Abstract
Blood-based brain biomarkers (BBM) such as glial fibrillary acidic protein (GFAP) and ubiquitin carboxy-terminal hydrolase L1 (UCH-L1) have potential to aid in the diagnosis of concussion. Recently developed point-of-care test devices would enable BBMs to be measured in field settings such military and sport environments within minutes of a suspicious head hit. However, head hits in these environments typically occur in the setting of vigorous physical exertion, which can itself increase BBMs levels. Thus, efforts to develop BBMs as acute concussion aids in field settings need to account for the effects of physical exertion. To determine the acute effects of physical exertion on the BBMs, we measured GFAP, UCH-L1, tau, and neurofilament light chain (NF-L) immediately before, immediately after, and 45 min after a single workout session consisting of aerobic and resistance exercises in 30 collegiate football players. Subjects wore body sensors measuring several aspects of exertion and underwent diffusion tensor imaging 24 h before and 48 h after exertion. All subjects were male with a mean age of 19.5 ± 1.2 years. The mean duration of activity during the workout session was 94 ± 31 min. There was a significant decrease in serum GFAP immediately after (median decrease of 27.76%, p < 0.0001) and a significant increase in serum UCH-L1 45 min after (median increase of 37.11%, p = 0.016) exertion, compared with pre-exertion baseline. No significant changes in tau or NF-L were identified. The duration of exertion had a significant independent linear correlation to the increase in serum UCHL1 from pre-exertion to 45 min after exertion (r = 0.68, p = 0.004). There were no significant pre- to post-exertional changes in any of the 39 examined brain white matter regions, and biomarker changes did not correlate to variation in white matter integrity in any of these regions. Thus, exertion appeared to be associated with immediate decreases in serum GFAP and very acute (45 min) increases in UCH-L1. These changes were related to the duration of exertion, but not to changes in brain white matter integrity. Our results have important implications for how these BBMs might be used to aid in the on-scene diagnosis of concussion occurring in the setting of physical exertion.
Keywords: brain biomarkers, concussion, glial fibrillary acidic protein, physical exertion, point of care test, ubiquitin carboxy-terminal hydrolase L1
Introduction
Despite the marked increase in scientific attention to concussion in the past decade, the diagnosis remains largely based on subjective reporting of symptoms, which are nonspecific and highly prevalent among those without concussion.1-3 Accumulating evidence suggests that blood-based brain biomarkers (BBMs) may be good injury classifiers and could be used to assist in concussion diagnosis.4–10 Although two BBMs (glial fibrillary acidic protein [GFAP] and ubiquitin carboxy-terminal hydrolase L1 [UCH-L1]) currently have U.S. Food and Drug Administration (FDA) clearance, their use is restricted to assisting in the decision to obtain a head CT scan, and not as a concussion diagnostic. The recent FDA clearance of a point-of-care test device for GFAP/UCH-L1 makes real the possibility that these BBMs, pending rigorous validation, could in the near future be used to aid concussion diagnosis in a field setting within minutes of a suspicious head hit,11 providing a practical breakthrough in acute concussion care. Recent evidence demonstrating elevations of GFAP within min of head impact underscore the feasibility of on-scene concussion diagnosis using BBMs.12
Situations in which a rapid and accurate point-of-care diagnostic are arguably the most critical include combat and contact sport environments. Missed concussion diagnoses in these settings have the potential to put others at risk due to impaired neurologic function and to increase the risk of more permanent sequalae should a second head hit occur. However, concussions in these environments typically occur in the setting of vigorous physical exertion, which can itself increase BBMs levels in blood. Thus, efforts to develop acute, point-of-care BBMs as aids to concussion diagnosis in military and sports settings need to account for the effects of physical exertion.
Several BBMs have been reported to be acutely elevated in peripheral blood after physical exertion (e.g., S100B, brain-derived neurotrophic factor [BDNF], total tau, neuron specific enolase [NSE], and visinin-like protein),13–19 although data related to GFAP and UCH-L1 are lacking. Mechanistic insight into exertional effects on brain proteins comes primarily from S100B and BDNF. Exertional increases in S100B are thought to result from stress-induced serotonin release activating astrocytes to express S100B via 5-HT1A receptors and increasing blood–brain barrier permeability.17,18,20–27 BDNF increases putatively emerge from upregulation of messenger RNA (mRNA) expression stimulated by exertion-related increases in ketogenic β-hydroxybutyrate.28,29 Both S100B and BDNF are expressed by cells outside the central nervous system, raising the possibility that non-brain effects of exercise may also contribute to post-exertional increases of these biomarkers.30–33 The degree of elevation of S100B and BDNF is related primarily to the intensity, duration, and type of physical exertion.34,35 For example, exertion involving running13,16,36 and weight lifting36 produce higher levels of S100B and BDNF than cycling35,37 and swimming.14 Despite this pattern, mechanical injury to neural elements due to running and jumping-related axial impacts were not thought to be a factor in exertional increases of these proteins,38 although this has not been explicitly investigated. Diffusion magnetic resonance imaging (MRI) studies have revealed beneficial white matter (WM) changes associated with aerobic exertion programs lasting 6-9 months (increased fractional anisotropy in uncinate fasciculus, fornix, and genu of corpus callosum),39-41 but to our knowledge the effects of single workout session—either beneficial or detrimental—have not been reported.
The extent to which exercise influences acute blood levels of brain biomarkers other than S100B and BDNF is relatively understudied. Post-exertional levels of serum GFAP were found to be elevated in one study42 and undetectable in another.19 To our knowledge, no studies have examined the effects of physical exertion on UCH-L1. Our primary objective was to determine the acute effects of physical exertion on GFAP and UCH-L1, as well as two additional widely researched BBMs (tau and neurofilament light chain [NF-L]), among a cohort of collegiate athletes in which each subject served as their own control. Our secondary objectives were to determine which aspects of physical exertion drive post-exertional BBM increases and the extent to which BBM increases reflect changes in brain WM integrity.
Methods
Study design
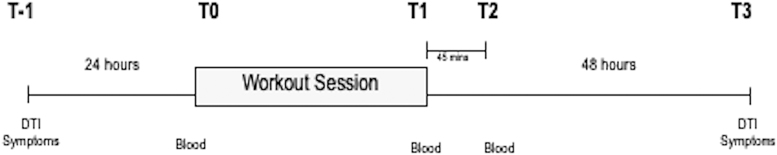
We conducted a prospective observational study of 30 collegiate football players before and after a single team workout session during the non-competitive spring season. Blood was collected immediately before (T0) and at two time-points after the session: immediately (T1) and 45 min (T2) post exertion. Diffusion tensor imaging (DTI) scanning was performed 24 h before (T-1) and 48 h after (T3) the workout session. (Fig. 1) All subjects wore a body-mounted sensor to measure several aspects of physical exertion during the workout session. Subjects were recruited from members of the University of Rochester (UR) Division III football team during the 2018-2019 academic year (n = 15), and the 2019-20 academic year (n = 15). Subjects were eligible for inclusion if they were >18 years old at the time of consent, an active member of the varsity football team, and expected to play in at least one football game over the course of the fall season. Subjects were excluded if they were diagnosed with a concussion within the month prior to study activities or if they had any contraindications to MRI scanning (e.g., dental braces, retained metallic foreign bodies, claustrophobia, etc.). The study protocol and the process of informed consent was reviewed and approved by the University of Rochester Research Subjects Review Board. Written informed consent was obtained from all participants prior to study activities.
FIG. 1.
Study overview. In all subjects (n = 30), blood samples were obtained immediately before (T0) and at two time-points after a workout session; immediately (T1) and 45 min (T2) after. Diffusion tensor imaging (DTI) and concussion symptom inventory were obtained in half the cohort (n = 15) 24 h before (T-1) and 48 h after (T3) the workout.
Workout session
The exertional protocol consisted of a mix of aerobic and resistance training. Athletes were instructed to perform a fixed series of exercises from a comprehensive list but were free to choose specific exercises within series. (Table 1) Workout sessions occurred in six groups of five subjects approximately 3 months after the last football game of the fall season. Each session took place in the same workout space (62′ × 46′) within the UR varsity training facilities and supervised by research study personnel. This space was temperature-controlled at 70°F for all sessions.
Table 1.
