Abstract
Background
Ferritin, an intracellular protein, has a pivotal role in immune dysregulation. Hyperferritinemia has been associated with higher disease severity and adverse clinical outcomes in COVID-19, including mortality. We aimed to study the association of serum ferritin levels with disease severity and clinical outcomes and its severity prediction potential in COVID-19 patients.
Methods
This retrospective study included 870 adult patients with symptomatic COVID-19 infection hospitalized between July 1, 2020 to December 21, 2020. All the patients had a positive polymerase chain reaction test result of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).
Results
The median age was 55 (IQR:40, 65) years with a male predominance [66.32% (n = 577)], among 870 COVID-19. Of these, 413 (47.47%) had mild COVID-19, and 457 (52.53%) had moderate plus severe COVID-19 disease. Median ferritin levels were significantly high in moderate to severe COVID-19 infection compared to mild [545.8 (326.0, 1046.0) vs 97.3 (52.65–155.5) (p = 0.001)], and in patients who developed a complication compared to without complications [380 (177.05, 863.15) vs 290 (110.9, 635) (p = 0.002). A slight elevation in median ferritin levels was observed in patients who had an ICU stay than non-ICU [326 (129.8, 655) vs 309 (119.1, 684) (p = 0.872)]. The cut-off for ferritin was identified at >287.4 ng/ml for mild versus moderate plus severe COVID-19 infections.
Conclusion
Moderate to severe COVID-19 patients have elevated ferritin levels. Patients with more than 287.4 ng/ml ferritin value would have greater chances of developing moderate to severe COVID-19 infections.
Keywords: COVID-19, Ferritin, Risk factor, SARS-CoV-2, Severity
1. Introduction
Human ferritin is a shell protein that has iron in its core.1 It is made up of variable ratios of the two types of subunits, ferritin light chain (FTL) and ferritin heavy chain (FTH).2, 3 This ratio is regulated in the body, and the FTH subunit increases during inflammatory responses, especially in the heart and kidney, and helps in converting the ferrous iron into the ferric form. The FTL subunit is more readily available in the liver and spleen and takes a lead role in storing iron.1 , 4
Elevated levels of ferritin in the systemic circulation are considered a double-edged sword in acute-phase response as well as in inflammation. Being an acute-phase reactant, hyperferritinemia can be noted in many acute and chronic inflammatory conditions, which may be due to the release from intracellular stores which have been damaged.5 Once released, ferritin is degraded and releases excess free iron, which favors the growth and replication of many viruses like the Hepatitis C Virus and Human Immunodeficiency Virus.2 , 6 Many regulatory and functional proteins of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus utilize iron.7 Ferritin level has been strongly correlated with COVID-19 disease severity.8 , 9 Hyperferritinemia has been observed as an independent factor associated with high mortality rates in COVID-19 infections.10 , 11 Studies have reported hyperferritinemia as a poor prognostic predictor in COVID-19 patients.9 , 12 , 13 Despite the strong association with mortality, it is not yet clear whether hyperferritinemia is a biomarker of disease progression or an essential modulator in the pathogenesis of the disease.5 Another argument is in spite of the availability and affordability of ferritin tests, it is often a neglected tool for predicting the prognosis of COVID-19.9 Many clinicians depend mainly on interleukin-6, C Reactive Protein, and D-dimer as a biomarker for severe COVID-19 disease [9]. Moreover, certain studies reported that although many COVID-19 patients have hyperferritinemia, increased levels of ferritin are not reliable outcome predictors.14 , 15 Therefore, this study was carried out to find the pragmatic utility of ferritin as a serum biomarker in COVID-19, which might help appropriately in triaging the patients at the time of admission. Moreover, the study fills the gap of the scarcity of data on ferritin application in COVID-19 disease from our study settings, especially in the Southern part of the country.
