Abstract
Objective
To estimate the incidence and time-to-classification of SLE by the 1997 ACR (ACR97) criteria, the SLICC criteria, and the European Alliance of Associations for Rheumatology/ACR (EULAR/ACR) criteria.
Methods
We identified all incident SLE cases from 2000–2018 in the well-defined Olmsted County population. Clinical data included in the ACR97, SLICC and EULAR/ACR criteria were manually abstracted from medical records. All incident cases met at least one of the three classification criteria. Time-to-classification was estimated from the first documented lupus-attributable disease manifestation to the time of criteria fulfilment by each of the three definitions. Annual incidence rates were age or age/sex adjusted to the 2000 US population.
Results
Of 139 incident cases there were 126 cases by the EULAR/ACR criteria, corresponding to an age/sex-adjusted incidence of 4.5 per 100 000 population (95% CI: 3.7, 5.2). The age/sex-incidence was higher than that of the SLICC criteria (113 cases; 4.0 per 100 000 [95% CI: 3.3, 4.7], P = 0.020) and the ACR97 (92 cases; 3.3 per 100 000 [95% CI: 2.6, 3.9], P < 0.001). The median time from first disease manifestation to criteria fulfilment was shorter for the EULAR/ACR criteria (29.4 months) than the ACR97 criteria (47.0 months, P < 0.001) and similar to the SLICC criteria (30.6 months, P = 0.83).
Conclusion
The incidence of SLE was higher by the EULAR/ACR criteria compared with the ACR97 and the SLICC criteria, and the EULAR/ACR criteria classified patients earlier that the ACR97 criteria but similar to the SLICC criteria.
Keywords: lupus, systemic lupus erythematosus, incidence, classification criteria, epidemiology
Rheumatology key messages.
In this population‐based study, the incidence of SLE was higher by the EULAR/ACR criteria compared to the ACR97 or the SLICC criteria.
The EULAR/ACR criteria classified patients earlier than the ACR97 criteria, but at a similar time than the SLICC criteria.
Introduction
SLE is a systemic autoimmune disease that can affect virtually any organ and requires a complex clinical workup to diagnose. The systemic nature of the disease, its numerous generic clinical manifestations and the heterogeneous US health system have made it difficult to perform population-based epidemiological studies to estimate the frequency of the disease. Through an initiative of the Centers for Disease Control and Prevention (CDC), five surveillance registries were created to provide incidence and prevalence of SLE across populations of different racial and ethnic groups: in 2002–2004 the Georgia Lupus Registry [1] and the Michigan Lupus Epidemiology and Surveillance Program [2]; and in 2007–2009 the California Lupus Surveillance Project [3], the Manhattan Lupus Surveillance Program [4] and the Indian Health Service Lupus registry [5].
Classification criteria are standardized definitions used for research purposes to identify well-defined cohorts of patients with heterogeneous clinical manifestations, as is the case in SLE. The aforementioned registries used the ACR 1997 (ACR97) classification criteria to define SLE cases [6]. Since the development of the registries, new classification criteria were developed and endorsed by the European Alliance of Associations for Rheumatology (EULAR) and the ACR [7]. The sensitivity and specificity of the new EULAR/ACR criteria are different from the ACR97 criteria and the SLICC criteria, which can impact the incidence estimates of the disease [8]. Furthermore, one of the reasons to develop the new EULAR/ACR criteria was to classify patients earlier in their disease process, but it has not been proven if this was accomplished.
In this study we aimed to estimate the incidence of SLE in Olmsted County, Minnesota using the ACR97, SLICC and EULAR/ACR criteria. A secondary aim was to estimate the time to classification among the three different classification criteria.
Methods
Study design
The Lupus Midwest Network (LUMEN) is a population-based study that utilized the resources of the Rochester Epidemiology Project (REP), a record-linkage system. This epidemiological study includes residents of Olmsted County, Minnesota, which has a >99% capture of the census population in the REP.
