Abstract
Erdheim-Chester disease is an uncommon non-Langerhans cell histiocytosis affecting multiple systems. There is limited knowledge on the imaging capabilities of this disease. We present an extremely rare case of Erdheim-Chester illness in a 67-year-old man with multisystem involvement, including the cardiovascular system, skeleton, retroperitoneum (renal and adrenal infiltration) and the neurologic system. The involvement of the various organs was thoroughly assessed using multimodal imaging modalities such as computed tomography, magnetic resonance imaging, positron emission tomography and bone scintigraphy. Erdheim-Chester illness was revealed by a bone biopsy. Especially when there is cardiac and cerebral involvement, Erdheim-Chester illness is a rare condition with a poor prognosis. Knowing the imaging characteristics of Erdheim-Chester disease may be helpful in understanding the radiological results of many organs affected by the disease as described and discussed in the current case report.
Keywords: Erdheim-Chester disease, Periadventitial tissue, Retroperitoneal fibrosis, Sclerotic bone lesions, Cerebellar atrophy
Introduction
Erdheim-Chester disease (ECD) is an uncommon non-Langerhans cell histiocytic proliferative disorder with multisystem involvement. Although children may occasionally also be affected, its occurrence peaks in the fifth to seventh decades of life with a slightly higher male incidence [1], [2], [3], [4]
Due to the condition's rarity, its precise etiopathogenesis is still under study, even if some recurrent activating mitogen-activated protein kinase (MAPK) pathway mutations have been identified, for example BRAF mutation [5]. Tissue damage on ECD is thought to be an immune-mediated condition. An excessive proliferation of mutated bone marrow stem cells Hematopoietic Stem Cells or myeloid progenitors occurs. Circulating monocytes, which have inherited the mutations, release pro-inflammatory cytokines like interferon alfa (INFalfa) and differentiates into foamy histiocytes and often Touton giant cells, that are attracted to the site of involvement, inducing cronic inflammatory response and fibrosis [6].
ECD symptoms vary depending on the site of involvement and are nonspecific [[1], [3], [6], [7]]. In the developing countries, common constitutional symptoms including fever, weight loss and night sweats might be mistaken for tuberculosis. Overall, skeletal involvement is the most frequent, and in many cases, bone pain is the initial presenting symptom [7], [8], [9], [10]. The prognosis in ECD depends on the sites and the degree of extraosseous involvement [11]. Although involvement of any organ system results in its dysfunction, the prognosis is unquestionably impacted by the involvement of specific sites like the central nervous system (CNS) and cardiovascular system (CVS) due to the poor response to chemotherapy [[6], [9]].
Although therapeutic breakthroughs have undoubtedly reduced morbidity, the impact on mortality has not improved that much, with reported 1- and 5-year overall survival rates of 96% and 68%, respectively [[1], [6], [9]].
Case report
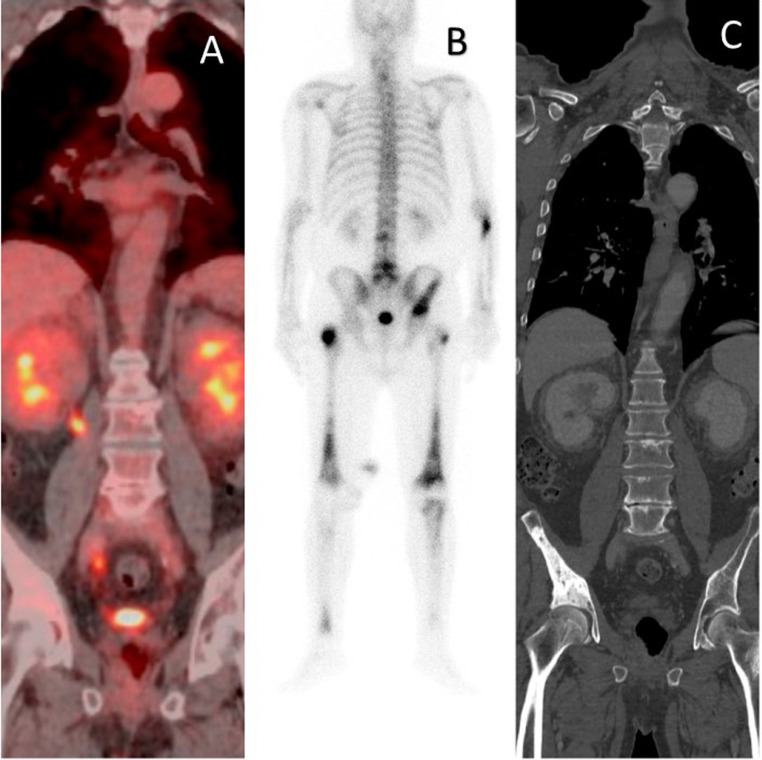
A 67-year-old man came to the Imaging Diagnostics Department of our Institute complaining pain in his pelvis and both thighs. The patient, a former smoker and hypertensive, reported a history of severe chronic multivessel vasculopathy and ischemic heart disease, treated by multiple coronary stents, right ileo-femoral bypass and femoral-femoral bypass. An X-ray of the pelvis was performed and showed multiple areas of osteosclerosis at the right iliac wing, the left acetabulum and both femoral heads. Positron emission tomography/computed tomography (PET-CT) with (18)F-fluorodeoxyglucose (18F-FDG) scan revealed typical bilateral and symmetric uptake of 18F-FDG at the pelvis and femoral heads (Fig. 1A), similar to that observed with radiography and technetium 99m (99mTc) bone scintigraphy (Fig. 1B). In the suspicion of metastatic disease, the patient was subjected a PET-CT scan with mdc, which confirmed the presence of sclerotic lesions (Fig. 1C); the lesions were found to have a modest increase in metabolic activity. Further alterations with the same densitometric and metabolic characteristics have been identified at the left scapula and sphenoid.
