Abstract
Lymphoma is the most common hematological malignancy and is among the 10 most prevalent cancers worldwide. Although survival has been improved by modern immunochemotherapeutic regimens, there remains a significant need for novel targeted agents to treat both B-cell and T-cell malignancies. Cytidine triphosphate synthase 1 (CTPS1), which catalyzes the rate-limiting step in pyrimidine synthesis, plays an essential and nonredundant role in B-cell and T-cell proliferation but is complemented by the homologous CTPS2 isoform outside the hemopoietic system. This report describes the identification and characterization of CTPS1 as a novel target in B- and T-cell cancers. A series of small molecules have been developed which show potent and highly selective inhibition of CTPS1. Site-directed mutagenesis studies identified the adenosine triphosphate pocket of CTPS1 as the binding site for this small molecule series. In preclinical studies, a potent and highly selective small molecule inhibitor of CTPS1 blocked the in vitro proliferation of human neoplastic cells, showing the highest potency against lymphoid neoplasms. Importantly, pharmacological CTPS1 inhibition induced cell death by apoptosis in the majority of lymphoid cell lines tested, thus demonstrating a cytotoxic mechanism of action. Selective CTPS1 inhibition also inhibited the growth of neoplastic human B- and T- cells in vivo. These findings identify CTPS1 as a novel therapeutic target in lymphoid malignancy. A compound from this series is in phase 1/2 clinical studies for the treatment of relapsed/refractory B- and T-cell lymphoma (NCT05463263).
INTRODUCTION
Cytotoxic chemotherapy has long been the mainstay of treatment for lymphoid cancers. Chemotherapy-induced inhibition of DNA synthesis has proven to be an effective means to kill tumor cells, as evidenced by the utility of drugs such as cytarabine and gemcitabine. More recently, advances in medicinal chemistry and understanding of cancer biology have started to move the field away from chemotherapy toward targeted agents, as exemplified by chronic lymphocytic leukemia where chemotherapy-free first line therapy is becoming a reality for many patients.1
To date, the development of targeted agents in hemato-oncology has mainly focused on cell surface markers (targeted by monoclonal antibodies) and cell signaling and survival pathways (targeted by small molecules). Selective targeting of DNA synthesis has the potential to recapitulate the therapeutic success of chemotherapy drugs and even surpass current levels of efficacy by avoiding some of their limitations, such as the need for intracellular metabolism to generate active forms and the often burdensome and dose-limiting toxicities. To date, however, attempts at targeting nucleotide synthesis pathways have been largely unsuccessful, with inhibitors of enzymes such as dihydroorotate dehydrogenase and cytidine triphosphate synthase (CTPS) showing limited efficacy or unexpected toxicity, respectively.2,3 Ultimately, inhibiting fundamental cellular processes such as nucleotide synthesis may not be associated with a therapeutic window for drug development.
A key bottleneck in the pyrimidine synthesis pathway is the enzymatic activity of CTPS which catalyzes the final step in the production of CTP.4,5 As well as being the rate-limiting step in pyrimidine synthesis, this process is unusual in the pyrimidine synthesis pathway in having 2 enzymes that catalyze the same reaction: CTPS1 and CTPS2, which share 74% amino acid identity at the protein level.6,7 Although both enzymes are ubiquitously expressed, human genetic studies have identified CTPS1 as critical for lymphoid cell proliferation. Individuals who are homozygous for an inherited CTPS1 splice site mutation, which leads to an 80%–90% reduction in functional CTPS1 activity, have normal numbers of major immune cell subsets but a marked defect in T- and B-cell proliferation. Of note, affected individuals show no demonstrable phenotype outside the hemopoietic system, suggesting that CTPS2 is able to support the proliferation of cells in nonhemopoietic tissues.8,9 Together, these findings characterize CTPS1 as playing an essential and nonredundant role in B- and T-cell proliferation and highlight this enzyme as a potential therapeutic target in lymphoid malignancy.
Using inhibitors of CTPS1 with high degrees of selectivity for CTPS1 over CTPS2, we demonstrate the therapeutic potential of CTPS1 inhibition in lymphoid cancers. CTPS1 inhibition reduces the proliferation of cancer cells in vitro, showing the highest potency against T-cell neoplasms. CTPS1 inhibition induces apoptosis of human neoplastic lymphoid cells in vitro and inhibits the growth of neoplastic B- and T- cells in vivo in xenotransplanted mice. Together, these findings identify CTPS1 as a novel therapeutic target in lymphoid malignancy and provide a strong rationale for the further exploration of this target in the clinic.
MATERIALS AND METHODS
Inhibition of CTPS1/2 in biochemical and cellular assays
The CTP synthase enzymatic activity of recombinant CTPS1 and CTPS2 proteins from different species was quantitated in the presence or absence of test compound using a cytidine triphosphate RapidFire mass spectrometry assay. Full length active C-terminal FLAG-His8-tagged CTPS1 (1-591) and CTPS2 (1-586) for all species were obtained from Proteros biostructures GmbH. Proteins were expressed in HEK293F cells and purified by nickel column affinity and size exclusion chromatography. Proteins were stored in 20 mM Tris/HCl, pH 7.9, 150 mM NaCl, 10% glycerol, 0.5 mM ethylenediaminetetraacetic acid, 2 mM dithiothreitol (DTT) at −80°C. Assay reagent conditions were as follows: assay buffer 50 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid pH 8.0, 5 mM KCl, 20 mM MgCl2, 1 mM DTT, 0.01% Tween-20; dimethyl sulfoxide (DMSO) content up to 2%; CTPS1 enzyme 100 nM; substrates 300 μM adenosine triphosphate (ATP), 200 μM uridine triphosphate (UTP), 70 μM guanosine triphosphate, 100 μM L-Glutamine; stop solution 1% formic acid with 0.5 μM 13C10-15N3-CTP in H2O. Incubation time was 30 minutes at room temperature. Representative half maximal inhibitory concentration (IC50) curves are shown in Suppl. Figure S1.
