Abstract
N-glycosylation is one of the most common types of protein modifications and it plays a vital role in normal physiological processes. However, aberrant N-glycan modifications are closely associated with the pathogenesis of diverse diseases, including processes such as malignant transformation and tumor progression. It is known that the N-glycan conformation of the associated glycoproteins is altered during different stages of hepatocarcinogenesis. Characterizing the heterogeneity and biological functions of glycans in liver cancer patients will facilitate a deeper understanding of the molecular mechanisms of liver injury and hepatocarcinogenesis. In this article, we review the role of N-glycosylation in hepatocarcinogenesis, focusing on epithelial-mesenchymal transition, extracellular matrix changes, and tumor microenvironment formation. We highlight the role of N-glycosylation in the pathogenesis of liver cancer and its potential applications in the treatment or diagnosis of liver cancer.
Subject terms: Cancer microenvironment, Diseases
Facts
N-glycosylation modifications mediate multiple biological functions such as cell recognition, signal transduction, and immune response.
The glycosylation pattern of tumor cells is often altered to facilitate cancer progression.
The N-glycan conformation of related proteins may change to different degrees during the pathogenesis of liver cancer.
Open questions
What physiological processes are involved in the pathogenesis of liver cancer?
What role does N-glycosylation play in the pathogenesis of liver cancer?
Does N-glycosylation modification have practical application prospects in the diagnosis and treatment of liver cancer?
Introduction
Liver cancer is the fifth most common cancer type in the world, and the second leading cause of cancer-related death, with more than 900,000 new cases and 830,000 deaths annually [1]. Liver cancer is a great threat to human health, especially among Asian people.
Primary liver cancer accounts for the largest proportion of liver cancer cases. The liver cancer involved in this review refers to primary liver cancer. According to different pathological features, primary liver cancer can be classified into hepatocellular carcinoma (HCC), intrahepatic cholangiocarcinoma, and the extremely uncommon combined hepatocellular cholangiocarcinoma [2]. Approximately 90% of these cases of conventional liver cancer are attributed to HCC [3]. HCC can develop in response to any cirrhosis risk factor, such as hepatitis B virus (HBV) infection, hepatitis C virus (HCV) infection, non-alcoholic steatohepatitis, aflatoxin exposure, obesity, and diabetes mellitus [4]. Liver cancer is aggressive, exhibiting rapid cell growth, effective angiogenesis, and a prominent anti-apoptosis phenotype—all driven by pathogenic mechanisms that remain unclear [5].
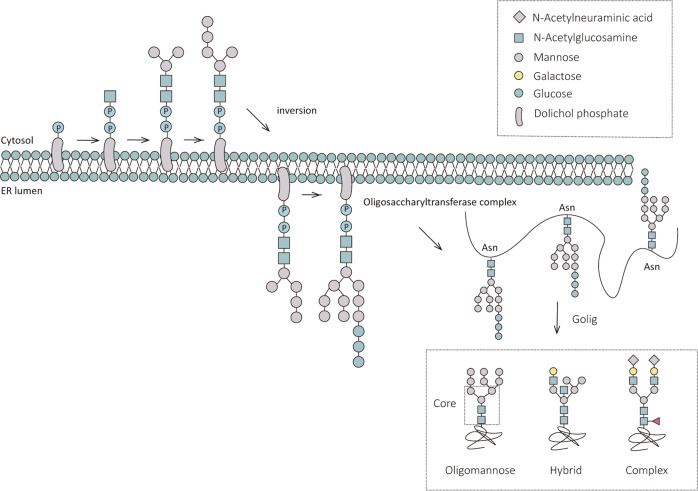
Glycosylation affects the biological functions of normal cells. It plays a vital role in the pathogenesis of cancer and is one of the most common types of post-translational protein modification [6]. Glycosylation modification can be classified as N-glycosylation and O-glycosylation based on the different types of amino acid residues linked with the glycan chain. N-glycosylation occurs at the amino acid sequence NXS/T(X ≠ P), and O-glycosylation occurs at the S/T site [7]. Glycosylated proteins, N-linked glycosylated proteins in particular, are predominant among proteins destined for extracellular environments. Here we focus on N-glycosylation modifications. First, Dolichol phosphate binds to the glycan chains to form the glycolipids Dolichol-linked oligosaccharides (DLOs), which serve as glycan donors to provide substrates for N-glycosylation [8]. The mature DLOs consist of two N-acetylglucosamines, nine mannoses and three glucoses. Subsequently, the N-glycan is recognized by the oligosaccharyltransferase (OST) complex, and the OST complex mediates its transfer to the N site on the nascent polypeptide chain that harbors an NXS/T(X ≠ P) sequence combination [7]. Finally, the N-glycan of the bound polypeptide chain will enter the Golgi for further processing and modification to form three different N-glycan classes: oligomannose, hybrid and complex [9]. The abovementioned is the main process of N-glycosylation. A schematic diagram is shown in Fig. 1.
Fig. 1. The process of N-glycosylation.
N-glycosylation modifications begin at the endoplasmic reticulum and end at the Golgi apparatus. The new oligosaccharide chains are initially processed in the endoplasmic reticulum and then transferred to the N-glycosylation sites of the new peptide chains by the action of the oligosaccharyltransferase complex (OST), and the attached polypeptide chains will undergo further processing and modification in the Golgi apparatus to form three N-glycan types, oligomannose, hybrid, and complex.
A slight change in the amount of glycosyltransferase or glycan donor in the body can lead to a great variation in the conformation of polysaccharides [10]; this is closely related to a massive change in life activities [11]. The association between glycoprotein levels and conformational variations and cancer development has been recognized for a long time. As early as 1978, Rostenberg et al. found statistically significant differences in alpha1-antitrypsin (alpha1-AT) glycosylation levels between patients with lung, prostate, and gastrointestinal cancers and control patients with benign tumors [12]. Although the authors did not conduct further studies on the differential glycoforms of alpha1-AT, their results clearly indicate that the glycosylation pattern of alpha1-AT is changed in different cancer types. In the 1990s, Hakomori systematically summarized the impact of abnormal glycosylation on tumorigenesis and tumor development, providing a framework for subsequent researchers [13]. During this period, studies on the connection between N-glycosylation status and the advancement of cancer have increased.
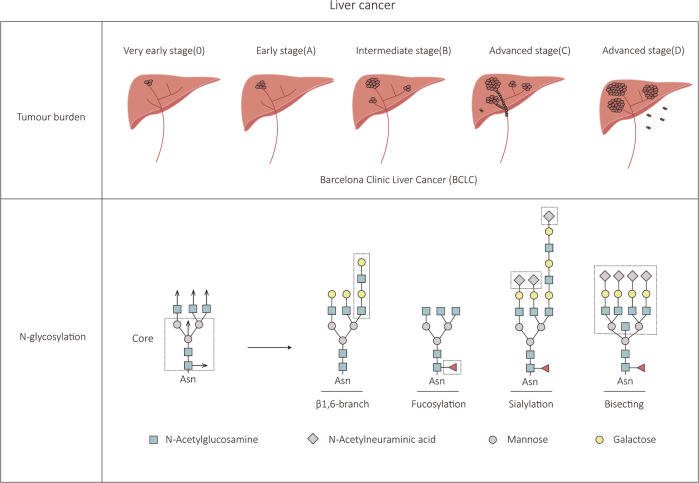
Liver cancer, one of the most prevalent malignancies, has also been a popular research subject. Certain N-glycosylation changes have been associated with liver cancer in particular. It is difficult to detect early-stage liver cancer because of a lack of reliable markers. The first study on the N-glycan chain categories of serum glycoproteins in patients with liver cancer was conducted by Turner in 1992, whose results showed that the levels of sialylation and fucosylation of N-glycan chains were strongly increased when liver lesions progressively developed into liver cancer [14] (Fig. 2). DelaCourt et al. further confirmed that there is a correlation between HCC genotype and N-glycosylation heterogeneity [15]. This suggests that targeting aberrant glycosylation modifications may facilitate disease diagnosis. Later research revealed that the N-glycosylation of envelope proteins is also necessary for the secretion of hepatitis virus particles [16, 17]. Jiang et al. discovered that the N-glycan chain on Golgi Protein 73 (GP73) Asn144 reduces the invasive ability of HCC cells by mediating intercellular adhesion [18]. Recently, it has been demonstrated that N-glycosylation of residues 294 and 454 of the Mer tyrosine kinase (MerTK), a crucial factor in the prognosis of HCC, can promote the proliferation and transformation of HCC cells [19]. It is clear that N-glycosylation alterations change the outcome of liver cancer development. The interest in investigating the glycosylation status of N-glycoproteins has remarkably increased in recent years due to its potential value for early diagnosis of liver cancer and effective therapy.
Fig. 2. Schematic diagram of liver cancer progression and associated N-glycan type changes.
Barcelona Clinic Liver Cancer (BCLC) is the most widely used clinical liver cancer staging system, which divides liver cancer into three stages, early (0/A), intermediate (B), and advanced (C/D), mainly based on the patient’s tumor status and liver function. N-glycan chains can participate in cell recognition, signal transduction, immune responses, and other life activities. Compared with normal cells, tumor cells, such as HCC cells, often exhibit abnormal changes in N-glycan conformation, such as abnormal branching chains and bisecting structures and altered levels of fucosylation and sialylation.
The pathogenesis of liver cancer involves biological processes related to epithelial-mesenchymal transition (EMT), extracellular matrix (ECM) changes, and tumor microenvironment (TME) formation [20]. The association between N-glycosylation upon HBV infection and during hepatocarcinogenesis has been generally described previously [21]. In this review, we discuss the physiological functions of N-glycosylation of related proteins in the abovementioned biological processes and propose potential strategies targeting N-glycosylation in the diagnosis and treatment of liver cancer.
Effect of N-glycosylation modification on epithelial-mesenchymal transition
Epithelial-mesenchymal transition (EMT) occurs when cells lose their epithelial properties and acquire mesenchymal properties which contribute to tumor progression [22].
