The SARS-CoV-2 omicron lineage (B.1.1.529) continues to proliferate and evolve, leading to subvariants adept at evading antibody responses.1 Bivalent mRNA vaccines expressing both the omicron BA.5 spike and the ancestral D614G spike were introduced in August, 2022 with the goal of boosting waning antibody titres and broadening coverage against emerging SARS-CoV-2 lineages.2 However, in early 2023, we and others reported that peak serum neutralising antibody (NAb) titres against SARS-CoV-2 variants following a bivalent vaccine booster were similar to peak titres following a monovalent booster.3, 4 Whether these antibody responses would diverge over time remains unknown.
We addressed this question by assessing serum virus-neutralising titres in 41 participants who received three monovalent mRNA vaccines followed by a bivalent booster, a monovalent booster, or a BA.5 breakthrough infection (appendix p 6). We collected serum samples at nearly 1 month and approximately 3 months following the last vaccine dose or breakthrough infection (appendix p 7) and determined their NAb titres using a pseudovirus neutralisation assay4 against the ancestral D614G strain and a panel of omicron subvariants (BA.2, BA.5, BQ.1·1, and XBB.1·5; appendix pp 2–3). Participants who received a monovalent booster were older (mean 55·3 years) than those who received a bivalent booster (mean 37·8 years) or those who had a breakthrough infection (mean 44·0 years; appendix p 7).
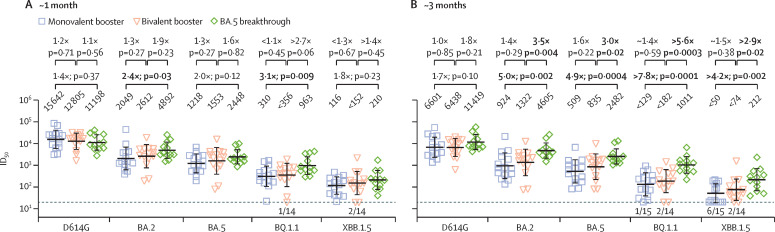
Each cohort exhibited the highest serum NAb titres (ie, the serum dilution at which 50% viral neutralisation occurs [ID50]) against D614G and substantially lower titres against the latest omicron subvariants. Consistent with our previous study,4 there was no significant difference at nearly 1 month after the last booster for the two vaccine cohorts (figure A ). At approximately 3 months after the last booster there were, again, no statistical differences between the two groups (figure B), although there was a trend toward higher titres in the bivalent group against omicron lineages (1·4–1·6 times higher; eg, 509 VS 835 against BA.5). The BA.5 breakthrough cohort exhibited significantly higher NAb titres at 3 months against all tested omicron subvariants when compared with both monovalent and bivalent booster cohorts (figure B). Over the approximately 2-month follow-up period, mean NAb titres in both vaccine cohorts decreased approximately two-fold (or 50%) against all tested viruses (eg, 1218 to 509 for monovalent cohorts against BA.5 and 1553 to 835 for bivalent cohorts against BA.5; appendix p 8), consistent with findings published in 2023.5 There was no discernible waning of antibody responses in the BA.5 breakthrough infection cases over the same observation period. This finding suggests that our vaccine cohorts were not affected by undetected breakthrough infections.
Figure.
SARS-CoV-2 NAb responses following bivalent or monovalent booster vaccination or BA.5 breakthrough infection
Bold values indicate significant differences. The number of samples at or below the assay limit of detection (ie, the dotted line) is denoted above the x-axis. (A) Peak serum-neutralising ID50 titres at nearly 1 month after a monovalent booster, bivalent booster, or BA.5 breakthrough. (B) Serum-neutralising ID50 titres at ∼ 3 months for the three cohorts.
These new results further strengthen our initial suggestion that boosting with the bivalent mRNA vaccines is not evidently better than boosting with the original monovalent vaccine, as judged by serum SARS-CoV-2-neutralising potency and breadth. Perhaps the inclusion of the ancestral spike in the bivalent vaccine exacerbates the challenge posed by immunological imprinting. Hope remains that a second bivalent vaccine booster could induce a superior NAb response against current and future viral variants.
QW and AB contributed equally. AG, LL, and DDH were joint senior authors. DDH is a cofounder of TaiMed Biologics and RenBio and a board director for Vicarious Surgical. DDH also serves as a consultant to WuXi Biologics, Brii Biosciences, and Very, and receives funding from Regeneron. AG is a member of a scientific advisory board for Janssen Pharmaceuticals. All other authors declare no competing interests.
Supplementary Material
References
- 1.Wang Q, Iketani S, Li Z, et al. Alarming antibody evasion properties of rising SARS-CoV-2 BQ and XBB subvariants. Cell. 2023;186:279–286. doi: 10.1016/j.cell.2022.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.US Food and Drug Administration Coronavirus (COVID-19) update: FDA authorizes Moderna, Pfizer-BioNTech bivalent COVID-19 vaccines for use as a booster dose. Aug 31, 2023. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-moderna-pfizer-biontech-bivalent-covid-19-vaccines-use
- 3.Collier AY, Miller J, Hachmann NP, et al. Immunogenicity of BA.5 bivalent mRNA vaccine boosters. N Engl J Med. 2023;388:565–567. doi: 10.1056/NEJMc2213948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang Q, Bowen A, Valdez R, et al. Antibody response to omicron BA.4-BA.5 bivalent booster. N Engl J Med. 2023;388:567–569. doi: 10.1056/NEJMc2213907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lasrado N, Collier AY, Miller J, et al. Waning immunity against XBB.1·5 following bivalent mRNA boosters. bioRxiv. 2023 doi: 10.1101/2023.01.22.525079. published online Jan 23. (preprint). [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.