Abstract
The production of urban waste has increased in the past decades leading to its mishandling. The effects on public health, economy, and wildlife that waste mismanagement can have are forcing governments to increase their efforts in detecting and mitigating the presence of waste. Identifying and monitoring sentinel species to assess the presence of urban litter could be a cost-effective option. Thus, analyzing the nest composition of yellow-legged gulls from an urban population inhabiting a very high populated city (Barcelona, Spain), and combining this information with accurate GPS tracking data, provides a potential tool to monitor the presence of marine and terrestrial litter over time. The results revealed the highest presence of debris in the nests of a seabird ever recorded. All the nests examined contained anthropogenic waste, with plastic items present in all of them. Crossing the nest composition with GPS tracking movements confirmed that the waste to build the nests was collected in the urban area and not in other environments surrounding the city. Then, the nest waste composition may be a good indicator of waste mismanagement and advise the municipalities to improve waste management and recycling strategies for the different types of litter. Using gulls breeding in cities as sentinel species and, in particular, the study of their nest composition, may provide essential data to decision-making stakeholders to adopt a One Health approach and help improve not only the environment’s health but also the health of those who live in it.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10661-023-11133-9.
Keywords: Coastal city, One health, Plastic, Sentinel species, Urban ecology, Waste management
Introduction
Coastal urban areas have increasingly grown in the last decades, and half of the population live in cities with over 100,000 people located within 100 km from the coast (Barragán & de Andrés, 2015). Accordingly, the production of municipal waste has been progressively increasing during the last decades, and it is predicted to double by 2025 (Hoornweg & Bhada-Tata, 2012). The correlation between municipal waste volume and resident population is linear; for each unit increase in population, the quantity of waste increases with an exponent of 1.06 (Wowrzeczka, 2021). As a result, the amount of urban waste grows faster than the number of inhabitants. This fact leads towards the generation of mismanaged urban waste, which is composed of urban litter (incorrectly disposed waste), and inadequately contained waste, which can be spread via wind and runoff from terrestrial to aquatic habitats (Ballatore et al., 2022; Lebreton & Andrady, 2019). Solid waste mismanagement is worsened by unsustainable practices that result in environmental contamination and the spread of diseases (Ferronato & Torretta, 2019). Consequently, this issue can have significant detrimental effects on public health, economy, and urban wildlife (Ballatore et al., 2022). To prevent these effects, governments are putting particular emphasis on detecting urban waste; therefore, identifying the use of sentinel species to monitor the presence of urban litter could be a cost-effective option (Acampora et al., 2016; Multisanti et al., 2022).
Sentinel organisms are a form of health indicators that serve as proxies for environment health signaling warnings, at different levels, about potential impacts on a specific ecosystem (Tabor & Alonso Aguirre, 2004). Several species have been identified to trace environmental pollution, including both land and aquatic ecosystems, and different types of pollution. For example, the osprey (Pandion haliaetus) has been used as sentinel species in freshwater habitats such as rivers, lakes, reservoirs, and estuaries to inform the effects and the presence of different environmental pollutants (Grove et al., 2009). Similarly, the loggerhead turtle (Caretta caretta) has been proposed as target indicator to monitor the impact of marine litter in aquatic ecosystems (Matiddi et al., 2017). To be considered a sentinel organism, it must meet particular requirements, including a well-known biology and natural history, a wide distribution, and availability in sufficient numbers, among others (Basu et al., 2007). Thus, in coastal cities, urban-dwelling wildlife can be used as litter sentinels. Among them, opportunistic large gulls could be good sentinel candidates to be used as tracers of marine and terrestrial litter due to their wide plastic behavior, foraging in both marine and urban habitats (Coccon et al., 2022; Gimeno et al., 2022; Langley et al., 2023; Méndez et al., 2020; Navarro et al., 2016; Spelt et al., 2019).
The availability of diverse food resources within cities has led gull populations to develop worldwide (Belant, 1997; Martín-Vélez et al., 2022; Meléndez-Arteaga et al., 2022; Spelt et al., 2019). As coastal and marine predators, gulls also obtain resources from fishing vessels, exploiting their discard fraction (Bartumeus et al., 2010; Gimeno et al., 2022; Pais de Faria et al., 2021). The increase of uncontrolled waste, including marine litter, in seaside cities, provides abundant anthropogenic material for gulls to be incorporated in their nests (Lato et al., 2021; Lopes et al., 2020; Thompson et al., 2020; Yaghmour & Al Marashda, 2020). This material can be quantifiable by researchers (Ryan, 2020; Tavares et al., 2020) which, along with the gulls’ behavior and the possibility to investigate the habitat used by urban gulls using accurate GPS instruments (Martín-Vélez et al., 2022; Rock et al., 2016; Spelt et al., 2019), allows a better understanding of the levels of mismanaged waste within their inhabiting ecosystems. Moreover, it may also allow inferring in the origin of the waste present in the nests.