The established notion is that EMT is correlated with tumor aggressiveness and poor outcome. A large amount of experimental evidence has shown that a variety of effectors are involved in the process of EMT in the development of HCC to promote the occurrence and pathogenesis of HCC [23]. E-cadherin, N-cadherin, Epithelial Cell Adhesion Molecule (EpCAM), vimentin, and other marker proteins that are frequently employed to identify the EMT process are crucial in stimulating normal liver cells to develop mesenchymal traits [24]. The investigators selected 145 clinical cases related to liver cancer, of which about 50% of cirrhotic patients were caused by HCV infection, and analyzed the expression levels of EMT-associated proteins in serum samples from patients in the normal, cirrhotic, and HCC groups. Comparing HCC patients to cirrhotic and healthy individuals, HCC patients showed the lowest levels of E-cadherin [25]. Moreover, deletion or downregulation of E-cadherin greatly promoted the invasive metastatic phenotype of liver cancer cells, conducive to the EMT process [26].
In HCC, related transcription factors such as Snail and Twist are often upregulated to accelerate the development of EMT [27]. In addition, some signaling pathways, such as the Wnt/β-catenin pathways that drive EMT, are also activated [28]. Under normal physiological conditions, β-catenin can bind to the intracellular structural domain of E-cadherin to enhance intercellular adhesion [29]. However, during the EMT process, the interaction between them is weakened; β-catenin dissociates from the calmodulin complex and is translocated to the nucleus, where it plays a regulatory role, such as increasing the expression levels of Snail and ZEB1 in HCC cells, which results in weakened cell adhesion and increased motility [30].
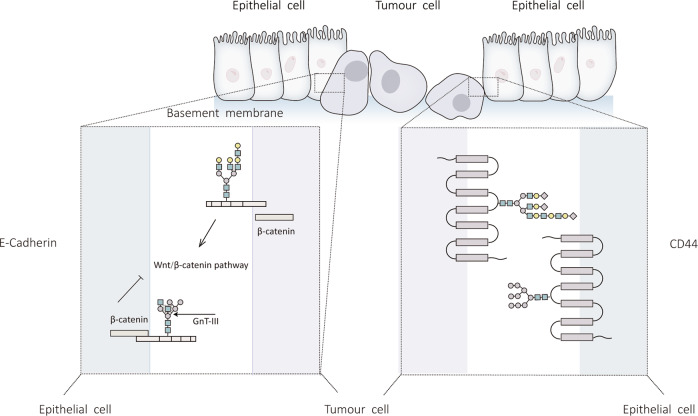
Therefore, EMT is a crucial process in the development of liver cancer. As previously discussed, tumor cells typically have varying degrees of glycosylation. In the following, we will elaborate on the influence of N-glycosylation modification of related proteins on the process of EMT in liver cancer (Fig. 3).
Fig. 3. Effect of N-glycosylation modification on EMT.
A large body of experimental evidence shows that EMT is an indispensable part of the occurrence and development of liver cancer, which involves the abnormal N-glycosylation of related proteins. This diagram summarizes the abnormal N-glycosylation of associated proteins during EMT in liver cancer.
E-cadherin
The N-glycan chain conformation of E-cadherin, a marker protein of epithelial cells and a transmembrane glycoprotein, greatly affects its adhesive properties [31], and in malignant tumors the N-glycan chains of E-cadherin tend to form a more complex branching chain structure [32], which promotes internalization of E-cadherin into the cytoplasm and disrupts intercellular adhesion, thereby disrupting the relevant signaling pathways and facilitating the occurrence of EMT [33].
As previously mentioned, β-catenin enters the nucleus following its dissociation from E-cadherin to activate the Wnt signaling pathway and promote the expression of target genes for EMT in HCC cells [30]. In addition, the N-glycan chain of E-cadherin controls the contact between E-cadherin and β-catenin. According to the findings of Xu et al., N-acetylglucosaminyltransferase 3 (GnT-III) is involved in this process [34]. GnT-III catalyzes the production of β-1,4 glycosidic linkages, resulting in the formation of the bisecting chain of E-cadherin. The results of Xu et al. further indicate that when TGF-β1 promotes EMT, the expression of GnT-III is downregulated, leading to a reduction of the bisecting N-glycan structure formed by E-cadherin and inhibition of the interplay between E-cadherin and β-catenin [35]. Then β-catenin stimulates the Wnt signaling pathway by nuclear translocation, accelerating EMT. The abovementioned conclusion clarified the value of the N-glycan structure of E-cadherin in EMT for the first time. Mo et al. confirmed this opinion in MHCC97-L cells, indicating that the mechanism may also be applicable to liver cancer [36].
This implies that important enzymes mediating the process of N-glycosylation modification, such as GnT-III, can be used as potential targets for liver cancer therapy.
EpCAM
Epithelial cell adhesion molecule (EpCAM) is a type I transmembrane glycoprotein that was once thought to be a tumor-associated antigen due to its high expression in quickly growing epithelial malignancies [37]. EpCAM has three N-glycosylation sites, which is Asn77, Asn111, and Asn198 [38]. It has been reported that when all the above sites are mutated, the half-life of EpCAM decreases from 21 to 7 h [39], which suggests that N-glycosylation can stabilize the protein.
EpCAM is present in a variety of cancer types and is crucial for tumor metastasis, cell proliferation, and adhesion. As one of the most important post-translational modification types, N-glycosylation is also involved in the abovementioned activities. There have been claims that when the N-glycan chain of EpCAM is disrupted in breast cancer cells, the expression of pro-apoptotic proteins Bax and Caspase 3 is increased, showing that EpCAM hinders apoptosis through its N-glycan [40]. Moreover, Liu et al. counseled that the N-glycan of EpCAM could enhance the adhesion of breast cancer cells [41]. When N-glycosylation sites were altered, the interaction between EpCAM and fibronectin (FN) was compromised and intercellular adhesion was decreased. Thus, N-glycosylation of EpCAM has extremely important physiological effects in breast cancer, which may also apply to other cancer types such as liver cancer.
In human liver cancer samples, EpCAM is often co-expressed with EMT marker proteins [42] and cancer stem cell biomarkers [43]. EpCAM-positive HCC cells are highly invasive [44]. Sancho-Bru et al. reported that EpCAM is considerably upregulated in patients with alcoholic hepatitis compared with the normal group [45]. When EpCAM was knocked down, the PI3K/Akt/mTOR signaling pathway was arrested, and the level of liver fibrosis in alcoholic hepatitis mice was improved [46]. These results indicate that EpCAM may serve as a target for liver cancer treatment. Based on the function of the N-glycan chain of EpCAM in breast cancer cells, it is reasonable to speculate that targeting the N-glycosylation of EpCAM may also have unexpected effects in liver cancer. More experimental evidence is needed to validate this conjecture.
CD44
Lymphocyte homing receptor (CD44) is a non-kinase transmembrane glycoprotein that is expressed in both embryonic stem cells and connective tissue [47]. CD44, as one of the common tumor stem cell biomarker, can regulate EMT and participate in the progression of tumors [48].
CD44 has a globular N-terminal hyaluronic acid binding domain (HABD), which binds specifically to hyaluronic acid [49]. This binding alters the conformation of CD44, activating various intracellular signaling pathways and regulating cell biological behaviors [50]. CD44 has been shown to participate in EMT by binding to its ligand hyaluronic acid [51]. The role of N-glycosylation in this process will be further elaborated below.
Specific residues on glycoproteins are important for recognition of other biomolecules. Changes in the glycan composition of glycoproteins greatly affect these interactions, such as the interaction between CD44 and hyaluronic acid. Their binding is highly regulated by glycosylation of the HABD [52]. Multiple N-glycosylation sites exist in the HABD of CD44 [53]. The interaction between CD44 and hyaluronic acid is highly influenced by the N-glycan conformation of HABD [54]. The complex N-glycan structure on HABD reduces the recognition ability between them. In addition, when N-glycan on the HABD is acidified by saliva, this also inhibits the binding of CD44 to its ligand, thus affecting the function of CD44 [55]. Studies have shown that the binding activity between CD44 and hyaluronic acid is notably enhanced after sialic acid molecules of the N-glycosylation chain on CD44 are specifically removed by treating HCC cells with sialidase NEU4, which inhibits the migration of HCC cells [56]. These results provide a new feasible idea for controlling HCC cell motility and metastasis.
Effect of N-glycosylation modification on the extracellular matrix
In terms of spatial distribution, the extracellular matrix (ECM) consists of two major components, the basement membrane, and the interstitial matrix, and the ECM is rich in fibronectin (FN), proteoglycans, and stromal cell proteins [57]. Under normal physiological conditions, these components are combined in a specific way to provide a suitable mechanical environment for cells, giving the matrix unique physical and biochemical characteristics, affecting cell morphology, metabolism, and migration [58].
In reality, the ECM is in a state of orderly reconstruction in normal organisms, and its components are constantly circulating in the three processes of secretion, modification, and degradation to adapt to and maintain tissue homeostasis [59]. However, during cancer development, this process is dysregulated, and although the ECM can act as a physical barrier to prevent tumor cell proliferation and invasion in the early stages, once tumor cells break through the basement membrane, prompting changes in the composition and structural properties of the ECM, the tumor stroma formed by ECM remodeling will facilitate tumor cell growth, survival, and invasion [60].
The liver has a pronounced capacity for regeneration in its role as a metabolite-detoxifying organ. This process generally starts with the activation of associated enzymes, such as MMPs, for the degradation of abnormal ECM components [61], followed by the stimulation of growth factors, such as TGF-β, to start proliferative remodeling [62]. Compared with other solid tumors, the degree of fibrosis in the liver is high after organic lesions, and excessive fibrosis can lead to cirrhosis, which eventually develops into HCC if not treated properly. Pathological damage to the liver occurs in response to various pathogenic factors and is essentially caused by excessive accumulation of ECM proteins [63]. In chronic liver injury, although the damaged liver will regenerate and rebuild, the new liver tissue no longer has its original structural features and functional characteristics [64]. The formation of aggregated fibrous bundles of ECM components that are difficult to degrade during the repair and reconstruction of multiple injuries, known as fibrous scars, which can obstruct blood flow of liver tissues and are the cause of liver tissue lesions, is the most common adverse effect of this repair method used to maintain homeostasis of the internal environment in chronic liver injury [65].
