Abstract
Mitochondrial energy conversion requires an intricate architecture of the inner mitochondrial membrane1. Here we show that a supercomplex containing all four respiratory chain components contributes to membrane curvature induction in ciliates. We report cryo-electron microscopy and cryo-tomography structures of the supercomplex that comprises 150 different proteins and 311 bound lipids, forming a stable 5.8-MDa assembly. Owing to subunit acquisition and extension, complex I associates with a complex IV dimer, generating a wedge-shaped gap that serves as a binding site for complex II. Together with a tilted complex III dimer association, it results in a curved membrane region. Using molecular dynamics simulations, we demonstrate that the divergent supercomplex actively contributes to the membrane curvature induction and tubulation of cristae. Our findings highlight how the evolution of protein subunits of respiratory complexes has led to the I–II–III2–IV2 supercomplex that contributes to the shaping of the bioenergetic membrane, thereby enabling its functional specialization.
Subject terms: Cryoelectron tomography, Cryoelectron microscopy, Structural biology
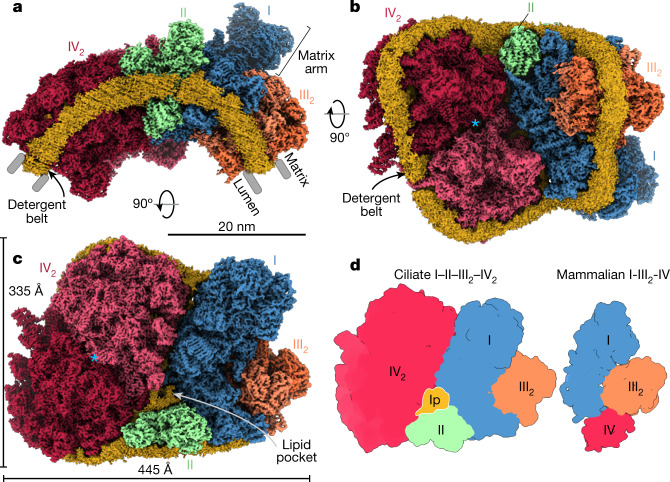
A supercomplex comprising all four respiratory chain components contributes to the induction of mitochondrial membrane curvature and tubulation of cristae.
Main
Mitochondrial energy conversion requires an electron transport chain (ETC) that generates a proton motive force across the inner mitochondrial membrane to drive the essential adenosine triphosphate (ATP) formation by F1Fo-ATP synthase. The ETC consists of four multisubunit membrane complexes: complex I (CI; NADH:ubiquinone (UQ) oxidoreductase), complex II (CII; succinate:UQ oxidoreductase), complex III (CIII; cytochrome bc1) and complex IV (CIV; cytochrome c oxidase). Biochemical and structural analyses have shown that these components can organize into supercomplexes containing CI, CIII dimer (CIII2) and CIV1,2. CII transfers electrons from succinate via its covalently bound flavin adenine dinucleotide (FAD) and iron–sulfur clusters to UQ and is also a component of the tricarboxylic acid cycle, making a functional link between the two central metabolic pathways3. Although CII has been suggested to interact with mammalian ETC complexes4–8, it was not experimentally found as part of any characterized supercomplex. In addition, for the bioenergetic process to occur, a specific topology of the crista membranes that form functionally distinct high-potential compartments is critical9. An established mechanism for maintenance of such a topology relies on oligomerization of ATP synthase and its specific interplay with lipids10–14. In ciliates, the inner mitochondrial membrane is organized as tubular cristae, which was previously explained by the helical row assembly of ATP synthase12,15,16.
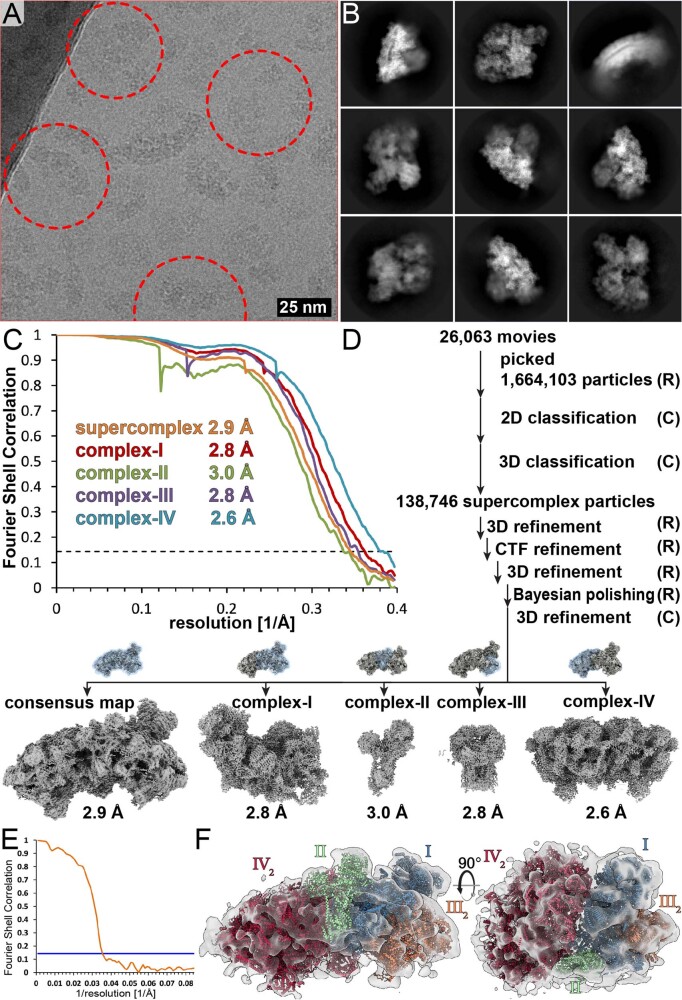
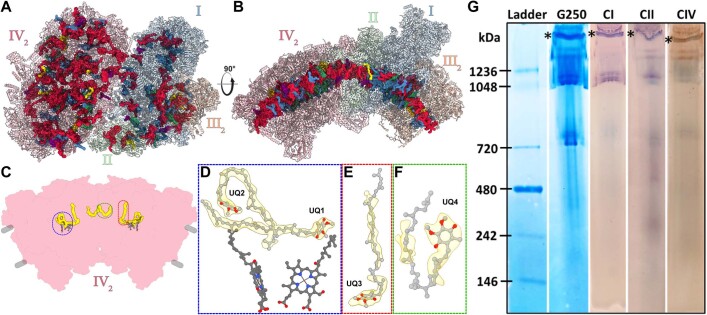
We purified the intact respiratory supercomplex from mitochondria of the ciliate protist Tetrahymena thermophila and determined its structure by single-particle cryo-electron microscopy (cryo-EM) (Extended Data Fig. 1 and Supplementary Table 1). At an overall resolution of 2.9 Å, the structure revealed that CI, CII, CIII2 and a CIV dimer (CIV2) associated into a 5.8-MDa supercomplex (Fig. 1). When viewed along the membrane plane, the assembly of more than 300 transmembrane helices displays a bent shape, indicating that the accommodating membrane adopts a local curvature with a radius of approximately 20 nm (Fig. 1). Masked refinements resolved individual structures that together form an assembly of 150 different protein subunits and 311 bound lipids (Extended Data Figs. 1 and 2a–f and Supplementary Tables 1 and 2). CIV2 is associated with the long side of the membrane region of CI, opposite CIII2. This arrangement is markedly different compared with known mammalian supercomplexes17,18 and correlates with the acquisition of four ciliate-specific CI subunits that would clash with the position of CIV as seen in mammals (Fig. 1d and Extended Data Fig. 3). CII is anchored between CI and CIV, highlighting the unique architecture and composition of the native supercomplex (Fig. 1). In-gel activity assays confirmed co-migration of functional CI, CII and CIV in a high-molecular-mass band, which we assigned to the intact supercomplex (Extended Data Fig. 2g; and see source data for Extended Data Fig. 2).
Extended Data Fig. 1. Cryo-EM and subtomogram averaging.
(A) A total of 26,063 movies were recorded and analyzed, a representative micrograph is shown. Particles that were not respiratory supercomplex were discarded by classification, since they cannot contribute to reconstruction. Cryo-EM structures were successfully obtained from three preliminary datasets. (B) 2D class averages. (C) Fourier Shell Correlation according to the 0.143 gold-standard criterion. (D) processing workflow in RELION-3.1 (R) and cryoSPARC2 (C). (E) Fourier Shell correlation of the subtomogram average indicating a resolution of 28 Å. (F) Subtomogram average map-model overlay.
Fig. 1. The supercomplex contains all four ETC components.
a, Side view of the supercomplex density showing the curved detergent micelle (yellow). b, Lumenal view illustrates how complexes CI, CII and CIV2 stabilize each other. The blue asterisk indicates the symmetry axis of CIV2. c, Matrix view shows CII binding in a wedge between CI and CIV2, resulting in the enclosure of a lipid pocket (lp). The blue asterisk indicates the same position as in b. d, Architecture comparison of the ciliate I–II–III2–IV2 supercomplex (this study; left) with the mammalian I–III2–IV respirasome (Protein Data Bank (PDB) accession 5J4Z; right), highlighting a different location of CIV2 that is correlated with acquisition of CI subunits that stabilize CIII2.
Extended Data Fig. 2. Bound native lipids of the ciliate I-II-III2-IV2 supercomplex reveal a curved membrane region.
Top view (A) and side view (B) with bound lipids. Cardiolipin (red), phosphatidylcholine (blue) phosphatidylethanolamine (green), phosphatidic acid (yellow), ubiquinone-8 (purple). (C-F) Location of the four ubiquinone-8 molecules with closeup views (D-F). (G) Coomassie-stained CN-PAGE and in-gel activity assay to visualize the purified I-II-III2-IV2 supercomplex. Individual lanes highlight protein bands with active CI, CII (purple) and CIV (brown), respectively. The band marked with an asterisk (*) was tentatively assigned as the intact supercomplex.
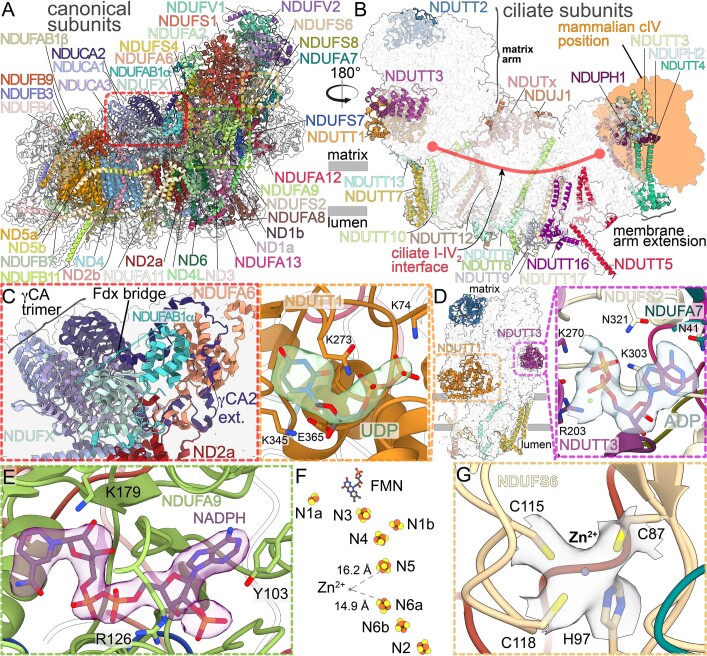
Extended Data Fig. 3. Lineage-specific structural features of CI dictate a divergent supercomplex architecture.
