Key Points
Question
Do individuals with bipolar disorder show aberrant brain activities in regions of the fronto-limbic network compared with healthy control individuals?
Findings
This systematic review and meta-analysis consisting of 49 functional magnetic resonance imaging studies (999 individuals with bipolar disorder and 1027 healthy control individuals) examining brain activity in 3 domains (emotion processing, reward processing, and working memory) found robust activity disturbances in brain regions, mostly within the fronto-limbic network.
Meaning
Aberrations in the fronto-limbic network may underlie cognitive and emotional dysfunctions in individuals with BD.
This systematic review and meta-analysis investigates the emotion processing, reward processing, and working memory brain domains of individuals with bipolar disorder vs those of healthy control individuals.
Abstract
Importance
Individuals with bipolar disorder (BD) experience cognitive and emotional dysfunctions. Various brain circuits are implicated in BD but have not been investigated in a meta-analysis of functional magnetic resonance imaging (fMRI) studies.
Objective
To investigate the brain functioning of individuals with BD compared with healthy control individuals in the domains of emotion processing, reward processing, and working memory.
Data Sources
All fMRI experiments on BD published before March 2020, as identified in a literature search of PubMed, Embase, Web of Science, Cochrane Library, PsycInfo, Emcare, Academic Search Premier, and ScienceDirect. The literature search was conducted on February 21, 2017, and March 2, 2020, and data were analyzed from January 2021 to January 2022.
Study Selection
fMRI experiments comparing adult individuals with BD and healthy control individuals were selected if they reported whole-brain results, including a task assessing at least 1 of the domains. In total, 2320 studies were screened, and 253 full-text articles were evaluated.
Data Extraction and Synthesis
A total of 49 studies were included after selection procedure. Coordinates reporting significant activation differences between individuals with BD and healthy control individuals were extracted. Differences in brain region activity were tested using the activation likelihood estimation method.
Main Outcomes and Measures
A whole-brain meta-analysis evaluated whether reported differences in brain activation in response to stimuli in 3 cognitive domains between individuals with BD and healthy control individuals were different.
Results
The study population included 999 individuals with BD (551 [55.2%] female) and 1027 healthy control individuals (532 [51.8%] female). Compared with healthy control individuals, individuals with BD showed amygdala and hippocampal hyperactivity and hypoactivation in the inferior frontal gyrus during emotion processing (20 studies; 324 individuals with BD and 369 healthy control individuals), hyperactivation in the orbitofrontal cortex during reward processing (9 studies; 195 individuals with BD and 213 healthy control individuals), and hyperactivation in the ventromedial prefrontal cortex and subgenual anterior cingulate cortex during working memory (20 studies; 530 individuals with BD and 417 healthy control individuals). Limbic hyperactivation was only found during euthymia in the emotion and reward processing domains; abnormalities in frontal cortex activity were also found in individuals with BD with mania and depression.
Conclusions and Relevance
This systematic review and meta-analysis revealed evidence for activity disturbances in key brain areas involved in cognitive and emotion processing in individuals with BD. Most of the regions are part of the fronto-limbic network. The results suggest that aberrations in the fronto-limbic network, present in both euthymic and symptomatic individuals, may be underlying cognitive and emotional dysfunctions in BD.
Introduction
Bipolar disorder (BD) is a severe psychiatric disorder characterized by recurrent depressive and manic episodes.1 These mood fluctuations contribute considerably to functional impairments, including dysfunction in education and work.2 In addition to affective symptoms, individuals with BD show cognitive impairments and emotion regulation deficits during episodes and also during euthymia.3,4,5 As a result, several of these deficits in individuals with BD may be considered trait-associated characteristics of BD. Abnormalities in a variety of brain regions and related circuitry could be underlying cognitive and emotion regulation deficits in individuals with BD, with studies in general describing predominantly aberrations in the fronto-limbic network,6,7,8,9 a group of interconnected neural regions including the prefrontal cortex (PFC), amygdala, anterior cingulate cortex (ACC), hippocampus, and nucleus accumbens.10
Individuals with BD appear to show amygdala hyperactivation during emotion processing across multiple tasks that evoke emotional responses.11 In addition, recent work shows reduced functional connectivity between the ventrolateral PFC, ACC, orbitofrontal cortex (OFC), and limbic areas, which points toward a more complex aberrant interplay between frontal and limbic structures instead of amygdala hyperactivity alone.10 In addition to emotion, individuals with BD show increased activity in fronto-limbic regions during reward processing, such as in the PFC, ACC, and striatum.12,13,14 Studies of working memory in individuals with BD have reported deviant frontal cortex activity,15,16,17 including medial frontal gyri hyperactivation18 and dorsolateral PFC (dlPFC) hypoactivation.19,20,21
Inconsistent findings are common across all emotional and cognitive domains. As a result, a robust hypothesis on fronto-limbic dysfunction in individuals with BD is lacking.22 Not surprisingly, and probably associated with aberrant brain activity, individuals with BD show behavioral deficiencies in terms of decreased task performance during emotion and reward processing, as well as during cognitive tasks that demand working memory activity.4,23,24,25,26 In addition to decreased task performances, fronto-limbic network deficiencies may also be involved in aberrant psychological mechanisms in individuals with BD, such as state-independent emotional hyper-reactivity, rumination, and the intense pursuit of goals and focus on achievement.27,28,29
Cognitive and emotion tasks that involve fronto-limbic brain activity have been intensively studied. Emotion processing involves attentional processes toward emotional stimuli, their interpretation, and the regulation of activated emotions (ie, the ability to monitor and modify the occurrence, intensity, and duration of an ongoing response to emotional stimuli).30 Activations in subcortical regions are associated with modulating and generating emotions, whereas frontal regions are involved in the evolution and regulation of emotional responses.10,26 The 3 primary functions of reward processing include associative learning (classical conditioning and operant reinforcement), incentive salience (motivation and desire), and positively valenced emotions (pleasure and hedonic).31 In particular, pleasure coding has been described in association with activation in the OFC,32 whereas ACC activity is associated with reward anticipation.33 In addition to the ACC, the ventral striatum has a key function in anticipation of reward stimuli and is part of a complex circuit involving limbic regions, such as the amygdala, attributing feelings toward the experienced reward.34 Working memory is seen as a platform where information temporarily can be held, manipulated, and then used to adjust goal-directed behavior.35,36 Studies of working memory have pointed to the involvement of the dlPFC, dorsal and anterior ACC, and parietal cortex.37 The dlPFC is associated with the integration and retrieval of information that is stored.38 The ACC is implicated in evaluative processes to adjust and adapt the received information depending on demand.39 In addition to fronto-limbic regions, the parietal cortex seems to be a workspace for processing sensory and perceptual information.40,41,42 In sum, tasks associated with emotion processing, reward processing, and working memory are all dependent on an adequate activation of fronto-limbic brain regions and can therefore be used to assess functioning in this network.