The REP allows ready access to the medical records from all healthcare providers for the local population, including the Mayo Clinic, the Olmsted Medical Center and their affiliated hospitals, local nursing homes and the few private practitioners. This system ensures virtually complete ascertainment of all clinically recognized cases of SLE among the residents of Olmsted County, Minnesota [9]. The characteristics and strengths of the REP, as well as its generalizability have been described elsewhere [10, 11]. The population of Olmsted County was 144 248 in 2010, with 74.7% in 2010 being ≥18 years of age. The ethnic distribution was 85.7% White, 4.2% Hispanic, 4.8% African American, 5.5% Asian/Native Hawaiian/Pacific Islander and 0.2% American Indian/Alaska Native [12]. The study was approved by the institutional review boards of the Mayo Clinic and Olmsted Medical Center.
Case finding definitions, and ascertainment
We identified all the potential SLE cases in Olmsted County using two strategies: (i) through International Classification of Diseases (ICD)-9 and ICD-10 codes for SLE, cutaneous lupus erythematosus, and other associated diseases (Supplementary Data Section S1, available at Rheumatology online), and (ii) laboratory measures associated with SLE such as anti-nuclear antibodies (≥1:80), low complement, anti-double stranded DNA, anti-Sm, lupus anticoagulant anticardiolipin (IgG, IgM, and IgA), and anti-beta-2 glycoprotein 1 (IgG, IgM and IgA) antibodies.
SLE cases were defined according to the ACR97 criteria (met at least 4 of the 11 classification criteria), the SLICC criteria (met at least 4 of 17 criteria, at least one of which must be clinical and one immunological, or the presence of biopsy proven lupus nephritis as well as antinuclear antibodies (ANA) or anti-double-stranded DNA antibodies), or the EULAR/ACR criteria (met at least 10 points, and at least one clinical criterion and ANA positivity) from 1 January 2000 to 31 December 2018 [6–8].
Clinical data for these criteria were thoroughly abstracted through medical record review. If a disease manifestation could be better explained by a condition other than SLE, it was not counted towards the criteria. The SLE incidence date was defined as the earliest date of criteria fulfilment for each criteria. A case was considered to be incident if the patient was an Olmsted County resident prior to the SLE incidence date. Data regarding age, sex, self-reported race and ethnicity (Hispanic, and non-Hispanic White, Asian and Black), date of first documentation of each manifestation, date of diagnosis, date of last follow-up, vital status, clinical characteristics and laboratory findings were recorded.
The review of all medical records and data extraction was performed using standardized Research Electronic Data Capture (REDCap) data capture tools hosted at Mayo Clinic [13, 14]. REDCap is a secure, web-based software platform designed to support data capture for research studies, providing (i) an interface for validated data capture, (ii) audit trails for tracking data manipulation and export procedures, and (iii) automated export procedures. Data abstractors were extensively trained; all the abstractors had a medical degree. All abstracted data were reviewed until each abstractor achieved 95% agreement with the first author. Audits of 10% random samples of the abstracted patients were performed throughout the data collection. The first author performed an independent review of all the patients who met at least one of the three criteria to confirm that the disease manifestations were correctly attributed to SLE.
Statistical analysis
Age- and sex-specific incidence rates were calculated for the ACR97, SLICC and EULAR/ACR SLE classification criteria by using the number of incident cases as the numerator and population counts from the REP census as the denominator [9]. Overall incidence rates were age- or age/sex-adjusted per 100 000 population to the 2000 projected US population [15]. To compute 95% CI for incidence rates, it was assumed that the number of incident cases followed a Poisson distribution. The time-to-classification was defined as the time from the first documented lupus-attributable disease manifestation to the time case criteria were fulfilled for each of the three classifications. Time-to-classification among different classification criteria was compared using the signed rank test. Comparisons of the agreement between patients identified by eachcriteria were performed using McNemar’s test. Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA) and R version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of incident SLE cases by three classification criteria
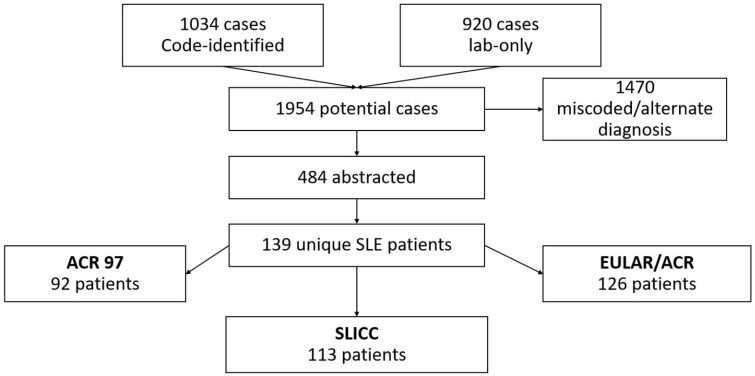
As shown in the flow diagram (Fig. 1), 1954 potential cases were identified, and 1470 were excluded due to miscoding, alternative diagnoses or diagnosis outside of Olmsted County or before year 2000. The remaining 484 cases underwent full abstraction. A total of 139 Olmsted County residents were identified that fulfilled one or more SLE classification criteria and were considered an incident case.
