Abstract
Behavioral and electrophysiological evidence suggests a gradual, experience-dependent specialization of cortical face processing systems that take place largely in the first year of life. To further investigate these findings, event-related potentials (ERPs) were collected from typically developing 9-month-old infants presented with pictures of familiar and unfamiliar monkey or human faces in two different orientations. Analyses revealed differential processing across changes in monkey and human faces. The N290 was greater for familiar compared to unfamiliar faces, regardless of species or orientation. In contrast, the P400 to unfamiliar faces was greater than to familiar faces, but only for the monkey condition. The P400 to human faces differentiated the orientation of both familiar and unfamiliar faces. These results suggest more specific processing of human compared to monkey faces in 9-month-olds.
Recent investigations into the nature and specificity of face perception suggest that faces may be processed differently from other objects (Kanwisher, 2000; Haxby et al, 2001). Both behavioral and neuroscientific investigations in adults reveal that humans have highly refined face processing abilities that may be restricted to certain areas of the occipital-temporal cortex (e.g. Kanwisher, McDermott, & Chun, 1997). There is, however, some debate over the nature of adult face processing. For example, some argue that face processing and the relatively focal brain regions involved in face processing are domain-specific, giving face perception special status compared to other perceptual processing (Kanwisher et al., 1997; Farah, 1996). Conversely, others argue that face processing is transformed into an “expert” perceptual ability over time and that face perception is simply a part of a more domain general system for expert perceptual processing (Gauthier, Williams, Tarr, & Tanaka, 1998; Gauthier, Skudlarski, Gore, & Anderson, 2000). Recent developmental investigations have begun to shed some light on this debate by elucidating the behavioral and electrophysiological correlates of face perception across development. The current study was designed to follow-up these investigations to further our understanding of the mechanisms involved in the formation of perceptual categories, such as face processing, during development.
Nelson (1993, 2001; 2003) posits that similar to the development of speech perception, the development of face perception undergoes changes characterized as becoming increasingly narrow and more specific. For example, in speech perception young infants have the ability to discriminate many different basic units of sounds or phonemes. However, with the experience of learning ones own native language comes a loss in the ability to discriminate certain sounds not represented in this language (Kuhl et al., 1997; Cheour et al., 1998; Werker & Tees, 1984). Nelson suggests that this type of ‘perceptual narrowing’ may also occur in face perception and that experience with or without different types of facial stimuli may shape the specificity of this system. A series of behavioral investigations not only supports the notion of perceptual narrowing in face processing, but has also found that experience influences the specificity of the face processing system (Pascalis, deHaan, & Nelson, 2002; Pascalis, et al., 2005). For example, 6-month-old infants have been found to discriminate monkey faces that 9-month-olds and adults do not, suggesting that younger infants exhibit a more broadly tuned face processing system than older infants and adults. Moreover, given 3 months of perceptual experience with monkey faces, 9-month-olds maintain the ability to discriminate monkey faces (Pascalis et al., 2005). Collectively these data suggest that, during the first 9 months of life, infants may be particularly sensitive to perceptual differences between different types of faces. This further support the notion that experience with faces during a “period of opportunity” may shape later discriminatory abilities. However, the mediating mechanisms and timeline of this sensitive period are still relatively unknown. For example, unlike the results with monkey faces, when infants are exposed to multiple other-race faces as opposed to a single other-race face, discrimination is demonstrated as young as 3-months-of-age (Sangrigoli & de Schonen, 2004). These results suggest that very brief exposure to unfamiliar types of faces may influence subsequent discriminatory abilities.
Several adult electrophysiological reports have identified a negatively peaked component of the event-related potential (ERP), occurring 170 milliseconds after stimulus onset, called the N170, that is thought to be an index of face processing (e.g. Bentin, Allison, Puce, Perez, & McCarthy, 1996; Carmel & Bentin, 2002; Jeffreys, 1996). This component is typically prominent over temporal-occipital electrode locations, it is larger in amplitude to faces compared to objects (Bentin et al., 1996, Carmel & Bentin, 2002; Itier & Taylor, 2004; Rossion et al., 2000), it is delayed (Bentin et al., 1996; Eimer, 2000; Rossion et al., 1999; 2000) and enhanced (Rossion et al., 1999; Eimer, 2000; Sagiv & Bentin, 2002) in response to inverted compared to upright faces, and it is typically not sensitive to changes in the familiarity of the face (Rossion et al., 1999; 2000; Eimer, 2000; Bentin & Deouell, 2000; Although see Jacques & Rossion, in press for alternate findings). Similar to the N170, the vertex positive potential (VPP) has also been associated with the early detection and encoding of faces (Botzel & Grusser, 1989; Jeffreys, 1989; Jeffreys, Tukmachi, & Rockley, 1992; Jeffreys & Tukmachi, 1992; Jeffreys, 1996). This positive component is commonly found over central and parietal scalp regions and occurs at approximately the same latency as the N170. This commonality, in addition to the similar functional response properties, has led to the hypothesis that the VPP and N170 arise from the same source(s), and that the VPP is the dipolar opposite of the N170 (Rossion, Joyce, Cottrell, & Tarr, 2004; Joyce & Rossion, 2005).
The results of several adult ERP studies report both amplitude and latency differences for the N170 and VPP components between human and monkey faces (Carmel & Bentin, 2002; de Haan, Pascalis, & Johnson, 2002; Scott, Shannon, & Nelson, in press). Scott et al., (in press) correlated amplitude of the VPP with behavioral accuracy in a monkey and human face recognition task and found that accuracy in the human face but not the monkey face task was positively related to the amplitude of the VPP. These findings reveal both behavioral and electrophysiological differences when adults view human versus monkey faces, suggesting that experience with faces significantly shapes the specificity of adult face processing.
