Abstract
Background: Men who have sex with men (MSM) living with HIV make up the majority of cases in the current Mpox outbreak. We aimed to investigate the perception of and vaccine readiness towards Mpox among MSM living with HIV in China. Methods: This cross-sectional online study was conducted from 10 August to 9 September 2022. Participants responded to survey questions about their socio-demographic information, HIV status, sexual behaviors, knowledge of Mpox, and attitudes towards Mpox vaccines. Results: A total of 577 MSM living with HIV participated in the study. A total of 37.6% expressed concerns about the Mpox epidemic in China, and 56.8% were willing to get the Mpox vaccine. Men who had > four sexual partners in the previous three months (aOR = 1.9 95% CI: 1.2–2.8 Ref: 0), had close contact with > four individuals in a day (3.1, 1.5–6.5 Ref: 0–3), were worried about the Mpox epidemic in China (1.6, 1.1–2.3 Ref: No), and believed that Mpox vaccines are safe (6.6, 2.7–16.4 Ref: No or not sure) and effective (1.9, 1.1–3.3 Ref: No) for people living with HIV were more likely to be willing to get the Mpox vaccine. MSM living with HIV with a high school education or below (0.5, 0.3–0.9 Ref: Postgraduate diploma), and sometimes (0.5, 0.3–0.8 Ref: Often), seldom, or never (0.5, 0.3–0.9 Ref: Often) followed news about Mpox were unwilling to get the Mpox vaccine. Conclusion: The ongoing Mpox pandemic has not attracted widespread concerns among MSM living with HIV in China. Having more sexual partners and close contacts, worrying about the Mpox epidemic, and believing in the vaccine’s safety and efficacy were predictors of their willingness to get the Mpox vaccine. Efforts should be made to raise awareness of the potential risk of Mpox in this at-risk population. Public health strategies should fully address predictors of vaccination willingness.
Keywords: Mpox, HIV, men who have sex with men, perception, willingness of vaccination
1. Introduction
Human Mpox is a zoonotic disease caused by the Mpox virus (MPXV), first recorded in the Democratic Republic of Congo in 1970 [1]. MPXV is a double-stranded DNA virus similar to smallpox and belongs to the orthopoxvirus [2,3]. Previously, the occurrence of Mpox outside Africa was mostly related to international travel and animal contact, and no secondary human-to-human transmission was confirmed [3,4]. However, since early May 2022, European and North American countries have reported a large number of Mpox cases [5,6,7]. This unusual Mpox outbreak has covered 103 countries and regions worldwide, 96 of which have historically never reported Mpox cases [8]. On 23 July 2022, the World Health Organization (WHO) issued the highest level of alert and declared that the global Mpox outbreak constitutes a Public Health Emergency of International Concern (PHEIC) [9].
As of 12 December 2022, a total of 82,503 confirmed cases, including 65 deaths, have been reported globally [8]. Although there is no clear evidence that the Mpox virus can be sexually transmitted, many recent cases in the current outbreak have been linked to sexual activities, especially among men who have sex with men (MSM) [7,10,11]. Potential sexual transmission is supported by lesions that initially occur at the site of sexual contact, including genital and anal lesions [12]. Surveillance data from multiple countries suggest that 28–51% of MSM patients with Mpox are people living with HIV (PLHIV) [13]. Although clinical data on Mpox in PLHIV are limited, studies have described that advanced or uncontrolled HIV infection may result in more severe outcomes. A study from Nigeria showed that PLHIV were more likely to have a larger skin rash, secondary bacterial infection, and longer duration of illness [14]. Recent reports from several countries with Mpox outbreaks indicate that a more severe disease course has not been described in PLHIV who have a robust immune system [15,16]. There is currently no specific treatment for Mpox, and vaccination is recommended for those at high risk of infection. There are two vaccines available to prevent orthopoxvirus infection [13]. JYNNEOS, a live virus vaccine, is licensed by the Food and Drug Administration to protect against Mpox in adults and has been evaluated to be safe and effective in PLHIV [17,18]. ACAM2000 is contraindicated in PLHIV because it may cause serious Mpox-related diseases in people with compromised immune systems [19].
Existing studies have found that many PLHIV continue to be sexually active and engage in risky sexual behaviors after their diagnosis [20,21,22]. We believe that MSM living with HIV are a high-risk population in the current Mpox outbreak. When vaccine resources are limited, MSM living with HIV may need to be considered as a priority group. In addition, previous studies have shown that despite specific guidelines, people living with HIV remain skeptical about the safety and effectiveness of vaccination because of their immunosuppressed status [23,24]. It is necessary to assess the perception of and vaccine readiness towards Mpox among this population who are at very high risk for Mpox transmission. Most studies have been based on the general population’s perception of Mpox, and little attention has been paid to MSM living with HIV [25,26], who comprise the majority of cases in the ongoing Mpox pandemic. This study is one of the first attempts to assess the perceptions of and vaccine readiness towards Mpox among MSM living with HIV in China. Correlates of the acceptance of Mpox vaccination were further tested in this population.
2. Methods
2.1. Study Design and Participants
This study is reported as per the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Table S1) [27].
This cross-sectional web-based study was conducted between 10 August and 9 September 2022. Participants were recruited by convenience sampling through Li Hui Shi Kong, an online WeChat official account with over 76,000 subscribers living with HIV from various regions in the country, and the estimated proportion of MSM is 75% [28]. When the survey QR code attached to the poster advertisement was scanned, a voluntary informed consent screen would be first opened. The voluntary informed consent also provided information about the purpose of the study, which was to investigate the perceptions of and vaccine readiness towards Mpox among MSM living with HIV. Participants who acknowledged reading the informed consent and providing consent electronically were directed to the formal questions of the questionnaire. The online questionnaire was programmed on Wenjuanxing, an online questionnaire survey platform with 82 million users in China. Inclusion criteria of individuals included the following: (1) ≥18 years, (2) identified as MSM, (3) self-identified as HIV-positive, and (4) had internet access. Study eligibilities were assessed automatically through the Wenjuanxing platform and double-checked during data cleaning.
2.2. Measures
A structured questionnaire was composed of six main sections addressing the following: socio-demographic information, HIV status, sexual behaviors, social life, knowledge of Mpox, and attitudes towards vaccines. The survey instruments used in this study were adapted from our previously published studies on COVID-19 and modified according to the questionnaire of other studies on the knowledge of and attitudes towards the current Mpox outbreak [25,29,30,31,32].