Although there is an increasing number of fMRI studies suggesting fronto-limbic functional abnormalities in individuals with BD, a meta-analysis specifically focusing on this brain network in individuals with BD, to our knowledge, has not yet been performed. A meta-analysis of fronto-limbic network activity in individuals with BD is important, as malfunctioning of this brain network can be considered as reflecting part of the pathophysiology of cognitive and emotional impairments in individuals with BD. Therefore, we conducted a systematic review and meta-analysis of fMRI studies in individuals with BD investigating emotion processing, reward processing, or working memory, domains that all rely on proper fronto-limbic network activity. By performing this systematic review and meta-analysis, we aimed to aggregate the evidence to be able to draw more rigorous conclusions regarding potential abnormalities in the fronto-limbic network in individuals with BD. Moreover, we wanted to elucidate whether trait (ie, euthymia) or state (ie, mania or depression) may be associated with potential fronto-limbic network alterations.
Methods
Literature Search and Selection
For the selection procedure, the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline43,44 was followed (eTable 1 in Supplement 1). The PRISMA flowchart depicting the process for the systematic literature search and selection of the studies is shown in the eFigure in Supplement 1. The literature search was conducted on February 21, 2017, and March 2, 2020, in the databases of PubMed, Embase, Web of Science, Cochrane Library, PsycInfo, Emcare, Academic Search Premier, and ScienceDirect. The following keywords were used in the literature search: bipolar disorder, manic depression, functional magnetic resonance imaging (fMRI), mania, and bipolar depression (eMethods in Supplement 1). Articles were eligible if written in English and with populations aged between 18 and 65 years. All control individuals were physically and neurologically healthy, with no current psychopathology. Exclusion criteria for all participants were medical or neurological illnesses that might influence brain function and any contraindications for receiving an MRI scan. Literature reviews, meta-analyses, methodological articles, case reports, letters, conference abstracts, and editorials were excluded. Selection of literature was conducted using 3 main inclusion criteria: task-related fMRI studies on BD with whole-brain analyses; studies had to include a task assessing at least 1 of the domains (ie, emotion processing, reward processing, or working memory); and studies that compared adult individuals with BD with healthy control individuals. fMRI studies using a region-of-interest analysis approach, those only assessing functional connectivity, and resting-state studies were excluded. Studies including only participants with high risk of BD, relatives with BD, offspring with BD, or BD in childhood (ie, younger than 18 years) were also excluded. Finally, the studies were grouped into at least 1 of the 3 domains. Data were analyzed from January 2021 to January 2022.
For each study, data were extracted (ie, first author, year of publication, mean age, sex, number of individuals with BD and healthy control individuals, mood state, contrasts, details about tasks, imaging results as coordinated clusters [x, y, z] in voxels, and details of the fMRI paradigm), which were used for description and further analyses. The fMRI studies were allocated to the 3 task domains (ie, emotion processing, reward processing, and working memory). Two fMRI studies examined 2 different domains and were therefore included in 2 different analyses.13,45
Statistical Analysis
Meta-analyses were conducted using GingerALE version 3.0.2.43,44 Differences in brain activation among regions associated with each domain were analyzed separately using the activation likelihood estimation method. The activation likelihood estimation approach uses modeling of activation locations (foci) by a 3-dimensional gaussian function, calculating the overlap of the distributions across experiments using the spatial uncertainty of the foci.44 It forms a probabilistic map of the likelihood that each voxel was activated by an experiment.
The analyses for each domain involved 2 analyses of contrasts. First, we analyzed the activation in brain areas that were more active in brains of individuals with BD compared to those of healthy control individuals, indicating hyperactivation in individuals with BD. Second, we analyzed the activation in brain areas that were more active in the brains of healthy control individuals compared to those of individuals with BD, indicating hypoactivation in individuals with BD. The voxel-level familywise error method (P = .05) was used for the correction of all analyses and contrasts of different domains. The number of threshold permutations was set at 1000, and the P value threshold at .05 with a minimum cluster size of suprathreshold voxels exceeding 100 mm3. Next, we performed sensitivity analyses focused on mood states. For each domain, we analyzed the effect of mood states separately (ie, euthymic, manic, and depressive). A similar procedure was followed for these analyses.
Results
In total, 49 whole-brain–based fMRI studies were included, which accrued 999 individuals with BD (551 [55.2%] female) and 1027 healthy control individuals (532 [51.8%] female). The included studies (per domain) and their clinical specifications are listed in eTable 2 in Supplement 1. All meta-analytical results are presented in Tables 1 and 2.
Table 1. Activation Likelihood Estimation Meta-analytical Results of Whole Brain–Based Studiesa.
| Anatomical label | Peak coordinates | BA | z Value | Cluster size, mm3 | ALE value | ||
|---|---|---|---|---|---|---|---|
| x | y | z | |||||
| Emotion processing domain | |||||||
| Bipolar disorder > healthy control | |||||||
| Left hippocampus | −18 | −14 | −10 | 28 | 6.70 | 864 | 0.029 |
| Right superior temporal gyrus | 48 | 14 | −10 | 38 | 6.06 | 176 | 0.025 |
| Left superior temporal gyrus | −56 | −16 | 6 | 41 | 5.86 | 176 | 0.025 |
| Left amygdala | −28 | −2 | −12 | NA | 5.52 | 160 | 0.022 |
| Healthy control > bipolar disorder | |||||||
| Right inferior frontal gyrus | 42 | 22 | 0 | 47 | 6.38 | 232 | 0.024 |
| Reward processing domain | |||||||
| Bipolar disorder > healthy control | |||||||
| Left orbitofrontal cortex | −46 | 30 | 0 | 47 | 6.22 | 344 | 0.024 |
| Working memory domain | |||||||
| Bipolar disorder > healthy control | |||||||
| Left subgenual anterior cingulate | −6 | 34 | −10 | 32 | 6.82 | 696 | 0.032 |
| Left ventromedial prefrontal cortex | −2 | 46 | −10 | 10 | 6.63 | 624 | 0.031 |
Abbreviations: ALE, activation likelihood estimation; BA, Brodmann area; NA, not applicable.