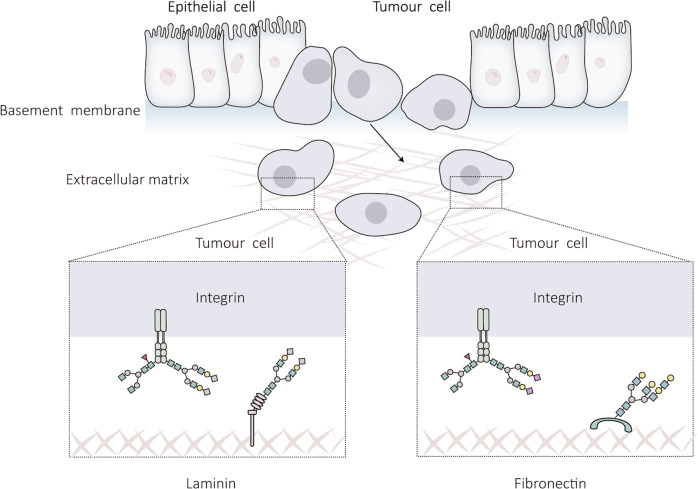
ECM dysregulation is associated with abnormal expression of its components [66], and the role of N-glycosylation modifications in this process will be discussed below (Fig. 4).
Fig. 4. Effect of N-glycosylation on the ECM.
Under normal physiological conditions, the components of the ECM can provide a suitable mechanical environment for cells. However, in liver cancer, the ECM is disordered, which is closely related to the N-glycosylation of ECM components. This figure outlines the effect of N-glycosylation on ECM homeostasis in liver cancer.
Integrin
Integrins are transmembrane glycoprotein receptors that bind to ECM components and trigger signaling cascades to regulate cellular homeostasis and normal developmental processes in the organism [67]. Integrins are composed of an α subunit and a β subunit. At present, 24 different integrin categories have been identified in vertebrates, with different ligand binding properties, serving as a bridge for information transmission between cells and the ECM [68]. For example, α5β1 integrin can recognize the Arg-Gly-Asp tripeptide sequence in FN and regulate intercellular adhesion [69], while α3β1 integrin can recognize its ligand-linked adhesion molecule (JAM-A) and promote the migration of polarized epithelial cells [70].
Abnormal glycosylation modifications, including sialylation and core fucosylation, are common in tumor cells and play a conspicuous role in cancer progression [71]. As the main N-glycan carrier, integrin has more than 20 potential glycosylation sites, and the core structure of N-glycan is crucial for the formation and function of heterodimers [72]. Han et al. revealed that miRNA can be employed as a tumor suppressor by regulating the sialic acid content of integrin 1 via the sialic acid transferase ST6Gal-I, preventing HCC cells from spreading [73]. In addition, it has been found that inhibition of the core fucosylation of integrin in HepG2 cells affects its downstream signaling pathway, resulting in significant suppression of cell proliferation and migration [74]. The abovementioned studies have shown that the abnormal N-glycan modification of the integrin subunit can affect its affinity to ligands, regulating cell function and making it tend to the malignant phenotype, suggesting that abnormal sialylation and fucosylation on targeted integrin N-glycan can be used as a potential way for HCC treatment.
Fibronectin
Fibronectin (FN) is a glycoprotein with a large molecular weight that is involved in wound healing and embryo development. It consists of two subunits, each of which is made up of three repeat modules with identical protein folding conformations, such as types I, II, and III [75]. Due to the selective splicing of mRNA, FN has different structural characteristics, which affects its solubility. Soluble FN exists in plasma and regulates cell adhesion, promoting wound healing, while insoluble FN exists in the ECM, mediating cell growth and differentiation [76].
Hsiao et al. reported the plasma FN N-glycan site and discovered that the N-glycan conformation of plasma FN influences its interaction with integrins, altering cell adhesion [77]. Therefore, N-glycan conformational changes could regulate the functional properties of plasma FN. Subsequently, Liu et al. analyzed the glycan types of human plasma FN, Mass spectrometry (MS) analysis revealed that 77.31 % of the six identified N-glycosides were sialic acidified [78], enriching our understanding of the site-specific glycosylation patterns of FN. Vascular infiltration is one of the main risk factors for recurrence and poor prognosis of liver cancer patients. It has been reported that FN is a biomarker of invasive HCC [79]. Linking FN with its N-glycosylation is helpful to find new therapeutic targets and improve the condition of patients.
Laminin
Laminin is an ECM glycoprotein found in the basement membrane that promotes cell adhesion and migration, maintains tissue structural stability and mediates specific physiological functions through interactions with cell surface receptors and stimulation of intracellular signaling cascade responses [80]. Laminins are heterogeneous trimers composed of five α-chains, four β-chains, and three γ-chains, but of the 60 possible heterogeneous trimers, only 16 have been confirmed biochemically [81]. Ln-332 is composed of α3, β3, and γ2 chains and mediates the adhesion between epithelial cells and the basement membrane. Ln-γ2, a component of Ln-332, is often expressed as a monomer in malignant tissues, and additionally, Ln-γ2 monomers can induce tumor cell proliferation and migration in vitro [82], suggesting that Ln-γ2 monomer can be used as a potential cancer detection marker to facilitate the screening of cancer and improve diagnosis. Yasuda et al. found that with the combined application of Ln-γ2 monomer and vitamin K deficiency-induced prothrombin (PIVKA-II) in clinical HCC detection, the diagnosis may be more reliable [83]. In addition, Ln-332 is a large heterogeneous trimeric glycoprotein, and relatively little is known about the functional impact of N-glycosylation on this class of proteins. Kariya et al. showed that cell adhesion was decreased when overexpression of N-acetylglucosaminyltransferase III (GnT-III), induced the formation of more diastereomeric N-glycan structures; however, intercellular adhesion was remarkably enhanced when overexpression of N-acetylglucosaminyltransferase V (GnT-V), induced the formation of more β1,6-branched N-glycan structures [84]. The abovementioned results demonstrated that N-glycosylation could regulate the biological function of Ln-332. This finding may lead to new therapeutic strategies for liver cancer.
Effect of N-glycosylation modification on tumor microenvironment
The tumor microenvironment (TME) is a dynamic system that surrounds a tumor inside the body that is widely implicated in tumorigenesis [85]. This system contains a variety of resident and infiltrating host cells, secreted factors, and ECM proteins [86]. Emerging evidence shows that TME-mediated changes in glycosylation play an important functional role in tumorigenesis.
The TME consists of a large number of immune cells. These immune cells can be split into two main types, tumor-antagonizing immune cells and tumor-promoting immune cells, based on their impact on the development of cancer. Tumor-antagonizing immune cells, such as CD8+ cytotoxic T cells, target and kill tumor cells in the early stages of cancer; tumor-promoting immune cells, such as Myeloid-Derived Suppressor Cells (MDSCs), suppress the immune response in the body and support cancer progression [87].
As an immune regulatory organ, the liver contains a variety of immune cells, such as natural killer cells and dendritic cells (DCs), which can mediate a series of immune response to eliminate pathogens and sustain internal environmental homeostasis [88]. The immune system of the body is out of balance in liver cancer, which causes excessive release of pro-inflammatory factors in the TME [89]. This causes a sustained inflammatory response in the liver, pushing the healthy liver in the direction of fibrosis and cirrhosis and ultimately leading to the development of liver cancer [90].
N-glycosylation is crucial for regulating immune cell differentiation and maturation [91]. DCs are the most powerful antigen presenting cells (APCs), mediating innate immunity and inducing adaptive immunity. Studies have revealed that sialylation levels drastically decrease during the maturation of DCs [92]. Further analysis unveiled that when mouse bone marrow-derived DCs (BMDCs) were treated with sialic acid blocking mimics, the viability and differentiation degree of BMDCs were not affected, but their maturation was obviously boosted, and the proliferation levels of BMDCs-induced antigen-specific CD8+ T cells were raised, impeding the progression of cancer development [93]. After stable overexpression of β-Galactoside α2-6-sialyltransferase 1 (ST6Gal-I) in HepG2 cells, the number of CD8+ T cells infiltrated in the TME was reduced, so the content of IFN-γ and TNF-α secreted by them were decreased, which is beneficial to liver cancer progression [94].
The impact of N-glycosylation on immune cells in the TME of liver cancer will be discussed below (Table 1).
Table 1.
Effect of N-glycosylation on immune cells in the TME of liver cancer.
 N-Acetylneuraminic acid
N-Acetylneuraminic acid  N-Acetylglucosamine
N-Acetylglucosamine  Mannose
Mannose
T cells
T cells are present in almost every organ and tissue of the body, including the liver. T cells are functionally diverse lymphocytes differentiated from hematopoietic stem cells in the bone marrow, which develop and mature in the thymus [95]. In the early stages of liver cancer, T cells are activated after receiving antigen-presenting signals from APCs, after which they infiltrate into the TME and fight against liver cancer cells, while the glycosylation patterns displayed by T cells are directly related to their maturation and activation [96].
As one of the crucial elements of T cell recognition of antigens, T cell receptor (TCR) plays a key role in T cell activation. This process involves the formation of immune synapses, where the TCR-antigenic peptide-major histocompatibility complex (MHC) forms the central region of the immune synapse, and the interaction of the abovementioned components stimulates the immune function of T cells [97]. The current findings suggest that the N-glycan chain on the TCR and MHC maintains the stability of related components in immune synapses, which inhibits their degradation by the proteasome [98, 99].
Tyrosinase is a common tumor antigen. When the N-glycan chain at its Asn317 site was disrupted, its presentation by MHC-I was greatly increased [100]. The recognition of antigenic peptides by TCR was much improved once the N-glycan chain was eliminated, which aided the ensuing immune response [100]. In addition, the antigenicity and immunogenicity of the hepatitis B surface antigen (HBsAg) are compromised with the attachment of extra N-glycan [101]. In this way, HBV molecules generate new N-glycan branches on HBsAg to obscure the original antigenic epitopes recognized by the TCR for immune escape [102]. This indicates that the N-glycan chain affects the recognition of antigenic peptides by the TCR. However, terminal N-glycan modifications on antigenic peptides or TCRs, such as fucosylation, are involved in the activation of T cells [103]. Sialylation is one of the classes of terminal N-glycosylated modifications and is essential for the regulation of immune function. According to a study by Oswald et al. [104], liver tissue exhibits a strong inflammatory response when 2,6-sialic acid branched chains are specifically removed from the surface of mouse liver cells. The T cell-mediated immune response is also triggered. This conclusion can be supported by the experimental data of Liu et al. [105]. The effect of N-glycosylation on the immune response is also correlated with terminal N-glycan modification.
Clarifying the aberrant N-glycosylation pattern of immune cells in the TME of liver cancer will help us better understand the pathogenesis of this cancer type.
