Abstract
Psoriasis is a chronic, immune-mediated, inflammatory disease primarily affecting the skin. It is currently coming to light that patients with psoriasis have disrupted intestinal barrier and often suffer from comorbidities associated with the gastrointestinal tract. Moreover, there is growing evidence of both cutaneous and intestinal paradoxical reactions during biologic treatment in patients with psoriasis. This review focuses on barrier defects and changes in immune responses in patients with psoriasis, which play an important role in the development of the disease but are also influenced by modern biological treatments targeting IL-17 and TNFα cytokines. Here, we highlight the relationship between the gut–skin axis, microbiota, psoriasis treatment, and the incidence of paradoxical reactions, such as inflammatory bowel disease in patients with psoriasis. A better understanding of the interconnection of these mechanisms could lead to a more personalized therapy and lower the incidence of treatment side effects, thereby improving the quality of life of the affected patients.
Keywords: Skin microbiota, Gut microbiota, Psoriasis, IBD, TNFα, IL-17, Biologics, Skin adverse events, Gut–skin axis
Key Summary Points
| The gut-skin axis is of critical importance in the pathogenesis of psoriasis. The connection between skin and gut is mediated by several mechanisms, where microbiota plays an irreplaceable role. |
| Psoriasis patients suffer from impaired gut barrier integrity and are therefore at high risk for comorbidities such as IBD, which disrupt their already delicate health balance. |
| Intensive efforts are underway to investigate what triggers the disruption of the gut barrier in psoriasis patients and whether this could be a cause or consequence of psoriasis manifestation. The involvement of the microbiota in the pathogenesis of psoriasis has been suggested. |
| Despite significant advances in modern biologic therapy for psoriasis, there are still patients who do not respond adequately to therapy or in whom have been the adverse effects observed. Personalized medicine should be the key to effective treatment. |
| Current scientific research seeks to identify biomarkers that can predict the occurrence of adverse events in psoriasis patients treated with biologics. We hypothesize that the composition and function of the microbiota may influence therapeutic efficacy and the development of adverse events. |
Introduction
Psoriasis is one of the most common chronic, immune-mediated, inflammatory diseases primarily affecting the skin. Despite intensive research, the exact etiology of psoriasis remains unknown. Its prevalence is estimated to be 2–3% worldwide. Clinical manifestations of psoriasis have up to five different variants, with the plaque type psoriasis accounting for more than 80% of all cases [1]. The development of psoriasis has a strong genetic background [2], and numerous external triggers contribute to disease development. These triggers include stress, unhealthy lifestyle, various medications, trauma, infections, and alterations in the composition of the microbiota [3].
Although psoriasis may also have an autoimmune origin, only a limited number of studies support this hypothesis [4]. The pathogenesis of psoriasis is most likely associated with a loss of immune tolerance to one of the three described potential psoriasis autoantigens (i.e., LL37/cathelicidin, ADAMTSL5, or neolipid antigens) [5–9]. With a better understanding of the immune system function and the discovery of new subpopulations of cells, psoriasis came to be perceived more as a systemic multifactorial disease associated mainly with Th17 and Th1 activation [6, 9].
Patients with psoriasis have a higher risk of developing other systemic diseases such as celiac disease or inflammatory bowel disease (IBD) [10, 11]. Moreover, patients with psoriasis are more likely to develop metabolic disorders such as type II diabetes, obesity, or dyslipidemia, and often show increased atherogenesis leading to a higher risk of myocardial infarction or stroke [12]. Up to 20% of patients with psoriasis also develop seronegative arthropathy [13]. Moreover, the physical symptoms of psoriasis often lead to patient stigmatization and further development of mental health comorbidities [14].
The use of targeted therapy, particularly TNFα and IL-17 inhibitors, has become a common practice in moderate-to-severe cases of psoriasis, IBD, and other inflammatory diseases. Although immensely helpful, modern biologic treatment comes with drawbacks, such as paradoxical side effects often manifested on the skin or in the intestine. This attests to the importance of the interconnection between the cutaneous and intestinal environment, referred to as the gut–skin axis.
This review discusses diverse aspects of the pathogenesis of psoriasis in relation to modern biologic treatment that are relevant for the development of paradoxical reactions. Understanding the role of microbiota in paradoxical reactions to targeted therapies will ultimately lead to better therapy management with direct potential for improving the patient's quality of life.
The Role of Barriers in Disease Pathogenesis
The concept of psoriasis as a systemic disease with malfunctioning barriers has emerged in recent years [10, 15]. Psoriasis is not only associated with compromised skin barrier function [16, 17], but it has been linked to an intestinal barrier malfunction as well [18, 19]. Impaired intestinal and skin barrier in patients with psoriasis could be responsible for circulating microbial antigens in the blood [20, 21], which likely potentiate inflammation [20, 22].
Changes in Skin Barrier Function
Apart from the visible manifestation of psoriatic plaques and well-described histological changes, impaired skin barrier functions include lower stratum corneum hydration, significantly higher transepidermal water loss (TEWL), and changes in skin surface pH, temperature, elasticity, or erythema index [23–25]. This goes hand in hand with decreased protection against environmental toxins and pathogens, which may then sensitize the immune system of patients with psoriasis. Moreover, the immune system primed against environmental agents may cross-react with various skin components, thereby disrupting the skin barrier from within. Another factor involved in compromising the skin barrier function in psoriasis might be the leaky gut syndrome common in many other chronic inflammatory diseases [26, 27].
Changes in Gut Barrier Function
There has been an intense effort to investigate what triggers intestinal barrier disruption in patients with psoriasis and to find out whether this is a cause or consequence of psoriasis manifestation. One of the factors contributing to an impaired intestinal barrier could be the systemic increase of cytokines such as IL-17 and TNFα. Inhibitors against these proinflammatory cytokines are commonly used as psoriasis and IBD treatment. However, many cytokines have a dual role. For instance, IL-17 is usually considered a proinflammatory cytokine but under physiological conditions acts at the mucosal interface, where it maintains and protects the epithelial barrier [28, 29].
The dual role of IL-17 is crucial for the optimal strategy treatment based on IL-17 inhibitors. A protective effect of this cytokine was demonstrated in mice deficient in IL-17R, who suffered from reduced neutrophil mobilization, making them more susceptible to infection by Porphyromonas gingivalis, an oral mucosa pathogen [28]. Thus, treatment with IL-17A inhibitors may interfere with the protective function of IL-17A in the intestine and IL-17F but not IL-17A deficiency leads to colitis reduction, as shown in a mouse model of colitis [30]. On the contrary, while protecting against bacterial infections, IL-17 also mediates several tissue pathologies in conditions such as P. aeruginosa mucoid infections [31], whooping cough [32], cystic fibrosis [33], or H. pylori infection in the gastrointestinal tract [34]. Therefore, IL-17RA- or IL-17A-knockout mice are resistant to IL-23-induced psoriasis-like epidermal hyperplasia and inflammation [35, 36].
TNFα increases intestinal permeability via modulation of tight junctions [37], whose important role in the formation of the epidermal and epithelial barrier is well documented. The overexpression of tight junctions in skin psoriatic lesions mainly compensates for the defective barrier. This is supported by the expression of occludin and zonulin (ZO-1) in acanthotic spinous cell layers and of claudin-5 in the granular cell layer [38]. Furthermore, a study by Sikora et al. [18] described elevated serum levels of claudin-3 in patients with psoriasis, which is typically connected to epithelial tightness [18]. Additionally, three times higher levels of serum zonulin were found in patients with psoriasis compared with healthy controls [39]. Although some studies have cautioned against using serum zonulin as a marker of intestinal mucosal barrier function [40], the positive correlation of serum zonulin with serum lipopolysaccharide suggests an impaired gut barrier function and subsequent bacterial translocation into the bloodstream [39].
Altered concentrations of serum markers in patients with psoriasis, such as the concentration of aforementioned claudin-3 or intestinal fatty-acid binding protein (I-FABP), indicate intestinal barrier damage in these patients [19, 41, 42] Interestingly, levels of I-FABP positively correlate with increased values of body mass index (BMI), psoriasis area and severity index (PASI), and neutrophil-to-lymphocyte ratio (NLR) indicating that intestinal integrity is affected by obesity, severity of the disease, and systemic inflammation [41]. There may be also other markers of intestinal barrier damage, nevertheless, when we assessed the ratio of cytokeratin 18 (CK18) and caspase-cleaved CK18 (ccCK18) as a marker of enterocyte damage, we did not find any significant differences between patients with psoriasis and healthy controls [19]. Other markers of intestinal barrier disruption in patients with psoriasis need to be investigated and their effects on disease pathogenesis examined.
The Gut–Skin Axis in the Pathogenesis of Psoriasis
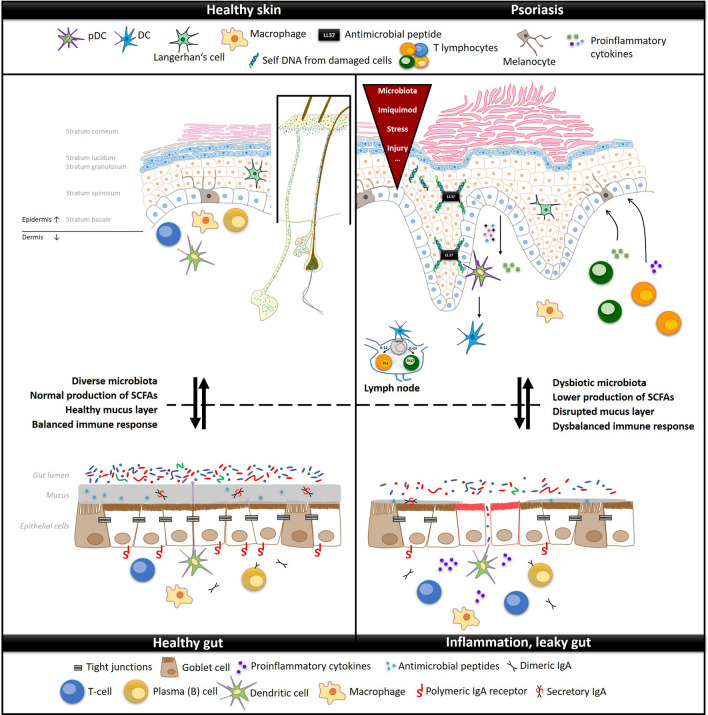
Skin and gut are in intimate contact with the underlying immune system and together orchestrate the host’s response to external antigens. The linkage between the skin and gut is mediated by several mechanisms, and the microbiota plays an irreplaceable role in this process. The interaction between these two organs probably plays an important role in the development of psoriasis as illustrated in Fig. 1.
Fig. 1.
Simplistic representation of skin and gut interconnection in health and during psoriasis. The diseased state is characterized by a disturbed barrier function of the skin and gut. In psoriatic skin, this is usually associated with perturbed composition of microbiota, increased production of antimicrobial peptides, increased transepidermal water loss, and overall inflammation, which manifests as skin redness, thickening, and accelerated cell turnover in psoriatic lesions. In the gut of patients with psoriasis, the diseased state is usually associated with microbiota dysbiosis, lower production of SCFAs, disrupted mucus layer, increased intestinal permeability, or decreased IgA secretion. DC dendritic cells, pDC plasmacytoid dendritic cell, SCFAs short-chain fatty acids
Adaptive immune system in both the skin and the gut is shaped by resident microbes. Commensal microbes interact with a subset of antigen presenting dendritic cells and the effector T-cell populations, controlling the immune response [43, 44]. As an example, the presence of segmented filamentous bacteria (SFB) in Peyer’s patches in the gut and their interactions with the immune system promote immune maturation and have been linked to the Th17 immune response and the development of Th17-mediated diseases [45–47]. However, to fully reach their proinflammatory potential, SFB bacteria might need the presence of other commensals [48]. As a Th17-mediated disease, it is highly possible that psoriasis is influenced by microbiota. We have shown that mice treated with broad-spectrum antibiotics have lower levels of local and systemic Th17 and are more resistant to skin psoriasis-like inflammation induced by imiquimod. In the same model of experimental psoriasis, mice receiving intestinal microbiota from patients with psoriasis have increased IL-17A expression in the gastrointestinal tract, delaying the recovery of psoriatic lesions. Thus, increased expression of IL-17A in the gut leads to systemic IL-17 production in the bloodstream, which in turn affects skin inflammation [49].
