Abstract
Hypertension (HTN) is a well-established risk factor for cardiovascular diseases (CVDs), including ischemic heart disease, stroke, heart failure, and atrial fibrillation. The prevalence of HTN, as well as mortality rates attributable to HTN, continue to increase, particularly in the United States and among Black populations. The risk of HTN involves a complex interaction of genetics and modifiable risk factors, including dietary patterns. In this regard, there is accumulating evidence that links dietary intake of red meat with a higher risk of poorly controlled blood pressure and HTN. However, research on this topic contains significant methodological limitations, which are described in the review. The report provided below also summarizes the available research reports, with an emphasis on processed red meat consumption and how different dietary patterns among certain populations may contribute to HTN-related health disparities. Finally, this review outlines potential mechanisms and provides recommendations for providers to counsel patients with evidence-based nutritional approaches regarding red meat and the risk of HTN, as well as CVD morbidity and mortality.
Keywords: blood pressure, hypertension, nutrition, public health, red meat, risk factor modification
INTRODUCTION: HYPERTENSION AND RED MEAT IN THE CURRENT CONTEXT
Hypertension (HTN) is a modifiable risk factor for cardiovascular disease (CVD), including ischemic heart disease, stroke, and heart failure.1–3 The prevalence of HTN has increased in the United States, where an estimated 44% of adults meet the current criteria for the diagnosis of this condition.4–7 Furthermore, between 2009 and 2019, US mortality rates attributable to HTN increased by an average of 34%, with significantly higher rates among Black individuals (56.7 per 10,000 non-Hispanic Black men) compared with White counterparts (25.7 per 10,000 non-Hispanic White men).7,8
The pathophysiology of HTN involves the complex interaction of genetics and lifestyle factors to include environment, physical activity, and dietary patterns. Even after accounting for potential confounders, accumulating evidence links the dietary consumption of red meat with a higher risk of HTN.9–38 Conversely, when combined with heart-healthy diets, other studies report no significant association with HTN for specific types of red meat.39–44 As such, it remains unclear how changes to contemporary red meat consumption patterns in the United States may serve as an applicable intervention to prevent and decrease HTN-related complications. Some variability in the findings may be attributable to inconsistent definitions of red meat. Based on the available literature, and for the purposes of this review, we defined and categorized “red meat” as unprocessed or processed and included pork-related products in the latter.
With approximately 74% of US adults reporting red meat intake per given day, red meat consumption in the United States is significantly higher compared to other countries.45,46 Indeed, the United States is the second-highest consumer of red meat globally.46 Among U.S populations, red meat consumption is particularly higher among adult men, those of lower educational and socioeconomic statuses (SES), and specific racial and ethnic groups.12–14,45,47 Moreover, these populations are also more likely to consume processed red meat, which is associated with worse CVD and HTN outcomes than the unprocessed type.45,47 Such differences in dietary patterns may perpetuate HTN-related health disparities and highlight the need for clear nutritional guidance related to red meat consumption.
THE CROSS-SECTION BETWEEN RED MEAT AND HTN
Extensive research demonstrates that blood pressure (BP) levels and HTN prevalence are distinctly lower among those who follow a diet devoid of meat.48–51 Cross-sectional studies that specifically assess the relation of red meat and HTN have observed a positive correlation.9–13 The association is reported for both systolic and diastolic BP parameters and a dose-dependent relation is suggested since higher tertiles of red meat consumption are associated with increasingly higher odds of HTN.9–13 Of note, the data also demonstrate that white meat intake (such as poultry) is associated with more favorable HTN outcomes than red meat consumption.9,27–29 Thus, studies that assess the exposure of general meat intake (which includes poultry and sometimes fish) must be carefully interpreted.
Data recently published from the U.S. National Health and Nutrition Examination Survey of 31,314 US adults found that, when compared with the lowest level of red meat intake, those in the fourth and fifth quintiles had a 29% and 39% higher odds of HTN, respectively (P-trend = 0.003).9 Moreover, substituting just one serving of red meat per day with poultry, fish, eggs, dairy, or plant-based protein was associated with an 8%–15% lower odds of HTN.9 Other cross-sectional studies found that red meat consumption is also associated with higher BP in general and more poorly controlled BP among those already with HTN.12,13 For example, a cross-sectional study of participants on hemodialysis with HTN found that red meat, and particularly processed red meat, was significantly associated with higher BP levels.13 Thus, red meat consumption, especially in certain populations, may confer a higher risk for uncontrolled HTN and associated CVD morbidity and mortality.
Since processed red meat has been consistently associated with a higher risk for HTN and CVD, it is critical to specify the type of red meat when evaluating studies that assess risk of HTN.12,13 The higher risk for processed red meat may be related to sodium content, additives, and gut microbiome metabolism into deleterious metabolites (see below). In cross-sectional studies, and when compared to unprocessed red meat, systolic BP was significantly higher with processed red meat consumption.12 Another important consideration throughout this review and among cross-sectional observations is the study sample, since, for example, Asian populations intake considerably less processed red meat compared to Western populations.12,22 As such, some cross-sectional studies have found a null relation among Asian sub-populations when red meat type was not defined.12,52 In contrast, the association is consistent among Black populations, which on average have significantly higher processed red meat intake than other cohorts.34,47,53
ASSOCIATION OF RED MEAT CONSUMPTION WITH CARDIOVASCULAR DISEASE, HTN, AND HTN OUTCOMES IN LONGITUDINAL COHORT STUDIES
Similar to cross-sectional studies, data from longitudinal studies indicate that increased red meat consumption is associated with a higher risk of HTN.27–35 A meta-analysis of cohort studies demonstrated a positive association between red meat intake and HTN risk with a pooled multivariable-adjusted relative risk (RR) of 1.22 (P < 0.001).30 Among the included studies, the Coronary Artery Risk Development in Young Adults (CARDIA) study followed participants for 15 years and found: (i) red meat consumption of ≥1–2 times/day was associated with a 20%–40% higher risk of HTN compared to <0.6 times/day and (ii) a positive dose-dependent relation across increasing quintiles of red meat consumption (p-trend = 0.004).34 Another study followed 80,426 adults on the Dietary Approach to Stop Hypertension (DASH) diet (already low in red meat) and found that after multivariable-adjustment (for body mass index, physical activity, alcohol, total energy intake, smoking, family history, and educational status), the degree of red meat consumption still conferred a significantly higher risk of HTN (first vs. fourth quartile multivariable-adjusted hazard ratio 1.25, 95% CI, 1.11–1.42).33 Importantly, red meat consumption among longitudinal studies has also been associated with increasing BP, more difficult to control HTN, and higher risk of stroke, heart failure, and mortality among those with HTN.23,27,54,55
Longitudinal studies that have assessed the risk of HTN have further demonstrated the importance of the type of red meat consumed (i.e., processed vs. unprocessed).24,25,56 For example, one study observed a significantly higher risk of HTN in those who consumed ≥5 vs. <1 serving/week of processed red meat (5.0 g serving, 17% higher risk), but not with unprocessed red meat.35 Similar to cross-sectional data, one cohort study did not observe a significant relation between red meat consumption and HTN; however, this study was among an Asian population and did not include data on processed vs. unprocessed red meat types.12,41,52 Given that consumption and preparation patterns vary significantly between Asian and Western populations, caution is warranted in generalizing such observations to US adults.12,24,25
The link between red meat consumption and incident CVD morbidity and mortality is well described across longitudinal studies, and supported by a positive dose-dependent association, even amid populations with relatively low intake.16–32,56–58 Recent systematic reviews of such cohort studies have also assessed evidence quality and concluded that red meat consumption is associated with adverse CVD outcomes, with one estimating a pooled RR of 1.37 for coronary heart disease per each additional daily serving.26,57 The association between red meat consumption and CVD outcomes is at times attenuated after accounting for BP or HTN, suggesting that HTN may mediate the link.21 Thus, cohort studies assessing red meat exposure specifically for BP and HTN outcomes provide valuable insight.