Variables in socio-demographics were as follows: age, gender identity, educational attainment, accommodation location, marital status, employment attainment, salary, sexual orientation, and disease history of chronic diseases and STD infections. People aged 18–25 are considered to be adolescents and young adults, while people over 40 are often classified as middle-aged, and may differ in their sexual behaviors, levels of knowledge, and risk awareness. Therefore, age groups were divided into three groups (≤25, 26–39, and >40) in this study. Chronic disease including cardiovascular disease, cancer, chronic respiratory disease, and other chronic diseases. For health conditions and sexual behaviors, participants reported information including their time since HIV diagnosis, latest HIV viral load results, latest CD+ 4 cell count (per uL), ART adherence (eight-item Morisky Medication Adherence Scale), vaccine history of smallpox, anal and oral sex in the past three months, number of anal sex partners in the past three months, and condom use. Inconsistent condom use was defined as reporting any frequency other than “always” during anal sex in the past three months. We also asked individuals whether they had recently experienced close daily contact, close daily conversations, or attended entertainment events. Close contact was defined as contact with body parts or bodily fluids (e.g., saliva, blood, sweat). Close conversations were defined as verbal communication within 1 m without a mask. Participants answered questions on their perceptions of Mpox, including their information sources and frequency of seeking information about Mpox, concerns about the Mpox epidemic, the safety and effectiveness of the Mpox vaccine, and perceived symptoms and transmission characteristics of Mpox. The willingness to adopt Mpox vaccine was measured by a five-point Likert scale (“Would you like to be vaccinated against Mpox?”) ranging from 1 (strongly disagree) to 5 (strongly agree). For this study, vaccine acceptance was re-categorized as willingness (4–5) and unwillingness (1–3). Participants who were unwilling to be vaccinated were asked to state their main reasons for their unwillingness. The seven-item Generalized Anxiety Disorder Scale (GAD-7) was used to assess anxiety status in this population [29]. The standardized Cronbach’s α of the GAD-7 in this study was 0.949. The Kaiser–Meyer–Olkin measure of sampling adequacy of the GAD-7 in this study was 0.945. Before the questionnaire was distributed, 54 MSM living with HIV participated in a pilot study to verify and modify the contents of the questionnaire. However, these participants in a pilot study were not included in the final survey.
2.3. Statistical Analysis
Descriptive analysis was performed for numerical and categorical variables. Additionally, median and interquartile range (IQR) or frequencies were calculated. Pearson’s chi-squared test was used to compare variables between MSM living with HIV willing to or refusing to get the Mpox vaccine. Two-sided p < 0.05 is considered statistically significant. Multivariable logistic regression was used to explore the associated factors of participants’ willingness to get the Mpox vaccine. Pearson’s chi-squared test was used to assess what variables could potentially explain participants’ support for vaccination against Mpox. Multivariate modeling was carried out using multiple logistic regression with the final choice of the model, including variables with p < 0.05 in univariate analysis. We also performed a multicollinearity diagnosis for the final logistic regression model by using the variance inflation factor (VIF). For any predictor variable, the square root of the VIF indicates the degree to which the confidence interval for that variable’s regression parameter is expanded relative to a model with uncorrelated predictors. As a general rule, a VIF > 10 indicates a multicollinearity problem. We used stepwise logistic regression to identify predictors of willingness to get the Mpox vaccine. Results of final logistic regression were reported as odds ratios (OR) and adjusted odds ratios (aOR) with corresponding 95% confidence intervals (95% CI). Analyses were performed using IBM SPSS Version 20.0 (SPSS, Inc. Chicago, IL, USA).
3. Results
3.1. Background Characteristics
The online survey was completed by 577 eligible MSM living with HIV, covering seven administrative divisions in mainland China (Table 1). Five hundred and twenty-three (90.6%) participants were cisgender men, and 54 (9.4%) participants were transgender women. The median age of the participants was 32 years (inter-quartile range: 28–37). Most of the participants were single (50.3%) and had a Bachelor’s degree (69.5%). A higher proportion of participants (46.1%) were employed, with most reporting a salary between CNY 5000 and 9999 (USD 722–1444). Seventy-two participants (12.5%) had a self-reported history of chronic disease; the most common sexually transmitted disease self-reported was syphilis (10.4%). More than half of the participants (56.8%) were ready to get the vaccine against Mpox at the current stage of the disease. The participants in the willing group were more likely to have a postgraduate diploma (p = 0.028).
Table 1.
Demographic characteristics of MSM living with HIV in China.
| Characteristics | Total N = 577 |
Willing to Receive Mpox Vaccination n = 328 |
Unwilling to Receive Mpox Vaccination n = 249 |
p Value |
|---|---|---|---|---|
| Gender Identity | 0.624 | |||
| Cisgender male | 523 (90.6) | 299 (91.2) | 224 (90.0) | |
| Transgender women | 54 (9.4) | 29 (8.8) | 25 (10.0) | |
| Age, year | 0.319 | |||
| ≤25 | 88 (15.3) | 44 (13.4) | 44 (17.7) | |
| 26–39 | 400 (69.3) | 230 (70.1) | 170 (68.2) | |
| ≥40 | 89 (15.4) | 54 (16.5) | 35 (14.1) | |
| Education | 0.028 | |||
| High school or below | 103 (17.9) | 55 (16.8) | 48 (19.3) | |
| Undergraduate diploma | 401 (69.5) | 221 (67.4) | 180 (72.3) | |
| Postgraduate diploma | 73 (12.7) | 52 (15.9) | 21 (8.4) | |
| Chinese geographical division | 0.177 | |||
| North China | 80 (13.9) | 50 (15.2) | 30 (12.0) | |
| Northeast China | 26 (4.5) | 17 (5.2) | 9 (3.6) | |
| East China | 221 (38.3) | 120 (36.6) | 101 (40.6) | |
| Central China | 64 (11.1) | 46 (14.0) | 22 (8.8) | |
| South China | 90 (15.6) | 43 (13.1) | 47 (18.9) | |
| Southwest China | 68 (11.8) | 17 (5.2) | 11 (4.4) | |
| Northwest China | 28 (4.9) | 35 (10.7) | 29 (11.6) | |
| Marital status | 0.412 | |||
| Single | 290 (50.3) | 156 (47.6) | 134 (53.8) | |
| Unmarried | 219 (38.0) | 132 (40.2) | 87 (34.9) | |
| Married | 50 (8.7) | 28 (8.5) | 22 (8.8) | |
| Other | 18 (3.1) | 12 (3.7) | 6 (2.4) | |
| Employment status | 0.674 | |||
| Full-time employment | 504 (87.3) | 290 (88.4) | 214 (85.9) | |
| Student | 40 (6.9) | 21 (6.4) | 19 (7.6) | |
| Unemployed | 33 (5.7) | 17 (5.2) | 16 (6.4) | |
| Salary (CNY) | 0.286 | |||
| 0–4999 | 201 (34.8) | 107 (32.6) | 94 (37.8) | |
| 5000–9999 | 237 (41.1) | 135 (41.2) | 102 (41.0) | |
| ≥10,000 | 139 (24.1) | 86 (26.2) | 53 (21.3) | |
| Region | 0.270 | |||
| Urban | 534 (92.5) | 307 (93.6) | 227 (91.2) | |
| Rural | 43 (7.5) | 21 (6.4) | 22 (8.8) | |
| Sexual orientation | 0.854 | |||
| Heterosexual or bisexual | 79 (13.7) | 47 (14.3) | 32 (12.9) | |
| Homosexual | 488 (84.6) | 275 (83.8) | 213 (85.5) | |
| Other or not sure | 10 (1.7) | 6 (1.8) | 4 (1.6) | |
| History of chronic diseases | 0.598 | |||
| No | 505 (87.5) | 285 (86.9) | 220 (88.4) | |
| Yes | 72 (12.5) | 43 (13.1) | 29 (11.6) | |
| Self-reported STD diagnoses | 0.845 | |||
| Yes | 83 (14.4) | 48 (14.6) | 35 (14.1) | |
| No | 494 (85.6) | 280 (85.4) | 214 (85.9) | |
| Level of anxiety severity, GAD-7 score | 0.428 | |||
| No or mild anxiety (<10) | 488 (84.6) | 274 (83.5) | 214 (85.9) | |
| Moderate to severe anxiety (≥10) | 89 (15.4) | 54 (16.5) | 35 (14.1) |
Abbreviations: CNY: Chinese Yuan; CNY 100 ≈ USD 15; ART: antiretroviral therapy; MSM: men who have sex with men; HIV: human immunodeficiency virus; STD: Sexually transmitted diseases. Chinese Geographical Division: North China (Beijing, Tianjin, Hebei, Shanxi, Inner Mongolia); Northeast China (Heilongjiang, Jilin, Liaoning); East China (Shanghai, Jiangsu, Zhejiang, Anhui, Jiangxi, Shandong, Fujian, Taiwan); Central China (Henan, Hubei, Hunan); South China (Guangdong, Guangxi, Hainan, Hong Kong, Macao); Southwest China (Chongqing, Sichuan, Guizhou, Yunnan, Tibet); Northwest China (Shaanxi, Gansu, Qinghai, Ningxia, Xinjiang). Chronic disease including cardiovascular disease, cancer, chronic respiratory disease, and other chronic diseases.