Exertional Workout Protocol
| Warm up (all) | ||||
|---|---|---|---|---|
| Burpees × 15 sec | Russian Hops × 15 sec | Mountain Climbers × 15 sec | ||
| Correctives (3-4 of the following) | ||||
|---|---|---|---|---|
| Goblet Squats × 10 Band pull-throughs × 10 Single leg glute bridge × 6 each |
Prone shoulder mobility Prone back and scapula (Y-T-W) × 5 each DBc 5-10 lbs | Standing pushups × 10 Laying shoulder press × 10 |
||
| Core lift (3) | ||||
|---|---|---|---|---|
| Squat 3 sets of 10-12 reps Clean Pull 4 sets of 3-5 reps RDLa Barbell 3 sets of 10 reps Dynamic Bench 8 sets of 3 reps |
Dynamic Squat 8 sets of 3 reps Bench press 3 sets of 10-12 reps BBb Overhead Press 3 sets of 6 reps BBb Power Shrugs 3 sets of 6 reps |
Seated Cable Row 3 sets of 8-12 reps Triceps Cable push-down 3 sets of 6-10 reps Skater Jumps 3 sets of 20-30 secs DBc Incline Press 3 sets of 6 reps |
||
| Main accessory lifts (2-3) | OR | Clean progression (all) | ||
|---|---|---|---|---|
| DBc lunge 3 sets of 8 reps DBc Shrug 3 sets of 12 reps Pull-ups 3 sets of 8-10 reps Bent over BB row 3 sets of 10 reps Landmines 1 set of 8 reps Negative Pull-ups 3 sets of 6 reps DBc Shoulder Side raises 3 sets of 8-12 reps |
Clean pulls Front squat Hang clean |
|||
| Triceps circuit (all) | OR | Accessory circuits (2-3) | OR | Bicep/deltoid circuit (all) |
|---|---|---|---|---|
| Half Full Skull crushers |
Hammer Curls Farmer Walk Rear foot elevated split squats Medicine Ball good mornings Farmer Plate carry |
Upright row Overhead press Bicep curl |
Romanian dead lift; bBarbell; cDumbbell.
Reps, repetitions.
Sensor-based measures of physical exertion
To capture the intensity and duration of physical exertion, each subject was outfitted with PlayerTek Wearable GPS Tracker (Catapult Sports, Melbourne, Australia) over the upper back, held securely within a lightweight vest fitted to each individual. This device is composed of a 10Hz Global Positioning/Global Navigation Satellite System, a 400 Hz triaxial accelerometer, gyroscope, and a 10 Hz triaxial magnetometer to track player movement and the intensity of physical exertion. Repeat measurements on the same device (intra-unit reliability) vary by 0.01% to <3.0%, with the majority of measurements varying by <1.0%. Within device (inter-unit) test-retest reliability revealed intraclass correlation coefficients ranging from 0.77 (95% confidence interval [CI]: 0.62–0.89) to 1.0 (95% CI: 0.99–1.0).43 Information from these four sensors wirelessly uploads to cloud-based software. When combined with manually input data on each subject's body weight, the software calculates total duration of exertion, distance traveled (km), energy expenditure (kilocalories), power score (watts/kg), player load (sum of all accelerations across all axes of the triaxial accelerometer), and work ratio (percentage of activity time during which player was moving at speeds >1.5 m/sec).44-46 It also detects body impacts >3 Gs,47–50 although the workout session did not involve any contact between athletes. Data collected by the PlayerTek device was downloaded from the cloud using the PlayerTek Sync Tool (version 5.68).
Blood sampling and analysis
Approximately 500 μL of capillary whole blood was obtained from each subject's fingertip using 14-gauge 2-mm incision depth BD Microtainer® contact-activated lancets (BD Biosciences, Franklin Lakes, NJ) directly into red stopper BD Microtainer non-sterile tubes (BD Biosciences). Samples were collected immediately prior to the workout session (T0), immediately following the workout session (T1), and 45 min following the workout session (T2). Samples were immediately placed on ice until centrifuged at 12,000 RPM for 7 min at room temperature within 1 h of collection. Aliquoted serum was immediately frozen and stored at -80°C until analysis.
Serum levels of NF-L, tau, UCH-L1, and GFAP were measured using a SIMOA® 4-plex assay kit (Quanterix Corp, Billerica, MA), which is a magnetic bead-based digital enzyme-linked immunosorbent assay (ELISA) that allows detection of biomarkers in femtomolar concentrations.51,52 All four biomarkers were measured from the same blood sample. Fifty microliters of serum were mixed with either anti-NF-L, anti-tau, anti-UCH-L1, or anti-GFAP antibody-coated paramagnetic capture beads and biotinylated detector antibody. Lower limits of detection were 0.104 pg/mL (NF-L), 0.024 pg/mL (tau), 0.221 pg/mL (GFAP), and 1.74 pg/mL (UCH-L1), and their respective intra-assay coefficients of variation are 5.4%, 6.7%, 3.7%, and 11.3%.
Assays were batched to minimize variability and technicians were blinded to clinical data and groups, with each batch run with appropriate standards and controls to ensure reliability. Longitudinal samples from the same individual were run on the same plate to reduce potential batch effects. Possible batch effects were minimized by including a standard case and control on all plates that are analyzed. All samples were analyzed in duplicate but could not be re-run in some instances due to the limited amount of serum derived from capillary whole blood. In cases where the analytical coefficient of variation (CV) exceeded 20%, values were included in our analysis if the CV for average enzyme per bead was less than 20%.52 Samples in which the analytical CV and bead count CV both exceeded 20% were excluded from further analysis. These results were considered missing at random and not imputed.
Neuroimaging
Fifteen of the 30 participating subjects were selected at random to undergo DTI neuroimaging 24 h before and 48 h after the workout session (T3). All images were obtained on a Siemens 3T MRI MAGNETOM PrismaFit whole-body scanner (Siemens Healthcare, Erlangen, Germany) running on software version VE11c, using a 64-channel phased array head coil. The imaging sequence consisted of a single-shot spin-echo echo planar imaging sequence with repetition time/echo time = 8000/89 msec, isotropic voxel 2 × 2 × 2 mm, iPAT (Generalized Autocalibrating Partially Parallel Acquisition) acceleration factor = 2, 60 directions of diffusion weighting, b = 1200 sec/mm2, one non-diffusion-weighted image (b = 0, denoted B0) with 10 averages. Three images at b = 0 sec/mm were acquired with reversed phase encoding to assist with distortion correction. Pre-processing included motion correction using rigid registration with the Advanced Normalization Tools (ANTS) software package,53 with adjustments to the gradient table performed based on patient position. Distortion correction with the Topup algorithm,54 as implemented in FSL (Functional MRI Brain Software Library),55 was performed using the b = 0 images with reverse phase encoding and the resulting transformation was applied to each gradient direction.
After distortion correction, robust estimation of tensors by outlier rejection (RESTORE) within the TORTOISE software package was performed,56 followed by computation of fractional anisotropy (FA), axial diffusivity (AD), radial diffusivity (RD), and mean diffusivity (MD). For cross-sectional analyses, measurements were averaged across voxels within 39 white matter regions of interest, defined automatically using the Diffusion-Oriented Tract Segmentation (DOTS) tract segmentation algorithm.
For longitudinal analyses, images were initially processed in the original acquisition space. Regions of interest were defined automatically using the DOTS tract segmentation on only the baseline image.57 To align longitudinal images across time-points, the baseline T1-w image for each subject was rigidly registered to Montreal Neurological Institute (MNI) space. The baseline motion and distortion corrected B0 image was then rigidly registered to the T1-w image, and the B0 images of each subsequent time-point were registered to the baseline B0 in MNI space. Finally, diffusion measurements and tract labels were transformed into MNI space for each time-point using concatenated spatial transformations where appropriate. Measurements were averaged across voxels within the 39 regions of interest for each subject.
Symptoms
Subjects were asked to self-report concussion-related symptoms at the time of DTI scanning 24 h before and 48 h after the workout session using the 22-item checklist in the Sport Concussion Assessment Tool (SCAT5)58 This checklist asks subjects to rate each symptom on a scale from 0 (none) to 6 (severe); thus, the total symptom score could range from 0-132. Because symptoms were assessed at the time of DTI, they were only obtained in the subset (n = 15) randomly selected for neuroimaging.
Statistical analysis
Biomarker changes (both absolute and relative) were described using means, standard deviations, medians, and interquartile ranges. Dependent sample t-tests were used to evaluate the statistical significance of longitudinal biomarker changes. Associations between exertional metrics and changes in biomarkers were evaluated Pearson's correlation coefficient r. The coefficient of determination (R2) was used to estimate the proportion of variation in biomarker changes explained by significant exertion. Changes in DTI metrics (i.e., regional FA and MD values) were evaluated using dependent sample t-tests, and these changes were associated with biomarker changes using Spearman correlation coefficients. Bonferroni corrections were made to limit inflation of the Type I error rates associated with multiple comparisons. This correction moved the p value indicating significance from p < 0.05 to <0.0063 for analyses involving the eight exertional metrics, and to <0.0013 for analyses involving the 39 brain white matter regions. We used SAS version 9.4 (SAS Institute, Cary, NC) for all statistical analyses.