Historically, longitudinal observational studies have provided evidence that vegetarian, lacto-ovo, pescatarian, and poultry-inclusive diets are associated with significantly decreased risk of HTN and other adverse CVD outcomes when compared to diets with red meat.30,34,48,59 More recent studies indicate that even in the context of a diet with some red meat consumption, the degree of plant-based intake may offset some of the adverse impact. Indeed, the CARDIA study with an omnivore diet found that plant-food was inversely associated with HTN risk in a dose-dependent manner (p-trend = 0.01).34
LIMITATIONS OF OBSERVATIONAL STUDIES: RED MEAT AND HTN
Observational studies can offer compelling evidence on the association between red meat consumption and HTN, but results must be interpreted in the light of limitations inherent to the methodology. Although such data can provide epidemiologic and diet-disease relation insight, limitations are often underappreciated, especially by the general public and media outlets.60
One potential limitation is reverse causality, which can occur when individuals receive a diagnosis (i.e., HTN), or notice health metric changes (such as worsening BP), then change their diet in attempt to reverse these findings.61 For example, initial epidemiological studies observed a J- or U-shaped association between alcohol use and BP, in which HTN risk was lower for light-to-moderate use but higher when alcohol was not consumed or consumed in high amounts.62 However, this is not supported by other studies that better probe for causality and directionality, and the phenomenon is attributed to those who decreased or ceased alcohol use after an HTN diagnosis.62 Given evidence that individuals tend to make beneficial lifestyle changes after an HTN diagnosis, it is possible that one dietary change would be a reduction in red meat.63 In this case, the association between red meat and HTN risk and morbidity would be attenuated.
A second and more prominent limitation to consider is that of residual confounding. Although longitudinal studies with sufficient multivariable analysis, matching, and follow-up may reduce the likelihood of residual confounding, this likely remains an issue. Indeed, red meat consumption correlates with several factors shown to independently increase the HTN risk, such as SES, gender, age, central obesity, physical activity, smoking, and nutritional knowledge.9,64,65 Moreover, associations may be influenced by wider dietary patterns, given that red meat consumption is also correlated with higher metabolic, saturated fat, and sodium intakes as well as with lower fruit, vegetable, whole grain, and fiber intakes.47,65 Indeed, there is significant evidence on the effects of sodium intake and BP that are difficult to separate from processed meat consumption. Even with large, comprehensive cohort studies, it remains challenging to account for all confounding factors, and thus, to interpret observed diet-disease associations. Such factors may falsely attenuate or strengthen the observed association between red meat consumption and HTN outcomes and should be considered within the context of individual studies.
A third limitation is reliance on self-reported dietary surveys to measure dietary exposures. Such methods must contend with limitations, including possible recall and reporting biases. The gold standard for dietary assessment among self-report options remains aggregating responses across several 24-hour periods (24HRs), with at least one occurring over a weekend. In a 24HR, participants are asked to list, with prompts, all foods consumed within the past 24 hours. Another method is the use of food frequency questionnaires (FFQs), which are appealing for their lower burden to participants and ability to measure habitual intake. Though most FFQs only ask about a limited number of foods, red meat is quite common, and FFQs may be particularly helpful when assessing the effect of diet on HTN since these accrue long-term. Among smaller studies, differences between 24HRs and FFQs results can be notable, but among larger populations, mean red meat intake is often similar, showing less than a 20% difference in results.66 As such, significant disparities can be noted by the dietary survey instrument utilized.
RANDOMIZED CONTROLLED TRIALS: RED MEAT AND HTN
Data from RCTs provide the strongest evidence for probing effects of specific exposures, such as red meat, on health outcomes. Although not all diet-disease relations can be practically evaluated by RCTs, those that have assessed the effect of red meat consumption on HTN outcomes provide insight to potential causality. It must be noted, though, that most of the presented RCTs, and nutritionally-based RCTs in general, do not have adequate follow-up to assess clinical outcomes such as incident CVD morbidity and mortality. Rather, currently available RCTs focus on risk factors and serial markers correlated with disease outcomes. With red meat consumption and HTN risk, the focus on BP changes during RCTs may reflect HTN risk and BP control. However, this may be influenced by bias including the inability to blind participants to diet and unanticipated changes related to the Hawthorne effect. Given these considerations, RCTs with a cross-over component, and that focus on event reduction after the intervention, are particularly useful.
