Abstract
Background:
The endocannabinoid (eCB) system plays an important role in homeostatic regulation of anxiety and stress responses; however, the eCB system can be disrupted following traumatic stressors. Additionally, traumatic or chronic stressors that occur during adulthood or early life can cause long-lasting disturbances in the eCB system. These alterations interfere with hypothalamic–pituitary–adrenal axis function and may be involved in lifelong increased fear and anxiety behaviors as well as increased risk for development of post-traumatic stress disorder (PTSD).
Methods:
This review focuses on the implications of trauma and significant stressors on eCB functionality and neural pathways, both in adolescence and into adulthood, as well as the current state of testing for CBD efficacy in treating pediatric and adult patients suffering from stress-induced eCB dysregulation. Articles were searched via Pubmed and included studies examining eCB modulation of stress-related disorders in both clinical settings and preclinical models.
Conclusion:
Given the potential for lifelong alterations in eCB signaling that can mediate stress responsiveness, consideration of pharmaceutical or nutraceutical agents that impact eCB targets may improve clinical outcomes in stress-related disorders. However, caution may be warranted in utilization of medicinal cannabinoid products that contain delta-9-tetrahydrocannabinol due to pronounced euphorigenic effects and potential to exacerbate stress-related behaviors. Other cannabinoid products, such as cannabidiol (CBD), have shown promise in reducing stress-related behaviors in pre-clinical models. Overall, pre-clinical evidence supports CBD as a potential treatment for stress or anxiety disorders resulting from previously stressful events, particularly by reducing fearful behavior and promoting extinction of contextual fear memories, which are hallmarks of PTSD. However, very limited clinical research has been conducted examining the potential effectiveness of CBD in this regard and should be examined further.
Keywords: cannabidiol, childhood trauma, neurogenesis, post-traumatic stress disorder, selective serotonin reuptake inhibitors
Introduction
Post-traumatic stress disorder (PTSD) is psychiatric disorder that can develop after either direct or indirect exposure to a traumatic event such as death, the threat of death, serious physical injury, or sexual violence.1 A diagnosis of PTSD requires the presence of multiple symptoms lasting at least 1 month following the traumatic event. One of the prominent symptoms is avoidant behavior toward stimuli associated with the trauma, which can include people, locations, situations, and even thoughts and memories.1
Intrusion symptoms such as involuntary reexperiencing of the traumatic event can include fear memories, recurrent memories, disassociation, and intense physical reactions related to triggering cues, which are other highly prominent PTSD symptoms.1–4 Numerous studies have further examined PTSD with symptoms of altered arousal and reactivity such as hypervigilance, altered sleep patterns, aggressive and irritable behavior, increased startle responses, and decreased concentration.1–4 Each symptom can be attributed to altered activity in numerous brain regions and dysfunction in neural circuits that underlie PTSD, and the duration and severity of the disorder are costly to both the individual and the society they live in.5–8
The prevalence of PTSD ranges from 1.3% to 12.2% depending on the country of residence and social background. Children are especially vulnerable to trauma as childhood abuse of any kind is associated with a 70% greater risk of developing PTSD.9 In 2019, an estimated 8.9 per 1000 children in the United States were victims of child abuse and/or neglect.9 While the overall rates of victimization have not changed in recent years, child mortality rates have increased by 10.8% in the period from 2015 to 2019, suggesting that victims may be exposed to more traumatic aspects of maltreatment.9 The effects of childhood trauma persist into adulthood likely due to trauma occurring during important brain development periods, which results in long-lasting effects.10
Currently, the only FDA-approved treatment for PTSD in adults is selective serotonin reuptake inhibitors (SSRIs), while the FDA has not approved any medications for PTSD in the pediatric population.11,12 Even as an approved treatment in adults, the successful response rate of SSRIs for patients rarely exceeds 60% and the full remission rate following treatment is less than 30%.4 Importantly, recent studies indicate that patients who experienced childhood trauma between the ages of 4 and 7 years had the lowest response to 8 weeks of SSRI treatment as adults.13
SSRIs also present unpleasant side effects, including sleep disturbances, headaches, nausea, appetite changes, and sexual dysfunction. These findings show a need for novel therapeutics that can assist with efficacious therapies, specifically trauma-focused cognitive-behavioral therapy.4,14,15
The endocannabinoid (eCB) system is an important component of stress response regulation that can be dysregulated in PTSD patients.16 eCBs such as N-arachidonoylethanolamine (AEA) and 2-arachidonoylglycerol (2-AG) play a key role in regulation of the stress response and maintenance of homeostatic states.17–21
Other less-studied eCBs may also produce additional effects, such as N-stearoylethanolamine (SEA) and N-palmitoylethanolamine (PEA), which have anti-inflammatory functions; N-oleoylethanolamide (OEA) regulates appetite and 1-arachidonoylglycerol (1-AG) is the biologically inactive form of 2-AG.22 Phytocannabinoids, which are extracted from the Cannabis sativa plant, can also interact with the eCB system and produce a variety of physical and psychological effects.23
Among the most prevalent and well-researched phytocannabinoids are the psychotropic delta-9-tetrahydrocannabinol (THC) and nonpsychotropic cannabidiol (CBD), the latter of which has been shown to reduce PTSD-related symptoms.24
In this review, we will discuss clinical and pre-clinical data examining the potential role of the eCB system in PTSD and neurotypical brain development, how eCB regulation of brain development may be altered by childhood traumas, and the potential use of CBD-based therapeutics in both adult and pediatric patients who present with PTSD symptoms.
Brief Overview of the eCB System and Its Relationship to Adaptive Stress Responses
The eCB system plays a significant role in modulation of the neuroendocrine stress response and maintains physiologic homeostasis by regulating physical and cognitive processes such as appetite, mood, energy usage, pain response, and cardiovascular and respiratory function.16 eCBs modulate synaptic function through retrograde signaling to cannabinoid receptor type 1 (CB1R) and cannabinoid receptor type 2 (CB2R) located at presynaptic terminals from numerous cell types, including glutamatergic, GABAergic, and monoaminergic neurons and neuroimmune cells typically inhibiting neurotransmitter release.25,26
Studies show that AEA, a fatty acid eCB, maintains the tone of neurotransmitter release during nonstress states.17,18 AEA has high affinity for CB1R and moderate affinity for CB2R; however, the downstream signals generated by AEA are moderate for CB1R and low for CB2R.27,28 2-AG, the other major fatty acid eCB, mediates the return to baseline after a stressful event takes place and contributes to synaptic plasticity.19–21 2-AG possesses moderate affinity for both CB1R and CB2R, but is thought to create stronger intracellular signals than AEA, indicating potential for higher efficacy of 2-AG.27,28
Hydrolysis of eCBs is undertaken by two major degradative enzymes: monoacylglycerol lipase (MAGL), which hydrolyzes 2-AG, and fatty acid amide hydrolase (FAAH), which hydrolyzes AEA and other N-acylethanolamines such as SEA, PEA, and OEA.29–31
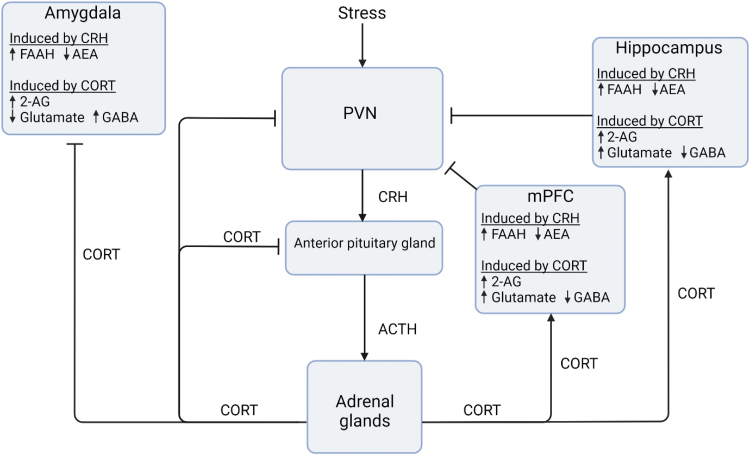
Specifically in terms of the stress response, CB1R is thought to play an inhibitory role in the hypothalamic–pituitary–adrenal (HPA) axis, the function of which is crucial for activation of classic neuroendocrine stress responses (Fig. 1).32,33 During an acute stress response, the corticotropin-releasing hormone (CRH) is released from neurons in the paraventricular nucleus (PVN) within the hypothalamus, initiating HPA responses.34,35 PVN CRH neurons respond to and integrate inputs from the amygdaloid complex, prefrontal cortex, hippocampus, and brain stem.36–38
FIG. 1.