Here, we aim to demonstrate how the analysis of the nest composition in combination with accurate GPS tracking information of an urban gull population could be used to monitor the presence of marine and terrestrial litter, not only on an ad hoc basis, but also over time to envisage trends or changes. In particular, we examined both the presence of anthropogenic material within the nests and the main habitats used by the urban population of yellow-legged gulls (Larus michahellis) inhabiting the crowded city of Barcelona (NE Spain). All this information highlighted the amount and type of litter in coastal cities, along with the potential habitats where this litter was collected by the urban gulls, supporting the value to use gulls as sentinel species to track mismanaged waste in cities.
Materials and methods
Fieldwork procedures
This study was developed during May and June 2021 in Barcelona (NE Iberian Peninsula, Spain, Fig. 1(a)), one of the largest cities of Europe (1.6 million of people; Eurostat). The urban population of yellow-legged gull of Barcelona has been estimated in ~ 500 pairs (Anton et al., 2017). The urban ecosystem of Barcelona surrounded by a fishing port, recreational harbors, urban waste installations, agricultural areas, and rivers provide easy-to-catch food to opportunistic gulls (Carmona et al., 2021; Méndez et al., 2020).
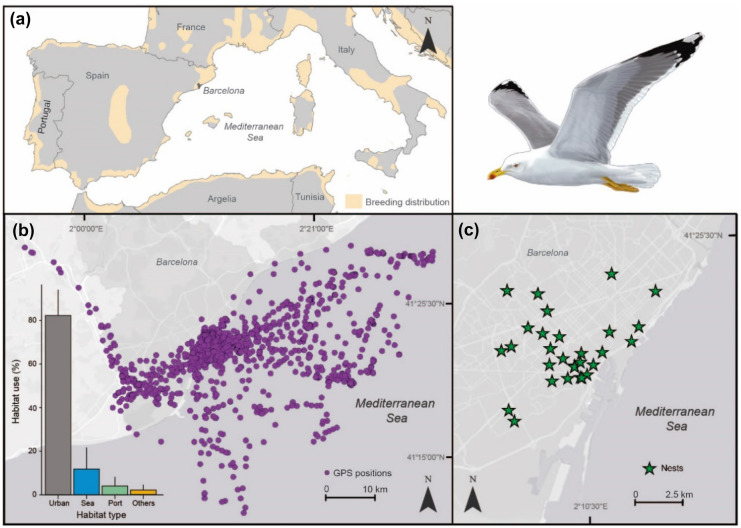
Fig. 1.
(a) Location of Barcelona city within the western Mediterranean Basin. (b) GPS positions and habitat use of 20 GPS-tracked yellow-legged gulls during 2021 breeding season in Barcelona. (c) Distribution of the 30 nests sampled in Barcelona during the 2021 breeding season to examine the presence of marine and terrestrial litter. Gull drawing by Martí Franch
To determine the presence of litter in the nests, we examined the composition of 30 nests of yellow-legged gull randomly collected throughout the city of Barcelona at the beginning of the incubation period (Fig. 1(c)). All the nests were collected by the Public Health Agency of Barcelona (following the Legislative Decree 2/2008, 15 April, DOGC). To investigate the spatial movements of breeding adults, 9 adult yellow-legged gulls were instrumented with GPS units (CatLog, Perthold Engineering LLC; 16 g of weight, corresponding to less than the 3% of the weight of yellow-legged gulls) that recorded the GPS position of each bird every 5 min, 24 h (Fig. 1(b)). The gulls were captured at the beginning of the breeding period in a trap (baited with fish) in a metropolitan park of Barcelona, and GPS devices were attached using a conventional Teflon harness (Thaxter et al., 2014). All these individuals had the presence of irrigated incubation patch, indicating that they were active breeders.
Nest litter quantification
All the litter from the nests was classified based on the Master List category of items from the European Marine Strategy Framework Directive (Galgani et al., 2013). In general, the list classifies the items according to material type, i.e., plastic, textile, paper, rubber, metal, glass and ceramics, processed wood, and others. However, after the analysis of the items found, the categories reported are as follows: plastic, paper, metal, and others (see Table 1). The other categories from the Master List were not present or were very scarce, thus were included in the category others. From each of these types of items, there are sub-categories, such a hygienic, which we considered important to report because it includes personal hygiene and health-related items such as disposable wet wipes or single-use gloves. The material studied did not include pellets that might have been regurgitated by the gulls, and only those items nicely packed in the nest were identified and quantified. All litter items from the nests were counted and weighed (in g), and three metrics were calculated: %FO (frequency of occurrence of each type of litter in relation to the total number of nests analyzed), %N (contribution by number of each type of litter in relation to the total number detected), and %W (contribution by weight of each type of litter in relation to the total weight of the nest). ANOVA and SNK post hoc tests were used to test for differences in weight among the different types of litter present in the nests. IBM SPSS v.28 was used to run the statistical tests.
