Abstract
Optimal growth and development in childhood and adolescence is crucial for lifelong health and well-being1–6. Here we used data from 2,325 population-based studies, with measurements of height and weight from 71 million participants, to report the height and body-mass index (BMI) of children and adolescents aged 5–19 years on the basis of rural and urban place of residence in 200 countries and territories from 1990 to 2020. In 1990, children and adolescents residing in cities were taller than their rural counterparts in all but a few high-income countries. By 2020, the urban height advantage became smaller in most countries, and in many high-income western countries it reversed into a small urban-based disadvantage. The exception was for boys in most countries in sub-Saharan Africa and in some countries in Oceania, south Asia and the region of central Asia, Middle East and north Africa. In these countries, successive cohorts of boys from rural places either did not gain height or possibly became shorter, and hence fell further behind their urban peers. The difference between the age-standardized mean BMI of children in urban and rural areas was <1.1 kg m–2 in the vast majority of countries. Within this small range, BMI increased slightly more in cities than in rural areas, except in south Asia, sub-Saharan Africa and some countries in central and eastern Europe. Our results show that in much of the world, the growth and developmental advantages of living in cities have diminished in the twenty-first century, whereas in much of sub-Saharan Africa they have amplified.
Subject terms: Public health, Paediatric research, Developing world, Nutrition
The advantage of living in cities compared with rural areas with respect to height and BMI in children and adolescents has generally become smaller globally from 1990 to 2020, except in sub-Saharan Africa.
Main
The growth and development of school-aged children and adolescents (ages 5–19 years) are influenced by their nutrition and environment at home, in the community and at school. Healthy growth and development at these ages help consolidate gains and mitigate inadequacies from early childhood and vice versa1, with lifelong implications for health and well-being2–6. Until recently, the growth and development of older children and adolescents received substantially less attention than in early childhood and adulthood7. Increasing attention on the importance of health and nutrition during school years has been accompanied by a presumption that differences in nutrition and the environment lead to distinct, and generally less healthy, patterns of growth and development at these ages in cities compared to rural areas8–17. This presumption is despite some empirical studies showing that food quality and nutrition are better in cities18,19.
Data on growth and developmental outcomes during school ages are needed, alongside data on the efficacy of specific interventions and policies, to select and prioritize policies and programmes that promote health and health equity, both for the increasing urban population and for children who continue to grow up in rural areas. Consistent and comparable global data also help benchmark across countries and territories and draw lessons on good practice. Yet, globally, there are fewer data on growth trajectories in rural and urban areas in these formative ages than for children under 5 years of age20 or for adults21. The available studies have been in one country, at one point in time and/or in one sex and narrow age groups. The few studies that covered more than one country22–24 mostly focused on older girls and used at most a few dozen data sources and hence could not systematically measure long-term trends. Consequently, many policies and programmes that aim to enhance healthy growth and development in school ages focus narrowly and generically on specific features of nutrition or the environment in either cities or rural areas10,13,25–28. Little attention has been paid to the similarities and differences between relevant outcomes in these settings or to the heterogeneity of the urban–rural differences across countries.
Here we report on the mean height and BMI of school-aged children and adolescents residing in rural and urban areas of 200 countries and territories (referred to as countries hereafter) from 1990 to 2020. Height and BMI are anthropometric measures of growth and development that are influenced by the quality of nutrition and healthiness of the living environment and are highly predictive of health and well-being throughout life in observational and Mendelian randomization studies2–6. These studies have shown that having low height and excessively low BMI increases the risk of morbidity and mortality, and low height impairs cognitive development and reduces educational performance and work productivity in later life2–4. A high BMI in these ages increases the lifelong risk of overweight and obesity and several non-communicable diseases, and might contribute to poor educational outcomes5,6.
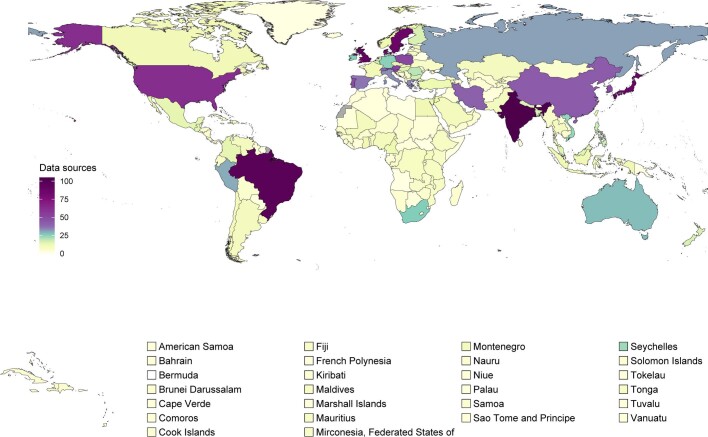
We used 2,325 population-based studies that measured height and weight in 71 million participants in 194 countries (Extended Data Fig. 1 and Supplementary Table 2). We used these data in a Bayesian hierarchical meta-regression model to estimate mean height and BMI of children and adolescents aged 5–19 years by rural and urban place of residence, year and age for 200 countries. Details of data sources and statistical methods are provided in the Methods. Our results represent the height and BMI for children and adolescents of the same age over time (that is, successive cohorts) in rural and urban areas of each country, and the difference between the two. For presentation, we summarize the 15 age-specific estimates, for single years of age from 5 to 19, through age standardization, which puts each country-year’s child and adolescent population on the same age distribution and enables comparisons to be made over time and across countries. We also show results, graphically and numerically, for index ages of 5, 10, 15 and 19 years in the Supplementary Information.
Extended Data Fig. 1. Number of data sources used in the analysis, by country.
.
In 1990, school-aged boys and girls who lived in cities had a height advantage (that is, were taller) compared with their rural counterparts. The exception was in high-income countries, where the urban height advantage was either negligible (<1.2 cm for age-standardized mean height; posterior probability (PP) for children living in urban areas being taller ranging from 0.51 to >0.99) or there was a small rural advantage (for example, Belgium, the Netherlands and the United Kingdom) (PP for children in rural areas being taller ranging from 0.53 to >0.95 where there was a rural height advantage) (Fig. 1 and Extended Data Fig. 2). The largest height differences between children and adolescents in cities and rural areas in 1990 occurred in some countries in Latin America (for example, Mexico, Guatemala, Panama and Peru), east and southeast Asia (China, Indonesia and Vietnam), central and eastern Europe (Bulgaria, Hungary and Romania) and sub-Saharan Africa (Democratic Republic of Congo (DR Congo) and Rwanda). The urban height advantage in boys and girls in the named countries ranged from 2.4 to 5.0 cm, and the PP of children living in urban areas being taller than children living in rural areas was >0.99 (see Supplementary Table 3 for country-specific numerical values of height in children living in rural versus urban areas, their difference and the corresponding credible intervals (CrIs)).
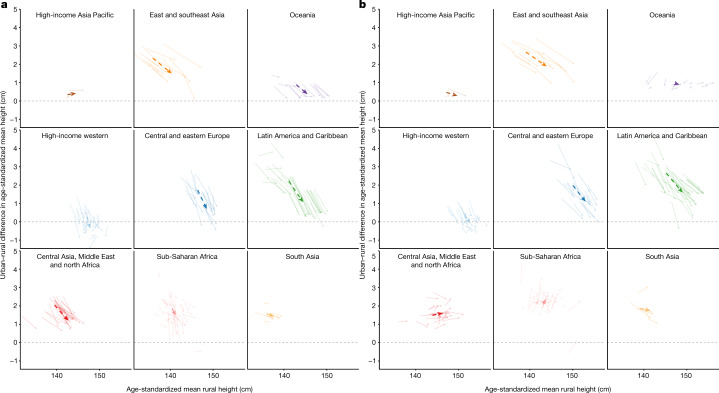
Fig. 1. Change in the urban–rural height difference from 1990 to 2020.
a,b, Change in the urban–rural difference in age-standardized mean height in relation to the change in age-standardized mean rural height in girls (a) and boys (b). Each solid arrow in lighter shade shows one country beginning in 1990 and ending in 2020. The dashed arrows in darker shade show the regional averages, calculated as the unweighted arithmetic mean of the values for all countries in each region along the horizontal and vertical axes. For the urban–rural difference, a positive number shows a higher urban mean height and a negative number shows higher rural mean height. See Extended Data Fig. 2 for urban–rural differences in age-standardized mean height and their change over time shown as maps, together with uncertainties in the estimates. See Supplementary Fig. 4a for results at ages 5, 10, 15 and 19 years. We did not estimate the difference between rural and urban height for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau).
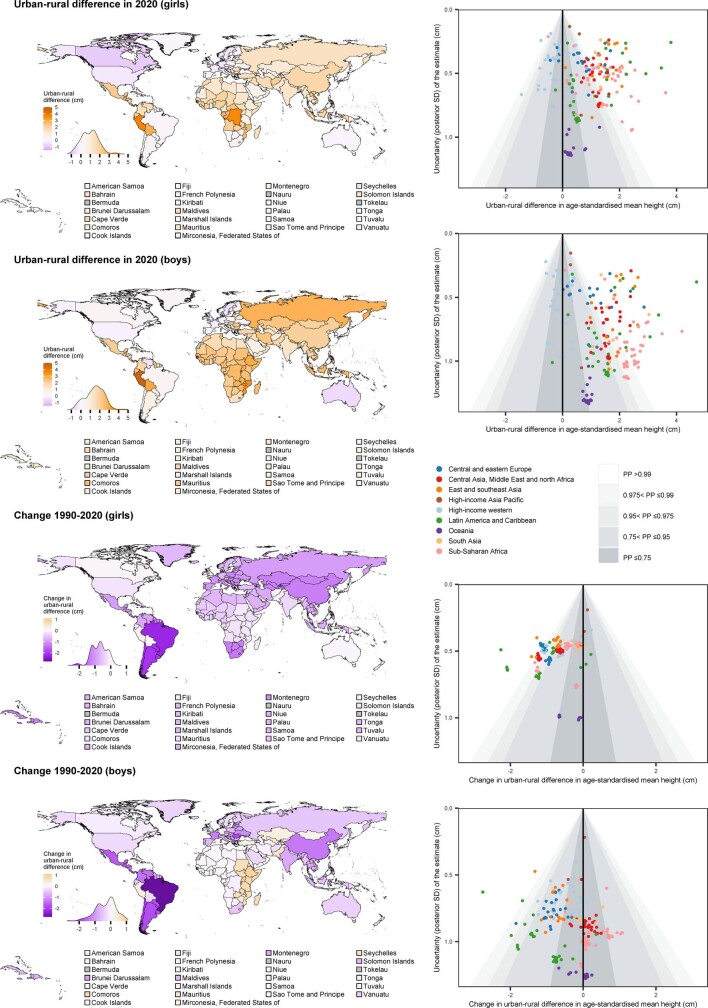
Extended Data Fig. 2. Urban-rural height difference in 2020 and change from 1990 to 2020.
The top two maps show the urban-rural difference in age-standardised mean height in 2020 for girls and boys resepectively. A positive number shows higher urban mean height and a negative number shows higher rural mean height. The bottom two maps show the change from 1990 to 2020. The density plot below each map shows the distribution of estimates across countries. The top two scatter plots show the urban-rural difference in age-standardised mean height in relation to the uncertainty of the difference measured by posterior s.d. The bottom two scatter plots show the change from 1990 to 2020 in urban-rural difference in mean height in relation to the uncertainty of the change measured by posterior s.d. Each point in the scatter plots shows one country. Shaded areas approximately show the posterior probability (PP) of a true difference (top two scatter plots) and of a true increase or decrease in difference (bottom two scatter plots). See Extended Data Fig. 8 for PPs of the urban-rural difference in age-standardised mean height and its change. See Supplementary Fig. 7 for results at ages 5, 10, 15 and 19 years. We did not estimate the difference between rural and urban height for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau), as indicated in grey.
The urban–rural height gap in the late twentieth century among low-income and middle-income countries was determined by how much children and adolescents in cities and rural areas had approached as opposed to fallen behind their peers in high-income countries, where there was little difference between urban and rural height. In countries such as Bulgaria, Hungary and Romania, the height of children and adolescents living in urban areas approached that of high-income countries, whereas children and adolescents in rural areas lagged behind, leading to a relatively large gap. In much of sub-Saharan Africa and south Asia, the height of children and adolescents lagged behind their peers in high-income countries regardless of where they lived, such that the urban–rural gap was relatively small. In a third group of low-income or middle-income countries that included Indonesia, Vietnam, Panama, Peru, DR Congo and Rwanda, children living in urban areas remained shorter than in high-income countries, but children from rural areas lagged even further behind, such that the urban–rural gap became large.
By 2020, the urban height advantage in school ages became smaller in much of the world. In many high-income western countries and some central European countries, it disappeared or reversed into a small (typically <1 cm) urban disadvantage (Fig. 1 and Extended Data Figs. 2 and 8). Countries with substantial convergence over these three decades were in central and eastern Europe (for example, Croatia), Latin America and the Caribbean (for example, Argentina, Brazil, Chile and Paraguay), east and southeast Asia (for example, Taiwan) and for girls in central Asia (for example, Kazakhstan and Uzbekistan). The urban height advantage in the named countries declined by around 1–2.5 cm from 1990 to 2020 (the PP of urban–rural height difference having declined ≥0.90 for the named countries). In many other middle-income countries (for example, China, Romania and Vietnam), the urban–rural height gaps declined, but children and adolescents living in cities remained taller than their rural counterparts (by 1.7–2.5 cm in the named countries for boys and girls; the PP of children in cities being taller than children in rural areas in 2020 >0.99). The exception to this convergence was for boys in most countries in sub-Saharan Africa and some countries in Oceania, south Asia and the region of central Asia, Middle East and north Africa, where the urban height advantage slightly increased over these three decades. The largest increase in the urban height advantage for boys occurred in countries in east Africa such as Ethiopia (0.9 cm larger height gap in 2020 than 1990; 95% CrI −0.9 to 2.9, and PP of an increase of 0.86), Rwanda (1.0 cm larger gap, 95% CrI −0.7 to 3.0, and PP 0.88) and Uganda (1.1 cm larger gap, 95% CrI of −0.6 to 3.1, and PP 0.89). For girls, the urban–rural gap remained largely unchanged in many countries in sub-Saharan Africa and south Asia.
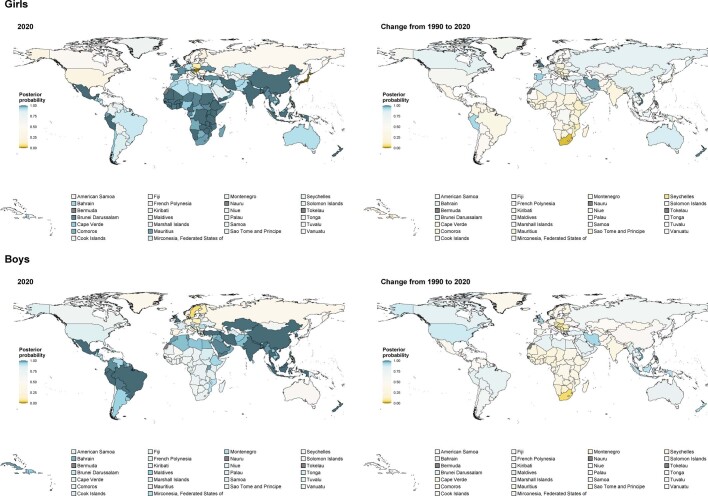
Extended Data Fig. 8. Posterior probability of urban-rural height difference in 2020 and its increase from 1990 to 2020.
The maps show the posterior probability (PP) that age-standardised mean height in 2020 in urban areas was higher than in rural areas (left-hand panels), and the PP that the urban-rural difference in age-standardised mean height increased from 1990 to 2020 (right-hand panels). For 2020, if estimated age-standardised mean urban height is statistically indistinguishable from rural height, the PP is 0.50. PPs closer to 0.50 indicate more uncertainty, those towards 1 indicate more certainty of urban children being taller, and those towards 0 indicate more certainty of rural being taller. For change, if an increase in urban-rural difference in mean height is statistically indistinguishable from a decrease, the PP is 0.50. PPs closer to 0.50 indicate more uncertainty, those towards 1 indicate more certainty of an increase in the urban-rural height difference, and those towards 0 indicate more certainty of a decrease. We did not estimate the PP for differences between rural and urban height for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau), as indicated in grey.
In middle-income countries and emerging economies (newly high-income and industrialized countries) where the height of children and adolescents residing in rural areas converged to those in cities, successive cohorts of children and adolescents living in rural areas outpaced their urban counterparts in becoming taller and attained heights that urban children in the same countries had done decades earlier: growing to heights closer to those seen in high-income countries (Figs. 2 and 3). Successive cohorts of children and adolescents residing in rural areas in sub-Saharan Africa did not experience the accelerated height gain seen in cohorts in rural areas of middle-income countries. Notably, in the case of boys living in sub-Saharan Africa, there was no gain, or possibly a decrease, in height, which in turn led to a persistence or even widening of the urban–rural gap. As a result of these global trends, by 2020, the largest urban–rural gaps in height were seen in Andean and central Latin America (for example, Bolivia, Panama and Peru, by up to 4.7 cm (95% CrI 4.0–5.5 cm) for boys and 3.8 cm (95% CrI 3.3–4.3 cm) for girls) and, especially for boys, in sub-Saharan Africa (for example, DR Congo, Ethiopia, Mozambique and Rwanda, by up to 4.2 cm (95% CrI 2.7–5.7 cm)).
