Abstract
Objective:
Determine whether variation in the HLA region is associated with the development of post-traumatic sepsis and septic shock.
Background:
Sepsis-related deaths remain a major source of mortality after traumatic injury. Genetic characteristics may contribute to susceptibility to adverse outcomes including sepsis and septic shock. Recent advances in next-generation sequencing technology now allow comprehensive genotyping of the HLA region.
Methods:
White adult trauma patients requiring more than 2 days of mechanical ventilation underwent HLA genotyping, and were followed for the development of sepsis and septic shock. Odds ratios (OR) for the associations between our outcomes and HLA variants were estimated, a correction for multiple comparisons was applied, and significant variants were included in regression models adjusting for potential confounders.
Results:
A total of 1184 patients were included. Patients were severely injured (median injury severity score 33); 33% developed sepsis, 6% septic shock, and in-hospital mortality was 14%. An amino acid variant (156Q) within the HLA-A peptide-binding groovewas associated with greater odds of sepsis [OR 1.50, (1.18–1.89)]. HLA-A*02:01 was associated with lower odds of septic shock [OR 0.52, (0.32–0.82)]. These associations remained significant after adjusting for potential confounders.
Conclusions:
This is the first study to apply next-generation sequencing techniques to evaluate associations between immunogenetic factors and post-traumatic sepsis and septic shock. Associations with class I HLA variants are novel as they implicate adaptive immunity in post-traumatic sepsis. These findings are a step towards developing a panel of genetic markers assessing risk of infection-related complications as we move towards more personalized medicine.
Keywords: HLA, human leukocyte antigen, sepsis, septic shock, trauma
Though most deaths related to traumatic injury occur shortly after injury, those who survive the initial physical and physiological insult are susceptible to the development of persistent organ dysfunction, inflammatory derangements, bacterial infection, and sepsis. Late phase deaths related to sepsis remain a major source of mortality after traumatic injury,1 and sepsis results in greater cost and added length of hospital stay.2–4 Clinical and demographic factors have been used to try and identify those at risk for the development of post-traumatic infection, and to explain differences in the severity of clinical manifestations of infection. However, unexplained and unpredictable variation remains.
Sepsis and septic shock are often described as a dysregulation of the immune system in response to infection. Greater susceptibility to infection after trauma may be related to a persistent state of immunoparalysis.5–7 Multiple genes mediate resistance to bacterial infection, and some data suggests that variability in susceptibility and response to infection are mediated in part by underlying genetic differences.8
The human leukocyte antigen (HLA) region is a highly polymorphic gene group encoding cell-surface proteins that play a role in the regulation of the immune system. Components of the HLA region have been implicated in many disease processes, including susceptibility to certain infections. In particular, specific HLA alleles have been associated with varying susceptibility to and severities of streptococcal infection,9,10 differential cytokine production and proliferation in response to foreign stimulus,11 and pneumonia-related septic shock.12 These data suggest that variability in the phenotypic manifestation of sepsis may be influenced by genotypic differences within the HLA region.
Using next-generation sequencing, we sought to determine whether variation in the HLA region was associated with the development of sepsis amongst critically injured patients.
METHODS
Study Design, Patient Recruitment, and Data Collection
We conducted a noninterventional, observational, prospective study at a large, level 1-trauma center in Seattle, WA (Harborview Medical Center), with approval from the University of Washington Institutional Review Board.
The details regarding patient recruitment and data collection methods were previously reported, and summarized below.13 Between August 2003 and November 2007, blunt or penetrating trauma patients admitted to the intensive care unit who required mechanical ventilation for more than 48 hours were eligible for this study. Patients were excluded if they were less than 18 years old or sustained a burn injury. To minimize confounding due to unmeasured genetic variation related to ancestry, this analysis was limited to individuals identifying as “white.” Demographic and clinical data were prospectively collected using the electronic medical record and an internal trauma registry. A conservative transfusion policy was in place to address anemia, and transfusion products were not explicitly leukodepleted for the purposes of this study.
