Abstract
Pneumonia is one of the most prevalent infections in the intensive care unit (ICU), where pneumonia may occur during hospitalization in the ICU as a complication. ICU patients with central nervous system (CNS) injuries are not an exception, and they may even be more susceptible to infections such as pneumonia due to issues such as swallowing difficulties, the requirement for mechanical ventilation, and extended hospital stay. Numerous common CNS injuries, such as ischemic stroke, traumatic brain injury, subarachnoid hemorrhage, and intracerebral hemorrhage, can prolong hospital stay and increase the risk of pneumonia. Multidrug-resistant (MDR) microorganisms are a common and significant concern, with increased mortality in nosocomial pneumonia. However, research on pneumonia due to MDR pathogens in patients with CNS injuries is limited. The aim of the present review was to provide the current evidence regarding pneumonia due to MDR pathogens in patients with CNS injuries. The prevalence of pneumonia due to MDR pathogens in CNS injuries differs among different settings, types of CNS injuries, geographical areas, and time periods in which the studies were performed. Specific risk factors for the emergence of pneumonia due to MDR pathogens have been identified in ICUs and neurological rehabilitation units. Antimicrobial resistance is currently a global issue, although using preventive measures, early diagnosis, and close monitoring of MDR strains may lessen its impact. Since there is a lack of information on these topics, more multicenter prospective studies are required to offer insights into the clinical features and outcomes of these patients.
Keywords: pneumonia, multidrug-resistance, intracerebral hemorrhage, traumatic brain injury, subarachnoid hemorrhage, neurointensive care unit, neurorehabilitation
1. Introduction
Nosocomial or hospital-acquired pneumonia (HAP) is an infection that appears during a hospital stay, usually 48 h or more after being admitted or within 14 days of discharge and was either absent or not incubating at the time of admission (1). HAP is the second most frequent nosocomial infection following urinary tract infections, with an incidence of 15-20%, according to a study from the United States (2). It is one of the primary causes of mortality in intensive care unit (ICU) patients (accounting for 25-50% of deaths) (1) and one of the causes of fatal hospital infections (mortality rate, 13%). Mechanical ventilation for >48 h (HAP incidence, 9-40%), length of hospital stay (HAP incidence, 3.3% until day 5; 1.3% at day 15), severity of underlying disease, Acute Physiology and Chronic Health Evaluation (APACHE) score (3), and the presence of comorbidities are the most important risk factors (4).
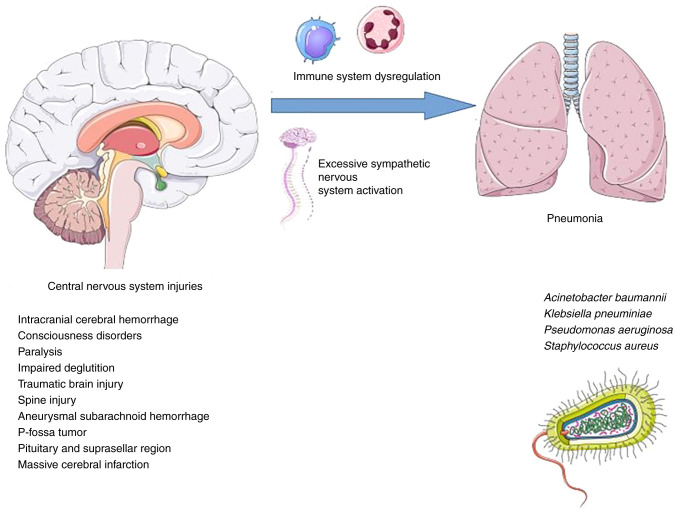
Hospitalized patients with central nervous system (CNS) injuries are particularly vulnerable to pneumonia, which can be exacerbated by bed rest, dysphagia, mental instability, or mechanical ventilation brought on by weak respiratory muscles (5). Pneumonia is one of the most common respiratory complications in stroke patients, affecting 5 to 9% of patients (6,7), and is much commoner in patients admitted to neuro-ICUs, which are ICUs devoted to the care of patients with immediately life-threatening neurological problems (incidence, 13-33%) (8). Due to the long time spent in the prone position and the risk of inhaling stomach contents that comes with it, a large number of people (up to 60%) with serious brain injuries develop pneumonia (9).
Immune system dysregulation due to persistent inflammatory response and excessive sympathetic activation are involved in the pathogenesis of pneumonia in individuals with CNS injuries. More specifically, in CNS injuries, secondary brain tissue damage is caused by the acute immune response, which is followed by immunosuppression caused by sympathetic nervous system activation. The latter raises the risk of infectious complications such as pneumonia. The inflammatory state caused by pneumonia can trigger a bystander autoimmune response against CNS antigens, resulting in a vicious cycle (10).
Nosocomial pneumonia affects ~36% of patients hospitalized for >48 h in neuro-ICUs (11). Other common infections in neuro-ICUs are urinary tract infections, bacteremia, and intracranial infections such as ventriculitis and meningitis (11). These infections can affect patient outcomes and increase mortality rates in critically ill patients. In addition, these infections increase the costs placed on healthcare systems (12,13).
