Abstract
Eugenol essential oil (EEO) is the major component in aromatic extracts of Syzygium aromaticum (clove) and has several biological properties, such as antibacterial, antioxidant, and anti-inflammatory activities, as well as controlling vomiting, coughing, nausea, flatulence, diarrhea, dyspepsia, stomach distension, and gastrointestinal spasm pain. It also stimulates the nerves. Therefore, the aim of this study was to extract and purify EEO from clove buds and assess its ability to combat resistant Helicobacter pylori. Additionally, EEO’s anti-inflammatory activity and its ability to suppress H. pylori biofilm formation, which is responsible for antibiotic resistance, was also investigated. Syzygium aromaticum buds were purchased from a local market, ground, and the EEO was extracted by using hydro-distillation and then purified and chemically characterized using gas chromatography–mass spectrometry (GC–MS). A disk-diffusion assay showed that Helicobacter pylori is sensitive to EEO, with an inhibition zone ranging from 10 ± 06 to 22 ± 04 mm. The minimum inhibition concentration (MIC) of EEO ranged from 23.0 to 51.0 μg/mL against both Helicobacter pylori clinical isolates and standard strains. In addition, EEO showed antibiofilm activity at 25 µg/mL and 50 µg/mL against various Helicobacter pylori strains, with suppression percentages of 49.32% and 73.21%, respectively. The results obtained from the anti-inflammatory assay revealed that EEO possesses strong anti-inflammatory activity, with human erythrocyte hemolysis inhibition percentages of 53.04, 58.74, 61.07, and 63.64% at concentrations of 4, 8, 16, and 32 μg/L, respectively. GC–MS analysis revealed that EEO is a major component of Syzygium aromaticum when extracted with a hydro-distillation technique, which was confirmed by its purification using a chemical separation process. EEO exhibited antibacterial action against resistant Helicobacter pylori strains, as well as antibiofilm and anti-inflammatory activities, and is a promising natural alternative in clinical therapy.
Keywords: eugenol, extraction, purification, Anti-H. pylori, anti-biofilm, anti-inflammatory, S. aromaticum, GC–MS main strategies
1. Introduction
Helicobacter pylori (H. pylori) is an important pathogenic microbe in the gastrointestinal tract that colonizes the mucus layer of the stomach in about 50% of humans worldwide and causes chronic gastritis, peptic ulcers, and gastric cancer. The increased rate of H. pylori resistance to antibiotics has created a dangerous situation. The eradication of H. pylori is an important goal in combating gastric diseases. Several regimens are currently available, but none of them can accomplish the eradication of H. pylori and the inflammation it causes. For this reason, the search for alternative and effective new therapeutic methods is critical [1]. Natural products of plant origin have been used throughout history as therapeutic bioresources and are described in texts from many nations. In ancient civilizations, plant food was used for medical therapy. Using natural products in therapeutic management against microbial infections such as H. pylori has advantages over therapies from synthetic sources. They are often chosen because they have fewer side effects when their toxicological and pharmacological activities are compared to those derived from chemical sources [2]. The decreased toxicity of natural products has prompted interest in the pharmacological activities that these products have against H. pylori. In the last decade, various studies have assessed the use of plants and plant extracts and constituents as gastroprotective agents against H. pylori activity [3]. Syzygium aromaticum, known as clove, is from the family Myrtaceae and has been shown to possess several biological activities [4]. S. aromaticum is a rich source of bioactive substances and has several therapeutic properties, including the control of vomiting, nausea, cough, flatulence, dyspepsia, diarrhea, stomach distension, relieving gastrointestinal spasm pain, relieving uterine contractions, and possessing anti-inflammatory activity [1,5,6]. Eugenol, a phenolic compound, is the main constituent of Syzygium aromaticum extract. It has antioxidant activity [7], includes a monoamine oxidase inhibitor, and has neuroprotective activity [8]. Additionally, eugenol exhibits excellent bactericidal activity against a broad range of bacteria, such as Staphylococcus aureus, Escherichia coli, H. pylori [1,9] and Listeria monocytogenes [10]. Moreover, some studies have suggested that the mode of antibacterial action of eugenol is through the disruption of the cytoplasmic membrane by increasing its non-specific permeability. Additionally, the hydrophobic nature of eugenol molecules helps to penetrate the lipopolysaccharide in the cell wall of Gram-negative bacteria and changes their cell structure, resulting in the infiltration of intracellular constituents [1,11,12]. Burt [11] revealed that the hydroxyl group on eugenol is associated with proteins that inhibit enzyme action in the bacterial cell. The antibacterial activity of several natural products has been reported against H. pylori. Eugenol is a major component of methanolic extracts of S. aromaticum and exhibits highly significant antibacterial activity against resistant strains of H. pylori [13]. Furthermore, eugenol prevents the growth of all 30 H. pylori strains at a concentration of 2 μg/mL. Additionally, H. pylori did not develop any resistance toward eugenol even after 10 passages grown at sub-inhibitory concentrations [14]. We aimed to extract the eugenol from an S. aromaticum extract and investigate its antibacterial, antibiofilm and anti-inflammatory activities against resistant H. pylori.
2. Results
2.1. Antibacterial Activity of EEO and MIC
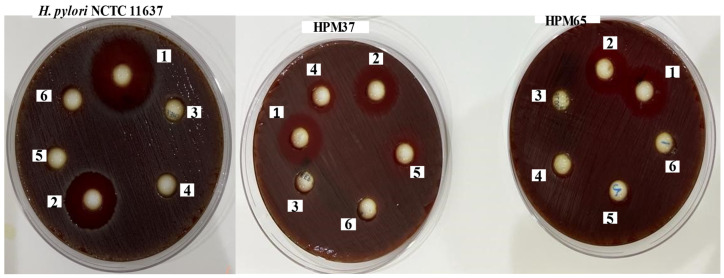
In the worldwide traditional medical system, several plants and plant products are known to possess potent medicinal advantages, suggesting that plants, plant products, and their extracts may be useful for specific medical cases. Hence, in our attempt to identify some active natural substances that have the ability to inhibit the growth of resistant H. pylori, we extracted EEO from S. aromaticum buds and assessed its ability to inhibit the growth of the bacterial strains under study. The results obtained from the extraction and purification process revealed a yield of 0.35% (w/w). The antibacterial activity of EEO assessed using the disc diffusion method showed that EEO displayed an inhibition zone diameter with mean ± SD (from 10 ± 06 to 22 ± 04 mm) against all isolates and the standard strain. These results were higher than that of amoxicillin (12.0, 12.0, 14.03, 16 ± 0.5) against the isolates of HPM 52, HPM65, HPM37, and standard strain NCTC 11637, respectively. The MIC of EEO ranged from 23.0 to 51 μg/mL against both. H. pylori isolates and standard strain NCTC 11637, as shown in Table 1 and Table 2 and Figure 1.