2. Methods
2.1. Patients and study design
This retrospective study was initiated after the approval from the Institutional Ethics Committee of Kasturba Medical College and Kasturba Hospital (IEC KMC&KH) with Reference number IEC 735/2020. The study has been registered in the Clinical Trials Registry of India (CTRI/2021/06/034428). We included patients who were symptomatic of COVID-19 infection and were hospitalized between July 1st, 2020 to December 21st, 2020 at our tertiary care center in the coastal part of South India. The study was completed in compliance with the World Medical Association Declaration of Helsinki. All patients selected in the study had a positive polymerase chain reaction (PCR) test result of SARS-CoV-2. Patients aged below 18, with iron deficiency anemia, anemia of chronic disease, liver cirrhosis, known or newly detected malignancies, acute myocardial infarction, acute inflammatory conditions like acute pancreatitis, sepsis due to any other cause, and pregnant women were excluded from our study. All the patients included in the study were classified as mild, moderate, severe, and critical COVID-19 at the time of admission, using the national guidelines provided by the Indian Council of Medical Research (ICMR) which were similar to the WHO guidelines. The patients with mild symptoms who did not require any oxygen support and oxygen saturation on room air >94% were taken as a non-severe category, while the patients with moderate, severe, and critical symptoms who required oxygen support and required intensive care unit admission at any time during their hospital stay were taken as a severe category.
2.2. Data collection
The history, demographic features, clinical characteristics, epidemiological information including recent contacts, any history of travel, duration of illness at admission, comorbidities such as type 2 diabetes mellitus (T2DM), hypertension (HTN), chronic kidney disease (CKD), etc., laboratory findings including hemoglobin (Hb), white blood cell count (WBC), platelets, absolute lymphocyte count (ALC), ferritin, blood and urine cultures, procalcitonin and treatment regimen were obtained from the hospital database. In addition, ECG, 2D ECHO, chest X-rays, and computer tomography (CT) scan reports if done, were retrieved from electronic medical records and analyzed. The details of hospital transfers and discharges were retrieved from medical records.
2.3. Laboratory confirmation and treatment
A nasopharyngeal throat swab for COVID-19 RT-PCR was done for all the patients in the study in the hospital virology lab using the TRUPCR RT qPCR kit. These RT-PCR assays performed were in accordance with the protocol established by the ICMR guidelines which were on par with the WHO guidelines regarding SARS-CoV-2 infection.
2.4. Statistical analysis
Descriptive statistics were used to summarize the data. Normality was assessed using the Kolmogorov-Smirnov test and Shapiro-Wilk test. Normally distributed data were expressed in mean +SD and a t-test was used to compare the mean. Non-normal distribution was expressed in median (25th and 75th quartiles) and the Mann-Whitney U test was used to analyze the difference between the medians of the two groups. Categorical variables were analyzed using the chi-square test. Multivariate analysis was performed using logistic regression to identify the risk factor that could influence the severity of COVID-19 infection. The severity of COVID-19 was categorized into two groups: mild and moderate plus severe. Age, gender, various comorbidities, and biochemical parameters were the variables considered for univariate analysis. For each independent variable, the odds ratio (OR, 95% CI) was determined. p-value <0.05 was deemed statistically significant. The receiver operating characteristic curve (ROC) was used to derive the cut-off value for ferritin levels in patients with mild versus moderate plus severe COVID-19 infection. A p-value <0.05 indicated statistical significance. Data were analyzed using Microsoft Excel, IBM Statistical Package for the Social Sciences (SPSS) statistics 22, and R v4.1.
3. Result
3.1. Patient characteristics with ferritin status
Eight hundred seventy patient data were collected, with a median age of 55 (40, 65) years and a male predominance of 66.32% (n = 577). On presentation, 58.85%, 36.21%, and 30.46% had fever, cough, and breathlessness, respectively. The average number of days since the onset of symptoms was 11.07 ± 6.41. Diabetes mellitus and hypertension were the two most common comorbid conditions, followed by ischemic heart disease (IHD) and chronic kidney disease (CKD). The median ferritin level in the study population was 315.55 ng/ml. Of the 870 patients, 413 (47.47%) had mild COVID-19, and 457 (52.53) had moderate plus severe COVID-19. Baseline demographic and clinical characteristics are given in Table 1 .
Table 1.
