D., Tripathi J., Tripathi Y.B., Tiwari S. In-vitro α amylase and glycosidase inhibitory effect of ethanolic extract of antiasthmatic drug—Shirishadi. J. Adv. Pharm. Technol. Res. 2013;4:206. doi: 10.4103/2231-4040.121415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ligresti A., Moriello A.S., Starowicz K., Matias I., Pisanti S., De Petrocellis L., Laezza C., Portella G., Bifulco M., Di Marzo V. Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma. J. Pharmacol. Exp. Ther. 2006;318:1375–1387. doi: 10.1124/jpet.106.105247. [DOI] [PubMed] [Google Scholar]
- 177.Sarfaraz S., Afaq F., Adhami V.M., Malik A., Mukhtar H. Cannabinoid receptor agonist-induced apoptosis of human prostate cancer cells LNCaP proceeds through sustained activation of ERK1/2 leading to G1 cell cycle arrest. J. Biol. Chem. 2006;281:39480–39491. doi: 10.1074/jbc.M603495200. [DOI] [PubMed] [Google Scholar]
- 178.Lukhele S.T., Motadi L.R. Cannabidiol rather than Cannabis sativa extracts inhibit cell growth and induce apoptosis in cervical cancer cells. BMC Complement. Altern. Med. 2016;16:1–16. doi: 10.1186/s12906-016-1280-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Marcu J.P., Christian R.T., Lau D., Zielinski A.J., Horowitz M.P., Lee J., Pakdel A., Allison J., Limbad C., Moore D.H. Cannabidiol Enhances the Inhibitory Effects of Δ9-Tetrahydrocannabinol on Human Glioblastoma Cell Proliferation and SurvivalCannabinoid Synergy Inhibits Glioblastoma Cell Growth. Mol. Cancer Ther. 2010;9:180–189. doi: 10.1158/1535-7163.MCT-09-0407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Cianchi F., Papucci L., Schiavone N., Lulli M., Magnelli L., Vinci M.C., Messerini L., Manera C., Ronconi E., Romagnani P. Cannabinoid receptor activation induces apoptosis through tumor necrosis factor α–mediated ceramide de novo synthesis in colon cancer cells. Clin. Cancer Res. 2008;14:7691–7700. doi: 10.1158/1078-0432.CCR-08-0799. [DOI] [PubMed] [Google Scholar]
- 181.McKallip R.J., Lombard C., Fisher M., Martin B.R., Ryu S., Grant S., Nagarkatti P.S., Nagarkatti M. Targeting CB2 cannabinoid receptors as a novel therapy to treat malignant lymphoblastic disease. Blood J. Am. Soc. Hematol. 2002;100:627–634. doi: 10.1182/blood-2002-01-0098. [DOI] [PubMed] [Google Scholar]
- 182.Armstrong J.L., Hill D.S., McKee C.S., Hernandez-Tiedra S., Lorente M., Lopez-Valero I., Anagnostou M.E., Babatunde F., Corazzari M., Redfern C.P. Exploiting cannabinoid-induced cytotoxic autophagy to drive melanoma cell death. J. Investig. Dermatol. 2015;135:1629–1637. doi: 10.1038/jid.2015.45. [DOI] [PubMed] [Google Scholar]
- 183.Borrelli F., Pagano E., Romano B., Panzera S., Maiello F., Coppola D., De Petrocellis L., Buono L., Orlando P., Izzo A.A. Colon carcinogenesis is inhibited by the TRPM8 antagonist cannabigerol, a Cannabis-derived non-psychotropic cannabinoid. Carcinogenesis. 2014;35:2787–2797. doi: 10.1093/carcin/bgu205. [DOI] [PubMed] [Google Scholar]
- 184.Galanti G., Fisher T., Kventsel I., Shoham J., Gallily R., Mechoulam R., Lavie G., Amariglio N., Rechavi G., Toren A. Δ9-Tetrahydrocannabinol inhibits cell cycle progression by downregulation of E2F1 in human glioblastoma multiforme cells. Acta Oncol. 2008;47:1062–1070. doi: 10.1080/02841860701678787. [DOI] [PubMed] [Google Scholar]
- 185.Galve-Roperh I., Sánchez C., Cortés M.L., del Pulgar T.G., Izquierdo M., Guzmán M. Anti-tumoral action of cannabinoids: Involvement of sustained ceramide accumulation and extracellular signal-regulated kinase activation. Nat. Med. 2000;6:313–319. doi: 10.1038/73171. [DOI] [PubMed] [Google Scholar]
- 186.McAllister S.D., Murase R., Christian R.T., Lau D., Zielinski A.J., Allison J., Almanza C., Pakdel A., Lee J., Limbad C. Pathways mediating the effects of cannabidiol on the reduction of breast cancer cell proliferation, invasion, and metastasis. Breast Cancer Res. Treat. 2011;129:37–47. doi: 10.1007/s10549-010-1177-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Solinas M., Massi P., Cinquina V., Valenti M., Bolognini D., Gariboldi M., Monti E., Rubino T., Parolaro D. Cannabidiol, a non-psychoactive cannabinoid compound, inhibits proliferation and invasion in U87-MG and T98G glioma cells through a multitarget effect. PLoS ONE. 2013;8:e76918. doi: 10.1371/journal.pone.0076918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Klein T.W. Cannabinoid-based drugs as anti-inflammatory therapeutics. Nat. Rev. Immunol. 2005;5:400–411. doi: 10.1038/nri1602. [DOI] [PubMed] [Google Scholar]