Table 1.
Antibacterial activity of EEO against twenty-five resistant H. pylori isolates and standard strain.
| No | Strains Code | Mean of Inhibition Zone Diameter mm (Mean ± SD) | No | Strains Code | Mean of Inhibition Zone Diameter mm (Mean ± SD) | ||
|---|---|---|---|---|---|---|---|
| EEO | Amoxicillin | EEO | Amoxicillin | ||||
| 1 | HPM4 | 13 ± 03 | 0.0 | 14 | HPM48 | 11 ± 15 | 0.0 |
| 2 | HPM7 | 12 ± 04 | 0.0 | 15 | HPM51 | 14 ± 10 | 0.0 |
| 3 | HPM9 | 11 ± 07 | 0.0 | 16 | HPM54 | 11 ± 10 | 0.0 |
| 4 | HPM12 | 10 ± 15 | 0.0 | 17 | HPM57 | 13 ± 20 | 0.0 |
| 5 | HPM15 | 14 ± 06 | 0.0 | 18 | HPM62 | 11 ± 00 | 0.0 |
| 6 | HPM16 | 12 ± 00 | 0.0 | 19 | HPM63 | 13 ± 00 | 0.0 |
| 7 | HPM19 | 14 ± 10 | 0.0 | 20 | HPM65 | 13 ± 00 | 12.0. |
| 8 | HPM26 | 11 ± 04 | 0.0 | 21 | HPM66 | 11 ± 20 | 0.0 |
| 9 | HPM37 | 15 ± 05 | 14.03 | 22 | HPM70 | 10 ± 21 | 0.0 |
| 10 | HPM44 | 10 ± 06 | 0.0 | 23 | HPM72 | 11 ± 16 | 0.0 |
| 11 | HPM48 | 14 ± 08 | 0.0 | 24 | HPM73 | 13 ± 15 | 0.0 |
| 12 | HPM52 | 11 ± 00 | 12.0 | 25 | HPM75 | 14 ± 08 | 0.0 |
| 13 | HPM56 | 13 ± 05 | 0.0 | 26 | H. pylori NCTC 11637 | 22 ± 04 | 16 ± 05 |
Table 2.
MIC of EEO against resistant H. pylori isolates and standard strain.
| No | Strains Code | Minimum Inhibitory Concentration (μg/mL) | No | Strains Code | Minimum Inhibitory Concentration (μg/mL) | ||
|---|---|---|---|---|---|---|---|
| EEO | Amoxicillin | EEO | Amoxicillin | ||||
| 1 | HPM4 | 27.0 | 28.0 | 14 | HPM48 | 40.40 | 36.0 |
| 2 | HPM7 | 29.0 | 30.0 | 15 | HPM51 | 29.0 | 47.0 |
| 3 | HPM9 | 23.0 | 35.0 | 16 | HPM54 | 24.60 | 35.0 |
| 4 | HPM12 | 24.0 | 27.0 | 17 | HPM57 | 29.90 | 37.0 |
| 5 | HPM15 | 30.50 | 34.0 | 18 | HPM62 | 39.10 | 40.0 |
| 6 | HPM16 | 33.60 | 42.0 | 19 | HPM63 | 51.0 | 49.0 |
| 7 | HPM19 | 24.0 | 31.0 | 20 | HPM65 | 28.0 | 3.0 |
| 8 | HPM26 | 24.40 | 29.0 | 21 | HPM66 | 32.50 | 50.0 |
| 9 | HPM37 | 35.0 | 2.0 | 22 | HPM70 | 48.0 | 44.0 |
| 10 | HPM44 | 27.50 | 30.0 | 23 | HPM72 | 45.0 | 31.0 |
| 11 | HPM48 | 33.0 | 36.0 | 24 | HPM73 | 36.0 | 48.0 |
| 12 | HPM52 | 39.0 | 2.0 | 25 | HPM75 | 50.0 | 43.0 |
| 13 | HPM56 | 46.20 | 37.0 | 26 | H. pylori NCTC 11637 | 21.0 | 2.0 |
Figure 1.
Antibacterial activity of purified eugenol (1), amoxicillin (2), water extract of S. aromaticum (3), Dimethyl sulfoxide (DMSO) (4), water (5) and mixed from water and DMSO (6) against resistant H. pylori isolates (HPM37 and HPM 65) and H. pylori NCTC 11637.
2.2. Biofilm Formation and Its Suppression by EEO
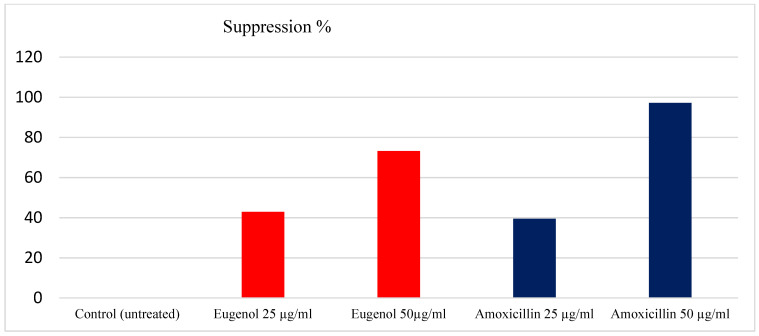
In this study, 76 H. pylori isolates were tested for their ability to form a biofilm. A total of 71 (93.36%) of the bacterial isolates formed biofilms with different degrees, the moderate degree of biofilm formed is reflected in the high frequency between H. pylori isolates under investigation. The bacterial strains formed a biofilm after the treatment with EEO at concentrations of 25 and 50 µg/mL with percentages of 36% (50.68) and 19% (26.75), respectively, as recorded in Table 3. Moreover, the results obtained from the antibiofilm activity of EEO showed suppression percentages of the formed biofilm with 49.32% and 73.21% at concentrations of 25 and 50 µg/mL, respectively. In contrast, amoxicillin yielded a suppression of biofilm formation with percentages of 43.46% and 92.37% at the same concentrations. The maximum suppression of biofilm formation by EEO was observed at 50 µg/mL, with (73.21%), as shown in Figure 2.
