Abstract
Background
Biologics have revolutionized the management of psoriasis, but response to treatment varies. Loss of treatment efficacy may occur over time, requiring treatment switching or escalation. Claims data on persistence may be informative of real-world treatment outcome. This analysis described persistence and rates of remission of patients with psoriasis initiated on current biologics.
Methods
Adults with psoriasis initiated (index date) on guselkumab, adalimumab, secukinumab, or ixekizumab between 07/13/2017 and 07/31/2020 were identified in the IBM MarketScan Databases. Discontinuation (or end of persistence) was defined as gaps in index biologic supply of more than twice the labelled dosing interval or mode days of supply (> 120 days for guselkumab and > 60 days for adalimumab, secukinumab, and ixekizumab). The proportion of patients reinitiating index therapy post-discontinuation and the proportion achieving remission (proxy definition: no claims for psoriasis-related treatment post-discontinuation among patients with ≥ 6 months of follow-up post-discontinuation) were assessed.
Results
There were 3408 patients in the guselkumab (mean age: 47.9 years old; female: 47.1%), 8017 in the adalimumab (47.4 years old; 54.1%), 6123 in the secukinumab (49.4 years old; 54.2%), and 3728 in the ixekizumab cohorts (49.1 years old; 50.3%). The median time to discontinuation was 26.2 months in the guselkumab cohort and 9.9, 12.4, and 12.5 months in adalimumab, secukinumab, and ixekizumab cohorts, respectively. Among those who discontinued index therapy, 22.9% in the guselkumab cohort and 21.1%, 31.9%, and 32.0% in the adalimumab, secukinumab, and ixekizumab cohorts reinitiated it. Remission rates were 17.2% in the guselkumab cohort and 12.4%, 10.5%, and 9.0% in adalimumab, secukinumab, and ixekizumab cohorts, respectively.
Conclusions
Patients on guselkumab showed trends toward better persistence and higher remission rates relative to other biologics. Finding patients who may be in remission suggests potential disease modification with current agents.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13555-023-00910-6.
Keywords: Biologics, Guselkumab, Persistence, Psoriasis, Remission, Treatment discontinuation
Key Summary Points
| Why carry out this study? (1–2 points for background; 1 point for hypothesis) |
| While biologics have revolutionized the management of psoriasis, response to treatment varies, and efficacy may decline over time, resulting in treatment discontinuation |
| Little is known about relative real-world performance of biologic agents for psoriasis, particularly after guselkumab, an interleukin-23 inhibitor, was approved for psoriasis in 2017 |
| This study aimed to describe real-world persistence, reinitiation, and possible remission of patients with psoriasis initiated on guselkumab, adalimumab, secukinumab, and ixekizumab using administrative claims data |
| What was learned from the study? (1 point for conclusions; 1–2 points for what was learned) |
| Guselkumab relative to other biologics showed trends toward better persistence and higher remission rates |
| Identification of patients who may have entered remission suggests potential for disease modification with existing biologics |
Introduction
Psoriasis is a chronic disease typically requiring ongoing treatment [1]. While mild disease may be sufficiently managed with topical agents [2], patients with moderate-to-severe psoriasis typically require systemic therapy, alone or in combination with topical agents or phototherapy [3]. However, conventional systemic therapies have been associated with safety concerns such as liver and renal toxicities [4]. With the approval of the first biologic in 2003 [5], the treatment landscape for moderate-to-severe psoriasis has dramatically improved, as long-term use of biologics is associated with better tolerability and safety compared with other systemic therapies [4, 6, 7]. Biologics commonly used for moderate-to-severe psoriasis in the USA include tumor necrosis factor (TNF) inhibitor adalimumab [US Food and Drug Administration (FDA) approval in 2008]; interleukin (IL)-17 inhibitors secukinumab (approval in 2015) and ixekizumab (approval in 2016); IL-12/23 inhibitor ustekinumab (approval in 2009); and IL-23 inhibitors guselkumab (approval in 2017) and risankizumab (approval in 2019) [3, 8].
Initial non-response to biologic treatment (primary failure) or loss of efficacy over time (secondary failure) may occur, prompting patients to discontinue treatment and switch to another treatment, escalate the dose, or add another treatment [3, 9, 10]. Drug survival, or treatment persistence, defined as the time between treatment start and treatment discontinuation, is a measure of overall biologic effectiveness, safety, and real-world utility [9–11]. In previous psoriasis research, drug survival was generally longer for ustekinumab than for secukinumab, adalimumab, and ixekizumab [12]. In the guselkumab era, the cumulative probability of drug survival at 18 months was 91.1% for guselkumab, 82.0% for ixekizumab, and 79.9% for secukinumab [13]. At the same time, data on persistence on guselkumab relative to other biologics remain scarce.
While treatment persistence is a potential indicator of patients being treated successfully, it is challenging to identify using claims data. Stopping treatment without a switch to any other treatment is intriguing as a potential sign of remission. Given the lack of validated definition of psoriasis remission in claims data, we sought to employ a proxy definition of remission on the basis of treatment patterns observed in the data. Thus, the aim of this study was to describe persistence on biologics, including the more recently approved guselkumab. To provide a more complete picture of disease control with various biologics in the real world, this study also attempted to describe possible remission on the basis of treatment patterns.
Methods
Data Source
Administrative claims data from the IBM MarketScan Commercial and Medicare Supplemental Databases ranging from 1 January 2016 to 31 January 2021 provided information on demographics, member health eligibility, and medical and prescription drug claims of commercially insured and Medicare beneficiaries nationwide. The data were de-identified and complied with the Health Insurance Portability and Accountability Act (HIPAA) regulations; therefore, no review by an institutional review board was required per Title 45 of CFR, Part 46.101(b)(4) [14]. The study was also in accordance with the 1964 Declaration of Helsinki.
Study Design
A retrospective descriptive design was used. The biologics in this analysis were guselkumab, adalimumab, secukinumab, and ixekizumab. These were the most commonly used biologics for psoriasis in the USA at the time of the study; risankizumab, tildrakizumab, and certolizumab pegol were not included because of few months of data available since their approval for psoriasis indication at the time this study was conducted [8, 15, 16]; and ustekinumab was not included because a similar study investigating treatment persistence among patients with psoriasis initiated on ustekinumab and other biologics has recently been published by our group [12]. The index window began on 13 July 2017 (i.e., FDA approval date of guselkumab for plaque psoriasis [17]) to ensure all four study biologics (i.e., guselkumab, adalimumab, secukinumab, and ixekizumab) were available on the market as treatment options for plaque psoriasis. The index window ended 6 months before the end of data (i.e., 31 July 2020) to allow for an equal opportunity for patients in all treatment cohorts to discontinue the index biologic. The index date was defined as the date of the first claim of a study biologic during the index window. The baseline period was defined as the 12-month period with continuous health plan eligibility before the index date. The follow-up period spanned from the index date to the earliest of the end of data or end of continuous health plan eligibility.