Endocannabinoid interactions with the HPA axis stress response. In response to stress, the PVN CRH is released, which binds to CRHR1 in the amygdala, hippocampus, mPFC, PVN, and other brain regions. CRH increases FAAH levels in most brain areas, which in turn reduce AEA and remove AEA-mediated inhibition of the HPA axis. CRH is also released to the anterior pituitary gland, which stimulates the release of ACTH into the circulation. ACTH at the adrenal glands induces CORT release into the circulation and provides direct negative feedback to the PVN to reduce production of CRH and ultimately ACTH. CORT also stimulates production of 2-AG in the amygdala, hippocampus, and mPFC, where it binds to CB1 and CB2 receptors on glutamatergic and GABAergic neurons. This inhibits amygdala activation and excites the hippocampus and mPFC activation, reducing HPA axis activity through the level of the PVN. Many of these endocannabinoid systems are disrupted in PTSD. Created with BioRender.com. 2-AG, 2-arachidonoyl glycerol; ACTH, adrenocorticotropic hormone; AEA, anandamide; CB1, cannabinoid type 1; CB2, cannabinoid type 2; CORT, cortisol/corticosterone; CRH, corticotropin-releasing hormone; CRHR1, corticotropin-releasing hormone receptor 1; eCB, endocannabinoid; FAAH, fatty acid amide hydrolase; HPA, hypothalamic–pituitary–adrenal; PFC, prefrontal cortex; PVN, paraventricular nucleus of hypothalamus.
CRH then stimulates pituitary adrenocorticotropic hormone (ACTH) release into the circulatory system,39 triggering synthesis of glucocorticoids from the adrenals and releasing them into circulation.40 Glucocorticoid signaling through glucocorticoid receptors in brain regions such as the hypothalamus, hippocampus, amygdala, and prefrontal cortex41,42 mediates physiological and psychological responses following stress, including suppression of the immune system, changes in memory, and mood regulation.43,44 Glucocorticoid receptors within the hypothalamus also initiate negative feedback regulation of CRH release and return HPA axis function to homeostatic levels34–35 (Fig. 1).
Importantly, stress-induced CRH also increases the activity of FAAH, reducing AEA levels and ultimately decreasing the inhibitory tone on CB1R signaling on the HPA axis. Similar effects may occur in the amygdala and hippocampus as well.45,46 AEA levels will remain low as long as the stressor persists, leading to an inability to maintain the homeostatic neurotransmitter tone and increasing the activity of the HPA axis to further extend the body's stress response.47,48
Counteracting AEA function, CRH-triggered release of ACTH and resulting glucocorticoid release stimulate the release of 2-AG within the hypothalamus and other areas of the brain.20,48,49 2-AG release increases CB1 signaling, which supports negative feedback regulation of stress responses in the hypothalamus, hippocampus, and prefrontal cortex.49–51
Given the critical role that the eCB system plays in regulation of synaptic function in the HPA axis and other brain regions regulating prototypical stress responses, harmful disruptions of this system resulting from trauma can reduce the negative feedback to the HPA axis and severely prolong a typically short stress response.16
Evidence of eCB-Mediated Changes in Brain Function Following Traumatic Stress Across the Life Span
Interest in the study of eCB signaling following traumatic stressors has increased in the last decade, both clinically and pre-clinically. In a clinical study investigating trauma-exposed individuals 4 to 6 years following the attack on the World Trade Center, subjects with PTSD had significantly lower circulating 2-AG levels than those who did not develop PTSD after controlling for factors such as stress from exposure to the attack, sex, alcohol abuse, and depression.
This same study showed that AEA levels had a negative correlation with intrusive PTSD symptoms.52 Additional studies have found that individuals with PTSD and a history of trauma have increased expression of CB1R across the brain as well as reduced AEA levels, compared with non-PTSD individuals who have experienced trauma or not.53
Each PTSD subject in these studies had experienced trauma tied to a single specific event, suggesting that an individual traumatic event is enough to cause pervasive and persistent changes to the eCB system years after the event has occurred.52,53 However, it is possible that lower endogenous levels of eCB signaling may be a risk factor for PTSD development, a question that should be examined further.
Other studies into the long-term effects of trauma on the eCB system show somewhat inconsistent results. One study found that the observed levels of PEA, OEA, and SEA in hair samples were negatively correlated with severity of PTSD symptoms, although AEA and 2-AG concentrations could not be reliably determined in this study.31 However, a study by Hauer et al54 examining trauma-exposed refugees found increased plasma levels of AEA, 2-AG, OEA, PEA, and SEA in PTSD individuals.
It should be noted that the number of subjects in the Hauer study54 (10 PTSD patients, 29 total subjects) and the Wilker study31 (38 PTSD patients, 76 total subjects) was low, and studies with larger populations and increased statistical power are needed. Another study found that 2-AG signaling, not AEA, in six healthy male astronauts was significantly reduced after 360 days of mission training under social isolation, suggesting that 2-AG may have a specific role in responding to chronic stressors.55
Additional studies show systemic and long-term changes on the eCB system following early life stressors. For instance, one study found higher levels of AEA and 2-AG in subjects with a history of childhood sexual abuse who later developed bipolar disorder as adults, while individuals who developed PTSD had increased levels of OEA.56 Another clinical study in pregnant mothers who had experienced childhood trauma found significantly higher levels of 1-AG and lower levels of SEA.22
Together, these studies suggest that the type and duration of stress and trauma may distinctly impact the eCB system, highlighting potentially complex effects on the eCB system from stress and trauma over varying time periods, which require further study.
In mouse and rat models, exposure to acute and chronic stress produces brain region-specific changes in eCB signaling in the medial prefrontal cortex, hippocampus, and amygdala. Both acute and chronic stressors, such as restraint or pain, typically reduce AEA levels in these three brain regions, while the effects on 2-AG vary.20,45,51,57,58 2-AG is increased by acute stress in the prefrontal cortex and hippocampus, but not the amygdala.