Table 3.
Detection of biofilm among H. pylori isolates before and after treatment with EEO.
| Treatment | Bacterial Biofilm Formation N (%) Total N = 76 |
Degree (%) N = 71 | |||
|---|---|---|---|---|---|
| Strong N (%) | Moderate N (%) | Weak N (%) | |||
| Control | -------- | 71 (93.36) | 17 (23.93) | 43 (60.54) | 11 (15.48) |
| EEO | 25 µg/mL | 36 (50.68) | 8 (22.21) | 11 (30.54) | 17 (47.20) |
| 50 µg/mL | 19 (26.75) | 0.0 | 9 (47.36) | 10 (52.63) | |
| Amoxicillin | 25 µg/mL | 43 (56.54) | 11 (25.57) | 19 (44.17) | 13 (30.22) |
| 50 µg/mL | 2 (2.63) | 0.0 | 2 (100%) | 0.0 | |
Figure 2.
Percentage of biofilm suppression with EEO and amoxicillin.
2.3. Development of Bacterial Resistance
In this study, we investigated the antibiotic resistance acquirement of H. pylori strains after exposure to subinhibitory concentrations of EEO. The antibiotic susceptibility of the bacterial isolates and standard NCTC 11637 strain before and after exposure to EEO several times revealed that antibiotic susceptibility had not changed, as shown in the results recorded in Table 4. This clarifies that the tested bacterial strains did not acquire resistance to antibiotics after exposure to EEO at the sub-inhibitory concentration (12 μg/mL) for 24 h several times.
Table 4.
Antibiotic susceptibility before (after) exposure to EEO.
| Bacterial Strains | Antibiotics Susceptibility before Exposure to EEO | Antibiotics Susceptibility before Exposure to EEO | ||||
|---|---|---|---|---|---|---|
| Clarithromycin | Metronidazole | Amoxicillin | Clarithromycin | Metronidazole | Amoxicillin | |
| NCTC 11637 | S | S | S | S | S | S |
| HPM52 | S | S | S | S | S | S |
| HPM56 | R | R | R | R | R | R |
| HPM637 | R | R | S | R | R | S |
| HPM65 | R | S | S | R | S | S |
S = Sensitive; R = Resistance.
2.4. Anti-Inflammatory Activity of EEO
Table 5 shows the inflammatory results obtained from the four different treatments with EEO and sodium diclofenac as a positive control. The purified EEO exhibited inhibition percentages of human erythrocyte hemolysis of 53.04%, 58.74%, 61.07%, and 63.64% at concentrations of 4, 8, 16, and 32 μg/L compared with inhibition by sodium diclofenac with 63.72%, 67.49%, 69.18%, and 71.43% at the same concentrations, respectively.
Table 5.
Assessment of anti-inflammatory activity of EEO.
| Treatment | Concentration (μg/mL) | Absorbance 560 nm | % Inhibition of Hemolysis |
|---|---|---|---|
| Control | 0.0 | 1.246 | 0.0% |
| EEO | 4 | 0.585 | 53.04% |
| 8 | 0.514 | 58.74% | |
| 16 | 0.485 | 61.07% | |
| 32 | 0.453 | 63.64% | |
| Sodium diclofenac | 4 | 0.452 | 63.72% |
| 8 | 0.405 | 67.49% | |
| 16 | 0.384 | 69.18% | |
| 32 | 0.357 | 71.43% |
2.5. GC–MS Analysis of EEO
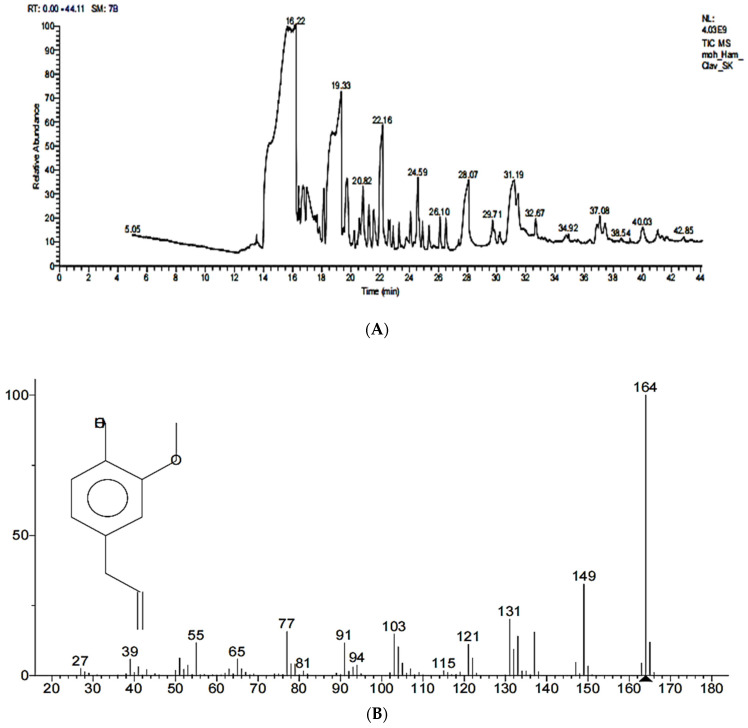
The GC–MS analysis of the methanolic extract of Syzygium aromaticum revealed that eugenol is the major component, with a percentage of 96.35%, while ethyl eugenol occupied 3.65%, which confirmed the extraction of EEO from Syzygium aromaticum buds with the hydro-distillation method. The purified EEO exhibited a maximum peak at 16.22, molecular formula C10H12O2 and a molecular weight of 164 m/z, as shown in Figure 3A,B.
