Abstract
Autism spectrum disorders (ASD) are a class of neurodevelopmental conditions with a large societal impact. Despite existing evidence suggesting a link between ASD pathogenesis and gut–brain axis dysregulation, there is no systematic review of the treatment of probiotics on ASD and its associated gastrointestinal abnormalities based on the gut–brain axis. Therefore, we performed an analysis for ASD based on preclinical and clinical research to give a comprehensive synthesis of published evidence of a potential mechanism for ASD. On the one hand, this review aims to elucidate the link between gastrointestinal abnormalities and ASD. Accordingly, we discuss gut microbiota dysbiosis regarding gut–brain axis dysfunction. On the other hand, this review suggests that probiotic administration to regulate the gut–brain axis might improve gastrointestinal symptoms, restore ASD-related behavioral symptoms, restore gut microbiota composition, reduce inflammation, and restore intestinal barrier function in human and animal models. This review suggests that targeting the microbiota through agents such as probiotics may represent an approach for treating subsets of individuals with ASD.
Keywords: autism spectrum disorders, probiotics, gut microbiota, gut–brain axis, gastrointestinal abnormalities
1. Introduction
Autism spectrum disorders (ASD) are severe neurodevelopmental disorders that first manifest in newborns and young children (Li and Zhou, 2016). It is marked by deficiencies in social and linguistic skills as well as repetitive behavior patterns (American Psychiatric Association, 2013). According to the Global Burden of Diseases, Injuries, and Risk Factors Study from 2016, 62.2 million individuals worldwide are considered to have ASD (Vos et al., 2017). In addition, its incidence appears to increase over time (Li et al., 2022). Therefore, research on ASD and development of clinical treatment for it are increasingly important.
Numerous comorbidities including epilepsy, anxiety, depression, Tourette syndrome, tic disorders (Howes et al., 2018), gastrointestinal (GI) problems (Chaidez et al., 2014), and intellectual disability are linked to ASD (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators, 2008). Among them, GI problems, such as abdominal pain, constipation, and diarrhea, are the common comorbidities affecting 9 to >70% of children with ASD (Frye and Rossignol, 2016). These GI disorders can be difficult to treat since they are often resistant to standard therapy (Frye and Rossignol, 2016). These GI problems are possibly linked to gut bacteria. The gut–brain axis, which describes the reciprocal interaction between the central nervous system (CNS) and the trillions of microorganisms that reside in the gut, is a potential pathway by which changes in gut microbiota may affect brain functions and development (Wang and Wang, 2016). Thus, the composition and function of gut microbiota can be important for ASD treatment. In this review, we focus on the applicable mechanisms whereby observe how probiotics can be used to treat GI symptoms and central symptoms of ASD through the gut–brain axis.
2. Gastrointestinal abnormalities in ASD
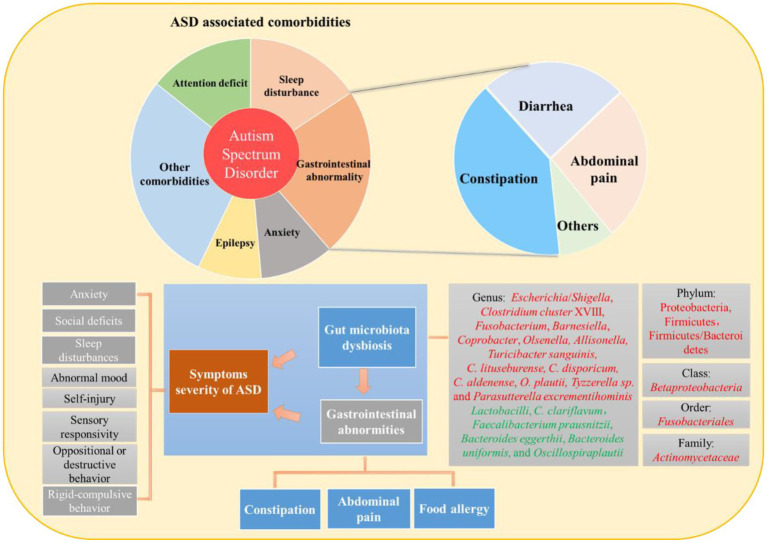
Numerous studies have suggested that patients with ASD often suffer from GI abnormalities; however, the pathogenesis of ASD-related GI problems is not yet fully understood. A recent study has reported two hypotheses for GI abnormalities in ASD (Navarro et al., 2016). One study hypothesized that GI abnormalities may be a manifestation of an underlying inflammatory process, which may be pathophysiologically related to abnormal microbiota. For example, gut microbiota dysbiosis contributes to the pathophysiology of many GI conditions such as inflammatory bowel disease and functional GI disease (Cammarota et al., 2014). The second hypothesis, the functional bowel disease hypothesis, considers that GI abnormalities in ASD may be simply a reflection of sensory over-responsivity to abdominal signals. Gut microbiota dysbiosis, GI abnormities, and ASD symptoms severity show strong relationships (Figure 1). Gastrointestinal abnormalities unrelated to any underlying anatomical or metabolic abnormalities often accompany ASD in humans (Gorrindo et al., 2012). According to a meta-analysis, children with ASD were four times more likely to experience general GI issues, three times more likely to experience constipation or diarrhea, and two times as likely to experience stomach pain (McElhanon et al., 2014). In most cases, the underlying cause for these symptoms was usually recognized as GI abnormalities.
Figure 1.
Interrelationship between gut microbiota dysbiosis, gastrointestinal abnormities, and symptoms severity of ASD.
2.1. Gastrointestinal abnormalities (abdominal pain and constipation) correlate with symptom severity of ASD in humans
The diagnosis of GI abnormalities is typically indicated by certain behavioral complications (Maenner et al., 2012). A previous study reported that GI abnormalities (assessed by the 6-GSI) significantly correlate with symptom severity in ASD (assessed by the autism treatment evaluation checklist) (Adams et al., 2011). Furthermore, constipation is the most common GI symptom observed in autistic children (Srikantha and Mohajeri, 2019). Moreover, the presence and intensity of abdominal pain have been directly associated with the severity of ASD core symptoms (Ding et al., 2017). Such findings suggested a gut–brain axis-mediated relationship between GI anomalies in ASD and behavioral output (Hsiao, 2014). In addition, GI abnormalities have shown a correlation with other ASD comorbidities, such as sleep difficulties, abnormal mood, and social deficits. In comparison with ASD patients without GI symptoms, it has been discovered that GI comorbidity in patients with ASD was associated with increased sleep issues, abnormal mood, argumentative, oppositional, defiant, or destructive behavior, anxiety, sensory responsiveness, rigid compulsive behaviors, self-injury, aggression, lack of expressive language, and social impairment (Nikolov et al., 2009).
2.2. Gut microbiota dysbiosis is associated with ASD-related GI symptoms (constipation, food allergy, and abdominal pain)
Increasing evidence has shown ASD children with constipation have higher relative abundances of Escherichia/Shigella and Clostridium cluster XVIII (Strati et al., 2017), the order Fusobacteriales, the family Actinomycetaceae, and the genera Fusobacterium, Barnesiella, Coprobacter, Olsenella, and Allisonella (Liu et al., 2019), as well as lower Faecalibacterium prausnitzii, Bacteroides eggerthii, Bacteroides uniformis, Oscillospira plautii, and Clostridium (C.) clariflavum amount (Luna et al., 2017). Moreover, the lower abundance of Lactobacilli (Iovene et al., 2017) could be related to constipation in patients with ASD because its depletion was connected with chronic constipation in non-ASD children (Kushak et al., 2017). Patients with ASD who also had allergies had higher relative abundances of the phylum Proteobacteria in their stools, previously linked to autoimmune diseases (Kong et al., 2019). In addition, cecal Betaproteobacteria, ileal and cecal Firmicutes, and the Firmicutes/Bacteroidetes ratio appear to increase in association with food allergies (Williams et al., 2011). It was found that Firmicutes/Bacteroidetes ratio is negatively correlated with allergy/immune function in feces in ASD children (Kong et al., 2019). Turicibacter sanguinis, C. lituseburense, C. disporicum, C. aldenense, and O. plautii levels were higher in ASD children who experienced GI discomfort. Some bacteria may be associated with >1 GI symptoms, for instance, C. aldenense and O. plautii have been also identified in ASD patients with constipation (Luna et al., 2017). Interestingly, some ASD children have extremely high levels of certain bacteria that are positively connected with GI symptoms (i.e., Turicibacter sanguinis) (Kang et al., 2013). More recently, Parracho et al. (2005) demonstrated that ASD children have higher fecal content of the C. histolyticum group-known toxin producers (Hatheway, 1990) than healthy unrelated controls but not than healthy siblings. In addition, high levels of Clostridium species were substantially related to GI issues in patients with ASD, including those with and without GI symptoms.
3. Impaired gut–brain axis in ASD
The hypothalamic–pituitary–adrenal axis, the vagus nerve, the sympathetic and parasympathetic nervous systems with the enteric nervous system, as well as the neuroendocrine and neuroimmune systems are considered to form the gut–brain axis, a biochemical bidirectional signaling pathway between the gut and the brain (Dinan and Cryan, 2015). A growing number of studies has demonstrated a role for it in the etiology of ASD (Li et al., 2017). Brain function was influenced by the gut microbiota via neuroendocrine, neuroimmune, and autonomic nervous systems (Mayer, 2011).
3.1. Gut microbiota dysbiosis leads to immune system dysregulation
The gut microbiota dysbiosis in autism usually results in immune system disorders (Doenyas, 2018). Interleukin-1 (IL-1), interleukin-6 (IL-6), interferon (INF), and tumor necrosis factor (TNF) are chemokines and cytokines that are released by the active immune system which may cross the blood–brain barrier. These mediators attach to brain endothelial cells, triggering immunological reactions (de Theije et al., 2011). A previous study found significantly higher IL-1, IL-6, and IL-8 plasma levels in the ASD group than in the typical development controls (Ashwood et al., 2011). In addition, the immune system is concentrated in and around the gut mucosa, where around 80% of it is located (Critchfield et al., 2011).