Among the most frequent types of infections in patients admitted to neuro-ICUs is ventilator-associated pneumonia (VAP), which appears in mechanically ventilated individuals at least 48 h after endotracheal intubation without any signs of a prior infection. It usually results from aspiration of oropharyngeal secretions into the tracheobronchial tree around the endotracheal cuff (14). Subarachnoid hemorrhage (SAH), traumatic brain injury (TBI), and stroke patients all require intensive care and may be admitted to neuro-ICUs, where they may be vulnerable to nosocomial infections such as pneumonia. Patients with subdural hematomas and intracerebral/intraventricular hemorrhages (IVH) have the greatest incidence rates of nosocomial infections, with rates of 21.3 and 21.1 cases per 1,000 days of hospitalization at the neuro-ICU, respectively (15).
Multidrug-resistant (MDR) microorganisms are defined as those that are resistant to at least one agent from three or more antimicrobial classes, including β-lactam/β-lactamase inhibitors; carbapenems; aminoglycosides; third- or fourth-generation cephalosporins, fluoroquinolones, and carbapenems for Gram-negative pathogens; non-susceptibility to oxacillin and/or cefoxitin (anti-staphylococcal β-lactams) for Gram-positive Staphylococcus aureus (S. aureus); and non-susceptibility to vancomycin and/or teicoplanin for Gram-positive Enterococcus spp. (16,17).
MDR microorganisms are a prevalent and serious concern with increased mortality in HAP and VAP (18). There is a scarcity of information on pneumonia due to MDR pathogens in patients with CNS injuries. The aim of the present review was to report the current evidence regarding pneumonia due to MDR pathogens in patients with CNS injuries.
2. Data extraction and synthesis
In order to provide insight regarding MDR pneumonia in patients with CNS injuries, an electronic search in PubMed and Google Scholar was performed with the keywords ‘multi-drug resistant pneumonia’ OR ‘MDR pneumonia’ OR ‘multi-drug resistant respiratory infections’ OR ‘MDR respiratory infections’ AND ‘central nervous injuries’ OR ‘brain injuries’ OR ‘stroke’ OR ‘intracranial hemorrhage’ OR ‘subarachnoid hemorrhage’ OR ‘neurorehabilitation unit’ OR ‘neurointensive care unit’ OR ‘neurological disorders’ OR ‘neurological injuries’, without language limitations in the selection of articles reporting data on MDR pneumonia in CNS injuries. Two authors thoroughly reviewed all articles. The reference list of each article that met the criteria was also hand-searched for other potentially relevant studies. Overall, 192 articles were found using the search criteria and the reference lists of previously identified documents. After eliminating duplicates, 119 were eliminated after title, abstract, or full text screening. Finally, nine articles presenting original studies providing data on MDR pneumonia in CNS injuries were included in data synthesis.
3. Mechanisms responsible for pneumonia development in CNS injuries
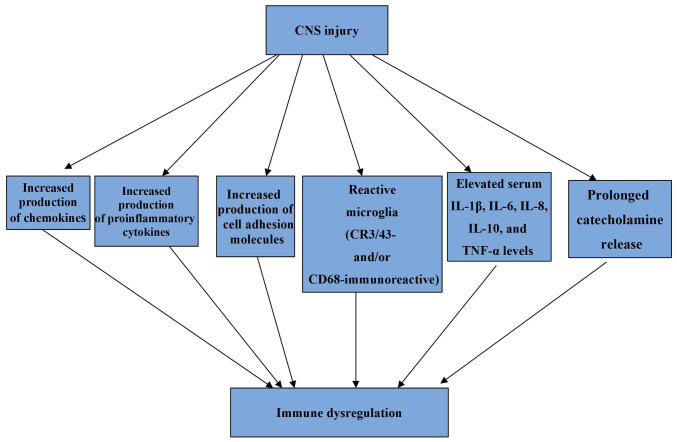
Critical illnesses of the CNS are more likely to result in pneumonia than in other illnesses in ICUs due to factors such as immunological dysregulation and immunosuppression resulting from brain injury, increased incidence of dysphagia, and the insertion of external ventricular drains (EVDs) (19). In patients with brain damage, immunological dysregulation is predominantly caused by a heightened inflammatory response that results in the production of chemokines, proinflammatory cytokines, and cell adhesion molecules both centrally and peripherally (20). These cytokines are produced to eliminate cellular debris in the CNS after injury, and an inflammatory response develops. However, a persistent and protracted inflammatory response can result in immune system dysregulation (21-23). More specifically, it has been found that three months after TBI, affected individuals frequently display extensive, densely packed, reactive microglia (CR3/43- and/or CD68-immunoreactive) and in the context of this inflammatory pathology, evidence of ongoing white matter degradation has also been observed (21). There is also evidence that increased microglial activation can be present up to 17 years after TBI (23). Moreover, TBI could be viewed as a condition with a persistent inflammatory state as elevated serum interleukin (IL)-1β, IL-6, IL-8, IL-10, and TNF-α levels over the first year post-injury have been detected (22).
The terms brain injury-induced immunodepression syndrome (BIIDS) and stroke-induced immunodepression syndrome (SIDS) refer to dysregulation occurring as a result of trauma, brain surgery, spinal cord injury (SCI), or SAH (23-25). SIDS is thought to have two phases. The early transitory activation of the first phase begins as soon as 12 h after the first injury and lasts for up to 24 h. The second phase is characterized by a systemic immunodepression that can last for many weeks (26-28).