Some of the earliest data come from RCTs that assessed the effect of plant-based diets on BP measures. For example, the Complete Health Improvement Program trial instructed participants on a plant-based diet, low in overall meat, and found that systolic and diastolic BP significantly decreased by 5.2% after just 30 days.67 Another study followed adults with HTN for 1 year after a vegan diet intervention and found 90% were adherent. Notably, not only did BP significantly decrease (by -9 and -5 mmHg for systolic and diastolic parameters, p<0.01 and <0.05, respectively), but 77% were able to discontinue anti-HTN medication during the trial with a sustained BP-lowering effect.68
Among RCTs that specifically consider red meat as the exposure and measure BP outcomes, the overall data appear inconclusive. Two meta-analyses of RCTs evaluated BP outcomes related to overall red meat consumption of ≥0.5 vs. <0.5 servings/day and concluded that there was no significant difference. However, limitations of these analyses included heterogeneity among the individual studies and lack of data regarding processed vs. unprocessed red meat type.43,44 When red meat type was accounted for in a different systemic review with meta-analysis, each additional 100g/day of overall red meat conferred a 14% higher risk of HTN (P-heterogeneity < 0.001; N = 7) and each additional just 50 g/day of processed red meat conferred a 12% higher risk of HTN (P-heterogeneity < 0.001; N = 4).69 Other studies further demonstrate the importance of specific red meat characteristics, such as the type of red meat (processed vs. unprocessed), degree of saturated fat, and preparation methods, in regards to BP and HTN outcomes.41,70
Some studies appear to suggest that red meat consumption may not be as associated with adverse BP outcomes when in conjunction with heart-healthy diets. For example, the OmniHeart study recruited adults with pre-HTN or HTN and compared the impact of a high-protein diet vs a high-carbohydrate diet on BP.39 However, although this study has been cited to support red meat intake to improve HTN risk, consumption amounts were similar between the groups (0.9 servings/day for the high-carbohydrate diet and 1.1 servings/day for the high-protein diet).39 Another RCT evaluated BP outcomes on a DASH-plus diet (DASH with 6 servings/week of lean red meat) vs. a non-DASH high-carbohydrate diet.40 DASH-plus was associated with greater systolic BP reductions compared with the non-DASH diet (−5.6 vs. −2.7 mmHg, P = 0.08 overall and −6.5 vs. −5.2 mmHg, P = 0.08 among those with HTN, respectively), but the mediation analysis suggested that the significant effect was due to weight loss.40 Furthermore, and when compared the standard DASH diet (systolic BP lowering of −7.1 overall and −11.5 mmHg among those with HTN), the results suggest that the DASH-plus addition of red meat consumption may have attenuated the beneficial BP-lowering effect, especially among those already with HTN.40,71
Such findings suggest that the variability among RCT results is in part attributable to the inconsistency of dietary compositions and methodologies, including red meat type and overall sodium intake. These characteristics are also important to consider when discussing possible underlying mechanisms for the relation between red meat consumption and BP, HTN, and adverse HTN-related outcomes.
POTENTIAL MECHANISMS: RED MEAT AND HTN
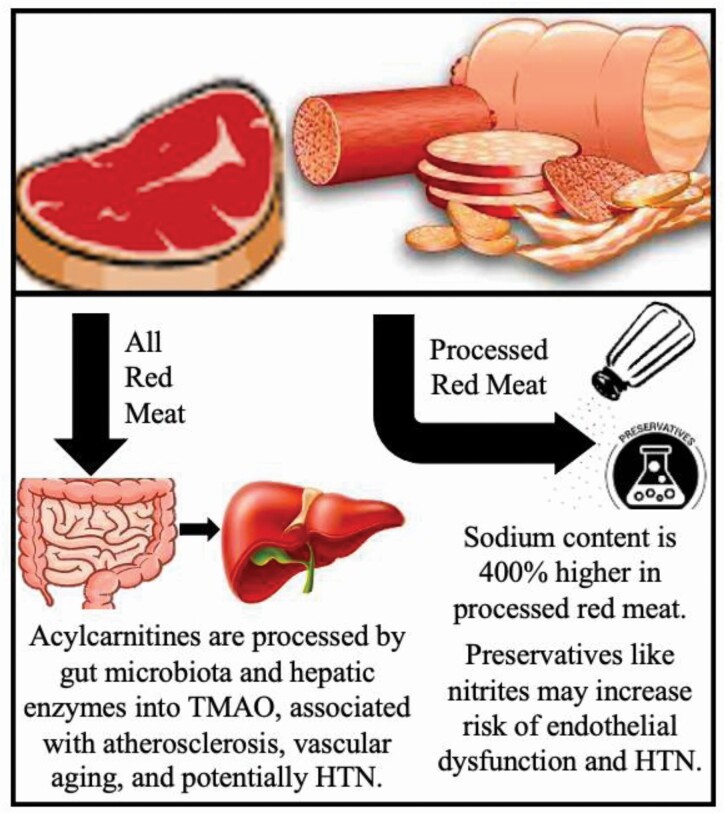
Many hypotheses exist regarding the association of red meat consumption, particularly that of processed red meat, with a higher risk of HTN and related complications (Figure 1). One mechanism that likely contributes is that of sodium content in red meat. It is well established that high sodium intake is associated with higher BP, risk of HTN, and poorly controlled HTN.71 As reviewed in this article, some cohort studies have found that processed, but not unprocessed red meat consumption, is associated with HTN incidence.24,25,56 Underlying these observations is that when compared to unprocessed meat, processed red meat contains approximately 400% more sodium content.12,25,37 In this regard, some studies found that after adjustment for urinary sodium excretion, the relation between red meat and HTN was attenuated or became non-significant, thus further suggesting that sodium content contributes.12 Indeed, the predicted BP effect from high sodium content is considered to account for more than two-thirds of the association between processed red meat consumption and coronary heart disease risk, and is also likely a mediator for the relation with heart failure.72,73
Figure 1.
Red meat consumption, particularly that of processed red meat, may confer an increased risk of HTN via mechanisms including the effects of sodium content, nitrite preservatives, and the metabolite trimethylamine N-oxide (TMAO). Abbreviation: HTN, hypertension.
Other studies suggest that sodium content may not entirely explain how processed red meat intake confers a higher risk for adverse CVD and HTN outcomes. Some literature indicates that red meat also contains high concentrations of nitrite additives for food preservation, which have been linked to endothelial dysfunction and thus may separately confer a higher risk of elevated and dysregulated BP.74 In the United States, processed red meats contain more nitrite additives than unprocessed red meats, perhaps up to an average of 50% more.37,75
Lastly, metabolomics may provide further insight into potential mechanisms that drive the association between red meat consumption and HTN as well as CVD. Small molecule metabolites in the serum can quantitively reflect dietary intake of specific foods and wider dietary patterns.76 There is strong evidence that red meat consumption results in discrete metabolomic signatures.77,78 In fact, red meat and processed red meat are rich in acylcarnitines, which are metabolized via intestinal microbiota and hepatic enzymes into trimethylamine-N-oxide (TMAO) (Figure 1).77,78 This results in higher serum and urine concentrations of the metabolite.79–81 Notably, TMAO is associated with several adverse effects (on fatty acid metabolism, endothelial dysfunction, atherosclerosis, oxidative stress, and vascular aging), and it is positively associated with the clinical Framingham risk score.82 In an RCT with crossover and washout components, red meat consumption was associated with a greater than 2-fold increase in TMAO plasma and urine levels, and discontinuation led to reduced levels within 4 weeks, suggesting a reversible effect.81 Red meat is associated with increased atherosclerosis, more than other foods high in saturated fat, and some studies suggest that this finding may be mediated in part by TMAO.10,83 Moreover, TMAO may lead to HTN via arterial stiffness, which is directly correlated with elevated BP and HTN.82,84
The idea that the relation between red meat consumption and adverse CVD outcomes—including stroke, heart failure, and mortality—may be in part driven by the underlying effect on BP has been postulated widely in the literature.21,23,38,43,54,55,85 Thus, the role of red meat on HTN is of particular importance. Future studies may further assess metabolomic signatures of red meat consumptions, including the processed and unprocessed types with specific measures of arterial stiffness, to better understand the association with BP and HTN outcomes.