(A) Canonical subunits of CI (coloured) include conserved antiporter-like folds (ND2a/b, ND4, ND5a/b), and γ-carbonic anhydrase trimer. (B) Augmented CI architecture occluding canonical CIV binding site. (C) The ferrodoxin bridge of NDUAB1-a and NDUFX connects ND2a of the membrane arm and NDUA6 of the matrix arm. (D) The matrix arm contains bound subunits NDUTT1 with UDP and NDUTT3 with ADP. (E) NDUFA9 contains a bound NADPH molecule close to the CI Q-tunnel. (F) Redox centers in CI matrix arm. (G) zinc ion coordinated by NDUS6.
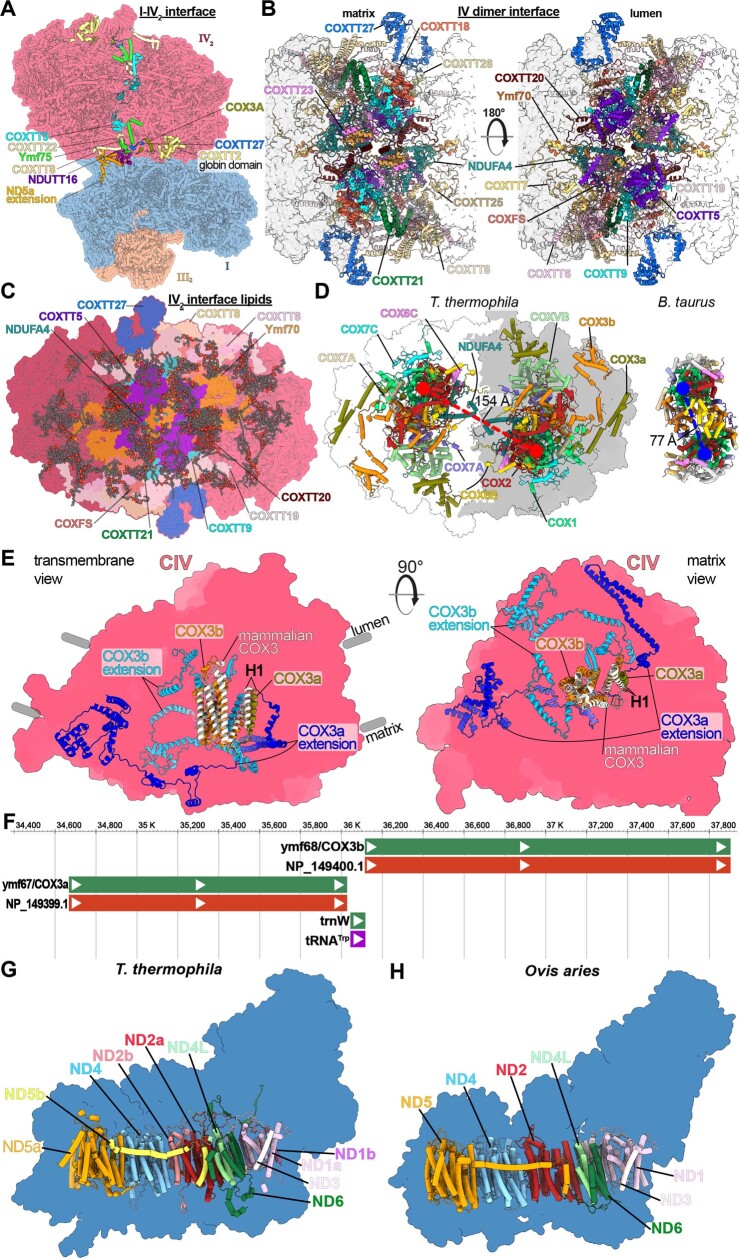
CIV2 is the most divergent of the four ETC complexes (Extended Data Figs. 4 and 5). We modelled 105 lipids, four UQs and 53 protein chains per monomer, of which four are mitochondrially encoded. We found that two of those subunits, previously annotated as ciliate-specific Ymf67 and Ymf68 (also known as COX3) represent complementary protein fragments, with coding genes split in the mitochondrial genome by a tRNATrp gene insertion (Extended Data Fig. 6). Each fragment is extended by over 400 and 200 residues, respectively. Together, they form a functional COX3 (COX3a and COX3b), including the conserved seven transmembrane helix fold (Extended Data Fig. 6e). In our structure, COX3a and COX3b extend throughout the CIV membrane region, and COX3b has evolved interactions with CI subunits on the matrix side, thereby mediating the supercomplex assembly (Fig. 2a,b). In particular, COX3b forms a contact with a peripheral amphipathic helix of NDUCA1, which is part of a zinc-free γ-carbonic anhydrase (γ-CA) heterotrimer (Fig. 2c). The γ-CA heterotrimer was previously reported in viridiplantae and ciliates (both diaphoretickes)8,19 and our structure demonstrates that it acts as a structural scaffold within the supercomplex architecture. Another CI–CIV contact at the same site involves COXTT2 with a N-terminal globin-like domain that interacts with NDUFA3 and was not resolved in the individual CIV2 structure8, suggesting that it becomes ordered to mediate supercomplex formation (Fig. 2a,c). The second major interaction site at the CI–CIV interface, which is on the lumenal side of the membrane, also involves a fragmented protein subunit, this time from CI. Consistent with the observation with respect to the protein splitting in CIV, we modelled the N-terminal extension of the ND5 fragment (ND5a), as well as the newly identified protein subunit NDUTT16 (from CI) (Fig. 2a,b,d). NDUTT16 engages in interactions with at least four subunits of CIV, as well as an interfacial CIV haem group (Fig. 2d and Extended Data Fig. 5f).
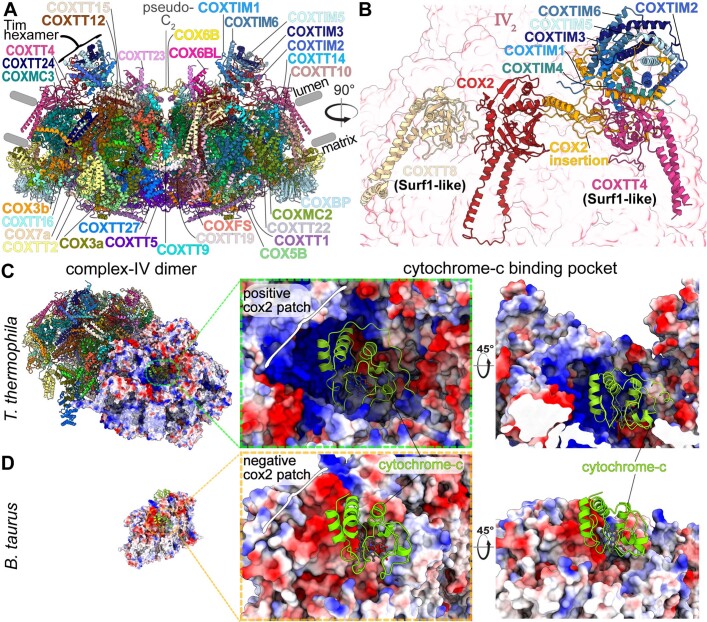
Extended Data Fig. 4. CIV2 contains a bound Tim hexamer and cytochrome-c binding site with inverted electrostatic charge.
(A) sideview of CIV2, containing numerous accessory subunits, including a Tim hexamer. (B) Closeup view of COX2, which contains an insertion that recruits the Tim hexamer to the CIV dimer. Furthermore, COX2 interacts with the two Surf1-like proteins COXTT8 and COXTT4. (C-D) ciliate and mammalian CIV2 structures. An overlay of a predicted Tt-cytochrome-c structure fits the cytochrome-c binding crater without clashes.
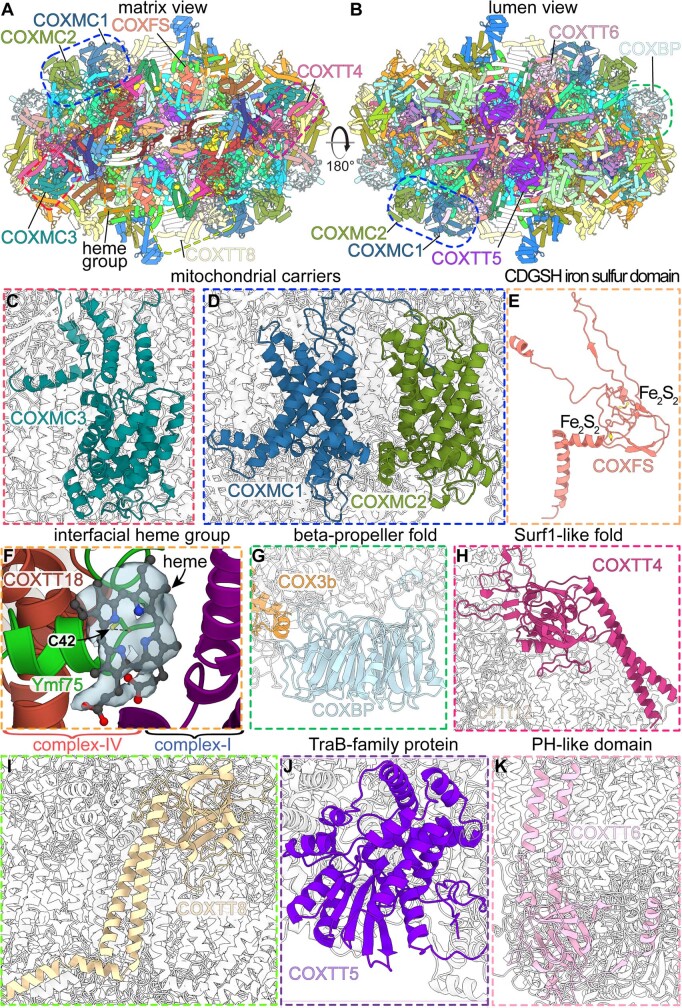
Extended Data Fig. 5. The augmented CIV2 contains numerous associated compact-fold subunits.
