Abstract
We describe the diagnosis and treatment of an atrioventricular accessory pathway (AP) in a horse using 3‐dimensional electro‐anatomical mapping (3D EAM) and radiofrequency catheter ablation (RFCA). During routine evaluation of the horse, intermittent ventricular pre‐excitation was identified on the ECG, characterized by a short PQ interval and abnormal QRS morphology. A right cranial location of the AP was suspected from the 12‐lead ECG and vectorcardiography. After precise localization of the AP using 3D EAM, ablation was performed and AP conduction was eliminated. Immediately after recovery from anesthesia an occasional pre‐excited complex still was observed, but a 24‐hour ECG and an ECG during exercise 1 and 6 weeks after the procedure showed complete disappearance of pre‐excitation. This case shows the feasibility of 3D EAM and RFCA to identify and treat an AP in horses.
Keywords: arrhythmia, electrophysiology, equine cardiology, ventricular pre‐excitation
Abbreviations
- 3D EAM
three‐dimensional electro‐anatomical mapping
- AP
accessory pathway
- AV
atrioventricular
- Bpm
beats per minute
- RF
radiofrequency
- RFCA
radiofrequency catheter ablation
- VCG
vectorcardiogram
1. CASE DESCRIPTION
Examination of a 10‐year old, 540 kg Andalusian gelding, used as teaching horse at the faculty of veterinary medicine, Ghent University, disclosed an intermittently increased first heart sound intensity on auscultation. A resting ECG at a heart rate of 43 ± 10 beats per minute (bpm) indicated that these heart sounds were associated with abnormal QRS complexes. Compared to normal sinus rhythm, these abnormal QRS complexes showed a short PQ interval (137 ± 16 ms; normal PQ interval, 230 ± 36 ms), short PQ segment (57 ms ± 14; normal PQ segment, 124 ± 10 ms) and increased QRS duration (201 ± 13 ms; normal QRS duration, 129 ± 5.4 ms; Figures 1 and 2A). Based on the ECG findings, a presumptive diagnosis of intermittent ventricular pre‐excitation over an accessory pathway (AP) was made. Continuous 24‐hour ECG recording indicated periods of normal sinus rhythm with intermittent ventricular pre‐excitation. During the recording, 1320 pre‐excited QRS complexes were found. Serum cardiac troponin I concentration was normal. Echocardiographic examination identified normal cardiac dimensions and function with a trace amount of aortic regurgitation, which was considered clinically unimportant. During exercise, pre‐excitation disappeared at heart rates >95 bpm. During recovery, pre‐excitation reappeared at a heart rate <95 bpm (Figure 2B).
FIGURE 1.
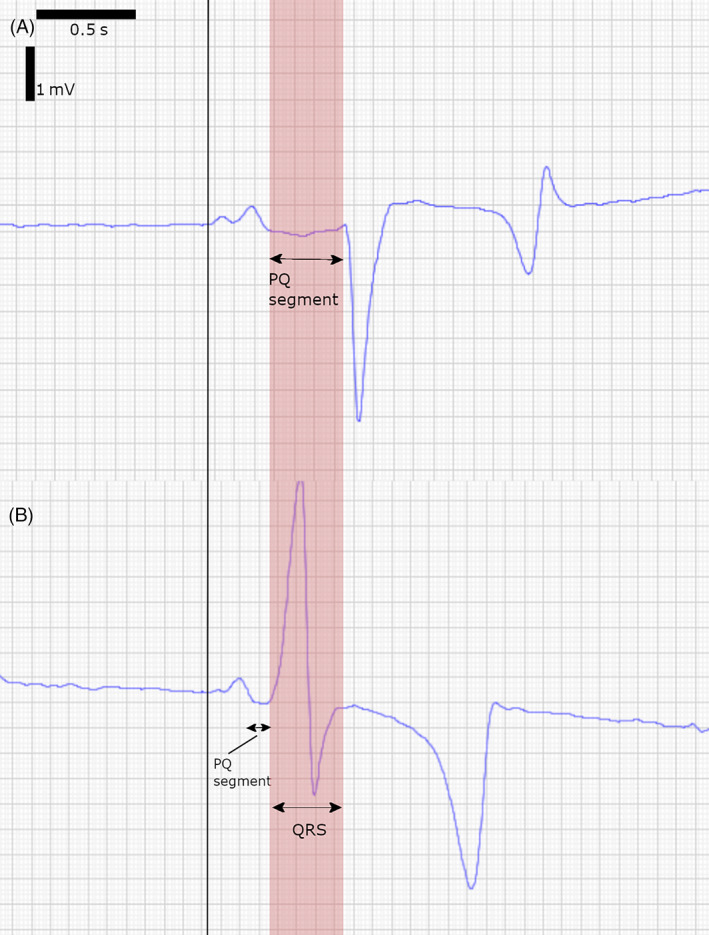
Comparison of a normal (A) and pre‐excitation (B) complex from a modified base‐apex electrocardiogram taken 5 s apart at a constant heart rate of 40 bpm. The black vertical line indicates the start of the P wave in both complexes. The red area indicates the PQ segment of the normal complex and how it relates to the pre‐excited QRS. (A) For the sinus beat, the PQ segment is 124 ms and QRS duration is 129 ms. (B) During pre‐excitation, the PQ segment is very short and immediately followed by the pre‐excited QRS complex, which is widened (201 ms) and shows a different morphology. A delta wave can be identified as a reduced dV/dt in the initial portion of the widened QRS complex. Comparison of both traces shows that during pre‐excitation, the entire pre‐excited ventricular depolarization is completed before the normal AV conduction reaches the ventricle.