Chronic exposure to the same type of stress increases 2-AG; however, chronic exposure to different types of stressors reduces 2-AG.49,57,59–62 Chronic repeated restraint or foot shock stress also alters CB1R signaling, creating deficits in learning, memory, and coping behaviors.63,64
Further studies indicate that trauma-induced eCB alteration is dependent on the age of exposure and that early life trauma is distinct from trauma experienced in adulthood.65,66 Importantly, the eCB system undergoes significant alterations during typical development. During early life, there is higher abundance of prefrontal cortex CB1R compared with adulthood.63,67
Since CB1R activation can extinguish fear memory and reduce aversive memories,68 stress-induced inhibition of CB1R signaling may increase the risk of PTSD and phobias.69 Conversely, repeated exposure to the same stressor can progressively increase 2-AG levels, likely by reduced MAGL expression, which causes progressively increased frequency of signaling through CB1Rs, leading to stress habituation.62
Additional pre-clinical research has found that maternal separation stress in rat pups resulted in decreased AEA levels and increased 2-AG levels in the amygdala and hippocampus.62,70 This early life stress can lead to long-term reductions in hippocampal AEA and 2-AG levels, compromising the eCB system into adulthood.
Early life stress in male mice impacts the eCB system by reducing CB1Rs across regions such as the prefrontal cortex, striatum, and amygdala.63,71 At the same time, maternal separation can lead to increased gene expression of FAAH and MAGL, further decreasing AEA and 2-AG levels in the amygdala, hippocampus, prefrontal cortex, and striatum.72
Results in female rodents have been mixed, with some studies indicating an increase in eCB degradation enzymes within the frontal cortex, striatum, hippocampus, and amygdala, while in other studies, these changes were restricted to just the hippocampus.72,73
During adolescence, the development of eCB activity parallels development of the HPA axis, resulting in changes that can influence fear learning and stress sensitivity.63,74 As discussed above, eCB signaling regulates HPA axis function, restricting its activity to short-term stress and maintaining low basal levels of glucocorticoids.18 Studies have emphasized the effects of stress and trauma over varying periods of time on the eCB system.75,76
Overall, both clinical and pre-clinical studies show that early life trauma leads to eCB system changes that can last into adulthood. Such alterations have strong implications for the possibility of subsequent development of PTSD and other stress-related disorders, as lifelong dysregulation of the eCB system by early life trauma may increase the risk that any future traumatic event would induce development of PTSD or reinforce existing PTSD symptoms.
CBD Effects on Stress Neurocircuits and Stress Behavior
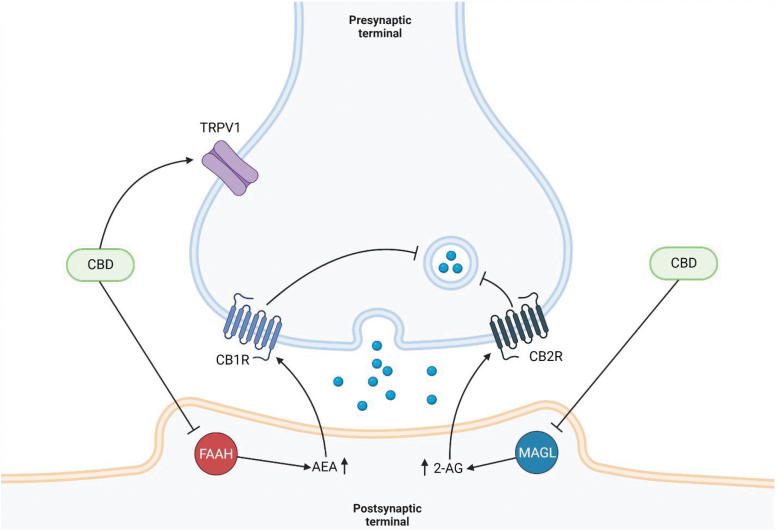
CBD may reduce the severity of PTSD-related symptoms by interacting with the HPA-eCB system.77,78 Rather than binding directly to CB1R and CB2R, as is the case with THC, CBD is a competitive inhibitor of FAAH and MAGL, binding to both enzymes at a higher affinity than either AEA or 2-AG.77,78 The resulting inhibition reduces the hydrolysis of eCBs, increasing circulating AEA and 2-AG levels throughout the body (Fig. 2).
FIG. 2.
CBD inhibition of synaptic signaling. CBD inhibits hydrolysis of AEA and 2-AG in the postsynaptic terminal by competitively binding to FAAH and MAGL, respectively. AEA and 2-AG then activate CB1R and CB2R on the presynaptic terminal, leading to suppression of neurotransmitter release. CBD can also act on the TRPV1 receptor, desensitizing TRPV1 channels and increasing Ca2+ concentrations in the presynaptic neuron. Created with BioRender.com. CB1R, cannabinoid receptor type 1; CB2R, cannabinoid receptor type 2; MAGL, monoacylglycerol lipase; TRPV1, transient receptor potential of vanilloid subtype 1.
This, in theory, means that CBD can combat dysregulation of the eCB system caused by trauma or prolonged stress and restore proper negative feedback of the HPA axis. Currently, limited clinical literature is available on utilization of CBD as a potential treatment for PTSD or other stress/anxiety-related disorders, particularly in patients who have experienced early life stress or traumas. However, some clinical studies have examined the utility of CBD in related contexts.
For example, CBD was shown to be effective in reducing anxiety in patients at high risk for psychosis, with a 600-mg dose of CBD every day for a week reducing anxiety from acute stress as well as attenuating abnormally low cortisol levels found in these patients, likely from a dysregulated HPA axis.79 Bergamaschi et al. found that a 600-mg dose of CBD reduced anxiety in participants with social anxiety disorder before they gave a public speech, compared with placebo.80
Another study found a similar reduction in anxiety in individuals with social anxiety disorder when treated with 400 mg of CBD for over 2 h.23 This reduction in anxiety correlated with decreased blood flow to brain regions associated with fear and anxiety.
While both these studies examined the effects of short-term CBD on anxiety, a longer study has also been conducted in which adolescents with social anxiety disorder received either placebo or 300 mg of CBD daily for 4 weeks.81 This study found that CBD significantly reduced anxiety symptoms compared with placebo, and self-reported levels of anxiety in the patients receiving CBD were similar to those reported in a separate study of patients taking paroxetine.82
More closely related to PTSD specifically, clinical studies into the effectiveness of CBD in fear extinction show promise, as a single dose of CBD (32 mg) given to healthy subjects postextinction significantly enhanced consolidation of extinction learning from conditioned shock.83
Given that the eCB system and fear extinction are altered in patients with PTSD, additional research will be needed to determine if CBD, potentially in conjunction with other phytocannabinoids (both synthetic and from plant extracts), may be effective in improving outcomes in PTSD patients.
Several pre-clinical studies have been conducted examining potential anxiolytic effects of CBD. A study by Uribe-Mariño et al investigated the effect of intraperitoneal administration of CBD in male Swiss mice exposed to an ethological model of panic attack caused by threatening stimuli from wild constrictor snakes.84 The results showed that pretreatment with CBD lowered active avoidance of the snake predator, indicating that CBD can reduce behavioral responses in environments containing clear and perceived danger following identification of threatening stimuli.