Figure 3.
(A) GC–MS of methanolic extract of Syzygium aromaticum. (B) GC–MS of purified EEO in methanolic alcohol.
3. Discussions
Helicobacter pylori is an important bacterial species in the gastrointestinal system that colonizes the mucoid layer of the stomach in about 50% of humans worldwide and causes several diseases, including chronic gastritis, peptic ulcers, and gastric cancer. The World Health Organization (WHO) recorded H. pylori as a class I carcinogen because of its role in cancer [1]. Currently, first-line treatment depends on the combination of two antibiotics, clarithromycin and amoxicillin or metronidazole with a proton pump inhibitor (triple therapy). Levofloxacin can be used as an alternative to clarithromycin in first-line therapy, with higher treatment rates. In recent years, with the rapidly increasing resistance of H. pylori to antibiotics, the treatment of this microbe has remained a major challenge to physicians [15]. For this reason, the eradication of H. pylori is very critical to patients worldwide. From this point, our study aims to research a new approach to eradicating resistant H. pylori and its virulence factors. In this context, we extracted EEO as an active compound from S. aromaticum buds to combat resistant H. pylori. In this study, the EEO extracted from S. aromaticum buds exhibited antibacterial activity against H. pylori isolates and standard strain NCTC 11637, with an inhibition zone diameter with a mean ± SD (from 10 ± 06 to 22 ± 04 mm), comparable to amoxicillin (from 0.0 to 16 ± 0.05). In addition, the MIC of EEO ranged from 23 to 51 μg/mL against both. H. pylori isolates and standard strain NCTC 11637. A previous study by Ali and his colleague revealed that the ability of eugenol inhibits the growth of thirty strains of H. pylori at a concentration of 2 μg/mL [14]. Moreover, H. pylori did not develop any resistance toward eugenol even after 10 passages grown at sub-inhibitory concentrations. Furthermore, El-Shouny et al. [13] reported that the methanolic extract of clove exhibited activity against resistant strains of H. pylori, with an inhibition zone ranging from 20 ± 0.57 to 25 ± 0.56 mm, where eugenol was detected as a major constituent, with a percentage (28.14%). Clove oil is recorded as a “generally regarded as safe compound” by the FDA when used at levels not exceeding 1500 ppm in food categories. In addition, the World Health Organization (WHO) and Expert Committee on Food Additives have established the acceptable daily human intake of clove oil at 2.5 mg/kg body weight for humans [16]. Several studies proposed that the mode of antibacterial action of eugenol is due to the disruption of the plasma membrane, which exceeds its non-specific permeability. Furthermore, the hydrophobic nature of eugenol enables it to permeate the lipopolysaccharide of the Gram-negative bacterial plasma membrane and modifies the cell structure, which then results in the infiltration of intracellular constituents [1,12]. The antibiofilm study revealed that EEO possesses strong antibiofilm activity against resistant H. pylori isolates with various degrees; the maximum suppression was observed (73.21%) at a concentration of 50 µg/mL. Previous studies have reported that eugenol can suppress and eradicate the biofilms caused by Vibrio parahaemolyticus [17] Staphylococcus aureus [18], Candida albicans [19], Candida tropicalis, Candida. dubliniensis [20], Porphyromonas gingivalis [21], Aggregatibacter actinomycetemcomitans ATCC 43718 [22], Staphylococcus aureus ATCC25923 [23], Escherichia coli O157:H7 [24], and Streptococci [25]. Additionally, significant biofilm reduction by clove extracts at various concentrations has been reported [26,27]. Our finding revealed that the sublethal dose of EEO did not develop bacterial resistance after exposure to EEO. These results are consistent with the results reported by Ali et al. [14]. H. pylori acquired resistance to antibiotics, such as clarithromycin and amoxicillin, after 10 sequential passages, as recorded previously [28]. In our study, we investigated the anti-inflammatory activity of EEO; the results obtained from the four treatments revealed the strong activity of EEO to inhibit human erythrocyte hemolysis with 53.04%, 58.74%, 61.07%, and 63.64% at concentrations of 4, 8, 16, and 32 μg/mL, respectively. The ability of drugs to stabilize erythrocyte membranes can also stabilize lysosomal membranes and, therefore, exhibit anti-inflammatory properties by changing the activity and release of cell mediators due to the resemblance between them [29]. Eugenol, as a natural compound with anti-inflammatory activity, has gained a great deal of attention in topical applications [30]. Capasso and his colleague reported eugenol to have both antiulcerogenic and anti-inflammatory activities [31]. In this context, eugenol was used before 1950 in the treatment of ulcer disease [32]. Previous studies have reported that eugenol has anti-inflammatory activities comparable with some NSAIDs, such as sodium diclofenac and indomethacin. Genetic studies of the anti-inflammatory activity of eugenol showed COX-2 inhibition without affecting COX-1 in mice macrophage cell cultures [33]. The GC–MS of methanolic extracts of S. aromaticum revealed that eugenol is a major compound, with a maximum peak of 16.4. The results obtained were consistent with DeFrancesco [34]. In Brazil, eugenol extracted from S. aromaticum essential oil was the predominate component, with a high percentage of (90.3%), in addition to eugenol acetate (1.87%) and β-caryophyllene (4.83%) [35]. S. aromaticum essential oil obtained in Italy [36] and China [21] also had eugenol as a major component, with 77.9% and 90.84%, respectively. These results agreed with the percentage obtained in the present study. The percentage of eugenol contained in the essential oil and the difference between the compounds can be directly related to the different geographic areas where the plant has been grown up, which can be affected or altered by abiotic and biotic factors such as the stage and age of plant growth, season, and climatic changes [37]. Moreover, the extraction method used to obtain the essential oil can also change its chemical constituents, as distillation and storage conditions can influence the content of its volatile oils. The change in chemical composition directly affects pharmacological and biological values, as noted in the anti-inflammatory and antimicrobial activities [38].
4. Materials and Methods
4.1. Plant Material
S. aromaticum was purchased from the local market of a Cairo governorate in Egypt. S. aromaticum was washed with distilled water, allowed to dry at room temperature, and powdered using an electric blender.
