Key Points
Question
What is the quality of life and functional outcome of patients with rectal cancer following a watch-and-wait approach after neoadjuvant chemoradiotherapy or radiotherapy?
Findings
In this cohort study of 278 patients with rectal cancer following a watch-and-wait approach, all reported good quality of life, approximately one-quarter reported bowel dysfunction during follow-up, 31.8% of male patients reported severe erectile dysfunction at 24 months, and female patients reported decreased sexual function during follow-up. Quality of life and functional outcome deteriorated when patients required surgery.
Meaning
Although functional problems were observed, patients with rectal cancer following a watch-and-wait approach after neoadjuvant chemoradiotherapy or radiotherapy reported good quality of life.
Abstract
Importance
A watch-and-wait approach for patients with rectal cancer and a clinical complete response after neoadjuvant chemoradiotherapy or radiotherapy is associated with better quality of life and functional outcome. Nevertheless, prospective data on both parameters are scarce.
Objective
To prospectively evaluate quality of life and functional outcome, including bowel, urinary, and sexual function, of patients following a watch-and-wait approach.
Design, Setting, and Participants
A total of 278 patients with rectal cancer and a clinical complete response or near-complete response after neoadjuvant chemoradiotherapy or radiotherapy were included in 2 prospective cohort studies: a single-center study (March 2014 to October 2017) and an ongoing multicenter study (from September 2017). Patients were observed by a watch-and-wait approach. Additional local excision or total mesorectal excision was performed for residual disease or regrowth. Data were analyzed between April 1, 2021, and August 27, 2021, for patients with a minimum follow-up of 24 months.
Main Outcomes and Measures
Quality of life was evaluated with the European Organisation for Research and Treatment of Cancer–Quality of Life Questionnaire–C30 (EORTC-QLQ-C30), EORTC-QLQ-CR38, or EORTC-QLQ-CR29 and 36-Item Short-Form Health Survey. The score for the questionnaires and 36-Item Short-Form Health Survey ranges from 0 to 100. For some scales, a high score indicates a high level of functioning, and for others it indicates a high level of complaints and symptomatology. Functional outcome was assessed by the Low Anterior Resection Syndrome score, Vaizey incontinence score, International Prostate Symptom Score, International Index of Erectile Function, and Female Sexual Function Index.
Results
Of 278 patients included, 187 were male (67%), and the median age was 66 years (range, 34-85 years). In the first 24 months, 221 patients (80%) were observed by a watch-and-wait approach without requiring surgery, 18 patients (6%) underwent additional local excision, and 39 patients (14%) underwent total mesorectal excision. In general, patients observed by a watch-and-wait approach reported good quality of life, with limited variation over time. At 3 months, 56 of 221 patients (25.3%) reported major bowel dysfunction; at 12 months, 53 patients (24.0%) reported it; and at 24 months, 55 patients (24.9%) reported it. At 24 months, 48 of 151 male patients (31.8%) reported severe erectile dysfunction. For female patients, sexual satisfaction and overall sexual function decreased during follow-up. Patients who underwent local excision reported more major bowel dysfunction (10 of 18 patients [55.6%]) compared with those without additional surgery. Quality-of-life scores, however, were comparable. After total mesorectal excision, patients scored significantly worse on several quality-of-life subscales.
Conclusions and Relevance
Results of this study suggest that patients with rectal cancer who were observed by a watch-and-wait approach had good quality of life, with some patients reporting bowel and sexual dysfunction. Quality of life and functional outcome deteriorated when patients required surgery. These data will be useful in daily care to counsel patients on what to expect from a watch-and-wait approach.
This cohort study assesses quality of life and functional outcome of patients with rectal cancer who are followed up via a watch-and-wait approach.
Introduction
In 2004, the first report appeared about the watch-and-wait (WW) approach for patients with rectal cancer and a clinical complete response after neoadjuvant chemoradiotherapy.1 Patients treated by this nonoperative approach were observed by strict surveillance, potentially avoiding total mesorectal excision (TME) and associated surgical risks. Excellent survival was seen for patients with a clinical complete response observed by the WW approach as local regrowth, occurring in 15% to 35% of patients, is generally amenable for curative treatment with TME.2,3 Since this first report, there has been increasing interest in WW and organ-preserving strategies, reflected by an increasing number of reports by centers and research groups.4,5,6,7,8,9,10
Patients observed by the WW approach presumably have a better quality of life (QoL) and functional outcome because they do not require a definitive colostomy and have less surgery-related morbidity. A small matched-controlled study revealed that patients observed by the WW approach scored better on several QoL domains and reported fewer bowel, urinary, and sexual dysfunctions compared with patients after TME.11 Nevertheless, prospective long-term data on QoL and functional outcome after the WW approach are limited. Most studies are of retrospective cohorts with small sample sizes.5,11,12,13,14,15,16,17,18 Moreover, most of these studies focused on anorectal function, whereas detailed data on QoL, urinary function, and sexual function are scarce.
Detailed data on QoL and functional outcome, including bowel, urinary, and sexual function, are necessary in daily practice for making an optimal treatment decision and counseling patients on the presumed benefits of the WW approach. Because randomized clinical trials for the WW approach are unethical and therefore unlikely to be conducted, the best evidence on QoL and functional outcome with the WW approach will come from large prospective cohort studies. The aim of this study was to provide these data by reporting clinical data on long-term QoL and functional outcome, including bowel, urinary, and sexual function, from a large prospective cohort of patients with rectal cancer who were observed by the WW approach.
Methods
Study Design and Participants
Quality of life and functional outcome in patients included in the Dutch Watch-and-Wait registry were assessed as part of 2 prospective cohort studies: a single-center study in a tertiary referral center from March 2014 to October 2017 and an ongoing multicenter study from September 2017 in 13 Dutch and Belgian centers. The studies were approved by the medical ethics committee of the Maastricht University Medical Centre (NCT02278653) and the Netherlands Cancer Institute (NCT03426397). Written informed consent was obtained for all patients. Both studies followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
For both studies, patients with nonmetastatic rectal cancer were eligible for the WW approach when a clinical complete response or near-complete response was established 8 to 12 weeks after chemoradiotherapy or radiotherapy, as previously defined.12 Neoadjuvant treatment could consist of long-course chemoradiotherapy (25 × 2 Gy or 28 × 1.8 Gy with concomitant capecitabine) or short-course radiotherapy (5 × 5 Gy). During follow-up, local excision or TME was performed for suspected residual tumor or regrowth.
Data Collection
Quality of life and functional outcome were assessed with validated questionnaires that were sent by mail at 3, 12, and 24 months after inclusion. Patients with a minimum follow-up of 24 months who completed at least 1 of the 3 questionnaires were selected for the present study.
General QoL was assessed by the European Organisation for Research and Treatment of Cancer–Quality of Life Questionnaire–C30 (EORTC-QLQ-C30) and 36-Item Short-Form Health Survey. The EORTC-QLQ-C30 compiles a global health scale, 5 functional scales, and 9 symptom scales or single items (range, 0-100). A high score on the global health scale and functional scales indicates a high level of functioning; a high score on the symptom scales or single items indicates a high level of symptomatology.19 By mistake, questions 6 to 10 were missing, and the subscales of role functioning and dyspnea could therefore not be calculated. The 36-Item Short-Form Health Survey comprises 8 multi-item scales (range, 0-100). A higher score indicates a higher level of functioning.20
Colorectal cancer–specific QoL was assessed with the EORTC-QLQ-CR38 or EORTC-QLQ-CR29. The EORTC-QLQ-CR38, used in the single-center study, compiles 4 functional scales or single items and 8 symptom scales or single items.21 The EORTC-QLQ-CR29, used in the multicenter study, incorporates 5 functional scales or single items and 18 symptom scales or single items.22 The principle of the scoring approach and interpretation of both questionnaires is identical to that of the EORTC-QLQ-C30.