Apart from immunological engagement, microbiota produces mammalian-like neurotransmitters and hormones, such as acetylcholine, histamine, serotonin, or corticotropin, as well as various products of dietary compound breakdown [50–52]. Microbial endocrinology lies at the intersection of the hormone production of both host and microbes and gut–skin axis, and may have general applications beyond a particular disease due to the neurochemicals shared between the host and microbiota [53].
The intestinal microbiota also produces metabolites that have the potential to modulate host immunity and alter the balance between tolerance and inflammation by influencing the differentiation of naïve T cells into the Th17 or Treg lineage [54]. Regulatory metabolites are molecules from the degradation of dietary components such as the short-chain fatty acids (SCFAs) propionate, butyrate or acetate. SCFAs are formed primarily by microbial digestion of prebiotics, dietary supplements indigestible to humans that selectively stimulate the growth of beneficial gut microbiota [55]. Recent research suggests that SCFAs such as butyrate inhibit the action of histone deacetylases, whereas inositol-3-phosphate (IP3), produced by microbial digestion of phytate, activates histone deacetylases in mammalian intestinal epithelial cells. As a result, microbial metabolites could balance the mucosal response to various microbial signals [56]. SCFAs also help to establish certain skin microbiota profiles, influencing cutaneous defense mechanisms [57]. Additionally, SCFAs bind to G-protein-coupled receptors, which can subsequently mediate systemic or skin anti-inflammatory responses and thus influence the pathogenesis of psoriasis [58].
Another link connecting the pathogenesis of psoriasis with the gut environment is the fact that patients with psoriasis are often diagnosed with small-intestinal bacterial overgrowth syndrome (SIBO). SIBO is described as microbial dysbiosis in the upper intestine, associated with intestinal discomfort of various kinds. Patients with psoriasis suffering from SIBO have experienced an improvement in the cutaneous symptoms of psoriasis after SIBO treatment with antibiotics and prebiotics that, apart from their main activity, also affect biofilm formation and intestinal motility [59].
The Composition and Function of the Skin and Gut Microbiota in Patients with Psoriasis
A great deal of effort is being made to uncover the relationship between the composition of the microbiota and the pathogenesis of psoriasis. To date, several features of a disturbed composition of the microbiota have been described as contributing to the pathogenic processes of psoriasis. Microbial infections have been linked to exacerbations of psoriasis already in the 1950s [60–62]. Tonsils of patients with psoriasis with a sore throat were more frequently infected by Group C Streptococcus and possess higher frequencies of skin-homing CD4+ and CD8+ T cells compared with infected tonsils of patients without psoriasis [63]. The onset of post-streptococcal psoriasis was attributed to the activation of T cells upon stimulation with keratin determinants that could be mistakenly recognized as streptococcal M proteins due to molecular mimicry [60]. Altered homeostasis of skin-resident Staphylococcus aureus, Streptococcus pyogenes, or Malassezia species could also contribute to the pathogenic processes of psoriasis [20, 64, 65]. Moreover, in patients with psoriasis, bacterial DNA may circulate in the blood and trigger a systemic inflammatory response [20, 21]. Two potential psoriasis cutaneotypes of pathophysiological significance have been described, the first dominated by Proteobacteria and associated with unaffected skin specimens, and the second dominated by Actinobacteria and Firmicutes, associated with lesioned specimens [66].
However, skin microbiome studies are strongly influenced by experimental design, such as different sampling methods (swabs, scrapings, biopsies), the choice of sequencing strategy (in the case of amplicon sequencing, the choice of 16S rRNA region), or DNA extraction strategy [19, 67, 68]. Each method has its strengths and weaknesses, making the study of the microbiome extremely challenging and the results difficult to compare. The choice of the 16S rRNA region for amplicon sequencing is especially important, as this can strongly affect the perceived diversity and composition of the microbial community [69–72]. The V1V3 region better distinguishes among Staphylococcus species [73, 74], and sequencing only the V4 region leads to an underrepresentation of Cutibacterium species [75]. The V3V4 region sufficiently covers skin microbial diversity [70, 76, 77], which was also confirmed by Teng et al. (2018), who compared the V3V4 and V1V3 regions of 16S rRNA [78]. By comparing the V1V2 and V3V4 regions in one dataset of patients with psoriasis, we showed that, although primers for the V1V2 regions were better at classifying Staphylococcus to the species level, sequencing of the V3V4 region yielded greater overall diversity [19].
Fungi on the human skin are an integral part of the whole microbiota community, yet studies concerning the composition of mycobiota in psoriasis are still scarce and inconsistent. The dominant genus in both healthy and psoriatic skin is Malassezia. We observed that psoriatic lesions on oily dorsal skin were dominated by Malassezia restricta, whereas dry elbow skin was dominated by Malassezia sympodialis [19]. In contrast, Paulino et al. (2006) described the opposite, i.e., M. restricta as the predominant species on dry elbow skin, followed by M. sympodialis [79]. We have observed a lower Malassezia globosa to Malassezia restricta ratio in samples from psoriatic lesions on the back compared with healthy skin, which is consistent with the finding of Takemoto et al. [80]. One of the factors potentially confounding results is ethnicity, as M. sympodialis was the predominant species of the skin mycobiota in a Polish patient cohort [81], whereas in Canadian patients it was M. globosa [82] and in Japanese patients M. restricta [83].
Specific associations between clusters of the bacterial genera Corynebacterium and Peptoniphilus have been identified in skin samples from patients with psoriasis [84], and we uncovered a similar pattern when we investigated bacteria–fungi interactions. We have shown that Corynebacterium and Peptoniphilus were positively correlated with Malasseziales in samples from healthy back skin of patients with psoriasis [19]. In psoriatic elbow lesions, we found Corynebacterium and Finegoldia clustering together with Aspergillus [19], which has been previously shown for Corynebacterium/Finegoldia clusters also in an American study by Chang et al. [84]. Additionally, mouse studies suggested that cutaneous fungi may exacerbate experimental skin inflammation by inducing the accumulation of IL17-A-producing Th, Tc, and γδ-T cells in the skin [85]. In our experiments, we manipulated the composition of mycobiota with antifungal agents, but have not observed any change in the severity of imiquimod-induced skin inflammation (IISI) between treated and control mice (unpublished data).
Dysbiosis of microbiota composition can shift the metabolic profile of the entire microbial community and is therefore an important factor underlying the altered immune response associated with a disease. Generally, loss of microbial diversity appears as the most constant finding among studies investigating microbiota composition during an illness [86]. However, dysbiosis does not necessarily mean the overall microbial diversity is reduced [22, 45, 87–90]. As an example, we have shown that patients with Crohn’s disease (CD) have increased skin microbiota richness and evenness and that CD is a stronger driver of skin microbiota than ulcerative colitis (UC). In addition to that, both CD and UC might influence various ecological niches on the skin [91].
Disturbances in the biodiversity and composition of the gut microbiota, even in less abundant species, have been associated with many diseases [27]. In psoriasis, no single pathogen has been identified to demonstrably contribute to psoriasis onset [22, 89, 92], even though the composition of gut microbiota of psoriasis patients differs from that of the healthy population [93]. Psoriatic gut microbiota displays a marked increase in Actinobacteria species and significant overrepresentation of Blautia, Coprococcus, Ruminococcus, or Dorea [89]. Tan et al. demonstrated that patients with psoriasis have a lower abundance of Akkermansia muciniphila, an important producer of SCFAs in the gut [90]. Other human studies reported a lack of A. muciniphila in other diseases, such as allergic asthma or ulcerative colitis [94, 95]. Patients with concomitant IBD and psoriasis had lower counts of F. prausnitzii in the gut than patients with psoriasis or IBD only [96]. F. prausnitzii could contribute to intestinal integrity via its production of anti-inflammatory peptides responsible for inhibition of the NF-κB pathway in intestinal epithelial cells [96].
Animal experimental models, although often expensive and difficult to carry out, offer great promise and could help us gain insights into different aspects of both balanced and dysbiotic human microbiota [97]. Despite the fact that no particular gut bacteria have been found to trigger systemic psoriatic inflammation or to worsen skin inflammation in human trials, several mouse studies were conducted to identify such taxa. Okada et al. (2020) showed that Staphylococcus aureus and Streptococcus danieliae orally administered to antibiotic-treated mice led to exacerbated skin lesions and elevated levels of TNFα, IL-17, and IL-22 cytokine in imiquimod induced model of skin inflammation (IISI) [98]. This is in line with our observation that there is a correlation between IISI improvement and decreased skin abundance of staphylococci and streptococci species after antibiotic treatment with metronidazole [99]. The antimicrobial effect of metronidazole and its subsequent effect on disease pathogenesis is further supported by studies showing the efficacy of metronidazole in alleviating experimental uveitis via changing the microbiota composition [100] or in improving the SIBO syndrome, which is primarily caused by small intestinal dysbiosis [101, 102].
We have shown that mice treated with a broad-spectrum antibiotic mix and germ-free mice had lower skin inflammation as manifested by reduced frequencies of γδ-T cells and Th17 cells in spleen and lymph nodes, in comparison with conventional mice treated with water [103]. Interestingly, even though the antibiotic administration did not change the bacterial load, it changed the bacterial community structure [99, 103]. In another study, neonatal vancomycin and polymyxin B treatment led to permanent compositional changes in cutaneous and intestinal microbiota in adult mice, which resulted in higher susceptibility to IISI experimental psoriasis [104]. A mouse study focused on wound healing revealed altered microbial density and composition on the skin after oral vancomycin treatment, specifically a reduced proportion of staphylococci-related sequences, as well as a lower IL-17 expression in the wounded skin [105].
Many studies have reported a beneficial effect of lactobacilli on cutaneous health [106–108], as well as their beneficial role in improving the intestinal barrier and decreasing sensitization to allergens [109]. In a mouse model of experimental colitis, the administration of Lactobacillus casei DN-114 001 lysate reduced the severity of colitis by increasing the numbers of Treg cells and decreasing the production of proinflammatory cytokines, while also changing the gut microbiota composition [110]. Mice monocolonized with Lactobacillus plantarum WCFS1 or with segmented filamentous bacteria (SFB) did not differ in IISI severity from germ-free mice [99].
Conducting microbiome research in humans has its advantages and disadvantages. Apart from the costly experiments, researchers have to deal with less predictable sample collection, generation, and analysis of data. Moreover, human microbiome studies are usually correlational and do not address causality. Combining mouse and human investigation approaches could give us a more comprehensive indication of the potential disease causality. From this point of view, the combined evidence supports the hypothesis that a specific composition of the gut microbiota could enhance the Th17 response and thus contribute to psoriasis exacerbation [99, 103].
Current Approaches to the Treatment of Psoriasis
A wide range of different therapies is currently used for psoriasis. The choice of treatment course depends on several factors including the severity of the disease, the concurrent presence of psoriatic arthritis, patient age, and the impact the disease has on the patient's life. First-line therapies include topical treatments such as emollients, topical corticosteroids, vitamin D3 derivatives, phototherapy, or nonsteroidal anti-inflammatory drugs. Mild to moderately severe forms of psoriasis require systemic treatments such as methotrexate or cyclosporine A. For more severe forms of psoriasis, targeted biological treatment is becoming increasingly common [111].
Biologics targeting TNFα (tumor necrosis factor alpha) are now often referred to as first-generation biologics. This group includes certolizumab, etanercept [112], infliximab [113], and adalimumab. Each of these TNFα inhibitors has a different structure, so the mechanisms of their immunological action differ. Therefore, each of them has different efficacy and causes different adverse events. Although anti-TNFα therapy is widely approved for the treatment of both psoriasis and IBD, 30–40% of patients do not respond or lose response during the therapy [114, 115]. Based on data from real-world psoriasis registries, the survival rate of infliximab or etanercept therapy is about 50% and 70% after 2 years of therapy, respectively [116].
