Abstract
Background
Sonography is often the first imaging procedure to be used in diagnostic investigation of the abdomen. The aim of this article is to provide a new interdisciplinary overview of recent groundbreaking advances in this modality.
Methods
A selective survey of the literature in PubMed was conducted. The literature search was carried out in 2021–2022 and included publications over the period 2004–2022.
Results
The novel sonographic software techniques can be divided into algorithms that deal with conventional B-scan optimization and new programs that extend the scope of sonographic examination. The latter include elastography, contrast-enhanced sonography, and image fusion in combination with other cross-sectional imaging modalities. Elastography can be used to assess the presence of steatosis, fibrosis, or cirrhosis in patients with liver disease. One study reported diagnostic accuracy of 84–87% for the diagnosis of significant fibrosis (F2), 89–91% for the diagnosis of severe fibrosis (F3), and 92–93% for the diagnosis of liver cirrhosis (F4). Contrast-enhanced sonography is used for evaluation of tumors and trauma. A prospective multicenter study found sensitivity of 95.8% for the characterization of malignant lesions and specificity of 83.1% for benign lesions. Image fusion has the potential to improve the diagnostic assessment of parenchymatous organs, vascular conditions, and the prostate.
Conclusion
With continuous improvement of the B-scan and the development of high-frequency probes and novel investigation techniques, sonography has become established as an increasingly autonomous examination procedure.
cme plus
This article has been certified by the North Rhine Academy for Continuing Medical Education. Participation in the CME certification program is possible only over the internet: cme.aerzteblatt.de. The deadline for submission is 26 January 2024.
Sonography is often the first imaging procedure to be used in the diagnostic investigation of the abdomen by representatives from different specialties, such as internal medicine, urology, gynecology, obstetrics, and radiology. New techniques in sonography are divided into software options that primarily aim to optimize B scans and those that extend the scope of sonographic examination. The term B scan is an abbreviation of “brightness scan.” Each investigation starts with optimization of the conventional B scan and is followed by additional examination protocols. To this end, in abdominal diagnostic investigation, multi frequency convex probes are habitually used. Structures or lesions localized on the surface can be examined by using higher frequency linear probes. A higher transmission frequency enables a more precise detail of the surface structures with the limitation that higher transmission frequencies have a shallower depth of penetration. Depending on the question and the location of the organ under examination, different probes and their available resolutions are used.
New techniques to optimize the B scan and examination techniques are discussed separately in the following article.
We carried out a selective literature search in PubMed in 2021–2022, which covered publications from 2004 to 2022.
Ultrasound technique
The basis of ultrasound diagnostics is the conventional B scan. The aim is therefore to improve the quality of the B scan individually by applying different software algorithms. The most commonly used algorithms are tissue harmonic imaging, spatial compounding, and speckle reduction. These techniques are explain in detail in the eMethods section (1, e19). All described techniques can simultaneously be used for B scan optimization (2, e2) (figure 1).
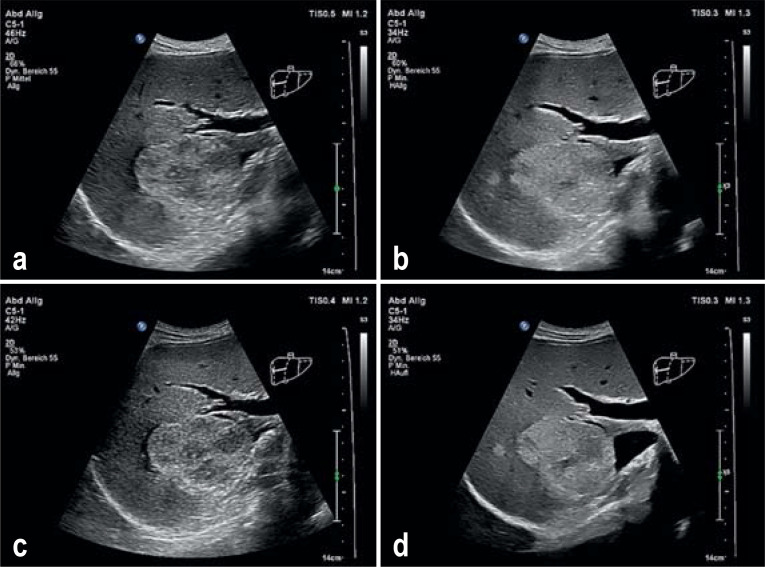
Figure 1.
a) Conventional B scan sonography of a solid echoic space occupying lesion of the liver; b) tissue harmonic imaging enables a better outline of the lesion through modification of the soft tissue exposure compared with the conventional B scan. c) Spatial compounding enables the sound waves to spread simultaneously into different directions and leads to an improved signal to noise ratio, thus reducing artefacts. d) Optimized exposure of the liver focus with simultaneous combination of tissue harmonic imaging, spatial compounding, and speckle reduction technique.
Elastography
Image acquisition by elastography entails as a first step the mechanical or acoustic stimulation of the tissue under study, which leads to a tissue shift (3, e7). An ultrasound impulse generates shear waves—also known as transverse waves. The speed at which the shear waves spread correlates with the deformation of the tissues that is to be examined (4, e8).
As an additional imaging modality for the characterization of significant fibrosis or cirrhosis ultrasound elastography is the first method for assessing liver stiffness. The method most commonly used in this setting is transient elastography, whose findings are highly consistent with histopathological findings after liver biopsy (e7).
Ferraioli et al. (5) studied the sensitivity and specificity of 2-D shear wave elastography in 121 HCV patients in order to grade the liver fibrosis compared with the gold standard of liver biopsy and transient elastography (TE). Liver fibrosis was categorized into significant fibrosis, severe fibrosis, and cirrhosis (table 1).
Table 1. SWE and TE compared with the METAVIR stage (meta-analysis of histological data in viral hepatitis) (5).
| Fibrosis stages | Sensitivity | Specificity | PPV | NPV |
| 2-D SWE | ||||
| F ≥2 | 90.0 (80.5–95.9) | 87.5 (74.8–95.3) | 91.3 (82.0–96.7) | 85.7 (72.8–94.1) |
| F ≥3 | 97.3 (85.8–99.9) | 95.1 (87.8–98.6) | 90.0 (76.3–97.2) | 98.7 (93.1–100) |
| F = 4 | 87.5 (67.6–97.3) | 96.8 (91.0–99.3) | 87.5 (67.6–97.3) | 96.8 (91.0–99.3) |
| TE | ||||
| F ≥2 | 69.6 (57.3–80.1) | 89.6 (77.3–96.5) | 90.6 (79.3–96.9) | 67.2 (54.3–78.4) |
| F ≥3 | 89.2 (74.6–97.0) | 88.8 (79.7–94.7) | 78.6 (63.2–89.7) | 94.7 (86.9–98.5) |
| F = 4 | 91.7 (73.0–99.0) | 96.8 (90.9–99.3) | 88.0 (68.8–97.5) | 97.8 (92.4–99.7) |
F, fibrosis; NPV, negative predictive value; PPV, positive predictive value; 2-D SWE, 2-D shearwave elastrography; TE, transient elastography
Friedrich-Rust et al. in their studies showed diagnostic accuracy of 84–87% for the diagnosis of significant fibrosis (F2), 89–91% for the diagnosis of sever fibrosis (F3), and 92–93% for the diagnosis of liver cirrhosis (f4) (6, 7).