Human embryonic kidney (HEK) cells lacking either CTPS1 or CTPS2 were generated using CRISPR technology, with lack of expression of the relevant protein confirmed by western blotting (Differential roles of CTPS1 and CTPS2 in cell proliferation, submitted for publication). Cells were cultured at 37°C with 5% CO2 in the presence or absence of test compound for either 3 days (HEK CTPS2 knockout) or 5 days (HEK CTPS1 knockout) to achieve equivalent cell growth (as the HEK CTPS1 knockout cells proliferate at a slower rate). Cell viability was measured using the CellTiter-Glo 2.0 reagent (Promega).
In silico modeling
Modeling was based on an initial assumption that the compounds bound at, or close to, the ATP/UTP binding site and was performed using ICM-Pro software (Molsoft LLC). Multispecies sequence alignments of CTPS1 and CTPS2, combined with a substrate-bound human CTPS1 filament cryoEM structure (5u03), indicated that the region within 8 Angstroms of the ATP/UTP binding site was highly conserved between species and CTPS isoforms, although a small number of residues showed limited variability and were investigated further. Hypotheses were made about the impact of each variable residue on species selectivity if they were implicated in ligand binding, and the observed loss of selectivity for rodent CTPS1 highlighted the residue I250 which is homologous to human CTPS1 in both rodent isoforms (Table 2). In the substrate-bound human CTPS1 structure, I250 was found to be in direct contact with the outer face of ATP, and, based on key pharmacophoric features of the compound series superposed onto adenine, it was postulated that the compounds bound with their terminal heterocycles at the ATP adenine site, H-bonding to V247. The precise pose was not known and was not used to drive potency.
Table 2.
Identification of Codon 250 as a Mediator of Isoform and Cross-species Selectivity
| Species ↓ residue → | 246 | 247 | 248 | 249 | 250 | 251 | 252 | 253 | 253 |
|---|---|---|---|---|---|---|---|---|---|
| CTPS1_Hs | D | V | S | S | I | Y | R | V | P |
| CTPS1_Cf | D | V | S | S | I | Y | R | V | P |
| CTPS1_Rn | D | V | S | S | I | Y | R | V | P |
| CTPS1_Mm | D | V | S | S | I | Y | R | V | P |
| CTPS2_Hs | D | V | S | S | T | Y | R | V | P |
| CTPS2_Cf | D | V | S | S | T | Y | R | V | P |
| CTPS2_Rn | D | V | S | S | I | Y | R | V | P |
| CTPS2_Mm | D | V | S | S | I | Y | R | V | P |
The table shows amino acid residues for positions around residue 250 which is predicted to project into the ATP binding pocket of the CTPS enzyme; alignment of CTPS1 and CTPS2 sequence across different species is shown.
Cf = Canis familiaris (dog); CTPS = cytidine triphosphate synthase; Hs = Homo sapiens (human); Mm = Mus musculus (mouse); Rn = Rattus norvegicus (rat).
CTP synthase complementation assay
The JURKAT cell line, derived from a male patient with T-cell acute lymphoblastic leukemia, expresses CTPS1 but not CTPS2 due to a hemizygous deletion of the CTPS2 gene on the X chromosome.9 JURKAT cells lacking CTPS1 were generated by knockout of CTPS1 by CRISPR as previously described, with lack of expression of both CTP synthase isoforms confirmed by western blotting.9 Knockout cells were cultured in medium containing supraphysiological concentrations of cytidine (200 μM) to enable cellular production of CTP via the salvage pathway and thus maintain cell viability. Wild-type and mutant CTPS1 and CTPS2 cDNAs from human and mouse were cloned into a pLVX-EF1α-IRES-mCherry backbone and used to generate lentiviral particles for cell transduction. Transduced cells were cultured in triplicate at 37°C with 5% CO2 in the presence or absence of test compound at 4-fold dilutions from 1600 to 1.5625 nM. Cell viability was measured after 3 days using the CellTiter-Blue cell viability reagent (Promega). The experiment was performed twice, and comparable results were obtained. A nonlinear regression model was used to calculate IC50 values (GraphPad Prism v9).
Human neoplastic lymphoid cell proliferation, cell death, and xenotransplantation assays
Human cancer cell lines were either provided by the NCI (Bethesda, MD) or Charles River Discovery Research Services (Freiburg, Germany), or acquired from ATCC (Rockville, MD), DSMZ (Braunschweig, Germany), JCRB (Japanese collection of research biosources, Japan), CLS (Cell Line Service, Heidelberg, Germany), ECACC (European collection of authenticated cell cultures), or KCLB (Korean cell line bank, Korea). The identity of the cell lines was confirmed by analysis of short tandem repeats.
For in vitro proliferation and cell death studies, human cancer cell lines were seeded in triplicate in 96-well plates and incubated at 37°C with 5% CO2. On the following day, test compound was added to the culture with final concentration of DMSO of 0.1%. Protein expression data were available for 48 of the 199 cell lines tested and were retrieved from the DepMap interface (https://depmap.org/portal/). For the cytidine rescue experiment (Figure 2E), cell culture medium was supplemented with cytidine (200 μM) to enable cellular production of CTP via the salvage pathway. After 72 hours of incubation with test compound, cell viability was assessed using the CellTiterGlo reagent (Promega) and cell death was assessed using the cell membrane permeability CellTox Green reagent (Promega). A nonlinear regression model was used to calculate IC50 values (GraphPad Prism v9).