Demographic and clinical characteristics of study participants.
| Parameters | Patients with normal ferritin levels (n = 303) | Patient with abnormal ferritin levels (n = 567) | p-value | Total patients (N = 870) |
|---|---|---|---|---|
| Agea | 50 (30, 64) | 57 (45, 65) | 0.001b | 55.0 (40, 65) |
| Gender | ||||
| Male | 181 (59.74) | 396 (69.84) | 0.003c | 577 |
| Female | 122 (40.26) | 171 (30.16) | 293 | |
| Comorbidities [n (%)] | ||||
| T2DM | 103 (33.99) | 284 (50.08) | 0.001c | 387 |
| HTN | 109 (35.97) | 272 (47.97) | 0.001c | 381 |
| IHD | 54 (17.82) | 98 (17.28) | 0.842c | 152 |
| CKD | 24 (7.92) | 83 (14.64) | 0.004c | 107 |
| COPD | 6 (1.98) | 19 (3.35) | 0.249c | 25 |
| Clinical feature [n (%)] | ||||
| Fever | 167 (55.11) | 345 (60.85) | 0.102c | 512 |
| Cough | 89 (29.37) | 226 (39.86) | 0.002c | 315 |
| Breathlessness | 64 (21.12) | 201 (35.25) | 0.001c | 265 |
| Myalgia | 34 (11.22) | 77 (13.85) | 0.320c | 111 |
| Loose stools | 32 (10.56) | 42 (7.41) | 0.112c | 74 |
| Sore throat | 33 (10.89) | 20 (3.53) | 0.001c | 53 |
| Anosmia | 14 (4.42) | 27 (4.76) | 0.925c | 41 |
| Ferritina (ng/ml) | 97.3 (52.65–155.5) | 545.8 (326.0, 1046.0) | 0.001b | 315.55 (122.52, 678) |
| Hba (g/dl) | 12.6 (11.45, 14.0) | 12.5 (10.6, 14.0) | 0.791b | 12.55 (10.9, 14.0) |
| WBCa (x 103/μL) | 6200 (4850,8500) | 8050 (5800, 12,100) | 0.180b | 7100 (5300, 10,800) |
| Plateletsa (Lakhs/ml) | 2.15 (1.75, 2.70) | 2.19 (1.56, 3.00) | 0.817b | 2.17 (1.61, 2.91) |
| ALCa (x 103/μL) | 1.33 (0.88, 1.89) | 0.94 (0.60, 1.46) | 0.390b | 1.08 (0.69, 1.65) |
Median (Interquartile range).
Mann Whitney U Test.
Chi-square Test.
3.2. Risk factors for severity of COVID-19
Multivariate analysis was performed to identify the risk factor that could influence the severity of COVID-19 infection. Patients were categorized as 18–64 and 65–81 years based on age. And based on ferritin levels, patients were grouped into the normal range (female:10–120 ng/ml, and male: 20–250 ng/ml) and the abnormal range. Age, ferritin levels, T2DM, CKD, and IHD were the only variables in our data that were significantly associated with the severity of COVID-19 infection (Table 2 ).
Table 2.
Independent risk factors of moderate plus severe COVID-19 using univariate and multivariate logistic regression analysis.
| Risk factors | COVID-19 infection severity |