FIGURE 2.
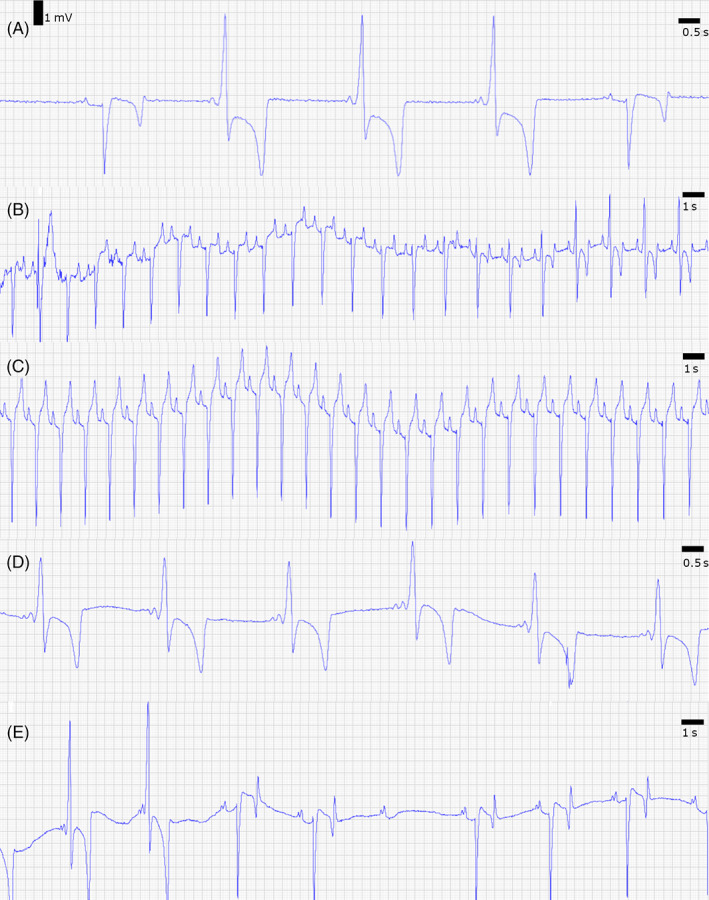
Modified base‐apex electrocardiogram (ECG) at rest (A), exercise (B), and after pharmacologic testing (C–E). 1 (A) An ECG at a resting heart rate of 44 bpm shows sinus rhythm (first and last complexes) and intermittent pre‐excitation (2rd‐4th complex). The normal complexes have a PQ interval and PQ segment of 230 and 124 ms, respectively, and a normal QRS morphology with a duration of 129 ms. The pre‐excitation complexes show a short PQ interval and PQ segment of 137 and 57 ms, respectively, and an abnormal QRS morphology with a prolonged QRS duration of 201 ms. (B) ECG during recovery after exercise at a heart rate of 88 bpm: there is a gradual transition from normal conduction to conduction along the accessory pathway. This can be observed by the progressive increase of the R wave amplitude and a shortening of the PQ intervals and segments. The pre‐excitation complexes have a PQ interval and PQ segment of 151 and 67 ms, respectively, and a QRS duration of 172 ms. (C) ECG during isoprenaline infusion (0.02 μg/kg/min) at a heart rate of 103 bpm. Isoprenaline induced sinus tachycardia with normal conduction via the AV node. These complexes have a PQ interval and PQ segment of 170 and 94 ms, respectively, and a QRS duration of 118 ms. (D) Intravenous administration of detomidine (7.5 μg/kg) IV induced ventricular pre‐excitation, with disappearance of the PQ segment. These complexes have a PQ interval of 141 ms; no PQ segment and a QRS duration of 197 ms. Blocked P waves were never observed immediately after detomidine administration. Mean heart rate is 35 bpm. (E) ECG 2 hours after detomidine injection. The first 2 QRS complexes are the result of pre‐excitation whereas the other complexes have normal PQ interval and QRS morphology. The second‐degree AV block is visible as a blocked P wave, which means that conduction over the accessory pathway also is blocked, which is rare in human patients.