Results
Subject characteristics
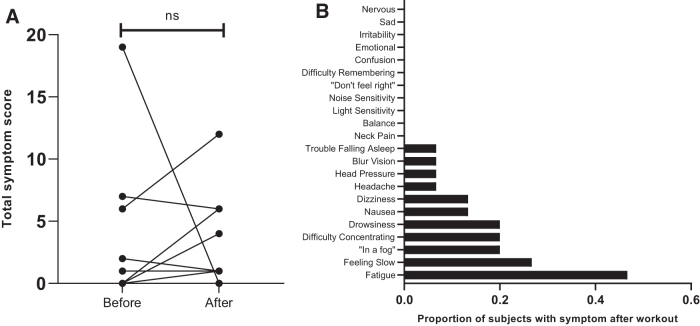
All subjects were male with a mean age of 19. 5 ± 1.2 years. (Table 2) Subjects represented an array of offensive and defensive football positions. The subset of 15 subjects who underwent DTI scanning and symptom evaluation were similar to the full cohort in terms of age (19.3 ± 1.3 years), race (93% white), ethnicity (100% non-Hispanic/Latino), and number of prior concussions (none: 66.7%, 1: 26.7%, >1: 6.7%). Compared with before the workout session, there was no significant change in mean total symptom score after the session (2.33 ± 5.1 vs. 2.73 ± 3.5; Fig. 2) The most frequently reported symptom after the workout session was fatigue (47%).
Table 2.
Subject Characteristics
| Characteristic | All subjects (n = 30) | Subjects undergoing DTI (n = 15) |
|---|---|---|
| Age, mean years (SD) | 19.5 (1.2) | 19.3 (1.3) |
| Sex, n male (%) | 30 (100) | 15 (100) |
| Race, n (%) | ||
| Black/AAa | 3 (10) | 0 |
| White | 24 (80) | 14 (93.3) |
| White + Asian or Black/AAa | 2(6.7) | 1 (6.7) |
| Other | 1 (3.3) | 0 |
| Ethnicity, n (%) | ||
| Hispanic/Latino | 0 | 0 |
| Not Hispanic/Latino | 30 (100) | 15 (100) |
| BMI, mean kg/m2 (SD) | 29.39 (5.0) | 28.48 (4.8) |
| Player position, n (%) | ||
| Center | 1 (3.3) | 1 (6.7) |
| Defensive back | 6 (20) | 2 (13.3) |
| Defensive line | 7 (23.3) | 3 (20) |
| Line backer | 7 (23.3) | 3 (20) |
| Offensive line | 4 (13.3) | 2 (13.3) |
| Running back | 2 (6.7) | 1 (6.7) |
| Tight end | 1 (3.3) | 1 (6.7) |
| Wide receiver | 2 (6.7) | 2 (13.3) |
| Number of prior concussionsb, n (%) | ||
| 0 | 18 (60) | 10 (66.7) |
| 1 | 7 (23.3) | 4 (26.7) |
| 2 | 3 (10) | 1 (6.7) |
| 3 | 2 (6.7) | 0 |
| > 3 | 0 | 0 |
AA, African American; b> 1 month before study.
DTI, diffusion tensor imaging; SD, standard deviation; BMI, body mass index.
FIG. 2.
Symptoms before and after exertion. (A) Total concussion symptom scores before and after workout session. Each black line represents a different subject. Value above the horizontal line represent p value from paired t-test, where statistical significance is defined as p < 0.05; values >0.05 are indicated by “ns” (non-significant). (B) Proportion of subjects reporting individual symptoms after workout session; n = 15 for both graphs.
Measures of exertion during the workout session
The mean duration of activity during the workout session was 94 ± 31 min during which athletes expended an average of 212 ± 79 kcal of energy (Supplementary Table S1). Although the workout did not involve physical contact between the athletes, the PlayerTek sensors detected a per-athlete average of 58.8 ± 67.7 impacts between 3-5 Gs and 2.9 ± 10.2 impacts between 5-10 Gs. Study coordinators present during the workout sessions did not observe any athlete-to-athlete contact. To understand why the sensors detected impacts in the absence of physical contact between players, we observed a single collegiate athlete performing individual elements of the workout session while wearing the PlayerTek sensor. Non-contact activities detected as impacts between 3 and 5 Gs on the PlayerTek sensor included hopping, box jumps, sensor contacting bench during dumbbell work, and sensor contacting the floor during weighted and unweighted crunches. Non-contact activities detected as impacts between 5 and 10 Gs included sensor contacting the floor during weighted crunches and off-body contact of the sensor (either inside or outside the vest) against a hard surface such as the floor. There were no impacts measured above 10 Gs.
Brain biomarker changes before and after workout session
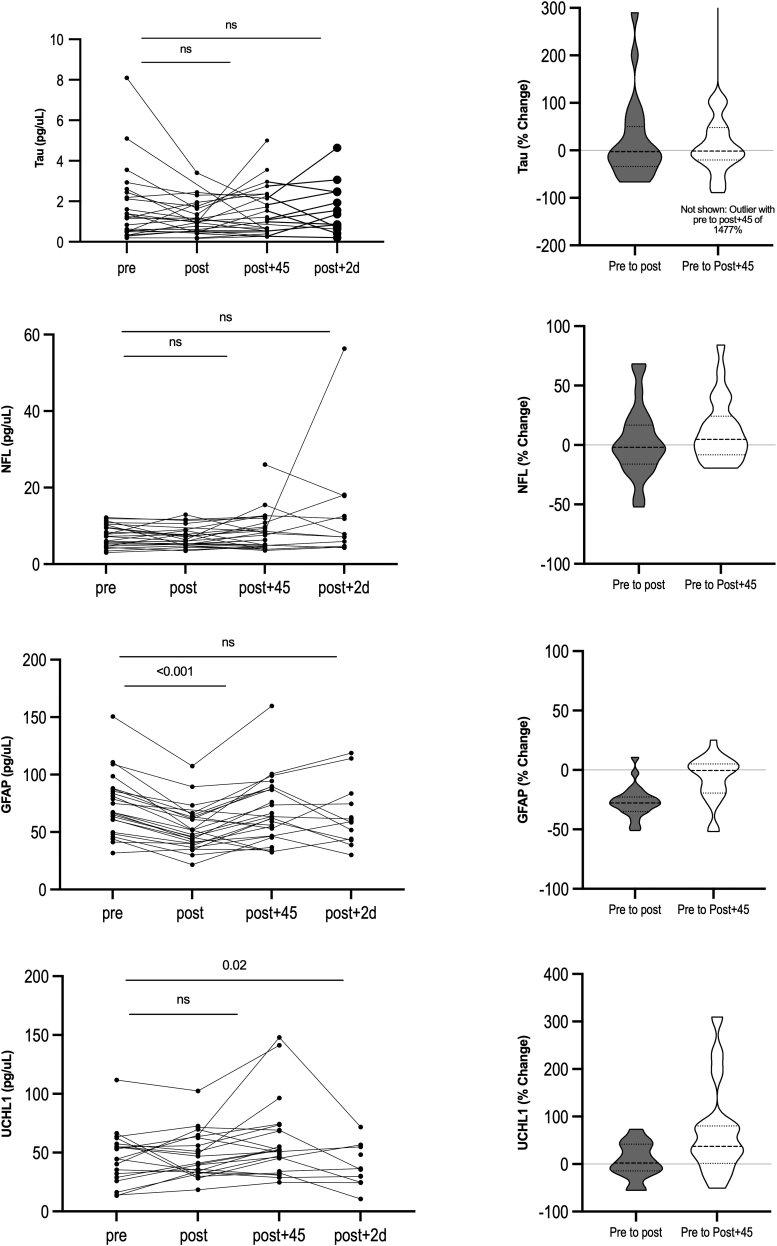
Some biomarker samples were excluded from analysis due to insufficient quantity of blood or analytical CV and bead count CV exceeding 20%. (Supplementary Tables S2 and S3) This resulted in some subjects not having BBM values from all three study time-points. (Supplementary Table S4) Relative to pre-exertion (T0), there was a significant decrease in serum GFAP immediately following exertion [T1; median decrease of 27.76%; t (22) = -9.72, p < 0.0001] that returned to baseline levels by 45 min following exertion (T2; Table 3; Fig. 3) There was a significant increase in serum UCH-L1 45 min after exertion [median increase of 37.11%; t (15) = 2.72, p = 0.016]. There was a small but statistically non-significant increase in NF-L 45 min after exertion (median and mean increase 4.7% and 12% respectively). There were sizable non-significant increases in mean tau at both time-points (likely due to a single outlier), however the median values were very close to zero. Biomarker levels at each study time-point are provided in Supplementary Table S5.