Bitencourt et al tested the effects of CBD on extinction of contextual fear memories in 3-month-old Wistar rats, using a conditioned foot shock model with 24-h intervals between each extinction event, and found that treatment with CBD before the extinction trials produced anxiolytic effects.85 Similar experiments were conducted by Lemos et al, with additional testing of neuronal activity in various brain regions.86
Between both these studies, mice given i.c.v. or i.v. injections of CBD (0.2, 1.0, and 2.0 μg/μL [1 μL injection volume] for Bitencourt et al; 10 mg/kg for Lemos et al) 5–30 min before an extinction trial displayed persistent reductions in percentage of freezing time compared with control mice with each successive extinction trial, with higher CBD dosages producing greater effects.85,86
The effects of CBD on fear memory extinction were antagonized by administration of a CB1 receptor antagonist 20 min before treatment, but not by a TRPV1 vanilloid receptor antagonist 5 min prior, indicating that CBD relies heavily on the CB1 receptor pathway to produce anxiolytic effects.86 Significant reductions in c-Fos expression were observed in the prelimbic and infralimbic regions of the prefrontal cortex and ventral bed nucleus of the stria terminalis, indicating reduction in neuronal activity.86
Direct microinjection of CBD into the prelimbic prefrontal cortex also reduced freezing behavior while inducing the opposite when injected into the infralimbic prefrontal cortex.86 These findings support the notion that the prelimbic prefrontal cortex is important in activation of contextual fear and represents a possible target for treatment.86
Another contextual fear memory paradigm conducted by Stern et al. found that CBD at a dose of 1.0 mg/kg i.p. administered immediately after memory retrieval could block reconsolidation of fear memories from 1 to 7 days prior, producing reduction in freezing behavior during reexposure to a foot shock-paired context.87 The same effect could not be achieved when CBD was administered 6 h or more after retrieval, nor did reduction in freezing behavior occur in the absence of memory activation, showing that the anxiolytic effects of CBD in this model are specific to reconsolidation of fear memory.
Furthermore, reconsolidation is permanently disrupted even 22 days later without reinstatement, indicating the long-term benefits of CBD treatment for fear memory extinction. The mechanisms for why this occurs are not fully understood, but may be related to increased neurogenesis in the hippocampus.88–91
Such findings are highly relevant to PTSD as PTSD patients have difficulty in extinction of fear memories related to the trauma exposure, suggesting that CBD may be an effective adjunctive to current psychiatric treatments for PTSD such as trauma-based cognitive-behavioral therapy.
Perspective: Is eCB a Viable Target for PTSD Treatment?
There has been a lot of concern regarding cannabinoids and their impact and long-term consequences on developing brains of children. Both CBD and THC are often purified from plant materials and utilized in clinical and pre-clinical studies.81 However, THC is the main euphorigenic component of cannabis and can produce negative psychotropic and anxiogenic effects, particularly at high doses.92 This may be due to THC increasing activation of the HPA axis, which results in increased levels of basal and stress-induced cortisol.18
There is especially a risk of adverse effects when administering treatments containing THC to pediatric patients as research has shown that THC administration in children reduces cognitive ability, object recognition memory, and spatial learning.93–95 CBD has been shown to counteract the negative effects of THC, such as memory impairment, anxiety, and psychotic-like symptoms, and is thought to produce stronger anxiolytic effects when in combination with THC.92 CBD may also be beneficial for healthy sleep patterns.96,97 Sleep is disrupted in PTSD, which may exacerbate symptoms, and may be worsened by THC.1,98
It should be noted that treatments utilizing purified CBD (without THC present) have been used clinically in pediatric patients with severe seizure-related disorders with minimal adverse side effects.99,100 Purified CBD has a very high safety profile and can be tolerated in doses of up to 6000 mg with minimal side effects when treating common ailments such as diarrhea, headaches, and nausea.101
CBD has also been shown to provide both antioxidant and anti-inflammatory effects, and in pre-clinical studies, it was found to work in combination with other phytocannabinoids and even different medications.102–105 However, many CBD-predominant preparations are largely unregulated and may contain THC at amounts above the lowest observed adverse effect level of 2.5 mg/day.106,107
Even a relatively low dosage of 50 mg of CBD, if given in a THC-to-CBD ratio of 1:20, can lead to undesirable side effects in patients.106 Any CBD-marketed treatment needs to be scrutinized for the presence of THC-like compounds, and treatments using both CBD and THC must consider the ratio of these two compounds to avoid the negative effects of THC while emphasizing the benefits from CBD, particularly if utilized in pediatric patient populations.
However, since early life trauma can inhibit eCB function, targeting this system using CBD or by other means may be of clinical benefit.
Summary
While much pre-clinical work has examined the effectiveness of CBD in treatment of PTSD-related behaviors, as well as in models for other psychiatric and neuropsychiatric disorders, there is a considerable dearth of clinical research in this area. Even less research has been conducted into any possible anxiolytic effects that CBD could have on symptoms caused by early life stress.
However, the research that has been performed indicates that CBD is likely to be an efficacious treatment that reduces avoidant and stress-related symptoms of PTSD while potentially minimizing adverse side effects of THC. There is more than sufficient evidence to show that the eCB system is intrinsically linked to regulation of stress responses and the associated brain regions.
Overall, pre-clinical evidence has demonstrated the need for further clinical trials into CBD as a potential treatment for PTSD and other psychiatric conditions that may be associated with disruption of the eCB system.
Acknowledgments
The authors thank Kent Vrana and the Penn State Medical Marijuana Academic Clinical Research Center for their continued support, contributions, and feedback on this article.
Abbreviations Used
- 1-AG
1-arachidonoylglycerol
- 2-AG
2-arachidonoylglycerol
- ACTH
adrenocorticotropic hormone
- AEA
N-arachidonoylethanolamine
- CB1R
cannabinoid receptor type 1
- CB2R
cannabinoid receptor type 2
- CBD
cannabidiol
- CRH
corticotropin-releasing hormone
- eCB
endocannabinoid
- FAAH
fatty acid amide hydrolase
- HPA
hypothalamic–pituitary–adrenal
- MAGL
monoacylglycerol lipase
- OEA
N-oleoylethanolamide
- PEA
N-palmitoylethanolamine
- PTSD
post-traumatic stress disorder
- PVN
paraventricular nucleus
- SEA
N-stearoylethanolamine
- SSRIs
selective serotonin reuptake inhibitors
- THC
delta-9-tetrahydrocannabinol
Author Disclosure Statement
The authors have no significant conflict of interests. Y.S. has minimal ownership of FLORA, a Vermont adult-use cannabis retailer.
Funding Information
This work was funded, in part, by an NIH grant, AA026865.
Cite this article as: Lookfong NA, Raup-Konsavage WM, Silberman Y (2023) Potential utility of cannabidiol in stress-related disorders, Cannabis and Cannabinoid Research 8:2, 230–240, DOI: 10.1089/can.2022.0130.