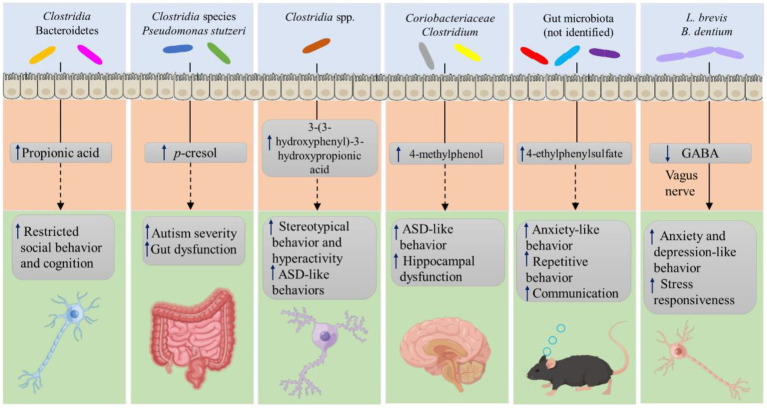
3.2. Gut microbiota metabolism dysbiosis contributes to ASD
Patients with ASD have variable bacterial diversity. According to several studies, they have significantly decreased species diversity and richness (Carissimi et al., 2019; Ma et al., 2019), whereas other studies found the opposite (Finegold et al., 2010; De Angelis et al., 2013). The gut microbiota affects brain physiology through its differential metabolites (Figure 2). Patients with ASD have been shown to have an increase in the level of metabolites including SCFAs, p-cresol, and ammonia, in serum, urine, and fecal samples, which can cause behavioral symptoms and symptoms resembling autism by the vagal pathway (Forsythe et al., 2014). Among these, SCFAs, including acetic acid, propionic acid, butyrate, isobutyric acid, valeric acid, and isovaleric acid, have been considered the major signaling metabolites, which play a critical role in regulating catecholamine production throughout life and in preserving the neurotransmitter phenotype after birth, and have been shown to be important in ASD (Wang et al., 2012). However, some studies found lower levels of these SCFAs, except for propionic and acetic acid, in children with ASD. Clostridium and Bacteroidetes can produce propionic acid, which can penetrate the blood–brain barrier and cause autism-like behaviors, such as impaired and restricted social, behavior, and cognition, by modulating 5-Hydroxytryptamine (5-HT) and dopamine (DA) in the brain (Thomas et al., 2012). In addition, propionic acid decreases the levels of intracellular antioxidants such as GSH and superoxide dismutase and the production of pro-inflammatory cytokines (Wajner et al., 2004). Increased oxidative stress and inflammation are known to play an important role in the pathogenesis of ASD (Bjørklund et al., 2020). Children with autism have been shown to have higher levels of the microbial metabolite p-cresol and its conjugate p-cresyl sulfate in their urine samples. Clostridia species and Pseudomonas stutzeri strains may explain the high p-cresol levels (Altieri et al., 2011). In addition, increasing serum levels of 4-methylphenol, a minor aromatic metabolite generated by gut bacteria, causes ASD-like behavior and hippocampus impairment (Liu et al., 2022). Moreover, ASD patients’ urine contains higher levels of 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, a phenylalanine metabolite generated by Clostridia spp., which may be responsible for the depletion of catecholamines that worsens stereotyped behavior and hyperactivity (Shaw, 2010). In addition, it has been connected to ASD-like behaviors in mouse models. Particularly, offspring of dams treated with the inflammatory molecule poly (I: C) show changes in gut microbiota composition and dysregulation of metabolite concentrations in the serum, including elevated levels of the microbial metabolite 4-ethylphenylsulfate, which led to anxiety-like behavior in mice otherwise untreated (Hsiao et al., 2013). In addition, 5-aminovaleric acid and taurine levels were reduced in recipient mice microbiota from persons with ASD, and both these metabolites can act as aminobutyric acid (GABA) receptor agonists (Sharon et al., 2019). In fact, in the BTBR T + Itpr3tf/J mouse model of ASD, treatment with these two metabolites was effective in reducing repetitive behaviors and improving sociability (Sharon et al., 2019). Tryptophan’s metabolite, indole, serves as a precursor for crucial chemicals including 5-HT and DA (De Angelis et al., 2013) and is able to be synthesized by Alistipes that are higher in individuals with anxiety and depression (Zhang et al., 2015), ultimately disrupting the serotonergic balance in the body. Therefore, an aberrant increase or decrease in gut microbiota-derived metabolites can worsen the symptoms of ASD.
Figure 2.
Gut microbiota-derived metabolites contribute to ASD.
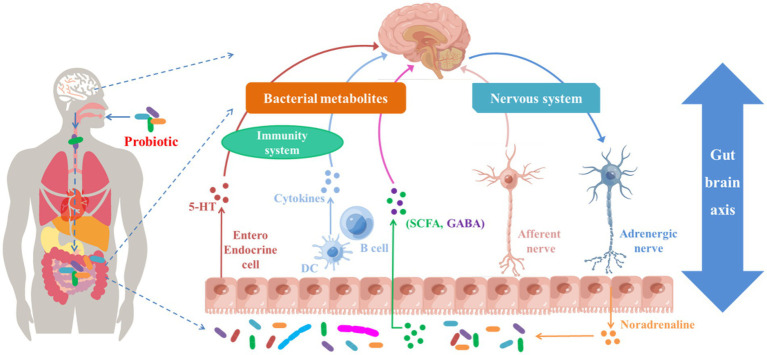
4. Probiotics improve ASD by regulating gut–brain axis
Hence, modulating the microbiota–gut–brain axis with probiotics could be an effective strategy for ASD improvement (Figure 3) and may alleviate GI dysfunction. Several trials have used probiotics to effectively treat GI disorders such as traveler’s diarrhea (McFarland, 2007) and irritable bowel syndrome (Saggioro, 2004). We consider the clinical trials using probiotics in children with ASD are justified based on the similar symptoms, the presence of toxin-producing Clostridium species in ASD persons, the evidence that the achievements in treating irritable bowel syndrome, and the suppression of Clostridium with probiotics. Recently, probiotic therapy has been described as an additional and alternative treatment for ASD (Tas, 2018; Cekici and Sanlier, 2019). Children with ASD aged 5–9 years who received probiotic supplements for 3 months showed improvements in their GI microbiota, GI symptoms, and the severity of their ASD symptoms, behaviors, and functioning (Shaaban et al., 2018). Similarly, a multi-strain combination of 10 probiotics administered for 4 weeks to a 12-year-old child with ASD decreased GI symptoms and improved ASD core symptoms (Grossi et al., 2016).
Figure 3.
Potential ASD treatment responses triggered by probiotics and their metabolites through gut–brain axis.
4.1. Clinical evidence that probiotics regulate gut–brain axis to alleviate ASD symptoms
There is evidence that probiotic supplementation improved the behavior of ASD children through the gut–brain axis (Table 1). The effect of probiotics on psychological conditions such as depression and anxiety is relatively well known (Ng et al., 2018). Children with autism who received vancomycin orally and probiotic Bifidobacterium supplements had significantly higher urine levels of 3-(3-hydroxyphenyl)-3-hydroxyproionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid (Xiong et al., 2016). The first metabolite can cause autistic symptoms by lowering catecholamine levels in the brain (Li and Zhou, 2016). Thus, the decreased levels of those metabolites may be responsible for improved eye contact and less constipation in children with autism (Xiong et al., 2016). A recent study found that probiotics could improve the brain activity of preschoolers with ASD. This was demonstrated by a reduction in frontopolar region power in the beta and gamma bands, a decrease in frontopolar region coherence in the same bands, and a change in frontal asymmetry using electroencephalography (EEG) (Billeci et al., 2022). Beta waves are connected to physiological activity, focus, analytical thought, and states of specific mental commitment or motor activities (Tallon-Baudry, 2003), whereas gamma waves are associated with working memory tasks and several early sensory reactions. When compared to typically developing persons, ASD brains’ resting EEGs frequently show enhanced beta and gamma spectral band activity (Nicotera et al., 2019). Abnormal GABAergic tone in the growth of plasticity and brain function is expected to be involved in the regulation of the EEG frequency bands, which may be partially responsible for the atypical increase in high-frequency bands in ASD (Baumgarten et al., 2016). One of the main features of the neurophysiology of ASD is an altered GABA (the CNS primary inhibitory neurotransmitter) pattern. Atypical brain excitation/inhibition balance, altered neuronal signaling, information processing, and responsive behavior, in particular, may be caused by the deficient inhibitory GABAergic signaling that characterizes patients with ASD (Foss-Feig et al., 2017). After probiotic supplementation, the brain activity of ASD children (showing an improvement in excitatory/inhibitory imbalance) suggested that probiotics can promote a change in brain activity in ASD children toward that of controls. Moreover, probiotic administration was found to promote a shift in brain connections toward a more typical pattern with respect to coherence and asymmetry. Importantly, probiotics could significantly improve the brain function of animals with ASD. For example, immunohistochemical analysis of brain tissues showed that B. longum CCFM1077 could ameliorate microglia activities in the cerebellum of autistic rats, as evidenced by the decreased IBA-1 protein expression (Kong et al., 2022). Furthermore, oral probiotics (containing B. bifidum, B. infantis, and L. helveticus) could inhibit MIA-induced decrease in PV+ neuron numbers in the PFC in adult offspring (Wang et al., 2019). In addition, treatment with Lactobacillus strains reversed the VPA-induced apoptosis and degeneration in the cerebellum (Sunand et al., 2020). All the aforementioned studies suggested that the recovery of brain function after probiotics treatment provides important evidence for the connection between the gut and the brain.