Second-generation biologics provide a more targeted therapy, focused on blocking the key cytokines in psoriasis, i.e., IL-23 and IL-17. These biologics include the IL-12/-23 inhibitor ustekinumab [117], and IL-17 inhibitors secukinumab and brodalumab. Anti-IL-17 drugs are currently biologics with the fastest onset of action and are effective in many immune-mediated inflammatory diseases, including psoriatic arthritis [1, 117, 118].
The latest psoriasis therapy works by blocking the regulatory cytokine IL-23. Three drugs blocking the IL-23 cytokine, specifically its p19 subunit, are currently available for psoriasis treatment: guselkumab, tildrakizumab, and risankizumab. IL-23 antagonists achieve very effective therapy of psoriasis with a quick onset of action and the highest long-term efficacy of all current biological drugs. Moreover, their safety profile is excellent, with only minor side effects such as nasopharyngitis, upper respiratory tract infection, or headache [117–119]. Anti-IL-23 therapy also has a great potential for the treatment of psoriatic arthritis and other inflammatory diseases such as IBD, but clinical trials for these diseases are still ongoing [1, 117].
Therapeutic Manipulation of the Microbiota of Patients with Psoriasis
The current body of literature suggests that biologic treatment may indeed affect the composition of the microbiota [93, 120] and that some patients may not respond to the treatment due to possible differences in microbiota composition [93, 121]. Altered microbiota composition changes microbiota interactions, which in turn influence the immune system and modify the body homeostasis. Yeh et al. [93] described changes in the gut microbiota composition of psoriasis patients after administration of IL-17 (secukinumab) but not IL12/23 (ustekinumab) inhibitors [93]. Six months after the treatment, patients receiving IL-17 inhibitors had shifted microbiota composition so that it resembled the microbiota composition of patients with IBD. In particular, these patients showed significantly increased abundance of Proteobacteria and decreased abundance of Bacteroidota and Firmicutes, which was not observed in patients treated with IL12/23 inhibitors. Interestingly, Coprococcus, a commensal bacterium decreased in patients with IBD, increased after treatment with IL12/23 inhibitors in patients with psoriasis. Moreover, the study described baseline differences between therapy responders and nonresponders in gut microbiota composition. This observation may be taken into account in future clinical practice when choosing the proper treatment for psoriatic patients that are genetically predisposed to the development of IBD [93].
A study dealing with the changes in skin microbiota after ustekinumab treatment showed that microbial communities diverged between lesional and nonlesional skin, and the distinction between body sites increased [120]. The skin microbiota of patients with psoriasis exhibited greater heterogeneity within skin lesions than in nonlesional skin before treatment initiation, and microbial variance increased as treatment progressed. The microbiota colonizing recurrent lesions did not overlap with microbiota residing there prior to the treatment, suggesting that colonization pattern varied between initial and recurrent psoriatic lesions. There is only one study dealing with mycobiota composition after the use of biologics, which shows that the psoriatic skin mycobiome composition is retained even after systemic anti-TNFα or anti-IL17 treatment [122].
Microbiota as a Therapeutic Tool in the Treatment of Psoriasis
The complex role the microbiota plays in shaping the immune system is increasingly being recognized. Its potential health impacts have motivated more practical applications in recent years. One approach is the intervention with either prebiotics, probiotics, or other commensal microbes that could be useful in restoring a protective microbiota composition and could thus serve as preventive medical therapy. Oral administration of probiotics together with beneficial prebiotics (i.e., in the form of synbiotics) could moderate the course of some dermatoses [123–125].
Despite the relative paucity of data regarding the therapeutic effect of microbiota in psoriasis, it has been shown that oral administration of Lactobacillus brevis SBC8803 for 12 weeks decreased TEWL and increased corneal hydration in human volunteers [126]. A phase 1b clinical trial, where patients with psoriasis were administered a high dose of EDP1815, a Prevotella histicola formulation, shows highly promising results [127]. Groeger et al. showed that daily administration of viable Bifidobacterium infantis 35264 for 6–8 weeks resulted in a significant decrease in plasma levels of C-reactive protein and TNFα, but not IL-6 concentration in patients with psoriasis [128]. Navarro-Lopez et al. evaluated the effect of a mixture of three probiotic strains in a 1:1:1 ratio, i.e., Bifidobacterium longum CECT 7347, B. lactis CECT 8145, and Lactobacillus rhamnosus CECT 8361, with a total of 1 × 109 CFU per capsule once a day for 12 weeks. This probiotic mixture was given as an adjunctive treatment along with topical steroids in patients with plaque psoriasis. The results showed a significant reduction in PASI score after 12 weeks in 66.7% of patients receiving probiotics and 41.9% of patients receiving placebo. Interestingly, after 6 months, patients who had received probiotics had a lower risk of psoriasis relapse compared with the placebo group [129].
Fecal microbiota transplantation (FMT) represents another widely tested approach to restoring balance to dysbiotic microbiota of the gut. Although it is officially recognized only for specific indications and its use is therefore mainly experimental, FMT offers a way of directly changing the gut microbiota to gain a therapeutic benefit via normalizing gut microbiota. To date, there is a limited number of studies using FMT for the treatment of psoriasis as the main diagnosis [130, 131]. The only clinical study shows that FMT is safe in psoriatic arthritis, but its efficacy with concomitant methotrexate treatment has not been established [130]. Taken together, these data underscore the importance of the gut–skin axis in the pathophysiology of psoriasis and imply the potential use of oral probiotics in the treatment of psoriasis.
Manipulation of the skin microbiota composition is also starting to gain attraction. The skin microbiota can be changed in several ways, i.e., skin microbiota transplantation, skin bacteriotherapy, or prebiotic stimulation. Skin microbiota transplantation (SMT) is an emerging concept in the treatment of skin diseases. However, it is technically challenging, as the skin microbiota composition is shaped by the distinct properties of the different skin microenvironments. Moreover, the dynamics of the skin microbiota profile differ between dry, moist, and sebaceous skin sites, with the latter two being considered quite stable over time [132]. SMT involves the transfer of the skin microbiota from a healthy donor to a disinfected skin area of a diseased person. Although the microbiota is transferred to its natural environment, this method is not widely applicable yet. In contrast to FMT, where the transmitted material undergoes a thorough control, in SMT, only a limited amount of material is transferred; hence, it is not possible to thoroughly investigate its composition without the need for cultivation. Therefore, potentially pathogenic microbes or viruses can be co-transferred and unintentionally harm the recipient [133]. Up till now, there is no established long-term treatment of psoriasis with bacterial substitution.
The Clinical Link between Psoriasis and IBD: Since Both Diseases Could Arise as an Adverse Effect of Biologic Treatment
IBD is a chronic recurrent inflammation of the gastrointestinal tract with two main forms: Crohn’s disease (CD) and ulcerative colitis (UC). IBD is a common comorbidity in patients with psoriasis and the pathogenesis of both diseases shows a similar pattern. In addition, IBD and psoriasis occasionally occur with each other as an adverse effect of the treatment of the respective disease. However, the relationship between psoriasis and IBD is still unclear. The prevalence of IBD in patients with psoriasis is about 1–2%, which is four times higher than the prevalence of IBD in the general population [134]. Conversely, the prevalence of psoriasis in IBD is 3.6% in CD and 2.8% in UC [135]. Both diseases are associated with increased intestinal permeability, an aberrant immune response against the microbiota, and activation of the Th17 pathway [27, 136, 137]. Moreover, according to genome-wide association studies (GWAS), IBD and psoriasis also share a pattern of susceptibility loci associated with the Th17 pathway, such as IL23R, IL12B, IL23A, TRAF3IP2, or innate immune responses [2, 138, 139]. Interestingly, psoriasis–CD patients are most often first diagnosed with CD, while psoriasis–UC patients are just as likely to be first diagnosed with UC as with psoriasis. Eppinga et al. 2017 hypothesize that the lower age of onset of CD and psoriasis–CD patients, the more closely are psoriasis and CD associated with genetic factors [140]. This is in contrast to the incidence of UC, where this finding has not been demonstrated [141]. Furthermore, patients with psoriasis often have features of latent IBD that could be masked due to the anti-inflammatory nature of psoriasis treatment [141]. Latent IBD features, such as elevated levels of anti-Saccharomyces cerevisiae antibodies (ASCA), may precede the manifestation of IBD in patients with psoriasis. Juzlova et al. 2016 described that positivity for ASCA preceded clinical manifestation by approximately 38 months in 10 out of 32 psoriatic patients with Crohn’s disease (31%) [142]. The association between psoriasis and IBD has been reinforced by the finding that one can appear as a paradoxical adverse event of the treatment for the other, as discussed later [143–145].
Paradoxical Adverse Events of Anti-TNFα Therapy
Adverse events including cutaneous and mucosal lesions have been described during anti-TNFα treatment. These immune-mediated inflammatory reactions represent a paradoxical event considering that biologics, especially anti-TNFα therapy, are commonly used in the management of immune-mediated inflammatory diseases such as severe psoriasis or IBD [114, 146–148]. Paradoxical events can be of diverse nature. For example, they can manifest as exacerbated skin lesions in patients with psoriasis, psoriasiform dermatitis in patients with IBD, or a new onset of CD or UC in patients with psoriasis [114, 115, 148]. In particular, patients with psoriasis treated with etanercept have an increased risk of developing CD and UC [149]. The reason why etanercept as opposed to other anti-TNF agents increases the risk of developing IBD in patients with psoriasis could lie in the different pattern of binding to TNF molecules. As mentioned before, infliximab binds specifically to all three binding sites of TNFα, whereas etanercept binds to both TNFα (to only two of the three binding sites) and TNFβ [112].
Paradoxical skin reactions affect up to 30% of patients with different diseases treated with anti-TNFα drugs, including patients treated for psoriasis, IBD, or rheumatoid arthritis [114, 115, 148]. Although paradoxical skin reactions typically do not require therapy cessation, 34% of the reactions are severe enough to warrant discontinuation of anti-TNFα therapy. The onset of lesion development can range widely from a few days to 4 years following the initiation of treatment [148]. The lesions occur most frequently in women and patients with a personal or familial history of inflammatory skin disease, and the progression of the cutaneous lesions does not correlate with intestinal disease activity [150, 151]. The formation of paradoxical skin lesions is probably triggered by increased reactivity of the immune system, as simultaneous administration of other immunosuppressants (methotrexate, corticosteroids) with anti-TNFα therapy decreases the occurrence of these paradoxical events [152]. Unfortunately, skin adverse events may significantly impair the quality of life in some patients despite the improvement of other symptoms [153, 154].
Around 20% of anti-TNFα-treated patients with IBD develop skin adverse events (SkAE) [155]. Classification of these manifestations is not uniform, but psoriasiform and eczematiform lesions are the most common manifestations associated with anti-TNFα therapies in patients with IBD [151, 156]. Even though the psoriasiform or eczematiform lesions appear to be morphologically and histologically similar to characteristic classical psoriasis or eczema manifestation, the underlying pathogenetic mechanism seems to be different [157]. Other paradoxical reactions, such as lichen planus-like eruptions, lichen planopilaris, or scleroderma-like changes are much less common [158–160].
While the etiology of paradoxical reactions remains unclear, several possible mechanisms may be involved, such as the deposition of immune complexes or shifts in cytokine production [156, 161]. The lack of proof about autoimmune origin of psoriasis does not exclude the possibility of the participation of autoimmune mechanisms in its pathogenesis, as well as in the development of SkAE after anti-TNFα treatment. This fact is supported by frequently occurring associations of psoriasis with various autoimmune diseases, including arthritis, celiac disease, thyroiditis, alopecia areata, and others [162]. These autoimmune diseases are not always accompanied by symptoms; hence, they are called subclinical forms of diseases. Genetic and epigenetic mechanisms participating in subclinical forms of diseases could be the reason for involvement in induction of paradoxical reactions to treatments targeting TNFα and IL-17 cytokines. .