Table 3.
Absolute and Relative Brain Biomarker Changes Before and After Workout Session
| |
|
Absolute change |
Relative change |
||||
|---|---|---|---|---|---|---|---|
| Biomarker | n | Mean pg/μL (SD) |
Median pg/μL | IQRe pg/μL |
Mean % (SD) |
Median % | IQRe % |
| Tau | |||||||
| ΔT0-T1a | 22 | -0.37 (1.23) | -0.015 | -0.70 – 0.26 | 19 (87) | -2.6 | -34 − 50 |
| ΔT0-T2b | 22 | -0.23 (2.03) | -0.015 | -0.50 – 0.29 | 73 (318) | -1.5 | -20 − 48 |
| NF-L | |||||||
| ΔT0-T1a | 23 | -0.18 (2.28) | -0.19 | -0.90 – 0.72 | 2.8 (30) | -1.9 | -16 − 17 |
| ΔT0-T2b | 22 | 0.81 (1.90) | 0.35 | -0.47 – 1.46 | 12 (27) | 4.7 | -8.3 − 24 |
| GFAP | |||||||
| ΔT0-T1a,c | 23 | -20.54 (12.15) | -19.54 | -23.26 – -15.31 | -26.68 (13.16) | -27.76 | -34.93 – -22.77 |
| ΔT0-T2b | 22 | -5.31 (13.43) | -0.29 | -14.5-3.05 | -7.25 (19.14) | -0.43 | -19.34 – 5.1 |
| UCH-L1 | |||||||
| ΔT0-T1a | 15 | -1.09 (18.02) | 1.16 | -9.35-12.18 | 6.38 (37.52) | 2.11 | -14.26 – 42.06 |
| ΔT0-T2b,d | 16 | 19.26 (30.17) | 19.57 | 1.68 – 30.73 | 68.82 (97.9) | 37.11 | 1.62 – 80.17 |
Change in biomarker concentration from immediately before workout to immediately after workout; bChange in biomarker concentration from immediately before workout to 45 min after workout; cp < 0.05; dp < 0.01; eInterquartile range.
SD, standard deviation; NF-L, neurofilament light chain; GFAP, glial fibrillary acidic protein; UCH-L, ubiquitin carboxy-terminal hydrolase L1.
FIG. 3.
Blood-based biomarker changes before and after a workout session. Left column: Absolute longitudinal changes in blood biomarker concentrations. Each black line represents a different subject. Values above the horizontal line represent p value from paired t-test, where statistical significance is defined as p < 0.05; values >0.05 are indicated by “ns” (non-significant). Right column. Relative changes in serum blood biomarker concentrations for entire cohort. Truncated violin plots display the rotated kernel density for each of the four biomarkers from pre to immediately post exertion (gray) and from pre-exertion to 45 min post-exertion (white). Dashed lines represent median, dotted lines represent interquartile range.
Relationship between brain biomarker changes and measures of exertion
Serum biomarker changes had a significant (p < 0.05) positive correlation with exercise duration, distance traveled, and kilocalories of energy expended, and a negative correlation with power expended and body impacts between 3 and 5 Gs (Table 4). However, only exercise duration (r = 0.004) remained significant after applying a Bonferroni-corrected p value of <0.0063. This variable accounted for 44% and 47%, respectively, of the variation in T0 to T2 change in serum concentrations of NF-L and UCH-L1.
Table 4.
Significant Correlations between Exercise Metrics and Brain Biomarker Changes Before and After Workout Session
| Biomarker | Correlation with exercise metrics, re |
|||||||
|---|---|---|---|---|---|---|---|---|
| Duration | Distance | Kilocalories | Power score | Player load | Work ratio | Impact 3-5 g | Impacts 5-10 g | |
| Tau ΔT0-T1a |
||||||||
| ΔT0-T2b | 0.50c | |||||||
| NF-L ΔT0-T1a |
||||||||
| ΔT0-T2b | 0.66 | -0.44c | ||||||
| GFAP ΔT0-T1a |
0.45c |
|||||||
| ΔT0-T2b | -0.48c | |||||||
| UCH-L1 ΔT0-T1a |
||||||||
| ΔT0-T2b | 0.68 | 0.55c | ||||||
Change in biomarker concentration from immediately before workout to immediately after workout; bChange in biomarker concentration from immediately before workout to 45 min after workout; cp < 0.05; dp < 0.01; ePearson correlation coefficient.
Bold: Bonferonni-corrected p value <0.0063.
NF-L, neurofilament light chain; GFAP, glial fibrillary acidic protein; UCH-L, ubiquitin carboxy-terminal hydrolase L1.
Brain WM integrity before and after the workout session
We identified pre- to post-exertional changes in FA and/or MD in 14 of the 39 examined WM regions that were significant at a p value of <0.05. However, none were significant after applying a Bonferroni-corrected p value of <0.0013 (Table 5).
Table 5.
Brain Regions with Significant Changes in White Matter Integrity Before and After Workout Session
| Brain region | Fractional anisotropy, median % Δ | Mean diffusivity, median % Δ |
|---|---|---|
| Right anterior thalamus radiation | 88.7a | |
| Posterior corpus callosum | 28.3a | |
| Superior corpus callosum | 77.6a | |
| Left cingulum | 17.9a | |
| Right fornix | -120.7a | |
| Left inferior fronto-occipital fasciculus | 54.7a | |
| Right inferior fronto-occipital fasciculus | 57.5a | |
| Middle cerebellar peduncle | -97.1a | |
| Left optic radiation | 77.5a | |
| Right optic tracts | -394.4b | 170.7a |
| Left superior fronto-occipital fasciculus | 71.3a | |
| Left superior longitudinal fasciculus | 120.0a | |
| Left uncinate fasciculus | -113.3a | 106.5a |
| Right uncinate fasciculus | -129.6b |
p < 0.05; bp < 0.01.
Bold: Bonferonni-corrected p value <0.0013.
Relationship between brain biomarker changes and changes in WM integrity
Brain biomarker changes significantly correlated with FA and/or MD in several white matter regions of interest (ROIs); in two ROIs these correlations had p values of <0.01. However, none of the correlations remained significant after applying a Bonferroni-corrected p value of <0.0013 (Table 6).
Table 6.
Brain Regions in which Brain Biomarker Changes were Significantly Correlated with Changes in White Matter Integrity Before and After a Workout Session
| Biomarker | Region and correlationa with FA Δ (T-1)-T3b | Region and correlationa with MD Δ (T-1)-T3c | ||
|---|---|---|---|---|
| Tau ΔT0-T1d |
Posterior CC: -0.68f |
Tapetum: -0.72f |
Left IFOC: -0.68f Left ILF: -0.68f |
|
| ΔT0-T2e | Left OT: 0.65f |
Right Cing: -0.67f Left IFOC: -0.67f Left ILF: -0.68f Left OR: -0.72f | Left PRT: -0.72f Left SLF: -0.71f Left STR: -0.71f Left UF: -0.65f | |
| NF-L ΔT0-T1d |
— |
Posterior CC: 0.78f Right CST: 0.67f |
Tapetum: 0.80g |
|
| ΔT0-T2e | — | Right SFOF: -0.71f | ||
| GFAP ΔT0-T1d |
Right ATR: 0.67f |
Left IFOC: 0.67f |
— |
|
| ΔT0-T2e | Left OT: 0.73f | Right UF: -0.64f | ||
| UCH-L1 ΔT0-T1d |
Right UF: -0.83g |
Right PTR: 0.74f |
||
| ΔT0-T2e | Posterior CC: -0.82f | Right UF: -0.86f | — | |
Spearman correlation coefficient; bChange in fractional anisotropy from 24 h before workout to 48 h after workout; cChange in mean diffusivity from 24 h before workout to 48 h after workout; dBiomarker change from immediately before workout to immediately after workout; eBiomarker change from immediately before workout to 45 min after workout; fp < 0.05, gp < 0.01.
Bold: Bonferonni-corrected p value <0.0013.