Recent developmental investigations have attempted to define the infant analog of these adult face-processing ERP components, in particular the N170 (de Haan, Pascalis, & Johnson, 2002; Halit, de Haan & Johnson, 2003; de Haan & Nelson, 1999; Scott & Nelson, in press). The infant N290 and P400 both exhibit sensitivity to faces (de Haan et al., 2002). Thus, the face effects seen for the N170 component in adults are not preceded by a single component in infants, but rather emerge from two distinct developmental components (de Haan et al., 2002; Halit et al., 2003). By 12 months of age the characteristics of the infant N290 are very similar to the adult N170. For example, similar to the N170, the 12-month N290 appears to be larger in response to the inversion of human, but not monkey faces (Halit et al., 2003). This adult-like sensitivity is not seen at 3- and 6 –months for the N290; however, an inversion effect is found for the later P400 component (de Haan et al., 2002; Halit et al., 2003). Furthermore, similar to the adult N170, the P400 in 6-month-olds peaks faster to faces than objects (de Haan & Nelson, 1999) and displays similar hemispheric differences to featural and configural face changes in 8-month-olds as adults (Scott & Nelson, in press). The above results suggest that the developmental precursor to the adult N170 is a combination of two distinct components. Furthermore there is evidence of a functional shift between 6 and 12 months of age, resulting in a more adult-like N290. The current investigation was designed to further investigate this shift by testing 9-month-old infants.
Further elucidating the development of face sensitive ERP components not only has the potential to inform our understanding of the development of face processing, but also the nature and specificity of adult face processing. In the current study we recorded ERPs while 9-month-old infants viewed different types of faces. Two groups of infants were tested, one viewed monkey faces and the other human faces. Infants were first habituated to a single face and then saw the habituated face in both the frontal and profile (head turned slightly to the side) orientations, as well as a novel face in both orientations. This study was designed to follow-up and extend previous infant ERP studies of face perception by habituating or familiarizing infants to a single face in one orientation and testing generalization to another orientation for both human and monkey faces. There were several specific hypotheses this study was designed to address. First, we expected to replicate previous findings showing electrophysiological differences between processing human and monkey faces in infants. Second, we expected to see more specific electrophysiological differences for human compared to monkey faces. Previous behavioral investigations suggest that very young infants are able generalize human identify across different orientations (e.g. Cohen & Strauss, 1979). However, it is not known whether infants can generalize across multiple orientations of faces of other species. Given the behavioral findings suggesting that 9-month-olds have lost the ability to discriminate monkey faces without training (Pascalis et al., 2005) we expected to see ERP differences analogous to these behavioral findings. More specifically, we expected ERP differences individuating human but not monkey faces. Finally, based on previous reports indicating that both the N290 and the P400 are candidate precursors to the adult N170, we expected the above differences to be distributed across both the N290 and P400 components. Previous electrophysiological findings show that 6-month-olds exhibit species differences for the N290 and orientation/inversion effects for the P400 (de Haan et al., 2002). However, by 12-months of age orientation/inversion effects have shifted from the P400 to the N290 (Halit et al., 2003). For the present experiment we expected to find species differences for the N290 and orientation differences for either the N290 or the P400 or both depending on whether 9-month-old infants are more similar to their 6- or 12- month-old counterparts.
Methods
Participants
The Institutional Review Board at the University of _____________approved all methods and procedures used in this study. Parents of all infants gave informed consent prior to testing. Participants were healthy, full-term 9-month-old infants (n=26; females=14) recruited from birth announcements. Each participant completed either a monkey (n=13; females= 6) or a human (n=13; females=8) face task while ERPs were recorded from the scalp. Parents of participants were paid $5 and all infants were given a small toy for participation. Approximately 93% of the participants were Caucasian. The data from an additional 16 participants were excluded due to experimenter error (n=2), or infant fussiness (n=14).
Stimuli
Stimuli included grayscale images of human and monkey faces presented on a blue background in different orientations. Stimuli were created using Adobe Photoshop 5.5. Stimuli were presented using ERPW, a software program designed for electrophysiological data collection and processing. Stimuli consisted of 160 trials of each of 4 conditions (randomly presented with equal probability) for either the monkey or the human task. All stimuli were presented on a blue screen. Participants were seated approximately 60–70 centimeters away from the computer monitor and the visual angle subtended by the stimuli was 13.30 degrees. See Figure 1 for example of monkey and human face stimuli.
Figure 1.

Stimuli presented to participants on computer screen for either a monkey or human discrimination task. Familiarized pictures (left) enlarged for clarity, followed by test pictures of equal probability (right) presented at random.
General Procedure
Infant participants completed either the monkey or the human face task and were randomly assigned to either group. Infants were seated in a high chair while pictures of the faces were displayed on a computer screen in front of them. Each task consisted of a habituation period, followed by test trials. During this habituation period, a single frontally oriented monkey or human face was presented to participants on a computer screen. During stimulus presentation an experimenter, discretely located behind a large black divider, recorded looking time. The habituation period consisted of multiple trials of the same picture. Trials were indicated by the infant’s individual looking times. For example, a trial started when the infant fixated on the image and ended when the infant looked away from the image for more than 1 second. The same picture was continuously presented until the total combined looking time of the previous two trials was less than fifty percent of the total time for the longest two consecutive trials. Infants in the monkey face group took approximately 8.77 (SD=3.72) trials to habituate and infants in the human face group took approximately 6.63 (SD=2.61) trials to habituate (the difference between groups was not significant).