In order to attempt localization of the AP, a 12‐lead ECG was recorded during pre‐excitation and vectorcardiograms (VCG) in different planes were calculated. 2 , 3 The VCG identified a left, caudoventral direction of the ventricular wavefront, suggesting an AP localized at the craniodorsal region of the right ventricular free wall. Right atrial pacing was performed to exactly localize the AP, because atrial pacing near the AP should result in maximal pre‐excitation. 4 In this patient, it was difficult to reach the right atrial free wall with the pacing catheter, and therefore it was not possible to localize the AP by means of atrial pacing. Pacing in the right ventricular apex did not result in retrograde conduction over the AP.
Because retrograde conduction was absent, radiofrequency catheter ablation (RFCA) would be more difficult if pre‐excitation was not continuously present. Therefore, drug testing was performed to stimulate pre‐excitation. First, isoprenaline was administered IV, because it is known to facilitate anterograde AP conduction in humans with intermittent ventricular pre‐excitation. 5 Constant rate infusion of isoprenaline (0.02 μg/kg/min; Isuprel, Hospira, Elsene) induced sinus tachycardia with normal atrioventricular (AV) conduction (Figure 2C). Because it is known to induce second‐degree AV block in the horse, 6 detomidine (7.5 μg/kg; Domidine, Dechra, Lille, Belgium) was administered IV during a separate procedure. Detomidine administration resulted in enhancement of AP conduction with continuous ventricular pre‐excitation (Figure 2D) for 2 hours. As the effect of detomidine decreased, ventricular pre‐excitation became intermittent again. In addition, the horse developed an occasional Mobitz type I second‐degree AV block at a heart rate of 35 bpm (Figure 2E). After 2 weeks, repeated 24‐hour ECG recordings confirmed the presence of intermittent ventricular pre‐excitation, which became continuous after another detomidine administration.
Subsequently, 3‐dimensional electro‐anatomical mapping (3D EAM) and RFCA, using the CARTO 3 System (Biosense Webster, Irvine, California, USA) were used to identify and treat the AP. First, in the standing horse, an 8.5F fixed curved sheath (HeartSpan, Merit Medical, Utah, USA) was inserted into the right jugular vein through which a decapolar steerable catheter (WEBSTER CS Bi‐Directional Catheter, Biosense Webster, Irvine, California, USA) was introduced and placed in the coronary sinus under echocardiographic guidance. The electrograms from this catheter served as a timing reference for atrial and ventricular activation during the mapping procedure. Subsequently, general anesthesia was administered and the horse was positioned in right lateral recumbency with 2 in‐parallel grounding patches on the right dorsal thorax. The magnetic field generator was placed in a wooden casing underneath the horse. During anesthesia, the horse showed normal AV conduction. Detomidine (7.5 μg/kg) was administered IV and initiated continuous pre‐excitation at a heart rate of 29 bpm. Anticoagulation treatment was implemented by administration of 60 IU/kg unfractionated heparin (Heparine LEO, LEO pharma, Lier, Belgium) IV at the start of the procedure, followed by 30 IU/kg 2 hours later. Through an 8.5F steerable sheath (Destino Reach, Oscor, Palm Harbor, Florida, USA) in the left jugular vein, an 8F contact force ablation catheter (Thermocool Smarttouch, Biosense Webster, Irvine, California, USA) was inserted to make a 3D EAM of the right atrium and right ventricle. The map was focused on the region where the abnormal bundle was suspected, as determined by 12‐lead ECG and VCG. This procedure identified an AP at the right cranial free wall between the right atrium and right ventricle (Figure 3A). The map identified the earliest ventricular activation, which defines the ventricular insertion of the AP. At this location, the electrograms recorded by the ablation catheter's tip, located at the right AV groove, showed an AP potential, also known as Kent potential (Figure 3B). These results indicate that the catheter is exactly located on the abnormal bundle, which is the correct location to perform ablation. The RF energy was delivered in power‐controlled mode (Stockert 70 generator, Biosense Webster, Irvine, California, USA) with a power of 40 W, an irrigation rate of 30 mL/min and a contact force of 15 g. Catheter tip temperature was monitored and power was manually decreased in the event that temperature reached 40°C. After applying 2 s of RF energy, loss of AP conduction occurred and energy delivery was continued for 29 s.
FIGURE 3.