Disruption in cytokine balance following TNFα inhibition seems to be one of the important immunological mechanisms in the development of paradoxical reactions. TNFα inhibition can lead to increased production of unopposed interferon-α (IFN-α) by plasmacytoid dendritic cells, which can in turn promote T-cell homing to epidermis and cause inflammation [156, 161]. In addition, serum levels of IL-17A and IL-23 are higher in patients with IBD who developed skin lesions under anti-TNFα therapy compared with patients without such lesions [163]. The skin lesions are infiltrated by IL-17A/IL-22-secreting Th17 cells, IFN-γ-secreting Th1 lymphocytes, and IFN-α-secreting cells [164]. Another mechanism significantly contributing to the development of psoriasis-like skin lesions may be dysbiosis, as changes in the gut or skin microbiota composition and function represent key steps in the pathogenesis of IBD or psoriasis [161, 165–167]. Shifts in the skin microbiome are also associated with flares of atopic eczema [168]. Therefore, by focusing on the role of the gut–skin axis in anti-TNFα therapies, we may be able to uncover the core pathogenetic mechanisms of these serious paradoxical adverse events and help to introduce a better-focused therapy in the future.
Anti-IL-17 Therapy as a Possible Trigger of IBD in Patients with Psoriasis
In recent years, IL-17 inhibitors (brodalumab, ixekizumab, secukinumab) have been established as effective and safe therapies for the treatment of severe psoriasis and psoriatic arthritis with a very rapid onset of action [117, 118, 169]. Although IL-17 is a key contributor to tissue repair on epithelial barriers and treatment with IL-17 inhibitors has undeniably pronounced positive effects, some side effects of this treatment have also been described [29, 117]. For example, the dysregulation of IL-17-producing cells, e.g., the blockade of IL-17, negatively affects the integrity of the intestinal barrier and exacerbates intestinal inflammation. This often increases the risk of fungal infections, especially upper respiratory tract infections and Candida infections [170]. Likewise, genetic defects in IL-17RA or IL-17F predispose the patients to chronic mucocutaneous candidiasis manifested as skin, nails, and mucosal infections [171]. Even though it does not influence the immune response, massive colonization with Candida is common in patients with psoriasis. For this reason, patients should be screened for candidiasis before and after treatment with IL-17 inhibitors [172].
Psoriasis and IBD share at least two common inflammatory pathways, Th1 and Th17. Biologics against TNFα or IL-12/IL-23 have been successfully used in the management of both diseases. Nevertheless, treatment with IL-17 inhibitors in IBD did not pass clinical trials [173, 174]. Notably, Crohn’s disease clinical trials with secukinumab and brodalumab failed to demonstrate efficacy and the condition worsened in some patients. An analysis of adverse events from 2017, which integrated data from seven randomized trials with ixekizumab in adult patients with psoriasis, showed that IBD cases were uncommon and occurred in < 1% of the patients [175], and new case studies still report manifestation of IBD in some patients [176, 177]. However, a potential causal relationship between IL-17 inhibition and IBD onset in patients with psoriasis has not yet been established. For now, it is recommended that the use of IL-17 inhibitors be avoided in patients with a personal history of or active IBD [117].
Most cases of CD incidence in patients on anti-IL-17 therapy described to date were patients > 40 years, which corresponds with the average age of patients with psoriasis. However, CD onset is most common in people aged 15–29 years in Europe [178]. Adolescents and young adults in particular have an increased risk of developing IBD during anti-IL-17 therapy or even many months after treatment cessation. It is crucial to clarify the role of IL-17 inhibitors in the pathogenesis of IBD, especially given that these new drugs can also be administered to pediatric patients with psoriasis. The current practice is merely to avoid IL-17 inhibitors in patients with active or past IBD, with no information about the potential risk of developing IBD in asymptomatic patients. There are no specific guidelines for physicians to facilitate therapeutic decisions, but caution should be exercised when prescribing IL-17 inhibitors to patients with psoriasis, especially in those with a family history of IBD.
Dysregulation of IL-17 production or function, e.g., by anti-IL-17 therapy, affects various microbial phyla in the gut. This, in turn, can promote intestinal dysbiosis and thus disrupt the integrity of mucosal barriers and exacerbate inflammation [37]. Treatment with IL-17 inhibitors in patients with psoriasis leads to an increase in Proteobacteria and a decrease in Ruminococcus or Firmicutes in the gut, the reduction of which correlates negatively with the amount of SCFAs produced [93]. Similar changes in the profile of the gut microbiota were described in patients with IBD. Moreover, one of the SCFA producers, Akkermansia muciniphila, was found to be significantly reduced in the gut microbiota of both psoriasis and IBD patients [90, 179, 180]. Although Akkermansia muciniphila degrades mucin, it is considered a beneficial bacterium that maintains intestinal integrity primarily through the production of SCFAs, some of which are essential for intestinal cell nutrition. New human studies investigating the cumulative incidence of risk factors of IL-17 inhibitors, as well as studies in mice examining the role of IL-17 in the pathogenesis of IBD or psoriasis are needed.
How to Choose the Most Effective Therapy for Patients with Psoriasis
Currently, physicians do not have predictive markers that would help them to choose the most effective and safest therapy for each individual patient. The choice of the treatment for the patient is thus mainly based on extensive data from clinical studies and psoriatic registries. For example, in patients with latent tuberculosis, the physicians rather choose therapies other than anti-TNF, similarly to how they avoid therapy with IL-17 blockers in patients who have been diagnosed with inflammatory bowel disease as these blockers can lead to IBD worsening or re-exacerbation [181]. Similarly to the selection of the most effective therapy, there are currently no predictive markers of paradoxical responses to biologic therapies to prevent their development. During the treatment itself, it is necessary to educate the patient about possible side effects and monitor them during regular check-ups.
These days we are talking about personalized psoriasis treatment when decisions are made on the following basis: (i) the patient’s state of health (gender, age, comorbidities, impact on quality of life, etc.), (ii) disease factors (type of psoriasis, extent/activity of psoriasis, involvement of difficult-to-treat locations, etc.), and (iii) knowledge of available therapies (safety, efficacy, drug survival, tolerability, effect on comorbidities). This approach significantly increases the chance of choosing the right treatment for the patient, but it is not personalized medicine in the true sense of the word. However, a standardized panel of biomarkers that merges genotype and phenotype of each patient would be more precise. Even though biomarkers will most likely play a very important role in the choice of treatment in the future, current knowledge is still very limited and that is why it is not reflected in clinical guidelines yet [182].
Conclusion
Patients with psoriasis suffer from impaired gut barrier integrity and are therefore at high risk for comorbidities such as IBD that disrupt their already delicate health balance. Despite significant advances in modern biological therapy for psoriasis, there remain patients who do not respond sufficiently to therapy or in whom the paradoxical adverse events have been observed. Therefore, it is crucial for clinicians to be able to target patients who are most likely to respond to the treatment without developing paradoxical adverse events, yet there are no specific guidelines to follow. To date, clinical trials of these biologics adopt the “one drug for all” strategy, although the therapy may be beneficial only in a subset of patients. Personalized medicine should be the key to treatment efficacy, since the mechanisms underlying primary or secondary nonresponse are multifactorial; nevertheless, current approaches are not yet a personalized medicine in the true sense of word. Factors underlying nonresponse to the treatment include the genetic predisposition and actual clinical status of the patient as well as previous medication and treatment. By studying how the gut and skin microbiota interact with the immune system in the setting of biological therapy, we may uncover new pathogenetic mechanisms of paradoxical skin reactions in patients treated with anti-TNF or anti-IL-17 biologics. The knowledge of pathogenic mechanisms may not only yield new biomarkers that will predict the adverse effects but also potential therapeutic targets, improving future care for patients with chronic inflammatory diseases treated with these drugs.
Acknowledgements
Funding
This research and the journal’s Rapid Service Fee were funded by the Ministry of Health of the Czech Republic, grant number NV18-09-00493, NU22J-05-00056, NU22-01-00077, and Institutional Research Concept (RVO: 61388971).
Author Contributions
Zuzana Jirásková Zákostelská, Zuzana Reiss, Helena Tlaskalová-Hogenová and Filip Rob had the original idea for the article. The first draft of the manuscript was written by Zuzana Jirásková Zákostelská and Zuzana Reiss and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Disclosures
Filip Rob has received honoraria as a speaker and/or consultant for AbbVie, Celgene, Eli Lilly, Janssen, Leo Pharma, MSD, Novartis, Pfizer, Sanofi Genzyme, UCB. Zuzana Jiraskova Zakostelska, Zuzana Reiss and Helena Tlaskalova-Hogenova declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Data availability
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
References
- 1.Armstrong AW, Read C. Pathophysiology, clinical presentation, and treatment of psoriasis: a review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 2.Tsoi LC, Stuart PE, Tian C, Gudjonsson JE, Das S, Zawistowski M, et al. Large scale meta-analysis characterizes genetic architecture for common psoriasis associated variants. Nat Commun. 2017;8:15382. doi: 10.1038/ncomms15382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Roszkiewicz M, Dopytalska K, Szymanska E, Jakimiuk A, Walecka I. Environmental risk factors and epigenetic alternations in psoriasis. Ann Agric Environ Med. 2020;27(3):335–342. doi: 10.26444/aaem/112107. [DOI] [PubMed] [Google Scholar]
- 4.Fry L, Baker BS, Powles AV, Engstrand L. Psoriasis is not an autoimmune disease? Exp Dermatol. 2015;24(4):241–244. doi: 10.1111/exd.12572. [DOI] [PubMed] [Google Scholar]
- 5.Arakawa A, Siewert K, Stohr J, Besgen P, Kim SM, Ruhl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med. 2015;212(13):2203–2212. doi: 10.1084/jem.20151093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boutet MA, Nerviani A, Gallo Afflitto G, Pitzalis C. Role of the IL-23/IL-17 axis in psoriasis and psoriatic arthritis: the clinical importance of its divergence in skin and joints. Int J Mol Sci. 2018;19(2):530. doi: 10.3390/ijms19020530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung KL, Jarrett R, Subramaniam S, Salimi M, Gutowska-Owsiak D, Chen YL, et al. Psoriatic T cells recognize neolipid antigens generated by mast cell phospholipase delivered by exosomes and presented by CD1a. J Exp Med. 2016;213(11):2399–2412. doi: 10.1084/jem.20160258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun. 2014;5:5621. doi: 10.1038/ncomms6621. [DOI] [PubMed] [Google Scholar]
- 9.Nestle FO, Kaplan DH, Barker J. Psoriasis. N Engl J Med. 2009;361(5):496–509. doi: 10.1056/NEJMra0804595. [DOI] [PubMed] [Google Scholar]
- 10.Singh S, Young P, Armstrong AW. An update on psoriasis and metabolic syndrome: A meta-analysis of observational studies. PLoS ONE. 2017;12(7):e0181039. doi: 10.1371/journal.pone.0181039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sundarrajan S, Arumugam M. Comorbidities of psoriasis—exploring the links by network approach. PLoS ONE. 2016;11(3):e0149175. doi: 10.1371/journal.pone.0149175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.rob f hj. Komorbidity psoriázy a jejich management. Remedia 2019;1/2019(29):56–60.