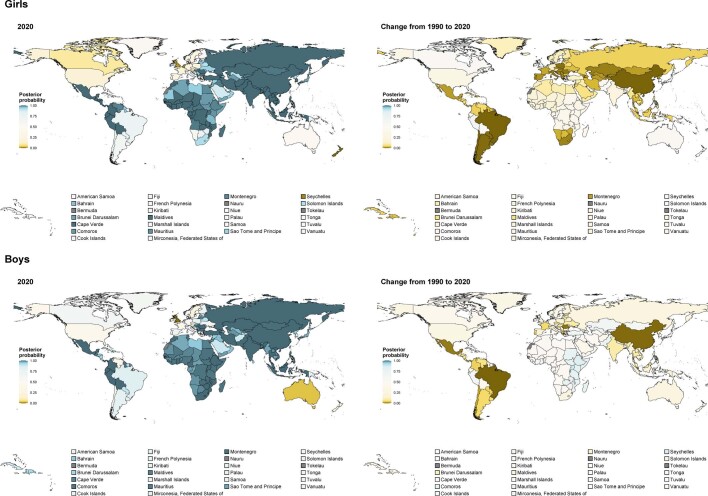
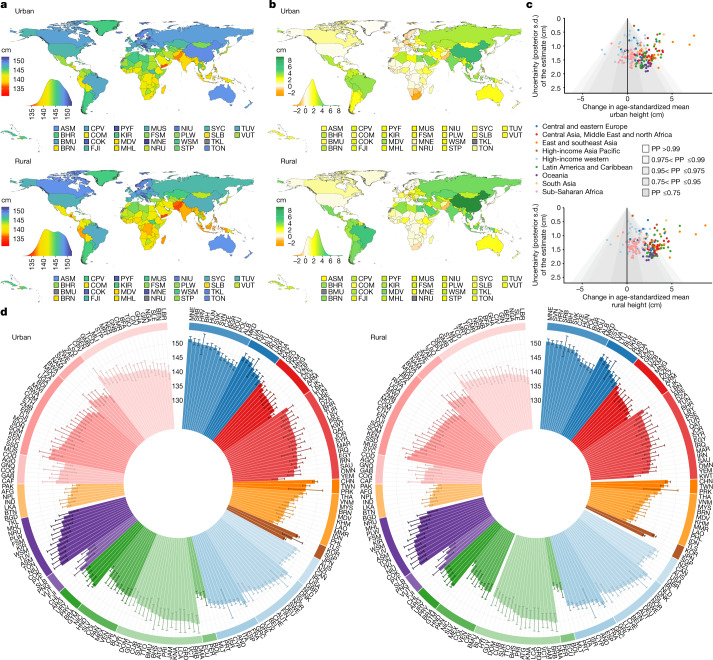
Fig. 2. Urban and rural height in 2020 and the change from 1990 to 2020 for girls.
a, Age-standardized mean height in 2020 by urban and rural place of residence for girls. The density plots show the distribution of estimates across countries. b, Age-standardized change in mean height from 1990 to 2020 by urban and rural place of residence for girls. The density plots show the distribution of estimates across countries. c, Change in mean height from 1990 to 2020 in relation to the uncertainty of the change measured by posterior standard deviation. Each point in the scatter plots shows one country. Shaded areas approximately show the PP of an estimated change being a true increase or decrease. The PP of a decrease is one minus that of an increase. If an increase in mean height is statistically indistinguishable from a decrease, the PP of an increase and a decrease is 0.50. PPs closer to 0.50 indicate more uncertainty, whereas those towards 1 indicate more certainty of change. d, Age-standardized mean height in 2020 for all countries. The height of each column is the posterior mean estimate shown together with its 95% CrI. Countries are ordered by region and super-region. See Extended Data Fig. 4 for a map of PP of the estimated change. See Supplementary Fig. 5 for results at ages 5, 10, 15 and 19 years. See Supplementary Table 3 for numerical results, including Crls, as age-standardized and at ages 5, 10, 15 and 19 years. We did not estimate mean rural height in countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore), mean urban height in countries classified as entirely rural (Tokelau) or their change over time in these countries, as indicated in grey. Countries are labelled using their International Organization for Standardization (ISO) 3166-1 alpha-3 codes. Afghanistan, AFG; Albania, ALB; Algeria, DZA; American Samoa, ASM; Andorra, AND; Angola, AGO; Antigua and Barbuda, ATG; Argentina, ARG; Armenia, ARM; Australia, AUS; Austria, AUT; Azerbaijan, AZE; Bahamas, BHS; Bahrain, BHR; Bangladesh, BGD; Barbados, BRB; Belarus, BLR; Belgium, BEL; Belize, BLZ; Benin, BEN; Bermuda, BMU; Bhutan, BTN; Bolivia, BOL; Bosnia and Herzegovina, BIH; Botswana, BWA; Brazil, BRA; Brunei Darussalam, BRN; Bulgaria, BGR; Burkina Faso, BFA; Burundi, BDI; Cabo Verde, CPV; Cambodia, KHM; Cameroon, CMR; Canada, CAN; Central African Republic, CAF; Chad, TCD; Chile, CHL; China, CHN; Colombia, COL; Comoros, COM; Congo, COG; Cook Islands, COK; Costa Rica, CRI; Cote d'Ivoire, CIV; Croatia, HRV; Cuba, CUB; Cyprus, CYP; Czechia, CZE; Denmark, DNK; Djibouti, DJI; Dominica, DMA; Dominican Republic, DOM; DR Congo, COD; Ecuador, ECU; Egypt, EGY; El Salvador, SLV; Equatorial Guinea, GNQ; Eritrea, ERI; Estonia, EST; Eswatini, SWZ; Ethiopia, ETH; Fiji, FJI; Finland, FIN; France, FRA; French Polynesia, PYF; Gabon, GAB; Gambia, GMB; Georgia, GEO; Germany, DEU; Ghana, GHA; Greece, GRC; Greenland, GRL; Grenada, GRD; Guatemala, GTM; Guinea Bissau, GNB; Guinea, GIN; Guyana, GUY; Haiti, HTI; Honduras, HND; Hungary, HUN; Iceland, ISL; India, IND; Indonesia, IDN; Iran, IRN; Iraq, IRQ; Ireland, IRL; Israel, ISR; Italy, ITA; Jamaica, JAM; Japan, JPN; Jordan, JOR; Kazakhstan, KAZ; Kenya, KEN; Kiribati, KIR; Kuwait, KWT; Kyrgyzstan, KGZ; Lao PDR, LAO; Latvia, LVA; Lebanon, LBN; Lesotho, LSO; Liberia, LBR; Libya, LBY; Lithuania, LTU; Luxembourg, LUX; Madagascar, MDG; Malawi, MWI; Malaysia, MYS; Maldives, MDV; Mali, MLI; Malta, MLT; Marshall Islands, MHL; Mauritania, MRT; Mauritius, MUS; Mexico, MEX; Micronesia (Federated States of), FSM; Moldova, MDA; Mongolia, MNG; Montenegro, MNE; Morocco, MAR; Mozambique, MOZ; Myanmar, MMR; Namibia, NAM; Nauru, NRU; Nepal, NPL; Netherlands, NLD; New Zealand, NZL; Nicaragua, NIC; Niger, NER; Nigeria, NGA; Niue, NIU; North Korea, PRK; North Macedonia, MKD; Norway, NOR; Occupied Palestinian Territory, PSE; Oman, OMN; Pakistan, PAK; Palau, PLW; Panama, PAN; Papua New Guinea, PNG; Paraguay, PRY; Peru, PER; Philippines, PHL; Poland, POL; Portugal, PRT; Puerto Rico, PRI; Qatar, QAT; Romania, ROU; Russian Federation, RUS; Rwanda, RWA; Saint Kitts and Nevis, KNA; Saint Lucia, LCA; Samoa, WSM; Sao Tome and Principe, STP; Saudi Arabia, SAU; Senegal, SEN; Serbia, SRB; Seychelles, SYC; Sierra Leone, SLE; Singapore, SGP; Slovakia, SVK; Slovenia, SVN; Solomon Islands, SLB; Somalia, SOM; South Africa, ZAF; South Korea, KOR; South Sudan, SSD; Spain, ESP; Sri Lanka, LKA; Saint Vincent and the Grenadines, VCT; Sudan, SDN; Suriname, SUR; Sweden, SWE; Switzerland, CHE; Syrian Arab Republic, SYR; Taiwan, TWN; Tajikistan, TJK; Tanzania, TZA; Thailand, THA; Timor-Leste, TLS; Togo, TGO; Tokelau, TKL; Tonga, TON; Trinidad and Tobago, TTO; Tunisia, TUN; Turkey, TUR; Turkmenistan, TKM; Tuvalu, TUV; Uganda, UGA; Ukraine, UKR; United Arab Emirates, ARE; United Kingdom, GBR; United States of America, USA; Uruguay, URY; Uzbekistan, UZB; Vanuatu, VUT; Venezuela, VEN; Vietnam, VNM; Yemen, YEM; Zambia, ZMB.
Fig. 3. Urban and rural height in 2020 and change from 1990 to 2020 for boys.
a–d, See the caption for Fig. 2 for descriptions of the contents of the figure and for definitions. We did not estimate mean rural height in countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore), mean urban height in countries classified as entirely rural (Tokelau) or their change over time, as indicated in grey.
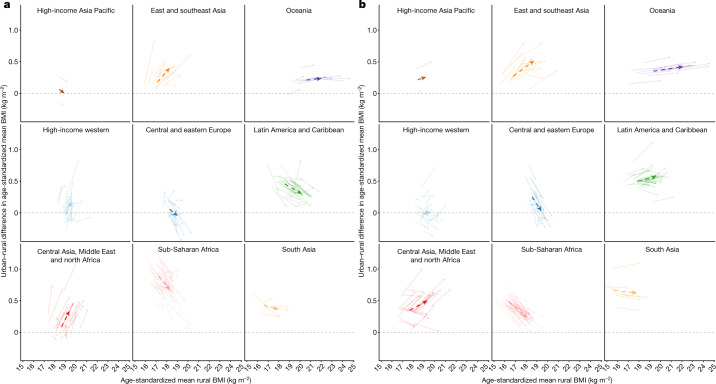
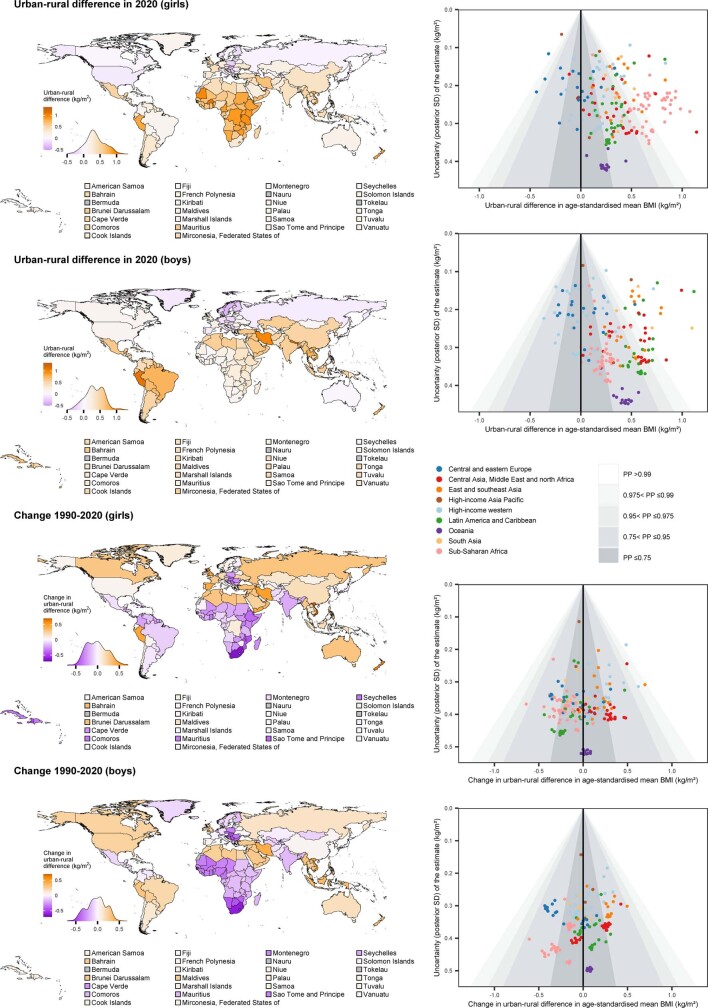
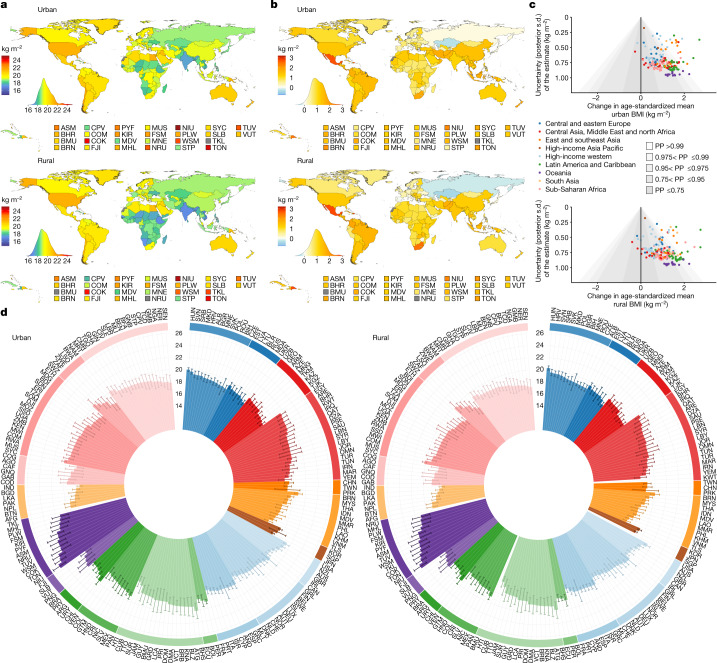
The urban–rural BMI difference was relatively small throughout these three decades, <1.4 kg m–2 in all countries and years and <1.1 kg m–2 in all but nine countries, for age-standardized mean BMI (Fig. 4 and Extended Data Figs. 3 and 9). In 1990, the urban–rural BMI gap was largest in sub-Saharan Africa (for example, Ethiopia, Kenya, Malawi, South Africa and Zimbabwe) and south Asia (for example, Bangladesh and India), followed by parts of Latin America (for example, Mexico and Peru). The urban–rural BMI gap in the two sexes in the named countries ranged from 0.4 to 1.2 kg m–2, and the PP of children and adolescents living in urban areas having a higher BMI than those in rural areas was ≥0.89. At that time, girls and/or boys in rural areas of some of these countries had mean BMI levels that were close to, and in some ages below, the thresholds of being underweight (>1 s.d. below the median of the World Health Organization (WHO) reference population).
Fig. 4. Change in the urban–rural BMI difference from 1990 to 2020.
a,b, Change in urban–rural difference in age-standardized mean BMI for girls (a) and boys (b) in relation to change in age-standardized mean rural BMI. See the caption for Fig. 1 for a description of the contents of this figure. See Extended Data Fig. 3 for urban–rural differences in age-standardized mean BMI and their change over time shown as maps, together with uncertainties in the estimates. See Supplementary Fig. 4b for results at ages 5, 10, 15 and 19 years. We did not estimate the difference between rural and urban BMI for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau).
Extended Data Fig. 3. Urban-rural body-mass index (BMI) difference in 2020 and change from 1990 to 2020.
See Extended Data Fig. 2 caption for descriptions of the contents of the figure and for definitions. See Extended Data Fig. 9 for PP of the urban-rural difference in age-standardised mean BMI and its change. See Supplementary Fig. 8 for results at ages 5, 10, 15 and 19 years. We did not estimate the difference between rural and urban BMI for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau), as indicated in grey.
Extended Data Fig. 9. Posterior probability of urban-rural body-mass index (BMI) difference in 2020 and its increase from 1990 to 2020.
The maps show the posterior probability (PP) that age-standardised mean BMI in 2020 in urban areas was higher than in rural areas (left-hand panels), and the PP that the urban-rural difference in mean BMI increased from 1990 to 2020 (right-hand panels). We did not estimate the PP for differences between rural and urban BMI for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau), as indicated in grey.
From 1990 to 2020, the BMI of successive cohorts of children and adolescents in both urban and rural areas increased in all but a few mostly high-income countries (for example, Denmark, Italy and Spain) (Figs. 5 and 6). There was heterogeneity in low-income and middle-income countries in how much the BMI increased in cities compared with rural areas. In the majority of countries in sub-Saharan Africa and south Asia, the BMI of successive cohorts of children and adolescents increased more in rural areas than in cities, leading to a closing of the urban–rural difference. The urban–rural BMI gap declined by up to 0.65 kg m–2 for both girls and boys, and the PP that the urban–rural BMI difference declined from 1990 to 2020 ranged from 0.52 to 0.95. In both sub-Saharan Africa and south Asia, these changes shifted the mean BMI of boys and girls in rural areas out of the range for being underweight. Moreover, in many countries in sub-Saharan Africa, this shift continued beyond the median of the WHO reference population and in some cases approached the threshold for being overweight (>1 s.d. above the median of the WHO reference population). The opposite, a larger increase in urban BMI, happened in most other low-income and middle-income countries, leading to a slightly larger urban BMI excess in 2020 than in 1990. High-income countries and those in central and eastern Europe experienced a mix of increasing and decreasing urban BMI excess, but remained within a small range (−0.3 to 0.6 kg m–2 for almost all countries) over the entire period of analysis. At the regional level, the urban–rural BMI difference changed by <0.25 kg m–2 in these regions.