(A,B) Matrix and lumen view of the CIV dimer, subunit locations of insets are indicated. (C,D) Subunits COXMC1, MC2 and MC3 form mitochondrial carrier folds. COXFS is a CDGSH iron sulfur domain (E). (F) Ymf75 coordinates a noncanonical heme group at the CIV periphery. (G-K) Compact folds of accessory subunits include a seven-bladed beta-propeller (G), two Surf1-like proteins (H, I), a TraB-family protein (J) and a Pleckstrin homology (PH) like domain (K).
Extended Data Fig. 6. Fragmentation and extension of COX3 and CI proton-pump subunits enables formation of a divergent supercomplex assembly.
(A) The supercomplex displays additional subunits and ordered structural elements (colored cartoons) compared to the amphipol-embedded CIV2 dimer (PDB 7W5Z). In the CI-IV interface this includes subunit NDUTT16 and a globin-like domain of COXTT2. (B) Matrix and lumenal views of the CIV2 interface include NDUFA4. (C) The CIV2 contains 210 lipids, many of which populate the dimer interface. (D) The T. thermophila dimer is augmented compared to the bovine dimer and contains the previously unassigned canonical subunit NDUFA4, which is absent in the bovine dimer (right, PDB 3X2Q). Vectors (red, blue) indicate different distances between COX1 centers. (E) Overlay of the mammalian COX3 (PDB 5IY5) and the Tt-COX3 (this study) shows that the N-terminal fragment (COX3a), corresponds to Helix-1 (H1) of the conserved structure. COX3b makes up most of the conserved COX3 fold and contains large extensions that contribute to supercomplex formation. (F) T. thermophila mitochondrial genome region showing the insertion of the tRNA-Trp gene in between ymf67/COX3a and ymf68/COX3b (genes in green, protein/RNA transcripts in red and purple). (G) ciliate CI outline (blue) with the antiporter-like subunits shown as cylinders. The canonical proton pumps ND1, ND2 and ND5 are encoded by split genes, resulting in chains a and b. (H) mammalian CI (blue, PDB 5LNK) with single-chain ND1-6 subunits color-coded as in G.
Fig. 2. The CI–CIV association and binding of CII.
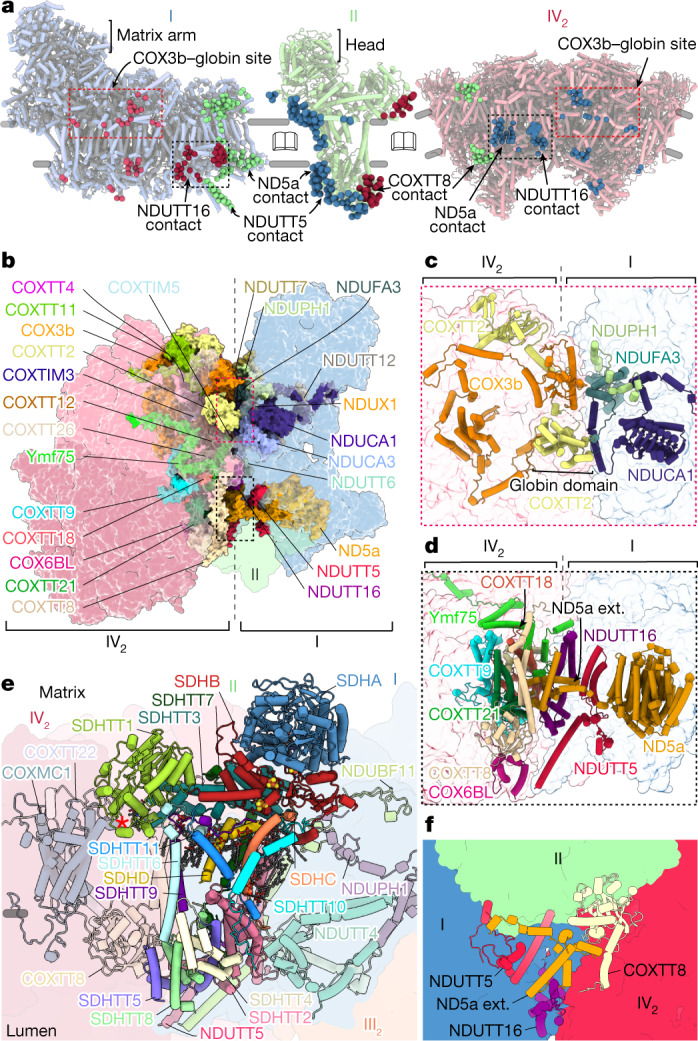
a, Open-book view with contact sites of CI with CII–CIV2 (left), CII with CI–CIV2 (middle) and CIV2 with CI–CII (right). Interactions are shown as spheres (CI in blue, CII in green, CIV2 in red or dark pink). Only one CIV monomer interacts with CII. The main interaction sites are indicated. b, The CI–CIV2 interacting subunits are shown in coloured surfaces. c, The COX3b–NDUCA1 contact site. d, The NDUTT16–ND5a contact site. ext., extension. e, CII binding to subunits of CI–CIV (transparent). The red asterisk marks C-type haem. f, CI, CII and CIV are connected via the membrane and lumenal regions.
Our finding of the split core subunits gaining a capacity of establishing intercomplex contacts to stabilize the supercomplex that curves the membrane suggests a putative mechanism by which gene fragmentation, followed by its expansion, might convey subunit function, which was also observed in mitoribosomes20,21. Overall, the CI–CIV2 interface involves 25 subunits, forming an extensive buried interface of approximately 2,300 Å2 with a curved membrane region (Fig. 2a,b), and thus a single mutation is unlikely to disrupt the curvature induction.
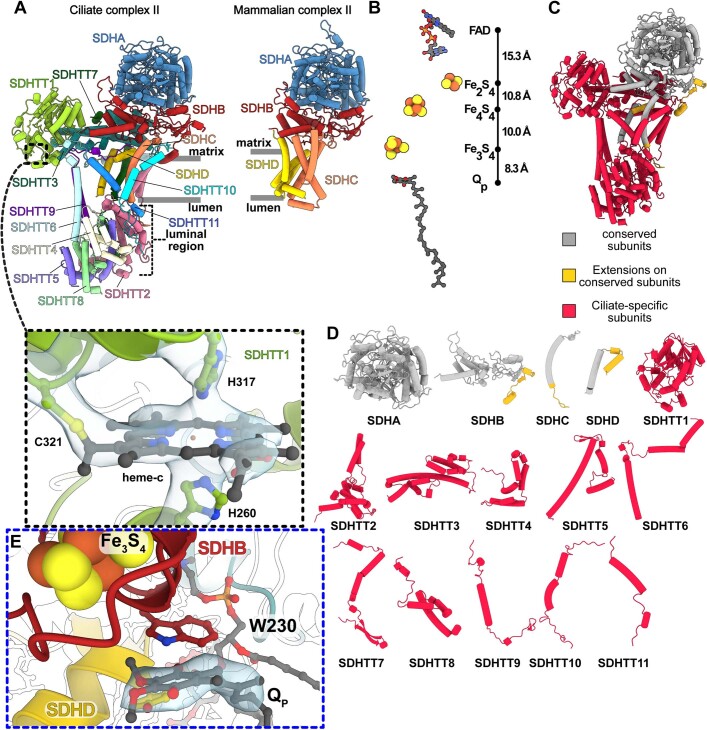
T. thermophila CII binds in a wedge-shaped gap formed by CI–CIV in our structure (Fig. 1a–c). In addition to the four canonical subunits (SDHA–D), it is composed of 11 ciliate-specific subunits SDHTT1–11 (Fig. 2e and Extended Data Fig. 7). The matrix module SDHA and SDHB forms a conserved head region, containing both the covalently bound FAD and the three iron–sulfur clusters (Extended Data Fig. 7b). The membrane anchor is formed by two small subunits, SDHC (7 kDa) and mitochondria-encoded SDHD (5 kDa), which could only be assigned by locating topologically conserved transmembrane helices in the map (Extended Data Fig. 7a,c,d). At the lumen, an approximately 70-kDa module (SDHTT2, SDHTT4, SDHTT5 and SDHTT8) anchors CII to CI–CIV (Fig. 2e,f and Extended Data Fig. 7a). SDHTT5 interacts with a helix of NDUTT5 protruding from the membrane arm of CI, and with the Surf1-like protein subunit COXTT8 (CIV) (Fig. 2a,e). At the same position, COXTT8 interacts with CI via the N-terminal ND5a extension, and with Ymf75, COXTT27 and COXTT18 at the CIV dimer interface. Thus, the three complexes—CI, CII and CIV—are connected in the lumen (Fig. 2f).
Extended Data Fig. 7. Conservation and divergence of ciliate CII.
(A) Ciliate CII (left) colored by subunits and shown in comparison to mammalian CII (right, PDB 1ZOY) with similar color scheme for subunits SDHA-D. SDHC and SDHD are substantially reduced and partially replaced by ciliate-specific subunits. Dashed box highlights a noncanonical cysteine-linked heme C that is coordinated by two axial histidines in SDHTT1. (B) Conserved electron transfer chain in CII. (C) CII colored by conserved subunits (grey), extensions (gold) and ciliate-specific subunits (red). (D) Individual CII subunits with similar color coding as in C. (E) CII contains a conserved proximal ubiquinone.
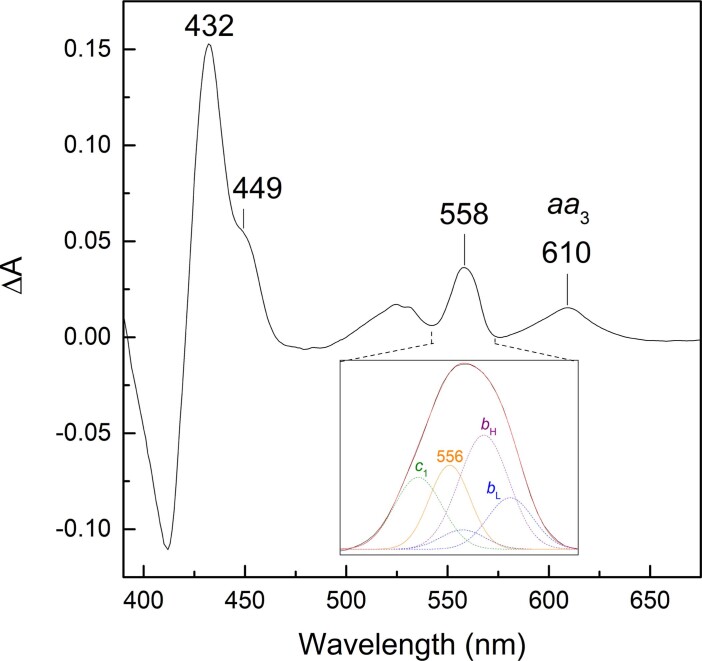
Between SDHB and SDHD, we identified a ligand, which we assign to ubiquinone bound to the conserved proximal Qp site22,23 (Extended Data Fig. 7e). On the matrix side, the 36-kDa soluble subunit SDHTT1 contains a bis-histidine C-type haem covalently bound by a single cysteine residue (Extended Data Fig. 7a). Although it is exposed to the membrane region, at a distance of approximately 60 Å from the Fe3S4 cluster, the non-canonical haem C is located too far to participate in direct CII electron transfer (Extended Data Fig. 7a). To provide further evidence of the presence of additional haem groups in the supercomplex, we recorded visible absorption redox spectra of the purified sample (Extended Data Fig. 8. Deconvolution of the merged absorption bands characteristic of B-type and C-type haems clearly revealed the contribution of at least one additional haem group in the supercomplex, in addition to those present in CIII, with specific absorption at 556 nm.