Fig. 1.
Flow diagram showing the screening process to identify patients with SLE in Olmsted County, Minnesota, 2000–2018
EULAR: European Alliance of Associations for Rheumatology.
Approximately 80% of the patients with SLE by at least one of the criteria were female, and mean age was 46 years. The total number of patients identified from minority groups including Asians, Blacks and Hispanics was similar across the different criteria, but the SLICC and EULAR/ACR criteria identified a higher proportion of non-Hispanic Whites than the ACR97 criteria. The median time from first disease manifestation to case criteria fulfilment was shorter for the EULAR/ACR criteria (29.4 months) than the ACR97 criteria (47.0 months, P < 0.001) and similar to the SLICC criteria (30.6 months, P = 0.83) (Table 1). When we examined which of the three classification criteria was fulfilled first, 30% of patients were classified by EULAR/ACR and SLICC criteria at the same time, 23% classified by EULAR/ACR criteria before ACR 97 or SLICC criteria, and 15% and 7% classified first by SLICC and ACR 97 criteria, respectively (Supplementary Table S1, available at Rheumatology online).
Table 1.
Overall case demographics and median time to criteria fulfilment for 139 patients with SLE, by different classification criteria, Olmsted County, Minnesota, 2000–2018
| Characteristic | ACR97 | SLICC | EULAR/ACR |
|---|---|---|---|
| (n = 92) | (n = 113) | (n = 126) | |
| Demographics | |||
| Agea, mean (s.d.), years | 46.5 (17.7) | 46.3 (18.1) | 46.9 (17.5) |
| Female sex, n (%) | 73 (79) | 94 (83) | 101 (80) |
| Race/ethnicity, n (%) | |||
| Asianb | 17 (18.5) | 19 (16.8) | 18 (14.3) |
| Blackb | 7 (7.6) | 8 (7.1) | 8 (6.3) |
| Whiteb | 63 (68.5) | 80 (70.8) | 94 (74.6) |
| Hispanic | 5 (5.4) | 6 (5.3) | 6 (4.8) |
| Time from first clinical criterion to criteria fulfilment, median (25th, 75th percentile), months | 47.0 (3.3, 108.7) | 30.6 (0.4, 102.9) | 29.4 (0.1, 102.9) |
At incidence.
Non-Hispanic. EULAR: European Alliance of Associations for Rheumatology.