When using elastography for evaluating the liver it should be borne in mind that the stiffness measurements can be affected negatively by patient dependent co-factors. Raised transaminases in the sense of active hepatitis, a postprandial examination, certain medications, and chronic heart failure are considered as disruptive factors in the assessment of liver stiffness (8, e9).
In one of the first publications on the subject of liver shear wave elastography, Friedrich-Rust et al. compared a conventional ultrasounds system with integrated shear wave elastography with the gold standard at the time, transient elastography (TE). They concluded that this new technique is a promising ultrasound based method to assess liver fibrosis in chronic viral hepatitis. The diagnostic accuracy in this preliminary study matched that of transient elastography (9).
Spoera et al. pointed out as early as 2014 that shear wave elastography as well as transient elastography yield reliable results in assessing liver stiffness. They can be used as a predictor for the severity of the fibrosis, and the need for liver biopsy in patients with chronic liver disease can be reduced (10).
Li et al. carried out a prospective study in 166 patients to investigate the diagnostic accuracy of 2-D shear waves to assess possible liver fibrosis in patients with chronic hepatitis B. The authors concluded that the values, correlated with histology, agree well for the differentiation of severe liver fibrosis and cirrhosis, but are of only limited value for diagnosing mild fibrosis (11), and no conclusion can be drawn about the genesis of liver damage.
Many studies have shown that 2-D shear wave elastography (SWE) and point-shear wave elastography are superior to transient elastography for assessing liver stiffness and fibrosis (12, e10-e13).
Point-shear wave elastography can acquire data only within a small region of interest; by comparison, 2-D shear wave elastography can utilize the entire B scan.
To evaluate current studies, Zhou et al. undertook a meta-analysis of 186 publications, aiming to find out which of the two shear wave modalities, 2-D SWE or point-SWE, is better at assessing liver fibrosis. They concluded that 2-D SWE as well as point-SWE have high sensitivity and specificity in detecting fibrosis, with 2-D SWE having higher sensitivity to detecting significant fibrosis and advanced fibrosis than pSWE (13). Table 2 shows the statistical analysis.
Table 2. Statistical summary of the meta-analysis by Zhou et al. (13).
| Fibrosis stages | Sensitivity | Specificity |
| 2-D SWE | ||
| F ≥2 | 0.84 (0.80–0.87) | 0.81 (0.75–0.85) |
| F ≥3 | 0.90 (0.86–0.93) | 0.87 (0.83– 0.91) |
| F = 4 | 0.89 (0.85–0.92) | 0.87 (0.83–0.90) |
| Point SWE | ||
| F ≥2 | 0.76 (0.73–0.80) | 0.79 (0.75–0.83) |
| F ≥3 | 0.83 (0.80–0.86) | 0.83 (0.80–0.86) |
| F = 4 | 0.85 (0.80–0.88) | 0.84 (0.81–0.87) |
Point SWE, Point shearwave elastography; 2-D SWE, 2-D shearwave elastography
The liver is a target organ for the use of shear wave elastography. The stiffness correlates with the grade of fibrosis and indirectly with the degree of portal hypertension and the risk of developing hepatocellular carcinoma (figure 2). Liver lesions can appear soft or hard on stiffness measurement, independent of their entity. Because of overlaps in the stiffness measurements, guidelines do not recommend using shear waves to distinguish between benign and malignant focal liver lesions (14, 15, e14– e16).
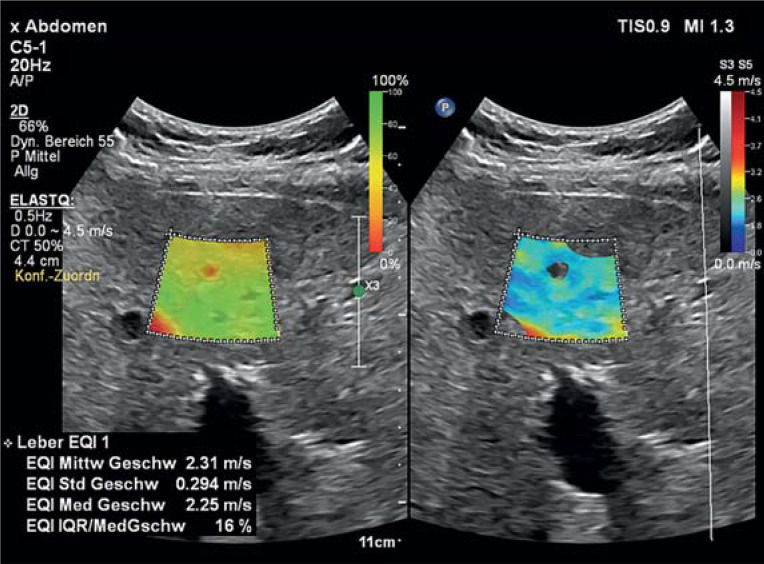
Figure 2.
2-D shearwave elastography of the liver. Tissue stiffness is determined within the selected region of interest and quantitatively reported with the unit m/s. The mean of the shearwaves is 2.31 m/s and therefore clearly raised, which matches the picture of cirrhosis.
Contrast enhanced ultrasound (CEUS)
The prevalence of focal liver lesions in the overall population is some 5%. They are often an incidental finding in the context of abdominal ultrasounds or targeted oncological staging (16).
Strobel et al. carried out a prospective study in a patient population of n=1349, investigating the diagnostic certainty of contrast enhanced ultrasound in characterizing space occupying lesions of the liver. Based on the gold standard, 573 benign lesions and 755 malignant lesions were included.
Compared with the gold standard, the diagnostic certainty of contrast enhanced ultrasound to assess a hepatic lesion was 90.3%.
Contrast enhanced sonography correctly identified 723/755 malignant lesions (sensitivity 95.8%) and 476/573 benign lesions (specificity 83.1%). The positive predictive value for the presence of a malignant tumor was 95.4%, the negative predictive value for the presence of a malignant tumor was 95.7% (17, e17) (efigure).
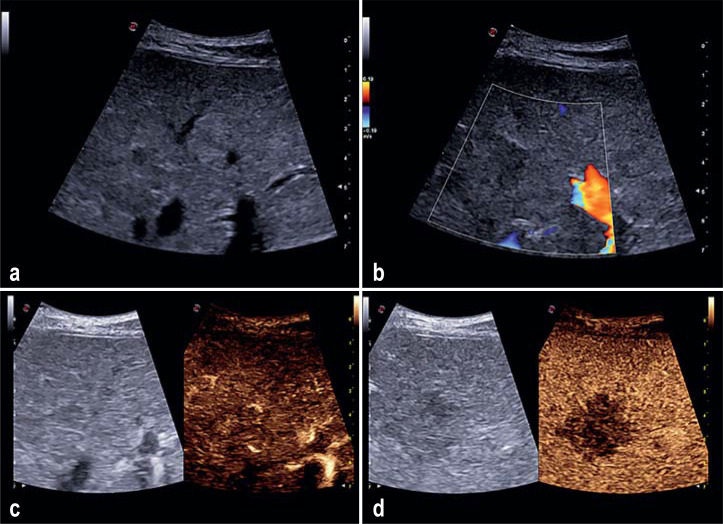
eFigure.