Extended Data Fig. 8. UV-visible redox absorption spectrum indicate activity of the supercomplex.
Dithionite minus air-oxidized difference spectrum. Peaks are labeled that correspond to the absorption of the A-type hemes present in CIV (449/610 nm, aa3) as well as merged absorption bands of other B- and C-type hemes (maxima at 432/558 nm). Inset: Deconvolution of the 558-nm absorption band highlighting the contribution of the B- and C-type hemes of CIII (bH at 561 nm, purple; bL at 558 and 565 nm, blue; c1 at 552 nm, green) and the presence of at least another heme-protein with absorption maximum at 556 nm (orange). The spectrum shown is representative of two separate supercomplex preparations.
The presence of a functional ETC with CII is consistent with previous observations that T. thermophila can utilize succinate to drive cellular respiration24. Our native structure with bound CII, which contributes to the ubiquinol pool, demonstrates that assembly of the supercomplex is not limited to the proton-pumping respiratory complexes (CI, CIII and CIV). Beyond decreasing cytochrome c transfer distance25, this suggests a potential role of supercomplex formation in mediating increased UQ diffusion, as suggested in analogous membrane systems with high protein to lipid ratios26,27. Furthermore, the tubular membrane morphology may require anchoring of CII to the curved supercomplex to retain it in the functionally relevant cristae and prevent diffusion to flat membrane regions.
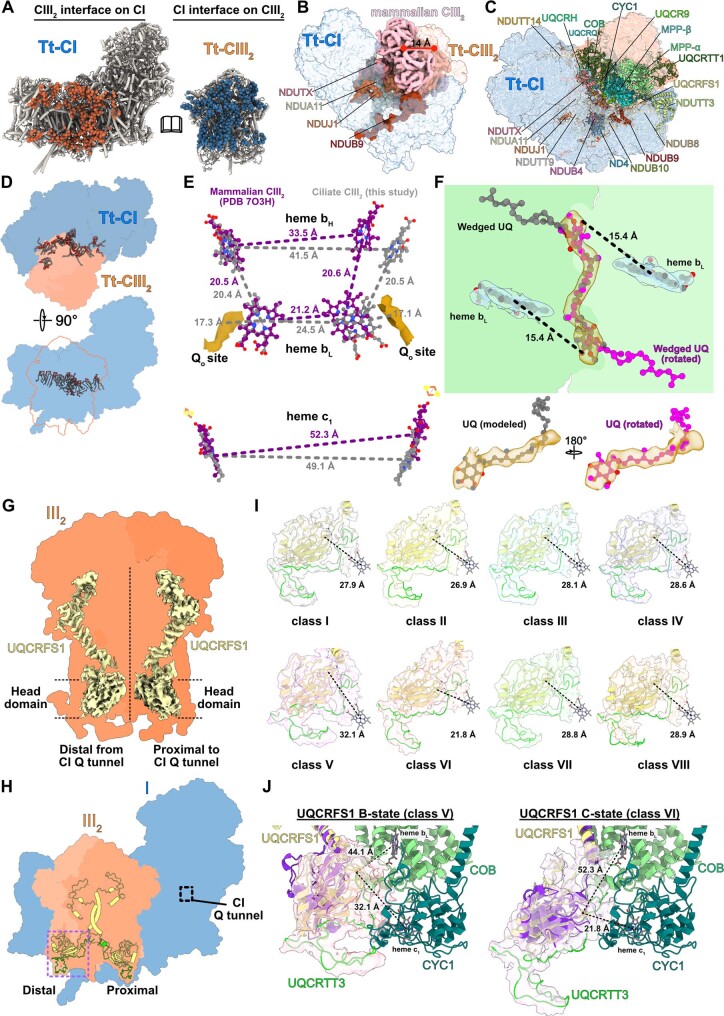
CIII2 in our structure is tilted with respect to CI by 37° (Fig. 3a). This tilted arrangement offsets the transmembrane region, consistent with its curved membrane environment. The interface involves 20 subunits and 19 bound native lipids interacting within the matrix, transmembrane and lumenal domains (Extended Data Fig. 9). When compared with the mammalian counterpart, CIII2 is rotated by 41° and shifted approximately 14 Å due to acquisition of four CI subunits, as well as the CIII subunit UQCRTT1 (refs. 17,28–30) (Fig. 3a and Extended Data Fig. 9a–c). This arrangement results in a specific CI–CIII2 contact with one copy of the Rieske iron–sulfur protein of CIII (UQCRFS1) interacting with the CI membrane arm (Fig. 3b). The interaction site is further augmented by a hitherto unidentified protein UQCRTT3, which interacts with the lumenal head domain of UQCRFS1 and wedges in between the interface to CYC1 (Fig. 3b).
Fig. 3. An altered CI–CIII2 interface maintains functional symmetry of CIII2.
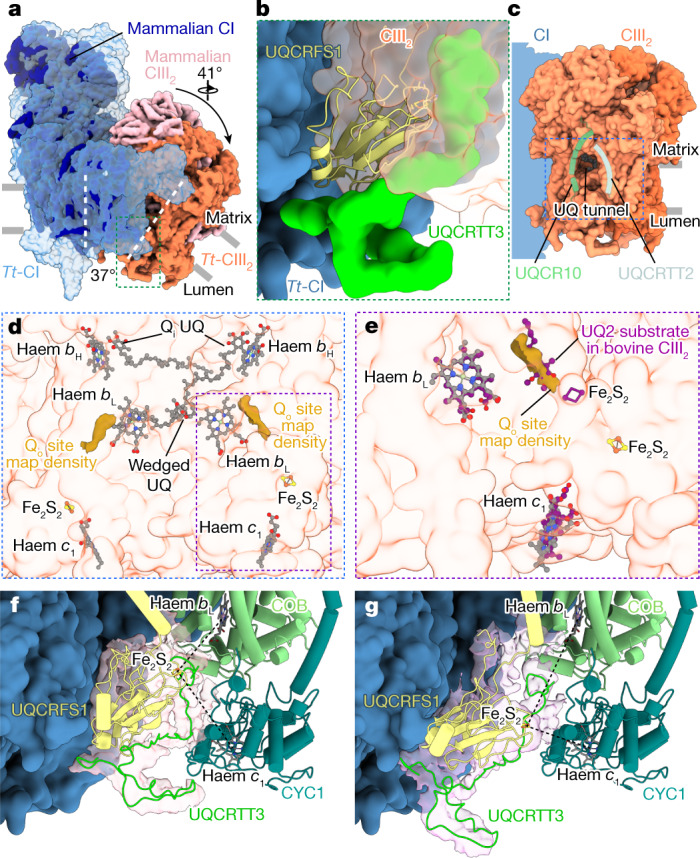
a, Superposition of T. thermophila CI–CIII2 (Tt-CI–Tt-CIII2) with mammalian CI–CIII2 (PDB accession 5J4Z) shows that T. thermophila CIII2 is tilted, rotated and displaced with respect to the CI membrane arm, as indicated by the white dashed lines and arrow, due to acquisition of new proteins. The green box view is shown in b. b, Tilted T. thermophila CIII2 results in an interaction between UQCRFS1 and the CI membrane arm together with UQCRTT3. c, T. thermophila CIII2 shows a membrane-accessible tunnel extending to the COB Qi sites. The blue box view is shown in d. d, The Qi UQs are located close to haem bH, with one UQ wedged in between the two haem bL molecules. The density map of T. thermophila CIII2 shows two features corresponding to Qo sites. The purple box view is shown in e. e, The Qo site density overlaps with ubiquinol in the bovine CIII2 structure (PDB accession 1NTZ). f,g, 3D maps showing B state (f) and C state (g) conformations of UQCRFS1 and UQCRTT3. Only COB and CYC1 proteins are shown for clarity, highlighting distances of Fe2S2 to haem c1 and bL (black dashes).
Extended Data Fig. 9. Extensive CI-CIII2 interface involves numerous lipids and accommodates flexible head domain of distal UQCRFS1.
(A) Buried surfaces in the CI-CIII2 interface. CIII2 contacts on CI (orange) and CI contact points on CIII2 (blue; >9,000 Å2 interface). (B) Four Tt-CI subunits would clash with mammalian CIII2 in canonical position, thus Tt-CIII2 is displaced 14 Å from Tt-CI. (C) Tt-CI (light blue) and Tt-CIII2 (light orange) with interface-forming subunits shown in colored ribbons. (D) Orthogonal views of bound native lipids in the I-III2 interface. (E) Distances between ciliate (grey) and mammalian (purple) heme bH, heme bL and heme c1 groups (measuring from Fe atoms) and the ciliate Qo sites. Superposing mammalian CIII2 (PDB 7O3H) shows that ciliate heme bH and heme bL are displaced further away from the symmetry-related hemes, whereas the ciliate heme c1 groups are slightly closer. Furthermore, the distance from Qo sites (dark gold) to heme bL is comparable to bovine CIII2 (17). (F) The CIII2 wedged ubiquinone (UQ, transparent gold density) in COB (green) is almost rotationally symmetric, showing planar head group density equally close to the heme bL molecules (transparent blue). (G) Cryo-EM density carved around the UQCRFS1 proteins in CIII2. Head domains show inherent flexibility. (H) Location of distal and proximal UQCRFS1 subunits (yellow ribbon) with respect to CI quinone tunnel (black box). The purple box highlights region for focused 3D classification. (I) 3D classes I-VIII. UQCRFS1 and UQCRTT3 were fitted to each map and distance measured from Fe2S2 cluster to heme c1. Only classes V and VI display markedly different distances. (J) Comparison of B-state class V (left) and C-state class VI (right). B-state UQCRFS1 superposes well with B-state UQCRFS1 from chicken CIII2 (purple, PDB 3BCC). C-state UQCRFS1 superposes almost perfectly with C-state UQCRFS1 from chicken CIII2 (purple, PDB 1BCC). Measured distances are from UQCRFS1 Fe2S2 to heme c1 and bL.
