Abstract
This expert group consensus statement emphasises the need for standardising the definition of progressive fibrosing interstitial lung diseases (F-ILDs), with an accurate initial diagnosis being of paramount importance in ensuring appropriate initial management. Equally, case-by-case decisions on monitoring and management are essential, given the varying presentations of F-ILDs and the varying rates of progression. The value of diagnostic tests in risk stratification at presentation and, separately, the importance of a logical monitoring strategy, tailored to manage the risk of progression, are also stressed. The term “progressive pulmonary fibrosis” (PPF) exactly describes the entity that clinicians often face in practice. The importance of using antifibrotic therapy early in PPF (once initial management has failed to prevent progression) is increasingly supported by evidence. Artificial intelligence software for high-resolution computed tomography analysis, although an exciting tool for the future, awaits validation. Guidance is provided on pulmonary rehabilitation, oxygen and the use of non-invasive ventilation focused specifically on the needs of ILD patients with progressive disease. PPF should be differentiated from acute deterioration due to drug-induced lung toxicity or other forms of acute exacerbations. Referral criteria for a lung transplant are discussed and applied to patient needs in severe diseases where transplantation is not realistic, either due to access limitations or transplantation contraindications. In conclusion, expert group consensus guidance is provided on the diagnosis, treatment and monitoring of F-ILDs with specific focus on the recognition of PPF and the management of pulmonary fibrosis progressing despite initial management.
Short abstract
Progressive pulmonary fibrosis (PPF) explains what clinicians increasingly face in practice. Assessing ILD progression, its risk and improved treatments based on current evidence for PPF (despite initial management) form the mainstay of this document. http://bit.ly/3GLdqfs
Introduction
The aim of this consensus statement is to provide expert guidance on the frequent management and monitoring uncertainties in fibrosing interstitial lung diseases (F-ILDs) other than idiopathic pulmonary fibrosis (IPF), with a particular focus on F-ILDs that continue to progress despite initial management. It is stressed that the statement should not be viewed as a guideline. Most of the questions explored are not amenable to formal guideline recommendations, but address situations that require clinicians to make decisions in routine practice in the absence of definitive evidence. Conclusions reached by an expert group consensus, based on a review of the literature, accumulated clinical experience and clinical reasoning, are intended to provide provisional guidance for clinicians, pending emergence of any in the next few years. (For details regarding working expert group methodology and the search terms used, refer to supplementary material S1 and S2.) In this statement, we suggest appropriate approaches in F-ILD from the time of presentation onwards, along with a discussion on initial and subsequent treatment and optimal monitoring for progression.
Many individual diseases falling under the umbrella of ILDs can also manifest as F-ILDs. In most cases, patients present because fibrotic disease is progressive. From this perspective of progression, recent terms used for this entity include “the progressive fibrotic phenotype” [1], “progressive fibrotic interstitial lung disease” [2] and “progressive pulmonary fibrosis” (PPF) [3]. All these terms are appropriate regarding the consideration of initial diagnosis at presentation, risk stratification and the identification of progression. However, none of these terms apply to that subgroup of non-IPF patients evaluated in recent antifibrotic trials; all omit the defining feature: progression of pulmonary fibrosis despite management considered to be appropriate for the ILD. Approximately 18–32% of non-IPF ILDs progress despite initial treatment within 61–80 months [4–9]. Thus, the various terms above, including “PPF”, used from a perspective of progression, fall short of defining a clear entity regarding antifibrotic usage.
There is a need to differentiate between PPF at first presentation (with worsening symptoms and, where available, worsening imaging features) and PPF occurring despite management. This distinction is critical in clinical practice. A “free-for-all” prescription of antifibrotic therapy for all patients with F-ILD at presentation fails to consider that traditional therapies meet the needs of a majority of non-IPF patients with F-ILD [10]. The danger of such an approach is chaotic management practice with failure to consider the relative benefits of individual therapies. This approach has been widely observed by group members, especially in the hands of less expert clinicians. The premature use of antifibrotic monotherapy therapy risks loss of the benefits of immunomodulation, applicable to most patients with non-IPF F-ILD at presentation. Thus, in this consensus statement, we refer to “PPF (despite management)” where appropriate and specifically to qualify the term “PPF” with regard to antifibrotic usage.
The problems widely perceived by clinicians include 1) the growing prevalence of ILDs and PPF despite management; 2) the need for unambiguous terminology in categorising progression despite initial management; 3) the need for clarity on risk stratification at presentation for future progression; 4) the need for alternative procedures to surgical lung biopsy (e.g. transbronchial cryobiopsy) to help initial diagnosis and risk stratification; 5) the restricted (and unrestricted) use of antifibrotics in various parts of the world; and 6) the burgeoning number of elderly patients with PPF (despite management) or with severe comorbidity.
Section 1: Definition and diagnosis
Question 1: What is the difference between F-ILD and a progressive F-ILD (i.e. PPF)?
ILDs are a heterogeneous group of diseases, mostly characterised by inflammation and fibrosis of the lung parenchyma, resulting in stiffening of the lungs [11, 12]. F-ILD is characterised by fibrosis detected on pathology, or by the presence of honeycombing and/or traction bronchiectasis on high-resolution computed tomography (HRCT) [13]. A definition that includes all patients with any fibrosis observed at biopsy, however minimal, is over-inclusive as F-ILD is generally identified using HRCT. However, biopsy occasionally establishes that HRCT abnormalities not considered to represent fibrosis (reticulation or ground-glass, without traction bronchiectasis) do represent fine intralobular fibrosis.
PPF denotes an ILD with radiological signs of fibrosis and evidence of progression over time. Patients with F-ILD generally have progressive disease, which is why they most often present with worsening symptoms as well as (where available) evidence of worsening imaging. The exceptions are diagnoses made by screening protocols (as in connective tissue disease (CTD) or lung cancer screening). It is estimated that over the subsequent 2 years, with management considered appropriate, only 13–40% of non-IPF F-ILDs will progress [10]. The recent non-IPF antifibrotic treatment data applied specifically to patients with F-ILD that continue to progress despite management tailored to the initial diagnosis [2]. We have therefore used the term “PPF” when discussing risk considerations at baseline and the identification of patients progressing during follow-up, but refer to “PPF (despite management)” when discussing the potential use of antifibrotic therapy.
Key conclusions
1) Established fibrosis refers to lung fibrosis evident on HRCT (honeycombing and/or traction bronchiectasis) at baseline or on serial lung imaging. Occasionally, biopsy establishes clinically important fibrotic disease that has not been identified by HRCT. The group feels that there is a clear distinction between F-ILDs, wherein there are signs of fibrosis at presentation, and an ILD with signs of fibrosis that continues to progress despite management (“progression” is defined in Section 2: Prognostication and monitoring).
2) The term “PPF”, accurately describing progressive disease both at presentation and subsequently, has sometimes been used as a term specifically describing disease progressing despite management.
3) Hence, to avoid confusion, F-ILD with clinical, radiological or physiological indicators of progression despite optimal management instituted for the underlying F-ILD, leading to consideration of antifibrotic therapy, is best designated as “PPF (despite management)”.
Question 2: What are the underlying diagnoses of patients with F-ILDs?
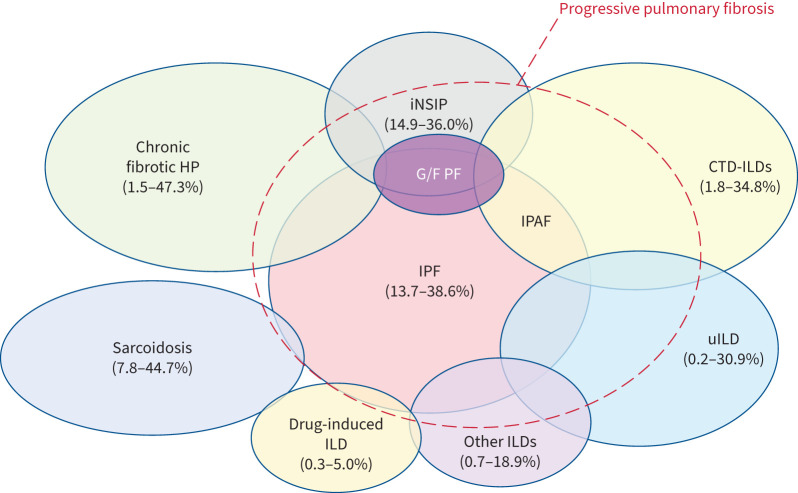
An accurate initial diagnosis is required to ensure that management is tailored for a particular F-ILD. Although it is beyond the scope of this document to provide an exhaustive list of F-ILDs that may progress, the most common underlying diagnoses include CTD-ILDs, fibrotic hypersensitivity pneumonitis (F-HP), idiopathic non-specific interstitial pneumonia (iNSIP), unclassifiable ILD (uILD), chronic sarcoidosis and others as listed in table 1. The global prevalence of F-ILDs is shown in figure 1 [14, 15]. The group believes that post-viral F-ILD needs to be better understood, with clearer knowledge of its prevalence (including persistent diffuse parenchymal lung abnormalities seen after recovery from acute coronavirus disease 2019 (COVID-19) [16]).
TABLE 1.
Underlying common diagnoses of patients with fibrosing interstitial lung diseases (ILDs)
| Idiopathic |
| Idiopathic interstitial pneumonia |
| Idiopathic non-specific interstitial pneumonia |
| Cryptogenic organising pneumonia with supervening fibrosis |
| Idiopathic desquamative interstitial pneumonia |
| Idiopathic lymphocytic interstitial pneumonia |
| Non-idiopathic |
| Connective tissue disease-ILDs |
| Fibrotic hypersensitivity pneumonitis |
| Exposure-related ILDs (asbestosis, silicosis, etc.) |
| Drug-induced ILDs (amiodarone, nitrofurantoin, etc.) |
| Sarcoidosis |
| Anti-neutrophilic cytoplasmic auto-antibody-associated vasculitis |
| Unclassifiable ILD |
Although idiopathic pulmonary fibrosis is a progressive and fibrosing ILD, it is a separate, well-defined entity and has not been considered as part of potentially progressive pulmonary fibrosis for this statement.
FIGURE 1.