Bowel function was assessed with the Low Anterior Resection Syndrome (LARS) score and Vaizey incontinence score. The LARS score (range, 0-42) assesses stool frequency, incontinence, and urge and is graded as no LARS (scores 0-20), minor LARS (scores 21-29), and major LARS (scores 30-42).23 The Vaizey score (range, 0-24) assesses the incidence of fecal incontinence and its effect on lifestyle. A score of 12 or higher indicates major incontinence.24,25 The LARS and Vaizey scores were not completed by patients who underwent a colostomy.
The International Prostate Symptom Score was used to measure urinary function in male patients. The score (range, 0-35) addresses incomplete emptying, frequency, intermittency, urgency, weak stream, straining, and nocturia, and it is categorized into mild dysfunction (scores 0-7), moderate dysfunction (scores 8-19), and severe urinary dysfunction (scores 20-35).26
Sexual function was evaluated with the International Index of Erectile Function questionnaire for male patients and the Female Sexual Function Index for female patients. The International Index of Erectile Function incorporates a total score and 5 subdomains. The subdomain erectile function is categorized into no dysfunction (scores 26-30), mild dysfunction (scores 22-25), mild to moderate dysfunction (scores 17-21), moderate dysfunction (scores 11-16), and severe erectile dysfunction (scores 1-10).27 The Female Sexual Function Index incorporates 6 subdomains (maximum score of 6) and a full-scale score (maximum score of 36). In both questionnaires, a higher score indicates better sexual function.27,28
Statistical Analysis
Statistical analyses were performed with SPSS, version 25.0 (IBM SPSS). Missing items within a questionnaire were handled according to the corresponding scoring manual. Multiple imputations with fully conditional specification were used to correct for missing data of the whole questionnaire at 1 or 2 time points. Values were drawn with predictive mean matching. The number of imputations was set to 10. Mean (SD) scores were calculated on QoL subscales and functional outcome scores because this is common for QoL studies.
Quality of life and functional outcome were analyzed for patients observed by the WW approach who did not require rectal surgery in the first 24 months after inclusion (WW group). Additionally, results were analyzed for the entire group of patients (patients in the WW group and patients after additional surgery); these results are provided in eTable 4 in Supplement 1. The paired-sample t test and McNemar test were used to test differences between time points. Clinical interpretation of changes over time for the EORTC-QLQ-C30 was reported according to the guideline of Cocks and colleagues.29
To explore associations, linear regression was used to test for the univariate association of sex, age (at inclusion), tumor distance, and neoadjuvant treatment on QoL and functional outcome of the WW group at 24 months. Tumor distance was measured on magnetic resonance imaging in centimeters from the anorectal junction to the distal tumor border. For neoadjuvant treatment, chemoradiotherapy was the reference standard. Linear regression and the Fisher exact test were used to test differences between the patients in the WW group and patients after additional surgery at 24 months. The analyses of the present study are exploratory; therefore, no adjustment for multiple testing was made, and 2-sided P ≤ .05 was considered statistically significant. Data analyses were conducted between April 1, 2021, and August 27, 2021.
Results
In April 2021, 289 patients with nonmetastatic rectal cancer with a clinical complete response or near-complete response after neoadjuvant chemoradiotherapy or radiotherapy who were prospectively included in the Dutch Watch-and-Wait registry had a minimum follow-up of 24 months: 91 patients in the single-center study and 198 in the multicenter study. The questionnaire response rates at 3, 12, and 24 months for the 289 patients were 89.3% (258 responders), 81.7% (236 responders), and 73.0% (211 responders), respectively. Eleven patients did not complete any questionnaire and were excluded from the present analysis. As a result, 278 patients were included in the analysis. Of these 278 patients, 187 were male (67%), 91 were female (33%), and the median age was 66 years (range, 34-85 years) at inclusion. Data on race and ethnicity are not recorded in the Dutch Watch-and-Wait registry and therefore were not available for this study.
At 24 months of follow-up, 221 of the 278 patients (80%) had a sustained clinical complete response and were observed by the WW approach, 18 (6%) were treated with local excision, and 39 (14%) were treated with TME. Seven of the 39 patients treated with TME were treated with local excision before TME. Patient and treatment characteristics are provided in the Table and the eFigure in Supplement 1.
Table. Patient Characteristics.
| Characteristic | Patients, No. (%) | |||
|---|---|---|---|---|
| Total | Treatment | |||
| WW | LE | TME | ||
| No. (%) | 278 (100) | 221 (79.5) | 18 (6.5) | 39 (14.0) |
| Age, median (range), y | 66 (34-85) | 66 (44-85) | 67 (48-85) | 62 (34-83) |
| <60 | 77 (27.7) | 59 (26.7) | 5 (27.8) | 13 (33.3) |
| 60-69 | 103 (37.1) | 81 (36.7) | 7 (38.9) | 15 (38.5) |
| ≥70 | 98 (35.3) | 81 (36.7) | 6 (33.3) | 11 (28.2) |
| Sex | ||||
| Male | 187 (67.3) | 151 (68.3) | 11 (61.1) | 25 (64.1) |
| Female | 91 (32.7) | 70 (31.7) | 7 (38.9) | 14 (35.9) |
| Clinical T stage | ||||
| cT1-2 | 55 (19.8) | 48 (21.7) | 3 (16.7) | 4 (10.3) |
| cT3 | 202 (72.7) | 157 (71.0) | 14 (77.8) | 31 (79.5) |
| cT4 | 21 (7.6) | 16 (7.2) | 1 (5.6) | 4 (10.3) |
| Clinical N stage | ||||
| cN0 | 72 (25.9) | 56 (25.3) | 4 (22.2) | 12 (30.8) |
| cN1 | 92 (33.1) | 70 (31.7) | 8 (44.4) | 16 (41.0) |
| cN2 | 114 (41.0) | 95 (43.0) | 6 (33.3) | 14 (35.9) |
| Tumor distance from anorectal junction, median (range), cm | 3.0 (0-15) | 3.0 (0-15) | 2.5 (0-10) | 3.5 (0-13) |
| <5 | 164 (59.0) | 133 (60.2) | 12 (66.7) | 19 (48.7) |
| ≥5 | 114 (41.0) | 88 (39.8) | 6 (33.3) | 20 (51.3) |
| Neoadjuvant treatment | ||||
| Short course of radiotherapy | 15 (5.4) | 8 (3.6) | 4 (22.2) | 3 (7.7) |
| Chemoradiotherapy | 263 (94.6) | 213 (96.4) | 14 (77.8) | 36 (92.3) |
| Responsea | ||||
| Clinical complete response | 153 (55.0) | 131 (59.3) | 6 (33.3) | 16 (41.0) |
| Clinical near-complete response | 125 (45.0) | 90 (40.7) | 12 (66.7) | 23 (59.0) |
| Colostomyb | ||||
| No | 242 (87.1) | 221 (100) | 18 (100) | 3 (7.7) |
| Yes | 36 (12.9) | 0 | 0 | 36 (92.3) |
Abbreviations: LE, local excision; TME, total mesorectal excision; WW, patients followed up by a watch-and-wait approach without requiring additional surgery.
Clinical response at restaging 8 to 12 weeks after chemoradiotherapy or radiotherapy.
Patients with or without a colostomy at 24 months.