Fig. 1.
18FDG PET-CT scan; 99mTc bone scintigraphy; CT abdomen: (A) bilateral uptake at the pelvis and femoral heads and (B) 99mTc bone scintigraphy, (C) corresponding to extensive sclerotic lesions at CT (C, white arrow).
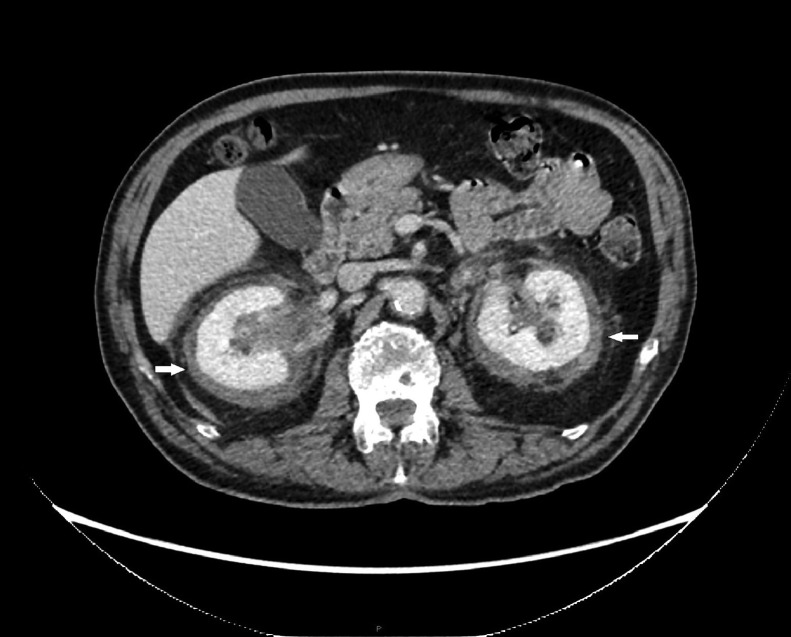
The abdomen CT scan revealed a soft tissue density lesion involving the retroperitoneal organs (bilateral adrenal glands and kidneys) as well as a fat gap and had an uneven contour and modest enhancement. It appeared to have "hairy kidneys" (Fig. 2) because of the invasion of perirenal fat and fascia.
Fig. 2.
Abdomen CT: proliferative tissue, seen as hypodense in CT, invade bilateral perirenal fat and fascia (white arrow).
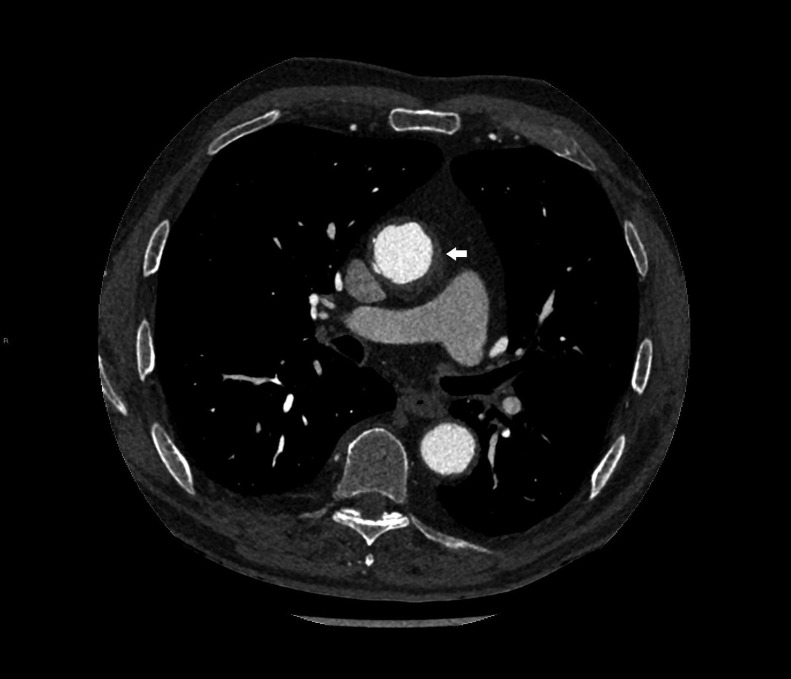
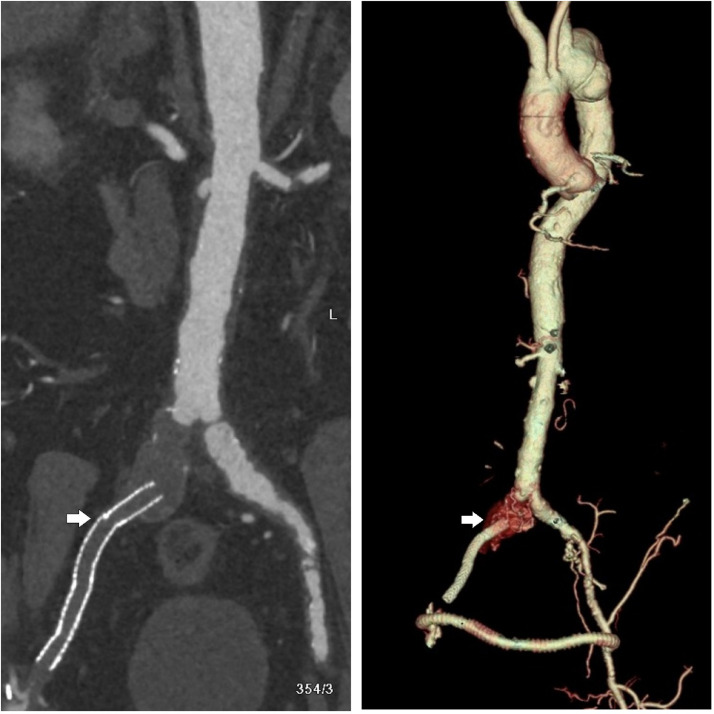
The angio-CT study documented the presence of periadventitial tissue at the level of the aortic arch, the descending thoracic aorta (Fig. 3), the abdominal aorta and the main splenic arterial vectors. Similar to what was seen in the perirenal site, the infiltration was hypoattenuated on CT and exhibits slight hypermetabolic activity on 18F-FDG PET-CT. Also, a thrombosed aneurysm of the right common iliac artery, an occlusion of the right iliac-femoral bypass (Fig. 4A) and a patency of the femoral-femoral bypass were reported (Fig. 4B).
Fig. 3.
Abdomen-thorax CT: aortic wall thickening (white arrow).
Fig. 4.
