Abstract
Successful pregnancies rely on sufficient energy and nutrient supply, which require the mother to metabolically adapt to support fetal needs. The placenta has a critical role in this process, as this specialized organ produces hormones and peptides that regulate fetal and maternal metabolism. The ability for the mother to metabolically adapt to support the fetus depends on maternal prepregnancy health. Two-thirds of pregnancies in the United States involve obese or overweight women at the time of conception. This poses significant risks for the infant and mother by disrupting metabolic changes that would normally occur during pregnancy. Despite well characterized functions of placental hormones, there is scarce knowledge surrounding placental endocrine regulation of maternal metabolic trends in pathological pregnancies. In this review, we discuss current efforts to close this gap of knowledge and highlight areas where more research is needed. As the intrauterine environment predetermines the health and wellbeing of the offspring in later life, adequate metabolic control is essential for a successful pregnancy outcome. Understanding how placental hormones contribute to aberrant metabolic adaptations in pathological pregnancies may unveil disease mechanisms and provide methods for better identification and treatment. Studies discussed in this review were identified through PubMed searches between the years of 1966 to the present. We investigated studies of normal pregnancy and metabolic disorders in pregnancy that focused on energy requirements during pregnancy, endocrine regulation of glucose metabolism and insulin resistance, cholesterol and lipid metabolism, and placental hormone regulation.
Keywords: energy homeostasis, pregnancy, placenta, metabolism, gestational diabetes
Maternal Metabolic Adaptations in Normal Pregnancy
Human pregnancy is an energetically demanding process and requires synchrony between the mother and fetus. Throughout gestation, maternal basal metabolic rate increases (1), which causes both the resting and total energy expenditure to increase to support fetal development and growth (2). Both fetal and placental development cause maternal energy intake and expenditure to increase each day by approximately 375 KJ (89 Kcal) in the first trimester, 1200 KJ (286 Kcal) in the second trimester, and 1950 KJ (466 Kcal) in the third trimester (3). A mother’s prepregnancy nutritional status, height, and weight determine her ability to energetically and metabolically adapt to fetal needs (4).
Placental hormones and growth factors regulate maternal metabolism to favor increased fat storage during the first and second trimester (5), representing an “anabolic” state (1). This anabolic phase is important because fetal energy demands are not met by only increasing energy intake during the third trimester (5) and, therefore, rely on fat storage that is accumulated early in the pregnancy. In the third trimester, increased lipolysis and the mobilization of fat stores occur (1), which is observed by increased blood plasma concentrations of fatty acids and glucose (6). This shift from anabolic to catabolic lipid metabolism allows lipids to be the main source for maternal energy and preserve glucose for the developing fetus (7).
Glucose metabolism
The maintenance of glucose metabolism is a key factor in a healthy pregnancy (7). The fetus is unable to undergo gluconeogenesis and it therefore relies on a supply of glucose from maternal blood plasma and the placenta (8). During the first trimester of pregnancy, maternal glucose homeostasis is regulated by several hormones, such as estrogen, insulin, and cortisol, which function to increase fat storage, decrease energy expenditure, and delay blood glucose clearance (9).
Fetal glucose demands increase around week 26 of gestation, which requires maternal basal endogenous glucose production to increase via hepatic gluconeogenesis (10). At the same time, increases in circulating insulin and decreased insulin sensitivity occur. Estrogen assists in the increased glucose production by enhancing cortisol-binding globulin production by the liver to promote gluconeogenesis (11). Despite a surge in glucose production, plasma glucose concentrations may simultaneously decrease (12), suggesting that the circulating glucose is supplied to the fetus and placenta (13). Riskin-Mashiah et al investigated normal fasting plasma glucose levels in a cohort of 7946 healthy, pregnant, hospitalized women (12). The team demonstrated that fasting glucose levels are critically maintained in order to remain constant throughout pregnancy (12, 14–19).
Insulin resistance
Glucose metabolism is also altered by increasing insulin resistance (20, 21), elevated plasma lipid concentrations (6), and pancreatic β-cell expansion due to maternal pancreatic islet hypertrophy (22, 23). Estrogen and progesterone regulate insulin resistance at week 6 of pregnancy (22). Prolactin and human placental lactogen (hPL) levels peak around week 10 (9), promoting β-cell proliferation and insulin production and secretion to meet higher insulin demands and further increase insulin resistance (20, 21). Insulin resistance continues to develop in the second trimester and peaks in the third trimester of pregnancy (24). Increased circulating progesterone, prolactin, cortisol, and hPL promote insulin resistance in adipocytes and skeletal muscles (24). High cortisol assists with the insulin resistance needed for delayed glucose clearance (25). Insulin initiates glucose uptake by binding to its receptor and through phosphorylation of the β-subunit, followed by phosphorylation of the insulin receptor substrate 1 (IRS-1) at a tyrosine residue, which is then primed for initiating signal transduction pathways (26, 27). In normal pregnancies, there is decreased insulin phosphorylation of the insulin receptor (28), and progesterone causes decreased IRS-1 expression, further decreasing the insulin-induced translocation of glucose transporter 4 (GLUT4) to the cell membrane to dampen glucose cellular uptake (26).
In addition to hormones, the cytokine tumor necrosis factor-α (TNFα) was identified to be a potential mediator for insulin resistance during later stages in pregnancy (29). Increases in circulating levels of TNFα have been associated with insulin resistance in obesity, sepsis, muscle damage, and even aging (30–32). It is also produced by the placenta and increased levels have been reported during pregnancy pathologies, such as preeclampsia and gestational diabetes (33, 34). In a prospective study, Kirwan et al showed that insulin resistance during late gestation is significantly correlated with changes in circulating TNFα, irrespective of fat mass (29).
Lipid metabolism
Pregnancy initiates substantial changes in maternal lipid metabolism that are supportive of fetal growth and development. The first and second trimesters are collectively referred to as the “anabolic phase” of pregnancy whereby increased estrogen, progesterone, and insulin concentrations favor lipid deposition and inhibit lipolysis (7). Changes in hormones like progesterone, growth hormone (GH), prolactin, and others increase maternal appetite to increase extra body fat (6). On average, pregnant women with a healthy BMI (body mass index; 18.5–24.9) gain 25–35 lbs of body weight throughout the entirety of pregnancy (35).
During the first 6 weeks of gestation, plasma lipid levels decrease (6). Increased insulin sensitivity at this time promotes fatty-acid (FA) synthesis and increases lipoprotein lipase, which facilitates the cellular uptake of circulating triacylglycerides (TAGs) (6). By week 10, higher levels of FAs, TAGs, cholesterol, and phospholipids are observed in the blood and this continues through the third trimester (6). At 30 weeks of gestation, a metabolic shift to a catabolic state occurs as lipids are used for maternal energy source, while glucose and amino acids are conserved for the fetus (7, 36). These changes are driven by insulin resistance, which promote lipid catabolism and decrease lipoprotein lipase levels during the third trimester (6, 36). Increased FAs are released and metabolized into TAGs before being absorbed by the syncytial layer of the placenta (6, 37).
Cholesterol is a major component of circulating lipids and is continuously recycled and delivered to sites throughout the body, including the placenta (6). The placenta utilizes cholesterol to synthesize approximately 400–500 mg of steroid hormones daily (6). Cholesterol is also important for placental oxidation and placental membrane formation (6). At week 12 of gestation, high density lipoprotein (HDL) cholesterol increases in response to estrogen and remains elevated throughout the pregnancy (6). TAGs are elevated by approximately 2-fold, and total and low density lipoprotein (LDL) cholesterol are increased by 30% to 50% in the third trimester (6).
Placental Hormones in Pregnancy
The human placenta has many functions: it regulates temperature, serves as a protective barrier against the maternal microenvironment and infection, helps to establish immunologic tolerance of the fetus, and provides exchange of gases, nutrients, and waste (38, 39). Among the many functions of the human placenta, the numerous hormones produced by this organ have significant influences on establishing and maintaining a healthy pregnancy (40). Altering energy homeostasis in pregnancy can damage the placenta, leading to inadequate function and subsequent pregnancy complications, which is observed in gestational diabetes mellitus (GDM). In the following sections we provide an overview of hormones and growth factors secreted by the placenta that assist in regulating metabolism throughout pregnancy.
