Abstract
The current study was planned to assess the neuropharmacological benefits of the Cucurbita maxima seed. These seeds have been conventionally used for the nutritional as well as amelioration of various diseases. However, there was a need to provide a pharmacological basis for such use. Four central nervous system-related functions, that is, anxiety, depression, memory, and motor coordination, were evaluated, and the levels of brain biogenic amines were also assessed. Anxiety was evaluated through selected experimental models, such as light and dark apparatus, elevated plus maze, head dip, and open field test. The head dip test was mainly used to assess exploratory behavior. Depression was assessed by two animal models, that is, the forced swim test and tail suspension test. Memory and learning ability were assessed by the passive avoidance test, stationary rod apparatus, and Morris's water maze test. Motor skilled learning was assessed by stationary rod and rotarod apparatus. Reversed phase high-pressure liquid chromatography was used to determine biogenic amine levels. Results reveal that C. maxima exhibited anxiolytic and antidepressant effects with memory improvement. There was a reduction in the weight of the animal following chronic administration. Furthermore, no remarkable effects were observed on motor coordination. Norepinephrine was found elevated, which may be linked to its antidepressant effects. These biological effects of C. maxima may be due to the presence of secondary metabolites, such as cucurbitacin, beta-sitosterol, polyphenolic compounds, citrulline, kaempferol, arginine, β-carotene, quercetin, and other antioxidants. The outcomes of the present study authenticate that the chronic use of C. maxima seeds reduces the intensity of neurological problems like anxiety and depression.
1. Introduction
Various neurological conditions, such as multiple sclerosis, traumatic brain injuries, and dementia, might be one of the causes of depression and anxiety. Dementia is a condition that affects the person's ability to execute routine life activities interfering with decisions of persons. To rule out the degree of dementia, the familiar paradigm is to evaluate one's ability to new learning and memory by certain models. Learning is an initial course of forming novel memories in the brain from an initial experience of previously learned events. It involves encrypting, storing, retrieving, and retaining the event in memory or forgetting [1]. Short-term memory is the storage of information for a short period without any repetition, whereas long-term memory is the preservation of data for an extended period due to recurrence. The usual learning procedure is based on the availability of neurotransmitters, such as dopamine, acetylcholine, and 5-hydroxytryptamine (5HT), which stimulate the hippocampus, amygdala, and other brain areas of the cerebral cortex like the sensory, visual, and auditory cortices [2]. Furthermore, several anxiolytics and antidepressant drugs alter learning memory or amnesia.
Cholinesterase inhibitors and dopamine agonists are considered the first line of treatment for the management of dementia [3]. Furthermore, some neurodegenerative disorders, such as Alzheimer's disease (AD), have progressive dementia either due to cholinergic neuronal loss or decreased levels of acetylcholine. The first management strategy for AD is to use acetylcholinesterase (AChE) inhibitors, which largely yield an effect by enhancing acetylcholine levels in the brain. Herbal remedies are traditionally popular for the treatment of various ailments because of the bioactive compounds in them.
Anxiety is a repeated episode of intense uncontrolled feelings of fear, confusion, tachycardia, and various panic symptoms. The short period of the depressive phase reaction is a normal reaction to everyday stress. However, if episodic symptoms of uneasiness continue that hinder the person's daily activity, then it is considered an anxiety disorder [4]. The major type of anxiety is generalized anxiety disorder. Furthermore, it is also associated with various depressive or behavioral disorders making management difficult [5]. There is a need to discover drug molecules having selectivity for 5-HT1A receptor antagonists and benzodiazepine type suppressant characteristics [6].
Depression is another psychological issue having persistent opposite depressive symptoms for more than 2 weeks. Depression is manifested by a feeling of sadness, guilt, tiredness, diminish concentration, loss of interest, low confidence, and disturbance in appetite or sleep. According to the Diagnostic and Statistical Manual, there are various types of depression, such as persistent depressive disorder, major depression, situational or seasonal affective disorder, postpartum depression, bipolar depression, psychotic depression, premenstrual dysphoric depression, and atypical depression [7].
Depression is a complex disorder that refers to the experience of feeling sadness and some heterogeneous associated symptoms that vary broadly characterized by loss of positive thoughts. Behavioral attributes of depression must incorporate the controlling variables at both interoceptive and environmental levels. These effects themselves are not problematic but rather adaptive, but if it becomes chronic, dysregulated, or maladaptive through several etiologies, then it needs to be treated. Moreover, depressed people commonly exhibit depressive-related behaviours including overthinking and social avoidance. This event may be negatively strengthened and create a cycle of increasing negative thoughts and avoidance, maliciously leading to depression [8]. According to the United States National Research Council and Institute of Medicine, various biological and environmental factors pose the person to depression including low age, genetic tendency, neurological or neuro-endocrinological, hormonal, and immunological. All these factors are cumulatively triggered by environmental factors such as stressful experiences of vulnerable persons due to psychosocial and biological circumstances. The presence of other medical and psychological disorders also worsens depression making it difficult to treat [9]. The initial antidepressant drugs were initially discovered based on hypotheses related to central nervous system (CNS) impairment of norepinephrine levels in the 1950s. After the revolution in drug discovery in neuroscience and genetics, new antidepressant drugs have been discovered, which cleared the basic mechanisms of depression and mechanism of the drug showing that the monoaminergic system is one of the bases of these mechanisms along with multiple interactions with other brain regulatory systems [10].
Cucurbita maxima was selected to confirm its memory-enhancing effect as several neuroprotective and antioxidant substances have been reported in the literature. C. maxima is a plant of Cucurbitaceae important for growth and immunity [11]. Besides its nutritional value, it is also a good source of important metabolites, such as phenolic glycosides [12], carotenoids, γ-aminobutyric acid [13, 14], flavonoids, alkaloids, tannins, saponins, and terpenoids [15]. It has been reported that it has hypocholesteremic, anthelmintic, hypotensive, hypoglycaemics, and antiperoxidative properties [16, 17]. Its use can also relieve symptoms of benign prostatic hyperplasia and some anxiety disorders [18]. Pectin isolated from C. maxima has the highest cytoprotective and antioxidant effect against reactive oxygen species generation in different cell lines [19, 20]. C. maxima seeds are supposed to have a high content of magnesium, which may behave as a N-methyl-D-aspartate (NMDA) receptor blocker, a very good pain killer specifically effective in nerve pain and have a euglycemic effect on diabetes decreasing type 2 diabetes generation in a healthy individual. Furthermore, it is also reported that a daily intake of 400 mg of magnesium oxide acts as a muscle relaxant. Therefore, it may relieve anxiety by relaxing the patients. Thus, due to these reported effects, C. maxima seeds were selected to evaluate their effects on the reduction of neurodegenerative disorders.
2. Materials and Methods
2.1. Collection of Seeds and Extraction
The seeds of C. maxima were bought from the local market and identified by the Plant Conservation center at the University of Karachi, Karachi, Pakistan, as GH #9501. The research was performed after approval of the advanced studies board, University of Karachi. The seeds of C. maxima were soaked in the proportion of 1 kg in 1.5 L using ethanol for 21 days. Seeds were kept in airtight ambered bottles during maceration followed by filtration and evaporation of the solvent. The extract was stored at 4°C till use after freeze-drying.
2.2. Experimental Design
The study was performed on healthy mice of either sex bred in the animal house, Department of Pharmacology, the University of Karachi, after acclimatization for 1 week. Mice were distributed into six groups having 10 mice per group kept in polycarbonate cages at 25 ± 2°C with access to food and water ad libitum. Animal of the control group received 5% Dimethyl sulfoxide (DMSO), two groups were given standard drugs diazepam 3 mg/kg [21] and imipramine 30 mg/kg [22], whereas mice of the test groups received ethanol extracts of C. maxima at doses of 50, 100, and 200 mg/kg for 30 days. The oral route of administration was selected to give DMSO, standard drugs, and seed extracts daily between the fixed time of 12 am to 1 pm. Behavioral experimentations were primarily conducted on days 1, 8, and 21 using different models for anxiety, depression, and memory. The behavioral sessions were recorded using an android mobile camera. At the end of the study, five mice from each group were sacrificed to determine the level of biogenic amines.
2.3. Chemicals
Chemicals purchased during the study were DMSO, imipramine hydrochloric acid (HCl; Sigma‑Aldrich, Headquarter Munich, Germany), Diazepam (Roche, Pakistan), some high-pressure liquid chromatography (HPLC) standards, such as homovanillic acid (HVA), noradrenaline (NA), adrenaline hydrochloride, 5HT, 5-hydroxy indole acetic acid, ethylene-diamine-tetra-acetic acid disodium salt, 3,4 dihydroxy-phenyl acetic acid (DOPAC), dopamine hydrochloride (DA), and HCl. Furthermore, some more chemicals were HPLC grade chemicals, such as methanol, acetonitrile, and deionized water. For disinfecting the apparatus after each reading, ethanol was used.