- 13.Alinaghi F, Calov M, Kristensen LE, Gladman DD, Coates LC, Jullien D, et al. Prevalence of psoriatic arthritis in patients with psoriasis: a systematic review and meta-analysis of observational and clinical studies. J Am Acad Dermatol. 2019;80(1):251–65 e19. doi: 10.1016/j.jaad.2018.06.027. [DOI] [PubMed] [Google Scholar]
- 14.Wu JJ, Feldman SR, Koo J, Marangell LB. Epidemiology of mental health comorbidity in psoriasis. J Dermatolog Treat. 2018;29(5):487–495. doi: 10.1080/09546634.2017.1395800. [DOI] [PubMed] [Google Scholar]
- 15.Korman NJ. Management of psoriasis as a systemic disease: what is the evidence? Br J Dermatol. 2020;182(4):840–848. doi: 10.1111/bjd.18245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guttman-Yassky E, Nograles KE, Krueger JG. Contrasting pathogenesis of atopic dermatitis and psoriasis—part I: clinical and pathologic concepts. J Allergy Clin Immunol. 2011;127(5):1110–1118. doi: 10.1016/j.jaci.2011.01.053. [DOI] [PubMed] [Google Scholar]
- 17.Montero-Vilchez T, Cuenca-Barrales C, Martinez-Lopez A, Molina-Leyva A, Arias-Santiago S. Skin adverse events related to personal protective equipment: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2021;35(10):1994–2006. doi: 10.1111/jdv.17436. [DOI] [PubMed] [Google Scholar]
- 18.Sikora M, Chrabaszcz M, Maciejewski C, Zaremba M, Waskiel A, Olszewska M, et al. Intestinal barrier integrity in patients with plaque psoriasis. J Dermatol. 2018;45(12):1468–1470. doi: 10.1111/1346-8138.14647. [DOI] [PubMed] [Google Scholar]
- 19.Stehlikova Z, Kostovcik M, Kostovcikova K, Kverka M, Juzlova K, Rob F, et al. Dysbiosis of skin microbiota in psoriatic patients: co-occurrence of fungal and bacterial communities. Front Microbiol. 2019;10:438. doi: 10.3389/fmicb.2019.00438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Munz OH, Sela S, Baker BS, Griffiths CE, Powles AV, Fry L. Evidence for the presence of bacteria in the blood of psoriasis patients. Arch Dermatol Res. 2010;302(7):495–498. doi: 10.1007/s00403-010-1065-0. [DOI] [PubMed] [Google Scholar]
- 21.Ramirez-Bosca A, Navarro-Lopez V, Martinez-Andres A, Such J, Frances R, Horga de la Parte J, et al. Identification of bacterial DNA in the peripheral blood of patients with active psoriasis. JAMA Dermatol. 2015;151(6):670–1. [DOI] [PubMed]
- 22.Codoner FM, Ramirez-Bosca A, Climent E, Carrion-Gutierrez M, Guerrero M, Perez-Orquin JM, et al. Gut microbial composition in patients with psoriasis. Sci Rep. 2018;8(1):3812. doi: 10.1038/s41598-018-22125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sano S, Chan KS, Carbajal S, Clifford J, Peavey M, Kiguchi K, et al. Stat3 links activated keratinocytes and immunocytes required for development of psoriasis in a novel transgenic mouse model. Nat Med. 2005;11(1):43–49. doi: 10.1038/nm1162. [DOI] [PubMed] [Google Scholar]
- 24.Maroto-Morales D, Montero-Vilchez T, Arias-Santiago S. Study of skin barrier function in psoriasis: The impact of emollients. Life (Basel). 2021;11(7). [DOI] [PMC free article] [PubMed]
- 25.Montero-Vilchez T, Segura-Fernandez-Nogueras MV, Perez-Rodriguez I, Soler-Gongora M, Martinez-Lopez A, Fernandez-Gonzalez A, et al. Skin barrier function in psoriasis and atopic dermatitis: transepidermal water loss and temperature as useful tools to assess disease severity. J Clin Med. 2021;10(2). [DOI] [PMC free article] [PubMed]
- 26.Fasano A. All disease begins in the (leaky) gut: role of zonulin-mediated gut permeability in the pathogenesis of some chronic inflammatory diseases. F1000Res. 2020;9:69. [DOI] [PMC free article] [PubMed]
- 27.Tlaskalova-Hogenova H, Stepankova R, Kozakova H, Hudcovic T, Vannucci L, Tuckova L, et al. The role of gut microbiota (commensal bacteria) and the mucosal barrier in the pathogenesis of inflammatory and autoimmune diseases and cancer: contribution of germ-free and gnotobiotic animal models of human diseases. Cell Mol Immunol. 2011;8(2):110–120. doi: 10.1038/cmi.2010.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yu JJ, Ruddy MJ, Conti HR, Boonanantanasarn K, Gaffen SL. The interleukin-17 receptor plays a gender-dependent role in host protection against Porphyromonas gingivalis-induced periodontal bone loss. Infect Immun. 2008;76(9):4206–4213. doi: 10.1128/IAI.01209-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.O'Connor W, Jr, Kamanaka M, Booth CJ, Town T, Nakae S, Iwakura Y, et al. A protective function for interleukin 17A in T cell-mediated intestinal inflammation. Nat Immunol. 2009;10(6):603–609. doi: 10.1038/ni.1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang C, Kakuta S, Shimizu K, Kadoki M, Kamiya T, Shimazu T, et al. Suppression of IL-17F, but not of IL-17A, provides protection against colitis by inducing Treg cells through modification of the intestinal microbiota. Nat Immunol. 2018;19(7):755–765. doi: 10.1038/s41590-018-0134-y. [DOI] [PubMed] [Google Scholar]
- 31.Dubin PJ, Kolls JK. IL-23 mediates inflammatory responses to mucoid Pseudomonas aeruginosa lung infection in mice. Am J Physiol Lung Cell Mol Physiol. 2007;292(2):L519–L528. doi: 10.1152/ajplung.00312.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dunne A, Ross PJ, Pospisilova E, Masin J, Meaney A, Sutton CE, et al. Inflammasome activation by adenylate cyclase toxin directs Th17 responses and protection against Bordetella pertussis. J Immunol. 2010;185(3):1711–1719. doi: 10.4049/jimmunol.1000105. [DOI] [PubMed] [Google Scholar]
- 33.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, et al. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nat Med. 2008;14(3):275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caruso R, Fina D, Paoluzi OA, Del Vecchio BG, Stolfi C, Rizzo A, et al. IL-23-mediated regulation of IL-17 production in Helicobacter pylori-infected gastric mucosa. Eur J Immunol. 2008;38(2):470–478. doi: 10.1002/eji.200737635. [DOI] [PubMed] [Google Scholar]
- 35.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, et al. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35(4):596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizzo HL, Kagami S, Phillips KG, Kurtz SE, Jacques SL, Blauvelt A. IL-23-mediated psoriasis-like epidermal hyperplasia is dependent on IL-17A. J Immunol. 2011;186(3):1495–1502. doi: 10.4049/jimmunol.1001001. [DOI] [PubMed] [Google Scholar]
- 37.Watson AJ, Duckworth CA, Guan Y, Montrose MH. Mechanisms of epithelial cell shedding in the mammalian intestine and maintenance of barrier function. Ann N Y Acad Sci. 2009;1165:135–142. doi: 10.1111/j.1749-6632.2009.04027.x. [DOI] [PubMed] [Google Scholar]
- 38.Peltonen S, Riehokainen J, Pummi K, Peltonen J. Tight junction components occludin, ZO-1, and claudin-1, -4 and -5 in active and healing psoriasis. Br J Dermatol. 2007;156(3):466–472. doi: 10.1111/j.1365-2133.2006.07642.x. [DOI] [PubMed] [Google Scholar]
- 39.Richetta AG, Grassi S, Moliterni E, Chello C, Calvieri C, Carnevale R, et al. Increased intestinal barrier permeability in patients with moderate to severe plaque-type psoriasis. J Dermatol. 2020;47(10):e366–e368. doi: 10.1111/1346-8138.15361. [DOI] [PubMed] [Google Scholar]
- 40.Ajamian M, Steer D, Rosella G, Gibson PR. Serum zonulin as a marker of intestinal mucosal barrier function: May not be what it seems. PLoS ONE. 2019;14(1):e0210728. doi: 10.1371/journal.pone.0210728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sikora M, Stec A, Chrabaszcz M, Waskiel-Burnat A, Zaremba M, Olszewska M, et al. Intestinal fatty acid binding protein, a biomarker of intestinal barrier, is associated with severity of psoriasis. J Clin Med. 2019;8(7):1021. doi: 10.3390/jcm8071021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sikora M, Chrabaszcz M, Waskiel-Burnat A, Rakowska A, Olszewska M, Rudnicka L. Claudin-3 - a new intestinal integrity marker in patients with psoriasis: association with disease severity. J Eur Acad Dermatol Venereol. 2019;33(10):1907–1912. doi: 10.1111/jdv.15700. [DOI] [PubMed] [Google Scholar]
- 43.Linehan JL, Harrison OJ, Han SJ, Byrd AL, Vujkovic-Cvijin I, Villarino AV, et al. Non-classical immunity controls microbiota impact on skin immunity and tissue repair. Cell. 2018;172(4):784–96 e18. doi: 10.1016/j.cell.2017.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Naik S, Bouladoux N, Linehan JL, Han SJ, Harrison OJ, Wilhelm C, et al. Commensal-dendritic-cell interaction specifies a unique protective skin immune signature. Nature. 2015;520(7545):104–108. doi: 10.1038/nature14052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen B, Chen H, Shu X, Yin Y, Li J, Qin J, et al. Presence of segmented filamentous bacteria in human children and its potential role in the modulation of human gut immunity. Front Microbiol. 2018;9:1403. doi: 10.3389/fmicb.2018.01403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stepankova R, Sinkora J, Hudcovic T, Kozakova H, Tlaskalova-Hogenova H. Differences in development of lymphocyte subpopulations from gut-associated lymphatic tissue (GALT) of germfree and conventional rats: effect of aging. Folia Microbiol (Praha) 1998;43(5):531–534. doi: 10.1007/BF02820814. [DOI] [PubMed] [Google Scholar]
- 47.Wu HJ, Ivanov II, Darce J, Hattori K, Shima T, Umesaki Y, et al. Gut-residing segmented filamentous bacteria drive autoimmune arthritis via T helper 17 cells. Immunity. 2010;32(6):815–827. doi: 10.1016/j.immuni.2010.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stepankova R, Powrie F, Kofronova O, Kozakova H, Hudcovic T, Hrncir T, et al. Segmented filamentous bacteria in a defined bacterial cocktail induce intestinal inflammation in SCID mice reconstituted with CD45RBhigh CD4+ T cells. Inflamm Bowel Dis. 2007;13(10):1202–1211. doi: 10.1002/ibd.20221. [DOI] [PubMed] [Google Scholar]
- 49.Chaonan Sun LC, Huan Yang, Hongjiang Sun, Zhen Xie, Bei Zhao, Xuemei Jiang, Bi Qin, Zhu Shen. Involvement of Gut Microbiota in the Development of Psoriasis Vulgaris. 2020. [DOI] [PMC free article] [PubMed]
- 50.Kawashima K, Misawa H, Moriwaki Y, Fujii YX, Fujii T, Horiuchi Y, et al. Ubiquitous expression of acetylcholine and its biological functions in life forms without nervous systems. Life Sci. 2007;80(24–25):2206–2209. doi: 10.1016/j.lfs.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 51.Masson F, Talon R, Montel MC. Histamine and tyramine production by bacteria from meat products. Int J Food Microbiol. 1996;32(1–2):199–207. doi: 10.1016/0168-1605(96)01104-X. [DOI] [PubMed] [Google Scholar]
- 52.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, et al. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS ONE. 2012;7(2):e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Neuman H, Debelius JW, Knight R, Koren O. Microbial endocrinology: the interplay between the microbiota and the endocrine system. FEMS Microbiol Rev. 2015;39(4):509–521. doi: 10.1093/femsre/fuu010. [DOI] [PubMed] [Google Scholar]
- 54.Omenetti S, Pizarro TT. The Treg/Th17 axis: a dynamic balance regulated by the gut microbiome. Front Immunol. 2015;6:639. doi: 10.3389/fimmu.2015.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–1412. doi: 10.1093/jn/125.6.1401. [DOI] [PubMed] [Google Scholar]