Quality of Life
Figure 1 presents the EORTC-QLQ-C30 and EORTC-QLQ-CR29 scores of the WW group. Compared with 3 months, the subscales emotional functioning (mean [SD] score, 85.9 [16.7] vs 88.1 [14.6]; P = .05) and social functioning (mean [SD] score, 89.0 [18.3] vs 92.9 [12.6]; P = .002) of the EORTC-QLQ-C30 improved at 24 months. Clinically, these differences were classified as trivial and small. Of the EORTC-QLQ-CR29, an improvement in the subscales dry mouth (mean [SD] score, 17.6 [28.2] vs 7.2 [15.2]; P < .001), hair loss (mean [SD] score, 6.8 [22.8] vs 1.9 [6.9]; P = .01), taste (mean [SD] score, 6.0 [19.0] vs 1.4 [5.7]; P = .004), anxiety (mean [SD] score, 74.9 [19.7] vs 82.3 [16.6]; P < .001), weight (mean [SD] score, 84.2 [22.4] vs 88.6 [18.7]; P = .04), and body image (mean [SD] score, 86.7 [22.1] vs 91.4 [12.1]; P = .02) was observed at 24 months. A deterioration in the subscales urinary incontinence (mean [SD] score, 22.9 [20.7] vs 25.0 [18.0]; P < .001) and embarrassment (mean [SD] score, 8.3 [17.1] vs 12.0 [17.1]; P = .05) was reported. Analyses of the 36-Item Short-Form Health Survey and EORTC-QLQ-CR38 are provided in eTable 1 and eTable 2 in Supplement 1.
Figure 1. Quality of Life of Patients Observed by the Watch-and-Wait Approach at 3, 12, and 24 Months After Inclusion.
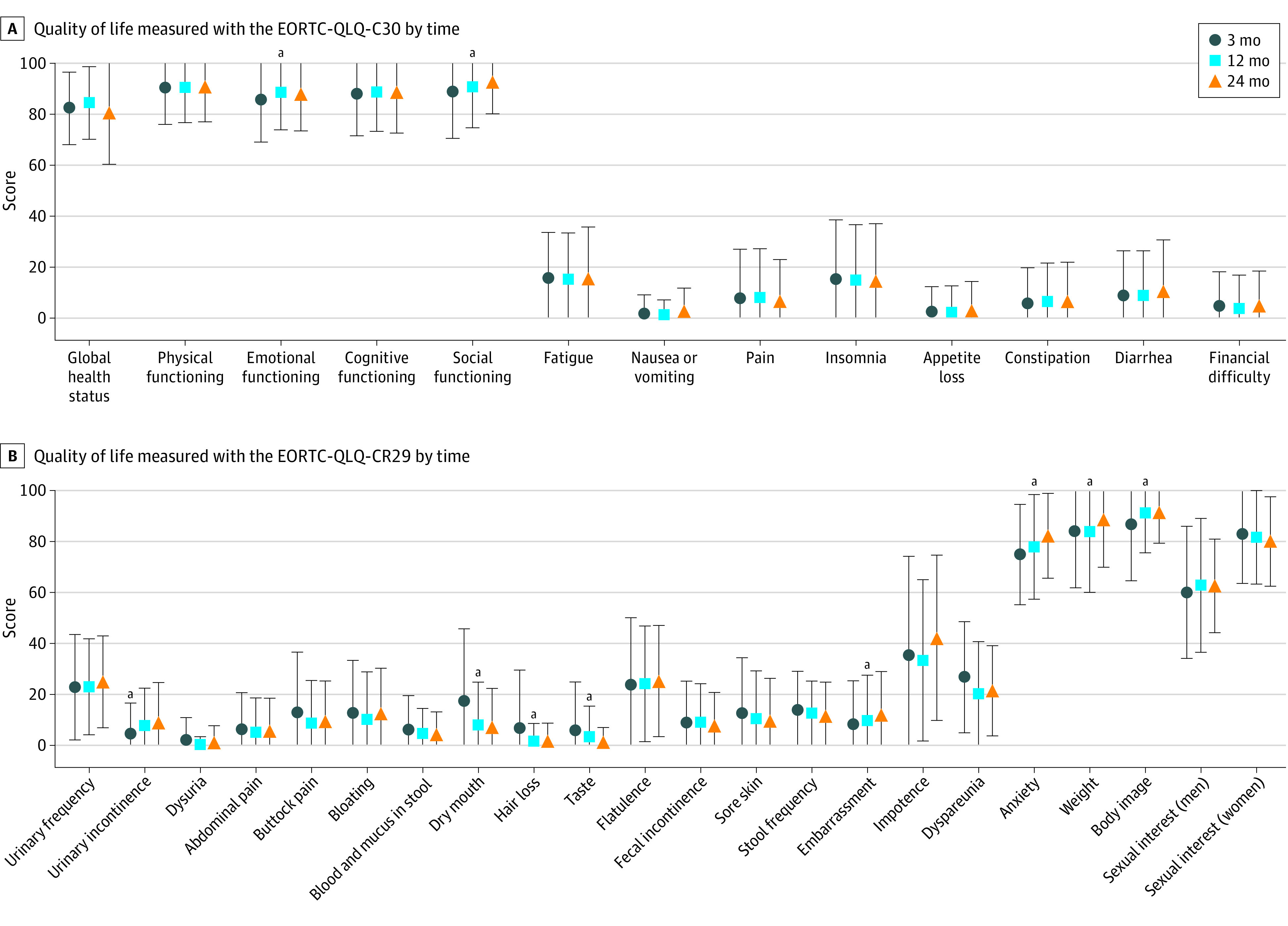
Quality of life measured with the EORTC-QLQ-C30 (A) and EORTC-QLQ-CR29 (B) of patients observed by the watch-and-wait approach without requiring additional surgery at 3, 12, and 24 months after inclusion. Data are mean (SD) scores. Error bars indicate SDs. EORTC-QLQ indicates European Organisation for Research and Treatment of Cancer–Quality of Life Questionnaire.
aStatistically significant difference between 3 and 24 months (P ≤ .05).
Compared with patients in the WW group, patients after local excision scored similarly on all EORTC-QLQ-C30 subscales at 24 months but reported more anxiety (mean [SD] score, 82.3 [16.6] vs 70.50 [23.7]; P = .02), measured with the EORTC-QLQ-CR29. Patients after TME scored worse on the EORTC-QLQ-C30 subscales global health status (mean [SD] score, 80.6 [20.0] vs 68.4 [28.4]; P = .001), physical functioning (mean [SD] score, 91.0 [13.8] vs 86.0 [16.6]; P = .05), emotional functioning (mean [SD] score, 88.1 [14.6] vs 81.6 [20.4]; P = .02), social functioning (mean [SD] score, 92.9 [12.6] vs 82.9 [20.8]; P < .001), fatigue (mean [SD] score, 15.7 [20.1] vs 23.1 [26.4]; P = .04), pain (mean [SD] score, 6.8 [16.2] vs 23.9 [27.5]; P < .001), appetite loss (mean [SD] score, 3.2 [11.2] vs 11.1 [22.1]; P = .001), and financial difficulties (mean [SD] score, 4.8 [13.7] vs 13.7 [23.8]; P = .001). Regarding the EORTC-QLQ-CR29 subscales, patients after TME reported worse scores on urinary frequency (mean [SD] score, 25.0 [18.1] vs 35.0 [21.1]; P = .006), urinary incontinence (mean [SD] score, 9.2 [15.5] vs 16.3 [24.8]; P = .04), abdominal pain (mean [SD] score, 5.8 [12.8] vs 12.8 [18.6]; P = .009), buttock pain (mean [SD] score, 9.6 [15.7] vs 18.5 [23.2]; P = .01), dry mouth (mean [SD] score, 7.2 [15.2] vs 15.3 [18.9]; P = .008), taste (mean [SD] score, 1.4 [5.7] vs 4.5 [10.6]; P = .02), and body image (mean [SD] score, 91.4 [12.1] vs 80.3 [25.4]; P < .001). Figure 2 presents the EORTC-QLQ-C30 and EORTC-QLQ-CR29 scores at 24 months of patients in the WW group and patients after surgery.
Figure 2. Quality of Life at 24 Months After Inclusion of Patients Observed by the Watch-and-Wait (WW) Approach, Patients After Local Excision (LE), and Patients After Total Mesorectal Excision (TME).