Determination of Sepsis and Septic Shock
Patients who met inclusion and exclusion criteria (outlined in Fig. 1) were followed until hospital discharge or death. The primary outcomes were the development of sepsis and septic shock according to the Sepsis-3 criteria.14 Sepsis was defined as an increase in Sequential Organ Failure Assessment (SOFA) score of 2 points or more in the setting of known or suspected infection occurring greater than 48 hours after injury. Infection etiology was based on positive culture results. Septic shock was defined as a subset of sepsis in which vasopressors were required to sustain a mean arterial pressure of 65 mm Hg or greater and a serum lactate greater than 2 mmol/L in the absence of hypovolemia. Patients with confirmed or suspected infection were treated with standard of care interventions (eg, antibiotics, fluid resuscitation, vasopressors, source control).
FIGURE 1.
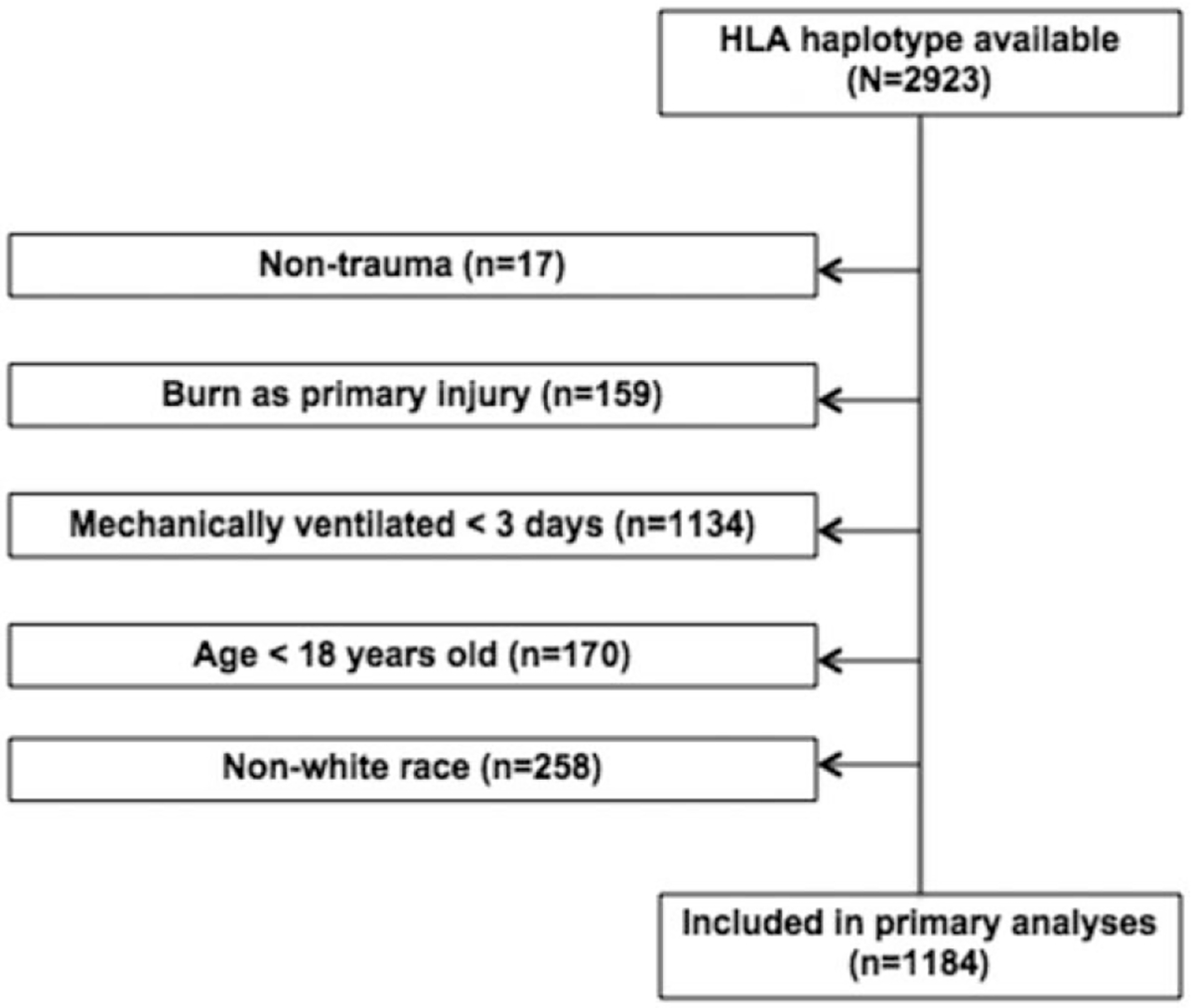
Flowchart outlining the inclusion and exclusion criteria for this study.