SPECIAL POPULATIONS: RED MEAT AND HTN HEALTH DISPARITIES
As previously described, there are significant racial and ethnic differences in HTN prevalence.7,8 Indeed, the prevalence of HTN in the United States is approximately 42% for those of African descent and 28% for White individuals.7 Moreover, Black populations not only tend to develop HTN earlier in life, leading to a longer time to develop HTN-related morbidity and mortality, but they also have higher average BP and worse HTN prognoses compared to White counterparts.86,87 Although poorly understood, pathophysiological differences in nocturnal BP, potassium levels, and salt loading have been implicated.88–91 Effects, though, are multifactorial and potentially include broader factors such as SES, access to medical care, stress, food insecurity, and dietary habits.9,64,87
Studies demonstrate clear differences in red meat consumption and preparation patterns by race and ethnicity. On average, Black individuals consume more red meat than Whites (129 vs. 92 g/week, respectively), especially more of the processed type, with bacon and sausage accounting for much of the difference.47,92,93 Black populations are also less likely to consume unprocessed beef and more likely to obtain fat intake from processed luncheon meats and fried preparations.94 Even among Black individuals who follow a more plant-based diet, the consumption of overall and processed red meat is still above evidence-based recommended levels and higher than in comparable White populations.95
Studies have also shown that the response to BP interventions is different by race and ethnicity. For example, the DASH diet has been shown to be more effective in lowering BP and controlling HTN among Black populations.96 Interestingly, serum levels of the metabolite 1-methylhystidine, a biomarker of red meat consumption in all populations, are only associated with BP in Black participants.53 Taken together, these findings suggest that differences in dietary patterns related to red meat consumption may contribute to HTN-related health disparities. This highlights the need for more research into more personalized dietary approaches which are tailored to individual sociodemographic factors.
EVIDENCE-BASED COUNSELING ON RED MEAT INTAKE AND HTN: NUTRITION AS AN INTERVENTION
The efficacy of antihypertensive medications is currently estimated at 40%–70% and the gap in HTN control is not solely explained by medication non-adherence.97,98 It is well established that diet can serve as an effective nonpharmacologic intervention.50 Vegetarian diets are associated with lower HTN risk, and even when omnivore diets are high in plant-based criteria, the association is modified by amount of red meat consumption.49–51 Although red meat can provide a source of protein, vitamins, micronutrients, and even satiety, the literature suggests it is not essential.58,71 Given that red meat consumption is associated with adverse CVD and HTN outcomes, dietary counseling, especially among specific populations, has important public health implications to bridge evidence-based findings.
The European EAT-Lancet commission guidelines and the Harvard School of Public Health advise against any processed red meat consumption.45,99 Regarding overall and unprocessed red meat consumption, the European, Canadian, and Mexican guidelines each recommend limiting intake to promote health, without more specific advice.45 Currently, the U.S. Dietary Guidelines for Americans treat protein-based foods similarly and do not provide recommendations related to amounts of red or processed meat intake.45 Based on observational studies presented in this review, it may be reasonable to recommend less than 50–100 g (one to two servings) per day of unprocessed red meat.36,69 More importantly, data support a recommendation of zero to less than 50 g (one serving) per day of processed red meat to reduce the risk of HTN and CVD.20,99 Still, RCT data on specific red meat consumption amounts and risk of HTN is currently inconclusive and many broader sociodemographic factors must be considered. Red meat is only one part of the diet, and as a whole, consumption should be generally decreased among US adults in regard to HTN.
Historically in the United States, red meat has been a more prominent source of protein intake compared to other countries, whereby nearly 75% of adults consume a serving of red meat daily. Contemporary research, mostly from observation cohort studies, suggests that consumption of red meat may be associated with adverse health outcomes to include HTN and the associated CVD morbidity and mortality. This higher risk may be due to added sodium content or the metabolism of red meat by the gut microbiome into deleterious metabolites. Additionally, consumption of processed, compared to unprocessed red meat, appears to incur a higher risk of HTN and CVD. Studies suggest that some of this risk can be mitigated by incorporating food choices that are not focused on red meat as the primary source of protein, such as vegetarian and pescatarian diets. Since there is limited clinical trial data to derive definitive conclusions, it is recommended that funding agencies prioritize this design so that clinicians, public health professionals, and the US population can be presented with robust information on the risks and benefits of red meat consumption. Finally, the effects of red meat consumption on the risk for HTN and subsequent CVD may be different by sex and race and ethnicity. Specifically, non-Hispanic Black men have the highest intakes of processed red meat as well as the highest rates of HTN and incident CVD. As such, this population may benefit the most from interventions to reduce red meat consumption.
Contributor Information
Tara Shrout Allen, Division of Preventive Medicine, Department of Family Medicine, University of California, San Diego, San Diego, California, USA.
Harpreet S Bhatia, Division of Cardiovascular Medicine, University of California, San Diego, San Diego, California, USA.
Alexis C Wood, USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, Houston, Texas, USA.
Shabnam R Momin, USDA/ARS Children’s Nutrition Research Center, Baylor College of Medicine, Houston, Texas, USA.
Matthew A Allison, Division of Preventive Medicine, Department of Family Medicine, University of California, San Diego, San Diego, California, USA.
ACKNOWLEDGMENTS
This work by Dr Allen and Dr Bhatia was partially supported by the National Institutes of Health, Grant 5T32HL079891, as part of the University of California, San Diego Integrated Cardiovascular Epidemiology Fellowship. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Work by Dr Wood’s on this project was funded, in part, by USDA/ARS cooperative agreement #58-3092-5-001. The contents of this publication do not necessarily reflect the views or policies of the U.S. Department of Agriculture, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. Dr Wood also reports receiving funding from the Hass Avocado Nutrition Board and the National Cattlemen’s Beef Association. The funders were not aware of nor had any role in the preparation of the manuscript or the decision to publish.
DISCLOSURE
Dr Wood reports receiving funding from the Hass Avocado Nutrition Board, and the National Cattlemen’s Beef Association. The funder was not aware of nor had any role in the preparation of the manuscript or the decision to publish. The other authors have no conflicts of interest to report.