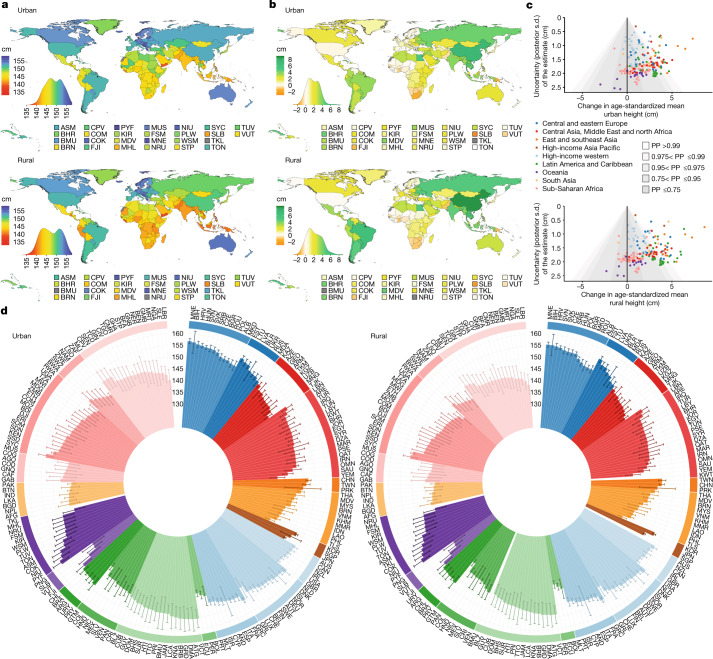
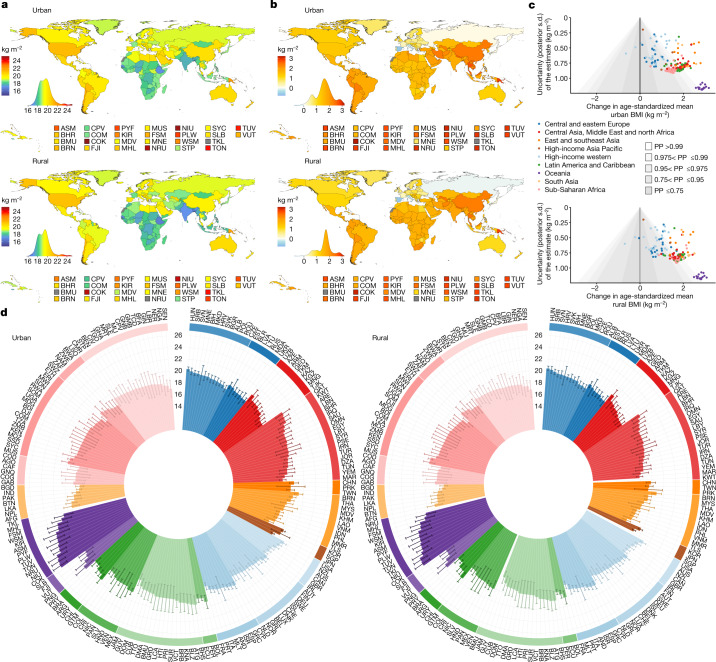
Fig. 5. Urban and rural BMI in 2020 and change from 1990 to 2020 for girls.
a–d, See the caption for Fig. 2 for descriptions of the contents of the figure and for definitions. See Extended Data Fig. 5 for a map of PP of the estimated change. See Supplementary Fig. 6 for results at ages 5, 10, 15 and 19 years. See Supplementary Table 4 for numerical results, including CrIs, as age-standardized and at ages 5, 10, 15 and 19 years. We did not estimate mean rural BMI in countries classified as entirely urban (Singapore, Bermuda and Nauru), mean urban BMI in areas classified as entirely countries (Tokelau) or their change over time, as indicated in grey.
Fig. 6. Urban and rural BMI in 2020 and change from 1990 to 2020 for boys.
a–d, See the caption for Fig. 2 for descriptions of the contents of the figure and for definitions. We did not estimate mean rural BMI in countries classified as entirely urban (Singapore, Bermuda and Nauru), mean urban BMI in countries classified as entirely rural (Tokelau) or their change over time, as indicated in grey.
The urban height advantage was larger in boys than girls in most countries (Supplementary Fig. 3). Urban excess BMI was larger in boys than girls in only about one-half of the countries. For the other half, mostly in high-income western countries and those in sub-Saharan Africa, urban excess BMI was larger in girls than boys. The urban height advantage was slightly larger at 5 years of age than at 19 years of age in most low-income and middle-income countries, especially for girls, but there was little difference across ages in high-income regions and in central and eastern Europe (Supplementary Fig. 4).
Since the introduction of modern sanitation in the nineteenth century, cities provided substantial nutritional and health advantages in high-income and subsequently low-income and middle-income countries19. Our results show that in the twenty-first century, during school ages, these advantages have disappeared in high-income countries and diminished in middle-income countries and emerging economies in Asia, Latin America and the Caribbean, and parts of Middle East and north Africa. Specifically, in these settings, successive cohorts of school-aged children and adolescents living in cities were outpaced by those in rural areas in terms of height gain but gained slightly more weight by 2020, typically in the unhealthy range (Fig. 7). This contrasted with the poorest region in the world: sub-Saharan Africa. In this region, the urban height advantage persisted or even expanded, whereas rural mean BMI went beyond remedying underweight and surpassed the median of the WHO reference population in 2020, hence consolidating the urban advantage. South Asia had a mixed pattern of urban versus rural trends from 1990 to 2020, with children and adolescents in rural areas gaining both more height and more weight for their height than those in cities. Notably, our results also show that differences in height and BMI between urban and rural populations within most countries are smaller than the differences across countries, even those in the same region.
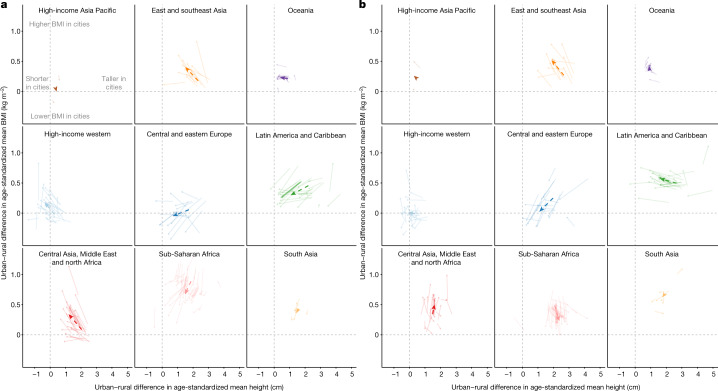
Fig. 7. Change in the urban–rural height and BMI difference from 1990 to 2020.
a,b, Change in the urban–rural difference in age-standardized mean height and the urban–rural difference in age-standardized mean BMI in girls (a) and boys (b). See the caption for Fig. 1 for a description of the contents of this figure. See Supplementary Fig. 4c for results at ages 5, 10, 15 and 19 years. We did not estimate the difference between rural and urban height and BMI for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or entirely rural (Tokelau).
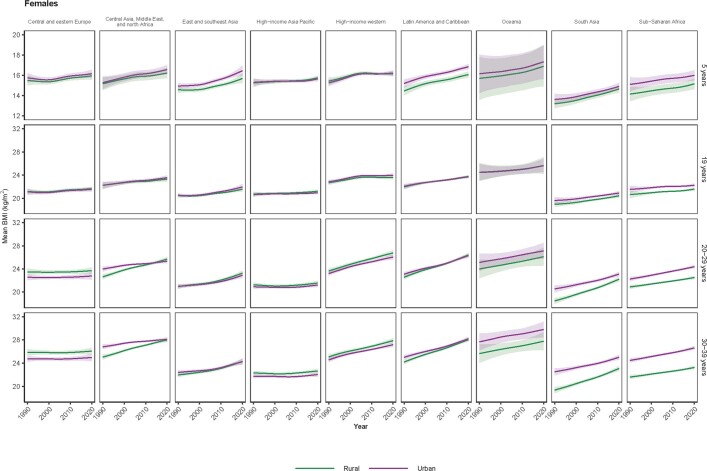
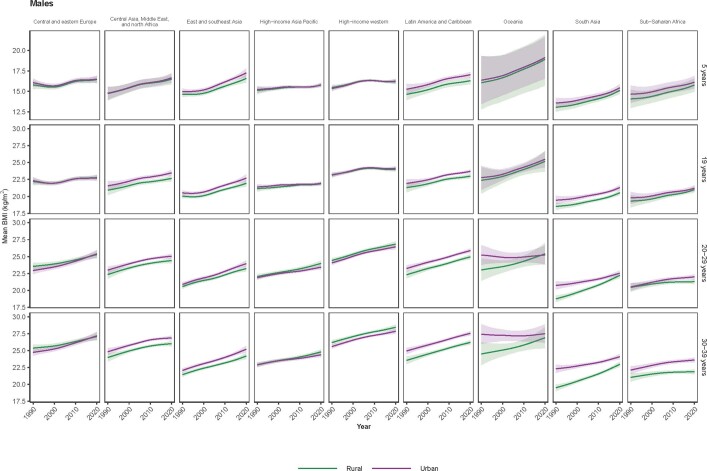
We also found that the urban–rural BMI gap, although dynamic, changed much less than the BMI of either subgroup of the population and less than commonly assumed when discussing the role of cities in the obesity epidemic8,10,12,13,15,16. Urban–rural BMI differences were especially small in high-income countries, which is consistent with evidence from a few countries that show diets and behaviours are affected more by household socioeconomic status than whether children and adolescents live in cities or rural areas29,30. Urban BMI excess increased slightly more in middle-income countries in east and southeast Asia, Latin America and the Caribbean, and Middle East and north Africa, a trend that was the opposite of the convergence in BMI of adults in these same regions21. Additional analyses of data collected by the NCD Risk Factor Collaboration (NCD-RisC) for young adults (20–29 and 30–39 years) showed that the shift from a small divergent trend to convergence of BMI between urban and rural areas happens in young adulthood (Extended Data Figs. 6 and 7), a period during which there is substantial, but variable, weight gain among population subgroups31. These shifts in trends from adolescence to young adulthood might be a result of changes in diet and energy expenditure that accompany changes in household structure, social and economic roles and the living environment32–34.
Extended Data Fig. 6. Trends in body-mass index (BMI) by place of residence for children, adolescents and young adults for females.
The figure shows trends in mean BMI at ages five and 19 years, and in age-standardised mean BMI for young adults (20–29 years and 30–39 years) for females. Shaded areas show the 95% CrIs. Trend for young adults were estimated using a model similar to the one described in Methods, where BMI-age patterns were allowed to vary flexibly via a cubic spline function without knots.
Extended Data Fig. 7. Trends in body-mass index (BMI) by place of residence for children, adolescents and young adults for males.
The figure shows trends in mean BMI at ages five and 19 years, and in age-standardised mean BMI for young adults (20–29 years and 30–39 years) for males. See Extended Data Fig. 6 caption for description of figure contents.
Long-term follow-up studies have shown that children and adolescents do not achieve their height potential if they do not consume sufficient and diverse nutritious foods or if they are exposed to repeated or persistent infections, which result in loss of nutrients2. Studies that use data on household socioeconomic and environmental factors have indicated that these physiological determinants of height are themselves affected by income, education, quality of the living environment and access to healthcare in rural as well as urban areas35. This evidence indicates that the relatively small urban–rural height differentials in high-income countries may be because of a greater abundance of nutritious foods, including some fortified foods, better education and healthcare and greater ability to finance programmes that promote healthy growth in countries with greater per-capita income and better infrastructure. Variations across these countries in the urban–rural height gap within this small range may be due to the extent of socioeconomic inequalities and poverty, differences in the availability and cost of nutritious foods between cities and rural areas and whether there are specific programmes (for example, food assistance or school food programmes) that improve nutrition in disadvantaged groups30,36,37. The more marked changes in height in urban versus rural areas took place in middle-income countries and emerging economies. Case studies in some countries where the heights of children and adolescents living in rural and urban areas converged show that the convergence was partly due to using the growth in national income towards programmes and services that helped close gaps in nutrition, sanitation and healthcare between different areas and social groups38–40. In countries in central and eastern Europe, transition to a market economy and increases in trade may have reduced the disparity in access to, and seasonality of, healthy foods between urban and rural areas41, and partly underlie the convergence of height seen in our results. By contrast, case studies in some countries have shown that where economic growth was accompanied by large inequalities in income, nutrition and/or services, the urban advantage persisted42–44.
The notable exception in the global trends was sub-Saharan Africa, where a stagnation or reversal of height gain in rural areas led to the persistence or widening of urban–rural height differences, whereas the opposite happened for BMI (Fig. 7). Case studies of specific countries have indicated that unfavourable trends in nutrition in rural Africa, where the majority of the poorest people in the world live, started from macroeconomic shocks in the late twentieth century45 and subsequent agriculture, trade and development policies that limited improvements in income and services, and emphasized agricultural exports over local food security and diversity45. These macroeconomic factors in turn led to less diverse diets, with higher caloric intake rather than a shift to protein-rich and nutrient-rich foods (for example, animal products, seafood, fruits and vegetables)46–48. Moreover, the slow expansion of infrastructure and services in rural areas restricted improvements in other determinants of healthy growth, such as clean water, sanitation and health care49.
Several other factors may have had a secondary role in the observed trends in height and BMI and their difference in rural and urban areas. First, weight gain during childhood may reduce the age of puberty onset, which in turn may limit height gain during adolescence50,51. No comparable global data currently exist on age at menarche and timing of pubertal growth, even at the national level. Second, rural-to-urban migration and reclassification of previously rural areas to urban as they grow and industrialize may account for some of the observed population-level trends. However, migration tends to be less common in childhood and adolescence than in adulthood in most countries. Finally, improvements in survival among children aged under 5 years in rural areas, particularly low-birthweight children, may have influenced the height and weight of those who survive beyond 5 years of age. However, current data on changes in child survival in rural and urban areas in sub-Saharan Africa are limited and inconclusive in terms of whether mortality declined faster in rural or urban areas52,53.
As attention in global health turns to children and adolescents, there is a need to consider and evaluate how growth and development in these formative ages may be affected both by social and economic policies that influence household income and poverty and by programmes that affect nutrition, health services, infrastructure and living environments in rural and urban areas. The need to identify, implement and evaluate policies and programmes that improve growth and development outcomes is particularly relevant as the increase in poverty and the cost of food, especially of nutrient-rich foods, as a result of the macroeconomic changes resulting from the COVID-19 pandemic and the war in Ukraine, may hinder further gains or even set back healthy growth and development in children and adolescents.
Methods
We estimated trends in mean height and BMI for children and adolescents aged 5–19 years from 1990 to 2020 by rural and urban place of residence for the 200 countries and territories listed in Supplementary Table 1. We pooled, in a Bayesian meta-regression, repeated cross-sectional population-based data on height and BMI. Our results represent estimates of height and BMI for children and adolescents of the same age over time (that is, for successive cohorts) in rural and urban settings for each country.
Data sources
We used a database on cardiometabolic risk factors collated by NCD-RisC. Data were obtained from publicly available multi-country and national measurement surveys, for example, Demographic and Health Surveys (DHS), WHO-STEPwise approach to Surveillance (STEPS) surveys, and those identified through the Inter-University Consortium for Political and Social Research, UK Data Service and European Health Interview & Health Examination Surveys Database. With the help of the WHO and its regional and country offices as well as the World Heart Federation, we identified and accessed population-based survey data from national health and statistical agencies. We searched and reviewed published studies as previously detailed54 and invited eligible studies to join NCD-RisC, as we did with data holders from earlier pooled analyses of cardiometabolic risk factors55–58. The NCD-RisC database is continuously updated through all the above routes and through periodic requests to NCD-RisC members to ask them to suggest additional sources in their countries.
We carefully checked that each data source met our inclusion criteria, as listed below. Potential duplicate data sources were first identified by comparing studies from the same country and year, followed by checking with NCD-RisC members that had provided data about whether the sources from the same country and year, with similar samples, were the same or distinct. If two sources were confirmed as duplicates, one was discarded. All NCD-RisC members were also periodically asked to review the list of sources from their country to verify that the included data met the inclusion criteria and were not duplicates.
For each data source, we recorded the study population, the sampling approach, the years of measurement and the measurement methods. Only data that were representative of the population were included. All data sources were assessed in terms of whether they covered the entire country, one or more subnational regions (that is one or more provinces or states, more than three cities, or more than five rural communities), or one or a small number of communities (limited geographical scope not meeting above national or subnational criteria), and whether participants in rural, urban or both areas were included. As stated in the sections on the statistical model, these study-level attributes were used in the Bayesian hierarchical model to estimate mean height and BMI by country, year, sex, age and place of residence using all available data while taking into account differences in the populations from which different studies had sampled. All submitted data were checked by at least two independent individuals. Questions and clarifications were discussed with NCD-RisC members and resolved before data were incorporated into the database.