HLA Genotyping
Discarded venous blood samples from study participants collected in ethylenediaminetetraacetic acid tubes were used for peripheral blood mononuclear cell isolation. Genomic DNA was isolated using the Qiagen QIAamp DNA Blood Midi Kit (Qiagen, Hilden, Germany). HLA typing was performed using the MIA FORA next generation sequencing (NGS) FLEX typing solution kit and analysis software (Immucor, Inc., Peachtree Corners, GA). The MIA FORA NGS FLEX typing solution is designed to interrogate all the major Class I (HLA-A, -B, -C) and class II (HLA -DPA1, -DPB1, -DQA1, -DQB1, and -DRB1, DRB3, DRB4, and DRB5) HLA genes using NGS platforms. Each sample was genotyped using locus specific long range polymerase chain reaction to appropriately select the genes of interest. For each sample all the individual polymerase chain reaction reactions were pool together at appropriate ratio using the SironaQuant software to ensure proper representation of each individual HLA gene. After pooling each sample was barcoded and multiple samples processed in parallel using automated devise and prepared for Illumina NGS sequencing. The data generated by the Illumina platform were analyzed using the MIA FORA software and the international ImMunoGeneTics (IMGT)/HLA 3.24.00 Database. To ensure the highest possible accuracy the MIA FORA software applies 3 independent algorithms—1 for mapping and 2 for phasing. At least 500,000 reads per sample were used to deliver 8 digits resolution per gene. Genotyping was performed by individuals blinded to clinical outcomes.
Statistical Analysis
Data were analyzed using R (Version 3.6.1). Categorical variables were reported as absolute values and percentages, and continuous variables as medians and interquartile ranges. Categorical data were compared using the Pearson Chi-square test, and continuous data were evaluated using the nonparametric Mann-Whitney U test.
Odds ratio (OR) estimates for patients with and without sepsis were calculated using the R package BIGDAWG (https://CRAN.R-project.org/package=BIGDAWG), which was specifically designed to identify associations within the HLA complex.15 Testing for Hardy-Weinberg equilibrium was performed as a primary quality control verification of the genotype data. Calculation of allele frequencies, binning of low frequency alleles, and Chi-square testing were performed as previously described.15 Alleles are “binned,” or combined into a single category if the expected count is less than 5 in either the cases or controls before calculation of the Chi-square statistic.16 Two-field allele resolutions were used for each analysis. The same binning and statistical calculations were then performed for estimated haplotypes. Protein sequences from the IMGT/HLA database (a publicly available database of HLA sequences), were used to run case-control association analyses for each polymorphic amino-acid position, and each amino-acid substitution. Strength of association was estimated by the OR and 95% confidence interval (CI). All P-values were 2 tailed. A correction for multiple comparisons was applied, and a P < 0.05 after correction was considered significant. Corrected P-values were determined by multiplying individual P-values by the number of comparisons made at the locus level as previously described.17–19
For each variant that was considered significantly associated with our outcomes using this method, multiple logistic regression was then employed to control for potential confounders. Pre-specified clinical variables—age, sex, abbreviated injury scale (AIS) scores for the head, spine, abdomen, and chest, and transfusion requirement—were included in the multiple logistic regression.
RESULTS
Demographics and Clinical Outcomes
We performed HLA genotyping for 2923 patients, of which 1184 patients met the inclusion and exclusion criteria outlined above, and were included in our primary analysis. Fig. 1 provides the flowchart of study population inclusion and exclusion criteria for this study.
Patient and injury characteristics, and clinical outcomes are provided in Table 1. Consistent with a typical trauma population, the majority of patients were male (74%), with a bimodal age distribution. Overall, patients were severely injured (median injury severity score 33), and had long hospital and intensive care unit stays (median 20 and 10 days, respectively). Thirty-three percent of patients developed sepsis and 6% developed septic shock; in-hospital mortality was 14% overall. Patients with sepsis and septic shock were slightly older, more likely to be male, have a high (>=3) abdominal AIS score, and require transfusion compared to those who did not develop sepsis. Pneumonia was the etiology of infection in 71% of septic patients (Table 1); similar proportions of infection etiologies were observed for septic patients who did and did not develop septic shock. Multiple organ dysfunction was highly prevalent amongst patients who met criteria for sepsis (90% with a SOFA score >=6, compared to 38% of nonseptic patients), and septic shock (99% with a SOFA score >=6).