Schematic representation of the prevalent spectrum of interstitial lung diseases (ILDs) that may be associated with “progressive pulmonary fibrosis (despite management)”. The lowest and highest prevalences across different countries are shown in brackets [14]. CTD: connective tissue disease; G/F PF: genetic and/or familial pulmonary fibrosis; HP: hypersensitivity pneumonitis; iNSIP: idiopathic non-specific interstitial pneumonia; IPAF: interstitial pneumonia with autoimmune features; IPF: idiopathic pulmonary fibrosis; uILD: unclassifiable ILD. Adapted with permission from [15].
Key conclusion
There are many ILD subtypes besides IPF that present as F-ILDs.
Question 3: Approximately what percentage of non-IPF ILDs progress despite management and are there any risk factors for progression or subsets that progress more often?
Based on three studies (n=473), 18–32% of patients with non-IPF ILDs develop “PPF (despite management)” within 61–80 months from the onset of symptoms [5, 7, 9].
A key risk factor for PPF is a usual interstitial pneumonia (UIP) pattern. Each ILD subtype is associated with different risks or odds of progression. Therefore, an accurate knowledge of the initial diagnosis of the ILD subtype is important to enable risk stratification. When an initial ILD diagnosis cannot be made (i.e. uILD), the risk of progression is higher than the other non-IPF ILD subtypes [17]. In the short term, once the disease progresses despite optimal management, the risk of further progression does not appear to be influenced by the ILD subtype [16]. However, in the long term, it has been observed with CTD-ILD, especially the idiopathic inflammatory myositis-ILD subtype, that progression can be significantly slower [18].
Risk factors for progression can be classified as general or disease-specific as listed in table 2 [2, 19–32].
TABLE 2.
Risk factors for the progression of non-idiopathic pulmonary fibrosis interstitial lung diseases (ILDs)
| Risk factor | First author (year) [ref.] | Hazard ratio (95% CI) | p-value |
| General risk factors | |||
| UIP | Flaherty (2019) [2] | 1.53 (−0.68–3.74) | NA |
| BMI | Alakhras (2007) [19] | 0.93 (0.89–0.97) | 0.002 |
| Oxygen desaturation during 6MWT# | Alfieri (2020) [20] | OR¶ 8.7 (4.42–17.3) | NA |
| Disease | |||
| Fibrotic hypersensitivity pneumonitis | Gimenez (2018) [21] | ||
| Decline in FVC by ≥10% | Gimenez (2018) [21] | 4.13 (1.96–8.70) | 0.005 |
| Lower baseline FVC % | Gimenez (2018) [21] | 1.03 (1.01–1.05) | 0.003 |
| Antigen identification | Gimenez (2018) [21] | 0.18 (0.04–0.77) | 0.021 |
| MUC5B+/TLD+ (gene variants) | Ley (2019) [22] | 3.52 (1.87–6.62) | 0.00009 |
| Rheumatoid arthritis-ILD | Zamora-Legoff (2017) [9] | ||
| UIP versus NSIP | Zamora-Legoff (2017) [9] | 3.29 (1.28–8.41) | 0.013 |
| High levels of CCP antibody/anti-CCP2 titres+ | Khan (2021) [23] | 1.05 (1.01–1.10) | 0.01 |
| Smoking, 30 pack-years | Kronzer (2021) [24] | OR¶ 6.06 (2.72–13.5) | NA |
| Fibrotic score on HRCT | Solomon (2016) [25] | 1.02 (1.01–1.03) | 0.0002 |
| Extent of fibrosis on HRCT | Solomon (2016) [25] | 1.12 (1.08–1.17) | <0.000006 |
| Systemic sclerosis | Goh (2017) [26] | ||
| Low baseline FVC <65% and low baseline DLCO ≤55% | Sánchez-Cano (2018) [27]; Hoffmann-Vold (2019) [28] | OR¶ 1.02 (1.01–1.03) | <0.001 |
| Decline in DLCO >15% | Le Gouellec (2017) [29] | 2.03 (1.25–3.29) | <0.005 |
| Decline in KCO >10% | Goh (2017) [26] | 2.35 (1.40–3.95) | <0.001 |
| Fibrotic score on HRCT | Ibrahim (2020) [30] | 2.52 (1.16–5.49) | 0.02 |
| Extent of fibrosis on HRCT (HRCT extent 10–30% and FVC <70%) | Goh (2008) [31] | 3.46 (2.19–5.46) | <0.0005 |
UIP: usual interstitial pneumonia; BMI: body mass index; 6MWT: 6-min walk test; NA: not available; FVC: forced vital capacity; NSIP: non-specific interstitial pneumonia; CCP: cyclic citrullinated peptide; HRCT: high-resolution computed tomography; DLCO: diffusing capacity of the lung for carbon monoxide; KCO: transfer coefficient of the lung for carbon monoxide. #: 6MWT correlates to some extent with DLCO levels, but should not be strictly viewed as a surrogate marker [32]; ¶: hazard ratio for the risk factor was not available in the literature; hence, odds ratio was considered; +: usefulness of assessing anti-citrullinated peptide antibody levels merits future research as this study was done only in women.
HRCT scans are often sufficient for diagnosis and to assess disease progression. However, knowledge of the histological phenotype adds value regarding the risk of progression when CT findings are inconclusive. Surgical lung biopsy is associated with significant risks and can be justified only if the benefits outweigh the risks. Diagnostic transbronchial cryobiopsy or transbronchial lung biopsy, with or without molecular analysis, may be a safer alternative in selected cases. The group does not advocate the performance of biopsy to evaluate the risk of progression.
Key conclusions
1) An accurate initial diagnosis is critical to understanding the likelihood of progression despite management (e.g. scleroderma-related ILD is more likely to progress than pulmonary sarcoidosis).
2) Risk stratification is based on identifying clinical, biochemical, molecular, physiological, histological and/or radiological features that indicate risk of progression.
3) In clinical practice, evaluating the risk of progression is important to better enable prognosis, clearer communication of future risk as well as decisions on monitoring frequency and, sometimes, as a guide to initial treatment.
4) The usefulness of a genetic classifier beyond histology remains open to research and debate.
Question 4: What is the importance of making an accurate initial F-ILD diagnosis?
An accurate initial diagnosis of F-ILD is of paramount importance to a) achieve accurate initial management, b) guide frequency and thoroughness of monitoring, and c) allow communication to the patient of the possible course of a particular ILD to prepare better for the future. In patients with IPF, there is no need to wait for progression to initiate antifibrotic therapy [33–36]. Based on recent data, in scleroderma with F-ILD, there may be benefits with upfront antifibrotic therapy in selected patients [37]. In other F-ILDs, the benefit of initial antifibrotic therapy is still to be evaluated. Many clinicians are attracted to antifibrotic therapy upfront in patients with a UIP pattern in rheumatoid arthritis or F-HP. There is an urgent need for trials to validate such an approach.
Key conclusions
1) An accurate initial ILD diagnosis should always be pursued; initiating inappropriate treatment carries an enhanced risk of treatment failure and adverse events without therapeutic benefit.
2) After establishing a firm diagnosis, the risk factors for progression should be ascertained (refer to table 2).
Question 5: What should be the minimum initial diagnostic work-up for a patient suspected to have F-ILD?
Any patient should undergo investigations to establish the underlying ILD subtype, rule out alternative diagnoses and determine prognosis. Providing an accurate diagnosis is a form of risk stratification. A baseline HRCT with end-inspiratory breath-hold, prone and expiratory images whenever indicated, absence of contrast and full pulmonary function tests (PFTs) are recommended in all patients with symptoms and evidence of progression on lung function [38]. Optimal HRCT scan criteria for PPF are listed in table 3. However, if the initial work-up is insufficient, a panel of tests may be used to better secure the initial diagnosis. Supplementary material S3 features a recommended/suggested panel of auto-antibodies, many providing information on the likelihood of future PPF, which will be useful not only when evaluating an autoimmune aetiology but also in risk stratification [39].
TABLE 3.
Optimal method for acquiring high-resolution computed tomography images for diagnosis and monitoring of fibrosing interstitial lung diseases
| Thin section (e.g. 1–2 mm) |
| Moderate-to-high-frequency or high-spatial-frequency algorithm |
| End inspiration |
| Without contrast |
| Supine±prone |
| Additional prone and expiratory values for monitoring (optional) |
Knowledge of the histological phenotype, whether by surgical lung biopsy or transbronchial cryobiopsy, in uILD may give clarity regarding the risk of progression even if it does not influence immediate therapeutic decisions [40].
Baseline lung function and HRCT results are required, close to the date of starting initial therapy in F-ILDs, to identify subsequent progression despite appropriate management.
Bronchoalveolar lavage cellular analysis could be considered at the outset. A lymphocytosis of <20% in F-HP has been associated with poorer prognosis and worse survival [41]. A precipitin screen is neither essential nor sufficient to make a diagnosis of F-HP. If an unusual antigen is identified at the outset, elimination can be subsequently considered. It is suggested that patients with F-HP in whom an offending antigen has been identified could have a better prognosis than when not identified [42].
Additionally, evaluation for other causes of dyspnoea, including coronary artery disease and pulmonary hypertension, may be appropriate at baseline as associated symptoms may be wrongly ascribed to the progression of ILDs.
Key conclusions
1) Baseline lung function and HRCT results should be available, prior to initiating therapy, to identify subsequent progression accurately.
2) Baseline echocardiography and serum N-terminal pro-brain natriuretic peptide (NT-proBNP) is justified.
Question 6: How should combined patient–doctor decisions on biopsies be made?