- 189.Nagarkatti P., Pandey R., Rieder S.A., Hegde V.L., Nagarkatti M. Cannabinoids as novel anti-inflammatory drugs. Future Med. Chem. 2009;1:1333–1349. doi: 10.4155/fmc.09.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wood C., Duparc N., Leblanc V., Cunin-Roy C. L’hypnose et la douleur. Médecine Clin. Pour Les Pédiatres. 2004;11:40–44. [Google Scholar]
- 191.Munch G. Le Cannabis, Les Deux Versants: Drogue et Médicament. Université de Lorraine; Lorraine, France: 2015. [Google Scholar]
- 192.Jeannin C. Master Thesis. University of Liege; Liege, Belgium: 2020. Évaluation et Prise en Charge de la Douleur Chez le Lapin de Compagnie: Comment les Optimiser en L’état Actuel des Connaissances? [Google Scholar]
- 193.Li J., Wang G., Qin Y., Zhang X., Wang H.-F., Liu H.-W., Zhu L.-J., Yao X.-S. Neuroprotective constituents from the aerial parts of Cannabis sativa L. subsp. sativa. RSC Adv. 2020;10:32043–32049. doi: 10.1039/D0RA04565A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Landucci E., Mazzantini C., Lana D., Davolio P.L., Giovannini M.G., Pellegrini-Giampietro D.E. Neuroprotective effects of cannabidiol but not Δ9-Tetrahydrocannabinol in rat hippocampal slices exposed to oxygen-glucose deprivation: Studies with Cannabis extracts and selected cannabinoids. Int. J. Mol. Sci. 2021;22:9773. doi: 10.3390/ijms22189773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Esposito G., Scuderi C., Savani C., Steardo Jr L., De Filippis D., Cottone P., Iuvone T., Cuomo V., Steardo L. Cannabidiol in vivo blunts β-amyloid induced neuroinflammation by suppressing IL-1β and iNOS expression. Br. J. Pharmacol. 2007;151:1272–1279. doi: 10.1038/sj.bjp.0707337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Perez M., Benitez S.U., Cartarozzi L.P., Del Bel E., Guimaraes F.S., Oliveira A.L. Neuroprotection and reduction of glial reaction by cannabidiol treatment after sciatic nerve transection in neonatal rats. Eur. J. Neurosci. 2013;38:3424–3434. doi: 10.1111/ejn.12341. [DOI] [PubMed] [Google Scholar]
- 197.Friedman L.K., Wongvravit J.P. Anticonvulsant and neuroprotective effects of cannabidiol during the juvenile period. J. Neuropathol. Exp. Neurol. 2018;77:904–919. doi: 10.1093/jnen/nly069. [DOI] [PubMed] [Google Scholar]
- 198.Devinsky O., Marsh E., Friedman D., Thiele E., Laux L., Sullivan J., Miller I., Flamini R., Wilfong A., Filloux F. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet Neurol. 2016;15:270–278. doi: 10.1016/S1474-4422(15)00379-8. [DOI] [PubMed] [Google Scholar]
- 199.Farrelly A.M., Vlachou S., Grintzalis K. Efficacy of phytocannabinoids in epilepsy treatment: Novel approaches and recent advances. Int. J. Environ. Res. Public Health. 2021;18:3993. doi: 10.3390/ijerph18083993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Stone N.L., Murphy A.J., England T.J., O’Sullivan S.E. A systematic review of minor phytocannabinoids with promising neuroprotective potential. Br. J. Pharmacol. 2020;177:4330–4352. doi: 10.1111/bph.15185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Dunn L.B., Damesyn M., Moore A.A., Reuben D.B., Greendale G.A. Does estrogen prevent skin aging?: Results from the first National Health and Nutrition Examination Survey (NHANES I) Arch. Dermatol. 1997;133:339–342. doi: 10.1001/archderm.1997.03890390077010. [DOI] [PubMed] [Google Scholar]
- 202.Gaisey J., Narouze S.N. Cannabinoids and Pain. Springer; Berlin/Heidelberg, Germany: 2021. Dronabinol (Marinol®) pp. 105–107. [Google Scholar]
- 203.Abuhasira R., Shbiro L., Landschaft Y. Medical use of cannabis and cannabinoids containing products–Regulations in Europe and North America. Eur. J. Intern. Med. 2018;49:2–6. doi: 10.1016/j.ejim.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 204.Babson K.A., Sottile J., Morabito D. Cannabis, cannabinoids, and sleep: A review of the literature. Curr. Psychiatry Rep. 2017;19:1–12. doi: 10.1007/s11920-017-0775-9. [DOI] [PubMed] [Google Scholar]
- 205.Sekar K., Pack A. Epidiolex as adjunct therapy for treatment of refractory epilepsy: A comprehensive review with a focus on adverse effects. F1000Research. 2019;8:8. doi: 10.12688/f1000research.16515.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.