- 56.Wu SE, Hashimoto-Hill S, Woo V, Eshleman EM, Whitt J, Engleman L, et al. Microbiota-derived metabolite promotes HDAC3 activity in the gut. Nature. 2020;586(7827):108–112. doi: 10.1038/s41586-020-2604-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Schwarz A, Bruhs A, Schwarz T. The short-chain fatty acid sodium butyrate functions as a regulator of the skin immune system. J Invest Dermatol. 2017;137(4):855–864. doi: 10.1016/j.jid.2016.11.014. [DOI] [PubMed] [Google Scholar]
- 58.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–1286. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Drago F, Ciccarese G, Indemini E, Savarino V, Parodi A. Psoriasis and small intestine bacterial overgrowth. Int J Dermatol. 2018;57(1):112–113. doi: 10.1111/ijd.13797. [DOI] [PubMed] [Google Scholar]
- 60.McFadden J, Valdimarsson H, Fry L. Cross-reactivity between streptococcal M surface antigen and human skin. Br J Dermatol. 1991;125(5):443–447. doi: 10.1111/j.1365-2133.1991.tb14769.x. [DOI] [PubMed] [Google Scholar]
- 61.Norrlind R. Psoriasis following infections with hemolytic streptococci. Acta Derm Venereol. 1950;30(1):64–72. [PubMed] [Google Scholar]
- 62.Perez-Lorenzo R, Zambrano-Zaragoza JF, Saul A, Jimenez-Zamudio L, Reyes-Maldonado E, Garcia-Latorre E. Autoantibodies to autologous skin in guttate and plaque forms of psoriasis and cross-reaction of skin antigens with streptococcal antigens. Int J Dermatol. 1998;37(7):524–531. doi: 10.1046/j.1365-4362.1998.00512.x. [DOI] [PubMed] [Google Scholar]
- 63.Sigurdardottir SL, Thorleifsdottir RH, Valdimarsson H, Johnston A. The association of sore throat and psoriasis might be explained by histologically distinctive tonsils and increased expression of skin-homing molecules by tonsil T cells. Clin Exp Immunol. 2013;174(1):139–151. doi: 10.1111/cei.12153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rudramurthy SM, Honnavar P, Chakrabarti A, Dogra S, Singh P, Handa S. Association of Malassezia species with psoriatic lesions. Mycoses. 2014;57(8):483–488. doi: 10.1111/myc.12186. [DOI] [PubMed] [Google Scholar]
- 65.Tomi NS, Kranke B, Aberer E. Staphylococcal toxins in patients with psoriasis, atopic dermatitis, and erythroderma, and in healthy control subjects. J Am Acad Dermatol. 2005;53(1):67–72. doi: 10.1016/j.jaad.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 66.Alekseyenko AV, Perez-Perez GI, De Souza A, Strober B, Gao Z, Bihan M, et al. Community differentiation of the cutaneous microbiota in psoriasis. Microbiome. 2013;1(1):31. doi: 10.1186/2049-2618-1-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fahlen A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304(1):15–22. doi: 10.1007/s00403-011-1189-x. [DOI] [PubMed] [Google Scholar]
- 68.Tett A, Pasolli E, Farina S, Truong DT, Asnicar F, Zolfo M, et al. Unexplored diversity and strain-level structure of the skin microbiome associated with psoriasis. NPJ Biofilms Microbiomes. 2017;3:14. doi: 10.1038/s41522-017-0022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bukin YS, Galachyants YP, Morozov IV, Bukin SV, Zakharenko AS, Zemskaya TI. The effect of 16S rRNA region choice on bacterial community metabarcoding results. Sci Data. 2019;6:190007. doi: 10.1038/sdata.2019.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Castelino M, Eyre S, Moat J, Fox G, Martin P, Ho P, et al. Optimisation of methods for bacterial skin microbiome investigation: primer selection and comparison of the 454 versus MiSeq platform. BMC Microbiol. 2017;17(1):23. doi: 10.1186/s12866-017-0927-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Willis C, Desai D, LaRoche J. Influence of 16S rRNA variable region on perceived diversity of marine microbial communities of the Northern North Atlantic. FEMS Microbiol Lett. 2019 doi: 10.1093/femsle/fnz152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang B, Wang Y, Qian PY. Sensitivity and correlation of hypervariable regions in 16S rRNA genes in phylogenetic analysis. BMC Bioinform. 2016;17:135. doi: 10.1186/s12859-016-0992-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Conlan S, Kong HH, Segre JA. Species-level analysis of DNA sequence data from the NIH human microbiome project. PLoS ONE. 2012;7(10):e47075. doi: 10.1371/journal.pone.0047075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jo JH, Kennedy EA, Kong HH. Research techniques made simple: bacterial 16S ribosomal RNA gene sequencing in cutaneous research. J Invest Dermatol. 2016;136(3):e23–e27. doi: 10.1016/j.jid.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Meisel JS, Hannigan GD, Tyldsley AS, SanMiguel AJ, Hodkinson BP, Zheng Q, et al. Skin microbiome surveys are strongly influenced by experimental design. J Invest Dermatol. 2016;136(5):947–956. doi: 10.1016/j.jid.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Grice EA, Kong HH, Renaud G, Young AC, Program NCS, Bouffard GG, et al. A diversity profile of the human skin microbiota. Genome Res. 2008;18(7):1043–1050. doi: 10.1101/gr.075549.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jumpstart Consortium Human Microbiome Project Data Generation Working G. Evaluation of 16S rDNA-based community profiling for human microbiome research. PLoS One. 2012;7(6):e39315. [DOI] [PMC free article] [PubMed]
- 78.Teng F, Darveekaran Nair SS, Zhu P, Li S, Huang S, Li X, et al. Impact of DNA extraction method and targeted 16S-rRNA hypervariable region on oral microbiota profiling. Sci Rep. 2018;8(1):16321. doi: 10.1038/s41598-018-34294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paulino LC, Tseng CH, Strober BE, Blaser MJ. Molecular analysis of fungal microbiota in samples from healthy human skin and psoriatic lesions. J Clin Microbiol. 2006;44(8):2933–2941. doi: 10.1128/JCM.00785-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Takemoto A, Cho O, Morohoshi Y, Sugita T, Muto M. Molecular characterization of the skin fungal microbiome in patients with psoriasis. J Dermatol. 2015;42(2):166–170. doi: 10.1111/1346-8138.12739. [DOI] [PubMed] [Google Scholar]
- 81.Jagielski T, Rup E, Ziolkowska A, Roeske K, Macura AB, Bielecki J. Distribution of Malassezia species on the skin of patients with atopic dermatitis, psoriasis, and healthy volunteers assessed by conventional and molecular identification methods. BMC Dermatol. 2014;14:3. doi: 10.1186/1471-5945-14-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gupta AK, Kohli Y, Summerbell RC, Faergemann J. Quantitative culture of Malassezia species from different body sites of individuals with or without dermatoses. Med Mycol. 2001;39(3):243–251. doi: 10.1080/mmy.39.3.243.251. [DOI] [PubMed] [Google Scholar]
- 83.Amaya M, Tajima M, Okubo Y, Sugita T, Nishikawa A, Tsuboi R. Molecular analysis of Malassezia microflora in the lesional skin of psoriasis patients. J Dermatol. 2007;34(9):619–624. doi: 10.1111/j.1346-8138.2007.00343.x. [DOI] [PubMed] [Google Scholar]
- 84.Chang HW, Yan D, Singh R, Liu J, Lu X, Ucmak D, et al. Alteration of the cutaneous microbiome in psoriasis and potential role in Th17 polarization. Microbiome. 2018;6(1):154. doi: 10.1186/s40168-018-0533-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hurabielle C, Link VM, Bouladoux N, Han SJ, Merrill ED, Lightfoot YL, et al. Immunity to commensal skin fungi promotes psoriasiform skin inflammation. Proc Natl Acad Sci U S A. 2020;117(28):16465–16474. doi: 10.1073/pnas.2003022117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mosca A, Leclerc M, Hugot JP. Gut microbiota diversity and human diseases: should we reintroduce key predators in our ecosystem? Front Microbiol. 2016;7:455. doi: 10.3389/fmicb.2016.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fredricks DN, Fiedler TL, Marrazzo JM. Molecular identification of bacteria associated with bacterial vaginosis. N Engl J Med. 2005;353(18):1899–1911. doi: 10.1056/NEJMoa043802. [DOI] [PubMed] [Google Scholar]
- 88.Srinivasan S, Hoffman NG, Morgan MT, Matsen FA, Fiedler TL, Hall RW, et al. Bacterial communities in women with bacterial vaginosis: high resolution phylogenetic analyses reveal relationships of microbiota to clinical criteria. PLoS ONE. 2012;7(6):e37818. doi: 10.1371/journal.pone.0037818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shapiro J, Cohen NA, Shalev V, Uzan A, Koren O, Maharshak N. Psoriatic patients have a distinct structural and functional fecal microbiota compared with controls. J Dermatol. 2019;46(7):595–603. doi: 10.1111/1346-8138.14933. [DOI] [PubMed] [Google Scholar]
- 90.Tan L, Zhao S, Zhu W, Wu L, Li J, Shen M, et al. The Akkermansia muciniphila is a gut microbiota signature in psoriasis. Exp Dermatol. 2018;27(2):144–149. doi: 10.1111/exd.13463. [DOI] [PubMed] [Google Scholar]
- 91.Reiss Z, Rob F, Kolar M, Schierova D, Kreisinger J, Jackova Z, et al. Skin microbiota signature distinguishes IBD patients and reflects skin adverse events during anti-TNF therapy. Front Cell Infect Microbiol. 2022;12:1064537. doi: 10.3389/fcimb.2022.1064537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yegorov S, Babenko D, Kozhakhmetov S, Akhmaltdinova L, Kadyrova I, Nurgozhina A, et al. Psoriasis is associated with elevated gut IL-1alpha and intestinal microbiome alterations. Front Immunol. 2020;11:571319. doi: 10.3389/fimmu.2020.571319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yeh NL, Hsu CY, Tsai TF, Chiu HY. Gut microbiome in psoriasis is perturbed differently during secukinumab and ustekinumab therapy and associated with response to treatment. Clin Drug Investig. 2019;39(12):1195–1203. doi: 10.1007/s40261-019-00849-7. [DOI] [PubMed] [Google Scholar]
- 94.Demirci M, Tokman HB, Uysal HK, Demiryas S, Karakullukcu A, Saribas S, et al. Reduced Akkermansia muciniphila and Faecalibacterium prausnitzii levels in the gut microbiota of children with allergic asthma. Allergol Immunopathol (Madr) 2019;47(4):365–371. doi: 10.1016/j.aller.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 95.Pittayanon R, Lau JT, Leontiadis GI, Tse F, Yuan Y, Surette M, et al. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: a systematic review. Gastroenterology. 2020;158(4):930–46 e1. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- 96.Eppinga H, Sperna Weiland CJ, Thio HB, van der Woude CJ, Nijsten TE, Peppelenbosch MP, et al. Similar depletion of protective Faecalibacterium prausnitzii in psoriasis and inflammatory bowel disease, but not in hidradenitis suppurativa. J Crohns Colitis. 2016;10(9):1067–1075. doi: 10.1093/ecco-jcc/jjw070. [DOI] [PubMed] [Google Scholar]
- 97.Fritz JV, Desai MS, Shah P, Schneider JG, Wilmes P. From meta-omics to causality: experimental models for human microbiome research. Microbiome. 2013;1(1):14. doi: 10.1186/2049-2618-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Okada K, Matsushima Y, Mizutani K, Yamanaka K. The role of gut microbiome in psoriasis: oral administration of Staphylococcus aureus and Streptococcus danieliae exacerbates skin inflammation of imiquimod-induced psoriasis-like dermatitis. Int J Mol Sci. 2020;21(9):3303. doi: 10.3390/ijms21093303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stehlikova Z, Kostovcikova K, Kverka M, Rossmann P, Dvorak J, Novosadova I, et al. Crucial role of microbiota in experimental psoriasis revealed by a gnotobiotic mouse model. Front Microbiol. 2019;10:236. doi: 10.3389/fmicb.2019.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Heissigerova J, Seidler Stangova P, Klimova A, Svozilkova P, Hrncir T, Stepankova R, et al. The microbiota determines susceptibility to experimental autoimmune uveoretinitis. J Immunol Res. 2016;2016:5065703. doi: 10.1155/2016/5065703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Garcia-Collinot G, Madrigal-Santillan EO, Martinez-Bencomo MA, Carranza-Muleiro RA, Jara LJ, Vera-Lastra O, et al. Effectiveness of Saccharomyces boulardii and metronidazole for small intestinal bacterial overgrowth in systemic sclerosis. Dig Dis Sci. 2020;65(4):1134–1143. doi: 10.1007/s10620-019-05830-0. [DOI] [PubMed] [Google Scholar]