Crude odds ratio (95% confidence interval) | Adjusted odds ratio (95% confidence interval); p-value | |
|---|---|---|---|---|
| Mild | Moderate plus Severe | |||
| Age | ||||
| 18-64 | 343 | 279 | 3.13 (2.27–4.30) | 2.31 (1.57–3.39); |
| 65-81 | 70 | 178 | 0.001 | |
| Ferritin | ||||
| Normal range | 231 | 72 | 6.79 (4.94–9.33) | 7.39 (5.15–10.60); |
| Abnormal range | 182 | 385 | 0.001 | |
| T2DM | ||||
| No | 290 | 193 | 3.23 (2.44–4.27) | 1.96 (1.39–2.79); |
| Yes | 123 | 264 | 0.001 | |
| CKD | ||||
| No | 389 | 374 | 3.59 (2.4–5.79) | 2.07 (1.204–3.575); |
| Yes | 24 | 83 | 0.009 | |
| IHD | ||||
| No | 373 | 345 | 3.03 (2.05–4.47) | 2.15 (1.33–3.47); |
| Yes | 40 | 112 | 0.002 | |
3.3. Ferritin levels and COVID-19
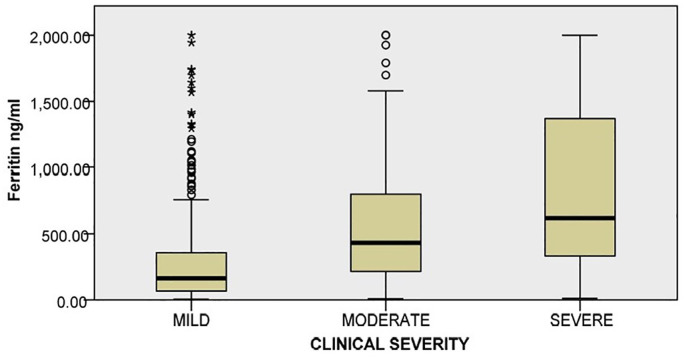
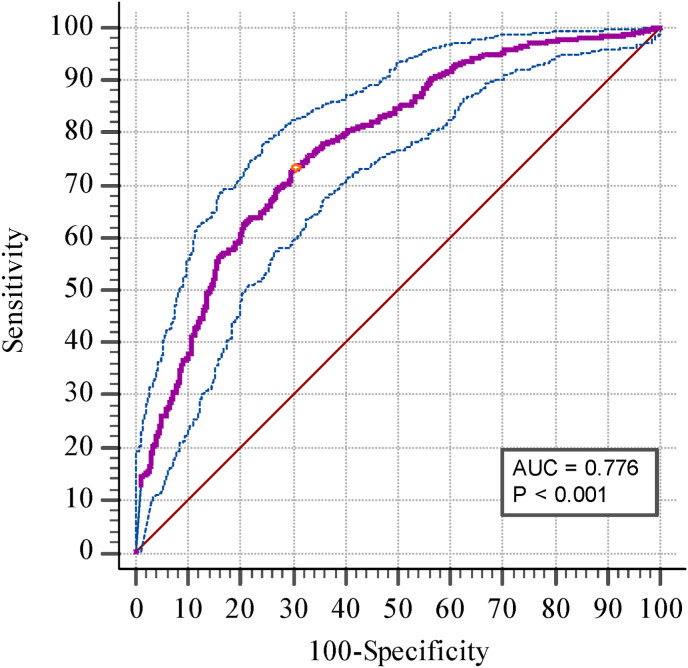
The median ferritin levels in the study population were 315.55 ng/ml, with 303 patients having ferritin levels within the normal range (female:10–120 ng/ml, and male: 20–250 ng/ml). A significant difference was observed in the median ferritin values between patients with mild (n = 413), moderate (n = 210), and severe (n = 247) COVID-19 infection (p = 0.001). Fig. 1 represents the correlation of ferritin levels with COVID-19 clinical severity. The cut-off for ferritin was identified at >287.4 ng/ml (Youden index: indicating the point of maximum sensitivity plus specificity) for mild versus moderate plus severe COVID-19 infections (Fig. 2 ).
Fig. 1.
Correlation of ferritin levels with severity of COVID-19.
Fig. 2.
Receiver operating characteristic curve analysis for ferritin levels in patients with mild versus moderate plus severe COVID-19 infection.
3.4. Ferritin levels and clinical outcomes
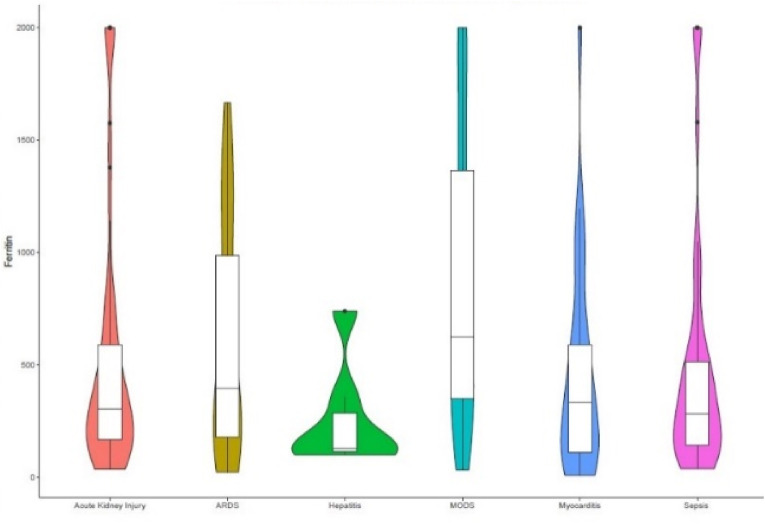
129 (14.83%) patients had died due to COVID-19 infection, and ICU admission was observed in 245 (28.16%) patients during the hospital stay with an average duration of 8.2 ± 4.92 days. 219 (25.17%) patients developed a complication during their hospital stay. Among the complications, 58 (26.48%), 52 (23.74%), and 40 (18.26%) patients developed multiple organ dysfunction (MODS), acute kidney injury (AKI), and sepsis with septic shock, respectively. Fig. 3 depicts the various complications and median ferritin levels observed in the patients. Table 3 shows the different clinical outcomes and their median ferritin levels.