Angio-CT: right iliac-femoral by-pass occlusion (A) and 3D-volume rendering (VR) reconstruction of femoral-femoral by-pass (white arrow).
A bone biopsy was performed and it documented the presence of CD68+, CD163+, and CD1- foamy histiocytes on immunostaining, pathognomonic for the diagnosis of ECD. The genomic evaluation revealed that BRAFV600E mutation was positive and the patient started the treatment with Vemurafenib, an oral tumor-inactivating agent, enable to inhibit several protein kinases, in particular the serine-threonine kinase BRAF. After 4 weeks of active treatment, the symptoms of the patient were relieved, and he asked to be discharged from the hospital.
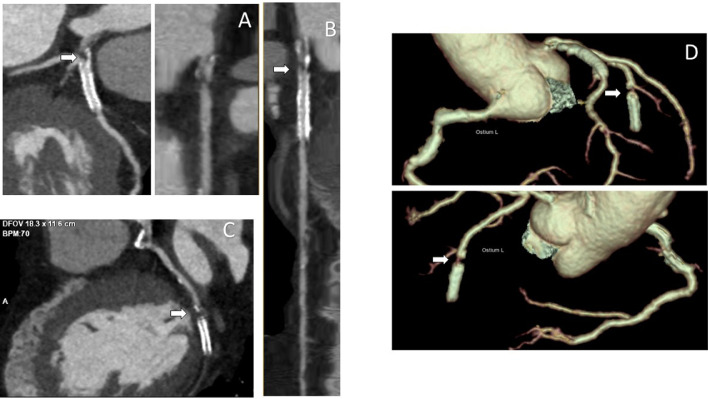
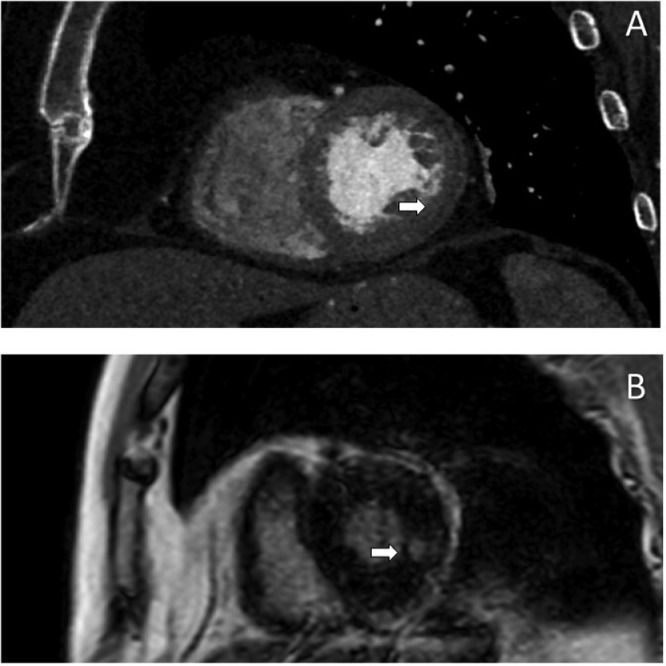
Three years later the patient was referred to a cardiology clinic due to the onset of new symptoms and the exacerbation of previous symptoms (mild evening fatigability in all 4 limbs, ataxia, dyskinesias, dyspnea and syncope). Following-up imaging revealed disease progression. In particular, the cardiac-CT angiography showed preocclusive stenosis of the left main coronary artery (Fig. 5A), severe intrastent stenosis of the proximal III of the anterior descending (Fig. 5B) and occlusion of the prestent tract of the obtuse marginal branch (Figs. 5C- and D). The morphologic study of the cardiac chambers showed an area of