Anonymized individual data from the studies in the NCD-RisC database were re-analysed according to a common protocol. We calculated the mean height and the mean BMI, and the associated standard errors, by sex, single year of age from 5 to 19 years and rural or urban place of residence. Additionally, for analysis of height, participants aged 20–30 years were included, assigned to their corresponding birth cohort, because mean height in these ages would be at least that when they were aged 19 years given that the decline in height with age begins in the third and fourth decades of life59. All analyses incorporated sample weights and complex survey design, when applicable, in calculating summary statistics. For studies that had used simple random sampling, we calculated the mean as the average of all individuals within the group and the associated standard error (s.d. divided by the square root of sample size); for studies that had used multistage (stratified) sampling, we accounted for survey design features, including clusters, strata and sample weights, to weight each observation by the inverse sampling probability and estimated standard error through Taylor series linearization, as implemented in the R ‘survey’ package60. Computer code was provided to NCD-RisC members who requested assistance. For surveys without information on the place of residence, we calculated summary statistics stratified by age and sex for the entire sample, which represented the population-weighted sum of rural and urban means; data on the share of population in urban and rural areas were from the United Nations Population Division61.
Additionally, summary statistics for nationally representative data from sources that were identified but not accessed using the above routes were extracted from published reports. Data were also extracted for two STEPS surveys that were not publicly available. We also included data from a previous global-data pooling study58, when not accessed through the above routes.
Data inclusion and exclusion
Data sources were included in the NCD-RisC height and weight database if the following criteria were met: measured data on height and weight were available; study participants were 5 years of age or older; data were collected using a probabilistic sampling method with a defined sampling frame; data were from population samples at the national, subnational or community level as defined above; and data were from the countries and territories listed in Supplementary Table 1.
We excluded all data sources that were solely based on self-reported weight and height without a measurement component because these data are subject to biases that vary by geography, time, age, sex and socioeconomic characteristics62–64. Owing to these variations, approaches to correcting self-reported data may leave residual bias. We also excluded data sources on population subgroups for which anthropometric status may differ systematically from the general population, including the following: studies that had included or excluded people based on their health status or cardiovascular risk; studies in which participants were only ethnic minorities; specific educational, occupational or socioeconomic subgroups (with the exception noted below); those recruited through health facilities (with the exception noted below); and females aged 15–19 years in surveys that sampled only ever-married women or measured height and weight only among mothers.
We used school-based data in countries and age–sex groups with school enrolment of 70% or higher. We used data for which the sampling frame was health insurance schemes in countries where at least 80% of the population were insured. Finally, we used data collected through general practice and primary care systems in high-income and central European countries with universal insurance because contact with the primary care systems tends to be as good as or better than response rates for population-based surveys.
We excluded participants whose age was <18 years and whose data were not reported by single year of age (<0.01% of all participants) because height and weight may have nonlinear age associations in these ages, especially during growth spurts. We excluded BMI data for females who were pregnant at the time of measurement (<0.01% of all participants). We excluded <0.2% of all participants who had recorded height: <60 cm or >180 cm for ages <10 years; <80 cm or >200 cm for ages 10–14 years; <100 cm or >250 cm for ages ≥15 years, or who had recorded weight: <5 kg or >90 kg for age <10 years; <8 kg or >150 kg for ages 10–14 years; <12 kg or >300 kg for ages ≥15 years, or who had recorded BMI: <6 kg m–2 or >40 kg m–2 for ages <10 years; <8 kg m–2 or >60 kg m–2 for ages 10–14 years; <10 kg m–2 or >80 kg m–2 for ages ≥15 years.
Conversion of BMI prevalence metrics to mean BMI
In 0.5% of our data points, mostly extracted from published reports or from a previous pooling analysis58, the mean BMI was not reported but data were available for the prevalence of one or more BMI categories, for example BMI ≥30 kg m–2. To use these data, we used previously validated conversion regressions65 to estimate the missing primary outcome from the available BMI prevalence metric or metrics. Additional details on regression model specifications along with the regression coefficients are reported at https://github.com/NCD-RisC/ncdrisc-methods/.
Statistical model overview
We used a Bayesian hierarchical meta-regression model to estimate the mean height and BMI by country, year, sex, age and place of residence using the aforementioned data. For presentation, we summarized the 15 age-specific estimates, for single years of age from 5 to 19 years, through age standardization, which puts the child and adolescent population for each country-year on the same age distribution, and hence enables comparisons to be made over time and across countries. We generated age-standardized estimates by taking weighted means of age-specific estimates using age weights from the WHO standard population66. We also show results, graphically and numerically, for index ages of 5, 10, 15 and 19 years in the Supplementary Information.
The statistical model is described in detail in statistical papers67,68, related substantive papers7,20,21,55–58,65,69 and in the section below on model specification. In summary, the model had a hierarchical structure in which estimates for each country and year were informed by its own data, if available, and by data from other years in the same country and from other countries, especially those in the same region and super-region, with data for similar time periods. The extent to which estimates for each country-year were influenced by data from other years and other countries depended on whether the country had data, the sample size of the data, whether they were national, and the within-country and within-region variability of the available data. For the purpose of hierarchical analysis, countries and territories were organized into 21 regions, mostly based on geography and national income (Supplementary Table 1). Regions were in turn organized into nine super-regions.
We used observation year, that is, the year in which data were collected, as the timescale for the analysis of BMI and birth year as the timescale for the analysis of height, consistent with previous analyses7,65,70. Time trends were modelled through a combination of a linear term, to capture gradual long-term change, and a second-order random walk, which allows for nonlinear trends71, both modelled hierarchically. The age associations of height and BMI were modelled, using cubic splines, to allow for nonlinear changes over age, including periods of rapid and slow rise. Periods of rapid rise representing adolescent growth spurts, which occur earlier in girls than boys72–74, were reflected in the placement of spline knots for boys and girls, respectively, as detailed in the section on model specification. Spline coefficients were allowed to vary across countries, informed by their own data as well as data from other countries as specified by a hierarchical structure, as previously described69.
The model also accounted for the possibility that height or BMI in subnational and community samples might differ systematically from nationally representative samples and have larger variation than in national studies. These features were accounted for through the inclusion of fixed-effect and random-effect terms for subnational and community data as detailed in the model specification section below. The fixed effects accounted for systematic differences between subnational or community studies and national studies. The inclusion of random effects allowed national data to have greater influence on the estimates than subnational or community data with similar sample sizes because the subnational and community data have additional variance from the random-effect terms. Both were estimated empirically as a part of model fitting.
Following the approach of previous papers20,21,67, the model included parameters representing the urban–rural height or BMI difference, which is empirically estimated and allowed to vary by country and year. We further expanded the model to allow urban–rural difference in height or BMI to vary by age, as height or weight with age may vary between children residing in rural versus urban areas. If data for a country-year-age group contained a mix of children living in urban and rural areas but were not stratified by place of residence (21% of all data sources), the estimated height or BMI difference was informed by stratified data from other age groups, years and countries, especially those in the same region with data from similar time periods and/or ages.
Statistical model specification
As stated earlier, for each data source, we calculated mean height and BMI, together with corresponding standard errors, stratified by sex, age and rural or urban place of residence. For sources that did not stratify the sample on the place of residence, we obtained age-and-sex-stratified data. Each study contributed up to 30 mean BMI data points or 32 mean height data points for each sex, with the exact number depending on how many age groups were represented in the study and whether the study provided data stratified on urban and rural place of residence. The likelihood for an observation at urbanicity level s (urban-only, rural-only or mixed; referred to as stratum hereafter) and age group h, with age zh, from study i, carried out in country j at time t is as follows:
where the country-specific intercept and linear time slope from the jth country (j = 1 … J, where J = 200 which is the total number of countries in our analysis) are denoted and , respectively. We describe the hierarchical model used for the s and in the section ‘Linear components of country time trends’. Letting T = 31 be the total number of years from 1990 to 2020, the T-length vector captures smooth nonlinear change over time in country j, as described in the section ‘Nonlinear change’. The age effects of the hth age group (with age ) in study are denoted by ; we describe the age model in the section ‘Age model’. The matrix contains terms describing whether studies were representative at the national, subnational or community level. In addition, a random effect, , is estimated for each study, described in the section ‘Study-level term and study-specific random effects’.
Linear components of country time trends
The model had a hierarchical structure, whereby studies were nested in countries, which were nested in regions (indexed by k), which were nested in super-regions (indexed by l), which were all nested in the globe (see Supplementary Table 1 for a list of countries and territories in each region, and regions in each super-region). This structure allowed the model to share information across units to a greater degree when data were non-existent or weakly informative (for example, had a small sample size or were not nationally representative) and, to a lesser extent, in data-rich countries and regions75.
The and terms are country-specific linear intercepts and time slopes with terms at each level of the hierarchy, denoted by the superscripts c, r, s and g, respectively:
where x = {c, r, s}.
The terms were each assigned a flat prior on the s.d. scale76. We also assigned flat priors to and .
Nonlinear change
Mean BMI or height may change nonlinearly over time7,54,58,65,70. We captured smooth nonlinear change in time in urban and rural strata of country j using the vector . Just as and are each defined as the sum of country, region, super-region and global components, we defined
To allow the model to differentiate between the degrees of nonlinearity that exist at the country, region, super-region and global levels, we assigned the four components of each a Gaussian autoregressive prior71,77. In particular, the vectors (j = 1 … J), (k = 1 … K), (l = 1 … L) and each have a normal prior with mean zero and precision , , and , respectively, where the scaled precision matrix in the Gaussian autoregressive prior penalizes first and second differences as follows:
P is multiplied by the estimated precision parameters , , and , thus upweighting or downweighting the strength of its penalties and ultimately determining the degree of smoothing at each level. For each of the four precision parameters, we used a truncated flat prior on the s.d. scale ()76. We truncated these priors such that log ≤ 20 for each of the four . This upper bound is enforced as a computational convenience, whereby models with log > 20 are treated as equivalent to a model with log = 20 as they essentially have no extralinear variability in time. In practice, this upper bound had little effect on the parameter estimates. Furthermore, we ordered the a priori as follows: < < < . This prior constraint conveys the natural expectation that, for example, the global height or BMI trend has less extralinear variability than the trend of any given region, which in turn has less variability than those of constituent countries.
The matrix has rank − 2, corresponding to a flat, improper prior on the mean and the slope of the ’s, the ’s and the ’s and , and is not invertible78. Thus, we had a proper prior in a reduced-dimension space71, with the prior expressed as follows:
Note that if had a non-zero mean, this would introduce non-identifiability with respect to . By the same token, would not be identifiable if had a non-zero time slope, and similarly for the other means and slopes. Thus, to achieve identifiability of the , , and , we constrained the mean and slope of and of each , and to be zero. Enforcing orthogonality between the linear and nonlinear portions of the time trends meant that each can be interpreted independently.
For the cases in which we have observations for at least two different time points, this improper prior will not lead to an improper posterior because the data will provide information about the mean and slope. In order to enforce the desired orthogonality between the linear and nonlinear portions of the model, we constrained the mean and slope of the ’s, ’s and ’s and of to be zero71.
For the six countries with no height data, and seven countries with no BMI data, we took the Moore–Penrose pseudoinverse of P 79, setting to infinity those eigenvalues that correspond to the non-identifiability. This effectively constrained the non-identified portions of the model to zero, as the corresponding variances are set to zero77; in this case the Rue and Held correction71 is not needed. An intermediate case occurs when data are observed for only one time point in a country. In this case, the full conditional precision has rank because the mean but not the linear trend of is identified by the data. We therefore constrained the linear trend of to zero by taking the generalized inverse of the full conditional precision. We then constrained the mean of to zero using the one-dimensional version of the Rue and Held correction71.
Age model
To capture sex-specific patterns of growth, especially adolescent growth spurts, we modelled age using cubic splines. The number and position of the knots of the splines were selected on the basis of a combination of physiological and statistical considerations, as described in a national level analysis7. For age group h with age zh, in study i, the age effect for height and BMI is given, respectively, as follows:
| height |
| BMI |
For height, four spline knots were placed at ages { for girls and at ages {, for boys. For BMI, we used two spline knots (at ages 10 and 15 years) because, at the population level, changes in BMI with age are smoother than those in height7,72,73. Each of the spline coefficients was allowed to vary across countries, with a hierarchical structure as described in a previous paper69, using the equation below, where is the global intercept, and are the country, region and super-region random intercepts, respectively. The kth age effect coefficients for study i () for each age group h, with age zh, are given as follows:
A flat improper prior was placed on each of the ’s .
Study-level term and study-specific random effects
Mean height or BMI from individual studies may deviate from the true country-year mean owing to factors associated with sampling, response or measurement. We used a study-level term to help account for potential systematic differences associated with data sources that are representative of subnational and community populations. Our model therefore included time-varying offsets (referred to as fixed effects above) for subnational and community data in the term :
where is the indicator for whether the coverage of study i, in country j and year t, is subnational or community.
Even after accounting for sampling variability, national studies may still not reflect the true mean height or BMI level of a country with perfect accuracy, and subnational and community studies have even larger variability. In study i, the study-specific random effect allows all age groups from the same study to have an unusually high or an unusually low mean after conditioning on the other terms in the model. Each is assigned a normal prior with variance depending on whether study i is representative at the national, subnational or community level. Random effects from national studies were constrained to have smaller variance () than random effects of subnational studies (), which were in turn constrained to have smaller variance than community studies (). To make country-level predictions, we set , thus not including random effects arising from imperfections and variations in study design and implementation and from within-country variability of height or BMI means.
Urban and rural strata
To model mean height and BMI by urban and rural places of residence, the model included offsets for the two strata. The offsets were captured by country-specific intercept, linear time and age effects, using a centred indicator term ():
where , with
In other words, for data not stratified by place of residence, the model treated the unstratified mean height or BMI as equivalent to the weighted sum of the (unobserved) urban sample mean height or BMI and rural sample mean height or BMI, with the weights based on the proportion of the population of that country living in urban areas in the year of the survey ().
The intercept () and slope () terms capture the country-to-country variation in the magnitude of the height or BMI difference between urban and rural populations and how the difference changes over time. The slope () captures the country-to-country variation in the BMI or height difference between urban and rural populations across age groups. These were specified with the same geographical hierarchy as the country-specific intercepts () and slopes () as follows:
where . The study random effect term incorporates deviations from the country-level urban–rural difference in each study and is analogous to .
Residual age-by-study variability
The age patterns across communities within a given country may differ from the overall age pattern of that country. This within-study variability cannot be captured by the terms, which are equal across age-specific observations in each study, so we included an additional variance component for each study, .
Model implementation
All analyses were done separately by sex because age, geographical and temporal patterns of height and BMI differ between girls and boys7,65. We fitted the statistical model using Markov chain Monte Carlo (MCMC). We started 35 parallel MCMC runs from randomly generated overdispersed starting values. For computational efficiency, each chain was run for a total of 75,000 iterations. All chains converged to the same target distribution within this number, but due to the overdispersed initial values, the length of burn-in required to converge to the target distribution varied. After the runs were completed, we used trace plots to monitor convergence and to select chains that had completed burn-in within 35,000 iterations. This resulted in 16 chains for boys and 17 for girls for BMI, and 14 chains for boys and 16 for girls for height. Within each of these chains, post-burn-in iterations were thinned by keeping every 10th iteration, which were then combined for all chains and further thinned to a final set of 5,000 draws of the model parameter estimates. We used the posterior distribution of the model parameters to obtain the posterior distributions of our outcomes: mean urban and rural height and BMI, and the urban–rural difference in mean height and BMI. Posterior estimates were made for one-year age groups from 5 to 19 years, as well as for age-standardized outcomes, by year. The reported Crls represent the 2.5th and the 97.5th percentiles of the posterior distributions. We also report the posterior s.d. of estimates, and PP that the estimated change in height or BMI in rural or urban areas, and in the urban–rural height or BMI difference over time, represents a true increase or decrease.
Convergence was confirmed for the country-sex specific posterior outcomes—namely mean urban height and BMI, mean rural height and BMI and the urban–rural difference in mean height and BMI—for reporting ages (5, 10, 15, 19 years and age-standardized) and years (1990 and 2020) using the R-hat diagnostic80,81. For height, the 2.5th to 97.5th percentiles of the R-hats for the reporting ages and years were 0.999–1.010 for girls and 0.999–1.004 for boys. For BMI, the 2.5th to 97.5th percentiles of the R-hats were 0.999–1.004 for girls and 0.999–1.005 for boys.
We applied the pool-adjacent-violators algorithm, a monotonic regression that uses an iterative algorithm based on least squares to fit a free-form line to a sequence of observations such that the fitted line is non-decreasing82,83, on the posterior height estimates to ensure that the height for each birth cohort increased monotonically with age. In practice, this had little effect on the results, with height at age 19 years adjusted by an average of 0.26 cm or less for both boys and girls. All analyses were conducting using the statistical software R (v.4.1.2)84.
Strengths and limitations
An important strength of our study is its novel scope of presenting consistent and comparable estimates of urban and rural height and BMI among school-aged children and adolescents, which is essential to formulate and evaluate policies that aim to improve health in these formative ages. We used a large number of population-based studies from 194 countries and territories covering around 99% of the population of the world. We maintained a high level of data quality and representativeness through repeated checks of study characteristics against our inclusion and exclusion criteria, and did not use any self-reported data to avoid bias in height and weight. Data were analysed according to a consistent protocol, and the characteristics and quality of data from each country were rigorously verified through repeated checks by NCD-RisC members. We used a statistical model that used all available data and took into account the epidemiological features of height and BMI during childhood and adolescence by using nonlinear time trends and age associations. The model used the available information on the urban–rural difference in height and BMI and estimated the age-varying and time-varying urban–rural difference for all countries and territories hierarchically.