Macrophages
Similar to T cells, macrophages are distributed throughout the body, but macrophages mediate the innate immune response. The majority of hepatic macrophages are Kupffer cells. When liver tissue is damaged, peritoneal and bone marrow-derived macrophages migrate to the liver, working with Kupffer cells and other immune cells to counteract the effects of the injury [106].
Based on their functional characteristics, macrophages can be divided into M1 and M2 macrophages. M1 macrophages positively regulate the immune response, while M2 macrophages secrete inflammatory inhibitory factors such as TGF-β, negatively controlling the immune response [107]. In liver cancer, the proportions of M1/M2 macrophages are always fluctuating, promoting or inhibiting TME formation [108]. Targeting macrophage subtypes to reverse the inflammatory response is a promising strategy for liver cancer treatment. During the differentiation and maturation of macrophages, their surface N-glycans undergo remodeling [109]. Yang et al. investigated the classes of N-glycans of mouse peritoneal macrophages and identified 587 N-glycoprotein components [110]. Their data showed that the N-glycosylation pattern of these proteins was notably remodeled during macrophage activation, and the level of core fucosylation expressed by mature macrophages was remarkably reduced [110]. CD14 is a macrophage marker protein, and its N-glycan depletion disrupts its membrane localization, thus affecting the polarization process of macrophages [110].
Galactose lectins are a class of proteins with a specific affinity for β-galactoside, which is a fundamental regulator of cell microenvironment remodeling, and its aberrant expression has been linked to a variety of clinical diseases [111, 112]. It has been reported that abnormal expression of Gal-1 and Gal-3 is closely related to the invasion and migration of liver cancer cells and poor prognosis of liver cancer patients [113, 114]. Gal9 mediates macrophage polarization as a ligand for N-glycosylated T-cell immunoglobulin mucin 3 (Tim-3) [115], which may lead to liver cancer cell death [116]. CD248 is a C-type lectin-like receptor, which is mostly expressed in cancer tissues and can bind to glycoprotein components in the TME to participate in related inflammatory reactions [117]. In HCC, CD248 is expressed on CAFs and interacts with the macrophage marker protein CD68, promoting macrophage M2 polarization [118]. IgG78 is a specific antibody targeting glycosylated CD248, which reduces the expression level of CD248 in CAFs and decreases the production of M2 macrophages, exerting anti-tumor effects [118]. While the targeting effect of IgG78 on CD248 is highly correlated with the level of N-glycan on CD248, the anti-tumor effect of IgG78 is diminished when the N-glycan chain of CD248 is disrupted [118].
The interaction between N-glycan proteins and their ligands or receptors tremendously influences the polarization of macrophages, and targeting the N-glycosylation regulatory mechanisms of the corresponding proteins would help to alleviate the inflammatory response in the liver.
Application of N-glycosylation in liver cancer diagnosis
The treatment of patients with HCC is closely related to their clinical diagnosis. Microwave ablation is typically used to treat early-stage patients; liver transplantation and hepatic resection are typically used to treat mid-stage patients; while advanced patients can only be treated with clinical systemic pharmacological treatments to slow down the disease process, commonly first-line drugs such as sorafenib [92].
For patients with early liver cancer, surgical treatment is the first choice, but for patients who need liver transplantation, it is not easy to find suitable liver donors [119]. Although drug therapy can improve the overall survival rate of advanced patients, the side effects of this treatment cannot be ignored [120]. This kind of risk could be avoided by techniques such as early screening. The establishment of early screening indicators could reduce the risk of death to a great extent, and improve patient survival. However, screening for liver cancer is not an easy task. Most patients are diagnosed in the middle to late stages and miss the best time for treatment, so it is important to establish accurate and reliable screening indicators [121].
Cancer development and N-glycosylation are tightly connected. Some N-glycosylated proteins are also common indicators for the diagnosis of liver cancer. Below, we will list the application of N-glycosylation of some proteins in the diagnosis of liver cancer (Box 1, Table 2).
Table 2.
Important N-glycosylation events in liver cancer.
| Protein | N-glycosylation events in liver cancer | Outcome/Application |
|---|---|---|
| E-Cadherin | Reduce bisecting N-glycan structure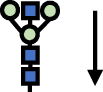
|
The interaction between E-Cadherin and β-catenin is weakened, and β-catenin enters the nucleus to activate the Wnt signaling pathway and promote the EMT process |
| CD44 | Increase sialylation levels
|
Promote the intercellular adhesion |
| Integrin | Raise sialylation and core fucosylation levels
|
Inhibit the proliferation of liver cancer cells |
| Fibronectin | Elevate sialylation levels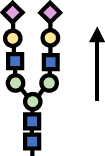
|
Influence its interaction with integrins, altering cell adhesion |
| Laminin | Increase the formation of β1, 6-branch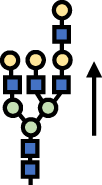
|
Enhance intercellular adhesion. |
| AFP | Expand core fucosylation levels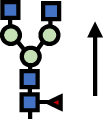
|
More reliable clinical diagnostic and prognostic indicator of liver cancer |
| AGP | Raise sialylation levels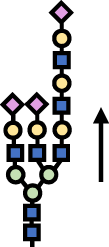
|
Increase diagnostic accuracy of liver cancer |
| Haptoglobin | Widen sialylation levels, lessen the formation of N-glycan branching structure
|
Reduce false negative results of diagnosis |
 N-Acetylneuraminic acid
N-Acetylneuraminic acid  N-Acetylglucosamine
N-Acetylglucosamine  Mannose
Mannose  Galactose
Galactose
Box 1 Application of N-glycosylation in liver cancer diagnosis.
AFP
AFP, a common liver cancer diagnostic indicator, is not sufficient by itself to determine whether a patient is diagnosed, but needs to be combined with Computed Tomography and other techniques [128]. Although most patients with liver cancer have high serum AFP levels, liver cancer is not the only cause of elevated AFP, as abnormalities in this indicator can also be detected in patients with lung cancer [129] or pancreatic cancer [130] and in pregnant women.
It has been established that abnormal glycosylation is a crucial component of carcinogenesis and cancer development, including HCC. We mentioned earlier that AFP is an N-glycosylation-modified protein with multiple N-glycosylation sites [131]. AFP can be classified into three groups, AFP-L1, AFP-L2, and AFP-L3, depending on how well they bind to the leptin Lens Culinaris Agglutinin (LCA). AFP-L1 is the main AFP type and does not bind specifically to LCA; AFP-L2 can be found in pregnant women and has an average binding ability to LCA; AFP-L3 has the strongest binding ability to LCA [132]. Core fucosylation at site 251 of AFP-L3 have been reported, and such abnormal N-glycosylation modifications are closely associated with malignant disease progression [133]. Serum AFP-L3 has also been used as a clinical diagnostic and prognostic indicator for the prevention and treatment of liver cancer, which is more reliable.
AGP
Similarly, abnormal levels of α1-acid glycoprotein (AGP) contribute to the pathogenesis of liver cancer and are a common diagnostic indicator for liver cancer [134]. AGP is synthesized by the liver and is primarily produced by macrophages and liver granulocytes, which are linked to the inflammatory reaction of the body [135]. At present, it has been confirmed that there are five N-glycosylation sites in AGP, which is a complex N-glycan and can form various types of branched conformations of sugar chains, with approximately 11% of the N-glycan chains undergoing sialylation [136]. It has been documented that the glycan conformation of AGP and other modification types on its N-glycan chain are highly correlated with the disease stage, and the level of N-glycan modification of AGP varies among different cancer types [137]. AGP can be used together with other marker proteins for the clinical diagnosis of liver cancer.
Nonglycopeptide-based mass spectrometry (NGP-MS) was used for high throughput screening of serum glycoproteins in patients with liver cancer. Several glycoproteins with potential application value were identified, and their practicability in the diagnosis of liver cancer was evaluated. The results showed that the diagnostic sensitivity of three candidate proteins including AGP was higher than that of AFP, and the combination of them would make the diagnosis more reliable [138].
Haptoglobin
Haptoglobin (Hp) is a plasma glycoprotein secreted by the liver, which is generally produced in the acute phase of the response to various stresses [139]. Hp can bind to hemoglobin (Hb), resulting in a new antigenic determinant, which is recognized and cleared by immune cells, thereby maintaining the stability of Hb content and reducing the cytotoxicity produced by Hb, and is an important antagonist of Hb toxicity [140]. Plasma Hp levels sharply rise during an inflammatory condition of the body.
Most serum tumor markers undergo aberrant glycosylation modifications, suggesting that such changes may be useful as a basis for disease diagnosis [141]. For diseases with high mortality rates, such as HCC, reasonable and reliable detection of markers in the early stages of cancer development can reduce false negative results and prevent misclassification to increase patient survival.
Hp is known to have four N-glycosylation sites with various types of sugar chains and mostly sialylation modifications [142]. It has been reported that the N-glycan type of Hp is altered in patients with liver cancer compared with normal adults [143]. Significant differences in the glycosylation levels of serum Hp between patients with cirrhosis and HCC have been reported. Quantitative analysis showed that the abnormal N-glycan structure of Hp occurred at the Asn207 site, as evidenced by the increased level of sialylation and the reduced branching chain structure [144]. Analysis of the structural specificity of glycosylation sites will help in clinical HCC diagnosis.
Conclusions
N-glycosylation can markedly affect biological processes such as cell adhesion, proliferation, and signal transduction, which in turn are closely related to the hepatocarcinogenesis. Genetic, metabolic, inflammatory responses and the ECM can lead to different degrees of alterations in the N-glycosylation conformation of proteins involved in hepatocarcinogenesis, thus driving the cancer development, including processes such as EMT, ECM changes, and TME formation.
Therefore, targeting N-glycosylation is a promising approach for cancer therapy. Cancer stages have been demonstrated to be closely related to the structural specificity of N-glycosylation, and identifying certain glycosylated epitopes in tumor tissue will aid in patient staging [122]. In addition, much evidence has supported the feasibility of targeting N-glycosylation in immunotherapy [123]. For example, to increase the efficacy of PD-1/PD-L1 immunotherapy, it is necessary to design specific antibodies that can recognize the complex N-glycan structure of PD-L1 itself [124], because the complex glycan structure formed by PD-L1 will obscure its conventional antigen epitopes, affect the binding between PD-L1 and anti-PD-L1 monoclonal antibodies, and increase the possibility of immune escape of cancer cells [125].