References
- 1. Ezzati M, Oza S, Danaei G, Murray CJ. Trends and cardiovascular mortality effects of state-level blood pressure and uncontrolled hypertension in the United States. Circulation 2008; 117:905–914. [DOI] [PubMed] [Google Scholar]
- 2. Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies C. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 2002; 360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 3. Kjeldsen SE. Hypertension and cardiovascular risk: general aspects. Pharmacol Res 2018; 129:95–99. [DOI] [PubMed] [Google Scholar]
- 4. Benjamin EJ, Muntner P, Alonso A, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Das SR, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Jordan LC, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, O’Flaherty M, Pandey A, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Spartano NL, Stokes A, Tirschwell DL, Tsao CW, Turakhia MP, VanWagner LB, Wilkins JT, Wong SS, Virani SS; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2019 update: a report from the American Heart Association. Circulation 2019; 139:e56–e528. [DOI] [PubMed] [Google Scholar]
- 5. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation 2020; 141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 6. Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernandez-Sola J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundstrom J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 Study. J Am Coll Cardiol 2020; 76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, Boehme AK, Buxton AE, Carson AP, Commodore-Mensah Y, Elkind MSV, Evenson KR, Eze-Nliam C, Ferguson JF, Generoso G, Ho JE, Kalani R, Khan SS, Kissela BM, Knutson KL, Levine DA, Lewis TT, Liu J, Loop MS, Ma J, Mussolino ME, Navaneethan SD, Perak AM, Poudel R, Rezk-Hanna M, Roth GA, Schroeder EB, Shah SH, Thacker EL, VanWagner LB, Virani SS, Voecks JH, Wang NY, Yaffe K, Martin SS. Heart disease and stroke statistics—2022 update: a report from the American Heart Association. Circulation 2022; 145:e153–e639. [DOI] [PubMed] [Google Scholar]
- 8. Muntner P, Carey RM, Gidding S, Jones DW, Taler SJ, Wright JT Jr, Whelton PK. Potential U.S. population impact of the 2017 ACC/AHA high blood pressure guideline. J Am Coll Cardiol 2018; 71:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ba DM, Gao X, Chinchilli VM, Liao D, Richie JP Jr, Al-Shaar L. Red and processed meat consumption and food insecurity are associated with hypertension; analysis of the national health and nutrition examination survey data, 2003–2016. J Hypertens 2022; 40:553–560. [DOI] [PubMed] [Google Scholar]
- 10. Atalic B, Toth J, Atalic V, Radanovic D, Miskulin M, Lucin A. Red and processed meat and cardiovascular risk factors. Acta Med Croatica 2013; 67:211–218. [PubMed] [Google Scholar]
- 11. Masala G, Bendinelli B, Versari D, Saieva C, Ceroti M, Santagiuliana F, Caini S, Salvini S, Sera F, Taddei S, Ghiadoni L, Palli D. Anthropometric and dietary determinants of blood pressure in over 7000 Mediterranean women: the European Prospective Investigation into Cancer and Nutrition-Florence cohort. J Hypertens 2008; 26:2112–2120. [DOI] [PubMed] [Google Scholar]
- 12. Oude Griep LM, Seferidi P, Stamler J, Van Horn L, Chan Q, Tzoulaki I, Steffen LM, Miura K, Ueshima H, Okuda N, Zhao L, Soedamah-Muthu SS, Daviglus ML, Elliott P, Group IR. Relation of unprocessed, processed red meat and poultry consumption to blood pressure in East Asian and Western adults. J Hypertens 2016; 34:1721–1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu PY, Yang SH, Wong TC, Chen TW, Chen HH, Chen TH, Chen YT. Association of processed meat intake with hypertension risk in hemodialysis patients: a cross-sectional study. PLoS One 2015; 10:e0141917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tzoulaki I, Brown IJ, Chan Q, Van Horn L, Ueshima H, Zhao L, Stamler J, Elliott P; International Collaborative Research Group on Macro-Micronutrients and Blood Pressure. Relation of iron and red meat intake to blood pressure: cross sectional epidemiological study. BMJ 2008; 337:a258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang J, Hayden K, Jackson R, Schutte R. Association of red and processed meat consumption with cardiovascular morbidity and mortality in participants with and without obesity: a prospective cohort study. Clin Nutr 2021; 40:3643–3649. [DOI] [PubMed] [Google Scholar]
- 16. Sheehy S, Palmer JR, Rosenberg L. High consumption of red meat is associated with excess mortality among African-American women. J Nutr 2020; 150:3249–3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zheng Y, Li Y, Satija A, Pan A, Sotos-Prieto M, Rimm E, Willett WC, Hu FB. Association of changes in red meat consumption with total and cause specific mortality among US women and men: two prospective cohort studies. BMJ 2019; 365:l2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alshahrani SM, Fraser GE, Sabate J, Knutsen R, Shavlik D, Mashchak A, Lloren JI, Orlich MJ. Red and processed meat and mortality in a low meat intake population. Nutrients 2019; 11:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bellavia A, Stilling F, Wolk A. High red meat intake and all-cause cardiovascular and cancer mortality: is the risk modified by fruit and vegetable intake? Am J Clin Nutr 2016; 104:1137–1143. [DOI] [PubMed] [Google Scholar]
- 20. Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012; 172:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhong VW, Van Horn L, Greenland P, Carnethon MR, Ning H, Wilkins JT, Lloyd-Jones DM, Allen NB. Associations of processed meat, unprocessed red meat, poultry, or fish intake with incident cardiovascular disease and all-cause mortality. JAMA Intern Med 2020; 180:503–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang X, Lin X, Ouyang YY, Liu J, Zhao G, Pan A, Hu FB. Red and processed meat consumption and mortality: dose-response meta-analysis of prospective cohort studies. Public Health Nutr 2016; 19:893–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bechthold A, Boeing H, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, De Henauw S, Michels N, Devleesschauwer B, Schlesinger S, Schwingshackl L. Food groups and risk of coronary heart disease, stroke and heart failure: a systematic review and dose-response meta-analysis of prospective studies. Crit Rev Food Sci Nutr 2019; 59:1071–1090. [DOI] [PubMed] [Google Scholar]