4.2. Extraction of EEO from S. aromaticum Buds
Eugenol was extracted from S. aromaticum buds using a hydro-distillation method with a Cleavenger-type apparatus for 2.5 h [39,40]. Approximately 300 g of powdered S. aromaticum was diluted in water in the proportion 1:10 (S. aromaticum + water) and extracted with the hydro-distillation method using a Clevenger system for 2.5 h at 100 °C. The EEO was collected and dried with anhydrous sodium sulfate (Na2SO4), and the final volume found was used to assess the yield through the mass/volume ratio by measuring the density. Mass/volume ratios were calculated from the mass (g) of the initial S. aromaticum material and the volume (mL) of obtained eugenol from the extraction process. The extracts were stored in sealed vials at 4 °C. for further studies.
4.3. Purification of Extracted EEO
An amount of 25 mL of EEO crude extract was transferred to a separating funnel, mixed with 50 mL of dichloromethane, and shaken well. The mixture was then allowed to separate into two distinct layers. In a 200 mL conical flask, the lower layer was collected, and 25 mL of dichloromethane was added a second time. The contents of the conical flask were transferred to a separating funnel, shaken well, and allowed to separate into two layers. The lower layer was collected in a conical flask and transferred to a clean separating funnel. Then, 40 mL of a 10% sodium hydroxide solution was added to the separating funnel to separate the eugenol acetate. The aqueous layer was collected in a conical flask, and the solution was acidified to a pH of less than 2.0 using conc. HCl. The pH was checked, and the aqueous layer was washed with a half-saturated sodium chloride solution. The organic phase was collected, and 25 mL of dichloromethane was added to it. The solution was dried with magnesium sulphate and filtered with filter paper. Finally, it was evaporated in a rotatory evaporator at a temperature of 60 °C until it became pure eugenol as a light-yellow oil [41]. The pure eugenol was characterized using GC–MS.
4.4. Anti-H. pylori Activity of EEO
The antibacterial activity of eugenol essential oil was determined against multidrug-resistant H. pylori isolates previously isolated from clinical samples and identified, as well as a standard strain of H. pylori NCTC 11637. Muller Hinton blood Agar (MHBA) medium was inoculated with 100 μL of bacterial growth (1.5 × 106 CFU/mL). Paper discs (8 mm) were saturated with 50 µL of eugenol extracts at a concentration of 10 mg/mL(eugenol/DMSO). The saturated paper discs were placed on the surface of agar plates inoculated with bacterial test strains; the antibiotic amoxicillin 25 µg/mL was used as a positive control on the same plates. The plates were incubated at 37 °C for 48 h under microaerophilic conditions (10% CO2, 5% O2, and 85% N2) using a gas pack system (Mitsubishi, Japan), and the inhibition zone diameter was estimated in millimeters (mm). This experiment was performed in three replicates [42,43].
4.5. Determination of Minimum Inhibitory Concentrations (MICs) of EEO
The MIC of EEO against H. pylori isolates and H. pylori NCTC 11637 was performed by the microdilution broth method using amoxicillin (HiMedia Laboratories Pvt. Ltd., Thane, India) as a positive control in a 96-well microplate. Muller Hinton broth (MHB) medium was inoculated with a cell suspension of H. pylori isolates and H. pylori NCTC 11637 (106 CFU/mL), and 100 µL of the inoculated medium was distributed in each well. The antibacterial substances (amoxicillin and EEO) were tested in a two-fold serial dilution, and the cultures were incubated at 35 °C for 3 days in a microaerophilic atmosphere (10% CO2, 5% O2, and 85% N2). EEO was investigated at concentrations of 0.0, 5.0, 10.0, 15.0, 20.0, 25.0, 30.0, 35.0, 40.0, 45.0, 50.0, 55.0, and 60.0 µg/mL and amoxicillin at 0.30, 0.613, 1.25, 2.5, 5.0, 10.0, 20.0, 40.0, and 60 µg/mL. The experiment was performed according to the criteria of (CLSI, 2020 guidelines (M7-A5) [42]. One of the negative control wells contained medium and EEO, while the other contained medium and amoxicillin at the tested concentrations, which were analyzed to determine the differences in optical density (O.D.) at 630 nm. MIC was defined as the lowest concentration of the EEO or amoxicillin, which can inhibit the visible growth of bacteria.
4.6. Antibiofilm Activity of EEO
A quantitative assessment of biofilm formation suppressed by EEO was performed using the micro-titer plate technique. Bacterial suspensions were prepared from 24-h-old cultures of each isolate. The turbidity of the initial suspension was adjusted by comparing it with a 0.5 McFarland standard. The initial bacterial suspensions contained approximately 108 CFU/mL, which were then diluted to 1:100 by sterile 0.85% saline solution. Then, 100 μL of this dilute was inoculated, in triplicate, in a 96-well flat-bottomed polystyrene plate (China) and incubated for 24 h at 37 °C. The content of each well was discharged, and the wells were washed several times with phosphate-buffered saline (PBS). After that, methanol fixation was conducted for 15 min, and the plate was then air-dried. Each well was stained with 100 μL of 1% crystal violet solution in water and incubated at room temperature for 30 min. Afterward, the stain was solubilized by 100 μL of glacial acetic acid (GAA) 33%; the plates were washed with distilled water three times and then dried. The optical density (OD) of each well at 570 nm was read using an enzyme-linked immunosorbent assay (ELISA). The cut-off OD control for the microtiter plate was defined as three standard deviations (SD) plus the mean OD of the negative control. Based on the OD average values, the results of the biofilm formation were interpreted as follows; (OD ≤ ODC) = negative (non-biofilm formation) ODC = optical density of control; (ODC ≤ OD ≤ 2ODC) = weak biofilm formation, (2ODC ≤ OD ≤ 4ODC) = moderate- biofilm formation and (4ODC ≤ OD) = strong biofilm formation [28].