Table 1.
Effect of probiotic supplementation on the health status of individuals with ASD.
| Probiotics | Species | Dose and duration | Effects | References |
|---|---|---|---|---|
| L. plantarum WCSF1 | Children with ASD, 4–16 years old | 4.5 × 1010 CFU per capsule per day for 3 weeks during the 12 weeks study duration | Improve behavioral scores and the stool consistency, increase Enterococci and Lactobacilli group, decreased Clostridium cluster XIVa | Parracho et al. (2010) |
| Any type of probiotic | Children with ASD, 2.5–18 years old | Daily usage (33%) | Lower levels of total SCFAs; Marginally elevate the level of Lactobacillus | Adams et al. (2011) |
| L. acidophilus Rosell-11 | Autistic children, 4–10 years old | 5 × 109 CFU per gram twice a day for 2 months | Decrease D-arabinitol and D-arabinitol/L-arabinitol ration in urine | Kałużna-Czaplińska and Błaszczyk (2012) |
| L. delbruecki, B. longum | 1010 CFU per capsule,3 times a day for 6 months | Decrease the ATEC score, improve speech/language communication, sociability, sensory cognitive awareness, and health/physical behavior | West et al. (2013) | |
| 3 Lactobacillus strains, 2 Bifidobacterium strains, and a Streptococcus strain (60:25:15 ratio) | Children with ASD, 2–9 years old | 3 capsules per day (1 capsule thrice a day) for 4 months | Normalize Bacteroidetes/Fircumutes ratio, increase Bifidobacterium, and reduce Desulfovibrio spp. and TNF-α level in feces | Tomova et al. (2015) |
| L. delbrueckii subsp. Bulgaricus, L. acidophilus, B. breve, B. longum, B. infantis, L. paracasei, L. plantarum, S. thermophiles | Children with ASD, 12 years old | 5 months of treatment period (4 weeks of initial treatment +4 months of follow up treatment);10 months of follow up period | Improve autistic core symptoms and abdominal symptoms | Grossi et al. (2016) |
| Saccharomyces boulardii | A 16-year-old boy with Autism | 3 × 109 CFU per capsule, initiated at 6 capsules daily (2 at breakfast, 2 at lunch, 1 at dinner, and 1 at bedtime), 12 capsules daily after 1 weeks, and 24 capsules after 3 months | Reduce obsessive compulsive disorder and self-injurious behavior | Kobliner et al. (2018) |
| B. longum, L. rhamnosus, L. acidophilus | Autistic children, age from 5–9 years old | 1 × 108 CFU per gram, 5 g per day for 3 months | Decrease severity of the ASD and GI symptoms; Increase abundances of Bifidobacteria and Lactobacillus | Shaaban et al. (2018) |
| L. rhamnosus, L. paracasei and B. longum | Autistic children aged between 9–12 years old | 2 × 1010 CFU, once daily for 6 weeks | Improve autistic symptoms (assessed by ATEC) | Tharawadeephimuk et al. (2019) |
| 6 bacteria (the strain was not shown) | Children with ASD, age from 3–8 years old | Each bacteria was 1 × 109 CFU/gram, 6 g per day, in combination with applied behavior analysis training for 4 weeks. | Alleviate the autism symptom (assessed by ATEC scores); Improve the GI symptom (assessed by a GI questionnaire) | Niu et al. (2019) |
| S. thermophilus, B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, L. delbrueckii subsp. bulgaricus | Children with ASD, age range from 18–72 months | 4.5 × 1011 bacteria each packet, 2 packets/day in the first month and 1packet/day in the following 5 months | Decline the ADOS scores in ASD children without GI symptoms; Improve GI symptoms, adaptive functioning, and sensory profiles in ASD children with GI symptoms; | Santocchi et al. (2020) |
| L. plantarum PS128 | Autistic children and adolescents aged 45–127 months | 3 × 1010 CFUs and 6 × 1010 CFUs of the probiotic if children weight was less than 30 kg and a higher weight, respectively. | Improve the Clinical Global Impression (CGI) scores | Mensi et al. (2021) |
| L. plantarum PS128 | Individuals with ASD aged 3–20 years | Combination therapy of daily 2 capsules (6 × 1010 CFUs) for 28 weeks and oxytocin starting on week 16 | Improve social and behavioral measurements, the ABC total score, ABC stereotyped behavior sub-score, and SRS cognition sub-score in a trend; Significantly improve Clinical Global Impression; enrich beneficial bacteria (Blautia, Barnesiella, ChristensenellaceaeR7, and Ruminococcaceae UCG-002) in the gut; decrease IL-1β in serum | Kong et al. (2021) |
| S. thermophilus, B. breve, B. longum, B. infantis, L. acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. Bulgaricus | Children aged 18–72 months diagnosed with ASD | A commercial probiotics formulation (the number of bacteria was not shown) | Decrease the power in frontopolar regions in β and γ bands, increase coherence in the same bands, and shift the frontal asymmetry | Billeci et al. (2022) |
| Bifidobacterium spp. and Lactobacillus spp. | Children with ASD aged 2–5 years | 108 bacteria/g, 10 grams daily for 3 months | Significantly increase Bifidobacterium spp. and Lactobacillus spp. in the stool; improve autism scale, sleep disturbances, communication to speak, social networking, and hyperactivity; reducing GI symptoms | Meguid et al. (2022) |
4.2. Preclinical evidence that probiotics regulate gut–brain axis to alleviate autism
There is no clear explanation for the regulatory effects of probiotic supplementation on the gut–brain axis in humans, but there are numerous preclinical studies in animal models of ASD (Table 2). Probiotics have been shown to prevent Candida from colonizing the stomach (Romeo et al., 2011), and Bifidobacterium (B.) longum BB536 could modulate Clostridium (decreased the harmful C. perfringens and increased Clostridium cluster IV) populations and rescue social impairment in a rodent model of autism induced by PPA (Abuaish et al., 2021). Some Clostridium species generate p-cresol, which has been suggested as a potential urine biomarker for autism (Persico and Napolioni, 2013). Moreover, Lactobacillus (L.) plantarum ST-III could ameliorate the social deficits, self-grooming, and freezing times and increase the abundance of the beneficial Lachnospiraceae and decrease that of Alistipes in a mouse model of ASD (offspring of pregnant mice exposure to triclosan) (Guo et al., 2022). The gut microbiota contains several members of the Lachnospiraceae family, which has beneficial effects on human health (David et al., 2014), as they can increase the synthesis of the SCFAs acetate and butyrate (Byndloss et al., 2017) as well as boost the conversion of primary to secondary bile acids and reduce the generation of pro-inflammatory cytokines, being also crucial in supplying energy to the host (Smith et al., 2013). Tryptophan is transformed into indoles by Alistipes, which ultimately throws off the body’s serotonergic equilibrium. A previous study found a higher presence of Alistipes in depressed and anxious individuals (Zhang et al., 2015). Treatment with L. helveticus CCFM1076 significantly reduced Turicibacter abundance in the gut and increased butyric acid levels in the cecum contents of valproic acid (VPA)-treated rats (Kong et al., 2021). In the BTBR mouse model of autism, probiotic L. rhamnosus therapy favorably influences the microbiota–gut–brain axis favorably (Pochakom et al., 2022), as indicated by a reduction in behavioral deficits in social novelty preference, increased microbial richness, phylogenetic diversity, presence of potential anti-inflammatory (Anaeroplasma and Christensenellaceae) and butyrate-producing taxa (Acetatifactor, Lachnospiraceae, and Butyricicoccus), and elevation of 5-aminovaleric acid and choline in serum and in the prefrontal cortex (PFC), respectively. Moreover, a mixture of probiotics VSL#3 significantly improved sociability, social interaction, anxiety-liked behavior, and behavioral despair, while restoring the Bacteroidetes/Firmicutes ratio induced by prenatal VPA exposure (Adıgüzel et al., 2022).
Table 2.
Effect of probiotic supplementation on the health status of animal models with ASD.