- 24. Abete I, Romaguera D, Vieira AR, Lopez de Munain A, Norat T. Association between total, processed, red and white meat consumption and all-cause, CVD and IHD mortality: a meta-analysis of cohort studies. Br J Nutr 2014; 112:762–775. [DOI] [PubMed] [Google Scholar]
- 25. Micha R, Michas G, Lajous M, Mozaffarian D. Processing of meats and cardiovascular risk: time to focus on preservatives. BMC Med 2013; 11:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Micha R, Shulkin ML, Penalvo JL, Khatibzadeh S, Singh GM, Rao M, Fahimi S, Powles J, Mozaffarian D. Etiologic effects and optimal intakes of foods and nutrients for risk of cardiovascular diseases and diabetes: systematic reviews and meta-analyses from the Nutrition and Chronic Diseases Expert Group (NutriCoDE). PLoS One 2017; 12:e0175149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhuang P, Jiao J, Wu F, Mao L, Zhang Y. Associations of meat consumption and changes with all-cause mortality in hypertensive patients during 11.4-year follow-up: findings from a population-based nationwide cohort. Clin Nutr 2021; 40:1077–1084. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Manson JE, Buring JE, Sesso HD. Meat intake and the risk of hypertension in middle-aged and older women. J Hypertens 2008; 26:215–222. [DOI] [PubMed] [Google Scholar]
- 29. Borgi L, Curhan GC, Willett WC, Hu FB, Satija A, Forman JP. Long-term intake of animal flesh and risk of developing hypertension in three prospective cohort studies. J Hypertens 2015; 33:2231–2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang Y, Zhang DZ. Red meat, poultry, and egg consumption with the risk of hypertension: a meta-analysis of prospective cohort studies. J Hum Hypertens 2018; 32:507–517. [DOI] [PubMed] [Google Scholar]
- 31. Miura K, Greenland P, Stamler J, Liu K, Daviglus ML, Nakagawa H. Relation of vegetable, fruit, and meat intake to 7-year blood pressure change in middle-aged men: the Chicago Western Electric Study. Am J Epidemiol 2004; 159:572–580. [DOI] [PubMed] [Google Scholar]
- 32. Ascherio A, Hennekens C, Willett WC, Sacks F, Rosner B, Manson J, Witteman J, Stampfer MJ. Prospective study of nutritional factors, blood pressure, and hypertension among US women. Hypertension 1996; 27:1065–1072. [DOI] [PubMed] [Google Scholar]
- 33. Lelong H, Blacher J, Baudry J, Adriouch S, Galan P, Fezeu L, Hercberg S, Kesse-Guyot E. Individual and combined effects of dietary factors on risk of incident hypertension: prospective analysis from the NutriNet-Sante cohort. Hypertension 2017; 70:712–720. [DOI] [PubMed] [Google Scholar]
- 34. Steffen LM, Kroenke CH, Yu X, Pereira MA, Slattery ML, Van Horn L, Gross MD, Jacobs DR Jr. Associations of plant food, dairy product, and meat intakes with 15-y incidence of elevated blood pressure in young black and white adults: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Am J Clin Nutr 2005; 82:1169–1177. [DOI] [PubMed] [Google Scholar]
- 35. Lajous M, Bijon A, Fagherazzi G, Rossignol E, Boutron-Ruault MC, Clavel-Chapelon F. Processed and unprocessed red meat consumption and hypertension in women. Am J Clin Nutr 2014; 100:948–952. [DOI] [PubMed] [Google Scholar]
- 36. Iqbal R, Dehghan M, Mente A, Rangarajan S, Wielgosz A, Avezum A, Seron P, AlHabib KF, Lopez-Jaramillo P, Swaminathan S, Mohammadifard N, Zatonska K, Bo H, Varma RP, Rahman O, Yusufali A, Lu Y, Ismail N, Rosengren A, Imeryuz N, Yeates K, Chifamba J, Dans A, Kumar R, Xiaoyun L, Tsolekile L, Khatib R, Diaz R, Teo K, Yusuf S. Associations of unprocessed and processed meat intake with mortality and cardiovascular disease in 21 countries [Prospective Urban Rural Epidemiology (PURE) Study]: a prospective cohort study. Am J Clin Nutr 2021; 114:1049–1058. [DOI] [PubMed] [Google Scholar]
- 37. Micha R, Wallace SK, Mozaffarian D. Red and processed meat consumption and risk of incident coronary heart disease, stroke, and diabetes mellitus: a systematic review and meta-analysis. Circulation 2010; 121:2271–2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yang C, Pan L, Sun C, Xi Y, Wang L, Li D. Red meat consumption and the risk of stroke: a dose-response meta-analysis of prospective cohort studies. J Stroke Cerebrovasc Dis 2016; 25:1177–1186. [DOI] [PubMed] [Google Scholar]
- 39. Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, MillerER, 3rd, Conlin PR, Erlinger TP, Rosner BA, Laranjo NM, Charleston J, McCarron P, Bishop LM, OmniHeart Collaborative Research G. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 2005; 294: 2455–2464. [DOI] [PubMed] [Google Scholar]
- 40. Nowson CA, Wattanapenpaiboon N, Pachett A. Low-sodium dietary approaches to stop hypertension-type diet including lean red meat lowers blood pressure in postmenopausal women. Nutr Res 2009; 29:8–18. [DOI] [PubMed] [Google Scholar]
- 41. Wang Z, Huang Q, Wang L, Jiang H, Wang Y, Wang H, Zhang J, Zhai F, Zhang B. Moderate intake of lean red meat was associated with lower risk of elevated blood pressure in Chinese women: results from the China Health and Nutrition Survey, 1991–2015. Nutrients 2020; 12:1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sayer RD, Wright AJ, Chen N, Campbell WW. Dietary approaches to stop hypertension diet retains effectiveness to reduce blood pressure when lean pork is substituted for chicken and fish as the predominant source of protein. Am J Clin Nutr 2015; 102:302–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guasch-Ferre M, Satija A, Blondin SA, Janiszewski M, Emlen E, O’Connor LE, Campbell WW, Hu FB, Willett WC, Stampfer MJ. Meta-analysis of randomized controlled trials of red meat consumption in comparison with various comparison diets on cardiovascular risk factors. Circulation 2019; 139:1828–1845. [DOI] [PubMed] [Google Scholar]
- 44. O’Connor LE, Kim JE, Campbell WW. Total red meat intake of >/=0.5 servings/d does not negatively influence cardiovascular disease risk factors: a systemically searched meta-analysis of randomized controlled trials. Am J Clin Nutr 2017; 105:57–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Frank SM, Jaacks LM, Batis C, Vanderlee L, Taillie LS. Patterns of red and processed meat consumption across North America: a nationally representative cross-sectional comparison of dietary recalls from Canada, Mexico, and the United States. Int J Environ Res Public Health 2021; 18:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Organisation for Economic Co-operation Development (OECD), Meat Consumption (Indicator) <https://data.oecd.org/agroutput/meat-consumption.htm> 2021. Accessed 13 February 2022. [Google Scholar]