We traced the membrane-accessible UQ tunnel, lined by UQCR10 and ciliate-specific subunit UQCRTT2 (Fig. 3c), leading to the conserved COB haem bH, where the density for a bound (semi)-UQ was observed in the Qi site31 (Fig. 3d and Extended Data Fig. 9e,f). Furthermore, we observed map density features close to the two conserved haem bL groups, which probably correspond to ubiquinols bound at the Qo sites (Fig. 3d,e and Extended Data Fig. 9e,f). The distances between the Qo site, haem bL and haem bH within each CIII monomer are consistent with those observed in mammalian CIII2, with the two haem bL in COB being bridged by a non-canonical UQ. We detected density for two copies of the flexible UQCRFS1 head domain (Extended Data Fig. 9g), which contrasts with recent work that found only the head domain proximal to the CI quinone tunnel to display flexibility, whereas the distal domain at the CI interface was proposed to be non-functional in electron transport8. Using focused 3D classification for the distal UQCRFS1 head domain, we then identified two classes probably representing the extremes of the head domain movement: from the B state where the Fe2S2 cluster is distanced from haem c1 to the C state where the Fe2S2 cluster is closest32,33 (Fig. 3f,g and Extended Data Fig. 9i). This movement of the UQCRFS1 head domain is coupled to conformational changes in the unidentified UQCRTT3 protein, suggesting a potential role for this subunit in regulation of CIII2 activity. Thus, our observation of the distal UQCRFS1 head domain flexibility, together with the haem bL-wedged UQ, suggests that functional symmetry is maintained in the ciliate CIII2 despite deviation from the structural symmetry.
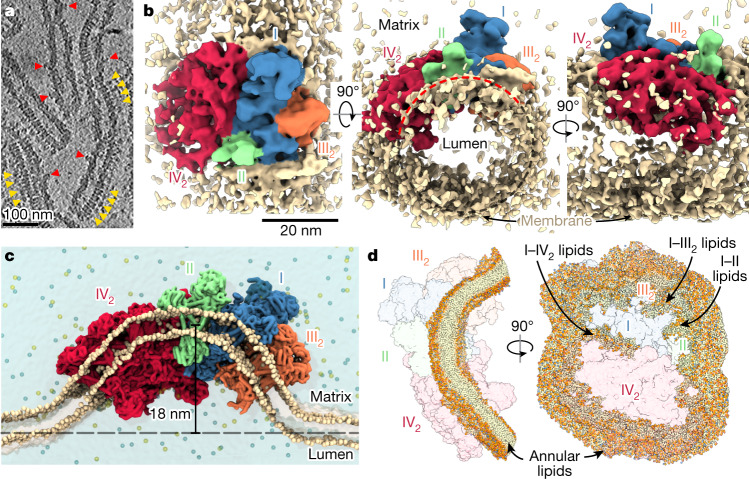
To investigate whether the membrane-bending capacity of the supercomplex is biologically relevant, we performed cryo-electron tomography of isolated mitochondrial membranes. Cryo-tomograms revealed approximately 40-nm tubular cristae densely packed with helical ATP synthase rows and supercomplexes, identified by the conspicuous CI matrix arm (Fig. 4a). To elucidate the supercomplex architecture in situ, we performed subtomogram averaging and obtained a map at 28 Å resolution (Extended Data Fig. 1e). The subtomogram average confirmed the presence of the supercomplex, which fits our atomic model (Extended Data Fig. 1f), suggesting that this is the most abundant form. The appearance of a tubular membrane density in the subtomogram average suggests that the supercomplex adopts a preferred orientation, with its CI–CIV2 interface approximately aligned with the long axis of the tube (Fig. 4b). Furthermore, the curved membrane region of the supercomplex subtends an angle of approximately 130°, indicating that it contributes to the tubular shape of the cristae.
Fig. 4. In situ structure and of the I–II–III2–IV2 supercomplex indicate a membrane-bending function.
a, Cryo-tomographic slice of tubular cristae with a diameter of approximately 40 nm. ATP synthase and the supercomplex are marked with yellow and red arrowheads, respectively. b, Subtomogram average of the I–II–III2–IV2 supercomplex revealing a preferred orientation in tubular membranes and an arc-shaped structure subtending approximately 130° (red dashes). c, Coarse-grained molecular dynamics simulation showing that the arched membrane region of the supercomplex generates a significant membrane curvature, resulting in an 18-nm local displacement of the membrane from the bilayer plane. d, Molecular dynamics simulation reveals the curved structure of the annular lipid shell surrounding the supercomplex, as well as lipid-filled subcomplex interfaces.
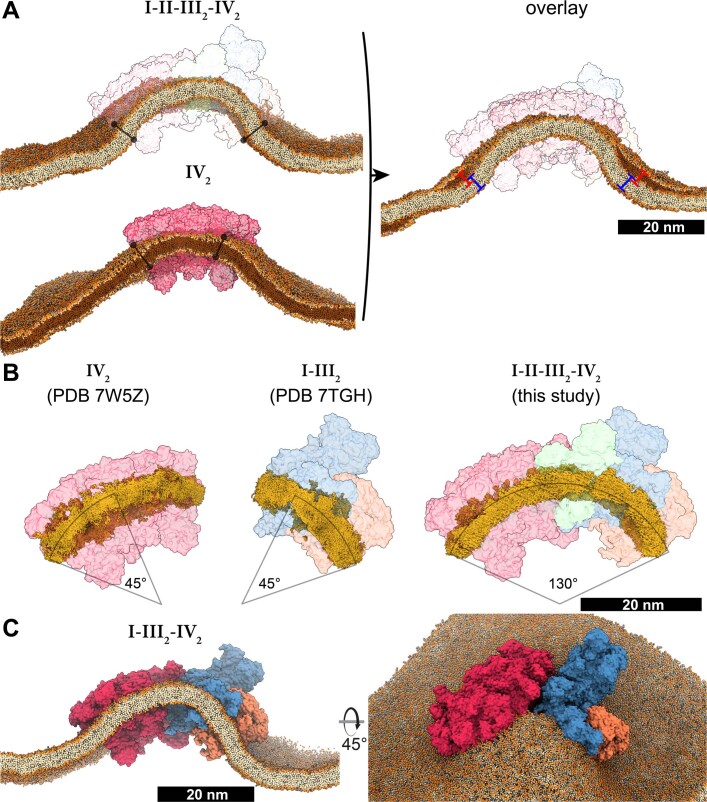
To elucidate the membrane-shaping activity of the supercomplex, we performed coarse-grained molecular dynamics simulations. When placed into a planar lipid bilayer, the supercomplex induces a curved membrane topology, displacing the membrane by 18 nm from the original plane (Fig. 4c and Supplementary Video 1), in contrast to the protein-free lipid bilayer, which remains relatively flat (Supplementary Video 2). Furthermore, the annular lipid shell surrounding the complex in the equilibrated system displays a highly curved architecture, supportive of an active role in membrane curvature induction (Fig. 4d and Supplementary Video 3). In addition, we observed lipid pockets in the transmembrane interfaces between subcomplexes, which suggests that their maintenance is crucial for the integrity of the supercomplex (Extended Data Fig. 2a,b), as was also shown for photosynthetic membranes34. Molecular dynamics simulations of CIV2 in a membrane suggest that it can also induce membrane bending in its immediate vicinity, similar to the supercomplex (Extended Data Fig. 10a,b). However, for the formation and stability of a tubular architecture, juxtapositioning of individual subcomplexes is probably necessary. This is also supported by the increased arc length of the detergent belt observed in the cryo-EM structure of the supercomplex, when compared with CIV2 (Extended Data Fig. 10b). Finally, molecular dynamics simulations of the supercomplex lacking CII showed a similar wrapping of the lipid bilayer as for the full supercomplex, with lipids filling the generated void (Extended Data Fig. 10c). This indicates that CII association may contribute to complex stability while retaining the enzyme in the tubular cristae regions, where ubiquinol is required as a substrate.
Extended Data Fig. 10. Membrane curvature induction by the supercomplex and subcomplexes.
(A) Left, selected frames of molecular dynamics simulations of the I-II-III2-IV2 supercomplex and CIV2, embedded in a lipid bilayer. Contact sites of protein complexes with sliced plane are shown by black markers. Right, overlay and zoom in of the frames of the I-II-III2-IV2 supercomplex and CIV2. The larger size of the supercomplex and its architecture bends the membrane (blue marker) more than CIV2 alone (red marker). (B) Detergent/saponin belts extracted from the cryo-EM structures of CIV2 (PDB 7W5Z), CI-CIII2 (PDB 7TGH) and of the I-II-III2-IV2 supercomplex. Lengths of curvature arcs when viewed along the membrane tube are indicated. (C) Selected frame of the molecular dynamics simulation of the I-III2-IV2 supercomplex (missing CII), displaying a similar curvature to the full supercomplex.
Our results indicate that cristae shaping involves both the respiratory supercomplex and the ATP synthase that together generate membrane tubulation. Although the coiled ATP synthase rows fix the helix diameter at 130 nm (refs. 12,15), supercomplexes serve the function of confining a narrow crista diameter of around 40 nm, which allows tight packing of cristae, thereby increasing the surface area of the bioenergetic membrane. This membrane-shaping organization of the respiratory supercomplex is markedly different from the mammalian homologue, which resides in the flat crista regions. Furthermore, because every crista represents an independent functional compartment35, restriction of the crista diameter by the respiratory supercomplex probably serves to minimize the volume, thereby potentially contributing to higher local concentration of electron carriers, ensuring that proton translocation results in an increased local proton motive force, ultimately optimizing conditions for ATP synthesis. This is consistent with the observation that mutant yeast strains with large, balloon-like cristae display respiratory defects36,37. Thus, our findings show how respiratory supercomplexes together with other factors can organize the architecture of the bioenergetic membrane, providing a mechanism for enabling its functional specialization.
Methods
Purification of T. thermophila supercomplex
T. thermophila cells were grown at 36 °C and harvested as previously described12,20, and cell pellets were resuspended in homogenization buffer (20 mM HEPES-KOH pH 7.5, 350 mM d-mannitol, 5 mM EDTA and 1x protease-inhibitor tablet) and lysed in a Dounce homogenizer on ice. Intact mitochondria were isolated first by differential centrifugation of the lysate; following membrane solubilization, the lysate was cleared at 30,000g for 30 min at 4 °C, and finally, on a discontinuous sucrose gradient with 15%, 23%, 32% and 60% w/v sucrose in buffer SEM (20 mM HEPES-KOH pH 7.5, 250 mM sucrose and 1 mM EDTA) at 14,1371g for 60 min at 4 °C in an SW28 rotor. Intact mitochondria sedimented to the interface between 32% and 60% sucrose and were collected from the gradient, snap-frozen in liquid nitrogen and stored at −80 °C. The isolated mitochondria were lysed in buffer A (25 mM HEPES-KOH pH 7.5, 25 mM KCl, 5 mM MgCl2 and 4% w/v digitonin) for 1 h on ice. This procedure was previously confirmed as a gentle solubilization method. Following mitochondrial membrane solubilization, cleared lysate was placed on a sucrose cushion (25 mM HEPES-KOH pH 7.5, 25 mM KCl, 5 mM MgCl2, 0.1% w/v digitonin and 30% w/v sucrose) in Ti70 tubes and centrifuged at 164,685g for 1 h. The pellet was gently washed and finally resuspended in buffer D (25 mM HEPES-KOH pH 7.5, 25 mM KCl, 5 mM MgCl2 and 0.1% w/v digitonin). Before loading sample material on a size-exclusion chromatography column, larger aggregates were pelleted at 30,000g for 20 min at 4 °C. Cleared sample was loaded on a Superose 6 Increase 3.2/300 column equilibrated in buffer D, collecting elution fractions of 100 µl throughout the run. Peak fractions were used immediately for cryo-grid preparation.