| Probiotics | Species | Dose and duration | Effects | References |
|---|---|---|---|---|
| L. rhamnosus JB-1 | Adult male BALB/c mice | 1 × 109 CFU of bacteria given orally every day for 28 days | Affect brain function through the vagus nerve | Bravo et al. (2011) |
| Bacteroides fragilis NCTC 9343 | Offspring of pregnant C57BL/6 N mice injected i.p. on E12.5 with 20 mg/kg viral mimic poly(I:C) | 1010 CFU in sugar-free applesauce over standard food pellets every other day for 6 days at weaning | Restore intestinal permeability, partly improve gut microbiota imbalance, improve communication, repetition, sensorimotor and anxiety-like behavioral abnormalities | Hsiao et al. (2013) |
| L. reuteri MM4-1A | Shank3 mutant mice | 109 bacteria reconstituted in a volume of 200 μL of PBS, twice a week for 3 weeks at 8 weeks of age | Attenuate unsocial behavior, decrease repetitive behaviors, and affect GABA receptor gene and protein levels in multiple brain regions | Tabouy et al. (2018) |
| L. reuteri MM4-1A | Offspring of C57Bl6/J mice access to HFD | 108 bacteria reconstituted in drinking water, access to water ad libitum for 4 weeks | Increase the oxytocin level of the hypothalamus and stimulate neurons in the ventral tegmental area of the midbrain | Buffington et al. (2016) |
| B. bifidum, B. infantis and L. helveticus | Offspring of pregnant C57BL/6 J mice injected i.p. on E12.5 with 20 mg/kg viral mimic poly(I:C) | 1.9 × 108 CFU/g Bifidobacteria and 6.4 × 109 CFU/g Lactobacillus reconstituted in drinking water at concentration of 1.5 g/100 mL, access to water from embryonic day 0.5 to postnatal day 21 | Restore MIA-induced weight loss in dams, social deficits, repetitive and stereotyped behaviors, depression-like behaviors, and anxiety-like behaviors in adult offspring; parvalbumin positive neuron loss; the decrease in levels of GABA in the PFC of adult offspring, and the decrease in proinflammatory cytokines (IL-6 and IL-17a) in both the maternal serum and fetal brain | Wang et al. (2019) |
| L. plantarum, L. casei, L. acidophilus, and L. bulgaricus | Offspring of the pregnant rats induced by VPA at a dose of 400 mg/kg, i.p. on an embryonic day 12 | 1 × 109 CFU/mL of probiotics given orally every day for 42 days | Significantly attenuate the behavioral anomalies; Decrease the 5-HT, increase BDNF, IL-6, and TNF-α levels in blood and brain; Reverse the VPA-induced apoptosis and degeneration in the cerebellum | Sunand et al. (2020) |
| L. helveticus CCFM1076 | Male offspring of pregnant Wistar rats injected i.p. on E12.5 with 500 mg/kg VPA | 109 CFU/mL bacteria daily gavage at age from 4 to 8 weeks | Improve social interaction, cognitive ability, and repetitive stereotyped behavior significantly; Up-regulate5-HT, L-Trp, and 5-HTP levels in the colon, feces, and serum; Balance excitatory and inhibitory neurotransmitter levels by restoring maternal VPA-induced decrease in GABA and Ach levels, and increase in Glu level and Glu/GABA in serum, the medial PFC or cerebellum of rats; Enhance oxytocin synthesis in the hypothalamus; Reduce the 5-HT associated Turicibacter in the gut; Increase butyric acid levels in the cecum contents | Kong et al. (2021) |
| B. longum BB536 | Young Sprague Dawley male rats, oral gavage of 250 mg/kg propionic acid dissolved in distilled water for 3 days | 2 × 109 CFU per 25 mg dissolved in a volume of 1 mL of sterile PBS, 0.5 mL daily by oral gavage for 22 days | Improve the social behavior impairment; Decrease the harmful C. perfringens and increase Clostridium cluster IV; Normalize the PPA-induced increase in Bdnf transcript levels in the hippocampus | Abuaish et al. (2021) |
| L. plantarum STIII | Offspring of pregnant ICR mice administered with triclosan dissolved in fresh corn oil at concentration of 50 mg/mL, intragastric gavage from the 7th day of pregnancy until the 21st day of weaning at a dose of 50 mg/kg | 5 × 108 CFU/g dissolved in PBS, 0.8 mL daily by intragastric gavage at the age of 7 weeks for 2 weeks | Ameliorate the social deficits, the self-grooming and freezing times; Increase the beneficial Lachnospiraceae abundance and decrease Alistipes abundance | Guo et al. (2022) |
| L. paracaseii LPC-37 | Male Wister albino rats treated with 250 mg PPA/kg BW/day for 3 days | 5 × 109 CFU dissolved in 1 mL of sterile PBS, 0.2 mL daily by oral gavage for 27 days before PPA exposure | Reverse PPA-induced decrease in α-MSH levels, neurotensin, and β-endorphin | Alghamdi et al. (2022) |
| B. infantis, B. breve, L. acidophilus, L. bulgaricus, L. casei, L. rhamnosus, and S. thermophiles | Male Wister albino rats treated with 250 mg PPA/kg BW/day for 3 days | 1 × 109 CFU/g dissolved in PBS, 0.2 g/kg BW daily by oral gavage for 27 days before PPA exposure | Reverse PPA-induced decrease in α-MSH levels, neurotensin, and β-endorphin | Alghamdi et al. (2022) |
| Four Lactobacillus spp. and Bifidobacterium spp. | Adult Wistar rats received broad-spectrum antibiotics mixture for 4 weeks at age of 10 weeks old | Daily oral gavage for 2 weeks | Improve the social behavior; restore antibiotics-induced decrease in SCFAs | Mintál et al. (2022) |
| Four Lactobacillus spp. and Bifidobacterium spp. | Male offspring of pregnant Wistar rat intraperitoneal injection of 500 mg/BW kg VPA on the 12.5th day of gestion | Daily oral gavage for 2 weeks | Improve the social behavior | Mintál et al. (2022) |
| S. thermophilus BT01, B. breve BB02, B. animalis subsp. lactis BL03, B. animalis subsp. lactis BL04, L. acidophilus BA05, L. plantarum BP06, L. paracasei BP07, L. helveticus BD08. | Male offspring of pregnant Wistar rat intraperitoneal injection of 500 mg/BW kg VPA on the embryonic day 12.5 | 2.25 × 1010 CFU/day probiotic was administered via orogastric gavage for 42 days | Improve the sociability, social interaction, anxiety-liked behavior, and behavioral despair; Significantly reverse the VPA-induced increase in serum IL-6 and decrease in serum IL-10; Restore the Bacteroidetes/Firmicutes ratio decreased by prenatal VPA exposure | Adıgüzel et al. (2022) |
| Lacticaseibacillus rhamnosus HA-114 | Male juvenile BTBR T+ Itpr3tf/J mouse | 1 × 109 CFU/ mL probiotic reconstituted in drinking water for 4 weeks | Reduce behavior deficits in social novelty preference; Increase microbial richness and phylogenetic diversity; increase the potential anti-inflammatory (Anaeroplasma, Christensenellaceae) and butyrate-producing taxa (Acetatifactor, Lachnospiraceae, and Butyricicoccus); Elevate levels of 5-aminovaleric acid and choline in serum and the PFC, respectively | Pochakom et al. (2022) |
Second, probiotics can modulate neuroactive compounds to attenuate ASD symptoms. Accumulating evidence has demonstrated that genetic and environmental risk factors converge to disturb the balance between glutamate (Glu)-mediated excitatory and γ-GABA-mediated inhibitory neurotransmission autism (Nelson and Valakh, 2015; Borisova, 2018). Probiotics can influence neurotransmitters such as γ-GABA, Glu, and 5-HT (Ng et al., 2018; Israelyan and Margolis, 2019). Tabouy et al. (2018) revealed that L. reuteri treatment decreased repetitive behaviors and increased GABA receptor gene expression (GABRA1, GABRA1, and GABRB1) and protein levels (GABRA1) in the hippocampus and the PFC of Shank3 mutant mice (a model of ASD). Moreover, treatment with Lactobacillus was shown to regulate emotional behavior and central GABA receptor expression via the vagus nerve (Bravo et al., 2011), which communicates connecting the brain and the gut, in a mouse. Probiotics that stimulate inhibitory neurotransmission (for example, by increasing GABA levels) may help restore the excitatory/inhibitory balance and recover the decreased social interaction associated with ASD (El-Ansary et al., 2018). In addition, daily L. helveticus CCFM1076 intake alleviates autistic-related features by regulating 5-HT anabolism and catabolism, balancing excitatory and inhibitory neurotransmitter release (as indicated by increased GABA in PFC and decreased Glu in serum, and PFC) in both the peripheral and CNS, and increasing oxytocin synthesis in the hypothalamus (Kong et al., 2021). 5-HT is produced in the gut and plays a central role in gut–brain connection (Owens and Nemeroff, 1994). Previously, 5-HT levels have been significantly correlated with GABA, Glu, and oxytocin, suggesting a vital role of 5-HT in the neuroendocrine network. Moreover, a single dose of oxytocin has been shown to regulate the 5-HT energy system, reduce anxiety (Neumann and Slattery, 2016), and help alleviate social dysfunction (Lawson et al., 2016). Another neuropsychiatric disease involves the altered neurotransmitter Glu (Shimmura et al., 2011). ACh is involved in learning and memory, attention, cognition, social interactions, and stereotypical behaviors (Avale et al., 2011; Karvat and Kimchi, 2014). In addition, L. reuteri treatment raised oxytocin levels in the brain, which improved behavioral aspects of brain function by stimulating the vagus nerve (Sgritta et al., 2019). Another study found that L. reuteri ingestion restored maternal high-fat diet-induced social deficits, oxytocin levels, and ventral tegmental area plasticity in offspring (Buffington et al., 2016). Furthermore, L. reuteri has been repeatedly shown to improve oxytocin-dependent behavior in several ASD mice models (Sgritta et al., 2019). Brain-derived neurotrophic factor (BDNF) is a neurotrophic factor that promotes the development and survival of cholinergic, dopaminergic, and serotonergic neurons in their mature and growing stages (Croen et al., 2008). Working memory, hippocampal learning, and brain plasticity are all influenced by BDNF (Leung and Thuret, 2015). In addition, BDNF impacts GABA inhibitory interneurons, ultimately causing cognitive deficits (Maqsood and Stone, 2016). One previous study reported that daily Lactobacillus strains supplementation reversed autistic deficits and decreased BDNF levels in serum and acetylcholinesterase (AChE) and 5-HT in the brain of the VPA-induced prenatal model of autism (Sunand et al., 2020). Acetylcholine (Ach), hydrolyzed by AChE in the synaptic cleft (Croen et al., 2008), is involved in learning and memory, attention, cognition, social interactions, and stereotypical behaviors (Karvat and Kimchi, 2014). In a recent report, both the pure and mixed probiotics had beneficial effects against PPA-induced neurotoxicity shown by increased levels of alpha-melanocyte-stimulating hormone (α-MSH) levels, neurotensin, and β-endorphin in ASD of rodent model (Alghamdi et al., 2022). A remarkable decrease in α-MSH in different brain regions has been involved in the pathogenesis of social isolation (Theoharides and Doyle, 2008); in fact, re-socialization fully recovered α-MSH immunoreactivity attenuating anxiety-and depression-like behaviors (Tejeda et al., 2012). Neurotensin may act on the CNS as atypical neuroleptics (Petrie et al., 2005). β-endorphin, endogenous opioid peptides, may alter social behavior and result in autistic-like features. A probiotic mixture was shown to attenuate both the antibiotics and VPA-induced autistic behavioral symptoms (Mintál et al., 2022). In the BTBR mouse model of autism, probiotic L. rhamnosus administration decreased behavioral abnormalities in social novelty preference and increased 5-aminovaleric acid and choline levels in serum and the PFC, respectively (Pochakom et al., 2022). The excitatory/inhibitory imbalance previously linked to the pathophysiology of ASD is attenuated by 5-aminovaleric acid, a GABA receptor agonist, of which persons with ASD have remarkably lower levels than non-ASD ones (Sharon et al., 2019). The social and behavioral impairments observed in ASD have been connected to cholinergic pathways through choline metabolism (Lam et al., 2006). Choline supplementation during pregnancy and blocking Ach the breakdown both helped BTBR mice with social and repetitive/restricted behavior deficiencies (Eissa et al., 2020).