- 47. Wang Y, Beydoun MA, Caballero B, Gary TL, Lawrence R. Trends and correlates in meat consumption patterns in the US adult population. Public Health Nutr 2010; 13:1333–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Chuang SY, Chiu TH, Lee CY, Liu TT, Tsao CK, Hsiung CA, Chiu YF. Vegetarian diet reduces the risk of hypertension independent of abdominal obesity and inflammation: a prospective study. J Hypertens 2016; 34:2164–2171. [DOI] [PubMed] [Google Scholar]
- 49. Appleby PN, Davey GK, Key TJ. Hypertension and blood pressure among meat eaters, fish eaters, vegetarians and vegans in EPIC-Oxford. Public Health Nutr 2002; 5:645–654. [DOI] [PubMed] [Google Scholar]
- 50. Yokoyama Y, Nishimura K, Barnard ND, Takegami M, Watanabe M, Sekikawa A, Okamura T, Miyamoto Y. Vegetarian diets and blood pressure: a meta-analysis. JAMA Intern Med 2014; 174:577–587. [DOI] [PubMed] [Google Scholar]
- 51. Lee KW, Loh HC, Ching SM, Devaraj NK, Hoo FK. Effects of vegetarian diets on blood pressure lowering: a systematic review with meta-analysis and trial sequential analysis. Nutrients 2020; 12:1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hidayat K, Zhu WZ, Peng SM, Ren JJ, Lu ML, Wang HP, Xu JY, Zhou H, Yu LG, Qin LQ. The association between meat consumption and the metabolic syndrome: a cross-sectional study and meta-analysis. Br J Nutr 2021:1–15. [DOI] [PubMed] [Google Scholar]
- 53. Razavi AC, Bazzano LA, He J, Whelton SP, Rebholz CM, Fernandez C, Krousel-Wood M, Li C, Shi M, Nierenberg JL, Li S, Kinchen J, Mi X, Kelly TN. Race modifies the association between animal protein metabolite 1-methylhistidine and blood pressure in middle-aged adults: the Bogalusa Heart Study. J Hypertens 2020; 38:2435–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Chen GC, Lv DB, Pang Z, Liu QF. Red and processed meat consumption and risk of stroke: a meta-analysis of prospective cohort studies. Eur J Clin Nutr 2013; 67:91–95. [DOI] [PubMed] [Google Scholar]
- 55. Ashaye A, Gaziano J, Djousse L. Red meat consumption and risk of heart failure in male physicians. Nutr Metab Cardiovasc Dis 2011; 21:941–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Rohrmann S, Overvad K, Bueno-de-Mesquita HB, Jakobsen MU, Egeberg R, Tjonneland A, Nailler L, Boutron-Ruault MC, Clavel-Chapelon F, Krogh V, Palli D, Panico S, Tumino R, Ricceri F, Bergmann MM, Boeing H, Li K, Kaaks R, Khaw KT, Wareham NJ, Crowe FL, Key TJ, Naska A, Trichopoulou A, Trichopoulos D, Leenders M, Peeters PH, Engeset D, Parr CL, Skeie G, Jakszyn P, Sanchez MJ, Huerta JM, Redondo ML, Barricarte A, Amiano P, Drake I, Sonestedt E, Hallmans G, Johansson I, Fedirko V, Romieux I, Ferrari P, Norat T, Vergnaud AC, Riboli E, Linseisen J. Meat consumption and mortality—results from the European Prospective Investigation into Cancer and Nutrition. BMC Med 2013; 11:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Miller V, Micha R, Choi E, Karageorgou D, Webb P, Mozaffarian D. Evaluation of the quality of evidence of the association of foods and nutrients with cardiovascular disease and diabetes: a systematic review. JAMA Netw Open 2022; 5:e2146705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Battaglia Richi E, Baumer B, Conrad B, Darioli R, Schmid A, Keller U. Health risks associated with meat consumption: a review of epidemiological studies. Int J Vitam Nutr Res 2015; 85:70–78. [DOI] [PubMed] [Google Scholar]
- 59. Papier K, Knuppel A, Syam N, Jebb SA, Key TJ. Meat consumption and risk of ischemic heart disease: a systematic review and meta-analysis. Crit Rev Food Sci Nutr 2021:1–12. [DOI] [PubMed] [Google Scholar]
- 60. Maki KC, Slavin JL, Rains TM, Kris-Etherton PM. Limitations of observational evidence: implications for evidence-based dietary recommendations. Adv Nutr 2014; 5:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sattar N, Preiss D. Reverse causality in cardiovascular epidemiological research: more common than imagined? Am Heart Assoc 2017:2369–2372. [DOI] [PubMed] [Google Scholar]
- 62. Sleight P. Short term and long term effects of alcohol on blood pressure, cardiovascular risk and all cause mortality. Blood Pressure 1996; 5:201–205. [DOI] [PubMed] [Google Scholar]
- 63. Neutel CI, Campbell NR. Changes in lifestyle after hypertension diagnosis in Canada. Can J Cardiol 2008; 24:199–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Clonan A, Roberts KE, Holdsworth M. Socioeconomic and demographic drivers of red and processed meat consumption: implications for health and environmental sustainability. Proc Nutr Soc 2016; 75:367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fogelholm M, Kanerva N, Männistö S. Association between red and processed meat consumption and chronic diseases: the confounding role of other dietary factors. Eur J Clin Nutr 2015; 69:1060–1065. [DOI] [PubMed] [Google Scholar]
- 66. Midthune D, Schatzkin A, Subar AF, Thompson FE, Freedman LS, Carroll RJ, Shumakovich MA, Kipnis V. Validating an FFQ for intake of episodically consumed foods: application to the National Institutes of Health–AARP Diet and Health Study. Public Health Nutr 2011; 14:1212–1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kent L, Morton D, Rankin P, Ward E, Grant R, Gobble J, Diehl H. The effect of a low-fat, plant-based lifestyle intervention (CHIP) on serum HDL levels and the implications for metabolic syndrome status—a cohort study. Nutr Metab (Lond) 2013; 10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lindahl O, Lindwall L, Spangberg A, Stenram A, Ockerman PA. A vegan regimen with reduced medication in the treatment of hypertension. Br J Nutr 1984; 52:11–20. [DOI] [PubMed] [Google Scholar]
- 69. Schwingshackl L, Schwedhelm C, Hoffmann G, Knuppel S, Iqbal K, Andriolo V, Bechthold A, Schlesinger S, Boeing H. Food groups and risk of hypertension: a systematic review and dose-response meta-analysis of prospective studies. Adv Nutr 2017; 8:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Montoro-Garcia S, Velasco-Soria A, Mora L, Carazo-Diaz C, Prieto-Merino D, Avellaneda A, Miranzo D, Casas-Pina T, Toldra F, Abellan-Aleman J. Beneficial impact of pork dry-cured ham consumption on blood pressure and cardiometabolic markers in individuals with cardiovascular risk. Nutrients 2022; 14:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sacks FM, Campos H. Dietary therapy in hypertension. N Engl J Med 2010; 362:2102–2112. [DOI] [PubMed] [Google Scholar]