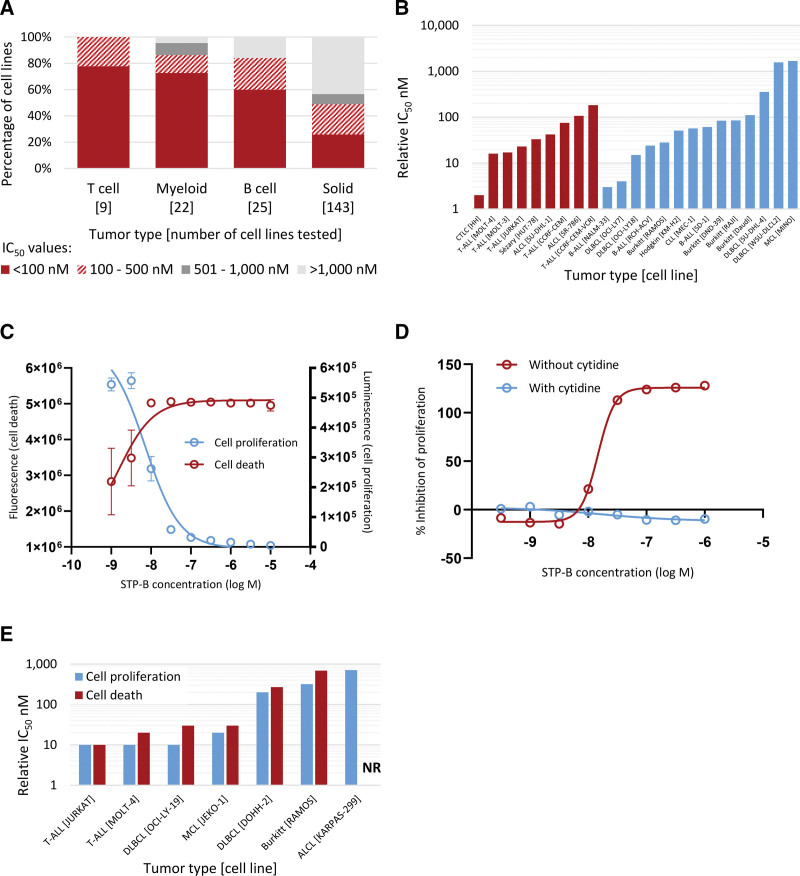
Figure 2.
Selective inhibition of CTPS1 inhibits the proliferation and induces dose-dependent death of human neoplastic cells in vitro. (A) Sensitivity of 199 human cancer cell lines to inhibition of proliferation by STP-B, a selective CTPS1 inhibitor. (B) Individual IC50 values for 9 cell lines derived from human T-cell malignancies (red) and 14 cell lines derived from human B-cell malignancies excluding myeloma (blue), showing nanomolar range sensitivity to STP-B for the majority of cell lines tested. (C) Dose-dependent inhibition of proliferation and induction of cell death by STP-B for JURKAT cells. (D) Inhibition of proliferation of human neoplastic T-cells (JURKAT) showing complete reversal of the antiproliferative effects of STP-B by the addition of cytidine, demonstrating that the block in proliferation is due to on-target inhibition of CTP synthase. (E) STP-B relative IC50 values for cell proliferation and cell death showing induction of cell death at nanomolar concentrations of STP-B in 6 of 7 cell lines tested. ALCL = anaplastic large cell lymphoma; B-ALL = B-cell acute lymphoblastic leukemia; CLL = chronic lymphocytic leukemia; CTCL = cutaneous T-cell lymphoma; CTPS = cytidine triphosphate synthase; DLBCL = diffuse large B-cell lymphoma; IC50 = half maximal inhibitory concentration; MCL = mantle cell lymphoma; NR = not reached; T-ALL = T-cell acute lymphoblastic leukemia.
Apoptosis induction was investigated in 5 lymphoma cell lines: DOHH-2 (ACC 47), JURKAT (ACC 282), MOLT-4 (ACC 362), JEKO-1 (ACC 553), and RAMOS (ACC 603). IC50 values for STP-B were calculated by exposing cells to serial dilutions of STP-B for 72 hours, followed by assessment of viable cells using the Cell Counting Kit-8 reagent (Dojindo Molecular Technologies) as per the manufacturer’s instructions. Cell lines were then exposed to STP-B at IC50 or ten times IC50 concentration for 24, 48, or 72 hours. Cell numbers were analyzed by manual counting using a Z2 coulter counter (Beckman Coulter). Apoptosis induction was measured by staining the cells with annexin V (BioLegend) and 7-aminoactinomycin D (7-AAD, Invitrogen) and analyzing the cells by flow cytometry. Apoptotic cells are defined as the sum of annexin V single and annexin V/7-AAD double positive cells. Cleavage of caspase 3 (BD Biosciences, #559341) was assessed using an appropriate isotype control (Invitrogen, #11-4614-80), with analysis by flow cytometry.
For the in vivo mantle cell lymphoma model, unirradiated female CB17-Prkdcscid severe combined immunodeficiency disease (SCID) mice were transplanted subcutaneously with 5 × 106 JEKO-1 cells. For the in vivo T-cell acute lymphoblastic leukemia model, sub-lethally irradiated (cobalt 60, 150 rad) female NOD.CB17-Prkdcscid/J nonobese diabetic, SCID mice were transplanted subcutaneously with 107 JURKAT cells embedded in Matrigel. Mice were randomized to treatment groups, n = 8 mice per group, when the tumors reached approximately 150 mm3 in volume. STP-B was administered subcutaneously either daily (JEKO-1) or on alternate days (JURKAT) at doses of 6 (JURKAT model only), 11, 18, 30, or 50 mg/kg for the duration of the study. Tumor volume was measured twice weekly using calipers. Animal studies were performed in an accredited facility (Crown Bioscience, Beijing) and overseen by an Ethical Review Committee comprising of the president, secretary, scientist, veterinarian, non-scientist, and lay members (members who have no relationship with the company and are not a breeder or user of laboratory animals), with responsibility for study conduct and approval, staff training, and animal facilities.