Table 1.
List of the different types of litter found in the yellow-legged gull’s nests from Barcelona in 2021 indicating the percentage of weight (% W) of each litter and the potential habitat where each type of litter could be present (X)
| Habitat | ||||||||
|---|---|---|---|---|---|---|---|---|
| Type of litter | %W | Urban | Port | Dump | Agriculture | Freshwater | Sea | Beach |
| Plastic | 68.83 | X | X | X | X | X | X | X |
| Cigarette buds | 0.29 | X | X | X | X | |||
| Bags (shopping, food, freezer) | 0.74 | X | X | X | X | |||
| Lids | 0.01 | X | X | X | X | |||
| Packaging related to candy | 0.00 | X | X | X | X | X | ||
| Food packaging (fast food) and cosmetics | 0.11 | X | X | |||||
| Packaging (cable ties, tapes, …) | 3.01 | X | X | X | X | |||
| Ropes/Strings | 4.66 | X | X | X | X | X | X | |
| Foam | 1.42 | X | X | X | X | X | ||
| Can holders | 0.05 | X | X | X | ||||
| Plastic pieces 0–2.5 cm (pellets) | 1.76 | X | X | X | X | X | X | X |
| Plastic pieces 2.5 cm-50 cm | 7.67 | X | X | X | ||||
| Other plastic objects (pens, lighters, …) | 0.30 | X | X | X | ||||
| Polystyrene | 0.00 | X | X | X | X | X | X | |
| All insulation materials | 46.96 | X | X | |||||
| Clothespin | 0.19 | X | ||||||
| Medical stuff | 0.18 | X | X | X | X | X | ||
| Plastic net (gardening) | 1.10 | X | X | |||||
| Paper/cardboard | 5.79 | X | X | X | X | X | X | X |
| Paper napkins, table cloths | 1.02 | X | X | |||||
| Boxes and box pieces | 2.20 | X | X | X | X | |||
| Drink cardboards | 0.06 | X | X | X | ||||
| Tobacco packs | 0.12 | X | X | X | ||||
| Paper pieces | 2.87 | X | X | X | X | |||
| Wood (processed) | 0.65 | X | X | X | X | X | X | X |
| Corks | 0.02 | X | ||||||
| Sticks | 0.11 | X | X | X | X | |||
| Other objects and pieces < 50 cm | 0.50 | X | X | X | X | X | X | X |
| Metal | 2.96 | X | X | X | X | X | X | X |
| Lids and covers for bottles and cans | 0.04 | X | X | X | ||||
| Aluminum foil | 0.32 | X | X | X | X | X | ||
| Other objects and pieces < 50 cm | 2.63 | X | X | X | X | X | ||
| Glass | 0.05 | X | X | X | X | X | X | X |
| Bottles and containers | 0.02 | X | X | X | ||||
| Other objects and pieces | 0.03 | X | X | X | X | |||
| Hygienic waste | 0.78 | X | X | X | X | X | X | X |
| Ear buds | 0.00 | X | X | X | ||||
| Wet wipes | 0.76 | X | X | X | X | |||
| Others | 20.95 | X | X | X | X | X | X | X |
| Rubber (balloons, balls, strings, valves, …) | 0.07 | X | X | X | X | X | X | X |
| Other textile | 14.97 | X | X | X | ||||
| Construction materials (bolts, nails, …) | 1.29 | X | X | X | X | |||
| Other objects and ceramic pieces | 0.10 | X | X | |||||
| Brushes (mixed materials) | 4.37 | X | X | X | ||||
Bold indicates % W and presence of items for the main categories
Spatial movement of gulls
To characterize the habitat utilized by the GPS-tracked yellow-legged gulls, we estimated the % of use of each habitat type by each GPS-tracked individual. Specifically, we calculated the % between the number of positions in each type of habitat divided by the total GPS fixes for each GPS-tracked gull. Habitat type was determined by overlapping the GPS with land-cover data (Urban Atlas, European Environment Agency; Copernicus Land Monitoring Services 2016; < 5 m resolution), aggregated into 7 categories: urban, beach, port, sea, freshwater, landfill, and agriculture. Each of these habitats could be the source of the different types of litter present in the nests (Table 1). To perform spatial analysis, we used QGIS 3.10. A Coruña (QGIS Development T 2019).