The reduction of gut inflammation (improved immune functions) may be another benefit of probiotic application for ASD. Several GI illnesses, including irritable bowel syndrome and inflammatory bowel disease, have been associated with increased mucosal inflammation (Ng et al., 2018). Children with ASD have been found to have greater levels of gut immune inflammation, which is linked to gut dysbiosis, as well as GI complaints (Hughes et al., 2018). In fact, 4 months of probiotic supplementation in children with ASD aged 2–9 years restored many of the abnormalities in their GI microbiota and reduced their intestinal inflammation (Tomova et al., 2015). Probiotics have been shown to reduce gut inflammation through numerous mechanisms including lowering gut barrier permeability, decreasing inflammatory cytokines, and other immunomodulatory effects. In pregnant female mice, maternal immune activation (MIA) results in impaired intestinal barrier integrity and symptoms like autism in the offspring, which are related to microbiome dysbiosis (Hsiao et al., 2013). After Bacteroidetes fragilis treatment, the repetitive behaviors were attenuated and intestinal permeability was restored, and the gut microbiota imbalance partially improved in the offspring (Hsiao et al., 2013). The probiotic mixture VSL#3 significantly improved sociability, social interaction, anxiety-liked behavior, and behavioral despair, while reversing the increase in serum IL-6 and decrease in serum IL-10 induced by prenatal VPA exposure (Adıgüzel et al., 2022). Moreover, daily Lactobacillus strain supplementation supports gut–brain axis in the VPA-induced prenatal model of autism by reversing autistic deficits and improving immune functions (Sunand et al., 2020). In their study, treatment with Lactobacillus strains decreased TNF-α levels in serum and IL-6 in the brain. TNF-and IL-1 attach to the brain’s endothelial cells to trigger immunological responses in the brain (de Theije et al., 2011). In addition, reduced IL-6 levels have been shown to enhance GABAergic interneuron activity, which in turn increases GAD65/67 levels, preventing the loss of parvalbumin-positive (PV+) neurons and GABA levels (Basta-Kaim et al., 2015).
5. Conclusion and future directions
In this review, we first showed the interrelationship between GI abnormality, gut microbiota dysbiosis, and ASD severity. Then, we presented how gut microbiota dysbiosis contributes to gut–brain axis dysfunction in patients with ASD. Finally, we indicated how probiotics affect the gut microbiota, leading to improvements in GI abnormalities and other behaviors by regulating the gut–brain axis.
Despite the encouraging preclinical and clinical results of probiotics supplementation, most accessible clinical studies had small sample sizes, most being single-center trials that enrolled only 20–30 children, and may use qualitative, self-reported questionnaires and surveys to measure treatment response in open-label trials, which might introduce bias. Due to the communication deficits that are common in children with ASD, the parents may also encounter several challenges while analyzing these aspects. The use of clinician ratings, more randomized, controlled research, and bigger study populations may produce more reliable findings. The long-term effects of probiotics in patients with ASD after cessation have not been investigated. Thus, it is necessary to prove the elution stage of probiotic administration in the future. Moreover, the lack of an established probiotic protocol results in a variety of probiotic strains, concentrations, and treatment times. Interestingly, probiotics were most useful when using certain strains and conditions (McFarland et al., 2018). Future research should consider using a standardized intervention plan. Mechanistic studies utilizing “multi-omics” may be used in the future. Recent technological advancements in the area of metabolomics have vastly improved the sensitivity and accuracy with which metabolites can be detected and characterized (Du et al., 2017; Wang et al., 2019). To progress the discipline even further, bigger studies using a defined intervention protocol and the development of metabolomics are also required. In summary, patients with neurodevelopmental disorders, such as ASD, may benefit from a well-chosen mix of probiotics as a potential non-invasive therapy.
Author contributions
PF and SZ co-wrote the manuscript. YZ revised the manuscript. EL supervised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Natural Science Foundation of Henan Province (No. 212300410399) and the Zhengzhou Major Collaborative Innovation Project (No. 18XTZX12003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abuaish S., Al-Otaibi N. M., Abujamel T. S., Alzahrani S. A., Alotaibi S. M., AlShawakir Y. A., et al. (2021). Fecal transplant and Bifidobacterium treatments modulate gut clostridium bacteria and rescue social impairment and hippocampal BDNF expression in a rodent model of autism. Brain Sci. 11:1038. doi: 10.3390/brainsci11081038, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J. B., Johansen L. J., Powell L. D., Quig D., Rubin R. A. (2011). Gastrointestinal flora and gastrointestinal status in children with autism–comparisons to typical children and correlation with autism severity. BMC Gastroenterol. 11:22. doi: 10.1186/1471-230X-11-22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adıgüzel E., Çiçek B., Ünal G., Aydın M. F., Barlak-Keti D. (2022). Probiotics and prebiotics alleviate behavioral deficits, inflammatory response, and gut dysbiosis in prenatal VPA-induced rodent model of autism. Physiol. Behav. 256:113961. doi: 10.1016/j.physbeh.2022.113961, PMID: [DOI] [PubMed] [Google Scholar]
- Alghamdi M. A., Al-Ayadhi L., Hassan W. M., Bhat R. S., Alonazi M. A., El-Ansary A. (2022). Bee pollen and probiotics may Alter brain neuropeptide levels in a rodent model of autism Spectrum disorders. Meta 12:562. doi: 10.3390/metabo12060562, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altieri L., Neri C., Sacco R., Curatolo P., Benvenuto A., Muratori F., et al. (2011). Urinary p-cresol is elevated in small children with severe autism spectrum disorder. Biomarkers 16, 252–260. doi: 10.3109/1354750X.2010.548010, PMID: [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . (2013). American Psychiatric Association: Diagnostic and statistical manual of mental disorders. Arlington, TX: American Psychiatric Association. [Google Scholar]
- Ashwood P., Krakowiak P., Hertz-Picciotto I., Hansen R., Pessah I., Van de Water J. (2011). Elevated plasma cytokines in autism spectrum disorders provide evidence of immune dysfunction and are associated with impaired behavioral outcome. Brain Behav. Immun. 25, 40–45. doi: 10.1016/j.bbi.2010.08.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avale M. E., Chabout J., Pons S., Serreau P., De Chaumont F., Olivo-Marin J. C., et al. (2011). Prefrontal nicotinic receptors control novel social interaction between mice. FASEB J. 25, 2145–2155. doi: 10.1096/fj.10-178558, PMID: [DOI] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance Year 2008 Principal Investigators . (2008). “Prevalence of autism spectrum disorders — autism and developmental disabilities monitoring network, 14 sites, United States, 2008,” in Morbidity and Mortality Weekly Report: Surveillance Summaries. Centers for Disease Control & Prevention (CDC), 61, 1–19. Available at: https://www.jstor.org/stable/24806043 [PubMed] [Google Scholar]
- Basta-Kaim A., Fijał K., Ślusarczyk J., Trojan E., Głombik K., Budziszewska B., et al. (2015). Prenatal administration of lipopolysaccharide induces sex-dependent changes in glutamic acid decarboxylase and parvalbumin in the adult rat brain. Neuroscience 287, 78–92. doi: 10.1016/j.neuroscience.2014.12.013, PMID: [DOI] [PubMed] [Google Scholar]
- Baumgarten T. J., Oeltzschner G., Hoogenboom N., Wittsack H.-J., Schnitzler A., Lange J. (2016). Beta peak frequencies at rest correlate with endogenous GABA+/Cr concentrations in sensorimotor cortex areas. PLoS One 11:e0156829. doi: 10.1371/journal.pone.0156829, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Billeci L., Callara A. L., Guiducci L., Prosperi M., Morales M. A., Calderoni S., et al. (2022). A randomized controlled trial into the effects of probiotics on electroencephalography in preschoolers with autism. Autism 27, 117–132. doi: 10.1177/13623613221082710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørklund G., Meguid N. A., El-Bana M. A., Tinkov A. A., Saad K., Dadar M., et al. (2020). Oxidative stress in autism spectrum disorder. Mol. Neurobiol. 57, 2314–2332. doi: 10.1007/s12035-019-01742-2 [DOI] [PubMed] [Google Scholar]