4.7. Development of Bacterial Resistance
Four H. pylori isolates and standard strain NCTC 11637 were used to evaluate the ability of bacterial strains to develop antibiotic resistance after exposure to EEO. The antibiotics susceptibility of these bacteria to three different antibiotics (clarithromycin, metronidazole and amoxicillin) were as follows: standard strain NCTC 11637 (sensitive to the three antibiotics), isolate HPM52 (sensitive to the three antibiotics), HPM56 (resistant to the three antibiotics), HPM37 (resistant to clarithromycin, metronidazole, and sensitive to amoxicillin), and HPM65 (resistant only to clarithromycin) and they were grown on agar at sub-inhibitory concentrations (12.0 μg/mL) of eugenol for 48 h. After the end of the incubation period, antibiotic susceptibility was retested again. This experiment was performed in three replicates [14].
4.8. Anti-Inflammatory Assay of EEO by Human RBCs
The anti-inflammatory activity of EEO was investigated using the human red blood cell membrane stabilization technique. The blood sample, collected from a healthy human volunteer who had not taken any NSAIDS 2 weeks prior to the experiment, was mixed with an equal volume of Alsever solution (2% dextrose, 0.8% sodium citrate, 0.5% citric acid and 0.42% NaCl), and centrifuged at 3000 rpm. The precipitated cells were washed with iso-saline and a 10% suspension was prepared. Different concentrations of EEO and sodium diclofenac were prepared viz., 4, 8, 16, and 32 μg/mL using dimethyl sulfoxide (DMSO). Then, 1 mL of phosphate buffer, 2 mL of hypo-saline, and 0.5 mL of human red blood cells (HRBC) suspension were added to the previous concentrations.
Then, they were incubated at 37 °C for 30 min and centrifuged at 3000 rpm for 20 min. The estimation of the inhibition of HRBC hemolysis was conducted through the estimation of supernatant hemoglobin content by using a spectrophotometer at 560 nm [44]. Percentage (%) inhibitions of hemolysis = (absorbance of control − absorbance of the sample)/(absorbance of control) “× 100”.
4.9. Identification of Eugenol Essential Oil by GC–MS
The eugenol essential oil compounds were analyzed and identified using GC–MS, as described by Zothanpuia et al. [45], with minor modifications. In brief, the EEO was dissolved in spectroscopy-grade methanol, and the GC–MS analysis was carried out using a Thermo Scientific trace GC1310-ISQ mass spectrometer (Austin, TX, USA) with a direct capillary column (length 30 m, thickness 0.25 µm, internal diameter 25 mm). The oven temperature was set to 50 °C for 5 min, then scaled up to 230 °C at a rate of 5 °C/min and maintained for 2 min; 1 μL of the sample was injected at 250 °C using helium as a carrier gas, divided at a ratio of 1:30. The mass spectrometer was set to scan from 40 to 1000 m/z in electron ionization (EI) mode at 200 °C and 70 eV. The observed compounds’ spectra were compared with the spectra of known compounds contained in the WILEY 09 (Wiley, New York, NY, USA) and NIST 11 libraries.
4.10. Statistical Analysis
The data were expressed as the mean ± SD value, which was calculated by using Minitab 18 software extended with a statistical package and Microsoft Excel 365.
5. Conclusions
The data obtained in this study show that EEO essential oil extracted from S. aromaticum possesses powerful antibacterial and antibiofilm activities against antibiotic-resistant H. pylori as well as anti-inflammatory activity. Therefore, we propose that EEO can be used to improve the treatment and eradication of H. pylori. In addition, the administration of eugenol in small amounts can protect against H. pylori biofilm-related infection.
Author Contributions
M.K.M.E. Conceptualization and investigation; G.M.E.-S. Conceptualization, writing—original draft preparation, investigation, supervision; S.A.M. Conceptualization. Writing review and editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.El-Sherbiny G.M., Elbestawy M.K.M. A review–plant essential oils active against Helicobacter Pylori. J. Essent. Oil Res. 2022;34:203–215. doi: 10.1080/10412905.2022.2025464. [DOI] [Google Scholar]
- 2.El-Sherbiny G.M., Gazelly A.M., Sharaf M.H., Moghannemm S.A., Shehata M.E., Ismail M.K., El-Hawary A.S. Exploitation of the antibacterial, antibiofilm and antioxidant activities of Salvadora Persica (Miswak) extract. J. Bioresour. Bioprod. 2023;8:59–65. doi: 10.1016/j.jobab.2022.11.006. [DOI] [Google Scholar]
- 3.Wang Y.-C. Medicinal plant activity on Helicobacter pylori related diseases. World J. Gastroenterol. 2014;20:10368–10382. doi: 10.3748/wjg.v20.i30.10368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alghazzaly A.M., El-Sherbiny G.M., Moghannemm S.A., Sharaf M.H. Antibacterial, antibiofilm, antioxidants and phytochemical profiling of Syzygium aromaticum extract. Egypt. J. Aquat. Biol. Fish. 2022;26:207–218. doi: 10.21608/ejabf.2022.260398. [DOI] [Google Scholar]
- 5.Foda A.M., Kalaba M.H., El-Sherbiny G.M., Moghannem S.A., El-Fakharany E.M. Antibacterial activity of essential oils for combating colistin-resistant bacteria. Expert Rev. Anti-Infect. Ther. 2022;20:1351–1364. doi: 10.1080/14787210.2022.2101997. [DOI] [PubMed] [Google Scholar]