Placental growth hormone
The placental growth hormone (PGH) is a growth hormone variant produced by the placenta, which regulates maternal gluconeogenesis and lipolysis to modulate maternal adaptations during pregnancy (41). Placental growth hormone replaces pituitary GH in the maternal circulation and its concentrations increase in maternal circulation throughout pregnancy until term (42). Placental growth hormone functions as an insulin antagonist and mediates insulin resistance by directly modulating insulin-like growth factor 1 (IGF-1) (36, 43) and also initates increased growth of maternal tissues (24). Placental growth hormone may act independently or dependently through IGF-1 to increase nutrient supply for the fetus (41).
Placental growth hormone is predominantly expressed in and secreted from placental syncytiotrophoblasts and, to a lesser extent, in extravillous trophoblasts (43). Placental growth hormone has a role in the placenta by acting in both a paracrine and autocrine manner to stimulate trophoblast invasion (44) and placental growth through its receptor, growth hormone receptor (GHR), on syncytiotrophoblasts (41). A study by Lacroix et al showed that PGH stimulates trophoblast invasiveness through activation of the Janus kinase-2/signal transducer and activator of the transcription factor-5 (JAK-STAT) signaling pathway (44) to initiate transcription of invasion-promoting genes (45). In a transgenic mice study, overexpression of PGH induced hyperinsulinemia, or severe insulin resistance (46). This is thought to result from maternal pancreatic β-cell expansion and a decrease in body fat, similar to conditions observed in the third trimester of human pregnancy (46).
Despite the many important functions of PGH during normal pregnancy, studies in pregnant women with diabetes have shown no correlation between changes in PGH levels and insulin levels (47). Additionally, women with deletions in the PGH gene were also reported to have pregnancies that resulted in children with normal birth weights (48). This could be explained by other hormones acting in overlapping pathways, which compensate for PGH insufficiency, such as GH or hPL (41).
Human placental lactogens
Human placental lactogens, also called chorionic somatotrophin hormone (CSH), are types of growth hormones that have several roles, including metabolic regulation by increasing maternal glucose levels, decreasing maternal glucose usage, and promoting lipolysis and insulin resistance (21, 36). Human placental lactogen is produced by syncytiotrophoblasts and secreted into maternal–fetal circulations after the sixth week of pregnancy (24). During early gestation, hPL exhibits anabolic activity by promoting glucose uptake and incorporation of glucose into glycogen, glycerol, and FAs (48).
Human placental lactogen concentrations rise in the third trimester and become an important contributor to insulin resistance (9, 24). During the third trimester, hPL augments lipolysis and fat mobilization, increasing free FA levels in maternal circulation (24). Human placental lactogen increases to 5000–7000 ng/ml at 32 to 35 weeks, then declines at term to approximately 20–50 ng/ml (49). Aside from its anabolic/catabolic activities, hPL indirectly controls insulin production and secretion by increasing human pancreatic β-cell replication and cell survival rates (50). Similar to PGH, women with deletions in the CSH gene experience normal pregnancy outcomes, suggesting that alterations in this hormone may not lead to pregnancy complications such as GDM (47, 48).
Ghrelin
Ghrelin, also known as growth hormone (GH)-releasing peptide, is a gastric-secreted acylated peptide hormone (51) that controls feeding behaviors by stimulating GH release through GH secretagogue receptors (GHSR) (52) and stimulating appetite to increase food intake (53). At the cellular level, ghrelin regulates energy balance and proliferation (52). Ghrelin also has a role in activating hepatic gluconeogenesis and inititates glucose uptake through phosphorylation of tyrosine molecules on IRS-1 (54). Ghrelin is also highly expressed in the first trimester of pregnancy by the human placenta—primarily in cytotrophoblasts and also in placental villi stroma (55). Ghrelin levels increase midpregnancy and decrease thereafter to undetectable levels in full-term human placenta (55).
The gestational stage dependent expression of ghrelin in the placenta overlaps with energy intake/expenditure requirement of the fetus. Nakahara et al used a rat model of pregnancy to show that ghrelin has a large effect on fetal growth (56). Their study shows that maternal treatment with ghrelin increased fetal birth weight, despite a restricted diet (56). This suggests that ghrelin may have physiological functions in homeostatic control of energy balance in pregnancy as well as in modulating fetal growth and development.
The role of ghrelin in the development of GDM still remains unclear. Women with GDM showed no significant differences in plasma levels of ghrelin compared to healthy pregnant women—although ghrelin mRNA was signficantly higher in the placenta of GDM women compared to healthy pregnancies (57). This suggests ghrelin may have a role in the placenta during GDM pregnancies, which needs to be further investigated. Interestingly, ghrelin knockout mice show normal fertility with no effect on growth or appetite (58). However, studies with ghrelin-receptor knockout mice revealed increased levels of IGF-1, suggesting that ghrelin-receptor signaling exerts a physiologic role in energy balance (58). Similar observations are observed in other rodent models and in humans who have deficiencies in grehlin-receptor function (59).
Leptin
Leptin is a hormone characterized by its roles in food intake regulation and energy expenditure in white adipose tissue (WAT), where it is secreted in response to increased energy storage (60). Leptin is also produced in, and modulates, a wide range of cellular functions in numerous tissues and organs, including the hypothalamus (61), gastric epithelium (62), and skeletal muscle (63). Leptin has recently emerged as an important player in reproductive health, from regulating the menstrual cycle and oocyte maturation (64) to embryo implantation and development (65, 66). Leptin expression in the placenta is regulated by exogenous 17beta-estradiol (E2) via crosstalk between estrogen receptor 1 and MAPK-PI3K signal transduction pathways (67, 68).
Leptin suppresses the appetite of healthy, nonpregnant (NP) individuals through its receptors (LRb) (69) on the hypothalamus located in the brain (70), where it also influences secretion of thyroid hormones, sex hormones, and growth hormones (69). Leptin binding to LRb causes transphosphorylation of intracellular LRb and activates the Jak kinase family 2 (Jak2) to intiate further signaling pathways (69). Despite its role in suppressing appetite, circulating levels of leptin gradually increase throughout gestation. Ladyman et al used a rat model of pregnancy to study the effects of leptin on feeding behavior during pregnancy. In their study, they treated NP and pregnant rats with leptin at gestation days 7 and 14 and measured food intake. They showed NP and gestation day-7 pregnant rats had a reduction in food eaten; however, the leptin did not effect feed behavior for gestation day-14 rats. Their results also revealed that leptin-induced STAT3 phosphorylation was reduced in the hypothalamic nuclei of pregnant rats, which could be the mechanism behind pregnancy-induced leptin resistance (71). Additionally, decreased mRNA of leptin receptor in the hypothalamus (70) inidicates that these rats experienced resistance to leptin (71). This finding is in coordinance with another murine study by Bates et al. They show that STAT3 activation occurs through the tyrosine 1138 residue on LRb (72). They replaced the tyrosine 1138 residue with a serine residue, and this inhibited STAT3 activation, resulting in hyperphagia and obesity (72).
A similar trend is observed during human pregnancy, where leptin levels and hyperphagia simultaneously increase throughout gestation. The study by Ladyman et al suggests that a similar mechanism occurs in humans that leads to resistance to the anorexigenic effects of leptin during pregnancy (71, 73). Leptin resistance during pregnancy is important to maintain increased energy intake to support fetal growth in the second and third trimester (70). This also contributes to adipose tissue storage in early and midpregnancy by hyperphagia to prepare for lipid mobilization during the catabolic phase of late pregnancy (74).
Besides its role in metabolism, studies identified autocrine/paracrine activities for leptin in the placenta due to its expression in placental trophoblasts and amnion cells (75, 76). These activities include positive regulation of trophoblast differentiation, promotion of placental angiogenesis and nutrient transport, and local immunomodulation at the maternal–fetal interface (77, 78). All such regulatory events are essential for fetal development and adequate placental function (77, 78).
Irisin
Irisin is a newly identified myokine that induces energy expenditure by converting WAT to brown adipose tissue (79). Irisin is also able to regulate glucose and lipid levels and improve insulin sensitivity (80, 81). Irisin consists of 112 amino acids and is produced by FNDC5 (fibronectin domain-containing protein 5) cleavage. Physical activity induces expression irisin through the activation of peroxisome proliferator-activated receptor-γ co-activator 1α (PGC1α), which transcriptionally upregulates FNDC5 (82). Irisin faciliates glucose uptake in skeletal muscle cells through activation of the AMP-activated protein kinase (AMPK) 2 pathways and translocation of GLUT4 to the plasma membrane (82). Murine studies have investigated effects of overexpression of irisin and found it decreases fasting insulin levels and improves glucose tolerance during high-fat diets (79). Irisin also increases expression of uncoupling protein 1, which initiates thermogenesis in adipocytes (79). It is also hypothesized that irisin can have anti-inflammatory affects through activation of peroxisome proliterator-activated receptor-α (PPARα) (82).