Decisions on biopsy procedures are, first and foremost, decisions made via joint doctor–patient discussion. Some patients prefer to have biopsy decisions made by the clinician and this view should always be respected. However, the group believes that patient participation should be actively encouraged, as this is likely to result in better biopsy decisions that take account of patient values regarding the balance of risk and benefit. It is suggested that provision of the following information is likely to empower patients in such discussions [43, 44].
a) The overall risk of the proposed procedure and the existing factors for that patient, which decrease or increase risk, must be explained. Patients should be fully informed of the low risk of mortality due to the procedure and other complications specific to the procedure (transbronchial cryobiopsy: pneumothorax and bleeding; surgical lung biopsy: prolonged air leakage and post-procedural chest pain).
b) It should be clearly indicated whether the biopsy decision is finely balanced medically based on risk/benefit considerations. The underlying premise is that if the decision is otherwise “a close call”, but the patient has a clear preference after an informed discussion, then the decision should no longer be considered as marginal.
c) When a biopsy is proposed, it should be clearly indicated whether the management is likely to change based on biopsy findings. If it is likely that the same management will be instituted with and without biopsy information, patients may choose to accept a diagnosis made with lower confidence and decline a biopsy procedure.
d) When there is diagnostic uncertainty and a biopsy is not recommended due to risk factors, this should be explained. In this way, it is understood that diagnostic certainty cannot be achieved without unacceptable risk.
e) The final combined decision should be supported by the clinician whenever possible when the patient views the result as a change in the initial recommendation. The group believes that this is almost invariably appropriate when biopsy decisions are finely balanced.
f) Occasionally, it is helpful to have an initial discussion and then to resume the discussion when the patient has had time to consider the pros and cons of biopsy.
Key conclusions
1) In the absence of a typical UIP pattern on HRCT, interventional diagnostic procedures should be considered on a case-by-case basis given that identification of UIP on histology is a major prognostic determinant even when a confident final diagnosis of ILD cannot be made.
2) The patient should be at the centre of decisions made on the interventional diagnostic procedures.
Section 2: Prognostication and monitoring
Question 7: How does one define progression?
Early recognition of “PPF (despite management)” is important, as there is insufficient evidence validating the use of antifibrotic therapy in F-ILDs at presentation [45]. No “one-size-fits-all” definition of progression exists. In the real world, various combinations of increasing respiratory symptoms, reductions in lung function (forced vital capacity (FVC) and diffusing capacity of the lung for carbon monoxide (DLCO)) and/or signs of increasing fibrosis on HRCT scan are used to identify progression. However, progression is progression, whether in 6 months or more insidiously. Rapid progression can reduce the threshold for introduction of antifibrotic therapy. Therefore, acute exacerbations need to be differentiated from progression of fibrosis. Although a 10% reduction in FVC was used as a stand-alone inclusion criterion in the INBUILD trial [2], smaller FVC reductions (5–10%) associated with symptomatic or radiological deterioration were the alternative inclusion criteria [46, 47].
In many cases, comparison of serial HRCT images may be enough to reliably determine the extent of progression of fibrosis. When comparing images side by side, it is critical to evaluate identical anatomical slices. Research is ongoing to understand whether these limitations can be overcome by automated methods of identifying disease progression [48].
Key conclusions
1) The frequency of PFTs in follow-up monitoring must be decided on a case-by-case basis. There is no “one-size-fits-all” approach with respect to the progression of F-ILDs because it varies from disease to disease.
2) Serial HRCT should be used to identify progressive fibrosis when serial symptomatic and pulmonary function data are inconclusive.
3) Patients with F-ILD should be monitored by HRCT only when clinically indicated. The interval for follow-up HRCT monitoring should be tailored to individual patient characteristics and the need for supplementary information on progression.
Question 8: Are there any radiological and/or histopathological patterns in PPF that are associated with a poor subsequent prognosis?
The process of follow-up HRCT is gaining more importance given the growing insights into PPF [49]. Changes in the HRCT patterns may be prognostically significant and indicate disease progression. For example, the progression of reticular opacities and/or traction bronchiectasis along with the development of honeycombing may all represent manifestations of PPF [50, 51].
The HRCT patterns can also progress over time in IPF. In one study, 47% of 68 patients with IPF and a probable UIP pattern on initial HRCT progressed to a definite UIP pattern [52].
Key conclusion
HRCT or symptoms alone should not be used as the “sole” markers for progression; instead, they should be used in conjunction with other parameters, especially lung function.
Question 9: Do clinically/radiologically derived scoring systems refine prognostic evaluation in F-ILDs?
In non-IPF F-ILDs, HRCT features at initial diagnosis, including the presence of UIP and/or the severity of traction bronchiectasis, have consistently predicted a higher likelihood of progression [1, 2, 9, 53–55]. HRCT has also been combined with PFTs in the staging system for systemic sclerosis-associated ILD (SSc-ILD) [31].
The clinical course of non-IPF progressive F-ILDs is variable and difficult to estimate. This has been addressed in studies, analysing different clinical, functional, radiological and biological variables, as well as examining the prognostic predictors and staging systems [56–58]. Various scoring systems such as the Composite Physiologic Index and Gender–Age–Physiology (GAP) provide statements of cohort risk but are not reliable in individual patients [59, 60].
Changes in the HRCT patterns and extent of fibrosis over serial scans can help prognostication in IPF [54]. Hwang et al. [61] found that increasing honeycombing in patients with F-ILD on serial HRCT (in addition to the existence of honeycombing at baseline HRCT) over 1 year predicted mortality, although the same was not denoted by lung function measurements.
Various scoring systems such as the modified Medical Research Council Dyspnoea scale, modified Rodnan skin score (for SSc-ILD), quantitative HRCT lung fibrosis score, ILD-GAP model, King's Brief Interstitial Lung Disease Questionnaire and St George's Respiratory Questionnaire have been used in progressive F-ILDs to help prognostication [62–65]. However, most studies are limited by their retrospective nature and small sample size. Until better validated systems are available, the group does not recommend using these routinely for prognosis.
Key conclusion
The group believes that validating a formal radiologically derived scoring system based on HRCT to predict F-ILD progression, both at baseline and in patients with PPF, is an important future research goal.
Question 10: Do blood biomarkers predict the risk of progression, acute exacerbations and mortality in patients with PPF?
Although many serum biomarkers have been suggested for monitoring the progression of PPF, validation in prospective studies has not been achieved [66].
At present, blood biomarker estimations are not suggested by this group, either for baseline diagnosis and prognostication or for monitoring.
Question 11: What are the most appropriate investigations and time frequencies for monitoring PPF?
Pulmonary function and HRCT have largely been used in clinical trials for monitoring progression in F-ILDs [2, 37, 67–69]. Sequential assessments and other appropriate investigations, when necessary, are recommended for a) detecting progression of disease and b) assessing complications and comorbidities.
Monitoring for progression of disease
Pulmonary function tests
PFTs (at least FVC and DLCO) are suggested in the first year at a frequency of at least every 3–4 months [69, 70]. The course of lung function decline in patients with IPF is progressive, but variable [38]. While those with F-HP and idiopathic interstitial pneumonias experienced similar rates of FVC decline when compared with placebo-treated patients in the subgroup analyses [71] in the INBUILD trial, the same is often variable in patients with CTD-ILD and sarcoidosis [18].
A decline in the FVC >10% predicted is a predictor of mortality [21, 25, 26, 72]. Smaller declines in FVC (5–10% predicted) have also been associated with a worse prognosis in IPF [47, 63, 73] and, in combination with symptomatic or imaging decline, they were a key inclusion criterion in the INBUILD trial [2]. Crucially, in the INBUILD trial, FVC progression in the placebo arm was virtually identical to FVC progression in untreated IPF in the INPULSIS cohorts. FVC appears to be a key predictor of future progression, provided it is integrated with symptomatic and imaging change [65]. The group suggests that marginal FVC trends might also be integrated with DLCO trends to increase confidence that FVC decline represents true progression [65, 74].
High-resolution computed tomography
Repeat HRCT scans should be directed by clinical context and lung function decline, and their frequency can vary from patient to patient. In the majority of patients, repeat imaging can be done annually (and even less frequently if the patient is clinically stable or improving), although certain situations may require more frequent HRCTs [69, 75].
Other investigations
A follow-up echocardiography, serum NT-proBNP, 6-min walk test (6MWT) and other serial investigations would be on a case-to-case basis, where progression of symptoms cannot be explained solely by pulmonary disease progression and may reflect worsening of cardiac disease, or the development or worsening of pulmonary hypertension.
Key conclusions
1) PFTs are recommended in the first year at least every 3–4 months, unless clinically indicated otherwise.
2) Serial HRCT is often required less frequently than regular symptom assessment and lung function monitoring.
3) If progression of symptoms cannot be explained solely on the pulmonary work-up, follow-up echocardiography and other investigations may be required on a case-to-case basis.
Question 12: What would be an appropriate way of defining acute exacerbations of F-ILDs?
Acute exacerbations of ILD are classically defined based on symptomatic and imaging changes, with new radiological abnormalities mostly seen in the form of ground-glass opacities that generally represent inflammation or lung injury [76]. The group considered a less explored concept of acute worsening (or exacerbation) of “fibrosis” per se that may be relevant in the context of non-IPF ILDs; however, data on the risk factors for acute exacerbation of PPF are currently inadequate. Once stable after an acute exacerbation of PPF (and drug-induced lung toxicity is also ruled out), fresh imaging and lung function tests are desirable to plan treatment ahead appropriately.
Section 3: Pharmacotherapies for the treatment of PPF
Question 13: What is “management” in PPF?
In this context, there is often a reference to progression despite “standard management” or “standard of care management”, but the use of these terms creates confusion. Plainly, there is no standardised regimen for diseases as disparate as SSc-ILD and asbestosis.
The management of F-HP is a helpful example. In choosing an initial treatment, clinicians should consider antigen status (and the ability to achieve antigen eviction), disease severity at presentation, existence of concurrent inflammation on CT, the distinction between rapid progression and long-term indolence before presentation, the distinction between a UIP pattern at presentation and other patterns of lung fibrosis, findings at bronchoalveolar lavage, age and comorbidity status, presence of side effects early in traditional management, and patient preferences.
In CTD-ILD, these variables (apart from antigen eviction) apply equally and, in addition, the need for systemic treatment must also be included, with discussions between pulmonologists and rheumatologists that include agreement on whether treatment imperatives are primarily systemic, pulmonary or both.
Thus, the initial management cannot be standardised but consists of individualising regimens, case by case and disease by disease.
Key conclusions
1) There is no standardised management regimen that can be applied to all PPF.
2) Initial management, even in individual ILD diagnoses, can vary widely depending on various factors.
Question 14: Is there any incremental benefit of immunosuppressive therapy when added to a background therapy of corticosteroids?