- 6.Sharaf M.H., El-Sherbiny G.M., Moghannem S.A., Abdelmonem M., Elsehemy I.A., Metwaly A.M., Kalaba M.H. New combination approaches to combat methicillin-resistant Staphylococcus aureus (MRSA) Sci. Rep. 2021;11:4240. doi: 10.1038/s41598-021-82550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jirovetz L., Buchbauer G., Stoilova I., Stoyanova A., Krastanov A., Schmidt E. Chemical Composition and Antioxidant Properties of Clove Leaf Essential Oil. J. Agric. Food Chem. 2006;54:6303–6307. doi: 10.1021/jf060608c. [DOI] [PubMed] [Google Scholar]
- 8.Kabuto H., Tada M., Kohno M. Eugenol [2-Methoxy-4-(2-propenyl)phenol] Prevents 6-Hydroxydopamine-Induced Dopamine Depression and Lipid Peroxidation Inductivity in Mouse Striatum. Biol. Pharm. Bull. 2007;30:423–427. doi: 10.1248/bpb.30.423. [DOI] [PubMed] [Google Scholar]
- 9.Walsh S.E., Maillard J.Y., Russell A.D., Catrenich C.E., Charbonneau D.L., Bartolo R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and Gram-negative bacteria. J. Appl. Microbiol. 2003;94:240–247. doi: 10.1046/j.1365-2672.2003.01825.x. [DOI] [PubMed] [Google Scholar]
- 10.Filgueiras C.T., Vanetti M.C.D. Effect of eugenol on growth and listeriolysin o production by Listeria monocytogenes. Braz. Arch. Biol. Technol. 2006;49:405–409. doi: 10.1590/S1516-89132006000400008. [DOI] [Google Scholar]
- 11.Burt S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004;94:223–253. doi: 10.1016/j.ijfoodmicro.2004.03.022. [DOI] [PubMed] [Google Scholar]
- 12.Devi K.P., Nisha S.A., Sakthivel R., Pandian S.K. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella typhi by disrupting the cellular membrane. J. Ethnopharmacol. 2010;130:107–115. doi: 10.1016/j.jep.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 13.El-Shouny W.A., Moawad M.S., Haider A.S., Ali S.S., Nouh S. Syzygium aromaticum L.: Traditional herbal medicine against cagA and vacA toxin genes-producing drug-resistant Helicobacter pylori. J. Tradit. Complement. Med. 2020;10:366–377. doi: 10.1016/j.jtcme.2019.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ali S.M., Khan A.A., Ahmed I., Musaddiq M., Ahmed K.S., Polasa H., Rao L.V., Habibullah C.M., A. Sechi L., Ahmed N. Antimicrobial activities of Eugenol and Cinnamaldehyde against the human gastric pathogen Helicobacter pylori. Ann. Clin. Microbiol. Antimicrob. 2005;4:20. doi: 10.1186/1476-0711-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korona-Glowniak I., Glowniak-Lipa A., Ludwiczuk A., Baj T., Malm A. The In Vitro Activity of Essential Oils against Helicobacter Pylori Growth and Urease Activity. Molecules. 2020;25:586. doi: 10.3390/molecules25030586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hassanien M.F.R. Composition and antiradical power of Syzygium aromaticum lipids. Chem. Nat. Compd. 2014;50:716–718. doi: 10.1007/s10600-014-1060-3. [DOI] [Google Scholar]
- 17.Ashrafudoullaa M., Furkanur M., Angela R.M., Park S.H., Ha S.D. Antibacterial and antibiofilm mechanism of eugenol against antibiotic resistance Vibrio parahaemolyticus. Food Microbiol. 2020;91:103500. doi: 10.1016/j.fm.2020.103500. [DOI] [PubMed] [Google Scholar]
- 18.Yadav M.K., Chae S.W., Im G.J., Chung J.W., Song J.J. Eugenol: A phyto- compound effective against methicillin-resistant and methicillin-sensitive Staphylococcus aureus clinical strain biofilms. PLoS ONE. 2015;10:e0119564. doi: 10.1371/journal.pone.0119564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan M.S.A., Ahmad I. Antibiofilm activity of certain phytocompounds and their synergy with fluconazole against Candida albicans biofilms. J. Antimicrob. Chemother. 2011;67:618–621. doi: 10.1093/jac/dkr512. [DOI] [PubMed] [Google Scholar]
- 20.de Paula S.B., Bartelli T.F., Di Raimo V., Santos J.P., Morey A.T., Bosini M.A., Nakamura C.V., Yamauchi L.M., Yamada-Ogatta S.F. Effect of eugenol on cell surface hydrophobicity, adhesion, and biofilm of Candida tropicalis and Candida dubliniensis isolated from oral cavity of HIV-infected patients. Evid. Based Complement. Altern. Med. 2014;2014:505204. doi: 10.1155/2014/505204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y., Wang Y., Zhu X., Cao P., Wei S., Lu Y. Antibacterial and antibiofilm activities of eugenol from essential oil of Syzygium aromaticum (L.) Merr. & L. M. Perry (clove) leaf against periodontal pathogen Porphyromonas gingivalis. Microb. Pathog. 2017;113:396–402. doi: 10.1016/j.micpath.2017.10.054. [DOI] [PubMed] [Google Scholar]
- 22.Utami D.T., Hertiani T., Pratiwi S.U.T., Randy A., Artanti A.N., Ermawati D.E., Rahmani S., Sasongko H., Kundarto W., Zulpadly M.F. Antibiofilm Activity of Eugenol against Aggregatibacter Actinomycetemcomitans ATCC 43718 (serotype B) Egypt. J. Chem. 2022. in press . [DOI]
- 23.Miladi H., Zmantar T., Kouidhi B., Al Qurashi Y.M.A., Bakhrouf A., Chaabouni Y., Mahdouani K., Chaieb K. Synergistic effect of eugenol, carvacrol, thymol, p- cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog. 2017;112:156–163. doi: 10.1016/j.micpath.2017.09.057. [DOI] [PubMed] [Google Scholar]
- 24.Kim B.R., Bae Y.M., Lee S.Y. Effect of environmental conditions on biofilm formation and related characteristics of Staphylococcus aureus. J. Food Saf. 2016;36:412–422. doi: 10.1111/jfs.12263. [DOI] [Google Scholar]
- 25.Yadav M.K., Park S.W., Chae S.W., Song J.J., Kim H.C. Antimicrobial activities of Eugenia caryophyllata extract and its major chemical constituent eugenol against Streptococcus pneumoniae. Apmis. 2013;121:1198–1206. doi: 10.1111/apm.12067. [DOI] [PubMed] [Google Scholar]