PK, and tumor growth inhibition modeling
A population pharmacokinetics (PK) model was developed with the aim of describing the PK of STP-B in healthy and tumor-bearing mice after subcutaneous drug administration, using nonlinear mixed effects modeling (NONMEM, version 7.4.4, ICON plc) with the first-order conditional estimation method with interaction. A 2-compartment disposition model with first-order absorption into and first-order elimination from the central compartment provided a consistent description of the observed data. The PK model allowed prediction of blood concentrations for each individual mouse in cohorts used for xenograft models, using the exact amount of drug administered based on daily body weight of each mouse along with the actual treatment schedule evaluated in each cohort. A Simeoni tumor growth inhibition model10 was developed to describe tumor growth characteristics; the PK model then allowed prediction of blood concentrations at the time of tumor volume measurements for different doses and dose regimens.
RESULTS
Identification of highly selective small molecule inhibitors of human CTPS1
Current understanding of the pyrimidine synthesis pathway indicates that selective targeting of CTPS1 may have utility in the treatment of lymphoid malignancies. Compound library screening was therefore undertaken to identify inhibitors of CTP synthase that show selectivity for the human CTPS1 isoform. Details of the initial medical chemistry approaches have been published elsewhere.11 In brief, a high throughput screening assay based on the production of ADP from ATP in the presence of purified human CTPS1 protein and the substrate UTP was used to screen a proprietary library of 240,000 compounds. Initial hits were further profiled with full dose response curves using recombinant enzyme and the JURKAT cell line, and differential inhibition of CTPS1 versus CTPS2 using recombinant enzyme assays. Compound optimization was then undertaken by iterative and empirical structure–activity relationship–based medicinal chemistry. This allowed the generation of small molecule compounds able to inhibit the CTPS1 enzyme at low nanomolar concentrations, with selectivity for human CTPS1 over CTPS2, as exemplified by 2 compounds shown in Figure 1A and described in Table 1. Selectively for CTPS1 inhibition was confirmed using HEK cells lacking either CTPS1 or CTPS2, and consequently dependent on CTPS2 or CTPS1, respectively, for proliferation. The enhanced sensitivity of CTPS1-dependent HEK cells to STP-B confirms the ability of this series of compounds to selectively inhibit CTPS1 in a cellular system (Figure 1B).
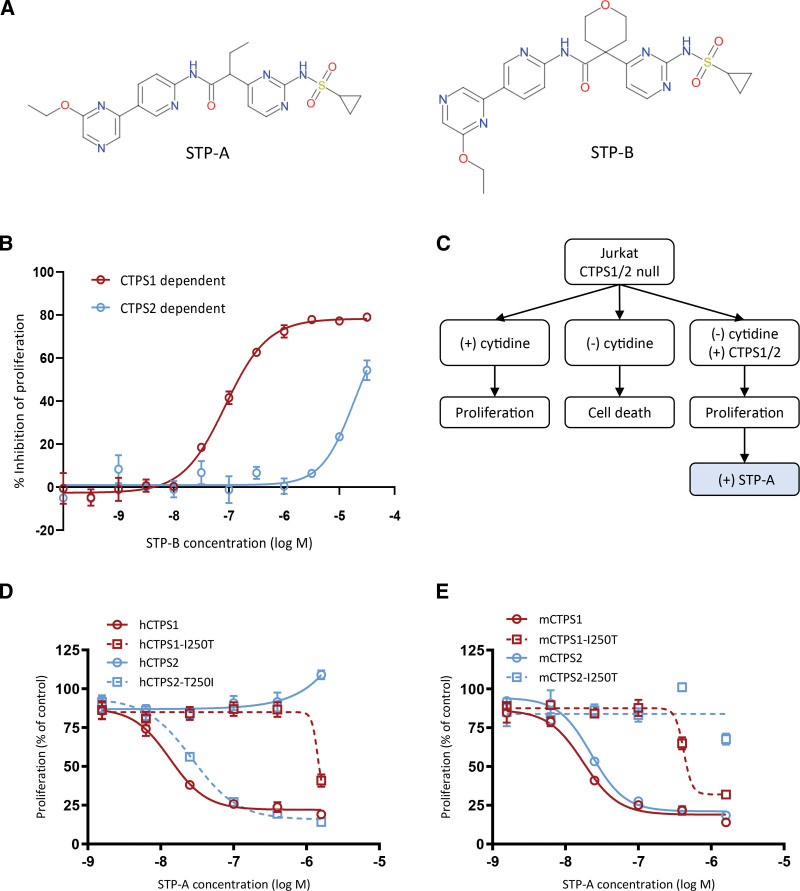
Figure 1.
Characterization of small molecule selective inhibitors of CTPS1. (A) Chemical structures of 2 selective CTPS1 inhibitors, STP-A and STP-B, derived from the same chemical series. (B) Proliferation of HEK cells deficient in CTPS2 or CTPS1 protein (and, therefore, dependent on CTPS1 or CTPS2, respectively, for proliferation) demonstrating the selectivity of STP-B for inhibition of CTPS1 over CTPS2. (C) Rationale and schema for CTPS1/2 complementation experiments. (D) Proliferation of cells complemented with native or mutant human CTPS1 or CTPS2 protein in the presence of increasing concentrations of STP-A, demonstrating the impact of residue 250 on the selectivity of the compound for CTPS1 over CTPS2. (E) Proliferation of cells complemented with native or mutant mouse CTPS1 or CTPS2 protein in the presence of increasing concentrations of STP-A, providing further support for the impact of residue 250 on the selectivity profile of these compounds. Graphs show means of triplicate values ± standard deviation. Data are representative of 2 independent experiments. CTPS = cytidine triphosphate synthase; HEK = human embryonic kidney.
Table 1.