Garcés et al reported that circulating irisin levels are higher in pregnant compared to NP women; its serum levels increase as gestation progresses, and its levels are significantly lower in preeclampsia (83). Further studies confirm that irisin is perturbed in other pregnancy complications, such as GDM, spontaneous preterm delivery, and intrauterine growth restriction (84–86). A study by Chen et al identified that irisin has a role in reducing oxidative stress and improving lipid metabolism in pregnancies complicated by liver dysfunction (87). Immunohistological evidence shows irisin is localized in cytotrophoblasts in the decidua and in synyctiotrophoblasts (82). Moreover, Drewlo et al recently showed that irisin may have a role in the placenta by regulating placental trophoblast differentiation in villous and extra villous cell models through activation of AMPK (88). The detailed physiological role of irisin in human pregnancy remains to be determined, although there are 2 key roles suggested for irisin during normal pregnancy: (1) to contribute to the development of normal gestational insulin resistance, and (2) to regulate body temperature (83).
Adiponectin
Adiponectin is an antidiabetic adipokine that serves important functions to regulate glucose metabolism and fatty acid oxidation (89). Adiponectin can promote pancreatic β-cell survival to prevent hepatic production of glucose (89) through AMPK mechanisms (28) and increase insulin sensitivity (90). It is produced in high amounts in lean women compared to overweight or obese NP women (91).
Adiponectin is produced in and secreted by maternal adipose tissue (89). Multiple studies described that adiponectin can also be secreted from the human placenta and that it acts in an autocrine/paracrine manner through adiponectin receptors 1 and 2 located on placental trophoblasts (91–93). However, other studies were unable to detect adiponectin expression in the term placenta (94–96) and therefore described adiponectin as an “adipose-specific secretory protein” (97), which is produced by maternal tissues during pregnancy. Depsite this, adiponectin is known to have significant roles in regulating insulin resistance and glucose homeostasis throughout pregnancy (98); it may function in a paracrine manner to increase adipocyte cell numbers, increase expression of lipid metabolism genes, and regulate local and systemic inflammation (97). As well, adiponectin decreases glucose production and lipogenesis, and its production is often decreased during unfavorable metabolic situations (97). Because of this, adiponectin is commonly used as a biomarker to understand metabolic states under certain conditions.
Abnormal Maternal Metabolic Adaptations in Gestational Diabetes
Gestational diabetes mellitus is a type of diabetes mellitus that develops only during pregnancy and usually disappears upon delivery. The American Diabetes Association classifies GDM as “diabetes first diagnosed in the second or third trimester of pregnancy that is not clearly either preexisting type 1 or type 2 diabetes” (99). It occurs in 15% to 20% of pregnancies and is associated with adverse outcomes, including macrosomia, neonatal hypoglycemia, and an increased rate of cesarean delivery (100). Gestational diabetes mellitus is generally characterized by maternal hyperglycemia, glucose intolerance, and high insulin resistance (101, 102).
In preconception, the median fasting plasma glucose (FPG) for women with normal pregnancies is 81 mg/dL and this slightly decreases to 80 mg/dL, 77 mg/dL, and 76 mg/dL in the first, second, and third trimesters, respectively (12), as shown in Fig. 1. Women are screened for GDM through a 1-step or 2-step oral glucose tolerance test (OGTT) at 24 to 28 weeks of gestation, unless they have risk factors for pregestational diabetes or hyperglycemia, in which case they will undergo OGTT at their first visit (99). The 1-step test involves a 75-gram OGTT that measures FPG, followed by blood glucose levels at 1 hour and 2 hours after glucose consumption (99). Based on this criteria, women are diagnosed with GDM if their blood glucose levels measure at or above at least 1 of the following: 92 mg/dL FPG, 180 mg/dL at 1 hour, or 153 mg/dL at 2 hours (99). The 2-step test does not require fasting and is conducted measuring glucose levels 1 hour after a 50-gram OGTT (99). If the patient measures ≥130, 135, or 140 mg/dL they are required to take a 100-gram OGTT after an 8-hour fast (99). Gestational diabetes mellitus is then diagnosed if the patient meets at least 2 of the following measurements: 95 mg/dL FPG, 180 mg/dL after 1 hour, 155 mg/dL after 2 hours, or 140 mg/dL after 3 hours (99).
Figure 1.
Fasting plasma glucose levels in normal pregnancy and GDM. In preconception, women who later go on to have normal pregnancies exhibit a mean FPG of 81 mg/dL (12). Mean FPG levels for women with normal pregnancies slightly decrease to 80 mg/dL, 77 mg/dL, and 76 mg/dL in the first, second, and third trimesters, respectively (12). A study by Sesmilo et al showed that first trimester FPG may vary, as their cohort of 6845 women had a mean FPG of 83 with a standard deviation of ± 7.3 mg/dL (132). Of these women, 10.2% developed GDM, which showed that women with a FPG ≥ 88 mg/dL in the first trimester were 1.82 times more likely to be diagnosed with GDM in the second trimester (132). Gestational diabetes mellitus is diagnosed in the first or second trimester if the patient measures a FPG ≥ 92 mg/dL by the 1-step OGTT (99). In a study by Seabra et al, they showed that GDM patients had a significantly higher FPG (90 mg/dL) compared to women without pregnancy complications (FPG 77.8 mg/dL, P = 0.016) in the third trimester (133).
Metabolic disorders in pregnancy, like GDM, often involve aberrant lipid metabolism. Bao et al investigated triglyceride, total cholesterol, LDL cholesterol, and HDL cholesterol levels in women with GDM (Fig. 2) and normal pregnancies (Fig. 3) (103). High levels of triglycerides and low levels of HDL cholesterol during early pregnancy was shown to increase the risk for developing GDM later on in pregnancy (103). These data also support the fact that hyperlipidemia is associated with GDM and, in some cases, is a precursor to this condition (6).
Figure 2.
Mean plasma lipid concentrations measured throughout GDM pregnancies. Gestational diabetes mellitus pregnancies often involve abnormal lipid concentrations throughout pregnancy. As well, infants born to mothers with GDM often have increased adipose tissue at birth. Bao et al identified that women with higher triglycerides in early pregnancy have an increased risk of developing GDM in later pregnancy (103). As well, lower HDL cholesterol in early pregnancy significantly increases the risk for developing GDM in later pregnancy (103). Changes in LDL cholesterol or total cholesterol throughout pregnancy was not shown to be significantly associated with risk of developing GDM (103). *P < 0.05 indicates significant differences in HDL, LDL cholesterol, or triglyceride concentration in GDM pregnancies compared to normal pregnancies during that gestation age (103). Image was adapted from Bao et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes. 2018;10(6):487–495.
Figure 3.
Mean plasma lipid concentrations measured throughout normal pregnancies. Healthy pregnancies typically show HDL cholesterol increases from 60–70 mg/dL in the late first trimester, and these levels decrease to below 60 mg/dL at the end of gestation (103). Low density lipoprotein cholesterol gradually increases across gestation from around 86–126 mg/dL (103). Triglycerides increase in the beginning of the second trimester, from around 130 mg/dL, and increase until the end of gestation to approximately 280 mg/dL (103). Total cholesterol increases from approximately 180 mg/dL at the end of the first trimester to around 230 mg/dL at the end of the third trimester (103). Image was adapted from Bao et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes. 2018;10(6):487–495.
Maternal obesity during pregnancy can involve greater lipolysis rates, which may cause lipotoxicity and can lead to maternal endothelial dysfunction (37). Elevated estrogens and abnormally high insulin resistance may also contribute to high lipid levels during pregnancy (6). Abnormal lipid metabolism can serve as a precursor to maternal metabolic disease postpartum (101, 104) and affect fetal birth weights, as the placenta may accumulate excessive amounts of lipids during obese pregnancies, altering delivery of FAs and TAGs to the fetus (37).