- Borisova T. (2018). Nervous system injury in response to contact with environmental, engineered and planetary micro-and nano-sized particles. Front. Physiol. 9:728. doi: 10.3389/fphys.2018.00728, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J. A., Forsythe P., Chew M. V., Escaravage E., Savignac H. M., Dinan T. G., et al. (2011). Ingestion of lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. 108, 16050–16055. doi: 10.1073/pnas.1102999108, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buffington S. A., Di Prisco G. V., Auchtung T. A., Ajami N. J., Petrosino J. F., Costa-Mattioli M. (2016). Microbial reconstitution reverses maternal diet-induced social and synaptic deficits in offspring. Cells 165, 1762–1775. doi: 10.1016/j.cell.2016.06.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byndloss M. X., Olsan E. E., Rivera-Chávez F., Tiffany C. R., Cevallos S. A., Lokken K. L., et al. (2017). Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575. doi: 10.1126/science.aam9949, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cammarota G., Ianiro G., Bibbo S., Gasbarrini A. (2014). Gut microbiota modulation: probiotics, antibiotics or fecal microbiota transplantation? Intern. Emerg. Med. 9, 365–373. doi: 10.1007/s11739-014-1069-4 [DOI] [PubMed] [Google Scholar]
- Carissimi C., Laudadio I., Palone F., Fulci V., Cesi V., Cardona F., et al. (2019). Functional analysis of gut microbiota and immunoinflammation in children with autism spectrum disorders. Dig. Liver Dis. 51, 1366–1374. doi: 10.1016/j.dld.2019.06.006, PMID: [DOI] [PubMed] [Google Scholar]
- Cekici H., Sanlier N. (2019). Current nutritional approaches in managing autism spectrum disorder: a review. Nutr. Neurosci. 22, 145–155. doi: 10.1080/1028415X.2017.1358481, PMID: [DOI] [PubMed] [Google Scholar]
- Chaidez V., Hansen R. L., Hertz-Picciotto I. (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. J. Autism Dev. Disord. 44, 1117–1127. doi: 10.1007/s10803-013-1973-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchfield J. W., Van Hemert S., Ash M., Mulder L., Ashwood P. (2011). The potential role of probiotics in the management of childhood autism spectrum disorders. Gastroenterol. Res. Pract. 2011, 1–8. doi: 10.1155/2011/161358, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croen L. A., Goines P., Braunschweig D., Yolken R., Yoshida C. K., Grether J. K., et al. (2008). Brain-derived neurotrophic factor and autism: maternal and infant peripheral blood levels in the early markers for autism (EMA) study. Autism Res. 1, 130–137. doi: 10.1002/aur.14, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- David L. A., Maurice C. F., Carmody R. N., Gootenberg D. B., Button J. E., Wolfe B. E., et al. (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature 505, 559–563. doi: 10.1038/nature12820, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Angelis M., Piccolo M., Vannini L., Siragusa S., De Giacomo A., Serrazzanetti D. I., et al. (2013). Fecal microbiota and metabolome of children with autism and pervasive developmental disorder not otherwise specified. PLoS One 8:e76993. doi: 10.1371/journal.pone.0076993, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Theije C. G., Wu J., Da Silva S. L., Kamphuis P. J., Garssen J., Korte S. M., et al. (2011). Pathways underlying the gut-to-brain connection in autism spectrum disorders as future targets for disease management. Eur. J. Pharmacol. 668, S70–S80. doi: 10.1016/j.ejphar.2011.07.013, PMID: [DOI] [PubMed] [Google Scholar]
- Dinan T. G., Cryan J. F. (2015). The impact of gut microbiota on brain and behaviour: implications for psychiatry. Curr. Opin. Clin. Nutr. Metab. Care 18, 552–558. doi: 10.1097/MCO.0000000000000221 [DOI] [PubMed] [Google Scholar]
- Ding H. T., Taur Y., Walkup J. T. (2017). Gut microbiota and autism: key concepts and findings. J. Autism Dev. Disord. 47, 480–489. doi: 10.1007/s10803-016-2960-9, PMID: [DOI] [PubMed] [Google Scholar]
- Doenyas C. (2018). Gut microbiota, inflammation, and probiotics on neural development in autism spectrum disorder. Neuroscience 374, 271–286. doi: 10.1016/j.neuroscience.2018.01.060, PMID: [DOI] [PubMed] [Google Scholar]
- Du C., Zhang B., He Y., Hu C., Ng Q. X., Zhang H., et al. (2017). Biological effect of aqueous C60 aggregates on Scenedesmus obliquus revealed by transcriptomics and non-targeted metabolomics. J. Hazard. Mater. 324, 221–229. doi: 10.1016/j.jhazmat.2016.10.052, PMID: [DOI] [PubMed] [Google Scholar]
- Eissa N., Jayaprakash P., Stark H., Łażewska D., Kieć-Kononowicz K., Sadek B. (2020). Simultaneous blockade of histamine H3 receptors and inhibition of acetylcholine esterase alleviate autistic-like behaviors in BTBR T+ tf/J mouse model of autism. Biomol. Ther. 10:1251. doi: 10.3390/biom10091251, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ansary A., Bacha A. B., Bjørklund G., Al-Orf N., Bhat R. S., Moubayed N., et al. (2018). Probiotic treatment reduces the autistic-like excitation/inhibition imbalance in juvenile hamsters induced by orally administered propionic acid and clindamycin. Metab. Brain Dis. 33, 1155–1164. doi: 10.1007/s11011-018-0212-8, PMID: [DOI] [PubMed] [Google Scholar]
- Finegold S. M., Dowd S. E., Gontcharova V., Liu C., Henley K. E., Wolcott R. D., et al. (2010). Pyrosequencing study of fecal microflora of autistic and control children. Anaerobe 16, 444–453. doi: 10.1016/j.anaerobe.2010.06.008, PMID: [DOI] [PubMed] [Google Scholar]
- Forsythe P., Bienenstock J., Kunze W. A. (2014). Vagal pathways for microbiome-brain-gut axis communication. Microb. Endocrinol. 817, 115–133. doi: 10.1007/978-1-4939-0897-4_5 [DOI] [PubMed] [Google Scholar]
- Foss-Feig J. H., Adkinson B. D., Ji J. L., Yang G., Srihari V. H., McPartland J. C., et al. (2017). Searching for cross-diagnostic convergence: neural mechanisms governing excitation and inhibition balance in schizophrenia and autism spectrum disorders. Biol. Psychiatry 81, 848–861. doi: 10.1016/j.biopsych.2017.03.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye R. E., Rossignol D. A. (2016). Identification and treatment of pathophysiological comorbidities of autism spectrum disorder to achieve optimal outcomes. Clin. Med. Insights Pediatr. 10, 43–56. doi: 10.4137/CMPed.S38337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorrindo P., Williams K. C., Lee E. B., Walker L. S., McGrew S. G., Levitt P. (2012). Gastrointestinal dysfunction in autism: parental report, clinical evaluation, and associated factors. Autism Res. 5, 101–108. doi: 10.1002/aur.237, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossi E., Melli S., Dunca D., Terruzzi V. (2016). Unexpected improvement in core autism spectrum disorder symptoms after long-term treatment with probiotics. SAGE Open Medical Case Reports 4:2050313X16666231. doi: 10.1177/2050313X16666231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Li R., Wang Y., Ma S., Zhang Y., Li S., et al. (2022). Lactobacillus plantarum ST-III modulates abnormal behavior and gut microbiota in a mouse model of autism spectrum disorder. Physiol. Behav. 257:113965. doi: 10.1016/j.physbeh.2022.113965, PMID: [DOI] [PubMed] [Google Scholar]
- Hatheway C. L. (1990). Toxigenic clostridia. Clin. Microbiol. Rev. 3, 66–98. doi: 10.1128/CMR.3.1.66, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes O. D., Rogdaki M., Findon J. L., Wichers R. H., Charman T., King B. H., et al. (2018). Autism spectrum disorder: consensus guidelines on assessment, treatment and research from the British Association for Psychopharmacology. J. Psychopharmacol. 32, 3–29. doi: 10.1177/0269881117741766, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E. Y. (2014). Gastrointestinal issues in autism spectrum disorder. Harv. Rev. Psychiatry 22, 104–111. doi: 10.1097/HRP.0000000000000029 [DOI] [PubMed] [Google Scholar]
- Hsiao E. Y., McBride S. W., Hsien S., Sharon G., Hyde E. R., McCue T., et al. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cells 155, 1451–1463. doi: 10.1016/j.cell.2013.11.024, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes H. K., Rose D., Ashwood P. (2018). The gut microbiota and dysbiosis in autism spectrum disorders. Curr. Neurol. Neurosci. Rep. 18:81. doi: 10.1007/s11910-018-0887-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iovene M. R., Bombace F., Maresca R., Sapone A., Iardino P., Picardi A., et al. (2017). Intestinal dysbiosis and yeast isolation in stool of subjects with autism spectrum disorders. Mycopathologia 182, 349–363. doi: 10.1007/s11046-016-0068-6, PMID: [DOI] [PubMed] [Google Scholar]
- Israelyan N., Margolis K. G. (2019). Reprint of: serotonin as a link between the gut-brain-microbiome axis in autism spectrum disorders. Pharmacol. Res. 140, 115–120. doi: 10.1016/j.phrs.2018.12.023, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kałużna-Czaplińska J., Błaszczyk S. (2012). The level of arabinitol in autistic children after probiotic therapy. Nutrition 28, 124–126. doi: 10.1016/j.nut.2011.08.002, PMID: [DOI] [PubMed] [Google Scholar]