A Novel Series of Small Molecules Show Selectivity for Inhibition of CTPS1 Over CTPS2 That Is Species Dependent
| STP-A | STP-B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species | CTPS1 IC50 (µM) | SD | N | CTPS2 IC50 (µM) | SD | N | Fold selectivity (CTPS1/CTPS2) | CTPS1 IC50 (µM) | SD | N | CTPS2 IC50 (µM) | SD | N | Fold selectivity (CTPS1/CTPS2) |
| Human | 0.0148 | 0.004 | 10 | 1.87 | 0.376 | 10 | 126 | 0.0093 | 0.0038 | 12 | 12.62 | 5.24 | 12 | 1,357 |
| Dog | 0.0293 | 0.002 | 4 | 4.14 | 1.481 | 4 | 141 | 0.0259 | 0.0058 | 8 | 21.75 | 37.75 | 7 | 840 |
| Mouse | 0.0267 | 0.008 | 5 | 0.126 | 0.020 | 4 | 5 | 0.0095 | 0.0030 | 13 | 1.33 | 0.64 | 12 | 140 |
| Rat | 0.0233 | 0.002 | 4 | 0.023 | 0.002 | 4 | 1 | 0.0095 | 0.0029 | 12 | 0.47 | 0.33 | 12 | 49 |
The table shows data for 2 exemplar compounds, shown in Figure 1A, consisting of geometric mean relative IC50 values for the inhibition of enzymatic activity in a recombinant protein assay.
CTP synthase 1 is a novel therapeutic target in lymphoma; IC50 = half maximal inhibitory concentration; SD = standard deviation.
Analysis of compound activity against recombinant CTPS1 and CTPS2 enzyme identified cross-species differences, with selectivity of STP-B for CTPS1 over CTPS2 being reduced in dogs, and the selectively of both compounds being much reduced in rodents, when compared with selectivity for the human enzyme (Table 1). Multispecies alignment was combined with in silico modeling to predict the mechanism of target binding for the compound series, based on the hypothesis that the CTPS1 ATP substrate pocket was the likely binding site for these compounds. The isoleucine 250 residue, which projects into the ATP pocket, was thus highlighted as a potential key determinant of selectivity. In humans, I250 is not conserved between CTPS1 and CTPS2 (T250 in the latter); codon 250 is also not conserved between human and rodent CTPS2 (T250 in the former, I250 in the latter) (Table 2). Together, these findings offer a potential explanation for both the CTPS1 selectivity in humans and the reduced selectivity in rodent species.
Mutagenesis studies identify the CTPS1 I250 residue as a key determinant of both CTPS isoform and cross-specifies selectivity
To test the hypothesis that the CTPS1 I250 residue dictates the isoform selectivity of these compounds, a cellular complementation assay was established using engineered human JURKAT cells lacking both CTPS1 and CTPS2. These CTPS null cells can be maintained in growth medium supplemented with supraphysiological concentrations of cytidine but undergo rapid apoptosis when exogenous cytidine is withdrawn. Cell proliferation can be rescued by expression of a functional CTPS protein.
The rationale for the experiments is summarized in Figure 1C. CTPS null cells were complemented with either human CTPS1 (hCTPS1) or human CTPS2 (hCTPS2) and IC50 values calculated using the selective CTPS1 inhibitor STP-A (Table 1). As expected, hCTPS1 cells were highly sensitive to inhibition by STP-A (IC50 13 nM), whereas the IC50 for hCTPS2 cells was at least 100-fold higher (IC50 not reached) consistent with the known selectivity profile of the compound series (Figure 1D).
To test the hypothesis that residue 250 is critical for the selectivity of STP-A for CTPS1 over CTPS2, site-directed mutagenesis was used to generate human CTPS1 harboring an I250T substitution (hCTPS1-I250T), and a reciprocal human CTPS2 harboring a T250I substitution (hCTPS2-T250I). Complementation of CTPS null cells with hCTPS1-I250T reduced the sensitivity of the cells to STP-A by >100-fold (IC50 1426 nM). In the reciprocal experiment, complementation of CTPS null cells with hCTPS2-T250I increased the sensitivity of the cells to STP-A such that the IC50 value (IC50 27 nM) was similar to hCTPS1 cells (Figure 1D). These experiments confirm a key role for the isoleucine at residue 250 in determining the selectivity of this compound series for CTPS1.
To further probe the binding and selectivity of the CTPS1 inhibitor series, experiments were designed to leverage the observed differences in selectivity between human and mouse CTPS enzymes. Cells complemented with either mouse CTPS1 (mCTPS1) or mouse CTPS2 (mCTPS2) were highly sensitive to inhibition by STP-A (IC50 18 nM and 23 nM, respectively; Figure 1E). These findings are in keeping with the reduced selectivity for mouse CTPS1 over CTPS2 observed in the recombinant enzyme studies (Table 1) and can potentially be explained by residue 250 being isoleucine in both mouse CTPS1 and CTPS2. Substitution of the isoleucine to threonine in either mouse CTPS1 or CTPS2 (mCTPS1-I250T and mCTPS2-I250T, respectively) reduced the sensitivity of the cells to STP-A (IC50 421 nM and not reached, respectively) (Figure 1E). These findings provide further evidence to support a pivotal role for residue 250 in determining both cross-species and CTPS1 versus CTPS2 selectivity.
Taken as a whole, these cross-species complementation experiments identify the presence of isoleucine at CTPS1 residue 250 as playing a key role in determining the high degree of selectivity of this compound series for human CTPS1 over CTPS2.
Selective CTPS1 inhibition induces death of human neoplastic cells with hematological cancers showing greatest sensitivity
The impairment of B- and T-cell proliferation observed in individuals with CTPS1 immunodeficiency8,9 suggests a potential role for targeting CTPS1 in lymphoid malignancy. The ability of STP-B to inhibit the proliferation of human neoplastic cells was assessed using a panel of 199 cancer cell lines covering both hematological malignancies and solid tumors (Suppl. Table S1). Cell lines derived from hematological malignancies were more sensitive to selective inhibition of CTPS1 than cell lines derived from solid tumors, with T-cell malignancies being the most sensitive (Figure 2A). Of note, sensitivity to CTPS1 inhibition was not associated with either CTPS1 or CTPS2 protein levels in the 48 cell lines where protein data were available (P = 0.66 and P = 0.35, respectively; linear regression analysis).