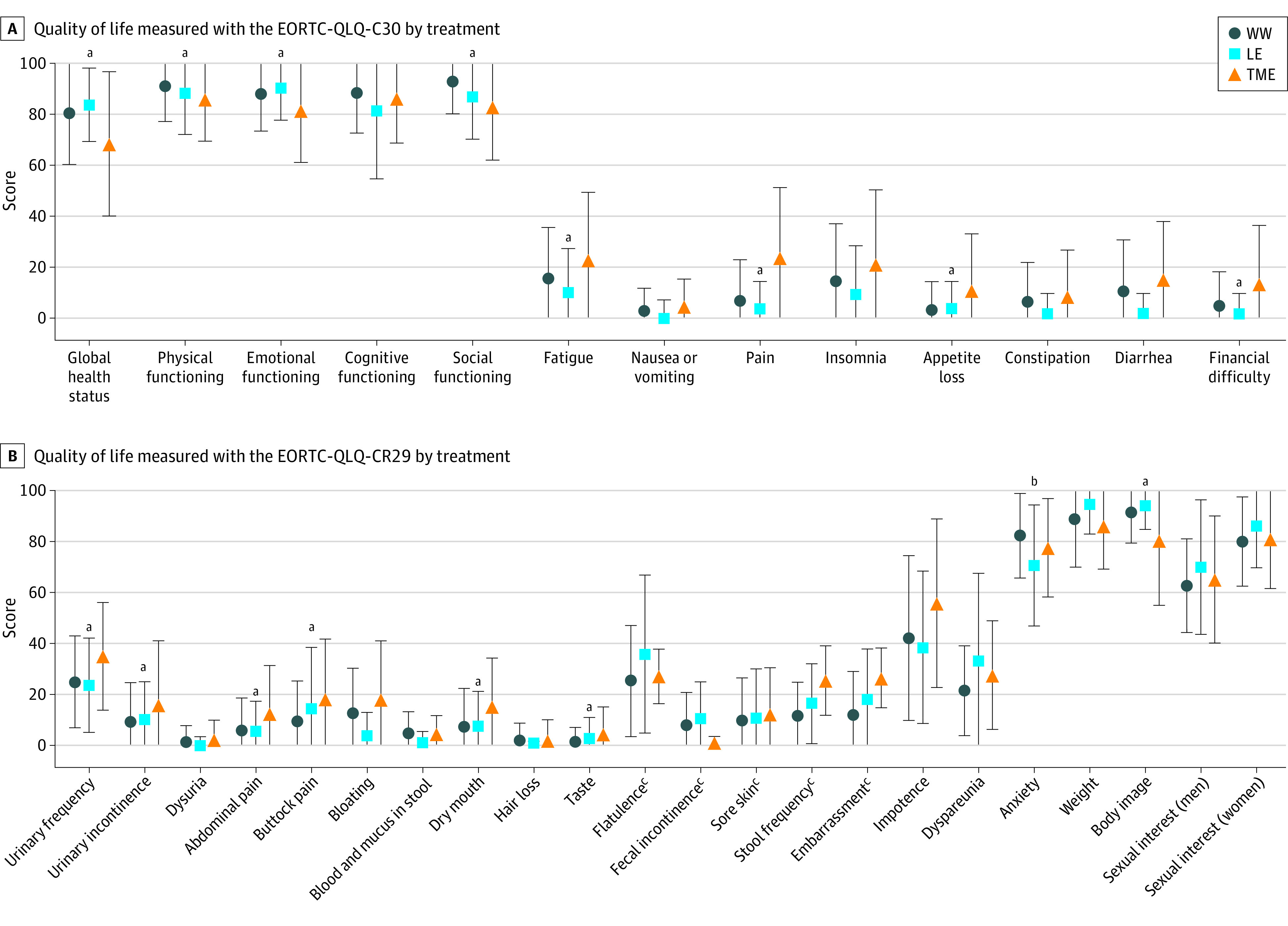
Quality of life at 24 months after inclusion measured with the EORTC-QLQ-C30 (A) and EORTC-QLQ-CR29 (B) of patients observed by the WW approach without requiring additional surgery, patients treated with LE, and patients treated with TME. EORTC-QLQ indicates European Organisation for Research and Treatment of Cancer–Quality of Life Questionnaire. Data are presented as mean (SD) scores. Error bars indicate SDs.
aStatistically significant difference between TME and WW (P ≤ .05).
bStatistically significant difference between LE and WW (P ≤ .05).
cSubscales were not completed by patients with a colostomy.
Linear regression indicated that women had worse outcomes on the subscales nausea (regression coefficient, 2.7; 95% CI, 0.1-5.2; P = .04), appetite loss (regression coefficient, 4.4; 95% CI, 1.3-7.6; P = .006), constipation (regression coefficient, 4.9; 95% CI, 0.6-9.3; P = .03), diarrhea (regression coefficient, 6.9; 95% CI, 1.2-12.5; P = .02), urinary incontinence (regression coefficient, 5.7; 95% CI, 0.2-11.3; P = .04), buttock pain (regression coefficient, 7.5; 95% CI, 1.9-13.0; P = .009), flatulence (regression coefficient, 8.9; 95% CI, 1.1-16.7; P = .03), and anxiety (regression coefficient, –6.9; 95% CI, –12.8 to –1.0; P = .02). Older age was associated with worse outcomes on the subscales physical functioning (regression coefficient, –0.3; 95% CI, –0.5 to –0.1; P = .002) and hair loss (regression coefficient, 0.2; 95% CI, 0.0-0.3; P = .01) but with better outcomes on the subscales fatigue (regression coefficient, –0.4; 95% CI, –0.7 to –0.1; P = .008), insomnia (regression coefficient, –0.4, 95% CI, –0.7 to 0.0; P = .03), financial difficulties (regression coefficient, –0.3; 95% CI, –0.5 to –0.1; P = .009), and anxiety (regression coefficient, 0.4; 95% CI, 0.1-0.7; P = .01). Older age in men was associated with better outcomes on the subscale sexual interest (regression coefficient, 0.7; 95% CI, 0.3-1.1; P = .001) but worse outcomes on the subscale impotence (regression coefficient, 1.7; 95% CI, 1.0-2.3; P < .001). Details of the linear regression analyses are provided in eTable 3 in Supplement 1.