ATR, anterior thalamus radiation; CC, corpus callosum; CPT, corticopontine tract; Cing, cingulum; CST, corticospinal tract; ICP, inferior cerebellar peduncle; IFOC, inferior fronto-occipital fasciculus; ILF, inferior longitudinal fasciculus; ML, medial lemniscus; OR, optic radiation; OT, optic tract; PTR, posterior thalamus radiation; SCP, superior cerebellar peduncle; SFOF, superior fronto-occipital fasciculus; SLF, superior longitudinal fasciculus; STR, superior thalamus radiation; UF, uncinate fasciculus.
Discussion
In the current study, we demonstrate that a single workout session consisting of aerobic and resistance training was associated with both increases and decreases in brain proteins in the peripheral circulation. We observed a 27.76% decrease in serum GFAP immediately after and a 37.11% increase in serum UCH-L1 45 min after exertion. There was a small but non-significant increase in NF-L 45 min after exertion, and no appreciable changes in tau.
Our study is novel in that we examined eight metrics of exertion that might be driving changes in biomarkers. We found that the duration of exertion had a significant independent linear correlation to increases in serum NF-L and UCH-L1 from pre-exertion to 45 min after exertion, accounting for 44% and 47%, respectively, of variation in changes. Kilocalories of energy expended may also be an important driver of BBM changes. The correlations involving kilocalories were in the expected direction (positive) and statistically significant at the p < 0.05 level, although nonsignificant applying Bonferroni correction for multiple comparisons. These results suggest that exercise plays an important role in changes in these brain proteins.
Our study is additionally novel in that we explored the relationship between biomarker changes and exercise-related changes in brain WM integrity. We found no significant pre- to post-exertional changes in any of the 39 examined brain WM regions, and biomarker changes did not correlate to variation in WM integrity in any of these ROIs. This suggests that a brief period of aerobic and resistance training neither improves WM integrity (increased FA) nor results in an injury pattern (decreased FA). These results also make it unlikely that the observed biomarker changes are due to changes in brain WM integrity.
Aside from S100B and BDNF, very few studies have explored the acute effects of physical exertion on brain proteins. In contrast to our results showing no exercise-related changes in tau, Di Battista and colleagues found an increase in tau (as well as in NSE, CK-BB, and neurogranin) immediately after high intensity interval training on a cycle ergometer in 11 adult males. GFAP was undetectable in 80% of subjects.19 Our results may have differed from theirs due to differences in exercise program (a mix of aerobic and resistance vs. aerobic only) and duration of exertion (90 vs. 22-31 min). GFAP may have been undetected because the employed analysis method (multiplex array format) is less sensitive to low GFAP concentrations than the single molecule array used in the current study.
Stefanus and colleagues found that 30 min of aerobic exertion on stationary bicycle among 22 healthy young adult males was associated with a decrease in mean GFAP of 40%, a finding similar to ours.42 GFAP levels did not change after 10 min of exertion. The authors speculated that aerobic exercise lasting at least 30 min stimulates glucocorticoids, which inhibits GFAP expression in astrocytes. This hypothesis is supported by studies in humans showing elevations in cortisol immediately after exertion, peaking at about 30-90 min and gradually declining and returning to baseline by about 3 h.59-61 It is further support by studies in rats showing reduced hippocampal astrogliosis (indicated by immunohistochemical quantification of GFAP expression) in association with increased serum corticosterone levels after treadmill walking.62,63 Thus, the immediate post-exertion decreases in GFAP we observed might be due to exercise-induced increases in cortisol, which suppresses astrocyte activation, while the return to baseline levels by 45 min might be due to declining cortisol levels and reduction in astrocyte suppression at this later time-point.
Interestingly, multiple studies suggest that post-exertional increases in cortisol are related to the intensity and duration of aerobic exertion.64 However, we did not find that any of the exertional metrics we examined were associated with this immediate decrease in GFAP, which might be expected based on the hypothesized relationship between exercise-induced cortisol elevation and GFAP levels. What might be the reason for this discrepancy? In our study, subjects performed both aerobic and resistance exercises, and it appears that resistance exercises result in much greater immediate cortisol elevations than aerobic exercises.59 Although our exercise sensor captured intensity and duration of aerobic activity, there are no metrics specifically dedicated to capturing the magnitude of resistance training.
Unlike GFAP, which decreased immediately post-exertion, UCH-L1 levels did not change until 45 min after exertion, at which point they increased. This increase correlated to several metrics of exertion, most notably duration of exertion, which explained nearly half the variation in levels. Pre-clinical studies suggest that exertion induces upregulation of genetic expression of UCH-L1. Several authors report increased striatal and hippocampal mRNA expression of UCH-L1 in rats undergoing aerobic exercise.65-67 Liu and colleagues speculated this might be due to exercise-induced signaling from mTOR or calcium/calmodulin dependent protein kinases.67 The time required for exercise to increase UCH-L1 expression and for this to be reflected in elevated serum levels was not reported. In contact and non-contact collegiate athletes undergoing blood sampling within 6 h of exertion without concussion, no significant changes in UCH-L1 levels from pre-season baseline were detected.7,10
Post-exertional increases in UCH-L1 might also arise from extracranial sources. Several human studies have reported mildly elevated serum UCH-L1 levels within 4 h of non-cranial trauma.6,68,69 UCH-L1 mRNA is known to be expressed in subcutaneous adipocytes, skin endothelial cells, skin smooth muscle cells (e.g., erector pili muscles), and skin fibroblasts.70 It is conceivable that the workout session might have caused microstress to these cell types. The recent discovery of acute post-concussion elevations of UCH-L1 in neuron-derived exosomes could potentially allow for future studies to determine if post-exertional increases in this protein are coming from the brain.71
Our results have important implications for how these blood-based biomarkers might be used to aid in the on-scene diagnosis of concussion occurring in the setting of physical exertion. Neither tau nor NF-L appear to be significantly impacted by exertion, making them potentially useful for this purpose. Unfortunately, neither protein has been found to accurately discriminate concussed from non-concussed subjects in the first hours after injury.72,73 GFAP and UCH-L1, on the other hand, have better classification accuracy in the acute time frame.73 To employ them in a very acute time frame (i.e., within minutes of a suspicious head hit), our results suggest that both the time after injury and the time after exertion must be considered.
In order to use UCH-L1 to assist in the diagnosis of concussion in the setting of vigorous exertion, one would need to measure it immediately to avoid the confounding effect of the exertion-related increase we observed at 45 min. If measuring UCH-L1 at 45 min, our results suggest that diagnostic accuracy could potentially be maintained if values were mathematically adjusted for the duration of exertion, although this needs to be empirically established. For GFAP, one would need to wait until 45 min after injury to measure levels. Exercised-induced reductions in immediate GFAP levels have potential to mask an increase due to brain injury, potentially resulting in false negative values. Although the GFAP decrease we observed was not significantly associated with any single exercise metric, there was a trend toward an association with kilocalories of energy expended, which explained 21% of the variation in immediate GFAP change. Again, it might be possible to maintain diagnostic accuracy of GFAP measured immediately after injury if values could be mathematically adjusted for kilocalories of energy expended. However, this requires measurement of this metric in all players with a sensor which may not be practical in all situations.
Our study has several limitations. There is a possibility that the exertion-related biomarker increases we observed during the off season are in part due to sub-clinical brain trauma experienced during the previous season. Prior studies suggest that a single season of contact sports can elevate brain protein biomarker levels74 and that these levels may remain elevated even into the off season.75 However, by focusing on subject-specific changes rather than group averages, we were able to isolate the effect of physical exertion on brain biomarker levels. Similarly, a prior history of concussion, which was present in one-third of athletes, could have potentially affected baseline DTI values (decreased FA and/or increased MD).76 However, analyzing subject-specific changes in FA and MD rather than group measures of central tendency minimizes the confounding effect of brain WM changes that occurred prior to the study.
Our use of capillary whole blood rather than venous blood might make it difficult to directly compare our results to previous studies, as marker levels tend to be higher in capillary whole blood.77,78 Pre and post exertional levels of tau, NF-L and UCH-L1 were higher than those previously reported in collegiate athletes 6 h after concussion and in athletes during the off-season.73 Pre-exertional and 45 min post-exertional GFAP levels were also higher than those reported in collegiate athletes during the off season but lower than those 6 h after concussion. Immediate post game GFAP levels were lower than 6 h post-SRC and off season.73
Due to the relatively small sample size, the power to detect small changes in biomarker values, as well as small changes in WM integrity (i.e., FA and MD), may have been limited. This problem was compounded somewhat by missing biomarker data (due to limited sample volume and/or high CV), and obtaining DTI in only half the cohort. Further, this study was conducted on males of a relatively narrow age range, limiting external generalizability. We did not collect information on use of supplements, some of which are known to affect cortisol levels,79 which could potentially impact peri-exertional GFAP concentrations.