Regarding their HIV statuses and sexual behaviors, 371 of the participants (64.3%) had been diagnosed with HIV for over three years, and 443 participants (76.8%) reported an undetectable viral load of HIV (Table 2). The majority of the participants (90.4%) had medium or high ART adherence. A total of 392 participants (67.9%) had anal sex in the past three months, 47.7% reported inconsistent condom use, and 18.9% reported more than four sexual partners. About half of the participants reported anal (45.1%) or oral (51.5%) sex more than once per month. Furthermore, a minority of participants (8.8%) reported having close contact with more than four individuals in a day, and 346 participants (60.0%) reported having close conversations with more than four individuals in a day. Participants in the willing group were more likely to have an undetectable HIV viral load (p < 0.001), high ART adherence (p = 0.040), fewer anal sex partners in the past three months (p < 0.001), and more close contacts daily (p = 0.018).
Table 2.
HIV status, sexual behaviors, and social contact variables related to willingness to get vaccinated among MSM living with HIV in China.
| Variables | Total N = 577 |
Willing to Receive Mpox Vaccination n = 328 |
Unwilling to Receive Mpox Vaccination n = 249 |
p Value |
|---|---|---|---|---|
| Time since HIV diagnosis (months) | 0.969 | |||
| ≤12 | 78 (13.5) | 44 (13.4) | 34 (13.7) | |
| 13–35 | 128 (22.2) | 74 (22.6) | 54 (21.7) | |
| ≥36 | 371 (64.3) | 210 (64.0) | 161 (64.7) | |
| Latest HIV viral load | <0.001 | |||
| Detectable | 82 (14.2) | 41 (12.5) | 41 (16.5) | |
| Undetectable | 443 (76.8) | 269 (82.0) | 174 (69.9) | |
| Not sure | 52 (9.0) | 18 (5.5) | 34 (13.7) | |
| Latest CD4 cell count (per uL) | 0.268 | |||
| <200 | 16 (2.8) | 6 (1.8) | 10 (4.0) | |
| 200 to 349 | 62 (10.7) | 35 (10.7) | 27 (10.8) | |
| 350 to 499 | 164 (28.4) | 97 (29.6) | 67 (26.9) | |
| ≥500 | 307 (53.2) | 178 (54.3) | 129 (51.8) | |
| Not sure | 28 (4.9) | 12 (3.7) | 16 (6.4) | |
| ART adherence, MMAS-8 score | 0.040 | |||
| Low (scores < 6) | 55 (9.6) | 24 (7.4) | 31 (12.6) | |
| Medium (6 ≤ scores < 8) | 187 (32.7) | 101 (31.1) | 86 (34.8) | |
| High (scores = 8) | 330 (57.7) | 200 (61.5) | 130 (52.6) | |
| Anal sex in the past three months | <0.001 | |||
| No | 185 (32.1) | 87 (26.5) | 98 (39.4) | |
| Yes | 392 (67.9) | 241 (73.5) | 151 (60.6) | |
| Frequency of anal sex in the past three months | 0.568 | |||
| ≥4 times per month | 72 (12.5) | 42 (12.8) | 30 (12.0) | |
| 1–3 times per month | 188 (32.6) | 112 (34.1) | 76 (30.5) | |
| <1 time per month | 317 (54.9) | 174 (53.0) | 143 (57.4) | |
| Frequency of oral sex in the past three months | 0.112 | |||
| ≥4 times per month | 84 (14.6) | 46 (14.0) | 38 (15.3) | |
| 1–3 times per month | 213 (36.9) | 133 (40.5) | 80 (32.1) | |
| <1 time per month | 280 (48.5) | 149 (45.4) | 131 (52.6) | |
| Number of anal sex partners in the past three months | <0.001 | |||
| ≥4 | 74 (12.8) | 37 (11.3) | 37 (14.9) | |
| 1–3 | 318 (55.1) | 204 (62.2) | 114 (45.8) | |
| 0 | 185 (32.1) | 87 (26.5) | 98 (39.4) | |
| Inconsistent condom use during anal sex | 0.683 | |||
| Yes | 187 (47.7) | 113 (46.9) | 74 (49.0) | |
| No | 205 (52.3) | 128 (53.1) | 77 (51.0) | |
| Experience community lockdown due to COVID-19 | 0.413 | |||
| Yes | 175 (30.3) | 95 (29.0) | 80 (32.1) | |
| No | 402 (69.7) | 233 (71.0) | 169 (67.9) | |
| Self-reported number of close contacts daily | 0.018 | |||
| 0–3 | 526 (91.2) | 291 (88.7) | 235 (94.4) | |
| ≥4 | 51 (8.8) | 37 (11.3) | 14 (5.6) | |
| Self-reported number of close conversations daily | 0.401 | |||
| 0–3 | 231 (40.0) | 138 (42.1) | 93 (37.3) | |
| 4–7 | 137 (23.7) | 72 (22.0) | 65 (26.1) | |
| ≥8 | 209 (36.2) | 118 (36.0) | 91 (36.5) | |
| Frequency of visiting entertainment venues in the past three months | 0.181 | |||
| ≥4 times per month | 24 (4.2) | 13 (4.0) | 11 (4.4) | |
| 1–3 times per month | 39 (6.8) | 27 (8.2) | 12 (4.8) | |
| <1 time per month | 103 (17.9) | 51 (15.5) | 52 (20.9) | |
| Never | 441 (71.2) | 237 (72.3) | 174 (69.9) |
Abbreviations: ART: antiretroviral therapy; MSM: men who have sex with men; HIV: human immunodeficiency virus. ART adherence information is missing for five individuals.
3.2. Knowledge about Mpox
As shown in Table 3, one in five (20.8%) participants followed news about Mpox frequently, and the main way they did so was through news media, such as the Internet (79.9%). Two hundred and seventeen participants (37.6%) were worried about the Mpox epidemic in China. Most participants agreed that Mpox could infect anyone through close contact (93.6%), and individuals living with HIV make up a large proportion of the current Mpox outbreak (80.1%). Regarding vaccines, 45.9% of the participants reported having received the smallpox vaccine, while 81.3% agreed that Mpox vaccines were effective against Mpox. Participants in the willing group were more likely to follow news about Mpox often (p < 0.001), get vaccinated for smallpox (p = 0.029), worry about the Mpox epidemic in China (p = 0.007), believe Mpox vaccines are safe for PLHIV (p < 0.001), and believe Mpox vaccines can be effective against Mpox (p < 0.001).
Table 3.