TABLE 1.
Patient Demographics, Injury Characteristics, Clinical Characteristics, and Outcomes Amongst Patients Who Did (n = 389) and Did Not (n = 795) Meet Criteria for Sepsis
| No Sepsis (n = 795) | Sepsis (n = 389) | |
|---|---|---|
|
| ||
| Patient demographics | ||
| Age, yr (median, [IQR]) | 40 [26, 52] | 46 [26, 58] |
| Male sex, n (%) | 575 (72.3) | 297 (76.3) |
| BMI, kg/m2 (median, [IQR]) | 26.0 [22.8, 30.1] | 27.4 [24.3, 31.4] |
| Injury characteristics | ||
| Blunt mechanism, n (%) | 738 (92.8) | 366 (94.1) |
| ISS, (median, [IQR]) | 33 [25, 41] | 33 [26, 41] |
| Head AIS >= 3, n (%) | 491 (61.8) | 199 (51.2) |
| Spine AIS >= 3, n (%) | 254 (31.9) | 149 (38.3) |
| Chest AIS >= 3, n (%) | 462 (58.1) | 266 (68.4) |
| Abdomen AIS >= 3, n (%) | 183 (23.0) | 142 (36.5) |
| Transfusion requirement, n (%) | 323 (40.6) | 232 (59.6) |
| Outcomes | ||
| Hospital LOS, d (median, [IQR]) | 17 [11, 25] | 28 [21, 42] |
| ICU LOS, d (median, [IQR]) | 7 [5, 12] | 19 [13, 27] |
| Mechanical ventilation, d (median, [IQR]) | 6 [4, 9] | 16 [10, 24] |
| Mortality, n (%) | 108 (13.6) | 59 (15.2) |
| Infection etiology | ||
| Bloodstream, n (%) | NA | 59 (15.2) |
| Surgical specimen, n (%) | NA | 38 (9.8) |
| Respiratory, n (%) | NA | 278 (71.5) |
| Other, n (%) | NA | 14 (3.6) |
| SOFA >= 6, n (%) | 294 (37.0) | 350 (90.0) |
| Missing | 14 (1.8) | 0(0) |
AIS indicates abbreviated injury scale; BMI, body mass index; ISS, injury severity score; LOS, length of stay; SOFA, sequential organ dysfunction assessment.
HLA Variants Associated With Development of Sepsis
ORs for 526 unique amino acid variants were calculated. The number of unique amino acid variants differed by loci (A: n = 102, B:n = 105, C: n = 55, DPA1: n = 10, DPB1: n = 38, DQA1: n = 56, DQB1: n = 69, DRB1: n = 91; a complete list of amino acid variants,their relative frequencies, associated ORs and adjusted P-values are provided in Supplementary Table 1, http://links.lww.com/SLA/C161. A single amino acid substitution (156Q) within the HLA-A protein peptide-binding groove was associated with greater odds of developing sepsis after correction for multiple comparisons and controlling for clinical confounders, including age, sex, transfusion requirement, and body-site specific AIS [OR 1.70, (95% CI 1.29–2.23)] (Table 2). No HLA alleles or haplotypes were associated with the development of sepsis.
TABLE 2.
Unadjusted and Adjusted Odds Ratio Estimates for the HLA Amino Acid Substitution Associated With the Development of Sepsis After Correction for Multiple Comparisons
| Unadjusted |
Adjusted* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Locus | Amino Acid | OR | 95% CI | P-value | Corrected P-value | aOR | 95% CI | P-value | Corrected P-value |
|
| |||||||||
| A | 156Q | 1.50 | 1.18–1.89 | 0.0005 | 0.048 | 1.70 | 1.29–2.23 | 0.0001 | 0.010 |
Adjusted for age; sex; high (>=3) AIS score for head, spine, abdomen, and chest; and transfusion requirement.
CI indicates confidence interval; HLA, human leukocyte antigen; OR, odds ratio.