Talairach coordinates are reported. The voxel-level familywise error method (P = .05) was used for the correction of all analyses and contrasts of different domains. The number of threshold permutations was set at 1000 and the P value threshold at .05, with a minimum cluster size of suprathreshold voxels exceeding 100 mm3.
Table 2. Activation Likelihood Estimation Meta-analytical Results of Whole Brain–Based Studies per Mood Statea.
| Anatomical label | Peak coordinates | BA | z Value | Cluster size, mm3 | ALE value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Emotion processing domain | |||||||
| Euthymia | |||||||
| Bipolar disorder > healthy control | |||||||
| Left parahippocampal gyrus | −24 | −20 | −10 | 28 | 5.62 | 216 | 0.019 |
| Healthy control > bipolar disorder | |||||||
| Left inferior frontal gyrus | −56 | 10 | 26 | 9 | 5.28 | 168 | 0.014 |
| Mania | |||||||
| Bipolar disorder > healthy control | |||||||
| Left thalamus | −4 | −32 | 10 | NA | 5.91 | 312 | 0.018 |
| Healthy control > bipolar disorder | |||||||
| Right inferior frontal gyrus | 46 | 26 | −2 | 47 | 5.31 | 224 | 0.014 |
| Depression | |||||||
| Bipolar disorder > healthy control | |||||||
| Left angular gyrus (parietal lobe) | −32 | −56 | 32 | 39 | 5.63 | 104 | 0.018 |
| Reward processing domain | |||||||
| Euthymia | |||||||
| Bipolar disorder > healthy control | |||||||
| Left parahippocampal gyrus | −22 | −42 | −10 | 36 | 5.81 | 152 | 0.019 |
| Left anterior cingulate | −16 | 42 | 0 | 32 | 5.81 | 152 | 0.019 |
| Left medial frontal gyrus | −16 | 48 | −6 | 10 | 5.81 | 152 | 0.019 |
| Right middle temporal gyrus | 60 | −4 | −8 | 21 | 5.81 | 152 | 0.019 |
| Depression | |||||||
| Bipolar disorder > healthy control | |||||||
| Left inferior frontal gyrus | −48 | 28 | −1 | 47 | 5.46 | 100 | 0.017 |
| Right superior temporal gyrus | 48 | −22 | −2 | 22 | 5.65 | 100 | 0.018 |
| Working memory domain | |||||||
| Mania | |||||||
| Bipolar disorder > healthy control | |||||||
| Left anterior cingulate | −6 | 40 | 4 | 32 | 6.26 | 400 | 0.022 |
| Healthy control > bipolar disorder | |||||||
| Left middle frontal gyrus | −30 | −6 | 56 | 6 | 8.30 | 624 | 0.037 |
| Depression | |||||||
| Bipolar disorder > healthy control | |||||||
| Left prefrontal cortex | −2 | 46 | −10 | 10 | 7.18 | 504 | 0.028 |
| Left anterior cingulate | −4 | 36 | −4 | 32 | 5.76 | 112 | 0.019 |
| Healthy control > bipolar disorder | |||||||
| Right parietal lobe | 8 | −66 | 56 | 7 | 5.70 | 112 | 0.018 |
| Left cerebellum | −30 | −52 | −32 | NA | 5.71 | 104 | 0.019 |
Abbreviations: ALE, activation likelihood estimation; BA, Brodmann area; NA, not applicable.
Talairach coordinates are reported. The voxel-level familywise error method (P = .05) was used for the correction of all analyses and contrasts of different domains. The number of threshold permutations was set at 1000 and the P value threshold at .05, with a minimum cluster size of suprathreshold voxels exceeding 100 mm3.
Emotion Processing
For emotion processing, 20 studies were included, with a total of 324 individuals with BD (155 [47.8%] female) and 369 healthy control individuals (170 [46.1%] female). Individuals with BD had different mood states; 116 (35.8%) were in a euthymic state, 70 (21.4%) were in a depressive state, 44 (13.6%) were in a manic state, 1 (0.3%) was in a mixed state, and state was not specified for the remaining 93 (28.7%). The emotion processing domain included emotion tasks with a variety of paradigms, including emotionally salient stimuli (eg, affect induction, emotional perception of facial emotions or prosody, emotion regulation, emotion recognition, emotion memory, and inhibition tasks).
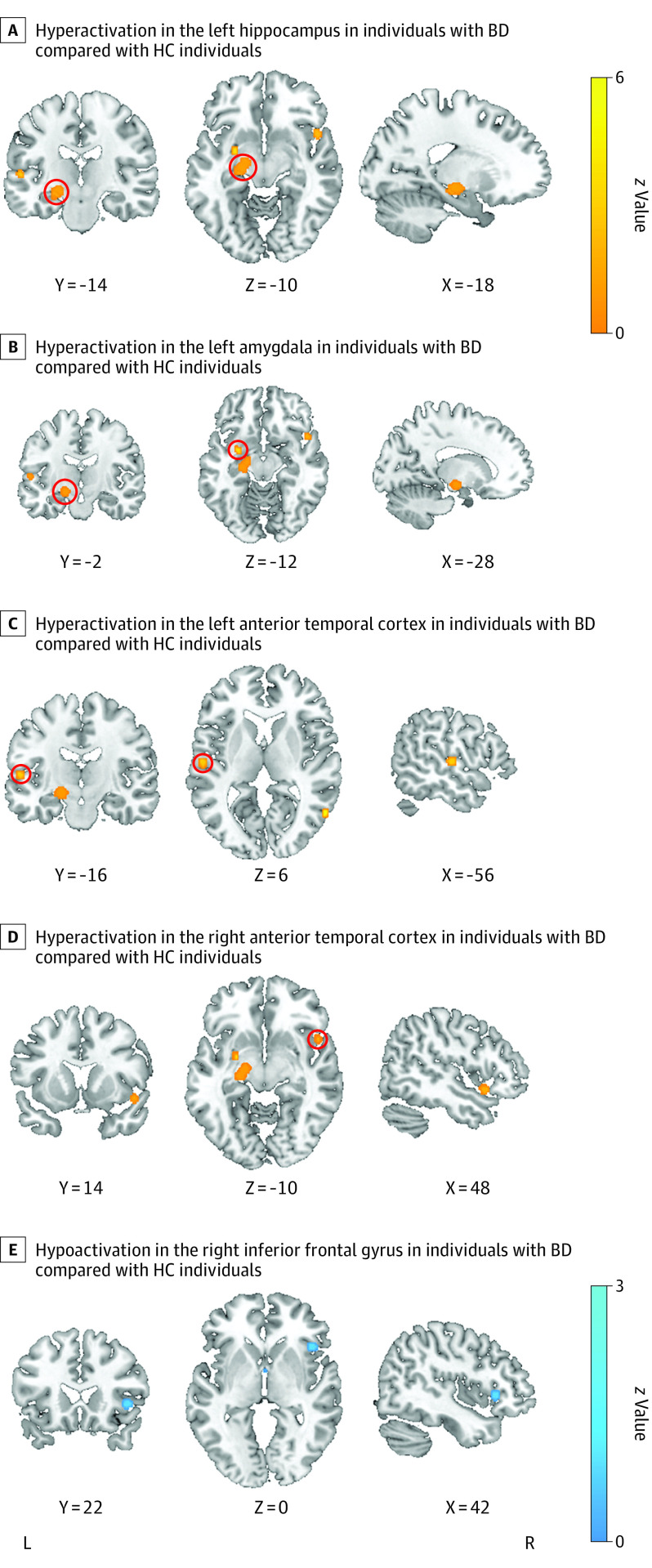
Hyperactivation was shown in all individuals with BD compared with healthy control individuals (Table 1) in part of the left hippocampus, the left and right anterior temporal cortices, and the left amygdala (Figure 1A-D). Hypoactivation in individuals with BD was found only in the right inferior frontal gyrus (IFG) compared to healthy control individuals (Figure 1E).