Knowledge about Mpox variables related to willingness to get vaccinated among MSM living with HIV in China.
| Variables | Total N = 577 |
Willing to Receive Mpox Vaccination n = 328 |
Unwilling to Receive Mpox Vaccination n = 249 |
p Value |
|---|---|---|---|---|
| Frequency of following information about the Mpox * | <0.001 | |||
| Often | 120 (20.8) | 89 (27.1) | 31 (12.4) | |
| Sometimes | 303 (52.5) | 163 (49.7) | 140 (56.2) | |
| Seldom or never | 154 (26.7) | 76 (23.2) | 78 (31.3) | |
| Source of Mpox information | ||||
| TV programs or Newspaper | 166 (28.8) | 95 (29.0) | 71 (28.5) | 0.906 |
| Internet | 461 (79.9) | 261 (79.6) | 200 (80.3) | 0.824 |
| Communication with friends and family | 25 (4.3) | 15 (4.6) | 10 (4.0) | 0.745 |
| Uptake smallpox vaccination | 0.029 | |||
| Yes | 265 (45.9) | 160 (48.8) | 105 (42.2) | |
| No | 213 (36.9) | 106 (32.3) | 107 (43.0) | |
| Not sure | 99 (17.2) | 62 (18.9) | 37 (14.9) | |
| Worry about the Mpox epidemic in China | 0.007 | |||
| Yes | 217 (37.6) | 139 (42.4) | 78 (31.3) | |
| No | 360 (62.4) | 189 (57.6) | 171 (68.7) | |
| I believe Mpox vaccines are safe for PLHIV | <0.001 | |||
| Yes | 519 (89.9) | 321 (97.9) | 198 (79.5) | |
| No | 21 (3.6) | 7 (2.1) | 14 (5.6) | |
| Not sure | 37 (6.4) | 0 (0.0) | 37 (14.9) | |
| Mpox can spread to anyone through close contact | 0.084 | |||
| Yes | 540 (93.6) | 312 (95.1) | 228 (91.6) | |
| No | 37 (6.4) | 16 (4.9) | 21 (8.4) | |
| Most people with Mpox recover fully within 2 to 4 weeks without the need for medical treatment | 0.646 | |||
| Yes | 333 (57.7) | 192 (58.5) | 141 (56.6) | |
| No | 244 (42.3) | 136 (41.5) | 108 (43.4) | |
| PLHIV make up a large proportion of current Mpox outbreak | 0.358 | |||
| Yes | 462 (80.1) | 267 (81.4) | 195 (78.3) | |
| No | 115 (19.9) | 61 (18.6) | 54 (21.7) | |
| ART may reduce the risk of severe illness from Mpox in PLHIV | 0.565 | |||
| Yes | 286 (49.6) | 166 (50.6) | 120 (48.2) | |
| No | 291 (50.4) | 162 (49.4) | 129 (51.8) | |
| Mpox vaccines can be effective against Mpox | <0.001 | |||
| Yes | 469 (81.3) | 293 (89.3) | 176 (70.7) | |
| No | 108 (18.7) | 35 (10.7) | 73 (29.3) |
Abbreviations: ART: antiretroviral therapy; MSM: men who have sex with men; HIV: human immunodeficiency virus; PLHIV: people living with HIV. * Participants could list all sources of information they used.
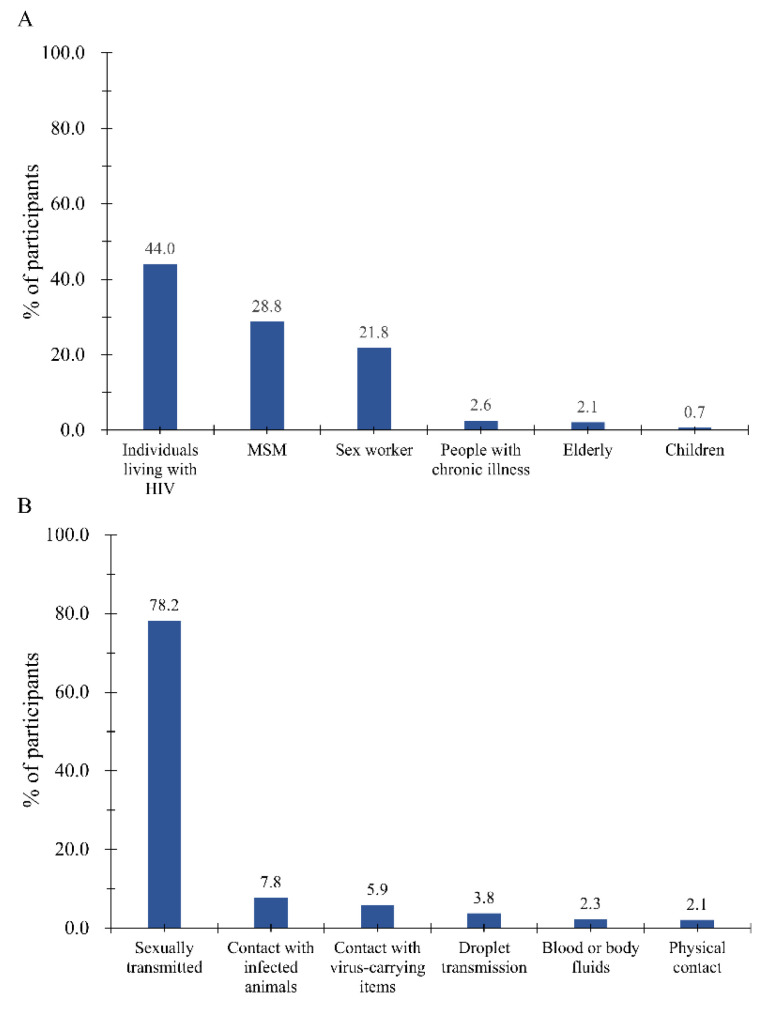
A total of 44.0% of the participants considered individuals living with HIV to be the highest risk group for Mpox (Figure 1A), followed by MSM (28.8%), sex workers (21.8%), people with chronic illnesses (2.7%), the elderly (2.1%), and children (0.7%). Regarding the dominant transmission route of Mpox in the current outbreak (Figure 1B), most participants considered this to be sexual transmission (78.2%), followed by contact with infected animals (7.8%), contact with virus-carrying items (5.9%), droplet transmission (3.8%), blood or body fluids (2.3%), and physical contact (2.1%).
Figure 1.
Participates’ perceived groups at high risk (A) and dominant transmission route of Mpox (B). An elderly person is defined as anyone over the age of 60. A child is defined as anyone under the age of 18.
3.3. Reasons for Unwillingness to Get the Mpox Vaccine
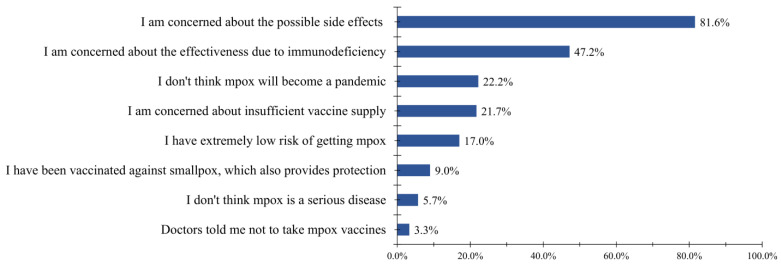
Figure 2 shows the reasons for the participants’ unwillingness to get vaccinated against Mpox. Most participants (81.6%) were concerned about the possible side effects of Mpox vaccines. A total of 47.2% of participants were concerned that the vaccine would not work in an immunocompromised population. Other reasons were as follows: believing that Mpox will not become a pandemic (22.2%), having concerns about insufficient vaccine supply (21.7%), having an extremely low risk of contracting Mpox (17.0%), having been vaccinated against smallpox which also provides protection (9.0%), believing that Mpox is not a serious disease (5.7%), and being told not to take the Mpox vaccine by a doctor (3.3%).