Study Cohorts
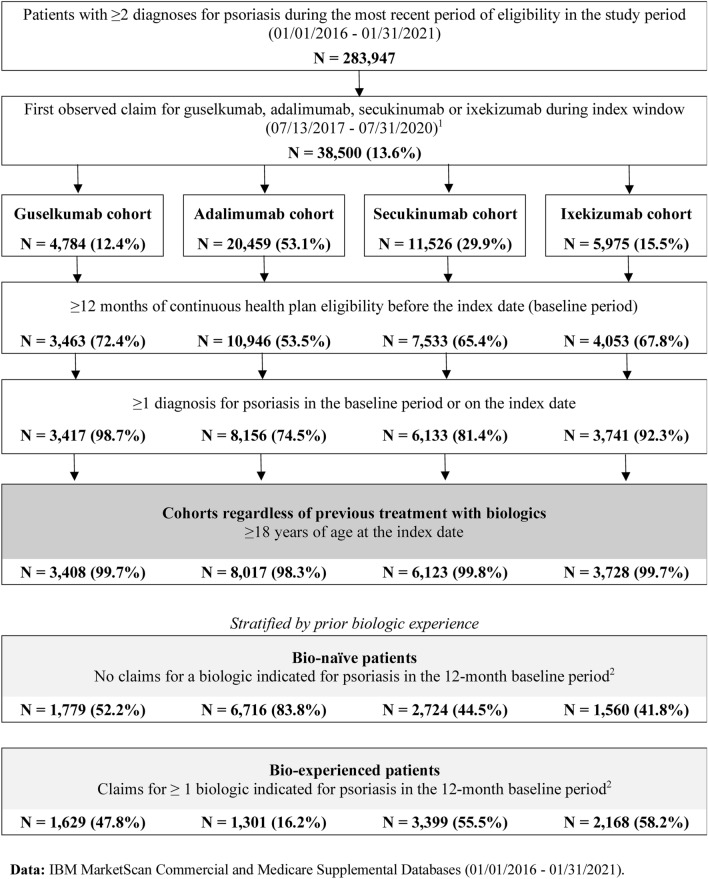
Patients were included in the study if they met the following criteria: (1) had ≥ 2 claims with a diagnosis for psoriasis [International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes: L40.0–L40.4, L40.8, L40.9] during the most recent period of continuous eligibility with ≥ 1 claim during the baseline period or on the index date; (2) had ≥ 1 claim for guselkumab, adalimumab, secukinumab, or ixekizumab, with the first observed claim on or after 13 July 2017 (guselkumab approval date for plaque psoriasis); (3) had ≥ 12 months of continuous health plan eligibility before the index date; and (4) were ≥ 18 years old on the index date. Patients were excluded from the study if they initiated multiple index biologics on the index date and/or had ≥ 1 claim for the index biologic any time before the index date.
Patients were classified into the following four cohorts on the basis of the biologic initiated during the index window: the guselkumab cohort, the adalimumab cohort, the secukinumab cohort, and the ixekizumab cohort. Patients who initiated more than one study biologic(s) at different times during the index window could be included in more than one cohort(s) with different index date(s). Non-mutually exclusive cohorts were permitted since the analysis was descriptive.
Study Outcomes
Discontinuation of the index biologic was defined as a therapy exposure gap in consecutive days of index biologic supply, or between the last day of supply and the end of the follow-up period. The discontinuation date was defined as the last day of supply prior to the therapy exposure gap. Two definitions of a therapy exposure gap (primary and secondary) were used to account for the differences in the post-induction dosing frequency indicated per label of the respective study biologic (i.e., guselkumab: 56 days; adalimumab: 14 days; secukinumab: 28 days; ixekizumab: 28 days) [18–21]. The primary definition of a therapy exposure gap was two times (2×) the per-label dosing frequency after the induction phase or the mode of days of supply observed in the data (i.e., guselkumab: > 120 days; adalimumab, secukinumab, ixekizumab: > 60 days [18, 19, 22, 23]; although adalimumab is indicated every 14 days, the mode of days of supply observed in the data was 28 days, as each dispensing typically contains two doses of adalimumab). The secondary definition of a therapy exposure gap was one time (1×) the per-label dosing frequency after the induction phase or the mode of days of supply observed in the data (i.e., guselkumab: > 60 days; adalimumab, secukinumab, ixekizumab: > 30 days) [18–21].
Reinitiation and remission were described among patients who discontinued the index biologic during the follow-up period. Reinitiation was defined as ≥ 1 claim for the index biologic after the end of the minimum therapy exposure gap (i.e., 120/60 days for guselkumab or 60/30 days for adalimumab, secukinumab, and ixekizumab). Remission was measured using a proxy definition during the follow-up period. Complete remission was defined as no claims for any psoriasis-related treatment after the index biologic discontinuation (using the primary definition) among patients with ≥ 6 months of follow-up post-discontinuation. Remission of moderate-to-severe disease allowed for patients to have claims for topical therapies after discontinuation of the index biologic.
As measures of persistence in claims data rely on information on days of medication supply, which is missing in medical claims and could be inconsistent with label recommendations in pharmacy claims, imputation of days of supply may be needed. For all treatment cohorts, the missing days of supply in medical claims were imputed as follows: guselkumab, 28 days of supply for the first claim and 56 days of supply for all subsequent claims, as per label [21]; adalimumab, 28 days of supply for all claims, which were based on the mode of the days of supply in pharmacy claims; secukinumab and ixekizumab, there were no medical claims (i.e., no claims with missing days of supply) in the data. For pharmacy claims, the days of supply for the second and subsequent guselkumab pharmacy claims coded as 28–30 days were imputed to 56 days of supply (per label and per the distribution of time between claims observed in the data); no imputations were made for pharmacy claims of adalimumab, secukinumab, and ixekizumab, as the observed days of supply were aligned with their respective label.