An incremental benefit of immunosuppressive therapy when added to a background therapy of corticosteroids was evaluated in certain types of non-IPF ILDs, including CTD-ILD and F-HP. These studies lack proper methodology (uncontrolled and non-randomised) and, thus, cannot result in firm conclusions. Current practice and accumulative experience over the years, however, reasonably justify this strategy.
Depending on the subtype of PPF, immunosuppression added to the background treatment of steroids may be of varying benefit. For example, this approach may have worthwhile benefits in patients with myositis or F-HP, with possibly less benefits in patients with cryptogenic organising pneumonia.
Key conclusions
1) The long-term side effects with systemic corticosteroid therapy are a cause for concern in the treatment of PPF; alternative long-term immunosuppressive agents may be associated with less side effects [77].
2) A case-by-case and disease-by-disease approach and review are required to assess the added effectiveness of immunosuppressants to the baseline steroid treatment.
Question 15: Is there any benefit of antifibrotic therapy when added to the background corticosteroid and/or immunosuppressive therapy?
Despite limited evidence, recent studies with nintedanib and pirfenidone show that both drugs reduce PPF progression when added to ongoing immunosuppressive therapy. Additionally, safety and tolerability of antifibrotics were acceptable and in line with data in IPF [2, 37, 71, 78, 79]. In the INBUILD trial, FVC decline was reduced by 57% with nintedanib compared with placebo [2]. However, background immunosuppression was not allowed in this trial, except for biologics in rheumatoid arthritis. Analysis of patients with uILD and SSc-ILD where pirfenidone was used in combination with mycophenolate mofetil showed that not only could patients benefit with pirfenidone but it also had an acceptable safety profile [68, 80].
In the SENSCIS trial, treatment with nintedanib over 52 weeks resulted in reduced decline in the annual adjusted mean FVC compared with placebo [37]. About half of the patients (48.3%) were also receiving mycophenolate. Interestingly, in a prespecified subgroup analysis, the annual rate of decline in FVC in the group that received both mycophenolate and nintedanib was lower (26.3 mL per year) than in those who received only nintedanib (55.4 mL per year) [81].
There remains a clinical dilemma regarding whether to intensify the immunosuppressive therapy, introduce an antifibrotic agent or use a combination of these two approaches. Many patients with SSc or other CTDs require immunosuppression for non-pulmonary reasons. More well-designed trials and expert guidance are clearly needed to study the incremental benefits of antifibrotic therapy when added to background immunosuppression [82]. With a UIP pattern on HRCT/pathology, the likelihood of progression is higher and, thus, there is also higher likelihood of these patients benefitting with antifibrotic treatment.
Key conclusions
1) Initial treatment should be based on the precise primary diagnosis.
2) Apart from IPF, SSc-ILD and, possibly, rheumatoid arthritis-associated ILD (UIP), antifibrotic medication should not be considered as a first-line therapy.
3) In PPF, there is growing evidence that antifibrotic therapy reduces lung function decline, regardless of background immunosuppressive therapy.
4) Careful monitoring for adverse events in the patient subgroup treated with combination therapy is advised.
Question 16: Can antifibrotics and immunosuppressants be combined upfront, or combined only sequentially in the context of ongoing disease progression?
Given the lack of evidence to support the use of combination therapy with antifibrotics and immunosuppressants at the initial diagnosis, sequential addition of the antifibrotic [2, 37, 80] is advisable, with an observation period of at least 3–6 months to determine progression despite adequate treatment for the initial diagnosis. Addition or intensification of immunosuppressants may be indicated prior to addition of antifibrotics in some patients with CTD-ILD. In some patients, immunosuppressants may be discontinued when initiating antifibrotic therapy, especially when no benefit has been observed in the course of disease and/or when tolerability is poor.
Key conclusions
1) The group does not advise general upfront combination therapy.
2) Antifibrotics may be administered sequentially in the context of PPF.
Question 17: Are there any issues regarding the tolerance and side effect profile when antifibrotics and immunosuppressants are prescribed together? Is there any blood biochemistry monitoring involved in this process?
In the INBUILD, SENSCIS and LOTUSS trials [2, 37, 80], there was no difference in the adverse events reported in the antifibrotics and immunosuppressants combination therapy group compared with the placebo group (p=0.58) [37, 68, 80]. The combination of antifibrotics and immunosuppressants is therefore considered reasonably safe and well tolerated [83].
There is currently no evidence suggesting a higher risk of hepatotoxicity when immunosuppressants and antifibrotics are combined [2, 77]. The basic mechanisms of liver toxicity for these two groups of drugs differ. Nevertheless, more frequent liver function tests and drug monitoring may be required during combination therapy.
Key conclusion
In patients receiving antifibrotic and immunosuppressant therapy, liver function and haemogram monitoring are advised monthly for the first 3 months, and then every 3 months or more frequently, on a patient-by-patient basis.
Question 18: What are the parameters to consider when choosing between nintedanib and pirfenidone?
Due to lack of head-to-head trials comparing the efficacy of nintedanib with pirfenidone, neither drug can be considered as more efficacious than the other [84].
Although there are more robust data on the efficacy of nintedanib in PPF, this group believes that based on the underlying design and the results of the studies conducted so far [2, 78, 79], as well as nintedanib's mechanism of action, the benefits of slowing progression should be applicable equally well to both pirfenidone and nintedanib (table 4) [85–94].
TABLE 4.
Comparison of antifibrotic agents
| Pirfenidone | Nintedanib | |
| Number of tablets [85 ] | 3–12# | 2 |
| Side effects [86–88] | Nausea, anorexia, weight loss, photosensitivity, rash, elevated liver enzymes | Diarrhoea, weight loss, elevated liver enzymes |
| Outdoor occupation/hobby [86] | + | |
| Anticoagulation therapy [89] | + | |
| Ischaemic heart disease [87, 89] | + | |
| Cost [90] ¶ | ||
| Newly planned/major surgery [91] | + | |
| Impact on quality of life [85] | + | + |
| Prevention of acute exacerbation and/or respiratory-related hospitalisations [92–94] | + | + |
A “+” possibly denotes the preferred drug of choice. #: pill burden will vary, depending on the strengths available in different countries; ¶: it is important to understand that the cost of these drugs varies across countries, insurance providers and healthcare providers (public versus private).
Question 19: For how long should the treatment with corticosteroids and immunosuppressive therapy be continued? What factors would determine the weaning process of these drugs?
There is no evidence regarding treatment duration of combined steroids and immunosuppressive therapy in PPF. There are no trials that have studied the combination of antifibrotics with corticosteroids and/or immunosuppressants to estimate the duration of the use of immunosuppressants. In most patients with an initial diagnosis of non-IPF F-ILD, the corticosteroid treatment has already been commenced or is in the process of being tapered. In specific PPF such as CTD-ILD and F-HP, the group is generally in favour of immunosuppressants (non-corticosteroid) being continued (table 5).
TABLE 5.
Likelihood of a benefit after prolonged therapy with immunosuppressants in progressive pulmonary fibrosis
| Increased likelihood of a benefit after prolonged therapy with immunosuppressants | Decreased likelihood of a benefit after prolonged therapy with immunosuppressants |
| OP pattern | UIP pattern |
| NSIP patterns | Older age |
| Myositis-ILD | Recurrent infections |
| Younger age | Absence of clinical or physiological improvement, with increased risk of side effects |
| Non-pulmonary symptoms that flare up on stoppage | |
| Previous objective response to corticosteroids and/or immunosuppressive therapy in the disease course |
OP: organising pneumonia; NSIP: non-specific interstitial pneumonia; ILD: interstitial lung disease; UIP: usual interstitial pneumonia.
Low-dose corticosteroids may need to be given indefinitely in the following situations:
a) Non-pulmonary symptoms that flare on cessation, including joint pains.
b) Steroid dependency of the lung disease, including sarcoidosis with clear functional decline on withdrawal.
Key conclusions
1) Every attempt should be made to minimise or withdraw regular corticosteroid therapy by using an alternative immunosuppressive agent.
2) The dose and duration of using immunosuppressive agents in PPF will vary from patient to patient.
Section 4: Pulmonary rehabilitation, oxygen and supportive care
Question 20: When should patients with PPF be referred to pulmonary rehabilitation?
This group believes that rehabilitation should be introduced, and subsequently modulated, depending on the course of the disease. The process of referral for rehabilitation in PPF is different when compared with COPD. When referring a patient for pulmonary rehabilitation, it is critical to examine: a) severity of the underlying disease, b) progression of the disease, c) patient's perspective and needs, d) comorbidities, and e) availability of rehabilitation services locally [95]. An initial pulmonary rehabilitation period of 8–12 weeks is likely to be sufficient, with maintenance rehabilitation being decided case by case. Additionally, pulmonary rehabilitation programmes often function as patient support groups.
Yoga is widely practised in many regions in the world within or outside of rehabilitation programmes and can be valuable in this setting, although yogic practices such as kapalabhati [96] can raise intrathoracic pressure, increasing the risk of lung barotrauma, and are preferably avoided.
Key conclusions
1) Pulmonary rehabilitation should begin as soon as possible in patients having PPF along with exercise intolerance.
2) Pulmonary rehabilitation largely aims to improve the quality of life and enable activities of daily living.
Question 21: When should long-term oxygen therapy or home non-invasive ventilation be initiated in patients with PPF?
Long-term oxygen therapy
Long-term oxygen therapy (LTOT) is appropriate in patients with ILD and significant resting hypoxaemia [70, 97–102]. Despite the lack of robust trials on LTOT in ILD, physicians reported symptomatic relief as the main indication for prescribing domiciliary oxygen therapy in ILD [103]. Ambulatory oxygen has been shown to be associated with an improved health-related quality of life in patients with ILD and exertional hypoxaemia [104].
In a randomised controlled trial, comparing palliative oxygen with room air for symptomatic treatment in patients with chronic breathlessness, the benefits of oxygen therapy increased as the hypoxia progressed [105]. Recent American Thoracic Society guidelines have given a conditional recommendation for ambulatory oxygen in patients with ILD having severe exertional hypoxaemia [106]. Further research is required to develop future guidelines for supplemental oxygen use in PPF.