UV–visible difference spectroscopy
The sample obtained from the sucrose cushion step was analysed for haem content and supercomplex composition using UV–visible difference spectroscopy and clear native-PAGE (CN-PAGE) combined with in-gel activity assays. UV–visible difference spectra were recorded between 390 nm and 675 nm using a home-built spectrophotometer. Protein samples were diluted as necessary in 50 mM HEPES and 0.1% digitonin at pH 8.0. Spectra were measured from sodium dithionite-reduced minus air-oxidized spectra. When multiple absorption bands overlapped, spectra were deconvoluted using the peak analysis function in OriginPro 2015 (OriginLab Corporation).
Gel electrophoresis
NativePAGE 3 to 12%, Bis-Tris, 1.0 mm, Mini Protein Gel (Invitrogen) pre-cast gels were used for CN-PAGE. The gels were loaded with a protein ladder (NativeMark, Invitrogen) and four identical sample lanes where the protein samples were mixed with NativePAGE Sample Buffer (final concentration, 50 mM BisTris, 6N HCl, 50 mM NaCl, 10% w/v glycerol and 0.001% Ponceau S, pH 7.2) as per the manufacturer’s instruction. Electrophoresis was conducted at 4 °C, first at 150 V for 30 min with NativePage Light Blue Cathode buffer (50 mM BisTris, 50 mM Tricine, pH 6.8, and 0.002% Coomassie G-250) and then at 250 V for 150 min with NativePAGE Anode Buffer (50 mM BisTris and 50 mM Tricine, pH 6.8). In-gel activity assays were performed following published protocols38. In brief, each sample lane from CN-PAGE was incubated with an aqueous solution to reveal (1) protein bands (0.02% Coomassie G-250, overnight) or the presence of active (2) CI (2 mM Tris pH 7.4, 2.5 mg ml−1 nitrotetrazolium blue chloride (NBT) and 0.1 mg ml−1 NADH, 15 min), (3) CII (5 mM Tris pH 7.4, 2.5 mg ml−1 NBT, 84 mM succinic acid and 0.2 mM phenazine methosulfate, 30–40 min) and (4) CIV (0.05 mM KPi pH 7.4, 0.5 mg ml−1 3,3′-diaminobenzidine (DAB) and 1 mg ml−1 cytochrome c from Saccharomyces cerevisiae, overnight). The reactions were stopped by incubation in 10% (v/v) acetic acid, followed by multiple exchanges of water. The gels shown in this paper are representative of two experiments from two separate supercomplex preparations.
Cryo-EM sample preparation and data collection
Supercomplex eluted at a concentration of approximately 10 mg ml−1. Aliquots of the peak fraction were diluted in buffer D to 0.75 mg ml−1 before applied to cryo-grids. Quantifoil R2/2-300 grids floated with a homemade 3-nm amorphous carbon layer were glow-discharged immediately before applying a 3 µl sample. Grids were vitrified using liquid ethane cooled by liquid nitrogen in a Vitrobot Mark IV, with a 30-s wait time before blotting grids for 3 s at blot force of 0. A total of 26,063 movies were collected on a Titan Krios (Thermo Fisher Scientific) operated at 300 kV at a nominal magnification of ×165,000 (0.83 Å per pixel) with a Quantum K2 camera (Gatan) using a slit width of 20 eV. An objective lens aperture of 70 µm was used, with an exposure rate of 4.26 electrons per pixel per second with 5-s exposure fractionated into 20 frames, and the defocus range was 0.6–2.6 μm.
Cryo-EM data processing
Motion correction was performed in the internal implementation of RELION-3.1 (ref. 39), followed by contrast transfer function estimation by CTFFIND4. Initial rounds of particle picking and 2D classification, followed by ab initio reconstruction, 3D classification and preliminary refinement of the supercomplex. Template-based particle picking in RELION was then used to pick and extract 1,664,103 particles. 2D classification and 3D heterogeneous refinement steps in cryoSPARC v2 (ref. 40) were then used to separate supercomplex particles from copurified ATP synthase, resulting in a final 138,746 supercomplex particles used for subsequent refinement. Following a consensus refinement in cryoSPARC, per-particle contrast transfer function refinement and Bayesian polishing were performed in RELION-3.1. For final refinements in cryoSPARC, particles were downsampled from a 724-pixel box to 480 pixels, resulting in a pixel size of 1.25 Å per pixel. Masked refinements of the respective supercomplex subregions resulted in map resolutions of 2.9 Å for the entire supercomplex, 2.8 Å for CI, 3.0 Å for CII, 2.8 Å for CIII and 2.6 Å for CIV2. Reported map resolutions are according to gold-standard Fourier shell correlation using the 0.143-criterion. To assess flexibility of the Rieske subunit wedged in the CI–CIII2 interface, we performed focused 3D classification in RELION-3.1 using pre-aligned particles with a mask on the extended area around the headgroup of the Rieske subunit. Classification into ten classes resulted in maps confirming flexibility of the structural element, with two classes corresponding closely to the previously reported B state and C state (Fig. 3f,g).
Cryo-electron tomography and subtomogram averaging
Crude mitochondrial pellets were resuspended in an equal volume of buffer containing 20 mM HEPES-KOH pH 7.4, 2 mM EDTA, 250 mM sucrose and mixed in a 1:1 ratio with 5-nm colloidal gold solution (Sigma Aldrich) and vitrified as described above on glow-discharged Quantifoil R2/2 Au 200 mesh grids. Tilt series were acquired on a Titan Krios operated at 300 kV with a K3 camera (slit width of 20 eV) using SerialEM or the EPU software (Thermo Fisher Scientific). Mitochondrial membranes were imaged at a nominal magnification of ×42,000 (2.11 Å per pixel) and an exposure rate of 19.5 electrons per pixel per second with a 3 e− Å−2 exposure per tilt fractionated into five frames, with tilt series acquired using the exposure-symmetric scheme41 to ±60° tilt and a 3° tilt increment. Following motion correction in motionCor2, tomographic reconstruction from the tilt series was performed in IMOD42 using phase-flipping and a binning factor of 2. Tomograms were contrast enhanced using nonlinear anisotropic diffusion filtering to facilitate manual particle picking of supercomplex particles based on the matrix arm of CI. Subtomogram averaging was performed in PEET43. Initial references were generated from the data by averaging after rotating subvolumes into a common orientation with respect to the membrane based on manually assigned vectors. Following initial rounds of averaging to generate a suitable reference, data were manually split into half-sets and refined independently, following low-pass filtering to 50 Å. Averaging of 360 particles from 12 tomograms resulted in a 28 Å subtomogram average.
Model building and refinement
Manual model building was performed in Coot44 and new subunits identified directly for the cryo-EM map. For identified canonical subunits, homology models were generated using SWISS-MODEL. Bound cardiolipins were unambiguously identified from their head group density. Other natively bound lipids were tentatively modelled as phosphatidylcholine, phosphatidylethanolamine or phosphatidic acid based on head group densities. Real-space refinement of atomic models was performed in PHENIX using secondary structure restraints45. Atomic model statistics were calculated using MolProbity46.
Given the mild solubilization conditions we used, for CIII2, the cryo-EM map showed density located on the pseudo-C2 symmetry axis between the two COB haem bL molecules, displaying planar map features consistent with the quinone moiety of UQ. The density clearly indicates that UQ can bind in two orientations, related by the symmetry rotation of the dimer. In either orientation, the quinone moiety is positioned close to a haem bL, where it potentially could accept electrons for transfer across the dimer axis. In the recent amphipol CIII2 structure8, the isoprenoid tail of UQ was modelled in the equivalent position; however, the planar density for the quinone was missing. This orientation-equivalent binding of UQ between the two COB haem bL molecules, together with the B-state and C-state Rieske conformations, suggest a maintained functional symmetry of ciliate CIII2 within the supercomplex.
In CI, we identified 49 canonical subunits and 21 subunits that we assign as phylum-specific. In each CIV monomer, we identified 11 subunits homologous to mammalian CIV (COX1, COX2, COX3a, COX3b, COX5B, COX6A, COX6B, COX6C, COX7A, COX7C and NDUFA4) and 42 ciliate-specific subunits, most of which are peripherally associated around the mitochondrial protein core. Three of the mammalian subunits missing in T. thermophila CIV (COX4, COX7B and COX8) are at the interface where two mitochondrial carriers are bound. The mitochondrially encoded core subunit COX3 is split into two fragments. Most of the TM helices are contributed by the C-terminal of COX3b, which is encoded by the mitochondrial ymf68 gene. The newly annotated Ymf68 is structurally conserved, apart from the missing helix (H1), which is structurally replaced by Ymf67. We therefore assign ymf67 and ymf68 of the ciliate mitochondrial genome as separately encoding the COX3a and COX3b subunit fragments, respectively. On the T. thermophila mitochondrial genome, ymf67 and ymf68 genes are located on the same strand, but are separated by the gene encoding tRNATrp, suggesting that a transposition event may have led to the fragmentation of the original gene encoding COX3. tRNA genes are known to be among the most motile elements in metazoan mitochondrial DNA. Both COX3a and COX3b fragments have evolved substantial subunit extensions that thread through the augmented CIV monomer unit to recruit lineage-specific subunits and mediate supercomplex assembly.
In the CIV dimer, the dimer interface of 17,000 Å2 is dominated by 16 species-specific subunits. Furthermore, when aligned on the CIV core, a comparison of the mammalian and ciliate structures reveals that the two dimers display markedly different architectures, dimer axes and distances between COX1 cores. This suggests that the dimerization of the ciliate CIV probably evolved through the acquisition of lineage-specific subunits and reflects the constraints of the unique tubular membrane environment.
In addition, each CIV monomer complex contains two different Surf1-like proteins, which were reported to complement defects causing Leigh syndrome in humans. In our structure, two Surf1-like proteins are permanently attached to CIV and display similar overall structures, consisting of a lumen-exposed soluble domain and a TM helix hairpin. The two Surf1 proteins are facing each other, bound on opposite sides of each CIV core.