Immunosuppression also develops from prolonged catecholamine release. Catecholamines are released after a brain injury because the hypothalamic-pituitary axis and sympathetic nervous system are engaged. Inflammatory response, as previously stated (29-31), can also be brought on by this. Additionally, the reaction is also mediated by 2-adrenergic receptors (32). In patients with brain injuries, infections are strongly correlated with increased sympathetic system activity, elevated catecholamine levels, and immunosuppression (33-35).
According to previous research (36), putamen and right frontal injuries render patients more vulnerable to infections. Due to their link to excessive sympathetic activation, which directly promotes cardiac and vascular alterations and leads to increased vigilance, heart rate, and blood flow to the skeletal muscles, insular brain strokes are associated with the highest risk of pneumonia (37).
The mechanisms responsible for pneumonia development in CNS injuries are illustrated in Fig. 1 and the mechanisms involved in immune system dysregulation caused by CNS injuries are illustrated in Fig. 2.
Figure 1.
Mechanisms responsible for pneumonia development in central nervous system injuries. Parts of the figure were drawn by using images from Servier Medical Art. Servier Medical Art by Servier is licensed under a Creative Commons Attribution 3.0 Unported License (https://creativecommons.org/licenses/by/3.0/).
Figure 2.
Mechanisms involved in immune system dysregulation caused by CNS injuries. CNS, central nervous system; IL, interleukin; TNF, tumor necrosis factor.
4. Prevalence of pneumonia due to MDR pathogens in CNS injuries
All the studies providing data regarding pneumonia due to MDR pathogens in CNS injuries are summarized in Table I (38-45). Regarding patients with TBI and pneumonia, the prevalence of MDR pneumonia ranges between 5.4 and 29.6%, according to different studies (39,40). In a study by Yang et al of the 324 patients with intracranial cerebral hemorrhage, 122 developed pneumonia, of whom 42 (34.2%) had MDR pathogen isolation (41). The reported incidence of MDR pneumonia among patients with various CNS injuries and pneumonia ranges between 8.5 and 42.2% (42,43). In a previous study including 89 patients with subarachnoid hemorrhage, intracerebral hemorrhage, and massive cerebral infarction, Teng et al found that among 40 patients who developed pneumonia, 15 (37.5%) had MDR pathogens (44).
Table I.
Studies describing patients with CNS injuries and pneumonia due to MDR pathogens.
| Study | CNS injury | Participants | Pneumonia | Pneumonia due to MDR pathogens | MDR pathogens |
|---|---|---|---|---|---|
| Yang et al, 2022(41) | Intracranial cerebral hemorrhage | 324 | 122 | 42/122 (34.2%) | A. baumannii (40.5%) |
| K. pneumoniae (26.2%) | |||||
| P. aeruginosa (23.8%) | |||||
| Jiang et al, 2022(45) | • Consciousness disorders | 575 | 427 | 79/427 (18.5%) | A. baumannii (45.3%) |
| • Paralysis | P. aeruginosa (36.8%) | ||||
| • Impaired deglutition | K. pneumoniae (12.6%) | ||||
| E. cloacae (1.1%) | |||||
| S. aureus (4.2%) | |||||
| Lee et al, 2019(42) | Various CNS illnesses | 277 | 351 | 148/351 (42.2%) | A. baumannii (23.6%) |
| S. pneumoniae (5.4%) | |||||
| P. aeruginosa (4%) | |||||
| K. pneumoniae (14.9%) | |||||
| K. aerogenes (1.4%) | |||||
| S. aureus (45.3%) | |||||
| Shrestha et al, 2019(43) | • Traumatic brain injury | 106 | 35 | 3/35 (8.5%) | K. pneumoniae (66.7%) |
| • Spine injury | Acinetobacter spp. (33.3%) | ||||
| • Aneurysmal SAH | |||||
| • Miscellaneous | |||||
| • P-fossa tumor | |||||
| • Pituitary and suprasellar region | |||||
| Beghi et al, 2018(4) | Traumatic brain injury | 61 | 8 | 6/8 (75%) | P. aeruginosa (33.3%) |
| K. pneumoniae (16.6%) | |||||
| P. mirabilis (16.6%) | |||||
| S. maltophilia (16.6%) | |||||
| Methicillin-resistant coagulase-negative | |||||
| Staphylococci (16.6%) | |||||
| Ye et al, 2022(40) | Traumatic brain injury | 230 | 230 | 68/230 (29.6%) | A. baumannii (45.2%) |
| K. pneumoniae (23.3%) | |||||
| P. aeruginosa (16.4%) | |||||
| S. aureus (15.1%) | |||||
| Leone et al, 2002(39) | Traumatic brain injury | 116 | 58 | 4/73 (5.4%) | S. aureus (100%) |
| Teng et al, 2022(44) | • SAH | 89 | 40 | 15/40 (37.5%) | S. aureus (20%) |
| • Intracerebral hemorrhage | B. cepacia (20%) | ||||
| • Massive cerebral infarction | K. pneumoniae (20%) | ||||
| C. striatum (20%) | |||||
| Acinetobacter spp (20%) | |||||
| Jovanovic et al, 2015(38) | Traumatic brain injury | 144 | 35 | 6/107 (5.6%) | S. aureus (100%) |
CNS, central nervous system; MDR, multidrug-resistance; A. baumannii, Acinetobacter baumannii; K. pneumoniae, Klebsiella pneumoniae; P. aeruginosa, Pseudomonas aeruginosa; E. cloacae, Enterobacter cloacae; S. aureus, Staphylococcus aureus; S. pneumoniae, Streptococcus pneumoniae; K. aerogenes, Klebsiella aerogenes; P. mirabilis, Proteus mirabilis; S. maltophilia, Stenotrophomonas maltophilia; B. cepacia, Burkholderia cepacia; C. striatum, Corynebacterium striatum; SAH, subarachnoid hemorrhage.