Sonography of a 63 year old female patient with newly diagnosed cervical carcinoma. She presented for a liver check-up. a) On conventional B scan sonography a definitely suspect hepatic lesion cannot be outlined. b) Color coded duplex sonography does not show up any atypical increased vascularization. c) On contrast enhanced sonography a hypervascularized hepatic lesion in the arterial phase cannot be exposed/captured. d) In the portal venous phase a 2 cm sized liver metastasis is demarcated.
In the setting of contrast enhanced diagnostic evaluation of the liver, Sono Vue (Bracco, Milan, Italy) is available in Germany. It consists of 1–10µm sized microbubbles that are filled with an inert gas (sulfur hexafluoride). The contrast medium is stabilized by an outer shell of phospholipids. Compared with contrast agent computed tomography (CT) or magnetic resonance imaging (MRI), no spillover into the interstitial space takes place. The ultrasound contrast medium remains in the vascular system and can therefore expose the capture of organ perfusion (18, e18).
No nephro-, hepato-, or cardiotoxic effects occur. Administration of the contrast medium does not affect thyroid function. The incidence of severe anaphylactic reactions is 1/10,000 applications (18, e19).
As with every application of contrast medium (CT, MRI, ultrasound) anaphylactoid reactions may develop in rare cases. As these are unpredictable, emergency medications should be ready in the examination room. In cardiovascular instability and severe cardiac arrhythmias, the indication should be stringently checked before every application of contrast medium and where needed should be defined cautiously or conservatively.
In contrast enhanced liver examinations, three different phases are considered that enable the detection and characterization of hepatic lesions (e18).
Torres et al. evaluated 287 CEUS examinations in children in a retrospective study. In this patient population, 36 children with unclear hepatic lesion were included. In a subgroup analysis the specificity for the correct categorization of a lesion as benign was 96% and the negative predictive value was 100%. No adverse effects were recorded for the CEUS examination (19).
Geyer et al. in a retrospective study investigated the diagnostic certainty of contrast enhanced ultrasound in the characterization of liver lesions and correlated these with the gold standard of histopathology.
Contrast enhanced ultrasound has a sensitivity of 94.5% and a specificity of 70.6%. The positive predictive value was 87.3% and the negative predictive values was 85.7% compared with histology as the gold standard. No adverse effects were observed (20). The authors concluded that contrast enhanced ultrasound is a safe imaging method with high diagnostic accuracy in the assessment of benign as well as malignant hepatic lesions.
In spite of the many advantages/benefits, the use of CEUS in the liver has limitations. Very small lesions below the detection threshold of 3–5 mm may be overlooked because of the limited resolution. Furthermore, one can assume that liver foci in liver localizations that are difficult to access—such as the subdiaphragmatic sections of segment VIII—cannot always be exposed. The depth of penetration of CEUS is limited, so deep seated lesions or lesions in steatosis are more difficult to capture (18). In addition to patient related parameters, such as meteorism or obesity, the diagnostic confidence of contrast enhanced ultrasounds depends on the experience of the doctors carrying it out (21, 22).
ESFUMB (the European Federation of Societies for Ultrasound in Medicine and Biology) in its recommendations explicitly points out that contrast enhanced liver sonography should be undertaken only by professionals who have sufficient experience in conventional as well as contrast enhanced ultrasound (21, 22).
Image fusion
To ensure dynamic image fusion between the ultrasound and a tomographic modality in real time, the required hardware consists of a magnetic field generator and a sound detection sensor. The software makes it possible to locate the transducer in the magnetic field and therefore to calculate the precise spatial position of the sensor in the room. Image formats that can be used are the established DICOM data of tomographic approaches (such as CT, MRI, positron emission tomography [PET]-CT, PET-MRI). The datasets can be fused manually or automatically and then move simultaneously with the sonographic scanning plane (23, 24). By simultaneous use of image fusion, a tumor related assessment can be done in the immediate comparison to CT or MRI (25, 26, e20).
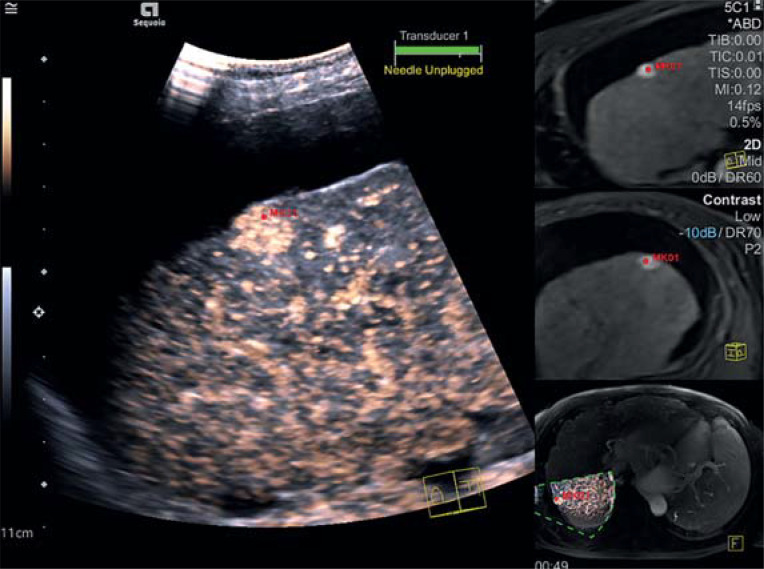
Combining contrast enhanced ultrasound with subsequent tomography can enable improved detection and characterization of liver lesions, with the option of consecutive therapy (26, 27) (figure 3).
Figure 3.
On the contrast enhanced MRI scan, a suspect liver lesion is visible (red marker) in a cirrhotic liver. In the same position and with a comparable size, this lesion shows increased early arterial contrast medium absorption on ultrasonography. MRI morphologically and sonographically the finding is consistent with a hepatocellular cancer about 1 cm in size.
Wobser et al. studied in a patient population (n=40) the response of hepatocellular carcinoma to the chemoembolization undertaken. Image fusion with CEUS rated the effectiveness of the treatment with a specificity of 100%, a sensitivity of 80%, and a positive predictive value of 1 (negative predictive value 0.63) (27).
In addition to the primary detection of lesions, image fusion enables an improved biopsy technique (e20). An example is detection of prostate cancer in transrectal ultrasound (TRUS). Prostate cancer foci are often anechoic, but they can also be isoechoic or hyperechoic (28– 31). New multimodal MRI examination protocols enable improved cancer detection (32– 34). The combination of B scan and MRI dataset in the context of image fusion makes it possible to undertake, in addition to a systematic biopsy, a targeted fusion MRI ultrasound biopsy of the suspect MRI lesion.
In addition to the improved detection rate of significant prostate cancers, this type of targeted biopsy is technically simpler than a biopsy undertaken directly in the MRI scanner. For this so called in bore biopsy, the patient is put in a prone position in the scanner tube and the biopsy is done as a rule via the transgluteal route, which requires more time.
Drost et al. in a Cochrane analysis studied the diagnostic accuracy of different biopsy methods for the detection of clinically significant prostate cancers and compared these. 13 of the 18 included studies were prospective. The authors maintain as their most important finding that using MRI/ultrasound fusion biopsy does actually detect more significant prostate cancers and that fewer punch specimens need to be taken to this end (35).