- 102.Soifer LO, Peralta D, Dima G, Besasso H. Comparative clinical efficacy of a probiotic vs. an antibiotic in the treatment of patients with intestinal bacterial overgrowth and chronic abdominal functional distension: a pilot study. Acta Gastroenterol Latinoam. 2010;40(4):323–7. [PubMed] [Google Scholar]
- 103.Zakostelska Z, Malkova J, Klimesova K, Rossmann P, Hornova M, Novosadova I, et al. Intestinal microbiota promotes psoriasis-like skin inflammation by enhancing Th17 response. PLoS ONE. 2016;11(7):e0159539. doi: 10.1371/journal.pone.0159539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zanvit P, Konkel JE, Jiao X, Kasagi S, Zhang D, Wu R, et al. Antibiotics in neonatal life increase murine susceptibility to experimental psoriasis. Nat Commun. 2015;6:8424. doi: 10.1038/ncomms9424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang M, Jiang Z, Li D, Jiang D, Wu Y, Ren H, et al. Oral antibiotic treatment induces skin microbiota dysbiosis and influences wound healing. Microb Ecol. 2015;69(2):415–421. doi: 10.1007/s00248-014-0504-4. [DOI] [PubMed] [Google Scholar]
- 106.Baba H, Masuyama A, Yoshimura C, Aoyama Y, Takano T, Ohki K. Oral intake of Lactobacillus helveticus-fermented milk whey decreased transepidermal water loss and prevented the onset of sodium dodecylsulfate-induced dermatitis in mice. Biosci Biotechnol Biochem. 2010;74(1):18–23. doi: 10.1271/bbb.90370. [DOI] [PubMed] [Google Scholar]
- 107.Gueniche A, Benyacoub J, Philippe D, Bastien P, Kusy N, Breton L, et al. Lactobacillus paracasei CNCM I-2116 (ST11) inhibits substance P-induced skin inflammation and accelerates skin barrier function recovery in vitro. Eur J Dermatol. 2010;20(6):731–737. doi: 10.1684/ejd.2010.1108. [DOI] [PubMed] [Google Scholar]
- 108.Ogawa M, Saiki A, Matsui Y, Tsuchimoto N, Nakakita Y, Takata Y, et al. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88) on dry skin conditions: A randomized, double-blind, placebo-controlled study. Exp Ther Med. 2016;12(6):3863–3872. doi: 10.3892/etm.2016.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kozakova H, Schwarzer M, Tuckova L, Srutkova D, Czarnowska E, Rosiak I, et al. Colonization of germ-free mice with a mixture of three lactobacillus strains enhances the integrity of gut mucosa and ameliorates allergic sensitization. Cell Mol Immunol. 2016;13(2):251–262. doi: 10.1038/cmi.2015.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zakostelska Z, Kverka M, Klimesova K, Rossmann P, Mrazek J, Kopecny J, et al. Lysate of probiotic Lactobacillus casei DN-114 001 ameliorates colitis by strengthening the gut barrier function and changing the gut microenvironment. PLoS ONE. 2011;6(11):e27961. doi: 10.1371/journal.pone.0027961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jenneck C, Novak N. The safety and efficacy of alefacept in the treatment of chronic plaque psoriasis. Ther Clin Risk Manag. 2007;3(3):411–420. [PMC free article] [PubMed] [Google Scholar]
- 112.Dai C, Jiang M, Sun MJ. Letter: increased risk of developing Crohn's disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNFalpha agents, particularly etanercept, for autoimmune diseases other than IBD. Aliment Pharmacol Ther. 2019;50(7):834–835. doi: 10.1111/apt.15460. [DOI] [PubMed] [Google Scholar]
- 113.Gall JS, Kalb RE. Infliximab for the treatment of plaque psoriasis. Biologics. 2008;2(1):115–124. doi: 10.2147/btt.s2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheum. 2010;40(3):233–240. doi: 10.1016/j.semarthrit.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 115.Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-alpha agents. Autoimmun Rev. 2014;13(1):15–19. doi: 10.1016/j.autrev.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 116.Warren RB, Smith CH, Yiu ZZN, Ashcroft DM, Barker J, Burden AD, et al. Differential drug survival of biologic therapies for the treatment of psoriasis: a Prospective observational cohort study from the British Association of Dermatologists Biologic Interventions Register (BADBIR) J Invest Dermatol. 2015;135(11):2632–2640. doi: 10.1038/jid.2015.208. [DOI] [PubMed] [Google Scholar]
- 117.Menter A, Strober BE, Kaplan DH, Kivelevitch D, Prater EF, Stoff B, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80(4):1029–1072. doi: 10.1016/j.jaad.2018.11.057. [DOI] [PubMed] [Google Scholar]
- 118.Ten Bergen LL, Petrovic A, Krogh Aarebrot A, Appel S. The TNF/IL-23/IL-17 axis-Head-to-head trials comparing different biologics in psoriasis treatment. Scand J Immunol. 2020;92(4):e12946. doi: 10.1111/sji.12946. [DOI] [PubMed] [Google Scholar]
- 119.Naik PP. Adverse effects of anti-interleukin-23 agents employed in patients with psoriasis: a systematic review. Dermatology. 2022;238(5):886–896. doi: 10.1159/000524199. [DOI] [PubMed] [Google Scholar]
- 120.Loesche MA, Farahi K, Capone K, Fakharzadeh S, Blauvelt A, Duffin KC, et al. Longitudinal study of the psoriasis-associated skin microbiome during therapy with ustekinumab in a randomized phase 3b clinical trial. J Invest Dermatol. 2018;138(9):1973–1981. doi: 10.1016/j.jid.2018.03.1501. [DOI] [PubMed] [Google Scholar]
- 121.Dei-Cas I, Giliberto F, Luce L, Dopazo H, Penas-Steinhardt A. Metagenomic analysis of gut microbiota in non-treated plaque psoriasis patients stratified by disease severity: development of a new Psoriasis-Microbiome Index. Sci Rep. 2020;10(1):12754. doi: 10.1038/s41598-020-69537-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Koike Y, Kuwatsuka S, Nishimoto K, Motooka D, Murota H. Skin mycobiome of psoriasis patients is retained during treatment with TNF and IL-17 inhibitors. Int J Mol Sci. 2020;21(11):3892. doi: 10.3390/ijms21113892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kalliomaki M, Salminen S, Arvilommi H, Kero P, Koskinen P, Isolauri E. Probiotics in primary prevention of atopic disease: a randomised placebo-controlled trial. Lancet. 2001;357(9262):1076–1079. doi: 10.1016/S0140-6736(00)04259-8. [DOI] [PubMed] [Google Scholar]
- 124.Pandey KR, Naik SR, Vakil BV. Probiotics, prebiotics and synbiotics—a review. J Food Sci Technol. 2015;52(12):7577–7587. doi: 10.1007/s13197-015-1921-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Volkova LA, Khalif IL, Kabanova IN. Impact of the impaired intestinal microflora on the course of acne vulgaris. Klin Med (Mosk) 2001;79(6):39–41. [PubMed] [Google Scholar]
- 126.Ogawa M, Saiki A, Matsui Y, Tsuchimoto N, Nakakita Y, Takata Y, et al. Effects of oral intake of heat-killed Lactobacillus brevis SBC8803 (SBL88™) on dry skin conditions: a randomized, double-blind, placebo-controlled study. Exp Ther Med. 2016;12(6):3863–3872. doi: 10.3892/etm.2016.3862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Evelo Biosciences Reports Positive EDP1815 Interim Clinical Data in Psoriasis Patients at Low Dose in Ongoing Phase 1b Tria. Retrieved from http://ir.evelobio.com/newsreleases/news-releasedetails/evelo-biosciencesreports-positive-edp1815- interim-clinical-data. 2019.
- 128.Groeger D, O'Mahony L, Murphy EF, Bourke JF, Dinan TG, Kiely B, et al. Bifidobacterium infantis 35624 modulates host inflammatory processes beyond the gut. Gut Microbes. 2013;4(4):325–339. doi: 10.4161/gmic.25487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Navarro-Lopez V, Martinez-Andres A, Ramirez-Bosca A, Ruzafa-Costas B, Nunez-Delegido E, Carrion-Gutierrez MA, et al. Efficacy and safety of oral administration of a mixture of probiotic strains in patients with psoriasis: a randomized controlled clinical trial. Acta Derm Venereol. 2019;99(12):1078–1084. doi: 10.2340/00015555-3305. [DOI] [PubMed] [Google Scholar]
- 130.Kragsnaes MS, Kjeldsen J, Horn HC, Munk HL, Pedersen FM, Holt HM, et al. Efficacy and safety of faecal microbiota transplantation in patients with psoriatic arthritis: protocol for a 6-month, double-blind, randomised, placebo-controlled trial. BMJ Open. 2018;8(4):e019231. doi: 10.1136/bmjopen-2017-019231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yin G, Li JF, Sun YF, Ding X, Zeng JQ, Zhang T, et al. Fecal microbiota transplantation as a novel therapy for severe psoriasis. Zhonghua Nei Ke Za Zhi. 2019;58(10):782–785. doi: 10.3760/cma.j.issn.0578-1426.2019.10.011. [DOI] [PubMed] [Google Scholar]
- 132.Oh J, Byrd AL, Park M, Kong HH, Segre JA, Program NCS. Temporal stability of the human skin microbiome. Cell. 2016;165(4):854–866. doi: 10.1016/j.cell.2016.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Callewaert C, Knödlseder N, Karoglan A, Güell M, Paetzold B. Skin microbiome transplantation and manipulation: Current state of the art. Comput Struct Biotechnol J. 2021;19:624–631. doi: 10.1016/j.csbj.2021.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Christophers E. Psoriasis—epidemiology and clinical spectrum. Clin Exp Dermatol. 2001;26(4):314–320. doi: 10.1046/j.1365-2230.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 135.Alinaghi F, Tekin HG, Burisch J, Wu JJ, Thyssen JP, Egeberg A. Global prevalence and bidirectional association between psoriasis and inflammatory bowel disease—a systematic review and meta-analysis. J Crohns Colitis. 2020;14(3):351–360. doi: 10.1093/ecco-jcc/jjz152. [DOI] [PubMed] [Google Scholar]
- 136.Coufal S, Galanova N, Bajer L, Gajdarova Z, Schierova D, Jiraskova Zakostelska Z, et al. Inflammatory bowel disease types differ in markers of inflammation, gut barrier and in specific anti-bacterial response. Cells. 2019;8(7):719. doi: 10.3390/cells8070719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Schierova D, Roubalova R, Kolar M, Stehlikova Z, Rob F, Jackova Z, et al. Fecal microbiome changes and specific anti-bacterial response in patients with IBD during anti-TNF therapy. Cells. 2021;10(11):3188. doi: 10.3390/cells10113188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tang H, Jin X, Li Y, Jiang H, Tang X, Yang X, et al. A large-scale screen for coding variants predisposing to psoriasis. Nat Genet. 2014;46(1):45–50. doi: 10.1038/ng.2827. [DOI] [PubMed] [Google Scholar]
- 139.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C, et al. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7(8):e1002254. doi: 10.1371/journal.pgen.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eppinga H, Poortinga S, Thio HB, Nijsten TEC, Nuij V, van der Woude CJ, et al. Prevalence and phenotype of concurrent psoriasis and inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(10):1783–1789. doi: 10.1097/MIB.0000000000001169. [DOI] [PubMed] [Google Scholar]