Home non-invasive ventilation
In patients with acute exacerbations of ILD, non-invasive ventilation (NIV) can provide vital support to the patient [107]. NIV plays a role in lowering breathlessness in patients with acute exacerbations of ILD, especially when there are clear advance directives against invasive ventilation [108, 109] and often against hospitalisation itself. Home NIV should be used only after obtaining consent and detailed discussions with the patient and his/her caregivers. However, in an actively dying patient, administering NIV may well prolong the process of dying, often adding little to the person's comfort.
Key conclusions
1) Patients with PPF could largely follow the LTOT protocols provided for patients with COPD and resting hypoxaemia [103].
2) Patients with exercise desaturation should receive ambulatory oxygen with the aim to increase mobility, exercise tolerance and improve the quality of life [103].
3) Patients with borderline hypoxaemia not meeting the requirements for LTOT may still benefit from LTOT due to reduction in breathlessness [110], but LTOT should be considered only after other causes of hypoxia have been excluded.
4) Home NIV for symptom management in ILD (including acute exacerbations of ILD) should be taken up after detailed discussion in advance with the patient and the caregivers, and should be continued only if improvement is seen in the quality of life.
Question 22: When would patients with PPF need referral to a lung transplant programme?
This group argues for early referral for lung transplant in PPF, as recommended in IPF. Transplant centres prefer to evaluate patients before the disease is at the end stage [111].
The timing of listing for transplantation must be carefully evaluated, considering the expected disease course and the risks associated with the transplantation. Earlier referral provides opportunities to address body mass index issues, treat/prevent osteoporosis, avoid deconditioning, and enable forming of relationships between transplant team personnel and patients, all aiding in improving outcomes.
Key conclusion
Refer early for lung transplant, if no overt contraindications exist.
Question 23: In the absence of a lung transplant programme being available or accessible to a patient, or in case of a contraindication to lung transplantation, what would be the best-care approach for further management of the patient?
Physicians must divide this approach into three domains: a) possible lifespan, b) quality of life and symptom management, and c) functional status. In PPF, the optimal way to deal with difficult-to-treat symptoms such as cough or breathlessness may necessitate a considerable shift in the attitude of the healthcare provider(s) towards symptom control. The treating physician may ask the patients certain questions such as:
What have you given up in life to avoid breathlessness?
Where would you like your care to be for the foreseeable future?
Where would you like your care to be when you are much sicker and weaker?
Refer to supplementary material S4 for more such relevant questions.
Non-pharmacological interventions such as positioning of the patient, relaxation techniques, massages and acupressure can be tried to alleviate persistent breathlessness. Low-dose opioids have proven to be safe and effective in reducing the sensation of dyspnoea in patients with chronic breathlessness syndrome [112]. A battery-operated handheld fan can significantly improve the sensation of breathlessness and physical activity [113].
Key conclusions
1) Patients with PPF should be asked about their specific healthcare needs and holistic care should be delivered via a palliative care team wherever available.
2) The goals of care should be decided in consultation with the patient, the patient's family, the treating physician and the palliative care team, as and when appropriate during the illness, and at an appropriate time [114]. This will significantly improve clarity regarding the treatment options available during end-of-life care.
3) In the context of this question, PPF is no different from IPF.
Question 24: When should advance directives be discussed in PPF with chronic respiratory failure?
Advance directive discussions are best guided by taking into consideration the patient's sensitivity regarding their condition and specific case requirements. Although not mandatory, these should be considered case by case; encouraged either at the outset or an appropriate time as rapport develops. Initiation of a conversation after a clinical decline following an acute exacerbation is one such situation. Honest, accurate and structured information regarding the prognosis and outcomes of aggressive medical therapy should be communicated to both the patient and caregivers. A link to an Indian Association of Palliative Care sample advance directive (supplementary material S5) is provided for clinicians interested in discussing advance directives with their patients: https://www.palliativecare.in/living-will/.
Key conclusions
1) Conversations on advance directives in case of PPF are, at the outset, not mandatory but should be encouraged at an appropriate time as and when a rapport develops between the patient and the healthcare provider(s).
2) When the goals of care are being discussed, all possible modalities of management should be highlighted with their risks and benefits to arrive at an informed shared decision.
3) In institutions or geographies where palliative care services are not available or easily accessible, respiratory physicians may be required to provide it themselves.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03187-2021.Supplement (166.5KB, pdf)
Shareable PDF
Acknowledgements
We are grateful to Brajesh Jha and B.K. Ravindra (both from Indegene, Bengaluru, India) for providing a structured literature search based on the keywords chosen by the authors and assembling the bibliography by topic. Indegene received unrestricted financial support from Cipla Ltd (Mumbai, India) to conduct this search. Neither Indegene nor Cipla were included in the evaluation of the literature. Cipla further extended its support by making this statement open access.
Footnotes
Conflict of interest: S.K. Rajan reports grants to their institution, consulting fees and lecture fees from Cipla and Boehringer Ingelheim; speaker fees from the Indian Chest Society; and support from Cipla to attend a European Respiratory Society meeting, all in the 36 months prior to manuscript submission. V. Cottin reports an unrestricted grant to their institution from Boehringer Ingelheim; consulting fees from Boehringer Ingelheim, Roche, Galapagos, Galecto, Shionogi, Fibrogen, RedX Pharma and PureTech; payment or honoraria and support for attending meetings from Boehringer Ingelheim and Roche; and participation on a data safety monitoring board or advisory board for Roche/Promedior, Celgene/Bristol Myers Squibb and Galapagos, all in the 36 months prior to manuscript submission. R. Dhar reports external expert contracts from Cipla and Boehringer Ingelheim; speaker fees from the Indian Chest Society, Cipla and Boehringer Ingelheim; and support for attendance at a European Respiratory Society meeting from Cipla. S. Danoff reports grant funding from Bristol Myers Squibb (Myositis ILD Trial), Boehringer Ingelheim (INBUILD and INBUILD-ON Trials) and Roche/Genentech (TRAIL Trial); royalties from UpToDate; advisory board fees from Boehringer Ingelheim and Lupin Pharma; payment for presentations from the France Foundation; funding for travel to present lectures from Boehringer Ingelheim; and participation on data safety monitoring boards for Galecto and Galapagos, all in the 36 months prior to manuscript submission; as well as a role as senior medical adviser and interim CMO for the Pulmonary Fibrosis Foundation and membership of the board of directors of the American Thoracic Society. K.R. Flaherty reports research grants paid to their institution by Boehringer Ingelheim; and consulting fees paid to them by Boehringer Ingelheim, Roche/Genentech, Blade Therapeutics, Shionogi, DevPro, AstraZeneca, Pure Health, Fibrogen, Sun Pharmaceuticals, Pliant, United Therapeutics, Arrowhead, Lupin, Polarean and PureTech, all in the 36 months prior to manuscript submission. K.K. Brown reports lung fibrosis research grants from the National Heart, Lung, and Blood Institute; being an external science advisor to AbbVie, CSL Behring, Dispersol, Huitai Biomedicine, Lilly, RedX Pharma, Theravance and Translate Bio; being a DMC chair for Biogen; scientific advisory board membership for Blade Therapeutics, Boehringer Ingelheim, Bristol Myers Squibb, DevPro Biopharma, Galapagos NV, Galecto, Open Source Imaging Consortium, Pliant, Sanofi and Third Pole; DMC membership for Humanetics; and a scientific collaboration with Sekisui Medical Co., all in the 36 months prior to manuscript submission; as well as leadership or fiduciary roles for the Fleischner Society and Open Source Imaging Consortium. A. Mohan declares no competing interests. E. Renzoni declares a research contract payment and advisory board meeting payment to their institution by Boehringer Ingelheim; lecture fees paid to their institution by Boehringer Ingelheim, Roche and Chiesi; and registration and travel expenses for the American Thoracic Society conference, paid by Boehringer Ingelheim, all in the 36 months prior to manuscript submission. M. Mohan reports no competing interests. Z. Udwadia reports no competing interests. P. Shenoy reports no competing interests. D. Currow reports intellectual property payments and royalties from Mayne Pharma International Pty Ltd, a manufacturer of sustained-release morphine; and consulting fees from Helsinn Pharmaceuticals, in the 36 months prior to manuscript submission; as well as leadership or fiduciary roles for the Dust Diseases Board of New South Wales and the Board of the Cancer Institute NSW. A. Devraj reports consulting fees from Boehringer Ingelheim, Roche, Galecto, Galapagos, Brainomix and Vicore, all in the 36 months prior to manuscript submission. B. Jankharia reports payment or honoraria to their institution from Cipla India, Lupin India, Boehringer Ingelheim and German Remedies India, all in the 36 months prior to manuscript submission; as well as being a past president of the Indian Musculoskeletal Society and the Indian Radiology and Imaging Association. R. Kulshrestha reports no competing interests. S. Jones reports grants to their institution from Boehringer Ingelheim and Trevi for being Chair of Action for Pulmonary Fibrosis (APF) and President of the European Pulmonary Fibrosis Federation (EU-IPFF) (both these organisations implement projects in partnership with industry; the author is not involved in these projects and does not gain personally); reports payment or honoraria from Boehringer Ingelheim, Vicore and Roche (paid to their institution); and participation on a data safety monitoring board or advisory board as Galecto DSMB for an anti-fibrotic therapy, in the 36 months prior to manuscript submission. C. Ravaglia reports no competing interests. S. Quadrelli reports speaker fees from Boehringer Ingelheim in the 36 months prior to manuscript submission. R. Iyer reports no competing interests. S. Dhooria reports no competing interests. M. Kolb reports research funding for pre-clinical work from Boehringer Ingelheim and Pieris; research funding for a clinical project from Roche; consulting fees from Boehringer Ingelheim, Roche, Horizon, Cipla, AbbVie, Belerophon, Algernon, CSL Behring, United Therapeutics and AstraZeneca; payment or honoraria from Novartis, Boehringer Ingelheim and Roche; payment for court testimony from Roche; participation on a data safety monitoring board or advisory board for Covance and United Therapeutics; and an allowance paid to their institution from the European Respiratory Society relating to their duties as European Respiratory Journal Chief Editor, all in the 36 months prior to manuscript submission. A.U. Wells reports payments or honoraria from Boehringer Ingelheim and Roche; and participation on a data safety monitoring board or advisory board for Boehringer Ingelheim, Roche and Veracyte, all in the 36 months prior to manuscript submission.