transmural hypodensity of the mid-basal infero-lateral wall (Fig. 6A) with corresponding late gadolinium enhancement on subsequent cardiac magnetic resonance imaging , compatible with ischemic necrosis (Fig. 6B). The angio-CT study of the aorta documented the appearance of a 39 × 34 mm sacciform aneurysm of the thoracic aorta (Fig. 7), subocclusive pathology of the femoral-femoral bypass, occlusion at the origin of the superficial femoral arteries bilaterally, occlusion of the right hypogastric artery and subocclusion of the left hypogastric artery.
Fig. 5.
Cardiac CT: (A) left main coronary artery subocclusive stenosis at CCTA seen in curved-reconstruction (white arrow) and lumen-reconstruction (white arrow). (B) Left anterior descending stent initimal hyperplasia (white arrow). (C) Prestent occlusion of the circumflex artery at CCTA (white arrow). (D) Prestent occlusion of the circumflex artery 3D-VR reconstruction (white arrow). CCTA, cardiac-CT angiography.
Fig. 6.
Cardiac CT and MRI: (A) ischemic necrosis in the mid-basal infero-lateral wall in short-axis view at CCTA (white arrow), (B) corresponding to transmural LGE at CMR (white arrow). CCTA, cardiac-CT angiography; CMR, cardiac magnetic resonance imaging; LGE, late gadolinium enhancement.
Fig. 7.
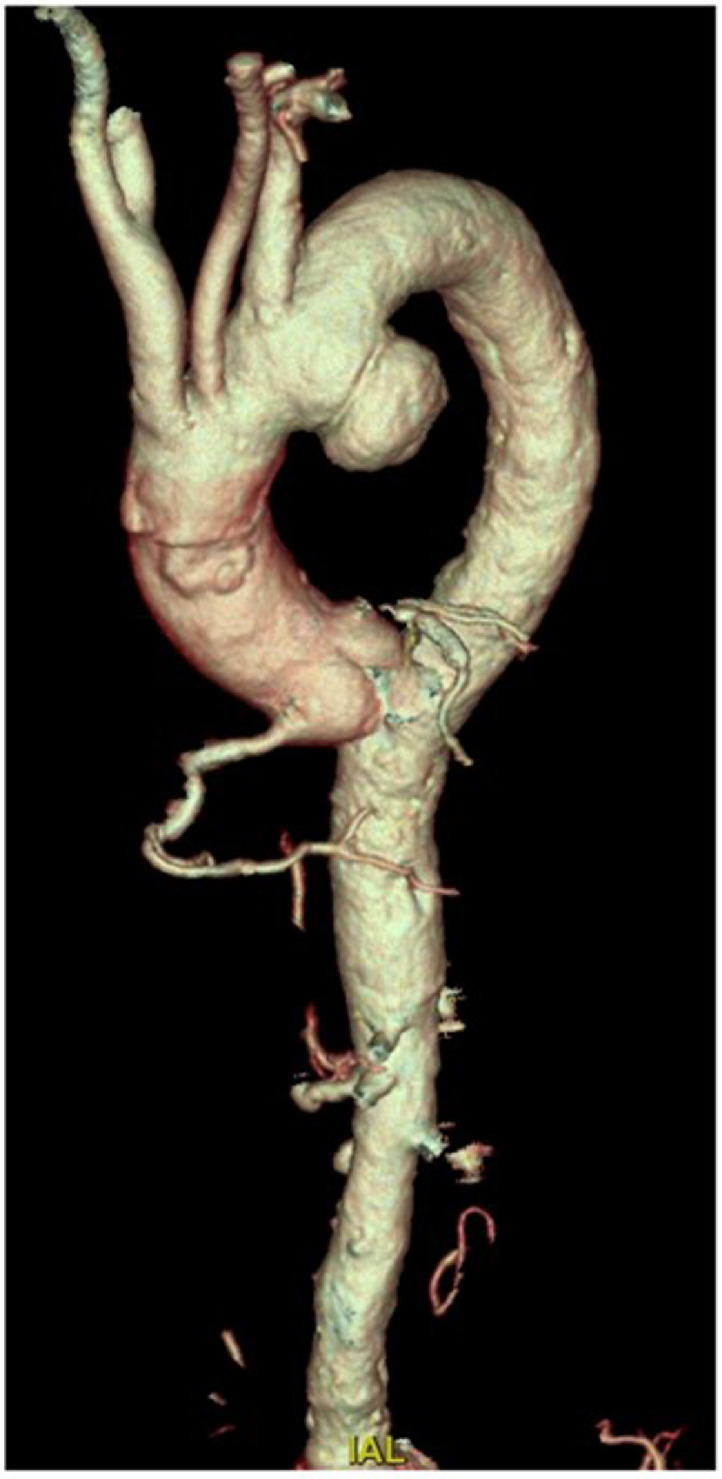
Cardiac CT sacciform aneurysm of the thoracic aorta on 3D-VR reconstruction.