Figure 1. Meta-analytic Maps of Brain Functional Changes in the Emotion Processing Domain.
Red circles show area of hyperactivation. BD indicates bipolar disorder; HC, healthy control.
The results regarding mood states (Table 2) revealed hyperactivation in the left parahippocampal gyrus and hypoactivation in the left IFG in individuals with BD who were in a euthymic state. During mania, we found hyperactivation in the left thalamus and hypoactivation in the right IFG in individuals with BD. Individuals with BD showed hyperactivation in the left parietal lobe during depressive state.
Reward Processing
Nine studies on reward processing were included, with a total of 195 individuals with BD (129 [66.2%] female) and 213 healthy control individuals (111 [52.1%] female). In this selection of studies, 117 individuals with BD (60.0%) were in a euthymic state, 65 (33.3%) were in a depressive state, 10 (5.1%) were in a manic state, and 3 (1.5%) were in a mixed state. The reward processing domain paradigms included monetary incentive, gambling, card number guessing, and Roulette tasks.
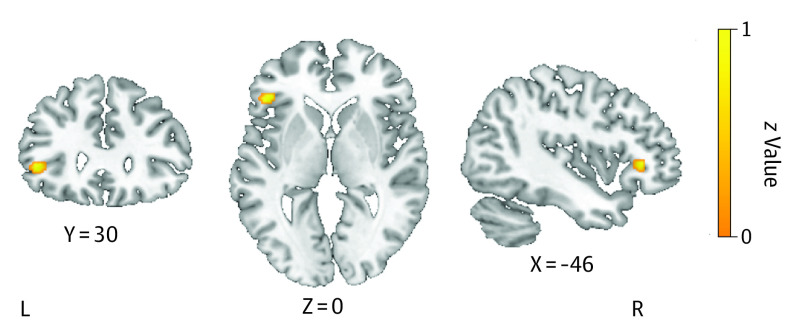
Taking all individuals with BD together (Table 1), only hyperactivation in the left OFC was shown compared to healthy control individuals (Figure 2). For the euthymic mood state in individuals with BD, hyperactivation was found in the left parahippocampal gyrus, ACC, MFG, and right temporal gyrus compared to healthy control individuals. Individuals who were in a depressive mood state showed hyperactivation in the left IFG and in the right superior temporal gyrus (Table 2). A meta-analysis in individuals with BD in a manic state could not be performed due to a lack of power.
Figure 2. Meta-analytic Maps of Brain Functional Changes in the Reward Processing Domain.
Hyperactivation in the left orbitofrontal cortex in individuals with bipolar disorder compared with healthy control individuals.
Working Memory
We included 20 studies with 530 individuals with BD (267 [50.4%] female) and 417 healthy control individuals (251 [60.2%] female) for working memory. There were 178 individuals (36.1%) in a euthymic state, 171 (34.7%) in a depressive state, 124 (25.1%) in a manic state, and state was not specified for 57 (10.8%). Most of the studies used a letter n–back task, and a few studies a delayed match to sample task.
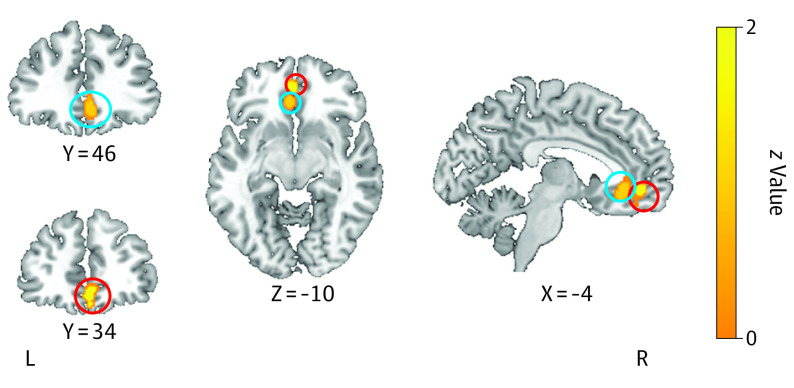
Overall, hyperactivation in individuals with BD was found in the left subgenual ACC and ventromedial PFC (Figure 3) compared to healthy control individuals (Table 1). With regard to mood states, no difference in individuals with BD in a euthymic state were found compared to healthy control individuals. Individuals who were in a manic state showed hyperactivity in the left ACC and hypoactivation in the left IFG during working memory. Individuals with BD in a depressed state showed hyperactivation in the left PFC and ACC, and hypoactivation was found in the right parietal lobe and left cerebellum (Table 2).
Figure 3. Meta-analytic Maps of Brain Functional Changes in the Working Memory Domain.
Hyperactivation in the left subgenual anterior cingulate (blue circles) and ventromedial prefrontal cortex (red circles) in individuals with bipolar disorder compared with healthy control individuals.
Discussion
In the current systematic review and meta-analysis, we investigated brain functioning in individuals with BD compared to that of healthy control individuals within cognitive domains related to emotion processing, working memory, and reward processing. Our findings revealed significant differences in brain activity in individuals with BD, mostly within the fronto-limbic network. Specifically, limbic activation alterations were only manifest in individuals with BD in a euthymic state, whereas more widespread frontal dysfunctions were also found during depression and mania. As such, aberrant limbic activity during cognitive and emotion processing may be a trait-associated BD characteristic; on the other hand, disruptions in frontal cortex activity may be associated with state-related factors.