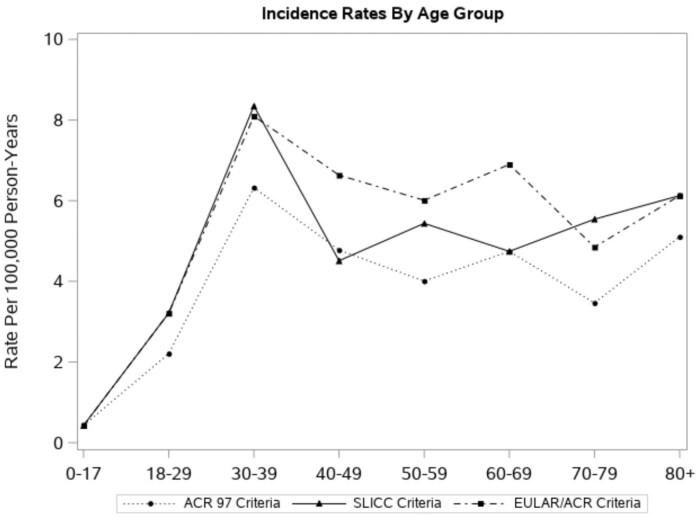
Table 2 details the difference in SLE incidence by each classification criteria. Of the 139 incident SLE cases, 92 were classified by ACR97, 113 by SLICC and 126 by EULAR/ACR criteria. The overall age- and sex-adjusted incidence rate by the EULAR/ACR criteria was 4.5 per 100 000 population (95% CI: 3.7, 5.2), which was higher than that of the ACR97 (3.3 per 100 000 [95% CI: 2.6, 3.9], P < 0.001) and the SLICC criteria (4.0 per 100 000 [95% CI: 3.3, 4.7], P = 0.02). Total age-adjusted incidence rates were 3–4 times higher in females than males across the three different classification criteria (females vs males was 5.0 vs 1.5 [ACR97], 6.4 vs 4.0 [SLICC], and 6.9 vs 4.5/100 000 [EULAR/ACR]) (Table 2). The EULAR/ACR and SLICC criteria identified the same number of patients younger than age 40 years. After age 40 years, the EULAR/ACR criteria identified more patients (Table 2). The ACR97 criteria classified fewer patients across all age groups compared with the EULAR/ACR criteria, but the patterns of the rates by age group for both criteria were similar (Fig. 2). As depicted in Fig. 3, within 1 year of meeting one of the classification criteria, 80 patients were classified for all three criteria, 24 for both SLICC and EULAR/ACR criteria, six for EULAR/ACR and ACR 97 criteria, and two for the SLICC and ACR 97 criteria. The EULAR/ACR criteria alone classified the majority of remaining cases.
Table 2.
Number of cases and incidence rate (per 100 000 population) of SLE by different classification criteria, overall and by age and sex groups, Olmsted County, Minnesota, 2000–2018a
| ACR 97 |
SLICC |
EULAR/ACR |
||||
|---|---|---|---|---|---|---|
| No. of cases | Incidence rate | No. of cases | Incidence rate | No. of cases | Incidence rate | |
| Overall by age group | ||||||
| 0–17 | 3 | 0.4 | 3 | 0.4 | 3 | 0.4 |
| 18–29 | 11 | 2.2 | 16 | 3.2 | 16 | 3.2 |
| 30–39 | 25 | 6.3 | 33 | 8.4 | 32 | 8.1 |
| 40–49 | 18 | 4.8 | 17 | 4.5 | 25 | 6.6 |
| 50–59 | 14 | 4.0 | 19 | 5.4 | 21 | 6.0 |
| 60–69 | 11 | 4.7 | 11 | 4.7 | 16 | 6.9 |
| 70–79 | 5 | 3.5 | 8 | 5.5 | 7 | 4.9 |
| ≥80 | 5 | 5.1 | 6 | 6.1 | 6 | 6.1 |
| Totalb | 92 | 3.3 (2.6, 3.9)d | 113 | 4.0 (3.3, 4.7)d | 126 | 4.5 (3.7, 5.2)d |
| Females by age group | ||||||
| 0–17 | 2 | 0.6 | 2 | 0.6 | 2 | 0.6 |
| 18–29 | 9 | 3.4 | 13 | 4.8 | 13 | 4.8 |
| 30–39 | 22 | 10.8 | 29 | 14.2 | 28 | 13.7 |
| 40–49 | 14 | 7.2 | 14 | 7.2 | 19 | 9.7 |
| 50–59 | 13 | 7.1 | 17 | 9.2 | 18 | 9.8 |
| 60–69 | 8 | 6.6 | 9 | 7.3 | 12 | 9.8 |
| 70–79 | 2 | 2.5 | 6 | 7.6 | 5 | 6.3 |
| ≥80 | 3 | 4.8 | 4 | 6.5 | 4 | 6.5 |
| Totalc | 73 | 5.0 (3.8, 6.2)d | 94 | 6.4 (5.1, 7.7)d | 101 | 6.9 (5.5, 8.2)d |
| Males by age group | ||||||
| 0–17 | 1 | 0.3 | 1 | 0.3 | 1 | 0.3 |
| 18–29 | 2 | 0.9 | 3 | 1.3 | 3 | 1.3 |
| 30–39 | 3 | 1.6 | 4 | 2.1 | 4 | 2.1 |
| 40–49 | 4 | 2.2 | 3 | 1.7 | 6 | 3.3 |
| 50–59 | 1 | 0.6 | 2 | 1.2 | 3 | 1.8 |
| 60–69 | 3 | 2.8 | 2 | 1.8 | 4 | 3.7 |
| 70–79 | 3 | 4.6 | 2 | 3.1 | 2 | 3.1 |
| ≥80 | 2 | 5.6 | 2 | 5.6 | 2 | 5.6 |
| Totalc | 19 | 1.5 (0.8, 2.2)d | 19 | 1.5 (0.8, 2.2)d | 25 | 1.9 (1.2, 2.7)d |
Rates are per 100 000 population. Denominator data are based on the Rochester Epidemiology Project census (see reference in text).