2.4. Model for Anxiety
2.4.1. Light and Dark Test
This model was employed to determine the anxiolytic ability of seed extracts by determining the preference of animals to be in the lighter compartment. This model has two equal compartments of 20 cm × 20 cm. The lighter compartment was brightened with a 100 V bulb, whereas the dark compartment had dark glasses that led to a darker region. The mice were introduced to a darker zone, and 5 minutes test sessions were performed during mice were freely moved between two compartments through an intermediate small gate. The anxiolytic activity was determined after measuring the time spent by mice in the light compartment, their percentages, and number of transitions. An increment in the percentage of time spent in the light area was suggestive of anxiolytic activity [23].
2.4.2. Elevated Plus Maze (EPM)
Subsequently, mice were employed in EPM on the eighth and twenty-first days of dosing. The EPM apparatus comprises two open arms (50 cm × 10 cm) and two closed arms (50 cm × 10 cm × 38 cm) linked to the central area (10 cm × 10 cm) at a height above 50 cm from the ground. Mice were placed in the central area towards the open arm and behavior was recorded for a 5-minute test session. Behavior measured were several entries into the open arm as well as the closed arm and time spent by the animal in the open arm and closed arm. An increment in time spent in the open area and the number of entries in the open arm was considered an anxiolytic activity. The anxiety index was calculated using the following formula. Anxiety in mice would be present if the result of the anxiety index was found from 0.6 to 1.0, whereas a reduction in the index exhibited an anxiolytic effect [24].
| (1) |
2.4.3. Head Dip Test
This test includes a white square wooden box of 35 cm × 45 cm × 45 cm containing equally spaced and sized three holes located at each side. Mice were introduced for 5 minutes session and several head dips through such holes were noted on the 8th and 21 days [25]. The increment in head dipping in this model reveals a rise in anxiety, whereas a decline in head dipping shows the anxiolytic effect of extracts [26].
2.4.4. Open Field Test Apparatus
It is a gold standard apparatus assessing different behaviors of rodents, such as anxiety, memory, exploration, and even depression. It consists of a plexiglass cube of 75 cm × 75 cm × 40 cm with marked flooring divided into 25 boxes, each of 15 cm × 15 cm with a central area demarcation of 30 cm × 30 cm. Mice were introduced in the center to explore the open field test (OFT) apparatus for 10 minutes session [27, 28]. During the test session, the parameters measured were the total distance covered by mice, the number of entries in the center, and their duration, rearing frequency, and duration. A reduction in the total distance shows a reduction in motor activity that may be due to sedative effects. An increment in time spent in center or center entries was revealing the anxiolytic activity. An increment in the frequency or duration of rearing shows high expressive and thoughtful behavior and an anxiety reduction. Recently, researchers related increment in rearing behavior with antidepressant effects [29].
2.5. Models for Memory
2.5.1. Passive Avoidance Test
Passive avoidance test (PAT) test is used for rapid assessment of the fear disinclination memory of mice. The escaping phenomenon was assessed using similar light and black apparatus having a grid floor and guillotine gate. The dark section's grid floor is attached to an electric source for giving tolerable foot electric shocks. The test was accomplished in three phases, i.e., habituation, education, and test periods. The mice were introduced in the illuminated section for 300 seconds on the first and second days of habituation facing the dark area with the guillotine door open. When the mice entered the dark booth, the door was shut down, and a foot jolt of 0.6 mA for 0.5 seconds was given through the grid floor [31]. During the test session, the mice were re-introduced into the apparatus after three hours, twenty-four hours, the eighth day, and the twenty-first day to assess short-term and long-term memories. Mice were placed in the illuminated section keeping the gate open to provide free access to the dark disinclination section. The latency to re-enter the black section was observed escaping electric shock with a maximum cut-off time of 300 seconds.
2.5.2. Morris Water Maze Test
This test was designed to evaluate the hippocampus-dependent acquisition of short- and long-term spatial learning and memory. This maize comprises rectangular water pool of dimension 60 cm × 30 cm. The test used water immersed fixed central stage of dimension 15 cm × 13 cm hidden by starchy at 25°C. In the education session, the stage was kept visible by maintaining the water below the stage. Mice were introduced in the pool and allowed to locate the stage. Four training sessions were performed for four consecutive days so that mice learned to discover the stage within a short period. The mice were tested on the first day, the eighth day, and the twenty-first day. A reduction in time was considered an improvement in special learning [33].
2.6. Motor Skilled Learning and Coordination
2.6.1. Effects on Motor Coordination through Rotarod
At present, anxiolytic drugs have effects on grip strength and muscle; therefore, in the current study, grip strength and muscular activity were examined via rotarod apparatus. The rotarod apparatus comprises an enclosed plastic rod of 8 cm × 3 cm. First, mice were given the training to walk on the stationary rods through four successive trails. The test session was performed at two speeds, i.e., 10 and 30 rpm on days 8th and 21st of administration of ethanol extracts. Latency time to keep on the movable rod until mice fall was noted with the cut-off time 180 seconds. An increment in time to keep on the rotating rod was considered an improvement in muscular activity. Drugs recognized to change neuromuscular coordination, such as diazepam, decrease the latency time of mice to keep on the rotating rod [30].
2.6.2. Stationary Rod Test
The education capability was assessed by stationary rod test (SRT) comprised raised steel rods with netted base. Mice were first taught by making them walk on the elevated rod with a networking stage. Animals were trained daily through four training sessions for four consecutive days with each trial of 120 seconds. After completing training, DMSO, standard drugs, and C. maxima seed extracts were administered orally for 30 days. The time required by animals to reach the stage was observed on the day first for short-term motor skilled learning, whereas on the eighth day and the twenty-first day for long-term motor skilled learning [32].
2.7. Models for Depression
2.7.1. Forced Swim Test
The test was employed to evaluate the anti-depressant effects of the extracts of the seeds on the 22nd day. The apparatus consists of rectangular plexiglass container of dimension 46 cm × 20 cm. Depression was induced in mice through the initial 2 minutes session by the learned helplessness phenomenon, whereas the last 4 minutes of mice's immobility time was noted as a sign of antidepressant activity [34]. Immobility is considered by the absence of the movement of mice except for those that required mice to keep their head out of water. Percent reduction in immobility time was used to assess the antidepressant effect of extracts [35, 36].
2.7.2. Tail Suspension Test
To verify the antidepressant effect, tail suspension method was used on the 22nd day. Animals were suspended with tape with their tails upside down for 6 minutes [37] inducing depression in the initial 2 minutes by the learned helplessness phenomenon. Immobility time was noted in the last 4 minutes, which was associated with antidepressant activity. The immobility time was measured as the absence of the movement of mice to upright themselves. Percent reduction in immobility was considered a degree of antidepressant effect of the extracts [35, 36]. Following the behavioral test, the mice were subsequently sacrificed via cervical dislocation. Their brains were taken out, stored at or below 80°C, and then processed for neurochemical analysis.
2.7.3. Neurochemical Analysis
Biogenic amines were determined by the reversed phase high-performance liquid chromatography or HPLC electrochemical detection (HPLC-EC) method using octadecylsilyl C18 column and methanol as a mobile phase [38]. These stored brains were initially defrosted, homogenized, and followed by extraction of biogenic amines using an extraction medium comprises of perchloric acid (70%). Consequently, two times centrifugations were performed that separated the homogenate. Homogenate was further separated by a reversed-phase column at a constant flow rate (1 mL/minutes) with the help of an HPLC pump. Electrochemical detection of separated biogenic amines was done using their correspondence standards that run simultaneously along with samples (Shimadzu, Kyoto, Japan) at 0.8 V of operating potential.
2.8. Statistical Analysis
All result values were calculated as Mean ± SEM by the SPSS statistical software package 26. One way analysis of variance was used followed by a post hoc Dunnet test at P < 0.5 and P < 0.1.
3. Results
3.1. Effect on Weight
Table 1 shows the effect of C. maxima on weight variation in mice. C. maxima seed extracts at 100 and 200 mg/kg exhibited an extremely substantial reduction in weight as compared with their initial weights on the 30th day with a percent reduction in weight by 11% at both doses.
Table 1.