Results and discussion
Anthropogenic waste in the nests
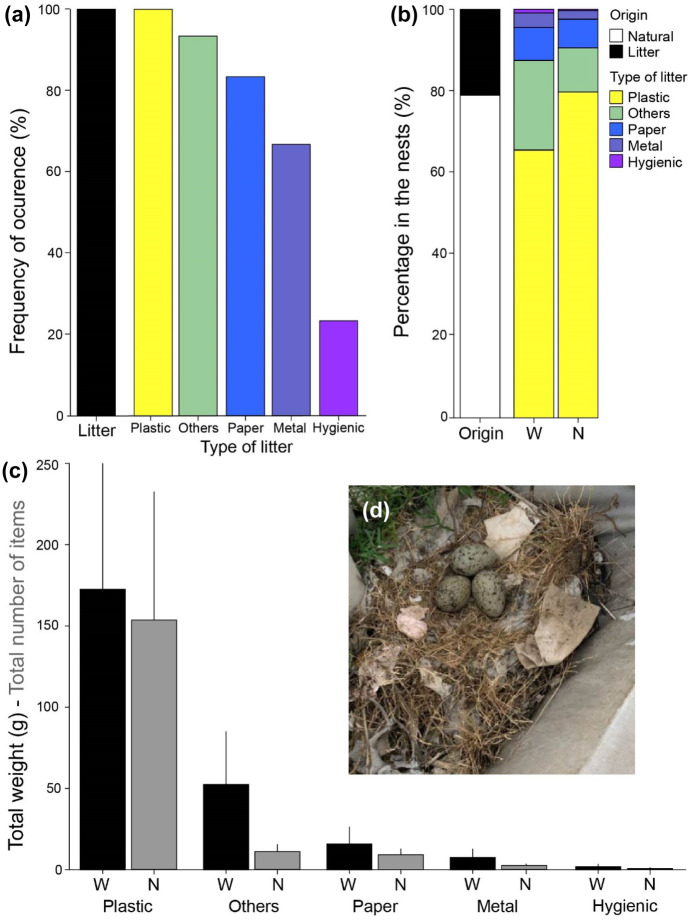
Recent studies have described the presence of anthropogenic waste in the nest of yellow-legged gulls and similar gulls (Battisti, 2020; Lato et al., 2021; Lopes et al., 2020; Pon & Pereyra, 2021; Thompson et al., 2020). These studies were mostly conducted in natural environments, with less availability of anthropogenic waste in the surroundings of the colonies, whereas we centered the study in an urban population inhabiting a very high–populated city. In particular, all the nests examined contained anthropogenic waste (Fig. 2a), accounting for the 21% of the total weight of the nests (Fig. 2b; Table 1). Regarding the type of waste found, plastic items were present in all of them (frequency of occurrence = 100%, Fig. 2a), followed by the categories others (93%), paper (83%), metal (66%), and hygienic waste (23%) (Fig. 2a). Statistically, plastic also showed the highest values in weight (ANOVA tests, F4, 29 = 14.14, p < 0.001) and number of items (F4, 29 = 17.87, p < 0.001) in comparison with the other type of waste, which did not differ between them (p > 0.05) (Fig. 2c, Table 1). The present study, then, reports the highest presence of debris in the nests of a seabird so far.
Fig. 2.
a Frequency of occurrence of litter and the different types of litter in the nests of yellow-legged gulls from Barcelona. b Percentage by the origin of the items (natural or litter), and by the type of litter present in the nests of the yellow-legged gulls from Barcelona by weight (W) and number (N). c Mean and 95% confidence interval of the presence of each type of litter in the nests of yellow-legged gulls from Barcelona by weight (W) and number (N). d Picture showing a yellow-legged gull nest located on one roof of Barcelona (picture by Tomás Montalvo)
From the items analyzed, it seems that the gulls probably collected some of the waste directly from the buildings’ roofs. The most evident hint was the presence of clothes (socks or panties) or clothespins in the nests (Table 1; Fig. S1) which the yellow-legged gulls most likely found in the clothing lines area of the buildings. Another example is the presence of a type of insulation material used on the roofs of the buildings (Fig. S1). A similar material was also found in urban yellow-legged gull nests in Porto, Portugal (Lopes et al., 2020). In fact, this is the main type of plastic found in the nests, accounting for the 46% of the weight (Table 1). As this type of waste is not considered an anthropogenic-origin litter per se, but an escape from the urban waste management, we suggest improving the fixation of this material to avoid the release of a high amount of this type of non-woven fabric, a type of plastic material, to the environment.
The presence of plastic, paper, metal, or glass in the nests mirrors the occurrence of this type of waste in the environment (Li et al., 2016), and thus, it is a sign of an incorrect municipal waste management. In this line, and with the final aim to prevent the harmful consequences of anthropogenic waste, the Barcelona City Council, similarly to initiatives from other European countries, has started a recycling improvement plan, with door-to-door collection campaigns aiming to improve the recycling figures that each citizen generates home (Barcelona City Hall waste management plan). This strategy has been proven very effective to increase household recycling and might help decrease waste mismanagement (Halvorsen, 2012), which may be traced with the composition of the gulls nests (Burger & Gochfeld, 2004; Lavers et al., 2013; Tavares et al., 2020).