Age and sex adjusted to the 2000 projected US population.
Age adjusted to the 2000 projected US population.
95% CIs.
Fig. 2.
Overall incidence rates of SLE, by age group and different classification criteria, Olmsted County, Minnesota, 2000–2018
EULAR: European Alliance of Associations for Rheumatology.
Fig. 3.
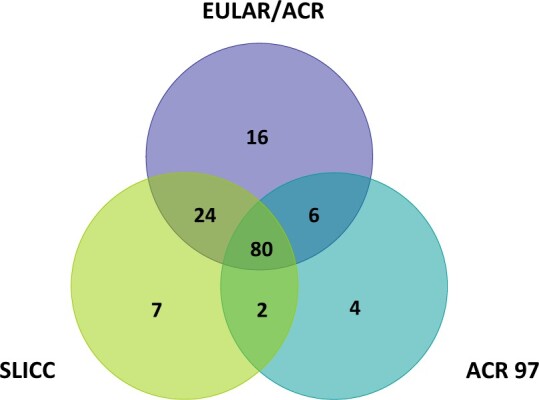
Venn diagram depicting the overlap of cases identified by the three classification criteria based on which criteria were met within 1 year of meeting the first set of criteria
EULAR: European Alliance of Associations for Rheumatology.
Table 3 displays the incidence rates across racial and ethnic groups. The age/sex adjusted incidence rate for non-Hispanic Asian (11.3–12.7/100 000), non-Hispanic Black (6.7–8.3/100 000) and Hispanic (5.2–5.5/100 000) patients was similar across the three different classification criteria, but the incidence in non-Hispanic White patients was lower by the ACR97 criteria (2.7/100 000) compared with SLICC (3.5/100 000, P = 0.003) and EULAR/ACR (4.1/100 000, P < 0.001) criteria.
Table 3.
Number of cases and age/sex adjusted incidence rate per 100 000 population of SLE, by different classification criteria and race/ethnicity, Olmsted County, Minnesota, 2000–2018a
| ACR 97 |
SLICC |
EULAR/ACR |
||||
|---|---|---|---|---|---|---|
| Race/ethnicityb | No. of cases | Incidence rate (95% CI) | No of cases | Incidence rate (95% CI) | No. of cases | Incidence rate (95% CI) |
| NH-Asian | 17 | 11.3 (5.8, 16.8) | 19 | 12.7 (6.8, 18.5) | 18 | 12.2 (6.4, 18.0) |
| NH-Black | 7 | 6.7 (1.5, 11.8) | 8 | 8.3 (1.5, 15.1) | 8 | 6.9 (2.0, 11.8) |
| Hispanic | 5 | 5.2 (0.0, 11.0) | 6 | 5.5 (0.0, 11.2) | 6 | 5.5 (0.0, 11.2) |
| NH-White | 63 | 2.7 (2.0, 3.4) | 80 | 3.5 (2.7, 4.2) | 94 | 4.1 (3.2, 4.9) |
Rates are per 100 000 population. Denominator data are based on the Rochester Epidemiology Project census (see reference in text). Rates are age- and sex-adjusted to the US standard population, 2000.
Cases were assigned to 1 of 4 mutually exclusive race/ethnicity categories: non-Hispanic White, non-Hispanic Black, Hispanic, non-Hispanic Asian. NH: non-Hispanic.