In a study by Beghi et al which included 61 individuals with TBI, 8 patients developed pneumonia in a rehabilitation unit, of whom 6 (75%) had an MDR pathogen isolation (4). In addition, Jiang et al in a recent study of 575 patients with consciousness disorders, paralysis, and impaired deglutition who were admitted to a rehabilitation unit, recorded 427 episodes of pneumonia, of which 79 (18.5%) were MDR pneumonia (45).
5. Risk factors for pneumonia due to MDR pathogens in CNS injuries
Given the worsening patient outcomes and the significant expenses affecting the healthcare system as a result of greater lengths of stay and prolonged duration of treatment, it is critical to be able to identify which patients are at higher risk of developing pneumonia due to MDR pathogens.
Patients with TBI acquire nosocomial infections at a rate of 41%, with pneumonia being the most frequent, with a prevalence rate of ≥30% (45). Surgical procedures, prolonged hospitalization, CNS injury, CSF leak, administration of barbiturates, nasal carriage of S. aureus, and the necessity for intubation and mechanical ventilation have all been found to play a significant role in pneumonia development in these patients (46). Furthermore, patients with the following features had a higher risk of developing pneumonia: Intubation on the scene or in the emergency department; younger age; lower Glasgow Coma Scale (GCS) score; males; prolonged mechanical ventilation; higher injury severity score (ISS), and additional brain injuries (47). Table II summarizes identified risk factors for pneumonia due to MDR pathogens in CNS injuries as well as their prevalence in cases of pneumonia due to MDR pathogens among various studies (40,42-45).
Table II.
Identified risk factors for MDR pneumonia in CNS injuries.
| Study | Type of CNS injury | Identified risk factors |
|---|---|---|
| Ye et al, 2022(40) | Traumatic brain injury | Age >60 years (67.4%) |
| Diabetes mellitus (45.7%) | ||
| Chronic obstructive pulmonary disease (34.8%) | ||
| Mechanical ventilation ≥7 days (69.6%) | ||
| Transfer from other hospitals (17.4%) | ||
| Lee et al, 2019(42) | Various CNS injuries | Risk factors for MDR i.e., antimicrobial agent use in the previous 90 days, hospitalization for 2 days in the previous 90 days, nursing home residency (61.6%) |
| Shrestha et al, 2019(43) | Various CNS injuries | Head injury (45.7%) |
| Spine injury (20%) | ||
| Teng et al, 2022(44) | Subarachnoid hemorrhage | Age >65 years (57.5%) |
| Intracerebral hemorrhage | Therapeutic hypothermia (32.5%) | |
| Massive cerebral infarction | ||
| Jiang et al, 2022(45) | Hospitalization in neurorehabilitation units | Recent antibiotic exposure (100%) |
| Low albumin level (52.1%) | ||
| Performance of tracheostomy (25%) |
MDR, multidrug-resistance; CNS, central nervous system.
6. Microbiological data
Common microorganisms involved in pneumonia due to MDR pathogens in CNS injuries are presented in Table I.
Rare causes of pneumonia in immunocompromised patients may include microorganisms which may rarely be encountered in immunocompetent patients (48). For example, cases of severe invasive infections such as pneumonia due to C. striatum may occur especially among immunocompromised patients who have a history of long hospital admissions, numerous courses of antibiotics, and/or those who have used invasive medical devices (49,50). According to some studies, Corynebacterium spp. should also be considered as potential pathogens, and suspicious isolates should be identified to the species level since C. striatum is frequently MDR (51,52).
Regarding Gram-positive MDR pathogens, of great interest is a study that examined the proportion of MDR of common bacteria isolated from hospitalized neurology patients with pneumonia in ICU and non-ICU settings, between the early and late years of the period 2007-2016. The prevalence of MDR among infections caused by S. aureus and S. pneumoniae did not differ significantly between the early and late study periods. S. pneumoniae exhibited sensitivity to penicillin, ceftriaxone and levofloxacin, especially during the late study period and resistance to tetracycline, erythromycin and trimethoprim-sulfamethoxazole. S. aureus exhibited sensitivity to vancomycin, quinupristin/dalfopristin, chloramphenicol, rifampicin and teicoplanin and resistance to clindamycin, ciprofloxacin, moxifloxacin, tetracycline, erythromycin and trimethoprim-sulfamethoxazole during the early and the late study period (42). In a study by Shrestha et al including patients with various CNS injuries with MDR VAP, all Gram-positive pathogens were sensitive to co-trimoxazole (43).