Functional Outcome
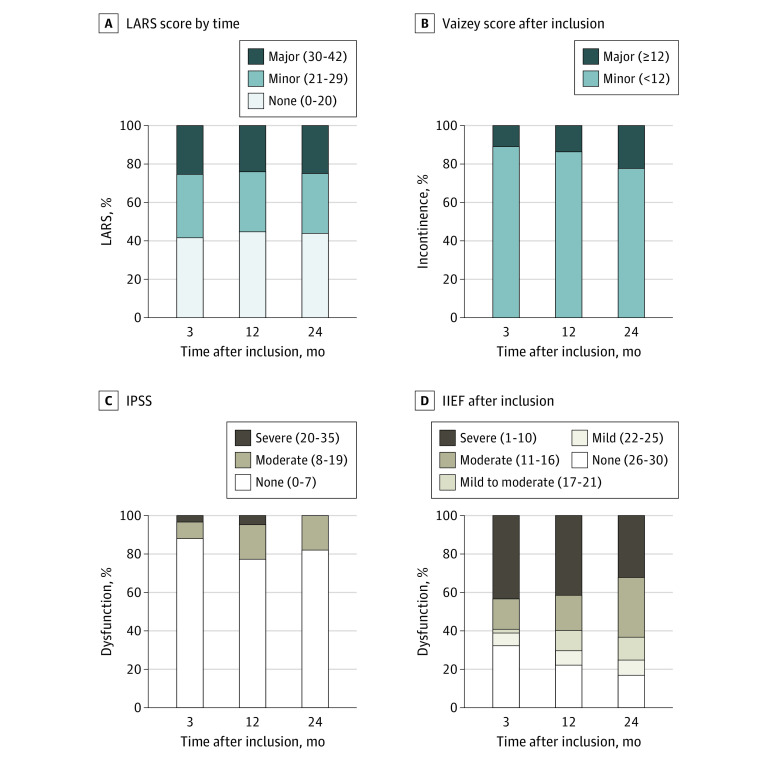
In the WW group, the LARS score (mean [SD] score, 20.9 [11.2] at 3 months and 20.6 [11.3] at 24 months) and percentage of major LARS (of 221 patients, 56 [25.3%] at 3 months and 55 [24.9%] at 24 months) did not change over time. The mean (SD) Vaizey score increased from 6.8 (4.3) at 3 months to 8.0 (4.4) at 24 months (mean [SD] difference, 1.3 [4.1]; 95% CI, 0.7-1.7; P < .001). Major incontinence increased from 10.9% (24 of 221 patients) at 3 months to 22.2% (49 patients) at 24 months (P = .001). Urinary function measured with the International Prostate Symptom Score (mean [SD] score, 5.2 [5.4] at 3 months and 5.5 [3.9] at 24 months) did not differ over time. At 24 months, 27 of 151 male patients (17.9%) in the WW group reported moderate urinary dysfunction. Regarding male sexual function, no differences in mean scores of all International Index of Erectile Function subscales were observed over time; however, the percentage of severe erectile dysfunction decreased from 43.0% (65 of 151 patients) at 3 months to 31.8% (48 patients) at 24 months (P = .03). Nevertheless, more than half of male patients reported severe or moderate erectile dysfunction at all time points. Regarding female sexual function, a decrease in the Female Sexual Function Index subscale satisfaction (mean [SD] score, 3.9 [1.5] at 3 months and 3.5 [1.4] at 24 months; P = .04) and the Female Sexual Function Index full-scale score (mean [SD] score, 18.2 [8.1] at 3 months and 15.6 [7.4] at 24 months; P = .002) was observed over time. Functional outcome data are provided in Figure 3 and eTable 1 in Supplement 1.
Figure 3. Functional Outcome of Patients Observed by the Watch-and-Wait Approach at 3, 12, and 24 Months After Inclusion.
Functional outcome according to the Low Anterior Resection Syndrome (LARS) score (A), Vaizey incontinence score (B), International Prostate Symptom Score (IPSS) (C), and International Index of Erectile Function (IIEF) score (D) of patients observed by the watch-and-wait approach without requiring additional surgery at 3, 12, and 24 months after inclusion. Mean (SD) scores for LARS were 20.9 (11.2) at 3 months, 21.0 (11.3) at 12 months, and 20.6 (11.3) at 24 months; for Vaizey, 6.8 (4.3) at 3 months, 7.1 (4.0) at 12 months, and 8.0 (4.4) at 24 months; for IPSS, 5.2 (5.4) at 3 months, 5.9 (5.8) at 12 months, and 5.5 (3.9) at 24 months; and for IIEF, 15.0 (11.5) at 3 months, 14.4 (10.1) at 12 months, and 14.5 (9.1) at 24 months.
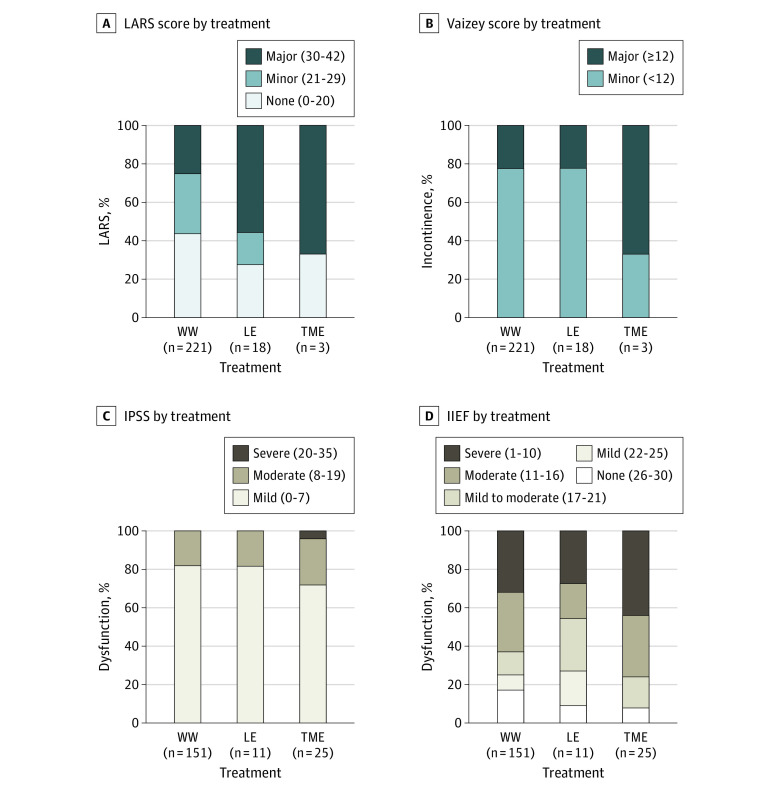
Compared with patients in the WW group, patients after local excision reported an increased LARS score (mean [SD] score, 28.2 [11.1] vs 20.6 [11.3]; regression coefficient, 7.6; 95% CI, 2.2-13.0; P = .006) and more major LARS (10 of 18 patients [55.6%] vs 55 of 221 patients [24.9%]; P = .008). No differences in urinary and sexual function measured with the International Prostate Symptom Score, International Index of Erectile Function, and Female Sexual Function Index score were observed. Male patients after TME reported more severe erectile dysfunction at 24 months (11 of 25 patients [44.0%] vs 48 of 151 patients [31.8%]; P = .26), although it was not statistically significant. No differences in urinary and sexual function measured with the International Prostate Symptom Score and Female Sexual Function Index score were observed. The LARS and Vaizey scores could not be compared because most patients after TME had a colostomy. Data on the functional outcomes of patients in the WW group and patients after surgery are provided in Figure 4 (eTable 2 in Supplement 1).
Figure 4. Functional Outcome at 24 Months After Inclusion of Patients Observed by the Watch-and-Wait (WW) Approach, Patients After Local Excision (LE), and Patients After Total Mesorectal Excision (TME).
Functional outcome at 24 months after inclusion according to the Low Anterior Resection Syndrome (LARS) score (A), Vaizey incontinence score (B), International Prostate Symptom Score (IPSS) (C), and International Index of Erectile Function (IIEF) score (D) of patients observed by the WW approach without requiring additional surgery, patients treated with LE, and patients treated with TME. The mean (SD) LARS score for WW was 20.6 (11.3); for LE, 28.2 (11.1); and for TME, 28.3 (12.2). The mean (SD) Vaizey incontinence score for WW was 8.0 (4.4); for LE, 8.7 (3.3); and for TME, 11.7 (3.2). Mean (SD) IPSS for WW was 5.5 (3.9); for LE, 5.7 (4.6); and for TME, 7.2 (6.2). The mean (SD) IIEF score for WW was 14.5 (9.1); for LE, 15.3 (9.4); and for TME, 11.3 (8.1).
Linear regression indicated that women had a higher LARS score (regression coefficient, 3.8; 95% CI, 0.6-6.9; P = .02). Older age in men was associated with a higher International Prostate Symptom Score and with worse outcomes on all International Index of Erectile Function subscales.