Despite our extensive efforts to identify and access data, some countries had fewer data, especially those in the Caribbean, Polynesia, Micronesia and sub-Saharan Africa. Of the studies used, fewer than half had data for children aged 5–9 years compared to nearly 90% with data for children and adolescents aged 10–19 years. The scarcity of data is reflected in the larger uncertainty of our estimates for these countries and regions, and younger age groups. This reflects the need to systematically include school-aged children in both health and nutrition surveys, and, especially in countries where school enrolment is high, to use schools as a platform for monitoring growth and developmental outcomes for entire national populations and key subgroups such as those in rural and urban areas. Although urban and rural classifications are commonly based on definitions by national statistical offices, classification of cities and rural areas may, appropriately, vary by country according to their demographic characteristics (for example, population size or density), economic activities, administrative structures, infrastructure and environment. Similarly, urbanization takes place through a variety of mechanisms such as changes in fertility in rural and urban areas, migration and reclassification of previously rural areas to urban as they grow and industrialize. Each of these mechanisms may have different implications for nutrition and physical activity, and hence height and/or BMI, and should be a subject of studies that follow individual participants and changes in their place of residence. Finally, there is variation in growth and development of children within rural or urban areas based on household socioeconomic status and community characteristics that affect access to and the quality of nutrition, the living environment and healthcare35,85,86. Among these, in some cities, a large number of families live in slums19,87. School-aged children and adolescents living in slums have nutrition, environment and healthcare access that is typically worse than other residents of the city, although often better than those in rural areas19,87–90.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-023-05772-8.
Supplementary information
Supplementary Tables 1–4, Supplementary Figs. 1–8 and Supplementary References; see contents page for details.
Acknowledgements
This study was funded by the UK Medical Research Council (grant number MR/V034057/1), the Wellcome Trust (Pathways to Equitable Healthy Cities grant 209376/Z/17/Z), the AstraZeneca Young Health Programme and the European Commission (STOP project through EU Horizon 2020 research and innovation programme under Grant Agreement 774548). For the purpose of open access, the author has applied a Creative Commons Attribution (CC BY) licence to the Author Accepted Manuscript version arising from this submission. We thank W. Dietz, L. Jaacks and W. Johnson for recommendations of relevant citations. The authors alone are responsible for the views expressed in this Article and they do not necessarily represent the views, decisions, or policies of the institutions with which they are affiliated.
Extended data figures and tables
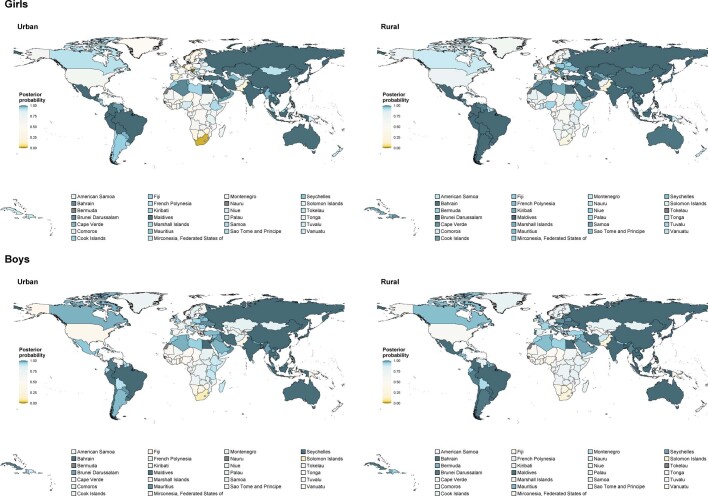
Extended Data Fig. 4. Posterior probability of increase in mean height in urban and rural areas from 1990 to 2020.
The maps show the PP that the age-standardised mean height increased from 1990 to 2020. The PP of a decrease is one minus that of an increase. If an increase in mean height is statistically indistinguishable from a decrease, the PP is 0.50. PPs closer to 0.50 indicate more uncertainty, those towards 1 indicate more certainty of an increase, and those towards 0 indicate more certainty of a decrease. We did not estimate PP for change in mean rural height for countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or change in mean urban height for countries classified as entirely rural (Tokelau), as indicated in grey.
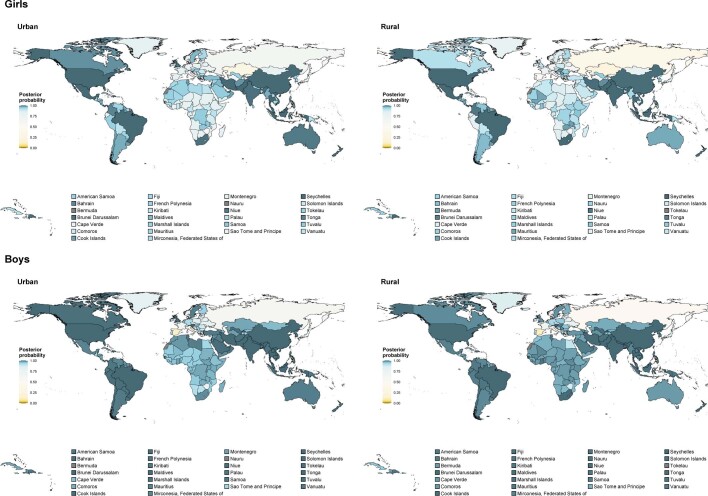
Extended Data Fig. 5. Posterior probability of increase in mean body-mass index (BMI) in urban and rural areas from 1990 to 2020.
The maps show the posterior probability (PP) that the age-standardised mean BMI increased from 1990 to 2020. The PP of a decrease is one minus that of an increase. We did not estimate PP for change in mean rural BMI in countries classified as entirely urban (Bermuda, Kuwait, Nauru and Singapore) or change in mean urban BMI in countries classified as entirely rural (Tokelau), as indicated in grey.
Author contributions
A.M., B.Z., A.R.M., H.B. and R.K.S. led the data collection and management. A.M., B.Z., A.R.M., H.B., C.J.P., J.E.B. and M.E. developed the statistical method. A.M., B.Z., A.R.M. and H.B. coded the statistical method. A.M. conducted analyses and prepared results. The other authors contributed to study design, and collected, reanalysed, checked and pooled data. M.E., A.M., B.Z., A.R.M. and H.B. wrote the first draft of the report. All other authors commented on the draft report.
Peer review
Peer review information
Nature thanks Sam Harper, Jessica Jones-Smith and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Estimates of mean BMI and height by country, year, sex, single year of age as well as age-standardized, and place of residence (urban and rural) will be available from https://www.ncdrisc.org in machine-readable numerical format and as visualizations upon publication of the paper. Input data from publicly available sources and contact information for data providers can be downloaded from https://www.ncdrisc.org and Zenodo (10.5281/zenodo.7355601).
Code availability
The computer code for the Bayesian hierarchical model and the code used to generate figures in this work will be available at https://www.ncdrisc.org and Zenodo (10.5281/zenodo.7355601).
Competing interests
M.E. reports a charitable grant from the AstraZeneca Young Health Programme.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anu Mishra, Bin Zhou, Andrea Rodriguez-Martinez, Honor Bixby
Deceased: Konrad Jamrozik, Altan Onat, Robespierre Ribeiro, Michael Sjöström, Agustinus Soemantri, Jutta Stieber, Dimitrios Trichopoulos
Contributor Information
NCD Risk Factor Collaboration (NCD-RisC):
Anu Mishra, Bin Zhou, Andrea Rodriguez-Martinez, Honor Bixby, Rosie K. Singleton, Rodrigo M. Carrillo-Larco, Kate E. Sheffer, Christopher J. Paciorek, James E. Bennett, Victor Lhoste, Maria L. C. Iurilli, Mariachiara Di Cesare, James Bentham, Nowell H. Phelps, Marisa K. Sophiea, Gretchen A. Stevens, Goodarz Danaei, Melanie J. Cowan, Stefan Savin, Leanne M. Riley, Edward W. Gregg, Wichai Aekplakorn, Noor Ani Ahmad, Jennifer L. Baker, Adela Chirita-Emandi, Farshad Farzadfar, Günther Fink, Mirjam Heinen, Nayu Ikeda, Andre P. Kengne, Young-Ho Khang, Tiina Laatikainen, Avula Laxmaiah, Jun Ma, Michele Monroy-Valle, Malay K. Mridha, Cristina P. Padez, Andrew Reynolds, Maroje Sorić, Gregor Starc, James P. Wirth, Leandra Abarca-Gómez, Ziad A. Abdeen, Shynar Abdrakhmanova, Suhaila Abdul Ghaffar, Hanan F. Abdul Rahim, Zulfiya Abdurrahmonova, Niveen M. Abu-Rmeileh, Jamila Abubakar Garba, Benjamin Acosta-Cazares, Ishag Adam, Marzena Adamczyk, Robert J. Adams, Seth Adu-Afarwuah, Kaosar Afsana, Shoaib Afzal, Valirie N. Agbor, Imelda A. Agdeppa, Javad Aghazadeh-Attari, Hassan Aguenaou, Carlos A. Aguilar-Salinas, Charles Agyemang, Mohamad Hasnan Ahmad, Ali Ahmadi, Naser Ahmadi, Nastaran Ahmadi, Imran Ahmed, Soheir H. Ahmed, Wolfgang Ahrens, Gulmira Aitmurzaeva, Kamel Ajlouni, Hazzaa M. Al-Hazzaa, Badreya Al-Lahou, Rajaa Al-Raddadi, Huda M. Al Hourani, Nawal M. Al Qaoud, Monira Alarouj, Fadia AlBuhairan, Shahla AlDhukair, Maryam A. Aldwairji, Sylvia Alexius, Mohamed M. Ali, Abdullah Alkandari, Ala’a Alkerwi, Buthaina M. Alkhatib, Kristine Allin, Mar Alvarez-Pedrerol, Eman Aly, Deepak N. Amarapurkar, Pilar Amiano Etxezarreta, John Amoah, Norbert Amougou, Philippe Amouyel, Lars Bo Andersen, Sigmund A. Anderssen, Odysseas Androutsos, Lars Ängquist, Ranjit Mohan Anjana, Alireza Ansari-Moghaddam, Elena Anufrieva, Hajer Aounallah-Skhiri, Joana Araújo, Inger Ariansen, Tahir Aris, Raphael E. Arku, Nimmathota Arlappa, Krishna K. Aryal, Nega Aseffa, Thor Aspelund, Felix K. Assah, Batyrbek Assembekov, Maria Cecília F. Assunção, May Soe Aung, Juha Auvinen, Mária Avdičová, Shina Avi, Ana Azevedo, Mohsen Azimi-Nezhad, Fereidoun Azizi, Mehrdad Azmin, Bontha V. Babu, Maja Bæksgaard Jørgensen, Azli Baharudin, Suhad Bahijri, Marta Bakacs, Nagalla Balakrishna, Yulia Balanova, Mohamed Bamoshmoosh, Maciej Banach, José R. Banegas, Joanna Baran, Rafał Baran, Carlo M. Barbagallo, Valter Barbosa Filho, Alberto Barceló, Maja Baretić, Amina Barkat, Joaquin Barnoya, Lena Barrera, Marta Barreto, Aluisio J. D. Barros, Mauro Virgílio Gomes Barros, Anna Bartosiewicz, Abdul Basit, Joao Luiz D. Bastos, Iqbal Bata, Anwar M. Batieha, Aline P. Batista, Rosangela L. Batista, Zhamilya Battakova, Louise A. Baur, Pascal M. Bayauli, Robert Beaglehole, Silvia Bel-Serrat, Antonisamy Belavendra, Habiba Ben Romdhane, Judith Benedics, Mikhail Benet, Gilda Estela Benitez Rolandi, Elling Bere, Ingunn Holden Bergh, Yemane Berhane, Salim Berkinbayev, Antonio Bernabe-Ortiz, Gailute Bernotiene, Ximena Berrios Carrasola, Heloísa Bettiol, Manfred E. Beutel, Augustin F. Beybey, Jorge Bezerra, Aroor Bhagyalaxmi, Sumit Bharadwaj, Santosh K. Bhargava, Hongsheng Bi, Yufang Bi, Daniel Bia, Katia Biasch, Elysée Claude Bika Lele, Mukharram M. Bikbov, Bihungum Bista, Dusko J. Bjelica, Anne A. Bjerregaard, Peter Bjerregaard, Espen Bjertness, Marius B. Bjertness, Cecilia Björkelund, Katia V. Bloch, Anneke Blokstra, Moran Blychfeld Magnazu, Simona Bo, Martin Bobak, Lynne M. Boddy, Bernhard O. Boehm, Jolanda M. A. Boer, Jose G. Boggia, Elena Bogova, Carlos P. Boissonnet, Stig E. Bojesen, Marialaura Bonaccio, Vanina Bongard, Alice Bonilla-Vargas, Matthias Bopp, Herman Borghs, Pascal Bovet, Khadichamo Boymatova, Lien Braeckevelt, Lutgart Braeckman, Marjolijn C. E. Bragt, Imperia Brajkovich, Francesco Branca, Juergen Breckenkamp, João Breda, Hermann Brenner, Lizzy M. Brewster, Garry R. Brian, Yajaira Briceño, Lacramioara Brinduse, Miguel Brito, Sinead Brophy, Johannes Brug, Graziella Bruno, Anna Bugge, Frank Buntinx, Marta Buoncristiano, Genc Burazeri, Con Burns, Antonio Cabrera de León, Joseph Cacciottolo, Hui Cai, Roberta B. Caixeta, Tilema Cama, Christine Cameron, José Camolas, Günay Can, Ana Paula C. Cândido, Felicia Cañete, Mario V. Capanzana, Naděžda Čapková, Eduardo Capuano, Rocco Capuano, Vincenzo Capuano, Marloes Cardol, Viviane C. Cardoso, Axel C. Carlsson, Esteban Carmuega, Joana Carvalho, José A. Casajús, Felipe F. Casanueva, Maribel Casas, Ertugrul Celikcan, Laura Censi, Marvin Cervantes‐Loaiza, Juraci A. Cesar, Snehalatha Chamukuttan, Angelique Chan, Queenie Chan, Himanshu K. Chaturvedi, Nish Chaturvedi, Norsyamlina Che Abdul Rahim, Miao Li Chee, Chien-Jen Chen, Fangfang Chen, Huashuai Chen, Shuohua Chen, Zhengming Chen, Ching-Yu Cheng, Yiling J. Cheng, Bahman Cheraghian, Angela Chetrit, Ekaterina Chikova-Iscener, Mai J. M. Chinapaw, Anne Chinnock, Arnaud Chiolero, Shu-Ti Chiou, María-Dolores Chirlaque, Belong Cho, Kaare Christensen, Diego G. Christofaro, Jerzy Chudek, Renata Cifkova, Michelle Cilia, Eliza Cinteza, Massimo Cirillo, Frank Claessens, Janine Clarke, Els Clays, Emmanuel Cohen, Laura-María Compañ-Gabucio, Hans Concin, Susana C. Confortin, Cyrus Cooper, Tara C. Coppinger, Eva Corpeleijn, Lilia Yadira Cortés, Simona Costanzo, Dominique Cottel, Chris Cowell, Cora L. Craig, Amelia C. Crampin, Amanda J. Cross, Ana B. Crujeiras, Juan J. Cruz, Tamás Csányi, Semánová Csilla, Alexandra M. Cucu, Liufu Cui, Felipe V. Cureau, Sarah Cuschieri, Ewelina Czenczek-Lewandowska, Graziella D’Arrigo, Eleonora d’Orsi, Liliana Dacica, Jean Dallongeville, Albertino Damasceno, Camilla T. Damsgaard, Rachel Dankner, Thomas M. Dantoft, Parasmani Dasgupta, Saeed Dastgiri, Luc Dauchet, Kairat Davletov, Maria Alice Altenburg de Assis, Guy De Backer, Dirk De Bacquer, Amalia De Curtis, Patrícia de Fragas Hinnig, Giovanni de Gaetano, Stefaan De Henauw, Pilar De Miguel-Etayo, Paula Duarte de Oliveira, David De Ridder, Karin De Ridder, Susanne R. de Rooij, Delphine De Smedt, Mohan Deepa, Alexander D. Deev, Vincent DeGennaro, Jr, Hélène Delisle, Francis Delpeuch, Stefaan Demarest, Elaine