Conclusions
Our results suggest that a single exertional workout session is associated with immediate decreases in serum GFAP and very acute (45 min) increases in UCH-L1. That these biomarker changes were found to be related to the duration of exertion and possibly to kilocalories of energy expended suggests that mathematical adjustment for these exertional metrics could maintain the diagnostic accuracy of GFAP and UCH-L1 in the setting of concussion. This hypothesis requires validation in a concussed cohort.
Supplementary Material
Acknowledgments
We greatly appreciate the participation of the athletes at the University of Rochester, without whom this research would not have been possible. The authors thank Sarah Dermady (Department of Emergency Medicine, University of Rochester) and Kourtney Korczak (Department of Emergency Medicine, University of Rochester) for their study coordination and management efforts.
Transparency, rigor, and reproducibility summary: This study was not formally registered as a clinical trial because it did not involve an intervention or evaluation of a drug, biologic or device. The analysis plan was not formally pre-registered. A sample size of 30 subjects was planned based on the availability of body sensors and staff to obtain blood at each of three time-points. Actual sample size was 30 subjects, and the observed effect sizes were tau: -0.42 and -0.18; NF-L: -0.20 and 0.25; GFAP: -0.86 and -0.19; and UCH-L1: 0.17 and 0.44. One hundred potential participants were screened, blood samples were obtained in 30, and successfully analyzed in 30. However, some biomarker samples were excluded from analysis due to insufficient quantity of blood or analytical CV and bead count CV exceeding 20%. (Supplementary Table 1 ).
All participants were blinded to results of the fluid biomarker measurements. Handling of blood samples was performed by team members blinded to the degree of physical exertion of the participants. Fluid biomarker quality control decisions and analyses were also performed by investigators blinded to exertional measures. Blood samples were acquired between January 2019 and January 2020 using methods described in the text and stored at -80°C until analysis, which occurred in March 2020. Assays were batched to minimize variability with each batch run with appropriate standards and controls to ensure reliability. Possible batch effects were minimized by including a standard case and control on all plates, and by analyzing longitudinal samples from the same individual on the same plate. No unexpected events occurred during the study. The analyses were validated for research use only.
Serum levels of NF-L, tau, UCH-L1, and GFAP were measured from the same blood sample using a SIMOA® 4-plex assay kit (Quanterix Corp).51,52 Lower limits of detection were 0.104 pg/mL (NF-L), 0.024 pg/mL (tau), 0.221 pg/mL (GFAP), and 1.74 pg/mL (UCH-L1), and their respective intra-assay coefficients of variation are 5.4%, 6.7%, 3.7%, and 11.3%. All equipment and analytical reagents used to perform measurements on the fluid biomarkers are widely available from Quanterix. The key inclusion criteria are established standards in the field. The correlational tests used were based on the assumptions of normality in the biomarker data (Pearson's) and non-normality in the DTI data (Spearman's). Missing data were considered missing at random and not imputed. Correction for multiple comparisons was performed using Bonferroni method. No replication or external validation studies have been performed or are planned/ongoing at this time to our knowledge.
De-identified data from this study are not available in a public archive, but will be made available (as allowable according to Institutional Review Board standards) by emailing the corresponding author as of July 1, 2022. There is no analytic code associated with this study. No future use of these biofluid samples is possible because insufficient quantities remain. The authors agree or have agreed to publish the manuscript using the Mary Ann Liebert Inc. “Open Access” option under appropriate license.
Authors' Contributions
JJB: Conceptualization (lead), writing-original draft (lead), supervision (equal), writing-review and editing (equal), funding acquisition (lead). BA: Formal analysis (lead), writing-review and editing (equal), funding acquisition (supporting). KMB: Conceptualization (supporting), project administration (lead), supervision (equal), visualization, writing-review and editing (equal), funding acquisition (supporting). DLP: Investigation (lead), writing-review and editing (equal), funding acquisition (supporting). ER: Resources (lead), funding acquisition (supporting). RM: Writing-review and editing (equal), formal analysis (supporting). KK: Writing-review and editing (equal). YC: Investigation (supporting). SS: Writing-review and editing (equal). JMG: Investigation (lead), funding acquisition (supporting).
Funding Information
This research was supported by the National Institute of Neurological Disorders and Stroke (Award Number R21NS10290). The funding agency/sponsor had no role in the study design, writing the manuscript, or in the decision to submit for publication.
Author Disclosure Statement
Dr. Bazarian reports research support from National Institute of Neurological Disorders and Stroke, BrainScope, Abbott and BrainBox.
For the other authors, no competing financial interests exist.
Supplementary Material
References
- 1. Iverson GL, Silverberg ND, Mannix R, et al. Factors associated with concussion-like symptom reporting in high school athletes. JAMA pediatrics 2015;169(12):1132–1140; doi: 10.1001/jamapediatrics.2015.2374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Iverson GL, Lange RT. Examination of “postconcussion-like” symptoms in a healthy sample. Appl Neuropsychol 2003;10(3):137–144; doi: 10.1207/S15324826AN1003_02 [DOI] [PubMed] [Google Scholar]
- 3. Asken BM, Snyder AR, Smith MS, et al. Concussion-like symptom reporting in non-concussed adolescent athletes. Clin Neuropsychol 2017;31(1):138–153; doi: 10.1080/13854046.2016.1246672 [DOI] [PubMed] [Google Scholar]
- 4. Diaz-Arrastia R, Wang KK, Papa L, et al. Acute biomarkers of traumatic brain injury: relationship between plasma levels of ubiquitin C-terminal hydrolase-L1 and glial fibrillary acidic protein. J Neurotrauma 2014;31(1):19–25; doi: 10.1089/neu.2013.3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Korley FK, Diaz-Arrastia R, Wu AH, et al. Circulating brain-derived neurotrophic factor has diagnostic and prognostic value in traumatic brain injury. J Neurotrauma 2016;33(2):215–225; doi: 10.1089/neu.2015.3949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Papa L, Brophy, G.M., Welch, R.D., et al. Time course and diagnostic accuracy of glial and neuronal blood biomarkers GFAP and UCH-L1 in a large cohort of trauma patients with and without mild traumatic brain injury. JAMA Neurol 2016;73(5):551–560; doi: 10.1001/jamaneurol.2016.0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meier T, Nelson, L.D., Huber, D.L., et al. Prospective assessment of acute blood markers of brain injury in sport-related concussion. J Neurotrauma 2017;34(22):3134–3142; doi: 10.1089/neu.2017.5046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Asken BM, Bauer RM, DeKosky ST, et al. Concussion BASICS III: serum biomarker changes following sport-related concussion. Neurology 2018;91(23):e2133–e2143; doi: 10.1212/WNL.0000000000006617 [DOI] [PubMed] [Google Scholar]
- 9. McCrea M, Broglio, S.P., McAllister, T.W., et al. ; CARE Consortium Investigators.. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: a prospective study from the NCAA-DoD CARE Consortium. JAMA Network Open 2020;3(1):e1919771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Meier TB, Huber DL, Bohorquez-Montoya L, et al. A prospective study of acute blood-based biomarkers for sport-related concussion. Ann Neurol 2020;87(6):907–920; doi: 10.1002/ana.25725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. AbbottMediaRoom. Abbott Receives FDA 510(k) Clearance for the First Rapid Handheld Blood Test for Concussions. Available from: https://abbott.mediaroom.com/2021-01-11-Abbott-Receives-FDA-510-k-Clearance-for-the-First-Rapid-Handheld-Blood-Test-for-Concussions [Last accessed July 7, 2021]. [Google Scholar]