As a positive side effect, fewer clinically non-significant cancers are found in this war, which reduces the risk of overtreatment for the affected patients. In the meantime, the now very good evidence for multiparametric MRI and fusion biopsy has led to a modification of the guideline recommendations. In the current version of the S3 guideline prostate carcinoma (version 6.2) (36), the use of multiparametric MRI is recommended not only after a negative biopsy finding but during the primary diagnostic evaluation.
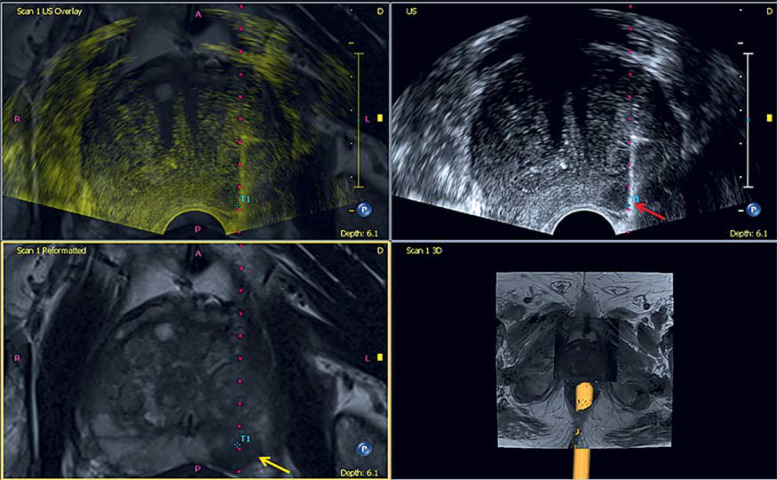
Different studies at our hospital have shown that combining data from prostate MRI and targeted B scan biopsy significantly improved the diagnostic accuracy compared with the gold standard of histology (35– 40) (figure 4). Schlenker et al. studied a patient population of n=408. In this population, 41 patients had a PIRADS-3 lesion on MRI, which was then biopsied in a targeted fashion, conventionally and fusion enhanced. On fusion biopsy, a relevantly increased score (Gleason 7a or above) was diagnosed (6/41 patients; 14.6%) than on conventional prostate biopsy (2/41 patients; 4.9%).
Figure 4.
On the MRI scan of the prostate a suspect PI-RADS-4- lesion is detected (yellow arrow) in the peripheral zone on the left side. Image fusion yields a fusion guided prostate biopsy (red arrow) of a suspect prostate lesion. GPS tracking (blue marker T1) correlates well with the lesion in both modalities. Histology confirmed a poorly differentiated acinar prostate carcinoma (Gleason score 3 + 4 = 7a).
Conclusions for clinical practice and outlook
Continuous improvements to B scans and novel diagnostic techniques have established sonography as an increasingly independent examination method. Ultrasound elastography is an established procedure that is easy to carry out and can be used as an add-on in the setting of routine ultrasound. Broad and comprehensive data of the use of elastography in patients with liver disorders already exist. The detection and characterization of hepatic lesions succeeds thanks to the use of contrast enhanced ultrasound and is therefore actually comparable with contrast enhanced MRI. Image fusion makes it possible to combine different tomography methods in the context of the primary detection of lesions or to plan an intervention. In routine clinical practice, this approach is now standard in large ultrasound centers.
Unfortunately, the same method-immanent limitations of ultrasonography apply for the techniques under discussion. Obesity, meteorism, or lacking compliance on the part of the patient can result in restricted interpretability of the results.
What can we expect in the near future increasingly as an additional diagnostic investigation in the setting of ultrasonography?
One focus is on the development of new broadband power Doppler or techniques that expose the flow in the vessels by means of vectors (e28, e29). The development of novel, contrast enhanced ultrasound techniques makes it possible to work with an image rate of up to 60 images/second and therefore notably improve the temporal resolution of contrast enhanced sonography.
Routine diagnostic evaluation has in the meantime become the standard in private practices. Special questions can only be answered by using high end equipment because of the substantial technical requirements, and they require a lot of time and effort. Under economic considerations this is akin to squaring the circle. For this reason it makes sense for particular clinical questions to refer the patient for further investigations in specialized institutions, in addition to basic diagnostic tests. In addition to the continuous further development of novel ultrasound techniques, the focus should be on comprehensive sonography training. This can be undertaken in centers locally or by means of the nationally available program of courses provided by the German Society of Ultrasound in Medicine (DEGUM).
Jointly with the International Contrast Ultrasound Society (ICUS) we have succeeded in developing and further developing continuing medical/professional development, by using social media, such as the Instagram account „ultrasound campus.“
Supplementary Material
eMethods
Tissue harmonic imaging
Tissue harmonic imaging has been used in the diagnostic evaluation of different organ systems for more than 20 years and it is being improved continually. Tissue harmonic imaging enables modification of tissue exposure, linked with improved capture of the tissue structure (e26). Defocusing and /or phase shifts owing to tissue inhomogeneities will usually lead to a significant loss of lateral resolution and reduced contrast exposure in conventional ultrasound examinations (e1, e3, e27).
Lateral resolution describes the resolution potential in the direction of the sounds spread/distribution and strongly depends on the shape/form of the sound field. The highest lateral resolution is found in the area of the focal point.
The compression and decompression of the ultrasound impulses within tissue bring about harmonic non-linear oscillations that correspond to double the frequency of the selected baseline frequency. In tissue harmonic imaging these harmonic echo components are additionally used for image construction/composition and lead to a better quality image (e2, e4-e5, e28).
Sodhi et al. showed in a cohort of 50 patients with focal hepatic lesions that using harmonic tissue imaging yielded additional information in 16% of cases; in 6% of cases these insights prompted treatment adjustments (e38).
Ranga et al. studied a patient population (n=100) with solid but also cystic hepatic lesions and assessed the overall image quality of the lesions on the basis of a point scale with different presets. The authors concluded that a combination of harmonic tissue imaging and spatial compounding yielded images of the highest quality. Using the mere B image had the lowest diagnostic value in characterizing the lesions (e29).
In the early years, tissue harmonic imaging was only temporarily used in the diagnostic evaluation of the breast as this technique was associated with a reduced frame rate (e1). The frame rate describes the number of images per second that are visible on the monitor. The fewer images are calculated the more the image acquisition is delayed. By using the higher frequencies, this technique is particularly advantageous in structures localized at the surface level. For lesions that require a great depth of penetration, the usefulness of this technique is limited (e1, e26).
Spatial compounding
The spatial compounding technique is based on the multidirectional electronic use of the transducer. This sends the sound waves into different directions at one level, and several of these images are subsequently composed into a single overlapping image in real time.
An improved signal to noise ratio, a reduction of artefacts, and a higher contrast resolution are the advantages of this technique compared with the conventional native B scan (e30– e33).
Different graded settings (low, medium, high, maximum) are available options for this technique, of which a low level has proved beneficial/advantageous on examination (e34).