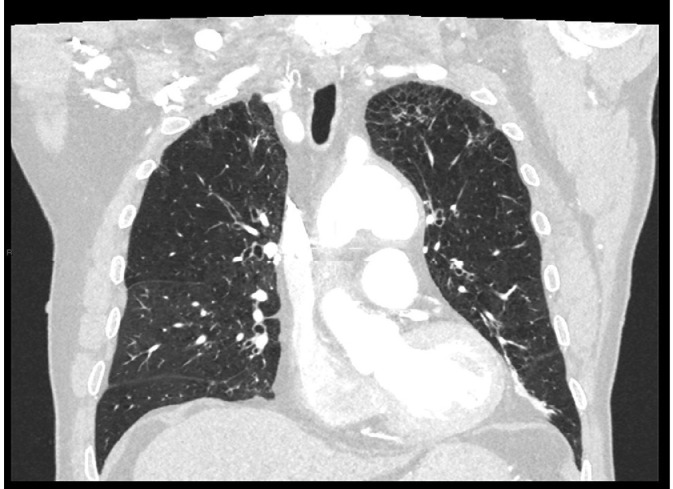
In the lung there was diffuse irregular thickening of the interlobular septa more evident in the apical region compatible with fibrotic interstitiopathy (Fig. 8).
Fig. 8.
Thorax CT pulmonary involvement.
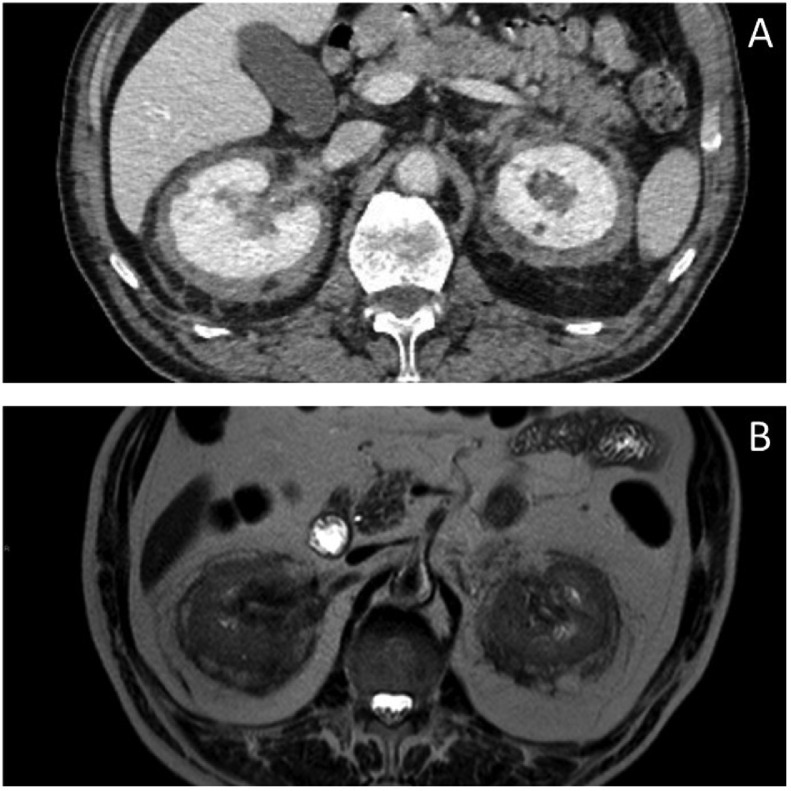
Abdominal CT showed a reduction in perirenal fibrotic tissue (Fig. 9A), as later confirmed by MRI examination (Fig. 9B), and a reduction in the extent of previous sclerotic bone lesions.
Fig. 9.
Abdomen CT pretherapy, abdomen MRI posttherapy. (A) Proliferative tissue reduction detected at CTand axial T2- weighed MRI sequence (B).