Effect of C. maxima seed extracts on weight in mice.
| Groups/dose (mg/kg) | Weight day 0 (g) | Weight day 8 (g) | Weight day 15 (g) | Weight day 30 (g) | % Change in weight |
|---|---|---|---|---|---|
| Control | 28 ± 0.26 | 27 ± 1.15 | 28 ± 0.47 | 26 ± 1.08 | −7 |
| C. maxima 50 | 26 ± 0.43 | 25 ± 0.43 | 28 ± 0.70 | 26 ± 0.60 | 0 |
| C. maxima 100 | 28 ± 0.40 | 26 ± 0.51 | 29 ± 0.49 | 25 ± 0.45∗∗ | −11 |
| C. maxima 200 | 27 ± 0.78 | 26 ± 1.05 | 27 ± 0.92 | 24 ± 0.78∗∗ | −11 |
| Diazepam 3 | 21 ± 0.62 | 26 ± 1.02 | 24 ± 1.43 | 24 ± 0.32∗∗ | 10 |
| Imipramine 30 | 26 ± 0.48 | 26 ± 1.37 | 30 ± 2.6 | 31 ± 2.6∗∗ | 16 |
n = 10, average ± SEM; ∗∗greatly substantial as compared with initial weight; − (ve) sign indicates a reduction in weight.
3.2. Effect on Memory and Learning
3.2.1. Passive Avoidance Test
Table 2 and Figure 1 reveal the effects of C. maxima seed extract on memory and learning by PAT. C. maxima 50 mg/kg exhibited a greatly substantial increment in latency time at 3 hours and 8th day. C. maxima 100 mg/kg revealed a greatly substantial increment in reaction time at 3 hours, 24 hours, 8th day, and 21st day. C. maxima 200 mg/kg revealed a substantial increment in reaction time at 3 hours and 21st day, whereas an extremely substantial increment in reaction time at 24 hours and 8th day.
Table 2.
Outcome of C. maxima seeds on memory by passive avoidance.
| Groups and doses (mg/kg) | Latency time (seconds) | |||
|---|---|---|---|---|
| 3 hours | 24 hours | 8th day | 21st day | |
| Control | 115 ± 34 | 99 ± 11.6 | 94 ± 15 | 55 ± 6.26 |
| C. maxima 50 | 253 ± 32∗∗ | 166 ± 45 | 286 ± 14∗∗ | 117 ± 35 |
| C. maxima 100 | 258 ± 28∗∗ | 282 ± 18.1∗∗ | 262 ± 26.3∗∗ | 253 ± 32∗∗ |
| C. maxima 200 | 243 ± 29∗ | 233 ± 36∗∗ | 300 ± 0.0∗∗ | 215 ± 32∗ |
| Diazepam 3 | 155 ± 65 | 86 ± 13.5 | 16 ± 3.1∗ | 32 ± 14∗ |
| Imipramine 30 | 257 ± 43∗ | 245 ± 54.6∗ | 269 ± 31∗ | 209 ± 39∗ |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 1.
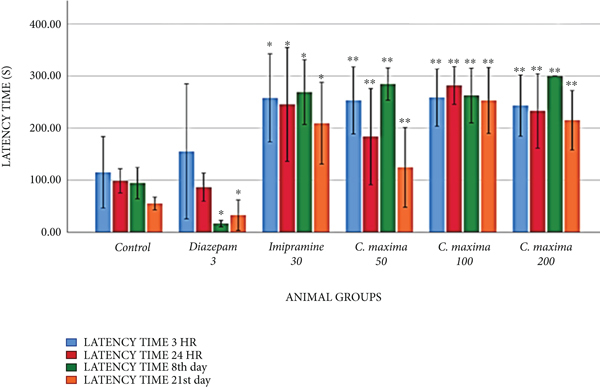
Effect of C. maxima on memory (passive avoidance test).
Diazepam 3 mg/kg exhibited a significant reduction in latency time on the 8th and 21st days, whereas imipramine 30 mg/kg exhibited a significant increment in latency time at 3 hours, 24 hours, 8th day, and 21st day.
3.2.2. Water Maze Test
Table 3 and Figure 2 reveal the effects of C. maxima seed extract on memory and learning by water maze test. C. maxima 50 and 100 mg/kg revealed a greatly substantial reduction in time to arrive at the central hidden stage on the eighth day and a substantial reduction in time to arrive at the central stage on the twenty-first day as compared with control. C. maxima 200 mg/kg showed a greatly substantial reduction in time to arrive at the stage at twenty-four hours and the eighth day, whereas the substantial reduction in time to arrive at the stage on the twenty-first day. Diazepam 3 mg/kg group revealed a greatly substantial increment in time to arrive at the central hidden stage on the eighth and twenty-first days, whereas imipramine 30 mg/kg revealed a greatly substantial reduction in time to arrive at the central hidden stage on the 8th and 21st days in comparison with control.
Table 3.
Effect of C. maxima seeds on the memory (water maze test).
| Groups and doses (mg/kg) | Time to arrive at the stage (seconds) | |||
|---|---|---|---|---|
| After learning | 24 hours | 8th day | 21st day | |
| Control | 14.4 ± 1.5 | 10.3 ± 0.7 | 19 ± 0.9 | 20.3 ± 0.7 |
| C. maxima 50 | 5.0 ± 0.7 | 2.5 ± 0.6∗∗ | 4.2 ± 0.5∗∗ | 4.8 ± 0.8∗ |
| C. maxima 100 | 6.1 ± 0.8 | 3.9 ± 0.7∗∗ | 5.5 ± 0.7∗∗ | 4.9 ± 1∗ |
| C. maxima 200 | 6.0 ± 0.5 | 3.0 ± 0.6∗∗ | 3.9 ± 0.5∗∗ | 4 ± 0.5∗ |
| Diazepam 3 | 15.2 ± 0.8 | 33.8 ± 2.7∗∗ | 36.6 ± 2.9∗∗ | 91.7 ± 13.1∗∗ |
| Imipramine 30 | 13.8 ± 1.3 | 11.9 ± 1.5 | 10.8 ± 1.7∗∗ | 6.2 ± 0.9∗∗ |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 2.
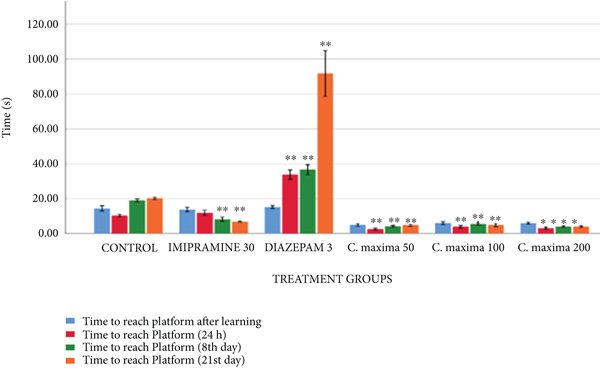
The outcome of C. maxima seeds on the memory (water maze test).
3.3. Effects on Motor Coordination and Motor Skilled Learning
3.3.1. Rotarod Test
C. maxima 100 and 200 mg/kg showed a substantial increment in fall time on the twenty-first day at low speed in comparison with control (Table 4). These C. maxima groups showed a greatly substantial increment in fall time on the 8th and 21st days at high speed in comparison with control. Diazepam 3 mg/kg exhibited a greatly substantial reduction in fall time on the 8th day at both rpm, whereas a highly significant reduction in fall on the 21st day at both rpm. Imipramine 20 mg/kg showed a greatly substantial increment in fall time on the 8th and 21st days at high speed in comparison with control.
Table 4.
Effect of C. maxima seed extracts on motor coordination by rotarod.
| Groups and doses (mg/kg) | Fall time(s) on 10 rpm | Fall time(s) on 30 rpm | ||
|---|---|---|---|---|
| 8th day | 21st day | 8th day | 21st day | |
| Control | 135.4 ± 7.2 | 123.83 ± 16.0 | 8.77 ± 0.7 | 4.37 ± 1.06 |
| C. maxima 50 | 154.6 ± 8.8 | 161.2 ± 7.8 | 23.9 ± 0.9∗∗ | 27.4 ± 1.5∗∗ |
| C. maxima 100 | 174.8 ± 2 | 176 ± 1.5∗ | 27.1 ± 1.3∗∗ | 29.7 ± 0.9∗∗ |
| C. maxima 200 | 167.4 ± 7.2 | 167.3 ± 7.3∗ | 36.2 ± 1.8∗∗ | 36.9 ± 1.4∗∗ |
| Diazepam 3 | 19.2 ± 2.5∗ | 40.4 ± 5.3∗∗ | 4.6 ± 0.9∗ | 4.40 ± 1.08∗∗ |
| Imipramine 30 | 139 ± 7.2 | 155.5 ± 1.7 | 26.3 ± 2.3∗ | 27.6 ± 3.7∗∗ |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
3.4. Stationary Rod Test
Table 5 and Figure 3 reveal the effects of C. maxima seed extract on memory and learning by SRT. C. maxima 50 mg/kg showed an extremely substantial reduction in time to reach the elevated stage at twenty-four hours and the eighth day, whereas a substantial reduction in time to reach the elevated stage on the 21st day in comparison with control. C. maxima 100 mg/kg showed a greatly substantial reduction in time to attain the elevated stage on the twenty-four hours, 8th day, and 21st day. C. maxima 200 mg/kg revealed a greatly substantial reduction in time to reach the stage on the eighth day and a substantial increment in time to reach the stage on the twenty-first day in comparison with the control. Animals who received diazepam 3 mg/kg exhibited a substantial increment in time to reach the elevated stage on the eighth and twenty-first days, whereas imipramine 30 mg/kg exhibited a substantial reduction in time to reach the elevated stage on the eighth and twenty-first days in comparison with control.