Dennison, Katarzyna Dereń, Valérie Deschamps, Meghnath Dhimal, Augusto Di Castelnuovo, Juvenal Soares Dias-da-Costa, María Elena Díaz-Sánchez, Alejandro Diaz, Pedro Díaz Fernández, María Pilar Díez Ripollés, Zivka Dika, Shirin Djalalinia, Visnja Djordjic, Ha T. P. Do, Annette J. Dobson, Liria Dominguez, Maria Benedetta Donati, Chiara Donfrancesco, Guanghui Dong, Yanhui Dong, Silvana P. Donoso, Angela Döring, Maria Dorobantu, Ahmad Reza Dorosty, Kouamelan Doua, Nico Dragano, Wojciech Drygas, Jia Li Duan, Charmaine A. Duante, Priscilla Duboz, Vesselka L. Duleva, Virginija Dulskiene, Samuel C. Dumith, Anar Dushpanova, Azhar Dyussupova, Vilnis Dzerve, Elzbieta Dziankowska-Zaborszczyk, Guadalupe Echeverría, Ricky Eddie, Ebrahim Eftekhar, Eruke E. Egbagbe, Robert Eggertsen, Sareh Eghtesad, Gabriele Eiben, Ulf Ekelund, Mohammad El-Khateeb, Laila El Ammari, Jalila El Ati, Denise Eldemire-Shearer, Marie Eliasen, Paul Elliott, Ronit Endevelt, Reina Engle-Stone, Rajiv T. Erasmus, Raimund Erbel, Cihangir Erem, Gul Ergor, Louise Eriksen, Johan G. Eriksson, Jorge Escobedo-de la Peña, Saeid Eslami, Ali Esmaeili, Alun Evans, David Faeh, Ildar Fakhradiyev, Albina A. Fakhretdinova, Caroline H. Fall, Elnaz Faramarzi, Mojtaba Farjam, Victoria Farrugia Sant’Angelo, Mohammad Reza Fattahi, Asher Fawwad, Wafaie W. Fawzi, Edit Feigl, Francisco J. Felix-Redondo, Trevor S. Ferguson, Romulo A. Fernandes, Daniel Fernández-Bergés, Daniel Ferrante, Thomas Ferrao, Gerson Ferrari, Marika Ferrari, Marco M. Ferrario, Catterina Ferreccio, Haroldo S. Ferreira, Eldridge Ferrer, Jean Ferrieres, Thamara Hubler Figueiró, Anna Fijalkowska, Mauro Fisberg, Krista Fischer, Leng Huat Foo, Maria Forsner, Heba M. Fouad, Damian K. Francis, Maria do Carmo Franco, Zlatko Fras, Guillermo Frontera, Flavio D. Fuchs, Sandra C. Fuchs, Isti I. Fujiati, Yuki Fujita, Matsuda Fumihiko, Viktoriya Furdela, Takuro Furusawa, Zbigniew Gaciong, Mihai Gafencu, Manuel Galán Cuesta, Andrzej Galbarczyk, Henrike Galenkamp, Daniela Galeone, Myriam Galfo, Fabio Galvano, Jingli Gao, Pei Gao, Manoli Garcia-de-la-Hera, María José García Mérida, Marta García Solano, Dickman Gareta, Sarah P. Garnett, Jean-Michel Gaspoz, Magda Gasull, Adroaldo Cesar Araujo Gaya, Anelise Reis Gaya, Andrea Gazzinelli, Ulrike Gehring, Harald Geiger, Johanna M. Geleijnse, Ronnie George, Ebrahim Ghaderi, Ali Ghanbari, Erfan Ghasemi, Oana-Florentina Gheorghe-Fronea, Alessandro Gialluisi, Simona Giampaoli, Francesco Gianfagna, Christian Gieger, Tiffany K. Gill, Jonathan Giovannelli, Glen Gironella, Aleksander Giwercman, Konstantinos Gkiouras, Natalya Glushkova, Natalja Gluškova, Ramesh Godara, Justyna Godos, Sibel Gogen, Marcel Goldberg, David Goltzman, Georgina Gómez, Jesús Humberto Gómez Gómez, Luis F. Gomez, Santiago F. Gómez, Aleksandra Gomula, Bruna Gonçalves Cordeiro da Silva, Helen Gonçalves, Mauer Gonçalves, Ana D. González-Alvarez, David A. Gonzalez-Chica, Esther M. González-Gil, Marcela Gonzalez-Gross, Margot González-Leon, Juan P. González-Rivas, Clicerio González-Villalpando, María-Elena González-Villalpando, Angel R. Gonzalez, Frederic Gottrand, Antonio Pedro Graça, Sidsel Graff-Iversen, Dušan Grafnetter, Aneta Grajda, Maria G. Grammatikopoulou, Ronald D. Gregor, Maria João Gregório, Else Karin Grøholt, Anders Grøntved, Giuseppe Grosso, Gabriella Gruden, Dongfeng Gu, Viviana Guajardo, Emanuela Gualdi-Russo, Pilar Guallar-Castillón, Andrea Gualtieri, Elias F. Gudmundsson, Vilmundur Gudnason, Ramiro Guerrero, Idris Guessous, Andre L. Guimaraes, Martin C. Gulliford, Johanna Gunnlaugsdottir, Marc J. Gunter, Xiu-Hua Guo, Yin Guo, Prakash C. Gupta, Rajeev Gupta, Oye Gureje, Enrique Gutiérrez González, Laura Gutierrez, Felix Gutzwiller, Xinyi Gwee, Seongjun Ha, Farzad Hadaegh, Charalambos A. Hadjigeorgiou, Rosa Haghshenas, Hamid Hakimi, Jytte Halkjær, Ian R. Hambleton, Behrooz Hamzeh, Willem A. Hanekom, Dominique Hange, Abu A. M. Hanif, Sari Hantunen, Jie Hao, Carla Menêses Hardman, Rachakulla Hari Kumar, Tina Harmer Lassen, Javad Harooni, Seyed Mohammad Hashemi-Shahri, Maria Hassapidou, Jun Hata, Teresa Haugsgjerd, Alison J. Hayes, Jiang He, Yuan He, Yuna He, Regina Heidinger-Felső, Margit Heier, Tatjana Hejgaard, Marleen Elisabeth Hendriks, Rafael dos Santos Henrique, Ana Henriques, Leticia Hernandez Cadena, Sauli Herrala, Marianella Herrera-Cuenca, Victor M. Herrera, Isabelle Herter-Aeberli, Karl-Heinz Herzig, Ramin Heshmat, Allan G. Hill, Sai Yin Ho, Suzanne C. Ho, Michael Hobbs, Doroteia A. Höfelmann, Michelle Holdsworth, Reza Homayounfar, Clara Homs, Wilma M. Hopman, Andrea R. V. R. Horimoto, Claudia M. Hormiga, Bernardo L. Horta, Leila Houti, Christina Howitt, Thein Thein Htay, Aung Soe Htet, Maung Maung Than Htike, Yonghua Hu, José María Huerta, Ilpo Tapani Huhtaniemi, Laetitia Huiart, Constanta Huidumac Petrescu, Martijn Huisman, Abdullatif Husseini, Chinh Nguyen Huu, Inge Huybrechts, Nahla Hwalla, Jolanda Hyska, Licia Iacoviello, Ellina M. Iakupova, Jesús M. Ibarluzea, Mohsen M. Ibrahim, Norazizah Ibrahim Wong, M. Arfan Ikram, Carmen Iñiguez, Violeta Iotova, Vilma E. Irazola, Takafumi Ishida, Godsent C. Isiguzo, Muhammad Islam, Sheikh Mohammed Shariful Islam, Duygu Islek, Ivaila Y. Ivanova-Pandourska, Masanori Iwasaki, Tuija Jääskeläinen, Rod T. Jackson, Jeremy M. Jacobs, Michel Jadoul, Tazeen Jafar, Bakary Jallow, Kenneth James, Kazi M. Jamil, Konrad Jamrozik, Anna Jansson, Imre Janszky, Edward Janus, Juel Jarani, Marjo-Riitta Jarvelin, Grazyna Jasienska, Ana Jelaković, Bojan Jelaković, Garry Jennings, Chao Qiang Jiang, Ramon O. Jimenez, Karl-Heinz Jöckel, Michel Joffres, Jari J. Jokelainen, Jost B. Jonas, Jitendra Jonnagaddala, Torben Jørgensen, Pradeep Joshi, Josipa Josipović, Farahnaz Joukar, Jacek J. Jóźwiak, Debra S. Judge, Anne Juolevi, Gregor Jurak, Iulia Jurca Simina, Vesna Juresa, Rudolf Kaaks, Felix O. Kaducu, Anthony Kafatos, Mónika Kaj, Eero O. Kajantie, Natia Kakutia, Daniela Kállayová, Zhanna Kalmatayeva, Ofra Kalter-Leibovici, Yves Kameli, Freja B. Kampmann, Kodanda R. Kanala, Srinivasan Kannan, Efthymios Kapantais, Eva Karaglani, Argyro Karakosta, Line L. Kårhus, Khem B. Karki, Philippe B. Katchunga, Marzieh Katibeh, Joanne Katz, Peter T. Katzmarzyk, Jussi Kauhanen, Prabhdeep Kaur, Maryam Kavousi, Gyulli M. Kazakbaeva, François F. Kaze, Calvin Ke, Ulrich Keil, Lital Keinan Boker, Sirkka Keinänen-Kiukaanniemi, Roya Kelishadi, Cecily Kelleher, Han C. G. Kemper, Maryam Keramati, Alina Kerimkulova, Mathilde Kersting, Timothy Key, Yousef Saleh Khader, Arsalan Khaledifar, Davood Khalili, Kay-Tee Khaw, Bahareh Kheiri, Motahareh Kheradmand, Alireza Khosravi, Ilse M. S. L. Khouw, Ursula Kiechl-Kohlendorfer, Sophia J. Kiechl, Stefan Kiechl, Japhet Killewo, Hyeon Chang Kim, Jeongseon Kim, Jenny M. Kindblom, Andrew Kingston, Heidi Klakk, Magdalena Klimek, Jeannette Klimont, Jurate Klumbiene, Michael Knoflach, Bhawesh Koirala, Elin Kolle, Patrick Kolsteren, Jürgen König, Raija Korpelainen, Paul Korrovits, Magdalena Korzycka, Jelena Kos, Seppo Koskinen, Katsuyasu Kouda, Éva Kovács, Viktoria Anna Kovacs, Irina Kovalskys, Sudhir Kowlessur, Slawomir Koziel, Jana Kratenova, Wolfgang Kratzer, Vilma Kriaucioniene, Susi Kriemler, Peter Lund Kristensen, Helena Krizan, Maria F. Kroker-Lobos, Steinar Krokstad, Daan Kromhout, Herculina S. Kruger, Ruan Kruger, Łukasz Kryst, Ruzena Kubinova, Renata Kuciene, Urho M. Kujala, Enisa Kujundzic, Zbigniew Kulaga, Mukhtar Kulimbet, R. Krishna Kumar, Marie Kunešová, Pawel Kurjata, Yadlapalli S. Kusuma, Vladimir Kutsenko, Kari Kuulasmaa, Catherine Kyobutungi, Quang Ngoc La, Fatima Zahra Laamiri, Carl Lachat, Karl J. Lackner, Youcef Laid, Lachmie Lall, Tai Hing Lam, Maritza Landaeta Jimenez, Edwige Landais, Vera Lanska, Georg Lappas, Bagher Larijani, Simo Pone Larissa, Tint Swe Latt, Martino Laurenzi, Laura Lauria, Maria Lazo-Porras, Gwenaëlle Le Coroller, Khanh Le Nguyen Bao, Agnès Le Port, Tuyen D. Le, Jeannette Lee, Jeonghee Lee, Paul H. Lee, Nils Lehmann, Terho Lehtimäki, Daniel Lemogoum, Branimir Leskošek, Justyna Leszczak, Katja B. Leth-Møller, Gabriel M. Leung, Naomi S. Levitt, Yanping Li, Merike Liivak, Christa L. Lilly, Charlie Lim, Wei-Yen Lim, M. Fernanda Lima-Costa, Hsien-Ho Lin, Xu Lin, Yi-Ting Lin, Lars Lind, Vijaya Lingam, Birgit Linkohr, Allan Linneberg, Lauren Lissner, Mieczyslaw Litwin, Jing Liu, Lijuan Liu, Wei-Cheng Lo, Helle-Mai Loit, Khuong Quynh Long, Guadalupe Longo Abril, Luis Lopes, Marcus V. V. Lopes, Oscar Lopes, Esther Lopez-Garcia, Tania Lopez, Paulo A. Lotufo, José Eugenio Lozano, Janice L. Lukrafka, Dalia Luksiene, Annamari Lundqvist, Nuno Lunet, Charles Lunogelo, Michala Lustigová, Edyta Łuszczki, Jean-René M’Buyamba-Kabangu, Guansheng Ma, Xu Ma, George L. L. Machado-Coelho, Aristides M. Machado-Rodrigues, Enguerran Macia, Luisa M. Macieira, Ahmed A. Madar, Anja L. Madsen, Gladys E. Maestre, Stefania Maggi, Dianna J. Magliano, Sara Magnacca, Emmanuella Magriplis, Gowri Mahasampath, Bernard Maire, Marjeta Majer, Marcia Makdisse, Päivi Mäki, Fatemeh Malekzadeh, Reza Malekzadeh, Rahul Malhotra, Kodavanti Mallikharjuna Rao, Sofia K. Malyutina, Lynell V. Maniego, Yannis Manios, Masimango Imani Manix, Jim I. Mann, Fariborz Mansour-Ghanaei, Taru Manyanga, Enzo Manzato, Anie Marcil, Paula Margozzini, Joany Mariño, Anastasia Markaki, Oonagh Markey, Eliza Markidou Ioannidou, Pedro Marques-Vidal, Larissa Pruner Marques, Jaume Marrugat, Yves Martin-Prevel, Rosemarie Martin, Reynaldo Martorell, Eva Martos, Katharina Maruszczak, Stefano Marventano, Giovanna Masala, Luis P. Mascarenhas, Shariq R. Masoodi, Ellisiv B. Mathiesen, Prashant Mathur, Alicia Matijasevich, Piotr Matłosz, Tandi E. Matsha, Victor Matsudo, Christina Mavrogianni, Artur Mazur, Jean Claude N. Mbanya, Shelly R. McFarlane, Stephen T. McGarvey, Martin McKee, Stela McLachlan, Rachael M. McLean, Scott B. McLean, Margaret L. McNairy, Breige A. McNulty, Sounnia Mediene Benchekor, Jurate Medzioniene, Parinaz Mehdipour, Kirsten Mehlig, Amir Houshang Mehrparvar, Aline Meirhaeghe, Jørgen Meisfjord, Christa Meisinger, Jesus D. Melgarejo, Marina Melkumova, João Mello, Fabián Méndez, Carlos O. Mendivil, Ana Maria B. Menezes, Geetha R. Menon, Gert B. M. Mensink, Maria Teresa Menzano, Indrapal I. Meshram, Diane T. Meto, Jie Mi, Kim F. Michaelsen, Nathalie Michels, Kairit Mikkel, Karolina Miłkowska, Jody C. Miller, Olga Milushkina, Cláudia S. Minderico, G. K. Mini, Juan Francisco Miquel, J. Jaime Miranda, Mohammad Reza Mirjalili, Daphne Mirkopoulou, Erkin Mirrakhimov, Marjeta Mišigoj-Duraković, Antonio Mistretta, Veronica Mocanu, Pietro A. Modesti, Sahar Saeedi Moghaddam, Bahram Mohajer, Mostafa K. Mohamed, Shukri F. Mohamed, Kazem Mohammad, Mohammad Reza Mohammadi, Zahra Mohammadi, Noushin Mohammadifard, Reza Mohammadpourhodki, Viswanathan Mohan, Salim Mohanna, Muhammad Fadhli Mohd Yusoff, Iraj Mohebbi, Farnam Mohebi, Marie Moitry, Line T. Møllehave, Niels C. Møller, Dénes Molnár, Amirabbas Momenan, Charles K. Mondo, Roger A. Montenegro Mendoza, Eric Monterrubio-Flores, Kotsedi Daniel K. Monyeki, Jin Soo Moon, Mahmood Moosazadeh, Hermine T. Mopa, Farhad Moradpour, Leila B. Moreira, Alain Morejon, Luis A. Moreno, Francis Morey, Karen Morgan, Suzanne N. Morin, Erik Lykke Mortensen, George Moschonis, Alireza Moslem, Malgorzata Mossakowska, Aya Mostafa, Seyed-Ali Mostafavi, Anabela Mota-Pinto, Jorge Mota, Mohammad Esmaeel Motlagh, Jorge Motta, Marcos André Moura-dos-Santos, Yeva Movsesyan, Kelias P. Msyamboza, Thet Thet Mu, Magdalena Muc, Florian Muca, Boban Mugoša, Maria L. Muiesan, Martina Müller-Nurasyid, Thomas Münzel, Jaakko Mursu, Elaine M. Murtagh, Kamarul Imran Musa, Sanja Musić Milanović, Vera Musil, Geofrey Musinguzi, Muel Telo M. C. Muyer, Iraj Nabipour, Shohreh Naderimagham, Gabriele Nagel, Farid Najafi, Harunobu Nakamura, Hanna Nalecz, Jana Námešná, Ei Ei K. Nang, Vinay B. Nangia, Martin Nankap, Sameer Narake, Paola Nardone, Take Naseri, Matthias Nauck, William A. Neal, Azim Nejatizadeh, Chandini Nekkantti, Keiu Nelis, Ilona Nenko, Martin Neovius, Flavio Nervi, Tze Pin Ng, Chung T. Nguyen, Nguyen D. Nguyen, Quang Ngoc Nguyen, Michael Y. Ni, Rodica Nicolescu, Peng Nie, Ramfis E. Nieto-Martínez, Yury P. Nikitin, Guang Ning, Toshiharu Ninomiya, Nobuo Nishi, Sania Nishtar, Marianna Noale, Oscar A. Noboa, Helena Nogueira, Maria Nordendahl, Børge G. Nordestgaard, Davide Noto, Natalia Nowak-Szczepanska, Mohannad Al Nsour, Irfan Nuhoğlu, Baltazar