References
- 1. Diagnostic and statistical manual of mental disorders: DSM-5-TR. 5th ed. American Psychiatric Association Publishing: Washington, DC; 2022. [Google Scholar]
- 2. Crum KI, Flanagan JC, Vaughan B, et al. Oxytocin, PTSD, and sexual abuse are associated with attention network intrinsic functional connectivity. Psychiatry Res Neuroimaging 2021;316:111345; doi: 10.1016/j.pscychresns.2021.111345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stein MB, Kline NA, Matloff JL. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: A double-blind, placebo-controlled study. Am J Psychiatry 2002;159(10):1777–1779; doi: 10.1176/appi.ajp.159.10.1777 [DOI] [PubMed] [Google Scholar]
- 4. Grillon C, Morgan CA 3rd. Fear-potentiated startle conditioning to explicit and contextual cues in Gulf War veterans with posttraumatic stress disorder. J Abnorm Psychol 1999;108(1):134–142; doi: 10.1037//0021-843x.108.1.134 [DOI] [PubMed] [Google Scholar]
- 5. Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999;156(11):1787–1795; doi: 10.1176/ajp.156.11.1787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sripada RK, Garfinkel SN, Liberzon I. Avoidant symptoms in PTSD predict fear circuit activation during multimodal fear extinction. Front Hum Neurosci 2013;7:672; doi: 10.3389/fnhum.2013.00672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bryant RA, Felmingham KL, Kemp AH, et al. Neural networks of information processing in posttraumatic stress disorder: A functional magnetic resonance imaging study. Biol Psychiatry 2005;58(2):111–118; doi: 10.1016/j.biopsych.2005.03.021 [DOI] [PubMed] [Google Scholar]
- 8. Kessler RC. Posttraumatic stress disorder: The burden to the individual and to society. J Clin Psychiatry 2000;61(Suppl 5):4–12; discussion 13–14. [PubMed] [Google Scholar]
- 9. Child Maltreatment 2019. Administration for Children & Families. Children's Bureau; 2021. [Google Scholar]
- 10. Jeong HJ, Durham EL, Moore TM, et al. The association between latent trauma and brain structure in children. Transl Psychiatry 2021;11(1):240; doi: 10.1038/s41398-021-01357-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Davidson J, Baldwin D, Stein DJ, et al. Treatment of posttraumatic stress disorder with venlafaxine extended release: A 6-month randomized controlled trial. Arch Gen Psychiatry 2006;63(10):1158–1165. [DOI] [PubMed] [Google Scholar]
- 12. Lee DJ, Schnitzlein CW, Wolf JP, et al. Psychotherapy versus pharmacotherapy for posttraumatic stress disorder: Systemic review and meta-analyses to determine first-line treatments. Depress Anxiety 2016;33(9):792–806; doi: 10.1002/da.22511 [DOI] [PubMed] [Google Scholar]
- 13. Williams LM, Debattista C, Duchemin AM, et al. Childhood trauma predicts antidepressant response in adults with major depression: Data from the randomized international study to predict optimized treatment for depression. Transl Psychiatry 2016;6(5):e799; doi: 10.1038/tp.2016.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Schnurr PP, Friedman MJ, Engel CC, et al. Cognitive behavioral therapy for posttraumatic stress disorder in women: A randomized controlled trial. JAMA 2007;297(8):820–830; doi: 10.1001/jama.297.8.820 [DOI] [PubMed] [Google Scholar]
- 15. Ferguson JM. SSRI antidepressant medications: adverse effects and tolerability. Prim Care Companion J Clin Psychiatry 2001;3(1):22–27; doi: 10.4088/pcc.v03n0105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Alteba S, Korem N, Akirav I. Cannabinoids reverse the effects of early stress on neurocognitive performance in adulthood. Learn Mem 2016;23(7):349–358; doi: 10.1101/lm.041608.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorzalka BB, Hill MN, Hillard CJ. Regulation of endocannabinoid signaling by stress: Implications for stress-related affective disorders. Neurosci Biobehav Rev 2008;32(6):1152–1160; doi: 10.1016/j.neubiorev.2008.03.004 [DOI] [PubMed] [Google Scholar]
- 18. Patel S, Roelke CT, Rademacher DJ, et al. Endocannabinoid signaling negatively modulates stress-induced activation of the hypothalamic-pituitary-adrenal axis. Endocrinology 2004;145(12):5431–5438; doi: 10.1210/en.2004-0638 [DOI] [PubMed] [Google Scholar]
- 19. Evanson NK, Tasker JG, Hill MN, et al. Fast feedback inhibition of the HPA axis by glucocorticoids is mediated by endocannabinoid signaling. Endocrinology 2010;151(10):4811–4819; doi: 10.1210/en.2010-0285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang M, Hill MN, Zhang L, et al. Acute restraint stress enhances hippocampal endocannabinoid function via glucocorticoid receptor activation. J Psychopharmacol 2012;26(1):56–70; doi: 10.1177/0269881111409606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wilson RI, Nicoll RA. Endocannabinoid signaling in the brain. Science 2002;296(5568):678–682; doi: 10.1126/science.1063545 [DOI] [PubMed] [Google Scholar]
- 22. Koenig AM, Gao W, Umlauft M, et al. Altered hair endocannabinoid levels in mothers with childhood maltreatment and their newborns. Biol Psychol 2018;135:93–101; doi: 10.1016/j.biopsycho.2018.03.006 [DOI] [PubMed] [Google Scholar]
- 23. Crippa JAS, Derenusson GN, Ferrari TB, et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J Psychopharmacol 2011;25(1):121–130; doi: 10.1177/0269881110379283 [DOI] [PubMed] [Google Scholar]
- 24. de Meijer EPM, Bagatta M, Carboni A, et al. The inheritance of chemical phenotype in Cannabis sativa L. Genetics 2003;163(1):335–346; doi: 10.1093/genetics/163.1.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hill EL, Gallopin T, Férézou I, et al. Functional CB1 receptors are broadly expressed in neocortical GABAergic and glutamatergic neurons. J Neurophysiol 2007;97(4):2580–2589; doi: 10.1152/jn.00603.2006 [DOI] [PubMed] [Google Scholar]
- 26. McLaughlin RJ, Hill MN, Gorzalka BB. Monoaminergic neurotransmission contributes to cannabinoid-induced activation of the hypothalamic-pituitary-adrenal axis. Eur J Pharmacol 2009;624(1–3):71–76; doi: 10.1016/j.ejphar.2009.09.055 [DOI] [PubMed] [Google Scholar]
- 27. Mechoulam R, Ben-Shabat S, Hanus L, et al. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol 1995;50(1):83–90; doi: 10.1016/0006-2952(95)00109-d [DOI] [PubMed] [Google Scholar]
- 28. Sugiura T, Kondo S, Sukagawa A, et al. 2-Arachidonoylglycerol: A possible endogenous cannabinoid receptor ligand in brain. Biochem Biophys Res Commun 1995;215(1):89–97; doi: 10.1006/bbrc.1995.2437 [DOI] [PubMed] [Google Scholar]
- 29. Mulder AM, Cravatt BF. Endocannabinoid metabolism in the absence of fatty acid amide hydrolase (FAAH): Discovery of phosphorylcholine derivatives of N-acyl ethanolamines. Biochemistry 2006;45(38):11267–11277; doi: 10.1021/bi061122s [DOI] [PubMed] [Google Scholar]