References
- 1.Wells AU, Brown KK, Flaherty KR, et al. What's in a name? That which we call IPF, by any other name would act the same. Eur Respir J 2018; 51: 1800692. doi: 10.1183/13993003.00692-2018 [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KR, Wells AU, Cottin V, et al. Nintedanib in progressive fibrosing interstitial lung diseases. N Engl J Med 2019; 381: 1718–1727. doi: 10.1056/NEJMoa1908681 [DOI] [PubMed] [Google Scholar]
- 3.Raghu G, Remy-Jardin M, Richeldi L, et al. Idiopathic pulmonary fibrosis (an update) and progressive pulmonary fibrosis in adults: an official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2022; 205: e18–e47. doi: 10.1164/rccm.202202-0399ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faverio P, Piluso M, De Giacomi F, et al. Progressive fibrosing interstitial lung diseases: prevalence and characterization in two Italian referral centers. Respiration 2020; 99: 838–845. doi: 10.1159/000509556 [DOI] [PubMed] [Google Scholar]
- 5.Guler SA, Winstone TA, Murphy D, et al. Does systemic sclerosis-associated interstitial lung disease burn out? Specific phenotypes of disease progression. Ann Am Thorac Soc 2018; 15: 1427–1433. doi: 10.1513/AnnalsATS.201806-362OC [DOI] [PubMed] [Google Scholar]
- 6.Nasser M, Larrieu S, Si-Mohamed S, et al. Progressive fibrosing interstitial lung disease: a clinical cohort (the PROGRESS study). Eur Respir J 2021; 57: 2002718. doi: 10.1183/13993003.02718-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reiseter S, Gunnarsson R, Mogens Aaløkken T, et al. Progression and mortality of interstitial lung disease in mixed connective tissue disease: a long-term observational nationwide cohort study. Rheumatology 2018; 57: 255–262. doi: 10.1093/rheumatology/kex077 [DOI] [PubMed] [Google Scholar]
- 8.Simpson T, Barratt SL, Beirne P, et al. The burden of progressive fibrotic interstitial lung disease across the UK. Eur Respir J 2021; 58: 2100221. doi: 10.1183/13993003.00221-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zamora-Legoff JA, Krause ML, Crowson CS, et al. Progressive decline of lung function in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol 2017; 69: 542–549. doi: 10.1002/art.39971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wijsenbeek M, Cottin V. Spectrum of fibrotic lung diseases. N Engl J Med 2020; 383: 958–968. doi: 10.1056/NEJMra2005230 [DOI] [PubMed] [Google Scholar]
- 11.American Lung Association . Interstitial lung disease. 2021. www.lung.org/lung-health-diseases/lung-disease-lookup/interstitial-lung-disease Date last accessed: 25 November 2022.
- 12.Wong AW, Ryerson CJ, Guler SA. Progression of fibrosing interstitial lung disease. Respir Res 2020; 21: 32. doi: 10.1186/s12931-020-1296-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Piciucchi S, Tomassetti S, Ravaglia C, et al. From “traction bronchiectasis” to honeycombing in idiopathic pulmonary fibrosis: a spectrum of bronchiolar remodeling also in radiology? BMC Pulm Med 2016; 16: 87. doi: 10.1186/s12890-016-0245-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dhooria S, Agarwal R, Sehgal IS, et al. Spectrum of interstitial lung diseases at a tertiary center in a developing country: a study of 803 subjects. PLoS One 2018; 13: e0191938. doi: 10.1371/journal.pone.0191938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cottin V. Treatment of progressive fibrosing interstitial lung diseases: a milestone in the management of interstitial lung diseases. Eur Respir Rev 2019; 28: 190109. doi: 10.1183/16000617.0109-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Udwadia ZF, Koul PA, Richeldi L. Post-COVID lung fibrosis: the tsunami that will follow the earthquake. Lung India 2021; 38: Suppl., S41–S47. doi: 10.4103/lungindia.lungindia_818_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryerson CJ, Urbania TH, Richeldi L, et al. Prevalence and prognosis of unclassifiable interstitial lung disease. Eur Respir J 2013; 42: 750–757. doi: 10.1183/09031936.00131912 [DOI] [PubMed] [Google Scholar]
- 18.Oldham JM, Lee CT, Wu Z, et al. Lung function trajectory in progressive fibrosing interstitial lung disease. Eur Respir J 2021; 59: 2101396. doi: 10.1183/13993003.01396-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alakhras M, Decker PA, Nadrous HF, et al. Body mass index and mortality in patients with idiopathic pulmonary fibrosis. Chest 2007; 131: 1448–1453. doi: 10.1378/chest.06-2784 [DOI] [PubMed] [Google Scholar]
- 20.Alfieri V, Crisafulli E, Visca D, et al. Physiological predictors of exertional oxygen desaturation in patients with fibrotic interstitial lung disease. Eur Respir J 2020; 55: 1901681. doi: 10.1183/13993003.01681-2019 [DOI] [PubMed] [Google Scholar]
- 21.Gimenez A, Storrer K, Kuranishi L, et al. Change in FVC and survival in chronic fibrotic hypersensitivity pneumonitis. Thorax 2018; 73: 391–392. doi: 10.1136/thoraxjnl-2017-210035 [DOI] [PubMed] [Google Scholar]
- 22.Ley B, Torgerson DG, Oldham JM, et al. Rare protein-altering telomere-related gene variants in patients with chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 2019; 200: 1154–1163. doi: 10.1164/rccm.201902-0360OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan T, Jose RJ, Renzoni EA, et al. A closer look at the role of anti-CCP antibodies in the pathogenesis of rheumatoid arthritis-associated interstitial lung disease and bronchiectasis. Rheumatol Ther 2021; 8: 1463–1475. doi: 10.1007/s40744-021-00362-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kronzer VL, Huang W, Dellaripa PF, et al. Lifestyle and clinical risk factors for incident rheumatoid arthritis-associated interstitial lung disease. J Rheumatol 2021; 48: 656–663. doi: 10.3899/jrheum.200863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Solomon JJ, Chung JH, Cosgrove GP, et al. Predictors of mortality in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2016; 47: 588–596. doi: 10.1183/13993003.00357-2015 [DOI] [PubMed] [Google Scholar]
- 26.Goh NS, Hoyles RK, Denton CP, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol 2017; 69: 1670–1678. doi: 10.1002/art.40130 [DOI] [PubMed] [Google Scholar]
- 27.Sánchez-Cano D, Ortego-Centeno N, Callejas JL, et al. Interstitial lung disease in systemic sclerosis: data from the Spanish Scleroderma Study Group. Rheumatol Int 2018; 38: 363–374. doi: 10.1007/s00296-017-3916-x [DOI] [PubMed] [Google Scholar]
- 28.Hoffmann-Vold AM, Weigt SS, Saggar R, et al. Endotype–phenotyping may predict a treatment response in progressive fibrosing interstitial lung disease. EBioMedicine 2019; 50: 379–386. doi: 10.1016/j.ebiom.2019.10.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Gouellec N, Duhamel A, Perez T, et al. Predictors of lung function test severity and outcome in systemic sclerosis-associated interstitial lung disease. PLoS One 2017; 12: e0181692. doi: 10.1371/journal.pone.0181692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ibrahim IMH, Gamal SM, Salama AM, et al. Systemic sclerosis: correlation between lung abnormalities on high-resolution computed tomography (HRCT) and pulmonary function tests (PFTs). Egypt J Radiol Nucl Med 2020; 51: 98. doi: 10.1186/s43055-020-00220-3 [DOI] [Google Scholar]
- 31.Goh NSL, Desai SR, Veeraraghavan S, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med 2008; 177: 1248–1254. doi: 10.1164/rccm.200706-877OC [DOI] [PubMed] [Google Scholar]
- 32.Deuschle K, Weinert K, Becker MO, et al. Six-minute walk distance as a marker for disability and complaints in patients with systemic sclerosis. Clin Exp Rheumatol 2011; 29: Suppl. 65, S53–S59. [PubMed] [Google Scholar]
- 33.King TE, Bradford WZ, Castro-Bernardini S, et al. A phase 3 trial of pirfenidone in patients with idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2083–2092. doi: 10.1056/NEJMoa1402582 [DOI] [PubMed] [Google Scholar]
- 34.Noble PW, Albera C, Bradford WZ, et al. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011; 377: 1760–1769. doi: 10.1016/S0140-6736(11)60405-4 [DOI] [PubMed] [Google Scholar]
- 35.Richeldi L, du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Engl J Med 2014; 370: 2071–2082. doi: 10.1056/NEJMoa1402584 [DOI] [PubMed] [Google Scholar]
- 36.Richeldi L, Costabel U, Selman M, et al. Efficacy of a tyrosine kinase inhibitor in idiopathic pulmonary fibrosis. N Engl J Med 2011; 365: 1079–1087. doi: 10.1056/NEJMoa1103690 [DOI] [PubMed] [Google Scholar]
- 37.Distler O, Highland KB, Gahlemann M, et al. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med 2019; 380: 2518–2528. doi: 10.1056/NEJMoa1903076 [DOI] [PubMed] [Google Scholar]
- 38.Raghu G, Remy-Jardin M, Myers JL, et al. Diagnosis of idiopathic pulmonary fibrosis. An official ATS/ERS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2018; 198: e44–e68. doi: 10.1164/rccm.201807-1255ST [DOI] [PubMed] [Google Scholar]
- 39.Jee AS, Adelstein S, Bleasel J, et al. Role of autoantibodies in the diagnosis of connective-tissue disease ILD (CTD-ILD) and interstitial pneumonia with autoimmune features (IPAF). J Clin Med 2017; 6: 51. doi: 10.3390/jcm6050051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ryerson CJ, Corte TJ, Myers JL, et al. A contemporary practical approach to the multidisciplinary management of unclassifiable interstitial lung disease. Eur Respir J 2021; 58: 2100276. doi: 10.1183/13993003.00276-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Patolia S, Tamae Kakazu M, Chami HA, et al. Bronchoalveolar lavage lymphocytes in the diagnosis of hypersensitivity pneumonitis among patients with interstitial lung disease. Ann Am Thorac Soc 2020; 17: 1455–1467. doi: 10.1513/AnnalsATS.202005-420OC [DOI] [PubMed] [Google Scholar]
- 42.Raghu G, Remy-Jardin M, Ryerson CJ, et al. Diagnosis of hypersensitivity pneumonitis in adults. An official ATS/JRS/ALAT clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e36–e69. doi: 10.1164/rccm.202005-2032ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cottin V. Lung biopsy in interstitial lung disease: balancing the risk of surgery and diagnostic uncertainty. Eur Respir J 2016; 48: 1274–1277. doi: 10.1183/13993003.01633-2016 [DOI] [PubMed] [Google Scholar]
- 44.King TE, Raj R. Role of lung biopsy in the diagnosis of interstitial lung disease – UpToDate. 2022. www.uptodate.com/contents/role-of-lung-biopsy-in-the-diagnosis-of-interstitial-lung-disease Date last accessed: 25 November 2022.