Habitat used by urban gulls
The analysis of the nest composition is an essential aspect to detect the presence of waste, but the possibility to identify the habitats used by yellow-legged gulls during their foraging movements is also important to relate where the waste was collected (Table 1). Here, by using GPS tracking data from nine breeding gulls, we were able to identify the main habitats covered by them and, thus, the potential origin of the litter found in the nests (Table 1). GPS-tracking data revealed that the main habitat used by adult yellow-legged gulls in Barcelona was the urban environment (mean ± standard deviation of the GPS positions = 82.21 ± 15.64%), followed by sea (11.81 ± 12.82%), port (4.03 ± 5.93%), agriculture (1.09 ± 3.23%), freshwater (0.73 ± 2.18%), dump (0.02 ± 0.07%), and beach (0.1 ± 0.28%) (Fig. 1b). Crossing the habitat use data with the waste found in the nests, this study suggests that almost all anthropogenic waste present in the nests was collected in the urban environment. Although we did not analyze the nests of the GPS-tracked gulls, this finding reinforces the idea that nesting material is collected locally by gulls (Thompson et al., 2020). Thus, long-term determination of the litter present in gull nests could be suitable to help in monitoring the efficiency of urban management and the amount of human-related waste present in the habitats where gulls are foraging (Burger & Gochfeld, 2004; Grant et al., 2018; Tavares et al., 2020).
Gulls as sentinel species
The use of gulls as a sentinel species for environmental health might not be a new concept (Burger & Gochfeld, 2004; Lavers et al., 2013). The analysis of the content of their nests, in combination with tracking information of breeding individuals, could open a new scenario to monitor the presence of anthropogenic waste in the environment (O’Hanlon et al., 2017). In particular, the current results open new possibilities to help detect and correct management of municipal waste. Areas such as dumpsters, constructions sites, gardens and parks, or beaches may be potential main sources of abandoned solid waste, where the local administration may need to focus to improve waste management strategies. The results also deepen in the knowledge of the impact that these residues can create in the environment. This approach complies with the “One Health” strategy that interrelates human, animal, and environmental health (Ewbank et al., 2021; Mackenzie & Jeggo, 2019). Measuring the variables that contribute to urban health is a challenge to promote healthier and more equitable cities. Seabirds can act, then, as sentinels of natural and anthropogenic changes in the health of the marine and terrestrial ecosystems (Thibault et al., 2019). Thus, there are compelling reasons to develop new approaches to help improve the detection, prevention, and monitoring of the parameters that affect global health. One of the objectives of the World Health Organization (WHO) is to have a Single Health Observatory to bring together data from different sources. That is why, using a species widely distributed in cities such as the yellow-legged gull, especially for the role it plays in terms of habitat use and nest composition, can provide information on the state of health of the environment. In this sense, further studies can also provide knowledge on animal health and the possible impacts on human welfare that may result. However, because the nest construction only occurs for a small period of time of the breeding season (i.e., 1 month), assembling these data with other types of information, such as non-breeding season tracking data and pellet analysis, for instance, could improve the understanding of the health state of the environment throughout the year. The integration, interpretation, and evaluation of the information obtained from monitoring the nests and other type of data should provide clues to the managers (ex. urban clean-up or recycling) on how citizens manage their waste. That would allow assistance in decision-making to adopt a One Health approach and thus achieving the goal to improve health and well-being, especially in overcrowded coastal cities.
Conclusions
The yellow-legged gulls from an urban population inhabiting a very high–populated city (Barcelona, Spain) had the highest presence of debris in the nests of a seabird so far. All the nests examined contained anthropogenic waste, accounting for the 21% of the total weight of the nests, with plastic items being present in all of them. When analyzing the composition combined with tracks, it was evidenced that the waste to build the nests was collected in the urban area and not in other environments surrounding the city. Then, the nest waste composition may be a good indicator of waste mismanagement and an advice to the municipalities to improve waste management and recycling strategies for the different types of litter. Proposing yellow-legged gulls as sentinel species, and in particular the study of their nest composition, may provide essential data to decision-making stakeholders to adopt a One Health approach and help improve not only the environment’s health but also the health for those who live in it.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Thanks to Camilo Iriondo (Zoo of Barcelona staff) for his help in the sampling procedures. This study is part of the Erasmus + project of Ilaria Martino, the BCN-Gulls project, and Intramural CSIC Project “Opportunistic gulls as sentinel species to monitor urban marine ecosystems.” We acknowledge the accreditation of the “Severo Ochoa Center of Excellence” (CEX2019-000928-S). All the experimental procedures were conducted in accordance with the EU and Spanish scientific legislation.