Apart from that, protein glycosylation increases molecular heterogeneity and functional diversity within cell populations. As mentioned above, the N-glycan conformation of related proteins is altered during the hepatocarcinogenesis, such as reduced diastereomeric N-glycan structure, abnormal fucosylation, and sialylation. For these aberrant types of N-glycosylation, reducing the aberrant glycosylation phenotype in tumor cells, starting with the relevant glycosyltransferases, may help restore the original cellular activity [126]. However, due to the limitation of technical means, it may also affect the normal expression and function of other N-glycosylated proteins [127].
Understanding the molecular basis behind these N-glycan modifications will help to further comprehend the molecular regulatory mechanisms underlying cancer. With the development of proteomics and other omics technologies, protein N-glycan biosynthesis and its recognition mechanism may become the main drug targets for liver cancer treatment. This innovative strategy is expected to overcome current limitations in the diagnosis, treatment, and prognosis of liver cancer patients.
Acknowledgements
We are grateful to Zhongpei Peng for instructing figure drawing. We thank LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
Author contributions
MH write the main manuscript. Dr Tang, Dr Zhou and Dr Zhang provide financial support for the publication of this review. Dr Tang, Dr Zhang, Dr Zhou, Dr Huang, Dr Lyu, Dr Xiao, Dr Guo, to review and modify the contents of the initial draft including pre-or post-publication stages. RY, CZ, and WL are responsible for some of the literature information collation.
Funding
This work was supported by the National Natural Science Foundation of China (32000523 to R.Z, 32070726 and 82273970 to J.F.T., 32270768 to C.F.Z.), National Natural Science Foundation of Hubei (2020CFA073 to J.F.T., 2022EHB038 to C.F.Z.), Wuhan Science and Technology Project (2022020801020272 to C.F.Z), the Hubei Province Science Foundation (2020CFB413 to R.Z).
Data availability
All data are available.
Competing interests
The authors declare no competing interests.
Ethics statement
No ethical statement is involved in this study.
Footnotes
Edited by Professor Francesca Pentimalli
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Mengyu Hu, Rui Zhang.
Contributor Information
Cefan Zhou, Email: cefan@hbut.edu.cn.
Jingfeng Tang, Email: Jingfeng_hut@163.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209–49. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Gao YX, Yang TW, Yin JM, Yang PX, Kou BX, Chai MY, et al. Progress and prospects of biomarkers in primary liver cancer (Review) Int J Oncol. 2020;57:54–66. doi: 10.3892/ijo.2020.5035. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Prim. 2021;7:7. doi: 10.1038/s41572-021-00245-6. [DOI] [PubMed] [Google Scholar]
- 4.Garrido A, Djouder N. Cirrhosis: A questioned risk factor for hepatocellular carcinoma. Trends Cancer. 2021;7:29–36. doi: 10.1016/j.trecan.2020.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Li X, Ramadori P, Pfister D, Seehawer M, Zender L, Heikenwalder M. The immunological and metabolic landscape in primary and metastatic liver cancer. Nat Rev Cancer. 2021;21:541–57. doi: 10.1038/s41568-021-00383-9. [DOI] [PubMed] [Google Scholar]
- 6.Pinho SS, Reis CA. Glycosylation in cancer: mechanisms and clinical implications. Nat Rev Cancer. 2015;15:540–55. doi: 10.1038/nrc3982. [DOI] [PubMed] [Google Scholar]
- 7.Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol. 2020;21:729–49. doi: 10.1038/s41580-020-00294-x. [DOI] [PubMed] [Google Scholar]
- 8.Russo D, Capolupo L, Loomba JS, Sticco L, D’Angelo G. Glycosphingolipid metabolism in cell fate specification. J Cell Sci. 2018;131:jcs219204. doi: 10.1242/jcs.219204. [DOI] [PubMed] [Google Scholar]
- 9.Ramírez AS, de Capitani M, Pesciullesi G, Kowal J, Bloch JS, Irobalieva RN, et al. Molecular basis for glycan recognition and reaction priming of eukaryotic oligosaccharyltransferase. Nat Commun. 2022;13:7296. doi: 10.1038/s41467-022-35067-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cymer F, Beck H, Rohde A, Reusch D. Therapeutic monoclonal antibody N-glycosylation - Structure, function and therapeutic potential. Biologicals. 2018;52:1–11. doi: 10.1016/j.biologicals.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Dube DH, Bertozzi CR. Glycans in cancer and inflammation-potential for therapeutics and diagnostics. Nat Rev Drug Discov. 2005;4:477–88. doi: 10.1038/nrd1751. [DOI] [PubMed] [Google Scholar]
- 12.Rostenberg I, Guízar-Vázquez J, Peñaloza R. Altered carbohydrate content of alpha1-antitrypsin in patients with cancer. J Natl Cancer Inst. 1978;61:961–5. [PubMed] [Google Scholar]
- 13.Hakomori S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996;56:5309–18. [PubMed] [Google Scholar]
- 14.Turner GA. N-glycosylation of serum proteins in disease and its investigation using lectins. Clin Chim Acta. 1992;208:149–71. doi: 10.1016/0009-8981(92)90073-Y. [DOI] [PubMed] [Google Scholar]
- 15.DelaCourt A, Black A, Angel P, Drake R, Hoshida Y, Singal A, et al. N-Glycosylation patterns correlate with hepatocellular carcinoma genetic subtypes. Mol Cancer Res. 2021;19:1868–77. doi: 10.1158/1541-7786.MCR-21-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu X, Mehta A, Dwek R, Butters T, Block T. Evidence that N-linked glycosylation is necessary for hepatitis B virus secretion. Virology. 1995;213:660–5. doi: 10.1006/viro.1995.0038. [DOI] [PubMed] [Google Scholar]
- 17.Wang CJ, Sung SY, Chen DS, Chen PJ. N-linked glycosylation of hepatitis B surface antigens is involved but not essential in the assembly of hepatitis delta virus. Virology. 1996;220:28–36. doi: 10.1006/viro.1996.0282. [DOI] [PubMed] [Google Scholar]
- 18.Jiang K, Li W, Zhang Q, Yan G, Guo K, Zhang S, et al. GP73 N-glycosylation at Asn144 reduces hepatocellular carcinoma cell motility and invasiveness. Oncotarget. 2016;7:23530–41. doi: 10.18632/oncotarget.8120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Y, Lan L, Li Y, Lu J, He L, Deng Y, et al. N-glycosylation stabilizes MerTK and promotes hepatocellular carcinoma tumor growth. Redox Biol. 2022;54:102366. doi: 10.1016/j.redox.2022.102366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tian H, Zhu X, Lv Y, Jiao Y, Wang G. Glucometabolic reprogramming in the hepatocellular carcinoma microenvironment: cause and effect. Cancer Manag Res. 2020;12:5957–74. doi: 10.2147/CMAR.S258196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dobrica MO, Lazar C, Branza-Nichita N. N-Glycosylation and N-Glycan processing in HBV biology and pathogenesis. Cells. 2020;9:1404. doi: 10.3390/cells9061404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- 23.Gurzu S, Kobori L, Fodor D, Jung I. Epithelial mesenchymal and endothelial mesenchymal transitions in hepatocellular carcinoma: a review. Biomed Res Int. 2019;2019:2962580. doi: 10.1155/2019/2962580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu GM, Li Q, Zhang PF, Shen SL, Xie WX, Chen B, et al. Restoration of FBP1 suppressed Snail-induced epithelial to mesenchymal transition in hepatocellular carcinoma. Cell Death Dis. 2018;9:1132. doi: 10.1038/s41419-018-1165-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Omran NM, El-Sherbini SM, Hegazy O, Elshaarawy AA, Talaat RM. Crosstalk between miR-215 and epithelial-mesenchymal transition specific markers (E-cadherin and N-cadherin) in different stages of chronic HCV Infection. J Med Virol. 2020;92:1231–8. doi: 10.1002/jmv.25637. [DOI] [PubMed] [Google Scholar]
- 26.Na TY, Schecterson L, Mendonsa AM, Gumbiner BM. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc Natl Acad Sci USA. 2020;117:5931–7. doi: 10.1073/pnas.1918167117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Feng M, Fang F, Fang T, Jiao H, You S, Wang X, et al. Sox13 promotes hepatocellular carcinoma metastasis by transcriptionally activating Twist1. Lab Invest. 2020;100:1400–10. doi: 10.1038/s41374-020-0445-0. [DOI] [PubMed] [Google Scholar]
- 28.Jiang Y, Han Q, Zhao H, Zhang J. Promotion of epithelial-mesenchymal transformation by hepatocellular carcinoma-educated macrophages through Wnt2b/β-catenin/c-Myc signaling and reprogramming glycolysis. J Exp Clin Cancer Res. 2021;40:13. doi: 10.1186/s13046-020-01808-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun Y, Yu M, Qu M, Ma Y, Zheng D, Yue Y, et al. Hepatitis B virus-triggered PTEN/β-catenin/c-Myc signaling enhances PD-L1 expression to promote immune evasion. Am J Physiol Gastrointest Liver Physiol. 2020;318:G162–G73. doi: 10.1152/ajpgi.00197.2019. [DOI] [PubMed] [Google Scholar]
- 30.Deldar Abad Paskeh M, Mirzaei S, Ashrafizadeh M, Zarrabi A, Sethi G. Wnt/β-Catenin signaling as a driver of hepatocellular carcinoma progression: an emphasis on molecular pathways. J Hepatocell Carcinoma. 2021;8:1415–44. doi: 10.2147/JHC.S336858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tamura K, Shan WS, Hendrickson WA, Colman DR, Shapiro L. Structure-function analysis of cell adhesion by neural (N-) cadherin. Neuron. 1998;20:1153–63. doi: 10.1016/S0896-6273(00)80496-1. [DOI] [PubMed] [Google Scholar]
- 32.Wang Y, Li Q, Niu L, Xu L, Guo Y, Wang L, et al. Suppression of G6PD induces the expression and bisecting GlcNAc-branched N-glycosylation of E-Cadherin to block epithelial-mesenchymal transition and lymphatic metastasis. Br J Cancer. 2020;123:1315–25. doi: 10.1038/s41416-020-1007-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao Y, Sato Y, Isaji T, Fukuda T, Matsumoto A, Miyoshi E, et al. Branched N-glycans regulate the biological functions of integrins and cadherins. FEBS J. 2008;275:1939–48. doi: 10.1111/j.1742-4658.2008.06346.x. [DOI] [PubMed] [Google Scholar]