Speckle reduction
The inherent occurrence/appearance of artefacts is known as “speckle” in sonography. These speckles are coupled with a reduction in contrast and spatial resolution; deeper-seated tissue structures appear darker. Speckle reduction technology allows smoothing and homogenizing of the surrounding structures in the image by suppressing the appearing artefacts. While differences in echogenicity are captured, the contours of the focal finding are maintained and a structural creation is prevented (e1-e26). Depending on the user choice, different pre-sets with different smoothing algorithms can be applied. These algorithms lead to increasing homogenization and smoothing of the surrounding tissue, comparable to imaging in modern MRI scans (e1-e26).
In different studies—such as that by Hyun et al—artificial neuronal networks were successfully trained with the aim to improve ultrasound imaging by optimizing speckle reduction imaging (e34– e35).
The use of new algorithmic filters while using speckle reduction technology leads to improved image quality, according to Choi et al. and Yu et al. (e6, e36).
AI algorithms are increasingly integrated into routine clinical practice.
In the area of endoscopic ultrasonography, this technique enables exact identification and sizing of lymph nodes for staging cancers of the gastrointestinal tract, according to Afshari et al. (e37).
Questions on the article in issue 4/2023:
Ultrasound—New Techniques Are Extending the Applications
The submission deadline is 26 January 2024. Only one answer is possible per question. Please select the answer that is most appropriate.
Question 1
What does the “B” in “B scan” stand for?
“better”
“bundle”
“black”
“brightness”
“budget”
Question 2
Which term—which is mentioned in the text of the article —can used as an alternative term for “shear waves” in ultrasound?
Beta waves
Longitudinal waves
Transverse waves
Gamma waves
Delta waves
Question 3
What distinguishes 2-D shear wave elastography from point shear wave elastography?
Lower sensitivity regarding the detection of advanced fibrosis
Use of the entire B scan
Lower sensitivity regarding the detection of significant fibrosis
Lower specificity regarding the detection of all fibrosis stages
Fibrosis cannot be diagnosed
Question 4
What, roughly, is the prevalence of focal hepatic lesions in the total population?
1 %
5 %
10 %
20 %
35 %
Question 5
Which inert gas is used as a contrast medium in contrast enhanced diagnostic evaluation of the liver?
Helium
Krypton
Nitrogen
Xenon
Sulfur hexafluoride
Question 6
Which statement regarding the mechanism/mode of action of the sonography contrast medium is correct?
It is hepatotoxic.
It affects thyroid function.
It is nephrotoxic.
It is cardiotoxic.
It remains in the vasculature
Question 7
In contrast enhanced sonography of the liver, what is the approximate estimated incidence of severe anaphylactic reactions?
1/100 applications
1/500 applications
1/1 000 applications
1/10 000 applications
1/100 000 applications
Question 8
Which recommendation does the S3 guideline prostate carcinoma make for the diagnostic evaluation of prostate cancer?
Use of mpMRT only after a negative biopsy result
Use of mpMRT only after a positive biopsy result
Use of mpMRT in the primary diagnostic evaluation
No use of mpMRT without prior biopsy
No use of mpMRT if the PSA value is abnormal
Question 9
Which patient dependent co-factor – which is mentioned in the text of the article – can negatively affect liver stiffness measurements taken by elastography ?
Ingestion of a meal
Thyroid function disorder
Respiratory infection
Gastritis
Diarrhea
Question 10
Approximately in which range is the detection limit for contrast enhanced sonography of the liver?
0.5–1 mm
1–2 mm
3–5 mm
5–8 mm
8–10 mm
Acknowledgments
Translated from the original German by Birte Twisselmann, PhD.
Footnotes
Conflict of interest statement
Prof. Clevert received honoraria for continuing medical educational events from Philips, Siemens, Esaote, Samsung, Mindray, und Bracco. He sits on the extended advisory board of Philips, Esaote, Samsung, Mindray, und Bracco.
The remaining authors declare that no conflict of interest exists.
References
- 1.Clevert DA, Helck A, Paprottka PM, Schwarz F, Reiser MF. Latest developments in ultrasound of the liver. Radiologe. 2011;51:661–670. doi: 10.1007/s00117-010-2124-4. [DOI] [PubMed] [Google Scholar]
- 2.Diesch ST, Jung F, Prantl L, Jung EM. Surface imaging of breast implants using modern high-frequency ultrasound technology in comparison to high-end sonography with power analyses for B-scan optimization. Clin Hemorheol Microcirc. 2022;80:487–495. doi: 10.3233/CH-219204. [DOI] [PubMed] [Google Scholar]
- 3.Piscaglia F, Salvatore V, Mulazzani L, Cantisani V, Schiavone C. Ultrasound shear wave elastography for liver disease A critical appraisal of the many actors on the stage. Ultraschall Med. 2016;37:1–5. doi: 10.1055/s-0035-1567037. [DOI] [PubMed] [Google Scholar]
- 4.Balleyguier C, Canale S, Ben Hassen W, et al. Breast elasticity: principles, technique, results: an update and overview of commercially available software. Eur J Radiol. 2013;82:427–430. doi: 10.1016/j.ejrad.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Ferraioli G, Tinelli C, Dal BB, Zicchetti M, Filice G, Filice C. Accuracy of real-time shear wave elastography for assessing liver fibrosis in chronic hepatitis C: a pilot study. Hepatology. 2012;56:2125–2133. doi: 10.1002/hep.25936. [DOI] [PubMed] [Google Scholar]
- 6.Friedrich-Rust M, Nierhoff J, Lupsor M, et al. Performance of acoustic radiation force impulse imaging for the staging of liver fibrosis: a pooled meta-analysis. J Viral Hepat. 2012;19:e212–e219. doi: 10.1111/j.1365-2893.2011.01537.x. [DOI] [PubMed] [Google Scholar]
- 7.Nierhoff J, Chavez Ortiz AA, Herrmann E, Zeuzem S, Friedrich-Rust M. The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a meta-analysis. Eur Radiol. 2013;23:3040–3053. doi: 10.1007/s00330-013-2927-6. [DOI] [PubMed] [Google Scholar]
- 8.Gersak MM, Badea R, Lenghel LM, Vasilescu D, Botar-Jid C, Dudea SM. Influence of food intake on 2-D shear wave elastography assessment of liver stiffness in healthy subjects. Ultrasound Med Biol. 2016;42:1295–1302. doi: 10.1016/j.ultrasmedbio.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Friedrich-Rust M, Wunder K, Kriener S, et al. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595–604. doi: 10.1148/radiol.2523081928. [DOI] [PubMed] [Google Scholar]
- 10.Sporea I, Gilja OH, Bota S, Şirli R, Popescu A. Liver elastography—an update. Med Ultrason. 2013;15:304–314. doi: 10.11152/mu.2013.2066.154.isp23. [DOI] [PubMed] [Google Scholar]
- 11.Li Y, Wu S. Liver stiffness measured with two-dimensional shear wave elastography comparable to histopathology falls dominantly on the severe liver fibrosis. Clin Hemorheol Microcirc. 2021;79:587–596. doi: 10.3233/CH-211223. [DOI] [PubMed] [Google Scholar]