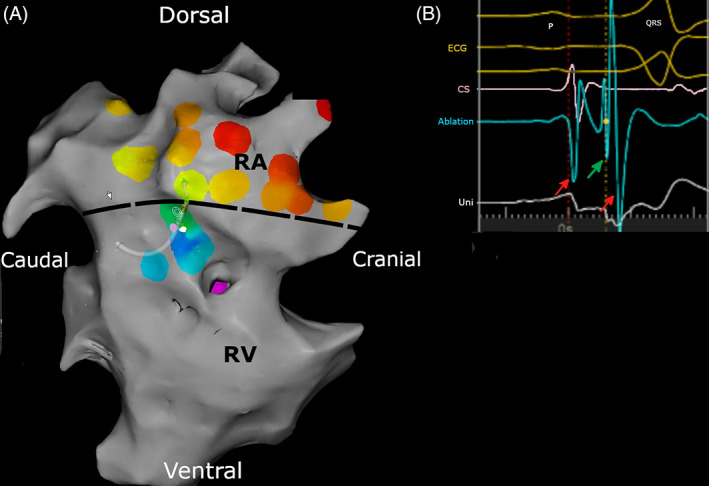
(A) Activation map of the right atrium (RA) and right ventricle (RV). Only a small part of the 3D anatomy (gray) has been created and only a few activation points (color) have been registered because the accessory pathway was quickly found. The color varies following the rainbow spectrum from red (earliest activation) to purple (latest activation) in relation to the electrograms from the coronary sinus, which served as a timing reference. The map demonstrates the earliest ventricular activation in continuation with atrial activation, which defines the ventricular insertion of the accessory pathway, located at the right cranial free wall between the right atrium and right ventricle. The dotted line indicates the demarcation between RA and RV. (B) Surface electrocardiogram (yellow traces), electrogram from the coronary sinus (CS) catheter (pink traces), recording from the ablation catheter at the accessory pathway (light blue trace) and unipolar recording from the ablation catheter (white trace), recorded at the same timing and catheter position as Figure 3A. The accessory pathway potential can be recognized as the sharp spike (green arrow) between the atrial (first red arrow) and ventricular (second red arrow) electrogram.
A 24‐hour ECG recorded immediately after the procedure showed 47 isolated pre‐excited complexes. Detomidine (7.5 μg/kg) administration 24 hours after the procedure no longer induced AP conduction, which proved successful elimination of the AP. Clinical evaluation, 24‐hour ECG monitoring and exercise ECG were repeated 1 and 6 weeks later, and showed complete disappearance of pre‐excitation and absence of complications. The horse was allowed to continue normal riding exercise.
2. DISCUSSION
In the normal heart, the annulus fibrosus electrically isolates the atria from the ventricles, and conduction to the ventricles only occurs via the AV node, bundle of His and Purkinje system. 7 An AP is a strand of myocardial cells that bypasses the AV conduction pathway and directly connects the atrial and ventricular myocardium. 8 These additional connections result in earlier ventricular activation compared to conduction via the AV node. This pathway leads to typical ECG findings of shortened PQ interval and segment, and different QRS morphology and duration. 9 The initial ventricular activation at the insertion of the AP typically results in a slurred upstroke of the QRS complex, the delta wave. In humans and dogs, the final QRS complex is the result of the fusion of the early ventricular depolarization by the AP and the slightly later ventricular depolarization via the AV node. In our horse, a delta wave was noticed as a decreased dV/dt in the initial portion of the pre‐excited complex. Analysis of the ECG (Figure 1) shows that almost the entire pre‐excited QRS complex occurred during the normal PQ segment and thus before the normal QRS. This finding indicates that almost the entire ventricle was activated by the AP. This situation contrasts to pre‐excitation in humans and dogs, and can be explained by the physiological AV conduction delay in horses, related to their high vagal tone.
Only a few cases of AP in horses have been described, 10 , 11 , 12 , 13 , 14 although the condition may be underreported because it can be easily missed or mistaken for other arrhythmias. Differential diagnoses for a widened QRS complex include ventricular premature complexes, bundle branch block, accelerated idioventricular rhythm and ventricular pre‐excitation. Ventricular premature complexes and accelerated idioventricular rhythm show no association with a P wave and therefore can be distinguished from pre‐excitation. 15 The widened QRS complexes caused by bundle branch block and ventricular pre‐excitation always are preceded by a P wave. However, bundle branch block is characterized by a normal PQ interval, 15 as opposed to the shortened PQ interval in pre‐excitation. An AP also can conduct retrogradely. Retrograde conduction leads to an ECG pattern with a normal QRS complex, normal PQ interval, but with a retrograde conducted P wave, typically within the ST segment, if retrograde AP conduction occurs.