Subgroup Analysis
All study outcomes (based on the primary definition of therapy exposure gap) were also assessed in two patient subgroups stratified by prior experience with biologics. The bio-naïve subgroup comprised patients without claims for biologics indicated for psoriasis during the 12-month baseline period. The bio-experienced subgroup comprised patients with claims for one or more unique biologics indicated for psoriasis during the 12-month baseline period. The biologics indicated for psoriasis considered were ustekinumab, adalimumab, secukinumab, ixekizumab, brodalumab, certolizumab pegol, etanercept, guselkumab, infliximab, risankizumab, and tildrakizumab.
Statistical Analysis
Patient baseline characteristics as well as reinitiation and remission outcomes were described using means, medians, and standard deviations (SDs) for continuous variables, and frequencies and proportions for binary variables. The probability of discontinuation of the index biologic was assessed using Kaplan–Meier (KM) survival analyses at 3, 6, 12, 18, and 24 months post-index. Patients for whom discontinuation was not observed during the follow-up time were censored on the last day of the index biologic supply before the end of follow-up. The median time to discontinuation estimated based on KM analysis was reported if available.
Results
Baseline Characteristics
The total number of patients was 3408 in the guselkumab cohort, 8017 in the adalimumab cohort, 6123 in the secukinumab cohort, and 3728 in the ixekizumab cohort (Fig. 1). Across cohorts, the mean age of patients was similar (ranging from 47.4 to 49.4 years old), and approximately half (47.1–54.2%) of the patients were female (Table 1). The majority (70.4–85.2%) of patients had at least one psoriasis-related comorbidity at baseline, with the most commonly observed conditions being hypertension and hyperlipidemia. Prior experience with biologics was observed in 47.8% of patients in the guselkumab cohort, 16.2% in the adalimumab cohort, 55.5% in the secukinumab cohort, and 58.2% in the ixekizumab cohort.
Fig. 1.
Sample selection of patients with psoriasis initiated on guselkumab, adalimumab, secukinumab, or ixekizumab. Notes: (1) Patients who initiated more than one study biologics will be observed in more than one cohort but at different times. Also excludes patients with initiation of multiple index biologics on the index date. (2) Biologics considered were guselkumab, adalimumab, secukinumab, ixekizumab, brodalumab, certolizumab pegol, etanercept, ustekinumab, infliximab, and risankizumab
Table 1.
Patient baseline characteristics
| Mean ± SD [median] or n (%) | Guselkumab cohort | Adalimumab cohort | Secukinumab cohort | Ixekizumab cohort |
|---|---|---|---|---|
| N = 3408 | N = 8017 | N = 6123 | N = 3728 | |
| Age at index date (years) | 47.9 ± 12.5 [48.9] | 47.4 ± 12.5 [48.9] | 49.4 ± 11.7 [50.5] | 49.1 ± 11.6 [50.7] |
| Female | 1606 (47.1) | 4338 (54.1) | 3319 (54.2) | 1874 (50.3) |
| Region of residence at index date | ||||
| South | 1701 (49.9) | 4083 (50.9) | 3072 (50.2) | 1860 (49.9) |
| Midwest | 739 (21.7) | 1288 (16.1) | 1138 (18.6) | 598 (16.0) |
| West | 619 (18.2) | 1695 (21.1) | 1152 (18.8) | 801 (21.5) |
| Northeast | 347 (10.2) | 937 (11.7) | 748 (12.2) | 467 (12.5) |
| Unknown | 2 (0.1) | 14 (0.2) | 13 (0.2) | 2 (0.1) |
| Year of index date | ||||
| 2017 | 286 (8.4) | 1538 (19.2) | 736 (12.0) | 424 (11.4) |
| 2018 | 1030 (30.2) | 2858 (35.6) | 2118 (34.6) | 1021 (27.4) |
| 2019 | 1025 (30.1) | 2549 (31.8) | 2163 (35.3) | 1492 (40.0) |
| 2020 | 1067 (31.3) | 1072 (13.4) | 1106 (18.1) | 791 (21.2) |
| Q-CCI1 | 0.7 ± 1.3 [0.0] | 0.8 ± 1.3 [0.0] | 0.9 ± 1.4 [0.0] | 0.8 ± 1.4 [0.0] |
| Selected comorbidities | 2398 (70.4) | 6506 (81.2) | 5218 (85.2) | 2980 (79.9) |
| Hypertension | 1211 (35.5) | 2923 (36.5) | 2420 (39.5) | 1450 (38.9) |
| Hyperlipidemia | 1130 (33.2) | 2601 (32.4) | 2178 (35.6) | 1366 (36.6) |
| Obesity | 879 (25.8) | 2248 (28.0) | 1807 (29.5) | 1113 (29.9) |
| Diabetes | 595 (17.5) | 1218 (15.2) | 1042 (17.0) | 721 (19.3) |
| Psoriatic arthritis | 570 (16.7) | 3272 (40.8) | 3320 (54.2) | 1429 (38.3) |
| Any psoriasis treatment | 2903 (85.2) | 6931 (86.5) | 5579 (91.1) | 3331 (89.4) |
| Topical therapies | 2320 (68.1) | 5672 (70.7) | 4014 (65.6) | 2518 (67.5) |
| Biologics | 1629 (47.8) | 1301 (16.2) | 3399 (55.5) | 2168 (58.2) |
| Anti-IL17a | 704 (20.7) | 305 (3.8) | 190 (3.1) | 670 (18.0) |
| Anti-IL-12/23 | 560 (16.4) | 183 (2.3) | 673 (11.0) | 498 (13.4) |
| Anti-TNF | 532 (15.6) | 877 (10.9) | 2633 (43.0) | 1204 (32.3) |
| Anti-IL-23 | 15 (0.4) | 44 (0.5) | 116 (1.9) | 103 (2.8) |
| 2+ biologics used | 242 (7.1) | 126 (1.6) | 469 (7.7) | 355 (9.5) |
| Conventional systemic therapies | 767 (22.5) | 2892 (36.1) | 2127 (34.7) | 1006 (27.0) |
| Apremilast | 465 (13.6) | 894 (11.2) | 1016 (16.6) | 485 (13.0) |
| Immunosuppressant agents | 298 (8.7) | 2083 (26.0) | 1224 (20.0) | 571 (15.3) |
| Retinoids | 60 (1.8) | 107 (1.3) | 93 (1.5) | 46 (1.2) |
| All-cause pharmacy and medical costs2 | 33,887 ± 38,970 [23,516] | 19,211 ± 32,270 [6988] | 39,703 ± 44,088 [33,159] | 39,467 ± 41,039 [33,245] |
| Pharmacy costs | 26,671 ± 30,290 [16,310] | 10,905 ± 19,631 [1654] | 29,452 ± 30,625 [23,739] | 30,310 ± 29,488 [25,302] |
| Medical costs | 7216 ± 23,235 [1551] | 8306 ± 24,668 [2244] | 10,251 ± 31,551 [2561] | 9156 ± 25,991 [2083] |
| Duration of follow-up period (months) | 16.5 ± 10.4 [13.5] | 19.3 ± 11.4 [18.5] | 17.9 ± 10.7 [16.4] | 17.1 ± 10.4 [15.6] |
ICD-10-CM International Classification of Diseases, 10th Revision, Clinical Modification, IL interleukin, Q-CCI Quan-Charlson Comorbidity Index, SD standard deviation, TNF tumor necrosis factor, USD United States dollar
1Quan H, Sundararajan V, Halfon P et al. Coding Algorithms for Defining Comorbidities in ICD-10-CM Administrative Data. Medical Care 2005; 43:1130–1139
2Healthcare costs are reported from a private payer’s perspective in 2021 USD adjusted for inflation using the US Consumer Price Index
Persistence to Index Therapy
The mean follow-up time was 16.5 months in the guselkumab cohort, 19.3 months in the adalimumab cohort, 17.9 months in the secukinumab cohort, and 17.1 months in the ixekizumab cohort (Table 1).