The most common clinical and immunological manifestations observed at the time patients met classification to one of the criteria were arthritis/synovitis, present in 50–60% across the three criteria, and leukopenia in 30% of the individuals (57% when combined with lymphopenia for the SLICC criteria). On the other hand, neurological involvement was very rare, affecting <2% of the incident cases. All of the incident cases were positive for ANA (based on classification criteria definition), around 60% were positive for anti-ds DNA and 30% were positive for anti-Sm. Close to 70% had low complement levels and 20% were positive for at least one antiphospholipid antibody (Supplementary Tables S2–S4, available at Rheumatology online).
Discussion
Conducting epidemiological studies of heterogeneous diseases such as SLE has been a public health challenge. In the past decade, a series of projects funded by the Centers of Disease Control and Prevention using similar methodology were able to obtain state-of-the-art epidemiological estimates of SLE across different racial/ethnic populations in the USA. Among the CDC-funded SLE registries, this study provides incidence and time to classification estimates using the newly developed and endorsed EULAR/ACR criteria as well as the previously used ACR97 and SLICC criteria.
In this population-based study, the incidence of SLE was higher by the EULAR/ACR criteria compared with the ACR97 and SLICC criteria. The EULAR/ACR criteria also classified patients earlier than the ACR97, but showed no difference compared with the SLICC criteria. The same number of minority patients was classified by the three classification criteria, but the SLICC and EULAR/ACR criteria classified more White patients than the ACR97 criteria.
Incidence rates using the ACR97 criteria (3.3/100 000) were lower in our study than those previously reported in the other CDC registries (4.6–5.6/100 000) [1–5]. However, the demographics of the populations across the different registries are different, which may explain the variation. When comparing the estimates in the White population only, the incidence was consistent between our study and the other four registries that included White patients, ranging between 2.7 and 3.7/100 000. The Manhattan registry had previously reported incidence estimates using the SLICC criteria, which were also similar (4.8/100 000 White population) to the ones reported in this study [4].
Olmsted County had a higher SLE incidence rate among Asian (11.3/100 000) patients than the San Francisco (4.1/100 000) and Manhattan registries (3.8/100 000) [3, 4]. However, the San Francisco registry Asian patients were predominantly from China and Japan while in our registry, the Asian patients were predominantly from India or Southeast Asia (Laotian, Burmese, Vietnamese, Cambodian and Hmong people). There is very little epidemiological data on the frequency of SLE in different Asian countries; a recent systematic review did not identify any studies from South or Southeast Asia [16, 17]. Given the genetic diversity in Asia, it is possible that SLE is more frequent in some Asian countries or regions, as is the case for other diseases [18].
Our study confirmed that the EULAR/ACR criteria classify patients sooner (median 29.4 months) after the initial symptom onset compared with the ACR97 criteria (median 47.0 months) but not compared with the SLICC criteria (median 30.6 months). Prior studies have provided conflicting results. A study using the LUMINA (Lupus in Minorities: Nature Versus Nurture) cohort found that only 13% of patients (vs ACR97 [15.3% vs SLICC criteria]) were classified earlier by the EULAR/ACR criteria. A similar study from a Latin-American multicentre SLE cohort (GLADEL) reported that 7.4% of patients (vs ACR97 [0.6% vs SLICC]) in their cohort were classified earlier by EULAR/ACR criteria [19, 20]. On the other hand, a recent study from Greece had findings similar to our study [21]. The discrepancies in the findings may be due to the populations and methodology. LUMINA enrolled patients up to 5 years after diagnosis, while GLADEL enrolled within 2 years of diagnosis, and some of the manifestations or laboratory values included (and heavily weighted) in the EULAR/ACR criteria were not recorded in these historical cohorts. When we performed similar analysis to these studies, the majority of patients were classified earlier by the SLICC and EULAR/ACR criteria (simultaneously), or by the EULAR/ACR criteria, and only 7% of the patients were classified by the ACR criteria first. The Greek inception cohort is still active; patients were enrolled at diagnosis and the cohort included patients with all the data elements for the analysis. In our study, the resources of the REP allowed us to identify the very first symptoms associated with SLE (e.g. first SLE-related complaint to primary care physicians or others even before diagnosis or testing) and follow the patients until meeting classification criteria, since we had access to the entirety of the medical record.