Figure 2.
Reasons for unwillingness to get vaccinated against Mpox.
3.4. Predictors of Willingness to Get the Mpox Vaccine
Table 4 presents the predictors of the participants’ willingness to get the Mpox vaccine among MSM living with HIV. In total, ten predictors of willingness to get the Mpox vaccine were included in the final model, including one sociodemographic variable (‘Education’), two HIV status variables (‘Latest HIV viral load’ and ‘ART adherence’), one sexual behaviors variable (‘Number of anal sex partners in the past three months’), one social contact variable (‘Self-reported number of close contacts daily’), and five knowledge variables (‘Frequency of following information about the Mpox’, ‘Uptake smallpox vaccination’, ‘Worry about the Mpox epidemic in China’, ‘I believe Mpox vaccines are safe for PLHIV’, and ‘Mpox vaccines can be effective against Mpox’). In the multivariate logistic regression analysis, men who had > four sexual partners in past three months (aOR = 1.9 95% CI: 1.2–2.8 Ref: 0), had close contact with > four individuals in a day (3.1, 1.5–6.5 Ref: 0–3), were worried about the Mpox epidemic in China (1.6, 1.1–2.3 Ref: No), and believed that the Mpox vaccine is safe (6.6, 2.7–16.4 Ref: No or not sure) and effective (1.9, 1.1–3.3 Ref: No) for PLHIV were more willing to get the vaccination. MSM living with HIV who had a high school education or below (0.5, 0.3–0.9 Ref: Postgraduate diploma), and sometimes (0.5, 0.3–0.8 Ref: Often), seldom, or never (0.5, 0.3–0.9 Ref: Often) followed news about Mpox were less willing to get the vaccination. Three variables were no longer statistically significant when entered into the multivariate logistic regression analysis.
Table 4.
Correlates of Mpox vaccination willingness among MSM living with HIV in China.
| Variables | Unadjusted OR (95% CI) |
p Value | aOR (95% CI) | p Value |
|---|---|---|---|---|
| Education | ||||
| High school or below | 0.5 (0.3–0.9) | 0.011 | 0.5 (0.3–0.9) | 0.041 |
| Undergraduate diploma | 0.5 (0.2–0.9) | 0.018 | 0.7 (0.3–1.5) | 0.356 |
| Postgraduate diploma | Ref | Ref | ||
| Latest HIV viral load | ||||
| Undetectable | Ref | Ref | ||
| Detectable or not sure | 0.6 (0.3–0.8) | 0.001 | 0.6 (0.4–1.0) | 0.063 |
| ART adherence, MMAS-8 score | ||||
| Low (scores < 6) | Ref | Ref | ||
| Medium (6 ≤ scores < 8) | 1.5 (0.8–2.8) | 0.177 | 1.1 (0.6–2.3) | 0.730 |
| High (scores = 8) | 2.0 (1.1–3.5) | 0.020 | 1.7 (0.9–3.2) | 0.128 |
| Uptake Smallpox vaccination | ||||
| No | Ref | Ref | ||
| Yes | 1.5 (1.1–2.2) | 0.021 | 1.3 (0.8–2.0) | 0.246 |
| Not sure | 1.7 (1.0–2.8) | 0.035 | 1.5 (0.9–2.7) | 0.127 |
| Number of anal sex partners in the past three months | ||||
| ≥4 | 2.0 (1.4–2.9) | <0.001 | 1.9 (1.2–2.8) | 0.003 |
| 1–3 | 1.1 (0.7–1.9) | 0.665 | 0.8 (0.4–1.5) | 0.490 |
| 0 | Ref | Ref | ||
| Self-reported number of close contacts daily | ||||
| 0–3 | Ref | Ref | ||
| ≥4 | 2.1 (1.1–4.0) | 0.020 | 3.1 (1.5–6.5) | 0.003 |
| Frequency of following information about the Mpox | ||||
| Often | Ref | Ref | ||
| Sometimes | 0.4 (0.3–0.6) | <0.001 | 0.5 (0.3–0.8) | 0.003 |
| Seldom or never | 0.3 (0.2–0.6) | <0.001 | 0.5 (0.3–0.9) | 0.021 |
| Worry about the Mpox epidemic in China | ||||
| Yes | 1.6 (1.1–2.3) | 0.007 | 1.6 (1.1–2.3) | 0.024 |
| No | Ref | Ref | ||
| I believe Mpox vaccines are safe for PLHIV | ||||
| Yes | 11.8 (5.3–26.5) | <0.001 | 6.6 (2.7–16.4) | <0.001 |
| No or not sure | Ref | Ref | ||
| The Mpox vaccine can be effective against Mpox | ||||
| Yes | 3.5 (2.2–5.4) | <0.001 | 1.9 (1.1–3.3) | 0.021 |
| No | Ref | Ref |
Abbreviations: ART: antiretroviral therapy; MSM: men who have sex with men; HIV: human immunodeficiency virus; PLHIV: people living with HIV; aOR: adjusted odds ratio; CI: confidence interval; Ref: reference.
4. Discussion
Our study found that 37.6% of MSM living with HIV in China expressed concerns about the potential Mpox epidemic in China and 56.8% were willing to get the Mpox vaccine. Willingness to get the Mpox vaccine was associated with educational level, number of sexual partners in the past three months, number of daily close contacts, frequency of seeking news about Mpox, and awareness of Mpox.
Our study showed that 73% of the participants sometimes or frequently followed the news about Mpox, and half of the participants agreed that PLHIV were at a higher risk of Mpox infection. China had not reported any Mpox cases before the current outbreak in 2022, as it used to be endemic to Africa [33]. The higher interest in Mpox among MSM living with HIV may be attributed to the fact that MSM make up the majority of cases in the current multinational Mpox outbreak. Although there is currently no evidence that HIV increases the likelihood of Mpox transmission, PLHIV accounted for approximately 40% of current Mpox cases [7]. However, higher interest in Mpox cannot be interpreted as a concern about the prevalence of Mpox. Our data showed that 37.6% of participants indicated concerns about the current Mpox outbreak spreading to China, which is similar than the findings of the general population in Saudi Arabia (37.4%) [25]. China has implemented strict entry screening measures, and screening for COVID-19 during the quarantine period is accompanied by Mpox testing [34]. Therefore, apart from two imported cases of Mpox in Hong Kong and Chongqing, there are currently no autochthonous cases of Mpox that have been reported in China, so there is no widespread concern [35,36]. Social restrictions have varied from province to province. These restrictions and measures may have prevented Mpox cases from entering the country. However, at the same time, it may have also led to us neglecting the risk of Mpox.