Our horse had intermittent pre‐excitation, the most commonly reported presentation of AP in horses. 11 , 12 , 13 , 14 In our horse, ventricular pre‐excitation disappeared during exercise, which can be explained by a change in autonomic tone, modulating AP conduction and enhancing AV nodal conduction. Rate‐dependent block is another potential mechanism in which the AP blocks conduction at higher heart rates and the AV node transmits the impulses instead. 16 The normalization of QRS complexes during exercise contrasts with previous case reports of APs in horses, in which AP conduction was enhanced during exercise, recovery or excitatory stimuli. 11 , 13 , 14 Exercise‐induced pre‐excitation has been reported in humans and is related to catecholamine‐sensitive APs. Increased catecholamine concentrations during exercise or stress activate beta‐adrenergic receptors in a catecholamine‐sensitive bypass tract and induce pre‐excitation. 17
Drugs were administered to assess the effect of AP conduction. In human medicine, IV administration of adenosine is commonly used, because it is a potent AV nodal blocking agent and thereby facilitates anterograde AP conduction. 18 , 19 , 20 Bolus administrations of adenosine are necessary to reach high local concentrations and transmural penetration at the AV node in order to induce AV block. 21 Adenosine has been administered to a single horse, but did not induce AV block (G. van Loon, personal communication). The dose needed in horses is unknown and the drug might fail to reach the AV node transmurally because of the thick myocardial wall. Therefore, we did not use adenosine in our case. Isoprenaline can be used in catecholamine‐sensitive APs to shorten the anterograde effective refractory period of the AP and thus improve AP conduction. 5 , 17 In our horse, isoprenaline infusion resulted in sinus tachycardia with normal AV conduction. This outcome could be expected because the AP in our horse was not dependent on sympathetic activation, which is supported by the fact that exercise abolished ventricular pre‐excitation. Isoprenaline might have produced a rate‐dependent block of the AP, an accelerated impulse conduction through the AV node, or both. 12 In our horse, detomidine increased AP conduction, which corresponds to the finding of increased pre‐excitation at rest and slow heart rate. A possible explanation is that both at rest and during detomidine sedation, the autonomic nervous system is predisposed toward increased vagal tone. Increased vagal tone decreases impulse generation at the sinus node and conduction along the AV node, thereby slowing the heart rate and favoring ventricular activation over the AP. 6 , 16 , 22 , 23 , 24 Another mechanism could be rate‐dependent block of the AP, in which impulses at higher heart rates are blocked. The lower heart rates during detomidine sedation and rest might promote ventricular activation along the AP. It is also possible that detomidine has a direct effect on the AP. Remarkably, on a few occasions after the detomidine effect decreased, the horse experienced a Mobitz type I second‐degree AV block without AP conduction. In humans, the occurrence of AV block in the presence of an AP is rare because the AP normally will conduct the atrial impulse when AV block occurs, resulting in pre‐excitation. 25 , 26 , 27 In our horse, AV block without pre‐excitation probably was related to impaired AP conduction because of changes in alpha‐2 function and autonomic tone.
The AP behavior and ECG characteristics were different in our horse compared to those observed in humans. Little is known about the risk of an AP and the effect on performance in horses. In humans and dogs, the risk lies in a high prevalence of associated arrhythmias. Conduction over the AV node returning to the atria via the AP can lead to AV reentrant tachycardia. Atrial fibrillation with anterograde conduction via the AP can be life‐threatening, because a rapid ventricular response can degenerate into ventricular fibrillation, causing sudden cardiac death. 9 , 28 , 29 , 30 , 31 , 32 , 33 In humans with asymptomatic pre‐excitation, risk stratification is performed by electrophysiological studies and noninvasive testing. 34 , 35 During an electrophysiology study, the effective refractory period of the AP is measured. A short anterograde effective refractory period of the AP is associated with an increased risk of sudden cardiac death, because it allows rapid conduction to the ventricles. Noninvasive testing consists of ECG monitoring and an exercise test. If these indicate intermittent ventricular pre‐excitation and abrupt complete disappearance of ventricular pre‐excitation during exercise, the risk is considered low. 34 , 35 Whether or not these criteria apply to horses is unknown, especially because APs in horses seem to behave differently. Based on these findings, and without further knowledge of the electrophysiological properties, horses with ventricular pre‐excitation probably cannot be considered safe to ride. Thorough electrophysiological studies to assess the refractory period of AP would be necessary to gain better insight into the potential risks in horses. To keep anesthesia time as short as possible, additional electrophysiological tests were not performed. Ideally, variables such as antegrade Wenckebach point during pre‐excitation and during AV conduction, retrograde Wenckebach point during pre‐excitation, antegrade effective refractory period during pre‐excitation and during AV conduction, retrograde effective refractory period during pre‐excitation, interval between the atrial electrogram of the His recording and the His signal (AH interval) and the ventricular electrogram and His signal (HV interval) would have been determined to better characterize the type of AP.