- 26.Chavan N.S., Phadtare R.D., Chavan T.B. Effect of aqueous extracts of different medicinal plants on control of Streptococcus mutans. Int. J. Curr. Microbiol. App. Sci. 2015;4:1072–1081. [Google Scholar]
- 27.Liaqat I., Pervaiz Q., Jamil B.S., Ahmed S.I., Jahan N. Investigation of Bactericidal Effects of Medicinal Plant Extracts on Clinical Isolates and Monitoring Their Biofilm Forming Potential. Pak Vet. J. 2016;36:159–164. [Google Scholar]
- 28.Nassar O., Desouky S.D., El-Sherbiny G.M., Abu-Elghait M. Correlation between phenotypic virulence traits and antibiotic resistance in Pseudomonas aeruginosa clinical isolates. Microb. Pathog. 2022;162:105339. doi: 10.1016/j.micpath.2021.105339. [DOI] [PubMed] [Google Scholar]
- 29.Omale J., Okafor P.N. Comparative antioxidant capacity, membrane stabilization, polyphenol composition and cytotoxicity of the leaf and stem of Cissus multistriata. Afr. J. Biotechnol. 2008;7:3129–3133. [Google Scholar]
- 30.Hong C.H., Hur S.K., Oh O.-J., Kim S.S., Nam K.A., Lee S.K. Evaluation of natural products on inhibition of inducible cyclooxygenase (COX-2) and nitric oxide synthase (iNOS) in cultured mouse macrophage cells. J. Ethnopharmacol. 2002;83:153–159. doi: 10.1016/S0378-8741(02)00205-2. [DOI] [PubMed] [Google Scholar]
- 31.Capasso R., Pinto L., Vuotto M., Di Carlo G. Preventive effect of eugenol on PAF and ethanol-induced gastric mucosal damage. Fitoterapia. 2000;71:S131–S137. doi: 10.1016/S0367-326X(00)00188-X. [DOI] [PubMed] [Google Scholar]
- 32.Bandes J., Samuels N.A., Hollander F., Goldfischer R.L., Winkelstein A. The clinical response of patients with a peptic ulcer to a topical mucilage (eugenol) Gastroenterology. 1951;18:391–399. doi: 10.1016/S0016-5085(51)80005-2. [DOI] [PubMed] [Google Scholar]
- 33.Kim S.S., Oh O.-J., Min H.-Y., Park E.-J., Kim Y., Park H.J., Han Y.N., Lee S.K. Eugenol suppresses cyclooxygenase-2 expression in lipopolysaccharide-stimulated mouse macrophage RAW264.7 cells. Life Sci. 2003;73:337–348. doi: 10.1016/S0024-3205(03)00288-1. [DOI] [PubMed] [Google Scholar]
- 34.DeFrancesco J.V. Extraction and Analysis of Eugenol from Cloves. J. Forensic Sci. Educ. 2021;3:9. [Google Scholar]
- 35.Silvestri J.D.F., Paroul N., Czyewski E., Lerin L., Rotava L., Cansian R.L., Mossi A., Toniazzo G., de Oliveira D., Treichel H. Perfil da composição química e atividades antibacteriana e antioxidante do óleo essencial do cravo-da-índia (Eugenia caryophyllata Thunb.) Rev. Ceres. 2018;57:589–594. doi: 10.1590/S0034-737X2010000500004. [DOI] [Google Scholar]
- 36.Ebani V.V., Najar B., Bertelloni F., Pistelli L., Mancianti F., Nardoni S. Chemical Composition and In Vitro Antimicrobial Efficacy of Sixteen Essential Oils against Escherichia coli and Aspergillus fumigatus Isolated from Poultry. Veter. Sci. 2018;5:62. doi: 10.3390/vetsci5030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Andrade B.F.M.T., Nunes Barbosa L., Probst I.P., Fernandes Júnior A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2017;26:34–40. doi: 10.1080/10412905.2013.860409. [DOI] [Google Scholar]
- 38.Besten M.A., Jasinski V.C.G., Costa Â.D.G.L.C., Nunes D.S., Sens S.L., Wisniewski A., Jr., Simionatto E.L., Riva D., Dalmarco J.B., Granato D. Chemical composition similarity between the essential oils isolated from male and female specimens of each five Baccharis species. J. Braz. Chem. Soc. 2012;23:1041–1047. doi: 10.1590/S0103-50532012000600007. [DOI] [Google Scholar]
- 39.Da Silva D.T., Bianchini N.H., Amaral L.D.P., Longhi S.J., Heinzmann B.M. Análise Do Efeito Da Sazonalidade Sobre O Rendimento Do Óleo Essencial Das Folhas De Nectandra grandiflora Nees. Rev. Árvore. 2015;39:1065–1072. doi: 10.1590/0100-67622015000600009. [DOI] [Google Scholar]
- 40.Ehlert P.A.D., Blank A.F., Arrigoni-Blank M.F., Paula J.W.A., Campos D.A., Alviano C.S. Tempo de hidrodestilação na extração de óleo essencial de sete espécies de plantas medicinais. Rev. Bras. de Plantas Med. 2006;8:79–80. [Google Scholar]
- 41.Bisergaeva R.A., Takaeva M.A., Sirieva Y.N. Extraction of eugenol, a natural product, and the preparation of eugenol benzoate. J. Phys. Conf. Ser. 2021;1889:022085. doi: 10.1088/1742-6596/1889/2/022085. [DOI] [Google Scholar]
- 42.CLSI . Performance Standards for Antimicrobial Susceptibility Testing. 28th ed. Clinical and Laboratory Standards Institute; Wayne, PA, USA: 2020. CLSI Supplement M100. [Google Scholar]
- 43.Ohno T., Kita M., Yamaoka Y., Imamura S., Yamamoto T., Mitsufuji S., Kodama T., Kashima K., Imanishi J. Antimicrobial Activity of Essential Oils against Helicobacter pylori. Helicobacter. 2003;8:207–215. doi: 10.1046/j.1523-5378.2003.00146.x. [DOI] [PubMed] [Google Scholar]
- 44.Rahman H., Eswaraiah M.C., Dutta A. In-vitro anti-inflammatory and anti-arthritic activity of Oryza sativa Var. joha rice (an aromatic indigenous rice of Assam) Am. Eurasian J. Agric. Environ. Sci. 2015;15:115–121. [Google Scholar]
- 45.Zothanpuia A.K., Passeri P., Chandra V.V., Leo V.K., Mishra B., Kumar B.P.S. Production of Potent Antimicrobial Compounds from Streptomyces cyaneofuscatus Associated with Fresh Water Sediment. Front. Microbiol. 2017;8:68. doi: 10.3389/fmicb.2017.00068. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.