After the habituation phase, each infant was immediately presented with the frontally oriented familiarized face, the familiarized face in a different orientation (e.g. tilted to the side), an unfamiliar face in the frontal orientation, and the same unfamiliar face in a different orientation (see Figure 1). There was a total of 160 randomly presented trials in each task, including 40 presentations of each condition. Infants were only presented trials when their eyes were fixated on the screen. Once an infant became fussy or inattentive, the study was ended. In the monkey face task infants completed an average of 94.4 (SD=20.5) trials, and in the human faces task infants completed an average of 100.6 (SD=11.8) trials (these are not significantly different from each other). Each stimulus was presented for 500 milliseconds, followed by an inter-stimulus-interval of between 1000 and 1200 milliseconds. During this interval infants viewed a blank blue screen.
Electrophysiological Procedure
ERPs were recorded from 29 tin electrodes sewn into a lycra cap using a modified 10/20 system (Electro-Cap International ©, Eaton Ohio). The following electrodes were included in this recording: Fz, Cz, Pz, F3, F4, F7, F8, FC1, FC2, FC5, FC6, C3, C4, CP1, CP2, CP5, CP6, P3, P4, PO3, PO4, PO7, PO8, T3, T4, T5, T6, A1, A2. Two separate tin electrodes were placed on the mastoid bones behind each ear, which were held in place by sticky foam. The electro-oculogram (EOG) was recorded from electrodes placed above and below the eyes. Impedances were accepted if they were below 10 kOhms. All scalp electrodes were referenced online to the scalp electrode Cz (located at the vertex) and then re-referenced offline to the average reference. EEG data were acquired with a Grass Neuro Acquisition System ® with Model 12A5 amplifiers. The gain was set to 20,000 for all scalp leads. The gain for EOG was set at 5,000. A bandpass filter was set to .1 to 30 Hz, and a 60 Hz notch filter was in place. EEG data was sampled at 200Hz.
During electrophysiological recording, an experimenter was seated to the side of the infant to redirect his/her attention to the stimuli if they became distracted. A second experimenter sat behind a black screen with a small hole and presented the stimuli when the infant’s eyes were fixated on the screen. After the data were collected, they were edited, averaged and analyzed using ERPW. Vertical eye blinks were regressed from the EEG using an algorithm developed by Gratton and colleagues (Gratton, Coles, & Donchin, 1983). Blinks were identified and corrected if there was more than a 100-microvolt change in amplitude within a 50 ms window. Trials were rejected for movement artifacts if the EEG response exceeded +/− 250 microvolts within a 50 ms window. ERPs were baseline corrected with respect to a 100 ms pre-stimulus recording interval.
Individual artifact-free trials were combined to form average waveforms for each subject and each condition. Grand averages were computed for each condition and also collapsing across conditions (e.g. all monkey trials and all human trials to look at possible main effects; all familiar and unfamiliar trials; all frontal and profile trials). An average of 20.31 (SD= 5.08) trials were included in each of the 4 conditions for the monkey face task and 19.53 (SD= 4.17) trials were included for each condition for the human faces task.
Based on previous research investigating face perception in infants, 2 components of interest were identified. The N290 and P400 are recently identified infant components associated with face processing (deHaan et al., 2002; deHaan & Nelson, 1999; Halit et al., 2003; Scott & Nelson, in press).
Results
For each component electrodes were selected for analyses based on previous developmental reports (e.g de Haan et al., 2002; Halit et al., 2003; Scott & Nelson, in press), and by identifying the electrode locations where each component was most prominent. Both the N290 and the P400 were most conspicuous at A2, T6, and O2. Electrodes in each hemisphere (T5, A1, and O1; T6, A2, and O2) were grouped and averaged for further analyses. For the N290 and the P400, average amplitude was measured within windows that best captured these components (N290:190–340 ms after stimulus onset; P400: 270–730 ms after stimulus onset). The windows chosen for analysis of these components was based on previous research (Scott & Nelson, in press) and an examination of the current waveforms. Averages were then submitted to a 2 × 2 × 2 × 2 MANOVA with two levels of familiarity (familiar, unfamiliar), two levels of orientation (frontal, profile) and two levels of hemisphere (left, right). Group (monkey faces task, human faces task) was submitted as a between-subjects factor. Only significant and marginally significant results are described, but all MANOVA results are presented in Tables 1 and 2.
Table 1:
MANOVA main effects and interactions with group (monkey versus human faces task). Items in bold are significant results.
| Main Effect | Group Interaction | |
|---|---|---|
| N290 Familiarity |
F(1, 24) = 6.05, p=.02
η2=.20 |
F(1, 24) = 3.63, p=.07 η2=.13 |
| N290 Orientation |
F(1, 24) = .04, p=.84 η2=.002 |
F(1, 24)=.09, p=.77 η2=.004 |
| N290 Hemisphere |
F(1, 24 = 1.24, p=.28 η2=.05 |
F(1, 24) =.29, p=.60 η2=.01 |
| P400 Familiarity |
F(1, 24) = 8.45, p=.01
η2=.26 |
F(1, 24) = 4.24, p=.05
η2=.15 |
| P400 Orientation |
F(1, 24) = 1.84, p=.18 η2=.07 |
F(1, 24) = 2.00, p=.17 η2=.08 |
| P400 Hemisphere |
F(1, 24) = .10, p=.75 η2=.004 |
F(1, 24) = .21, p=.66 η2=.01 |
Table 2:
MANOVA interaction effects. Items in bold are significant results.