STP-B inhibited the proliferation, as measured by metabolic activity, of all 9 neoplastic T-cell lines tested at nanomolar concentrations, with IC50 values ranging from 2 to 183 nM. STP-B inhibited the proliferation of 12 of 14 neoplastic B- cell lines tested, with IC50 values for sensitive lines ranging from 3 to 356 nM (Figure 2B). Exposure of JURKAT neoplastic T-cells to increasing concentrations of STP-B resulted in a dose-dependent inhibition of proliferation and induction of cell death (Figure 2C). This inhibition of proliferation was fully reversible on addition of supraphysiological concentrations of cytidine to the growth medium (Figure 2D). High concentrations of cytidine are able to bypass CTPS1 and produce CTP via the salvage pathway; therefore, these findings confirm that growth inhibition is due to on-target inhibition of CTP synthase activity by STP-B. Importantly, STP-B induced cell death in 6 of 7 neoplastic B- or T-cell lines tested at IC50 values that were similar to those observed for the antiproliferative effects (Figure 2E).
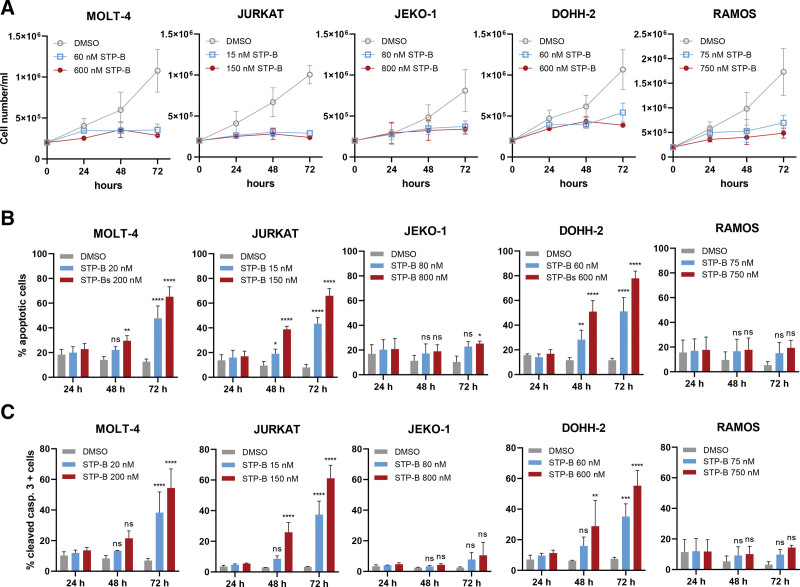
To understand the mechanisms of cell death, further analysis was undertaken in 5 lymphoma cell lines. STP-B inhibited cell proliferation of all of these lines, as measured by cell counts, at low nanomolar concentrations (Figure 3A). This was associated with a time-dependent induction of apoptosis in 3 of the lines tested, as demonstrated by both annexin V and cleaved caspase (Figure 3B and C, respectively).
Figure 3.
Selective inhibition of CTPS1 inhibits lymphoma cell line growth and induces apoptosis in a dose- and time-dependent manner. (A) Growth curves of lymphoma cell lines exposed to STP-B, as assessed by cell counts. (B) Flow cytometric analysis of apoptotic cell fraction (sum of annexin V positive and annexin V/7-AAD double positive cells). (C) Flow cytometric analysis of the proportion of cells positive for cleaved caspase 3. In all experiments shown in this figure, cells were exposed to STP-B at IC50 (blue) or 10 times the IC50 (red) using the relevant concentration of DMSO as a control (gray). Graphs show means of 3 independent experiments ± standard deviation. Statistical comparison vs control was performed using 2-way ANOVA with Bonferroni correction; *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001; ****P ≤ 0.0001. ANOVA = analysis of variance; CTPS = cytidine triphosphate synthase; DMSO = dimethyl sulfoxide; IC50 = half maximal inhibitory concentration; ns = not significant.
Together, these findings demonstrate broad antitumor activity of STP-B at nanomolar concentrations against human lymphoid cancers and establish a cytotoxic (rather than cytostatic) mechanism of action.
STP-B, a potent and selective CTPS1 inhibitor, inhibits the in vivo growth of neoplastic human lymphoid cells in preclinical disease models
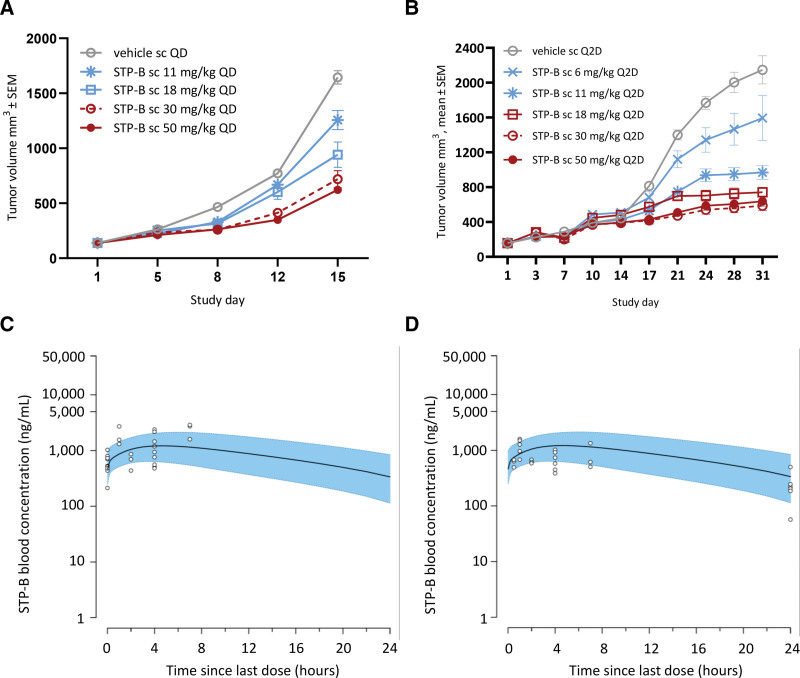
The ability of STP-B to inhibit the growth of malignant lymphoid cells in vivo was assessed in 2 xenotransplantation models using either JEKO-1 mantle cell lymphoma cells or JURKAT T-cell acute lymphoblastic leukemia cells. For both models, the respective cancer cell line was transplanted subcutaneously into immunodeficient mice. STP-B was administered subcutaneously either daily or on alternate days across a range of doses. Alternate day dosing was well tolerated, whereas daily dosing resulted in dose-dependent body weight loss which limited the duration of continual daily dosing to 2 weeks (Suppl. Figures S2A and S2B, respectively). Administration of STP-B to tumor-bearing mice inhibited tumor growth in models of B- and T-cell neoplasia (Figure 4A and B, respectively), both of which showed a clear dose–response relationship.