Table 5.
Effect of C. maxima seed extract on motor skilled learning and memory (stationary rod).
| Groups and doses mg/kg | Time to reach the stage (seconds) | |||
|---|---|---|---|---|
| After learning | 24 hours | 8th day | 21st day | |
| Control | 12.0 ± 1.0 | 19.5 ± 1.1 | 25.9 ± 0.8 | 26.3 ± 1.1 |
| C. maxima 50 | 11.9 ± 0.3 | 8.8 ± 1.1∗∗ | 15 ± 1.4∗∗ | 11 ± 0.9∗ |
| C. maxima 100 | 14.8 ± 0.8 | 9.2 ± 0.8∗∗ | 12 ± 0.6∗∗ | 11 ± 0.9∗∗ |
| C. maxima 200 | 16.2 ± 0.8 | 13.1 ± 0.8 | 11.9 ± 0.6∗∗ | 64.1 ± 9.2∗ |
| Diazepam 3 | 16.3 ± 0.6 | 28 ± 5 | 63.4 ± 8.5∗∗ | 82.9 ± 10.5∗∗ |
| Imipramine 30 | 13.9 ± 1.6 | 11.9 ± 1.8 | 8.2 ± 1.5∗∗ | 6.2 ± 0.9∗∗ |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 3.
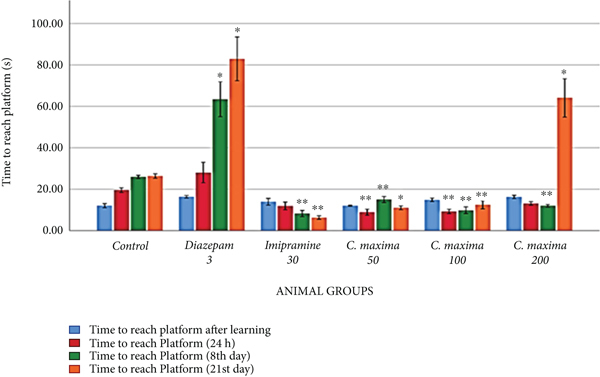
The outcome of C. maxima seeds on motor skilled learning and memory (stationary rod).
3.5. Effect on Anxiety
3.5.1. Light and Dark Model
C. maxima 50 mg/kg showed a greatly substantial increment in transitions on the 8th and 22nd day, whereas a greatly substantial increment in the percentage of time spent in the light area on the 21st day in comparison with control.
C. maxima 100 200 mg/kg showed an extremely substantial increment in the percentage of time spent in the light area on the 8th and 21st days, whereas C. maxima 100 mg/kg showed a greatly substantial increment in transitions on the 8th day in comparison with the control. Animals who received diazepam 3 mg/kg showed a greatly substantial increment in the percentage of time spent in light area and transitions on the 8th and 21st days in comparison with control. Animals given imipramine 30 mg/kg showed a substantial increment in transitions on the 8th day. Table 6 showed the effects of C. maxima seed extracts on anxiety by light and dark models.
Table 6.
Effect of C. maxima seeds on anxiety by light and dark model.
| Groups and doses (mg/kg) | No of transitions | % Time in light | ||
|---|---|---|---|---|
| 8th day | 21st day | 8th day | 21st day | |
| Control | 1.5 ± 0.2 | 2.4 ± 0.5 | 6.7 ± 1.8 | 10.6 ± 1.7 |
| C. maxima 50 | 6.2 ± 0.9∗∗ | 7.6 ± 0.7∗∗ | 30.9 ± 9.6 | 34.3 ± 3.7∗∗ |
| C. maxima 100 | 4.9 ± 0.3∗∗ | 4.6 ± 0.4 | 58.9 ± 7∗∗ | 55 ± 7∗∗ |
| C. maxima 200 | 3.8 ± 0.3 | 3.5 ± 0.3 | 40.3 ± 4.5∗∗ | 40.5 ± 4.5∗∗ |
| Diazepam 3 | 4.5 ± 0.5 | 0.7 ± 0.7 | 33.5 ± 8.5∗∗ | 54.3 ± 3.9∗∗ |
| Imipramine 30 | 5.6 ± 0.9∗ | 3.8 ± 1.1 | 10.8 ± 2.7 | 6.3 ± 2 |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
3.5.2. Elevated plus Maze
Table 7 and Figure 4 show the effects of C. maxima seed extracts on anxiety by eEPM. C. maxima 100 and 200 mg/kg showed an extremely substantial and substantial increment in the percentage of time spent in an open area on the 8th and 22nd days, whereas exhibiting a significant increment in transitions on the 8th day as compared with control. These two groups also showed a greatly substantial and substantial reduction in time spent and transitions in closed arms on the eighth and twenty-first days, respectively. C. maxima 100 and 200 mg/kg also exhibited a reduction in anxiety index on the eighth and twenty-first days, respectively.
Table 7.
Effect of C. maxima seed extracts on anxiety (elevated plus maze).
| Groups and doses (mg/kg) | Open arm's time (seconds) | Closed arm's time (seconds) | Closed-arm entries | Open arm entries | Anxiety index | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | |
| Control | 11 ± 1.28 | 27 ± 3.82 | 289 ± 1.3 | 270.8 ± 4.5 | 2.9 ± 0.5 | 3.6 ± 0.4 | 3.3 ± 0.6 | 3.8 ± 0.6 | 0.71 | 0.68 |
| C. maxima 50 | 70 ± 8 | 23 ± 3 | 230 ± 8 | 277 ± 3 | 4.4 ± 0.8 | 2.5 ± 0.4 | 7.4 ± 1.3 | 4.3 ± 0.7 | 0.58 | 0.65 |
| C. maxima 100 | 187 ± 28.5∗∗ | 91.6 ± 14.5∗ | 113 ± 8.5∗∗ | 209 ± 14.5∗ | 3.8 ± 0.6 | 4 ± 0.71 | 8.5 ± 1.4∗∗ | 8.0 ± 1.3 | 0.33 | 0.51 |
| C. maxima 200 | 214 ± 22∗∗ | 93 ± 8.5∗ | 86 ± 22∗∗ | 206.9 ± 8.5∗ | 2.7 ± 0.6 | 4.0 ± 0.7 | 8.4 ± 1.3∗∗ | 8.4 ± 1.7 | 0.26 | 0.49 |
| Diazepam 3 | 181 ± 24∗∗ | 192 ± 35∗∗ | 119 ± 24∗∗ | 107.6 ± 35∗∗ | 2.8 ± 0.5 | 6.3 ± 0.5∗ | 5.3 ± 0.5 | 10.0 ± 1.2∗ | 0.37 | 0.39 |
| Imipramine 30 | 26 ± 6.5 | 47 ± 15 | 266.2 ± 12 | 253 ± 15 | 4.5 ± 1.3 | 4.0 ± 1.9 | 7.8 ± 2 | 8.0 ± 1.6 | 0.63 | 0.59 |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 4.
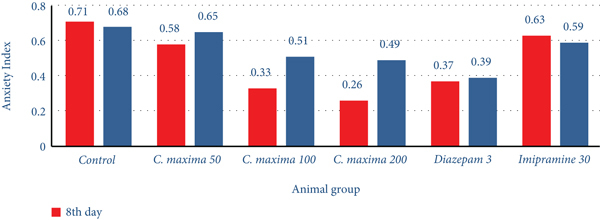
The outcome of C. maxima seeds on anxiety index (EPM).
Animals who received diazepam 3 mg/kg exhibited a greatly significant increment in time spent in the open arm on the eighth and twenty-first days as compared with control, whereas exhibiting a significant increment in transitions on the 21st day. Imipramine 30 mg/kg exhibited an increment in time spent in the open arm as compared with the control. The seed extract of C. maxima 100 and 200 mg/kg on the 8th day exhibited a significant reduction in the anxiety index as per standard. Table 6 showed the effects of C. maxima seed extracts on anxiety by EPM.