Gestational diabetes mellitus may also develop due to the dysregulation of pancreatic β-cell function against a background of insufficient insulin action (both insulin sensitivity and secretion defects) and abnormal secretion of pregnancy hormones (20, 23, 105). Leptin, irisin, and adiponectin are among the many placental hormones that are found to be dysregulated in GDM. In this section, we discuss their roles in GDM development.
Leptin involvement during GDM
Leptin and leptin receptor expression are found to be increased in the placenta of women with GDM, and this may result from hyperinsulemia to increase leptin levels (106). These conditions also promote increases in proinflammatory proteins, which further increase the production of leptin (106). Besides this, studies have shown that specific single nucleotide polymorphisms in the leptin gene (LEP-2548G/A) predisposes risk for developing GDM (106). Interestingly, leptin levels measured in early pregnancy have been used as predictive biomarkers for later development of GDM (107) and in pregnancies complicated by type 1 diabetes (108). Kautzky-Willer found that pregnant women have higher blood leptin levels than NP women, which peak at around 28 weeks into gestation (109). In the third trimester, leptin levels start declining and are significantly lowered postpartum in healthy pregnancies (109). While this trend is similar in women who develop GDM, leptin levels are significantly higher in GDM when compared to normal pregnancies and NP women, as shown in Fig. 4 (109). It is also reported that leptin levels remain high postpartum in women with GDM, which negatively correlates with placental function and birth weight (109).
Figure 4.
Leptin measurements in the second trimester of pregnancy. Kautzky-Willer et al showed that there is a significant increase in plasma leptin levels in women who have GDM (24.9 ng/mL) compared to normal pregnancies (18.2 ng/mL) (109). Leptin levels during nonpregnancy are 8.2 ng/mL (109). ***P < 0.001.
A similar observation was reported by Qiu et al (110), which investigated a cohort of 823 women and measured plasma leptin in the first trimester of pregnancy. They found that women with higher leptin levels (31.0 ng/ml) had a 4.7% increased risk of developing GDM compared to women with normal or lower leptin levels (≤14.3 ng/ml) (110). Qiu et al further showed that every 10 ng/ml increase in leptin concentration increased the risk of developing GDM by 20% (110). A meta-analysis study in 2015 by Bao et al reported similar trends for leptin (111).
Interestingly, studies by Festa et al and Mosavat et al report significantly lowered leptin levels in women with GDM compared to control (112, 113), which is opposite from Qiu et al (110). Each of these studies compared GDM women with normal pregnant women who have a comparable BMI, maternal age, gestational age, gestational weight gain, and in some cases ethnicity. The opposite results may be attributed to the sampling method, as both Festa et al and Mosavat et al used blood serum to measure leptin levels, while Qiu et al measured leptin from blood plasma. The method and time required for serum and plasma separation from whole blood is shown to have an effect on the levels of certain metabolites such as insulin, peptide C, and other factors such as vascular endothelial growth factor (VEGF) (114-116). These studies highlight the importance of potential errors introduced in the measurement of clinical samples, and future surveys of blood leptin levels during pregnancy warrant a more critical assessment of specimen collection.
Irisin involvement during GDM
Many reports describe GDM patients as having lower irisin levels compared to healthy, age-matched controls (117, 118), as shown in Fig. 5. A study by Seven et al measured irisin levels in pregnant women with GDM, obese without GDM, and control pregnancies and identified that women with GDM have significantly lower irisin levels, while obese non-GDM women had higher irisin levels (both compared to control pregnancies) (119). These results point out that irisin likely has different pathogenic effects in GDM and non-GDM obesity during pregnancy (119, 120).
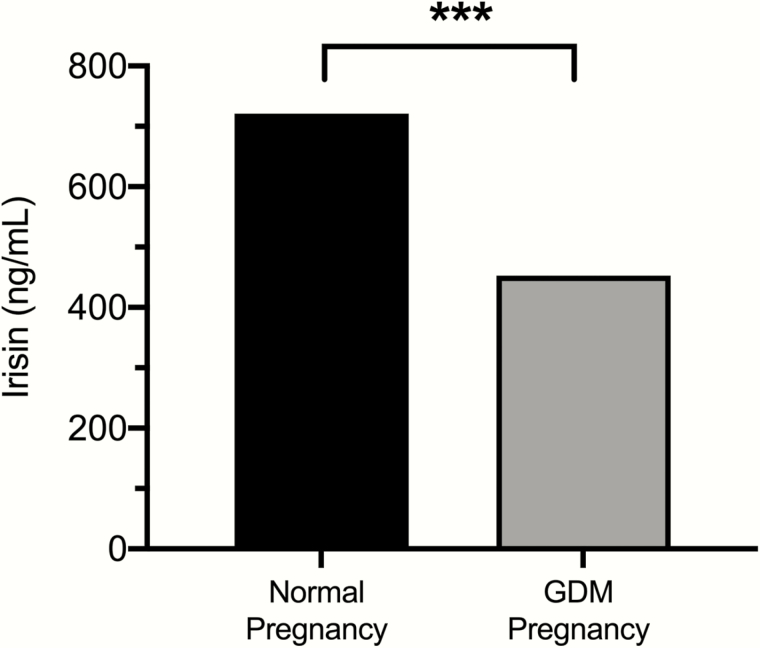
Figure 5.
Irisin measurements in the first trimester of pregnancy. Erol et al showed that there is a decrease in irisin levels in women who later developed GDM (452 ng/mL) compared to normal pregnancies (752 ng/mL) (121). ***P < 0.001.
Erol et al also found that GDM patients had significantly lower irisin levels in the first trimester (453 ng/ml in GDM vs. 721 ng/ml in controls) and slightly lower levels during the second trimester (749 ng/ml in GDM vs. 757 ng/ml in controls) (121). Similarly, Ural et al showed significantly lower irisin levels in blood serum during the second trimester in a cohort of 45 women with GDM and 41 matched controls (122). However, Ural et al reported values of 1 ug/l (or 1 ng/mL) in comparison to the median value of 749 ng/ml reported by Erol et al. The specificity and sensitivity of ELISA kits used in these studies might contribute to the differences in irisin levels reported by the 2 groups. Both Erol et al and Ural et al report a positive correlation of irisin with fasting insulin levels in these women, which may suggest a role for irisin in glucose and energy metabolism during pregnancy. Moreover, studies have shown that exercise prevents fetal overgrowth (123), which often occurs during GDM pregnancies. Exercise increases irisin secretion (124) and insulin sensitivity, decreases plasma glucose concentrations, and increases angiogenic factors in the blood (123), further showing the importance of irisin during pregnancy.
Current approaches in the field of GDM aim to establish a biomarker panel for diagnosis in the first or second trimester (22). It is important to indentify women who are at a greater risk for developing GDM in later pregnancy and if their infant is at risk of developing comorbidities as a result (22). Wang et al suggestes that irisin measurements in the first trimester may serve as a significant risk factor for developing GDM later in the pregnancy (125). However, a recent study by Jedrychowski et al showed that ELISA-based detection of irisin might not be sufficient and that a more sensitive technology like mass spectrophotometry might be required to correctly assess irisin levels during pregnancy (126). These results warrant a more thorough investigation into irisin detection and the mechanisms by which irisin contributes to GDM pathology before its development as a biomarker.
Adiponectin involvement in GDM
Adiponectin is known to have major roles regulating energy homeostasis. Multiple reports show decreased levels of adiponectin during GDM pregnancies (127–129). Adiponectin expression in adipocytes is negatively regulated by proinflammatory factors, like TNFα, which are highly expressed during GDM pregnancies and could explain why adiponectin is decreased in GDM (28). Using a transgenic knockout mouse model, a recent study revealed that adiponectin-deficient mice developed glucose intolerance and hyperlipidemia in the later stages of pregnancy and their offspring exhibited increased fetal weight (130). These adiponectin-deficient mice also show increased production of hepatic glucose and triglycerides and decreased β-cell mass compared to the normal pregnant mice, which are characteristic of GDM pregnancies. Interestingly, when these adiponectin knockout mice were then administered adiponectin, it reversed the glucose intolerance and prevented fetal overgrowth. These results show the high impact of adiponectin on maintaining energy homeostasis during pregnancy (130).