| Interaction Effects | Group Interactions | |
|---|---|---|
| N290 Familiarity * Orientation |
F(1, 24) = 2.02, p=.17 η2=.08 |
F(1, 24) = 2.84, p=.11 η2=.11 |
| N290 Familiarity * Hemisphere |
F(1, 24) = 2.68, p=.12 η2=.10 |
F(1, 24) = .56, p=.46 η2=.02 |
| N290 Orientation * Hemisphere |
F(1, 24) =.12, p=.73 η2=.01 |
F(1, 24) = 1.53, p=. 23 η2=.06 |
| N290 Familiarity * Orientation * Hemisphere |
F(1, 24) = .43, p=.52 η2=.02 |
F(1, 24) = .96, p=.34 η2=.04 |
| P400 Familiarity * Orientation | F(1, 24) = 3.22, p=.09 η2=.12 |
F(1, 24) = 5.54, p= .03
η2=.18 |
| P400 Familiarity * Hemisphere |
F(1, 24) = 5.41, p=.03
η2=.18 |
F(1, 24) = 1.09, p=.31 η2=.04 |
| P400 Orientation * Hemisphere | F(1, 24) = .001, p=.99 η2=.001 |
F(1, 24) =2.18, p=.15 η2=.08 |
| P400 Familiarity * Orientation * Hemisphere | F(1, 24) = 1.19, p=.29 η2=.05 |
F(1, 24) =.02, p=.89 η2=.001 |
N290.
As illustrated in Figure 2, the average amplitude of the N290 was significantly greater for familiar compared to unfamiliar faces. This difference marginally interacted with group (monkey, human), but did not interact with orientation (frontal, profile) or hemisphere (left, right). The marginal interaction with group was due to an enhanced response to familiar compared to unfamiliar human faces.
Figure 2.
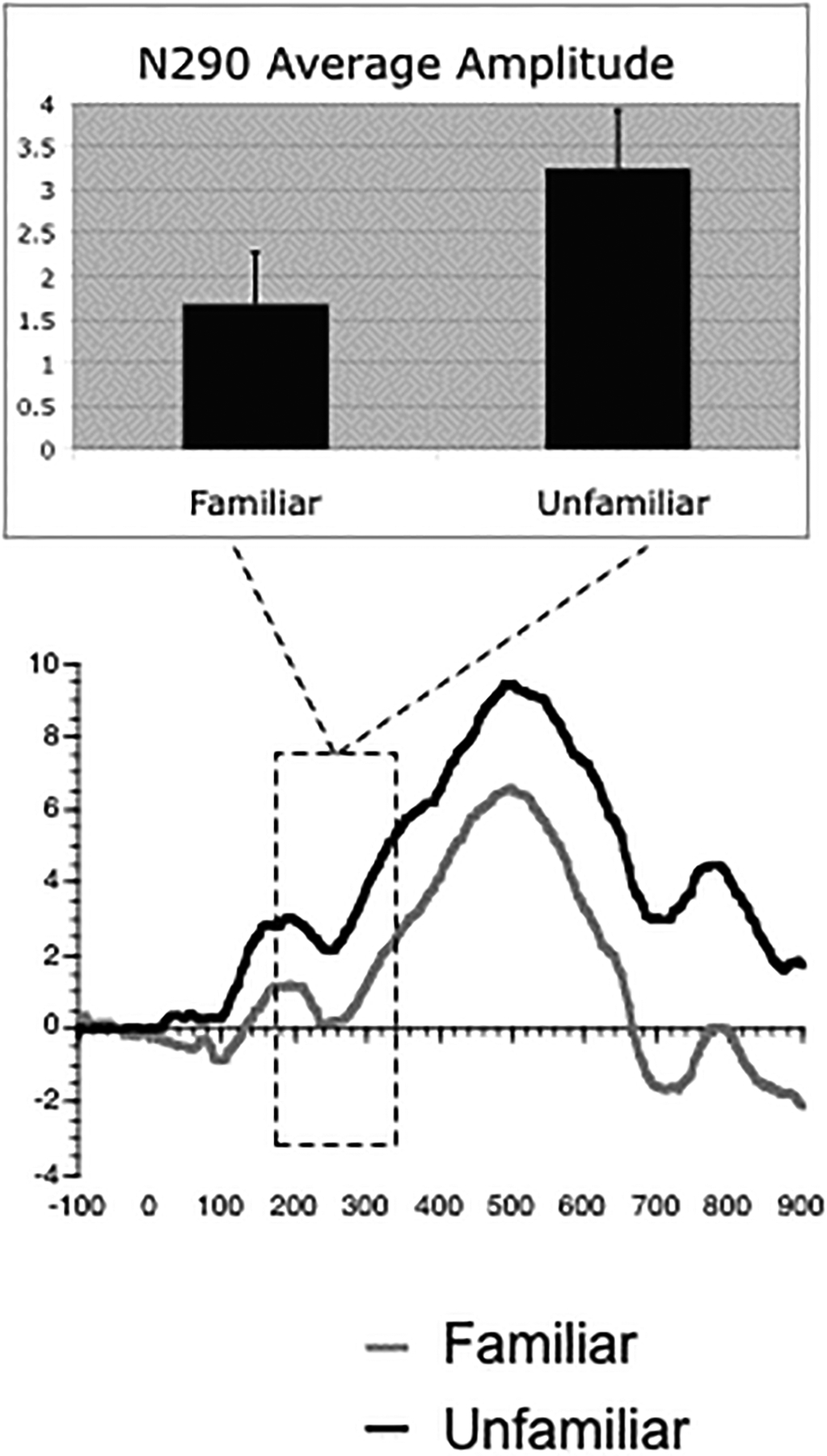
ERP results for the N290. Familiar faces (collapsed across orientations) elicited greater negative amplitude N290 than unfamiliar faces (collapsed across orientations). Means (+/− 1 SE) of these effects are illustrated in the top panels. Please note that the greater negativity is shown by a smaller mean average to familiar compared to unfamiliar faces because the average of this negative going component is a positive number. An average of electrodes A1, A2, T5, T6, O1, and O2 are pictured. Boxed regions indicate the time windows used for analyses.