3.5.3. Head Dip Test
C. maxima 100 mg/kg showed a greatly substantial reduction in the number of head dips on the 21st day. C. maxima 200 mg/kg showed an extremely substantial reduction in the number of head dip on the eighth and twenty-first days in comparison with control animals.
Animals given the diazepam 3 mg/kg exhibited a greatly substantial reduction in the number of head dip on the eighth day while displaying a greatly substantial reduction in the number of head dips on the twenty-first day in comparison with the control. Animals that received imipramine 30 mg/kg revealed a greatly substantial reduction in head dip in comparison with the control group. Table 8 reveals the effect of C. maxima seed extract on several head dips.
Table 8.
Effect of C. maxima seed extracts on head dips.
| Groups and doses (mg/kg) | No. of head dips | |
|---|---|---|
| 8th day | 21st day | |
| Control | 31.5 ± 0.9 | 29.7 ± 0.6 |
| C. maxima 50 | 28 ± 1.5 | 28.5 ± 1.3 |
| C. maxima 100 | 26.3 ± 1.5 | 15.5 ± 2.2∗∗ |
| C. maxima 200 | 24 ± 1.4∗ | 17 ± 1.9∗∗ |
| Diazepam 3 | 39.8 ± 1.7∗ | 21 ± 1.8∗∗ |
| Imipramine 30 | 19.4 ± 2.9∗∗ | 17.4 ± 2.0∗∗ |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
3.5.4. Open Field Test
Table 9 and Figure 5 reveal the effects of C. maxima seed extract on anxiety by OFT. C. maxima 50 mg/kg showed a greatly substantial rise in the total distance on the eighth day; significant and greatly substantial rise in center entries on the 8th and 21st days, respectively; significant and greatly significant increment in the duration of rearing's at the 8th and 21st days in comparison with control, respectively (Table 8). C. maxima 100 and 200 mg/kg showed an extremely substantial increment in the total distance on the 8th day; greatly significant increment in center entries, center time; number, and duration of rearing 8th and 21st days in comparison with control. Diazepam 3 mg/kg exhibited a significant reduction in the total distance on the 21st day; a greatly significant increment in center entries along with center time on the 21st day; a significant reduction in the number and duration of rearing's on the 21st day as compared with control. Imipramine 30 mg/kg exhibited an extremely significant reduction in center entries on the 21st day and an extremely significant increment in numbers of rearing's at the 8th and 21st days in comparison with the control.
Table 9.
Outcome of C. maxima seeds in open field test.
| Groups and doses (mg/kg) | Total distance travelled (cm) | No. of center entries | Centre time(s) | No. of rearing | Duration of rearing (s) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | 8th day | 21st day | |
| Control | 1904 ± 81 | 2273 ± 14 | 2.8 ± 0.3 | 2.7 ± 0.3 | 3.4 ± 0.5 | 3.5 ± 0.6 | 4.3 ± 0.5 | 11 ± 1 | 4.5 ± 0.5 | 13 ± 1 |
| C. maxima 50 | 3007 ± 11∗∗ | 2140 ± 15 | 5.8 ± 0.7∗ | 8 ± 0.9∗∗ | 5 ± 1 | 8 ± 1.2s | 4.7 ± 1 | 12 ± 1.5 | 17 ± 1.2∗∗ | 24 ± 1.2∗ |
| C. maxima 100 | 2642 ± 14∗ | 2412 ± 16 | 8 ± 0.6∗∗ | 8.6 ± 0.6∗∗ | 14 ± 1∗∗ | 14 ± 1.1∗∗ | 16.3 ± 0.8∗∗ | 25 ± 1∗∗ | 16 ± 1∗ | 32 ± 1.8∗∗ |
| C. maxima 200 | 2941 ± 11∗∗ | 2572 ± 11 | 7.8 ± 0.3∗∗ | 11 ± 0.7∗∗ | 15 ± 1∗∗ | 17 ± 1.6∗∗ | 26.5 ± 0.9∗∗ | 24 ± 1∗∗ | 12.8 ± 1∗∗ | 32 ± 4.2∗∗ |
| Diazepam 3 | 2452 ± 22 | 1246 ± 18∗ | 6.25 ± 1 | 10 ± 1∗∗ | 6 ± 2 | 23 ± 2∗∗ | 2 ± 0.5 | 1 ± 0.3∗∗ | 3 ± 0.9 | 5.5 ± 1∗ |
| Imipramine 30 | 1503 ± 80 | 1591 ± 83 | 2 ± 0.28 | 6.5 ± 0.6∗∗ | 3 ± 0.6 | 7.32 ± 0.4 | 15 ± 1∗∗ | 22 ± 0.7∗∗ | 8 ± 1.9 | 11 ± 2 |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 5.
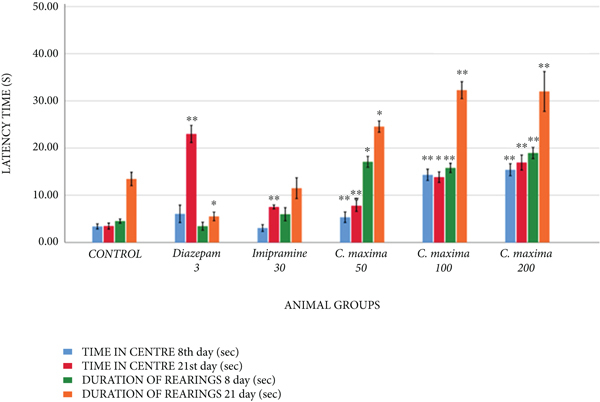
The outcome of C. maxima seeds in OFT.
3.5.5. Effect on Depression
When evaluating the antidepressive research, two models were used. During this examination, every mouse survived. None of the mice drowned when tested using the forced swim test or the tail flick method.
3.5.6. Forced Swim Test
C. maxima 50, 100, and 200 mg/kg showed an extremely substantial reduction in immobility time with a percent reduction of 36%, 35.4%, and 49.3%, respectively, on the 22nd day in comparison with the control. Imipramine 30 mg/kg shows maximum antidepressant activity, that us, 54.1% as compared with control in FST.
3.5.7. Tail Suspension Test
C. maxima 50, 100, and 200 mg/kg showed an extremely substantial reduction in immobility time with a percent reduction of 34.3%, 35.4%, and 42%, respectively, on the 22nd day in comparison with control. While imipramine 30 mg/kg showed an extremely substantial reduction in immobility time with a percent reduction of 52.5% on the 22nd day as compared with the control (Table 10 and Figure 6).
Table 10.
Effect of C. maxima seed extracts on depression by forced swim test and tail suspension test.
| Groups and doses (mg/kg) | Forced swim test | Tail suspension test | ||
|---|---|---|---|---|
| Immobility time (seconds) 22nd day | % Reduction immobility | Immobility time (seconds) 22nd day | % Reduction immobility | |
| Control | 200 ± 2.3 | — | 211.2 ± 4.8 | — |
| C. maxima 50 | 154 ± 7.8∗∗ | 36 | 158 ± 1.7∗∗ | 34.3 |
| C. maxima 100 | 155 ± 8.7∗∗ | 35.4 | 155 ± 2.5∗∗ | 35.4 |
| C. maxima 200 | 122 ± 7.8∗∗ | 49.3 | 139 ± 4.1∗∗ | 42 |
| Diazepam 3 | 218 ± 14.8 | 9.19 | 224 ± 4.1 | 2.5 |
| Imipramine 30 | 110 ± 6.3∗∗ | 54.1 | 113.9 ± 8.6∗∗ | 52.5 |
n = 10, average ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control.
Figure 6.
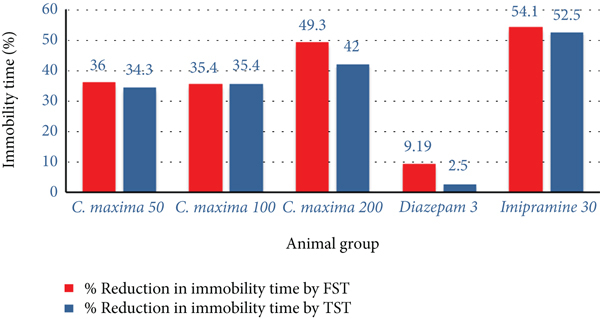
Comparison of reduction in immobility by C. maxima seed extract (FST and TST).