During emotion processing, we found that individuals with BD showed hyperactivation in the hippocampus, parts of the temporal cortex, and amygdala and hypoactivation in the right IFG (as part of the PFC) when all affective states were pooled together. This is in line with an earlier meta-analysis7 and a systematic review11 that focused on emotion processing and found abnormal activity in the fronto-limbic network. Our results regarding the emotion processing domain can be functionally interpreted. For instance, amygdala hyperactivation may be interpreted as reflecting a state of oversensitivity, resulting in increased amygdala responses even when there is no need for such a response. The amygdala has a crucial role in emotion generation (ie, perception and arousal), identification of emotional stimuli,46,47,48 and emotion regulation.49 In addition to amygdala hyperactivation during emotion processing, we also found increased activation in the hippocampus. Besides its crucial role in memory, this region is also involved in socioemotional processing and the production of affective states.48,50 Further, hyperactivation was found in the temporal cortex, which is notably involved in social and emotion processing, recognition, and semantic memory.51 Our results regarding emotion processing also encompassed hypoactivation in the IFG, which is known to be associated with inhibitory control.52 Inhibition is a major subcomponent of executive function and is defined as the ability to suppress the process of irrelevant stimuli and dominant response when appropriate.53 The inability to inhibit responses is, among other things, associated with impulsive behavior.54 Moreover, individuals with BD might have difficulties in the identification of emotional stimuli (either negative or positive), leading to increased arousal and making it more difficult to regulate emotions; this in turn may provoke mood episodes.55 From a network perspective, hypoactivity in inhibitory structures, such as the IFG, might be related to hyperactivity in the whole network related to the fronto-limbic system (as found here in the hippocampus, parts of the temporal cortex, and amygdala) that would normally be inhibited.56
For reward processing, our results showed hyperactivation in the OFC in individuals with BD. OFC activity is important for pleasure coding as well as reward outcome and for processing the experience of hyperhedonia.32 This region forms the key in a hypersensitivity model of reward processing that was introduced based on a behavioral approach system hypothesis for individuals with BD.57 The model of the behavioral approach system refers to the hypothesis that individuals with BD have a hypersensitive reward system that leads to overreaction or underreaction to reward-related cues. It states that excessive reward system activation leads to manic symptoms, whereas excessive deactivation gives rise to depressive symptoms.57 This model is thought to be associated with hyperdopaminergia, which leads to high-risk, high–reward-seeking behavior observed during mania.58 Our finding of OFC hyperactivation in individuals with BD is in line with this reward hypersensitivity model of BD.
To our best knowledge, no previous meta-analyses of neuroimaging studies have focused on the reward system in individuals with BD. To date, only a systematic review on imaging findings during reward processing in unaffected first-degree relatives has been performed.59 Although relatives are nonaffected and do not have symptoms, these genetically at-risk individuals seem to show reward-related activity dysfunction similar to individuals with BD, that is, increased activity in the OFC in response to reward.59 The fact that the current systematic review and meta-analysis found a similar OFC activity pattern in a large sample of individuals with BD as found in healthy relatives underlines the importance of these aberrations, as they may serve as an important element in the pathological pathway toward BD.
For working memory tasks, our results showed hyperactivation in the subgenual ACC and ventromedial PFC in individuals with BD compared to healthy control individuals. Interestingly, these regions are connected to limbic structures and are functionally involved in reward valuation and emotion regulation, but recent studies highlight their role in working memory as well. Both subgenual ACC and ventromedial PFC play an important role in integrating cognitive and emotional stimuli. For example, the ventromedial PFC structurally connects the amygdala with the dlPFC and functionally regulates the influencing effects of capacity-exceeding working memory load from the dlPFC and the mediating deleterious effects of emotional interference on cognitive processing in the amygdala.60,61 In addition, the subgenual ACC is seen as another bridge between the dlPFC and amygdala and plays a role in emotion processing and attention.62 The interconnection of these 2 regions to the dlPFC and the amygdala facilitates interactions between emotion and cognition.61 Our results provide further support for the potential role of dysregulated mPFC and subgenual ACC activity as a direct contributor to poor working memory performance and deficiencies in emotional processing in individuals with BD.
In addition to the whole BD group, analyses were also performed by mood state. Limbic hyperactivity was only found in individuals with BD in a euthymic state (parahippocampal), whereas abnormalities in frontal activation, although with a more widespread pattern, were also revealed during states of depression and mania. A tentative conclusion can be drawn that limbic hyperactivation during emotion and cognitive processing in individuals with BD may be a trait-dependent characteristic, whereas frontal cortex dysfunction may also be affected during states in individuals with BD. Functionally, failed frontal inhibitory control may be more pronounced when individuals experience a depressive or manic episode, while the aforementioned increased limbic oversensitivity may only occur during euthymia and may be a risk factor for provoking mood states.55 Given power constraints, these conclusions should be interpreted with caution. It can therefore not be ruled out that increased limbic activity could be the case during mania or depression; however, the current systematic review and meta-analysis shows that frontal hypoactivation may be a more robust state-dependent finding. One could hypothesize that individuals with BD take high-dose or other medication during affective states compared to when they are in a euthymic state, which may normalize limbic activity.63
Two earlier meta-analyses with smaller numbers of inclusions investigated task fMRI studies in individuals with BD.7,64 Our findings regarding emotional processing are consistent with limbic hyperactivation and IFG hypoactivation as found in 1 meta-analysis.7 However, all kinds of paradigms related to a variety of cognitive functions were included, while the current systematic review and meta-analysis focused on working memory and emotion and reward processing with regard to the hypothesis of impaired fronto-limbic network activity in individuals with BD specifically. One other meta-analysis focused on the comparison between youth with BD and adults with BD.64 Similar amygdala hyperactivation during emotional tasks and ACC hyperactivation during nonemotional tasks was found in youth with BD, which underlines the important role of these brain areas in the psychopathology of BD and suggests common trait-like abnormalities.
To the best of our knowledge, this is the first systematic review and meta-analysis showing robust fronto-limbic network abnormalities during emotion and cognitive functioning. The differentiation of 3 cognitive domains in association with fronto-limbic network functioning in individuals with BD allowed a better perspective on how neurocognitive abnormalities can coexist in parallel.