Because detomidine consistently activated the AP, it was used to induce pre‐excitation during the 3D EAM. The 3D EAM was essential for accurate catheter manipulation around the tricuspid annulus and quickly showed the AP potential. At that time, only a rudimentary anatomical map and only a few activation points were collected. However, this information was enough to determine the AP location. Radiofrequency catheter ablation is the treatment of choice for APs in humans and dogs. 29 , 30 It has a high success rate of almost 100% and a low complication rate. 29 , 30 , 33 , 36 In horses, RFCA has been used successfully for the treatment of atrial tachycardia, 37 but so far not for an AP. The principle of mapping anterograde conducting pathways is to find the earliest ventricular activation site or AP potential. While mapping the tricuspid annulus with the ablation catheter, the AP potential (a sharp, rapid deflection between the atrial and ventricular electrogram 38 ) could be identified in our horse. The pathway potential represents direct registration of the AP activation and is an important indicator of a successful ablation site. 39 , 40 , 41 The AP ablations are performed along the AV annulus. In our horse, the slightly larger ventricular compared to atrial signal suggested good position on the AV annulus. Within 2 s of RF energy delivery, the pathway potential disappeared and ablation was continued for 29 s. This outcome is consistent with results in humans and dogs, where good catheter position should eliminate AP conduction within 1 to 6 s, after which RF delivery is continued for up to 60 s. 4 , 30 , 33 , 42 The occasional AP conduction in the first hours after ablation, followed by complete conduction block also has been described in humans. The reason for this delayed cure is not clear, but progression of inflammatory injury and necrosis might be an explanation. 43 , 44 , 45 , 46
In conclusion, the AP in our horse had features that are not commonly observed in humans and dogs, such as the absence of retrograde conduction. Further research is necessary to elucidate AP behavior in the horse. Our case shows that it is feasible to identify an AP by 3D EAM and to achieve permanent correction by RFCA in an adult horse.
CONFLICT OF INTEREST DECLARATION
Authors declare no conflict of interest.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
Authors declare no IACUC or other approval was needed.
HUMAN ETHICS APPROVAL DECLARATION
Authors declare human ethics approval was not needed for this study.
ACKNOWLEDGMENT
Eva Buschmann and Ingrid Vernemmen are PhD fellows funded by the Research Foundation Flanders (FWO‐Vlaanderen), grant numbers 1SE9122N and 1S71521N.
Buschmann E, Van Steenkiste G, Boussy T, et al. Three‐dimensional electro‐anatomical mapping and radiofrequency ablation as a novel treatment for atrioventricular accessory pathway in a horse: A case report. J Vet Intern Med. 2023;37(2):728‐734. doi: 10.1111/jvim.16668
REFERENCES
- 1. Verheyen T, Decloedt A, de Clerq D, et al. Electrocardiography in horses ‐ Part 1: how to make a good recording. Vlaams Diergeneeskundige Tijdschrift. 2010;79:331‐336. [Google Scholar]
- 2. Van Steenkiste G, Delhaas T, Hermans B, et al. An exploratory study on vectorcardiographic identification of the site of origin of focally induced premature depolarizations in horses, Part I: The Atria. Animals. 2022;12:549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Steenkiste G, Delhaas T, Hermans B, Vera L, Decloedt A, van Loon G. An exploratory study on vectorcardiographic identification of the site of origin of focally induced premature depolarizations in horses, Part II: The ventricles. Animals. 2022;12:550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Issa Z, Miller J, Zipes D. Typical atrioventricular bypass tracts. Clinical Arrhythmiology and Electrophysiology. 3th ed. Philadelphia, PA: Elsevier; 2019:599‐676. [Google Scholar]
- 5. Yamamoto T, Yeh SJ, Lin FC, Wu D. Effects of isoproterenol on accessory pathway conduction in intermittent or concealed Wolff‐Parkinson‐White syndrome. Am J Cardiol. 1990;65:1438‐1442. [DOI] [PubMed] [Google Scholar]