The presence of subunit extension and accessory subunits in CIV generates a pronounced cavity around the cytochrome c-binding site. However, overlaying of a T. thermophila cytochrome c homology model suggests that the canonical binding site is not obstructed. Cytochrome c binding is known to be driven by electrostatic interactions with the CuA domain of COX2, which forms a negatively charged patch in mammals. This structural feature is positively charged in T. thermophila, interacting mainly with H1 of cytochrome c, which displays a flipped polarity. We conclude that the experimentally observed functional incompatibility of T. thermophila CIV and mammalian cytochrome c is not due to divergent architecture, but to an inverted surface charge of the binding pocket.
Molecular dynamics simulations
We performed coarse-grained (CG) molecular dynamics simulations on the entire T. thermophila supercomplex structure using Martini3 forcefield47 to study the rearrangement of the lipid bilayer around the highly bent protein assembly. Using the martinize2 (version 2.6) tool, we transformed the atomistic structure into a CG model (atoms clustered into Martini beads)48. Using the small molecules database and the existing topologies of phospholipids available in the Martini3 forcefield47, we generated the force-field parameters for cardiolipin. Cofactors and resolved lipids in the structure were not included in the simulated model system, and only protein was simulated to study the dynamics of lipid molecules around it. First, the CG model of the protein structure was minimized for 100 steps in vacuum to remove possible steric clashes. Then, the minimized CG supercomplex was embedded in a large (75 nm × 75 nm) hybrid membrane slab (POPE:POPC:CL in 4:2:1 ratio) using the insane.py script49. The CG protein–membrane system was solvated using standard Martini3 water beads and 100 mM Na+ and Cl− ions. Starting from this initial position, the simulation system was minimized keeping all beads free, first in double precision to resolve steric clashes between the lipids (maximum of 500 steps) and then in regular single precision (maximum of 10,000 steps). After minimization, with 4,000 kJ mol−1 nm−2 harmonic constraints on the backbone beads, the system was equilibrated using velocity-rescaling thermostat50 and Berendsen barostat51 for 10 ns. During production runs, the 4,000 kJ mol−1 nm−2 harmonic constraints on the backbone beads were maintained. Velocity-rescaling thermostat50 and Parrinello–Rahman barostat52 were used for temperature (310 K) and pressure (1 bar) control in the production phase. Coulombic interactions were treated with the reaction-field algorithm using εr = 15 (ref. 53). The Verlet cut-off scheme was implemented with a Lennard–Jones cut-off of 1.1 nm (ref. 54). The time step of the CG molecular dynamics simulations was 20 fs. Initial simulation replicas showed incomplete or unstable wrapping of the membrane around the protein, so we translated the lipid bilayer patch in z-direction and altered the insertion angle of the supercomplex to find an initial position that allowed the membrane to equilibrate and wrap fully around the protein (Systems T1-T7, simulation lengths of 0.9–2.8 µs, total of 9.6 µs). After finding the correct insertion of the protein into the membrane, we initiated three independent simulation replicas (Systems P1-P3, simulation lengths of 10 µs each, total of 30 µs). The simulations were performed using the Gromacs software (version 2021)55. In addition to molecular dynamics simulations of the entire supercomplex in the lipid bilayer, we also performed molecular dynamics simulations of the pure lipid bilayer (see above; three replicas, 10 µs each) and of CIV2, CI–CIII2 and CI–CIII2–CIV2 subcomplexes (1–3 replicas, 1–5 µs each).
Data visualization and analysis
Images and videos were rendered using ChimeraX56 and Visual Molecular Dynamics57. To analyse the T. thermophila cytochrome c-binding site, the mammalian cytochrome c-bound CIV structure (Protein Data Bank ID: 5IY5) was overlaid to the T. thermophila structure. Using AlphaFold2 (ref. 58), a T. thermophila cytochrome c structure was predicted and overlaid to both the mammalian structure and the T. thermophila structures. The composite map of the complete respiratory supercomplex was generated in ChimeraX56. This map was only used for visualization, and not for atomic model refinement; instead, a consensus map was used. The buried areas of the CI–CII–CIII2–CIV2 supercomplex and the CIV2 interface were calculated in ChimeraX56.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-05817-y.
Supplementary information
This file contains Supplementary Table 1: Data collection and model statistics; Supplementary Table 2: List of proteins and comments; and Source Data for Extended Data Fig. 2.
Coarse-grained molecular dynamics simulation of the T. thermophila supercomplex. First 800 ns of the molecular dynamics simulation starting from an initially planar membrane reveals a deformation of the bilayer into a curved topology to accommodate the membrane protein complex.
Coarse-grained molecular dynamics simulation of pure lipid bilayer. Membrane shows fluctuations, however, a stable curved architecture (as in the case of supercomplex) is not observed.
Annular lipid shell of the T. thermophila supercomplex. Final frame of the coarse-grained molecular dynamics simulation with supercomplex and surrounding annular lipids shown, highlighting the curved shape of the lipid belt.
Acknowledgements
We thank SciLifeLab EM facility (funded by the KAW, EPS and Kempe foundations), the Center for Scientific Computing, Finland for high-performance supercomputing resources, the EMBO Young Investigator Program, the Swedish Foundation for Strategic Research (FFL15:0325), the Ragnar Söderberg Foundation (M44/16), Cancerfonden (2017/1041), the European Research Council (ERC-2018-StG-805230) and the Knut and Alice Wallenberg Foundation (2018.0080). V.S. was supported by the Academy of Finland, the Sigrid Jusélius Foundation, the Jane and Aatos Erkko Foundation, the Magnus Ehrnrooth Foundation and the University of Helsinki. A.Maréchal was supported by the Medical Research Council (CDA MR/M00936X/1, transition support MR/T032154/1). We also thank M. Kozlov, B. Bruininks, W. Kulig, P. C. T. Souza and M. Girych for discussions on molecular dynamics simulations.
Extended data figures and tables
Source data
Author contributions
A. Mühleip, R.K.F. and A.A. designed the project. V.T. performed cell culturing and isolation of mitochondria. A. Mühleip and R.K.F. prepared the sample and collected cryo-EM data. A. Mühleip, R.K.F. and R.B. processed cryo-EM data and built the model. A.Mühleip performed cryo-electron tomography and subtomogram averaging. O.H. and V.S. performed molecular dynamics simulations. T.G., A. Maréchal and A.A. performed biochemical and spectroscopic analyses. A. Mühleip, R.K.F. and A.A. wrote the manuscript with contributions from O.H., V.S. and A.Maréchal. All authors contributed to revising the manuscript.
Peer review
Peer review information
Nature thanks Adam Frost and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Funding
Open access funding provided by Stockholm University.
Data availability
The atomic coordinates were deposited in the Protein Data Bank (PDB) under accession numbers 8BQS (supercomplex), 8B6F (CI), 8B6G (CII), 8B6H (CIV) and 8B6J (CIII). The cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under the respective accession numbers: EMD-16184, EMD-15865, EMD-15866, EMD-15867 and EMD-15868. The subtomogram averages have been deposited under EMD-15900. The atomic coordinates that were used in this study are: 1NTZ (cytochrome bc1), 5IY5 (cytochrome c) and 5J4Z (ovine supercomplexes). The full versions of all gels are provided in the source file. An Excel file containing the visible absorption spectroscopy data and their analysis has been added. All the data will be publicly available. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Alexander Mühleip, Rasmus Kock Flygaard
Extended data
is available for this paper at 10.1038/s41586-023-05817-y.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-05817-y.
References
- 1.Schägger H, Pfeiffer K. Supercomplexes in the respiratory chains of yeast and mammalian mitochondria. EMBO J. 2000;19:1777–1783. doi: 10.1093/emboj/19.8.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Caruana NJ, Stroud DA. The road to the structure of the mitochondrial respiratory chain supercomplex. Biochem. Soc. Trans. 2020;48:621–629. doi: 10.1042/BST20190930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bezawork-Geleta A, Rohlena J, Dong L, Pacak K, Neuzil J. Mitochondrial complex II: at the crossroads. Trends Biochem. Sci. 2017;42:312–325. doi: 10.1016/j.tibs.2017.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Acin-Perez R, Fernandez-Silva P, Peleato ML, Perez-Martos A, Enriquez JA. Respiratory active mitochondrial supercomplexes. Mol. Cell. 2008;32:529–539. doi: 10.1016/j.molcel.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 5.Jiang C, et al. Regulation of mitochondrial respiratory chain complex levels, organization, and function by arginyltransferase 1. Front. Cell Dev. Biol. 2020;8:603688. doi: 10.3389/fcell.2020.603688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lapuente-Brun E, et al. Supercomplex assembly determines electron flux in the mitochondrial electron transport chain. Science. 2013;340:1567–1570. doi: 10.1126/science.1230381. [DOI] [PubMed] [Google Scholar]
- 7.Schon EA, Dencher NA. Heavy breathing: energy conversion by mitochondrial respiratory supercomplexes. Cell Metab. 2009;9:1–3. doi: 10.1016/j.cmet.2008.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Zhou L, Maldonado M, Padavannil A, Guo F, Letts JA. Structures of Tetrahymena’s respiratory chain reveal the diversity of eukaryotic core metabolism. Science. 2022;376:831–839. doi: 10.1126/science.abn7747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colina-Tenorio L, Horten P, Pfanner N, Rampelt H. Shaping the mitochondrial inner membrane in health and disease. J. Intern. Med. 2020;287:645–664. doi: 10.1111/joim.13031. [DOI] [PubMed] [Google Scholar]
- 10.Blum TB, Hahn A, Meier T, Davies KM, Kühlbrandt W. Dimers of mitochondrial ATP synthase induce membrane curvature and self-assemble into rows. Proc. Natl Acad. Sci. USA. 2019;116:4250–4255. doi: 10.1073/pnas.1816556116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mühleip A, McComas SE, Amunts A. Structure of a mitochondrial ATP synthase with bound native cardiolipin. eLife. 2019;8:e51179. doi: 10.7554/eLife.51179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kock Flygaard R, Mühleip A, Tobiasson V, Amunts A. Type III ATP synthase is a symmetry-deviated dimer that induces membrane curvature through tetramerization. Nat. Commun. 2020;11:5342. doi: 10.1038/s41467-020-18993-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pinke G, Zhou L, Sazanov LA. Cryo-EM structure of the entire mammalian F-type ATP synthase. Nat. Struct. Mol. Biol. 2020;27:1077–1085. doi: 10.1038/s41594-020-0503-8. [DOI] [PubMed] [Google Scholar]