Author contribution
Eve Galimany: formal analysis, investigation, methodology, writing-original draft; Joan Navarro: fieldwork, formal analysis, investigation, methodology, resources, writing-original draft; Ilaria Martino: formal analysis, writing; Raül Aymí: fieldwork, writing—review and editing; Pablo Cermeño: fieldwork, writing—review and editing; Tomas Montalvo: fieldwork, investigation, methodology, review and editing.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors declare that Ilaria Martino received financial support provided by Erasmus +.
Data availability
Data will be made available on request.
Declarations
Ethics approval
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Acampora H, Lyashevska O, Van Franeker JA, O'Connor I. The use of beached bird surveys for marine plastic litter monitoring in Ireland. Marine Environmental Research. 2016;120:122–129. doi: 10.1016/j.marenvres.2016.08.002. [DOI] [PubMed] [Google Scholar]
- Anton M, Herrando S, Garcia D, Ferrer X, Cebrian R. Atles dels ocells nidificants de Barcelona. Barcelona: Barcelona/ICO/UB/Zoo Ad, editor; 2017. [Google Scholar]
- Ballatore A, Verhagen TJ, Li Z, Cucurachi S. This city is not a bin: Crowdmapping the distribution of urban litter. Journal of Industrial Ecology. 2022;26:197–212. doi: 10.1111/jiec.13164. [DOI] [Google Scholar]
- Barragán JM, de Andrés M. Analysis and trends of the world's coastal cities and agglomerations. Ocean Coastal Management. 2015;114:11–20. doi: 10.1016/j.ocecoaman.2015.06.004. [DOI] [Google Scholar]
- Bartumeus F, Giuggioli L, Louzao M, Bretagnolle V, Oro D, Levin SA. Fishery discards impact on seabird movement patterns at regional scales. Current Biology. 2010;20:215–222. doi: 10.1016/j.cub.2009.11.073. [DOI] [PubMed] [Google Scholar]
- Basu N, Scheuhammer AM, Bursian SJ, Elliott J, Rouvinen-Watt K, Chan HM. Mink as a sentinel species in environmental health. Environmental Research. 2007;103:130–140. doi: 10.1016/j.envres.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Battisti C. Heterogeneous composition of anthropogenic litter recorded in nests of Yellow-legged gull (Larusmichahellis) from a small Mediterranean island. Marine Pollution Bulletin. 2020;150:110682. doi: 10.1016/j.marpolbul.2019.110682. [DOI] [PubMed] [Google Scholar]
- Belant JL. Gulls in urban environments: Landscape-level management to reduce conflict. Landscape and Urban Planning. 1997;38:245–258. doi: 10.1016/S0169-2046(97)00037-6. [DOI] [Google Scholar]
- Burger J, Gochfeld M. Marine birds as sentinels of environmental pollution. EcoHealth. 2004;1:263–274. doi: 10.1007/s10393-004-0096-4. [DOI] [Google Scholar]
- Carmona M, Aymí R, Navarro J. Importance of predictable anthropogenic food subsidies for an opportunistic gull inhabiting urban ecosystems. European Journal of Wildlife Research. 2021;67:9. doi: 10.1007/s10344-020-01446-2. [DOI] [Google Scholar]
- Coccon F, Vanni L, Dabalà C, Giunchi D. The abundance of yellow-legged gulls Larusmichahellis breeding in the historic centre of Venice, Italy and the initial effects of the new waste collection policy on the population. Urban Ecosystems. 2022;25:643–656. doi: 10.1007/s11252-021-01175-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewbank AC, Esperón F, Sacristán C, Sacristán I, Krul R, de Macedo EC, Calatayud O, Bueno I, de Francisco Strefezzi R, Catão-Dias JL. Seabirds as anthropization indicators in two different tropical biotopes: A One Health approach to the issue of antimicrobial resistance genes pollution in oceanic islands. The Science of the Total Environment. 2021;754:142141. doi: 10.1016/j.scitotenv.2020.142141. [DOI] [PubMed] [Google Scholar]
- Ferronato N, Torretta V. Waste mismanagement in developing countries: A review of global issues. International Journal of Environmental Research and Public Health. 2019;16:1060. doi: 10.3390/ijerph16061060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galgani, F., Hanke, G., Werner, S., Oosterbaan, L., Nilsson, P., Fleet, D., Kinsey, S., Thompson, R. C., van Franeker, J., Vlachogianni, T., Scoullos, M., Mira Veiga, J., Palatinus, A., Matiddi, M., Maes, T., Korpinen, S., Budziak, A., Leslie, H., Gago, J., et al. (2013). Monitoring guidance for marine litter in European seas. MSFD GES Technical Subgroup on Marine Litter (TSG-ML). DRAFT REPORT. p. 120. http://hdl.handle.net/10508/1649
- Gimeno, M., García, J. A., Afán, I., Aymí, R., Montalvo, T., & Navarro, J. (2022). Age-related differences in foraging behaviour at sea and interactions with fishing vessels in an opportunistic urban gull. ICES Journal of Marine Science, fsac120.