Nunes, Eha Nurk, Fred Nuwaha, Moffat Nyirenda, Terence W. O’Neill, Dermot O’Reilly, Galina Obreja, Caleb Ochimana, Angélica M. Ochoa-Avilés, Eiji Oda, Augustine N. Odili, Kyungwon Oh, Kumiko Ohara, Claes Ohlsson, Ryutaro Ohtsuka, Örn Olafsson, Maria Teresa A. Olinto, Isabel O. Oliveira, Mohd Azahadi Omar, Saeed M. Omar, Altan Onat, Sok King Ong, N. Charlotte Onland-Moret, Lariane M. Ono, Pedro Ordunez, Rui Ornelas, Ana P. Ortiz, Pedro J. Ortiz, Merete Osler, Clive Osmond, Sergej M. Ostojic, Afshin Ostovar, Johanna A. Otero, Kim Overvad, Ellis Owusu-Dabo, Fred Michel Paccaud, Ioannis Pagkalos, Elena Pahomova, Karina Mary de Paiva, Andrzej Pająk, Alberto Palloni, Luigi Palmieri, Wen-Harn Pan, Songhomitra Panda-Jonas, Arvind Pandey, Francesco Panza, Mariela Paoli, Sousana K. Papadopoulou, Dimitrios Papandreou, Rossina G. Pareja, Soon-Woo Park, Suyeon Park, Winsome R. Parnell, Mahboubeh Parsaeian, Ionela M. Pascanu, Patrick Pasquet, Nikhil D. Patel, Marcos Pattussi, Halyna Pavlyshyn, Raimund Pechlaner, Ivan Pećin, Mangesh S. Pednekar, João M. Pedro, Nasheeta Peer, Sergio Viana Peixoto, Markku Peltonen, Alexandre C. Pereira, Marco A. Peres, Cynthia M. Pérez, Valentina Peterkova, Annette Peters, Astrid Petersmann, Janina Petkeviciene, Ausra Petrauskiene, Olga Petrovna Kovtun, Emanuela Pettenuzzo, Niloofar Peykari, Norbert Pfeiffer, Modou Cheyassin Phall, Son Thai Pham, Rafael N. Pichardo, Daniela Pierannunzio, Iris Pigeot, Hynek Pikhart, Aida Pilav, Lorenza Pilotto, Francesco Pistelli, Freda Pitakaka, Aleksandra Piwonska, Andreia N. Pizarro, Pedro Plans-Rubió, Alina G. Platonova, Bee Koon Poh, Hermann Pohlabeln, Nadija S. Polka, Raluca M. Pop, Stevo R. Popovic, Miquel Porta, Georg Posch, Anil Poudyal, Dimitrios Poulimeneas, Hamed Pouraram, Farhad Pourfarzi, Akram Pourshams, Hossein Poustchi, Rajendra Pradeepa, Alison J. Price, Jacqueline F. Price, Antonio Prista, Rui Providencia, Jardena J. Puder, Iveta Pudule, Maria Puiu, Margus Punab, Muhammed S. Qadir, Radwan F. Qasrawi, Mostafa Qorbani, Hedley K. Quintana, Pedro J. Quiroga-Padilla, Tran Quoc Bao, Stefan Rach, Ivana Radic, Ricardas Radisauskas, Salar Rahimikazerooni, Mahfuzar Rahman, Mahmudur Rahman, Olli Raitakari, Manu Raj, Tamerlan Rajabov, Sherali Rakhmatulloev, Ivo Rakovac, Sudha Ramachandra Rao, Ambady Ramachandran, Otim P. C. Ramadan, Virgílio V. Ramires, Jacqueline Ramke, Elisabete Ramos, Rafel Ramos, Lekhraj Rampal, Sanjay Rampal, Lalka S. Rangelova, Vayia Rarra, Ramon A. Rascon-Pacheco, Cassiano Ricardo Rech, Josep Redon, Paul Ferdinand M. Reganit, Valéria Regecová, Jane D. P. Renner, Judit A. Repasy, Cézane P. Reuter, Luis Revilla, Abbas Rezaianzadeh, Yeunsook Rho, Lourdes Ribas-Barba, Robespierre Ribeiro, Elio Riboli, Adrian Richter, Fernando Rigo, Attilio Rigotti, Natascia Rinaldo, Tobias F. Rinke de Wit, Ana I. Rito, Raphael M. Ritti-Dias, Juan A. Rivera, Reina G. Roa, Louise Robinson, Cynthia Robitaille, Romana Roccaldo, Daniela Rodrigues, Fernando Rodríguez-Artalejo, María del Cristo Rodriguez-Perez, Laura A. Rodríguez-Villamizar, Andrea Y. Rodríguez, Ulla Roggenbuck, Peter Rohloff, Fabian Rohner, Rosalba Rojas-Martinez, Nipa Rojroongwasinkul, Dora Romaguera, Elisabetta L. Romeo, Rafaela V. Rosario, Annika Rosengren, Ian Rouse, Vanessa Rouzier, Joel G. R. Roy, Maira H. Ruano, Adolfo Rubinstein, Frank J. Rühli, Jean-Bernard Ruidavets, Blanca Sandra Ruiz-Betancourt, Maria Ruiz-Castell, Emma Ruiz Moreno, Iuliia A. Rusakova, Kenisha Russell Jonsson, Paola Russo, Petra Rust, Marcin Rutkowski, Marge Saamel, Charumathi Sabanayagam, Hamideh Sabbaghi, Elena Sacchini, Harshpal S. Sachdev, Alireza Sadjadi, Ali Reza Safarpour, Sare Safi, Saeid Safiri, Mohammad Hossien Saghi, Olfa Saidi, Nader Saki, Sanja Šalaj, Benoit Salanave, Eduardo Salazar Martinez, Calogero Saleva, Diego Salmerón, Veikko Salomaa, Jukka T. Salonen, Massimo Salvetti, Margarita Samoutian, Jose Sánchez-Abanto, Inés Sánchez Rodríguez, Sandjaja, Susana Sans, Loreto Santa Marina, Ethel Santacruz, Diana A. Santos, Ina S. Santos, Lèlita C. Santos, Maria Paula Santos, Osvaldo Santos, Rute Santos, Tamara R. Santos, Jouko L. Saramies, Luis B. Sardinha, Nizal Sarrafzadegan, Thirunavukkarasu Sathish, Kai-Uwe Saum, Savvas Savva, Mathilde Savy, Norie Sawada, Mariana Sbaraini, Marcia Scazufca, Beatriz D. Schaan, Angelika Schaffrath Rosario, Herman Schargrodsky, Anja Schienkiewitz, Karin Schindler, Sabine Schipf, Carsten O. Schmidt, Ida Maria Schmidt, Andrea Schneider, Peter Schnohr, Ben Schöttker, Sara Schramm, Stine Schramm, Helmut Schröder, Constance Schultsz, Matthias B. Schulze, Aletta E. Schutte, Sylvain Sebert, Moslem Sedaghattalab, Rusidah Selamat, Vedrana Sember, Abhijit Sen, Idowu O. Senbanjo, Sadaf G. Sepanlou, Guillermo Sequera, Luis Serra-Majem, Jennifer Servais, Ľudmila Ševčíková, Svetlana Shalnova, Teresa Shamah-Levy, Seyed Morteza Shamshirgaran, Coimbatore Subramaniam Shanthirani, Maryam Sharafkhah, Sanjib K. Sharma, Jonathan E. Shaw, Amaneh Shayanrad, Ali Akbar Shayesteh, Lela Shengelia, Zumin Shi, Kenji Shibuya, Hana Shimizu-Furusawa, Tal Shimony, Rahman Shiri, Namuna Shrestha, Khairil Si-Ramlee, Alfonso Siani, Rosalynn Siantar, Abla M. Sibai, Labros S. Sidossis, Natalia Silitrari, Antonio M. Silva, Caroline Ramos de Moura Silva, Diego Augusto Santos Silva, Kelly S. Silva, Xueling Sim, Mary Simon, Judith Simons, Leon A. Simons, Agneta Sjöberg, Michael Sjöström, Natalia A. Skoblina, Gry Skodje, Tatyana Slazhnyova, Jolanta Slowikowska-Hilczer, Przemysław Slusarczyk, Liam Smeeth, Hung-Kwan So, Fernanda Cunha Soares, Grzegorz Sobek, Eugène Sobngwi, Morten Sodemann, Stefan Söderberg, Moesijanti Y. E. Soekatri, Agustinus Soemantri, Reecha Sofat, Vincenzo Solfrizzi, Mohammad Hossein Somi, Emily Sonestedt, Yi Song, Sajid Soofi, Thorkild I. A. Sørensen, Elin P. Sørgjerd, Charles Sossa Jérome, Victoria E. Soto-Rojas, Aïcha Soumaré, Alfonso Sousa-Poza, Slavica Sovic, Bente Sparboe-Nilsen, Karen Sparrenberger, Phoebe R. Spencer, Angela Spinelli, Igor Spiroski, Jan A. Staessen, Hanspeter Stamm, Kaspar Staub, Bill Stavreski, Jostein Steene-Johannessen, Peter Stehle, Aryeh D. Stein, George S. Stergiou, Jochanan Stessman, Ranko Stevanović, Jutta Stieber, Doris Stöckl, Jakub Stokwiszewski, Ekaterina Stoyanova, Gareth Stratton, Karien Stronks, Maria Wany Strufaldi, Lela Sturua, Ramón Suárez-Medina, Machi Suka, Chien-An Sun, Liang Sun, Johan Sundström, Yn-Tz Sung, Jordi Sunyer, Paibul Suriyawongpaisal, Nabil William G. Sweis, Boyd A. Swinburn, Rody G. Sy, René Charles Sylva, Moyses Szklo, Lucjan Szponar, Lorraine Tabone, E. Shyong Tai, Konstantinos D. Tambalis, Mari-Liis Tammesoo, Abdonas Tamosiunas, Eng Joo Tan, Xun Tang, Maya Tanrygulyyeva, Frank Tanser, Yong Tao, Mohammed Rasoul Tarawneh, Jakob Tarp, Carolina B. Tarqui-Mamani, Radka Taxová Braunerová, Anne Taylor, Julie Taylor, Félicité Tchibindat, Saskia Te Velde, William R. Tebar, Grethe S. Tell, Tania Tello, Yih Chung Tham, K. R. Thankappan, Holger Theobald, Xenophon Theodoridis, Nihal Thomas, Barbara Thorand, Betina H. Thuesen, Ľubica Tichá, Erik J. Timmermans, Dwi H. Tjandrarini, Anne Tjonneland, Hanna K. Tolonen, Janne S. Tolstrup, Murat Topbas, Roman Topór-Mądry, Liv Elin Torheim, María José Tormo, Michael J. Tornaritis, Maties Torrent, Laura Torres-Collado, Stefania Toselli, Giota Touloumi, Pierre Traissac, Thi Tuyet-Hanh Tran, Mark S. Tremblay, Areti Triantafyllou, Dimitrios Trichopoulos, Antonia Trichopoulou, Oanh T. H. Trinh, Atul Trivedi, Yu-Hsiang Tsao, Lechaba Tshepo, Maria Tsigga, Panagiotis Tsintavis, Shoichiro Tsugane, John Tuitele, Azaliia M. Tuliakova, Marshall K. Tulloch-Reid, Fikru Tullu, Tomi-Pekka Tuomainen, Jaakko Tuomilehto, Maria L. Turley, Gilad Twig, Per Tynelius, Evangelia Tzala, Themistoklis Tzotzas, Christophe Tzourio, Peter Ueda, Eunice Ugel, Flora A. M. Ukoli, Hanno Ulmer, Belgin Unal, Zhamyila Usupova, Hannu M. T. Uusitalo, Nalan Uysal, Justina Vaitkeviciute, Gonzalo Valdivia, Susana Vale, Damaskini Valvi, Rob M. van Dam, Bert-Jan van den Born, Johan Van der Heyden, Yvonne T. van der Schouw, Koen Van Herck, Wendy Van Lippevelde, Hoang Van Minh, Natasja M. Van Schoor, Irene G. M. van Valkengoed, Dirk Vanderschueren, Diego Vanuzzo, Anette Varbo, Gregorio Varela-Moreiras, Luz Nayibe Vargas, Patricia Varona-Pérez, Senthil K. Vasan, Daniel G. Vasques, Tomas Vega, Toomas Veidebaum, Gustavo Velasquez-Melendez, Biruta Velika, Maïté Verloigne, Giovanni Veronesi, W. M. Monique Verschuren, Cesar G. Victora, Giovanni Viegi, Lucie Viet, Frøydis N. Vik, Monica Vilar, Salvador Villalpando, Jesus Vioque, Jyrki K. Virtanen, Sophie Visvikis-Siest, Bharathi Viswanathan, Mihaela Vladulescu, Tiina Vlasoff, Dorja Vocanec, Peter Vollenweider, Henry Völzke, Ari Voutilainen, Martine Vrijheid, Tanja G. M. Vrijkotte, Alisha N. Wade, Thomas Waldhör, Janette Walton, Elvis O. A. Wambiya, Wan Mohamad Wan Bebakar, Wan Nazaimoon Wan Mohamud, Rildo de Souza Wanderley Júnior, Ming-Dong Wang, Ningli Wang, Qian Wang, Xiangjun Wang, Ya Xing Wang, Ying-Wei Wang, S. Goya Wannamethee, Nicholas Wareham, Adelheid Weber, Karen Webster-Kerr, Niels Wedderkopp, Daniel Weghuber, Wenbin Wei, Aneta Weres, Bo Werner, Leo D. Westbury, Peter H. Whincup, Kremlin Wickramasinghe, Kurt Widhalm, Indah S. Widyahening, Andrzej Więcek, Philipp S. Wild, Rainford J. Wilks, Johann Willeit, Peter Willeit, Julianne Williams, Tom Wilsgaard, Rusek Wojciech, Bogdan Wojtyniak, Kathrin Wolf, Roy A. Wong-McClure, Andrew Wong, Emily B. Wong, Jyh Eiin Wong, Tien Yin Wong, Jean Woo, Mark Woodward, Frederick C. Wu, Hon-Yen Wu, Jianfeng Wu, Li Juan Wu, Shouling Wu, Justyna Wyszyńska, Haiquan Xu, Liang Xu, Nor Azwany Yaacob, Uruwan Yamborisut, Weili Yan, Ling Yang, Xiaoguang Yang, Yang Yang, Nazan Yardim, Tabara Yasuharu, Martha Yépez García, Panayiotis K. Yiallouros, Agneta Yngve, Moein Yoosefi, Akihiro Yoshihara, Qi Sheng You, San-Lin You, Novie O. Younger-Coleman, Yu-Ling Yu, Yunjiang Yu, Safiah Md Yusof, Ahmad Faudzi Yusoff, Luciana Zaccagni, Vassilis Zafiropulos, Ahmad A. Zainuddin, Seyed Rasoul Zakavi, Farhad Zamani, Sabina Zambon, Antonis Zampelas, Hana Zamrazilová, Maria Elisa Zapata, Abdul Hamid Zargar, Ko Ko Zaw, Ayman A. Zayed, Tomasz Zdrojewski, Magdalena Żegleń, Kristyna Zejglicova, Tajana Zeljkovic Vrkic, Yi Zeng, Luxia Zhang, Zhen-Yu Zhang, Dong Zhao, Ming-Hui Zhao, Wenhua Zhao, Yanitsa V. Zhecheva, Shiqi Zhen, Wei Zheng, Yingfeng Zheng, Bekbolat Zholdin, Maigeng Zhou, Dan Zhu, Marie Zins, Emanuel Zitt, Yanina Zocalo, Nada Zoghlami, Julio Zuñiga Cisneros, Monika Zuziak, Zulfiqar A. Bhutta, Robert E. Black, and Majid Ezzati
Extended data
is available for this paper at 10.1038/s41586-023-05772-8.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-023-05772-8.
References
- 1.Prentice AM, et al. Critical windows for nutritional interventions against stunting. Am. J. Clin. Nutr. 2013;97:911–918. doi: 10.3945/ajcn.112.052332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanner JM. Growth as a mirror of the condition of society: secular trends and class distinctions. Acta Paediatr. Jpn. 1987;29:96–103. doi: 10.1111/j.1442-200X.1987.tb00015.x. [DOI] [PubMed] [Google Scholar]
- 3.Strauss J, Thomas D. Health, nutrition, and economic development. J. Econ. Lit. 1998;36:766–817. [Google Scholar]
- 4.Nüesch E, et al. Adult height, coronary heart disease and stroke: a multi-locus Mendelian randomization meta-analysis. Int. J. Epidemiol. 2016;45:1927–1937. doi: 10.1093/ije/dyv074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park MH, Falconer C, Viner RM, Kinra S. The impact of childhood obesity on morbidity and mortality in adulthood: a systematic review. Obes. Rev. 2012;13:985–1000. doi: 10.1111/j.1467-789X.2012.01015.x. [DOI] [PubMed] [Google Scholar]
- 6.Caird J, et al. Does being overweight impede academic attainment? A systematic review. Health Educ. J. 2014;73:497–521. doi: 10.1177/0017896913489289. [DOI] [Google Scholar]
- 7.NCD Risk Factor Collaboration (NCD-RisC). Height and body-mass index trajectories of school-aged children and adolescents from 1985 to 2019 in 200 countries and territories: a pooled analysis of 2181 population-based studies with 65 million participants. Lancet. 2020;396:1511–1524. doi: 10.1016/S0140-6736(20)31859-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Global Report on Urban Health: Equitable Healthier Cities for Sustainable Development (World Health Organization & UN-Habitat, 2016).