- Kang D.-W., Park J. G., Ilhan Z. E., Wallstrom G., LaBaer J., Adams J. B., et al. (2013). Reduced incidence of Prevotella and other fermenters in intestinal microflora of autistic children. PLoS One 8:e68322. doi: 10.1371/journal.pone.0068322, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karvat G., Kimchi T. (2014). Acetylcholine elevation relieves cognitive rigidity and social deficiency in a mouse model of autism. Neuropsychopharmacology 39, 831–840. doi: 10.1038/npp.2013.274, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobliner V., Mumper E., Baker S. M. (2018). Reduction in obsessive compulsive disorder and self-injurious behavior with Saccharomyces boulardii in a child with autism: a case report. Integr. Med. 17:38. [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Chen Q., Mao X., Wang G., Zhao J., Zhang H., et al. (2022). Bifidobacterium longum CCFM1077 ameliorated neurotransmitter disorder and neuroinflammation closely linked to regulation in the kynurenine pathway of autistic-like rats. Nutrients 14:1615. doi: 10.3390/nu14081615, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X., Liu J., Cetinbas M., Sadreyev R., Koh M., Huang H., et al. (2019). New and preliminary evidence on altered oral and gut microbiota in individuals with autism spectrum disorder (ASD): implications for ASD diagnosis and subtyping based on microbial biomarkers. Nutrients 11:2128. doi: 10.3390/nu11092128, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X.-J., Liu J., Liu K., Koh M., Sherman H., Liu S., et al. (2021). Probiotic and oxytocin combination therapy in patients with autism spectrum disorder: a randomized, double-blinded, placebo-controlled pilot trial. Nutrients 13:1552. doi: 10.3390/nu13051552, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Q., Wang B., Tian P., Li X., Zhao J., Zhang H., et al. (2021). Daily intake of lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 12, 2591–2604. doi: 10.1039/D0FO02375B, PMID: [DOI] [PubMed] [Google Scholar]
- Kushak R. I., Winter H. S., Buie T. M., Cox S. B., Phillips C. D., Ward N. L. (2017). Analysis of the duodenal microbiome in autistic individuals: association with carbohydrate digestion. J. Pediatr. Gastroenterol. Nutr. 64, e110–e116. doi: 10.1097/MPG.0000000000001458, PMID: [DOI] [PubMed] [Google Scholar]
- Lam K. S., Aman M. G., Arnold L. E. (2006). Neurochemical correlates of autistic disorder: a review of the literature. Res. Dev. Disabil. 27, 254–289. doi: 10.1016/j.ridd.2005.03.003, PMID: [DOI] [PubMed] [Google Scholar]
- Lawson S. K., Gray A. C., Woehrle N. S. (2016). Effects of oxytocin on serotonin 1B agonist-induced autism-like behavior in mice. Behav. Brain Res. 314, 52–64. doi: 10.1016/j.bbr.2016.07.027, PMID: [DOI] [PubMed] [Google Scholar]
- Leung K., Thuret S. (2015). Gut microbiota: a modulator of brain plasticity and cognitive function in ageing. Healthcare 3, 898–916. doi: 10.3390/healthcare3040898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q., Han Y., Dy A. B. C., Hagerman R. J. (2017). The gut microbiota and autism spectrum disorders. Front. Cell. Neurosci. 11:120. doi: 10.3389/fncel.2017.00120, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Liao Y., Zhou Q., Qu Q., Sheng M., Lv L., et al. (2022). Changes of gut microbiota in autism spectrum disorders and common probiotics & Chinese herbal medicine therapeutic mechanisms: a review. Adv. Neurodev. Disord. 6, 290–303. doi: 10.1007/s41252-022-00266-6 [DOI] [Google Scholar]
- Li Q., Zhou J.-M. (2016). The microbiota–gut–brain axis and its potential therapeutic role in autism spectrum disorder. Neuroscience 324, 131–139. doi: 10.1016/j.neuroscience.2016.03.013 [DOI] [PubMed] [Google Scholar]
- Liu S., Li E., Sun Z., Fu D., Duan G., Jiang M., et al. (2019). Altered gut microbiota and short chain fatty acids in Chinese children with autism spectrum disorder. Sci. Rep. 9, 1–9. doi: 10.1038/s41598-018-36430-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu G., Yu Q., Tan B., Ke X., Zhang C., Li H., et al. (2022). Gut dysbiosis impairs hippocampal plasticity and behaviors by remodeling serum metabolome. Gut Microbes 14:2104089. doi: 10.1080/19490976.2022.2104089, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna R. A., Oezguen N., Balderas M., Venkatachalam A., Runge J. K., Versalovic J., et al. (2017). Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell. Mol. Gastroenterol. Hepatol. 3, 218–230. doi: 10.1016/j.jcmgh.2016.11.008, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma B., Liang J., Dai M., Wang J., Luo J., Zhang Z., et al. (2019). Altered gut microbiota in Chinese children with autism spectrum disorders. Front. Cell. Infect. Microbiol. 9:40. doi: 10.3389/fcimb.2019.00040, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner M. J., Arneson C. L., Levy S. E., Kirby R. S., Nicholas J. S., Durkin M. S. (2012). Brief report: association between behavioral features and gastrointestinal problems among children with autism spectrum disorder. J. Autism Dev. Disord. 42, 1520–1525. doi: 10.1007/s10803-011-1379-6, PMID: [DOI] [PubMed] [Google Scholar]
- Maqsood R., Stone T. W. (2016). The gut-brain axis, BDNF, NMDA and CNS disorders. Neurochem. Res. 41, 2819–2835. doi: 10.1007/s11064-016-2039-1, PMID: [DOI] [PubMed] [Google Scholar]
- Mayer E. A. (2011). Gut feelings: the emerging biology of gut–brain communication. Nat. Rev. Neurosci. 12, 453–466. doi: 10.1038/nrn3071, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhanon B. O., McCracken C., Karpen S., Sharp W. G. (2014). Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics 133, 872–883. doi: 10.1542/peds.2013-3995, PMID: [DOI] [PubMed] [Google Scholar]
- McFarland L. V. (2007). Meta-analysis of probiotics for the prevention of traveler's diarrhea. Travel Med. Infect. Dis. 5, 97–105. doi: 10.1016/j.tmaid.2005.10.003, PMID: [DOI] [PubMed] [Google Scholar]
- McFarland L. V., Evans C. T., Goldstein E. J. (2018). Strain-specificity and disease-specificity of probiotic efficacy: a systematic review and meta-analysis. Front. Med. 5:124. doi: 10.3389/fmed.2018.00124, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguid N. A., Mawgoud Y. I. A., Bjørklund G., Mehanne N. S., Anwar M., Effat B. A. E.-K., et al. (2022). Molecular characterization of probiotics and their influence on children with autism Spectrum disorder. Mol. Neurobiol. 59, 6896–6902. doi: 10.1007/s12035-022-02963-8, PMID: [DOI] [PubMed] [Google Scholar]
- Mensi M. M., Rogantini C., Marchesi M., Borgatti R., Chiappedi M. (2021). Lactobacillus plantarum PS128 and other probiotics in children and adolescents with autism spectrum disorder: a real-world experience. Nutrients 13:2036. doi: 10.3390/nu13062036, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintál K., Tóth A., Hormay E., Kovács A., László K., Bufa A., et al. (2022). Novel probiotic treatment of autism spectrum disorder associated social behavioral symptoms in two rodent models. Sci. Rep. 12:5399. doi: 10.1038/s41598-022-09350-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro F., Liu Y., Rhoads J. M. (2016). Can probiotics benefit children with autism spectrum disorders? World J. Gastroenterol. 22, 10093–10102. doi: 10.3748/wjg.v22.i46.10093, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson S. B., Valakh V. (2015). Excitatory/inhibitory balance and circuit homeostasis in autism spectrum disorders. Neuron 87, 684–698. doi: 10.1016/j.neuron.2015.07.033, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann I. D., Slattery D. A. (2016). Oxytocin in general anxiety and social fear: a translational approach. Biol. Psychiatry 79, 213–221. doi: 10.1016/j.biopsych.2015.06.004, PMID: [DOI] [PubMed] [Google Scholar]
- Ng Q. X., Peters C., Ho C. Y. X., Lim D. Y., Yeo W.-S. (2018). A meta-analysis of the use of probiotics to alleviate depressive symptoms. J. Affect. Disord. 228, 13–19. doi: 10.1016/j.jad.2017.11.063, PMID: [DOI] [PubMed] [Google Scholar]
- Ng Q. X., Soh A. Y. S., Loke W., Lim D. Y., Yeo W.-S. (2018). The role of inflammation in irritable bowel syndrome (IBS). J. Inflamm. Res. 11, 345–349. doi: 10.2147/JIR.S174982, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera A. G., Hagerman R. J., Catania M. V., Buono S., Di Nuovo S., Liprino E. M., et al. (2019). EEG abnormalities as a neurophysiological biomarker of severity in autism spectrum disorder: a pilot cohort study. J. Autism Dev. Disord. 49, 2337–2347. doi: 10.1007/s10803-019-03908-2, PMID: [DOI] [PubMed] [Google Scholar]
- Nikolov R. N., Bearss K. E., Lettinga J., Erickson C., Rodowski M., Aman M. G., et al. (2009). Gastrointestinal symptoms in a sample of children with pervasive developmental disorders. J. Autism Dev. Disord. 39, 405–413. doi: 10.1007/s10803-008-0637-8, PMID: [DOI] [PubMed] [Google Scholar]
- Niu M., Li Q., Zhang J., Wen F., Dang W., Duan G., et al. (2019). Characterization of intestinal microbiota and probiotics treatment in children with autism spectrum disorders in China. Front. Neurol. 10:1084. doi: 10.3389/fneur.2019.01084, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens M. J., Nemeroff C. B. (1994). Role of serotonin in the pathophysiology of depression: focus on the serotonin transporter. Clin. Chem. 40, 288–295. doi: 10.1093/clinchem/40.2.288, PMID: [DOI] [PubMed] [Google Scholar]
- Parracho H. M., Bingham M. O., Gibson G. R., McCartney A. L. (2005). Differences between the gut microflora of children with autistic spectrum disorders and that of healthy children. J. Med. Microbiol. 54, 987–991. doi: 10.1099/jmm.0.46101-0 [DOI] [PubMed] [Google Scholar]