- Grant ML, Lavers JL, Stuckenbrock S, Sharp PB, Bond AL. The use of anthropogenic marine debris as a nesting material by brown boobies (Sula leucogaster) Marine Pollution Bulletin. 2018;137:96–103. doi: 10.1016/j.marpolbul.2018.10.016. [DOI] [PubMed] [Google Scholar]
- Grove RA, Henny CJ, Kaiser JL. Osprey: Worldwide sentinel species for assessing and monitoring environmental contamination in rivers, lakes, reservoirs, and estuaries. Journal of Toxicology and Environmental Health Part B. 2009;12:25–44. doi: 10.1080/10937400802545078. [DOI] [PubMed] [Google Scholar]
- Halvorsen B. Effects of norms and policy incentives on household recycling: An international comparison. Resources, Conservation and Recycling. 2012;67:18–26. doi: 10.1016/j.resconrec.2012.06.008. [DOI] [Google Scholar]
- Hoornweg D, Bhada-Tata P. What a waste: a global review of solid waste management. Washington, DC: © World Bank; 2012. [Google Scholar]
- Langley, L. P., Bearhop, S., Burton, N. H., Banks, A. N., Frayling, T., Thaxter, C. B., Clewley, G. D., Scragg, E., & Votier, S. C. (2023). Urban and coastal breeding lesser black‐backed gulls (Larus fuscus) segregate by foraging habitat. Ibis, 165, 214–230.
- Lato KA, Thorne LH, Fuirst M, Brownawell BJ. Microplastic abundance in gull nests in relation to urbanization. Marine Pollution Bulletin. 2021;164:112058. doi: 10.1016/j.marpolbul.2021.112058. [DOI] [PubMed] [Google Scholar]
- Lavers JL, Hodgson JC, Clarke RH. Prevalence and composition of marine debris in brown booby (Sula leucogaster) nests at Ashmore Reef. Marine Pollution Bulletin. 2013;77:320–324. doi: 10.1016/j.marpolbul.2013.09.026. [DOI] [PubMed] [Google Scholar]
- Lebreton L, Andrady A. Future scenarios of global plastic waste generation and disposal. Palgrave Communications. 2019;5:1–11. doi: 10.1057/s41599-018-0212-7. [DOI] [Google Scholar]
- Li WC, Tse HF, Fok L. Plastic waste in the marine environment: A review of sources, occurrence and effects. The Science of the Total Environment. 2016;566–567:333–349. doi: 10.1016/j.scitotenv.2016.05.084. [DOI] [PubMed] [Google Scholar]
- Lopes CS, Pais de Faria J, Paiva VH, Ramos JA. Characterization of anthropogenic materials on yellow-legged gull (Larusmichahellis) nests breeding in natural and urban sites along the coast of Portugal. Environmental Science and Pollution Research. 2020;27:36954–36969. doi: 10.1007/s11356-020-09651-x. [DOI] [PubMed] [Google Scholar]
- Mackenzie JS, Jeggo M. The One Health approach—why is it so important? Tropical Medicine and Infectious Disease. 2019;4:88. doi: 10.3390/tropicalmed4020088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-Vélez V, Montalvo T, Afán I, Sánchez-Márquez A, Aymí R, Figuerola J, Lovas-Kiss Á, Navarro J. Gulls living in cities as overlooked seed dispersers within and outside urban environments. The Science of the Total Environment. 2022;823:153535. doi: 10.1016/j.scitotenv.2022.153535. [DOI] [PubMed] [Google Scholar]
- Matiddi M, Hochsheid S, Camedda A, Baini M, Cocumelli C, Serena F, Tomassetti P, Travaglini A, Marra S, Campani T, Scholl F, Mancusi C, Amato E, Briguglio P, Maffucci F, Fossi MC, Bentivegna F, de Lucia GA. Loggerhead sea turtles (Carettacaretta): A target species for monitoring litter ingested by marine organisms in the Mediterranean Sea. Environmental Pollution. 2017;230:199–209. doi: 10.1016/j.envpol.2017.06.054. [DOI] [PubMed] [Google Scholar]
- Meléndez-Arteaga J, Bregnballe T, Frederiksen M. Identifying spatial drivers of long-term population growth in three large gull species: The importance of mink farms and urban areas. Avian Conservation & Ecology. 2022;17:10. doi: 10.5751/ACE-02233-170210. [DOI] [Google Scholar]
- Méndez A, Montalvo T, Aymí R, Carmona M, Figuerola J, Navarro J. Adapting to urban ecosystems: Unravelling the foraging ecology of an opportunistic predator living in cities. Urban Ecosystems. 2020;23:1117–1126. doi: 10.1007/s11252-020-00995-3. [DOI] [Google Scholar]