Fig. 3.
Ferritin levels in the various clinical complications of COVID-19.
Table 3.
The various clinical outcomes of COVID-19 with median ferritin levels.
| Clinical outcome | No. of patients | Median ferritin | p-value |
|---|---|---|---|
| With complications | n = 219 | 380 (177.05, 863.15) | p = 0.002 |
| Without complications | n = 651 | 290 (110.9, 635) | |
| With ICU stay | n = 245 | 326 (129.8, 655) | p = 0.872 |
| Without ICU stay | n = 625 | 309 (119.1, 684) | |
| Non-survivors | n = 129 | 309.9 (132.7, 660) | p = 0.798 |
| Survivors | n = 741 | 315.7 (121.5, 678) |
4. Discussion
In this study, the median age of the COVID-19 patients was 55 years with a male predominance, similar to various other studies. The possible reasons for the gender discrepancy could include differences in social habits, comorbid conditions, and psychological and social characteristics.16 The results of our study show that increasing age and the presence of comorbidities such as T2DM, CKD, and IHD can significantly influence the severity of the infection.
The major finding of our study show that median ferritin levels were significantly high in moderate to severe COVID-19 infection compared to mild [545.8 (326.0, 1046.0) vs 97.3 (52.65–155.5) (p = 0.001)], and in patients who developed a complication [380 (177.05, 863.15) vs 290 (110.9, 635) (p = 0.002)]. Slight elevation in median ferritin levels was observed in patients who had an ICU stay [326 (129.8, 655) vs 309 (119.1, 684) (p = 0.872)]. A recent systematic review of 163 studies concluded that high serum ferritin levels were associated with more severe disease and poor outcomes in COVID-19 infection.2 Pasini et al., 2021 studied the various metabolic parameters in patients who suffer from long- COVID syndrome (fatigue, muscle weakness, dyspnoea). They identified several parameters including ferritin, D-dimer, and erythrocyte sedimentation rates were elevated.17 Elevated ferritin levels were also identified in the autopsy reports of COVID-19 patients.18 Although several other studies report an association between ferritin levels and survival, we found no association between the two.19, 20, 21
Iron in the body is primarily stored in the form of ferritin which is an intracellular protein. Ferritin being a positive acute phase reactant, hyperferritinemia could be used to assess the physiological response to inflammation. In the presence of hyperferritinemia, ferritin could be a reason for immune dysregulation by promoting an inflammatory response and immune suppression that leads to cytokine storm.22 Although the exact cause for elevated ferritin in COVID-19 infection is unknown, it could be influenced by cytokine release or cellular damage that results in the leakage of intracellular ferritin.23, 24 It has been previously shown that ferritin is a direct indicator of cellular damage suggestive of an association between organ damage and ferritin production. This could later cause cell death, known as ferroptosis. It is suggested that inflammation associated with sepsis could alter iron metabolism and deficiency to facilitate the immune system, which could be an early sign of COVID.24 The excess iron can also lead to fibrin polymerization and induce a pro-coagulant state.2 Moreover, elevated ferritin levels may result in excess production of reactive oxygen species and oxidative stress that can cause tissue toxicity.25 Ferritin not only enhances inflammation, but it also has a pathogenic role in the process of inflammation by binding with the mucin domain 2 and T-cell immunoglobulin and encouraging the expression of various pro-inflammatory mediators. In addition, some research has shown that the H chain of ferritin facilitates the release of inflammatory cytokines by the macrophages.26
We report a ferritin cut-off value of 287.4 ng/ml with a sensitivity of 73.3% and specificity of 69.73% (AUC 0.776). Indicating that people having ferritin values more than 287.4 ng/ml would have greater chances of developing moderate to severe COVID-19 infections. Similarly, Bozkurt et al., 2021 reported that in ROC analysis, the level of ferritin ≥264.5 ng/ml predicted severe COVID-19 with 73.9% sensitivity and 94.2% specificity.24 Likewise, Cao et al. identified a ferritin cut-off value at 272.5 ng/ml with 96% sensitivity and 70% specificity.27 Considering this, it could be said that hyperferritinemia is a risk factor for increased severity of COVID-19 infection. Therefore, appropriate measures such as the use of iron chelators, and dietary iron intake alterations could be considered in patients presenting with COVID-19.