Discussion
This large prospective cohort study provides important information on QoL and functional outcome, including bowel, urinary, and sexual function, of patients with rectal cancer who were observed by the WW approach after neoadjuvant chemoradiotherapy or radiotherapy. Patients observed by the WW approach who did not require additional surgery reported good QoL, with limited variation over time. Major LARS was observed in approximately one-quarter of patients at all time points (at 3 months, 56 of 221 patients [25.3%]; at 12 months, 53 patients [24.0%]; and at 24 months, 55 patients [24.9%]). Male patients reported severe erectile dysfunction (48 of 151 patients [31.8%]) and moderate urinary dysfunction (27 of 141 patients [19.1%]) at 24 months. Sexual satisfaction and overall sexual function of female patients decreased during follow-up. After local excision, patients had QoL scores comparable to those of patients in the WW group (Figure 2 and eTable 2 in Supplement 1) but reported more major LARS (10 of 18 patients [55.6%]) at 24 months. After TME, patients scored worse on several QoL subscales. Linear regression indicated that women had worse outcomes on several QoL subscales and higher LARS score at 24 months. Older age at inclusion was particularly associated with more urinary and sexual dysfunction in men at 24 months.
Regarding general QoL, no differences over time were observed except for an improvement in emotional and social functioning. Improvement in emotional functioning may reflect that insecurities that come with WW may decrease over time as patients become more used to frequent follow-up visits and the knowledge that the risk of recurrence decreases.30 In comparison with studies on QoL after chemoradiotherapy or radiotherapy and TME, the patients in the WW group in the present study scored better on almost all subscales regarding general QoL and the majority of subscales regarding cancer-specific QoL.10,31,32
Regarding gastrointestinal function, approximately 25% of patients in the WW group reported major LARS (at 3 months, 56 of 221 patients [25.3%]; at 12 months, 53 patients [24.0%]; and at 24 months, 55 patients [24.9%]), which is in the same range as a previous study that reported 33% of patients with major LARS after WW.11 However, this contrasts favorably with the higher rate in the literature of up to 66% of patients with major LARS after TME.33,34 Regarding urinary function, 27 of 151 male patients (17.9%) in the WW group reported moderate urinary dysfunction at 24 months. Although urinary dysfunction after rectal cancer treatment is mainly attributed to surgical autonomic nerve damage,35 higher levels of dysfunction are reported after neoadjuvant radiotherapy.36,37 Radiotherapy is also associated with a decline in sexual function,37,38 which is reflected in the present study because most male patients reported moderate or severe erectile dysfunction at all time points. For female patients, a deterioration in sexual satisfaction and overall sexual function was observed during follow-up. The negative association between radiotherapy and female sexual function has been previously attributed to neurovascular damage, vaginal fibrosis, and atrophy of vaginal mucosa.39,40,41
Compared with data on QoL in the general Dutch population, the WW group had similar scores on almost all EORTC-QLQ-C30 subscales at 24 months.42,43 On some subscales, the WW group seemed to score better. The phenomenon of patients with rectal cancer scoring better than the general population has been noticed previously.44 It is hypothesized that patients adapt to changes and limitations after their diagnosis and treatment. The standards on which patients base their QoL may change.45 Successful treatment of a potentially life-threatening disease may therefore lead to higher QoL scores.
Within the first 24 months of follow-up, 57 patients were treated with additional local excision or TME. After local excision, more patients reported major LARS (10 of 18 patients [55.6%]) at 24 months, without a clear association with QoL. This relatively high prevalence of major LARS aligns with the 50% that was previously reported.9 Patients in the present study who underwent additional TME scored worse on several QoL subscales. Nevertheless, in general, these QoL scores are similar to the scores described in the literature of patients after neoadjuvant radiotherapy and TME.10,32 Compared with the QoL of the Dutch population, after TME, patients in the present study scored worse on more than half of the EORTC-QLQ-C30 subscales, albeit the differences were small.42,43 Therefore, the general QoL of the entire group of patients in this cohort can be considered good whether or not they required additional surgery.
In the present study, women had a worse outcome on several QoL subscales, which was also observed in the Dutch population.46 In addition, women had higher LARS scores, which has been reported previously34,47,48 and has been attributed to the combination of differences in pelvic anatomy, preexistent pelvic floor dysfunction, and previous obstetric trauma.34,47,49,50 As might be expected, older age in men was associated with more urinary and sexual dysfunction. No major differences were found between patients after short-course radiotherapy and patients after long-course chemoradiotherapy, which is similar to the results of previous studies on these 2 radiotherapy regimens followed by surgery.51,52,53
Limitations
This study has several limitations. First, the initial questionnaire was sent 3 months after inclusion; therefore, early associations of treatment with QoL and functional outcome were not reflected within our data. In addition, there are no baseline data to refer to. However, the rectal tumor was still in situ at baseline, and we do not know exactly how QoL and functional outcome are associated with the tumor itself. Second, there was no comparator group, a limitation that is common in studies on WW because randomization between TME and WW is unethical and patients with a clinical complete response generally object to randomization. Third, the sample size of the group of patients after surgery was small; therefore, the comparison made with the WW group has to be interpreted with caution. Fourth, as pointed out in the Methods, the subscales role functioning and dyspnea of the EORTC-QLQ-C30 could not be analyzed.
Conclusions
This study provides important clinical data on the QoL and functional outcome of patients with rectal cancer who were observed by the WW approach after neoadjuvant chemoradiotherapy or radiotherapy. Data on QoL and functional outcome of patients in the WW group, as well as patients treated with additional surgery, are provided. As stated previously, these data from a large prospective study will be the best evidence on QoL and functional outcome of patients after the WW approach because randomized clinical trials are unethical. The data provided by the present study are essential for shared decision-making and for counseling patients on what to expect with the WW approach. With expanding treatment options available for patients with rectal cancer, making the optimal treatment decision for the individual patient is an increasingly challenging task. It requires a good understanding of all treatment options, including the associated oncologic outcome, as well as data on QoL and functional outcome.
eFigure. Treatment Details of 278 Patients Included for the Current Analysis
eTable 1. Quality of Life and Functional Outcome of Patients Followed by Watch and Wait Without Requiring Additional Surgery at 3, 12, and 24 Months Following Inclusion