- 34.Xu Q, Akama R, Isaji T, Lu Y, Hashimoto H, Kariya Y, et al. Wnt/beta-catenin signaling down-regulates N-acetylglucosaminyltransferase III expression: the implications of two mutually exclusive pathways for regulation. J Biol Chem. 2011;286:4310–8. doi: 10.1074/jbc.M110.182576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xu Q, Isaji T, Lu Y, Gu W, Kondo M, Fukuda T, et al. Roles of N-acetylglucosaminyltransferase III in epithelial-to-mesenchymal transition induced by transforming growth factor β1 (TGF-β1) in epithelial cell lines. J Biol Chem. 2012;287:16563–74. doi: 10.1074/jbc.M111.262154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mo C, Liu T, Zhang S, Guo K, Li M, Qin X, et al. Reduced N-acetylglucosaminyltransferase III expression via Smad3 and Erk signaling in TGF-β1-induced HCC EMT model. Disco Med. 2017;23:7–17. [PubMed] [Google Scholar]
- 37.Chen G, Yang Y, Liu W, Huang L, Yang L, Lei Y, et al. EpCAM is essential for maintenance of the small intestinal epithelium architecture via regulation of the expression and localization of proteins that compose adherens junctions. Int J Mol Med. 2021;47:621–32. doi: 10.3892/ijmm.2020.4815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brown TC, Sankpal NV, Gillanders WE. Functional Implications of the Dynamic Regulation of EpCAM during Epithelial-to-Mesenchymal Transition. Biomolecules. 2021;11:956. doi: 10.3390/biom11070956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schnell U, Cirulli V, Giepmans BN. EpCAM: structure and function in health and disease. Biochim Biophys Acta. 2013;1828:1989–2001. doi: 10.1016/j.bbamem.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Liu X, Gao J, Sun Y, Liu T, Yan Q, et al. The role of epithelial cell adhesion molecule N-glycosylation on apoptosis in breast cancer cells. Tumour Biol. 2017;39:1010428317695973. doi: 10.1177/1010428317695973. [DOI] [PubMed] [Google Scholar]
- 41.Liu X, Gao J, Sun Y, Zhang D, Liu T, Yan Q, et al. Mutation of N-linked glycosylation in EpCAM affected cell adhesion in breast cancer cells. Biol Chem. 2017;398:1119–26. doi: 10.1515/hsz-2016-0232. [DOI] [PubMed] [Google Scholar]
- 42.Fan QM, Jing YY, Yu GF, Kou XR, Ye F, Gao L, et al. Tumor-associated macrophages promote cancer stem cell-like properties via transforming growth factor-beta1-induced epithelial-mesenchymal transition in hepatocellular carcinoma. Cancer Lett. 2014;352:160–8. doi: 10.1016/j.canlet.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 43.Kim H, Choi GH, Na DC, Ahn EY, Kim GI, Lee JE, et al. Human hepatocellular carcinomas with “Stemness”-related marker expression: keratin 19 expression and a poor prognosis. Hepatology. 2011;54:1707–17. doi: 10.1002/hep.24559. [DOI] [PubMed] [Google Scholar]
- 44.Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55:838–45. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sancho-Bru P, Altamirano J, Rodrigo-Torres D, Coll M, Millán C, José Lozano J, et al. Liver progenitor cell markers correlate with liver damage and predict short-term mortality in patients with alcoholic hepatitis. Hepatology. 2012;55:1931–41. doi: 10.1002/hep.25614. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Z, Wen H, Weng J, Feng L, Liu H, Hu X, et al. Silencing of EPCAM suppresses hepatic fibrosis and hepatic stellate cell proliferation in mice with alcoholic hepatitis via the PI3K/Akt/mTOR signaling pathway. Cell Cycle. 2019;18:2239–54. doi: 10.1080/15384101.2019.1642067. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 47.Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: A Multifunctional Mediator of Cancer Progression. Biomolecules. 2021;11:1850. doi: 10.3390/biom11121850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xu H, Tian Y, Yuan X, Wu H, Liu Q, Pestell RG, et al. The role of CD44 in epithelial-mesenchymal transition and cancer development. Onco Targets Ther. 2015;8:3783–92. doi: 10.2147/OTT.S95470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaudhry GE, Akim A, Naveed Zafar M, Safdar N, Sung YY, Muhammad TST. Understanding Hyaluronan Receptor (CD44) Interaction, HA-CD44 activated potential targets in cancer therapeutics. Adv Pharm Bull. 2021;11:426–38. doi: 10.34172/apb.2021.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Karousou E, Misra S, Ghatak S, Dobra K, Götte M, Vigetti D, et al. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017;59:3–22. doi: 10.1016/j.matbio.2016.10.001. [DOI] [PubMed] [Google Scholar]
- 51.Park GB, Ko HS, Kim D. Sorafenib controls the epithelial‑mesenchymal transition of ovarian cancer cells via EGF and the CD44‑HA signaling pathway in a cell type‑dependent manner. Mol Med Rep. 2017;16:1826–36. doi: 10.3892/mmr.2017.6773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vuorio J, Škerlová J, Fábry M, Veverka V, Vattulainen I, Řezáčová P, et al. N-Glycosylation can selectively block or foster different receptor-ligand binding modes. Sci Rep. 2021;11:5239. doi: 10.1038/s41598-021-84569-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Han H, Stapels M, Ying W, Yu Y, Tang L, Jia W, et al. Comprehensive characterization of the N-glycosylation status of CD44s by use of multiple mass spectrometry-based techniques. Anal Bioanal Chem. 2012;404:373–88. doi: 10.1007/s00216-012-6167-4. [DOI] [PubMed] [Google Scholar]
- 54.Guvench O. Revealing the mechanisms of protein disorder and N-Glycosylation in CD44-Hyaluronan binding using molecular simulation. Front Immunol. 2015;6:305. doi: 10.3389/fimmu.2015.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faller CE, Guvench O. Terminal sialic acids on CD44 N-glycans can block hyaluronan binding by forming competing intramolecular contacts with arginine sidechains. Proteins. 2014;82:3079–89. doi: 10.1002/prot.24668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang X, Dou P, Akhtar ML, Liu F, Hu X, Yang L, et al. NEU4 inhibits motility of HCC cells by cleaving sialic acids on CD44. Oncogene. 2021;40:5427–40. doi: 10.1038/s41388-021-01955-7. [DOI] [PubMed] [Google Scholar]
- 57.Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Deliv Rev. 2016;97:4–27. doi: 10.1016/j.addr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 58.Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J. 2021;288:6850–912. doi: 10.1111/febs.15776. [DOI] [PubMed] [Google Scholar]
- 59.Mohan V, Das A, Sagi I. Emerging roles of ECM remodeling processes in cancer. Semin Cancer Biol. 2020;62:192–200. doi: 10.1016/j.semcancer.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 60.Eble JA, Niland S. The extracellular matrix in tumor progression and metastasis. Clin Exp Metastasis. 2019;36:171–98. doi: 10.1007/s10585-019-09966-1. [DOI] [PubMed] [Google Scholar]
- 61.Geervliet E, Moreno S, Baiamonte L, Booijink R, Boye S, Wang P, et al. Matrix metalloproteinase-1 decorated polymersomes, a surface-active extracellular matrix therapeutic, potentiates collagen degradation and attenuates early liver fibrosis. J Control Rel. 2021;332:594–607. doi: 10.1016/j.jconrel.2021.03.016. [DOI] [PubMed] [Google Scholar]
- 62.Michalopoulos GK, Bhushan B. Liver regeneration: biological and pathological mechanisms and implications. Nat Rev Gastroenterol Hepatol. 2021;18:40–55. doi: 10.1038/s41575-020-0342-4. [DOI] [PubMed] [Google Scholar]
- 63.Tang H, You T, Sun Z, Bai C, Wang Y. Extracellular matrix-based gene expression signature defines two prognostic subtypes of hepatocellular carcinoma with different immune microenvironment characteristics. Front Mol Biosci. 2022;9:839806. doi: 10.3389/fmolb.2022.839806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friedman SL, Pinzani M. Hepatic fibrosis 2022: Unmet needs and a blueprint for the future. Hepatology. 2022;75:473–88. doi: 10.1002/hep.32285. [DOI] [PubMed] [Google Scholar]
- 65.Wu Y, Cao Y, Xu K, Zhu Y, Qiao Y, Chen J, et al. Dynamically remodeled hepatic extracellular matrix predicts prognosis of early-stage cirrhosis. Cell Death Dis. 2021;12:163. doi: 10.1038/s41419-021-03443-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Loft A, Alfaro AJ, Schmidt SF, Pedersen FB, Terkelsen MK, Puglia M, et al. Liver-fibrosis-activated transcriptional networks govern hepatocyte reprogramming and intra-hepatic communication. Cell Metab. 2021;33:1685–700.e9. doi: 10.1016/j.cmet.2021.06.005. [DOI] [PubMed] [Google Scholar]
- 67.Cooper J, Giancotti FG. Integrin signaling in cancer: mechanotransduction, stemness, epithelial plasticity, and therapeutic resistance. Cancer Cell. 2019;35:347–67. doi: 10.1016/j.ccell.2019.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michael M, Parsons M. New perspectives on integrin-dependent adhesions. Curr Opin Cell Biol. 2020;63:31–7. doi: 10.1016/j.ceb.2019.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou J, Yan D, Liu Y, Huang P, Cui H. The roles of Integrin α5β1 in human cancer. Onco Targets Ther. 2020;13:13329–44. doi: 10.2147/OTT.S273803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Thölmann S, Seebach J, Otani T, Florin L, Schnittler H, Gerke V, et al. JAM-A interacts with α3β1 integrin and tetraspanins CD151 and CD9 to regulate collective cell migration of polarized epithelial cells. Cell Mol Life Sci. 2022;79:88. doi: 10.1007/s00018-022-04140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Silsirivanit A. Glycosylation markers in cancer. Adv Clin Chem. 2019;89:189–213. doi: 10.1016/bs.acc.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 72.Janik ME, Lityńska A, Vereecken P. Cell migration-the role of integrin glycosylation. Biochim Biophys Acta. 2010;1800:545–55. doi: 10.1016/j.bbagen.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 73.Han Y, Liu Y, Fu X, Zhang Q, Huang H, Zhang C, et al. miR-9 inhibits the metastatic ability of hepatocellular carcinoma via targeting beta galactoside alpha-2,6-sialyltransferase 1. J Physiol Biochem. 2018;74:491–501. doi: 10.1007/s13105-018-0642-0. [DOI] [PubMed] [Google Scholar]