Summary and Conclusion
Maternal physiology and overall health prior to pregnancy determine the ability to metabolically adapt to support fetal development. In normal pregnancy, maternal carbohydrate and lipid metabolism and its regulation are altered with advancing gestation. These trends are characterized by a progressive decrease in maternal glucose sensitivity coinciding with increasing lipolysis, indicating a shift from an anabolic state to catabolic state in late pregnancy. Numerous placental hormones—including placental growth hormone, placental lactogens, leptin, ghrelin, irisin, and adiponectin—regulate glucose and lipid metabolism, as well as the insulin sensitivity/resistance that occurs throughout anabolic and catabolic states of pregnancy (36).
Preconception maternal obesity is a risk factor for placental dysfunction, which drives aberrant metabolic control (37). Altering the normal trends of maternal energy homeostasis can lead to pregnancy-related metabolic disease, like GDM. This is concerning, as two-thirds of pregnancies in the United States involve overweight or obese women, and conditions like GDM can have lasting effects on maternal–fetal health (37). Gestational diabetes mellitus leads to insufficient insulin levels and aberrant blood glucose concentrations that impair cognitive, neurological, and endocrine development in the fetus, which negatively impact the offspring in later life. Besides maternal–fetal comorbidities, these pregnancy complications pose a large economic burden accounting for approximately 1.6 billion dollars per year in United States (131).
It is also important to acknowledge that molecular pathways mediated by placental hormones driving the changes in metabolism during normal pregnancy and GDM are not completely elucidated. This is further complicated by the unavailability of sensitive methods to detect targets like irisin. Understanding the mechanisms that regulate metabolic trends during pregnancy is critical for better identification, treatment, and prevention of metabolic-related pregnancy complications.
The placenta may additionally secrete yet undetected factors that might contribute to this process. Future research should focus on determining accurate levels of known factors and global screening approaches that detect novel factors might be beneficial. It is also crucial to determine the full extent to which aberrant hormone expression may be harmful to the placenta, including the regulation of placental function at the molecular level. Finally, research into means of damping effects of abnormal metabolic trends during metabolic-related pregnancy complications would greatly impact the current means of treatment to alleviate these complications.
Acknowledgments
We thank Dr. Brian Knight’s team for editoral assistance and Ken Provost from Xavier Studio for the scientific illustrations.
Financial Support: Funding for this article was received from the Department of Obstetrics, Gynecology and Reproductive Biology in the College of Human Medicine at Michigan State University.
Author Contributions: All authors contributed to the article design, literature analysis, and drafting of the article. B.A. served as primary editor of this article.
Contributor Information
Brooke Armistead, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Eugenia Johnson, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Robert VanderKamp, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Elzbieta Kula-Eversole, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Leena Kadam, Department of Obstetrics and Gynecology, Wayne State University, Detroit, Michigan.
Sascha Drewlo, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Hamid-Reza Kohan-Ghadr, Department of Obstetrics, Gynecology and Reproductive Biology, College of Human Medicine, Michigan State University, Grand Rapids, Michigan.
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
References
- 1. King JC, Butte NF, Bronstein MN, Kopp LE, Lindquist SA. Energy metabolism during pregnancy: influence of maternal energy status. Am J Clin Nutr. 1994;59(2 Suppl):439S–445S. [DOI] [PubMed] [Google Scholar]
- 2. Abeysekera MV, Morris JA, Davis GK, O’Sullivan AJ. Alterations in energy homeostasis to favour adipose tissue gain: a longitudinal study in healthy pregnant women. Aust N Z J Obstet Gynaecol. 2016;56(1):42–48. [DOI] [PubMed] [Google Scholar]
- 3. Butte NF, King JC. Energy requirements during pregnancy and lactation. Public Health Nutr. 2005;8(7A):1010–1027. [DOI] [PubMed] [Google Scholar]
- 4. King JC. Physiology of pregnancy and nutrient metabolism. Am J Clin Nutr. 2000;71(5 Suppl):1218S–1225S. [DOI] [PubMed] [Google Scholar]
- 5. Jebeile H, Mijatovic J, Louie JCY, Prvan T, Brand-Miller JC. A systematic review and metaanalysis of energy intake and weight gain in pregnancy. Am J Obstet Gynecol. 2016;214(4):465–483. [DOI] [PubMed] [Google Scholar]
- 6. Grimes SB, Wild R. Effect of pregnancy on lipid metabolism and lipoprotein levels. In: Feingold KR, Anawalt B, Boyce A, et al. , eds. Endotext. South Dartmouth, MA: MDText.com, Inc; 2000. [PubMed] [Google Scholar]
- 7. Butte NF. Carbohydrate and lipid metabolism in pregnancy: normal compared with gestational diabetes mellitus. Am J Clin Nutr. 2000;71(5 Suppl):1256S–1261S. [DOI] [PubMed] [Google Scholar]
- 8. Cowett RM, Farrag HM. Selected principles of perinatal-neonatal glucose metabolism. Semin Neonatol. 2004;9(1):37–47. [DOI] [PubMed] [Google Scholar]
- 9. Kumar P, Magon N. Hormones in pregnancy. Niger Med J. 2012;53(4):179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bell AW, Bauman DE. Adaptations of glucose metabolism during pregnancy and lactation. J Mammary Gland Biol Neoplasia. 1997;2(3):265–278. [DOI] [PubMed] [Google Scholar]
- 11. Kumari P, Sharma SN, Kumar S, Kumar M. A comparative study of blood pressure in normal and pregnancy induced hypertensive cases for early diagnosis of hypertensive disorders in a tertiary care hospital. Int J Sci Study. 2014;2(3):33–37. [Google Scholar]
- 12. Riskin-Mashiah S, Damti A, Younes G, Auslander R. Normal fasting plasma glucose levels during pregnancy: a hospital-based study. J Perinat Med. 2011;39(2):209–211. [DOI] [PubMed] [Google Scholar]
- 13. Baumann MU, Deborde S, Illsley NP. Placental glucose transfer and fetal growth. Endocrine. 2002;19(1):13–22. [DOI] [PubMed] [Google Scholar]
- 14. Knopp RH, Montes A, Childs M, Li JR, Mabuchi H. Metabolic adjustments in normal and diabetic pregnancy. Clin Obstet Gynecol. 1981;24(1):21–49. [DOI] [PubMed] [Google Scholar]
- 15. Silverstone FA, Solomons E, Rubricius J. The rapid intravenous glucose tolerance test in pregnancy. J Clin Invest. 1961;40:2180–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lind T, Billewicz WZ, Brown G. A serial study of changes occurring in the oral glucose tolerance test during pregnancy. J Obstet Gynaecol Br Commonw. 1973;80(12):1033–1039. [DOI] [PubMed] [Google Scholar]
- 17. Victor A. Normal blood sugar variation during pregnancy. Acta Obstet Gynecol Scand. 1974;53(1):37–40. [DOI] [PubMed] [Google Scholar]
- 18. Hornnes PJ, Kühl C. Cortisol and glucose tolerance in normal pregnancy. Diabetes Metab. 1984;10(1):1–6. [PubMed] [Google Scholar]
- 19. Campbell DM, Sutherland HW, Pearson DW. Maternal glucose response to a standardized test meal throughout pregnancy and postnatally. Am J Obstet Gynecol. 1994;171(1):143–146. [DOI] [PubMed] [Google Scholar]
- 20. Sorenson RL, Brelje TC. Prolactin receptors are critical to the adaptation of islets to pregnancy. Endocrinology. 2009;150(4):1566–1569. [DOI] [PubMed] [Google Scholar]