In a study by Lee et al (42), both the ICU and non-ICU settings have experienced an increase in the proportion of cases with Gram-negative bacteria that are resistant to various antibiotics and in both settings, the percentage of non-susceptibility to amikacin and colistin remained low between the early and late years of the period 2007-2016(42). In the study by Shrestha et al all Gram-negative bacterial strains were sensitive to colistin (43). Gram-negative organisms have recently been reported to be dominant in neurorehabilitation ward patients, with Acinetobacter baumannii (A. baumannii) being almost universally resistant to ciprofloxacin, imipenem, piperacillin, piperacillin/tazobactam and meropenem and Klebsiella pneumoniae (K. pneumoniae) being resistant to numerous antibiotics, except tigecycline, cefoperazone/sulbactam, sulfonamide, cefepime, and piperacillin/tazobactam (44).
7. Imaging data
Imaging data on patients with pneumonia due to MDR pathogens and CNS injuries is limited. A study investigating the pathogen distribution and imaging characteristics in patients with severe craniocerebral injuries with pneumonia due to MDR pathogens reported that the imaging features included consolidation, pleural effusion, and ground-glass opacities accounting for 63.24, 72.06 and 45.59%, respectively (40).
8. Prevention
In the neuro-ICU, proposed VAP prevention strategies include daily sedation interruption and a readiness-to-extubate evaluation; facilitation of early morbidity, elevating the head of the bed to 30-45˚; utilization of endotracheal tubes with subglottic secretion drainage ports and a closed/in-line endotracheal suctioning system; substituting the ventilator circuit if visibly soiled or malfunctioning; monitoring of residual gastric volume; early parenteral nutrition; and deep venous thrombosis prophylaxis (38). Other suggested preventive measures for VAP include antiseptic mouth wash, spontaneous breathing trial, and early extubation (15).
In addition, comprehensive rehabilitation approaches, including secretion management, training of respiratory muscles, airway clearance techniques, swallowing exercises, and pharyngeal electrical stimulation have been suggested for tracheotomized patients in neurorehabilitation units for the prevention of infections (47). Moreover, short-duration, high-dose antibiotic regimens appear to be effective in reducing the risk of antibiotic resistance (41).
An additional prevention approach is addressing brain injury-induced immunosuppression, which is mainly induced by sympathetic nervous system activation (53). With regard to microorganism infection in humans, the human host immunity response must be taken into account. Owing to the host immune defenses, most of the viral and bacterial infections are self-limiting to an immunocompetent host (54), and the microorganisms become commensal microorganisms if they can co-exist with human beings. In addition as indicated, MDR only becomes a significant issue when there is immunological dysregulation and immunosuppression in the host. Thus, eliminating immunological dysregulation and immunosuppression in patients with CNS injuries may provide a more feasible therapeutic solution than targeting MDR microorganisms and eliminating these microorganisms.
The pivotal role of immunity in acquiring essential nutrition from the human microbiota (55-57) should also be considered. The human microbiome is essential to the health and wellbeing of individuals, as they are the indispensable source of metabolites for the body (57). In the case of acute infection, with the help of a special pathway of the innate immune defense, programmed cell death, such as apoptosis, necroptosis, and pyroptosis (58,59), both the microorganisms and damaged host cells will be destroyed and become a source of nutrition for healing.
By using these preventive strategies, the reduction of all pulmonary infections will result in the reduction of pneumonia caused by MDR bacteria.
9. Conclusions
The incidence of pneumonia due to MDR pathogens in CNS injuries varies among different settings, underlying injuries, and countries in which the studies were performed. Certain risk factors for the development of MDR pneumonia in ICUs and in neurorehabilitation units have been identified. The most frequently isolated microorganisms in pneumonia due to MDR pathogens in CNS injuries are A. baumannii, K. pneumoniae, Pseudomonas aeruginosa, and S. aureus with various patterns of resistance. The application of preventive strategies and the early detection and close monitoring of MDR strains may reduce the burden of antimicrobial resistance, which is now a global issue. Further multicenter prospective studies are needed to provide data on the clinical characteristics and outcomes of these patients as the data concerning these issues are limited.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