- 45.Distler O, Brown KK, Distler JHW, et al. Design of a randomised, placebo-controlled clinical trial of nintedanib in patients with systemic sclerosis-associated interstitial lung disease (SENSCISTM). Clin Exp Rheumatol 2017; 35: Suppl. 106, 75–81. [PubMed] [Google Scholar]
- 46.Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005; 26: 153–161. doi: 10.1183/09031936.05.00034505 [DOI] [PubMed] [Google Scholar]
- 47.Zappala CJ, Latsi PI, Nicholson AG, et al. Marginal decline in forced vital capacity is associated with a poor outcome in idiopathic pulmonary fibrosis. Eur Respir J 2010; 35: 830–835. doi: 10.1183/09031936.00155108 [DOI] [PubMed] [Google Scholar]
- 48.Bartholmai BJ, Raghunath S, Karwoski RA, et al. Quantitative computed tomography imaging of interstitial lung diseases. J Thorac Imaging 2013; 28: 298–307. doi: 10.1097/RTI.0b013e3182a21969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Torres PPTES, Rabahi MF, Moreira MdC, et al. Importance of chest HRCT in the diagnostic evaluation of fibrosing interstitial lung diseases. J Bras Pneumol 2021; 47: e20200096. doi: 10.36416/1806-3756/e20200096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Johkoh T, Müller NL, Cartier Y, et al. Idiopathic interstitial pneumonias: diagnostic accuracy of thin-section CT in 129 patients. Radiology 1999; 211: 555–560. doi: 10.1148/radiology.211.2.r99ma01555 [DOI] [PubMed] [Google Scholar]
- 51.Nishimura K, Kitaichi M, Izumi T, et al. Usual interstitial pneumonia: histologic correlation with high-resolution CT. Radiology 1992; 182: 337–342. doi: 10.1148/radiology.182.2.1732946 [DOI] [PubMed] [Google Scholar]
- 52.Salvatore M, Singh A, Yip R, et al. Progression of probable UIP and UIP on HRCT. Clin Imaging 2019; 58: 140–144. doi: 10.1016/j.clinimag.2019.07.003 [DOI] [PubMed] [Google Scholar]
- 53.Kim EJ, Elicker BM, Maldonado F, et al. Usual interstitial pneumonia in rheumatoid arthritis-associated interstitial lung disease. Eur Respir J 2010; 35: 1322–1328. doi: 10.1183/09031936.00092309 [DOI] [PubMed] [Google Scholar]
- 54.Walsh SLF, Sverzellati N, Devaraj A, et al. Connective tissue disease related fibrotic lung disease: high resolution computed tomographic and pulmonary function indices as prognostic determinants. Thorax 2014; 69: 216–222. doi: 10.1136/thoraxjnl-2013-203843 [DOI] [PubMed] [Google Scholar]
- 55.Cottin V, Hirani NA, Hotchkin DL, et al. Presentation, diagnosis and clinical course of the spectrum of progressive-fibrosing interstitial lung diseases. Eur Respir Rev 2018; 27: 180076. doi: 10.1183/16000617.0076-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kapnadak SG, Raghu G. Lung transplantation for interstitial lung disease. Eur Respir Rev 2021; 30: 210017. doi: 10.1183/16000617.0017-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Laporta Hernandez R, Aguilar Perez M, Lázaro Carrasco MT, et al. Lung transplantation in idiopathic pulmonary fibrosis. Med Sci 2018; 6: E68. doi: 10.3390/medsci6030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leard LE, Holm AM, Valapour M, et al. Consensus document for the selection of lung transplant candidates: an update from the International Society for Heart and Lung Transplantation. J Heart Lung Transplant 2021; 40: 1349–1379. doi: 10.1016/j.healun.2021.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SH, Park JS, Kim SY, et al. Comparison of CPI and GAP models in patients with idiopathic pulmonary fibrosis: a nationwide cohort study. Sci Rep 2018; 8: 4784. doi: 10.1038/s41598-018-23073-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wells AU, Desai SR, Rubens MB, et al. Idiopathic pulmonary fibrosis: a composite physiologic index derived from disease extent observed by computed tomography. Am J Respir Crit Care Med 2003; 167: 962–969. doi: 10.1164/rccm.2111053 [DOI] [PubMed] [Google Scholar]
- 61.Hwang JH, Misumi S, Curran-Everett D, et al. Longitudinal follow-up of fibrosing interstitial pneumonia: relationship between physiologic testing, computed tomography changes, and survival rate. J Thorac Imaging 2011; 26: 209–217. doi: 10.1097/RTI.0b013e3181e35823 [DOI] [PubMed] [Google Scholar]
- 62.Du Bois RM, Albera C, Bradford WZ, et al. 6-minute walk distance is an independent predictor of mortality in patients with idiopathic pulmonary fibrosis. Eur Respir J 2014; 43: 1421–1429. doi: 10.1183/09031936.00131813 [DOI] [PubMed] [Google Scholar]
- 63.Reichmann WM, Yu YF, Macaulay D, et al. Change in forced vital capacity and associated subsequent outcomes in patients with newly diagnosed idiopathic pulmonary fibrosis. BMC Pulm Med 2015; 15: 167. doi: 10.1186/s12890-015-0161-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rosa R, Santos AS, Coelho R, et al. The relation of six-minute walk test and lung function in interstitial lung disease. Eur Respir J 2013; 42: Suppl. 57, P2353. [Google Scholar]
- 65.Wells AU. Forced vital capacity as a primary end point in idiopathic pulmonary fibrosis treatment trials: making a silk purse from a sow's ear. Thorax 2013; 68: 309–310. doi: 10.1136/thoraxjnl-2012-202640 [DOI] [PubMed] [Google Scholar]
- 66.Inoue Y, Kaner RJ, Guiot J, et al. Diagnostic and prognostic biomarkers for chronic fibrosing interstitial lung diseases with a progressive phenotype. Chest 2020; 158: 646–659. doi: 10.1016/j.chest.2020.03.037 [DOI] [PubMed] [Google Scholar]
- 67.Buzan MTA, Pop CM. State of the art in the diagnosis and management of interstitial lung disease. Clujul Med 2015; 88: 116–123. doi: 10.15386/cjmed-457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med 2020; 8: 147–157. doi: 10.1016/S2213-2600(19)30341-8 [DOI] [PubMed] [Google Scholar]
- 69.Takizawa A, Kamita M, Kondoh Y, et al. Current monitoring and treatment of progressive fibrosing interstitial lung disease: a survey of physicians in Japan, the United States, and the European Union. Curr Med Res Opin 2021; 37: 327–339. doi: 10.1080/03007995.2020.1860920 [DOI] [PubMed] [Google Scholar]
- 70.Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. doi: 10.1164/rccm.2009-040GL [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wells AU, Flaherty KR, Brown KK, et al. Nintedanib in patients with progressive fibrosing interstitial lung diseases-subgroup analyses by interstitial lung disease diagnosis in the INBUILD trial: a randomised, double-blind, placebo-controlled, parallel-group trial. Lancet Respir Med 2020; 8: 453–460. doi: 10.1016/S2213-2600(20)30036-9 [DOI] [PubMed] [Google Scholar]
- 72.Jo HE, Glaspole I, Grainge C, et al. Baseline characteristics of idiopathic pulmonary fibrosis: analysis from the Australian Idiopathic Pulmonary Fibrosis Registry. Eur Respir J 2017; 49: 1601592. doi: 10.1183/13993003.01592-2016 [DOI] [PubMed] [Google Scholar]
- 73.Du Bois RM, Weycker D, Albera C, et al. Forced vital capacity in patients with idiopathic pulmonary fibrosis: test properties and minimal clinically important difference. Am J Respir Crit Care Med 2011; 184: 1382–1389. doi: 10.1164/rccm.201105-0840OC [DOI] [PubMed] [Google Scholar]
- 74.George PM, Spagnolo P, Kreuter M, et al. Progressive fibrosing interstitial lung disease: clinical uncertainties, consensus recommendations, and research priorities. Lancet Respir Med 2020; 8: 925–934. doi: 10.1016/S2213-2600(20)30355-6 [DOI] [PubMed] [Google Scholar]
- 75.Wijsenbeek M, Kreuter M, Olson A, et al. Progressive fibrosing interstitial lung diseases: current practice in diagnosis and management. Curr Med Res Opin 2019; 35: 2015–2024. doi: 10.1080/03007995.2019.1647040 [DOI] [PubMed] [Google Scholar]
- 76.Collard HR, Ryerson CJ, Corte TJ, et al. Acute exacerbation of idiopathic pulmonary fibrosis. An international working group report. Am J Respir Crit Care Med 2016; 194: 265–275. doi: 10.1164/rccm.201604-0801CI [DOI] [PubMed] [Google Scholar]
- 77.Brown KK, Rajan SK, Shenoy P, et al. The emerging role of mycophenolate mofetil in interstitial lung diseases. Expert Rev Respir Med 2021; 15: 1539–1549. doi: 10.1080/17476348.2021.2001331 [DOI] [PubMed] [Google Scholar]
- 78.Behr J, Prasse A, Kreuter M, et al. Pirfenidone in patients with progressive fibrotic interstitial lung diseases other than idiopathic pulmonary fibrosis (RELIEF): a double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir Med 2021; 9: 476–486. doi: 10.1016/S2213-2600(20)30554-3 [DOI] [PubMed] [Google Scholar]
- 79.Maher TM, Corte TJ, Fischer A, et al. Pirfenidone in patients with unclassifiable progressive fibrosing interstitial lung disease: design of a double-blind, randomised, placebo-controlled phase II trial. BMJ Open Respir Res 2018; 5: e000289. doi: 10.1136/bmjresp-2018-000289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Khanna D, Albera C, Fischer A, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol 2016; 43: 1672–1679. doi: 10.3899/jrheum.151322 [DOI] [PubMed] [Google Scholar]