Figure 4.
Selective inhibition of CTPS1 inhibits the growth of human neoplastic lymphoid cells in vivo. (A) Tumor growth curves for neoplastic human B- cells (JEKO-1 mantle cell lymphoma) transplanted subcutaneously in SCID mice (n = 8 per group) showing dose-dependent inhibition of tumor growth by STP-B. (B) Tumor growth curves for neoplastic human T-cells (JURKAT T-cell acute lymphoblastic leukemia) transplanted subcutaneously in NOD-SCID mice (n = 8 per group) showing a dose-dependent inhibition of tumor growth by STP-B. (C/D) STP-B blood concentration vs time profiles at steady state predicted from a PK model (daily subcutaneous administration of STP-B at 30 mg/kg) overlaid with observed blood concentrations measured in satellite JEKO-1 (C) or JURKAT (D) tumor-bearing mice. Black line: median blood concentrations predicted by the PK model; blue shaded area: 90% prediction intervals; open circles: observed blood concentrations from sparse PK samples collected from tumor-bearing mice in a satellite cohort to the main efficacy study. CTPS = cytidine triphosphate synthase; PK = pharmacokinetics; NOD-SCID = nonobese diabetic, severe combined immunodeficiency disease; SEM = standard error of the mean.
A population PK model allowed the prediction of blood concentrations in experimental tumor-bearing mice (Figure 4C and D). The population PK model, combined with a model of tumor growth inhibition, identified target drug concentrations for tumor inhibition of 2.7 and 1.1 μg/mL for the JEKO-1 and JURKAT models, respectively. After correction for plasma protein binding and blood to plasma ratio, this equates to a free drug plasma concentration of 382 and 156 nM, respectively, for the 2 models. For comparison, the in vitro free drug IC90 concentrations for the JEKO-1 and JURKAT cell lines were 72 and 83 nM, respectively. Together, these results demonstrate the ability of STP-B to inhibit the in vivo growth of neoplastic B- and T-cells at drug concentrations that are likely to be achievable in human patients.
DISCUSSION
The last decade has witnessed a shift in the treatment paradigm for lymphoid cancers, as cytotoxic chemotherapy is replaced by targeted small molecules and monoclonal antibodies. In B-cell lymphoma, agents that target molecular pathways have delivered improvements in both efficacy and tolerability, as exemplified by approvals for drugs targeting molecules such as CD20, BCL2, and BTK. By contrast, the treatment of T-cell lymphoma is still dominated by chemotherapy, although early reports of efficacy from targeted agents have raised hopes that a similar targeted therapy paradigm might be achievable. Overall, there remains a need for the discovery and validation of new targets in both B- and T-cell cancers, focused on novel and divergent pathways such that new agents can be combined with existing drugs to maximize the benefit to patients.
In this report, we describe the identification and characterization of a novel class of compounds that deliver selective inhibition of CTPS1, the rate-limiting enzyme in the pyrimidine synthesis pathway. CTPS1 was identified as a potential therapeutic target from genetic studies of families with immunodeficiency characterized by impairment of T- and B-cell proliferation.8,9 Importantly, families with germline mutations of CTPS1 have no identifiable phenotype outside of the hemopoietic system, raising the expectation that selective targeting of CTPS1 may deliver antilymphoma activity in the absence of significant mechanism-based toxicity. In the preclinical studies described herein, selective inhibitors of CTPS1 induced cell death of neoplastic lymphoid cells in vitro and demonstrated antitumor activity in vivo.
Although families with inherited CTPS1 deficiency have no nonhematological phenotype, body weight loss was observed in mice administered STP-B, a selective CTPS1 inhibitor. One potential explanation is the reduced selectivity of the compound series for rodent CTPS1. Indeed, isoleucine 250 was identified as a key residue involved in compound selectivity in humans, whereas isoleucine 250 is replaced by threonine in mouse CTPS1. This reduced selectivity may result in inhibition of mouse CTPS2 in vivo with consequent sequalae due to impaired proliferation of nonhematopoietic cells including those of the gastrointestinal tract. Another possibility is the use of the SCID strain of mice for the efficacy studies. It was important to perform the efficacy studies using human cancer cells, due to the reduced selectivity of these compounds for mouse CTPS1, thus necessitating the use of immunodeficient mice. SCID mice lack DNA-PKcs, a kinase enzyme that plays a critical role in the repair of double-stranded DNA breaks. This generalized DNA repair defect, when combined with CTPS1 inhibition, might impair the growth of constantly self-renewing intestinal epithelial cells, and thus render SCID mice susceptible to gastrointestinal side effects resulting in weight loss. Body weight loss limited the duration and dosing regimen in the in vivo xenograft studies; nonetheless, STP-B was able to show a clear dose response associated with significant suppression of tumor growth in both model systems.