Limitations
Some limitations need to be noted. First, although significant results were revealed in the analyses per mood state, the number of individuals with BD for the different states was limited. As mentioned above, this may result in the negative finding of unincreased limbic activity during affective states. Future studies specifically focusing on state-associated emotional and cognitive functioning are required to increase the power of meta-analyses. Second, we were unable to perform sensitivity analyses and disentangle the potential effects of psychopharmaceuticals due to heterogeneity in medication.65 Most individuals were treated with mood stabilizers as monotherapy or in combination with other psychotropics. However, a review in individuals with BD found no significant effect of medication on brain activation.66 Third, clinical heterogeneity and demographic features often make it complicated to compare across studies. To obtain generalizable results, we included a broad range of studies, conditions, and multiple contrasts in 3 domains of interest, although individuals with cognitive impairment were excluded. Meta-analytic results help one to draw overriding conclusions and identify consistencies in the literature despite heterogeneity, though they might lack specificity as to the nature of any aberration. A further limitation is that we could not correct for specific participant factors, including symptomatology, such as psychosis. A few studies measured and reported psychotic symptoms, while others did not. Because of the lack of information, we did not model sex, medication, and comorbidity, factors that might be associated with brain activity in individuals with BD. However, it is known that particular mood states are associated with confounding fMRI results, specifically in the fronto-limbic network.67 In the current analyses, we were able to tackle this important factor.
Conclusion
To our knowledge, the current study is the first systematic review and meta-analysis in individuals with BD investigating brain activity during cognitive and emotional tasks that demand proper fronto-limbic functioning. Studies have reported that the fronto-limbic network was affected in individuals with BD, both in the euthymic state and in symptomatic individuals. Regarding reward processing specifically, more studies are needed to replicate and expand our findings. Moreover, fMRI studies in individuals with BD would benefit from the standardization of reward paradigms. The field may be furthered by using novel approaches, such as multimodal analyses and pattern-recognition techniques. These advances have the potential to increase the clinical and scientific relevance of reward-processing fMRI paradigms in individuals with BD, which may result in their use during diagnostics or in investigating therapeutic targets. Longitudinal fMRI studies monitoring at-risk individuals as well as individuals with first-onset BD are needed to examine the development of cognitive impairment and its association with fronto-limbic findings over the course of BD.
eMethods. Literature search
eTable 1. PRISMA 2020 Checklist
eFigure. PRISMA Flow chart
eTable 2. Descriptives of included fMRI studies
eReferences
Data sharing statement
References
- 1.Judd LL, Akiskal HS, Schettler PJ, et al. The comparative clinical phenotype and long term longitudinal episode course of bipolar I and II: a clinical spectrum or distinct disorders? J Affect Disord. 2003;73(1-2):19-32. doi: 10.1016/S0165-0327(02)00324-5 [DOI] [PubMed] [Google Scholar]
- 2.Grande I, Berk M, Birmaher B, Vieta E. Bipolar disorder. Lancet. 2016;387(10027):1561-1572. doi: 10.1016/S0140-6736(15)00241-X [DOI] [PubMed] [Google Scholar]
- 3.Martínez-Arán A, Vieta E, Colom F, et al. Cognitive impairment in euthymic bipolar patients: implications for clinical and functional outcome. Bipolar Disord. 2004;6(3):224-232. doi: 10.1111/j.1399-5618.2004.00111.x [DOI] [PubMed] [Google Scholar]
- 4.Bora E, Yucel M, Pantelis C. Cognitive endophenotypes of bipolar disorder: a meta-analysis of neuropsychological deficits in euthymic patients and their first-degree relatives. J Affect Disord. 2009;113(1-2):1-20. doi: 10.1016/j.jad.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 5.Robinson LJ, Thompson JM, Gallagher P, et al. A meta-analysis of cognitive deficits in euthymic patients with bipolar disorder. J Affect Disord. 2006;93(1-3):105-115. doi: 10.1016/j.jad.2006.02.016 [DOI] [PubMed] [Google Scholar]
- 6.Kohn N, Eickhoff SB, Scheller M, Laird AR, Fox PT, Habel U. Neural network of cognitive emotion regulation–an ALE meta-analysis and MACM analysis. Neuroimage. 2014;87:345-355. doi: 10.1016/j.neuroimage.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen CH, Suckling J, Lennox BR, Ooi C, Bullmore ET. A quantitative meta-analysis of fMRI studies in bipolar disorder. Bipolar Disord. 2011;13(1):1-15. doi: 10.1111/j.1399-5618.2011.00893.x [DOI] [PubMed] [Google Scholar]
- 8.Strakowski SM, Adler CM, Holland SK, Mills NP, DelBello MP, Eliassen JC. Abnormal fMRI brain activation in euthymic bipolar disorder patients during a counting Stroop interference task. Am J Psychiatry. 2005;162(9):1697-1705. doi: 10.1176/appi.ajp.162.9.1697 [DOI] [PubMed] [Google Scholar]
- 9.Strakowski SM, Delbello MP, Adler CM. The functional neuroanatomy of bipolar disorder: a review of neuroimaging findings. Mol Psychiatry. 2005;10(1):105-116. doi: 10.1038/sj.mp.4001585 [DOI] [PubMed] [Google Scholar]
- 10.Bi B, Che D, Bai Y. Neural network of bipolar disorder: Toward integration of neuroimaging and neurocircuit-based treatment strategies. Transl Psychiatry. 2022;12(1):143. doi: 10.1038/s41398-022-01917-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Townsend J, Altshuler LL. Emotion processing and regulation in bipolar disorder: a review. Bipolar Disord. 2012;14(4):326-339. doi: 10.1111/j.1399-5618.2012.01021.x [DOI] [PubMed] [Google Scholar]
- 12.Chase HW, Nusslock R, Almeida JR, Forbes EE, LaBarbara EJ, Phillips ML. Dissociable patterns of abnormal frontal cortical activation during anticipation of an uncertain reward or loss in bipolar versus major depression. Bipolar Disord. 2013;15(8):839-854. doi: 10.1111/bdi.12132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jogia J, Dima D, Kumari V, Frangou S. Frontopolar cortical inefficiency may underpin reward and working memory dysfunction in bipolar disorder. World J Biol Psychiatry. 2012;13(8):605-615. doi: 10.3109/15622975.2011.585662 [DOI] [PubMed] [Google Scholar]