- 12. Seidenfaden S-C, Kjerulff JL, Juul N, et al. Diagnostic accuracy of prehospital serum S100B and GFAP in patients with mild traumatic brain injury: a prospective observational multicenter cohort study–“the PreTBI I study.” Scand J Trauma Resusc Emerg Med 2021;29(1):75: doi: 10.1186/s13049-021-00891-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otto M, Holthusen S, Bahn E, et al. Boxing and running lead to a rise in serum levels of S-100B protein. Int J Sports Med 2000;21(8):551–555; doi: 10.1055/s-2000-8480 [DOI] [PubMed] [Google Scholar]
- 14. Dietrich MO, Tort AB, Schaf DV, et al. Increase in serum S100B protein level after a swimming race. Can J Appl Physiol 2003;28(5):710–716; doi: 10.1139/h03-054 [DOI] [PubMed] [Google Scholar]
- 15. Watson P, Black KE, Clark SC, et al. Exercise in the heat: effect of fluid ingestion on blood-brain barrier permeability. Med Sci Sports Exerc 2006;38(12):2118–2124; doi: 10.1249/01.mss.0000235356.31932.0a [DOI] [PubMed] [Google Scholar]
- 16. Hasselblatt M, Mooren FC, Von Ahsen N, et al. Serum S100beta increases in marathon runners reflect extracranial release rather than glial damage. Neurology 2004;62(9):1634–1636; doi: 10.1212/01.wnl.0000123092.97047.b1 [DOI] [PubMed] [Google Scholar]
- 17. Spiropoulos A, Goussetis E, Margeli A, et al. Effect of inflammation induced by prolonged exercise on circulating erythroid progenitors and markers of erythropoiesis. Clin Chem Lab Med 2010;48(2):199–203; doi: 10.1515/CCLM.2010.034 [DOI] [PubMed] [Google Scholar]
- 18. Knaepen K, Goekint M, Heyman EM, et al. Neuroplasticity—exercise-induced response of peripheral brain-derived neurotrophic factor. Sports Med 2010;40(9):765–801; doi: 10.2165/11534530-000000000-00000 [DOI] [PubMed] [Google Scholar]
- 19. Di Battista AP, Moes KA, Shiu MY, et al. High-intensity interval training is associated with alterations in blood biomarkers related to brain injury. Front Physiol 2018;9:1367; doi: 10.3389/fphys.2018.01367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ding Q, Vaynman S, Souda P, et al. Exercise affects energy metabolism and neural plasticity-related proteins in the hippocampus as revealed by proteomic analysis. Eur J Neurosci 2006;24(5):1265–1276; doi: 10.1111/j.1460-9568.2006.05026.x [DOI] [PubMed] [Google Scholar]
- 21. Saur L, Baptista PP, de Senna PN, et al. Physical exercise increases GFAP expression and induces morphological changes in hippocampal astrocytes. Brain Struct Funct 2014;219(1):293–302; doi: 10.1007/s00429-012-0500-8 [DOI] [PubMed] [Google Scholar]
- 22. Schulte S, Schiffer T, Sperlich B, et al. The impact of increased blood lactate on serum S100B and prolactin concentrations in male adult athletes. Eur J Appl Physiol 2013;113(3):811–817; doi: 10.1007/s00421-012-2503-9 [DOI] [PubMed] [Google Scholar]
- 23. Michetti F, Bruschettini M, Frigiola A, et al. Saliva S100B in professional sportsmen: High levels at resting conditions and increased after vigorous physical activity. Clin Biochem 2011;44(2-3):245–247; doi: 10.1016/j.clinbiochem.2010.10.007 [DOI] [PubMed] [Google Scholar]
- 24. Sharma HS, Cervos-Navarro J, Dey PK. Increased blood-brain barrier permeability following acute short-term swimming exercise in conscious normotensive young rats. Neurosci Res 1991;10(3):211–221; doi: 10.1016/0168-0102(91)90058-7 [DOI] [PubMed] [Google Scholar]
- 25. Watson P, Shirreffs SM, Maughan RJ. Blood-brain barrier integrity may be threatened by exercise in a warm environment. Am J Physiol Regul Integr Comp Physiol 2005;288(6):R1689–1694; doi: 10.1152/ajpregu.00676.2004 [DOI] [PubMed] [Google Scholar]
- 26. Koh SX, Lee JK. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med 2014;44(3):369–385; doi: 10.1007/s40279-013-0119-9 [DOI] [PubMed] [Google Scholar]
- 27. Nierwińska K; Chalimoniuk, M. Żebrowska, A., et al. Blood-brain barrier and exercise—a short review. J Hum Kinet 2008;19:83–92. [Google Scholar]
- 28. Rasmussen P, Brassard P, Adser H, et al. Evidence for a release of brain-derived neurotrophic factor from the brain during exercise. Expe Physiol 2009;94(10):1062–1069; doi: 10.1113/expphysiol.2009.048512 [DOI] [PubMed] [Google Scholar]
- 29. Sleiman SF, Henry J, Al-Haddad R, et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. Elife 2016;5:e15092; doi: 10.7554/eLife.15092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Matthews VB, Åström M-B, Chan M, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia 2009;52(7):1409–1418; doi: 10.1007/s00125-009-1364-1 [DOI] [PubMed] [Google Scholar]
- 31. Fujimura H, Altar CA, Chen R, et al. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb Haemost 2002;87(04):728–734. [PubMed] [Google Scholar]
- 32. Gonçalves CA, Leite MC, Guerra MC. Adipocytes as an important source of serum S100B and possible roles of this protein in adipose tissue. Cardiovasc Psychiatry Neurol 2010;2010: 790431l; doi: 10.1155/2010/790431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Petersson S, Shubbar E, Enerbäck L, et al. Expression patterns of S100 proteins in melanocytes and melanocytic lesions. Melanoma Res 2009;19(4):215–225; doi: 10.1097/CMR.0b013e32832c6358 [DOI] [PubMed] [Google Scholar]
- 34. Koh SX, Lee JK. S100B as a marker for brain damage and blood–brain barrier disruption following exercise. Sports Med 2014;44(3):369–385; doi: 10.1007/s40279-013-0119-9 [DOI] [PubMed] [Google Scholar]
- 35. Gustafsson G, Lira CM, Johansson J, et al. The acute response of plasma brain-derived neurotrophic factor as a result of exercise in major depressive disorder. Psychiatry Res 2009;169(3):244–248; doi: 10.1016/j.psychres.2008.06.030 [DOI] [PubMed] [Google Scholar]
- 36. Rogatzki MJ, Keuler SA, Harris AE, et al. Response of protein S100B to playing American football, lifting weights, and treadmill running. Scand J Med Sci Sports 2018;28(12):2505–2514; doi: 10.1111/sms.13297 [DOI] [PubMed] [Google Scholar]
- 37. Schulte S, Sperlich T, Kleinoder H, et al. Serum concentrations of S100B are not affected by cycling to exhaustion with or without vibration. J Hum Kinet 2011;30:59–63; doi: 10.2478/v10078-011-0073-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schulte S, Podlog LW, Hamson-Utley JJ, et al. A systematic review of the biomarker S100B: implications for sport-related concussion management. J Athl Train 2014;49(6):830–850; doi: 10.4085/1062-6050-49.3.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Predovan D, Gazes Y, Lee S, et al. Effect of aerobic exercise on white matter tract microstructure in young and middle-aged healthy adults. Fron Hum Neurosci 2021; 15:681634; doi: 10.3389/fnhum.2021.681634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Burzynska AZ, Jiao Y, Knecht AM, et al. White matter integrity declined over 6-months, but dance intervention improved integrity of the fornix of older adults. Front Aging Neurosci 2017;9:59; doi: 10.3389/fnagi.2017.00059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chaddock-Heyman L, Erickson KI, Kienzler C, et al. Physical activity increases white matter microstructure in children. Front Neurosci 2018;12:950; doi: 10.3389/fnins.2018.00950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stefanus R, Yolanda S, Antarianto RD. Comparison of GFAP and HSP27 concentrations in acute moderate-intensity aerobic exercise of different duration. Med J Indonesia 2016;25(2):112–117/ [Google Scholar]
- 43. Nicolella DP, Torres-Ronda L, Saylor KJ, et al. Validity and reliability of an accelerometer-based player tracking device. PLoS One 2018;13(2):e0191823; doi: 10.1371/journal.pone.0191823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Catapultsports. What is Player Load. Available from: https://support.catapultsports.com/hc/en-us/articles/360000574716-What-is-Player-Load- [Last Accessed January 7, 2022]. [Google Scholar]
- 45. Catapultsports. Volume Metrics. Available from: https://support.catapultsports.com/hc/en-us/articles/360000648316-Volume-Metrics [Last Accessed; January 7, 2022]. [Google Scholar]
- 46. Catapultsports. Intensity Metrics. Available from: https://support.catapultsports.com/hc/en-us/articles/360000648336-Intensity-Metrics [Last Accessed; January 7, 2022]. [Google Scholar]