Discontinuation of Index Biologic Based on Primary Therapy Exposure Gap Definition (2× Per-Label Dosing Frequency/Mode of Days of Supply)
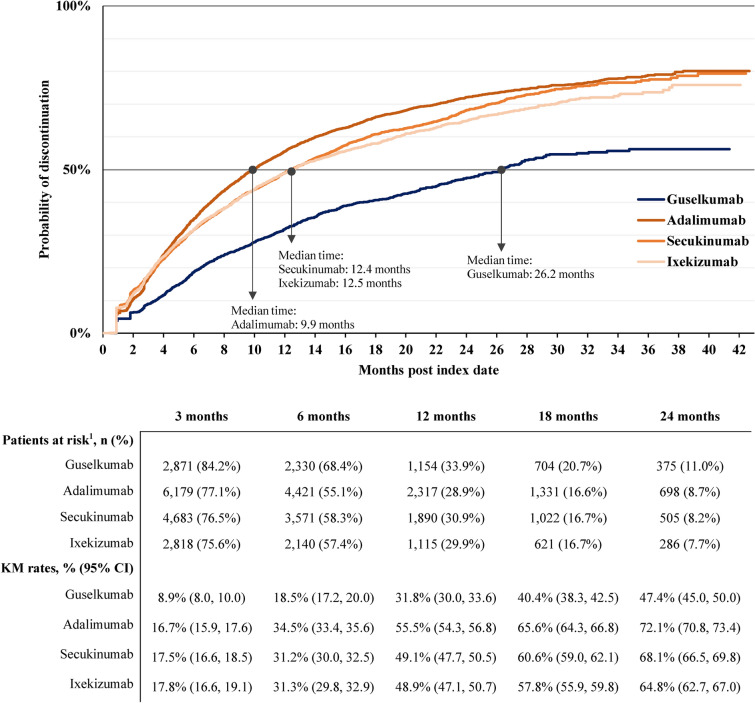
Using the primary definition, the probability of index biologic persistence trended longer in the guselkumab cohort relative to other cohorts at all timepoints (Fig. 2). At 24 months, the probability of discontinuation was 47.4% in the guselkumab cohort, 72.1% in the adalimumab cohort, 68.1% in the secukinumab cohort, and 64.8% in the ixekizumab cohort. The median time to discontinuation was 26.2 months with guselkumab, 9.9 months with adalimumab, 12.4 months with secukinumab, and 12.5 months with ixekizumab.
Fig. 2.
Time to discontinuation based on therapy exposure gap of more than 2× per-label dosing frequency or mode of days of supply for each cohort. CI confidence interval; KM Kaplan–Meier. Notes: (1) Patients at risk of having the event are patients who have not had the event and have not been lost to follow-up at that point in time
Among the subset of patients who discontinued index therapy any time during the follow-up period, the index therapy was reinitiated in 22.9% (257 of 1124) patients in the guselkumab cohort, 21.1% (972 of 4610) patients in the adalimumab cohort, 31.9% (1020 of 3201) patients in the secukinumab cohort, and 32.0% (596 of 1861) patients in the ixekizumab cohort. The mean [median] time to reinitiation of index therapy, among those who discontinued, was 4.0 [2.6] months in the guselkumab cohort, 3.3 [1.6] months in the adalimumab cohort, 2.4 [1.3] months in the secukinumab cohort, and 2.4 [1.3] months in the ixekizumab cohort.
Discontinuation of Index Biologic Based on Secondary Therapy Exposure Gap Definition (1× Per-Label Dosing Frequency/Mode of Days of Supply)
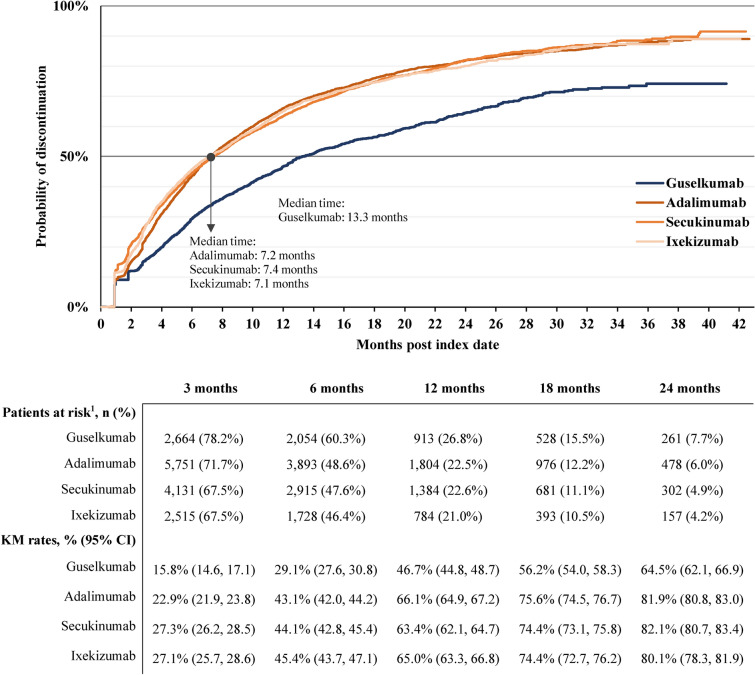
Using the secondary definition with a shorter therapy exposure gap, persistence trended longer in the guselkumab cohort relative to other cohorts at all timepoints assessed (Fig. 3). At 24 months, the probability of discontinuation was 64.5% in the guselkumab cohort, 81.9% in the adalimumab cohort, 82.1% in the secukinumab cohort, and 80.1% in the ixekizumab cohort. The median time to discontinuation was 13.3 months with guselkumab, 7.2 months with adalimumab, 7.4 months with secukinumab, and 7.1 months with ixekizumab.