- 21. Goyvaerts L, Lemaire K, Arijs I, et al. Prolactin receptors and placental lactogen drive male mouse pancreatic islets to pregnancy-related mRNA changes. Plos One. 2015;10(3):e0121868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lorenzo-Almorós A, Hang T, Peiró C, et al. Predictive and diagnostic biomarkers for gestational diabetes and its associated metabolic and cardiovascular diseases. Cardiovasc Diabetol. 2019;18(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rieck S, Kaestner KH. Expansion of beta-cell mass in response to pregnancy. Trends Endocrinol Metab. 2010;21(3):151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Handwerger S, Freemark M. The roles of placental growth hormone and placental lactogen in the regulation of human fetal growth and development. J Pediatr Endocrinol Metab. 2000;13(4):343–356. [DOI] [PubMed] [Google Scholar]
- 25. Walejko JM, Antolic A, Koelmel JP, Garrett TJ, Edison AS, Keller-Wood M. Chronic maternal cortisol excess during late gestation leads to metabolic alterations in the newborn heart. Am J Physiol Endocrinol Metab. 2019;316(3):E546–E556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sonagra AD, Biradar SM, K D, Murthy D S J. Normal pregnancy – a state of insulin resistance. J Clin Diagn Res. 2014;8(11):CC01–CC03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kampmann U, Knorr S, Fuglsang J, Ovesen P. Determinants of maternal insulin resistance during pregnancy: an updated overview. J Diabetes Res. 2019;2019:5320156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Barbour LA, McCurdy CE, Hernandez TL, Kirwan JP, Catalano PM, Friedman JE. Cellular mechanisms for insulin resistance in normal pregnancy and gestational diabetes. Diabetes Care. 2007;30(Suppl 2):S112–S119. [DOI] [PubMed] [Google Scholar]
- 29. Kirwan JP, Hauguel-De Mouzon S, Lepercq J, et al. TNF-alpha is a predictor of insulin resistance in human pregnancy. Diabetes. 2002;51(7):2207–2213. [DOI] [PubMed] [Google Scholar]
- 30. Del Aguila LF, Krishnan RK, Ulbrecht JS, et al. Muscle damage impairs insulin stimulation of IRS-1, PI 3-kinase, and Akt-kinase in human skeletal muscle. Am J Physiol Endocrinol Metab. 2000;279(1):E206–E212. [DOI] [PubMed] [Google Scholar]
- 31. Kirwan JP, Krishnan RK, Weaver JA, Del Aguila LF, Evans WJ. Human aging is associated with altered TNF-α production during hyperglycemia and hyperinsulinemia. Am J Physiol Endocrinol Metab. 2001;281(6):E1137–E1143. [DOI] [PubMed] [Google Scholar]
- 32. Rigato O, Ujvari S, Castelo A, Salomão R. Tumor necrosis factor alpha (TNF-alpha) and sepsis: evidence for a role in host defense. Infection. 1996;24(4):314–318. [DOI] [PubMed] [Google Scholar]
- 33. Laham N, Brennecke SP, Bendtzen K, Rice GE. Tumour necrosis factor alpha during human pregnancy and labour: maternal plasma and amniotic fluid concentrations and release from intrauterine tissues. Eur J Endocrinol. 1994;131(6):607–614. [DOI] [PubMed] [Google Scholar]
- 34. Beckmann I, Visser W, Struijk PC, van Dooren M, Glavimans J, Wallenburg HC. Circulating bioactive tumor necrosis factor-alpha, tumor necrosis factor-alpha receptors, fibronectin, and tumor necrosis factor-alpha inducible cell adhesion molecule VCAM-1 in uncomplicated pregnancy. Am J Obstet Gynecol. 1997;177(5):1247–1252. [DOI] [PubMed] [Google Scholar]
- 35.In: Rasmussen KM, Yaktine AL, eds. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: National Academy of Sciences; 2009. [PubMed] [Google Scholar]
- 36. Freemark M. Placental hormones and the control of fetal growth. J Clin Endocrinol Metab. 2010;95(5):2054–2057. [DOI] [PubMed] [Google Scholar]
- 37. León-Aguilar LF, Croyal M, Ferchaud-Roucher V, et al. Maternal obesity leads to long-term altered levels of plasma ceramides in the offspring as revealed by a longitudinal lipidomic study in children. Int J Obes (Lond). 2019;43(6):1231–1243. [DOI] [PubMed] [Google Scholar]
- 38. Hayder H, O’Brien J, Nadeem U, Peng C. MicroRNAs: crucial regulators of placental development. Reproduction. 2018;155(6):R259–R271. [DOI] [PubMed] [Google Scholar]
- 39. Armistead B, Kadam L, Drewlo S, Kohan-Ghadr HR. The role of NFκB in healthy and preeclamptic placenta: trophoblasts in the spotlight. Int J Mol Sci. 2020;21(5):1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burton GJ, Fowden AL. The placenta: a multifaceted, transient organ. Philos Trans R Soc Lond B Biol Sci. 2015;370(1663):20140066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Velegrakis A, Sfakiotaki M, Sifakis S. Human placental growth hormone in normal and abnormal fetal growth. Biomed Rep. 2017;7(2):115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Napso T, Yong HEJ, Lopez-Tello J, Sferruzzi-Perri AN. The role of placental hormones in mediating maternal adaptations to support pregnancy and lactation. Front Physiol. 2018;9:1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scippo ML, Frankenne F, Hooghe-Peters EL, Igout A, Velkeniers B, Hennen G. Syncytiotrophoblastic localization of the human growth hormone variant mRNA in the placenta. Mol Cell Endocrinol. 1993;92(2):R7–13. [DOI] [PubMed] [Google Scholar]
- 44. Lacroix MC, Guibourdenche J, Fournier T, et al. Stimulation of human trophoblast invasion by placental growth hormone. Endocrinology. 2005;146(5):2434–2444. [DOI] [PubMed] [Google Scholar]
- 45. Gupta SK, Malhotra SS, Malik A, Verma S, Chaudhary P. Cell signaling pathways involved during invasion and syncytialization of trophoblast cells. Am J Reprod Immunol. 2016;75(3):361–371. [DOI] [PubMed] [Google Scholar]
- 46. Barbour LA, Shao J, Qiao L, et al. Human placental growth hormone causes severe insulin resistance in transgenic mice. Am J Obstet Gynecol. 2002;186(3):512–517. [DOI] [PubMed] [Google Scholar]
- 47. Fuglsang J, Lauszus F, Flyvbjerg A, Ovesen P. Human placental growth hormone, insulin-like growth factor I and -II, and insulin requirements during pregnancy in type 1 diabetes. J Clin Endocrinol Metab. 2003;88(10):4355–4361. [PubMed] [Google Scholar]
- 48. Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res. 2006;65(Suppl 3):41–49. [DOI] [PubMed] [Google Scholar]
- 49. Newbern D, Freemark M. Placental hormones and the control of maternal metabolism and fetal growth. Curr Opin Endocrinol Diabetes Obes. 2011;18(6):409–416. [DOI] [PubMed] [Google Scholar]
- 50. Lombardo MF, De Angelis F, Bova L, et al. Human placental lactogen (hPL-A) activates signaling pathways linked to cell survival and improves insulin secretion in human pancreatic islets. Islets. 2011;3(5):250–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–660. [DOI] [PubMed] [Google Scholar]
- 52. Harrison JL, Adam CL, Brown YA, et al. An immunohistochemical study of the localization and developmental expression of ghrelin and its functional receptor in the ovine placenta. Reprod Biol Endocrinol. 2007;5:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Patterson M, Bloom SR, Gardiner JV. Ghrelin and appetite control in humans–potential application in the treatment of obesity. Peptides. 2011;32(11):2290–2294. [DOI] [PubMed] [Google Scholar]
- 54. Palik E, Baranyi E, Melczer Z, et al. Elevated serum acylated (biologically active) ghrelin and resistin levels associate with pregnancy-induced weight gain and insulin resistance. Diabetes Res Clin Pract. 2007;76(3):351–357. [DOI] [PubMed] [Google Scholar]
- 55. Gualillo O, Caminos J, Blanco M, et al. Ghrelin, a novel placental-derived hormone. Endocrinology. 2001;142(2):788–794. [DOI] [PubMed] [Google Scholar]
- 56. Nakahara K, Nakagawa M, Baba Y, et al. Maternal ghrelin plays an important role in rat fetal development during pregnancy. Endocrinology. 2006;147(3):1333–1342. [DOI] [PubMed] [Google Scholar]
- 57. Telejko B, Kuzmicki M, Zonenberg A, et al. Ghrelin in gestational diabetes: serum level and mRNA expression in fat and placental tissue. Exp Clin Endocrinol Diabetes. 2010;118(2):87–92. [DOI] [PubMed] [Google Scholar]