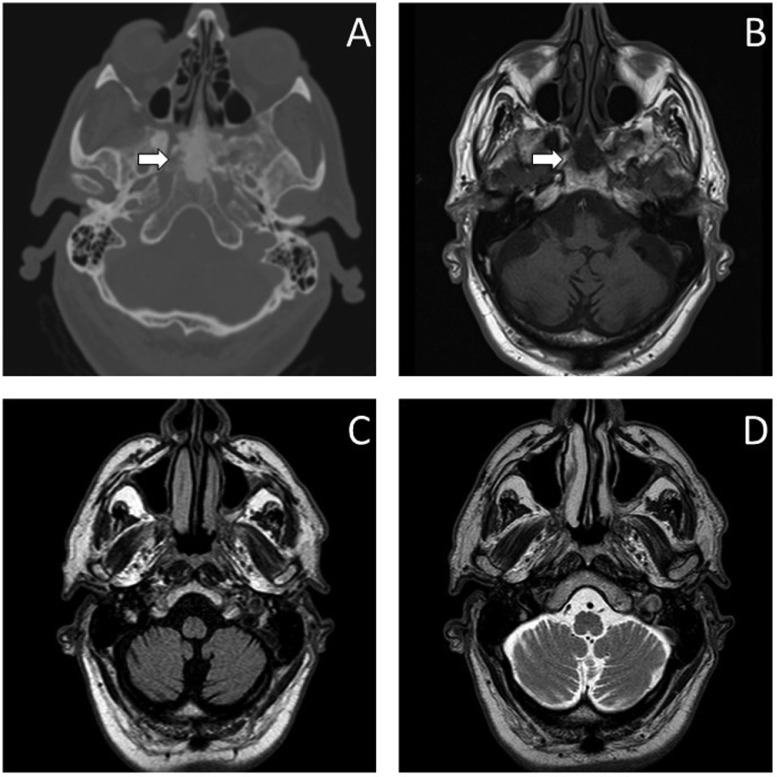
Brain magnetic resonance imaging (MRI) confirmed the presence of sclerotic tissue at the level of sphenoid corpus (Figs. 10A and B) and documented diffuse degenerative phenomena in the cerebellar hemispheres and subtentorial encephalic tissue (Figs. 10C and D). Severe atrophy of the cerebellar hemispheres, temporo-mesial regions and brainstem emerged at the subsequent brain PET scan.
Fig. 10.
Head CT, brain MRI (A) sphenoid involvement at CT (white arrow) and axial T1-weighed MRI sequence(white arrow) (B); cerebellar degeneration at MRI in axial FLAIR (C) and T2-weighed sequences (D).
Discussion
ECD is a rare, non-(LCH) Langerhans cell histiocytosis histiocytic proliferative disorder with a systemic predisposition that typically affects the elderly [[2], [3], [9], [12]]. Proper identification of this disorder among the plethora of nonspecific symptoms requires a multidisciplinary approach based on the clinical symptoms, radiological findings, and at last from histopathological confirmation [3]. Despite not being a cancerous disease, the disorder has a poor prognosis and is greatly influenced by the locations and levels of extraosseous involvement [[9], [10]]. Chemotherapy has a poor outcome and an unstoppable downward trajectory when the CNS and CVS are involved [[1], [6]]. In order to avoid misdiagnosis, knowledge of its imaging findings is crucial.
Imaging, in all its forms, is essential to the care of this difficult patient population. Knowing the imaging results of ECD multisystemic lesions can help with early diagnosis, more rapid multidisciplinary input, and, if possible, faster therapy with new drugs, potentially lowering morbidity and death [[7], [9], [10]].
Depending on the site of involvement, a variety of imaging modalities including radiography, ultrasonographyCT, MRI, and nuclear imaging may be used [[6], [10], [13]]. PET—Imaging is employed to identify the sites of involvement, define the extention, and follow evolution over time in ECD because it is a systemic illness with osseous and extraosseous symptoms [[7], [9], [13], [14]].
To identify skeletal involvement, plain radiography and 99mTc bone scintigraphy are helpful [[3], [9]]. Cross-sectional imaging methods like CT or MRI are highly helpful for the assessment of thoraco-abdominal and CNS involvement [[11], [15]]. When evaluating CVS and CNS involvement, MRI is favored over CT due to its greater contrast resolution [9].
The diagnosis of ECD is based on histologic findings that are supported by the right clinical signs and radiologic context [[1], [2], [9], [13]]. The afflicted organs mostly determined the clinical symptoms [6].
In our case, multisystem cardiovascular disease was the first manifestation of ECD. Regular circumferential periaortic infiltration that gives the appearance of a “coated aorta” is a characteristic sign of this disease [[4], [6], [16], [17]]. The main differential diagnosis is Takayasu's illness. In contrast to ECD, Takayasu's disease affects the entire artery wall, resulting in concentric thickening, thrombosis, stenosis, and occlusion, as well as vascular ectasia, aneurysms, and ulcers [[9], [13]].