Fig. 3.
Time to discontinuation based on therapy exposure gap of over 1× per-label dosing frequency or mode of days of supply for each cohort. CI confidence interval, KM Kaplan–Meier. Notes: (1) Patients at risk of having the event are patients who have not had the event and have not been lost to follow-up at that point in time
Among patients who discontinued index therapy any time during the follow-up period, the index therapy was reinitiated in 43.1% (692 of 1607) patients in the guselkumab cohort, 37.2% (2011 of 5405) patients in the adalimumab cohort, 50.8% (2045 of 4025) patients in the secukinumab cohort, and 52.3% (1262 of 2412) patients in the ixekizumab cohort. The mean [median] time to reinitiation of index therapy, among those who discontinued, was 2.4 [1.1] months in the guselkumab cohort, 2.0 [0.7] months in the adalimumab cohort, 1.6 [0.7] months in the secukinumab cohort, and 1.6 [0.7] months in the ixekizumab cohort.
Rates of Remission
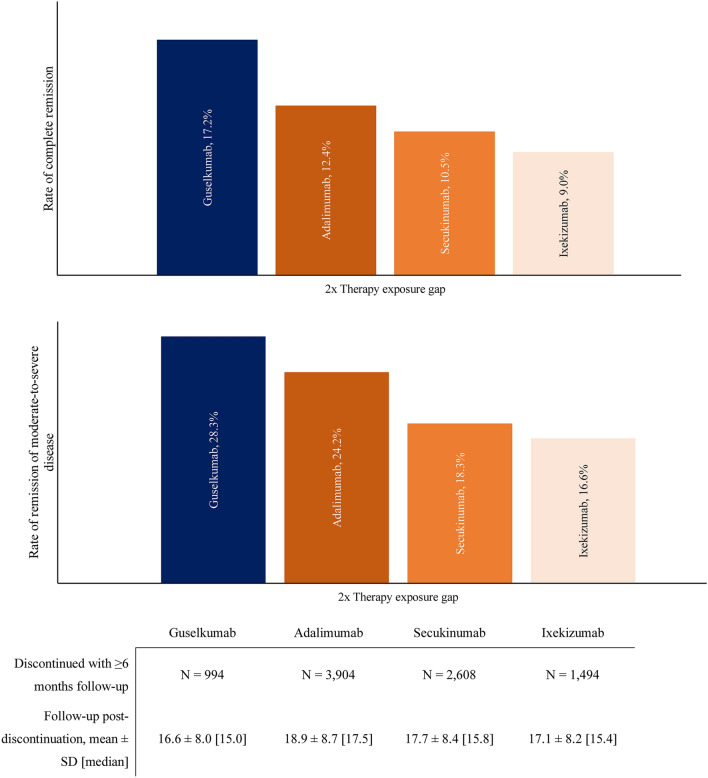
Among patients who discontinued index therapy on the basis of the primary definition and who had ≥ 6 months of follow-up, complete remission per proxy definition was achieved in 17.2% of the guselkumab, 12.4% of the adalimumab, 10.5% of the secukinumab, and 9.0% of the ixekizumab cohorts (Fig. 4). Remission of moderate-to-severe disease was achieved in 28.3% of the guselkumab, 24.2% of the adalimumab, 18.3% of the secukinumab, and 16.6% of the ixekizumab cohorts.
Fig. 4.
Rates of remission among patients who discontinued index therapy and had ≥ 6 months of follow-up post-discontinuation. SD standard deviation
Subgroup Analyses
Bio-naïve Subgroup
The mean [median] follow-up time of the bio-naïve subgroup was 14.4 [11.7] months in the guselkumab cohort (N = 1779), 19.2 [18.4] months in the adalimumab cohort (N = 6716), 17.0 [15.2] months in the secukinumab cohort (N = 2724), and 15.5 [14.5] months in the ixekizumab cohort (N = 1560).
The probability of persistence at 24 months was higher and median time to discontinuation was longer in the guselkumab cohort than in other cohorts (Supplementary Figure S1a).
Among patients who discontinued the index therapy any time during the follow-up period, 24.1% reinitiated the index therapy in the guselkumab cohort and 21.2%, 34.6%, and 38.6% reinitiated it in the adalimumab, secukinumab, and ixekizumab cohorts, respectively. The mean [median] time to reinitiation of index therapy, among those who discontinued, was 4.4 [3.6] months in the guselkumab cohort and 3.4 [1.7], 2.5 [1.3], and 2.3 [1.2] months in the adalimumab, secukinumab, and ixekizumab cohorts, respectively.
Complete remission and remission of moderate-to-severe disease was achieved in a higher proportion of patients in the guselkumab cohort than other cohorts (Supplementary Figure S2).
Bio-experienced Subgroup
The mean [median] available follow-up time of the bio-experienced subgroup was 18.9 [18.1] months in the guselkumab cohort (N = 1629), 19.8 [18.8] months in the adalimumab cohort (N = 1301), 18.6 [17.5] months in the secukinumab cohort (N = 3399), and 18.3 [16.7] months in the ixekizumab cohort (N = 2168).
The probability of persistence at 24 months was higher and median time to discontinuation was longer in the guselkumab cohort than in other cohorts (Supplementary Figure S1b).
Among patients who discontinued index therapy any time during the follow-up period, 21.8% reinitiated the index therapy in the guselkumab cohort and 20.5%, 29.8%, and 27.7% reinitiated it in the adalimumab, secukinumab, and ixekizumab cohorts, respectively. The mean [median] time to reinitiation of index therapy, among those who discontinued, was 3.6 [2.1] months in the guselkumab cohort and 2.8 [1.3], 2.4 [1.2], and 2.5 [1.3] months in the adalimumab, secukinumab, and ixekizumab cohorts, respectively.