- 14.Nusslock R, Almeida JR, Forbes EE, et al. Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord. 2012;14(3):249-260. doi: 10.1111/j.1399-5618.2012.01012.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler CM, Holland SK, Schmithorst V, Tuchfarber MJ, Strakowski SM. Changes in neuronal activation in patients with bipolar disorder during performance of a working memory task. Bipolar Disord. 2004;6(6):540-549. doi: 10.1111/j.1399-5618.2004.00117.x [DOI] [PubMed] [Google Scholar]
- 16.Drapier D, Surguladze S, Marshall N, et al. Genetic liability for bipolar disorder is characterized by excess frontal activation in response to a working memory task. Biol Psychiatry. 2008;64(6):513-520. doi: 10.1016/j.biopsych.2008.04.038 [DOI] [PubMed] [Google Scholar]
- 17.Thermenos HW, Goldstein JM, Milanovic SM, et al. An fMRI study of working memory in persons with bipolar disorder or at genetic risk for bipolar disorder. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(1):120-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Monks PJ, Thompson JM, Bullmore ET, et al. A functional MRI study of working memory task in euthymic bipolar disorder: evidence for task-specific dysfunction. Bipolar Disord. 2004;6(6):550-564. doi: 10.1111/j.1399-5618.2004.00147.x [DOI] [PubMed] [Google Scholar]
- 19.Townsend J, Bookheimer SY, Foland-Ross LC, Sugar CA, Altshuler LL. fMRI abnormalities in dorsolateral prefrontal cortex during a working memory task in manic, euthymic and depressed bipolar subjects. Psychiatry Res. 2010;182(1):22-29. doi: 10.1016/j.pscychresns.2009.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamilton LS, Altshuler LL, Townsend J, et al. Alterations in functional activation in euthymic bipolar disorder and schizophrenia during a working memory task. Hum Brain Mapp. 2009;30(12):3958-3969. doi: 10.1002/hbm.20820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frangou S, Kington J, Raymont V, Shergill SS. Examining ventral and dorsal prefrontal function in bipolar disorder: a functional magnetic resonance imaging study. Eur Psychiatry. 2008;23(4):300-308. doi: 10.1016/j.eurpsy.2007.05.002 [DOI] [PubMed] [Google Scholar]
- 22.Townsend JD, Torrisi SJ, Lieberman MD, Sugar CA, Bookheimer SY, Altshuler LL. Frontal-amygdala connectivity alterations during emotion downregulation in bipolar I disorder. Biol Psychiatry. 2013;73(2):127-135. doi: 10.1016/j.biopsych.2012.06.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krabbendam L, Arts B, van Os J, Aleman A. Cognitive functioning in patients with schizophrenia and bipolar disorder: a quantitative review. Schizophr Res. 2005;80(2-3):137-149. doi: 10.1016/j.schres.2005.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Arts B, Jabben N, Krabbendam L, van Os J. Meta-analyses of cognitive functioning in euthymic bipolar patients and their first-degree relatives. Psychol Med. 2008;38(6):771-785. doi: 10.1017/S0033291707001675 [DOI] [PubMed] [Google Scholar]
- 25.Koenders MA, Dodd AL, Karl A, Green MJ, Elzinga BM, Wright K. Understanding bipolar disorder within a biopsychosocial emotion dysregulation framework. J Affect Disord Rep. 2020;2:100031. doi: 10.1016/j.jadr.2020.100031 [DOI] [Google Scholar]
- 26.Dickstein DP, Finger EC, Skup M, Pine DS, Blair JR, Leibenluft E. Altered neural function in pediatric bipolar disorder during reversal learning. Bipolar Disord. 2010;12(7):707-719. doi: 10.1111/j.1399-5618.2010.00863.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuhr K, Hautzinger M, Meyer TD. Implicit motives and cognitive variables: specific links to vulnerability for unipolar or bipolar disorder. Psychiatry Res. 2014;215(1):61-68. doi: 10.1016/j.psychres.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 28.Johnson SL, Carver CS. Extreme goal setting and vulnerability to mania among undiagnosed young adults. Cognit Ther Res. 2006;30(3):377-395. doi: 10.1007/s10608-006-9044-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nusslock R, Young CB, Damme KSF. Elevated reward-related neural activation as a unique biological marker of bipolar disorder: assessment and treatment implications. Behav Res Ther. 2014;62:74-87. doi: 10.1016/j.brat.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Morawetz C, Bode S, Derntl B, Heekeren HR. The effect of strategies, goals and stimulus material on the neural mechanisms of emotion regulation: a meta-analysis of fMRI studies. Neurosci Biobehav Rev. 2017;72:111-128. doi: 10.1016/j.neubiorev.2016.11.014 [DOI] [PubMed] [Google Scholar]
- 31.Schultz W. Neuronal reward and decision signals: from theories to data. Physiol Rev. 2015;95(3):853-951. doi: 10.1152/physrev.00023.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berridge KC, Kringelbach ML. Pleasure systems in the brain. Neuron. 2015;86(3):646-664. doi: 10.1016/j.neuron.2015.02.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219-1236. doi: 10.1016/j.neubiorev.2010.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haber SN, Knutson B. The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology. 2010;35(1):4-26. doi: 10.1038/npp.2009.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chatham CH, Badre D. Multiple gates on working memory. Curr Opin Behav Sci. 2015;1:23-31. doi: 10.1016/j.cobeha.2014.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vogel EK, Machizawa MG. Neural activity predicts individual differences in visual working memory capacity. Nature. 2004;428(6984):748-751. doi: 10.1038/nature02447 [DOI] [PubMed] [Google Scholar]
- 37.Dima D, Jogia J, Frangou S. Dynamic causal modeling of load-dependent modulation of effective connectivity within the verbal working memory network. Hum Brain Mapp. 2014;35(7):3025-3035. doi: 10.1002/hbm.22382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai WJ, Abd Hamid AI, Abdullah JM. Working memory from the psychological and neurosciences perspectives: a review. Front Psychol. 2018;9:401. doi: 10.3389/fpsyg.2018.00401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Osaka M, Osaka N, Kondo H, et al. The neural basis of individual differences in working memory capacity: an fMRI study. Neuroimage. 2003;18(3):789-797. doi: 10.1016/S1053-8119(02)00032-0 [DOI] [PubMed] [Google Scholar]
- 40.Braver TS, Bongiolatti SR. The role of frontopolar cortex in subgoal processing during working memory. Neuroimage. 2002;15(3):523-536. doi: 10.1006/nimg.2001.1019 [DOI] [PubMed] [Google Scholar]