Retroperitoneal involvement was asymptomatic as often reported in previous literature [9]. Renovascular involvement, however, may be the source of subsequent systemic hypertension [13]. In our case, there was not only renovascular involvement but also periaortic and other vascular infiltration, which may be risk factors for hypertension.
According to reports, up to 96% of those with ECD will have skeletal involvement, but only 50% will have symptoms [[1], [9], [10]]. The femur, tibia, and fibula are the most commonly impacted bones; the ulna, radius, and humerus are less frequently affected ][[3], [10]]. As in our case, pain is typically felt in the knees and ankles.
Though the signs of pulmonary involvement in ECD are not-specific, they are highly suggestive of the diagnosis when present. These signs include smooth thickening of the pleura and fissures, smooth septal thickening, small centrilobular nodules, parenchymal consolidation, cystic lesions, and mosaic “ground glass” opacities [[9], [10], [11], [15]].
ECD can also affect the meninges, the orbits, and the facial bones in addition to the CNS [[18], [19]]. It can also cause a variety of symptoms, including diabetes insipidus, exophthalmos, cerebellar ataxia, panhypopituitarism, and papilledema [[1], [4], [6], [20]]. The main symptom of our patient was mild evening fatigue in all 4 limbs, almost 3 years after diagnosis. A diagnosis of ECD, in fact, should be considered in patients with myasthenia-like symptoms who have tested negative for myasthenia gravis and neurodegenerative diseases including hereditary and autoimmune cerebellar ataxia and disorders of the hypothalamus.
As well as the genetic basis, therapy is also actively under investigation [[2], [21]]. Protocols include the use of interferon and, for those patients carrying the BRAF mutation, Vemurafenib, also as first line treatment [[6], [22]]. In our case, we observed that after 3 years of therapy there was a response to treatment only at the level of retroperitoneal fibrosis and bone localizations. Cardiovascular and neurological manifestations were still present and had progressed. We hypothesized that this was imputable to the fact that these were chronic inflammatory processes leading to tissue remodeling and atrophy and, when a point of no return is reached, with current therapies the course cannot be changed. Therefore, it will be an important focus of future studies to go after the causes of this nonresponse and possibly find other drugs capable of improving it.
Conclusion
ECD is still a rare instance of an infiltrative disease although it has a high morbidity and mortality rate [[4], [10]]
The key to stopping this disease's progression is still early detection and prompt treatment. For proper differential diagnosis, it is important to know the main radiological findings of this pathology, as most patients undergoing imaging present nonspecific symptoms, such as bone pain or cardiovascular or neurologic involvement [10].
It has not yet been thoroughly determined how to treat ECD. According to the literature Interferon was the most frequently used treatment [1], and consensus guidelines said that starting therapy was advised for all patients, barring those who were asymptomatic [[10], [13]].
Therapy with Vemurafenib remains reserved for patients with a BRAF gene mutation [[5], [6]]. Although good results were obtained on bone lesions and retroperitoneal fibrosis, neurologic and cardiovascular involvement does not seem to benefit from therapy and remains the worst prognostic factor [[3], [10]].
So, multimodality imaging is even more important for the multidisciplinary team to analyze the cohort of signs, symptoms and radiological findings that allow to better frame the patient with ECD and to direct him as soon as possible to biopsy, or to an earliest diagnosis.
Patient consent
The authors declare that this report does not contain any personal information that could lead to the identification of the patient. Informed consent was obtained from the patient.
Footnotes
Competing Interests: The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Haroche J., Cohen-Aubart F., Charlotte F., Maksud P., Grenier P. Cluzel P et al., The histiocytosis Erdheim-Chester disease is an inflammatory myeloid neoplasm. Expert Rev Clin Immunol, 11. Taylor and Francis Ltd.; 2015. p. 1033–1042. [DOI] [PubMed]
- 2.Liu J., Gao L., Pu H., He W., Peng L. Erdheim-Chester disease with multisystem involvement evaluated by multimodal imaging: a case report. Radiol Case Rep. 2022;17(3):784–789. doi: 10.1016/j.radcr.2021.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alduraibi S.K. A case of Erdheim-Chester disease initially mistaken for retroperitoneal lymphoma. Radiol Case Rep. 2020;15(5):570–575. doi: 10.1016/j.radcr.2020.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krishna V.V.R., James T.E.L.H., Chang K.T.E. Erdheim-Chester disease with rare radiological features in a 14-year old girl with pre-B acute lymphocytic leukemia and diabetes mellitus. J Radiol Case Rep. 2014;8(8):7–15. doi: 10.3941/jrcr.v8i8.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haroche J., Charlotte F., Arnaud L., von Deimling A., Hélias-Rodzewicz Z., Hervier B., et al. High prevalence of BRAF V600E mutations in Erdheim-Chester disease but not in other non-Langerhans cell histiocytoses. Blood. 2012;120(13):2700–2703. doi: 10.1182/blood-2012-05-430140. [DOI] [PubMed] [Google Scholar]