Complete remission and remission of moderate-to-severe disease was achieved in a higher proportion of patients in the guselkumab cohort than other cohorts (Supplementary Figure S2).
Discussion
In this U.S. retrospective cohort study, persistence to guselkumab trended higher than on other common biologics for psoriasis, including adalimumab, secukinumab, and ixekizumab. When discontinuation was defined using 1× the per-label dosing frequency (or mode of days of supply, for adalimumab) as therapy exposure gap, almost 40–50% of patients were observed to have reinitiated index therapy across cohorts, suggesting that this definition may have captured a large portion of patients with delayed prescription fills (e.g., due to access/insurance issues).
Using the proxy definition of remission, a numerically higher proportion of patients who initiated guselkumab met the complete remission and remission of moderate-to-severe psoriasis criteria relative to those who initiated adalimumab, secukinumab, or ixekizumab. The observation that some patients remained off biologic therapy post-discontinuation may be suggestive of potential disease modification with the study biologics; nonetheless, other reasons for remaining off biologic therapy may include pregnancy, upcoming surgery, cancer diagnosis, or other patient factors. Subgroup analyses among bio-naïve and bio-experienced patients showed similar trends in all outcomes assessed.
Several studies evaluated persistence on biologics in psoriasis, although few were conducted in U.S. clinical practice settings [13, 24–27]. Among U.S. studies, Xu et al. assessed the persistence of commercially insured patients with psoriasis and comorbid psoriatic arthritis who initiated a biologic of interest, including all four of the current study biologics [25]. Certain differences in the study designs exist between the Xu et al. and the current study. For instance, that study required patients to have comorbid psoriatic arthritis, which affects approximately 30% of patients with psoriasis [28, 29]; and persistence outcomes were assessed using the proportions of patients with continuous use of treatment during a fixed 9-month follow-up period [25], whereas the current study employed KM analyses. Nonetheless, the results of the two studies were consistent in that more patients remained on treatment with guselkumab than with other study biologics at all timepoints assessed.
In another claims analysis comparing persistence to ixekizumab and guselkumab among U.S. patients with psoriasis [26], persistence estimates at 6 and 12 months based on KM analyses were numerically higher with ixekizumab than with guselkumab for a 60-day allowable treatment gap; and the persistence at the same timepoints was comparable between the two biologics for a 90-day allowable treatment gap. Notably, that study used the same allowable treatment gap definition (i.e., either 60 or 90 days) for both ixekizumab and guselkumab, which have different label-indicated dosing frequencies. That approach could have advantaged ixekizumab, which has a shorter dosing interval than guselkumab, when assessing persistence. Even in that study, more patients switched to another treatment from ixekizumab (17.6%) than from guselkumab (12.9%) by 12 months [26].
The current findings on persistence based on a U.S. claims database also align with non-U.S. nationwide registry-based studies [e.g., studies that used the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR) and the Danish national registry (DERMBIO)], which reported that guselkumab was associated with the longest drug survival relative to other biologics examined (including anti-TNF agents, secukinumab, ixekizumab, and ustekinumab) in patients with psoriasis [24, 30]. The current study has covered a relatively complete treatment landscape of biologics for psoriasis in the USA, including guselkumab, for which few data on persistence exist. Given the approval of newer biologics for psoriasis since the approval of guselkumab in 2017, future investigation including more recently approved biologics may be warranted.
The different dosing schedules of the study biologics could have contributed to the persistence patterns observed in the current study. Survey studies have reported that patients with psoriasis generally prefer biologics with the least frequent dosing [31, 32]. Although these studies did not evaluate the reasons behind the preference, patients with other chronic diseases prefer longer dosing intervals owing to convenience, reduced medication burden, and the medication schedule being easier to remember and fitting better with their lifestyle [33–35]. Thus, the less frequent dosing requirement of guselkumab may have partially contributed to the longer persistence observed. In addition to dosing convenience, other factors may also affect patient persistence to treatments, including ineffectiveness, secondary failure, side effects (which may or may not be treatment-related), reimbursement policies, and availability of new treatment options [13, 36, 37].
Dermatologists generally agree that treatment persistence, commonly referred to as drug survival for biologics, is a comprehensive measure that reflects treatment effectiveness and safety as well as patient and physician preferences of biologics [36, 38]. On the other hand, suboptimal persistence to biologics is associated with poorer clinical outcomes in psoriasis [39–41]. Therefore, biologics associated with higher persistence may have the potential to improve long-term clinical outcomes of patients with psoriasis.
Limitations
The analysis was descriptive in nature, and baseline differences between cohorts were not accounted for; therefore, the results should be interpreted with caution, and future studies among matched cohorts are warranted to confirm that these will remain robust after accounting for differences in baseline patient characteristics. Second, the results may not be generalizable to uninsured patients or patients without commercial health insurance. Third, as with all claims analyses, prescription fills captured did not account for whether the medication dispensed was taken as prescribed. Fourth, the proxy definition of remission used in this study is an exploratory approach that requires validation. Fifth, reasons for discontinuation of index biologic and those for remaining off biologic therapy are unknown. Finally, guselkumab was a new drug at the time this study was conducted and may have been reserved for patients with more refractory disease; this might be expected to bias the findings toward lower persistence with guselkumab than would be the case in a population not selected for more severe disease.
Conclusions
In this retrospective cohort study, patients with psoriasis initiated on guselkumab showed trends toward lower discontinuation rates, longer median time to discontinuation, and higher remission rates relative to patients initiated on other biologics. Patients remaining off treatment or just on topical therapies post-treatment discontinuation is suggestive of potential disease modification. Similar patterns were observed in both bio-naïve and bio-experienced patients.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study was sponsored by Janssen Scientific Affairs, LLC. The study sponsor also funded the journal’s Rapid Service Fees.
Medical Writing and Editorial Assistance
Medical writing assistance was provided by Christine Tam, MSc, MWC, and Flora Chik, PhD, MWC, both employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC which funded the development and conduct of this study and manuscript.
Author Contributions
Timothy Fitzgerald and Steven R. Feldman contributed to study conception and design, data analysis and interpretation. Maryia Zhdanava, Dominic Pilon, Aditi Shah, Annalise Hilts, and Patrick Lefebvre contributed to study conception and design, collection and assembly of data, and data analysis and interpretation. All authors reviewed and approved the final content of this manuscript. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Prior Presentation
Part of the results were included in a presentation at the Society of Dermatology Physician Assistants Annual Summer Dermatology Conference, June 16–19, 2022, in Austin, Texas. Results for the bio-naïve and bio-experienced subgroups were included in a presentation at the Maui Derm NP + PA Summer 2022 Conference, June 22–25, 2022, in Colorado Springs, Colorado.