- 141.Triantafillidis JK, Malgarinos G. Severe psoriasis preceding diagnosis of large bowel Crohn's disease for 15 years. J Crohns Colitis. 2013;7(2):e80. doi: 10.1016/j.crohns.2012.07.014. [DOI] [PubMed] [Google Scholar]
- 142.Juzlova K, Votrubova J, Dzambova M, Gopfertova D, Hercogova J, Smerhovsky Z. Gastrointestinal comorbidities in patients with psoriasis in the Czech Republic: The results of 189 patients with psoriasis and 378 controls. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2016;160(1):100–105. doi: 10.5507/bp.2015.048. [DOI] [PubMed] [Google Scholar]
- 143.Lolli E, Saraceno R, Calabrese E, Ascolani M, Scarozza P, Chiricozzi A, et al. Psoriasis phenotype in inflammatory bowel disease: a case-control prospective study. J Crohns Colitis. 2015;9(9):699–707. doi: 10.1093/ecco-jcc/jjv068. [DOI] [PubMed] [Google Scholar]
- 144.Nehring P, Przybyłkowski A. Is psoriasis treatment a risk factor for inflammatory bowel disease? Pharm Med. 2020;34:257–262. doi: 10.1007/s40290-020-00340-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Vlachos C, Gaitanis G, Katsanos KH, Christodoulou DK, Tsianos E, Bassukas ID. Psoriasis and inflammatory bowel disease: links and risks. Psoriasis (Auckland, NZ) 2016;6:73. doi: 10.2147/PTT.S85194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mocci G, Marzo M, Papa A, Armuzzi A, Guidi L. Dermatological adverse reactions during anti-TNF treatments: Focus on inflammatory bowel disease. J Crohns Colitis. 2013;7(10):769–779. doi: 10.1016/j.crohns.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 147.Fiorino G, Danese S, Pariente B, Allez M. Paradoxical immune-mediated inflammation in inflammatory bowel disease patients receiving anti-TNF-alpha agents. Autoimmun Rev. 2014;13(1):15–19. doi: 10.1016/j.autrev.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 148.Ko JM, Gottlieb AB, Kerbleski JF. Induction and exacerbation of psoriasis with TNF-blockade therapy: a review and analysis of 127 cases. J Dermatolog Treat. 2009;20(2):100–108. doi: 10.1080/09546630802441234. [DOI] [PubMed] [Google Scholar]
- 149.Korzenik J, Larsen MD, Nielsen J, Kjeldsen J, Norgard BM. Increased risk of developing Crohn's disease or ulcerative colitis in 17 018 patients while under treatment with anti-TNFalpha agents, particularly etanercept, for autoimmune diseases other than inflammatory bowel disease. Aliment Pharmacol Ther. 2019;50(3):289–294. doi: 10.1111/apt.15370. [DOI] [PubMed] [Google Scholar]
- 150.Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF-alpha induced psoriasis in patients with inflammatory bowel disease. Aliment Pharm Ther. 2009;29(9):921–927. doi: 10.1111/j.1365-2036.2009.03955.x. [DOI] [PubMed] [Google Scholar]
- 151.Rahier JF, Buche S, Peyrin-Biroulet L, Bouhnik Y, Duclos B, Louis E, et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol. 2010;8(12):1048–1055. doi: 10.1016/j.cgh.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 152.Pugliese D, Guidi L, Ferraro PM, Marzo M, Felice C, Celleno L, et al. Paradoxical psoriasis in a large cohort of patients with inflammatory bowel disease receiving treatment with anti-TNF alpha: 5-year follow-up study. Aliment Pharm Ther. 2015;42(7):880–888. doi: 10.1111/apt.13352. [DOI] [PubMed] [Google Scholar]
- 153.Rahier JF, Buche S, Peyrin-Biroulet L, Bouhnik Y, Duclos B, Louis E, et al. Severe skin lesions cause patients with inflammatory bowel disease to discontinue anti-tumor necrosis factor therapy. Clin Gastroenterol Hepatol. 2010;8(12):1048–1055. doi: 10.1016/j.cgh.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 154.Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthritis Rheum. 2008;59(7):996–1001. doi: 10.1002/art.23835. [DOI] [PubMed] [Google Scholar]
- 155.Nigam GB, Bhandare AP, Antoniou GA, Limdi JK. Systematic review and meta-analysis of dermatological reactions in patients with inflammatory bowel disease treated with anti-tumour necrosis factor therapy. Eur J Gastroenterol Hepatol. 2021;33(3):346–357. doi: 10.1097/MEG.0000000000001917. [DOI] [PubMed] [Google Scholar]
- 156.Collamer AN, Guerrero KT, Henning JS, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: a literature review and potential mechanisms of action. Arthrit Rheum-Arthr. 2008;59(7):996–1001. doi: 10.1002/art.23835. [DOI] [PubMed] [Google Scholar]
- 157.Gatzka M. Skin under Tnf influence: how regulatory T cells work against macrophages in psoriasis. J Pathol. 2017;241(1):3–5. doi: 10.1002/path.4820. [DOI] [PubMed] [Google Scholar]
- 158.Jayasekera PSA, Walsh ML, Hurrell D, Parslew RAG. Case report of lichen planopilaris occurring in a pediatric patient receiving a tumor necrosis factor alpha inhibitor and a review of the literature. Pediatr Dermatol. 2016;33(2):E143–E146. doi: 10.1111/pde.12768. [DOI] [PubMed] [Google Scholar]
- 159.Andrade P, Lopes S, Albuquerque A, Osorio F, Pardal J, Macedo G. Oral lichen planus in IBD patients: a paradoxical adverse effect of anti-TNF-alpha therapy. Digest Dis Sci. 2015;60(9):2746–2749. doi: 10.1007/s10620-015-3680-2. [DOI] [PubMed] [Google Scholar]
- 160.Inoue-Nishimoto T, Hanafusa T, Igawa K, Azukizawa H, Yokomi A, Yokozeki H, et al. Possible association of anti-tumor necrosis factor-alpha antibody therapy with the development of scleroderma-like changes with lichen planus. Eur J Dermatol. 2015;25(5):513–515. doi: 10.1684/ejd.2015.2631. [DOI] [PubMed] [Google Scholar]
- 161.Collamer AN, Battafarano DF. Psoriatic skin lesions induced by tumor necrosis factor antagonist therapy: clinical features and possible immunopathogenesis. Semin Arthritis Rheu. 2010;40(3):233–240. doi: 10.1016/j.semarthrit.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 162.Furue K, Ito T, Tsuji G, Kadono T, Nakahara T, Furue M. Autoimmunity and autoimmune co-morbidities in psoriasis. Immunology. 2018;154(1):21–27. doi: 10.1111/imm.12891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Wlodarczyk M, Sobolewska A, Wojcik B, Loga K, Fichna J, Wisniewska-Jarosinska M. Correlations between skin lesions induced by anti-tumor necrosis factor-alpha and selected cytokines in Crohn's disease patients. World J Gastroentero. 2014;20(22):7019–7026. doi: 10.3748/wjg.v20.i22.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tillack C, Ehmann LM, Friedrich M, Laubender RP, Papay P, Vogelsang H, et al. Anti-TNF antibody-induced psoriasiform skin lesions in patients with inflammatory bowel disease are characterised by interferon-gamma-expressing Th1 cells and IL-17A/IL-22-expressing Th17 cells and respond to anti-IL-12/IL-23 antibody treatment. Gut. 2014;63(4):567–577. doi: 10.1136/gutjnl-2012-302853. [DOI] [PubMed] [Google Scholar]
- 165.Manichanh C, Rigottier-Gois L, Bonnaud E, Gloux K, Pelletier E, Frangeul L, et al. Reduced diversity of faecal microbiota in Crohn's disease revealed by a metagenomic approach. Gut. 2006;55(2):205–211. doi: 10.1136/gut.2005.073817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fahlen A, Engstrand L, Baker BS, Powles A, Fry L. Comparison of bacterial microbiota in skin biopsies from normal and psoriatic skin. Arch Dermatol Res. 2012;304(1):15–22. doi: 10.1007/s00403-011-1189-x. [DOI] [PubMed] [Google Scholar]
- 167.Fry L, Baker BS, Powles AV, Fahlen A, Engstrand L. Is chronic plaque psoriasis triggered by microbiota in the skin? Brit J Dermatol. 2013;169(1):47–52. doi: 10.1111/bjd.12322. [DOI] [PubMed] [Google Scholar]
- 168.Kong HDH, Oh J, Deming C, Conlan S, Grice EA, Beatson MA, et al. Temporal shifts in the skin microbiome associated with disease flares and treatment in children with atopic dermatitis. Genome Res. 2012;22(5):850–859. doi: 10.1101/gr.131029.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dong J, Goldenberg G. New biologics in psoriasis: an update on IL-23 and IL-17 inhibitors. Cutis. 2017;99(2):123–127. [PubMed] [Google Scholar]
- 170.Fauny M, Moulin D, D'Amico F, Netter P, Petitpain N, Arnone D, et al. Paradoxical gastrointestinal effects of interleukin-17 blockers. Ann Rheum Dis. 2020;79(9):1132–1138. doi: 10.1136/annrheumdis-2020-217927. [DOI] [PubMed] [Google Scholar]
- 171.Huppler AR, Bishu S, Gaffen SL. Mucocutaneous candidiasis: the IL-17 pathway and implications for targeted immunotherapy. Arthritis Res Ther. 2012;14(4):217. doi: 10.1186/ar3893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Elsner K, Holstein J, Hilke FJ, Blumenstock G, Walker B, Schmidt S, et al. Prevalence of candida species in psoriasis. Mycoses. 2022;65(2):247–254. doi: 10.1111/myc.13399. [DOI] [PubMed] [Google Scholar]
- 173.Targan SR, Feagan B, Vermeire S, Panaccione R, Melmed GY, Landers C, et al. A randomized, double-blind, placebo-controlled phase 2 study of brodalumab in patients with moderate-to-severe Crohn's disease. Am J Gastroenterol. 2016;111(11):1599–1607. doi: 10.1038/ajg.2016.298. [DOI] [PubMed] [Google Scholar]
- 174.Hueber W, Sands BE, Lewitzky S, Vandemeulebroecke M, Reinisch W, Higgins PD, et al. Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn's disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut. 2012;61(12):1693–1700. doi: 10.1136/gutjnl-2011-301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Reich K, Leonardi C, Langley RG, Warren RB, Bachelez H, Romiti R, et al. Inflammatory bowel disease among patients with psoriasis treated with ixekizumab: A presentation of adjudicated data from an integrated database of 7 randomized controlled and uncontrolled trials. J Am Acad Dermatol. 2017;76(3):441–8 e2. doi: 10.1016/j.jaad.2016.10.027. [DOI] [PubMed] [Google Scholar]
- 176.Smith MK, Pai J, Panaccione R, Beck P, Ferraz JG, Jijon H. Crohn's-like disease in a patient exposed to anti-Interleukin-17 blockade (Ixekizumab) for the treatment of chronic plaque psoriasis: a case report. BMC Gastroenterol. 2019;19(1):162. doi: 10.1186/s12876-019-1067-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nazarian A, Grin A, Wijeratne DT. Ixekizumab associated new-onset inflammatory bowel disease. ACG Case Rep J. 2020;7(2):e00316. doi: 10.14309/crj.0000000000000316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Johnston RD, Logan RF. What is the peak age for onset of IBD? Inflamm Bowel Dis. 2008;14(Suppl 2):S4–5. doi: 10.1002/ibd.20545. [DOI] [PubMed] [Google Scholar]
- 179.Earley H, Lennon G, Balfe A, Coffey JC, Winter DC, O'Connell PR. The abundance of Akkermansia muciniphila and its relationship with sulphated colonic mucins in health and ulcerative colitis. Sci Rep. 2019;9(1):15683. doi: 10.1038/s41598-019-51878-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, et al. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol. 2020;104(23):10203–10215. doi: 10.1007/s00253-020-10948-7. [DOI] [PubMed] [Google Scholar]
- 181.Elmets CA, Lim HW, Stoff B, Connor C, Cordoro KM, Lebwohl M, et al. Joint American Academy of Dermatology-National Psoriasis Foundation guidelines of care for the management and treatment of psoriasis with phototherapy. J Am Acad Dermatol. 2019;81(3):775–804. doi: 10.1016/j.jaad.2019.04.042. [DOI] [PubMed] [Google Scholar]
- 182.Camela E, Potestio L, Fabbrocini G, Ruggiero A, Megna M. New frontiers in personalized medicine in psoriasis. Expert Opin Biol Ther. 2022;22(12):1431–1433. doi: 10.1080/14712598.2022.2113872. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.