3.5.8. Brain Biogenic Amines Evaluation
Table 11 and Figure 7 show the effects of C. maxima seeds on brain biogenic amines at selected doses. C. maxima 50 mg/kg revealed an extremely substantial increment in concentration of NA and an extremely significant reduction in the concentration of HVA in the brain on the 30th day in comparison with control. C. maxima 100 mg/kg revealed an extremely substantial increment in concentration of NA, DOPAC, and DA, whereas an extremely significant reduction in the concentration of HVA in the brain on the 30th day in comparison with the control. C. maxima 200 mg/kg revealed a greatly substantial increment in concentration of NA, DOPAC, DA, and 5HT in the brain on the 30th day in comparison with the control.
Table 11.
Outcome of C. maxima seed extracts on brain biogenic amines.
| Groups and doses (mg/kg) | NA | HVA | DA | DOPAC | 5HT | 5-HIAA |
|---|---|---|---|---|---|---|
| (pg/mg of wet tissue) | ||||||
| Control | 56 ± 4.4 | 487 ± 9.5∗∗ | 357 ± 128.5 | 172 ± 29.8 | 97 ± 29.5 | 333 ± 7.8 |
| C. maxima 50 | 347 ± 10.5∗∗ | 298.8 ± 18.5∗∗ | 435.7 ± 17.2 | 130.2 ± 12.5 | 313 ± 29.2 | 325 ± 53.3 |
| C. maxima 100 | 573 ± 21∗∗ | 264.5 ± 41∗∗ | 632 ± 13.5∗∗ | 421 ± 13∗∗ | 382 ± 83.2 | 269.2 ± 75.2 |
| C. maxima 200 | 697.8 ± 35∗∗ | 423.2 ± 74 | 883.1 ± 58∗∗ | 443.9 ± 52∗∗ | 452.6 ± 86∗∗ | 296.0 ± 34 |
n = 10, mean ± SEM; ∗P < 0.05 substantial; ∗∗P < 0.01 greatly substantial in comparison with control; NA = norepinephrine; DOPAC = dioxyphenylacetic acid; DA = dopamine; 5-HIAA = 5-hydroxyindoleaceticacid; HVA = homovanillic acid; 5HT = 5-hydroxytryptamine.
Figure 7.
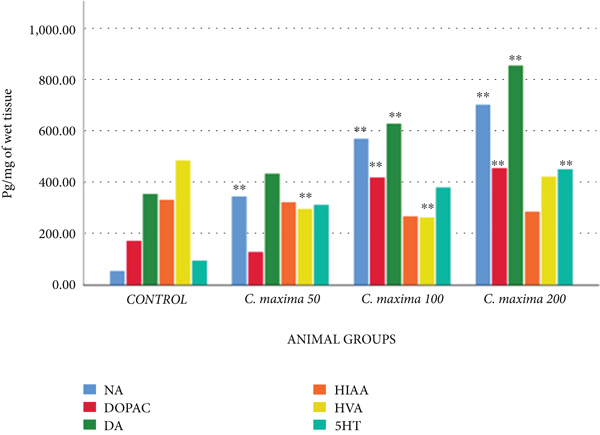
Levels of biogenic amines in the brain.
4. Discussion
Herbal medicines are playing a crucial role in the prevention and treatment of various disorders as these are the main sources of secondary metabolites. The current study was designed to evaluate the pharmacological activity of C. maxima seeds extract to rule out their role in weight reduction, anxiety, depression, and memory amelioration. Diazepam 3 mg/kg exhibited weight gain, whereas weight gained was more pronounced in the case of imipramine 30 mg/kg (16%). A reduction in weight by more than 10% from initial body weight is a remarkable change in weight which was observed by C. maxima at 100 and 200 mg/kg. The weight reduction by C. maxima may be due to the presence of high content of steroidal anti-inflammatory substances like cucurbitacin A, B, C, and D and the absence of cholesterol and saturated fatty acids. Furthermore, C. maxima seed extracts also revealed a marked reduction in spontaneous activity, pain response, touch response, corneal and light responses, grip strength, at 50, 100, and 200 mg/kg doses, whereas there was marked increment in the balance beam light in the light and dark model. C. maxima at 100 mg/kg showed maximum anxiolytic activity, that is, 58.9% and 55% on the 8th and 21st days, respectively, equivalent to diazepam (54%). In the EPM model, the maximum reduction in anxiety index was observed at 200 mg/kg dose on the 8th day, that is, 0.26, whereas diazepam shows a significant reduction in anxiety index, that is, 0.37 and 0.39 at the 8th and the 21st day. Imipramine exhibited no remarkable reduction in anxiety in any model of anxiety.
In the OFT model, the effects of C. maxima were almost comparable with the control in terms of total distance covered. Diazepam exhibited a significant reduction in total distance covered by animals on the 21st day, whereas C maxima extract and imipramine have no effect on the reduction of total distance covered by the animal on both the 8th and 21st days. Despite the reduction of total distance, diazepam significantly increased the center time and center entries verifying its anxiolytic effect. Imipramine only increases center entries as well as rearing showing improved learning and exploration. However, C. maxima exhibited more rearing duration as compared with the imipramine 3 mg/kg. An increase in rearing behaviors shows improved learning, whereas an increase in center activity shows an anxiolytic effect.
In the PAT, C. maxima at 50 mg/kg displayed an extremely substantial rise in latency time to enter the punished area at 3 hours and 8th day. C. maxima at 100 mg/kg displayed an extremely substantial rise in reaction time at 3 hours, 24 hours, 8th day, and 21st day. C. maxima at 200 mg/kg revealed a substantial rise in reaction time at 3 hours and 21st day, whereas a greatly substantial increase in reaction time at 24 hours and 8th day. Diazepam exhibited a significant reduction in latency time to enter a punished dark box due to its amnesic effect. C. maxima extracts revealed no such effects. Imipramine 30 mg/kg showed a significant increment in latency time at 8 hours, 24 hours, 8th day and 21st day.
In the water maze test, C. maxima 50 and 100 mg/kg showed an extremely substantial fall in time to arrive at the stage on the eighth day and a substantial fall in time to attain the stage on the twenty-first day in comparison with the control. C. maxima 200 mg/kg showed an extremely significant decrease in time to reach the stage at a time at 24 hours, and 8th day, whereas a significant decrease in time to reach the stage on the 21st day in comparison with control that is comparable with imipramine. Diazepam significantly increases the time to reach the platform highlighting its amnesic effect.
As far as motor coordination was evaluated, C. maxima 100 and 200 mg/kg showed improvement in muscle activity by an increase in the fall time in rotarod. Diazepam 3 mg/kg exhibited a greatly substantial reduction in fall time on the 8th day at both rpm, whereas a highly substantial reduction in fall on the 21st day at both rpm. Imipramine 20 mg/kg showed a greatly substantial increment in fall time on the 8th and 21st days at high speed in comparison with control.
In the SRT, the animals given C. maxima seed extract revealed an extremely substantial decline in the time to attain the stage at 24 hours and eighth day, whereas a substantial reduction in the time to achieve the stage on the twenty-first day in comparison with the control. C. maxima at 100 mg/kg showed a greatly substantial fall in time to attain the stage at 24 hours, 8th day, and 21st day in comparison with control. C. maxima at 200 mg/kg showed a greatly substantial increment in time to attain the stage on the eighth day and a substantial fall in time to attain the stage on the twenty-first day in comparison with the control. Diazepam showed a greatly substantial increase in time to attain the stage highlighting its effect on reduction in motor learning due to muscle relaxant activity.
The antidepressant effects of seeds were determined and evaluated by the forced swimming and tail suspension tests in mice. C. maxima extracts showed maximum antidepressant effects at 200 mg/kg comparable with imipramine. This may be due to increased concentration of NA and dopamine and a decrease in the metabolism of NA indicated by decreasing concentration of HVA in the brain, whereas at 200 mg/kg C. maxima also revealed an elevation in 5HT level. Dopamine metabolism was also found to increase after C. maxima dosing. The reason may be due to CNS active metabolites, such as polyphenols and β-carotene [39].
Sinha et al. [40] reported that ethanol extract of C. maxima is a cholinesterase inhibitor, hence producing a neuroprotective effect since the hydrolysis of the acetylcholine by cholinesterase has been linked with cognition impairment. Increased activity of the brain AChE causes fast hydrolysis of acetylcholine in turn increases the risk for the progression of dementia. Therefore, there is a growing interest in novel cholinesterase inhibitors for the management of cognition impairment [41]. In addition, higher inhibition of butyrylcholinesterase (BChE) activity is often desirable in humans. Any mutilation of the monoaminergic neurotransmission by monoamine oxidase (MAO) inhibitor has been implicated in the pathogenesis and progression of several neurodegenerative diseases, especially Parkinson's and Alzheimer's [42]. Hence, inhibition of MAO activities by suitable agents, particularly plant-derived molecules/extracts, may provide a useful therapeutic strategy in the management of cognitive impairment. Many previous investigations have reported that plant extracts are potent inhibitors of MAO activity. In this study, the ability of the tested extracts of C. maxima, to inhibit MAO activity could be a probable mechanism, contributing to their neuroprotective properties [40]. Hence, inhibition of AChE and BChE activities and stimulation of Na+/K+-ATPase activity by tested extracts can provide an avenue for the development of effective drugs of plant origin for the management of cognitive disorders.