- 14.Mühleip A, et al. ATP synthase hexamer assemblies shape cristae of Toxoplasma mitochondria. Nat. Commun. 2021;12:120. doi: 10.1038/s41467-020-20381-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mühleip AW, et al. Helical arrays of U-shaped ATP synthase dimers form tubular cristae in ciliate mitochondria. Proc. Natl Acad. Sci. USA. 2016;113:8442–8447. doi: 10.1073/pnas.1525430113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Allen RD, Schroeder CC, Fok AK. An investigation of mitochondrial inner membranes by rapid-freeze deep-etch techniques. J. Cell Biol. 1989;108:2233–2240. doi: 10.1083/jcb.108.6.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu J, et al. The architecture of the mammalian respirasome. Nature. 2016;537:639–643. doi: 10.1038/nature19359. [DOI] [PubMed] [Google Scholar]
- 18.Guo R, Zong S, Wu M, Gu J, Yang M. Architecture of human mitochondrial respiratory megacomplex I2III2IV2. Cell. 2017;170:1247–1257.e12. doi: 10.1016/j.cell.2017.07.050. [DOI] [PubMed] [Google Scholar]
- 19.Klusch N, Senkler J, Yildiz O, Kühlbrandt W, Braun HP. A ferredoxin bridge connects the two arms of plant mitochondrial complex I. Plant Cell. 2021;33:2072–2091. doi: 10.1093/plcell/koab092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tobiasson V, Amunts A. Ciliate mitoribosome illuminates evolutionary steps of mitochondrial translation. eLife. 2020;9:e59264. doi: 10.7554/eLife.59264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tobiasson, V., Berzina, I. & Amunts, A. Structure of a mitochondrial ribosome with fragmented rRNA in complex with membrane-targeting elements. Nat. Comm.13, 6132 (2022). [DOI] [PMC free article] [PubMed]
- 22.Hagerhall C. Succinate: quinone oxidoreductases. Variations on a conserved theme. Biochim. Biophys. Acta. 1997;1320:107–141. doi: 10.1016/S0005-2728(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 23.Sun F, et al. Crystal structure of mitochondrial respiratory membrane protein complex II. Cell. 2005;121:1043–1057. doi: 10.1016/j.cell.2005.05.025. [DOI] [PubMed] [Google Scholar]
- 24.Balabaskaran Nina P, et al. Highly divergent mitochondrial ATP synthase complexes in Tetrahymena thermophila. PLoS Biol. 2010;8:e1000418. doi: 10.1371/journal.pbio.1000418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berndtsson J, et al. Respiratory supercomplexes enhance electron transport by decreasing cytochrome c diffusion distance. EMBO Rep. 2020;21:e51015. doi: 10.15252/embr.202051015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tremmel IG, Kirchhoff H, Weis E, Farquhar GD. Dependence of plastoquinol diffusion on the shape, size, and density of integral thylakoid proteins. Biochim. Biophys. Acta. 2003;1607:97–109. doi: 10.1016/j.bbabio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 27.Kirchhoff H. Diffusion of molecules and macromolecules in thylakoid membranes. Biochim. Biophys. Acta. 2014;1837:495–502. doi: 10.1016/j.bbabio.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 28.Letts JA, Fiedorczuk K, Sazanov LA. The architecture of respiratory supercomplexes. Nature. 2016;537:644–648. doi: 10.1038/nature19774. [DOI] [PubMed] [Google Scholar]
- 29.Althoff T, Mills DJ, Popot JL, Kuhlbrandt W. Arrangement of electron transport chain components in bovine mitochondrial supercomplex I1III2IV1. EMBO J. 2011;30:4652–4664. doi: 10.1038/emboj.2011.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu M, Gu J, Guo R, Huang Y, Yang M. Structure of mammalian respiratory supercomplex I1III2IV1. Cell. 2016;167:1598–1609.e10. doi: 10.1016/j.cell.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 31.Gao X, et al. Structural basis for the quinone reduction in the bc1 complex: a comparative analysis of crystal structures of mitochondrial cytochrome bc1 with bound substrate and inhibitors at the Qi site. Biochemistry. 2003;42:9067–9080. doi: 10.1021/bi0341814. [DOI] [PubMed] [Google Scholar]
- 32.Zhang Z, et al. Electron transfer by domain movement in cytochrome bc1. Nature. 1998;392:677–684. doi: 10.1038/33612. [DOI] [PubMed] [Google Scholar]
- 33.Rajagukguk S, et al. Effect of mutations in the cytochrome b ef loop on the electron-transfer reactions of the Rieske iron–sulfur protein in the cytochrome bc1 complex. Biochemistry. 2007;46:1791–1798. doi: 10.1021/bi062094g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen, M. et al. Distinct structural modulation of photosystem I and lipid environment stabilizes its tetrameric assembly. Nat. Plants6, 314–320 (2020). [DOI] [PubMed]
- 35.Wolf DM, et al. Individual cristae within the same mitochondrion display different membrane potentials and are functionally independent. EMBO J. 2019;38:e101056. doi: 10.15252/embj.2018101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Davies KM, et al. Macromolecular organization of ATP synthase and complex I in whole mitochondria. Proc. Natl Acad. Sci. USA. 2011;108:14121–14126. doi: 10.1073/pnas.1103621108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paumard P, et al. The ATP synthase is involved in generating mitochondrial cristae morphology. EMBO J. 2002;21:221–230. doi: 10.1093/emboj/21.3.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jha P, Wang X, Auwerx J. Analysis of mitochondrial respiratory chain supercomplexes using blue native polyacrylamide gel electrophoresis (BN-PAGE) Curr. Protoc. Mouse Biol. 2016;6:1–14. doi: 10.1002/9780470942390.mo150182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zivanov J, et al. New tools for automated high-resolution cryo-EM structure determination in RELION-3. eLife. 2018;7:e42166. doi: 10.7554/eLife.42166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Punjani A, Rubinstein JL, Fleet DJ, Brubaker MA. cryoSPARC: algorithms for rapid unsupervised cryo-EM structure determination. Nat. Methods. 2017;14:290–296. doi: 10.1038/nmeth.4169. [DOI] [PubMed] [Google Scholar]
- 41.Hagen WJH, Wan W, Briggs JAG. Implementation of a cryo-electron tomography tilt-scheme optimized for high resolution subtomogram averaging. J. Struct. Biol. 2017;197:191–198. doi: 10.1016/j.jsb.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kremer JR, Mastronarde DN, McIntosh JR. Computer visualization of three-dimensional image data using IMOD. J. Struct. Biol. 1996;116:71–76. doi: 10.1006/jsbi.1996.0013. [DOI] [PubMed] [Google Scholar]
- 43.Nicastro D, et al. The molecular architecture of axonemes revealed by cryoelectron tomography. Science. 2006;313:944–948. doi: 10.1126/science.1128618. [DOI] [PubMed] [Google Scholar]
- 44.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr. D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 45.Afonine PV, et al. Real-space refinement in PHENIX for cryo-EM and crystallography. Acta Crystallogr. D. 2018;74:531–544. doi: 10.1107/S2059798318006551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen VB, et al. MolProbity: all-atom structure validation for macromolecular crystallography. Acta Crystallogr. D. 2010;66:12–21. doi: 10.1107/S0907444909042073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Souza PCT, et al. Martini 3: a general purpose force field for coarse-grained molecular dynamics. Nat. Methods. 2021;18:382–388. doi: 10.1038/s41592-021-01098-3. [DOI] [PubMed] [Google Scholar]
- 48.de Jong DH, et al. Improved parameters for the Martini coarse-grained protein force field. J. Chem. Theory Comput. 2013;9:687–697. doi: 10.1021/ct300646g. [DOI] [PubMed] [Google Scholar]
- 49.Wassenaar TA, Ingolfsson HI, Bockmann RA, Tieleman DP, Marrink SJ. Computational lipidomics with insane: a versatile tool for generating custom membranes for molecular simulations. J. Chem. Theory Comput. 2015;11:2144–2155. doi: 10.1021/acs.jctc.5b00209. [DOI] [PubMed] [Google Scholar]
- 50.Bussi G, Donadio D, Parrinello M. Canonical sampling through velocity rescaling. J. Chem. Phys. 2007;126:014101. doi: 10.1063/1.2408420. [DOI] [PubMed] [Google Scholar]
- 51.Berendsen HJC, Postma JPM, Vangunsteren WF, Dinola A, Haak JR. Molecular-dynamics with coupling to an external bath. J. Chem. Phys. 1984;81:3684–3690. doi: 10.1063/1.448118. [DOI] [Google Scholar]
- 52.Parrinello M, Rahman A. Polymorphic transitions in single-crystals—a new molecular-dynamics method. J. Appl. Phys. 1981;52:7182–7190. doi: 10.1063/1.328693. [DOI] [Google Scholar]
- 53.Tironi IG, Sperb R, Smith PE, Van Gunsteren WF. A generalized reaction field method for molecular-dynamics simulations. J. Chem. Phys. 1995;102:5451–5459. doi: 10.1063/1.469273. [DOI] [Google Scholar]
- 54.Grubmüller H, Heller H, Windemuth A, Schulten K. Generalized Verlet algorithm for efficient molecular dynamics simulations with long-range interactions. Mol. Simul. 1991;6:121–142. doi: 10.1080/08927029108022142. [DOI] [Google Scholar]
- 55.Abraham MJ, et al. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/j.softx.2015.06.001. [DOI] [Google Scholar]
- 56.Goddard TD, et al. UCSF ChimeraX: meeting modern challenges in visualization and analysis. Protein Sci. 2018;27:14–25. doi: 10.1002/pro.3235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Humphrey W, Dalke A, Schulten K. VMD: visual molecular dynamics. J. Mol. Graphics. 1996;14:33–38. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 58.Jumper J, et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
This file contains Supplementary Table 1: Data collection and model statistics; Supplementary Table 2: List of proteins and comments; and Source Data for Extended Data Fig. 2.
Coarse-grained molecular dynamics simulation of the T. thermophila supercomplex. First 800 ns of the molecular dynamics simulation starting from an initially planar membrane reveals a deformation of the bilayer into a curved topology to accommodate the membrane protein complex.
Coarse-grained molecular dynamics simulation of pure lipid bilayer. Membrane shows fluctuations, however, a stable curved architecture (as in the case of supercomplex) is not observed.
Annular lipid shell of the T. thermophila supercomplex. Final frame of the coarse-grained molecular dynamics simulation with supercomplex and surrounding annular lipids shown, highlighting the curved shape of the lipid belt.
Data Availability Statement
The atomic coordinates were deposited in the Protein Data Bank (PDB) under accession numbers 8BQS (supercomplex), 8B6F (CI), 8B6G (CII), 8B6H (CIV) and 8B6J (CIII). The cryo-EM maps have been deposited in the Electron Microscopy Data Bank (EMDB) under the respective accession numbers: EMD-16184, EMD-15865, EMD-15866, EMD-15867 and EMD-15868. The subtomogram averages have been deposited under EMD-15900. The atomic coordinates that were used in this study are: 1NTZ (cytochrome bc1), 5IY5 (cytochrome c) and 5J4Z (ovine supercomplexes). The full versions of all gels are provided in the source file. An Excel file containing the visible absorption spectroscopy data and their analysis has been added. All the data will be publicly available. Source data are provided with this paper.