- 41.Rodriguez Merzagora AC, Izzetoglu M, Onaral B, Schultheis MT. Verbal working memory impairments following traumatic brain injury: an fNIRS investigation. Brain Imaging Behav. 2014;8(3):446-459. doi: 10.1007/s11682-013-9258-8 [DOI] [PubMed] [Google Scholar]
- 42.Andersen RA, Cui H. Intention, action planning, and decision making in parietal-frontal circuits. Neuron. 2009;63(5):568-583. doi: 10.1016/j.neuron.2009.08.028 [DOI] [PubMed] [Google Scholar]
- 43.Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. Neuroimage. 2012;59(3):2349-2361. doi: 10.1016/j.neuroimage.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp. 2009;30(9):2907-2926. doi: 10.1002/hbm.20718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Moser DA, Doucet GE, Lee WH, et al. Multivariate associations among behavioral, clinical, and multimodal imaging phenotypes in patients with psychosis. JAMA Psychiatry. 2018;75(4):386-395. doi: 10.1001/jamapsychiatry.2017.4741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Anderson AK. Feeling emotional: the amygdala links emotional perception and experience. Soc Cogn Affect Neurosci. 2007;2(2):71-72. doi: 10.1093/scan/nsm022 [DOI] [Google Scholar]
- 47.Bradley MM, Sabatinelli D, Lang PJ, Fitzsimmons JR, King W, Desai P. Activation of the visual cortex in motivated attention. Behav Neurosci. 2003;117(2):369-380. doi: 10.1037/0735-7044.117.2.369 [DOI] [PubMed] [Google Scholar]
- 48.Phillips ML, Drevets WC, Rauch SL, Lane R. Neurobiology of emotion perception II: implications for major psychiatric disorders. Biol Psychiatry. 2003;54(5):515-528. doi: 10.1016/S0006-3223(03)00171-9 [DOI] [PubMed] [Google Scholar]
- 49.Banks SJ, Eddy KT, Angstadt M, Nathan PJ, Phan KL. Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci. 2007;2(4):303-312. doi: 10.1093/scan/nsm029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Amunts K, Kedo O, Kindler M, et al. Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps. Anat Embryol (Berl). 2005;210(5-6):343-352. doi: 10.1007/s00429-005-0025-5 [DOI] [PubMed] [Google Scholar]
- 51.Ding SL, Van Hoesen GW, Cassell MD, Poremba A. Parcellation of human temporal polar cortex: a combined analysis of multiple cytoarchitectonic, chemoarchitectonic, and pathological markers. J Comp Neurol. 2009;514(6):595-623. doi: 10.1002/cne.22053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Green MJ, Cahill CM, Malhi GS. The cognitive and neurophysiological basis of emotion dysregulation in bipolar disorder. J Affect Disord. 2007;103(1-3):29-42. doi: 10.1016/j.jad.2007.01.024 [DOI] [PubMed] [Google Scholar]
- 53.Logan GD, Cowan WB. On the ability to inhibit thought and action: a theory of an act of control. Psychol Rev. 1984;91(3):295. doi: 10.1037/0033-295X.91.3.295 [DOI] [PubMed] [Google Scholar]
- 54.Morsel AM, Dhar M, Hulstijn W, Temmerman A, Morrens M, Sabbe B. Inhibitory control in euthymic bipolar disorder: event related potentials during a go/nogo task. Clin Neurophysiol. 2017;128(4):520-528. doi: 10.1016/j.clinph.2016.12.006 [DOI] [PubMed] [Google Scholar]
- 55.García-Blanco AC, Perea M, Salmerón L. Attention orienting and inhibitory control across the different mood states in bipolar disorder: an emotional antisaccade task. Biol Psychol. 2013;94(3):556-561. doi: 10.1016/j.biopsycho.2013.10.005 [DOI] [PubMed] [Google Scholar]
- 56.Zhang L, Wu H, Zhang A, et al. Aberrant brain network topology in the frontoparietal-limbic circuit in bipolar disorder: a graph-theory study. Eur Arch Psychiatry Clin Neurosci. 2021;271(7):1379-1391. doi: 10.1007/s00406-020-01219-7 [DOI] [PubMed] [Google Scholar]
- 57.Alloy LB, Olino T, Freed RD, Nusslock R. Role of reward sensitivity and processing in major depressive and bipolar spectrum disorders. Behav Ther. 2016;47(5):600-621. doi: 10.1016/j.beth.2016.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Burdick KE, Braga RJ, Gopin CB, Malhotra AK. Dopaminergic influences on emotional decision making in euthymic bipolar patients. Neuropsychopharmacology. 2014;39(2):274-282. doi: 10.1038/npp.2013.177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miskowiak KW, Kjærstad HL, Meluken I, et al. The search for neuroimaging and cognitive endophenotypes: a critical systematic review of studies involving unaffected first-degree relatives of individuals with bipolar disorder. Neurosci Biobehav Rev. 2017;73:1-22. doi: 10.1016/j.neubiorev.2016.12.011 [DOI] [PubMed] [Google Scholar]
- 60.Zhang L, Ai H, Opmeer EM, et al. Distinct temporal brain dynamics in bipolar disorder and schizophrenia during emotion regulation. Psychol Med. 2020;50(3):413-421. doi: 10.1017/S0033291719000217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Farruggia MC, Laird AR, Mattfeld AT. Common default mode network dysfunction across psychopathologies: a neuroimaging meta-analysis of the n-back working memory paradigm. bioRxiv. Published online January 31, 2020:2020.01.30.927210. doi: 10.1101/2020.01.30.927210 [DOI]
- 62.Zhang FF, Peng W, Sweeney JA, Jia ZY, Gong QY. Brain structure alterations in depression: psychoradiological evidence. CNS Neurosci Ther. 2018;24(11):994-1003. doi: 10.1111/cns.12835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet. 2002;359(9302):241-247. doi: 10.1016/S0140-6736(02)07450-0 [DOI] [PubMed] [Google Scholar]
- 64.Wegbreit E, Cushman GK, Puzia ME, et al. Developmental meta-analyses of the functional neural correlates of bipolar disorder. JAMA Psychiatry. 2014;71(8):926-935. doi: 10.1001/jamapsychiatry.2014.660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Phillips ML, Swartz HA. A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am J Psychiatry. 2014;171(8):829-843. doi: 10.1176/appi.ajp.2014.13081008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Phillips ML, Travis MJ, Fagiolini A, Kupfer DJ. Medication effects in neuroimaging studies of bipolar disorder. Am J Psychiatry. 2008;165(3):313-320. doi: 10.1176/appi.ajp.2007.07071066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Norwood MF, Lakhani A, Maujean A, Zeeman H, Creux O, Kendall E. Brain activity, underlying mood and the environment: a systematic review. J Environ Psychol. 2019;65:101321. doi: 10.1016/j.jenvp.2019.101321 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Literature search
eTable 1. PRISMA 2020 Checklist
eFigure. PRISMA Flow chart
eTable 2. Descriptives of included fMRI studies
eReferences
Data sharing statement