P400.
For the P400, the average amplitude to unfamiliar faces was greater than to familiar faces. This difference between familiar and unfamiliar faces interacted with group and as can bee seen in Figure 3a, was specific to the participants who completed the monkey faces task. However, unlike participants in the monkey faces group, participants in the human faces group had an enhanced amplitude P400 to familiar frontal compared to familiar profile faces and to unfamiliar profile compared to unfamiliar frontal faces. This interaction is illustrated in Figure 3b. In addition, in the right hemisphere, unfamiliar faces elicited a greater response than familiar faces (See Figure 4). This hemisphere difference did not interact with group or orientation.
Figure 3.
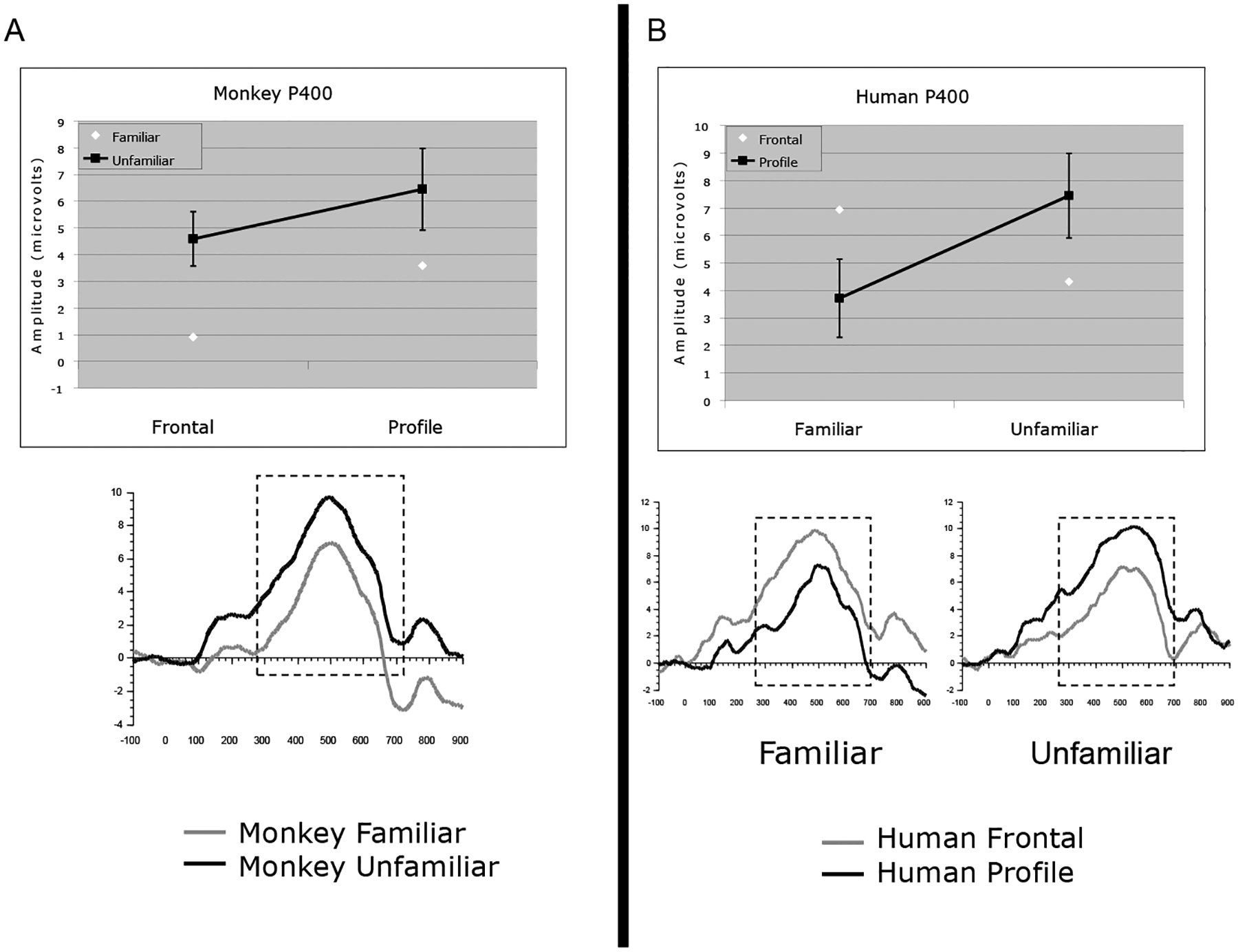
ERP results for the P400. The left panel illustrates the ERP effects for monkey faces and the right panel illustrates the effects for human faces. Means (+/− 1 SE) of these effects are illustrated in the top two panels. An average of electrodes A1, A2, T5, T6, O1, and O2 are pictured. Boxed regions indicate the time windows used for analyses.
Figure 4.