Arora et al. [43] revealed prominent anxiolytic activity of Cucurbita moschata seed extracts at 200 mg/kg which was comparable with the standard drug alprazolam in both models. Moreover, an alteration was also observed in motor coordination by the ethanol extract at the same dose. The probable mechanism suggested was an increase in chloride ion influx suggesting a γ-Aminobutyric acid type A (GABAA) receptors-mediated mechanism of action. Hence, it may be assumed that C. maxima extract may have produced the effect in a similar pattern.
Antidepressant and anxiolytic drugs have a memory suppressing effect [44]. They emphasize the discovery of newer therapies without adverse effects. Hence in the current study, nootropic effects of C. maxima were measured using three different models stationary rod, passive avoidance, and water maze test. C. maxima seed extract exhibited an increasing effect on the memory-recalling process both at short-term and long-term levels in comparison with control in all three selected models.
C. maxima is popular for the amelioration of various diseases since contain different secondary polyphenolic molecules, such as quercetin and p-coumaric acid [40]. Several biological activities of C. maxima have been reported due to the presence of these metabolites. It behaves like a potent antioxidant because of a high percentage of β-carotene that enhances immunity and decreases the incidence of other medical problems, such as cancer and the progression of heart disease.
Hence in the present study, it is justified to conclude that the reduction in anxiety and depression may be linked with changes in levels of biogenic amines. Recently, a study was conducted on the neuroprotective effect of C. maxima ether seed extracts on ethidium bromide-induced demyelination in Wistar rats. The results of this study are concurrent to our study, which shows that C. maxima seeds have a potential neuroprotective effect in rat-induced demyelination with an improvement in muscle strength and coordination [45]. Sinha et al. [40] reveal that modulation of MAOs, cholinesterase, and sodium–potassium ATPase activities in the brain through phyto-molecules has been effective in the management of cognitive disorders. Sinha et al. [40] also showed that ethanol and hexane extracts of C. maxima at 50 μg/ml concentration inhibited the AChE and BChE activities compared with the standard drug, donepezil which was linked to the presence of quercetin in Cucurbita species. Quercetin is a plant-derived polyphenol having anti-carcinogenic, anti-inflammatory, and antiviral properties, as well as the capacity to reduce lipid peroxidation, platelet aggregation, and capillary permeability [46].
5. Conclusion
Neurological problems are an increasing trend these days. Therefore, it is a need for time to identify natural products with neuropharmacological benefits. C. maxima has now been regarded as an important neuroprotective since several investigators have reported that it contains many neuroprotective and anti-inflammatory metabolites. Thus, it should be included in our daily diet not only to enhance memory, but also to reduce the symptoms of anxiety and depression. Present work authenticates that the chronic use of C. maxima seed in neurological problems has been very efficacious in ameliorating such problems. However, before being used widely, adequate clinical trials are crucial. Moreover, without appropriate coverage by reputable worldwide venues, the advantages of these studies cannot be widely distributed.
Acknowledgments
Research grant from the Department of Pharmacology, University of Karachi has been utilized to complete this piece of work.
Data Availability
Data supporting this research article are available from the corresponding author or first author on reasonable request.
Ethical Approval
This study was supported by approval from the Board of Advance Studies and Research, University of Karachi. Reference no. 03297/Pharm was granted by the Board on April 20, 2017, to conduct the study. This approval was followed by the permission of the Ethical Committee, Department of Pharmacology for the use of animals as per the National Institute of Health guidelines for the care and usage of laboratory animals.
Conflicts of Interest
The author(s) declare(s) that they have no conflicts of interest.
Authors' Contributions
All authors have contributed to the writing of the manuscript. Dr. Shahana Wahid contributed to the material preparation, data collection, experimental work, and analysis. Dr. Ali Alqahtani contributed to the review and editing of the manuscript. Dr. Rafeeq Alam Khan contributed to the conception and design of the study and finalized the manuscript. All authors read and approved the final manuscript.
References
- 1.Okano H., Hirano T., Balaban E. Learning and memory. Proceedings of the National Academy of Sciences of the United States of America . 2000;97(23):12403–12404. doi: 10.1073/pnas.210381897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wiltgen B., Sanders M., Anagnostaras S., Sage J., Fanselow M. Context fear learning in the absence of the hippocampus. The Journal of Neuroscience . 2006;26(20):5484–5491. doi: 10.1523/JNEUROSCI.2685-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luanaigh C. O., Lawlor B. Drugs that affect competence. In: Maeck L., Stoppe G., editors. Competence Assessment in Dementia . Wien: Springer-Verlag; 2008. pp. 209–226. [DOI] [Google Scholar]
- 4.Raikhy S., Gautam S., Kanodia S. Pattern and prevalence of psychiatric disorders among patients attending dermatology OPD. Asian Journal of Psychiatry . 2017;29:85–88. doi: 10.1016/j.ajp.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 5.Ballenger J. C., Davidson J. R., Lecrubier Y., et al. Consensus statement on posttraumatic stress disorder from the international consensus group on depression and anxiety. The Journal of Clinical Psychiatry . 2000;61:60–66. [PubMed] [Google Scholar]
- 6.Pytka K., Podkowa K., Rapacz A., et al. The role of serotonergic, adrenergic and dopaminergic receptors in antidepressant-like effect. Pharmacological Reports . 2015;68:263–274. doi: 10.1016/j.pharep.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Rihmer Z., Xenia G. Antidepressant-resistant depression and antidepressant-associated suicidal behaviour: the role of underlying bipolarity. Depression Research and Treatment . 2011;2011:p. 5. doi: 10.1155/2011/906462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kanter J. W., Busch A. M., Weeks C. E., Landes S. J. The nature of clinical depression: symptoms, syndromes, and behavior analysis. The Behavior Analyst . 2008;31(1):1–21. doi: 10.1007/BF03392158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.National Research Council and Institute of Medicine (US) Committee on depression, parenting practices, and the healthy development of children. In: England M. J., Sim L. J., editors. Depression in Parents, Parenting, and Children: Opportunities to Improve Identification, Treatment, and Prevention . Washington, DC: National Academies Press; 2009. 3, The Etiology of Depression. Available from: https://www.ncbi.nlm.nih.gov/books/NBK215119. [PubMed] [Google Scholar]
- 10.Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues in Clinical Neuroscience . 2002;4(1):7–20. doi: 10.31887/DCNS.2002.4.1/bbondy. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mansour E. H., Dworschak E., Pollhamer Z., Gergely A., Hovari J. C. maxima and canola seed proteins and bread quality. Acta Alimentaria . 1999;28:59–70. [Google Scholar]
- 12.Glew R. H., Glew R. S., Chuang L. T., et al. Amino acid, mineral and fatty acid content of pumpkin seeds (Cucurbita spp.) and Cyperus esculentus nuts in the Republic of Niger. Plant Foods for Human Nutrition . 2006;61(2):51–56. doi: 10.1007/s11130-006-0010-z. [DOI] [PubMed] [Google Scholar]
- 13.Matus Z., Molnar P., Szabo L. G. Main carotenoids in pressed seeds (Cucurbitae semen) of oil pumpkin (Cucurbita pepo convar. pepo var. styriaca) Acta Pharmaceutica Hungarica . 1993;63(5):247–256. [PubMed] [Google Scholar]
- 14.Murkovic M., Mulleder U., Neunteufl H. Carotenoid content in different varieties of pumpkins. Journal of Food Composition and Analysis . 2002;15(6):633–638. doi: 10.1006/jfca.2002.1052. [DOI] [Google Scholar]
- 15.Muchirah P. N., Waihenya R., Muya S., Abubakar L., Ozwara H., Makokha A. Characterization and antioxidant activity of Cucurbita maxima Duchesne pulp and seed extracts. The Journal of Phytopharmacology . 2018;7(2):134–140. doi: 10.31254/phyto.2018.7206. [DOI] [Google Scholar]