- 9.Healthy Environments for Healthy Children: Key Messages for Action. Report No. 924159988X (World Health Organization, 2010).
- 10.Report of the Commission on Ending Childhood Obesity (World Health Organization, 2016).
- 11.Smith DM, Cummins S. Obese cities: how our environment shapes overweight. Geogr. Compass. 2009;3:518–535. doi: 10.1111/j.1749-8198.2008.00198.x. [DOI] [Google Scholar]
- 12.Fraser B. Latin America’s urbanisation is boosting obesity. Lancet. 2005;365:1995–1996. doi: 10.1016/S0140-6736(05)66679-2. [DOI] [PubMed] [Google Scholar]
- 13.Pirgon Ö, Aslan N. The role of urbanization in childhood obesity. J. Clin. Res. Pediatr. Endocrinol. 2015;7:163–167. doi: 10.4274/jcrpe.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.The State of the World’s Children 2012: Children in an Urban World (UNICEF, 2012).
- 15.Kirchengast, S. & Hagmann, D. “Obesity in the city”—urbanization, health risks and rising obesity rates from the viewpoint of human biology and public health. Hum. Biol. Public Health.2, 1–21 (2021).
- 16.Congdon P. Obesity and urban environments. Int. J. Environ. Res. 2019;16:464. doi: 10.3390/ijerph16030464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gong P, et al. Urbanisation and health in China. Lancet. 2012;379:843–852. doi: 10.1016/S0140-6736(11)61878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liese AD, Weis KE, Pluto D, Smith E, Lawson A. Food store types, availability, and cost of foods in a rural environment. J. Am. Diet. Assoc. 2007;107:1916–1923. doi: 10.1016/j.jada.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 19.Krumdiek CL. The rural-to-urban malnutrition gradient: a key factor in the pathogenesis of urban slums. JAMA. 1971;215:1652–1654. doi: 10.1001/jama.1971.03180230062012. [DOI] [PubMed] [Google Scholar]
- 20.Paciorek CJ, Stevens GA, Finucane MM, Ezzati M. Children’s height and weight in rural and urban populations in low-income and middle-income countries: a systematic analysis of population-representative data. Lancet Glob. Health. 2013;1:e300–e309. doi: 10.1016/S2214-109X(13)70109-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.NCD Risk Factor Collaboration (NCD-RisC). Rising rural body-mass index is the main driver of the global obesity epidemic in adults. Nature. 2019;569:260–264. doi: 10.1038/s41586-019-1171-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jaacks LM, Slining MM, Popkin BM. Recent trends in the prevalence of under- and overweight among adolescent girls in low- and middle-income countries. Pediatr. Obes. 2015;10:428–435. doi: 10.1111/ijpo.12000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Monteiro C, Popkin BM. Trends of obesity and underweight in older children and adolescents in the United States, Brazil, China, and Russia. Am. J. Clin. Nutr. 2002;75:971–977. doi: 10.1093/ajcn/75.6.971. [DOI] [PubMed] [Google Scholar]
- 24.Neuman M, Kawachi I, Gortmaker S, Subramanian SV. Urban–rural differences in BMI in low- and middle-income countries: the role of socioeconomic status. Am. J. Clin. Nutr. 2013;97:428–436. doi: 10.3945/ajcn.112.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidden Cities: Unmasking and Overcoming Health Inequities in Urban Settings. Report No. 9241548037 (World Health Organization & Centre for Health Development, 2010).
- 26.Population-based Approaches to Childhood Obesity Prevention. Report No. 9789241504782 (World Health Organization, 2012).
- 27.Prevention of Overweight and Obesity in Children and Adolescents: UNICEF Programming Guidance (UNICEF, 2019).
- 28.de Sa TH, et al. Urban design is key to healthy environments for all. Lancet Glob. Health. 2022;10:e786–e787. doi: 10.1016/S2214-109X(22)00202-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelly C, Callaghan M, Molcho M, Nic Gabhainn S, Alforque Thomas A. Food environments in and around post-primary schools in Ireland: associations with youth dietary habits. Appetite. 2019;132:182–189. doi: 10.1016/j.appet.2018.08.021. [DOI] [PubMed] [Google Scholar]
- 30.Conrad D, Capewell S. Associations between deprivation and rates of childhood overweight and obesity in England, 2007–2010: an ecological study. BMJ Open. 2012;2:e000463. doi: 10.1136/bmjopen-2011-000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Katsoulis M, et al. Identifying adults at high-risk for change in weight and BMI in England: a longitudinal, large-scale, population-based cohort study using electronic health records. Lancet Diabetes Endocrinol. 2021;9:681–694. doi: 10.1016/S2213-8587(21)00207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arnett JJ. Emerging adulthood: a theory of development from the late teens through the twenties. Am. Psychol. 2000;55:469–480. doi: 10.1037/0003-066X.55.5.469. [DOI] [PubMed] [Google Scholar]
- 33.Poobalan AS, Aucott LS, Clarke A, Smith WCS. Diet behaviour among young people in transition to adulthood (18–25 year olds): a mixed method study. Health Psychol. Behav. Med. 2014;2:909–928. doi: 10.1080/21642850.2014.931232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Winpenny EM, et al. Changes in diet through adolescence and early adulthood: longitudinal trajectories and association with key life transitions. Int. J. Behav. Nutr. Phys. Act. 2018;15:86. doi: 10.1186/s12966-018-0719-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith LC, Ruel MT, Ndiaye A. Why is child malnutrition lower in urban than in rural areas? Evidence from 36 developing countries. World Develop. 2005;33:1285–1305. doi: 10.1016/j.worlddev.2005.03.002. [DOI] [Google Scholar]
- 36.Lundborg P, Rooth D-O, Alex-Petersen J. Long-term effects of childhood nutrition: evidence from a school lunch reform. Rev. Econ. Stud. 2022;89:876–908. doi: 10.1093/restud/rdab028. [DOI] [Google Scholar]
- 37.Graça P, Gregório MJ, Freitas MG. A decade of food and nutrition policy in Portugal (2010–2020) Port. J. Public Health. 2020;38:94–118. doi: 10.1159/000510566. [DOI] [Google Scholar]
- 38.Winichagoon P. Thailand nutrition in transition: situation and challenges of maternal and child nutrition. Asia Pac. J. Clin. Nutr. 2013;22:6–15. doi: 10.6133/apjcn.2013.22.1.17. [DOI] [PubMed] [Google Scholar]
- 39.Paes-Sousa R, Vaitsman J. The Zero Hunger and Brazil without Extreme Poverty programs: a step forward in Brazilian social protection policy. Cien Saude Colet. 2014;19:4351–4360. doi: 10.1590/1413-812320141911.08812014. [DOI] [PubMed] [Google Scholar]
- 40.Núñez J, Pérez G. The escape from malnutrition of Chilean boys and girls: height-for-age Z scores in late XIX and XX centuries. Int. J. Environ. Res. Public Health. 2021;18:10436. doi: 10.3390/ijerph181910436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Herrmann, R., Möser, A. & Weber, S. A. Grocery retailing in Germany: Situation, development and pricing strategies PDF Logo (Justus-Liebig-Universität Gießen, 2009).
- 42.Thang NM, Popkin B. Child malnutrition in Vietnam and its transition in an era of economic growth. J. Hum. Nutr. Diet. 2003;16:233–244. doi: 10.1046/j.1365-277X.2003.00449.x. [DOI] [PubMed] [Google Scholar]
- 43.Subramanyam MA, Kawachi I, Berkman LF, Subramanian SV. Socioeconomic inequalities in childhood undernutrition in India: analyzing trends between 1992 and 2005. PLoS ONE. 2010;5:e11392. doi: 10.1371/journal.pone.0011392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jingzhong Y, Lu P. Differentiated childhoods: impacts of rural labor migration on left-behind children in China. J. Peasant Stud. 2011;38:355–377. doi: 10.1080/03066150.2011.559012. [DOI] [PubMed] [Google Scholar]
- 45.Sundberg, S. Agriculture, poverty and growth in Africa: linkages and policy challenges. CABI Rev. (2009).
- 46.Bentham J, et al. Multidimensional characterization of global food supply from 1961 to 2013. Nat. Food. 2020;1:70–75. doi: 10.1038/s43016-019-0012-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Micha R, et al. Global, regional and national consumption of major food groups in 1990 and 2010: a systematic analysis including 266 country-specific nutrition surveys worldwide. BMJ Open. 2015;5:e008705. doi: 10.1136/bmjopen-2015-008705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fraval, S. et al. Food access deficiencies in sub-Saharan Africa: prevalence and implications for agricultural interventions. Front. Sustain. Food Syst.3, 104 (2019).
- 49.Thomson M, Kentikelenis A, Stubbs T. Structural adjustment programmes adversely affect vulnerable populations: a systematic-narrative review of their effect on child and maternal health. Public Health Rev. 2017;38:13. doi: 10.1186/s40985-017-0059-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Frisch RE, Revelle R. Height and weight at menarche and a hypothesis of critical body weights and adolescent events. Science. 1970;169:397–399. doi: 10.1126/science.169.3943.397. [DOI] [PubMed] [Google Scholar]
- 51.Holmgren A, et al. Pubertal height gain is inversely related to peak BMI in childhood. Pediatr. Res. 2017;81:448–454. doi: 10.1038/pr.2016.253. [DOI] [PubMed] [Google Scholar]
- 52.Alhassan JAK, Adeyinka DA, Olakunde BO. Equity dimensions of the decline in under-five mortality in Ghana: a joinpoint regression analysis. Tropi. Med. Int. Health. 2020;25:732–739. doi: 10.1111/tmi.13391. [DOI] [PubMed] [Google Scholar]
- 53.Wolde KS, Bacha RH. Trend and correlates of under-5 mortality in Ethiopia: a multilevel model comparison of 2000–2016 EDHS data. SAGE Open Med. 2022;10:20503121221100608. doi: 10.1177/20503121221100608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult body–mass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19·2 million participants. Lancet. 2016;387:1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Danaei G, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- 56.Danaei G, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378:31–40. doi: 10.1016/S0140-6736(11)60679-X. [DOI] [PubMed] [Google Scholar]
- 57.Farzadfar F, et al. National, regional, and global trends in serum total cholesterol since 1980: systematic analysis of health examination surveys and epidemiological studies with 321 country-years and 3·0 million participants. Lancet. 2011;377:578–586. doi: 10.1016/S0140-6736(10)62038-7. [DOI] [PubMed] [Google Scholar]
- 58.Finucane MM, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9·1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cline MG, Meredith KE, Boyer JT, Burrows B. Decline of height with age in adults in a general population sample: estimating maximum height and distinguishing birth cohort effects from actual loss of stature with aging. Hum. Biol. 1989;61:415–425. [PubMed] [Google Scholar]
- 60.Lumley T. Analysis of complex survey samples. J. Stat. Softw. 2004;9:1–19. doi: 10.18637/jss.v009.i08. [DOI] [Google Scholar]
- 61.United Nations Department of Economic and Social Affairs, Population Division. World Urbanization Prospects: The 2018 Revision (United Nations, 2019).
- 62.Hayes AJ, Clarke PM, Lung TW. Change in bias in self-reported body mass index in Australia between 1995 and 2008 and the evaluation of correction equations. Popul. Health Metr. 2011;9:53. doi: 10.1186/1478-7954-9-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Connor Gorber S, Tremblay M, Moher D, Gorber B. A comparison of direct vs. self-report measures for assessing height, weight and body mass index: a systematic review. Obes. Rev. 2007;8:307–326. doi: 10.1111/j.1467-789X.2007.00347.x. [DOI] [PubMed] [Google Scholar]
- 64.Ezzati M, Martin H, Skjold S, Vander Hoorn S, Murray CJL. Trends in national and state-level obesity in the USA after correction for self-report bias: analysis of health surveys. J. R. Soc. Med. 2006;99:250–257. doi: 10.1177/014107680609900517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ahmad, O. B. et al. Age Standardization of Rates: A New WHO Standard (World Health Organization, 2001).
- 67.Finucane MM, Paciorek CJ, Stevens GA, Ezzati M. Semiparametric Bayesian density estimation with disparate data sources: a meta-analysis of global childhood undernutrition. J. Am. Stat. Assoc. 2015;110:889–901. doi: 10.1080/01621459.2014.937487. [DOI] [Google Scholar]
- 68.Finucane MM, Paciorek CJ, Danaei G, Ezzati M. Bayesian estimation of population-level trends in measures of health status. Stat. Sci. 2014;29:18–25. doi: 10.1214/13-STS427. [DOI] [Google Scholar]
- 69.NCD Risk Factor Collaboration (NCD-RisC). Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet. 2021;398:957–980. doi: 10.1016/S0140-6736(21)01330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.NCD Risk Factor Collaboration (NCD-RisC). A century of trends in adult human height. eLife. 2016;5:e13410. doi: 10.7554/eLife.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rue, H. V. & Held, L. Gaussian Markov Random Fields: Theory and Applications (Chapman & Hall/CRC, 2005).
- 72.Cameron, N. L. Human Growth and Development (Academic Press, 2002).
- 73.de Onis M, et al. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tanner JM, Whitehouse RH, Marubini E, Resele LF. The adolescent growth spurt of boys and girls of the Harpenden growth study. Ann. Hum. Biol. 1976;3:109–126. doi: 10.1080/03014467600001231. [DOI] [PubMed] [Google Scholar]
- 75.Gelman, A. & Jill, J. Data Analysis Using Regression and Multilevel/Hierarchical Models (Cambridge Univ. Press, 2007).
- 76.Gelman A. Prior distributions for variance parameters in hierarchical models. Bayesian Anal. 2006;1:515–533. doi: 10.1214/06-BA117A. [DOI] [Google Scholar]
- 77.Breslow NE, Clayton DG. Approximate inference in generalized linear mixed models. J. Am. Stat. Assoc. 1993;88:9–25. [Google Scholar]
- 78.Wood, S. N. Generalized Additive Models: An Introduction with R (Chapman & Hall/CRC, 2006).
- 79.Harville, D. A. Matrix Algebra from a Statistician’s Perspective (Springer-Verlag, 2008).
- 80.Vehtari A, Gelman A, Simpson D, Carpenter B, Bürkner P-C. Rank-normalization, folding, and localization: an improved R for assessing convergence of MCMC (with discussion) Bayesian Anal. 2021;16:667–718. doi: 10.1214/20-BA1221. [DOI] [Google Scholar]
- 81.Stan Development Team. RStan: the R interface to Stanhttps://mc-stan.org/ (2022).
- 82.Best MJ, Chakravarti N. Active set algorithms for isotonic regression; a unifying framework. Math. Program. 1990;47:425–439. doi: 10.1007/BF01580873. [DOI] [Google Scholar]
- 83.De Leeuw J, Hornik K, Mair P. Isotone optimization in R: pool-adjacent-violators algorithm (PAVA) and active set methods. J. Stat. Softw. 2010;32:1–24. [Google Scholar]
- 84.The R Development Core Team. R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2021).
- 85.Huang R, Moudon AV, Cook AJ, Drewnowski A. The spatial clustering of obesity: does the built environment matter? J. Hum. Nutr. Diet. 2015;28:604–612. doi: 10.1111/jhn.12279. [DOI] [PubMed] [Google Scholar]
- 86.Menon P, Ruel MT, Morris SS. Socio-economic differentials in child stunting are consistently larger in urban than in rural areas. Food Nutr. Bull. 2000;21:282–289. doi: 10.1177/156482650002100306. [DOI] [Google Scholar]
- 87.Ezeh A, et al. The history, geography, and sociology of slums and the health problems of people who live in slums. Lancet. 2017;389:547–558. doi: 10.1016/S0140-6736(16)31650-6. [DOI] [PubMed] [Google Scholar]
- 88.Fink G, Günther I, Hill K. Slum residence and child health in developing countries. Demography. 2014;51:1175–1197. doi: 10.1007/s13524-014-0302-0. [DOI] [PubMed] [Google Scholar]
- 89.Jonah CMP, May JD. The nexus between urbanization and food insecurity in South Africa: does the type of dwelling matter? Int. J. Urban Sustain. Dev. 2020;12:1–13. doi: 10.1080/19463138.2019.1666852. [DOI] [Google Scholar]
- 90.Progress Report: Sustainable Development Goal 11 (Target 11.1) (Habitat for Humanity International, 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1–4, Supplementary Figs. 1–8 and Supplementary References; see contents page for details.
Data Availability Statement
Estimates of mean BMI and height by country, year, sex, single year of age as well as age-standardized, and place of residence (urban and rural) will be available from https://www.ncdrisc.org in machine-readable numerical format and as visualizations upon publication of the paper. Input data from publicly available sources and contact information for data providers can be downloaded from https://www.ncdrisc.org and Zenodo (10.5281/zenodo.7355601).
The computer code for the Bayesian hierarchical model and the code used to generate figures in this work will be available at https://www.ncdrisc.org and Zenodo (10.5281/zenodo.7355601).