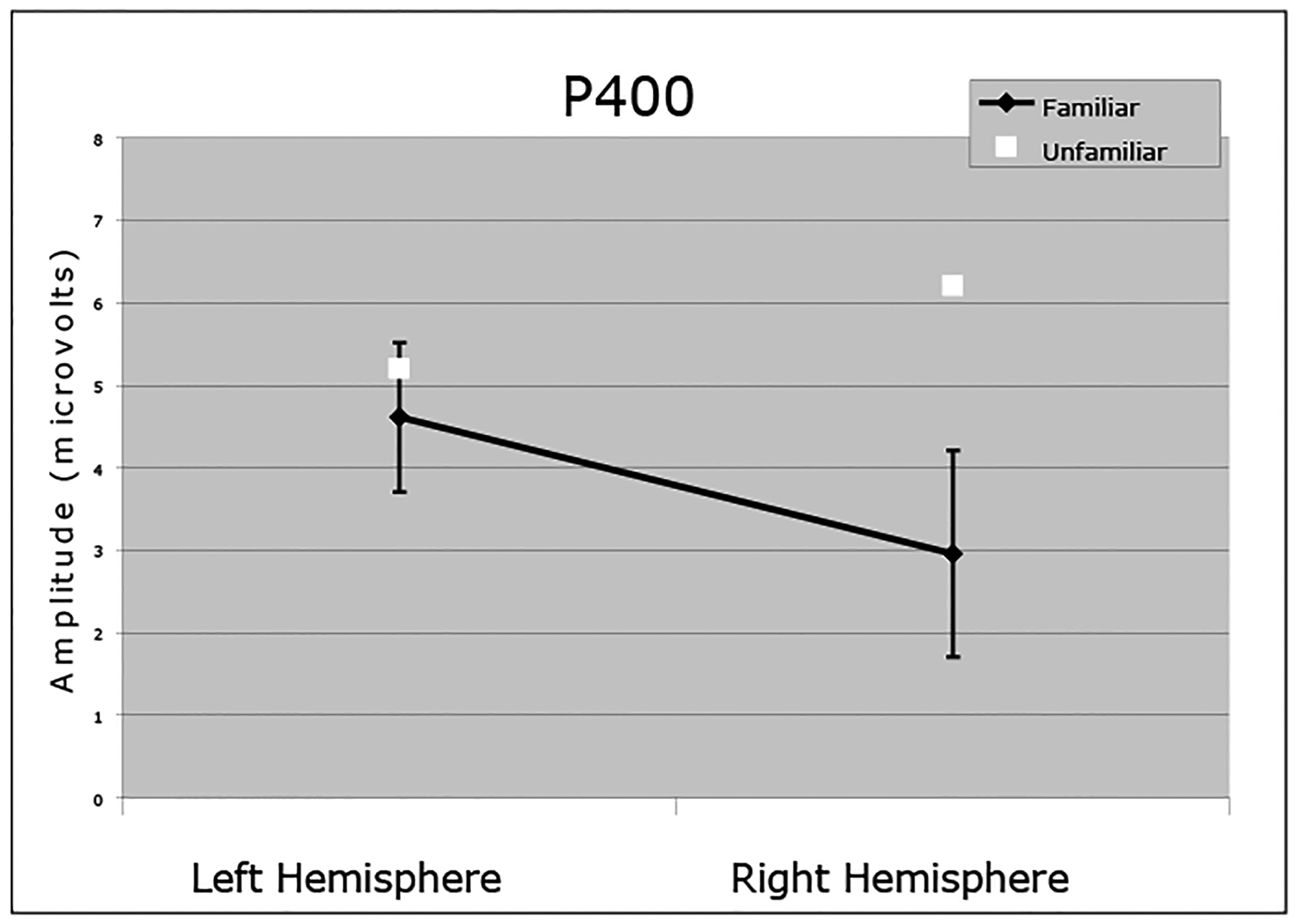
Means (+/− 1 SE) of the P400 hemisphere differences for familiar and unfamiliar faces.
Discussion
In brief, the 9-month-old ERP waveform appears to be differentially sensitive to human and monkey faces. Infants who completed the monkey face task showed amplitude differences for both the N290 (See Figure 2) and P400 (See Figure 3) to familiar versus unfamiliar faces. Infants in the human faces condition also showed familiarity differences as indexed by the N290. However, for the P400, amplitude was greater for frontal versus profile orientated familiar faces, and greater for profile versus frontal orientated unfamiliar faces (See Figure 3). Finally, for infants in both groups, unfamiliar faces elicited a greater amplitude response than familiar faces in the right hemisphere. This difference was not apparent in the left hemisphere (See Figure 4). These results are consistent with reports indicating larger right compared to left hemisphere neural responses to faces compared to other objects (e.g. Gauthier et al., 1999; Kanwisher et al., 1997).
These data suggest that the early perceptual processing of monkey and human faces in 9-month-olds, as indexed by the N290, is not yet completely tuned to pick up species differences (as the adult N170 is). However, there was a trend revealing an interaction between familiarity and species for the N290 (See Table 1). Although not significantly greater, there is an enhanced N290 to familiar compared to unfamiliar human, but not monkey faces. These findings suggest that 9-month-old infants may be beginning to have a more specialized and specific electrophysiological response to human faces compared to monkey faces. It is notable that for both human and monkey faces; infants are generalizing across orientations for the habituated or familiar and novel or unfamiliar faces. These data are consistent with recent reports, in adults, finding face familiarity and identity effects for the N170 (e.g. Jacques & Rossion, in press).
Contrary to expectations, the 9-month old electrophysiological response maintained sensitivity to monkey identity by showing similar enhancements for frontal and profile images of the same monkey. Previous behavioral data suggest that by 9-months-of-age the ability to discriminate monkey faces, using the VPC paradigm, is lost (Pascalis et al., 2002; Pascalis et al., 2005). However, untrained 6-month-olds and 9-month-olds, who have been trained with monkey faces, maintain the ability to discriminate these same faces. Thus without experience, the ability to behavioral discriminate monkey faces is lost. The results from the present experiment reveal a disconnect between behavioral looking time findings, indicating that 9-month-old infants do not discriminate monkey faces without previous experience, and the present electrophysiological findings. These findings may be due to methodological differences between the different tasks used (ERP-adapted habituation versus visual paired comparison tasks). Indeed, Sangrigoilo and de Schonen (2004) used a habituation procedure and found that increasing the number of exemplars to which infants are exposed during habituation to leads to better discrimination. Future research should examine the methodological differences between habituation and visual-paired-comparison procedures. The use of ERPs in the present study appears to provide a more sensitive measure than previous behavioral methods revealing that, although past findings suggest that 9-month-olds do not behaviorally discriminate monkey faces, part of the ventral visual stream may be processing this information.