Disclosures
Timothy Fitzgerald is an employee of Janssen Scientific Affairs, LLC, and holds stock in Johnson & Johnson. Maryia Zhdanava, Dominic Pilon, Aditi Shah, Annalise Hilts, and Patrick Lefebvre are employees of Analysis Group, Inc., a consulting company that has provided paid consulting services to Janssen Scientific Affairs, LLC. Steven R. Feldman received research, speaking and/or consulting support from Janssen Scientific Affairs, LLC and is the founder and majority owner of www.DrScore.com [drscore.com] and founder and part owner of Causa Research.
Compliance with Ethics Guidelines
All data were de-identified and complied with HIPAA regulations; therefore, institutional review board approval was not required per Title 45 of CFR, Part 46.101(b)(4) [14]. The study was also in accordance with the 1964 Declaration of Helsinki.
Data Availability
The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions on the availability of these data, which were obtained under license for the current study. Any researchers interested in obtaining the data used in this study can access database through IBM MarketScan, under a license agreement, including the payment of appropriate license fee.
References
- 1.Ramirez-Fort MK, Levin AA, Au SC, Gottlieb AB. Continuous versus intermittent therapy for moderate-to-severe psoriasis. Clin Exp Rheumatol. 2013;31(4 Suppl 78):S63–70. [PubMed] [Google Scholar]
- 2.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 3.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 4.Gisondi P, Del Giglio M, Girolomoni G. Treatment approaches to moderate to severe psoriasis. Int J Mol Sci. 2017;18(11):2427. doi: 10.3390/ijms18112427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Center for Drug Evaluation and Research U.S. Food and Drug Administration. Alefacept Product Approval Information - Licensing Action 2003. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2003/alefbio013003L.htm. Accessed Aug 13, 2019.
- 6.Tsai YC, Tsai TF. Anti-interleukin and interleukin therapies for psoriasis: current evidence and clinical usefulness. Ther Adv Musculoskelet Dis. 2017;9(11):277–294. doi: 10.1177/1759720X17735756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sbidian E, Chaimani A, Garcia-Doval I, Do G, Hua C, Mazaud C, et al. Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev. 2017;12:CD011535. doi: 10.1002/14651858.CD011535.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AbbVie Inc. AbbVie Expands Immunology Portfolio in the U.S. with FDA Approval of SKYRIZI™ (risankizumab-rzaa) for Moderate to Severe Plaque Psoriasis North Chicago, IL updated April 23, 2019. Available from: https://news.abbvie.com/news/press-releases/abbvie-expands-immunology-portfolio-in-us-with-fda-approval-skyrizi-risankizumab-rzaa-for-moderate-to-severe-plaque-psoriasis.htm. Accessed: January 23, 2023.
- 9.Sutaria N, Au SC. Failure rates and survival times of systemic and biologic therapies in treating psoriasis: a retrospective study. J Dermatolog Treat. 2021;32(6):617–620. doi: 10.1080/09546634.2019.1688756. [DOI] [PubMed] [Google Scholar]
- 10.Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker J, Burden AD, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 11.Mourad AI, Gniadecki R. Biologic drug survival in psoriasis: A systematic review & comparative meta-analysis. Front Med (Lausanne) 2020;7:625755. doi: 10.3389/fmed.2020.625755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pilon D, Fitzgerald T, Zhdanava M, Teeple A, Morrison L, Shah A, et al. Risk of treatment discontinuation among patients with psoriasis initiated on ustekinumab and other biologics in the USA. Dermatol Ther (Heidelb) 2022;12(4):971–987. doi: 10.1007/s13555-022-00707-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Torres T, Puig L, Vender R, Lynde C, Piaserico S, Carrascosa JM, et al. Drug survival of IL-12/23, IL-17 and IL-23 inhibitors for psoriasis treatment: a retrospective multi-country, multicentric cohort study. Am J Clin Dermatol. 2021;22(4):567–579. doi: 10.1007/s40257-021-00598-4. [DOI] [PubMed] [Google Scholar]
- 14.U.S. Department of Health and Human Services. 45 CFR 46: pre-2018 requirements. Available from: https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html#46.101. Accessed Oct 16 2020.
- 15.Sun Pharmaceutical Industries Ltd. Sun Pharma Announces U.S. FDA Approval of ILUMYA™ (tildrakizumab-asmn) for the Treatment of Moderate-to-Severe Plaque Psoriasis Mumbai, India and Princeton, NJ updated March 21, 2018. Available from: https://sunpharma.com/wp-content/uploads/2020/12/Sun-Pharma-Announces-U.S.-FDA-Approval-of-ILUMYA.pdf. Accessed Jan 23 2023.
- 16.UCB. UCB Announces the Approval of CIMZIA® (certolizumab pegol) for Moderate-to-Severe Plaque Psoriasis, Representing an Important New Option for Patients in the U.S. Brussels, Belgium updated May 29, 2018. Available from: https://www.ucb-usa.com/stories-media/UCB-U-S-News/detail/article/Press-Release. Accessed Jan 23 2023.
- 17.Johnson & Johnson. Janssen Announces U.S. FDA Approval of Tremfya™ (guselkumab) for the treatment of moderate to severe plaque psoriasis Horsham, PA: Janssen Biotech, Inc.; 2017. Available from: https://www.jnj.com/media-center/press-releases/janssen-announces-us-fda-approval-of-tremfya-guselkumab-for-the-treatment-of-moderate-to-severe-plaque-psoriasis. Accessed Dec 3 2020.
- 18.Eli Lilly and Company. Taltz (ixekizumab) Prescribing Information. Indianapolis, IN; May 2022.
- 19.Novartis Pharmaceuticals Corporation. Cosentyx (secukinumab) Prescribing Information. East Hanover, NJ; December 2021.
- 20.AbbVie Inc. Humira (adalimumab) Prescribing Information. North Chicago, IL; February 2021.
- 21.Janssen Biotech Inc. Tremfya (guselkumab) Prescribing Information. Horsham, PA; July 2020.