- 6. Yamashita K, Tsubakishita S, Futaoka S, et al. Cardiovascular effects of medetomidine, detomidine and xylazine in horses. J Vet Med Sci. 2000;62:1025‐1032. [DOI] [PubMed] [Google Scholar]
- 7. Anderson RH, Ho SY, Gillette PC, Becker AE. Mahaim, Kent and abnormal atrioventricular conduction. Cardiovasc Res. 1996;31:480‐491. [PubMed] [Google Scholar]
- 8. Ho SY. Accessory atrioventricular pathways: getting to the origins. Circulation. 2008;117:1502‐1504. [DOI] [PubMed] [Google Scholar]
- 9. van Loon G. Cardiac arrhythmias in horses. Vet Clin North Am Equine Pract. 2019;35:85‐102. [DOI] [PubMed] [Google Scholar]
- 10. Jesty SA, Kraus MS, Johnson AL, Gelzer ARM, Bartol J. An accessory bypass tract masked by the presence of atrial fibrillation in a horse. J Vet Cardiol. 2011;13:79‐83. [DOI] [PubMed] [Google Scholar]
- 11. Viu J, Armengou L, Decloedt A, Jose‐Cunilleras E. Investigation of ventricular pre‐excitation electrocardiographic pattern in two horses: clinical presentation and potential causes. J Vet Cardiol. 2018;20:213‐221. [DOI] [PubMed] [Google Scholar]
- 12. Muir WW, McGuirk SM. Ventricular preexcitation in two horses. J Am Vet Med Assoc. 1983;183:573‐576. [PubMed] [Google Scholar]
- 13. Cooper SA. Ventricular pre‐excitation (Wolff‐Parkinson‐White syndrome) in a horse. Vet Rec. 1962;74:527‐530. [Google Scholar]
- 14. Senta T, Amada A. Wolff‐Parkinson‐White (ventricular pre‐excitation syndrome) in a thoroughbred. Japan Racing Association Racehorse Health Research Institute Report. 1967;4:129–136. [Google Scholar]
- 15. Schwarzwald CC. Disorders of the cardiovascular system. In: Reed SM, Warwick BM, Debra SC, eds. Equine Internal Medicine. 4th ed. St. Louis, Missouri: Elsevier; 2018:387‐541. [Google Scholar]
- 16. German LD, Gallagher JJ, Broughton A, Guarnieri T, Trantham JL. Effects of exercise and lsoproterenol during atrial fibrillation In patients with Wolff‐Parkinson‐White syndrome. Am J Cardiol. 1983;51:1203‐1206. [DOI] [PubMed] [Google Scholar]
- 17. Horio Y, Matsuyama K, Morikami Y, et al. Blocking effect of verapamil on conduction over a catecholamine‐sensitive bypass tract in exercise‐induced Wolff‐Parkinson‐White syndrome. J Am Coll Cardiol. 1984;4:186‐191. [DOI] [PubMed] [Google Scholar]
- 18. Cohen TJ, Tucker KJ, Abbott JA, et al. Usefulness of adenosine in augmenting ventricular preexcitation for noninvasive localization of accessory pathways. Am J Cardiol. 1992;69:1178‐1185. [DOI] [PubMed] [Google Scholar]
- 19. Ali H, Lupo P, Foresti S, et al. Adenosine and preexcitation variants: reappraisal of electrocardiographic changes. Ann Noninvasive Electrocardiol. 2016;21:420‐424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Belardinelli L, Linden J, Berne RM. The cardiac effects of adenosine. Prog Cardiovasc Dis. 1989;32:73‐97. [DOI] [PubMed] [Google Scholar]
- 21. Camm J, Garrat C. Adenosine and supraventricular tachycardia. N Engl J Med. 1991;325:1621‐1629. [DOI] [PubMed] [Google Scholar]
- 22. Wakita R, Takahashi M, Ohe C, Kohase H, Umino M. Occurrence of intermittent Wolff‐Parkinson‐White syndrome during intravenous sedation. J Clin Anesth. 2008;20:146‐149. [DOI] [PubMed] [Google Scholar]
- 23. German LD, Gallagher JJ. Functional properties of accessory atrioventricular pathways in Wolff‐Parkinson‐White syndrome: clinical implications. Am J Med. 1984;76:1079‐1086. [DOI] [PubMed] [Google Scholar]
- 24. Ergul Y, Unsal S, Ozyilmaz I, et al. Electrocardiographic and electrophysiologic effects of dexmedetomidine on children. Pace. 2015;38:682‐687. [DOI] [PubMed] [Google Scholar]
- 25. Palanca V, Quesada A, Roda J, Villalba S, Mihi N, Velasco J. Intermittent atrioventricular block in an accessory pathway associated with complete Infra‐Hisian block. Revista Española de Cardiología (English Edition). 2004;57:363‐366. [PubMed] [Google Scholar]
- 26. Dinckal MH, Davutoglu VD, Bayata S, Yesil M. Masked complete atrioventricular block in a patient with ventricular preexcitation. J Interv Card Electrophysiol. 2004;11:33‐35. [DOI] [PubMed] [Google Scholar]
- 27. Sacher F, Douard H, Wright M, et al. Compensatory accessory pathway for complete atrioventricular block: 28‐year follow‐up. Pace. 2009;32:952‐956. [DOI] [PubMed] [Google Scholar]