HLA Variants Associated With the Development of Septic Shock
Two hundred sixty-six HLA alleles were binned for low counts before analysis (Supplementary Table 2, http://links.lww.com/SLA/C161). ORs for 61 HLA alleles were calculated. The number of unique alleles evaluated differed by locus (A: n = 7, B: n = 10,C: n = 10, DPA1: n = 2, DPB1: n = 5, DQA1: n = 9, DQB1: n = 9, DRB1: n = 9); a complete list of these alleles, their relative frequencies, associated ORs and adjusted P-values are provided in Supplementary Table 3, http://links.lww.com/SLA/C161. HLA-A*02:01 was associated with lower odds of septic shock [OR 0.44, (95% CI 0.26–0.74)] after correcting for multiple comparisons and adjusting for clinical confounders (Table 3). This association was noted in both the overall population, and amongst the subset of patients with sepsis (data not shown). No amino acid variants or HLA haplotypes were associated with the development of septic shock.
TABLE 3.
Unadjusted and Adjusted Odds Ratio Estimates for the HLA Allele Associated With the Development of Septic Shock After Correction for Multiple Comparisons
| Unadjusted |
Adjusted* |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Locus | Allele | OR | 95% CI | P-value | Corrected P-value | aOR | 95% CI | P-value | Corrected P-value |
|
| |||||||||
| A | 02:01 | 0.52 | 0.32–0.82 | 0.0034 | 0.024 | 0.43 | 0.24–0.76 | 0.0038 | 0.027 |
Adjusted for age; sex; high (>=3) AIS score for head, spine, abdomen, and chest; and transfusion requirement.
CI indicates confidence interval; HLA, human leukocyte antigen; OR, odds ratio.
DISCUSSION
Sepsis is a complex multi-genic process. The physiologic response to infection is a function of both the invading pathogen and the host. Genetic variation within the host may predispose individuals not only to infection, but the altered immune responses that have become synonymous with sepsis and septic shock. Efforts to describe associations between genetic characteristics and the development of sepsis have identified numerous candidate genes, however, the majority of available studies focus on primary infection, rather than post-traumatic infection.
We observed that post-traumatic sepsis and septic shock were associated with HLA-A variants. A single, nonsynonymous amino acid substitution within the peptide-binding groove of HLA-A was associated with greater odds of sepsis. HLA-A*02:01 was associated with the lower odds of developing septic shock. Both remained significantly associated after controlling for age, sex, body site-specific AIS scores, and transfusion requirement. No haplotypes were significantly associated with the outcomes of interest.
Prior research evaluating the HLA region and its relationship to sepsis and septic shock focused primarily on differences in expression rather than sequence variability. Amongst trauma patients, studies of the HLA region have demonstrated associations between both impaired surface expression of HLA-DR, and persistently depressed HLA-DR expression and major sepsis.20–22 One study evaluating the association between the HLA region and sepsis in a small mixed surgical and medical population identified HLA-A*31 as a risk factor the development of sepsis.23 However, this allele was too rare to be evaluated in our study.
The associations between class I HLA molecules and sepsis and septic shock was a particularly unexpected finding, and the mechanisms by which these findings are mediated are not well understood. The innate immune response is central to early containment of infection, and class II HLA molecules are classically thought to mediate the early immune response to bacterial infection. However, some data suggest a potential role for class I molecules—including HLA-A.27 Cross-presentation of exogenously derived peptides by class I molecules is one possible mechanism by which class I molecules may contribute to the immune response to bacterial infection. In addition, previous research has shown that binding of superantigens is influenced by allelic and polymorphic differences in HLA molecules28; differential activation by superantigens may in part explain why variability in the HLA-A molecule is associated with differential susceptibility to sepsis and septic shock. Finally, the specific amino acid substitution identified in this study (156Q) is located within the peptide-binding groove of the HLA-A molecule (Fig. 2),29 and may impact both peptide binding and interactions with T-cell receptors.30 Polymorphisms at this position have previously been implicated in transplant rejection and acute graft versus host disease.30 This particular substitution is not present in the HLA-A*02:01 which may in part explain the differential associations observed.
FIGURE 2.