- 22.Arnold T, Brinkley-Rubinstein L, Chan PA, Perez-Brumer A, Bologna ES, Beauchamps L, et al. Social, structural, behavioral and clinical factors influencing retention in Pre-Exposure Prophylaxis (PrEP) care in Mississippi. PLoS ONE. 2017;12(2):e0172354. doi: 10.1371/journal.pone.0172354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pochwat B, Nowak G, Szewczyk B. An update on NMDA antagonists in depression. Expert Rev Neurother. 2019;19(11):1055–1067. doi: 10.1080/14737175.2019.1643237. [DOI] [PubMed] [Google Scholar]
- 24.Egeberg A, Roseno NAL, Aagaard D, Lorup EH, Nielsen ML, Nymand L, et al. Drug survival of biologics and novel immunomodulators for rheumatoid arthritis, axial spondyloarthritis, psoriatic arthritis, and psoriasis - a nationwide cohort study from the DANBIO and DERMBIO registries. Semin Arthritis Rheum. 2022;53:151979. doi: 10.1016/j.semarthrit.2022.151979. [DOI] [PubMed] [Google Scholar]
- 25.Xu C, Teeple A, Wu B, Fitzgerald T, Feldman SR. Treatment adherence and persistence of seven commonly prescribed biologics for moderate to severe psoriasis and psoriatic arthritis in a U.S. commercially insured population. J Dermatolog Treat. 2021;33(4):2270–7. doi: 10.1080/09546634.2021.1950600. [DOI] [PubMed] [Google Scholar]
- 26.Blauvelt A, Burge R, Gallo G, Charbonneau B, Malatestinic W, Zhu B, et al. A retrospective cohort analysis of treatment patterns over 1 year in patients with psoriasis treated with ixekizumab or guselkumab. Dermatol Ther (Heidelb) 2022;12(3):701–714. doi: 10.1007/s13555-022-00686-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmitt-Egenolf M, Freilich J, Stelmaszuk-Zadykowicz NM, Apol E, Hansen JB, Levin LA. Drug persistence of biologic treatments in psoriasis: a Swedish national population study. Dermatol Ther (Heidelb) 2021;11(6):2107–2121. doi: 10.1007/s13555-021-00616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.FitzGerald O, Ogdie A, Chandran V, Coates LC, Kavanaugh A, Tillett W, et al. Psoriatic arthritis. Nat Rev Dis Primers. 2021;7(1):59. doi: 10.1038/s41572-021-00293-y. [DOI] [PubMed] [Google Scholar]
- 29.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65.e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 30.Yiu ZZN, Becher G, Kirby B, Laws P, Reynolds NJ, Smith CH, et al. Drug survival associated with effectiveness and safety of treatment with guselkumab, ixekizumab, secukinumab, ustekinumab, and adalimumab in patients with psoriasis. JAMA Dermatol. 2022;158(10):1131–1141. doi: 10.1001/jamadermatol.2022.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M, Brenneman SK, Carter CT, Essoi BL, Farahi K, Johnson MP, et al. Patient-reported treatment satisfaction and choice of dosing frequency with biologic treatment for moderate to severe plaque psoriasis. Patient Prefer Adherence. 2015;9:777–784. doi: 10.2147/PPA.S85773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang M, Carter C, Olson WH, Johnson MP, Brennem SK, Lee S, et al. Patient preference for dosing frequency based on prior biologic experience. J Drugs Dermatol. 2017;16(3):220–226. [PubMed] [Google Scholar]
- 33.Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38(4 Suppl 1):S2–6. doi: 10.1016/j.bone.2006.01.150. [DOI] [PubMed] [Google Scholar]
- 34.Sakai A, Ikeda S, Okimoto N, Matsumoto H, Teshima K, Okazaki Y, et al. Clinical efficacy and treatment persistence of monthly minodronate for osteoporotic patients unsatisfied with, and shifted from, daily or weekly bisphosphonates: the BP-MUSASHI study. Osteoporos Int. 2014;25(9):2245–2253. doi: 10.1007/s00198-014-2756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hugo C, Weihprecht H, Banas B, Schroppel B, Jank S, Arns W, et al. Renal function and patient-reported outcomes in stable kidney transplant patients following conversion from twice-daily immediate-release tacrolimus to once-daily prolonged-release tacrolimus: a 12-month observational study in routine clinical practice in Germany (ADAGIO) Transplant Proc. 2021;53(5):1484–1493. doi: 10.1016/j.transproceed.2021.01.034. [DOI] [PubMed] [Google Scholar]
- 36.van den Reek J, Kievit W, de Jong E. Comment on “Drug survival analysis is not a good method for assessing the safety or effectiveness of systemic therapies in psoriasis”. Actas Dermosifiliogr. 2017;108(7):695–696. doi: 10.1016/j.ad.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 37.Yeung H, Wan J, Van Voorhees AS, Callis Duffin K, Krueger GG, Kalb RE, et al. Patient-reported reasons for the discontinuation of commonly used treatments for moderate to severe psoriasis. J Am Acad Dermatol. 2013;68(1):64–72. doi: 10.1016/j.jaad.2012.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de la Cueva DP, Notario J, Ferrandiz C, Lopez Estebaranz JL, Alarcon I, Sulleiro S, et al. Expert consensus on the persistence of biological treatments in moderate-to-severe psoriasis. J Eur Acad Dermatol Venereol. 2019;33(7):1214–1223. doi: 10.1111/jdv.15600. [DOI] [PubMed] [Google Scholar]
- 39.Menter A, Feldman SR, Weinstein GD, Papp K, Evans R, Guzzo C, et al. A randomized comparison of continuous vs intermittent infliximab maintenance regimens over 1 year in the treatment of moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2007;56(1):31.e1–31.e15. doi: 10.1016/j.jaad.2006.07.017. [DOI] [PubMed] [Google Scholar]
- 40.Menter A, Tyring SK, Gordon K, Kimball AB, Leonardi CL, Langley RG, et al. Adalimumab therapy for moderate to severe psoriasis: a randomized, controlled phase III trial. J Am Acad Dermatol. 2008;58(1):106–115. doi: 10.1016/j.jaad.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 41.Gottlieb AB, Chaudhari U, Mulcahy LD, Li S, Dooley LT, Baker DG. Infliximab monotherapy provides rapid and sustained benefit for plaque-type psoriasis. J Am Acad Dermatol. 2003;48(6):829–835. doi: 10.1067/mjd.2003.307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are not publicly available due to restrictions on the availability of these data, which were obtained under license for the current study. Any researchers interested in obtaining the data used in this study can access database through IBM MarketScan, under a license agreement, including the payment of appropriate license fee.