- 16.Nkosi C. Z., Opoku A. R., Terblanche S. E. Antioxidative effects of pumpkin seed (Cucurbita pepo) protein isolate in CCl4-Induced liver injury in low-protein fed rats. Phytotherapy Research . 2006;20(11):935–940. doi: 10.1002/ptr.1977. [DOI] [PubMed] [Google Scholar]
- 17.Aguilar Y. M., Yero O. M., López J. C., Navarro M. V., Espinosa E. M. Phytosterols and squalene as hypocholesterolemic substances in five varieties of Cucurbita maxima and Cucurbita moschata (C. maxima) seeds. Revista Cubana de Plantas Medicinales . 2011;16:72–81. [Google Scholar]
- 18.Gossell-Williams G., Davis A., O‘Connor N. Inhibition of testosterone-induced hyperplasia of the prostate of Sprague-Dawley rats by pumpkin seed oil. Journal of Medicinal Food . 2006;9(2):284–286. doi: 10.1089/jmf.2006.9.284. [DOI] [PubMed] [Google Scholar]
- 19.Caili F., Huan S., Quanhong L. A review on pharmacological activities and utilization technologies of C. maxima. Plant Foods for Human Nutrition . 2006;61(2):70–77. doi: 10.1007/s11130-006-0016-6. [DOI] [PubMed] [Google Scholar]
- 20.Torkova A. A., Lisitskaya K. V., Filimonov I. S., et al. Physicochemical and functional properties of Cucurbita maxima pumpkin pectin and commercial citrus and apple pectins: a comparative evaluation. PLoS One . 2018;13(9):p. e0204261. doi: 10.1371/journal.pone.0204261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cheng L., Pan G. F., Xiao-bo S., Yun-xiang H., You-shun P., Lin-yan Z. Evaluation of anxiolytic-like effect of aqueous extract of Asparagus Stem in mice. Evidence-Based Complementary and Alternative Medicine . 2013;2013 doi: 10.1155/2013/587260.587260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Podolan M., Santos J. D., Walber T., Possamai F., Viola G. G., Linode O. C. A single injection of imipramine affected proliferation in the hippocampus of adult Swiss mice depending on the route of administration, doses, survival time and lodging conditions. Journal of Chemical Neuroanatomy . 2019;100:p. 101655. doi: 10.1016/j.jchemneu.2019.101655. [DOI] [PubMed] [Google Scholar]
- 23.Michel B., Martine H. The mouse light/dark box test. European Journal of Pharmacology . 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 24.Contreras C. M., Rodriguez-Landa J. F., Garcia-Rios R. I., Cueto-Escobedo J., Guillen-Ruiz G., Bernal-Morales B. Myristic acid produces anxiolytic-like effects in Wistar rats in the elevated plus-maze. BioMed Research International . 2014;2014 doi: 10.1155/2014/492141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kliethermes C. L., Crabbe J. C. Pharmacological and genetic influences on hole-board behaviors in mice. Pharmacology, Biochemistry, and Behavior . 2006;85(1):57–65. doi: 10.1016/j.pbb.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Aslam M., Najam R. A review of pharmacognostical, phytochemical and pharmacological properties of Lagenaria siceraria: a miracle herb. International Journal of Biomedical Advance Research . 2013;4:266–274. doi: 10.7439/ijbar.v4i5.329. Archives 4. [DOI] [Google Scholar]
- 27.Crupi R., Mazzon E., Marino A., et al. Melatonin treatment mimics the antidepressant action in chronic corticosterone-treated mice. Journal of Pineal Research . 2010;49(2):123–129. doi: 10.1111/j.1600-079X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 28.Russo E., Chimirri S., Aiello R., et al. Lamotrigine positively affects the development of psychiatric comorbidity in epileptic animals, while psychiatric comorbidity aggravates seizures. Epilepsy & Behavior . 2013;28(2):232–240. doi: 10.1016/j.yebeh.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 29.Rizwana B., Maheshwari K. K. Evaluation of the antidepressant activity of Acyranthes aspera by using open field test in rats. World Journal of Pharmaceutical Research . 2019;8:1183–1195. doi: 10.20959/wjpr20196-14912. [DOI] [Google Scholar]
- 30.Asgharzade S., Rabiei Z., Rafieian-Kopaei M. Effects of Matricaria chamomilla extract on motor coordination impairment induced by scopolamine in rats. Asian Pacific Journal of Tropical Biomedicine . 2015;5(10):829–833. doi: 10.1016/j.apjtb.2015.06.006. [DOI] [Google Scholar]
- 31.Cho J., Kang J. S., Long P. H., Jing J., Back Y., Chung K. S. Antioxidant and memory-enhancing effects of purple sweet potato anthocyanin and cordyceps mushroom extract. Archives of Pharmacal Research . 2003;26(10):821–825. doi: 10.1007/BF02980027. [DOI] [PubMed] [Google Scholar]
- 32.Lundquist A. J., Llewellyn G. N., Kishi S. H., et al. Knockdown of astrocytic monocarboxylate transporter 4 in the motor cortex leads to loss of dendritic spines and a deficit in motor learning. Molecular Neurobiology . 2022;59(2):1002–1017. doi: 10.1007/s12035-021-02651-z. [DOI] [PubMed] [Google Scholar]
- 33.Gallivan L. M., Schmitzer-Torbert N. A low-cost Morris water maze for undergraduate research: construction and demonstration in a rat model of obesity-induced diabetes. Journal of Undergraduate Neuroscience Education . 2018;16(2):A143–A151. [PMC free article] [PubMed] [Google Scholar]
- 34.Aiello M., Salvatore E., Cachia A., et al. Relationship between simultaneously acquired resting-state regional cerebral glucose metabolism and functional MRI: a PET/MR hybrid scanner study. NeuroImage . 2015;113:111–121. doi: 10.1016/j.neuroimage.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 35.Planchez B., Surget A., Belzung C. Animal models of major depression: drawbacks and challenges. Journal of Neural Transmission . 2019;126(11):1383–1408. doi: 10.1007/s00702-019-02084-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adachi N., Sakhri F. Z., Ikemoto H., et al. Kamikihito rescued depressive-like behaviors and hippocampus neurogenesis in chronic restraint stress rats. Journal of Traditional and Complementary Medicine . 2022;12(2):172–179. doi: 10.1016/j.jtcme.2021.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steru L., Chermat R., Thierry B., Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology . 1985;85(3):367–370. doi: 10.1007/BF00428203. [DOI] [PubMed] [Google Scholar]
- 38.Tahira P., Roomana R., Sadia H., Darakhsahan J. H. Increased serotonergic functions following administration of 1-(1-naphthyl) piperazine in propranolol injected rats. Pakistan Journal of Pharmaceutical Sciences . 2006;19:190–194. [PubMed] [Google Scholar]
- 39.Kistler L., Newsom L. A., Ryan T. M., Clarke A. C., Smith B. D., Perry G. H. Gourds and squashes (Cucurbita spp.) adapted to megafaunal extinction and ecological anachronism through domestication. Proceedings of the National Academy of Sciences of the United States of America . 2015;112(49):15107–15112. doi: 10.1073/pnas.1516109112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sinha S., Kumar B., Luqman S., Singh D. K. Neuroprotective potential of Cucurbita maxima Duchesne ex Poir, Caeselpenia bunduc (L.) Roxb and Bombax ceiba Linn extracts. South African Journal of Botany . 2019;120, article S0254629918310184 doi: 10.1016/j.sajb.2018.09.027. [DOI] [Google Scholar]
- 41.Mehta M., Adem A., Sabbagh M. New acetylcholinesterase inhibitors for Alzheimer’s disease. International Journal of Alzheimer’s Disease . 2012;2012 doi: 10.1155/2012/728983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Behl T., Makkar R., Sehgal A., et al. Current trends in neurodegeneration: cross talks between oxidative stress, cell death, and inflammation. International Journal of Molecular Sciences . 2021;22(14):p. 7432. doi: 10.3390/ijms22147432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arora I., Behl T., Groverc M., Sachdeva M., Pal G., Khan N. Study of anxiolytic and motor co-ordination activity of Cucurbita moschata and its possible mechanism through GABA receptors. Obesity Medicine . 2020;18:p. 100204. doi: 10.1016/j.obmed.2020.100204. [DOI] [Google Scholar]
- 44.Nicolas A., Ruby P. M. Dreams, sleep, and psychotropic drugs. Frontiers in Neurology . 2020;11 doi: 10.3389/fneur.2020.507495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Neelamma G., Duraiswamy B., Suresh N. S., Vadivelan C., Sandhya R., Justin C. Evaluation of protective neuro pharmacological activity of seeds of Cucurbita maxima against ethidium bromide induced demyelination in a rat model. Internation Journal of Pharmaceutical Sciences Review and Research . 2018;48:83–91. [Google Scholar]
- 46.Li Y., Yao J., Han C., et al. Quercetin, inflammation, and immunity. Nutrients . 2016;8(3):p. 167. doi: 10.3390/nu8030167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data supporting this research article are available from the corresponding author or first author on reasonable request.