Overall, the present data suggest that, in 9-month-olds, the processing of human faces is more specialized than the processing of monkey faces. Nine-month olds are able to break apart familiar and unfamiliar frontal and profile faces for human but not for monkey faces as reflected in the P400 component. These results provide further support for previous findings suggesting a gradual specialization of infant ERP face components (de Haan, et al., 2002; Halit et al., 2003). Moreover, the results reported here are partially consistent with previous adult studies suggesting differential processing of human and monkey faces for the N170 and VPP (de Haan et al., 2002; Scott et al., in press). This suggests that by 9-months-of-age, the neural systems that underlie face processing have begun to specialize based on differential experience with human versus monkey faces. The data presented here provide insight into the development of the visual system and suggest that the 9-month old ventral visual stream has become tuned for processing familiarity and orientation to human faces but only familiarity for monkey faces. Thus, by this age the importance of human over monkey faces is clearly delineated in the visual system. These results are also supported by the increased habituation time for monkey compared to human faces (although this difference was not significant). This is somewhat intuitive in light of the importance of recognizing human faces across many views/perspectives in everyday human interactions.
The current study is an important first step toward understanding the development and electrophysiological correlates of perceptual narrowing and the development of perceptual expertise. The present results provide further evidence for the relation of the N290 and P400 in the development of face processing abilities. Similar to previous reports (e.g. de Haan et al., 2002; Halit et al., 2003), the N290 and P400 in the present study appear to be differentially modulated by different stimulus manipulations. The N290 is modulated by changes in familiarity for both human and monkey faces, but more so for human faces. The P400 is sensitive to changes in familiarity for monkey faces, and both familiarity and orientation for human faces. Previously, 6-month-olds have been reported as processing the species and orientation of a stimulus at separate ERP components (the N290 and P400, respectively). This shifts to a single negative component by 12 months of age and into adulthood. The present data suggest that unlike 6-month-olds (de Haan et al., 2002), modulations of species are present for the P400 and to some extent for the N290. However, differences in the orientation of the faces are only present for the P400 and only for human faces.
In the context of previous behavioral studies (Pascalis et al., 2002; Pascalis et al., 2005) it would be informative to replicate the present study using concurrent behavioral and electrophysiological measures with younger infants to determine if there are brain changes associated with the loss of behavioral discriminatory abilities, and to determine whether younger infants show an increased ability to parse orientation in monkey faces compared to 9-month-olds. A particular challenge will be to detect in the brain how the loss of an ability is manifested; for example, if the ability to recognize monkey faces is entirely lost, how will this appear in scalp-recorded brain activity? Conversely, if this function is not lost but rather, inhibited or relegated to a non-functional circuit, might ERPs be able to detect such a circuit? These are just some of the challenges that await future research.
Contributor Information
Lisa Scott, University of Colorado.
Robert W. Shannon, University of Minnesota
Charles A. Nelson, Harvard Medical School
References
- Bentin S, Allison T, Puce A, Perez E, & McCarthy G (1996). Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience, 8, 551–565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentin S & Deouell LY (2000). Structural encoding and identification in face processing: ERP evidence for separate mechanisms. Cognitive Neuropsychology, 17, 35–54. [DOI] [PubMed] [Google Scholar]
- Botzel K, & Grusser O-J (1989). Electric brain potentials evoked by pictures of faces and non-faces: A search for “face-specific” EEG potentials. Experimental Brain Research, 77, 349–360. [DOI] [PubMed] [Google Scholar]
- Carmel D & Bentin S (2002). Domain specificity versus expertise: Factors influencing distinct processing of faces. Cognition, 83, 1–29. [DOI] [PubMed] [Google Scholar]
- Cheour M, Ceponiene R, Lehtokoski A, Luuk A, Allik J, Alho K, Näätänen R (1998). Development of language-specific phoneme representations in the infant brain. Nature Neuroscience, 5, 351–353. [DOI] [PubMed] [Google Scholar]
- Cohen LB & Strauss MS (1979). Concept acquisition in the human infant. Child Development, 50, 419–424. [PubMed] [Google Scholar]
- de Haan M, Johnson M, and Halit H (2003). Development of face-sensitive event-related potentials during infancy: a review. International Journal of Psychophysiology, 51, 45–58. [DOI] [PubMed] [Google Scholar]
- de Haan M & Nelson CA (1999). Brain Activity Differentiates Face and Object Processing in 6-Month-Old Infants. Developmental Psychology., 34, 1114–1121. [DOI] [PubMed] [Google Scholar]
- de Haan M, Pascalis O, & Johnson MH (2002). Specialization of neural mechanisms underlying face recognition in human infants. Journal of Cognitive Neuroscience, 12(2), 199–209. [DOI] [PubMed] [Google Scholar]