- 30. Nomura DK, Hudak CSS, Ward AM, et al. Monoacylglycerol lipase regulates 2-arachidonoylglycerol action and arachidonic acid levels. Bioorg Med Chem Lett 2008;18(22):5875–5878; doi: 10.1016/j.bmcl.2008.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wilker S, Pfeiffer A, Elbert T, et al. Endocannabinoid concentrations in hair are associated with PTSD symptom severity. Psychoneuroendocrinology 2016;67:198–206; doi: 10.1016/j.psyneuen.2016.02.010 [DOI] [PubMed] [Google Scholar]
- 32. Herkenham M, Lynn AB, Johnson MR, et al. Characterization and localization of cannabinoid receptors in rat brain: A quantitative in vitro autoradiographic study. J Neurosci 1991;11(2):563–583; doi: 10.1523/JNEUROSCI.11-02-00563.1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wittmann G, Deli L, Kalló I, et al. Distribution of type 1 cannabinoid receptor (CB1)-immunoreactive axons in the mouse hypothalamus. J Comp Neurol 2007;503(2):270–279; doi: 10.1002/cne.21383 [DOI] [PubMed] [Google Scholar]
- 34. Rivier C, Vale W. Modulation of stress-induced ACTH release by corticotropin-releasing factor, catecholamines and vasopressin. Nature 1983;305(5932):325–327; doi: 10.1038/305325a0 [DOI] [PubMed] [Google Scholar]
- 35. Vale W, Spiess J, Rivier C, et al. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981;213(4514):1394–1397; doi: 10.1126/science.6267699 [DOI] [PubMed] [Google Scholar]
- 36. Hurley KM, Herbert H, Moga MM, et al. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol 1991;308(2):249–276; doi: 10.1002/cne.903080210 [DOI] [PubMed] [Google Scholar]
- 37. McEwen BS, Weiss JM, Schwartz LS. Selective retention of corticosterone by limbic structures in rat brain. Nature 1968;220(5170):911–912; doi: 10.1038/220911a0 [DOI] [PubMed] [Google Scholar]
- 38. Sesack SR, Deutch AY, Roth RH, et al. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: An anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. J Comp Neurol 1989;290(2):213–242; doi: 10.1002/cne.902900205 [DOI] [PubMed] [Google Scholar]
- 39. Lowry PJ, Estivariz FE, Gillies GE, et al. CRF: Its regulation of ACTH and pro-opiomelanocortin peptide release and its extra hypothalamic occurrence. Acta Endocrinol Suppl (Copenh) 1986;276:56–62; doi: 10.1530/acta.0.111s0056 [DOI] [PubMed] [Google Scholar]
- 40. Gorrigan RJ, Guasti L, King P, et al. Localisation of the melanocortin-2-receptor and its accessory proteins in the developing and adult adrenal gland. J Mol Endocrinol 2011;46(3):227–232; doi: 10.1530/JME-11-0011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience 1990;39(3):579–604; doi: 10.1016/0306-4522(90)90244-x [DOI] [PubMed] [Google Scholar]
- 42. Herman JP, Patel PD, Akil H, et al. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol Endocrinol 1989;3(11):1886–1894; doi: 10.1210/mend-3-11-1886 [DOI] [PubMed] [Google Scholar]
- 43. Krugers HJ, Douma BR, Andringa G, et al. Exposure to chronic psychosocial stress and corticosterone in the rat: Effects on spatial discrimination learning and hippocampal protein kinase Cgamma immunoreactivity. Hippocampus 1997;7(4):427–436; doi: [DOI] [PubMed] [Google Scholar]
- 44. Roozendaal B, de Quervain DJ, Ferry B, et al. Basolateral amygdala-nucleus accumbens interactions in mediating glucocorticoid enhancement of memory consolidation. J Neurosci 2001;21(7):2518–2525; doi: 10.1523/JNEUROSCI.21-07-02518.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gray JM, Vecchiarelli HA, Morena M, et al. Corticotropin-releasing hormone drives anandamide hydrolysis in the amygdala to promote anxiety. J Neurosci 2015;35(9):3879–3892; doi: 10.1523/JNEUROSCI.2737-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Gray JM, Wilson CD, Lee TTY, et al. Sustained glucocorticoid exposure recruits cortico-limbic CRH signaling to modulate endocannabinoid function. Psychoneuroendocrinology 2016;66:151–158; doi: 10.1016/j.psyneuen.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bhatnagar S, Huber R, Nowak N, et al. Lesions of the posterior paraventricular thalamus block habituation of hypothalamic-pituitary-adrenal responses to repeated restraint. J Neuroendocrinol 2002;14(5):403–410; doi: 10.1046/j.0007-1331.2002.00792.x [DOI] [PubMed] [Google Scholar]
- 48. Hill MN, Karatsoreos IN, Hillard CJ, et al. Rapid elevations in limbic endocannabinoid content by glucocorticoid hormones in vivo. Psychoneuroendocrinology 2010;35(9):1333–1338; doi: 10.1016/j.psyneuen.2010.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Hill MN, McLaughlin RJ, Pan B, et al. Recruitment of prefrontal cortical endocannabinoid signaling by glucocorticoids contributes to termination of the stress response. J Neurosci 2011;31(29):10506–10515; doi: 10.1523/JNEUROSCI.0496-11.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci 1993;13(9):3839–3847; doi: 10.1523/JNEUROSCI.13-09-03839.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hill MN, McLaughlin RJ, Bingham B, et al. Endogenous cannabinoid signaling is essential for stress adaptation. Proc Natl Acad Sci U S A 2010;107(20):9406–9411; doi: 10.1073/pnas.0914661107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hill MN, Bierer LM, Makotkine I, et al. Reductions in circulating endocannabinoid levels in individuals with post-traumatic stress disorder following exposure to the World Trade Center attacks. Psychoneuroendocrinology 2013;38(12):2952–2961; doi: 10.1016/j.psyneuen.2013.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Neumeister A, Normandin MD, Pietrzak RH, et al. Elevated brain cannabinoid CB1 receptor availability in post-traumatic stress disorder: A positron emission tomography study. Mol Psychiatry 2013;18(9):1034–1040; doi: 10.1038/mp.2013.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hauer D, Schelling G, Gola H, et al. Plasma concentrations of endocannabinoids and related primary fatty acid amides in patients with post-traumatic stress disorder. PLoS One 2013;8(5):e62741; doi: 10.1371/journal.pone.0062741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Yi B, Nichiporuk I, Nicolas M, et al. Reductions in circulating endocannabinoid 2-arachidonoylglycerol levels in healthy human subjects exposed to chronic stressors. Prog Neuropsychopharmacol Biol Psychiatry 2016;67:92–97; doi: 10.1016/j.pnpbp.2016.01.004 [DOI] [PubMed] [Google Scholar]
- 56. Schaefer C, Enning F, Mueller JK, et al. Fatty acid ethanolamide levels are altered in borderline personality and complex posttraumatic stress disorders. Eur Arch Psychiatry Clin Neurosci 2014;264(5):459–463; doi: 10.1007/s00406-013-0470-8 [DOI] [PubMed] [Google Scholar]