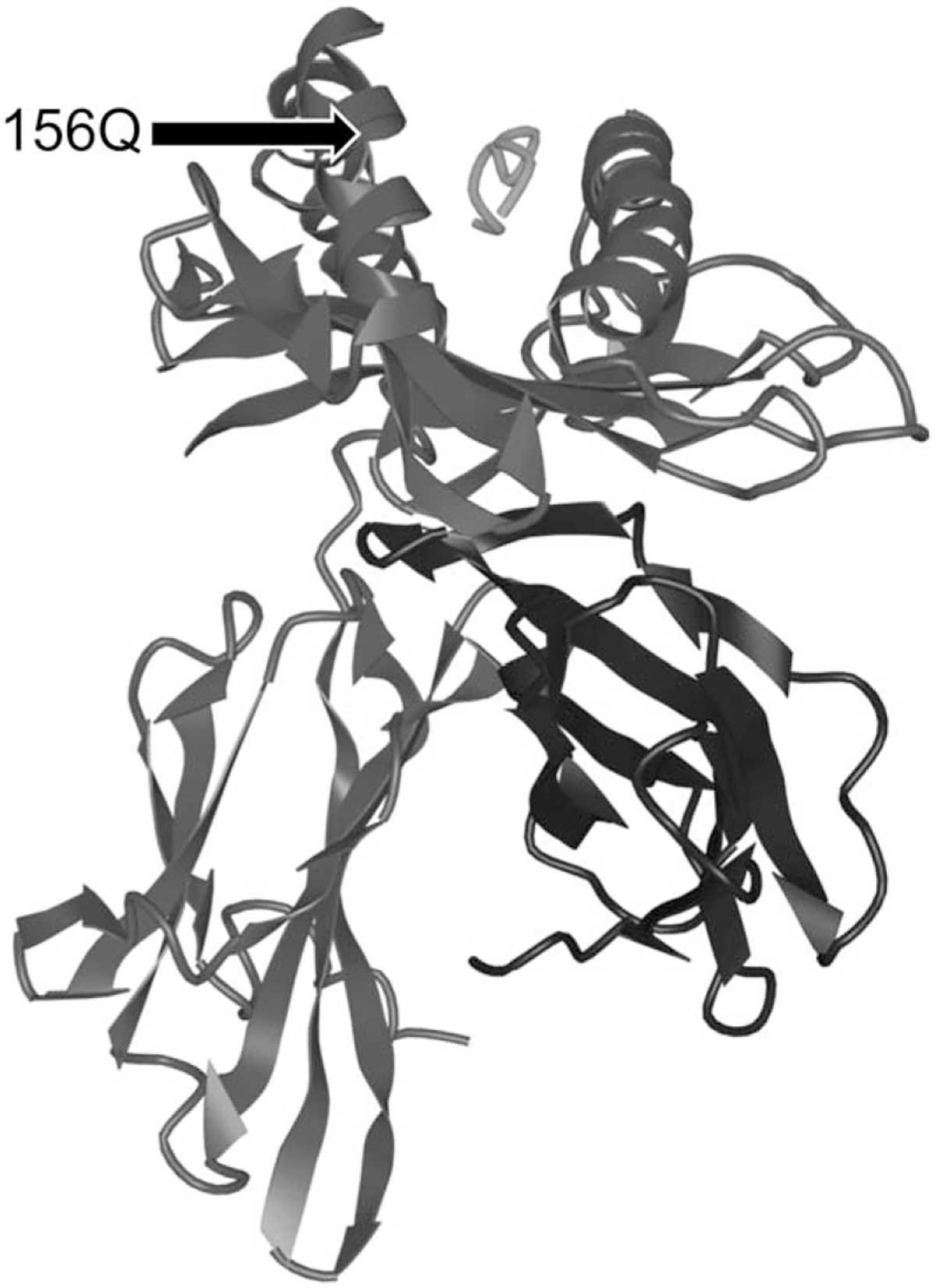
3D ribbon diagram of the HLA molecule with an antigen complexed within the peptide-binding groove and the position of 156Q amino acid substitution indicated (adapted from iCn3D, Wang et al).
This study has a few limitations. The cohort studied in this population was collected over a decade ago, and the management of both critically injured and septic patients has changed in this time. This may alter outcomes and susceptibility to infection after trauma. Relying on older criteria, during the period of patient recruitment, the incidence of sepsis amongst traumatically injured patients was approximately 9.7%.31 More modern series utilizing Sepsis-3 criteria estimate an incidence of approximately 3%.24 Our cohort is not directly comparable as the inclusion criteria were designed to enrich for patients at high risk for the development of sepsis. Despite these differences in incidence and management, the genetic characteristics being compared are relatively time-invariant and should transcend changes in clinical practice patterns.
The use of race as a surrogate for ancestry is wrought with challenges,32 and is an additional limitation of this study. Geographic heterogeneity and residual population stratification may introduce additional, unaccounted for genetic factors. However, no deviations from Hardy-Weinberg Equilibrium were observed in this cohort.