AG and VEG conceptualized the study. VEG, AAF, IT, GF, KF, KP and NT reviewed the data for inclusion in the review, and wrote and prepared the draft of the manuscript. VEG and GF provided critical revisions. Data authentication is not applicable. All authors contributed to manuscript revision and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the infectious diseases society of America and the American Thoracic Society. Clin Infect Dis. 2016;63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Magill SS, O'Leary E, Janelle SJ, Thompson DL, Dumyati G, Nadle J, Wilson LE, Kainer MA, Lynfield R, Greissman S, et al. Changes in prevalence of health care-associated infections in U.S. Hospitals. N Engl J Med. 2018;379:1732–1744. doi: 10.1056/NEJMoa1801550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 4.Beghi G, De Tanti A, Serafini P, Bertolino C, Celentano A, Taormina G. Monitoring of Hospital acquired pneumonia in patients with severe brain injury on first access to intensive neurological rehabilitation: First year of observation. Monaldi Arch Chest Dis. 2018;88(888) doi: 10.4081/monaldi.2018.888. [DOI] [PubMed] [Google Scholar]
- 5.Zhang X, Xiao L, Niu L, Tian Y, Chen K. Comparison of six risk scores for stroke-associated pneumonia in patients with acute ischemic stroke: A systematic review and Bayesian network meta-analysis. Front Med (Lausanne) 2022;9(964616) doi: 10.3389/fmed.2022.964616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlayson O, Kapral M, Hall R, Asllani E, Selchen D, Saposnik G. Risk factors, inpatient care, and outcomes of pneumonia after ischemic stroke. Neurology. 2011;77:1338–1345. doi: 10.1212/WNL.0b013e31823152b1. Canadian Stroke Network; Stroke Outcome Research Canada (SORCan) Working group. [DOI] [PubMed] [Google Scholar]
- 7.Ingeman A, Andersen G, Hundborg HH, Svendsen ML, Johnsen SP. In-hospital medical complications, length of stay, and mortality among stroke unit patients. Stroke. 2011;42:3214–3218. doi: 10.1161/STROKEAHA.110.610881. [DOI] [PubMed] [Google Scholar]
- 8.Juan W, Zhen H, Yan-Ying F, Hui-Xian Y, Tao Z, Pei-Fen G, Jian-Tian H. A comparative study of two tube feeding methods in patients with dysphagia after stroke: A randomized controlled trial. J Stroke Cerebrovasc Dis. 2020;29(104602) doi: 10.1016/j.jstrokecerebrovasdis.2019.104602. [DOI] [PubMed] [Google Scholar]
- 9.Lee K, Rincon F. Pulmonary complications in patients with severe brain injury. Crit Care Res Pract. 2012;2012(207247) doi: 10.1155/2012/207247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winklewski PJ, Radkowski M, Demkow U. Cross-talk between the inflammatory response, sympathetic activation and pulmonary infection in the ischemic stroke. J Neuroinflammation. 2014;11(213) doi: 10.1186/s12974-014-0213-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Busl KM. Healthcare-associated infections in the neurocritical care unit. Curr Neurol Neurosci Rep. 2019;19(76) doi: 10.1007/s11910-019-0987-y. [DOI] [PubMed] [Google Scholar]
- 12.Chacón-Aponte AA, Durán-Vargas ÉA, Arévalo-Carrillo JA, Lozada-Martínez ID, Bolaño-Romero MP, Moscote-Salazar LR, Grille P, Janjua T. Brain-lung interaction: A vicious cycle in traumatic brain injury. Acute Crit Care. 2022;37:35–44. doi: 10.4266/acc.2021.01193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gonçalves B, Rynkowski C, Turon R, Charris N, Miranda F, de Caro V, Prazeres M, Santos T, Greer DM, Sharshar T, et al. doi: 10.1007/s12028-022-01629-6. Clinical characteristics and outcomes of patients with aneurysmal subarachnoid hemorrhage: A prospective multicenter study in a middle-income country. Neurocrit Care: Nov 2, 2022. doi: 10.1007/s12028-022-01629-6 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 14.Rafa E, Kołpa M, Wałaszek MZ, Domański A, Wałaszek MJ, Różańska A, Wójkowska-Mach J. Healthcare-Acquired infection surveillance in neurosurgery patients, incidence and microbiology, five years of experience in two polish units. Int J Environ Res Public Health. 2022;19(7544) doi: 10.3390/ijerph19127544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abulhasan YB, Rachel SP, Châtillon-Angle MO, Alabdulraheem N, Schiller I, Dendukuri N, Angle MR, Frenette C. Healthcare-associated infections in the neurological intensive care unit: Results of a 6-year surveillance study at a major tertiary care center. Am J Infect Control. 2018;46:656–662. doi: 10.1016/j.ajic.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 16.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 17.Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. JAMA. 2016;316:1193–1204. doi: 10.1001/jama.2016.11764. [DOI] [PubMed] [Google Scholar]
- 18.Sfeir MM. Diagnosis of multidrug-resistant pathogens of pneumonia. Diagnostics (Basel) 2021;11(2287) doi: 10.3390/diagnostics11122287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lord AS, Nicholson J, Lewis A. Infection prevention in the neurointensive care unit: A systematic review. Neurocrit Care. 2019;31:196–210. doi: 10.1007/s12028-018-0568-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Le Thuc O, Blondeau N, Nahon JL, Rovère C. The complex contribution of chemokines to neuroinflammation: Switching from beneficial to detrimental effects. Ann N Y Acad Sci. 2015;1351:127–140. doi: 10.1111/nyas.12855. [DOI] [PubMed] [Google Scholar]