- 81.Highland KB, Distler O, Kuwana M, et al. Efficacy and safety of nintedanib in patients with systemic sclerosis-associated interstitial lung disease treated with mycophenolate: a subgroup analysis of the SENSCIS trial. Lancet Respir Med 2021; 9: 96–106. doi: 10.1016/S2213-2600(20)30330-1 [DOI] [PubMed] [Google Scholar]
- 82.Wells AU. Pirfenidone in patients with non-IPF progressive fibrotic interstitial lung diseases: expert guidance is urgently needed. Lancet Respir Med 2021; 9: 437–438. doi: 10.1016/S2213-2600(21)00173-9 [DOI] [PubMed] [Google Scholar]
- 83.Cottin V, Richeldi L, Rosas I, et al. Nintedanib and immunomodulatory therapies in progressive fibrosing interstitial lung diseases. Respir Res 2021; 22: 84. doi: 10.1186/s12931-021-01668-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ogura T, Taniguchi H, Azuma A, et al. Safety and pharmacokinetics of nintedanib and pirfenidone in idiopathic pulmonary fibrosis. Eur Respir J 2015; 45: 1382–1392. doi: 10.1183/09031936.00198013 [DOI] [PubMed] [Google Scholar]
- 85.Graney BA, Lee JS. Impact of novel antifibrotic therapy on patient outcomes in idiopathic pulmonary fibrosis: patient selection and perspectives. Patient Relat Outcome Meas 2018; 9: 321–328. doi: 10.2147/PROM.S144425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azuma A. Pirfenidone: antifibrotic agent for idiopathic pulmonary fibrosis. Expert Rev Respir Med 2010; 4: 301–310. doi: 10.1586/ers.10.32 [DOI] [PubMed] [Google Scholar]
- 87.Gulati S, Luckhardt TR. Updated evaluation of the safety, efficacy and tolerability of pirfenidone in the treatment of idiopathic pulmonary fibrosis. Drug Healthc Patient Saf 2020; 12: 85–94. doi: 10.2147/DHPS.S224007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kato M, Sasaki S, Nakamura T, et al. Gastrointestinal adverse effects of nintedanib and the associated risk factors in patients with idiopathic pulmonary fibrosis. Sci Rep 2019; 9: 12062. doi: 10.1038/s41598-019-48593-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kolonics-Farkas AM, Šterclová M, Mogulkoc N, et al. Anticoagulant use and bleeding risk in central European patients with idiopathic pulmonary fibrosis (IPF) treated with antifibrotic therapy: real-world data from EMPIRE. Drug Saf 2020; 43: 971–980. doi: 10.1007/s40264-020-00978-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hadda V, Guleria R. Antifibrotic drugs for idiopathic pulmonary fibrosis: what we should know? Indian J Med Res 2020; 152: 177–180. doi: 10.4103/ijmr.IJMR_90_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-de la Mora DA, Sanchez-Roque C, Montoya-Buelna M, et al. Role and new insights of pirfenidone in fibrotic diseases. Int J Med Sci 2015; 12: 840–847. doi: 10.7150/ijms.11579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Azuma A, Nukiwa T, Tsuboi E, et al. Double-blind, placebo-controlled trial of pirfenidone in patients with idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2005; 171: 1040–1047. doi: 10.1164/rccm.200404-571OC [DOI] [PubMed] [Google Scholar]
- 93.Ley B, Swigris J, Day BM, et al. Pirfenidone reduces respiratory-related hospitalizations in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2017; 196: 756–761. doi: 10.1164/rccm.201701-0091OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Iwata T, Yoshino I, Yoshida S, et al. A phase II trial evaluating the efficacy and safety of perioperative pirfenidone for prevention of acute exacerbation of idiopathic pulmonary fibrosis in lung cancer patients undergoing pulmonary resection: West Japan Oncology Group 6711 L (PEOPLE Study). Respir Res 2016; 17: 90. doi: 10.1186/s12931-016-0398-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nici L, Donner C, Wouters E, et al. American Thoracic Society/European Respiratory Society statement on pulmonary rehabilitation. Am J Respir Crit Care Med 2006; 173: 1390–1413. doi: 10.1164/rccm.200508-1211ST [DOI] [PubMed] [Google Scholar]
- 96.Johnson DB, Tierney MJ, Sadighi PJ. Kapalabhati pranayama: breath of fire or cause of pneumothorax? A case report. Chest 2004; 125: 1951–1952. doi: 10.1378/chest.125.5.1951 [DOI] [PubMed] [Google Scholar]
- 97.Bradley B, Branley HM, Egan JJ, et al. Interstitial lung disease guideline: the British Thoracic Society in collaboration with the Thoracic Society of Australia and New Zealand and the Irish Thoracic Society. Thorax 2008; 63: Suppl. 5, v1–v58. doi: 10.1136/thx.2007.086215 [DOI] [PubMed] [Google Scholar]
- 98.Cottin V, Crestani B, Valeyre D, et al. Diagnosis and management of idiopathic pulmonary fibrosis: French practical guidelines. Eur Respir Rev 2014; 23: 193–214. doi: 10.1183/09059180.00001814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Funke-Chambour M, Azzola A, Adler D, et al. Idiopathic pulmonary fibrosis in Switzerland: diagnosis and treatment. Respiration 2017; 93: 363–378. doi: 10.1159/000464332 [DOI] [PubMed] [Google Scholar]
- 100.Hardinge M, Annandale J, Bourne S, et al. British Thoracic Society guidelines for home oxygen use in adults. Thorax 2015; 70: Suppl. 1, i1–i43. doi: 10.1136/thoraxjnl-2015-206865 [DOI] [PubMed] [Google Scholar]
- 101.Magnet FS, Schwarz SB, Callegari J, et al. Long-term oxygen therapy: comparison of the German and British Guidelines. Respiration 2017; 93: 253–263. doi: 10.1159/000455879 [DOI] [PubMed] [Google Scholar]
- 102.McDonald CF, Whyte K, Jenkins S, et al. Clinical practice guideline on adult domiciliary oxygen therapy: executive summary from the Thoracic Society of Australia and New Zealand. Respirology 2016; 21: 76–78. doi: 10.1111/resp.12678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Khor YH, Renzoni EA, Visca D, et al. Oxygen therapy in COPD and interstitial lung disease: navigating the knowns and unknowns. ERJ Open Res 2019; 5: 00118-2019. doi: 10.1183/23120541.00118-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Visca D, Mori L, Tsipouri V, et al. Effect of ambulatory oxygen on quality of life for patients with fibrotic lung disease (AmbOx): a prospective, open-label, mixed-method, crossover randomised controlled trial. Lancet Respir Med 2018; 6: 759–770. doi: 10.1016/S2213-2600(18)30289-3 [DOI] [PubMed] [Google Scholar]
- 105.Abernethy AP, McDonald CF, Frith PA, et al. Effect of palliative oxygen versus room air in relief of breathlessness in patients with refractory dyspnoea: a double-blind, randomised controlled trial. Lancet 2010; 376: 784–793. doi: 10.1016/S0140-6736(10)61115-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jacobs SS, Krishnan JA, Lederer DJ, et al. Home oxygen therapy for adults with chronic lung disease. An official American Thoracic Society clinical practice guideline. Am J Respir Crit Care Med 2020; 202: e121–e141. doi: 10.1164/rccm.202009-3608ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cottin V, Wollin L, Fischer A, et al. Fibrosing interstitial lung diseases: knowns and unknowns. Eur Respir Rev 2019; 28: 180100. doi: 10.1183/16000617.0100-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Moderno EV, Yamaguti WPS, Schettino GPP, et al. Effects of proportional assisted ventilation on exercise performance in idiopathic pulmonary fibrosis patients. Respir Med 2010; 104: 134–141. doi: 10.1016/j.rmed.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 109.Quill CM, Quill TE. Palliative use of noninvasive ventilation: navigating murky waters. J Palliat Med 2014; 17: 657–661. doi: 10.1089/jpm.2014.0010 [DOI] [PubMed] [Google Scholar]
- 110.Koczulla AR, Schneeberger T, Jarosch I, et al. Long-term oxygen therapy. Dtsch Ärztebl Int 2018; 115: 871–877. doi: 10.3238/arztebl.2018.0871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Glanville AR, Estenne M. Indications, patient selection and timing of referral for lung transplantation. Eur Respir J 2003; 22: 845–852. doi: 10.1183/09031936.03.00039003 [DOI] [PubMed] [Google Scholar]
- 112.Brighton LJ, Miller S, Farquhar M, et al. Holistic services for people with advanced disease and chronic breathlessness: a systematic review and meta-analysis. Thorax 2019; 74: 270–281. doi: 10.1136/thoraxjnl-2018-211589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Barnes-Harris M, Allgar V, Booth S, et al. Battery operated fan and chronic breathlessness: does it help? BMJ Support Palliat Care 2019; 9: 478–481. doi: 10.1136/bmjspcare-2018-001749 [DOI] [PubMed] [Google Scholar]
- 114.Grossman D, Grossman Y, Nadler E, et al. Integrating palliative care assessment tools to enhance understanding of illness trajectory in post-acute care and long-term care. Am J Hosp Palliat Care 2022; 39: 340–344. doi: 10.1177/10499091211018193 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material ERJ-03187-2021.Supplement (166.5KB, pdf)
This one-page PDF can be shared freely online.
Shareable PDF ERJ-03187-2021.Shareable (432.3KB, pdf)