- 12.Long H, Xu W, Zhong X, et al. Feasibility of liver stiffness measured using two-dimensional shear wave elastography in assessing preoperative liver function for patients with hepatocellular carcinoma. Abdom Radiol (NY) 2022;47:664–671. doi: 10.1007/s00261-021-03374-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhou X, Rao J, Wu X, Deng R, Ma Y. Comparison of 2-D shear wave elastography and point shear wave elastography for assessing liver fibrosis. Ultrasound Med Biol. 2021;47:408–427. doi: 10.1016/j.ultrasmedbio.2020.11.013. [DOI] [PubMed] [Google Scholar]
- 14.Barr RG, Ferraioli G, Palmeri ML, et al. Elastography assessment of liver fibrosis: Society of Radiologists in Ultrasound Consensus Conference Statement. Radiology. 2015;276:845–861. doi: 10.1148/radiol.2015150619. [DOI] [PubMed] [Google Scholar]
- 15.Ferraioli G, Barr RG, Farrokh A, et al. How to perform shear wave elastography. Part I. Med Ultrason. 2022;24:95–106. doi: 10.11152/mu-3217. [DOI] [PubMed] [Google Scholar]
- 16.Strobel, D, Bernatik T. Diagnosis of focal liver lesions. Dtsch Arztebl. 2006;103 A-789. [Google Scholar]
- 17.Strobel D, Seitz K, Blank W, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions—diagnostic accuracy in clinical practice (DEGUM multicenter trial) Ultraschall Med. 2008;29:499–505. doi: 10.1055/s-2008-1027806. [DOI] [PubMed] [Google Scholar]
- 18.Greis C. Ultrasound contrast agents as markers of vascularity and microcirculation. Clin Hemorheol Microcirc. 2009;43:1–9. doi: 10.3233/CH-2009-1216. [DOI] [PubMed] [Google Scholar]
- 19.Torres A, Koskinen SK, Gjertsen H, Fischler B. Australas contrast-enhanced ultrasound is useful for the evaluation of focal liver lesions in children. J Ultrasound Med. 2021;27(24):143–150. doi: 10.1002/ajum.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geyer T, Clevert DA, Schwarz S, et al. Diagnostic value of CEUS prompting liver biopsy: histopathological correlation of hepatic lesions with ambiguous imaging characteristics. Diagnostics (Basel) 2020;27:11–35. doi: 10.3390/diagnostics11010035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for contrast enhanced ultrasound (CEUS) in the liver—update 2020—WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultraschall Med. 2020;41:562–585. doi: 10.1055/a-1177-0530. [DOI] [PubMed] [Google Scholar]
- 22.Chiorean L, Tana C, Braden B, et al. Advantages and limitations of focal liver lesion assessment with ultrasound contrast agents: comments on the European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) Guidelines. Med Princ Pract. 2016;25:399–407. doi: 10.1159/000447670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clevert DA, D‘Anastasi M, Jung EM. Contrast-enhanced ultrasound and microcirculation: efficiency through dynamics–current developments. Clin Hemorheol Microcirc. 2013;53:171–186. doi: 10.3233/CH-2012-1584. [DOI] [PubMed] [Google Scholar]
- 24.Jung EM, Clevert DA. Possibilities of sonographic image fusion: current developments. Radiologe. 2015;55:937–948. doi: 10.1007/s00117-015-0025-2. [DOI] [PubMed] [Google Scholar]
- 25.Clevert DA, Helck A, Paprottka PM, et al. Ultrasound-guided image fusion with computed tomography and magnetic resonance imaging Clinical utility for imaging and interventional diagnostics of hepatic lesions. Radiologe. 2018;58:538–544. doi: 10.1007/s00117-011-2252-5. [DOI] [PubMed] [Google Scholar]
- 26.Jung EM, Friedrich C, Hoffstetter P, et al. Volume navigation with contrast enhanced ultrasound and image fusion for percutaneous interventions: first results. PLoS One. 2012;7 doi: 10.1371/journal.pone.0033956. e33956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wobser H, Wiest R, Salzberger B, et al. Evaluation of treatment response after chemoembolisation (TACE) in hepatocellular carcinoma using real time image fusion of contrast-enhanced ultrasound (CEUS) and computed tomography (CT)—preliminary results. Clin Hemorheol Microcirc. 2014;57:191–201. doi: 10.3233/CH-141830. [DOI] [PubMed] [Google Scholar]
- 28.Schlenker B, Clevert DA, Salomon G. Sonographic imaging of the prostate. Urologe A. 2014;53:1052–1060. doi: 10.1007/s00120-014-3533-1. [DOI] [PubMed] [Google Scholar]
- 29.Frauscher F, Klauser A, Halpern EJ. Advances in ultrasound for the detection of prostate cancer. Ultrasound Q. 2002;18:135–142. doi: 10.1097/00013644-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Dahnert WF, Hamper UM, Eggleston JC, Walsh PC, Sanders RC. Prostatic evaluation by transrectal sonography with histopathologic correlation: the echopenic appearance of early carcinoma. Radiology. 1986;158:97–102. doi: 10.1148/radiology.158.1.3510032. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara K, Wheeler TM, Scardino PT. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol. 1989;142:76–82. doi: 10.1016/s0022-5347(17)38666-4. [DOI] [PubMed] [Google Scholar]
- 32.Junker D, Schafer G, Edlinger M, et al. Evaluation of the PI-RADS scoring system for classifying mpMRI findings in men with suspicion of prostate cancer. Biomed Res Int. 2013;2013 doi: 10.1155/2013/252939. 252939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int. 2013;112:1080–1087. doi: 10.1111/bju.12259. [DOI] [PubMed] [Google Scholar]
- 35.Drost FH, Osses DF, Nieboer D, et al. Prostate MRI, with or without MRI-targeted biopsy, and systematic biopsy for detecting prostate cancer. Cochrane Database Syst Rev. 2019;4 doi: 10.1002/14651858.CD012663.pub2. CD012663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leitlinienprogramm Onkologie. S3-Leitlinie Prostatakarzinom (Version 6.2) www.leitlinienprogramm-onkologie.de/leitlinien/prostatakarzinom (last accessed on 30 November 2022) [Google Scholar]
- 37.Apfelbeck M, Tritschler S, Clevert DA, et al. Postoperative change in Gleason score of prostate cancer in fusion targeted biopsy: a matched pair analysis. Scand J Urol. 2021;55:27–32. doi: 10.1080/21681805.2020.1849390. [DOI] [PubMed] [Google Scholar]
- 38.Chaloupka M, Apfelbeck M, Pfitzinger P, et al. Multiparametric magnetic resonance imaging and multiparametric magnetic resonance imaging-guided biopsy in the diagnostic pathway of prostate cancer. Radiologe. 2020;60(Suppl 1):63–69. doi: 10.1007/s00117-020-00716-z. [DOI] [PubMed] [Google Scholar]
- 39.Schlenker B, Apfelbeck M, Armbruster M, Chaloupka M, Stief CG, Clevert DA. Comparison of PIRADS 3 lesions with histopathological findings after MRI-fusion targeted biopsy of the prostate in a real world-setting. Clin Hemorheol Microcirc. 2019;71:165–170. doi: 10.3233/CH-189407. [DOI] [PubMed] [Google Scholar]
- 40.Schlenker B, Apfelbeck M, Buchner A, Stief C, Clevert DA. MRI-TRUS fusion biopsy of the prostate: quality of image fusion in a clinical setting. Clin Hemorheol Microcirc. 2018;70:433–440. doi: 10.3233/CH-189308. [DOI] [PubMed] [Google Scholar]