- 6.Papo M., Emile J.F., Trovati T., Bay P., Alistair Baber, Hermine O. Erdheim-Chester Disease: a Concise Review. Curr Rheumatol Rep. 2019;21(12):66. doi: 10.1007/s11926-019-0865-2. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z., Yu W., Guan W., Lin Q., Guermazi A. Atypical skeletal involvement in patients with Erdheim–Chester disease: CT imaging findings. Orphanet J Rare Dis. 2022;17(1):34. doi: 10.1186/s13023-022-02185-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Veyssier-Belot C., Cacoub P., Caparros-Lefebvre D., Wechsler B., Brun B., Remy M., et al. Erdheim-Chester disease clinical and radiologic characteristics of 59 cases. Medicine. 1996;75(3):157–169. doi: 10.1097/00005792-199605000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Kumar P., Singh A., Gamanagatti S., Kumar S., Chandrashekhara S.H. Imaging findings in erdheim-chester disease: what every radiologist needs to know. Pol J Radiol. 2018;83:e54–e62. doi: 10.5114/pjr.2018.73290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lodhi U., Sarmast U., Khan S., Yaddanapudi K. Multisystem radiologic manifestations of Erdheim-Chester disease. Case Rep Radiol. 2016;2016:1–6. doi: 10.1155/2016/2670495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.J.P. Das, L. Xie, C.C. Riedl, S.A. Hayes, M.S. Ginsberg, and D.F. Halpenny, ‘Cardiothoracic manifestations of Erdheim-Chester disease’, 2019. [DOI] [PMC free article] [PubMed]
- 12.Calcaterra G., Bassareo P.P., Barillà F., Sergi D., Chiocchi M., Romeo F., et al. The deadly quartet (Covid-19, old age, lung disease, and heart failure) explains why coronavirus-related mortality in Northern Italy was so high. Curr Cardiol Rev. 2021;17(1):74–77. doi: 10.2174/1573403X16666200731162614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes C., Graça B., Donato P. Thoracic, abdominal and musculoskeletal involvement in Erdheim-Chester disease: CT, MR and PET imaging findings. Insights Imaging. 2014;5(4):473–482. doi: 10.1007/s13244-014-0331-7. Springer Verlag. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirchner J., Hatzoglou V, Buthorn J, Bossert D, Siglerb A M, Reiner A S. etal. 18F-FDG PET/CT versus anatomic imaging for evaluating disease extent and clinical trial eligibility in Erdheim-Chester disease: results from 50 patients in a registry study. Eur J Nucl Med Mol Imaging. 2021;48:1154–1165. doi: 10.1007/s00259-020-05047-8/Published. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wittemberg K.H., Swensen S.J., Myers J.L. Pulmonary involvement with Erdheim-Chester disease: radiographic and CT findings. AJR. 2000;174:1327–1331. doi: 10.2214/ajr.174.5.1741327. www.ajronline.org [Online] [DOI] [PubMed] [Google Scholar]
- 16.Yoon M., Lee S.H., Shim H.S., Kang S.M. Erdheim-Chester disease presenting as an intracardiac mass and pericardial effusion confirmed by biopsy: a case report. Eur Heart J Case Rep. 2021;5(10) doi: 10.1093/ehjcr/ytab351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gandini R., Chiocchi M., Maresca L., Pipitone V., Messina M., Simonetti G. Chronic contained rupture of an abdominal aortic aneurysm: from diagnosis to endovascular resolution. Cardiovasc Intervent Radiol. 2008;31(S2):62–66. doi: 10.1007/s00270-007-9154-y. [DOI] [PubMed] [Google Scholar]
- 18.Martiniuc G., Turliuc D.M., Miron M., Martiniuc C. A possible case of Erdheim–Chester disease. Rom J Ophthalmol. 2018;62(4):308–311. doi: 10.22336/rjo.2018.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chiocchi M., Luciano A., De Stasio V., Pugliese L., Di Donna C., Cerocchi M., et al. Lipomatous hypertrophy of the interatrial septum: A potential pitfall resolved on comparing previous PET/CT. Radiol Case Rep. 2022;18(1):222–225. doi: 10.1016/j.radcr.2022.09.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiocchi M., Di Donna C., Intorcia A., Pugliese L., De Stasio V., Di Tosto F. A rare case of a giant circumflex coronary artery aneurysm 10 years after bentall surgery. Radiol Case Rep. 2021;16(7):1749–1753. doi: 10.1016/j.radcr.2021.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mollace V, Rosano GMC, Anker SD, Coats AJS, Seferovic P, Mollace R, et al. Pathophysiological Basis for Nutraceutical Supplementation in Heart Failure: A Comprehensive Review. Nutrients. 2021;13(1):257. doi: 10.3390/nu13010257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gandini R, Chiocchi M, Loreni G, Del Giudice C, Morosetti D, Chiaravalloti A, et al. Treatment of type II endoleak after endovascular aneurysm repair: the role of selective vs. nonselective transcaval embolization. J Endovasc Ther. 2014;21(5):714–722. doi: 10.1583/14-4571MR.1. [DOI] [PubMed] [Google Scholar]