Respiratory infections were particularly common in this cohort compared to other studies.24 This is likely related to the inclusion criteria requiring ventilator use for greater than 48 hours for enrollment. This may limit generalizability to non-ventilator dependent critically injured patient populations.
Organ dysfunction has been used as a marker of the heterogeneity of response to injury and a marker of poor outcomes.25 Measures of organ dysfunction differed between patients who did and did not develop sepsis, though neither the amino acid variant 156Q nor the HLA-A*02:01 allele were associated with higher SOFA scores. Over a third of patients who did not develop sepsis had evidence of persistent multiple organ dysfunction, likely related to their initial injury. Thus, in our sepsis cohort, it is unclear whether organ dysfunction proceeded, or was a direct result of, severe infection. Though depressed HLA-DR expression has been associated with greater odds of organ failure in patients with community-acquired pneumonia,26 there is a little data describing associations between specific HLA variants and the development of organ dysfunction outside of transplant and autoimmune disease literature.
Despite these limitations, our large sample size and complete HLA genotyping allowed for more accurate ascertainment of potential associations, and the prospective study design was important for reducing phenotypic misclassification.
These findings may help explain variability in the severity of clinical manifestations of infection. Prompt diagnosis and treatment of infection is critical to improving mortality, and early identification of populations at greater risk for developing sepsis before infection would be ideal. Though genotyping is not currently part of the standard evaluation of critically injured and ill patients, these findings are a step towards developing a panel of genetic markers to assess risk of infection-related complications as we move towards more personalized medicine. Further research focused on confirming these associations in a validation cohort, and identifying potential mechanistic explanations are needed.
Supplementary Material
Acknowledgments
This paper was supported by DOD-HDTRAI1-11-1-005 Accurate Immune Typing using High-throughput Sequencing grant to M. Mindrinos. D. Horn is supported by the NIH T32 Postdoctoral Research Fellowship in Trauma, Injury, and Inflammation (5T32GM121290). G. O’Keefe is supported by an NIH R01 grant, R01GM066946. In addition, M. Mindrinos, S. Krishnakumar, and M. Li were employed by Immucor when they performed the HLA genotyping for this study.
Footnotes
The authors report no conflicts of interest.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
REFERENCES
- 1.Raju R. Immune and metabolic alterations following trauma and sepsis – an overview. Biochim Biophys Acta - Mol Basis Dis. 2017;1863:2523–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dombrovskiy VY, Martin AA, Sunderram J, et al. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003*. Crit Care Med. 2007;35:1244–1250. [DOI] [PubMed] [Google Scholar]
- 3.Lagu T, Rothberg MB, Shieh M-S, et al. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit Care Med. 2012;40:754–761. [DOI] [PubMed] [Google Scholar]
- 4.Ingraham AM, Xiong W, Hemmila MR, et al. The attributable mortality and length of stay of trauma-related complications. Ann Surg. 2010;252:358–362. [DOI] [PubMed] [Google Scholar]
- 5.Kimura F, Shimizu H, Yoshidome H, et al. Immunosuppression following surgical and traumatic injury. Surg Today. 2010;40:793–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almansa R, Wain J, Tamayo E, et al. Immunological monitoring to prevent and treat sepsis. Crit Care. 2013;17:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Skrupky LP, Kerby PW, Hotchkiss RS. Advances in the management of sepsis and the understanding of key immunologic defects. Anesthesiology. 2011;115:1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villar J, Maca-Meyer N, Pérez-Méndez L, et al. Bench-to-bedside review: understanding genetic predisposition to sepsis. Crit Care. 2004;8:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kotb M, Norrby-Teglund A, McGeer A, et al. An immunogenetic and molecular basis for differences in outcomes of invasive group A streptococcal infections. Nat Med. 2002;8:1398–1404. [DOI] [PubMed] [Google Scholar]
- 10.Parks T, Elliott K, Lamagni T, et al. Elevated risk of invasive group A streptococcal disease and host genetic variation in the human leucocyte antigen locus. Genes Immun. 2019;21:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso C, Candore G, Modica MA, et al. Major histocompatibility complex regulation of cytokine production. J Interf Cytokine Res. 1996;16:983–988. [DOI] [PubMed] [Google Scholar]