Changes in transmission patterns of the current Mpox outbreak may also partly explain the lower concerns of this population. Previous studies have confirmed the presence of the Mpox virus in semen [37,38,39]. Heskin et al. [12] recently reported a case of Mpox with documented transmission through sex. Furthermore, studies from Italy have documented Mpox co-infection with other sexually transmitted infections (STIs), supporting the hypothesis of sexual transmission [38,40]. The impact of Mpox appears to be more limited than that of COVID-19, which is transmitted through respiratory droplets. Most participants in our survey believed that in this outbreak, the highest risk of Mpox transmission was through sexual encounters, suggesting that this population might have knowledge of novel transmission routes on top of the classic transmission routes identified in this outbreak.
Vaccination is an important tool in preventing the spread of Mpox. A total of 56.8% of MSM living with HIV in our study were willing to get the Mpox vaccine. Similar to our findings, 59.8% of MSM living with HIV in France indicated their acceptance towards Mpox vaccination [41]. Dukers-Muijrers and colleagues found that 81.5% of the MSM surveyed in the Netherlands were willing to receive an Mpox vaccine [42]. However, the willingness rate of Mpox vaccination varied among different populations and regions [43]. In the general population, the willingness rate to get the Mpox vaccine ranged from 29% in Romania to 50.6% in Saudi Arabia [25,44]. Among healthcare workers, the rate of willingness to get the Mpox vaccine varied from 8.8% in the Czech Republic to 90.1% in China [45,46].
Concerns about the possible side effects of Mpox vaccines and concerns about its effectiveness due to immunodeficiency were considered critical to participants’ unwillingness to get vaccinated against Mpox in our study. Lack of faith in the efficacy and safety of vaccines is a major challenge in achieving vaccine coverage among PLHIV, which has been widely reported [47]. Zheng et al. [29] reported that 47.2% of MSM living with HIV had a high level of COVID-19 vaccine hesitancy. A cohort study of HIV patients found that only about 39% of PLHIV got vaccined against influenza [47]. This concern is understandable, given the lack of a global consensus among experts on Mpox vaccination for PLHIV. The interim guidelines for the prevention and treatment of Mpox in person with HIV infection issued by the United States CDC showed that JYNNEOS was well tolerated with similar immunogenicity in individuals with CD4 cell count of 200–750/μL, regardless of HIV infection status [13]. However, immunogenicity remains unknown in PLHIV with CD4 cell counts <100/µL or those who are not virologically suppressed. Due to the current limited availability of the Mpox vaccine, the CDC recommends prioritizing vaccination for those who have been exposed to suspected or confirmed cases of Mpox [48]. For patients living with HIV at risk of severe Mpox infection, clinicians should make reasonable prevention and treatment recommendations based on their clinical status, viral suppression, and CD4 cell count.
Our results demonstrated that participants who had multiple sexual partners and daily close contacts in the past three months were more willing to receive the Mpox vaccine. Ibuka et al. [49] also reported that social contact might affect influenza vaccination status and the probability of vaccination increased with the number of contacts. As Mpox spreads through close contact, participants who have more sexual partners and close contacts were perceived to confront a higher risk of infection. They should take additional precautions to reduce these potential risks or to protect others. This study also showed that participants who believed the Mpox vaccines were safe and effective were more likely to be vaccinated. The frequency of following Mpox news was a predictor of willingness to get the Mpox vaccine, and the Internet was the main source of information. Participants who sometimes and rarely paid attention to information about Mpox were less likely to get vaccinated than those who frequently paid attention. Our colleagues found similar results in COVID-19 vaccine uptake and hesitancy among this population [29]. This may be attributable to limited access to online health resources and caring more for one’s own health [50]. This study also showed that participants with a high school education or below were more likely not to be vaccinated. A recent meta-analysis reported that educational level was a strong predictor of COVID-19 vaccination willingness [51]. Lower education may limit people’s access to health information and may interfere with their judgments.
The current study is one of the few studies that provide insight into Mpox perception and vaccine readiness among MSM living with HIV in China. The pilot study conducted in this study helps to strengthen the logic of the questionnaire and can allow us to more comprehensively understand the suggestions of the population on the questionnaire, with a good quality control effect. Several limitations should be noted. First, the cross-sectional design of this study limits the determination of causality. Second, convenience sampling and non-participation could have introduced some selection bias. The sample in this study might not fully represent all MSM living with HIV in China, and the generalizability of the findings needs to be interpreted with caution. Third, self-reported data may lead to recall and social desirability biases, especially regarding data on sensitive information and on smallpox vaccination. By distributing an anonymous questionnaire, social expectation bias might be reduced. Forth, some measurements of Mpox knowledge and attitudes in this study did not use a scale and the questionnaire did not contain reliability and validity measurements, but referred to those of previous studies. Fifth, the data collection occurred during the COVID-19 pandemic, and this context should be taken into consideration when interpreting the results. Sixth, our study included only participants with Internet access, who may have better educational and economic conditions, which may have led to an overestimation of the willingness to get vaccinated. Finally, participants’ perceptions of the Mpox threat and willingness to get the Mpox vaccine may change as the situation evolves. It is worth noting that there were no Mpox cases reported in mainland China at the time of data collection, until the first imported case was reported in Chongqing, China on 16 September 2022 [36]. Future studies could explore the impact of this factor on Mpox perception in this population.
5. Conclusions
The ongoing Mpox pandemic has not attracted widespread concerns among MSM living with HIV in China. Just over half of MSM living with HIV were willing to get the Mpox vaccine. Efforts should be made to raise awareness of the potential risk of Mpox in this at-risk population. Public health strategies should fully address predictors of vaccination willingness.
Acknowledgments
The authors would like to thank all participants for participating in this study.
Abbreviations
| aOR | adjusted odds ratio |
| ART | antiretroviral therapy |
| CDC | Center for Disease Control and Prevention |
| CI | confidence interval |
| HIV | human immunodeficiency virus |
| MSM | men who have sex with men |
| PLHIV | people living with HIV |
| PHEIC | Public Health Emergency of International Concern |
| Ref | reference |
| WHO | World Health Organization |
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/vaccines11030528/s1, Table S1: STROBE Statement—Checklist of items that should be included in reports of cross-sectional studies.
Author Contributions
Conceptualization, H.Z.; Methodology, L.F. and Y.S.; Software, L.F. and Y.S.; Validation, L.F., Y.S. and Y.L.; Formal Analysis, L.F., Y.S. and Y.L.; Investigation, L.F., Y.S., Y.L. and H.L.; Data Curation, H.Z., L.F., Y.S. and Y.L.; Writing—Original Draft Preparation, L.F.; Writing—Review and Editing, L.F., Y.S., Y.L., B.W., L.Y., T.T., X.W., X.P., Q.L., Y.C., Y.-F.L., H.L., X.M. and H.Z.; Visualization, L.F., Y.S., Y.L. and B.W.; Supervision, H.Z., L.F. and Y.S.; Project Administration, H.Z.; Funding Acquisition, H.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study was conducted with the approval of the Sun Yat-sen University Ethics Committee (SYSU-PHS-2022051).
Informed Consent Statement
Informed consent was obtained from all participants online before proceeding with the questionnaire.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, H.Z., upon reasonable request.
Conflicts of Interest
We declare no competing interests.