- 58. Sun Y, Ahmed S, Smith RG. Deletion of ghrelin impairs neither growth nor appetite. Mol Cell Biol. 2003;23(22):7973–7981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Chanoine JP. Ghrelin in growth and development. Horm Res. 2005;63(3):129–138. [DOI] [PubMed] [Google Scholar]
- 60. Perry RJ. Leptin revisited: the role of leptin in starvation. Mol Cell Oncol. 2018;5(5):e1435185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Bellefontaine N, Chachlaki K, Parkash J, et al. Leptin-dependent neuronal NO signaling in the preoptic hypothalamus facilitates reproduction. J Clin Invest. 2014;124(6):2550–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Azuma T, Suto H, Ito Y, et al. Gastric leptin and Helicobacter pylori infection. Gut. 2001;49(3):324–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulates leptin gene expression in muscle and fat. Nature. 1998;393(6686):684–688. [DOI] [PubMed] [Google Scholar]
- 64. Sylvia KE, Lorenz TK, Heiman JR, Demas GE. Physiological predictors of leptin vary during menses and ovulation in healthy women. Reprod Biol. 2018;18(1):132–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Herrid M, Palanisamy SK, Ciller UA, et al. An updated view of leptin on implantation and pregnancy: a review. Physiol Res. 2014;63(5):543–557. [DOI] [PubMed] [Google Scholar]
- 66. Su RW, Fazleabas AT. Implantation and establishment of pregnancy in human and nonhuman primates. Adv Anat Embryol Cell Biol. 2015;216:189–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Gambino YP, Maymó JL, Pérez-Pérez A, et al. 17Beta-estradiol enhances leptin expression in human placental cells through genomic and nongenomic actions. Biol Reprod. 2010;83(1):42–51. [DOI] [PubMed] [Google Scholar]
- 68. Gambino YP, Pérez Pérez A, Dueñas JL, Calvo JC, Sánchez-Margalet V, Varone CL. Regulation of leptin expression by 17beta-estradiol in human placental cells involves membrane associated estrogen receptor alpha. Biochim Biophys Acta. 2012;1823(4):900–910. [DOI] [PubMed] [Google Scholar]
- 69. Myers MG, Cowley MA, Münzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. [DOI] [PubMed] [Google Scholar]
- 70. Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol. 2010;22(7):805–817. [DOI] [PubMed] [Google Scholar]
- 71. Ladyman SR, Grattan DR. Region-specific reduction in leptin-induced phosphorylation of signal transducer and activator of transcription-3 (STAT3) in the rat hypothalamus is associated with leptin resistance during pregnancy. Endocrinology. 2004;145(8):3704–3711. [DOI] [PubMed] [Google Scholar]
- 72. Bates SH, Stearns WH, Dundon TA, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421(6925):856–859. [DOI] [PubMed] [Google Scholar]
- 73. Ladyman SR, Grattan DR. Suppression of leptin receptor messenger ribonucleic acid and leptin responsiveness in the ventromedial nucleus of the hypothalamus during pregnancy in the rat. Endocrinology. 2005;146(9):3868–3874. [DOI] [PubMed] [Google Scholar]
- 74. Hauguel-de Mouzon S, Lepercq J, Catalano P. The known and unknown of leptin in pregnancy. Am J Obstet Gynecol. 2006;194(6):1537–1545. [DOI] [PubMed] [Google Scholar]
- 75. Masuzaki H, Ogawa Y, Sagawa N, et al. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3(9):1029–1033. [DOI] [PubMed] [Google Scholar]
- 76. Challier J, Galtier M, Bintein T, Cortez A, Lepercq J, Hauguel-de Mouzon S. Placental leptin receptor isoforms in normal and pathological pregnancies. Placenta. 2003;24(1):92–99. [DOI] [PubMed] [Google Scholar]
- 77. Jansson T, Wennergren M, Illsley NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab. 1993;77(6):1554–1562. [DOI] [PubMed] [Google Scholar]
- 78. Zhang Y, Olbort M, Schwarzer K, et al. The leptin receptor mediates apparent autocrine regulation of leptin gene expression. Biochem Biophys Res Commun. 1997;240(2):492–495. [DOI] [PubMed] [Google Scholar]
- 79. Boström P, Wu J, Jedrychowski MP, et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481(7382):463–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Swick AG, Orena S, O’Connor A. Irisin levels correlate with energy expenditure in a subgroup of humans with energy expenditure greater than predicted by fat free mass. Metabolism. 2013;62(8):1070–1073. [DOI] [PubMed] [Google Scholar]
- 81. Gizaw M, Anandakumar P, Debela T. A review on the role of irisin in insulin resistance and type 2 diabetes mellitus. J Pharmacopuncture. 2017;20(4):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 82. Arhire LI, Mihalache L, Covasa M. Irisin: a hope in understanding and managing obesity and metabolic syndrome. Front Endocrinol (Lausanne). 2019;10:524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Garcés MF, Peralta JJ, Ruiz-Linares CE, et al. Irisin levels during pregnancy and changes associated with the development of preeclampsia. J Clin Endocrinol Metab. 2014;99(6):2113–2119. [DOI] [PubMed] [Google Scholar]
- 84. Pavlova T, Zlamal F, Tomandl J, Hodicka Z, Gulati S, Bienertova-Vasku J. Irisin maternal plasma and cord blood levels in mothers with spontaneous preterm and term delivery. Dis Markers. 2018;2018:7628957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Ebert T, Stepan H, Schrey S, et al. Serum levels of irisin in gestational diabetes mellitus during pregnancy and after delivery. Cytokine. 2014;65(2):153–158. [DOI] [PubMed] [Google Scholar]
- 86. Briana DD, Boutsikou M, Athanasopoulos N, Marmarinos A, Gourgiotis D, Malamitsi-Puchner A. Implication of the myokine irisin in maternal energy homeostasis in pregnancies with abnormal fetal growth. J Matern Fetal Neonatal Med. 2016;29(21):3429–3433. [DOI] [PubMed] [Google Scholar]
- 87. Chen JQ, Li Q, Ma JR. Maternal serum, placental, and umbilical venous blood irisin levels in intrahepatic cholestasis of pregnancy. J Matern-Fetal Neo M. 2019:1–8. [DOI] [PubMed] [Google Scholar]
- 88. Drewlo S, Johnson E, Kilburn BA, Kadam L, Armistead B, Kohan-Ghadr HR. Irisin induces trophoblast differentiation via AMPK activation in the human placenta. J Cell Physiol. 2020. [DOI] [PubMed] [Google Scholar]
- 89. Bozkurt L, Göbl CS, Baumgartner-Parzer S, Luger A, Pacini G, Kautzky-Willer A. Adiponectin and leptin at early pregnancy: association to actual glucose disposal and risk for GDM-A prospective cohort study. Int J Endocrinol. 2018;2018:5463762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Retnakaran R. Adiponectin and β-cell adaptation in pregnancy. Diabetes. 2017;66(5):1121–1122. [DOI] [PubMed] [Google Scholar]
- 91. Chen J, Tan B, Karteris E, et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia. 2006;49(6):1292–1302. [DOI] [PubMed] [Google Scholar]
- 92. Caminos JE, Nogueiras R, Gallego R, et al. Expression and regulation of adiponectin and receptor in human and rat placenta. J Clin Endocrinol Metab. 2005;90(7):4276–4286. [DOI] [PubMed] [Google Scholar]
- 93. Lappas M, Yee K, Permezel M, Rice GE. Release and regulation of leptin, resistin and adiponectin from human placenta, fetal membranes, and maternal adipose tissue and skeletal muscle from normal and gestational diabetes mellitus-complicated pregnancies. J Endocrinol. 2005;186(3):457–465. [DOI] [PubMed] [Google Scholar]
- 94. McDonald EA, Wolfe MW. Adiponectin attenuation of endocrine function within human term trophoblast cells. Endocrinology. 2009;150(9):4358–4365. [DOI] [PubMed] [Google Scholar]
- 95. Ichida K, Moriyama T, Morita H, et al. Plasma adiponectin concentrations and placental adiponectin expression in pre-eclamptic women. Gynecol Endocrinol. 2007;23(4):238–243. [DOI] [PubMed] [Google Scholar]
- 96. Corbetta S, Bulfamante G, Cortelazzi D, et al. Adiponectin expression in human fetal tissues during mid- and late gestation. J Clin Endocrinol Metab. 2005;90(4):2397–2402. [DOI] [PubMed] [Google Scholar]