While the EULAR/ACR and the SLICC criteria identified more White patients than the ACR97 criteria, the three criteria identified the same number of minority patients. A recent study demonstrated that the EULAR/ACR criteria performed well across different racial and ethnic groups [22]. The EULAR/ACR criteria have been shown in validation cohorts to be more sensitive than the ACR97 criteria (96% vs 83%) and as sensitive as the SLICC criteria (97%), but more specific than the SLICC criteria (93% EULAR/ACR vs 84% SLICC) and as specific as the ACR97 criteria (93%) [23]. Studies have shown that minority patients have more severe disease than Whites, so it is possible that the EULAR/ACR criteria may classify patients with milder disease, thus classifying more White patients [24]. In our study as in others, minority patients had more severe disease (data not shown) and therefore were more likely to be classified by all three criteria. However, given the small numbers of minority patients, it is possible that our study did not have adequate power to identify differences across the different criteria.
Our study does have at least three limitations. First, ∼30% of the patients identified were from a racial or ethnic minority, and therefore our results may not apply in population with higher representation of minorities. Second, it is uncertain how findings from this geographically limited study apply to larger (e.g. national) populations. Third, our study is based on medical record review, and therefore case ascertainment depends on the completeness of the documentation and workup done by clinicians. The major strengths of this study are the long-standing record-linkage system of the REP, which provides more clinical detail than in the other CDC-funded registries, being population-based, which helps find the full spectrum of all clinically detected cases of SLE, and the ability to compare retrospectively the different classification criteria in the same patients.
In conclusion, the results from this population‐based study revealed that the incidence of SLE was higher by the EULAR/ACR criteria compared with the ACR97 or the SLICC criteria, and the EULAR/ACR criteria classified patients earlier than the ACR97 criteria, but at a similar time to the SLICC criteria.
Supplementary Material
Acknowledgements
The study team would like to thank Barbara Abbott for her support to access the Rochester Epidemiology Project data. The Rochester Epidemiology Project was supported by the National Institute on Aging of the National Institutes of Health under Award Number R01AG034676, and Grant Number UL1 TR002377 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: The Lupus Midwest Network (LUMEN) project is supported by the Centers for Disease Control and Prevention of the U.S. Department of Health and Human Services (HHS) under Grant number U01 DP006491 as part of a financial assistance award totalling $1 750 000 with 100% funding by CDC/HHS. The contents are those of the author(s) and do not necessarily represent the official views of, nor an endorsement, by CDC/HHS, or the U.S. Government.
Disclosure statement: A.D.-G. is supported by the Rheumatology Research Foundation Scientist Development Award and the Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery. The rest of the authors have declared no conflicts of interest.
Data availability statement
The data underlying this article will be shared on reasonable request to the corresponding author.
Supplementary data
Supplementary data are available at Rheumatology online.
Contributor Information
Alí Duarte-García, Division of Rheumatology, Department of Medicine; Robert D. and Patricia E. Kern Center for the Science of Health Care Delivery.
Mehmet Hocaoglu, Division of Rheumatology, Department of Medicine.
Shirley-Ann Osei-Onomah, Division of Rheumatology, Department of Medicine.
Jesse Y Dabit, Division of Rheumatology, Department of Medicine.
Rachel E Giblon, Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN.
Charles G Helmick, Centers for Diseases Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Division of Population Health, Atlanta, GA, USA.
Cynthia S Crowson, Division of Rheumatology, Department of Medicine; Division of Clinical Trials and Biostatistics, Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN.