- 74.Zhou Y, Fukuda T, Hang Q, Hou S, Isaji T, Kameyama A, et al. Inhibition of fucosylation by 2-fluorofucose suppresses human liver cancer HepG2 cell proliferation and migration as well as tumor formation. Sci Rep. 2017;7:11563. doi: 10.1038/s41598-017-11911-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patten J, Wang K. Fibronectin in development and wound healing. Adv Drug Deliv Rev. 2021;170:353–68. doi: 10.1016/j.addr.2020.09.005. [DOI] [PubMed] [Google Scholar]
- 76.Dalton CJ, Lemmon CA. Fibronectin: Molecular structure, fibrillar structure and mechanochemical signaling. Cells. 2021;10:2443. doi: 10.3390/cells10092443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hsiao CT, Cheng HW, Huang CM, Li HR, Ou MH, Huang JR, et al. Fibronectin in cell adhesion and migration via N-glycosylation. Oncotarget. 2017;8:70653–68. doi: 10.18632/oncotarget.19969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Liu D, Wang S, Zhang J, Xiao W, Miao CH, Konkle BA, et al. Site-Specific N- and O-Glycosylation analysis of human plasma fibronectin. Front Chem. 2021;9:691217. doi: 10.3389/fchem.2021.691217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Krishnan MS, Rajan KdA, Park J, Arjunan V, Garcia Marques FJ, Bermudez A, et al. Genomic analysis of vascular invasion in HCC reveals molecular drivers and predictive biomarkers. Hepatology. 2021;73:2342–60. doi: 10.1002/hep.31614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goddi A, Schroedl L, Brey EM, Cohen RN. Laminins in metabolic tissues. Metabolism. 2021;120:154775. doi: 10.1016/j.metabol.2021.154775. [DOI] [PubMed] [Google Scholar]
- 81.Yap L, Tay HG, Nguyen MTX, Tjin MS, Tryggvason K. Laminins in cellular differentiation. Trends Cell Biol. 2019;29:987–1000. doi: 10.1016/j.tcb.2019.10.001. [DOI] [PubMed] [Google Scholar]
- 82.Kiyokawa H, Yasuda H, Oikawa R, Okuse C, Matsumoto N, Ikeda H, et al. Serum monomeric laminin-γ2 as a novel biomarker for hepatocellular carcinoma. Cancer Sci. 2017;108:1432–9. doi: 10.1111/cas.13261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yasuda H, Nakagawa M, Kiyokawa H, Yoshida E, Yoshimura T, Koshikawa N, et al. Unique biological activity and potential role of monomeric Laminin-γ2 as a novel biomarker for hepatocellular carcinoma: a review. Int J Mol Sci. 2019;20:226. doi: 10.3390/ijms20010226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kariya Y, Kato R, Itoh S, Fukuda T, Shibukawa Y, Sanzen N, et al. N-Glycosylation of laminin-332 regulates its biological functions. A novel function of the bisecting GlcNAc. J Biol Chem. 2008;283:33036–45. doi: 10.1074/jbc.M804526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sacks D, Baxter B, Campbell BCV, Carpenter JS, Cognard C, Dippel D, et al. Multisociety consensus quality improvement revised consensus statement for endovascular therapy of acute ischemic stroke. Int J Stroke. 2018;13:612–32. doi: 10.1177/1747493018778713. [DOI] [PubMed] [Google Scholar]
- 86.De Palma M, Biziato D, Petrova TV. Microenvironmental regulation of tumour angiogenesis. Nat Rev Cancer. 2017;17:457–74. doi: 10.1038/nrc.2017.51. [DOI] [PubMed] [Google Scholar]
- 87.Lei X, Lei Y, Li JK, Du WX, Li RG, Yang J, et al. Immune cells within the tumor microenvironment: Biological functions and roles in cancer immunotherapy. Cancer Lett. 2020;470:126–33. doi: 10.1016/j.canlet.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 88.Zheng Y, Wang S, Cai J, Ke A, Fan J. The progress of immune checkpoint therapy in primary liver cancer. Biochim Biophys Acta Rev Cancer. 2021;1876:188638. doi: 10.1016/j.bbcan.2021.188638. [DOI] [PubMed] [Google Scholar]
- 89.Guo S, Wang X, Zhou H, Gao Y, Wang P, Zhi H, et al. PD-L1- Biomolecules. 2022;12:1226. doi: 10.3390/biom12091226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Chen Y, Tian Z. HBV-induced immune imbalance in the development of HCC. Front Immunol. 2019;10:2048. doi: 10.3389/fimmu.2019.02048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Comelli EM, Sutton-Smith M, Yan Q, Amado M, Panico M, Gilmartin T, et al. Activation of murine CD4+ and CD8+ T lymphocytes leads to dramatic remodeling of N-linked glycans. J Immunol. 2006;177:2431–40. doi: 10.4049/jimmunol.177.4.2431. [DOI] [PubMed] [Google Scholar]
- 92.Silva Z, Ferro T, Almeida D, Soares H, Ferreira JA, Deschepper FM, et al. MHC Class I stability is modulated by cell surface sialylation in human dendritic cells. Pharmaceutics. 2020;12:249. doi: 10.3390/pharmaceutics12030249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Balneger N, Cornelissen LAM, Wassink M, Moons SJ, Boltje TJ, Bar-Ephraim YE, et al. Sialic acid blockade in dendritic cells enhances CD8(+) T cell responses by facilitating high-avidity interactions. Cell Mol Life Sci. 2022;79:98. doi: 10.1007/s00018-021-04027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang L, Li S, Yu X, Han Y, Wu Y, Wang S, et al. α2,6-Sialylation promotes immune escape in hepatocarcinoma cells by regulating T cell functions and CD147/MMP signaling. J Physiol Biochem. 2019;75:199–207. doi: 10.1007/s13105-019-00674-8. [DOI] [PubMed] [Google Scholar]
- 95.Zhang M, Lin X, Yang Z, Li X, Zhou Z, Love PE, et al. Metabolic regulation of T cell development. Front Immunol. 2022;13:946119. doi: 10.3389/fimmu.2022.946119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chapman NM, Boothby MR, Chi H. Metabolic coordination of T cell quiescence and activation. Nat Rev Immunol. 2020;20:55–70. doi: 10.1038/s41577-019-0203-y. [DOI] [PubMed] [Google Scholar]
- 97.Douanne T, Griffiths GM. Cytoskeletal control of the secretory immune synapse. Curr Opin Cell Biol. 2021;71:87–94. doi: 10.1016/j.ceb.2021.02.008. [DOI] [PubMed] [Google Scholar]
- 98.Gómez-Henao W, Tenorio EP, Sanchez FRC, Mendoza MC, Ledezma RL, Zenteno E. Relevance of glycans in the interaction between T lymphocyte and the antigen presenting cell. Int Rev Immunol. 2021;40:274–88. doi: 10.1080/08830185.2020.1845331. [DOI] [PubMed] [Google Scholar]
- 99.Ryan SO, Bonomo JA, Zhao F, Cobb BA. MHCII glycosylation modulates Bacteroides fragilis carbohydrate antigen presentation. J Exp Med. 2011;208:1041–53. doi: 10.1084/jem.20100508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Chiritoiu GN, Jandus C, Munteanu CV, Ghenea S, Gannon PO, Romero P, et al. Epitope located N-glycans impair the MHC-I epitope generation and presentation. Electrophoresis. 2016;37:1448–60. doi: 10.1002/elps.201500449. [DOI] [PubMed] [Google Scholar]
- 101.Kang Y, Li F, Guo H, Yang S, Zhang Y, Zhu H, et al. Amino acid substitutions Q129N and T131N/M133T in hepatitis B surface antigen (HBsAg) interfere with the immunogenicity of the corresponding HBsAg or viral replication ability. Virus Res. 2018;257:33–9. doi: 10.1016/j.virusres.2018.08.019. [DOI] [PubMed] [Google Scholar]
- 102.Salpini R, Piermatteo L, Battisti A, Colagrossi L, Aragri M, Yu La Rosa K, et al. A hyper-glycosylation of HBV surface antigen correlates with HBsAg-negativity at immunosuppression-driven HBV reactivation in vivo and hinders HBsAg recognition in vitro. Viruses. 2020;12:251. doi: 10.3390/v12020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Alatrash G, Qiao N, Zhang M, Zope M, Perakis AA, Sukhumalchandra P, et al. Fucosylation enhances the efficacy of adoptively transferred antigen-specific cytotoxic T lymphocytes. Clin Cancer Res. 2019;25:2610–20. doi: 10.1158/1078-0432.CCR-18-1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Oswald DM, Zhou JY, Jones MB, Cobb BA. Disruption of hepatocyte Sialylation drives a T cell-dependent pro-inflammatory immune tone. Glycoconj J. 2020;37:395–407. doi: 10.1007/s10719-020-09918-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Liu R, Cao X, Liang Y, Li X, Jin Q, Li Y, et al. Downregulation of ST6GAL1 promotes liver inflammation and predicts adverse prognosis in hepatocellular carcinoma. J Inflamm Res. 2022;15:5801–14. doi: 10.2147/JIR.S385491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Dou L, Shi X, He X, Gao Y. Macrophage phenotype and function in liver disorder. Front Immunol. 2019;10:3112. doi: 10.3389/fimmu.2019.03112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Guilliams M, Scott CL. Liver macrophages in health and disease. Immunity. 2022;55:1515–29. doi: 10.1016/j.immuni.2022.08.002. [DOI] [PubMed] [Google Scholar]
- 108.Wang C, Ma C, Gong L, Guo Y, Fu K, Zhang Y, et al. Macrophage polarization and its role in liver disease. Front Immunol. 2021;12:803037. doi: 10.3389/fimmu.2021.803037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang L, Zhang Q, Lin L, Xu Y, Huang Y, Hu Z, et al. Microarray investigation of glycan remodeling during macrophage polarization reveals α2,6 sialic acid as an anti-inflammatory indicator. Mol Omics. 2021;17:565–71. doi: 10.1039/D0MO00192A. [DOI] [PubMed] [Google Scholar]