- E1.Wöhrle NK, Hellerhoff K, Reiser MF, Clevert DA. Modern gray-scale sonography of the breast. Radiologe. 2010;50:964–966-72. doi: 10.1007/s00117-010-2007-8. [DOI] [PubMed] [Google Scholar]
- E2.Clevert DA, Jung EM, Pfister K, et al. Modern ultrasound diagnostics of deep vein thrombosis in lung embolism of unknown origin. Radiologe. 2007;47:673–684. doi: 10.1007/s00117-007-1530-8. [DOI] [PubMed] [Google Scholar]
- E3.Jung EM, Wiggermann P, Stroszczynski C, Reiser MF, Clevert DA. Ultrasound diagnostics of diffuse liver diseases. Radiologe. 2012;52:706–716. doi: 10.1007/s00117-012-2307-2. [DOI] [PubMed] [Google Scholar]
- E4.Bottenus N, LeFevre M, Cleve J, Crowley AL, Trahey G. Resolution and speckle reduction in cardiac imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68:1131–1143. doi: 10.1109/TUFFC.2020.3034518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E5.Khor HG, Ning G, Zhang X, Liao H. Ultrasound speckle reduction using wavelet-based generative adversarial network. IEEE J Biomed Health Inform. 2022;26:3080–3091. doi: 10.1109/JBHI.2022.3144628. [DOI] [PubMed] [Google Scholar]
- E6.Yu H, Ding M, Zhang X, Wu J. PCANet based nonlocal means method for speckle noise removal in ultrasound images. PLoS One. 2018;13 doi: 10.1371/journal.pone.0205390. e0205390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E7.Cosgrove D, Piscaglia F, Bamber J, et al. EFSUMB guidelines and recommendations on the clinical use of ultrasound elastography Part 2: clinical applications. Ultraschall Med. 2013;34:238–253. doi: 10.1055/s-0033-1335375. [DOI] [PubMed] [Google Scholar]
- E8.Fischer T, Sack I, Thomas A. Characterization of focal breast lesions by means of elastography. RoFo. 2013;185:816–823. doi: 10.1055/s-0033-1335939. [DOI] [PubMed] [Google Scholar]
- E9.Reiberger T, Ferlitsch A, Payer BA, et al. Non-selective beta-blockers improve the correlation of liver stiffness and portal pressure in advanced cirrhosis. J Gastroenterol. 2012;47:561–568. doi: 10.1007/s00535-011-0517-4. [DOI] [PubMed] [Google Scholar]
- E10.Dong DR, Hao MN, Li C, et al. Acoustic radiation force impulse elastography, FibroScan, Forns‘ index and their combination in the assessment of liver fibrosis in patients with chronic hepatitis B, and the impact of inflammatory activity and steatosis on these diagnostic methods. Mol Med Rep. 2015;11:4174–4182. doi: 10.3892/mmr.2015.3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E11.Liu Y, Dong CF, Yang G, et al. Optimal linear combination of ARFI, transient elastography and APRI for the assessment of fibrosis in chronic hepatitis B. Liver Int. 2015;35:816–825. doi: 10.1111/liv.12564. [DOI] [PubMed] [Google Scholar]
- E12.Zeng J, Zheng J, Huang Z, et al. Comparison of 2-D shear wave elastography and transient elastography for assessing liver fibrosis in chronic hepatitis B. Ultrasound Med Biol. 2017;43:1563–1570. doi: 10.1016/j.ultrasmedbio.2017.03.014. [DOI] [PubMed] [Google Scholar]
- E13.Ren X, Xia S, Ni Z, Zhan W, Zhou J. Analysis of three ultrasound elastography techniques for grading liver fibrosis in patients with chronic hepatitis B. Radiol Med. 2018;123:735–741. doi: 10.1007/s11547-018-0905-4. [DOI] [PubMed] [Google Scholar]
- E14.Yu H, Wilson SR. Differentiation of benign from malignant liver masses with acoustic radiation force impulse technique. Ultrasound Q. 2011;27:217–223. doi: 10.1097/RUQ.0b013e318239422e. [DOI] [PubMed] [Google Scholar]
- E15.Dietrich CF, Shi L, Wei Q, et al. What does liver elastography measure? Technical aspects and methodology. Minerva Gastroenterol Dietol. 2021;67:129–140. doi: 10.23736/S2724-5985.20.02787-7. [DOI] [PubMed] [Google Scholar]
- E16.Dong Y, Sirli R, Ferraioli G, et al. Shear wave elastography of the liver—review on normal values. Z Gastroenterol. 2017;55:153–166. doi: 10.1055/s-0042-117226. [DOI] [PubMed] [Google Scholar]
- E17.Kaltenbach TE, Engler P, Kratzer W, et al. Prevalence of benign focal liver lesions: ultrasound investigation of 45,319 hospital patients. Abdom Radiol (NY) 2016;41:25–32. doi: 10.1007/s00261-015-0605-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E18.Dietrich CF, Nolsøe CP, Barr RG, et al. Guidelines and good clinical practice recommendations for contrast-enhanced ultrasound (CEUS) in the liver-update 2020 WFUMB in cooperation with EFSUMB, AFSUMB, AIUM, and FLAUS. Ultrasound Med Biol. 2020;46:2579–2604. doi: 10.1016/j.ultrasmedbio.2020.04.030. [DOI] [PubMed] [Google Scholar]
- E19.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol. 2006;32:1369–1375. doi: 10.1016/j.ultrasmedbio.2006.05.031. [DOI] [PubMed] [Google Scholar]
- E20.Rennert J, Georgieva M, Schreyer AG, et al. Image fusion of contrast enhanced ultrasound (CEUS) with computed tomography (CT) or magnetic resonance imaging (MRI) using volume navigation for detection, characterization and planning of therapeutic interventions of liver tumors. Clin Hemorheol Microcirc. 2011;49:67–81. doi: 10.3233/CH-2011-1458. [DOI] [PubMed] [Google Scholar]
- E21.Dahnert WF, Hamper UM, Eggleston JC, Walsh PC, Sanders RC. Prostatic evaluation by transrectal sonography with histopathologic correlation: the echopenic appearance of early carcinoma. Radiology. 1986;158:97–102. doi: 10.1148/radiology.158.1.3510032. [DOI] [PubMed] [Google Scholar]
- E22.Shinohara K, Wheeler TM, Scardino PT. The appearance of prostate cancer on transrectal ultrasonography: correlation of imaging and pathological examinations. J Urol. 1989;142:76–82. doi: 10.1016/s0022-5347(17)38666-4. [DOI] [PubMed] [Google Scholar]
- E23.Junker D, Schafer G, Edlinger M, et al. Evaluation of the PI-RADS scoring system for classifying mpMRI findings in men with suspicion of prostate cancer. Biomed Res Int. 2013;2013 doi: 10.1155/2013/252939. 252939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E24.Barentsz JO, Richenberg J, Clements R, et al. ESUR prostate MR guidelines 2012. Eur Radiol. 2012;22:746–757. doi: 10.1007/s00330-011-2377-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E25.Kuru TH, Roethke MC, Rieker P, et al. Histology core-specific evaluation of the European Society of Urogenital Radiology (ESUR) standardised scoring system of multiparametric magnetic resonance imaging (mpMRI) of the prostate. BJU Int. 2013;112:1080–1087. doi: 10.1111/bju.12259. [DOI] [PubMed] [Google Scholar]
- E26.Bleck JS. Basic principles of ultrasonography and its relevance for internal medicine. Internist (Berl) 2012;53:251–260. doi: 10.1007/s00108-011-2955-8. [DOI] [PubMed] [Google Scholar]
- E27.Clevert D, Jung EM, Jungius KP, Ertan K, Kubale R. Value of tissue harmonic imaging (THI) and contrast harmonic imaging (CHI) in detection and characterisation of breast tumours. Eur Radiol. 2007;17:1–10. doi: 10.1007/s00330-006-0325-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E28.Choi BI. The current status of imaging diagnosis of hepatocellular carcinoma. Liver Transpl. 2004;10(2 Suppl 1):20–25. doi: 10.1002/lt.20038. [DOI] [PubMed] [Google Scholar]