eTable 2. Quality of Life and Functional Outcome of Patients Treated With Local Excision (LE) and Patients Treated With Total Mesorectal Excision (TME) Compared to Patients Followed by Watch-and-Wait Without Requiring Additional Surgery (W&W) at 24 Months Following Inclusion
eTable 3. Association of Sex, Age, Tumor Distance, and Neoadjuvant Treatment on Quality of Life and Functional Outcome at 24 Months Following Inclusion
eTable 4. Quality of Life and Functional Outcome of All Patients Included in the Dutch Watch-and-Wait Registry at 3, 12, and 24 Months Following Inclusion
Nonauthor Collaborators
Data Sharing Statement
References
- 1.Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg. 2004;240(4):711-717. doi: 10.1097/01.sla.0000141194.27992.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Valk MJM, Hilling DE, Bastiaannet E, et al. ; IWWD Consortium . Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet. 2018;391(10139):2537-2545. doi: 10.1016/S0140-6736(18)31078-X [DOI] [PubMed] [Google Scholar]
- 3.Dossa F, Chesney TR, Acuna SA, Baxter NN. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2017;2(7):501-513. doi: 10.1016/S2468-1253(17)30074-2 [DOI] [PubMed] [Google Scholar]
- 4.Renehan AG, Malcomson L, Emsley R, et al. Watch-and-wait approach versus surgical resection after chemoradiotherapy for patients with rectal cancer (the OnCoRe project): a propensity-score matched cohort analysis. Lancet Oncol. 2016;17(2):174-183. doi: 10.1016/S1470-2045(15)00467-2 [DOI] [PubMed] [Google Scholar]
- 5.Maas M, Beets-Tan RG, Lambregts DM, et al. Wait-and-see policy for clinical complete responders after chemoradiation for rectal cancer. J Clin Oncol. 2011;29(35):4633-4640. doi: 10.1200/JCO.2011.37.7176 [DOI] [PubMed] [Google Scholar]
- 6.Appelt AL, Pløen J, Harling H, et al. High-dose chemoradiotherapy and watchful waiting for distal rectal cancer: a prospective observational study. Lancet Oncol. 2015;16(8):919-927. doi: 10.1016/S1470-2045(15)00120-5 [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Aguilar J, Renfro LA, Chow OS, et al. Organ preservation for clinical T2N0 distal rectal cancer using neoadjuvant chemoradiotherapy and local excision (ACOSOG Z6041): results of an open-label, single-arm, multi-institutional, phase 2 trial. Lancet Oncol. 2015;16(15):1537-1546. doi: 10.1016/S1470-2045(15)00215-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rullier E, Rouanet P, Tuech JJ, et al. Organ preservation for rectal cancer (GRECCAR 2): a prospective, randomised, open-label, multicentre, phase 3 trial. Lancet. 2017;390(10093):469-479. doi: 10.1016/S0140-6736(17)31056-5 [DOI] [PubMed] [Google Scholar]
- 9.Stijns RCH, de Graaf EJR, Punt CJA, et al. ; CARTS Study Group . Long-term oncological and functional outcomes of chemoradiotherapy followed by organ-sparing transanal endoscopic microsurgery for distal rectal cancer: the CARTS study. JAMA Surg. 2019;154(1):47-54. doi: 10.1001/jamasurg.2018.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bach SP, Gilbert A, Brock K, et al. ; TREC Collaborators . Radical surgery versus organ preservation via short-course radiotherapy followed by transanal endoscopic microsurgery for early-stage rectal cancer (TREC): a randomised, open-label feasibility study. Lancet Gastroenterol Hepatol. 2021;6(2):92-105. doi: 10.1016/S2468-1253(20)30333-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hupkens BJP, Martens MH, Stoot JH, et al. Quality of life in rectal cancer patients after chemoradiation: watch-and-wait policy versus standard resection—a matched-controlled study. Dis Colon Rectum. 2017;60(10):1032-1040. doi: 10.1097/DCR.0000000000000862 [DOI] [PubMed] [Google Scholar]
- 12.Martens MH, Maas M, Heijnen LA, et al. Long-term outcome of an organ preservation program after neoadjuvant treatment for rectal cancer. J Natl Cancer Inst. 2016;108(12):djw171. doi: 10.1093/jnci/djw171 [DOI] [PubMed] [Google Scholar]
- 13.Habr-Gama A, Lynn PB, Jorge JM, et al. Impact of Organ-Preserving Strategies on Anorectal Function in Patients with Distal Rectal Cancer Following Neoadjuvant Chemoradiation. Dis Colon Rectum. 2016;59(4):264-269. doi: 10.1097/DCR.0000000000000543 [DOI] [PubMed] [Google Scholar]
- 14.van der Sande ME, Hupkens BJP, Berbée M, et al. Impact of radiotherapy on anorectal function in patients with rectal cancer following a watch and wait programme. Radiother Oncol. 2019;132:79-84. doi: 10.1016/j.radonc.2018.11.017 [DOI] [PubMed] [Google Scholar]
- 15.Dizdarevic E, Frøstrup Hansen T, Pløen J, et al. Long-term patient-reported outcomes after high-dose chemoradiation therapy for nonsurgical management of distal rectal cancer. Int J Radiat Oncol Biol Phys. 2020;106(3):556-563. doi: 10.1016/j.ijrobp.2019.10.046 [DOI] [PubMed] [Google Scholar]
- 16.Haak HE, Maas M, Lambregts DMJ, Beets-Tan RGH, Beets GL; Dutch Watch-and-Wait Consortium . Is watch and wait a safe and effective way to treat rectal cancer in older patients? Eur J Surg Oncol. 2020;46(3):358-362. doi: 10.1016/j.ejso.2020.01.005 [DOI] [PubMed] [Google Scholar]
- 17.Quezada-Diaz FF, Smith JJ, Jimenez-Rodriguez RM, et al. Patient-reported bowel function in patients with rectal cancer managed by a watch-and-wait strategy after neoadjuvant therapy: a case-control study. Dis Colon Rectum. 2020;63(7):897-902. doi: 10.1097/DCR.0000000000001646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones HJS, Al-Najami I, Cunningham C. Quality of life after rectal-preserving treatment of rectal cancer. Eur J Surg Oncol. 2020;46(11):2050-2056. doi: 10.1016/j.ejso.2020.07.018 [DOI] [PubMed] [Google Scholar]
- 19.Aaronson NK, Ahmedzai S, Bergman B, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365-376. doi: 10.1093/jnci/85.5.365 [DOI] [PubMed] [Google Scholar]
- 20.Ware JE Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36), I: conceptual framework and item selection. Med Care. 1992;30(6):473-483. doi: 10.1097/00005650-199206000-00002 [DOI] [PubMed] [Google Scholar]
- 21.Sprangers MA, te Velde A, Aaronson NK; European Organization for Research and Treatment of Cancer Study Group on Quality of Life . The construction and testing of the EORTC colorectal cancer–specific quality of life questionnaire module (QLQ-CR38). Eur J Cancer. 1999;35(2):238-247. doi: 10.1016/S0959-8049(98)00357-8 [DOI] [PubMed] [Google Scholar]
- 22.Gujral S, Conroy T, Fleissner C, et al. ; European Organisation for Research and Treatment of Cancer Quality of Life Group . Assessing quality of life in patients with colorectal cancer: an update of the EORTC quality of life questionnaire. Eur J Cancer. 2007;43(10):1564-1573. doi: 10.1016/j.ejca.2007.04.005 [DOI] [PubMed] [Google Scholar]
- 23.Emmertsen KJ, Laurberg S. Low Anterior Resection Syndrome score: development and validation of a symptom-based scoring system for bowel dysfunction after low anterior resection for rectal cancer. Ann Surg. 2012;255(5):922-928. doi: 10.1097/SLA.0b013e31824f1c21 [DOI] [PubMed] [Google Scholar]
- 24.Vaizey CJ, Carapeti E, Cahill JA, Kamm MA. Prospective comparison of faecal incontinence grading systems. Gut. 1999;44(1):77-80. doi: 10.1136/gut.44.1.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hupkens BJP, Breukink SO, Olde Reuver of Briel C, et al. Dutch validation of the Low Anterior Resection Syndrome score. Colorectal Dis. 2018;20(10):881-887. doi: 10.1111/codi.14228 [DOI] [PubMed] [Google Scholar]
- 26.Barry MJ, Fowler FJ Jr, O’Leary MP, et al. ; The Measurement Committee of the American Urological Association . The American Urological Association symptom index for benign prostatic hyperplasia. J Urol. 1992;148(5):1549-1557. doi: 10.1016/S0022-5347(17)36966-5 [DOI] [PubMed] [Google Scholar]
- 27.Rosen RC, Riley A, Wagner G, Osterloh IH, Kirkpatrick J, Mishra A. The International Index of Erectile Function (IIEF): a multidimensional scale for assessment of erectile dysfunction. Urology. 1997;49(6):822-830. doi: 10.1016/S0090-4295(97)00238-0 [DOI] [PubMed] [Google Scholar]