- Farah MJ (1996). Is face recognition ‘special’? Evidence from neuropsychology. Behavioral Brain Research, 76, 181–189. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Skudlarski P, Gore JC, & Anderson AW (2000). Expertise for cars and birds recruits brain areas involved in face recognition. Nature Neuroscience, 3, 191–197. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, & Gore JC (1999). Activation of the middle fusiform ‘face area’ increases with expertise in recognizing novel objects. Nature Neuroscience, 2, 568–573. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Williams P, Tarr MJ, & Tanaka J (1998). Training ‘greeble’ experts: a framework for studying expert object recognition processes. Vision Research, 38, 2401–2428. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MGH & Donchin E (1983). A new method of off-line removal of ocular artifact. Electroencephalography and Clinical Neurophysiology 55, 468–484. [DOI] [PubMed] [Google Scholar]
- Halit H, de Haan M, and Johnson MH (2003). Cortical specialisation for face processing: Face-sensitive event-related potential components in 3 and 12 month-old infants. NeuroImage, 1,9 1180–1193. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, & Pietrini P (2001). Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science, 293, 2425–2430. [DOI] [PubMed] [Google Scholar]
- Itier R, & Taylor MJ (2004). Source analysis of the N170 to faces and objects. Neuroreport, 15, 1261–1265. [DOI] [PubMed] [Google Scholar]
- Jacques C & Rossion B (in press). The speed of individual face categorization. Psychological Science. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA (1989). A face-responsive potential recorded from the human scalp. Experimental Brain Research 78, 193–202. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA (1996). Evoked potential studies of face and object processing. Visual Cognition, 3, 1–38. [Google Scholar]
- Jeffreys DA & Tukmachi ESA (1992). The vertex-positive scalp potential evoked by faces and objects. Experimental Brain Research, 91, 340–350. [DOI] [PubMed] [Google Scholar]
- Jeffreys DA, Tukamachi ESA, Rockley G (1992). Evoked potentials evidence for human brain mechanisms that respond to single, fixated faces. Experimental Brain Research, 91, 351–362. [DOI] [PubMed] [Google Scholar]
- Joyce C, & Rossion B (2005) The face-sensitive N170 and VPP components manifest the same brain processes: The effect of reference electrode site. Clinical Neurophysiology, 116, 2613–2631. [DOI] [PubMed] [Google Scholar]
- Kanwisher N (2000). Domain Specificity in Face Perception. Nature Neuroscience, 3, 759–763. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, & Chun MM. (1997). The fusiform face area: A module in human extrastriate cortex specialized for face perception. Journal of Neuroscience, 17, 4302–4311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl PK, Andruski JE, Chistovich IA, Chistovich LA, Kozhevnikova EV, Ryskina VL, Stolyarova EI, Sundberg U, & Lacerda F (1997). Cross-language analysis of phonetic units in language addressed to infants. Science, 277, 684–686. [DOI] [PubMed] [Google Scholar]
- Nelson CA (1993). The recognition of facial expressions in infancy: Behavioral and electrophysiological correlates. In de Boysson-Bardies B, de Schonen S, Jusczyk P, MacNeilage P, & Morton J (Eds.), Developmental Neurocognition: Speech and Face Processing in the First Year of Life (p. 187–193). The Netherlands: Kluwer Academic Press. [Google Scholar]
- Nelson CA (2001). The development and neural bases of face recognition. Infant and Child Development, 10, 3–18. [Google Scholar]
- Nelson CA (2003). The Development of Face Recognition Reflects an Experience-Expectant and Activity-Dependent Process. In Pascalis O & Slater A (Eds.), The Development of Face Processing in Infancy and Early Childhood. (p. 79–97). Nova Science Publishers, Inc. [Google Scholar]
- Pascalis O, deHaan M, & Nelson CA (2002). Is face processing species-specific during the first year of life. Science, 296, 1321–1323. [DOI] [PubMed] [Google Scholar]
- Pascalis O, Scott LS, Kelly DJ, Shannon RW, Nicholson E, Coleman M, & Nelson CA (2005). Plasticity of face processing in infancy. Proceedings of the National Academy of Sciences, 102, 5297–5300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossion B, Delvenne J-F, Debatisse D, Goffaux V, Bruyer R, Crommelinck M, & Guerit J-M (1999). Spatio-temporal localization of the face inversion effect: An event-related potentials study. Biological Psychology, 50, 173–189. [DOI] [PubMed] [Google Scholar]
- Rossion B, Joyce C, Cottrell G, & Tarr MJ (2004). Early lateralization and orientation tuning for face, word and object processing in the visual cortex. Neuroimage. [DOI] [PubMed] [Google Scholar]
- Rossion B, Dricot L, Devolder A, Bodart JM, Crommelinck M, de Gelder B, et al. (2000). Hemispheric asymmetries for whole-based and part-based face processing in the human fusiform gyrus. Journal of Cognitive Neuroscience, 12(5), 793–802. [DOI] [PubMed] [Google Scholar]
- Sagiv N & Bentin S (2001). Structural encoding of human and schematic faces: Holistic and part-based processes. Journal of Cognitive Neuroscience, 13, 937–951. [DOI] [PubMed] [Google Scholar]
- Sangrgoli S & de Schonen S (2004). Recognition of own-race and other-race faces by three-month-old infants. Journal of Child Psychology and Psychiatry, 45, 1219–1227. [DOI] [PubMed] [Google Scholar]
- Scott LS & Nelson CA (in press). Featural and configural face processing in adults and infants: A behavioral and electrophysiological investigation. Perception. [DOI] [PubMed] [Google Scholar]
- Scott LS, Shannon RW, & Nelson CA (in press) Behavioral and electrophysiological evidence of species-specific face processing. Cognitive, Affective and Behavioral Neuroscience. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker DM (1993). Spatial sampling of head electrical fields: the geodesic sensory net. Electroencephalography and Clinical Neurophysiology, 87, 145–163. [DOI] [PubMed] [Google Scholar]
- Werker JH & Tees RC (1984). Cross language speech percpetion: Evidence for perceptual reorganization during the first year of life. Infant Behavior and Development, 7, 49–63. [Google Scholar]