Funding Statement
This study was supported by the Natural Science Foundation of China Excellent Young Scientists Fund [82022064], Natural Science Foundation of China International/Regional Research Collaboration Project [72061137001], and Program from Wuxi Science and Technology Bureau [Y20222006]. The funding parties did not have any role in the design of the study or in the explanation of the data.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Marennikova S.S., Seluhina E.M., Mal’Ceva N.N., Cimiskjan K.L., Macevic G.R. Isolation and properties of the causal agent of a new variola-like disease (monkeypox) in man. Bull. World Health Organ. 1972;46:599–611. [PMC free article] [PubMed] [Google Scholar]
- 2.Mccollum A.M., Damon I.K. Human Monkeypox. Clin. Infect. Dis. 2013;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 3.Ahmed S.K., Rashad E.A.A., Mohamed M.G., Ravi R.K., Essa R.A., Abdulqadir S.O., Khdir A.A. The global human monkeypox outbreak in 2022: An overview. Int. J. Surg. 2022;104:106794. doi: 10.1016/j.ijsu.2022.106794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bunge E.M., Hoet B., Chen L., Lienert F., Weidenthaler H., Baer L.R., Steffen R. The changing epidemiology of human monkeypox—A potential threat? A systematic review. PLOS Neglected Trop. Dis. 2022;16:e0010141. doi: 10.1371/journal.pntd.0010141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Adalja A., Inglesby T. A Novel International Monkeypox Outbreak. Ann. Intern. Med. 2022;175:1175–1176. doi: 10.7326/M22-1581. [DOI] [PubMed] [Google Scholar]
- 6.Girometti N., Byrne R., Bracchi M., Heskin J., McOwan A., Tittle V., Gedela K., Scott C., Patel S., Gohil J., et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: An observational analysis. Lancet Infect. Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. New Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention 2022 Monkeypox Outbreak Global Map. [(accessed on 15 December 2022)];2022 Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html.
- 9.World Health Organization 2022. [(accessed on 15 December 2022)]. Available online: https://www.who.int/emergencies/situations/monkeypox-oubreak-2022.
- 10.Orviz E., Negredo A., Ayerdi O., Vázquez A., Muñoz-Gomez A., Monzón S., Clavo P., Zaballos A., Vera M., Sánchez P., et al. Monkeypox outbreak in Madrid (Spain): Clinical and virological aspects. J. Infect. 2022;85:412–417. doi: 10.1016/j.jinf.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Patel A., Bilinska J., Tam J.C.H., Fontoura D.D.S., Mason C.Y., Daunt A., Snell L.B., Murphy J., Potter J., Tuudah C., et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: Descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heskin J., Belfield A., Milne C., Brown N., Walters Y., Scott C., Bracchi M., Moore L.S., Mughal N., Rampling T., et al. Transmission of monkeypox virus through sexual contact–A novel route of infection. J. Infect. 2022;85:334–363. doi: 10.1016/j.jinf.2022.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O’Shea J., Filardo T.D., Morris S.B., Weiser J., Petersen B., Brooks J.T. Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection—United States, August 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:1023–1028. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogoina D., Iroezindu M., James H.I., Oladokun R., Yinka-Ogunleye A., Wakama P., Otike-Odibi B., Usman L.M., Obazee E., Aruna O., et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 15.Duque M.P., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., Mansinho K., Duque L.M., Fernandes C., Cordeiro R., et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance. 2022;27:2200424. doi: 10.2807/1560-7917.es.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martínez J.I., Gil Montalbán E., Bueno S.J., Martínez F.M., Juliá A.N., Díaz J.S., Marín N.G., Deorador E.C., Forte A.N., García M.A., et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance. 2022;27:2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Overton E.T., Stapleton J.T., Frank I., Hassler S., Goepfert P.A., Barker D., Wagner E., Von Krempelhuber A., Virgin G., Weigl J., et al. Safety and Immunogenicity of Modified Vaccinia Ankara-Bavarian Nordic Smallpox Vaccine in Vaccinia-Naive and Experienced Human Immunodeficiency Virus-Infected Individuals: An Open-Label, Controlled Clinical Phase II Trial. Open Forum Infect. Dis. 2015;2:ofv040. doi: 10.1093/ofid/ofv040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Greenberg R.N., Overton E.T., Haas D.W., Frank I., Goldman M., Von Krempelhuber A., Virgin G., Bädeker N., Vollmar J., Chaplin P. Safety, Immunogenicity, and Surrogate Markers of Clinical Efficacy for Modified Vaccinia Ankara as a Smallpox Vaccine in HIV-Infected Subjects. J. Infect. Dis. 2012;207:749–758. doi: 10.1093/infdis/jis753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rao A.K., Petersen B.W., Whitehill F., Razeq J.H., Isaacs S.N., Merchlinsky M.J., Campos-Outcalt D., Morgan R.L., Damon I., Sánchez P.J., et al. Use of JYNNEOS (Smallpox and Monkeypox Vaccine, Live, Nonreplicating) for Preexposure Vaccination of Persons at Risk for Occupational Exposure to Orthopoxviruses: Recommendations of the Advisory Committee on Immunization Practices—United States, 2022. MMWR. Morb. Mortal. Wkly. Rep. 2022;71:734–742. doi: 10.15585/mmwr.mm7122e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilson P.A., Kahana S.Y., Fernandez M.I., Harper G.W., Mayer K., Wilson C.M., Hightow-Weidman L.B. Sexual Risk Behavior Among Virologically Detectable Human Immunodeficiency Virus–Infected Young Men Who Have Sex With Men. JAMA Pediatr. 2016;170:125–131. doi: 10.1001/jamapediatrics.2015.3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy D.A., Durako S.J., Moscicki A.-B., Vermund S.H., Ma Y., Schwarz D.F., Muenz L.R. No change in health risk behaviors over time among HIV infected adolescents in care: Role of psychological distress. J. Adolesc. Health. 2001;29:57–63. doi: 10.1016/S1054-139X(01)00287-7. [DOI] [PubMed] [Google Scholar]
- 22.Tanney M.R., Naar-King S., Murphy D.A., Parsons J.T., Janisse H. Multiple Risk Behaviors Among Youth Living with Human Immunodeficiency Virus in Five U.S. Cities. J. Adolesc. Health. 2010;46:11–16. doi: 10.1016/j.jadohealth.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shrestha R., Meyer J.P., Shenoi S., Khati A., Altice F.L., Mistler C., Aoun-Barakat L., Virata M., Olivares M., Wickersham J.A. COVID-19 Vaccine Hesitancy and Associated Factors among People with HIV in the United States: Findings from a National Survey. Vaccines. 2022;10:424. doi: 10.3390/vaccines10030424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao Y., Chai R., Yang J., Zhang X., Huang X., Yu M., Fu G.-F., Lan G., Qiao Y., Zhou Q., et al. Reasons for COVID-19 Vaccine Hesitancy Among Chinese People Living With HIV/AIDS: Structural Equation Modeling Analysis. JMIR Public Health Surveill. 2022;8:e33995. doi: 10.2196/33995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Temsah M.-H., Aljamaan F., Alenezi S., Alhasan K., Saddik B., Al-Barag A., Alhaboob A., Bahabri N., Alshahrani F., Alrabiaah A., et al. Monkeypox caused less worry than COVID-19 among the general population during the first month of the WHO Monkeypox alert: Experience from Saudi Arabia. Travel Med. Infect. Dis. 2022;49:102426. doi: 10.1016/j.tmaid.2022.102426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alshahrani N.Z., Alzahrani F., Alarifi A.M., Algethami M.R., Alhumam M.N., Ayied H.A.M., Awan A.Z., Almutairi A.F., Bamakhrama S.A., Almushari B.S., et al. Assessment of Knowledge of Monkeypox Viral Infection among the General Population in Saudi Arabia. Pathogens. 