- 57. Dubreucq S, Matias I, Cardinal P, et al. Genetic dissection of the role of cannabinoid type-1 receptors in the emotional consequences of repeated social stress in mice. Neuropsychopharmacology 2012;37(8):1885–1900; doi: 10.1038/npp.2012.36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. McLaughlin RJ, Hill MN, Bambico FR, et al. Prefrontal cortical anandamide signaling coordinates coping responses to stress through a serotonergic pathway. Eur Neuropsychopharmacol 2012;22(9):664–671; doi: 10.1016/j.euroneuro.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hill MN, Carrier EJ, McLaughlin RJ, et al. Regional alterations in the endocannabinoid system in an animal model of depression: Effects of concurrent antidepressant treatment. J Neurochem 2008;106(6):2322–2336; doi: 10.1111/j.1471-4159.2008.05567.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Hill MN, McLaughlin RJ, Morrish AC, et al. Suppression of amygdalar endocannabinoid signaling by stress contributes to activation of the hypothalamic-pituitary-adrenal axis. Neuropsychopharmacology 2009;34(13):2733–2745; doi: 10.1038/npp.2009.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lomazzo E, Bindila L, Remmers F, et al. Therapeutic potential of inhibitors of endocannabinoid degradation for the treatment of stress-related hyperalgesia in an animal model of chronic pain. Neuropsychopharmacology 2015;40(2):488–501; doi: 10.1038/npp.2014.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sumislawski JJ, Ramikie TS, Patel S. Reversible gating of endocannabinoid plasticity in the amygdala by chronic stress: A potential role for monoacylglycerol lipase inhibition in the prevention of stress-induced behavioral adaptation. Neuropsychopharmacology 2011;36(13):2750–2761; doi: 10.1038/npp.2011.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lee TT-Y, Hill MN, Hillard CJ, et al. Disruption of peri-adolescent endocannabinoid signaling modulates adult neuroendocrine and behavioral responses to stress in male rats. Neuropharmacology 2015;99:89–97; doi: 10.1016/j.neuropharm.2015.07.021 [DOI] [PubMed] [Google Scholar]
- 64. Spiacci GBL, Antero LS, Reis DG, et al. Dorsal hippocampus cannabinoid type 1 receptors modulate the expression of contextual fear conditioning in rats: Involvement of local glutamatergic/nitrergic and GABAergic neurotransmissions. Eur Neuropsychopharmacol 2016;26(10):1579–1589; doi: 10.1016/j.euroneuro.2016.08.010 [DOI] [PubMed] [Google Scholar]
- 65. Lee TT, Hill MN. Age of stress exposure modulates the immediate and sustained effects of repeated stress on corticolimbic cannabinoid CB1 receptor binding in male rats. Neuroscience 2013;249:106–114; doi: 10.1016/j.neuroscience.2012.11.017 [DOI] [PubMed] [Google Scholar]
- 66. Reich CG, Mihalik GR, Iskander AN, et al. Adolescent chronic mild stress alters hippocampal CB1 receptor-mediated excitatory neurotransmission and plasticity. Neuroscience 2013;253:444–454; doi: 10.1016/j.neuroscience.2013.08.066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heng L, Beverley JA, Steiner H, et al. Differential developmental trajectories for CB1 cannabinoid receptor expression in limbic/associative and sensorimotor cortical areas. Synapse 2011;65(4):278–286; doi: 10.1002/syn.20844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Litvin Y, Phan A, Hill MN, et al. CB1 receptor signaling regulates social anxiety and memory. Genes Brain Behav 2013;12(5):479–489; doi: 10.1111/gbb.12045 [DOI] [PubMed] [Google Scholar]
- 69. Campos AC, Ferreira FR, da Silva WA, et al. Predator threat stress promotes long lasting anxiety-like behaviors and modulates synaptophysin and CB1 receptors expression in brain areas associated with PTSD symptoms. Neurosci Lett 2013;533:34–38; doi: 10.1016/j.neulet.2012.11.016 [DOI] [PubMed] [Google Scholar]
- 70. Hill MN, Eiland L, Lee TTY, et al. Early life stress alters the developmental trajectory of corticolimbic endocannabinoid signaling in male rats. Neuropharmacology 2019;146:154–162; doi: 10.1016/j.neuropharm.2018.11.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Vangopoulou C, Bourmpoula MT, Koupourtidou C, et al. Effects of an early life experience on rat brain cannabinoid receptors in adolescence and adulthood. IBRO Rep 2018;5:1–9; doi: 10.1016/j.ibror.2018.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Marco EM, Echeverry-Alzate V, López-Moreno JA, et al. Consequences of early life stress on the expression of endocannabinoid-related genes in the rat brain. Behav Pharmacol 2014;25(5–6):547–556; doi: 10.1097/FBP.0000000000000068 [DOI] [PubMed] [Google Scholar]
- 73. Dow-Edwards D, Frank A, Wade D, et al. Sexually-dimorphic alterations in cannabinoid receptor density depend upon prenatal/early postnatal history. Neurotoxicol Teratol 2016;58:31–39; doi: 10.1016/j.ntt.2016.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rubino T, Realini N, Castiglioni C, et al. Role in anxiety behavior of the endocannabinoid system in the prefrontal cortex. Cereb Cortex 2008;18(6):1292–1301; doi: 10.1093/cercor/bhm161 [DOI] [PubMed] [Google Scholar]
- 75. Pignatelli D, Xiao F, Gouveia AM, et al. Adrenarche in the rat. J Endocrinol 2006;191(1):301–308; doi: 10.1677/joe.1.06972 [DOI] [PubMed] [Google Scholar]
- 76. Romeo RD, Bellani R, Karatsoreos IN, et al. Stress history and pubertal development interact to shape hypothalamic-pituitary-adrenal axis plasticity. Endocrinology 2006;147(4):1664–1674; doi: 10.1210/en.2005-1432 [DOI] [PubMed] [Google Scholar]
- 77. Bisogno T, Hanus L, De Petrocellis L, et al. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br J Pharmacol 2001;134(4):845–852; doi: 10.1038/sj.bjp.0704327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. De Petrocellis L, Ligresti A, Moriello AS, et al. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol 2011;163(7):1479–1494; doi: 10.1111/j.1476-5381.2010.01166.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Appiah-Kusi E, Petros N, Wilson R, et al. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology (Berl) 2020;237(4):1121–1130; doi: 10.1007/s00213-019-05442-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bergamaschi MM, Queiroz RHC, Chagas MHN, et al. Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 2011;36(6):1219–1226; doi: 10.1038/npp.2011.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Masataka N. Anxiolytic effects of repeated cannabidiol treatment in teenagers with social anxiety disorders. Front Psychol 2019;10:2466; doi: 10.3389/fpsyg.2019.02466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Nordahl HM, Vogel PA, Morken G, et al. Paroxetine, cognitive therapy or their combination in the treatment of social anxiety disorder with and without avoidant personality disorder: A randomized clinical trial. Psychother Psychosom 2016;85(6):346–356; doi: 10.1159/000447013 [DOI] [PubMed] [Google Scholar]