- 21.Johnson VE, Stewart JE, Begbie FD, Trojanowski JQ, Smith DH, Stewart W. Inflammation and white matter degeneration persist for years after a single traumatic brain injury. Brain. 2013;136:28–42. doi: 10.1093/brain/aws322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar RG, Boles JA, Wagner AK. Chronic inflammation after severe traumatic brain injury: Characterization and associations with outcome at 6 and 12 months postinjury. J Head Trauma Rehabil. 2015;30:369–381. doi: 10.1097/HTR.0000000000000067. [DOI] [PubMed] [Google Scholar]
- 23.Ramlackhansingh AF, Brooks DJ, Greenwood RJ, Bose SK, Turkheimer FE, Kinnunen KM, Gentleman S, Heckemann RA, Gunanayagam K, Gelosa G, Sharp DJ. Inflammation after trauma: Microglial activation and traumatic brain injury. Ann Neurol. 2011;70:374–383. doi: 10.1002/ana.22455. [DOI] [PubMed] [Google Scholar]
- 24.Shim R, Wong CH. Ischemia, immunosuppression and infection-tackling the predicaments of post-stroke complications. Int J Mol Sci. 2016;17(64) doi: 10.3390/ijms17010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cui P, McCullough LD, Hao J. Brain to periphery in acute ischemic stroke: Mechanisms and clinical significance. Front Neuroendocrinol. 2021;63(100932) doi: 10.1016/j.yfrne.2021.100932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chamorro A, Urra X, Planas AM. Infection after acute ischemic stroke: A manifestation of brain-induced immunodepression. Stroke. 2007;38:1097–103. doi: 10.1161/01.STR.0000258346.68966.9d. [DOI] [PubMed] [Google Scholar]
- 27.Chang L, Chen Y, Li J, Liu Z, Wang Z, Chen J, Cao W, Xu Y. Cocaine-and amphetamine-regulated transcript modulates peripheral immunity and protects against brain injury in experimental stroke. Brain Behav Immun. 2011;25:260–269. doi: 10.1016/j.bbi.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 29.Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–786. doi: 10.1038/nrn1765. [DOI] [PubMed] [Google Scholar]
- 30.Prass K, Braun JS, Dirnagl U, Meisel C, Meisel A. Stroke propagates bacterial aspiration to pneumonia in a model of cerebral ischemia. Stroke. 2006;37:2607–2612. doi: 10.1161/01.STR.0000240409.68739.2b. [DOI] [PubMed] [Google Scholar]
- 31.Wong CHY. Effects of stroke beyond the brain. Nat Rev Immunol. 2019;19(719) doi: 10.1038/s41577-019-0234-4. [DOI] [PubMed] [Google Scholar]
- 32.Mracsko E, Liesz A, Karcher S, Zorn M, Bari F, Veltkamp R. Differential effects of sympathetic nervous system and hypothalamic-pituitary-adrenal axis on systemic immune cells after severe experimental stroke. Brain Behav Immun. 2014;41:200–209. doi: 10.1016/j.bbi.2014.05.015. [DOI] [PubMed] [Google Scholar]
- 33.Chamorro A, Amaro S, Vargas M, Obach V, Cervera A, Gómez-Choco M, Torres F, Planas AM. Catecholamines, infection, and death in acute ischemic stroke. J Neurol Sci. 2007;252:29–35. doi: 10.1016/j.jns.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Haeusler KG, Schmidt WU, Föhring F, Meisel C, Helms T, Jungehulsing GJ, Nolte CH, Schmolke K, Wegner B, Meisel A, et al. Cellular immunodepression preceding infectious complications after acute ischemic stroke in humans. Cerebrovasc Dis. 2008;25:50–58. doi: 10.1159/000111499. [DOI] [PubMed] [Google Scholar]
- 35.Klehmet J, Harms H, Richter M, Prass K, Volk HD, Dirnagl U, Meisel A, Meisel C. Stroke-induced immunodepression and post-stroke infections: Lessons from the preventive antibacterial therapy in stroke trial. Neuroscience. 2009;158:1184–1193. doi: 10.1016/j.neuroscience.2008.07.044. [DOI] [PubMed] [Google Scholar]
- 36.Pluta R, Januszewski S, Czuczwar SJ. Neuroinflammation in Post-ischemic neurodegeneration of the brain: Friend Foe or Both? Int J Mol Sci. 2021;22(4405) doi: 10.3390/ijms22094405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Walter U, Kolbaske S, Patejdl R, Steinhagen V, Abu-Mugheisib M, Grossmann A, Zingler C, Benecke R. Insular stroke is associated with acute sympathetic hyperactivation and immunodepression. Eur J Neurol. 2013;20:153–159. doi: 10.1111/j.1468-1331.2012.03818.x. [DOI] [PubMed] [Google Scholar]
- 38.Jovanovic B, Milan Z, Markovic-Denic L, Djuric O, Radinovic K, Doklestic K, Velickovic J, Ivancevic N, Gregoric P, Pandurovic M, et al. Risk factors for ventilator-associated pneumonia in patients with severe traumatic brain injury in a Serbian trauma centre. Int J Infect Dis. 2015;38:46–51. doi: 10.1016/j.ijid.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 39.Leone M, Bourgoin A, Giuly E, Antonini F, Dubuc M, Viviand X, Albanèse J, Martin C. Influence on outcome of ventilator-associated pneumonia in multiple trauma patients with head trauma treated with selected digestive decontamination. Crit Care Med. 2002;30:1741–1746. doi: 10.1097/00003246-200208000-00011. [DOI] [PubMed] [Google Scholar]
- 40.Ye YQ, He LL, Liu GL, Zhang J, Long LS. Pathogen distribution, imaging characteristics, and establishment and verification of risk prediction model of pulmonary infection with multi-drug resistant organism in patients with severe craniocerebral injury. Zhongguo Yi Xue Ke Xue Yuan Xue Bao. 2022;44:636–642. doi: 10.3881/j.issn.1000-503X.14670. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 41.Yang H, Fan Y, Li C, Zhang M, Liu W. A retrospective study on risk factors and disease burden for Hospital-acquired pneumonia caused by multi-drug-resistant bacteria in patients with intracranial cerebral hemorrhage. Neurol Sci. 2022;43:2461–2467. doi: 10.1007/s10072-021-05721-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee HS, Moon J, Shin HR, Ahn SJ, Kim TJ, Jun JS, Lee ST, Jung KH, Park KI, Jung KY, et al. Pneumonia in Hospitalized neurologic patients: Trends in pathogen distribution and antibiotic susceptibility. Antimicrob Resist Infect Control. 2019;8(25) doi: 10.1186/s13756-019-0475-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shrestha DK, Rajbhandari B, Pradhanang A, Sedain G, Shilpakar SK, Pradhan S. Ventilator-associated pneumonia in neurosurgical patients: A tertiary care center study. J Inst Med. 2019;41:40–44. [Google Scholar]