The initial clinical development of this compound series will focus on B- and T-cell lymphoma. Preclinical studies presented herein also suggest potential utility for CTPS1 inhibition in myeloid malignancies and solid tumors. In keeping with the latter, several recent preclinical papers have described a role for CTPS1 in cancer, and highlighted selective CTPS1 inhibition as a potential antitumor target.12–14 Key to unlocking CTPS1 as a cancer target was the development of a chemical series with selectivity for CTPS1 over CTPS2. A combination of site-directed mutagenesis and interspecies comparisons identified the isoleucine 250 residue of CTPS1 as critical to the selectivity of the compound series described herein. These findings also localize the binding of compounds from this chemical series to the ATP substrate pocket of CTPS1. This is consistent with recent work in which the binding to CTPS1 of compounds from this series that are closely related to STP-A and STP-B was investigated by cryogenic electron microscopy.15 To our knowledge, the compound series exemplified in this report is the first described to demonstrate significant selectivity for CTPS1 over CTPS2.
Taken as a whole, the findings presented herein identify CTPS1 as a potential novel therapeutic target in lymphoid malignancies and characterize a small molecule series that is able to potently and selectively inhibit this target. It is hoped that the precise targeting of CTPS1 will offer a novel therapeutic option for patients with lymphoma. The high degree of selectivity of these compounds also makes them ideal candidates for combining with other targeted therapies, to contribute towards the goal of moving lymphoma therapy away from cytotoxic chemotherapy and towards precision treatments. A compound from this series has recently entered clinical development for patients with relapsed or refractory B- or T-cell lymphoma (NCT05463263).
AUTHOR CONTRIBUTIONS
HA, NM, CP, EH, and AEP designed experiments and analyzed data; RL, DL, LB, GJ, and AN designed and optimized the compound series and performed experiments; HL and AF supervised research and analyzed data; SL supervised research, analyzed data, and wrote the paper; PAB analyzed data and wrote the paper. All authors reviewed, edited, and approved the final manuscript.
DISCLOSURES
HA, EH, AEP, and PAB are employees of Step Pharma. All the other authors have no conflicts of interest to disclose.
SOURCES OF FUNDING
This work was partially supported by grants from Agence Nationale de la Recherche ANR-18-CE15-0025-01 (France) (to SL) and ANR-10-IAHU-01 (to Imagine Institute) and Proof of Concept ERC-2015-PoC_Master/ERC-2015-PoC_680465_SAFEIMMUNOSUPPRESS (to SL). The research in this article was partially funded by Step Pharma. NM is a fellowship recipient of the Association Nationale de la Recherche Technologique (ANRT, France) under agreement with the industrial partner Step Pharma. SL is a senior scientist of the Centre de la Recherche Nationale (CNRS, France).
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
REFERENCES
- 1.Rhodes JM, Barrientos JC. Chemotherapy-free frontline therapy for CLL: is it worth it? Hematol Am Soc Hematol Educ Program. 2020;2020:24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Tao L, Zhou X, et al. DHODH and cancer: promising prospects to be explored. Cancer Metab. 2021;9:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schimmel KJM, Gelderblom H, Guchelaar HJ. Cyclopentenyl cytosine (CPEC): an overview of its in vitro and in vivo activity. Curr Cancer Drug Targets. 2007;7:504–509. [DOI] [PubMed] [Google Scholar]
- 4.van Kuilenburg AB, Meinsma R, Vreken P, et al. Isoforms of human CTP synthetase. Adv Exp Med Biol. 2000;486:257–261. [DOI] [PubMed] [Google Scholar]
- 5.van Kuilenburg AB, Meinsma R, Vreken P, et al. Identification of a cDNA encoding an isoform of human CTP synthetase. Biochim Biophys Acta. 2000;1492:548–552. [DOI] [PubMed] [Google Scholar]
- 6.Kursula P, Flodin S, Ehn M, et al. Structure of the synthetase domain of human CTP synthetase, a target for anticancer therapy. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2006;62(Pt 7):613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lynch EM, Kollman JM. Coupled structural transitions enable highly cooperative regulation of human CTPS2 filaments. Nat Struct Mol Biol. 2020;27:42–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin E, Palmic N, Sanquer S, et al. CTP synthase 1 deficiency in humans reveals its central role in lymphocyte proliferation. Nature. 2014;510:288–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin E, Minet N, Boschat AC, et al. Impaired lymphocyte function and differentiation in CTPS1-deficient patients result from a hypomorphic homozygous mutation. JCI Insight. 2020;5:e133880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Simeoni M, Magni P, Cammia C, et al. Predictive pharmacokinetic-pharmacodynamic modeling of tumor growth kinetics in xenograft models after administration of anticancer agents. Cancer Res. 2004;64:1094–1101. [DOI] [PubMed] [Google Scholar]
- 11.Novak A, Laughton D, Lane R, et al. Discovery and optimization of potent and orally available CTP synthetase inhibitors for use in treatment of diseases driven by aberrant immune cell proliferation [Published online November 30, 2022]. J Med Chem. 2022;65:16640–16650. [DOI] [PubMed] [Google Scholar]
- 12.Sun Z, Zhang Z, Wang QQ, et al. Combined inactivation of CTPS1 and ATR is synthetically lethal to MYC-overexpressing cancer cells. Cancer Res. 2022;82:1013–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y, Zhang J, Li Y, et al. CTPS1 promotes malignant progression of triple-negative breast cancer with transcriptional activation by YBX1. J Transl Med. 2022;20:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu F, Mao Y, Ma T, et al. CTPS1 suppresses proliferation and migration in colorectal cancer cells [Published online August 1, 2022]. Cell Cycle Georget Tex. 2022;21:2563–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lynch EM, DiMattia MA, Albanese S, et al. Structural basis for isoform-specific inhibition of human CTPS1. Proc Natl Acad Sci U S A. 2021;118:e2107968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.