- 83. Das RK, Kamboj SK, Ramadas M, et al. Cannabidiol enhances consolidation of explicit fear extinction in humans. Psychopharmacology (Berl) 2013;226(4):781–792; doi: 10.1007/s00213-012-2955-y [DOI] [PubMed] [Google Scholar]
- 84. Uribe-Mariño A, Francisco A, Castiblanco-Urbina MA, et al. Anti-aversive effects of cannabidiol on innate fear-induced behaviors evoked by an ethological model of panic attacks based on a prey vs the wild snake Epicrates cenchria crassus confrontation paradigm. Neuropsychopharmacology 2012;37(2):412–421; doi: 10.1038/npp.2011.188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Bitencourt RM, Pamplona FA, Takahashi RN. Facilitation of contextual fear memory extinction and anti-anxiogenic effects of AM404 and cannabidiol in conditioned rats. Eur Neuropsychopharmacol 2008;18(12):849–859; doi: 10.1016/j.euroneuro.2008.07.001 [DOI] [PubMed] [Google Scholar]
- 86. Lemos JI, Resstel LB, Guimarães FS. Involvement of the prelimbic prefrontal cortex on cannabidiol-induced attenuation of contextual conditioned fear in rats. Behav Brain Res 2010;207(1):105–111; doi: 10.1016/j.bbr.2009.09.045 [DOI] [PubMed] [Google Scholar]
- 87. Stern CAJ, Gazarini L, Takahashi RN, et al. On disruption of fear memory by reconsolidation blockade: Evidence from cannabidiol treatment. Neuropsychopharmacology 2012;37(9):2132–2142; doi: 10.1038/npp.2012.63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Campos AC, Ortega Z, Palazuelos J, et al. The anxiolytic effect of cannabidiol on chronically stressed mice depends on hippocampal neurogenesis: Involvement of the endocannabinoid system. Int J Neuropsychopharmacol 2013;16(6):1407–1419; doi: 10.1017/S1461145712001502 [DOI] [PubMed] [Google Scholar]
- 89. Fogaça MV, Campos AC, Coelho LD, et al. The anxiolytic effects of cannabidiol in chronically stressed mice are mediated by the endocannabinoid system: Role of neurogenesis and dendritic remodeling. Neuropharmacology 2018;135:22–33; doi: 10.1016/j.neuropharm.2018.03.001 [DOI] [PubMed] [Google Scholar]
- 90. Xu C, Chang T, Du Y, et al. Pharmacokinetics of oral and intravenous cannabidiol and its antidepressant-like effects in chronic mild stress mouse model. Environ Toxicol Pharmacol 2019;70:103202; doi: 10.1016/j.etap.2019.103202 [DOI] [PubMed] [Google Scholar]
- 91. Wolf SA, Bick-Sander A, Fabel K, et al. Cannabinoid receptor CB1 mediates baseline and activity-induced survival of new neurons in adult hippocampal neurogenesis. Cell Commun Signal 2010;8:12; doi: 10.1186/1478-1811X-8-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Todd SM, Arnold JC. Neural correlates of interactions between cannabidiol and Δ(9)-tetrahydrocannabinol in mice: Implications for medical cannabis. Br J Pharmacol 2016;173(1):53–65; doi: 10.1111/bph.13333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Cha YM, White AM, Kuhn CM, et al. Differential effects of delta9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 2006;83(3):448–455; doi: 10.1016/j.pbb.2006.03.006 [DOI] [PubMed] [Google Scholar]
- 94. Quinn HR, Matsumoto I, Callaghan PD, et al. Adolescent rats find repeated delta(9)-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 2008;33(5):1113–1126; doi: 10.1038/sj.npp.1301475 [DOI] [PubMed] [Google Scholar]
- 95. Stiglick A, Kalant H. Residual effects of chronic cannabis treatment on behavior in mature rats. Psychopharmacology (Berl) 1985;85(4):436–439; doi: 10.1007/BF00429660 [DOI] [PubMed] [Google Scholar]
- 96. Chagas MH, Crippa JA, Zuardi AW, et al. Effects of acute systemic administration of cannabidiol on sleep-wake cycle in rats. J Psychopharmacol 2013;27(3):312–316; doi: 10.1177/0269881112474524 [DOI] [PubMed] [Google Scholar]
- 97. Hsiao YT, Yi PL, Li CL, et al. Effect of cannabidiol on sleep disruption induced by the repeated combination tests consisting of open field and elevated plus-maze in rats. Neuropharmacology 2012;62(1):373–384; doi: 10.1016/j.neuropharm.2011.08.013 [DOI] [PubMed] [Google Scholar]
- 98. Nicholson AN, Turner C, Stone BM, et al. Effect of delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults. J Clin Psychopharmacol 2004;24(3):305–313; doi: 10.1097/01.jcp.0000125688.05091.8f [DOI] [PubMed] [Google Scholar]
- 99. Devinsky O, Cross JH, Wright S. Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med 2017;377(7):699–700; doi: 10.1056/NEJMc1708349 [DOI] [PubMed] [Google Scholar]
- 100. Devinsky O, Patel AD, Cross JH, et al. Effect of cannabidiol on drop seizures in the Lennox-Gastaut syndrome. N Engl J Med 2018;378(20):1888–1897; doi: 10.1056/NEJMoa1714631 [DOI] [PubMed] [Google Scholar]
- 101. Taylor L, Gidal B, Blakey G, et al. A phase I, randomized, double-blind, placebo-controlled, single ascending dose, multiple dose, and food effect trial of the safety, tolerability and pharmacokinetics of highly purified cannabidiol in healthy subjects. CNS Drugs 2018;32(11):1053–1067; doi: 10.1007/s40263-018-0578-5.Erratumin:CNSDrugs2019;33(4):397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. da Silva VK, de Freitas BS, Garcia RCL, et al. Antiapoptotic effects of cannabidiol in an experimental model of cognitive decline induced by brain iron overload. Transl Psychiatry 2018;8(1):176; doi: 10.1038/s41398-018-0232-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Petrosino S, Verde R, Vaia M, et al. Anti-inflammatory properties of cannabidiol, a nonpsychotropic cannabinoid, in experimental allergic contact dermatitis. J Pharmacol Exp Ther 2018;365(3):652–663; doi: 10.1124/jpet.117.244368 [DOI] [PubMed] [Google Scholar]
- 104. Wong H, Cairns BE. Cannabidiol, cannabinol and their combinations act as peripheral analgesics in a rat model of myofascial pain. Arch Oral Biol 2019;104:33–39; doi: 10.1016/j.archoralbio.2019.05.028 [DOI] [PubMed] [Google Scholar]
- 105. Neelakantan H, Tallarida RJ, Reichenbach ZW, et al. Distinct interactions of cannabidiol and morphine in three nociceptive behavioral models in mice. Behav Pharmacol 2015;26(3):304–314; doi: 10.1097/FBP.0000000000000119 [DOI] [PubMed] [Google Scholar]
- 106. Lachenmeier DW, Habel S, Fischer B, et al. Are adverse effects of cannabidiol (CBD) products caused by tetrahydrocannabinol (THC) contamination? F1000Res 2019;8:1394; doi: 10.12688/f1000research.19931.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Bonn-Miller MO, Loflin MJE, Thomas BF, et al. Labeling accuracy of cannabidiol extracts sold online. JAMA 2017;318(17):1708–1709; doi: 10.1001/jama.2017.11909 [DOI] [PMC free article] [PubMed] [Google Scholar]