- Multisanti CR, Merola C, Perugini M, Aliko V, Faggio C. Sentinel species selection for monitoring microplastic pollution: A review on one health approach. Ecological Indicators. 2022;145:109587. doi: 10.1016/j.ecolind.2022.109587. [DOI] [Google Scholar]
- Navarro J, Grémillet D, Afán I, Ramirez F, Bouten W, Forero MG. Feathered detectives: Real-time GPS tracking of scavenging gulls pinpoints illegal waste dumping. PLoS ONE. 2016;11:e0159974. doi: 10.1371/journal.pone.0159974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hanlon NJ, James NA, Masden EA, Bond AL. Seabirds and marine plastic debris in the northeastern Atlantic: A synthesis and recommendations for monitoring and research. Environmental Pollution. 2017;231:1291–1301. doi: 10.1016/j.envpol.2017.08.101. [DOI] [PubMed] [Google Scholar]
- Pais de Faria J, Vaz PT, Lopes CS, Calado JG, Pereira JM, Veríssimo SN, Paiva VH, Gonçalves AMM, Ramos JA. The importance of marine resources in the diet of urban gulls. Marine Ecology Progress Series. 2021;660:189–201. doi: 10.3354/meps13599. [DOI] [Google Scholar]
- Pon JPS, Pereyra PJ. First evidence of anthropogenic debris in nests of the Kelp Gull (Larusdominicanus) from a small semi-desert Argentinean coastal ecosystem. Marine Pollution Bulletin. 2021;170:112650. doi: 10.1016/j.marpolbul.2021.112650. [DOI] [PubMed] [Google Scholar]
- Rock P, Camphuysen CJ, Shamoun-Baranes J, Ross-Smith VH, Vaughan IP. Results from the first GPS tracking of roof-nesting herring gulls Larusargentatus in the UK. Ringing & Migration. 2016;31:47–62. doi: 10.1080/03078698.2016.1197698. [DOI] [Google Scholar]
- Ryan PG. Using photographs to record plastic in seabird nests. Marine Pollution Bulletin. 2020;156:111262. doi: 10.1016/j.marpolbul.2020.111262. [DOI] [PubMed] [Google Scholar]
- Spelt A, Williamson C, Shamoun-Baranes J, Shepard E, Rock P, Windsor S. Habitat use of urban-nesting lesser black-backed gulls during the breeding season. Scientific Reports. 2019;9:10527. doi: 10.1038/s41598-019-46890-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabor GM, Alonso Aguirre A. Ecosystem health and sentinel species: Adding an ecological element to the proverbial ‘‘Canary in the mineshaft’’. EcoHealth. 2004;1:226–228. doi: 10.1007/s10393-004-0092-8. [DOI] [Google Scholar]
- Tavares DC, Moura JF, Acevedo-Trejos E, Crawford RJ, Makhado A, Lavers JL, Witteveen M, Ryan PG, Merico A. Confidence intervals and sample size for estimating the prevalence of plastic debris in seabird nests. Environmental Pollution. 2020;263:114394. doi: 10.1016/j.envpol.2020.114394. [DOI] [PubMed] [Google Scholar]
- Thaxter CB, Ross-Smith VH, Clark JA, Clark NA, Conway GJ, Marsh M, Leat EHK, Burton NH. A trial of three harness attachment methods and their suitability for long-term use on Lesser Black-backed Gulls and Great Skuas. Ringing & Migration. 2014;29:65–76. doi: 10.1080/03078698.2014.995546. [DOI] [Google Scholar]
- Thibault M, Houlbrèque F, Lorrain A, Vidal E. Seabirds: Sentinels beyond the oceans. Science. 2019;366:813. doi: 10.1126/science.aaz7665. [DOI] [PubMed] [Google Scholar]
- Thompson DL, Ovenden TS, Pennycott T, Nager RG. The prevalence and source of plastic incorporated into nests of five seabird species on a small offshore island. Marine Pollution Bulletin. 2020;154:111076. doi: 10.1016/j.marpolbul.2020.111076. [DOI] [PubMed] [Google Scholar]
- Wowrzeczka B. City of waste—importance of scale. Sustainability. 2021;13:3909. doi: 10.3390/su13073909. [DOI] [Google Scholar]
- Yaghmour F, Al Marashda AS. Frequency and composition of anthropogenic debris in the nests of sooty gulls Larus (Adelarus) hemprichii Bruch, 1853 from Sir Bu Na’ir Island United Arab Emirates. Marine Pollution Bulletin. 2020;150:110715. doi: 10.1016/j.marpolbul.2019.110715. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.