2022;11:904. doi: 10.3390/pathogens11080904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Von Elm E., Altman D.G., Egger M., Pocock S.J., Gøtzsche P.C., Vandenbroucke J.P., Initiative S. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: Guidelines for reporting observational studies. Lancet. 2007;370:1453–1457. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- 28.Fu L., Zhao J., Zheng W., Sun Y., Tian T., Wang B., Yang L., Zhou X., Lin Y.F., Yang Z., et al. Oral Sexual Behavior Among HIV-Infected Men Who Have Sex with Men-China, February 2021. China CDC Wkly. 2022;4:541–548. doi: 10.46234/ccdcw2022.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng W., Sun Y., Li H., Zhao H., Zhan Y., Gao Y., Hu Y., Li P., Lin Y.-F., Chen H., et al. COVID-19 vaccine uptake and hesitancy among HIV-infected men who have sex with men in mainland China: A cross-sectional survey. Hum. Vaccines Immunother. 2021;17:4971–4981. doi: 10.1080/21645515.2021.1996152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao H., Wang H., Li H., Zheng W., Yuan T., Feng A., Luo D., Hu Y., Sun Y., Lin Y.-F., et al. Uptake and adverse reactions of COVID-19 vaccination among people living with HIV in China: A case–control study. Hum. Vaccines Immunother. 2021;17:4964–4970. doi: 10.1080/21645515.2021.1991183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xiu X., Wang A., Qian Q., Wu S. The US Public’s Perception of the Threat of COVID-19 During the Rapid Spread of the COVID-19 Outbreak: Cross-Sectional Survey Study. J. Med. Internet Res. 2021;23:e23400. doi: 10.2196/23400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang B., Peng X., Li Y., Fu L., Tian T., Liang B., Sun Y., Chen Y., Wu X., Liu Q., et al. Perceptions, precautions, and vaccine acceptance related to monkeypox in the public in China: A cross-sectional survey. J. Infect. Public Health. 2023;16:163–170. doi: 10.1016/j.jiph.2022.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mileto D., Riva A., Cutrera M., Moschese D., Mancon A., Meroni L., Giacomelli A., Bestetti G., Rizzardini G., Gismondo M.R., et al. New challenges in human monkeypox outside Africa: A review and case report from Italy. Travel Med. Infect. Dis. 2022;49:102386. doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Health Commission of the People’s Republic of China [(accessed on 17 December 2022)];2022 Available online: https://www.gov.cn/fuwu/2022-07/01/content_5698882.htm.
- 35.China News Service 2022. [(accessed on 17 December 2022)]. Available online: https://vs.cns.com.cn/video/detailTemp/395115.html?id=395115.
- 36.Zhao H., Wang W.L., Zhao L., Ye S., Song J.D., Lu R.J., Zong H., Wu C., Huang W., Huang B., et al. The First Imported Case of Monkeypox in the Mainland of China—Chongqing Municipality, China, September 16, 2022. China CDC Wkly. 2022;4:853–854. doi: 10.46234/ccdcw2022.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peiró-Mestres A., Fuertes I., Camprubí-Ferrer D., Marcos M., Vilella A., Navarro M., Rodriguez-Elena L., Riera J., Català A., Martínez M.J., et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Eurosurveillance. 2022;27:2200503. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., D’Abramo A., Cicalini S., Lapa D., Pittalis S., et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Eurosurveillance. 2022;27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lapa D., Carletti F., Mazzotta V., Matusali G., Pinnetti C., Meschi S., Gagliardini R., Colavita F., Mondi A., Minosse C., et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect. Dis. 2022;22:1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raccagni A.R., Mileto D., Canetti D., Tamburini A.M., Rizzo A., Bruzzesi E., Castagna A., Nozza S. Monkeypox and pan-resistant Campylobacter spp infection in Entamoeba histolytica and Chlamydia trachomatis re-infection in a man who have sex with men. J. Infect. 2022;85:436–480. doi: 10.1016/j.jinf.2022.06.028. [DOI] [PubMed] [Google Scholar]
- 41.Zucman D., Fourn E., Touche P., Majerholc C., Vallée A. Monkeypox Vaccine Hesitancy in French Men Having Sex with Men with PrEP or Living with HIV in France. Vaccines. 2022;10:1629. doi: 10.3390/vaccines10101629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dukers-Muijrers N.H.T.M., Evers Y., Widdershoven V., Davidovich U., Adam P.C.G., de Coul E.L.M.O., Zantkuijl P., Matser A., Prins M., de Vries H.J.C., et al. Mpox vaccination willingness, determinants, and communication needs in gay, bisexual, and other men who have sex with men, in the context of limited vaccine availability in the Netherlands (Dutch Mpox-survey) Front. Public Health. 2023;10:1058807. doi: 10.3389/fpubh.2022.1058807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lounis M., Riad A. Monkeypox (MPOX)-Related Knowledge and Vaccination Hesitancy in Non-Endemic Countries: Concise Literature Review. Vaccines. 2023;11:229. doi: 10.3390/vaccines11020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peptan C., Băleanu V.D., Mărcău F.C. Study on the Vaccination of the Population of Romania against Monkeypox in Terms of Medical Security. Vaccines. 2022;10:1834. doi: 10.3390/vaccines10111834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Riad A., Drobov A., Rozmarinová J., Drapáčová P., Klugarová J., Dušek L., Pokorná A., Klugar M. Monkeypox Knowledge and Vaccine Hesitancy of Czech Healthcare Workers: A Health Belief Model (HBM)-Based Study. Vaccines. 2022;10:2022. doi: 10.3390/vaccines10122022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hong J., Pan B., Jiang H., Zhang Q., Xu X., Jiang H., Ye J., Cui Y., Yan X., Zhai X., et al. The willingness of Chinese healthcare workers to receive monkeypox vaccine and its independent predictors: A cross-sectional survey. J. Med. Virol. 2022;95:e28294. doi: 10.1002/jmv.28294. [DOI] [PubMed] [Google Scholar]
- 47.Tsachouridou O., Georgiou A., Naoum S., Vasdeki D., Papagianni M., Kotoreni G., Forozidou E., Tsoukra P., Gogou C., Chatzidimitriou D., et al. Factors associated with poor adherence to vaccination against hepatitis viruses, streptococcus pneumoniae and seasonal influenza in HIV-infected adults. Hum. Vaccines Immunother. 2018;15:295–304. doi: 10.1080/21645515.2018.1509644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention [(accessed on 15 December 2022)];2022 Available online: https://www.cdc.gov/poxvirus/monkeypox/index.html.
- 49.Ibuka Y., Ohkusa Y., Sugawara T., Chapman G.B., Yamin D., Atkins K.E., Taniguchi K., Okabe N., Galvani A.P. Social contacts, vaccination decisions and influenza in Japan. J. Epidemiology Community Health. 2015;70:162–167. doi: 10.1136/jech-2015-205777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Benis A., Khodos A., Ran S., Levner E., Ashkenazi S. Social Media Engagement and Influenza Vaccination During the COVID-19 Pandemic: Cross-sectional Survey Study. J. Med. Internet Res. 2021;23:e25977. doi: 10.2196/25977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Q., Yang L., Jin H., Lin L. Vaccination against COVID-19: A systematic review and meta-analysis of acceptability and its predictors. Prev. Med. 2021;150:106694. doi: 10.1016/j.ypmed.2021.106694. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, H.Z., upon reasonable request.