- E29.Ranga U, Kalra N, Saxena AK, et al. Focal hepatic lesions characterisation by different sonographic techniques: a prospective analysis. J Ultrasound. 2015;19:15–23. doi: 10.1007/s40477-015-0172-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E30.Lin DC, Nazarian LN, O‘Kane PL, McShane JM, Parker L, Merritt CR. Advantages of real-time spatial compound sonography of the musculoskeletal system versus conventional sonography. AJR Am J Roentgenol. 2002;179:1629–1631. doi: 10.2214/ajr.179.6.1791629. [DOI] [PubMed] [Google Scholar]
- E31.Afshari P, Zakian C, Bachmann J, Ntziachristos V. Speckle reduction in ultrasound endoscopy using refraction based elevational angular compounding. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97717-2. 183708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E32.Baad M, Lu ZF, Reiser I, Paushter D. Clinical significance of US artifacts. Radiographics. 20173;7:1408–1423. doi: 10.1148/rg.2017160175. [DOI] [PubMed] [Google Scholar]
- E33.Bottenus N, LeFevre M, Cleve J, Crowley AL, Trahey G. Resolution and speckle reduction in cardiac imaging. IEEE Trans Ultrason Ferroelectr Freq Control. 2021;68:1131–1143. doi: 10.1109/TUFFC.2020.3034518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E34.Hyun D, Brickson LL, Looby KT, Dahl JJ. Beamforming and speckle reduction using neural networks. IEEE Trans Ultrason Ferroelectr Freq Control. 2019;66:898–910. doi: 10.1109/TUFFC.2019.2903795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E35.Khor HG, Ning G, Zhang X, Liao H. Ultrasound speckle reduction using wavelet-based generative adversarial network. IEEE J Biomed Health Inform. 2022;26:3080–3091. doi: 10.1109/JBHI.2022.3144628. [DOI] [PubMed] [Google Scholar]
- E36.Choi H, Jeong J. Speckle noise reduction for ultrasound images by using speckle reducing anisotropic diffusion and Bayes threshold. J Xray Sci Technol. 2019;27:885–898. doi: 10.3233/XST-190515. [DOI] [PubMed] [Google Scholar]
- E37.Afshari P, Zakian C, Bachmann J, Ntziachristos V. Speckle reduction in ultrasound endoscopy using refraction based elevational angular compounding. Sci Rep. 2021;11 doi: 10.1038/s41598-021-97717-2. 183708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E38.Sodhi KS, Sidhu R, Gulati M, Saxena A, Suri S, Chawla Y. Role of tissue harmonic imaging in focal hepatic lesions: comparison with conventional sonography. Gastroenterol Hepatol. 2005;20:1488–1593. doi: 10.1111/j.1440-1746.2005.03780.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods
Tissue harmonic imaging
Tissue harmonic imaging has been used in the diagnostic evaluation of different organ systems for more than 20 years and it is being improved continually. Tissue harmonic imaging enables modification of tissue exposure, linked with improved capture of the tissue structure (e26). Defocusing and /or phase shifts owing to tissue inhomogeneities will usually lead to a significant loss of lateral resolution and reduced contrast exposure in conventional ultrasound examinations (e1, e3, e27).
Lateral resolution describes the resolution potential in the direction of the sounds spread/distribution and strongly depends on the shape/form of the sound field. The highest lateral resolution is found in the area of the focal point.
The compression and decompression of the ultrasound impulses within tissue bring about harmonic non-linear oscillations that correspond to double the frequency of the selected baseline frequency. In tissue harmonic imaging these harmonic echo components are additionally used for image construction/composition and lead to a better quality image (e2, e4-e5, e28).
Sodhi et al. showed in a cohort of 50 patients with focal hepatic lesions that using harmonic tissue imaging yielded additional information in 16% of cases; in 6% of cases these insights prompted treatment adjustments (e38).
Ranga et al. studied a patient population (n=100) with solid but also cystic hepatic lesions and assessed the overall image quality of the lesions on the basis of a point scale with different presets. The authors concluded that a combination of harmonic tissue imaging and spatial compounding yielded images of the highest quality. Using the mere B image had the lowest diagnostic value in characterizing the lesions (e29).
In the early years, tissue harmonic imaging was only temporarily used in the diagnostic evaluation of the breast as this technique was associated with a reduced frame rate (e1). The frame rate describes the number of images per second that are visible on the monitor. The fewer images are calculated the more the image acquisition is delayed. By using the higher frequencies, this technique is particularly advantageous in structures localized at the surface level. For lesions that require a great depth of penetration, the usefulness of this technique is limited (e1, e26).
Spatial compounding
The spatial compounding technique is based on the multidirectional electronic use of the transducer. This sends the sound waves into different directions at one level, and several of these images are subsequently composed into a single overlapping image in real time.
An improved signal to noise ratio, a reduction of artefacts, and a higher contrast resolution are the advantages of this technique compared with the conventional native B scan (e30– e33).
Different graded settings (low, medium, high, maximum) are available options for this technique, of which a low level has proved beneficial/advantageous on examination (e34).
Speckle reduction
The inherent occurrence/appearance of artefacts is known as “speckle” in sonography. These speckles are coupled with a reduction in contrast and spatial resolution; deeper-seated tissue structures appear darker. Speckle reduction technology allows smoothing and homogenizing of the surrounding structures in the image by suppressing the appearing artefacts. While differences in echogenicity are captured, the contours of the focal finding are maintained and a structural creation is prevented (e1-e26). Depending on the user choice, different pre-sets with different smoothing algorithms can be applied. These algorithms lead to increasing homogenization and smoothing of the surrounding tissue, comparable to imaging in modern MRI scans (e1-e26).
In different studies—such as that by Hyun et al—artificial neuronal networks were successfully trained with the aim to improve ultrasound imaging by optimizing speckle reduction imaging (e34– e35).
The use of new algorithmic filters while using speckle reduction technology leads to improved image quality, according to Choi et al. and Yu et al. (e6, e36).
AI algorithms are increasingly integrated into routine clinical practice.
In the area of endoscopic ultrasonography, this technique enables exact identification and sizing of lymph nodes for staging cancers of the gastrointestinal tract, according to Afshari et al. (e37).