- 97. Turer AT, Scherer PE. Adiponectin: mechanistic insights and clinical implications. Diabetologia. 2012;55(9):2319–2326. [DOI] [PubMed] [Google Scholar]
- 98. Mazaki-Tovi S, Kanety H, Sivan E. Adiponectin and human pregnancy. Curr Diab Rep. 2005;5(4):278–281. [DOI] [PubMed] [Google Scholar]
- 99. American Diabetes A. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S14–S31. [DOI] [PubMed] [Google Scholar]
- 100. Metzger BE, Lowe LP, Dyer AR, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358(19):1991–2002. [DOI] [PubMed] [Google Scholar]
- 101. Chiefari E, Arcidiacono B, Foti D, Brunetti A. Gestational diabetes mellitus: an updated overview. J Endocrinol Invest. 2017;40(9):899–909. [DOI] [PubMed] [Google Scholar]
- 102. Dickens LT, Thomas CC. Updates in gestational diabetes prevalence, treatment, and health policy. Curr Diab Rep. 2019;19(6):33. [DOI] [PubMed] [Google Scholar]
- 103. Bao W, Dar S, Zhu Y, et al. Plasma concentrations of lipids during pregnancy and the risk of gestational diabetes mellitus: a longitudinal study. J Diabetes. 2018;10(6):487–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wadhwa PD, Buss C, Entringer S, Swanson JM. Developmental origins of health and disease: brief history of the approach and current focus on epigenetic mechanisms. Semin Reprod Med. 2009;27(5):358–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Powe CE, Allard C, Battista MC, et al. Heterogeneous contribution of insulin sensitivity and secretion defects to gestational diabetes mellitus. Diabetes Care. 2016;39(6):1052–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Pérez-Pérez A, Toro A, Vilariño-García T, et al. Leptin action in normal and pathological pregnancies. J Cell Mol Med. 2018;22(2):716–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Powe CE. Early pregnancy biochemical predictors of gestational diabetes mellitus. Curr Diab Rep. 2017;17(2):12. [DOI] [PubMed] [Google Scholar]
- 108. Iciek R, Wender-Ozegowska E, Zawiejska A, et al. Placental leptin and its receptor genes expression in pregnancies complicated by type 1 diabetes. J Physiol Pharmacol. 2013;64(5):579–585. [PubMed] [Google Scholar]
- 109. Kautzky-Willer A, Pacini G, Tura A, et al. Increased plasma leptin in gestational diabetes. Diabetologia. 2001;44(2):164–172. [DOI] [PubMed] [Google Scholar]
- 110. Qiu C, Williams MA, Vadachkoria S, Frederick IO, Luthy DA. Increased maternal plasma leptin in early pregnancy and risk of gestational diabetes mellitus. Obstet Gynecol. 2004;103(3):519–525. [DOI] [PubMed] [Google Scholar]
- 111. Bao W, Baecker A, Song Y, Kiely M, Liu S, Zhang C. Adipokine levels during the first or early second trimester of pregnancy and subsequent risk of gestational diabetes mellitus: a systematic review. Metabolism. 2015;64(6):756–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Festa A, Shnawa N, Krugluger W, Hopmeier P, Schernthaner G, Haffner SM. Relative hypoleptinaemia in women with mild gestational diabetes mellitus. Diabet Med. 1999;16(8):656–662. [DOI] [PubMed] [Google Scholar]
- 113. Mosavat M, Omar SZ, Tan PC, Razif MFM, Sthaneshwar P. Leptin and soluble leptin receptor in association with gestational diabetes: a prospective case-control study. Arch Gynecol Obstet. 2018;297(3):797–803. [DOI] [PubMed] [Google Scholar]
- 114. Liu L, Aa J, Wang G, et al. Differences in metabolite profile between blood plasma and serum. Anal Biochem. 2010;406(2):105–112. [DOI] [PubMed] [Google Scholar]
- 115. McIlhenny C, George WD, Doughty JC. A comparison of serum and plasma levels of vascular endothelial growth factor during the menstrual cycle in healthy female volunteers. Br J Cancer. 2002;86(11):1786–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Oddoze C, Lombard E, Portugal H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clin Biochem. 2012;45(6):464-–469. [DOI] [PubMed] [Google Scholar]
- 117. Du XL, Jiang WX, Lv ZT. Lower circulating irisin level in patients with diabetes mellitus: a systematic review and meta-analysis. Horm Metab Res. 2016;48(10):644–652. [DOI] [PubMed] [Google Scholar]
- 118. Yuksel MA, Oncul M, Tuten A, et al. Maternal serum and fetal cord blood irisin levels in gestational diabetes mellitus. Diabetes Res Clin Pract. 2014;104(1):171–175. [DOI] [PubMed] [Google Scholar]
- 119. Seven A, Yalinbas E, Kucur SK, et al. Comprehensive evaluation of irisin levels in fetomaternal circulation of pregnant women with obesity or gestational diabetes mellitus. Ir J Med Sci. 2019;188(4):1213–1219. [DOI] [PubMed] [Google Scholar]
- 120. Pardo M, Crujeiras AB, Amil M, et al. Association of irisin with fat mass, resting energy expenditure, and daily activity in conditions of extreme body mass index. Int J Endocrinol. 2014;2014:857270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Erol O, Erkal N, Ellidağ HY, et al. Irisin as an early marker for predicting gestational diabetes mellitus: a prospective study. J Matern Fetal Neonatal Med. 2016;29(22):3590–3595. [DOI] [PubMed] [Google Scholar]
- 122. Ural UM, Sahin SB, Tekin YB, Cüre MC, Sezgin H. Alteration of maternal serum irisin levels in gestational diabetes mellitus. Ginekol Pol. 2016;87(5):395–398. [DOI] [PubMed] [Google Scholar]
- 123. Son JS, Liu X, Tian Q, et al. Exercise prevents the adverse effects of maternal obesity on placental vascularization and fetal growth. J Physiol. 2019;597(13):3333–3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Daskalopoulou SS, Cooke AB, Gomez YH, et al. Plasma irisin levels progressively increase in response to increasing exercise workloads in young, healthy, active subjects. Eur J Endocrinol. 2014;171(3):343–352. [DOI] [PubMed] [Google Scholar]
- 125. Wang P, Ma HH, Hou XZ, Song LL, Song XL, Zhang JF. Reduced plasma level of irisin in first trimester as a risk factor for the development of gestational diabetes mellitus. Diabetes Res Clin Pract. 2018;142:130–138. [DOI] [PubMed] [Google Scholar]
- 126. Jedrychowski MP, Wrann CD, Paulo JA, et al. Detection and quantitation of circulating human irisin by tandem mass spectrometry. Cell Metab. 2015;22(4):734–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Worda C, Leipold H, Gruber C, Kautzky-Willer A, Knöfler M, Bancher-Todesca D. Decreased plasma adiponectin concentrations in women with gestational diabetes mellitus. Am J Obstet Gynecol. 2004;191(6):2120–2124. [DOI] [PubMed] [Google Scholar]
- 128. Ranheim T, Haugen F, Staff AC, Braekke K, Harsem NK, Drevon CA. Adiponectin is reduced in gestational diabetes mellitus in normal weight women. Acta Obstet Gynecol Scand. 2004;83(4):341–347. [DOI] [PubMed] [Google Scholar]
- 129. Retnakaran R, Hanley AJ, Raif N, Connelly PW, Sermer M, Zinman B. Reduced adiponectin concentration in women with gestational diabetes: a potential factor in progression to type 2 diabetes. Diabetes Care. 2004;27(3):799–800. [DOI] [PubMed] [Google Scholar]
- 130. Qiao L, Wattez JS, Lee S, et al. Adiponectin deficiency impairs maternal metabolic adaptation to pregnancy in mice. Diabetes. 2017;66(5):1126–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Dall TM, Yang W, Gillespie K, et al. The economic burden of elevated blood glucose levels in 2017: diagnosed and undiagnosed diabetes, gestational diabetes, and prediabetes. Diabetes care. 2019;42(9):1661–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sesmilo G, Prats P, Garcia S, et al. First-trimester fasting glycemia as a predictor of gestational diabetes (GDM) and adverse pregnancy outcomes. Acta Diabetol. 2020;57(6):697–703. [DOI] [PubMed] [Google Scholar]
- 133. Seabra G, Saunders C, de Carvalho Padilha P, Zajdenverg L, da Silva LB, de Souza Santos MM. Association between maternal glucose levels during pregnancy and gestational diabetes mellitus: an analytical cross-sectional study. Diabetol Metab Syndr. 2015;7:17. [DOI] [PMC free article] [PubMed] [Google Scholar]