- 28.Rosen R, Brown C, Heiman J, et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J Sex Marital Ther. 2000;26(2):191-208. doi: 10.1080/009262300278597 [DOI] [PubMed] [Google Scholar]
- 29.Cocks K, King MT, Velikova G, et al. Evidence-based guidelines for interpreting change scores for the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Eur J Cancer. 2012;48(11):1713-1721. doi: 10.1016/j.ejca.2012.02.059 [DOI] [PubMed] [Google Scholar]
- 30.Pennings AJ, Kimman ML, Gielen AHC, Beets GL, Melenhorst J, Breukink SO. Burden of disease experienced by patients following a watch-and-wait policy for locally advanced rectal cancer: a qualitative study. Colorectal Dis. 2021;23(11):2870-2878. doi: 10.1111/codi.15838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiltink LM, Chen TY, Nout RA, et al. Health-related quality of life 14 years after preoperative short-term radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomised trial. Eur J Cancer. 2014;50(14):2390-2398. doi: 10.1016/j.ejca.2014.06.020 [DOI] [PubMed] [Google Scholar]
- 32.Pucciarelli S, Giandomenico F, De Paoli A, et al. Bowel function and quality of life after local excision or total mesorectal excision following chemoradiotherapy for rectal cancer. Br J Surg. 2017;104(1):138-147. doi: 10.1002/bjs.10318 [DOI] [PubMed] [Google Scholar]
- 33.Chen TY, Wiltink LM, Nout RA, et al. Bowel function 14 years after preoperative short-course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer. 2015;14(2):106-114. doi: 10.1016/j.clcc.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 34.Sandberg S, Asplund D, Bisgaard T, et al. Low anterior resection syndrome in a Scandinavian population of patients with rectal cancer: a longitudinal follow-up within the QoLiRECT study. Colorectal Dis. 2020;22(10):1367-1378. doi: 10.1111/codi.15095 [DOI] [PubMed] [Google Scholar]
- 35.Lange MM, Maas CP, Marijnen CA, et al. ; Cooperative Clinical Investigators of the Dutch Total Mesorectal Excision Trial . Urinary dysfunction after rectal cancer treatment is mainly caused by surgery. Br J Surg. 2008;95(8):1020-1028. doi: 10.1002/bjs.6126 [DOI] [PubMed] [Google Scholar]
- 36.Pollack J, Holm T, Cedermark B, et al. Late adverse effects of short-course preoperative radiotherapy in rectal cancer. Br J Surg. 2006;93(12):1519-1525. doi: 10.1002/bjs.5525 [DOI] [PubMed] [Google Scholar]
- 37.Bregendahl S, Emmertsen KJ, Lindegaard JC, Laurberg S. Urinary and sexual dysfunction in women after resection with and without preoperative radiotherapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2015;17(1):26-37. doi: 10.1111/codi.12758 [DOI] [PubMed] [Google Scholar]
- 38.Bruheim K, Guren MG, Dahl AA, et al. Sexual function in males after radiotherapy for rectal cancer. Int J Radiat Oncol Biol Phys. 2010;76(4):1012-1017. doi: 10.1016/j.ijrobp.2009.03.075 [DOI] [PubMed] [Google Scholar]
- 39.Rodrigues AC, Teixeira R, Teixeira T, Conde S, Soares P, Torgal I. Impact of pelvic radiotherapy on female sexuality. Arch Gynecol Obstet. 2012;285(2):505-514. doi: 10.1007/s00404-011-1988-5 [DOI] [PubMed] [Google Scholar]
- 40.Viswanathan AN, Lee LJ, Eswara JR, et al. Complications of pelvic radiation in patients treated for gynecologic malignancies. Cancer. 2014;120(24):3870-3883. doi: 10.1002/cncr.28849 [DOI] [PubMed] [Google Scholar]
- 41.Svanström Röjvall A, Buchli C, Bottai M, et al. Effect of radiotherapy for rectal cancer on female sexual function: a prospective cohort study. Br J Surg. 2020;107(5):525-536. doi: 10.1002/bjs.11373 [DOI] [PubMed] [Google Scholar]
- 42.Mols F, Husson O, Oudejans M, Vlooswijk C, Horevoorts N, van de Poll-Franse LV. Reference data of the EORTC QLQ-C30 questionnaire: five consecutive annual assessments of approximately 2000 representative Dutch men and women. Acta Oncol. 2018;57(10):1381-1391. doi: 10.1080/0284186X.2018.1481293 [DOI] [PubMed] [Google Scholar]
- 43.Nolte S, Liegl G, Petersen MA, et al. ; EORTC Quality of Life Group . General population normative data for the EORTC QLQ-C30 health-related quality of life questionnaire based on 15,386 persons across 13 European countries, Canada and the United States. Eur J Cancer. 2019;107:153-163. doi: 10.1016/j.ejca.2018.11.024 [DOI] [PubMed] [Google Scholar]
- 44.Giandomenico F, Gavaruzzi T, Lotto L, et al. Quality of life after surgery for rectal cancer: a systematic review of comparisons with the general population. Expert Rev Gastroenterol Hepatol. 2015;9(9):1227-1242. doi: 10.1586/17474124.2015.1070667 [DOI] [PubMed] [Google Scholar]
- 45.Carr AJ, Gibson B, Robinson PG. Measuring quality of life: is quality of life determined by expectations or experience? BMJ. 2001;322(7296):1240-1243. doi: 10.1136/bmj.322.7296.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van de Poll-Franse LV, Mols F, Gundy CM, et al. Normative data for the EORTC QLQ-C30 and EORTC-sexuality items in the general Dutch population. Eur J Cancer. 2011;47(5):667-675. doi: 10.1016/j.ejca.2010.11.004 [DOI] [PubMed] [Google Scholar]
- 47.van Heinsbergen M, Van der Heijden JAG, Stassen LP, et al. The low anterior resection syndrome in a reference population: prevalence and predictive factors in the Netherlands. Colorectal Dis. 2020;22(1):46-52. doi: 10.1111/codi.14790 [DOI] [PubMed] [Google Scholar]
- 48.Juul T, Elfeki H, Christensen P, Laurberg S, Emmertsen KJ, Bager P. Normative data for the Low Anterior Resection Syndrome score (LARS score). Ann Surg. 2019;269(6):1124-1128. doi: 10.1097/SLA.0000000000002750 [DOI] [PubMed] [Google Scholar]
- 49.Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population-based cross-sectional study. Colorectal Dis. 2013;15(9):1130-1139. doi: 10.1111/codi.12244 [DOI] [PubMed] [Google Scholar]
- 50.Soerensen MM, Buntzen S, Bek KM, Laurberg S. Complete obstetric anal sphincter tear and risk of long-term fecal incontinence: a cohort study. Dis Colon Rectum. 2013;56(8):992-1001. doi: 10.1097/DCR.0b013e318299c209 [DOI] [PubMed] [Google Scholar]
- 51.Pietrzak L, Bujko K, Nowacki MP, et al. ; Polish Colorectal Study Group . Quality of life, anorectal and sexual functions after preoperative radiotherapy for rectal cancer: report of a randomised trial. Radiother Oncol. 2007;84(3):217-225. doi: 10.1016/j.radonc.2007.07.007 [DOI] [PubMed] [Google Scholar]
- 52.McLachlan SA, Fisher RJ, Zalcberg J, et al. The impact on health-related quality of life in the first 12 months: a randomised comparison of preoperative short-course radiation versus long-course chemoradiation for T3 rectal cancer (Trans-Tasman Radiation Oncology Group Trial 01.04). Eur J Cancer. 2016;55:15-26. doi: 10.1016/j.ejca.2015.10.060 [DOI] [PubMed] [Google Scholar]
- 53.Erlandsson J, Fuentes S, Radu C, et al. Radiotherapy regimens for rectal cancer: long-term outcomes and health-related quality of life in the Stockholm III trial. BJS Open. 2021;5(6):zrab137. doi: 10.1093/bjsopen/zrab137 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Treatment Details of 278 Patients Included for the Current Analysis
eTable 1. Quality of Life and Functional Outcome of Patients Followed by Watch and Wait Without Requiring Additional Surgery at 3, 12, and 24 Months Following Inclusion
eTable 2. Quality of Life and Functional Outcome of Patients Treated With Local Excision (LE) and Patients Treated With Total Mesorectal Excision (TME) Compared to Patients Followed by Watch-and-Wait Without Requiring Additional Surgery (W&W) at 24 Months Following Inclusion
eTable 3. Association of Sex, Age, Tumor Distance, and Neoadjuvant Treatment on Quality of Life and Functional Outcome at 24 Months Following Inclusion
eTable 4. Quality of Life and Functional Outcome of All Patients Included in the Dutch Watch-and-Wait Registry at 3, 12, and 24 Months Following Inclusion
Nonauthor Collaborators
Data Sharing Statement