The major advantage of our study is the sample size of 870 patients. While the limitations of our study are, mainly the retrospective nature of the study. Also, only the baseline ferritin levels were estimated, and did not assess the subsequent levels or availability of the pre-COVID-19 ferritin levels.
5. Conclusions
Early ferritin estimation could indicate the severity of COVID infection and outcomes. Ferritin being a commonly tested biomarker is readily available in most laboratory settings and relatively inexpensive. We suggest measurement of ferritin levels on presentation with the disease, which could help understand the intensity of the disease and enhance treatment outcomes. The clinical implication of our study is that ferritin could serve as a very simple and cost-effective indicator to detect severe COVID-19 patients in the early phase itself as well as help to predict their clinical outcome. Therefore, serum ferritin can be considered a reliable tool to provide guidance for subsequent treatment.
Funding Sources
Nil.
CRediT authorship contribution statement
Shilia Jacob Kurian: conducted the literature search and wrote the manuscript, performed statistical tests, All the authors participated e in literature collection and review. All the authors approved the final draft of the manuscript. Sara Poikayil Mathews: collected the data, All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript. Abin Paul: collected the data, All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript. Subeesh K. Viswam: Kurian performed statistical tests, All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript. Dr. Shivashankara Kaniyoor Nagri: critically evaluated the manuscript. All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript. Sonal Sekhar Miraj: conducted the literature search and wrote the manuscript, All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript. Shubhada Karanth: designed the study, conducted the literature search and wrote the manuscript, All the authors participated in literature collection and review. All the authors approved the final draft of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the review work in this paper.
Acknowledgment
Authors express their gratitude to Kasturba Medical College, Manipal, Manipal Academy of Higher Education, Manipal, India for the research support and facilities.
References
- 1.Kernan K.F., Carcillo J.A. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaushal K., Kaur H., Sarma P., et al. Serum ferritin as a predictive biomarker in COVID-19. A systematic review, meta-analysis and meta-regression analysis. J Crit Care. 2022;67:172–181. doi: 10.1016/j.jcrc.2021.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Plays M., Müller S., Rodriguez R. Chemistry and biology of ferritin. Metallomics. 2021;13(5) doi: 10.1093/mtomcs/mfab021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen L.A., Gutierrez L., Weiss A., et al. Serum ferritin is derived primarily from macrophages through a nonclassical secretory pathway. Blood. 2010;116(9):1574–1584. doi: 10.1182/blood-2009-11-253815. [DOI] [PubMed] [Google Scholar]
- 5.Edeas M., Saleh J., Peyssonnaux C. Iron: innocent bystander or vicious culprit in COVID-19 pathogenesis? Int J Infect Dis. 2020;97:303–305. doi: 10.1016/j.ijid.2020.05.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Drakesmith H., Prentice A. Viral infection and iron metabolism. Nat Rev Microbiol. 2008;6(7):541–552. doi: 10.1038/nrmicro1930. [DOI] [PubMed] [Google Scholar]
- 7.Liu W., Zhang S., Nekhai S., et al. Depriving iron supply to the virus represents a promising adjuvant therapeutic against viral survival. Curr Clin Microbiol Rep. 2020;7(2):13–19. doi: 10.1007/s40588-020-00140-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lalani K., Seshadri S., Samanth J., et al. Cardiovascular complications and predictors of mortality in hospitalized patients with COVID-19: a cross-sectional study from the Indian subcontinent. Trop Med Health. 