- 72. Micha R, Michas G, Mozaffarian D. Unprocessed red and processed meats and risk of coronary artery disease and type 2 diabetes—an updated review of the evidence. Curr Atheroscler Rep 2012; 14:515–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kaluza J, Akesson A, Wolk A. Long-term processed and unprocessed red meat consumption and risk of heart failure: a prospective cohort study of women. Int J Cardiol 2015; 193:42–46. [DOI] [PubMed] [Google Scholar]
- 74. Kleinbongard P, Dejam A, Lauer T, Jax T, Kerber S, Gharini P, Balzer J, Zotz RB, Scharf RE, Willers R, Schechter AN, Feelisch M, Kelm M. Plasma nitrite concentrations reflect the degree of endothelial dysfunction in humans. Free Radic Biol Med 2006; 40:295–302. [DOI] [PubMed] [Google Scholar]
- 75. Kim Y, Keogh J, Clifton P. A review of potential metabolic etiologies of the observed association between red meat consumption and development of type 2 diabetes mellitus. Metabolism 2015; 64:768–779. [DOI] [PubMed] [Google Scholar]
- 76. O’Sullivan A, Gibney MJ, Brennan L. Dietary intake patterns are reflected in metabolomic profiles: potential role in dietary assessment studies. Am J Clin Nutr 2011; 93:314–321. [DOI] [PubMed] [Google Scholar]
- 77. Thogersen R, Rasmussen MK, Sundekilde UK, Goethals SA, Van Hecke T, Vossen E, De Smet S, Bertram HC. Background diet influences TMAO concentrations associated with red meat intake without influencing apparent hepatic TMAO-related activity in a porcine model. Metabolites 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med 2013; 368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Abbasi J. TMAO and heart disease: the new red meat risk? JAMA 2019; 321:2149–2151. [DOI] [PubMed] [Google Scholar]
- 80. Wedekind R, Kiss A, Keski-Rahkonen P, Viallon V, Rothwell JA, Cross AJ, Rostgaard-Hansen AL, Sandanger TM, Jakszyn P, Schmidt JA, Pala V, Vermeulen R, Schulze MB, Kuhn T, Johnson T, Trichopoulou A, Peppa E, La Vechia C, Masala G, Tumino R, Sacerdote C, Wittenbecher C, de Magistris MS, Dahm CC, Severi G, Mancini FR, Weiderpass E, Gunter MJ, Huybrechts I, Scalbert A. A metabolomic study of red and processed meat intake and acylcarnitine concentrations in human urine and blood. Am J Clin Nutr 2020; 112:381–388. [DOI] [PubMed] [Google Scholar]
- 81. Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, Koeth RA, Li L, Wu Y, Tang WHW, Krauss RM, Hazen SL. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J 2019; 40:583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Beckman JA, Shibao CA. Trimethylamine-N-Oxide, more red meat for the vascular scientists. Hypertension 2020; 76:40–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bergeron N, Chiu S, Williams PT, S MK, Krauss RM. Effects of red meat, white meat, and nonmeat protein sources on atherogenic lipoprotein measures in the context of low compared with high saturated fat intake: a randomized controlled trial. Am J Clin Nutr 2019; 110:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. O’Rourke M. Arterial stiffness, systolic blood pressure, and logical treatment of arterial hypertension. Hypertension 1990; 15:339–347. [DOI] [PubMed] [Google Scholar]
- 85. Larsson SC, Virtamo J, Wolk A. Red meat consumption and risk of stroke in Swedish men. Am J Clin Nutr 2011; 94:417–421. [DOI] [PubMed] [Google Scholar]
- 86. Yano Y, Reis JP, Tedla YG, Goff DC Jr, Jacobs DR Jr, Sidney S, Ning H, Liu K, Greenland P, Lloyd-Jones DM. Racial differences in associations of blood pressure components in young adulthood with incident cardiovascular disease by middle age: Coronary Artery Risk Development in Young Adults (CARDIA) Study. JAMA Cardiol 2017; 2:381–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104. [DOI] [PubMed] [Google Scholar]
- 88. Profant J, Dimsdale JE. Race and diurnal blood pressure patterns. A review and meta-analysis. Hypertension 1999; 33:1099–1104. [DOI] [PubMed] [Google Scholar]
- 89. Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: results from a 15-year longitudinal study in youth and young adults. Circulation 2006; 114:2780–2787. [DOI] [PubMed] [Google Scholar]
- 90. Andrew ME, Jones DW, Wofford MR, Wyatt SB, Schreiner PJ, Brown CA, Young DB, Taylor HA. Ethnicity and unprovoked hypokalemia in the Atherosclerosis Risk in Communities Study. Am J Hypertens 2002; 15:594–599. [DOI] [PubMed] [Google Scholar]
- 91. Wright JT Jr, Rahman M, Scarpa A, Fatholahi M, Griffin V, Jean-Baptiste R, Islam M, Eissa M, White S, Douglas JG. Determinants of salt sensitivity in black and white normotensive and hypertensive women. Hypertension 2003; 42:1087–1092. [DOI] [PubMed] [Google Scholar]
- 92. Howard G, Cushman M, Moy CS, Oparil S, Muntner P, Lackland DT, Manly JJ, Flaherty ML, Judd SE, Wadley VG, Long DL, Howard VJ. association of clinical and social factors with excess hypertension risk in black compared with white US adults. JAMA 2018; 320:1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Rodriguez C, McCullough ML, Mondul AM, Jacobs EJ, Chao A, Patel AV, Thun MJ, Calle EE. Meat consumption among Black and White men and risk of prostate cancer in the Cancer Prevention Study II Nutrition Cohort. Cancer Epidemiol Biomarkers Prev 2006; 15:211–216. [DOI] [PubMed] [Google Scholar]
- 94. An R, Nickols-Richardson S, Alston R, Shen S, Clarke C. Total, fresh, lean, and fresh lean beef consumption in relation to nutrient intakes and diet quality among U.S. adults, 2005–2016. Nutrients 2019; 11:563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Judd SE, Letter AJ, Shikany JM, Roth DL, Newby PK. Dietary patterns derived using exploratory and confirmatory factor analysis are stable and generalizable across race, region, and gender subgroups in the REGARDS study. Front Nutr 2015; 1:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Svetkey LP, Simons-Morton D, Vollmer WM, Appel LJ, Conlin PR, Ryan DH, Ard J, Kennedy BM. Effects of dietary patterns on blood pressure: subgroup analysis of the Dietary Approaches to Stop Hypertension (DASH) randomized clinical trial. Arch Intern Med 1999; 159:285–293. [DOI] [PubMed] [Google Scholar]
- 97. Egan BM, Li J, Hutchison FN, Ferdinand KC. Hypertension in the United States, 1999 to 2012: progress toward healthy people 2020 goals. Circulation 2014; 130:1692–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nwankwo T, Yoon SS, Burt V, Gu Q. Hypertension among adults in the United States: National Health and Nutrition Examination Survey, 2011–2012. NCHS Data Brief 2013:1–8. [PubMed] [Google Scholar]
- 99. Health HSoP. Five Quick Tips for Following the Healthy Eating Plate and Healthy Eating Pyramid. 2013. [Google Scholar]