References
- 1. Lim SS, Bayakly AR, Helmick CG et al. The incidence and prevalence of systemic lupus erythematosus, 2002-2004: the Georgia Lupus Registry. Arthritis Rheumatol 2014;66:357–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Somers EC, Marder W, Cagnoli P et al. Population-based incidence and prevalence of systemic lupus erythematosus: the Michigan Lupus Epidemiology and Surveillance program. Arthritis Rheumatol 2014;66:369–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dall'Era M, Cisternas MG, Snipes K et al. The incidence and prevalence of systemic lupus erythematosus in San Francisco County, California: the California Lupus Surveillance Project. Arthritis Rheumatol 2017;69:1996–2005. [DOI] [PubMed] [Google Scholar]
- 4. Izmirly PM, Wan I, Sahl S et al. The incidence and prevalence of systemic lupus erythematosus in New York County (Manhattan), New York: the Manhattan Lupus Surveillance Program. Arthritis Rheumatol 2017;69:2006–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ferucci ED, Johnston JM, Gaddy JR et al. Prevalence and incidence of systemic lupus erythematosus in a population-based registry of American Indian and Alaska Native people, 2007-2009. Arthritis Rheumatol 2014;66:2494–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum 1997;40:1725. [DOI] [PubMed] [Google Scholar]
- 7. Aringer M, Costenbader K, Daikh D et al. 2019 European League Against Rheumatism/American College of Rheumatology classification criteria for systemic lupus erythematosus. Arthritis Rheumatol 2019;71:1400–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Petri M, Orbai AM, Alarcon GS et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum 2012;64:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. St Sauver JL, Grossardt BR, Yawn BP, Melton LJ 3rd, Rocca WA. Use of a medical records linkage system to enumerate a dynamic population over time: the Rochester epidemiology project. Am J Epidemiol 2011;173:1059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. St Sauver JL, Grossardt BR, Yawn BP et al. Data resource profile: the Rochester Epidemiology Project (REP) medical records-linkage system. Int J Epidemiol 2012;41:1614–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. St Sauver JL, Grossardt BR, Leibson CL et al. Generalizability of epidemiological findings and public health decisions: an illustration from the Rochester Epidemiology Project. Mayo Clin Proc 2012;87:151–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Population of Olmsted County, Minnesota: Census 2010 and 2000 interactive map, demographics, statistics, graphs, quick facts. http://censusviewer.com/county/MN/Olmsted (1 July 2021, date last accessed).
- 13. Harris PA, Taylor R, Thielke R et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Harris PA, Taylor R, Minor BL et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Klein RJ, Schoenborn CA. Age adjustment using the 2000 projected U.S. population. Healthy People 2010 Stat Notes 2001:1–10. [PubMed] [Google Scholar]
- 16. Tanaka Y, O'Neill S, Li M, Tsai IC, Yang YW. Systemic lupus erythematosus: targeted literature review of the epidemiology, current treatment and disease burden in the Asia Pacific region. Arthritis Care Res (Hoboken) (in press), doi: 10.1002/acr.24431. [DOI] [PubMed] [Google Scholar]
- 17. Jakes RW, Bae SC, Louthrenoo W et al. Systematic review of the epidemiology of systemic lupus erythematosus in the Asia-Pacific region: prevalence, incidence, clinical features, and mortality. Arthritis Care Res (Hoboken) 2012;64:159–68. [DOI] [PubMed] [Google Scholar]
- 18. GenomeAsia KC. The GenomeAsia 100K Project enables genetic discoveries across Asia. Nature 2019;576:106–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ugarte-Gil MF, Pons-Estel GJ, Harvey GB et al. Applying the 2019 EULAR/ACR lupus criteria to patients from the LUMINA cohort. Arthritis Care Res (Hoboken) 2021;73:1451–5. [DOI] [PubMed] [Google Scholar]
- 20. Pons-Estel GJ, Ugarte-Gil MF, Harvey GB et al. Applying the 2019 EULAR/ACR lupus criteria to patients from an established cohort: a Latin American perspective. RMD Open 2020;6:e001097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Adamichou C, Nikolopoulos D, Genitsaridi I et al. In an early SLE cohort the ACR-1997, SLICC-2012 and EULAR/ACR-2019 criteria classify non-overlapping groups of patients: use of all three criteria ensures optimal capture for clinical studies while their modification earlier classification and treatment. Ann Rheum Dis 2020;79:232–41. [DOI] [PubMed] [Google Scholar]
- 22. Johnson SR, Brinks R, Costenbader KH et al. Performance of the 2019 EULAR/ACR classification criteria for systemic lupus erythematosus in early disease, across sexes and ethnicities. Ann Rheum Dis 2020;79:1333–9. [DOI] [PubMed] [Google Scholar]
- 23. Aringer M, Petri M. New classification criteria for systemic lupus erythematosus. Curr Opin Rheumatol 2020;32:590–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maningding E, Dall'Era M, Trupin L, Murphy LB, Yazdany J. Racial and ethnic differences in the prevalence and time to onset of manifestations of systemic lupus erythematosus: the California Lupus Surveillance Project. Arthritis Care Res 2020;72:622–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article will be shared on reasonable request to the corresponding author.