- 47. Cummins C, Orr R. Analysis of physical collisions in elite National Rugby League match play. Int J Sports Physiol Perform 2015;10(6):732–739; doi: 10.1123/ijspp.2014-0541 [DOI] [PubMed] [Google Scholar]
- 48. Gabbett TJ. Relationship between accelerometer load, collisions, and repeated high-intensity effort activity in rugby league players. J Strength Cond Res 2015;29(12):3424–34231; doi: 10.1519/JSC.0000000000001017 [DOI] [PubMed] [Google Scholar]
- 49. Gastin PB, McLean O, Spittle M, et al. Quantification of tackling demands in professional Australian football using integrated wearable athlete tracking technology. J Sci Med Sport 2013;16(6):589–593; doi: 10.1016/j.jsams.2013.01.007 [DOI] [PubMed] [Google Scholar]
- 50. Gastin PB, McLean OC, Breed RV, et al. Tackle and impact detection in elite Australian football using wearable microsensor technology. J Sports Sci 2014;32(10):947-953; doi: 10.1080/02640414.2013.868920 [DOI] [PubMed] [Google Scholar]
- 51. Kuhle J, Barro C, Andreasson U, et al. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin Chem Lab Med 2016;54(10):1655–1661; doi: 10.1515/cclm-2015-1195 [DOI] [PubMed] [Google Scholar]
- 52. Wilson DH, Rissin DM, Kan CW, et al. The Simoa HD-1 analyzer: a novel fully automated digital immunoassay analyzer with single-molecule sensitivity and multiplexing. J Lab Autom 2016;21(4):533–547; doi: 10.1177/2211068215589580 [DOI] [PubMed] [Google Scholar]
- 53. Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044; doi: 10.1016/j.neuroimage.2010.09.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Andersson JL, Skare S, Ashburner J. How to correct susceptibility distortions in spin-echo echo-planar images: application to diffusion tensor imaging. Neuroimage 2003;20(2):870–888; doi: 10.1016/S1053-8119(03)00336-7 [DOI] [PubMed] [Google Scholar]
- 55. Jenkinson M, Beckmann CF, Behrens TE, et al. Fsl. Neuroimage 2012;62(2):782–790; doi: 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- 56. Pierpaoli C, Walker L, Irfanoglu M, et al. TORTOISE: an integrated software package for processing of diffusion MRI data. 2010. Available from: https://www.nichd.nih.gov/sites/default/files/inline-files/TORTOISE_MRI_software%20_package.pdf [Last accessed October 25, 2022]. [Google Scholar]
- 57. Bazin PL, Ye C, Bogovic JA, et al. Direct segmentation of the major white matter tracts in diffusion tensor images. NeuroImage 2011;58(2):458–468; doi: 10.1016/j.neuroimage.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McCrory P, Meeuwisse W, Dvorak J, et al. Consensus statement on concussion in sport—the 5th international conference on concussion in sport held in Berlin, October 2016. Br J Sports Med 2017; 51(11):838-847; doi: 10.1136/bjsports-2017-097699 [DOI] [PubMed] [Google Scholar]
- 59. Tremblay MS, Copeland JL, Van Helder W. Effect of training status and exercise mode on endogenous steroid hormones in men. J Appl Physiol 2004;96(2):531–539; doi: 10.1152/japplphysiol.00656.2003 [DOI] [PubMed] [Google Scholar]
- 60. Daly W, Seegers C, Timmerman S, et al. Peak cortisol response to exhausting exercise: effect of blood sampling schedule. Med Sportiva 2004;8(1):17–20. [PMC free article] [PubMed] [Google Scholar]
- 61. Kanaley JA, Weltman JY, Pieper KS, et al. Cortisol and growth hormone responses to exercise at different times of day. J Clin Endocrinol Metab 2001;86(6):2881–2889; doi: 10.1210/jcem.86.6.7566 [DOI] [PubMed] [Google Scholar]
- 62. Kim K, Shin MS, Cho HS, et al. Effects of endurance exercise on expressions of glial fibrillary acidic protein and myelin basic protein in developing rats with maternal infection-induced cerebral palsy. J Exerc Rehabil 2014;10(1):9–14; doi: 10.12965/jer.140084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bernardi C, Tramontina AC, Nardin P, et al. Treadmill exercise induces hippocampal astroglial alterations in rats. Neural Plast 2013;2013:709732; doi: 10.1155/2013/709732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Chen C, Nakagawa S, An Y, et al. The exercise-glucocorticoid paradox: How exercise is beneficial to cognition, mood, and the brain while increasing glucocorticoid levels. Front Neuroendocrinol 2017;44:83–102; doi: 10.1016/j.yfrne.2016.12.001 [DOI] [PubMed] [Google Scholar]
- 65. Liu W, Li L, Kuang H, et al. Proteomic profile of carbonylated proteins screen regulation of apoptosis via CaMK signaling in response to regular aerobic exercise. Biomed Res Int 2018;2018:2828143; doi: 10.1155/2018/2828143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kirchner L, Chen WQ, Afjehi-Sadat L, et al. Hippocampal metabolic proteins are modulated in voluntary and treadmill exercise rats. Exp Neurol 2008;212(1):145–151; doi: 10.1016/j.expneurol.2008.03.014 [DOI] [PubMed] [Google Scholar]
- 67. Liu W, Li L, Liu S, et al. MicroRNA expression profiling screen miR-3557/324-targeted CaMK/mTOR in the rat striatum of Parkinson's disease in regular aerobic exercise. Biomed Res Int 2019;2019:7654798; doi: 10.1155/2019/7654798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Papa L, Lewis LM, Silvestri S, et al. Serum levels of ubiquitin C-terminal hydrolase distinguish mild traumatic brain injury from trauma controls and are elevated in mild and moderate traumatic brain injury patients with intracranial lesions and neurosurgical intervention. J Trauma Acute Care Surg 2012;72(5):1335–1344; doi: 10.1097/TA.0b013e3182491e3d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Papa L, Zonfrillo MR, Welch RD, et al. Evaluating glial and neuronal blood biomarkers GFAP and UCH-L1 as gradients of brain injury in concussive, subconcussive and non-concussive trauma: a prospective cohort study. BMJ Paediatr Open 2019 Aug 25;3(1):e000473; doi: 10.1136/bmjpo-2019-000473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Uhlén M, Fagerberg L, Hallström BM, et al. Tissue-based map of the human proteome. Science 2015;347(6220):1260419/ [DOI] [PubMed] [Google Scholar]
- 71. Goetzl EJ, Elahi FM, Mustapic M, et al. Altered levels of plasma neuron-derived exosomes and their cargo proteins characterize acute and chronic mild traumatic brain injury. FASEB J 2019;33(4):5082–5088; doi: 10.1096/fj.201802319R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Gill J, Merchant-Borna K, Jeromin A, et al. Acute plasma tau relates to prolonged return to play after concussion. Neurology 2016;88(6):595–602; doi: 10.1212/WNL.0000000000003587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. McCrea M, Broglio SP, McAllister TW, et al. Association of blood biomarkers with acute sport-related concussion in collegiate athletes: findings from the NCAA and Department of Defense CARE Consortium. JAMA Netw Open 2020;3(1):e1919771; doi: 10.1001/jamanetworkopen.2019.19771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Oliver JM, Jones, M.T., Kirk, K.M., et al. Serum neurofilament light in American football athletes over the course of a season. J Neurotrauma 2016;33(19):1784–1789; doi: 10.1089/neu.2015.4295 [DOI] [PubMed] [Google Scholar]
- 75. Shahim P, Zetterberg H, Tegner Y, et al. Serum neurofilament light as a biomarker for mild traumatic brain injury in contact sports. Neurology 2017;88(19):1788–1794; doi: 10.1212/WNL.0000000000003912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Churchill N, Hutchison M, Richards D, et al. Brain structure and function associated with a history of sport concussion: a multi-modal magnetic resonance imaging study. J Neurotrauma 2017 Feb 15;34(4):765–771; doi: 10.1089/neu.2016.4531 [DOI] [PubMed] [Google Scholar]
- 77. Astrand R, Romner B, Reinstrup P, et al. Comparison between capillary, venous and arterial levels of protein S100B in patients with severe brain pathology. Clin Chem Lab Med 2012;50(6):1055–1061; doi: 10.1515/cclm-2011-0639 [DOI] [PubMed] [Google Scholar]
- 78. Siart B, de Oliveira FMS, Shen Q, et al. Protein measurements in venous plasma, earlobe capillary plasma and in plasma stored on filter paper. Anal Biochem 2019;566:146–150; doi: 10.1016/j.ab.2018.11.016 [DOI] [PubMed] [Google Scholar]
- 79. Stachowicz M, Lebiedzińska A. The effect of diet components on the level of cortisol. Eur Food Res Technol 2016;242(12):2001–2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.