- Parracho H. M., Gibson G. R., Knott F., Bosscher D., Kleerebezem M., McCartney A. L. (2010). A double-blind, placebo-controlled, crossover-designed probiotic feeding study in children diagnosed with autistic spectrum disorders. Int. J. Probiot. Prebiot. 5:69. [Google Scholar]
- Persico A. M., Napolioni V. (2013). Urinary p-cresol in autism spectrum disorder. Neurotoxicol. Teratol. 36, 82–90. doi: 10.1016/j.ntt.2012.09.002, PMID: [DOI] [PubMed] [Google Scholar]
- Petrie K. A., Schmidt D., Bubser M., Fadel J., Carraway R. E., Deutch A. Y. (2005). Neurotensin activates GABAergic interneurons in the prefrontal cortex. J. Neurosci. 25, 1629–1636. doi: 10.1523/JNEUROSCI.3579-04.2005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pochakom A., Mu C., Rho J. M., Tompkins T. A., Mayengbam S., Shearer J. (2022). Selective probiotic treatment positively modulates the microbiota–gut–brain Axis in the BTBR mouse model of autism. Brain Sci. 12:781. doi: 10.3390/brainsci12060781, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romeo M., Romeo D., Trovato L., Oliveri S., Palermo F., Cota F., et al. (2011). Role of probiotics in the prevention of the enteric colonization by Candida in preterm newborns: incidence of late-onset sepsis and neurological outcome. J. Perinatol. 31, 63–69. doi: 10.1038/jp.2010.57, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saggioro A. (2004). Probiotics in the treatment of irritable bowel syndrome. J. Clin. Gastroenterol. 38, S104–S106. doi: 10.1097/01.mcg.0000129271.98814.e2 [DOI] [PubMed] [Google Scholar]
- Santocchi E., Guiducci L., Prosperi M., Calderoni S., Gaggini M., Apicella F., et al. (2020). Effects of probiotic supplementation on gastrointestinal, sensory and Core symptoms in autism Spectrum disorders: a randomized controlled trial. Front. Psych. 11:550593. doi: 10.3389/fpsyt.2020.550593, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sgritta M., Dooling S. W., Buffington S. A., Momin E. N., Francis M. B., Britton R. A., et al. (2019). Mechanisms underlying microbial-mediated changes in social behavior in mouse models of autism spectrum disorder. Neuron 101, 246–259.e6. doi: 10.1016/j.neuron.2018.11.018, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaaban S. Y., El Gendy Y. G., Mehanna N. S., El-Senousy W. M., El-Feki H. S., Saad K., et al. (2018). The role of probiotics in children with autism spectrum disorder: a prospective, open-label study. Nutr. Neurosci. 21, 676–681. doi: 10.1080/1028415X.2017.1347746, PMID: [DOI] [PubMed] [Google Scholar]
- Sharon G., Cruz N. J., Kang D.-W., Gandal M. J., Wang B., Kim Y.-M., et al. (2019). Human gut microbiota from autism spectrum disorder promote behavioral symptoms in mice. Cells 177, 1600–1618.e17. doi: 10.1016/j.cell.2019.05.004, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. (2010). Increased urinary excretion of a 3-(3-hydroxyphenyl)-3-hydroxypropionic acid (HPHPA), an abnormal phenylalanine metabolite of clostridia spp. in the gastrointestinal tract, in urine samples from patients with autism and schizophrenia. Nutr. Neurosci. 13, 135–143. doi: 10.1179/147683010X12611460763968, PMID: [DOI] [PubMed] [Google Scholar]
- Shimmura C., Suda S., Tsuchiya K. J., Hashimoto K., Ohno K., Matsuzaki H., et al. (2011). Alteration of plasma glutamate and glutamine levels in children with high-functioning autism. PLoS One 6:e25340. doi: 10.1371/journal.pone.0025340, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith P. M., Howitt M. R., Panikov N., Michaud M., Gallini C. A., Bohlooly-y M., et al. (2013). The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 341, 569–573. doi: 10.1126/science.1241165, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikantha P., Mohajeri M. H. (2019). The possible role of the microbiota-gut-brain-axis in autism spectrum disorder. Int. J. Mol. Sci. 20:2115. doi: 10.3390/ijms20092115, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F., Cavalieri D., Albanese D., De Felice C., Donati C., Hayek J., et al. (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome 5, 1–11. doi: 10.1186/s40168-017-0242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunand K., Mohan G. K., Bakshi V. (2020). Supplementation of lactobacillus probiotic strains supports gut-brain-axis and defends autistic deficits occurred by valproic acid-induced prenatal model of autism. Pharm. J. 12, 1658–1669. doi: 10.5530/pj.2020.12.226 [DOI] [Google Scholar]
- Tabouy L., Getselter D., Ziv O., Karpuj M., Tabouy T., Lukic I., et al. (2018). Dysbiosis of microbiome and probiotic treatment in a genetic model of autism spectrum disorders. Brain Behav. Immun. 73, 310–319. doi: 10.1016/j.bbi.2018.05.015, PMID: [DOI] [PubMed] [Google Scholar]
- Tallon-Baudry C. (2003). Oscillatory synchrony and human visual cognition. J. Physiol. 97, 355–363. doi: 10.1016/j.jphysparis.2003.09.009 [DOI] [PubMed] [Google Scholar]
- Tas A. A. (2018). Dietary strategies in autism spectrum disorder (ASD). Prog. Nutr. 20, 554–562. doi: 10.23751/pn.v20i4.6693 [DOI] [Google Scholar]
- Tejeda H., Shippenberg T., Henriksson R. (2012). The dynorphin/κ-opioid receptor system and its role in psychiatric disorders. Cell. Mol. Life Sci. 69, 857–896. doi: 10.1007/s00018-011-0844-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharawadeephimuk W., Chaiyasut C., Sirilun S., Sittiprapaporn P.. (2019). Preliminary study of probiotics and kynurenine pathway in autism spectrum disorder. 2019 16th international conference on electrical engineering/electronics, computer, telecommunications and information technology (ECTI-CON), Pattaya, Chonburi, Thailand: IEEE. pp. 425–428. [Google Scholar]
- Theoharides T. C., Doyle R. (2008). Autism, gut-blood-brain barrier, and mast cells. J. Clin. Psychopharmacol. 28, 479–483. doi: 10.1097/JCP.0b013e3181845f48, PMID: [DOI] [PubMed] [Google Scholar]
- Thomas R. H., Meeking M. M., Mepham J. R., Tichenoff L., Possmayer F., Liu S., et al. (2012). The enteric bacterial metabolite propionic acid alters brain and plasma phospholipid molecular species: further development of a rodent model of autism spectrum disorders. J. Neuroinflammation 9, 1–18. doi: 10.1186/1742-2094-9-153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomova A., Husarova V., Lakatosova S., Bakos J., Vlkova B., Babinska K., et al. (2015). Gastrointestinal microbiota in children with autism in Slovakia. Physiol. Behav. 138, 179–187. doi: 10.1016/j.physbeh.2014.10.033, PMID: [DOI] [PubMed] [Google Scholar]
- Vos T., Abajobir A. A., Abate K. H., Abbafati C., Abbas K. M., Abd-Allah F., et al. (2017). Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the global burden of disease study 2016. Lancet 390, 1211–1259. doi: 10.1016/S0140-6736(17)32154-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wajner M., Latini A., Wyse A., Dutra-Filho C. (2004). The role of oxidative damage in the neuropathology of organic acidurias: insights from animal studies. J. Inherit. Metab. Dis. 27, 427–448. doi: 10.1023/B:BOLI.0000037353.13085.e2, PMID: [DOI] [PubMed] [Google Scholar]
- Wang L., Christophersen C. T., Sorich M. J., Gerber J. P., Angley M. T., Conlon M. A. (2012). Elevated fecal short chain fatty acid and ammonia concentrations in children with autism spectrum disorder. Dig. Dis. Sci. 57, 2096–2102. doi: 10.1007/s10620-012-2167-7, PMID: [DOI] [PubMed] [Google Scholar]
- Wang P., Ng Q. X., Zhang B., Wei Z., Hassan M., He Y., et al. (2019). Employing multi-omics to elucidate the hormetic response against oxidative stress exerted by nC60 on Daphnia pulex. Environ. Pollut. 251, 22–29. doi: 10.1016/j.envpol.2019.04.097, PMID: [DOI] [PubMed] [Google Scholar]
- Wang H.-X., Wang Y.-P. (2016). Gut microbiota-brain axis. Chin. Med. J. 129, 2373–2380. doi: 10.4103/0366-6999.190667, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X., Yang J., Zhang H., Yu J., Yao Z. (2019). Oral probiotic administration during pregnancy prevents autism-related behaviors in offspring induced by maternal immune activation via anti-inflammation in mice. Autism Res. 12, 576–588. doi: 10.1002/aur.2079, PMID: [DOI] [PubMed] [Google Scholar]
- West R., Roberts E., Sichel L., Sichel J. (2013). Improvements in gastrointestinal symptoms among children with autism spectrum disorder receiving the Delpro® probiotic and immunomodulator formulation. J. Prob. Health 1, 1–6. [Google Scholar]
- Williams B. L., Hornig M., Buie T., Bauman M. L., Cho Paik M., Wick I., et al. (2011). Impaired carbohydrate digestion and transport and mucosal dysbiosis in the intestines of children with autism and gastrointestinal disturbances. PLoS One 6:e24585. doi: 10.1371/journal.pone.0024585, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X., Liu D., Wang Y., Zeng T., Peng Y. (2016). Urinary 3-(3-hydroxyphenyl)-3-hydroxypropionic acid, 3-hydroxyphenylacetic acid, and 3-hydroxyhippuric acid are elevated in children with autism spectrum disorders. Biomed Res. Int. 2016:9485412. doi: 10.1155/2016/9485412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Yin A., Li H., Wang R., Wu G., Shen J., et al. (2015). Dietary modulation of gut microbiota contributes to alleviation of both genetic and simple obesity in children. EBioMedicine 2, 968–984. doi: 10.1016/j.ebiom.2015.07.007, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]