2022;50(1):55. doi: 10.1186/s41182-022-00449-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hulkoti V.S., Acharya S., Kumar S., et al. Association of serum ferritin with COVID-19 in a cross-sectional study of 200 intensive care unit patients in a rural hospital: is ferritin the forgotten biomarker of mortality in severe COVID-19? J Fam Med Prim Care. 2022;11(5):2045–2050. doi: 10.4103/jfmpc.jfmpc_1921_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mehta P., McAuley D.F., Brown M., et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Phua J., Weng L., Ling L., et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tiwari L., Gupta P., Yankappa N., et al. Clinicodemographic profile and predictors of poor outcome in hospitalised COVID-19 patients: a single-centre, retrospective cohort study from India. BMJ Open. 2022 Jun 1;12(6) doi: 10.1136/bmjopen-2021-056464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parhad P., Galhotra A., Jindal A., et al. An assessment of the profile and predictors of outcomes in COVID-19 patients hospitalized in a tertiary care institute in Central India. Cureus. 2022;14(7) doi: 10.7759/cureus.26909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feld J., Tremblay D., Thibaud S., et al. Ferritin levels in patients with COVID-19: a poor predictor of mortality and hemophagocytic lymphohistiocytosis. Int J Lab Hematol. 2020;42(6):773–779. doi: 10.1111/ijlh.13309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figliozzi S., Masci P.G., Ahmadi N., et al. Predictors of adverse prognosis in COVID-19: a systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10) doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- 16.Emezue C. Digital or digitally delivered responses to domestic and intimate partner violence during COVID-19. JMIR Public Health Surveill. 2020;6(3) doi: 10.2196/19831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pasini E., Corsetti G., Romano C., et al. Serum metabolic profile in patients with long-Covid (PASC) syndrome: clinical implications. Front Med. 2021;8:1182. doi: 10.3389/fmed.2021.714426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fox S.E., Akmatbekov A., Harbert J.L., et al. Pulmonary and cardiac pathology in African American patients with COVID-19: an autopsy series from New Orleans. Lancet Respir Med. 2020;8(7):681–686. doi: 10.1016/S2213-2600(20)30243-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alroomi M., Rajan R., Omar A.A., et al. Ferritin level: a predictor of severity and mortality in hospitalized COVID‐19 patients. Immun Inflamm Dis. 2021;9(4):1648–1655. doi: 10.1002/iid3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Habib H.M., Ibrahim S., Zaim A., et al. The role of iron in the pathogenesis of COVID-19 and possible treatment with lactoferrin and other iron chelators. Biomed Pharmacother. 2021;136 doi: 10.1016/j.biopha.2021.111228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vargas-Vargas M., Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Públic. 2020;44:e72. doi: 10.26633/RPSP.2020.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujani M., Raychaudhuri S., Singh M., et al. An analysis of hematological, coagulation and biochemical markers in COVID-19 disease and their association with clinical severity and mortality: an Indian outlook. Am J Blood Res. 2021;11(6):580. [PMC free article] [PubMed] [Google Scholar]
- 24.Bozkurt F.T., Tercan M., Patmano G., et al. Can ferritin levels predict the severity of illness in patients with COVID-19? Cureus. 2021;13(1) doi: 10.7759/cureus.12832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bianconi V., Mannarino M.R., Figorilli F., et al. The detrimental impact of elevated Ferritin to Iron ratio on in-hospital prognosis of patients with COVID-19. Expert Rev Mol Diagn. 2022;22(4):469–478. doi: 10.1080/14737159.2022.2052047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng L., Li H., Li L., et al. Ferritin in the coronavirus disease 2019 (COVID‐19): a systematic review and meta‐analysis. J Clin Lab Anal. 2020;34(10) doi: 10.1002/jcla.23618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cao P., Wu Y., Wu S., et al. Elevated serum ferritin level effectively discriminates severity illness and liver injury of coronavirus disease 2019 pneumonia. Biomarkers. 2021;26(3):207–212. doi: 10.1080/1354750X.2020.1861098. [DOI] [PMC free article] [PubMed] [Google Scholar]