- 44.Teng G, Wang N, Nie X, Zhang L, Liu H. Analysis of risk factors for early-onset ventilator-associated pneumonia in a neurosurgical intensive care unit. BMC Infect Dis. 2022;22(66) doi: 10.1186/s12879-022-07053-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang W, Li L, Wen S, Song Y, Yu L, Tan B. Gram-negative multidrug-resistant organisms were dominant in neurorehabilitation ward patients in a general Hospital in southwest China. Sci Rep. 2022;12(11087) doi: 10.1038/s41598-022-15397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kourbeti IS, Vakis AF, Ziakas P, Karabetsos D, Potolidis E, Christou S, Samonis G. Infections in patients undergoing craniotomy: Risk factors associated with post-craniotomy meningitis. J Neurosurg. 2015;122:1113–119. doi: 10.3171/2014.8.JNS132557. [DOI] [PubMed] [Google Scholar]
- 47.Kesinger MR, Kumar RG, Wagner AK, Puyana JC, Peitzman AP, Billiar TR, Sperry JL. Hospital-acquired pneumonia is an independent predictor of poor global outcome in severe traumatic brain injury up to 5 years after discharge. J Trauma Acute Care Surg. 2015;78:396–402. doi: 10.1097/TA.0000000000000526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shi MM, Monsel A, Rouby JJ, Xu YP, Zhu YG, Qu JM. Inoculation pneumonia caused by coagulase negative Staphylococcus. Front Microbiol. 2019;10(2198) doi: 10.3389/fmicb.2019.02198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva-Santana G, Silva CMF, Olivella JGB, Silva IF, Fernandes LMO, Sued-Karam BR, Santos CS, Souza C, Mattos-Guaraldi AL. Worldwide survey of Corynebacterium striatum increasingly associated with human invasive infections, nosocomial outbreak, and antimicrobial multidrug-resistance, 1976-2020. Arch Microbiol. 2021;203:1863–1880. doi: 10.1007/s00203-021-02246-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Clariot S, Constant O, Lepeule R, Fihman V, Razazi K, Cook F, Attias A, Merle JC, Hemery F, Levesque E, et al. Clinical relevance and impact of Corynebacterium isolation in lower respiratory tract of critically ill patients requiring mechanical ventilation. Infection. 2020;48:413–420. doi: 10.1007/s15010-020-01411-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milosavljevic MN, Milosavljevic JZ, Kocovic AG, Stefanovic SM, Jankovic SM, Djesevic M, Milentijevic MN. Antimicrobial treatment of Corynebacterium striatum invasive infections: A systematic review. Rev Inst Med Trop Sao Paulo. 2021;63(e49) doi: 10.1590/S1678-9946202163049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shariff M, Aditi A, Beri K. Corynebacterium striatum: An emerging respiratory pathogen. J Infect Dev Ctries. 2018;12:581–586. doi: 10.3855/jidc.10406. [DOI] [PubMed] [Google Scholar]
- 53.Erfani Z, Jelodari Mamaghani H, Rawling JA, Eajazi A, Deever D, Mirmoeeni S, Azari Jafari A, Seifi A. Pneumonia in nervous system injuries: An analytic review of literature and recommendations. Cureus. 2022;14(e25616) doi: 10.7759/cureus.25616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Levin BR, Baquero F, Ankomah PP, McCall IC. Phagocytes, antibiotics, and self-limiting bacterial infections. Trends Microbiol. 2017;25:878–892. doi: 10.1016/j.tim.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 55.Georgakopoulou VE, Mantzouranis K, Damaskos C, Karakou E, Melemeni D, Mermigkis D, Petsinis G, Sklapani P, Trakas N, Tsiafaki X. Correlation between serum levels of 25-Hydroxyvitamin D and severity of community-acquired pneumonia in hospitalized patients assessed by pneumonia severity index: An observational descriptive study. Cureus. 2020;12(e8947) doi: 10.7759/cureus.8947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yu L. Restoring good health in elderly with diverse gut microbiome and food intake restriction to combat COVID-19. Indian J Microbiol. 2021;61:104–107. doi: 10.1007/s12088-020-00913-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McFall-Ngai M, Hadfield MG, Bosch TC, Carey HV, Domazet-Lošo T, Douglas AE, Dubilier N, Eberl G, Fukami T, Gilbert SF, et al. Animals in a bacterial world, a new imperative for the life sciences. Proc Natl Acad Sci USA. 2013;110:3229–3236. doi: 10.1073/pnas.1218525110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tanouchi Y, Lee AJ, Meredith H, You L. Programmed cell death in bacteria and implications for antibiotic therapy. Trends Microbiol. 2013;21:265–270. doi: 10.1016/j.tim.2013.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Jorgensen I, Rayamajhi M, Miao EA. Programmed cell death as a defence against infection. Nat Rev Immunol. 2017;17:151–164. doi: 10.1038/nri.2016.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.