- 110.Yang L, Gong T, Shen H, Pei J, Zhang L, Zhang Q, et al. Precision N-Glycoproteomic profiling of murine peritoneal macrophages after different stimulations. Front Immunol. 2021;12:722293. doi: 10.3389/fimmu.2021.722293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zhao Y, Guo C, Zeng L, Li J, Liu X, Wang Y, et al. Mesenchymal stem cells ameliorate fibrosis by enhancing autophagy via inhibiting Galectin-3/Akt/mTOR pathway and by alleviating the EMT via Inhibiting Galectin-3/Akt/GSK3β/Snail pathway in NRK-52E Fibrosis. Int J Stem Cells. 2022;16:52–65. doi: 10.15283/ijsc22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bacigalupo ML, Manzi M, Rabinovich GA, Troncoso MF. Hierarchical and selective roles of galectins in hepatocarcinogenesis, liver fibrosis and inflammation of hepatocellular carcinoma. World J Gastroenterol. 2013;19:8831–49. doi: 10.3748/wjg.v19.i47.8831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leung Z, Ko FCF, Tey SK, Kwong EML, Mao X, Liu BHM, et al. Galectin-1 promotes hepatocellular carcinoma and the combined therapeutic effect of OTX008 galectin-1 inhibitor and sorafenib in tumor cells. J Exp Clin Cancer Res. 2019;38:423. doi: 10.1186/s13046-019-1402-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Song M, Pan Q, Yang J, He J, Zeng J, Cheng S, et al. Galectin-3 favours tumour metastasis via the activation of β-catenin signalling in hepatocellular carcinoma. Br J Cancer. 2020;123:1521–34. doi: 10.1038/s41416-020-1022-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang W, Zhang Y, He Y, Wang X, Fang Q. Lipopolysaccharide mediates time-dependent macrophage M1/M2 polarization through the Tim-3/Galectin-9 signalling pathway. Exp Cell Res. 2019;376:124–32. doi: 10.1016/j.yexcr.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 116.Fujita K, Iwama H, Sakamoto T, Okura R, Kobayashi K, Takano J, et al. Galectin-9 suppresses the growth of hepatocellular carcinoma via apoptosis in vitro and in vivo. Int J Oncol. 2015;46:2419–30. doi: 10.3892/ijo.2015.2941. [DOI] [PubMed] [Google Scholar]
- 117.Teicher BA. CD248: A therapeutic target in cancer and fibrotic diseases. Oncotarget. 2019;10:993–1009. doi: 10.18632/oncotarget.26590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Yang F, Wei Y, Han D, Li Y, Shi S, Jiao D, et al. Interaction with CD68 and Regulation of GAS6 expression by endosialin in fibroblasts drives recruitment and polarization of macrophages in hepatocellular carcinoma. Cancer Res. 2020;80:3892–905. doi: 10.1158/0008-5472.CAN-19-2691. [DOI] [PubMed] [Google Scholar]
- 119.Couri T, Pillai A. Goals and targets for personalized therapy for HCC. Hepatol Int. 2019;13:125–37. doi: 10.1007/s12072-018-9919-1. [DOI] [PubMed] [Google Scholar]
- 120.Zhao Y, Zhang YN, Wang KT, Chen L. Lenvatinib for hepatocellular carcinoma: From preclinical mechanisms to anti-cancer therapy. Biochim Biophys Acta Rev Cancer. 2020;1874:188391. doi: 10.1016/j.bbcan.2020.188391. [DOI] [PubMed] [Google Scholar]
- 121.Sartoris R, Gregory J, Dioguardi Burgio M, Ronot M, Vilgrain V. HCC advances in diagnosis and prognosis: Digital and Imaging. Liver Int. 2021;41:73–7. doi: 10.1111/liv.14865. [DOI] [PubMed] [Google Scholar]
- 122.Fang P, Ji Y, Oellerich T, Urlaub H, Pan KT. Strategies for proteome-wide quantification of glycosylation macro- and micro-heterogeneity. Int J Mol Sci. 2022;23:1609. doi: 10.3390/ijms23031609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Huang Y, Zhang HL, Li ZL, Du T, Chen YH, Wang Y, et al. FUT8-mediated aberrant N-glycosylation of B7H3 suppresses the immune response in triple-negative breast cancer. Nat Commun. 2021;12:2672. doi: 10.1038/s41467-021-22618-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sun L, Li CW, Chung EM, Yang R, Kim YS, Park AH, et al. Targeting glycosylated PD-1 induces potent antitumor immunity. Cancer Res. 2020;80:2298–310. doi: 10.1158/0008-5472.CAN-19-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Lee HH, Wang YN, Xia W, Chen CH, Rau KM, Ye L, et al. Removal of N-linked glycosylation enhances PD-L1 detection and predicts Anti-PD-1/PD-L1 therapeutic efficacy. Cancer Cell. 2019;36:168–78.e4. doi: 10.1016/j.ccell.2019.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lopez Sambrooks C, Baro M, Quijano A, Narayan A, Cui W, Greninger P, et al. Oligosaccharyltransferase inhibition overcomes therapeutic resistance to EGFR Tyrosine Kinase inhibitors. Cancer Res. 2018;78:5094–106. doi: 10.1158/0008-5472.CAN-18-0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Cao X, Meng P, Shao Y, Yan G, Yao J, Zhou X, et al. Nascent glycoproteome reveals that N-linked Glycosylation Inhibitor-1 Suppresses Expression of Glycosylated Lysosome-Associated Membrane Protein-2. Front Mol Biosci. 2022;9:899192. doi: 10.3389/fmolb.2022.899192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang T, Zhang KH. New blood biomarkers for the diagnosis of AFP-Negative Hepatocellular Carcinoma. Front Oncol. 2020;10:1316. doi: 10.3389/fonc.2020.01316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tang J, Ge QM, Huang R, Shu HY, Su T, Wu JL, et al. Clinical Significance of CYFRA21-1, AFP, CA-153, CEA, and CA-199 in the diagnosis of lung cancer ocular metastasis in hypertension population. Front Cardiovasc Med. 2021;8:670594. doi: 10.3389/fcvm.2021.670594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Belur S, Jagadeesh N, Swamy BM, Inamdar SR. A core fucose specific lectin from Cephalosporium curvulum induces cellular apoptosis in hepatocellular and pancreatic cancer cells and effective in detecting AFP. Glycoconj J. 2020;37:435–44. doi: 10.1007/s10719-020-09921-3. [DOI] [PubMed] [Google Scholar]
- 131.Wu M, Liu H, Liu Z, Liu C, Zhang A, Li N. Analysis of serum alpha-fetoprotein (AFP) and AFP-L3 levels by protein microarray. J Int Med Res. 2018;46:4297–305. doi: 10.1177/0300060518789304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wang K, Li Y, Wang X, Jiao J, Gu W, Liang X. Automatic time-resolved fluorescence immunoassay of Serum Alpha Fetoprotein-L3 Variant via LCA magnetic cationic polymeric liposomes improves the diagnostic accuracy of liver cancer. Int J Nanomed. 2020;15:4933–41. doi: 10.2147/IJN.S242527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Lee J, Yeo I, Kim Y, Shin D, Kim J, Lim YS. Comparison of Fucose-specific lectins to improve quantitative AFP-L3 assay for diagnosing hepatocellular carcinoma using mass spectrometry. J Proteome Res. 2022;21:1548–57. doi: 10.1021/acs.jproteome.2c00196. [DOI] [PubMed] [Google Scholar]
- 134.Liang J, Zhu J, Wang M, Singal AG, Odewole M, Kagan S, et al. Evaluation of AGP fucosylation as a marker for hepatocellular carcinoma of three different etiologies. Sci Rep. 2019;9:11580. doi: 10.1038/s41598-019-48043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ruiz M. Into the Labyrinth of the Lipocalin α1-Acid Glycoprotein. Front Physiol. 2021;12:686251. doi: 10.3389/fphys.2021.686251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Keser T, Tijardović M, Gornik I, Lukić E, Lauc G, Gornik O, et al. High-throughput and site-specific N-Glycosylation analysis of human Alpha-1-Acid Glycoprotein offers a great potential for new biomarker discovery. Mol Cell Proteom. 2021;20:100044. doi: 10.1074/mcp.RA120.002433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sumanth MS, Jacob SP, Abhilasha KV, Manne BK, Basrur V, Lehoux S, et al. Different glycoforms of alpha-1-acid glycoprotein contribute to its functional alterations in platelets and neutrophils. J Leukoc Biol. 2021;109:915–30. doi: 10.1002/JLB.3A0720-422R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cao WQ, Jiang BY, Huang JM, Zhang L, Liu MQ, Yao J, et al. Straightforward and highly efficient strategy for hepatocellular carcinoma glycoprotein biomarker discovery using a nonglycopeptide-Based Mass Spectrometry Pipeline. Anal Chem. 2019;91:12435–43. doi: 10.1021/acs.analchem.9b03074. [DOI] [PubMed] [Google Scholar]
- 139.Zhou S, Li Y, He L, Chen M, Li W, Xiao T, et al. Haptoglobin is an early indicator of survival after radiation-induced severe injury and bone marrow transplantation in mice. Stem Cell Res Ther. 2022;13:461. doi: 10.1186/s13287-022-03162-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Bellelli A, Tame JRH. Hemoglobin allostery and pharmacology. Mol Asp Med. 2022;84:101037. doi: 10.1016/j.mam.2021.101037. [DOI] [PubMed] [Google Scholar]
- 141.Radovani B, Gudelj I. N-Glycosylation and Inflammation; the Not-So-Sweet Relation. Front Immunol. 2022;13:893365. doi: 10.3389/fimmu.2022.893365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Lin Y, Zhu J, Zhang J, Dai J, Liu S, Arroyo A, et al. Glycopeptides with Sialyl Lewis antigen in serum haptoglobin as candidate biomarkers for nonalcoholic steatohepatitis hepatocellular carcinoma using a higher-energy collision-induced dissociation parallel reaction monitoring-mass spectrometry method. ACS Omega. 2022;7:22850–60. doi: 10.1021/acsomega.2c02600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng X, Shu H, Zhang S, Peng Y, Zhang L, Cao X, et al. Relative quantification of N-Glycopeptide Sialic acid linkage isomers by ion mobility mass spectrometry. Anal Chem. 2021;93:15617–25. doi: 10.1021/acs.analchem.1c02803. [DOI] [PubMed] [Google Scholar]
- 144.Gutierrez Reyes CD, Huang Y, Atashi M, Zhang J, Zhu J, Liu S, et al. PRM-MS Quantitative Analysis Of Isomeric. Metabolites. 2021;11:563. doi: 10.3390/metabo11080563. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available.