- 12.Aladzsity I, Madách K, Szilágyi Á, et al. Analysis of the 8.1 ancestral MHC haplotype in severe, pneumonia-related sepsis. Clin Immunol. 2011;139: 282–289. [DOI] [PubMed] [Google Scholar]
- 13.Shalhub S, Junker CE, Imahara SD, et al. Variation in the TLR4 gene influences the risk of organ failure and shock posttrauma: a cohort study. 2009;66:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankar-Hari M, Phillips GS, Levy ML, et al. Developing a new definition and assessing new clinical criteria for septic shock. JAMA. 2016;315: 775–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pappas DJ, Marin W, Hollenbach JA, et al. Bridging ImmunoGenomic data analysis workflow gaps (BIGDAWG): an integrated case-control analysis pipeline. Hum Immunol. 2016;77:283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hollenbach JA, Mack SJ, Thomson G, et al. Analytical methods for disease association studies with immunogenetic data. Methods Mol Biol. 2012;882:245–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lagha A, Messadi A, Boussaidi S, et al. HLA DRB1/DQB1 alleles and DRB1-DQB1 haplotypes and the risk of rheumatoid arthritis in Tunisians: a population-based case-control study. HLA. 2016;88:100–109. [DOI] [PubMed] [Google Scholar]
- 18.Kuranov A, Kötter I, Henes J, et al. Behçet’s disease in HLA-B*51 negative Germans and Turks shows association with HLA-Bw4–80I. Arthritis Res Ther. 2014;16:R116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stevens AM, Kanaan SB, Torok KS, et al. Brief report: HLA-DRB1, DQA1, and DQB1 in juvenile-onset systemic sclerosis. Arthritis Rheumatol. 2016;68:2772–2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershman MJ, Cheadle WG, Wellhausen SR, et al. Monocyte HLA-DR antigen expression characterizes clinical outcome in the trauma patient. Br J Surg. 1990;77:204–207. [DOI] [PubMed] [Google Scholar]
- 21.Cheron A, Floccard B, Allaouchiche B, et al. Lack of recovery in monocyte human leukocyte antigen-DR expression is independently associated with the development of sepsis after major trauma. Crit Care. 2010;14:R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ditschkowski M, Kreuzfelder E, Rebmann V, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. 1999;229:246–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.da Silva FP, Preuhs Filho G, Finger E, et al. HLA-A*31 as a marker of genetic susceptibility to sepsis. Rev Bras Ter Intensiva. 2013;25:284–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eguia E, Cobb AN, Baker MS, et al. Risk factors for infection and evaluation of Sepsis-3 in patients with trauma. 2019;218:851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shepherd JM, Cole E, Brohi K. Contemporary patterns of multiple organ dysfunction in trauma. Shock. 2017;47:429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menéndez R, Méndez R, Almansa R, et al. Simultaneous depression of immunological synapse and endothelial injury is associated with organ dysfunction in community-acquired pneumonia. J Clin Med. 2019;8:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haffner AC, Zepter K, Elmetst CA. Major histocompatibility complex class I molecule serves as a ligand for presentation of the superantigen staphylococcal enterotoxin B to T cells. Proc Natl Acad Sci. 1996;93:3037–3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Llewelyn M, Sriskandan S, Peakman M, et al. HLA class II polymorphisms determine responses to bacterial superantigens. J Immunol. 2004;172:1719–1726. [DOI] [PubMed] [Google Scholar]
- 29.Wang J, Youkharibache P, Zhang D, et al. iCn3D, a web-based 3D viewer for sharing 1D/2D/3D representations of biomolecular structures. Bioinformatics. 2020;36:131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Badrinath S, Kunze-Schumacher H, Blasczyk R, et al. A micropolymorphism altering the residue triad 97/114/156 determines the relative levels of tapasin independence and distinct peptide profiles for HLA-A* 24 allotypes. J Immunol Res. 2014;2014:298145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wafaisade A, Lefering R, Bouillon B, et al. Epidemiology and risk factors of sepsis after multiple trauma: an analysis of 29,829 patients from the Trauma Registry of the German Society for Trauma Surgery. Crit Care Med. 2011;39:621–628. [DOI] [PubMed] [Google Scholar]
- 32.Mersha TB, Abebe T. Self-reported race/ethnicity in the age of genomic research: its potential impact on understanding health disparities. Hum Genomics. 2015;9:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