- 28. Centurión OA, Shimizu A, Isomoto S, et al. Mechanisms for the genesis of paroxysmal atrial fibrillation in the Wolff ‐ Parkinson ‐ White syndrome: intrinsic atrial muscle vulnerability vs. electrophysiological properties of the accessory pathway. Europace. 2008;10:294‐302. [DOI] [PubMed] [Google Scholar]
- 29. Brugada J, Katritsis DG, Arbelo E, et al. 2019 ESC Guidelines for the management of patients with supraventricular tachycardia. Eur Heart J. 2020;41:655‐720. [DOI] [PubMed] [Google Scholar]
- 30. Santilli RA, Mateos Pañero M, Porteiro Vázquez DM, Perini A, Perego M. Radiofrequency catheter ablation of accessory pathways in the dog: the Italian experience (2008–2016). J Vet Cardiol. 2018;20:384‐397. [DOI] [PubMed] [Google Scholar]
- 31. Santilli RA, Caivano D, Pariaut R, et al. Low‐energy ablation of anteroseptal accessory pathways in two dogs. J Vet Cardiol. 2018;20:285‐293. [DOI] [PubMed] [Google Scholar]
- 32. Miyazaki S, Hocini M, Linton N, et al. Initial results of efficacy of left linear ablation using a novel simultaneous multielectrode ablation catheter. J Cardiovasc Electrophysiol. 2011;22:739‐745. [DOI] [PubMed] [Google Scholar]
- 33. Wright KN, Connor CE, Irvin HM, Knilans TK, Webber D, Kass PH. Atrioventricular accessory pathways in 89 dogs: clinical features and outcome after radiofrequency catheter ablation. J Vet Intern Med. 2018;32:1517‐1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Katritsis DG, Morady F. Wolff‐Parkinson‐White‐syndrome‐and‐atrioventricular reentrant tachycardias. Clinical Cardiac Electrophysiology: A Practical Guide. 1st ed. Philadelphia, PA: Elsevier; 2021:276‐305. [Google Scholar]
- 35. Pelliccia A, Sharma S, Gati S, et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur Heart J. 2021;42:17‐96. [DOI] [PubMed] [Google Scholar]
- 36. Leung LWM, Gallagher MM. Review paper on WPW and athletes: let sleeping dogs lie? Clin Cardiol. 2020;43:897‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Van Steenkiste G, Boussy T, Duytschaever M, et al. Detection of the origin of atrial tachycardia by 3D electro‐anatomical mapping and treatment by radiofrequency catheter ablation in horses. J Vet Intern Med. 2022;36:1481‐1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Josephson ME. Preexcitation syndromes. Josephon's Clinical Cardiac Electrophysiology: Techniques and Interpretations. 6th ed. Philadelphia, PA: Wolters Kluwer; 2016:339‐446. [Google Scholar]
- 39. Calkins H, Kim YN, Schmaltz S, et al. Electrogram criteria for identification of appropriate target sites for radiofrequency catheter ablation of accessory atrioventricular connections. Circulation. 1991;85:565‐573. [DOI] [PubMed] [Google Scholar]
- 40. Kuck KH, Schluter M. Single‐catheter approach to radiofrequency current ablation of left‐sided accessory pathways in patients with Wolff‐Parkinson‐White syndrome. Circulation. 1991;6:2366‐2375. [DOI] [PubMed] [Google Scholar]
- 41. Murgatroyd FD, Krahn AD, Klein GJ, et al. Handbook of Cardiac Electrophysiology: A Practical Guide to Invasive EP Studies and Catheter Ablation. 1st ed. London: Remedica Medical Education and Publishing; 2002. [Google Scholar]
- 42. Wright KN, Knilans TK, Irvin HM. When, why, and how to perform cardiac radiofrequency catheter ablation. J Vet Cardiol. 2006;8:95‐107. [DOI] [PubMed] [Google Scholar]
- 43. Issa ZF, Miller JM, Zipes DP. Ablation energy sources. Clinical Arrhythmology and Electrophysiology. 3th ed. Philadelphia, PA: Elsevier; 2019:206‐237. [Google Scholar]
- 44. Nath S, Whayne JG, Kaul S, Goodman NC, Jayaweera AR, Haines DE. Effects of radiofrequency catheter ablation on regional myocardial blood flow: possible mechanism for late electrophysiological outcome. Circulation. 1994;89:2667‐2672. [DOI] [PubMed] [Google Scholar]
- 45. Wagshal AB, Pires LA, Mittleman RS, Cuello C, Bonavita GJ, Stephen Huang SK. Early recurrence of accessory pathways after radiofrequency catheter ablation does not preclude long‐term cure. Am J Cardiol. 1993;72:843‐846. [DOI] [PubMed] [Google Scholar]
- 46. Sarubbi B, D'alto M, Calvanese R, et al. Late cure after radiofrequency catheter ablation in a pediatric patient. J Cardiovasc Med. 2006;7:356‐361. [DOI] [PubMed] [Google Scholar]


